Abstract
Apolipoprotein C3 (apoC3) and angiopoietin-like protein 3 (ANGPTL3) inhibit lipolysis by lipoprotein lipase and may influence the secretion and uptake of various lipoproteins. Genetic studies show that depletion of these proteins is associated with improved lipid profiles and reduced cardiovascular events so it was anticipated that drugs which mimic the effects of loss-of-function mutations would be useful lipid treatments. ANGPTL3 inhibitors were initially developed as a treatment for severe hypertriglyceridaemia including familial chylomicronaemia syndrome (FCS), which is usually not adequately controlled with currently available drugs. However, it was found ANGPTL3 inhibitors were also effective in reducing low-density lipoprotein cholesterol (LDL-C) and they were studied in patients with homozygous familial hypercholesterolaemia (FH). Evinacumab targets ANGPTL3 and reduced LDL-C by about 50% in patients with homozygous FH and it has been approved for that indication. The antisense oligonucleotide (ASO) vupanorsen targeting ANGPTL3 was less effective in reducing LDL-C in patients with moderate hypertriglyceridaemia and its development has been discontinued but the small interfering RNA (siRNA) ARO-ANG3 is being investigated in Phase 2 studies. ApoC3 can be inhibited by the ASO volanesorsen, which reduced triglycerides by >70% in patients with FCS and it was approved for FCS in Europe but not in the United States because of concerns about thrombocytopaenia. Olezarsen is an N-acetylgalactosamine-conjugated ASO targeting apoC3 which appears as effective as volanesorsen without the risk of thrombocytopaenia and is undergoing Phase 3 trials. ARO-APOC3 is an siRNA targeting apoC3 that is currently being investigated in Phase 3 studies.
Keywords: Angiopoietin-like protein 3, Antisense oligonucleotides, Apolipoprotein C-III, Monoclonal antibody, Triglycerides
INTRODUCTION
Dyslipidaemia is a major risk factor for atherosclerotic cardiovascular disease (ASCVD).1 Low-density lipoprotein cholesterol (LDL-C) is the primary target for treatment as the evidence from genetic, epidemiologic, and clinical studies overwhelmingly supports it being a cause of ASCVD.2 The initial approach for all dyslipidaemias should involve dietary and lifestyle modification and optimal control of predisposing conditions, particularly obesity and diabetes. All current guidelines for the management of dyslipidaemias recommend treatment to reduce the LDL-C level to a target level depending on the overall cardiovascular risk, using statins initially and if necessary the addition of ezetimibe and/or proprotein convertase subtilisin/kexin type 9 inhibitors.1,3 Bempedoic acid, which inhibits ATP-citrate lyase, an enzyme upstream of 3-hydroxy-3-methyl-glutaryl-coenzyme A, the target of statins, has recently been shown to reduce cardiovascular events in statin intolerant patients.4 This offers an alternative treatment to statins or could be used in addition to statins and other medications if the LDL-C target has not been achieved.
Other lipid fractions which have been shown to contribute to ASCVD include triglyceride-rich lipoproteins (TRL) and lipoprotein(a) [Lp(a)] and various treatments are available or in development to target these.5 Genetic and epidemiologic studies have shown TRL and their remnants are important contributors to ASCVD whereas severe hypertriglyceridaemia, with serum triglycerides (TG) >10 mmol/L (>880 mg/dL) in European guidelines, is a risk for acute pancreatitis.6,7 Mendelian randomization studies showed that genetic scores composed of TG-lowering variants in the lipoprotein lipase (LPL) gene and LDL-C-lowering variants in the LDL receptor (LDLR) gene were associated with similar lower risk of coronary heart disease (CHD) per 10 mg/dL lower level of apolipoprotein B (apoB), suggesting the clinical benefit of lowering TG and LDL-C levels may be proportional to the absolute change in apoB, which reflects the number of atherogenic particles.8
Similarly, a meta-regression analysis of 3 classes of lipid-lowering therapies that reduce TGs to a greater extent than they do LDL-C, fibrates, niacin, and marine-derived omega-3 fatty acids, showed the reduction for major vascular events was related to the change in non-high-density lipoprotein cholesterol (non-HDL-C), which represents the cholesterol carried in LDL particles together with that in TRL particles and is usually strongly correlated with apoB levels.9 The benefits of omega-3 fatty acids, particularly high-dose eicosapentaenoic acid in the REDUCE-IT study,10 appeared to be greater than predicted from their lipid-lowering effects in that analysis.
Fasting TG levels >1.7 mmol/L (>150 mg/dL) are considered abnormal by most lipid guidelines, but in the European lipid guidelines pharmacotherapy is only recommended when TGs are >2.3 mmol/L (>200 mg/dL) for primary prevention patients or high-risk patients who are at LDL-C goal and then fenofibrate or bezafibrate may be considered in combination with statins.1 In high-risk patients with persistently elevated TGs at 1.5–5.6 mmol/L (135–499 mg/dL) despite statin treatment, omega-3 fatty acids (icosapent ethyl 2×2 g daily) are recommended.1 American guidelines recommend adding a fibrate or omega-3 fatty acid to reduce the risk of acute pancreatitis in patients with severe hypertriglyceridaemia if TGs remain ≥5.6 mmol/L (≥500 mg/dL) after managing underlying conditions and following a very low fat diet.11
Genetic studies, such as the Mendelian randomization study mentioned above,8 have identified the genes for several proteins that influence TG levels and the risk of ASCVD. Many of these affect the activity of LPL and two that have emerged as targets for inhibition are apolipoprotein C3 (apoC3) and angiopoietin-like protein 3 (ANGPTL3).12
ANGPTL3
1. Function of ANGPTL3
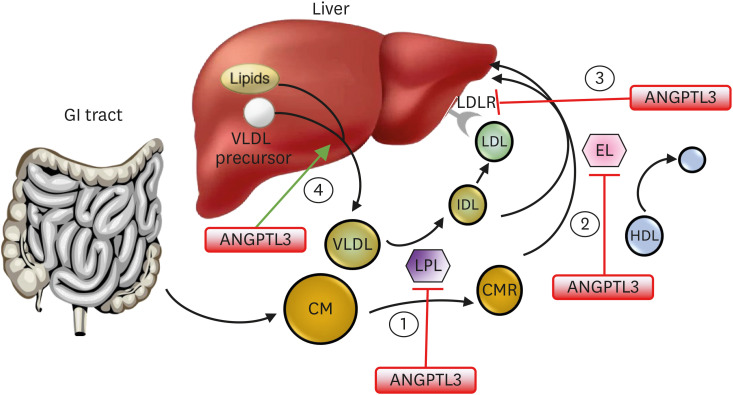
ANGPTL3 is a 460-amino-acid polypeptide that is expressed principally in the liver and was first identified in 1999.13 It was shown to inhibit LPL activity and increase TG-rich very low-density lipoprotein (VLDL).14 It can also increase high-density lipoprotein cholesterol (HDL-C) levels by inhibiting endothelial lipase (EL, encoded by LIPG) activity.15 ANGPTL3 may also influence liver secretion of TG in VLDL and hepatic removal of LDL particles via either the LDLR or through endothelial lipase-dependent clearance of VLDL and remnant particles (Fig. 1).12,16,17
Fig. 1. Action of ANGPTL3 in lipoprotein metabolism. ANGPTL3 inhibits 1) lipoprotein lipase and 2) endothelial lipase. It may also 3) inhibit the liver uptake of LDL particles and 4) stimulate release of VLDL particles from the liver. Inhibition of ANGPTL3 reduces triglycerides from chylomicron and VLDL remnants, reduces HDL-C, and in the absence of functioning LDL receptors, remnant particles and LDL can be taken up by the liver via an endothelial lipase-dependent mechanism.
ANGPTL3, angiopoietin-like protein 3; CM, chylomicron; CMR, chylomicron remnant; EL, endothelial lipase; HDL, high-density lipoprotein; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; LPL, lipoprotein lipase; VLDL, very low-density lipoprotein.
The reduced-function N396S mutation in LIPG results in a large increase in HDL-C and a much smaller increase in LDL-C and this was associated with a significant reduction in CAD in a recent Mendelian randomization study.18 Therefore, increasing activity of EL by removing ANGPTL3 inhibition should reduce LDL-C but may also reduce HDL-C levels so the overall benefit may vary in different patient groups.
Other angiopoietin-like proteins also influence LPL activity in an organ specific manner. Angiopoietin-like protein 4 (ANGPTL4) is produced in adipose tissue during fasting and inhibits the lipolytic action of LPL and the uptake of fatty acids in adipose tissue.19 Angiopoietin-like protein 8 (ANGPTL8) is synthesized in the liver and adipose tissue and levels are low during fasting and increase during feeding, mediated by insulin, when it blocks the inhibitory effect of ANGPTL4 on adipose tissue LPL through formation of ANGPTL4/ANGPTL8 complexes, and at the same time, ANGPTL8 increases the inhibitory effect of ANGPTL3 on LPL in muscle by forming ANGPTL3/ANGPTL8 complexes consequently increasing the uptake of fatty acids in adipose tissue and reducing uptake in muscle in the postprandial state.20
Loss-of-function variants in the ANGPTL3 gene are associated with lower plasma levels of TGs, LDL-C and HDL-C and decreased odds of ASCVD, so it was assumed that drugs that inhibited ANGPTL3 would simulate the effects of these genetic variants and would be clinically useful, although the effect on HDL-C might reduce the benefit in some people.21,22
Carriers of inactivating mutations in the ANGPTL4 gene had lower levels of TGs and a lower risk of CHD than did noncarriers,23 and inhibition of Angptl4 in mice and monkeys with the monoclonal antibody (mAb) REGN1001 also resulted in corresponding reductions in these lipid values. However, in mice and monkeys treated with REGN1001 there was accumulation of lipids in the mesenteric lymph nodes with abdominal lymphadenopathy secondary to granulomatous lipid accumulation. Previous studies of Angptl4 knockout mice or treating mice with the anti-Angptl4 mAb 14D12 identified lipogranulomatous lesions of the intestines and their draining lymphatics and mesenteric lymph nodes.24 These findings suggested that ANGPTL4 may not be a useful target for therapy.
