Summary
Background
Pre-exposure vaccination with MVA-BN has been widely used against mpox to contain the 2022 outbreak. Many countries have defined prioritized strategies, administering a single dose to those historically vaccinated for smallpox, to achieve quickly adequate coverage in front of low supplies. Using epidemiological models, real-life effectiveness was estimated at approximately 36%–86%, but no clinical trials were performed. Few data on MVA-BN immunogenicity are currently available, and there are no established correlates of protection. Immunological response in PLWH in the context of the 2022 outbreak was also poorly described.
Methods
Blood samples were collected from participants eligible for pre-exposure MVA-BN vaccination before (T1) receiving a full course of vaccine (single-dose for vaccine-experienced or smallpox-primed and two-dose for smallpox vaccine-naïve or smallpox non-primed) and one month after the last dose (T2 and T3, respectively). MPXV-specific IgGs were measured by in-house immunofluorescence assay, using 1:20 as screening dilution, MPXV-specific nAbs by 50% plaque reduction neutralization test (PRNT50, starting dilution 1:10), and IFN-γ-producing specific T cells to MVA-BN vaccine, by ELISpot assay. Paired or unpaired t-test and Wilcoxon or Mann–Whitney test were used to analyse IgG and nAbs, and T-cell response, as appropriate. The probability of IgG and nAb response in vaccine-experienced vs. vaccine-naïve was estimated in participants not reactive at T1. The McNemar test was used to evaluate vaccination's effect on humoral response both overall and by smallpox vaccination history. In participants who were not reactive at T1, the proportion of becoming responders one month after full-cycle completion by exposure groups was compared by logistic regression and then analysed by HIV status strata (interaction test). The response was also examined in continuous, and the Average Treatment Effect (ATE) of the difference from baseline to schedule completion according to previous smallpox vaccination was estimated after weighting for HIV using a linear regression model. Self-reports of adverse effects following immunization (AEFIs) were prospectively collected after the first MVA-BN dose (T1). Systemic (S-AEFIs: fatigue, myalgia, headache, GI effects, chills) and local (L-AEFIs: redness, swelling, pain) AEFIs were graded as absent (grade 0), mild (1), moderate (2), or severe (3). The maximum level of severity for S-AEFIs and L-AEFIs ever experienced over the 30 days post-dose by vaccination exposure groups were analysed using a univariable multinomial logistic regression model and after adjusting for HIV status; for each of the symptoms, we also compared the mean duration by exposure group using an unpaired t-test.
Findings
Among the 164 participants included, 90 (54.8%) were smallpox vaccine-experienced. Median age was 49 years (IQR 41–55). Among the 76 (46%) PLWH, 76% had a CD4 count >500 cells/μL. There was evidence that both the IgG and nAbs titers increased after administration of the MVA-BN vaccine. However, there was no evidence for a difference in the potential mean change in humoral response from baseline to the completion of a full cycle when comparing primed vs. non-primed participants. Similarly, there was no evidence for a difference in the seroconversion rate after full cycle vaccination in the subset of participants not reactive for nAbs at T1 (p = 1.00 by Fisher's exact test). In this same analysis and for the nAbs outcome, there was some evidence of negative effect modification by HIV (interaction p-value = 0.17) as primed people living with HIV (PLWH) showed a lower probability of seroconversion vs. non-primed, and the opposite was seen in PLWoH. When evaluating the response in continuous, we observed an increase in T-cell response after MVA-BN vaccination in both primed and non-primed. There was evidence for a larger increase when using the 2-dose vs. one-dose strategy with a mean difference of −2.01 log2 (p ≤ 0.0001), after controlling for HIV. No evidence for a difference in the risk of developing any AEFIs of any grade were observed by exposure group, except for the lower risk of grade 2 (moderate) fatigue, induration and local pain which was lower in primed vs. non-primed [OR 0.26 (0.08–0.92), p = 0.037; OR 0.30 (0.10–0.88), p = 0.029 and OR 0.19 (0.05–0.73), p = 0.015, respectively]. No evidence for a difference in symptom duration was also detected between the groups.
Interpretation
The evaluation of the humoral and cellular response one month after the completion of the vaccination cycle suggested that MVA-BN is immunogenic and that the administration of a two-dose schedule is preferable regardless of the previous smallpox vaccination history, especially in PLWH, to maximize nAbs response. MVA-BN was safe as well tolerated, with grade 2 reactogenicity higher after the first administration in vaccine-naïve than in vaccine-experienced individuals, but with no evidence for a difference in the duration of these adverse effects. Further studies are needed to evaluate the long-term duration of immunity and to establish specific correlates of protection.
Funding
The study was supported by the National Institute for Infectious Disease Lazzaro Spallanzani IRCCS “Advanced grant 5 × 1000, 2021” and by the Italian Ministry of Health “Ricerca Corrente Linea 2”.
Keywords: mpox, MVA-BN immunogenicity, Reactogenicity, Cellular response, Humoral response, HIV, PLWH, Vaccination, Vaccine, Orthopox, MVA-BN
Research in context.
Evidence before this study
The third-generation vaccine MVA-BN was licensed against smallpox on the basis of data from studies on animals showing that the vaccine elicits robust humoral and cellular immune responses and clinical protection against severe mpox and randomized clinical trials in humans that demonstrated the safety of MVA-BN and its immunogenicity comparable to that of ACAM 2000.
Data from 2022 real-world studies estimated the adjusted vaccine effectiveness ranging from 36% to 86% and the humoral response to MVA-BN vaccination was described as moderate in terms of antibody (IgG) serum response or low regarding the neutralizing response against mpox, in contrast to a strong specific MPXV cellular response. To date, no immunological correlates of protection have been identified.
Added value of this study
This study represents the first prospective evaluation of early humoral and cellular immune response to MVA-BN in more than 150 high-risk people for mpox infection during the 2022 vaccination campaign, and the analysis was focused on estimating the causal effect of the vaccination strategy (one dose vs. two doses) and role of HIV infection on the immune response.
Data showed that a two-dose course in naïve donors elicited a stronger stimulus to the cellular response compared to a single-dose course in vaccine-experienced, while there was no evidence for a difference in IgG and nAbs response by vaccination strategy. In contrast, in the subset of PLWH, a single-dose cycle in smallpox-primed elicited a detectable MPXV-specific nAb response only in less than half of the participants with a reduced probability of becoming reactive for nAbs, compared to the two-shot vaccination in smallpox non-primed.
Finally, our study showed that MVA-BN vaccination was globally well tolerated, and grade 2 reactogenicity seemed to be higher after the first dose of the two-dose course than the single-dose while the data carried no evidence for a difference in mean duration of symptoms between the exposure groups.
Implications of all the available evidence
Data from our evaluation of the humoral and cellular response one month after the completion of the vaccination cycle suggest that MVA-BN is safe and immunogenic and that the administration of a two-dose schedule is preferable regardless of the previous smallpox vaccination history, especially in PLWH to maximize their nAbs response. In the absence of randomized clinical trials, immunobridging studies are of special importance.
Introduction
As of May 2022, a mpox epidemic had rapidly spread across many non-endemic countries, causing more than 90,000 cases worldwide to date.1 As reported by the major global case series,2,3 gay, bisexual, and other men who have sex with men (GBMSM) have been most affected during the outbreak. A transmission route mainly during sexual intercourse has been hypothesized4 and supported by the isolation of the replication-competent virus in seminal fluid.5
From July 23rd, 2022 to May 10th, 2023, the World Health Organization (WHO) declared mpox a public health emergency of international concern (PHEIC) and, among the various tools to contain the epidemic, recommended vaccination both as post-exposure prophylaxis for contacts and as pre-exposure prophylaxis for high-risk groups.6
Consequently, the European Medicines Agency (EMA)7 and the Food and Drug Administration (FDA)8 authorized the use of modified vaccinia Ankara-Bavarian Nordic (MVA-BN) for the prevention of mpox.
