Abstract
The effectiveness of pasteurization and the concentration of Mycobacterium avium subsp. paratuberculosis in raw milk have been identified in quantitative risk analysis as the most critical factors influencing the potential presence of viable Mycobacterium paratuberculosis in dairy products. A quantitative assessment of the lethality of pasteurization was undertaken using an industrial pasteurizer designed for research purposes with a validated Reynolds number of 62,112 and flow rates of 3,000 liters/h. M. paratuberculosis was artificially added to raw whole milk, which was then homogenized, pasteurized, and cultured, using a sensitive technique capable of detecting one organism per 10 ml of milk. Twenty batches of milk containing 103 to 104 organisms/ml were processed with combinations of three temperatures of 72, 75, and 78°C and three time intervals of 15, 20, and 25 s. Thirty 50-ml milk samples from each processed batch were cultured, and the logarithmic reduction in M. paratuberculosis organisms was determined. In 17 of the 20 runs, no viable M. paratuberculosis organisms were detected, which represented >6-log10 reductions during pasteurization. These experiments were conducted with very heavily artificially contaminated milk to facilitate the measurement of the logarithmic reduction. In three of the 20 runs of milk, pasteurized at 72°C for 15 s, 75°C for 25 s, and 78°C for 15 s, a few viable organisms (0.002 to 0.004 CFU/ml) were detected. Pasteurization at all temperatures and holding times was found to be very effective in killing M. paratuberculosis, resulting in a reduction of >6 log10 in 85% of runs and >4 log10 in 14% of runs.
Mycobacterium paratuberculosis causes chronic, infectious enteritis known as Johne's disease, or paratuberculosis, in cattle and other ruminants. The organism is shed in feces and has also been isolated from milk. M. paratuberculosis has been proposed as a potential cause of Crohn's disease in humans, with consumption of milk being suggested as a possible source of infection. However, the cause of Crohn's disease is still uncertain, and there is no substantiated causal link between Johne's disease and Crohn's disease.
In 1996, a survey of whole, pasteurized cows' milk obtained from retail outlets in England and Wales was conducted to determine the presence of M. paratuberculosis (15). The IS900 PCR product was detected in 7% (22 of 312) of milk samples tested, and the presence of acid-fast organisms with typical M. paratuberculosis morphology in long-term cultures was described. The cultures were IS900 PCR positive but were overgrown with other organisms. More recently, the isolation of M. paratuberculosis from 1.8% (10 of 567) of pasteurized milk samples in the United Kingdom was reported (8).
Laboratory simulation methods have been used to investigate the effectiveness of the pasteurization of milk at 63°C for 30 min (low temperature, long time) and 72 to 75°C for 15 to 20 s (high temperature, short time [HTST]) in killing M. paratuberculosis. The most common methods for measuring thermal susceptibility employ batch heating in open vials, closed vials, or a Franklin pasteurizing unit (2, 6, 7, 12, 19, 23). Laboratory simulation of pasteurization has been criticized as a method for evaluating the heat resistance of microorganisms, due to differential heat distribution (1).
The effects of pasteurization on M. paratuberculosis have also been investigated in a few studies using pilot-scale commercial HTST simulation methods (10, 11, 18-20). Small-scale units may have laminar flow in the holding tube, and correction factors are used to ensure that minimum holding times are achieved, whereas commercial pasteurizers use continuous turbulent-flow pasteurization. M. paratuberculosis was not recovered using HTST conditions (72°C at an equivalent of 15 s) when milk was inoculated with 102 to 106 CFU of different strains of M. paratuberculosis per ml or when milk was inoculated with mammary gland macrophages containing ingested M. paratuberculosis (10, 11, 18, 20). Culture techniques for isolating M. paratuberculosis from pasteurized milk have been criticized for low recovery of mycobacteria due to harsh decontamination of samples (5). A minimum reduction of >4 log10 M. paratuberculosis organisms was achieved using a pilot-scale turbulent-flow pasteurizer with a maximum flow rate of 200 kg/h, heat treatment at 72°C for 15 s, and mild sample decontamination (17).