Studies with REGN3776, a fully human mAb that can bind monkey and human ANGPTL8 with a high affinity, showed it could reduce plasma TG by up-regulating LPL activity in humanized ANGPTL8 mice and reduced body weight and fat content.25 In addition, blocking ANGPTL8 by this antibody reduced serum TG and increased serum HDL-C in dyslipidaemic cynomolgus monkeys suggesting that inhibition of ANGPTL8 may provide a useful therapeutic target.
Various strategies have been developed to pharmacologically inactivate ANGPTL3 for treatment of dyslipidaemia, which include the blocking mAb evinacumab, the antisense oligonucleotide (ASO) vupanorsen, and the small interfering RNA (siRNA) ARO-ANG3 (Table 1). Other strategies for pharmacological inactivation of ANGPTL3 might include a CRISPR genome editing system, such as base editor 3, and the development of oral, small-molecule inhibitors may be possible in the future.26
Table 1. Inhibitors of angiopoietin-like protein 3.
| Agent | Mode of action | Dose | Efficacy | Comments |
|---|---|---|---|---|
| Evinacumab | Monoclonal antibody | 15 mg/kg IV Q4W | ↓ LDL-C up to 56% | Approved for HoFH |
| ↓ TG up to 88% | ||||
| Vupanorsen | ASO | 80–160 mg SC Q4W | ↓ LDL-C up to 33% | Development discontinued |
| 60–160 mg SC Q2W | ↓ TG up to 63% | |||
| ARO-ANG3 | RNAi | 50–300 mg SC Q12W or less | ↓ LDL-C up to 54% | Phase 2 studies ongoing |
| ↓ TG up to 65% |
QnW, given every n weeks; IV, intravenous; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; HoFH, homozygous familial hypercholesterolaemia; ASO, antisense oligonucleotide; SC, subcutaneous; RNAi, RNA interference.
2. Evinacumab
Evinacumab (REGN1500, Evkeeza®, Regeneron Pharmaceuticals, Inc.) is a fully human mAb with a human IgG4 constant region. It was developed using Regeneron’s Velocimmune® technology platform.27 It has high affinity to ANGPTL3 from mouse, rat, monkey, and humans. It binds to ANGPTL3 in the circulation creating immune complexes and it is likely that the primary mechanism of action would be potentiation of plasma lipase activity.28 Clinical studies at Phase 1 to 3 have been completed (Table 2).
Table 2. Evinacumab clinical development program.
| Study name, reference | Patients and sample size | Treatment | Change in LDL-C (%) or other outcome | |
|---|---|---|---|---|
| Phase 1 | ||||
| Dewey et al., 2017 (NCT01749878)22 | Healthy with high TG and/or LDL-C n=83. SAD | SC 75, 150, 250 mg, IV 5, 10, 20 mg/kg, SC or IV placebo | −5.25% to −23.19% placebo corrected LS mean at day 15 | |
| Ahmad et al., 2019 (NCT02107872)29 | Healthy with high TG and/or LDL-C n=56. MAD | SC 150, 300, 450 mg Q1W | −22.0% with 300 mg Q1W SC, −25.1% with 20 mg/kg IV Maximum placebo corrected LS mean | |
| SC 300, 450 mg Q2W | ||||
| IV 20 mg/kg Q4W | ||||
| Harada-Shiba M et al., 2020 (NCT03146416)30 | Healthy subjects 12 Japanese; 12 Caucasian per cohort×4 | SC 300 mg×1 | −18.5%/−14.2% | |
| SC 300 mg Q1W×8 | −49.8%/−35.8% | |||
| SC placebo | −5.0%/+11.0% | |||
| IV 5 mg/kg Q4W×2 | −17.7%/−18.9% | |||
| IV 15 mg/kg Q4W×2 | −28.6%/−40.2% | |||
| IV placebo | +4.9%/13.7% | |||
| Japanese/Caucasian at wk 8 | ||||
| Phase 2 | ||||
| Gaudet et al., 2017 (NCT02265952)31 | 9 HoFH | 250 mg SC on day 1, 15 mg/kg IV on day 15 | −49% (range, 25% to 90%) at wk 4 | |
| Rosenson et al., 2023 (NCT03452228)32 | Severe hypertriglyceridaemia. n=51 | 15 mg/kg Q4W for 12 wk | Variable response depending on LPL mutations | |
| Phase 3 | ||||
| Raal et al., 2020, ELIPSE-HoFH (NCT03399786)33 | HoFH n=65 | IV 15 mg/kg Q4W placebo | −49.0% (95% CI, −65.0% to −33.1%) | |
| Rosenson et al., 2020 (NCT03175367)34 | HeFH or refractory high LDL-C n=272 | SC 450 mg Q1W | −56.0% vs. placebo | |
| SC 300 mg Q1W | −52.9% vs. placebo | |||
| SC 300 mg Q2W SC placebo | −38.5% vs. placebo | |||
| IV 15 mg/kg Q4W | −50.5% vs. placebo | |||
| IV 5 mg/kg Q4W IV placebo | −24.2% vs. placebo | |||
Values given as mean with range or 95% confidence intervals where available.
LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; SAD, single ascending dose; SC, subcutaneous; IV, intravenous; LS, least square; MAD, multiple ascending dose; QnW, every n weeks; HoFH, homozygous familial hypercholesterolemia; HeFH, heterozygous familial hypercholesterolemia.
A Phase 1 single ascending dose (SAD) study in 83 human volunteers with moderately high TG >1.7 but ≤5.1 mmol/L (>150 but ≤450 mg/dL) and/or LDL-C ≥2.6 mmol/L (≥100 mg/dL) showed that evinacumab produced dose-dependent reductions in TG up to 76%, in LDL-C up to 23% and in HDL-C up to 25% compared to placebo (NCT01749878).22 Results of the SAD study in 83 subjects and a Phase 1 multiple ascending dose (MAD) study in 56 dyslipidaemic subjects (NCT02107872) were reported by Ahmad et al.29 Evinacumab 150/300/450 mg once weekly, and 300/450 mg every 2 weeks was given by subcutaneous (SC) injection or 20 mg/kg once every 4 weeks by intravenous (IV) injection. The maximum reduction in TG was 83.1% at day 2 with 20 mg/kg IV once every 4 weeks.29 There were reductions in LDL-C up to 25.1% in the MAD study and HDL-C was reduced to a variable extent with the SC doses and by 6.2% versus placebo with evinacumab IV at 20 mg/kg Q4W.
In a Phase 1 study comparing the pharmacokinetics, pharmacodynamics, safety, and tolerability of evinacumab in four dose cohorts between healthy Japanese and Caucasian adults with LDL-C ≥2.6 and <4.1 mmol/L (≥100 and <160 mg/dL), there were comparable pharmacokinetic profiles for both SC and IV doses of evinacumab in both ethnic groups and it was generally well tolerated with similar dose-related reductions in LDL-C and TGs in both groups.30
In a Phase 2 proof-of-concept, open-label study in 9 adults with homozygous familial hypercholesterolemia (HoFH) who were already taking aggressive lipid-lowering therapy, evinacumab was given as 250 mg SC x 1 on day 1, 15 mg/kg IV × 1 on day 15, and 450 mg SC weekly × 4, starting on day 85 (NCT02265952).31 At week 4, the mean (±SD) reductions in lipids levels were 49±23% in LDL-C, 46±18% in apoB, 49±22% in non-HDL cholesterol, 36±16% in HDL-C and a median of 47% (interquartile range, 38% to 57%) in TGs. Further analysis of this study showed that evinacumab was effective in lowering LDL-C in the patients with HoFH irrespective of the type of mutation in the LDLR and the mechanism appeared to be independent of the LDLR as LDLR activity was not affected.35
A Phase 2 trial in patients with severe hypertriglyceridaemia, with fasting TG ≥5.6 mmol/L (≥500 mg/dL) and with a history of hospitalization for acute pancreatitis, involved 17 patients with familial chylomicronaemia syndrome (FCS), 15 patients with multifactorial chylomicronaemia syndrome with heterozygous loss-of-function LPL pathway mutations and 19 patients with multifactorial chylomicronemia syndrome without LPL pathway mutations (NCT03452228).32 Treatment with evinacumab 15 mg /kg IV every 4 weeks for 12 weeks reduced TG to a variable extent in the 3 cohorts having the greatest effect versus placebo in those with no LPL mutations of median –81.7% versus +80.9%, a smaller effect in those with heterozygous LPL pathway mutations of –64.8% versus +9.4%, and no significant effect in those with FCS of −27.7% versus −22.9%.32
In the Phase 3 ELIPSE HoFH (Efficacy and Safety of Evinacumab in Patients With Homozygous Familial Hypercholesterolemia) trial, the efficacy of evinacumab was evaluated in 65 patients with HoFH on multiple lipid-lowering therapies (NCT03399786).33 Evinacumab 15 mg/kg every 4 weeks (Q4W) reduced LDL-C by a mean of 49% compared with placebo after 24 weeks. There was also a 55% decrease in TG levels with evinacumab compared to a 4.6% reduction in the placebo group and a 30% reduction in HDL-C levels was seen with evinacumab. The effects on LDL-C were similar in patients with complete LDLR deficiency (null-null) as in those with residual LDLR activity (non-null). Adverse event rates were similar in the evinacumab and placebo groups. Based on this study the FDA approved evinacumab for HoFH, but it was noted that it is a very expensive treatment and may not be suitable for a wider application.36
In a substudy from ELIPSE HoFH, four patients with HoFH underwent apoB kinetic analyses which showed an increase in the fractional catabolic rate of IDL apoB and LDL apoB, suggesting that evinacumab lowers LDL-C predominantly by increasing apoB-containing lipoprotein clearance from the circulation (NCT03409744).37 Another substudy in two severely affected young HoFH patients who underwent coronary computed tomography angiography (CCTA) before and after 6 months of treatment, showed profound plaque reduction after intensive lipid lowering therapy with statins, ezetimibe, LDL apheresis, and evinacumab.38
In a meta-analysis of five randomized controlled trials (RCTs) with a total of 568 subjects, treatment with evinacumab, as compared with placebo, significantly reduced LDL-C by 33%.39 Evinacumab reduced HDL-C by 12.8% compared with placebo which may limit the potential for wider application in dyslipidaemia.
