Summary
Non-alcoholic fatty liver disease (NAFLD), which has a prevalence of over 25% in adults, encompasses a wide spectrum of liver diseases. Metabolic-dysfunction associated steatotic liver disease (MASLD), the new term for NAFLD, is characterized by steatotic liver disease accompanied by cardiometabolic criteria, showing a strong correlation with metabolic diseases. Polycystic ovary syndrome (PCOS) is a common reproductive endocrine disease affecting 4–21% of women of reproductive age. Numerous studies have indicated that NAFLD and PCOS often occur together. However, as MASLD is a new term, there is still a lack of reports describing the effects of MASLD on the development of PCOS. In this review article, we have summarized the complex and multifaceted connections between MASLD and PCOS. Understanding the pathogenesis and treatment methods could not only guide the clinical prevention, diagnosis, and treatment of PCOS in patients with MASLD, but also increase the clinical attention of reproductive doctors to MASLD.
Subject areas: Health sciences, Medicine, Medical specialty, Endocrinology, Reproductive medicine
Graphical abstract
Health sciences; Medicine; Medical specialty; Endocrinology; Reproductive medicine
Introduction
Non-alcoholic fatty liver disease (NAFLD) encompasses a range of hepatic conditions, including simple steatosis, non-alcoholic steatohepatitis (NASH), and progressed fibrosis. This definition requires the exclusion of other chronic liver disease and steatosis causes, as well as the absence of excessive alcohol consumption. The prevalence of NAFLD has risen significantly, linked to the increasing rates of obesity and type 2 diabetes.1,2 A systematic review and meta-analysis found an overall prevalence of 39.7% in men and 25.6% in women.3 Given the presence of other potential pathogenesis mechanisms such as alcoholism and viral hepatitis, a new terminology has been proposed: metabolic-dysfunction associated steatotic liver disease (MASLD), which includes clear diagnostic criteria for both adults and children.4 They also suggested a comprehensive nomenclature, steatotic liver disease (SLD), to cover various causes of steatosis. The diagnosis of MASLD includes SLD coexisting with at least one of five cardiometabolic criteria embracing overweight, hyperglycemia, hypertension, and dyslipidemia. Furthermore, the term metabolic dysfunction-associated steatohepatitis (MASH) has been proposed to replace NASH. They demonstrated that 98% of patients with NAFLD meet the MASLD criteria.
Polycystic ovary syndrome (PCOS) is a complex syndrome with pathophysiology linked to metabolic, genetic, epigenetic, and environmental factors. It is one of the most common reproductive endocrine diseases, characterized by hyperandrogenemia (HA), anovulation, increased luteinizing hormone (LH) levels, and polycystic ovary. According to Rotterdam 2003 criteria, a meta-analysis reviewed the prevalence of PCOS as 10%.5
PCOS patients have a higher prevalence of NAFLD, more serious hepatic steatosis, and advanced liver fibrosis compared to those without PCOS.6,7,8 The prevalence of NAFLD in patients with PCOS is 34–70%, while in general women, the prevalence is 14–34%.9 Moreover, Vassilatou et al. have shown that NAFLD women have a higher PCOS prevalence than non-NAFLD women in a clinical study.10 Furthermore, Dong Liu et al. confirmed that individuals with genetically predicated NAFLD are more susceptible to PCOS, with implications on insulin and bioavailable testosterone levels.11 The pathogenesis of liver lipid accumulation and PCOS share many risk factors, most of which are metabolism-associated, as shown in Figures 1 and 2. The theory of the hepato-ovarian axis was proposed in recent years and recent evidence suggests that PCOS is an independent risk factor for NAFLD, and vice versa.12 As the nomenclature changes, there is controversy over the usability of previous research results. However, in a study by Hannes Hagström, over 99.5% of patients with NAFLD fulfilled MASLD criteria,13 indicating that previous studies on NAFLD are still valid.14 This review summarizes the underlying association mechanisms and potential treatment methods between MASLD and PCOS using previous data on NAFLD.
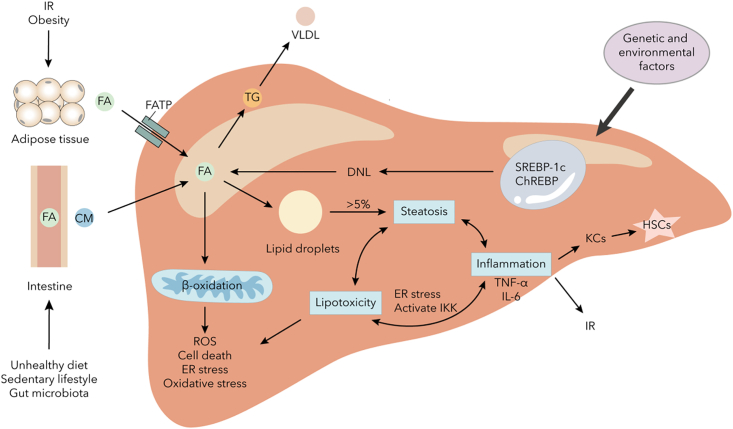
Figure 1.
The pathogenesis of lipid accumulation in liver
A high-fat diet and altered gut microbiota can increase lipid absorption in the intestine. Insulin resistance can lead to increased lipolysis in adipose tissue. Elevated levels of free fatty acids and de novo lipogenesis can enhance fat content in the liver. This can then drive β-oxidation stress in mitochondria and enhance lipid droplet accumulation. The accumulation of lipids can accelerate hepatic steatosis and further aggravate lipotoxicity and inflammation. These changes can impair mitochondrial function, drive advanced ER stress, and lead to the release of pro-inflammatory cytokines, which can further exacerbate metabolic dysfunction and advance fibrosis by activating Kupffer cells and hepatic stellate cells. Abbreviations: IR, insulin resistance; FA, fatty acid; CM, chylomicron; TG, triglyceride; VLDL, very low-density lipoprotein; DNL, de novo lipogenesis; ER, endoplasmic reticulum; ROS, reactive oxygen species; SREBP-1c, sterol regulatory element binding protein 1c; ChREBP, carbohydrate responsive element binding protein; KC, Kupffer cell; HSC, hepatic stellate cell.
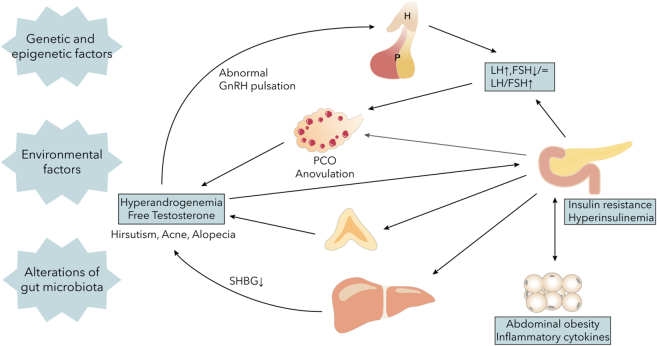
Figure 2.