MVA-BN is a third-generation vaccine based on the replication-deficient modified vaccinia Ankara, licensed against smallpox9 with a subcutaneous administration schedule of two doses delivered at least 28 days apart. In the non-human primate model, the vaccine elicited a robust humoral and cellular immune response and clinical protection against severe mpox diseases and death.10 In humans, randomized clinical trials (RCTs) demonstrated the safety and immunogenicity of MVA-BN as comparable to that of previously available second-generation smallpox vaccine ACAM 2000.11 Differently from the first- and second-generation smallpox vaccines, MVA-BN can be administered to immunocompromised patients due to its non-replicating mechanism and RCTs have previously shown in people living with HIV (PLWH) a humoral response comparable to that in people living without HIV (PLWoH).12,13
Due to the limited supplies to vaccinate, during the 2022 mpox outbreak, all risk groups with an adequate number of doses, vaccination with at least one dose has been prioritized in many countries, reserving the two-dose cycle for vaccinia-naïve individuals. Moreover, the EMA and FDA issued a recommendation to support vaccination dose-sparing strategies by administering the vaccine through the intradermal route, which has been demonstrated to be as safe and immunogenic as the subcutaneous route.14, 15, 16
Data from real-world studies conducted during the 2022 outbreak through epidemiological models estimated a lower rate of mpox infection in recipients of the MVA-BN vaccination compared to the unvaccinated, with different estimates of effectiveness varying from 36% to 86%17, 18, 19, 20
Despite these data, no immunological correlates of protection have yet been identified. The humoral response to MVA-BN vaccination was recently described as moderate in terms of antibody (IgG) serum response21 or low regarding the neutralizing response against mpox,22 in contrast to a strong specific MPXV cellular response.21,23 Conversely, another study24 demonstrated a substantial and durable humoral response (against the vaccinia virus) both in primed receiving one dose and in not-primed receiving one or two doses of MVA-BN.
The aim of this study was to evaluate the immunogenicity, in terms of the humoral and cellular immune response, and reactogenicity according to previous smallpox vaccination and HIV infection, after the administration of MVA-BN delivered as pre-exposure prophylaxis to high-risk groups by a vaccination program of an Italian Region during the 2022 mpox outbreak.
Methods
Patients’ enrollment
The Lazio Region vaccination campaign started in August 8th, 2022, following recommendations from the Ministry of Health.25 All eligible persons in the Lazio region received the vaccine in a hospital setting at the Lazzaro Spallanzani National Institute for Infectious Diseases, which was identified, therefore, as the only vaccine center in the entire region. The target population was GBMSM at high risk, defined as reporting multiple sexual partners, participation in group sex events, sexual encounters in clubs/cruising/saunas, recent sexually transmitted infections (STIs), or sexual acts associated with the use of chemical drugs (Chemsex), together with laboratory personnel with possible direct exposure to orthopoxviruses (OPXV). The vaccination schedule consisted of a two-dose cycle administered at least 28 days apart for individuals who had never received the smallpox vaccine (vaccine-naïve or non-primed) and a single-dose cycle for persons who had received the smallpox vaccine (vaccine-experienced or primed) in the past. Of note, in Italy, the smallpox vaccination campaign was stopped in 1977 and officially abrogated in 1981.26 For the first two weeks of the vaccination campaign, MVA-BN was administered subcutaneously, then the intradermal route was adopted, following the ministerial indications.27 A prospective observational cohort was integrated into the framework of this vaccination campaign.
Study protocol
Mpox-Vac protocol (“Studio prospettico osservazionale per monitorare aspetti relativi alla sicurezza, all'efficacia, all'immunogenicità e all'accettabilità della vaccinazione anti Monkeypox con vaccino MVA-BN (JYNNEOS) in persone ad alto rischio”) was approved by the INMI Lazzaro Spallanzani Ethical Committee (approval number 41z, Register of Non-Covid Trials 2022). All subjects eligible for mpox vaccination according to the ministerial guidelines and who signed a written informed consent were enrolled in the study. Laboratory personnel were excluded from the analysis. Timepoints of the study were scheduled at each vaccine administration (T1-T2), one month after the completion of the cycle (T3), and then six months, one, two, and three years from vaccination. For the vaccine-experienced individuals who received a single dose, T2 was the time point corresponding to one month after vaccination completion. At baseline, subjects were evaluated for demographic and behavioural characteristics linked to mpox exposure. The immunogenicity study consists of random samples stratified by historical smallpox vaccination, HIV infection and mode of administration (subcutaneous or intradermal).
Vaccination was not recommended in people with acute or prior mpox infection or who had contact with a known mpox case. Participants who were reactive for IgG or nAbs even before the mpox vaccination (titre >1:20 for IgG and >1:10 for nAbs at T1) were excluded from the analysis. Information regarding HIV status and CD4 count, previous sexually transmitted infections (STIs), and HIV pre-exposure prophylaxis (PrEP) uptake was collected. Blood samples (sera and peripheral blood mononuclear cells -PBMCs) were collected for the immune response assessment at each time point. In this paper, we showed the analysis of the data collected from T1 to T3 (Supplementary Figure S1).
MPXV-specific IgG and neutralization assays
MPXV-specific IgGs and neutralizing antibodies in serum were measured as previously described.28 Briefly, anti-MPXV IgGs were assessed on immunofluorescence slides prepared in-house with Vero E6 cells (ATCC) infected with an MPXV isolate from the skin lesion of a mpox patient infected during the 2022 outbreak (GenBank: ON745215.1, referred to the clinical sample). Serum samples were tested with a starting dilution of 1:20, and serial two-fold dilution were performed to determine anti-MPXV IgG titer. MPXV-specific neutralizing antibodies (nAbs) were measured by 50% plaque-reduction-neutralization test (PRNT50) with a starting dilution of 1:10. Specifically, serum samples were heat-inactivated at 56 °C for 30 min and titrated in duplicate in 4 four-fold serial dilutions. Each serum dilution was added to the same volume (1:1) of a solution containing 100 TCID50 MPXV isolate (GenBank: ON745215.1, referred to the clinical sample) and incubated at 37 °C for 2 h. Subconfluent Vero E6 cells were infected with virus/serum mixtures and incubated at 37 °C and 5% CO2. After 5 days, the supernatant was carefully discarded, and a crystal violet solution (Diapath S.P.A.) containing 10% formaldehyde (Sigma–Aldrich) was added. After 30 min, the fixing solution was removed, and cells were washed with phosphate-buffered saline (PBS, 1X; Sigma–Aldrich). The number of plaques was counted using the Cytation 5 reader (Biotek). The neutralizing titers were estimated by measuring the plaques number reduction as compared to the control virus wells. The highest serum dilution showing at least 50% of the plaques number reduction was indicated as the 50% neutralization titer (PRNT 50%). Each test included serum control (1:10 dilution of each sample tested without virus), cell control (Vero E6 cells alone), and virus control (100 TCID50 MPXV in octuplicate).
PBMC isolation
Briefly, peripheral blood mononuclear cells (PBMC) were isolated by using Ficoll density gradient centrifugation (Pancoll human, PAN Biotech) and frozen in fetal bovine serum addictioned of 10% of DMSO (Merck Life sciences, Milan, Italy) at vapors of liquid nitrogen.
Elispot assay
The frequency of T cell-specific responses to the MVA-BN vaccine was assessed by interferon-γ ELISpot assay. Briefly, PBMC were thawed and suspended in complete medium [RPMI-1640 added of 10% fetal bovine serum, 1% l-glutamine, and 1% penicillin/streptomycin (Euroclone S.p.A, Italy)]. Live PBMC were counted by Trypan blue exclusion, plated at 3 × 105 cells per well in ELISpot plates (Human IFN-γ ELISpot plus kit; Mabtech, Nacka Strand, Sweden), and stimulated for 18–20 h with MOI 1 of the MVA-BN vaccine suspension [JYNNEOS (Smallpox and Monkeypox Vaccine, Live, non-replicating)] and aCD28/aCD49d (1 μg/ml, BD Biosciences) at 37 °C (5% CO2). A T cell superantigen (SEB 200 nM, Sigma) was added as a positive control. At the end of incubation, the ELISpot assay was developed according to the manufacturer's instructions. Results are expressed as spot-forming cells per 106 PBMCs (SFC/106 PBMCs) in stimulating cultures after subtracting the background (unstimulated culture).