There are ongoing open-label trials to assess the long-term safety and efficacy of evinacumab in adult and adolescent patients with HoFH (NCT03409744) and pediatric patients with HoFH (NCT04233918).
3. Vupanorsen
Vupanorsen (IONIS-ANGPTL3-LRx/AKCEA-ANGPTL3-LRx/ISIS 703802/ PF-07285557, Ionis Pharmaceuticals, Akcea Therapeutics, and Pfizer) is a N-Acetylgalactosamine (GalNAc)-modified second generation ASO that selectively inhibits ANGPTL3 protein synthesis targeted to the liver. It was developed using Ionis’ advanced LIgand Conjugated Antisense (LICA) technology platform.40 An earlier non-GalNAc conjugated ANGPTL3 ASO was studied in Phase 1 at doses up to 400 mg weekly but vupanorsen has about 30-fold higher potency and can be administered at a less frequent dosing interval and in lower doses.41
Phase 1 studies in human volunteers with elevated TG levels showed that multiple doses of 10, 20, 40, or 60 mg given SC once weekly for 6 weeks resulted in reductions in levels of ANGPTL3 protein from baseline of 46.6 to 84.5%, along with reductions of TG of 33.2 to 63.1%, reductions of LDL-C of 1.3 to 32.9%, VLDL cholesterol of 27.9 to 60.0%, non-HDL cholesterol of 10.0 to 36.6%, apoB of 3.4 to 25.7%, and apoC3 of 18.9 to 58.8% (NCT02709850).42 There were no serious adverse events.
In a Phase 2 study in 105 patients with elevated fasting TG >1.7 mmol/L (>150 mg/dL), type 2 diabetes, and hepatic steatosis treated for 6 months with 40 or 80 mg every 4 weeks or 20 mg every week of SC vupanorsen, there were significant reductions in ANGPTL3 of 41%, 59%, 56%, in the three groups, respectively, compared with 8% reduction with placebo and significant reductions in fasting TG of 36%, 53%, 47%, at six months in the three groups, respectively, compared with 16% reduction with placebo.43 There were also significant reductions in apoC3 (58%), remnant cholesterol (38%), total cholesterol (19%), non-HDL-C (18%), HDL-C (24%), and apoB (9%) compared to placebo, but there was no improvement in glycaemic parameters, or hepatic fat fraction. The most common adverse events were injection site reactions that were generally mild.
In the Phase 2b Vupanorsen in Statin-Treated Patients With Elevated Cholesterol: TaRgeting ANGPTL3 with an aNtiSense oLigonucleotide in AdulTs with dyslipidEmia (TRANSLATE-TIMI 70, NCT04516291) study, adults on statin therapy with combined hyperlipidaemia (non-HDL-C ≥2.6 mmol/L [≥100 mg/dL] and TG 1.7 to 5.6 mmol/L [150 to 500 mg/dL]) were randomized to 7 vupanorsen dose regimens (80, 120, or 160 mg SC every 4 weeks, or 60, 80, 120, or 160 mg SC every 2 weeks or placebo.44 There were dose-dependent reductions in non-HDL-C over placebo ranging from 22.0% to 27.7% and in TG ranging from 41.3% to 56.8%. There were more modest reductions in LDL-C (7.9%–16.0%) and ApoB (6.0%–15.1%). There were elevations in liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and increased hepatic fat.
Vupanorsen was also studied in various other patient groups including patients with familial partial lipodystrophy (NCT03514420), FCS (NCT03360747) and patients diagnosed with type 2 diabetes, hypertriglyceridaemia and nonalcoholic fatty liver disease (NCT03371355) as well as a Phase 1 study in healthy, adult Japanese participants (NCT04459767) and in patients with FH (NCT02709850).
Although the TRANSLATE-TIMI 70 study met its primary endpoint of a significant reduction in non-HDL-C and showed significant reductions in TG and ANGPTL3, the magnitude of non-HDL-C and TG reduction observed did not support continuation of the clinical development program for CV risk reduction or severe hypertriglyceridaemia and there were also dose-dependent increases in liver fat, and higher doses were associated with elevations in ALT and AST. In January 2022 Pfizer announced that they would terminate the Pfizer-led clinical development program and return the rights for vupanorsen to Ionis.45
4. ARO-ANG3
ARO-ANG3 (Arrowhead Pharmaceuticals) is an RNAi-based therapy using Arrowhead Pharmaceuticals’ TRiM™ platform to durably reduce ANGPTL3 expression by hepatocytes. In a Phase 1 study (AROANG31001, NCT03747224) single doses of ARO-ANG3 35, 100, 200 or 300 mg or placebo were given SC to healthy volunteers who were followed for at least 4 months post dose.46 In 24 subjects there were dose-dependent reductions from baseline in ANGPTL3 with mean maximal reductions of 79%–83% for the 200 and 300 mg doses. Mean maximal reductions in TG were up to 67% and in LDL-C up to 30%. HDL-C was reduced up to 19%. The changes were durable up to 16 weeks and there were no major adverse events and no discontinuations or dose related adverse laboratory changes.
The study was continued with doses of 100, 200 or 300 mg given SC to healthy volunteers on days 1 and 29.47 The mean nadir for ANGPTL3 levels of -83 to -93% occurred 2 weeks after the second dose and there was minimal change for 200 and 300 mg doses at week 16. The nadir for TG of -61% occurred at 3 weeks and that for LDL-C of −45% occurred 4–6 weeks after the second dose and the effects continued through week 16. HDL-C was reduced by 14%–37% at week 16. The drug was well tolerated without serious or severe adverse events or dropouts related to the drug.
Patients with heterozygous FH were also included in the Phase 1 study and the 3 doses of ARO-ANG3 reduced mean ANGPTL3 levels in a dose-dependent manner between 62%–92% at week 16 and there were significant reductions in LDL-C (23%–37%) and TG (25%–43%).48
Arrowhead recently completed enrolment in the Phase 2b study in more than 180 patients with elevated LDL-C and TG (ARCHES-2, AROANG3-2001, NCT04832971) to evaluate the efficacy and safety of ARO-ANG3 in participants with mixed dyslipidaemia. Participants will receive 2 SC injections of ARO-ANG3 or placebo.
An open-label Phase 2 clinical study (GATEWAY, AROANG3-2003, NCT05217667) is evaluating the efficacy and safety of ARO-ANG3 in 18 subjects with HoFH with two dose levels of ARO-ANG3 (200 mg and 300 mg) on day 1 and day 84 on top of continued standard of care. Initial data have been presented showing that at study week 20, with the two doses of ARO-ANG3 there were mean reductions in ANGPTL3 of 82.7% and 80.1%, in LDL-C of 48.1% and 44.0%, and in ApoB of 39.2% and 34.5%, respectively, along with reductions in HDL-C, non-HDL-C, and TG.49 The treatment was well tolerated.
5. Other treatments targeting ANGPTL3
Various other treatments targeting ANGPTL3 are in development. Solbinsiran (LY3561774, Eli Lilly) is another GalNAc-conjugated siRNA that specifically targets ANGPTL3 expression in the liver.50 A Phase 1 study of LY3561774 in participants with dyslipidemia (NCT04644809) was completed and a Phase 2b study in participants with mixed dyslipidaemia and on a stable dose of a statin (PROLONG-ANG3, NCT05256654) is in progress.