The pathogenesis of PCOS
PCOS is a systemic disease associated with the entire body. Initially, elevated GnRH levels result in increased LH pulsatile release and an increased LH:FSH ratio, leading to anovulation and androgen abnormalities. This, in turn, induces insulin resistance and promotes abnormal GnRH release. Insulin resistance reduces SHBG synthesis and secretion in the liver, leading to increased androgen levels from the adrenal glands and ovaries, ultimately resulting in high free testosterone levels. Additionally, insulin resistance exacerbates inflammation in adipose and hepatic tissues, and throughout the body, further worsening PCOS symptoms. Abbreviations: H, hypothalamus; P, pituitary; PCO, polycystic ovary; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; FSH, follicle-stimulating hormone; SHBG, sex hormone-binding globulin.
Underlying mechanisms
Insulin resistance
Accumulating evidence indicates that insulin resistance (IR) is closely related to the development of MASLD and PCOS. Patients with MASLD often experience IR under high-calorie intake triggers, leading to an increased risk of progression from steatosis to MASH and fibrosis. IR is one of the “multiple hits” that predispose patients to the development of hepatic steatosis and progression to MASH by enhancing de novo lipogenesis and fibrotic response of hepatic stellate cells.15 IR is also a typical feature of PCOS. The correlation between HA and hyperinsulinemia was first reported as early as 1980. IR can induce endocrine and reproductive features of PCOS by acting on the theca cells and reducing sex hormone-binding globulin (SHBG) production in the liver to stimulate androgen production.16 In turn, HA impairs insulin sensitivity and exacerbates visceral obesity. About 75% of PCOS cases have impaired insulin action.17 Thus, IR shows reciprocal causation with MASLD and PCOS. The associated mechanisms are as follows.
Firstly, in adipose tissue, IR leads to adipocyte dysfunction, including impaired lipolysis and lipid synthesis, increased circulating FFAs, lower adiponectin secretion, and enhanced release of pre-inflammatory cytokines. Preliminary experimental observations have shown that abnormal lipolysis and high circulating FFAs levels progress lipid overload and accumulation in the liver, causing lipotoxicity and inflammatory cytokines release to exacerbate hepatic steatosis and IR.18 Lower levels of adiponectin and leptin are associated with insulin sensitivity impairment.19 Furthermore, the altered secretion of adipokines impacts eating and metabolism like adiponectin and leptin. Secondly, in the liver, IR interferes with the secretion of hepatokines. For example, decreased SHBG secretion leads to increased free testosterone levels in the serum of PCOS patients.20 IR can also stimulate androgen production through a direct or indirect effect on the pituitary.21 Furthermore, increased serum-free androgen attenuated insulin clearance, resulting in hyperinsulinemia.17 Thirdly, systematic inflammation is another reason. Systematic inflammation is pervasive in MASLD and PCOS. Activation of NF-κB and elevation of proinflammatory cytokines exacerbate the progression from steatosis to steatohepatitis. Cytokines can activate various stress-related protein kinases and then induce serine/threonine-mediated phosphorylation of insulin receptor substrate (IRS)-1, resulting in attenuation of IRS-1-mediated insulin signaling.22 Pre-inflammatory cytokines and oxidative stress inhibit IRS-1 and IRS-2 insulin signaling and activate inflammatory reactions leading to aggravated IR via activating different pathways.19 Furthermore, in the liver, IR induces IRS-2 signaling down-regulation, SREBP-1 overexpression, DNL pathway up-regulation, and increased serum triglyceride levels.23 Enhanced TGs, FFAs, and other abnormal lipid metabolite levels lead to mitochondrial dysfunctions and oxidative stress, and then exacerbate hepatic steatosis and inflammation.
In summary, IR contributes to dyslipidemia, HA, abnormal hepatokines levels, and inflammation in patients with MASLD and PCOS, inducing reproductive and endocrine symptoms like expressions of metabolic dysfunctions and clinical HA manifestations. The main mechanism is shown in Figure 3.
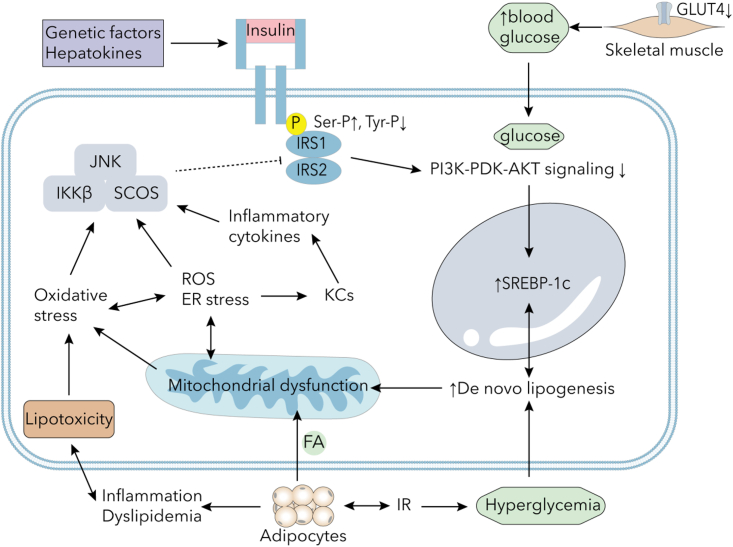
Figure 3.
The main mechanisms of IR
IR and reduced GLUT4 expression in skeletal muscle tissue result in increased circulating glucose levels. Genetic factors and hepatokines may influence insulin signaling by promoting serine/threonine phosphorylation of insulin receptor substrates and inhibiting tyrosine phosphorylation. Upregulation of SREBP-1c collaborates with hyperglycemia to facilitate de novo lipogenesis. Hyperinsulinemia can lead to adipose tissue expansion and promote obesity, while IR can stimulate lipolysis and inflammatory cytokines in adipocytes, causing dyslipidemia, inflammation, and lipotoxicity. Lipotoxicity and mitochondrial dysfunction may induce oxidative stress, ER stress, and ROS elevation in the liver, activating Kupffer cells to produce inflammatory cytokines. Abbreviations: IR, insulin resistance; FA, fatty acid; ER, endoplasmic reticulum; ROS, reactive oxygen species; IRS, insulin receptor substrate; GLUT, glucose transporter; KC, Kupffer cells; JNK, c-Jun N-terminal kinase 1; IKK, inhibitor of the nuclear factor-κB kinase; SCOS, suppressor of cytokine signaling; SREBP-1c, sterol regulatory element binding protein 1c; PI3K, phophatidylinostitol 3-kinase; AKT, protein kinase B.