Assessment of adverse reactions
Self-reported adverse effects following immunization (AEFIs) were prospectively collected for 30 days from vaccine recipients at the time of any dose (T1 and T2) through a symptoms’ diary. Participants reported the presence of systemic symptoms (S-AEFIs) classified as fatigue, muscle pain, headache, gastrointestinal effects, and chills, and local ones (L-AEFIs), such as redness, induration, and pain. AEFIs were graded by the vaccinees as absent (grade 0), mild,1 moderate,2 or severe.3 Participants enrolled in the immunogenicity analysis who completed data for the symptom diary were included in the analysis evaluating the safety of the vaccine.
Statistical analysis
Demographic and epidemiological characteristics of the patients were described using the median and Interquartile Range (IQR) for continuous parameters and absolute and relative (percentage) frequencies for categorical variables, overall and stratified by our exposure group of interest: history of smallpox vaccination (naïve vs. experienced). Several outcomes were defined to quantify the level of immunogenicity after mpox vaccination. First, we analyzed the probability of seroconversion for MPXV-specific IgG and nAbs post-vaccination, overall and after stratification by previous smallpox vaccination. The McNemar test for paired binary data was used to test whether the proportion of participants who seroconverted after the vaccination was significantly higher than that which would have been observed by chance alone overall and after stratification by smallpox vaccination history. The proportion of seroconverters according to smallpox vaccination was then compared using the Chi-square test or Fisher exact test when appropriate. Odd ratios of seroconversion by the history of smallpox vaccination were calculated in standard univariable logistic regression models after controlling for HIV status. In the attempt to inform guidelines for which vaccine strategy to use in PLWH we also hypothesized effect measure modification by HIV for the humoral response. This was tested in the logistic model both in the multiplicative (OR) and additive (risk difference) estimand scale (which provided similar results), and for convenience, only the results for the multiplicative scale have been shown by means of a forest plot. Secondly, we also used a quantitative outcome defined as the average change in MPXV-specific IgG and nAb titers from baseline (T1) to one month after the completion of the vaccination schedule (T2 for vaccine-experienced and T3 for vaccine-naïve). Again, we first conducted an analysis to evaluate whether there was an overall increase in the average levels of these titers and the completion of the vaccination schedule above the level that was expected by chance alone using a paired t-test for the means (using the responses in the log2 scale) and the equivalent non-parametric Wilcoxon signed-rank test for the rank's distribution (when using the raw scale). In univariable analyses, we also compared the average levels of these markers by exposure vaccination group by means of an unpaired t-test (log scale) and the non-parametric equivalent of the Mann–Whitney U test (natural raw scale). Finally, we repeated this comparison analysis after controlling for HIV using a counterfactual framework and by estimating the average treatment effect (ATE) associated with vaccination exposure using a linear regression model. Of note, the aim of this analysis was to evaluate what would have been the response to MVA-BN vaccine had everybody in the sample received the single dose vs., the two doses. We used a doubly robust method (using augmented inverse probability weighting–AIPW) to obtain unbiased estimates of the ATE even when either the propensity model or the outcome model was not correctly specified. Both the propensity and outcome models included HIV as a single confounding variable (Supplementary Figure S2) and for the outcome model we also used the saturated model including the interaction parameter between exposure and HIV-status (results were similar, not shown). In the Supplementary material graphs, we show the crude median changes in T-cell responses by vaccination strategies and stratified by HIV-status. Finally, we also investigated the safety of MVA-BN vaccination overall and by exposure groups. Again, two main outcomes were considered. First, for each participant, we calculated the maximum level of severity ever experienced over the 30 days past the first dose of vaccination and for each of the systemic and local reactions, a categorical variable with 4 levels (no symptoms, mild, moderate, and severe symptoms). We have shown the raw proportions of participants falling into these categories by vaccination exposure groups. This outcome was also analyzed using an univariable multinomial logistic regression model and after adjusting for HIV status. In this model, the no-symptoms category was chosen as the reference, and the estimated odds ratios show the risk of falling into one of the symptomatic categories if participants were smallpox vaccine-experienced vs. vaccine-naïve. As a second outcome, we calculated the average number of days in which participants experienced each of the 4-levels of symptoms over the 30 days past the first dose of the MVA-BN vaccine. For this outcome, we limited the analysis to the unadjusted comparisons of the mean days by exposure group stratified by individual symptoms (both systemic and local reactions).
Role of funding
The study was supported by the National Institute for Infectious Disease Lazzaro Spallanzani IRCCS “Advanced grant 5 × 1000, 2021” and by the Italian Ministry of Health “Ricerca Corrente Linea 2”.
Results
The process leading to the selection of the study population is reported in a flowchart (Supplementary Figure S3). The main characteristics of the study population are reported in Table 1. Among the 164 participants enrolled, 90 (54.9%) were vaccine-experienced, and 74 (45.1%) were vaccine-naïve. All were male, and the majority (90.2%) self-identified as MSM. Overall, the median age was 49 years (IQR 41–55), 53 (50–57) years in the vaccine-experienced and 40 (34–45) years in the naïve group, respectively (p < 0.0001) and there was no evidence for a difference in any other characteristic between the two groups.
Table 1.
Main characteristics of the study population according to previous smallpox vaccination.
| Characteristics | Previous vaccination against smallpox |
||||
|---|---|---|---|---|---|
| Smallpox vaccine-experienced |
Smallpox vaccine-naive |
p-valuef | p-valueg | Total |
|
| N = 90 | N = 74 | N = 164 | |||
| Sexual orientation, n (%) | 0.149 | ||||
| Bisexual | 9 (10.0%) | 4 (5.4%) | 13 (7.9%) | ||
| Transgender | 3 (3.3%) | 0 (0.0%) | 3 (1.8%) | ||
| MSM | 78 (86.7%) | 70 (94.6%) | 148 (90.2%) | ||
| Age, years | <0.0001 | <0.0001 | |||
| Median (IQR) | 53 (50, 57) | 40 (34, 45) | 49 (41, 55) | ||
| PrEPausers, n (%) | 0.210 | ||||
| No | 67 (74.4%) | 53 (71.6%) | 120 (73.2%) | ||
| Yes | 16 (17.8%) | 19 (25.7%) | 35 (21.3%) | ||
| Unknown | 7 (7.8%) | 2 (2.7%) | 9 (5.5%) | ||
| STIsbover the previous year, n (%) | |||||
| Yes | 15 (16.7%) | 16 (21.6%) | 0.421 | 31 (18.9%) | |
| Syphilis | 8 (53.3%) | 6 (37.5%) | 0.384 | 14 (45.2%) | |
| Gonorrhoea | 5 (33.3%) | 6 (37.5%) | 0.812 | 11 (35.5%) | |
| Chlamydia | 1 (6.7%) | 7 (43.8%) | 0.020 | 8 (25.8%) | |
| HPVc | 2 (13.3%) | 0 (0.0%) | 0.137 | 2 (6.5%) | |
| Chemsex use, n (%) | 0.341 | ||||
| Yes | 5 (5.6%) | 7 (9.5%) | 12 (7.3%) | ||
| PLWHd | 45 (50.0%) | 31 (41.9%) | 0.302 | 76 (46.3%) | |
| On effective HAART | 45 (100.0%) | 31 (100.0%) | 76 (100.0%) | ||
| CD4 counte, cells/μL | 0.863 | ||||
| 0–200 | 2 (4.4%) | 2 (6.5%) | 4 (5.3%) | ||
| 201–500 | 9 (20.0%) | 5 (16.1%) | 14 (18.4%) | ||
| >501 | 34 (75.6%) | 24 (77.4%) | 58 (76.3%) | ||
| Mode of administration of the first dose, n (%) | 0.164 | ||||
| Subcutaneous | 50 (55.6%) | 33 (44.6%) | 83 (50.6%) | ||
Pre-exposure prophylaxis for HIV infection.
Sexually transmitted infections.
Human papilloma virus.
People living with HIV.
With reference only to PLWH.
Chi-square or Mann–Whitney test as appropriate.
Unpaired t-test.