Only one study has evaluated HTST pasteurization conditions (73°C for 15 or 25 s) using a commercial-scale pasteurizer (9). It was reported that M. paratuberculosis was isolated from 6.9% (10 of 144) of pasteurized milk samples. The raw milk had been obtained from two herds with an unknown prevalence of Johne's disease, and the concentration of M. paratuberculosis organisms in the bulk raw milk was not determined.
Our study evaluated the efficacy of pasteurization in killing M. paratuberculosis by measuring the reduction of viable M. paratuberculosis organisms following homogenization and heat treatment using a commercial-scale pasteurization unit with the capacity to process 3,000 liters/h. Different temperatures and holding times were evaluated, and a mild decontamination culture protocol was used to recover M. paratuberculosis from heat-treated milk containing known concentrations of M. paratuberculosis organisms.
MATERIALS AND METHODS
Industrial pasteurizer.
The industrial pasteurizer was designed for research purposes to simulate a commercial, turbulent, continuous-flow milk pasteurizer (22) with a Reynolds number of 62,112. The holding tube consisted of three sections to achieve holding times of 15, 20, and 25 s.
Holding tube test.
The residence time distribution of the holding tubes was tested once a stable flow rate had been achieved on heated water. A salt solution was injected into the holding tube, and the conductivity was measured to determine the amount of time that the milk would be held in the holding tubes. Each tube was tested at least seven times.
Plate heat exchanger test.
The plate heat exchanger in the pasteurizer used for heating the milk to the pasteurizing temperature and to cool it down after the holding period was tested for leaks to ensure that pasteurized milk could not be contaminated by raw milk. A salt solution was passed through one section, and water was passed through the partitioned section, both under pressure. The conductivity of both sections was measured for 15 min.
Pasteurizer operation.
The pasteurizer was sterilized using hot water heated to 85°C just prior to use. The production mode started once the pasteurizer had stabilized at the chosen temperature in the 15-s holding tube. The milk was processed, and prior to the commencement of sampling from the outlet hose, allowance was made for the residence volume of the unit. For the longer holding times of 20 and 25 s, the holding tube was lengthened and sterilized and the temperature was stabilized before the production mode commenced. The inlet and outlet holding tube temperatures and flow rate were recorded every 10 s during plant operation.
Raw milk.
Four batches of fresh raw milk were delivered on the mornings of the trials. Each batch of milk was tested by the supplier for butterfat, protein, nonfat solids, lactose, antibiotics, and direct microscopic counts of the number of bacteria present. A sample of raw milk was collected prior to inoculation with M. paratuberculosis for a total plate count to estimate the number of contaminating bacteria present.
Inoculation of raw milk.
Raw milk was inoculated with either a mixture of four field isolates of M. paratuberculosis restriction fragment length polymorphism strain C1 or a mixture of five field isolates of M. paratuberculosis restriction fragment length polymorphism strain C3 (Table 1) (25). All isolates of M. paratuberculosis were grown separately in static cultures with modified Watson and Reid media (16) and harvested after several months of incubation at 37°C when a confluent pellicle of cells had formed. M. paratuberculosis isolates were washed twice with phosphate-buffered saline (PBS; pH 7.2), the C1 isolates were combined, and the C3 isolates were combined. C1 and C3 isolates were stored at −80°C prior to use. Organisms were resuspended in about 500 ml of milk and then added to the vat while the milk in the vat was mixed thoroughly. Five samples were collected for culture from each batch of prehomogenized inoculated milk to determine the concentration of organisms in the raw milk (21).
TABLE 1.
Temperatures and holding times used to process four batches of milk inoculated with M. paratuberculosis strain C1 or C3 on four separate days
| Temp (°C) | Holding time (s) | Day milk collected and inoculated with M. paratuberculosis strain:
|
|||
|---|---|---|---|---|---|
| C1 | C1 | C3 | C3 | ||
| 72 | 15 | 1 | 2 | 3 | 4 |
| 20 | 1 | 3 | |||
| 25 | 2 | 4 | |||
| 75 | 15 | 1 | 3 | ||
| 20 | 1 | 3 | |||
| 25 | 2 | 4 | |||
| 78 | 15 | 2 | 4 | ||
| 20 | 1 | 3 | |||
| 25 | 2 | 4 | |||
Homogenization conditions.