Obesity
Obesity is a common risk factor for hepatic steatosis and PCOS. Initially, obesity plays a key role in the development of hepatic isolated steatosis and advanced MASH. Approximately 50% of patients with NAFLD and 80% of patients with MASH are overweight or obese.24 People with NAFLD and obesity can be diagnosed as MASLD. A meta-analysis indicated that among obese/overweight people, the prevalence of MASLD was as high as 50.7%.25 Additionally, another meta-analysis shows that approximately 50% of women with PCOS are overweight or obese, and in overweight or obese patients, the prevalence of PCOS is higher than in those with normal weight.26 Furthermore, overweight and obesity are linked to several shared cardiovascular risk factors in patients with MASLD and PCOS.27,28
Adipose tissue acts as an endocrine organ that secretes adipokines and cytokines such as leptin, adiponectin, TNF-α, IL-1β, and IL-6. A high-fat diet promotes fat accumulation by expansion of adipose tissue through the hyperplasia and hypertrophy of adipocytes. This process results in changes in the secretion of adipokines and cytokines, such as increased circulating FFAs and leptin, and decreased adiponectin, which further promotes IR and induces metabolic disorders and androgen secretion. Adipokines interact with hepatokines to cause metabolism disorders. Obesity also leads to alterations in the microenvironment of follicular fluid. Robker et al. reported that in overweight women, higher BMI were associated with higher insulin levels in follicular fluid, and lower SHBG, glucose transporter 4 (GLUT-4), and IRS-2 levels in granulosa and cumulus cells, resulting in IR and fat accumulation.29 Furthermore, IR and androgen excess in women may further contribute to abdominal adipose tissue deposition, creating a positive feedback cycle. Obesity in MASLD promotes the occurrence of PCOS by facilitating IR and endocrine alterations.
SHBG
SHBG, a transporter of sex steroids, has a high affinity for testosterone in circulating plasma which controls the bioavailability of testosterone to the target tissues. Low SHBG levels are considered as a biomarker in women with PCOS, leading to HA symptoms such as hirsutism, alopecia, and acne.30 Decreases SHBG levels, a sign of PCOS in patients, is also linked to a higher risk of PCOS. Lower SHBG levels in adolescents with PCOS are associated with an increased risk of NAFLD.31 In individuals with NAFLD, SHBG levels in the liver and blood are lower compared to those without NAFLD, and lower SHBG levels are connected to the development and regression of NAFLD.32,33 SHBG has a positive impact on metabolism. SHBG levels are linked to a lower likelihood of obesity, T2DM, metabolic syndrome, and hepatic steatosis.34 Moreover, in cellular model, reduced SHBG expression is associated with lower mRNA and protein levels of IRS-1, IRS-2, and PI3K, suggesting that SHBG may be involved in the insulin signaling pathway and IR.35 IR, in turn, acts on the theca cells along with increased LH to increase ovarian androgen production and decrease hepatic SHBG production, leading to higher levels of free or biologically active testosterone.36 SHBG, HA and IR formed a vicious cycle. Therefore, circulating levels of SHBG are negatively linked to the prevalence and severity of fatty liver and PCOS.
Genetic factors
Many genes play crucial roles in the development and progression of NAFLD by modulating metabolism, including mutations in the endocannabinoid receptor 1 (CNR1) gene, patatin-like phospholipase domain-containing protein 3 (PNPLA3), and fat mass and obesity-associated gene (FTO). Given their influence on metabolism, we can consider them MASLD-related mutations.
The endocannabinoid system is reported to have a major role in energy balance regulation. Endocannabinoid system activity is significantly higher in overweight, obese, PCOS, and metabolic dysfunction patients than in healthy individuals.37 Some mutations of CNR1 overexpression affect the occurrence of MetS and PCOS. For example, CNR1 rs12720071 is associated with a 3-fold higher risk of PCOS (OR: 3.01), and CNR1 rs806368 is correlated with an 8-fold higher risk of PCOS (OR: 8.81). Moreover, patients with CNR1 rs12720071 have a 3.6-fold higher risk of HA.38 Activation of the endocannabinoid system induces abdominal obesity, dyslipidemia, and IR in patients with MASLD,39 while CNR1 blockade reduces serum alanine transaminase (ALT) levels and hepatic inflammation markers in women with PCOS.40 Furthermore, CNR1 overexpression not only modulated glucose and lipid metabolism but also modified inflammation and androgen levels, showing a close correlation between MASLD and PCOS.
NAFLD and PCOS are both accompanied by abnormal FTO expression. FTO expression is related to hepatic fat accumulation and the expression of lipogenic genes, which is increased in MASLD patients and mice models.41 Experiments on mouse models have shown that increased FTO expression potentially increases body weight, affects metabolism and oxidative stress, and leads to IR,42 while decreased FTO expression can reverse hyperglycemia and glucose tolerance impairment.43 The common FTO rs9939609 variant is related to PCOS susceptibility, possibly via mediating by BMI and IR.44 In women with PCOS, FTO mutation showed a greater effect on the BMI, IR, and other obese-related traits than that of control patients.45,46
The I148M variant of PNPLA3, the specific SNP rs738409 (rs738409 C>G), is strongly associated with fat accumulation, hepatic steatosis, and inflammation in hepatocytes. PNPLA3 I148M mutation reduces PNPLA3 expression and fat mobilization and promotes hepatic steatosis in experiments on a mouse model.47 According to the study on 208 patients with NAFLD, compared with non-carriers, patients with I148M mutations have a higher risk of liver disease, IR, and metabolic syndromes.48 Furthermore, the mutation is an independent risk factor for progression from cirrhosis to HCC which can impair retinol release of hepatic stellate cells to facilitate inflammation and fibrosis.49,50 PNPLA3 I148M is also closely related to increased serum leptin levels and decreased serum adiponectin levels, resulting in a predisposition to IR.51 The effect of the PNPLA3 I148M variant is larger in women than in men.52 Given that PNPLA3 I148M is the most robust genetic variant associated with MAFLD, and there is strong association between this mutation and metabolism, it suggests a potential connection with metabolic symptoms in patients with PCOS. However, due to the lack of related research, more studies are needed to identify the underlying links. We expect the associations between PNPLA3 and PCOS be detected to provide a new means for prevention and therapy.
Hepatokines
Hepatokines, secreted proteins derived from the liver, can communicate with distant organs to regulate metabolism through endocrine functions. In cases of obesity, IR, dyslipidemia, and other conditions that may increase metabolic stress, the expression levels of certain hepatokines are deregulated. In individuals with MASLD, liver steatosis can influence the secretion of hepatokines, which in turn can regulate lipid metabolism and insulin action. While many hepatokines have been identified and studied, we specifically focused on metabolism-associated hepatokines in this section, which may contribute to obesity, IR, dyslipidemia, and PCOS, in order to characterize the potential mechanisms of MASLD and PCOS.
Angiopoietin-like proteins (ANGPTL) primarily regulate lipid metabolism. ANGPTL3 is elevated in patients with NASH but not in patients with simple steatosis, while ANGPTL8 concentrations are increased in patients with T2DM, obesity, and hepatic steatosis.53,54 Overexpression of ANGPTL8 inhibits triglyceride clearance and leads to higher serum triglyceride levels and liver fat accumulation, whereas ablation of ANGPTL8 reduces plasma triglyceride concentrations in a rat model.55 Wang et al. reported that women with PCOS also have higher concentrations of ANGPTL8 compared to healthy controls.56 ANGPTL8 is an indicator of different stages of NAFLD due to its positive association with NAFLD severity. These studies demonstrate that elevated ANGPTL8 levels are linked to MASLD and PCOS through modulation of metabolism. The next step is to investigate the impact of ANGPTL8 inhibition on the symptoms of MASLD and PCOS.