An anti-ANGPTL3/8 mAb developed by Eli Lilly and Company and acquired by Kyttaro was reported to be in development.51 Studies with an anti-ANGPTL3/8 antibody showed it reduced circulating TG in vivo in hypertriglyceridaemic transgenic mice.52 Vaccines targeting ANGPTL3 have also been used in preclinical studies, including a virus-like particle-based vaccine targeting the LPL-binding domain of ANGPTL3 which reduced TGs in mice,53 and a peptide vaccine against ANGPTL3 which reduced TG, LDL-C levels, hepatic steatosis and atherosclerosis in mice.54 Gene editing therapy has also been tested in mice including a variation of the CRISPR/Cas9 genome editing delivered using an adenoviral vector,55 and a lipid nanoparticle delivery platform carrying Cas9 mRNA and guide RNA for CRISPR-Cas9-based genome editing of ANGPTL3.56
APOC3
1. Function of apoC3
ApoC3 is a 79 amino acid glycoprotein of ~8.8 kDa synthesized principally in the liver and to a lesser extent in the intestine. It circulates on most lipid particles including VLDL, LDL, Lp(a), and HDL particles and can be present in multiple copies per particle. Plasma apoC3 concentrations correlate with TG levels and are associated with increased cardiovascular risk. ApoC3 expression is suppressed by insulin and is induced by glucose via transcriptional regulatory elements in the gene promoter region.57,58 Activation of peroxisome proliferator–activated receptor-α also reduces apoC3 expression, accounting in part for the hypotriglyceridaemic action of fibrates.59
Genetic studies show that loss-of-function mutations in APOC3 are associated with improved lipid metabolism and reduced cardiovascular risk.60,61,62,63,64 In the Exome Sequencing Project, heterozygous carriers of at least one of four mutations that disrupted APOC3 function were associated with a 46% lowering of apoC3 levels, a 39% lowering of TGs, a 25% increase in HDL-C, and a 40% reduction in CVD risk.63
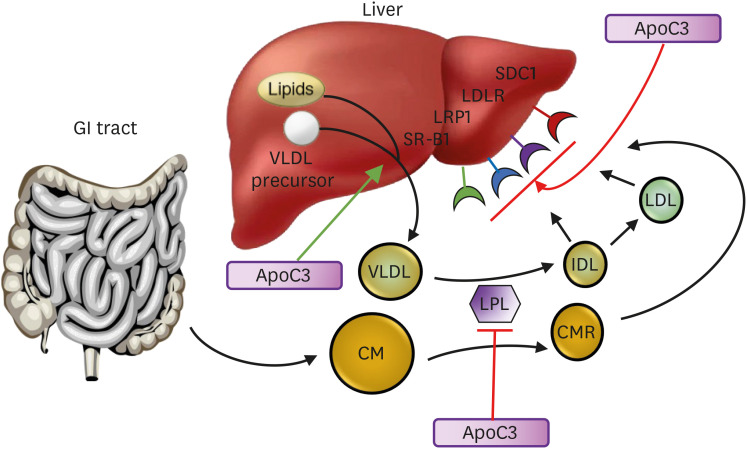
ApoC3 not only inhibits the activity of LPL, but it also reduces hepatic clearance of TRL remnants via the LDLR, LDLR-related protein (LRP) and the heparin sulfate proteoglycan (HSPG) syndecan-1 (SDC1) (Fig. 2).65 This appears to involve an interaction with apoE on TRL remnants.66 The scavenger receptor B1 may also be involved in the liver uptake of TRL remnants which may be inhibited by apoC3.67 ApoC3 may also enhance the hepatic assembly and secretion of VLDL under certain conditions.68
Fig. 2. Action of apoC3 in lipoprotein metabolism. ApoC3 inhibits lipoprotein lipase and inhibits the liver uptake of remnants and LDL particles by the LDLR or LRP1, and possibly SDC1 and SRB1, by interfering with the binding of apoE to those receptors. It may also stimulate release of VLDL particles from the liver. When LPL activity is absent, inhibition of apoC3 facilitates hepatic uptake of particles by the LDLR and LRP1.
ApoC3, apolipoprotein C3; CM, chylomicron; CMR, chylomicron remnant; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; LPL, lipoprotein lipase; LRP1, LDLR-related protein 1; SDC1, syndecan-1; SR-B1, scavenger receptor B1; VLDL, very low-density lipoprotein.
Various inhibitors of apoC3 are in development including ASOs and an RNA interference compound (Table 3).
Table 3. Inhibitors of apolipoprotein C3.
| Agent | Mode of action | Dose | Efficacy | Comments |
|---|---|---|---|---|
| Volanesorsen | ASO | 300 mg SC Q1W | ↓ TG 50%–90% | Approved for FCS (Europe) |
| Olezarsen | ASO | 10–50 mg SC Q12W | ↓ TG 50%–90% | GalNAc linked |
| ARO-APOC3 | RNAi | 50 mg SC Q12W | ↓ TG 50%–90% | GalNAc linked |
| Phase 3 studies |
ASO, antisense oligonucleotide; SC, subcutaneous; QnW, given every n weeks; TG, triglycerides; FCS, familial chylomicronaemia syndrome; GalNAc, N-Acetylgalactosamine; RNAi, RNA interference.
2. Volanesorsen
Volanesorsen (ISIS 304801/ISIS-APOCIIIRx/IONIS-APOCIIIRx, Waylivra®, Akcea/Ionis) is a 20-nucleotide second generation 2’-O-methoxyethyl chimeric antisense inhibitor of apoC3 mRNA developed for the very rare condition of FCS, as well as potentially for other forms of hypertriglyceridaemia and familial partial lipodystrophy.69 It was approved in the EU in May 2019 for the treatment of adult patients with FCS based on positive results from the multinational, Phase 3 APPROACH and COMPASS studies.70 It was not approved by the US FDA in 2018, mainly because of the high incidence of thrombocytopaenia.71 The clinical trials with volanesorsen are summarised in Table 4.
Table 4. Clinical trials with volanesorsen.
| Study name, reference | Patients and sample size | Treatment | Change in TG (%) or other outcome | |
|---|---|---|---|---|
| Phase 1 | ||||
| Graham et al., 201372 | 33 healthy subjects | 50-, 100-, 200-, and 400-mg SC, SAD and MAD | Up to −43.8% 1 wk after last dose compared to +28.5% with placebo | |
| Phase 2 | ||||
| Gaudet et al., 201473 | 3 FCS | 300-mg Q1W ×13 | −56% to −86% | |
| Gaudet et al., 2015 (NCT01529424)74 | TG 350–2,000 or 225–2,000 mg/dL on fibrate. n=57 | 100-, 200-, 300-mg or placebo Q1W ×13 | Up to −70.9% compared to +20.1% with placebo and −64.0% compared to −7.7% in the fibrate group | |
| Digenio et al., 2016 (NCT01647308)75 | T2D TG 200–500 mg/dL. n=15 | SC 300 mg Q1W | −69.1% | |
| SC placebo ×15 | −9.9% | |||
| Phase 3 | ||||
| APPROACH (NCT02211209)76 | FCS. n=66 | SC 300 mg Q1W | −77% at 3 mon | |
| SC placebo | +18% | |||
| COMPASS (NCT02300233)77 | FCS or TG ≥500 mg/dL. n=113 | SC 300 mg Q1W | −71% at 3 mon | |
| SC placebo | +1% | |||
Values given as means.
TG, triglycerides; SC, subcutaneous; SAD, single ascending dose; MAD, multiple ascending dose; FCS, familial chylomicronaemia syndrome; QnW, given every n weeks.
Pharmacokinetic studies show that volanesorsen is highly bound to plasma proteins and plasma concentrations declined in a multiphasic fashion, characterized by a relatively fast initial distribution phase and then a much slower terminal elimination phase following SC bolus administration.78 Volanesorsen is the major component in plasma, and it undergoes endo- and exonuclease-mediated metabolism with urinary excretion being the major elimination pathway for volanesorsen and its metabolites. It has been associated with thrombocytopaenia although the mechanism is not known.76 Patients with FCS may have splenomegaly which may predispose to thrombocytopaenia.
In a Phase 1 dose escalation study in 33 healthy volunteers, 6 multiple SC doses of volanesorsen at 50, 100, 200 or 400 mg over a five-week period resulted in dose-dependent reductions in plasma apoC3 up to >77.5% with concomitant lowering of TG levels by up to 43.8% (ISIS 304801-CS1).72 There were small reductions in LDL-C with the 3 highest doses and variable increases in HDL-C.
In an open-label Phase 2 trial in three patients with FCS, including those with LPL-null mutations, volanesorsen reduced plasma ApoC3 levels by 71 to 90% and TG levels by 56 to 86%.73 Studies in mice lacking LPL showed the mechanism of reduction in TG with an ApoC3 ASO was dependent on LDLRs and LDLR-related protein 1 (LRP1).65 Hepatic HSPG receptors, predominantly SDC1 can also be involved in clearing TRLs.
In a Phase 2 trial in untreated patients with elevated fasting TG levels of 4.0–22.6 mmol/L (350–2,000 mg/dL) and in patients receiving stable fibrate therapy with fasting TG levels of 2.5–22.6 mmol/L (225–2,000 mg/dL) volanesorsen, 100, 200 or 300 mg given SC weekly produced dose-dependent reductions in apoC3 up to 80% in the single treatment group and up to 71% as an add-on to fibrates along with reductions of 31.3 to 70.9% in TG levels (ISIS 304801-CS2, NCT01529424).74 There were dose-dependent increases in HDL-C up to 45.7% and significant dose-dependent LDL-C elevations and no overall change in non-HDL-C or ApoB levels.
In a Phase 2 placebo-controlled study, 15 patients with type 2 diabetes and hypertriglyceridaemia were given weekly SC volanesorsen 300 mg or placebo for 15 doses (ISIS 304801-CS4, NCT01647308).75 The mean reductions in ApoC3 and TG with volanesorsen were 87.5% and 69.1%, respectively, compared with 7.3% and 9.9% with placebo. There was a 57% improvement in whole-body insulin sensitivity and 3 months after the last dose there was 0.44% reduction in HbA1c with volanesorsen.