Regarding the high-risk behavior categories, 35 participants (21.3%) were on PrEP, 31 (18.9%) reported at least one STI diagnosed in the last year, and 76 (46.3%) were PLWH, all on highly active antiretroviral therapy (HAART). CD4 count was lower than 200 cells/μL only in 4 participants (5.3%), while it was higher than 500 cells/μL in 58 (76.3%). Overall, 83 (50.6%) subjects received the first dose by the subcutaneous route, of whom 50 as a single dose among vaccine-experienced (55.6% of this group) and 33 as the first dose of the two-dose schedule in naïve group (44.6% of them). All the second doses were administered intradermically.
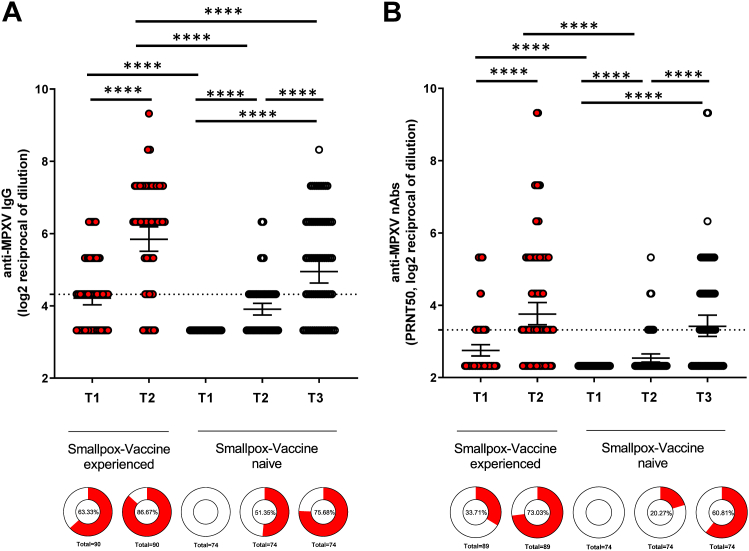
There was evidence that the IgG and nAbs titers increase by a larger amount that what was expected by chance alone after the administration of the MVA-BN vaccine regardless of the dose, and an inter-group comparison showed evidence for a higher GMT only for IgG when comparing primed vs. non-primed participants (p < 0.0001) (Fig. 1).
Fig. 1.
MPXV-specific antibodies (IgG) and MPXV-specific neutralizing antibodies (nAbs) response in smallpox vaccine experienced (red plot) vs. smallpox vaccine-naïve (black plot) Panel A. Titers of MPXV-specific IgG were measured by immunofluorescence (1:20) starting dilution. Intra-group comparisons were performed with paired t-test; inter-group comparisons with unpaired t-test. Panel B. Titers of MPXV-specific nAbs were measured by 50% plaque reduction neutralization test (1:10 starting dilution). Intra-group comparisons were performed with paired t-test, and inter-group comparisons with unpaired t-test. Titers are expressed as Geometric Mean Titres (GMT) of the reciprocal serum dilution (log2 scale). Error bars refer to the 95% confidence interval of GMT. ∗∗∗∗p < 0.0001.
However, when we compared potential outcomes (i.e. comparing what would have happened if everybody had received one dose vs. two doses) and after controlling for HIV status, there was no evidence for a difference in the change of both IgG and nAbs titers from baseline to one month after the completion of the full vaccination schedule between vaccination strategies (Table 2).
Table 2.
Potential average change after full vaccination cycle and average treatment effect (ATE) from fitting a linear regression model (log2 scale).
| Potential average change at post full vaccine cycle and ATEa from fitting a linear regression model (log2 scale) |
||||
|---|---|---|---|---|
| Mean (log2) in exposed (95% CI) | Mean (log2) in unexposed (95% CI) | ATEb (95% CI) | p-value | |
| Smallpox vaccine-experienced vs. vaccine-naïve | ||||
| Elispot | ||||
| Double robust | 0.69 (0.26, 1.12) | 2.70 (2.20, 3.20) | −2.01 (−2.65, −1.36) | <0.0001 |
| IgG | ||||
| Double robust | 1.75 (1.54, 1.97) | 1.83 (1.51, 2.16) | −0.08 (−0.47, 0.31) | 0.6932 |
| nAbs | ||||
| Double robust | 1.21 (0.93, 1.49) | 1.34 (1.01, 1.67) | −0.13 (−0.57, 0.31) | 0.5572 |
Average Treatment Effect.
Weighted for HIV.
Among the subgroup of the vaccinia-experienced, the proportion of reactivity significantly increased after the single-dose administration from 57/90 (63.3%) at T1 to 78/90 (86.7%) at T2 for IgG, and from 30/89 (33.7%) to 65/89 (73.0%), for nAbs, respectively (p < 0.0001, McNemar test).
Among the subgroup of the vaccinia-naïve, the proportion of seroconversion before and after the second dose varied from 38/74 (51.4%) to 56/74 (75.7%) for IgG (p = 0.0001, McNemar test) and from 15/74 (20.3%) to 45/74 (60.8%) for nAbs (p < 0.0001, McNemar test), respectively (Fig. 1-donut graphs).
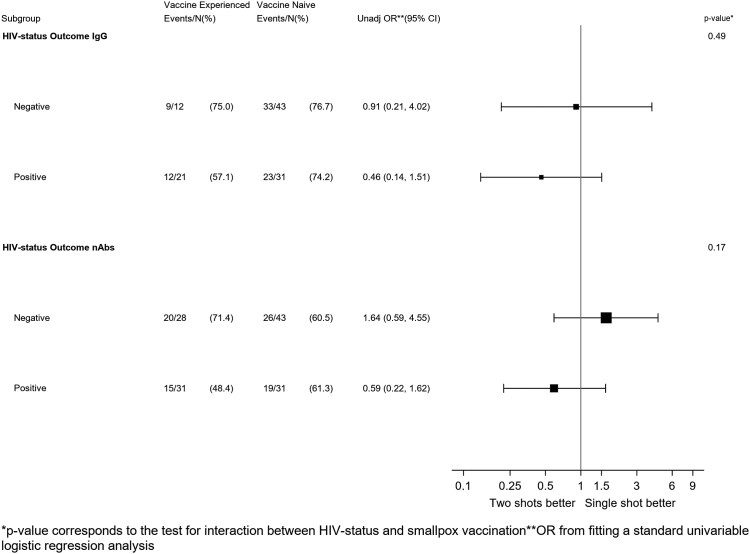
In the subset of participants who were not reactive at T1, we found no evidence that the proportion of those who became reactive after the full cycle of MVA-BN in the overall population was different by smallpox vaccination history (Fisher's exact test p = 0.25 for IgG and p = 1.00 for nAbs, respectively) (Table 3). Among the vaccine-experienced individuals and according to HIV strata, the rate of IgG and nAbs seroconversion varied from 75% in PLWoH to 57% in PLWH for IgG, and from 71% to 48% for nAbs, respectively (Table 3).
Table 3.
Proportion of participants who were non-reactive at T1 and who were reactive for IgG or nAbs, after the completion of the full cycle MVA-BN vaccination, according to HIV status and previous smallpox vaccination (vaccine-experienced or primed) or not (vaccine-naïve or non-primed).
| Anti-MPXV IgG |
Anti-MPXV nAbs |
|||
|---|---|---|---|---|
| vaccine-naïve | vaccine-experienced | vaccine-naïve | vaccine-experienced | |
| Participants not reactive at T1 (n) | 74a | 33a | 74b | 59b |
| Reactive after full cycle n (%) | 56 (75.7) | 21 (63.6) | 45 (60.8) | 35 (59.3) |
| HIV negative | 43 | 12 | 43 | 28 |
| Reactive after full cycle n (%) | 33 (76.7) | 9 (75.0) | 26 (60.5) | 20 (71.4) |
| HIV positive | 31 | 21 | 31 | 31 |
| Reactive n (%) | 23 (74.2) | 12 (57.1) | 19 (61.3) | 15 (48.4) |
Participants with IgG titers <1:20 at T1.
Participants with nAbs titers <1:10 at T1.
In a logistic regression model testing the effect-measure modification by HIV for the humoral response, there was little evidence for an interaction for the IgG response with little difference in rate of seroconversion between primed and non-primed according to HIV-status (p = 0.49). In contrast, primed showed a lower chance of seroconversion than the non-primed (OR = 0.59; 95% CI: 0.22–1.62) while the opposite was seen in the PLWoH group (OR = 1.64; 95% 0.59–4.55, interaction p = 0.17, Fig. 2).