Fibroblast growth factor (FGF) is a large family of proteins that regulate body development and metabolism. Among them, the FGF19 subfamily includes FGF19, FGF21, and FGF23. FGF19 has an impact on lipid and glucose metabolism. In patients with NAFLD, the interaction between FGF19 and FGFR4 is heightened, and this enhanced interaction is strongly linked to the occurrence and progression of NAFLD.57 Additionally, serum FGF21 levels are higher in patients with obese NAFLD compared to healthy controls. This higher concentration is associated with obesity, BMI, metabolic syndrome, and the accumulation of hepatic TG based on a longitudinal analysis.58 Patients accompanied IR consistently exhibit higher FGF21 levels and FGF21 resistance, alongside serum hyperinsulinemia and IR. Treatment with FGF21 enhances insulin sensitivity and reduces glucose levels. Similarly, in FGF21 whole-body knockout mice, insulin sensitivity was impaired and hepatic glucose production increased.59 The rationale behind this is that FGF21 leads to weight loss and improvement in hepatic steatosis, therefore, in individuals with MASLD and obesity, there is a compensatory elevation in serum FGF21 levels due to FGF21 resistance.60 In patients with PCOS, both FGF19 and FGF21 levels were found to be elevated and correlated with lipid and glucose metabolism.61 However, another study reported no relationship between FGF21 and metabolic syndrome in women with PCOS.62 These conflicting findings require further validation.
Fetuin-A and fetuin-B are both positively associated with liver fat content. Levels of fetuin-A are significantly elevated in patients with PCOS and MASLD and are positively correlated with IR, T2DM, dyslipidemia, steatosis severity, and HA.63,64 Additionally, fetuin-A acts as an endogenous ligand of toll-like receptor 4, which is involved in the process of lipid-induced IR.65 Furthermore, initial experimental observations suggest that hyperglycemia and hyperlipidemia can induce endoplasmic reticulum stress and subsequently increase fetuin-A secretion through the ERK1/2 pathway.66 An innovative treatment approach involving low-dose spironolactone, pioglitazone, and metformin combination has been shown to normalize fetuin-A levels and improve symptoms in women with PCOS.67 The action of fetuin-B is similar to that of fetuin-A. Moreover, serum fetuin-B concentration is associated with impaired glucose intolerance and is negatively related to first-stage insulin secretion β-cell function, as well as hepatic steatosis.68,69 Fetuin-A and fetuin-B are hepatokines that are associated with MASLD and PCOS by affecting glucose and lipid metabolism.
The HMGB1 gene in humans encodes the high mobility group box 1 (HMGB1) protein, which was initially believed to only function as a nuclear protein for regulating gene transcription. However, it is now known to also act as a cytokine mediator of inflammation, leading to tissue injury, elevated blood glucose and FFAs levels, and increased serum HMBG1 levels.70,71 Zhang et al. explored the potential connection between HMGB1 and IR in women with PCOS, showing that HMGB1 abundance increased in the follicular fluid and induced insulin signaling pathway impairments and autophagy activation in granulosa cells.72 Inhibition of autophagy consistently alleviated IR induced by HMGB1.73
Dipeptidyl peptidase-4 (DPP-4), a serine protease, can inactivate glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which stimulates insulin secretion. In individuals with fatty liver, DPP-4 activity is increased and GLP-1 levels are low, leading to IR and progression of hepatic steatosis.74 However, in women with PCOS, we observed similar DPP-4 activity compared to healthy controls, whereas DPP-4 inhibitors were effective in treating metabolic symptoms of PCOS.75 Therefore, the activity of DPP-4 should be further evaluated.
Selenoprotein P (SeP), a liver-derived protein, has been found to be positively associated with hyperglycemia, IR, and T2DM. Previous reports have shown abnormally high SeP levels in patients with obesity, T2DM, PCOS and fatty liver.76 Islets volume of α- and β-cells decreased after high-SeP treatment. Similarly, preliminary experiments observations indicated that SeP-neutralizing antibodies can improve IR, and protect islets and insulin secretion.77 Targeting SeP in treatment offers a new approach to treating PCOS and MASLD.
Growth differentiation factor 15 (GDF-15), a member of the TGF-β superfamily, is expressed at low concentration in many organs and is present in elevated levels in the liver under conditions such as obesity, liver diseases, chronic kidney disease, and cardiovascular disease. Serum GDF-15 levels have been found to predict liver fibrosis in people with MASLD.78 Experiments have shown that GDF-15 can protect against steatosis and fibrosis by inhibiting mitochondrial damage, reducing oxidative stress, downregulating ROS and dsDNA release, and reducing inflammatory cytokine secretion.79 In patients with PCOS, GDF-15 overexpressed in response to increased oxidative stress and ROS levels.80 Elevated GDF-15 levels have favorable effects on metabolic dysfunction in MASLD, MASH, and PCOS. People with relatively low GDF-15 levels are more susceptible to liver steatosis and PCOS. Therefore, GDF-15 may be a therapeutic target for the treatment of patients with MASLD and PCOS, which requires further clinical experiments due to its essential role in metabolism.
Inflammation
Lipid metabolism disorder and IR in patients with MASLD lead to inflammation due to increased oxidative stress, ER stress, and ROS levels. The lipotoxicity and metabolite toxicity activate Kupffer cells, resulting in chronic inflammation, and then activate hepatic stellate cells and immune cells to promote fibrosis. Moreover, elevated ROS leads to chronic hepatic cell death, compensatory proliferation, increased immune cell activation, and worsening inflammation.81 As hepatic steatosis progresses, inflammation continues to deteriorate.
Chronic low-grade inflammation has been reported in patients with PCOS, with elevated inflammatory marker concentrations such as white blood cell counts, C-reactive protein, and cytokines.82 PCOS is often associated with obesity and IR, showing a much greater elevation of inflammatory markers. Even after excluding obesity and elevated BMI, these markers still exist, indicating that the pro-inflammatory effects of HA also exist in patients with PCOS.83 IR in adipose tissue caused chronic inflammation and stimulated macrophage recruitment and activation, leading to systematic inflammation. Obesity-induced systemic inflammation promotes the development of IR and diabetes by triggering inflammation in the pancreatic islets and causing β-cell apoptosis.84
Furthermore, the activation of pro-inflammatory pathways, such as the NF-kB signaling pathway, results in the secretion of inflammatory substances and the activation of macrophages, leading to systemic inflammation and impaired insulin sensitivity.85 Studies have shown that mice with fatty liver induced by a high-fat diet exhibit increased NF-κB signaling. Initial experiments have revealed that mice with selective activation of the NF-κB pathway in hepatocytes experience worsening IR in the liver and the entire body due to the upregulation of cytokine expression, including IL-6.86 Conversely, inhibiting IKK-β and the NF-κB pathway can reverse IR induced by obesity and a high-fat diet.87 Moreover, alterations of gut microbiota lead to dysfunctions of lipopolysaccharide (LPS) metabolism, resulting in elevated levels of LPS in the plasma, leading to systemic inflammation and contributing to the development of MASLD and PCOS.88
Moreover, in patients with PCOS of normal weight, inflammation in adipose tissue worsens impaired insulin signaling. Similarly, PCOS is not only caused by inflammation but also triggers inflammation through obesity, IR, and HA. Therefore, inflammation, closely linked to metabolic disorders, is both the cause and result of MASLD and PCOS. Thus, inflammation serves as a mechanistic connection between the onset and negative effects of PCOS and MASLD.