The APPROACH (A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study of ISIS 304801 Administered Subcutaneously to Patients With Familial Chylomicronemia Syndrome [FCS]) study included 66 patients with FCS randomised to receive volanesorsen 300 mg SC weekly or placebo over 52 weeks (ISIS 304801-CS6, NCT02211209).76 Mean baseline fasting TG levels were 25.0±13.6 mmol/L (2,209±1,199 mg/dL) and mean plasma apoC3 levels were 30.2±14.2 mg/dL (normal <20 mg/dL). After three months of treatment, apoC3 levels were reduced by >80% and TG levels were lowered by 77% (mean reduction of 19.3 mmol/L [1,700 mg/dL]) with volanesorsen. Injection site reactions were common and about half the patients had a mild degree of thrombocytopaenia (platelet count <100×109/L) and 2/33 had platelet count <25×109/L.
In the COMPASS (Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia) Phase 3 trial, volanesorsen 300 mg or placebo SC once-weekly for 13 weeks then every 2 weeks for a total of 26 weeks was given to 114 patients with fasting TG ≥5.7 mmol/L (≥500 mg/dL) (ISIS 304801-CS16, NCT02300233).77 At 3 months, mean plasma TG were reduced by 71·2% with volanesorsen compared with an increase of 0.9% in the placebo group (p<0.0001). Injection-site reactions occurred in 24% with volanesorsen compared with 0.2% with placebo and one subject had a platelet count reduction to <50×109/L in the volanesorsen group.
After completion of the APPROACH study, patients had the option of enrolling in the APPROACH open label extension (OLE) study (ISIS 304801-CS7, NCT2658175). This was completed with participants with FCS rolling over from the APPROACH (ISIS 304801-CS6, NCT02211209) index study and participants with FCS rolling over from the COMPASS (ISIS 304801-CS16, NCT02300233) index study. There were sustained reductions of plasma TG levels and common adverse events were injection site reactions and platelet count decrease, consistent with previous studies.79 In a combined analysis of these studies, volanesorsen showed a significant reduction in pancreatitis, with 1 occurring pancreatitis in the volanesorsen group versus 9 cases of pancreatitis in the placebo group.
The Investigation of Findings and Observations Captured in Burden of Illness Survey in FCS Patients (IN-FOCUS) was a multinational web-based survey in 60 patients which showed they had multiple symptoms of a physical, emotional and cognitive nature and the diagnosis of FCS was often delayed.80 The Retrospective Findings and Observations Captured in Burden of Illness Survey in FCS Patients (ReFOCUS) study was a retrospective global web-based survey in 22 patients with FCS who received volanesorsen for ≥3 months in the APPROACH OLE study.81 The various symptoms were significantly reduced with volanesorsen treatment.
The long-term efficacy and safety of volanesorsen was assessed in the VOL4002 study in 22 adults with genetically confirmed FCS with or without previous treatment in the APPROACH and/or APPROACH-OLE volanesorsen Phase 3 studies.82 The subjects self-administered volanesorsen 285 mg SC once every 2 weeks with individual patient volanesorsen exposure ranging from 6 to 51 months. In 12 treatment-naive patients, volanesorsen treatment resulted in a sustained median 52% reduction in TG levels and in 10 prior-exposure patients there was a 51% reduction from pre-treatment baseline. There was a 74% reduction in pancreatitis event rates found during volanesorsen treatment compared with the 5-year period before. Platelet declines were consistent with observations in Phase 3 clinical trials and no patient recorded a platelet count <50×109 /L.
A Phase 2 study was performed in 5 patients with partial lipodystrophy in a 16-week placebo-controlled, randomized, double blind study of volanesorsen, 300 mg weekly, followed by 1-year open label extension (NCT02639286).83 Volanesorsen decreased apoC3 and TG, partly through an LPL dependent mechanism, and it appeared to improve insulin resistance and hepatic steatosis.
The BROADEN Study (A Study of Volanesorsen (Formerly IONIS-APOCIIIRx) in Participants With Familial Partial Lipodystrophy) was a Phase 2/Phase 3 study that included 40 patients with familial partial lipodystrophy and concomitant hypertriglyceridaemia and diabetes treated with weekly SC volanesorsen 300 mg or placebo for 52 weeks (NCT02527343).84 TG were reduced from baseline at 3 months by least squares mean (LSM) −88% in the volanesorsen group versus −22% in the placebo group, with a difference in LSM of −67%. There was a significant LSM relative reduction in hepatic fat fraction (HFF) of 53% at month 12 with volanesorsen versus placebo. Data evaluated from the COMPASS, APPROACH, and BROADEN trials showed that volanesorsen reduced HFF and there was a strong inverse correlation between the baseline HFF and the change in HFF in the volanesorsen groups.85
3. Olezarsen
Olezarsen (ISIS 678354, IONIS-APOCIII-LRx, AKCEA-APOCIII-LRx, Akcea/Ionis) is a GalNAc-conjugated ASO targeted to hepatic APOC3 mRNA to inhibit apoC3 production.
In a Phase 1/2a study in healthy volunteers with elevated TG it was well tolerated and effectively reduced apoC3 and TG levels (NCT02900027).86 Single SC doses of 10, 30, 60, 90, or 120 mg of olezarsen resulted in median reductions of in apoC3 of 0, −42%, −73%, −81%, and −92%, respectively, and reductions in TG of −12%, −7%, −42%, −73%, and −77%, respectively, at 14 days after dosing. Multiple SC doses of 15 and 30 mg weekly and 60 mg every 4 weeks resulted in median reductions in apoC3 of −66%, −84%, and −89%, respectively, and in TG of −59%, −73%, and −66%, respectively, at 1 week after the last dose. There were also significant reductions in total cholesterol, apoB, non-HDL-C, VLDL-C and increases in HDL-C.
In a Phase 2, dose-ranging, randomised, double-blind, placebo-controlled study in 114 participants with raised fasting serum TG of 2.26–5.65 mmol/L (200–500 mg/dL) and established CVD or at a high risk for CVD, doses of SC olezarsen (10 or 50 mg every 4 weeks, 15 mg every 2 weeks, or 10 mg every week) or placebo were given for 6–12 months (ISIS 678354-CS2, NCT03385239).87 Reductions in TG were 23% with 10 mg every 4 weeks, 56% with 15 mg every 2 weeks, 60% with 10 mg every week, and 60% with 50 mg every 4 weeks, compared with an increase by 6% in the pooled placebo group. There were significant decreases in apoC3, VLDL-C, non-HDL-C, and apoB and there were no platelet count, liver, or renal function changes in any of the olezarsen groups.
There are several ongoing Phase 3 studies with olezarsen. The BALANCE study (ISIS 678354-CS3, NCT04568434) is an ongoing Phase 3 clinical study evaluating olezarsen SC once every 4 weeks in 66 people with FCS. The CORE study (ISIS 678354-CS5, NCT05079919) is a Phase 3 study in up to approximately 540 participants with severe hypertriglyceridaemia and fasting TG ≥5.65 mmol/L (500 mg/dL). ISIS 678354-CS6 (NCT05552326) is a Phase 3, multi-center, randomised, double-blind, placebo-controlled study in up to approximately 390 participants with severe hypertriglyceridaemia with a 53-week treatment period, and a 13-week post-treatment evaluation period or transition to OLE study with up to 1-year treatment. ISIS 678354-CS7 (NCT05185843) is a study with olezarsen in up to 30 participants with FCS previously treated with volanesorsen.
ISIS 678354-CS8 (NCT05355402) is a placebo-controlled study in approximately 112 participants with hypertriglyceridaemia and established or at increased risk for ASCVD, and/or with severe hypertriglyceridaemia. ISIS 678354-CS9 (NCT05610280) is a Phase 3, multi-center, placebo-controlled study with a 53-week treatment period in up to 1312 participants with hypertriglyceridaemia and ASCVD.
ISIS 678354-CS13 (NCT05130450) is a multi-center, open-label extension study with a 53-week treatment period in up to 60 participants with FCS rolling-over from Study ISIS 678354-CS3 (NCT04568434). ISIS 678354-CS15 (NCT05681351) is a multi-center, open-label study in up to 700 participants with severe hypertriglyceridaemia who would be rolled over from studies ISIS 678354-CS5 (NCT05079919) or ISIS 678354-CS6 (NCT05552326) with a 53-week treatment period.
4. ARO-APOC3
ARO-APOC3 (Arrowhead Pharmaceuticals) is an investigational RNAi therapeutic targeting APOC3 being developed as a treatment for patients with severe hypertriglyceridaemia (SHTG), mixed dyslipidaemia (MD), and FCS. ARO-APOC3 is being investigated in the Phase 2 SHASTA-2 clinical study (NCT04720534) in patients with SHTG, the Phase 2 MUIR clinical study (NCT04998201) in patients with MD, and in the Phase 3 PALISADE clinical study (NCT05089084) in patients with FCS.
A Phase 1 study of ARO-APOC3 in healthy volunteers involved single SC doses of 10, 25, 50 or 100 mg ARO-APOC3 or placebo and participants were followed for 16 weeks post dose (AROAPOC31001, NCT03783377).88 The treatment was well tolerated and there were reductions from baseline in apoC3 by ≥87% and TG by a maximum of 64%. Stable reductions lasted 12 weeks after the dose. There was a mean maximal increase in HDL-C of up to 69%. The study continued with doses of 10, 25 or 50 mg given SC to healthy volunteers on days 1 and 29 with follow up for up to 16 weeks.88 There were dose-dependent reductions in apoC3 up to 94% and TG were reduced up to 74% with smaller reductions in LDL-C. HDL-C increased up to 84%.