Following inoculation, each batch of milk was homogenized using a two-stage homogenizer. The homogenizer processed milk at a rate of 1,050 liters/h under a pressure of 27,000 kg/cm2 to reduce fat globules to 1 μm in diameter. Each batch of homogenized milk was thoroughly mixed while five samples were collected from each batch (21). These samples were cultured to enumerate the concentration of M. paratuberculosis organisms prior to pasteurization and to monitor dispersal and mixing of the organisms in the milk.
Pasteurization conditions.
Four batches of raw milk containing M. paratuberculosis strain C1 or strain C3 were processed on four separate days using nine combinations of three heat and three holding times, with additional runs of 72°C for 15 s (Table 1). About 200 liters of milk was processed for each run. Ten samples of milk were collected from each run directly into containers from the outlet flow of the pasteurizer.
Analytical sensitivity of culture.
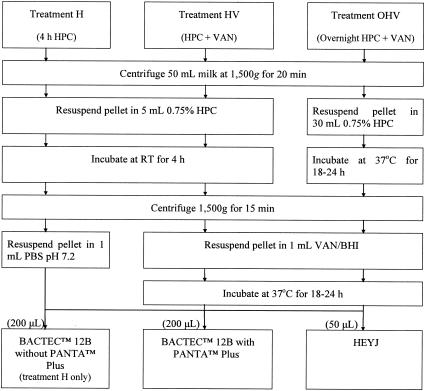
Three culture treatments were used to culture the raw and pasteurized milk samples (Fig. 1). The treatments consisted of the following components: hexadecylpyridinium chloride (HPC) for 4 h (treatment H), HPC (4 h) plus 100 μl each of vancomycin, amphotericin B, and nalidixic acid (VAN) (treatment HV), and HPC (overnight) plus VAN (treatment OHV). The detection limits of two of these culture treatments, H and OHV, were determined by using a C1 field strain of M. paratuberculosis. The field strain was harvested from Watson and Reid media after growth at 37°C for several months had produced a confluent pellicle of cells. Harvested cells were washed three times in PBS, pH 7.2, and resuspended in 5 ml of PBS, pH 7.2. Inoculum concentrations were determined by culturing 10-fold dilutions. Ultrahigh-temperature-processed milk was inoculated with the suspension of the C1 field strain of M. paratuberculosis to a final concentration of 7.3 × 105 CFU/ml, and 10-fold dilutions were prepared in triplicate. The samples were processed using treatments H and OHV and inoculated into supplemented BACTEC 12B bottles (BD, North Ryde, New South Wales, Australia). All cultures were incubated at 37°C and monitored for growth for at least 8 weeks. BACTEC 12B cultures with positive growth indices were subcultured to Herrold's egg yolk slopes supplemented with mycobactin J (HEYJ; Allied Monitor, Fayette, Mo.) (14). Growth on HEYJ was confirmed as M. paratuberculosis by PCR using primers IS900/150C and IS900/921 (24). The concentration of M. paratuberculosis organisms was determined by colony counting and the most-probable-number method by using Genstat 6 (VSN International Ltd.).
FIG. 1.
Procedure for decontamination and preparation of milk samples for culture using treatments H, HV, and OHV.
Processing and culture of milk samples.
Milk samples collected prehomogenization, posthomogenization, and following pasteurization were transported on ice and processed for culture the same day. Each milk sample was mixed thoroughly, divided into three 50-ml volumes, and prepared for culture by using treatments H, HV, and OHV (Fig. 1). Following decontamination, the resuspended pellet and serial 10-fold dilutions prepared from the pellet were inoculated into BACTEC 12B (BD) (treatment H), into BACTEC 12B supplemented with PANTA Plus (BD) (treatments H, HV, and OHV), and onto HEYJ (treatments H, HV, and OHV).