Gut microbiota
The human gut microbiota composition is linked to various diseases, such as metabolic disorders and hepatic steatosis. Diet is a crucial factor that directly influences gut microbiota, impacting its composition and leading to dysfunction. Mice fed a high-calorie diet, as opposed to a conventional one, experience increased body weight, impaired lipid metabolism, and hepatic steatosis.89 The underlying reason is that high-fat diet-induced obesity, which impairs the gut microbiota’s composition and function, leading to increased lipid absorption and obesity, partly due to IR and inflammation.90 Additionally, PCOS stool microbiota exhibits reduced diversity and an altered phylogenetic profile, contributing to reproductive and metabolic issues.91 Exposure to healthy gut microbiota prevented abnormal metabolic phenotypes, reduced weight gain and abdominal obesity, and improved IR in PCOS mice models.92
Gut microbiota plays a crucial role in regulating the development and progression of MASLD. The composition and metabolites of gut microbiota can impact metabolism, the immune system, redox homeostasis, and mitochondrial function.93 Germ-free mice fed a high-fat diet and then given microbiota from patients with MASH exhibited worsened MASH symptoms, including increased hepatic steatosis and inflammation.94 However, when these mice inoculated with microbiota from the same donor lost weight, they showed normal liver physiology, aligning with the role of weight loss in hepatic steatosis in patients.95 Additionally, microbiota promotes the conversion of primary bile acids into secondary bile acids. Dysregulation of microbiota induced by hepatic steatosis impairs bile acid metabolism, which can impact PCOS through the gut microbiota-bile acid-interleukin-22 axis in mouse PCOS models.96 Furthermore, a high-fat diet significantly increased intestinal permeability by reducing the expression of epithelial tight junction proteins and disrupting intestinal barrier function, leading to metabolic disorders, endotoxemia, and inflammation.97 Other experiments suggested that microbiota can mitigate metabolic symptoms by inducing Th17 cells to regulate intestinal lipid absorption, offering a potential therapeutic target.98
In summary, the gut microbiota is a metabolic factor linking MASLD and PCOS by modulating absorption and metabolism in the intestine. Restoring gut microbiota is a potential therapeutic approach. However, further studies are needed to clarify the specific role of the gut microbiota and establish personalized treatments targeting the gut microbiota.
Hyperandrogenemia
HA, one of the diagnostic criteria of PCOS, is induced by IR, lower SHBG, and other metabolic and endocrine factors. In women, androgens are mainly produced by the ovaries and adrenal glands. Previous studies have suggested the co-existence of abnormalities in both sources of androgen overproduction. One study found that excessive adrenal-derived androgen secretion negatively regulates the hypothalamus-pituitary-ovary axis in a feedback loop, and disrupts the rhythm of GnRH release.99 Persistent high-frequency GnRH pulses in patients with PCOS lead to increased LH pulse amplitudes, resulting in hypersecretion of LH and relative deficiency of follicle-stimulating hormone (FSH), which causes HA, anovulation, and polycystic ovary.100 Excessive androgen also stimulates inflammation, cumulus cell apoptosis, follicular dysplasia, and liver and abdominal fat accumulation.101 HA is an independent risk factor for hepatic steatosis and metabolic syndrome in patients with PCOS after adjusting for age and BMI.6 Patients with hyperandrogenic PCOS have a higher liver fat content than those with normal-androgenic PCOS.102 Androgens enhance serine phosphorylation of insulin receptors, thereby impacting insulin signaling pathways and activating IR according to the initial experiments.22 Additionally, dehydroepiandrosterone can reduce membrane GLUT4 expression in skeletal muscle tissue and disrupt mitochondrial and glucose balance, leading to the development of IR.103 Moreover, androgens promote de novo lipogenesis and hepatic steatosis by inducing IR and increasing SREBP-1c levels.104 As a result, HA is influenced by endocrine dysfunction, which is associated with reduced SHBG and insulin sensitivity. Subsequently, HA negatively affects glucose and lipid metabolism, contributing to the symptoms of hyperglycemia, IR, obesity, and fatty liver.
Other factors
The close links between metabolic dysfunction and PCOS and MASLD suggest that environmental factors contributing to overweight and IR may be the main cause of these conditions. For instance, a sedentary lifestyle and unhealthy eating habits can lead to obesity and IR, resulting in metabolic and reproductive issues, and ultimately raising the likelihood of PCOS and hepatic steatosis. Jensen et al. reported that high-sugar diets also raise the risk of fatty liver and accelerated MASH development.105
Exposure to some endocrine disruptors may also contribute to PCOS and MASLD. Serum bisphenol A (BPA) is one such disruptor that can interfere with normal biological endocrine functions, either directly or indirectly. Recent reports have raised concern about its potential impact on the development of obesity, diabetes, and other chronic diseases. Studies have shown a positive association between BPA and the occurrence of PCOS, as well as its involvement in the development of IR and HA.106 Additionally, BPA also disrupts various processes including adipogenesis, inflammation, and oxidative stress, which are thought to be plausibly linked to MASLD.
Moreover, about 1/6 of patients with NAFLD are diagnosed with subclinical hypothyroidism, characterized by elevated levels of thyroid-stimulating hormone (TSH).107 Elevated TSH levels are correlated with obesity, dyslipidemia, IR, fatty liver, and higher plasma concentrations of total cholesterol. The prevalence of MASLD rises steadily with increasing TSH levels. Moreover, among women of reproductive age, hypothyroidism is another common endocrinological disorder. In line with this, women with PCOS have a significantly higher prevalence of subclinical hypothyroidism compared to the control group.108 Elevated TSH levels impair oocyte maturation and fertilization in patients with PCOS undergoing in vitro fertilization, and are also positively associated with overall mortality and cardiovascular mortality in patients with MASLD.109,110 In conclusion, thyroid dysfunction impairs metabolic processes, leading to hyperglycemia and lipid accumulation, making it a potential target for therapy.