The same study involved participants with hypertriglyceridaemia (fasting TG ≥3.4 mmol/L [300 mg/dL]) or multifactorial chylomicronaemia (MCM), with fasting TG ≥9.9 mmol/L (880 mg/dL) and without biallelic pathogenic variants, who received single SC doses of 50 mg ARO-APOC3.89 Preliminary results in 9 subjects showed mean reductions in apoC3 levels by 96% and in TG by 78% 4 weeks after treatment. Two MCM patients had a transient elevation in ALT to >3 × ULN.
Four genetically confirmed FCS patients were given 50 mg ARO-APOC3 and 26 patients with MCM received 10, 25, 50, or 100 mg ARO-APOC3 on Days 1 and 29.90 Mean apoC3 was reduced by 98% in FCS patients and by 96% in MCM patients and both groups had similar maximum median reductions in TG of 91% and 90%, respectively. HDL-C was markedly increased and the doses were well tolerated.
In the Phase 3 study in patients with FCS (PALISADE, A Phase 3 Study to Evaluate the Efficacy and Safety of ARO-APOC3 in Adults With Familial Chylomicronemia Syndrome, AROAPOC3-3001, NCT05089084), participants will be randomized to receive 4 SC doses of ARO-APOC3 or matching placebo and those who complete the randomized period will continue in a 2-year open-label extension period where all participants will receive ARO-APOC3.
5. Other treatments targeting apoC3
Lilly have APOC3 siRNA which is being studied for the treatment of CVD. A mAb to apoC3 has also been described which could inhibit apoC3 from the liver and the intestine but apoC3 is present in large amounts in the plasma which may make the mAb approach difficult.91
CONCLUSION
Drugs with various modes of action are in development to target apoC3 and ANGPTL3 and some have been approved for limited indications. Whilst these inhibitors might be expected to primarily reduce TG, the ANGPTL3 inhibitors are effective in reducing LDL-C in HoFH and evinacumab has been approved for this indication. Whether evinacumab will be suitable for more common types of dyslipidaemia remains to be seen and it is currently very expensive, which would limit its usage. Vupanorsen was studied in mixed hyperlipidaemia and reductions in LDL-C and apoB were modest and there was a tendency for liver fat accumulation and increase in liver enzymes, so the development program was halted. Also, ANGPTL3 inhibitors generally reduce HDL-C, the significance of which remains to be determined.
The inhibitors of apoC3 are effective in reducing TG and the rate of acute pancreatitis in the severe hypertriglyceridaemia of FCS and volanesorsen has been approved in Europe for this indication. Thrombocytopaenia is a concern with this ASO which is not conjugated with GalNAc and olezarsen appears not to cause this adverse effect and may have a wider indication. Volanesorsen treatment did increase LDL-C in some patients with high TG with no overall change in non-HDL-C or apoB, which might suggest there would be a lack of benefit in reducing ASCVD. This has not been reported with olezarsen but it may depend on the type of patient treated and the results of ongoing studies are awaited. The siRNA compounds inhibiting both ANGPTL3 and apoC3 look promising and further study results should help to define their role. All of these drugs are likely to be expensive which will limit their application.
Footnotes
Funding: This manuscript was funded by a grant for the “Overseas Famous Teachers” Project of Guangdong Provincial Department of Science and Technology and Research Funding from the Faculty of Medicine, Macau University of Science and Technology.
Conflict of Interest: Brian Tomlinson is the editor of Journal of LIpid and Atherosclerosis. However, he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Data Availability Statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
- Conceptualization: Tomlinson B.
- Visualization: Wu QY, Zhong YM, Li YH.
- Writing - original draft: Tomlinson B.
- Writing - review & editing: Wu QY, Zhong YM, Li YH.
References
- 1.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 2.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1046–e1081. doi: 10.1161/CIR.0000000000000624. [DOI] [PubMed] [Google Scholar]
- 4.Nissen SE, Lincoff AM, Brennan D, Ray KK, Mason D, Kastelein JJ, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388:1353–1364. doi: 10.1056/NEJMoa2215024. [DOI] [PubMed] [Google Scholar]
- 5.Tokgözoğlu L, Libby P. The dawn of a new era of targeted lipid-lowering therapies. Eur Heart J. 2022;43:3198–3208. doi: 10.1093/eurheartj/ehab841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laufs U, Parhofer KG, Ginsberg HN, Hegele RA. Clinical review on triglycerides. Eur Heart J. 2020;41:99–109c. doi: 10.1093/eurheartj/ehz785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginsberg HN, Packard CJ, Chapman MJ, Borén J, Aguilar-Salinas CA, Averna M, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J. 2021;42:4791–4806. doi: 10.1093/eurheartj/ehab551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ference BA, Kastelein JJ, Ray KK, Ginsberg HN, Chapman MJ, Packard CJ, et al. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA. 2019;321:364–373. doi: 10.1001/jama.2018.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marston NA, Giugliano RP, Im K, Silverman MG, O’Donoghue ML, Wiviott SD, et al. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials. Circulation. 2019;140:1308–1317. doi: 10.1161/CIRCULATIONAHA.119.041998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tall AR, Thomas DG, Gonzalez-Cabodevilla AG, Goldberg IJ. Addressing dyslipidemic risk beyond LDL-cholesterol. J Clin Invest. 2022;132:e148559. doi: 10.1172/JCI148559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conklin D, Gilbertson D, Taft DW, Maurer MF, Whitmore TE, Smith DL, et al. Identification of a mammalian angiopoietin-related protein expressed specifically in liver. Genomics. 1999;62:477–482. doi: 10.1006/geno.1999.6041. [DOI] [PubMed] [Google Scholar]
- 14.Shimizugawa T, Ono M, Shimamura M, Yoshida K, Ando Y, Koishi R, et al. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J Biol Chem. 2002;277:33742–33748. doi: 10.1074/jbc.M203215200. [DOI] [PubMed] [Google Scholar]
- 15.Shimamura M, Matsuda M, Yasumo H, Okazaki M, Fujimoto K, Kono K, et al. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler Thromb Vasc Biol. 2007;27:366–372. doi: 10.1161/01.ATV.0000252827.51626.89. [DOI] [PubMed] [Google Scholar]
- 16.Adam RC, Mintah IJ, Alexa-Braun CA, Shihanian LM, Lee JS, Banerjee P, et al. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J Lipid Res. 2020;61:1271–1286. doi: 10.1194/jlr.RA120000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, Soundarapandian MM, Castoreno AB, Millar JS, Rader DJ. LDL-cholesterol reduction by ANGPTL3 inhibition in mice is dependent on endothelial lipase. Circ Res. 2020;127:1112–1114. doi: 10.1161/CIRCRESAHA.120.317128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas DG, Wei Y, Tall AR. Lipid and metabolic syndrome traits in coronary artery disease: a Mendelian randomization study. J Lipid Res. 2021;62:100044. doi: 10.1194/jlr.P120001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruppert PM, Michielsen CC, Hazebroek EJ, Pirayesh A, Olivecrona G, Afman LA, et al. Fasting induces ANGPTL4 and reduces LPL activity in human adipose tissue. Mol Metab. 2020;40:101033. doi: 10.1016/j.molmet.2020.101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YQ, Pottanat TG, Siegel RW, Ehsani M, Qian YW, Zhen EY, et al. Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids. J Lipid Res. 2020;61:1203–1220. doi: 10.1194/jlr.RA120000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Athyros VG, Katsiki N, Dimakopoulou A, Patoulias D, Alataki S, Doumas M. Drugs that mimic the effect of gene mutations for the prevention or the treatment of atherosclerotic disease: from PCSK9 inhibition to ANGPTL3 inactivation. Curr Pharm Des. 2018;24:3638–3646. doi: 10.2174/1381612824666181009100517. [DOI] [PubMed] [Google Scholar]