The cultures taken from the milk prior to pasteurization were monitored for growth for at least 20 weeks, and the cultures from the pasteurized milk were monitored for growth for at least 28 weeks. BACTEC cultures with a growth index of >500 were subcultured onto solid media. Cultures showing growth with the colony morphology typical of M. paratuberculosis, dependence on mycobactin J, and the presence of IS900 were classified as M. paratuberculosis. The concentration of M. paratuberculosis was determined by counting colonies on HEYJ and most-probable-number calculations of positive growth in the BACTEC dilution series, using Genstat 6. The results of the different culture treatments used for prehomogenized and posthomogenized milk were compared using Kruskal-Wallis one-way analysis of variance in Genstat 6.
RESULTS
Validation of pasteurizer operating conditions.
No leaks were detected in the plate heat exchanger. The holding tubes were calibrated and standardized. Flow rates of 2,715 to 2,730 liters/h for the short tube, 2,816 to 2,840 liters/h for the medium tube, and 2,885 to 2,935 liters/h for the long tube produced average holding times of 15.11 ± 0.04, 20.36 ± 0.10, and 25.16 ± 0.03 s, respectively. The temperature of the milk at the start and end of each holding tube and following pasteurization was monitored and recorded every 10 s during each trial (Table 2). The flow rate for each tube was also monitored and recorded every 10 s. The flow rates ranged from 2,694 to 2,734 liters/h for the short tube, 2,816 to 2,798 liters/h for the medium tube, and 2,837 to 2,894 liters/h for the long tube. For each run programmed at 72°C for 15 s, the mean temperature of the holding tube ranged from 72.1 to 74.3°C, and the mean flow rate ranged from 2,711 to 2,718 liters/h. The mean temperature of the milk following pasteurization ranged from 5.5 to 8.4°C for each trial immediately before the milk was placed on ice.
TABLE 2.
Monitored temperature profiles of holding tubes for the pasteurization runs
| Programmed temp (°C) | Holding tube temp (°C)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Inlet
|
Outlet
|
|||||||
| Mean | Minimum | Maximum | SD | Mean | Minimum | Maximum | SD | |
| 72 | 73.3 | 72.6 | 74.4 | 0.4 | 72.5 | 72.1 | 73.8 | 0.5 |
| 75 | 76.0 | 75.3 | 76.7 | 0.4 | 75.6 | 75.0 | 76.5 | 0.5 |
| 78 | 79.1 | 78.6 | 79.6 | 0.3 | 78.3 | 77.9 | 78.6 | 0.2 |
Raw milk.
Prior to inoculation of the milk with M. paratuberculosis, the number of bacteria in each batch of raw milk ranged from 4 × 104 to 1.2 × 105 organisms/ml, as estimated by direct microscopic count. The number of viable bacteria estimated by total plate counts ranged from 4.7 × 103 to 1.4 × 105 CFU/ml. No antibiotics were detected in the milk batches, and the milk contained 3.86 ± 0.01 g of butterfat per kg, 3.48 ± 0.02 g of protein per kg, 9.12 ± 0.03 g of nonfat solids per kg, and 4.85 ± 0.01 g of lactose per kg.
Analytical sensitivity of culture.
For culture treatment OHV, which employed two overnight decontamination steps (one in HPC and one in half-strength brain heart infusion broth containing VAN), the lower detection limit for M. paratuberculosis was 1 CFU/ml of milk. The more sensitive detection limit for treatment H, which used a milder decontamination step of HPC for 4 h, was 1 CFU/10 ml of milk.
Concentrations of M. paratuberculosis organisms in prehomogenized and posthomogenized milk.
The M. paratuberculosis concentration was 1 log10 less in prehomogenized milk than in posthomogenized milk by use of treatment H and HEYJ slopes, but there was no significant difference between prehomogenized and posthomogenized milk samples with BACTEC 12B (P = 0.916). The M. paratuberculosis concentration in posthomogenized milk ranged from 4 × 102 to 8 × 103 CFU/ml on HEYJ slopes and from 8 × 101 to 2 × 103 CFU/ml in BACTEC 12B.