Fig. 2.
Forest-Plot of the effect on seroconversion of the type of vaccination course by HIV strata.
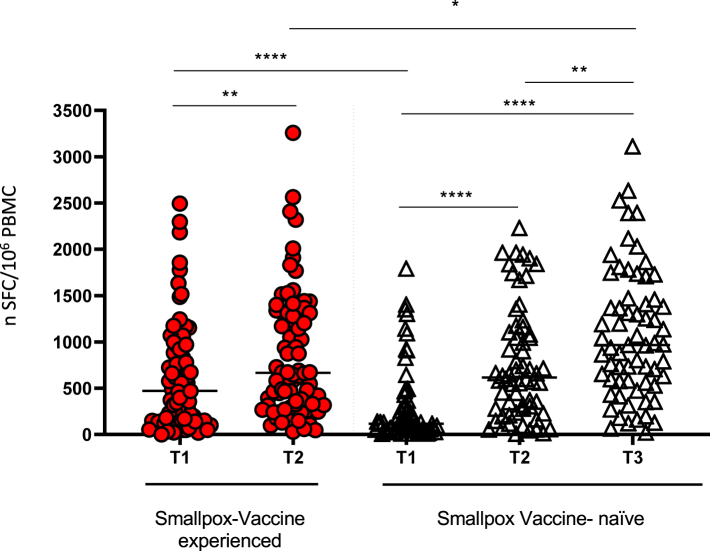
Overall, in the continuous scale, we observe an increase in T-cell response after MVA-BN vaccination both in primed and not-primed individuals. Inter-group comparisons by univariable analysis showed strong evidence that the T-cell response was higher in non-primed vs. primed participants (Fig. 3, p = 0.04)). We also described the raw data after stratification for HIV status (Supplementary Figure S4). These plots show that PLWoH had a higher level of T-cell response before MVA-BN vaccination (time T1) compared to PLWH individuals. Consequently, PLWoH appeared to have a larger benefit from the two-dose vs. one-dose vaccination, although this additional increase was not required (as the response level appeared sufficiently high even before vaccination). On the contrary, PLWH had clearly lost immunization at T1 and, therefore, truly benefited from the MVA-BN vaccination, especially those who received two doses. Finally, when we re-analyzed the data using a counterfactual framework (therefore essentially comparing vaccination strategies and using potential outcomes) and after controlling for HIV-status, we estimated a mean difference of—2.01 log2 SFC/106 PBMCs (95% CI: −2.65, −1.36; p ≤ 0.0001) when comparing the one dose strategy vs. the two-dose strategy (Table 2). No breakthrough infections or re-infections episodes have been observed during the study period.
Fig. 3.
Frequency of T cells responding to MVA-BN vaccine expressed as the number of SFC/106 PBMC tested by interferon-γ ELISpot assay. Intra-group comparisons were performed with the Wilcoxon test. Inter-group comparisons were performed by the Mann–Whitney test. The horizontal lines refer to the median of the SFC/106 PBMC. ∗p = 0.04; ∗∗p = 0.001; ∗∗∗∗p < 0.0001.
Reactogenicity
Among the participants enrolled for the immunogenicity analysis, 121 fulfilled the symptom diary delivered after the administration of the first or single dose, 58 (47.9%) were vaccine-experienced and 63 (52.1%) vaccine-naïve. No serious adverse events were observed.
Overall systemic reactions occurred in 58 (48%) participants: 26 (45%) were vaccine-experienced, and 32 (55%) were vaccine-naïve. Details of the occurrence of systemic reaction are shown in Table 4. After adjusting for HIV status, we found no evidence for a difference in the risk of developing any systemic reaction of any grade between vaccine-experienced and vaccine-naïve, except for a lower risk of grade 2 (moderate) fatigue which were lower in vaccine-experienced (OR 0.26; 95% CI: 0.08–0.92, p = 0.037).
Table 4.
Systemic adverse reactions after the first/single dose of MVA-BN vaccination according to previous smallpox vaccination and odds ratio of experiencing reaction of each grade in the 121 participants who fulfilled the symptoms diary.
| Systemic reactions | Vaccine-experienced (n = 58) |
Vaccine- naïve (n = 63) |
p-valuea | Adj ORb (CI 95%) | p-valuec |
|---|---|---|---|---|---|
| n (%) | n (%) | ||||
| Chills | 5 (8.6) | 8 (13) | 0.011 | ||
| Absent | 53 (92) | 55 (87) | 1 | ||
| Mild | 2 (3) | 7 (11) | 0.27 (0.05–1.37) | 0.11 | |
| Moderate | 2 (3) | 1 (2) | 0.006 | 1.91 (0.17–21.99) | 0.60 |
| Severe | 1 (2) | 0 | Ndd | 0.95 | |
| Nausea | 4 (7) | 9 (14) | 0.012 | ||
| Absent | 54 (94) | 54 (86) | 1 | ||
| Mild | 2 (3) | 6 (9) | 0.34 | 0.20 | |
| Moderate | 2 (3) | 3 (5) | 0.011 | 0.68 | 0.68 |
| Severe | 0 | 0 | – | – | |
| Vomit | 0 | 1 (2) | 0.007 | ||
| Absent | 58 (100) | 62 (98) | 1 | ||
| Mild | 0 | 0 | – | – | |
| Moderate | 0 | 1 (2) | 0.007 | Ndd | 0.95 |
| Severe | 0 | 0 | – | – | |
| Muscle pain | 13 (22) | 14 (22) | 0.046 | ||
| Absent | 45 (78) | 49 (78) | 1 | ||
| Mild | 8 (14) | 7 (11) | 1.24 (0.41–3.71) | 0.70 | |
| Moderate | 2 (3) | 4 (6) | 0.010 | 0.50 (0.09–2.88) | 0.43 |
| Severe | 3 (5) | 3 (5) | 0.99 (0.19–5.27) | 0.99 | |
| Fatigue | 23 (40) | 27 (43) | 0.004 | ||
| Absent | 35 (60) | 36 (57) | 1 | ||
| Mild | 15 (26) | 9 (14) | 1.68 (0.65–4.38) | 0.28 | |
| Moderate | 4 (7) | 13 (21) | 0.002 | 0.26 (0.08–0.92) | 0.04 |
| Severe | 4 (7) | 5 (8) | 0.76 (0.18–3.11) | 0.70 | |
| Headache | 16 (28) | 18 (29) | 0.026 | ||
| Absent | 42 (73) | 45 (71) | |||
| Mild | 13 (22) | 11 (17) | 1.17 (0.47–2.94) | 0.73 | |
| Moderate | 2 (3) | 6 (10) | 0.005 | 0.30 (0.6–1.63) | 0.16 |
| Severe | 1 (2) | 1 (2) | 0.83 (0.05–14.02) | 0.89 |
Chi-square test.
Odds ratio for the vaccine-experienced to develop adverse reaction compared to vaccine-naïve.
Logistic regression model adjusted for HIV.
Nd: not determined.
Among the 113 (93%) participants reporting any local reactions, 53 (47%) were vaccine-experienced, and 60 (53%) were vaccine-naïve. Again, the risk of occurrence of grade 2 (moderate) induration and local pain was lower in vaccine-experienced than in vaccine-naïve (OR 0.30, 95% CI: 0.10–0.88, p = 0.03 and OR 0.19, 95% CI: 0.05–0.73, p = 0.01, respectively) (Table 5).
Table 5.