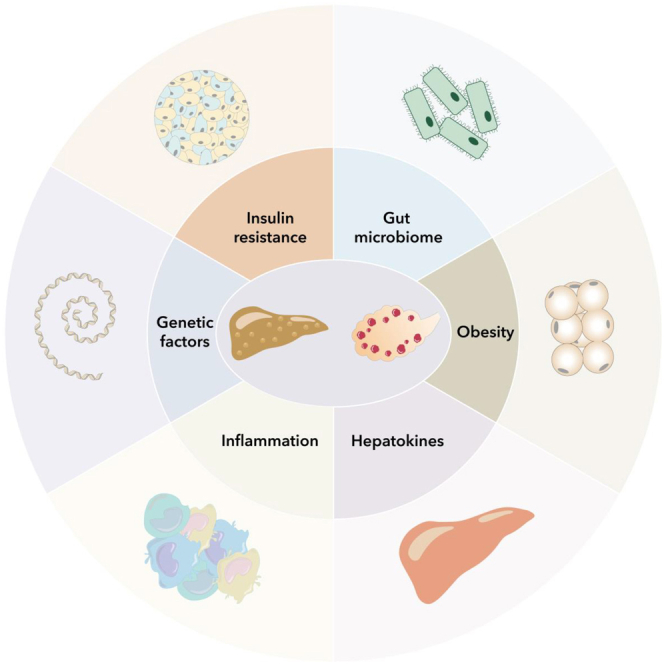
Risk factors can also interact with each other, such as genetic factors, hepatokines, IR, SHBG, gut microbiota, inflammation, and obesity, which correlate and promote PCOS occurrence by inducing metabolic dysfunction. Figure 4 shows some possible associated factors. HA, induced by these factors, exacerbates metabolic dysfunctions and promotes the progression of MASLD. However, the mechanism causing these dysfunctions and impairments is still unresolved and requires further research.
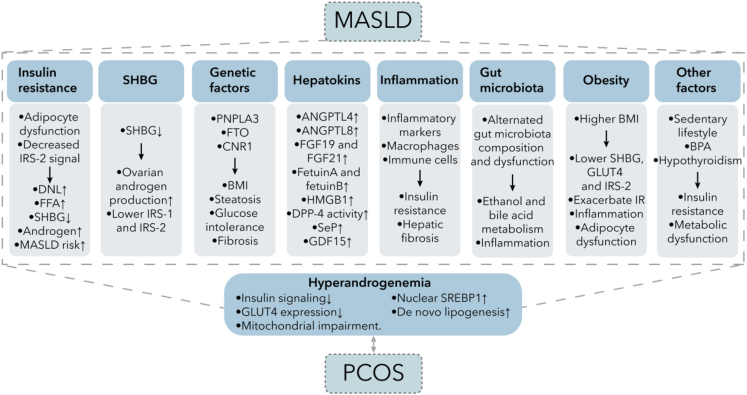
Figure 4.
Underlying associated mechanisms between MASLD and PCOS
The connection between MASLD and PCOS involves complex mechanisms. Many of these factors are related to metabolism, such as insulin resistance, SHBG, obesity, genetic factors, and hepatokines, which can impact glucose and lipid metabolism. Inflammation is also a significant factor that can contribute to insulin resistance and hepatic fibrosis. In recent years, the gut microbiota has garnered attention for its diverse effects on the body, including promoting inflammation and metabolic abnormalities. All of these factors are correlated with hyperandrogenemia and PCOS through their influence on metabolism. As a result, there are multiple shared metabolic symptoms in both MASLD and PCOS. Abbreviations: IRS, insulin receptor substrate; DNL, de novo lipogenesis; FFA, free fatty acid; SHBG, sex hormone-binding globulin; MASLD, metabolic-dysfunction associated steatotic liver disease; PNPLA3, patatin-like phospholipase domain-containing protein 3; FTO, fat mass and obesity associated gene; CNR1, endocannabinoid receptor 1; BMI, body mass index; ANGPTL, angiopoietin-like protein; FGF, fibroblast growth factor ; HMGB, high mobility group box; DPP-4, Dipeptidyl peptidase-4; SeP, slenoprotein P; GDF-15, growth differentiation factor 15; GLUT, glucose transporter; BPA, bisphenol A; SREBP-1c, sterol regulatory element binding protein 1c.
Therapeutic strategies
There is currently no proven drug treatment for MASLD, and there is no universal treatment for MASLD in patients with PCOS. Due to the increased occurrence of complications, clinicians should prioritize regular follow-up visits and monitoring of relevant indicators, including weight, serum glucose, HbA1c, blood lipid parameters, and glucose tolerance tests. The primary management of patients with MASLD and PCOS mainly focuses on lifestyle regulation. Since metabolism plays a key role, therapy should be symptom-oriented, and potential optional treatments include metabolism-related pharmacological treatments, nutritional preparations, and bariatric surgery.
Lifestyle regulation
The first-line treatment for MASLD and PCOS is weight loss achieved through reducing calorie intake, exercise, and a healthy diet, including reduced fructose and a Mediterranean diet. Lifestyle regulation is the fundamental treatment for MASLD and PCOS through a healthy diet with reduced calories and increased exercise. A 7% weight loss can reduce fat accumulation, relieve inflammation, and improve liver histological impairment.111 Based on preliminary experiments, lifestyle regulation can reduce weight, increase circulating SHBG levels, decrease free testosterone levels, and improve insulin sensitivity and HA-related symptoms in patients with PCOS.112,113 Women with PCOS who are overweight (BMI ≥ 25 kg/m2) may experience increased rates of conception and live births following a substantial weight loss (>10%).114 Time-restricted feeding, a type of intermittent fasting, is beneficial in reducing body fat, improving menstruation and HA, decreasing IR and reducing alanine aminotransferase and high-sensitivity C-reactive protein levels in women with PCOS.112 Furthermore, the Mediterranean diet can improve IR and IR-related diseases such as obesity, T2DM, hepatic steatosis, and PCOS.115
Hakimi et al. found that exercise can lower insulin and free androgen levels, which helps restore the regulation of ovulation by the hypothalamic-pituitary-ovarian axis.116 Aerobic training can reduce IR and inflammatory markers in obese mice through the PPAR-α pathway.117 Alternate-day fasting combined with aerobic exercise can significantly reduce body weight, fat mass, waist circumference, and ALT levels while increasing insulin sensitivity.118 Additionally, a combination of tea and saponin seed diet and aerobic exercise can decrease oxidative stress and improve metabolism in high-fat diet-induced MASLD mice.119 Although there is no clear evidence that exercise alone can improve PCOS, it is clear that moderate BMI reduction can improve metabolic syndromes. Long-term lifestyle changes are the simplest and healthiest way to improve metabolic dysfunction, and persistence and adherence are crucial for long-term regulation.
Pharmacological treatments
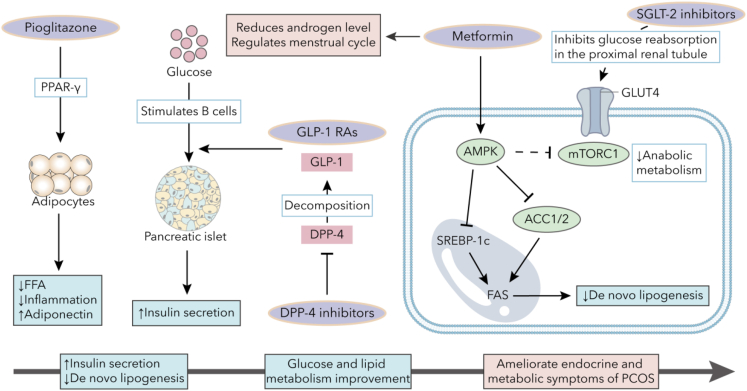
While there are no approved drugs for MASLD, potential optional symptom-oriented pharmacological therapies for MASLD in PCOS treatment should not only ameliorate liver fat accumulation, IR, and liver injury but also regulate ovulation and menstruation. In this regard, we outline the significant mechanisms of action of commonly used drugs (see Figure 5).