Pasteurized milk.
In 17 of 20 pasteurization runs at 72, 75, and 78°C for 15, 20, and 25 s, there was a >6-log10 reduction in numbers of M. paratuberculosis organisms, as no viable M. paratuberculosis cells were detected in 1.5 liters of pasteurized milk cultured and inoculated with 4 × 102 to 8 × 103 CFU of M. paratuberculosis per ml. M. paratuberculosis C1 or C3 was recovered only from milk from three runs with heat treatments of 72°C for 15 s, 75°C for 25 s, and 78°C for 15 s at concentrations of 0.004 and 0.002 CFU/ml, which correspond to 4- to 6-log10 reductions. M. paratuberculosis was recovered from samples subjected to these three heat treatments when culture treatment H and BACTEC 12B supplemented with and without PANTA Plus were used.
DISCUSSION
This study used large volumes of milk that were inoculated with known concentrations of M. paratuberculosis organisms, homogenized, and pasteurized with an industrial pasteurizer with a continuous turbulent flow of 3,000 liters/h to represent large-scale dairy processing operations. Only one other study has evaluated the effect of pasteurization on M. paratuberculosis by use of a commercial-scale, turbulent-flow pasteurizer, but concentrations of organisms were not determined (9). Most previous studies have used small volumes of milk and laboratory simulation or small-scale units (2, 4, 6, 7, 10-12, 17-20, 23).
Heat treatments of 72 to 78°C for 15 to 25 s effectively killed >6 log10 M. paratuberculosis cells in 85% of runs and >4 log10 M. paratuberculosis cells in the remaining runs. Our findings that were obtained by using an industrial pasteurizer with continuous turbulent flow for the heat treatments were consistent with those of previous reports. Other studies that have employed pilot-scale pasteurizers have not recovered M. paratuberculosis from milk loaded with 102 to 106 CFU/ml and heated at 72°C for 15 s (11, 17, 18, 20). The extrapolated reduction by heat treatment at 72°C for 15 s was reported to be a >7-log10 kill (17). In our study, M. paratuberculosis was recovered in very low numbers (1 CFU/250 to 500 ml) from 3 of 20 (15%) pasteurization runs at 72°C for 15 s, 75°C for 25 s, and 78°C for 15 s. Other studies have also recovered M. paratuberculosis from milk pasteurized at higher temperatures and longer times than minimum pasteurization conditions of 72°C for 15 s, particularly when high concentrations of M. paratuberculosis cells were inoculated (7, 8, 10, 15). Corrections for nonturbulent flow, however, are critical. It was reported that, in experiments performed with a pilot-scale pasteurizer that produced logarithmic reductions of 2 to 6, the average residence time of 18 s was calculated to be borderline for achieving a minimum residence time of 15 s (10).
The raw milk was inoculated with M. paratuberculosis field isolates that had been subcultured on fewer than three occasions in our laboratory. It has been suggested that different strains of M. paratuberculosis and laboratory-adapted strains may vary in their heat susceptibility (13). There appeared to be no difference in heat susceptibility between the two cattle strains (C1 and C3) of M. paratuberculosis used in this study.
The concentration of M. paratuberculosis organisms in prehomogenized milk was 1 log10 lower than in posthomogenized milk as determined by colony counting on HEYJ, which indicates that there might have been some dispersal of M. paratuberculosis aggregates in the inoculum. It has been suggested that declumped M. paratuberculosis organisms were more susceptible to heat treatment than clumped organisms, due to mechanical damage (7). However, other studies have shown no difference in the heat susceptibility of clumped and declumped M. paratuberculosis cells (12, 23). Since most retail milk in Australia is homogenized prior to pasteurization, any effect of homogenization on the survival of M. paratuberculosis during processing would reflect commercial treatment and conditions.