Local adverse reactions after the first/single dose of MVA-BN vaccination according to previous smallpox vaccination and odds ratio of experiencing reaction of each grade in the 121 participants who fulfilled the symptoms diary.
| Local reactions | Vaccine-experienced (N = 58) | Vaccine- naïve (N = 63) | p-valuea | Adj ORb (CI 95%) | p-valuec |
|---|---|---|---|---|---|
| Redness | 41 (70.7) | 48 (76.2) | 0.028 | ||
| Absent | 17 (29.3) | 15 (23.8) | 1 | ||
| Mild | 13 (22.4) | 17 (27.1) | 0.69 (0.25–1.89) | 0.47 | |
| Moderate | 15 (25.9) | 21 (33.3) | 0.011 | 0.66 (0.25–1.73) | 0.40 |
| Severe | 13 (22.4) | 10 (15.9) | 1.15 (0.40–3.40) | 0.80 | |
| Induration | 38 (65.5) | 50 (79.4) | 0.006 | ||
| Absent | 20 (34.5) | 13 (20.6) | 1 | ||
| Mild | 21 (36.2) | 24 (38.1) | 0.57 (0.23–1.43) | 0.23 | |
| Moderate | 8 (13.8) | 18 (28.6) | 0.004 | 0.30 (0.10–0.88) | 0.03 |
| Severe | 9 (15.5) | 8 (12.7) | 0.76 (0.23–2.46) | 0.64 | |
| Pain | 38 (65.5) | 50 (79.4) | 0.003 | ||
| Absent | 20 (34.5) | 13 (20.6) | 1 | ||
| Mild | 31 (53.4) | 31 (49.2) | 0.6 (0.25–1.44) | 0.25 | |
| Moderate | 4 (6.9) | 13 (20.6) | 0.001 | 0.19 (0.05–0.73) | 0.01 |
| Severe | 3 (5.2) | 6 (9.5) | 0.31 (0.06–1.48) | 0.14 |
Chi-square test.
Odds ratio for the vaccine-experienced to develop adverse reaction compared to vaccine-naïve.
Logistic regression model adjusted for HIV.
Local redness and induration were the most long-lasting symptoms (on average, 12.0 and 9.2 days for vaccine-experienced and 11.1 and 10.2 for vaccine-naïve, respectively). Conversely, the systemic reactions were short, with a maximum mean duration for fatigue of at least mild grade of 1.45 and 1.30 days for vaccine-experienced and vaccine-naïve, respectively. Overall, no evidence for a difference in symptom duration were detected between primed and not-primed participants (Table 6).
Table 6.
Duration of systemic and local adverse reactions according to previous smallpox vaccination.
| Systemic reactions | Duration of reactions |
||||
|---|---|---|---|---|---|
| Duration (days) |
p-valuea | ||||
| Smallpox vaccine-experienced |
Smallpox vaccine-naïve |
||||
| Mean Median |
SD IQR |
Mean Median |
SD IQR |
||
| Chills | |||||
| At least mild | 2.7 2.0 |
3.2 1.0, 3.0 |
1.4 1.0 |
0.7 1.0, 1.5 |
0.0533 |
| Moderate or severe | 2.2 1.0 |
2.9 1.0, 2.0 |
1.0 1.0 |
1.0, 1.0 | <0.0001 |
| Nausea | |||||
| At least mild | 2.5 1.0 |
3.4 1.0, 2.0 |
2.1 1.0 |
1.5 1.0, 3.0 |
0.4654 |
| Moderate or severe | 1.9 1.0 |
2.4 1.0, 2.0 |
1.7 1.0 |
1.2 1.0, 3.0 |
0.8703 |
| Vomit | |||||
| At least mild | 2.3 1.0 |
3.4 1.0, 2.0 |
|||
| Muscle pain | |||||
| At least mild | 3.7 2.0 |
4.1 1.0, 4.0 |
3.1 3.0 |
1.9 1.0, 4.0 |
0.5815 |
| Moderate or severe | 2.8 2.0 |
3.2 1.0, 3.0 |
2.1 2.0 |
1.5 1.0, 3.0 |
0.2590 |
| Fatigue | |||||
| At least mild | 4.3 3.0 |
4.9 1.0, 5.0 |
3.0 2.0 |
2.0 1.0, 4.0 |
0.4824 |
| Moderate or severe | 2.7 2.0 |
3.2 1.0, 3.0 |
2.2 1.0 |
1.9 1.0, 3.0 |
0.1394 |
| Headache | |||||
| At least mild | 3.1 2.0 |
3.6 1.0, 4.0 |
2.0 1.0 |
1.7 1.0, 2.0 |
0.7115 |
| Moderate or severe | 2.2 1.0 |
2.8 1.0, 2.0 |
2.1 1.0 |
2.3 1.0, 3.0 |
0.5709 |
| Local Reactions | |||||
| Redness | |||||
| At least mild | 16.7 17.0 |
9.4 9.0, 27.0 |
14.6 13.5 |
10.0 5.5, 24.5 |
0.2898 |
| Moderate or severe | 6.3 5.0 |
5.5 2.0, 9.0 |
6.3 4.0 |
7.0 2.0, 5.0 |
0.8982 |
| Induration | |||||
| At least mild | 14.0 12.0 |
9.2 6.0, 22.0 |
12.9 9.5 |
9.9 5.0, 23.0 |
0.5496 |
| Moderate or severe | 5.4 3.0 |
5.2 2.0, 7.0 |
4.0 3.0 |
4.0 2.0, 4.0 |
0.4702 |
| Local pain | |||||
| At least mild | 6.4 5.0 |
5.4 2.0, 8.0 |
4.3 4.5 |
2.8 2.0, 6.0 |
0.1168 |
| Moderate or severe | 3.9 3.0 |
3.9 1.0, 5.0 |
2.5 2.0 |
1.4 1.0, 3.0 |
0.2651 |
Analysis was performed for each symptom of any grade and for each symptom of grades 3–4 (moderate or severe).
Unpaired t-test.
Discussion
Due to limited data on the immunogenicity and efficacy of the MVA-BN vaccine against mpox, recommendations on vaccination have differed worldwide in terms of the number of doses, the route of administration and the indication for use as pre- or post-exposure prophylaxis. Many countries adopted a single vaccination schedule, especially for smallpox vaccine-experienced individuals, because of low stockpiles of the MVA-BN vaccine and the need to roll out an expanded vaccination campaign that could rapidly reach all the high-risk people by administering at least one dose. This prioritizing strategy was supported by the first UK report on vaccine efficacy,19 which estimated the protection offered by a single dose of MVA-BN to be around 78% and by findings that a single dose of MVA-BN in previously smallpox vaccinated people aged 56–80 years old elicited a rapid boost of B cell response.29 Moreover, the protective effect of the first-generation smallpox vaccine against moderate/severe mpox was estimated to have approximately 58% effectiveness.30 The use of stockpiles of third-generation vaccines (although less consistent) has been preferred over second-generation ones because MVA-BN can also be administered to individuals for whom the second-generation vaccine was contraindicated, such as immunocompromised people. Unfortunately, half of the breakthrough infections observed in the real-world study of single-dose vaccination effectiveness19 occurred in PLWH.31
Recent RCTs showed that two doses of MVA-BN elicited a comparable humoral response between PLWH and PLWoH but with lower GMTs in PLWH and that a two-dose schedule of MVA-BN was immunogenic in people with AIDS history.32 Although PLWH enrolled in these studies had a CD4 count higher than 350 cells/μL, stratification for CD4 count showed that GMTs in PLWH tended to be lower with decreasing CD4 count, although not statistically significant.
Based on these data, the British HIV Association (BHIVA) and the NIH, CDC, and IDSA in the US recommended two full vaccine doses for PLWH,33,34 but these recommendations have not been adopted globally, and the single-dose schedule was still used in smallpox primed individuals.
We established that MVA-BN vaccination was able to increase both humoral and T-cell responses in all study participants. Estimated rates of IgG and nAbs seroconversions (>61%) and the average increases in T-cell response on the continuous scale were consistent with those previously shown.21, 22, 23
Our counterfactual analysis suggested that the two-dose strategy led to a better average T-cell response at the end of the full cycle than the one-dose strategy, regardless of HIV status.
Of note, the increased T-cell response in smallpox-primed PLWH was observed despite PLWH retaining a lower level of residual specific T-cells from historical smallpox vaccination than PLWoH.
People historically vaccinated for smallpox can maintain a residual T-cell response for decades after vaccination,35 and although a progressive decline across the years has been suggested,36 a residual T-cell response to Orthopox and Mpox peptides was estimated in around 30% of vaccine-experienced individuals.37
Albeit primed PLWoH retained a T-cell response from the historical smallpox vaccination and did not show a clear benefit after a single dose, the administration of MVA-BN is useful as it is effective in eliciting a neutralizing antibody response.