- 22.Dewey FE, Gusarova V, Dunbar RL, O’Dushlaine C, Schurmann C, Gottesman O, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 2017;377:211–221. doi: 10.1056/NEJMoa1612790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewey FE, Gusarova V, O’Dushlaine C, Gottesman O, Trejos J, Hunt C, et al. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med. 2016;374:1123–1133. doi: 10.1056/NEJMoa1510926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai U, Lee EC, Chung K, Gao C, Gay J, Key B, et al. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci U S A. 2007;104:11766–11771. doi: 10.1073/pnas.0705041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gusarova V, Banfi S, Alexa-Braun CA, Shihanian LM, Mintah IJ, Lee JS, et al. ANGPTL8 blockade with a monoclonal antibody promotes triglyceride clearance, energy expenditure, and weight loss in mice. Endocrinology. 2017;158:1252–1259. doi: 10.1210/en.2016-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen PY, Gao WY, Liou JW, Lin CY, Wu MJ, Yen JH. Angiopoietin-like protein 3 (ANGPTL3) modulates lipoprotein metabolism and dyslipidemia. Int J Mol Sci. 2021;22:7310. doi: 10.3390/ijms22147310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy AJ, Macdonald LE, Stevens S, Karow M, Dore AT, Pobursky K, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A. 2014;111:5153–5158. doi: 10.1073/pnas.1324022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gusarova V, Alexa CA, Wang Y, Rafique A, Kim JH, Buckler D, et al. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J Lipid Res. 2015;56:1308–1317. doi: 10.1194/jlr.M054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad Z, Banerjee P, Hamon S, Chan KC, Bouzelmat A, Sasiela WJ, et al. Inhibition of angiopoietin-like protein 3 with a monoclonal antibody reduces triglycerides in hypertriglyceridemia. Circulation. 2019;140:470–486. doi: 10.1161/CIRCULATIONAHA.118.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harada-Shiba M, Ali S, Gipe DA, Gasparino E, Son V, Zhang Y, et al. A randomized study investigating the safety, tolerability, and pharmacokinetics of evinacumab, an ANGPTL3 inhibitor, in healthy Japanese and Caucasian subjects. Atherosclerosis. 2020;314:33–40. doi: 10.1016/j.atherosclerosis.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Gaudet D, Gipe DA, Pordy R, Ahmad Z, Cuchel M, Shah PK, et al. ANGPTL3 inhibition in homozygous familial hypercholesterolemia. N Engl J Med. 2017;377:296–297. doi: 10.1056/NEJMc1705994. [DOI] [PubMed] [Google Scholar]
- 32.Rosenson RS, Gaudet D, Ballantyne CM, Baum SJ, Bergeron J, Kershaw EE, et al. Evinacumab in severe hypertriglyceridemia with or without lipoprotein lipase pathway mutations: a phase 2 randomized trial. Nat Med. 2023;29:729–737. doi: 10.1038/s41591-023-02222-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJ, Rubba P, et al. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med. 2020;383:711–720. doi: 10.1056/NEJMoa2004215. [DOI] [PubMed] [Google Scholar]
- 34.Rosenson RS, Burgess LJ, Ebenbichler CF, Baum SJ, Stroes ES, Ali S, et al. Evinacumab in patients with refractory hypercholesterolemia. N Engl J Med. 2020;383:2307–2319. doi: 10.1056/NEJMoa2031049. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee P, Chan KC, Tarabocchia M, Benito-Vicente A, Alves AC, Uribe KB, et al. Functional analysis of LDLR (low-density lipoprotein receptor) variants in patient lymphocytes to assess the effect of evinacumab in homozygous familial hypercholesterolemia patients with a spectrum of LDLR activity. Arterioscler Thromb Vasc Biol. 2019;39:2248–2260. doi: 10.1161/ATVBAHA.119.313051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuehn BM. Evinacumab approval adds a new option for homozygous familial hypercholesterolemia with a hefty price tag. Circulation. 2021;143:2494–2496. doi: 10.1161/CIRCULATIONAHA.121.055463. [DOI] [PubMed] [Google Scholar]
- 37.Reeskamp LF, Millar JS, Wu L, Jansen H, van Harskamp D, Schierbeek H, et al. ANGPTL3 inhibition with evinacumab results in faster clearance of IDL and LDL apoB in patients with homozygous familial hypercholesterolemia-brief report. Arterioscler Thromb Vasc Biol. 2021;41:1753–1759. doi: 10.1161/ATVBAHA.120.315204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reeskamp LF, Nurmohamed NS, Bom MJ, Planken RN, Driessen RS, van Diemen PA, et al. Marked plaque regression in homozygous familial hypercholesterolemia. Atherosclerosis. 2021;327:13–17. doi: 10.1016/j.atherosclerosis.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Jin M, Meng F, Yang W, Liang L, Wang H, Fu Z. Efficacy and safety of evinacumab for the treatment of hypercholesterolemia: a meta-analysis. J Cardiovasc Pharmacol. 2021;78:394–402. doi: 10.1097/FJC.0000000000001073. [DOI] [PubMed] [Google Scholar]
- 40.Ruscica M, Zimetti F, Adorni MP, Sirtori CR, Lupo MG, Ferri N. Pharmacological aspects of ANGPTL3 and ANGPTL4 inhibitors: New therapeutic approaches for the treatment of atherogenic dyslipidemia. Pharmacol Res. 2020;153:104653. doi: 10.1016/j.phrs.2020.104653. [DOI] [PubMed] [Google Scholar]
- 41.Brandt TA, Lee RG, Digenio A, Graham MG, Crooke RM, Hughes SG, et al. ISIS-ANGPTL3RX, an antisense inhibitor to angiopoietin-like 3, reduces plasma lipid levels in mouse models and in healthy human volunteers. Atherosclerosis. 2015;241:e30–e31. [Google Scholar]
- 42.Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med. 2017;377:222–232. doi: 10.1056/NEJMoa1701329. [DOI] [PubMed] [Google Scholar]
- 43.Gaudet D, Karwatowska-Prokopczuk E, Baum SJ, Hurh E, Kingsbury J, Bartlett VJ, et al. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur Heart J. 2020;41:3936–3945. doi: 10.1093/eurheartj/ehaa689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergmark BA, Marston NA, Bramson CR, Curto M, Ramos V, Jevne A, et al. Effect of vupanorsen on non-high-density lipoprotein cholesterol levels in statin-treated patients with elevated cholesterol: TRANSLATE-TIMI 70. Circulation. 2022;145:1377–1386. doi: 10.1161/CIRCULATIONAHA.122.059266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfizer. Pfizer and Ionis Announce Discontinuation of Vupanorsen Clinical Development Program [Internet] Pfizer; 2022. [cited 2023 Jun 20]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-ionis-announce-discontinuation-vupanorsen. [Google Scholar]
- 46.Watts GF, Scott R, Gladding P, Sullivan D, Baker J, Clifton P, et al. RNA interference targeting hepatic angiopoietin-like protein 3 results in prolonged reductions in plasma triglycerides and LDL-C in human subjects. Circulation. 2019;140:E987–E988. [Google Scholar]
- 47.Watts GF, Schwabe C, Scott R, Gladding P, Sullivan D, Baker J, et al. RNAi inhibition of angiopoietin-like protein 3 (ANGPTL3) with ARO-ANG3 mimics the lipid and lipoprotein profile of familial combined hypolipidemia. Eur Heart J. 2020;41(Suppl 2):ehaa946.3331 [Google Scholar]
- 48.Watts GF, Schwabe C, Scott R, Gladding P, Sullivan D, Baker J, et al. Abstract 15751: pharmacodynamic effect of ARO-ANG3, an investigational RNA interference targeting hepatic angiopoietin-like protein 3, in patients with hypercholesterolemia. Circulation. 2020;142(Suppl 3):A15751 [Google Scholar]
- 49.Raal F, Bergeron J, Watts GF, Gaudet D, Sullivan D, Turner T, et al. ARO-ANG3, an Investigational RNAi Therapeutic, Decreases Serum LDL-Cholesterol, Apolipoprotein B, and Angiopoietin-like Protein 3 in Patients with Homozygous Familial Hypercholesterolaemia [Internet] European Atherosclerosis Society; 2023. [cited 2023 Jul 18]. Available from: https://ir.arrowheadpharma.com/static-files/93c32012-148d-4cb6-9a2c-e42caf345805. [Google Scholar]
- 50.Lilly. Medicines in Development [Internet] Lilly; 2023. [cited 2023 Jun 20]. Available from: https://www.lilly.com/discovery/clinical-development-pipeline. [Google Scholar]
- 51.Business Wire. Kyttaro Announces Worldwide Exclusive Licensing Agreement with Lilly for Antibody Therapeutic Program. Business Wire; 2023. [cited 2023 Jun 20]. Available from: https://www.businesswire.com/news/home/20220411005206/en/Kyttaro-Announces-Worldwide-Exclusive-Licensing-Agreement-With-Lilly-for-Antibody-Therapeutic-Program. [Google Scholar]
- 52.Balasubramaniam D, Schroeder O, Russell AM, Fitchett JR, Austin AK, Beyer TP, et al. An anti-ANGPTL3/8 antibody decreases circulating triglycerides by binding to a LPL-inhibitory leucine zipper-like motif. J Lipid Res. 2022;63:100198. doi: 10.1016/j.jlr.2022.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fowler A, Sampson M, Remaley AT, Chackerian B. A VLP-based vaccine targeting ANGPTL3 lowers plasma triglycerides in mice. Vaccine. 2021;39:5780–5786. doi: 10.1016/j.vaccine.2021.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fukami H, Morinaga J, Nakagami H, Hayashi H, Okadome Y, Matsunaga E, et al. Vaccine targeting ANGPTL3 ameliorates dyslipidemia and associated diseases in mouse models of obese dyslipidemia and familial hypercholesterolemia. Cell Rep Med. 2021;2:100446. doi: 10.1016/j.xcrm.2021.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chadwick AC, Evitt NH, Lv W, Musunuru K. Reduced blood lipid levels with in vivo CRISPR-Cas9 base editing of ANGPTL3. Circulation. 2018;137:975–977. doi: 10.1161/CIRCULATIONAHA.117.031335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiu M, Glass Z, Chen J, Haas M, Jin X, Zhao X, et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3 . Proc Natl Acad Sci U S A. 2021;118:e2020401118. doi: 10.1073/pnas.2020401118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li WW, Dammerman MM, Smith JD, Metzger S, Breslow JL, Leff T. Common genetic variation in the promoter of the human apo CIII gene abolishes regulation by insulin and may contribute to hypertriglyceridemia. J Clin Invest. 1995;96:2601–2605. doi: 10.1172/JCI118324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caron S, Verrijken A, Mertens I, Samanez CH, Mautino G, Haas JT, et al. Transcriptional activation of apolipoprotein CIII expression by glucose may contribute to diabetic dyslipidemia. Arterioscler Thromb Vasc Biol. 2011;31:513–519. doi: 10.1161/ATVBAHA.110.220723. [DOI] [PubMed] [Google Scholar]