Comparing the ability of culture to recover M. paratuberculosis from milk using the decontamination treatments in this study, there was 1-log10-greater sensitivity for the treatment with minimum decontamination. The most sensitive culture treatment for isolating M. paratuberculosis, with a detection limit of 1 CFU/10 ml, was achieved using a short decontamination time in HPC at room temperature. Similar findings in sensitivity have been reported for treatment of milk with 0.75% HPC for 5 h, compared with treatment of milk with 0.75% HPC and VAN (3). The use of chemicals and antibiotics to remove contaminating microbes would potentially kill some M. paratuberculosis cells. The harshness of the chemical and antibiotic treatments required depends on the number and type of contaminating microbes present in the sample. In our study, a mild decontamination treatment was used. This type of treatment had no impact on the measurement of the logarithmic reduction, because the reduction was determined from comparisons of cultures and enumerations of organisms in milk before and after heat treatment using the same culture methods.
The focus of this study was to determine the effectiveness of heat treatment in a continuous, turbulent-flow pasteurizer in killing M. paratuberculosis by using sensitive culture methods. Pasteurization was determined to reduce the number of CFU of M. paratuberculosis by at least 4 log10, and in most processing runs, reductions were by >6 log10 M. paratuberculosis CFU at temperatures of 72 to 78°C and holding times of 15 to 25 s by a culture protocol with reduced decontamination and large volumes of milk.
Acknowledgments
This study was supported by Dairy Australia.
We express our appreciation to Nick Kydas from the Dairy Process Engineering Centre for commissioning and operating the pasteurization unit and to John Near for providing equipment, facilities, and assistance.
REFERENCES
- 1.Cerf, O., and M. W. Griffiths. 2000. Mycobacterium paratuberculosis heat resistance. Lett. Appl. Microbiol. 30:341-344. [DOI] [PubMed] [Google Scholar]
- 2.Chiodini, R. J., and J. Hermon-Taylor. 1993. The thermal resistance of Mycobacterium paratuberculosis in raw milk under conditions simulating pasteurization. J. Vet. Diagn. Investig. 5:629-631. [DOI] [PubMed] [Google Scholar]
- 3.Dundee, L., I. R. Grant, H. J. Ball, and M. T. Rowe. 2001. Comparative evaluation of four decontamination protocols for the isolation of Mycobacterium avium subsp. paratuberculosis from milk. Lett. Appl. Microbiol. 33:173-177. [DOI] [PubMed] [Google Scholar]
- 4.Gao, A., L. Mutharia, S. Chen, K. Rahn, and J. Odumeru. 2002. Effect of pasteurization on survival of Mycobacterium paratuberculosis in milk. J. Dairy Sci. 85:3198-3205. [DOI] [PubMed] [Google Scholar]
- 5.Grant, I. R., and J. R. Stabel. 1998. Does Mycobacterium paratuberculosis survive current pasteurization conditions? Appl. Environ. Microbiol. 64:2760-2761. (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant, I. R., H. J. Ball, S. D. Neill, and M. T. Rowe. 1996. Inactivation of Mycobacterium paratuberculosis in cows' milk at pasteurization temperatures. Appl. Environ. Microbiol. 62:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant, I. R., H. J. Ball, and M. T. Rowe. 1999. Effect of higher pasteurization temperatures, and longer holding times at 72 degrees C, on the inactivation of Mycobacterium paratuberculosis in milk. Lett. Appl. Microbiol. 28:461-465. [DOI] [PubMed] [Google Scholar]
- 8.Grant, I. R., H. J. Ball, and M. T. Rowe. 2002. Incidence of Mycobacterium paratuberculosis in bulk raw and commercially pasteurized cows' milk from approved dairy processing establishments in the United Kingdom. Appl. Environ. Microbiol. 68:2428-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant, I. R., E. I. Hitchings, A. McCartney, F. Ferguson, and M. T. Rowe. 2002. Effect of commercial-scale high-temperature, short-time pasteurization on the viability of Mycobacterium paratuberculosis in naturally infected cows' milk. Appl. Environ. Microbiol. 68:602-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammer, P., C. Kiesner, H.-G. Walte, K. Knappstein, and P. Teufel. 2002. Heat resistance of Mycobacterium avium subsp. paratuberculosis in raw milk. Kiel. Milchwirtsch. Forschungsber. 54:275-303. [Google Scholar]
- 11.Hope, A. F., P. A. Tulk, and R. J. Condron. 1996. Commercial pasteurization of Mycobacterium paratuberculosis in whole milk, p. 377-382. In R. J. Chiodini, M. E. Hines II, and M. T. Collins (ed.), Proceedings of the Fifth International Colloquium on Paratuberculosis. International Association for Paratuberculosis, Madison, Wis.