We also found evidence that HIV infection was a negative effect measure modifier for the nAbs seroconversion response. Essentially, smallpox primed PLWH, receiving a single dose, showed lower seroconversion rates than the non-primed PLWH, receiving two doses. Thus, considering that nAbs response could be one of the possible immunologic correlates of protection against mpox infection, as demonstrated for smallpox,38 our data suggested that the administration of two doses of MVA-BN should be always recommended in PLWH.
This recommendation is in line with the BHIVA and the US NIH/CDC/IDSA statement on mpox vaccination in PLWH14,15 and represents the immunogenicity counterpart of the real-world assessment of reduced effectiveness of partial (75.2%) compared to full (85.9%) vaccination, especially in immunocompromised people (51.0% vs. 70.2%, respectively).39 Moreover, recent data have confirmed that a second dose of MVA-BN could be necessary to achieve levels of nAbs comparable to those elicited by natural infection, whose immune response is considered stronger and more rapid than that elicited after vaccination.29
The analysis of adverse reactions in our population showed that the administration of MVA-BN is usually safe and well-tolerated. According to a previous report,40 reactogenicity seemed higher after the first dose of the two-dose course than the single-dose, in particular for the occurrence of moderate fatigue, local induration, and pain. The systemic adverse reaction lasted a few days, while the local ones had a more clinically relevant duration, as previously described16, but with no statistical evidence for a difference between primed and not primed.
The strength of our study is that it represents the first prospective evaluation of immunogenicity (humoral and cellular) on blood samples longitudinally collected from more than one hundred high-risk people for mpox infection during the 2022 vaccination campaign. Moreover, comprehensive statistical analyses were performed to try to minimize bias due to confounding by HIV and one of these attempted to estimate the causal effect of the vaccination strategy (one dose vs. two doses). In addition, our study informs about reactogenicity induced by the first dose in each vaccination strategy.
However, some limitations need to be mentioned. First, the observational nature of the study so that conclusions regarding the efficacy of the vaccination strategies are valid under the assumption that HIV is the only confounder, there are no other unmeasured confounding pathways and at least one of the models (propensity or outcome model) have been correctly specified. However, due to the difficulties in realizing clinical trials on the effectiveness and immunogenicity of vaccines against mpox, immunobridging studies as well as analysis involving counterfactuals aiming to estimate the causal effect of different strategies, are of special importance and should also be repeated in special populations, such as immunocompromised patients.41
Second, it is difficult to make direct comparisons with previously reported results because different methods for antibody detection have been used. Also, because no commercial mpox-specific peptides were available, the T-cell response was evaluated using the MVA-BN vaccine, which could elicit the innate response. This could explain the baseline reactivity of non-primed participants. We also cannot rule out that some participants may have gotten infected prior to vaccination. Additionally, our analysis does not provide information regarding the immunogenicity of the MVA-BN vaccine in severely immunocompromised people because only a minority of the PLWH included had a CD4 count lower than 200 cells/μL. Furthermore, the study was conducted in a non-endemic setting, and the applicability of these results in the endemic zone remains unknown.
Our counterfactual evaluation of the humoral and cellular response one month after the completion of the vaccination cycle suggested that MVA-BN is immunogenic and that the administration of a two-dose schedule is preferable regardless of the previous smallpox vaccination history, especially in PLWH to maximize nAbs seroconversion rates. MVA-BN was safe and well tolerated, with reactogenicity higher but not severe after the first administration in vaccine-naïve than in vaccine-experienced individuals. These data are important as, in the absence of randomized trials, provide the only source of data to base recommendations for any future use of the MVA-BN vaccine to increase coverage against mpox, which, in endemic regions, has been hypothesized to occupy the ecological niche left vacant by now-eradicated smallpox.42 Further studies are needed to evaluate the long-term duration of immunity and attempt to establish specific correlates of protection.
Contributors
VM, AA, and ACL conceived the study; VM, GM, EC, and SL wrote the protocol. VM wrote the first draft of the manuscript. AA, ACL, EC, GM, FM, AV, and CA revised the manuscript. EN, ML, LS, ET, CM, CP, AM, PP, and RG contributed to the final version of the manuscript. PP was responsible for data management. VM, ACL, PP, GM, EC, AA accessed and verify the data. ACL, GM, and EC performed the statistical analysis. VM, CA, SL, AO, and GMo enrolled and followed the patients during the study time points. VMo, PG, AS, GR, and AB contributed to the realization of the study. GM, FC, SM, AB, DL, and FM provided serological assays on samples. EC, SN, RC, GG, ET, and CF provided tests for T-cell response. AA, EG, and FV reviewed and supervised the manuscript. AA provided the grant for funding the study. All authors gave their final approval of the version to be submitted and were responsible for the decision to submit the manuscript.
Data sharing statement
Data will be available upon request from the corresponding author.
Declaration of interests
The authors declare that no conflicting financial interests or other competing relationships exist.
Acknowledgements
We thank the participants who gave their time to the project, the nurse, the laboratory staff, and the bio-banking personnel: G Prota, A Rossi, V Antonelli, M Baroni, and M Properzi. Thanks to all the members of the Mpox Vaccine Lazio Study Group.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102420.
Contributor Information
Valentina Mazzotta, Email: valentina.mazzotta@inmi.it.
the Mpox Vaccine Lazio Study Group:
Enza Anzalone, Marta Camici, Fabio Cannone, Priscilla Caputi, Claudia Cimaglia, Rita Corso, Flavia Cristofanelli, Stefania Cruciani, Nicola De Marco, Chiara De Ponte, Giulia Del Duca, Paolo Faccendini, Francesca Faraglia, Augusto Faticoni, Marisa Fusto, Saba Gebremeskel, Maria Letizia Giancola, Giuseppina Giannico, Simona Gili, Maria Rosaria Iannella, Angela Junea, Alessandra Lamonaca, Alessandra Marani, Erminia Masone, Ilaria Mastrorosa, Stefania Mazzotta, Alessandra Nappo, Giorgia Natalini, Alfredo Parisi, Sara Passacantilli, Jessica Paulicelli, Maria Maddalena Plazzi, Adriano Possi, Gianni Preziosi, Silvia Rosati, Marika Rubino, Pietro Scanzano, Laura Scorzolini, Virginia Tomassi, Maurizio Vescovo, Serena Vita, Luciano Caterini, Luigi Coppola, Dimitra Kontogiannis, Gabriella D'Ettorre, Marco Ridolfi, Simona Di Giambenedetto, Damiano Farinacci, Alessandra Latini, Mauro Marchili, and Raffaella Marocco
Appendix A. Supplementary data
References
- 1.Centers and Disease Control and Prevention . 2022. Monkeypox outbreak global map. 2023. Data as of October 25th 2023 at 5:30 pm EDT. [Google Scholar]
- 2.Thornhill J.P., Barkati S., Walmsley S., et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med. 2022;387(8):679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 3.Tarín-Vicente E.J., Alemany A., Agud-Dios M., et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022;400(10353):661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antinori A., Mazzotta V., Vita S., et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lapa D., Carletti F., Mazzotta V., et al. Monkeypox virus isolation from a semen sample collected in the early phase of infection in a patient with prolonged seminal viral shedding. Lancet Infect Dis. 2022;22(9):1267–1269. doi: 10.1016/S1473-3099(22)00513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern Accessed on July 13rd, 2023.
- 7.https://www.ema.europa.eu/en/news/ema-recommends-approval-imvanex-prevention-monkeypox-disease Accessed on July 13rd, 2023.
- 8.https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply Accessed on July 13rd, 2023.