Figure 5.
Molecular mechanisms of anti-diabetic drugs
Pioglitazone, an agonist for PPAR-γ, reduces circulating FFA levels and inflammation cytokines while increasing adiponectin expression. DPP-4 inhibitors enhance GLP-1 function by inhibiting DPP-4, which promotes GLP-1 decomposition. Metformin can activate AMPK to inhibit ACC1/2 and SREBP-1c, leading to the suppression of de novo lipogenesis. GLP-1 RAs can enhance glucose-stimulated insulin secretion and protect pancreatic β cells. SGLT-2 inhibitors may induce hypoglycemic effects by inhibiting glucose reabsorption in the proximal renal tubule. These medications can improve endocrine and metabolic symptoms of PCOS by partly increasing insulin secretion, alleviating inflammation, and reducing androgen levels. Abbreviations: FFA, free fatty acid; PPAR, peroxisome proliferator-activated receptor; GLP-1, glucagon-like peptide 1; GLP-1 RA, Glucagon-like peptide-1 receptor agonist; DPP-4, dipeptidyl peptidase-4; SGLT2, sodium-glucose cotransporter 2; GLUT, glucose transporter; SREBP-1c, sterol regulatory element binding protein 1c; AMPK, AMP-activated protein kinase; mTOR, mammalian target of rapamycin; ACC, acetyl-CoA carboxylase; PCOS, polycystic ovary syndrome.
Metformin
Metformin, a biguanide, is widely used to regulate blood glucose. AMPK is a key regulator of glucose, lipid, and energy metabolism, catalyzing glucose and lipid metabolic dysfunction improvement. Previous experiments have shown that metformin can inhibit gluconeogenesis by activating AMPK through a lysosomal pathway, inhibiting CREB binding protein phosphorylation and suppressing gluconeogenic gene expression.120,121 Additionally, AMPK activation can inhibit hepatic mammalian target of rapamycin complex (mTORC) 1 signaling to inhibit anabolic metabolism.122 Furthermore, activated AMPK can lead to acetyl-CoA carboxylase (ACC) 1/2 phosphorylation, inhibiting SREBP-1c phosphorylation and de novo lipogenesis.123 Metformin can also stimulate GLP-1 release to enhance insulin secretion, increase GLUT4 translocation in skeletal muscles, and improve insulin sensitivity.124 Moreover, metformin affects the composition and function of the gut microbiota, restores abnormal mitochondrial life cycles, and increases mitochondrial density in the livers of obese mice.125,126
Metformin is beneficial for patients with PCOS, improving IR, hirsutism, and normalizing menstrual cycles. Metformin treatment also lowers the risk of ovarian hyperstimulation syndrome in women with PCOS undergoing assisted reproduction.127 Additionally, the use of metformin in patients with PCOS has been linked to higher pregnancy rates and more favorable pregnancy outcomes compared to the control group.128 In the case of patients with MASLD, metformin not only improves IR but also reduces lipid accumulation in hepatic cells and adipocytes, as shown in in vitro studies.129 Preliminary experimental observations have consistently demonstrated that metformin has a positive effect on MASLD, leading to improvements in liver histology and fat content.130 Furthermore, metformin has been found to inhibit diet-induced MASH-related hepatocellular carcinoma progression in zebrafish and counteract the effects of a high-fat diet on hepatic steatosis, inflammation, and tumorigenesis.131 Recent studies have also indicated that metformin can reduce fatty liver, ameliorate MASH, inhibit the development of HCC, and normalize menstrual cycles and ovulation.132 Consequently, metformin can be considered a viable treatment for both MASLD and PCOS.
Glucagon-like peptide-1 receptor agonists
GLP-1 may enhance glycemic control, stimulate glucose-stimulated insulin secretion, protect β-cells, and reduce bodyweight. GLP-1 receptor agonists (GLP-1 RAs) have demonstrated strong effects in increasing insulin sensitivity, reducing cardiovascular risk, promoting weight loss, increasing SHBG levels, and improving reproductive function.133 GLP-1 RAs may also decrease steatosis, alleviate inflammation in MASH patients, and slow down fibrosis progression.134 For women with PCOS, GLP-1 RAs, either alone or in combination with other metabolic regulators, are emerging as potential treatments for metabolic dysfunctions. For instance, GLP-1 RA alone or combined with metformin can significantly reduce weight and testosterone levels in obese patients with PCOS.135 However, in patients with MASLD, serum GLP-1 levels are similar to those of normal individuals, while liver GLP-1R expression is significantly down-regulated, which is induced by high-fat diet feeding and elevated free fatty acids. Therefore, GLP-1 RAs can be a potential treatment option for MASLD/MASH in patients with PCOS, through promoting weight loss, ameliorating HA and IR, and reducing the inflammation reaction.
Preliminary studies have shown that exendin-4, a GLP-1 RA, can decrease lipoprotein synthesis caused by IR and reverse dyslipidemia and hepatic steatosis in mice.136 Liraglutide may help improve liver enzyme levels, reduce liver fat accumulation, and improve parameters related to glucose metabolism.137,138 Semaglutide has been found to lower alanine aminotransferase and inflammatory marker levels, and contribute to the improvement of symptoms in patients with MASH.139 Liraglutide and semaglutide also have positive effects on cardiovascular outcomes. Another GLP-1 RA tirzepatide has been shown to increase adiponectin and decrease biomarkers associated with MASH, such as ALT, AST, and procollagen III.140 Therefore, GLP-1 RAs may be promising agents for treating MASLD and PCOS, especially in patients with IR, T2DM, and dyslipidemia.
Dipeptidyl peptidase-4 inhibitor
DPP-4 inhibitors increase GLP-1 and GIP levels by inhibiting DPP-4, which could switch them from high-affinity ligands to low-affinity ligands. A prior clinical study on 30 patients with PCOS showed that sitagliptin, a DPP4 inhibitor, increased β-cells function and prevented the transition from impaired glucose tolerance to T2DM in obese patients with PCOS.141 Sitagliptin can also decrease visceral fat and improve glucose levels by boosting early insulin secretion in overweight women with PCOS.142 Furthermore, linagliptin has been demonstrated to lower the expression of SREBP1c and FAS, both associated with de novo lipogenesis, and increase the expression of PPARα, leading to an improvement in hepatic steatosis in mouse experiments.143 In addition, in mouse PCOS models induced by letrozole, linagliptin treatment improved dyslipidemia and decreased inflammatory markers and free testosterone levels, in part by inhibiting the NF-κB pathway.144 Therefore, DPP-4 inhibitors may alleviate symptoms associated with glucose and lipid metabolism and prevent the progression of MASLD.
PPARs agonists
PPARs are transcription factors that regulate various metabolic pathways, including glucose and lipid metabolism. They are potential therapeutic targets for inflammation, dyslipidemia, and IR. PPARα, mainly expressed in hepatocytes, regulates lipid metabolism by controlling fatty acid intake and β-oxidation. It also exerts anti-inflammatory effects by inhibiting NF-κB.145 PPARδ is primarily expressed in skeletal muscle and inhibiting it in vitro reduces inflammation and steatosis associated with MASLD.146 PPARγ, mainly found in adipose tissue, is the most extensively studied form and has been linked to steatosis and insulin sensitivity.