- 12.Keswani, J., and J. F. Frank. 1998. Thermal inactivation of Mycobacterium paratuberculosis in milk. J. Food Prot. 61:974-978. [DOI] [PubMed] [Google Scholar]
- 13.Klijn, N., A. A. Herrewegh, and P. de Jong. 2001. Heat inactivation data for Mycobacterium avium subsp. paratuberculosis: implications for interpretation. J. Appl. Microbiol. 91:697-704. [DOI] [PubMed] [Google Scholar]
- 14.Merkal, R. S. 1970. Diagnostic methods for detection of paratuberculosis (Johne's disease), p. 620-623. In Proceedings of the 74th Annual Meeting of the U.S. Animal Health Association. USAHA, Philadelphia, Pa.
- 15.Millar, D., J. Ford, J. Sanderson, S. Withey, M. Tizard, T. Doran, and J. Hermon-Taylor. 1996. IS900 PCR to detect Mycobacterium paratuberculosis in retail supplies of whole pasteurized cows' milk in England and Wales. Appl. Environ. Microbiol. 62:3446-3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison, N. E. 1965. Circumvention of the mycobactin requirement of Mycobacterium paratuberculosis. J. Bacteriol. 89:762-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearce, L. E., H. T. Truong, R. A. Crawford, G. F. Yates, S. Cavaignac, and G. W. de Lisle. 2001. Effect of turbulent-flow pasteurization on survival of Mycobacterium avium subsp. paratuberculosis added to raw milk. Appl. Environ. Microbiol. 67:3964-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stabel, J. R. 2000. Johne's disease and milk: do consumers need to worry? J. Dairy Sci. 83:1659-1663. [DOI] [PubMed] [Google Scholar]
- 19.Stabel, J. R., E. M. Steadham, and C. A. Bolin. 1996. Heat inactivation of Mycobacterium paratuberculosis in raw milk using holder-test tube method and lab-scale industrial pasteurization method, p. 331-333. In Proceedings of the 100th Annual Meeting of the U.S. Animal Health Association, Little Rock, Ark.
- 20.Stabel, J. R., E. M. Steadham, and C. A. Bolin. 1997. Heat inactivation of Mycobacterium paratuberculosis in raw milk: are current pasteurization conditions effective? Appl. Environ. Microbiol. 63:4975-4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Standards Australia. 1992. Milk and milk products—methods of sampling. Australian standard 1166. Standards Australia, Sydney, Australia.
- 22.Standards Australia. 1992. Equipment for the pasteurization of milk and other liquid dairy products. Part 1. Continuous-flow systems. Australian standard 3993.1. Standards Australia, Sydney, Australia.
- 23.Sung, N., and M. T. Collins. 1998. Thermal tolerance of Mycobacterium paratuberculosis. Appl. Environ. Microbiol. 64:999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vary, P. H., P. R. Andersen, E. Green, J. Hermon-Taylor, and J. J. McFadden. 1990. Use of highly specific DNA probes and the polymerase chain reaction to detect Mycobacterium paratuberculosis in Johne's disease. J. Clin. Microbiol. 28:933-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whittington, R. J., A. F. Hope, D. J. Marshall, C. A. Taragel, and I. Marsh. 2000. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis: IS900 restriction fragment length polymorphism and IS1311 polymorphism analyses of isolates from animals and a human in Australia. J. Clin. Microbiol. 38:3240-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]