- 9.Volz A., Sutter G. Modified vaccinia virus Ankara: history, value in basic research, and current perspectives for vaccine development. Adv Virus Res. 2017;97:187–243. doi: 10.1016/bs.aivir.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatch G.J., Graham V.A., Bewley K.R., et al. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J Virol. 2013;87(14):7805–7815. doi: 10.1128/JVI.03481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pittman P.R., Hahn M., Lee H.S., et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897–1908. doi: 10.1056/NEJMoa1817307. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg R.N., Overton E.T., Haas D.W., et al. Safety, immunogenicity, and surrogate markers of clinical efficacy for modified vaccinia Ankara as a smallpox vaccine in HIV-infected subjects. J Infect Dis. 2013;207(5):749–758. doi: 10.1093/infdis/jis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overton E.T., Stapleton J., Frank I., et al. Safety and immunogenicity of modified vaccinia ankara-bavarian nordic smallpox vaccine in vaccinia-naive and experienced human immunodeficiency virus-infected individuals: an open-label, controlled clinical phase II trial. Open Forum Infect Dis. 2015;2(2):ofv040. doi: 10.1093/ofid/ofv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frey S.E., Wald A., Edupuganti S., et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33(39):5225–5234. doi: 10.1016/j.vaccine.2015.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frey S.E., Goll J.B., Beigel J.H. Erythema and induration after mpox (JYNNEOS) vaccination revisited. N Engl J Med. 2023;388(15):1432–1435. doi: 10.1056/NEJMc2215846. [DOI] [PubMed] [Google Scholar]
- 16.Mazzotta V., Piselli P., Cozzi-Lepri A., et al. 2023. Comparison of subcutaneous versus intradermal route of administration of MVA vaccine. Poster number 00375, Conference on Retroviruses and Opportunistic Infections (CROI) [Google Scholar]
- 17.Deputy N.P., Deckert J., Chard A.N., et al. Vaccine effectiveness of JYNNEOS against mpox disease in the United States. N Engl J Med. 2023;388(26):2434–2443. doi: 10.1056/NEJMoa2215201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payne A.B., Ray L.C., Cole M.M., et al. Reduced risk for mpox after receipt of 1 or 2 doses of JYNNEOS vaccine compared with risk among unvaccinated persons - 43 U.S. Jurisdictions, july 31-october 1, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(49):1560–1564. doi: 10.15585/mmwr.mm7149a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertran M., Andrews N., Davison C., et al. Effectiveness of one dose of MVA–BN smallpox vaccine against mpox in England using the case-coverage method: an observational study. Lancet Infect Dis. 2023;23(7):828–835. doi: 10.1016/S1473-3099(23)00057-9. https://www.thelancet.com/journals/laninf/article/PIIS1473- 3099(23)00057-9/fulltext Available from: [DOI] [PubMed] [Google Scholar]
- 20.Wolff Sagy Y., Zucker R., Hammerman A., et al. Real- world effectiveness of a single dose of mpox vaccine in males. Nat Med. 2023;29(3):748–752. doi: 10.1038/s41591-023-02229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohn H., Bloom N., Cai G.Y., et al. Mpox vaccine and infection-driven human immune signatures: an immunological analysis of an observational study [published online ahead of print, 2023 Jul 17] Lancet Infect Dis. 2023;23(11):1302–1312. doi: 10.1016/S1473-3099(23)00352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaeck L.M., Lamers M.M., Verstrepen B.E., et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat Med. 2023;29(1):270–278. doi: 10.1038/s41591-022-02090-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Leeuwen L., Shamier M., Geurtsvankessel C. Humoral and cellular responses of mpox vaccination in at-risk individuals. ECCMID. 2023 Oral presentation O0412. [Google Scholar]
- 24.Ilchmann H., Samy N., Reichhardt D., et al. One- and two-dose vaccinations with modified vaccinia ankara-bavarian nordic induce durable B-cell memory responses comparable to replicating smallpox vaccines. J Infect Dis. 2023;227(10):1203–1213. doi: 10.1093/infdis/jiac455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministero della Salute 05/08/2022 CIRCOLARE del Ministero della Salute n. 35365. Indicazioni ad interim sulla strategia vaccinale contro il vaiolo delle scimmie (MPX) https://www.salute.gov.it/portale/malattieInfettive/archivioNormativaMalattieInfettive.jsp Accessed on July 13rd, 2023.
- 26.Istituto Superiore di Sanità, epicentro sul vaiolo. https://www.epicentro.iss.it/vaiolo/ Accessed on July 13rd, 2023.
- 27.Ministero della Salute 23/08/2022 CIRCOLARE del Ministero della Salute n. 36865. Aggiornamento sulla modalità di somministrazione del vaccino JYNNEOS (MVA-BN) https://www.salute.gov.it/portale/malattieInfettive/archivioNormativaMalattieInfettive.jsp Accessed on July 13rd, 2023.
- 28.Colavita F., Mazzotta V., Rozera G., et al. Kinetics of viral DNA in body fluids and antibody response in patients with acute monkeypox virus infection. iScience. 2023;26(3) doi: 10.1016/j.isci.2023.106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg R.N., Hay C.M., Stapleton J.T., et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia Ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0157335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Ewijk C.E., Miura F., van Rijckevorsel G., et al. Mpox outbreak in The Netherlands, 2022: public health response, characteristics of the first 1,000 cases and protection of the first-generation smallpox vaccine. Euro Surveill. 2023;28(12) doi: 10.2807/1560-7917.ES.2023.28.12.2200772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogoina D., Strub-Wourgaft N. Can a single dose of Modified Vaccinia Ankara-Bavarian Nordic vaccine protect against mpox? Lancet Infect Dis. 2023;23(7):768–769. doi: 10.1016/S1473-3099(23)00115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Overton E.T., Lawrence S.J., Stapleton J.T., et al. A randomized phase II trial to compare safety and immunogenicity of the MVA-BN smallpox vaccine at various doses in adults with a history of AIDS. Vaccine. 2020;38:2600–2607. doi: 10.1016/j.vaccine.2020.01.058. [DOI] [PubMed] [Google Scholar]
- 33.BHIVA rapid guidance on Mpox (formerly monkeypox) virus. https://www.bhiva.org/BHIVA-rapid-guidance-on-monkeypox-virus Accessed on July 13rd, 2023.
- 34.Panel on guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. National institutes of health, centers for disease control and prevention, HIV medicine association, and infectious diseases society of America. https://clinicalinfo.hiv.gov/en/guidelines/adult-and- adolescent-opportunistic-infection Available at: Accessed (September 10, 2023) [V1-20]
- 35.Sammartino J.C., Cassaniti I., Ferrari A., et al. Characterization of immune response against monkeypox virus in cohorts of infected patients, historic and newly vaccinated subjects. J Med Virol. 2023;95(5) doi: 10.1002/jmv.28778. [DOI] [PubMed] [Google Scholar]
- 36.Hammarlund E., Lewis M.W., Hansen S.G., et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9(9):1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 37.Matusali G., Petruccioli E., Cimini E., et al. Evaluation of cross-immunity to the mpox virus due to historic smallpox vaccination. Vaccines. 2023;11(10):1541. doi: 10.3390/vaccines11101541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lum F.M., Torres-Ruesta A., Tay M.Z., et al. Monkeypox: disease epidemiology, host immunity and clinical interventions. Nat Rev Immunol. 2022;22(10):597–613. doi: 10.1038/s41577-022-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalton A.F., Diallo A.O., Chard A.N., et al. Estimated effectiveness of JYNNEOS vaccine in preventing mpox: a multijurisdictional case-control study — United States, August 19, 2022–March 31, 2023. MMWR Morb Mortal Wkly Rep. 2023;72:553–558. doi: 10.15585/mmwr.mm7220a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng L., Lopez L.K., Glover C., et al. Short-term adverse events following immunization with modified vaccinia ankara-bavarian nordic (MVA-BN) vaccine for mpox. JAMA. 2023;329(23):2091–2094. doi: 10.1001/jama.2023.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazzotta V., Matusali G., Oliva A., Maggi F., Antinori A. Progress in the evaluation of modified vaccinia Ankara vaccine against mpox. Lancet Infect Dis. 2023;23(11):1214–1215. doi: 10.1016/S1473-3099(23)00369-9. [DOI] [PubMed] [Google Scholar]
- 42.Adetifa I., Muyembe J.J., Bausch D.G., Heymann D.L. Mpox neglect and the smallpox niche: a problem for Africa, a problem for the world. Lancet. 2023;401(10390):1822–1824. doi: 10.1016/S0140-6736(23)00588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.