- 59.Forcheron F, Cachefo A, Thevenon S, Pinteur C, Beylot M. Mechanisms of the triglyceride- and cholesterol-lowering effect of fenofibrate in hyperlipidemic type 2 diabetic patients. Diabetes. 2002;51:3486–3491. doi: 10.2337/diabetes.51.12.3486. [DOI] [PubMed] [Google Scholar]
- 60.Gibson WT. Beneficial metabolic phenotypes caused by loss-of-function APOC3 mutations. Clin Genet. 2015;87:31–32. doi: 10.1111/cge.12483. [DOI] [PubMed] [Google Scholar]
- 61.Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371:32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 62.Natarajan P, Kohli P, Baber U, Nguyen KH, Sartori S, Reilly DF, et al. Association of APOC3 loss-of-function mutations with plasma lipids and subclinical atherosclerosis: the multi-ethnic bioimage study. J Am Coll Cardiol. 2015;66:2053–2055. doi: 10.1016/j.jacc.2015.08.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wulff AB, Nordestgaard BG, Tybjærg-Hansen A. APOC3 loss-of-function mutations, remnant cholesterol, low-density lipoprotein cholesterol, and cardiovascular risk: mediation- and meta-analyses of 137 895 individuals. Arterioscler Thromb Vasc Biol. 2018;38:660–668. doi: 10.1161/ATVBAHA.117.310473. [DOI] [PubMed] [Google Scholar]
- 65.Gordts PL, Nock R, Son NH, Ramms B, Lew I, Gonzales JC, et al. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J Clin Invest. 2016;126:2855–2866. doi: 10.1172/JCI86610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramms B, Patel S, Nora C, Pessentheiner AR, Chang MW, Green CR, et al. ApoC-III ASO promotes tissue LPL activity in the absence of apoE-mediated TRL clearance. J Lipid Res. 2019;60:1379–1395. doi: 10.1194/jlr.M093740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gugliucci A. Triglyceride-rich lipoprotein metabolism: key regulators of their flux. J Clin Med. 2023;12:4399. doi: 10.3390/jcm12134399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sundaram M, Zhong S, Bou Khalil M, Links PH, Zhao Y, Iqbal J, et al. Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J Lipid Res. 2010;51:150–161. doi: 10.1194/jlr.M900346-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Milonas D, Tziomalos K. Experimental therapies targeting apolipoprotein C-III for the treatment of hyperlipidemia - spotlight on volanesorsen. Expert Opin Investig Drugs. 2019;28:389–394. doi: 10.1080/13543784.2019.1582028. [DOI] [PubMed] [Google Scholar]
- 70.Paik J, Duggan S. Volanesorsen: first global approval. Drugs. 2019;79:1349–1354. doi: 10.1007/s40265-019-01168-z. [DOI] [PubMed] [Google Scholar]
- 71.Medscape. FDA Rejects Volanesorsen (Waylivra) for Rare Triglyceride Disorder [Internet] Medscape; 2019. [cited 2023 Jul 18]. Available from: https://www.medscape.com/viewarticle/901515. [Google Scholar]
- 72.Graham MJ, Lee RG, Bell TA, 3rd, Fu W, Mullick AE, Alexander VJ, et al. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res. 2013;112:1479–1490. doi: 10.1161/CIRCRESAHA.111.300367. [DOI] [PubMed] [Google Scholar]
- 73.Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med. 2014;371:2200–2206. doi: 10.1056/NEJMoa1400284. [DOI] [PubMed] [Google Scholar]
- 74.Gaudet D, Alexander VJ, Baker BF, Brisson D, Tremblay K, Singleton W, et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015;373:438–447. doi: 10.1056/NEJMoa1400283. [DOI] [PubMed] [Google Scholar]
- 75.Digenio A, Dunbar RL, Alexander VJ, Hompesch M, Morrow L, Lee RG, et al. Antisense-mediated lowering of plasma apolipoprotein C-III by volanesorsen improves dyslipidemia and insulin sensitivity in type 2 diabetes. Diabetes Care. 2016;39:1408–1415. doi: 10.2337/dc16-0126. [DOI] [PubMed] [Google Scholar]
- 76.Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med. 2019;381:531–542. doi: 10.1056/NEJMoa1715944. [DOI] [PubMed] [Google Scholar]
- 77.Gouni-Berthold I, Alexander VJ, Yang Q, Hurh E, Steinhagen-Thiessen E, Moriarty PM, et al. Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia (COMPASS): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021;9:264–275. doi: 10.1016/S2213-8587(21)00046-2. [DOI] [PubMed] [Google Scholar]
- 78.Post N, Yu R, Greenlee S, Gaus H, Hurh E, Matson J, et al. Metabolism and disposition of volanesorsen, a 2′-O-(2 methoxyethyl) antisense oligonucleotide, across species. Drug Metab Dispos. 2019;47:1164–1173. doi: 10.1124/dmd.119.087395. [DOI] [PubMed] [Google Scholar]
- 79.Witztum JL, Gaudet D, Arca M, Jones A, Soran H, Gouni-Berthold I, et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome: long-term efficacy and safety data from patients in an open-label extension trial. J Clin Lipidol. 2023;17:342–355. doi: 10.1016/j.jacl.2023.03.007. [DOI] [PubMed] [Google Scholar]
- 80.Davidson M, Stevenson M, Hsieh A, Ahmad Z, Roeters van Lennep J, Crowson C, et al. The burden of familial chylomicronemia syndrome: Results from the global IN-FOCUS study. J Clin Lipidol. 2018;12:898–907.e2. doi: 10.1016/j.jacl.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 81.Arca M, Hsieh A, Soran H, Rosenblit P, O’Dea L, Stevenson M. The effect of volanesorsen treatment on the burden associated with familial chylomicronemia syndrome: the results of the ReFOCUS study. Expert Rev Cardiovasc Ther. 2018;16:537–546. doi: 10.1080/14779072.2018.1487290. [DOI] [PubMed] [Google Scholar]
- 82.Jones A, Peers K, Wierzbicki AS, Ramachandran R, Mansfield M, Dawson C, et al. Long-term effects of volanesorsen on triglycerides and pancreatitis in patients with familial chylomicronaemia syndrome (FCS) in the UK Early Access to Medicines Scheme (EAMS) Atherosclerosis. 2023;375:67–74. doi: 10.1016/j.atherosclerosis.2023.05.008. [DOI] [PubMed] [Google Scholar]
- 83.Lightbourne M, Startzell M, Bruce KD, Brite B, Muniyappa R, Skarulis M, et al. Volanesorsen, an antisense oligonucleotide to apolipoprotein C-III, increases lipoprotein lipase activity and lowers triglycerides in partial lipodystrophy. J Clin Lipidol. 2022;16:850–862. doi: 10.1016/j.jacl.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oral EA, Garg A, Tami J, Huang EA, O’Dea LS, Schmidt H, et al. Assessment of efficacy and safety of volanesorsen for treatment of metabolic complications in patients with familial partial lipodystrophy: Results of the BROADEN study: Volanesorsen in FPLD; The BROADEN Study. J Clin Lipidol. 2022;16:833–849. doi: 10.1016/j.jacl.2022.08.008. [DOI] [PubMed] [Google Scholar]
- 85.Prohaska TA, Alexander VJ, Karwatowska-Prokopczuk E, Tami J, Xia S, Witztum JL, et al. APOC3 inhibition with volanesorsen reduces hepatic steatosis in patients with severe hypertriglyceridemia. J Clin Lipidol. 2023;17:406–411. doi: 10.1016/j.jacl.2023.04.007. [DOI] [PubMed] [Google Scholar]
- 86.Alexander VJ, Xia S, Hurh E, Hughes SG, O’Dea L, Geary RS, et al. N-acetyl galactosamine-conjugated antisense drug to APOC3 mRNA, triglycerides and atherogenic lipoprotein levels. Eur Heart J. 2019;40:2785–2796. doi: 10.1093/eurheartj/ehz209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tardif JC, Karwatowska-Prokopczuk E, Amour ES, Ballantyne CM, Shapiro MD, Moriarty PM, et al. Apolipoprotein C-III reduction in subjects with moderate hypertriglyceridaemia and at high cardiovascular risk. Eur Heart J. 2022;43:1401–1412. doi: 10.1093/eurheartj/ehab820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schwabe C, Scott R, Sullivan D, Baker J, Clifton P, Hamilton J, et al. RNA interference targeting apolipoprotein C-III with ARO-APOC3 in healthy volunteers mimics lipid and lipoprotein findings seen in subjects with inherited apolipoprotein C-III deficiency. Eur Heart J. 2020;41:ehaa946.3330 [Google Scholar]
- 89.Clifton P, Sullivan D, Baker J, Schwabe C, Thackwray S, Scott R, et al. Abstract 12594: pharmacodynamic effect of ARO-APOC3, an investigational hepatocyte-targeted RNA interference therapeutic targeting apolipoprotein C3, in patients with hypertriglyceridemia and multifactorial chylomicronemia. Circulation. 2020;142(Suppl 3):A12594 [Google Scholar]
- 90.Clifton P, Sullivan D, Baker J, Schwabe C, Thackwray S, Scott R, et al. Abstract 10357: ARO-APOC3, an investigational RNAi therapeutic, shows similar efficacy and safety in genetically confirmed FCS and non-FCS participants with severe hypertriglyceridemia. Circulation. 2021;144(Suppl 1):A10357 [Google Scholar]
- 91.Khetarpal SA, Zeng X, Millar JS, Vitali C, Somasundara AV, Zanoni P, et al. A human APOC3 missense variant and monoclonal antibody accelerate apoC-III clearance and lower triglyceride-rich lipoprotein levels. Nat Med. 2017;23:1086–1094. doi: 10.1038/nm.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]