Thiazolidinediones (TZDs) are selective PPARγ agonists that stimulate PPARγ in adipocytes, improving insulin sensitivity, reducing free fatty acid levels, and increasing adiponectin levels.147 Pioglitazone can reduce cardiovascular risk, improve steatosis and inflammation, improve liver histology biopsies, and has antiatherogenic properties.148 Pioglitazone treatment has been observed to result in decreased concentrations of TGs and low-density lipoprotein (LDL), as well as an increase in high-density lipoprotein (HDL). This effect may be attributed to the activation of PPARα.149 A combination of DPP-4 inhibitor and PPARγ agonist improved the structure of the intestinal barrier and reduced liver steatosis in experiments on obese mice.150 However, additional studies are required to conclusively identify their mechanisms of action. TZDs are linked to weight gain, higher risk of fractures, and increased heart failure.151 Despite these side effects, the benefits of TZDs outweigh them, making them still recommended for treating hepatic steatosis in diabetes. Preliminary studies indicated that in women with PCOS, TZDs effectively decreased insulin and fasting blood glucose levels and improved menstrual cycle and ovulation.152,153 However, due to safety concerns, they are only used as a second-line treatment. Elafibranor, a new PPARα/δ agonist, exhibits therapeutic efficacy on IR, inflammation, lipid accumulation, and fibrosis in rodent MASLD models via reducing the expression of enzymes correlated with gluconeogenesis.154,155 However, further clinical application is needed. In general, TZDs can be considered as an alternative treatment option for MASLD, improving IR, reducing hepatic steatosis and inflammation, promoting weight loss, normalizing menstrual cycles, and relieving PCOS symptoms.
Sodium-glucose cotransporter 2 inhibitor
Sodium-glucose cotransporter (SGLT) has two subtypes. SGLT1 is found in the small intestine, lung, heart, and the S3 segment of the proximal tubule, while SGLT2 is exclusively expressed in the S1 and S2 segments of the proximal tubule. SGLT2 inhibitors reduce serum glucose by inhibiting glucose and sodium reabsorption in the proximal renal tubule, leading to increased urinary glucose and sodium excretions. They can also reduce body weight, ameliorate diet-induced hepatic steatosis, prevent the progression of MASH, reduce the risks of cardiovascular disease, and improve blood pressure.156,157 Moreover, SGLT1 can reabsorb glucose in an acute compensatory manner, resulting in a very low risk of hypoglycemia.158 Trials are currently underway to assess the histological benefits of SGLT-2 inhibitors in patients with MASH. Therefore, SGLT2 inhibitors are considered the most promising treatment option for patients with MASLD. In a randomized controlled study, a 12-week empagliflozin treatment significantly improved anthropometric and physical parameters (i.e., waist circumference, hip circumference, BMI, and fat-free mass) for women with PCOS, but not hormonal or metabolic variables.159 However, SGLT2 inhibitors have adverse effects such as promoting mycotic genital infections and urinary tract infections, attributed to the presence of glucosuria.156 Furthermore, therapy with SGLT2 inhibitor is associated with increased risks for diabetic ketoacidosis in diabetes patients, as reported in many studies.160 These adverse actions still need to be addressed.
Nutritional preparations
Recent studies have shown that certain nutritional supplements can improve both MASLD and PCOS. For example, omega-3 fatty acid supplementation can significantly reduce liver fat content, TGs, and blood pressure, resulting in positive effects on fatty liver and cardiometabolic events.161 Vitamin E can also improve MASLD and MASH by ameliorating hepatic steatosis and hepatocellular ballooning.162 Co-supplementation of omega-3 fatty acids and vitamin E in patients with PCOS can decrease serum TGs, LDL, very low-density lipoprotein (VLDL), TC, insulin, serum total testosterone and free testosterone levels, and liver fat accumulation.163,164 Additionally, cinnamon supplementation can decrease serum fasting blood glucose, fasting insulin, TC, and LDL and improved IR in women with PCOS.165 Vitamin D supplementation has also been shown to result in modest improvements in ALT and IR when compared to a placebo.166 All of these nutritional supplements can help improve lipid accumulation by relieving glucose metabolism, but further studies are needed to confirm their therapeutic values for patients with MASLD and PCOS.
Bariatric surgery
Bariatric surgery is commonly used to treat obesity and related diseases. The most frequently used procedures are Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy. According to a meta-analysis shows, bariatric surgery can improve endocrine dysregulation and reduce serum total testosterone levels, serum free testosterone levels, BMI, and the incidence of menstrual abnormalities, alopecia, and T2DM in women with PCOS.167 For patients with MASH and severe obesity, bariatric surgery effectively improves steatosis, hepatic inflammation, and fibrosis.168 Lassailly et al. monitored MASH patients who had undergone bariatric surgery 5 years ago and discovered that MASH resolution was noticeable after 1 year, and fibrosis started to improve after 1 year and continued to improve until 5 years later. Bariatric surgery significantly improved histology and liver function in morbidly obese patients by reducing oxidative stress and inflammation. The study found a noteworthy decrease in liver markers for oxidative stress, inflammation, and fibrosis.169 Moreover, following bariatric surgery, the gut microbial signatures become more similar to those of lean, healthy individuals than those of patients with fatty liver.170 However, the implement of bariatric surgery still needs to be evaluated on an individual basis. Therefore, additional research is still necessary to validate the recommendation of bariatric surgery as a treatment option for MASLD in patients with PCOS.
Other treatment options
Statins can improve steatosis and steatohepatitis, but their main function is to lower plasma cholesterol and reduce the risk of cardiovascular disease, the leading cause of death in patients with MASLD and MASH.171 The combined oral contraceptive pill (COCP) is beneficial for reducing HA and regulating menstruation. Combining metformin with COCP may help manage overweight and metabolic dysfunctions. Assisted reproductive technology, including ovulation-inducing drugs, artificial insemination, and in vitro fertilization, can be attempted by patients with PCOS experiencing infertility. The combined use of sitagliptin and metformin can improve egg quality, enhance the probability of fertilization and pregnancy, and improve pregnancy outcomes. Furthermore, adjusting the gut microbiota has been proposed as beneficial for restoring metabolism, so FMT is currently undergoing clinical testing. Probiotics and antibiotics can partially improve gut microbiota disorders. Genetic factors, promising targets for the future, are still being discovered. Additionally, herbs targeting the pathogenesis processes of “multiple hits” could help improve hepatic steatosis and metabolic dysfunction to some extent. Furthermore, a previous study proposed that miR-615-5p can reduce mammalian target of rapamycin (mTOR) and SREBP-1c mRNA and protein levels to decrease TGs and lipid droplet accumulation.172 However, there is still a lack of studies related to their impact on PCOS symptoms, and more treatment options are still being explored.
Conclusion
The change from NAFLD to MASLD allows for a closer correlation between MASLD and PCOS due to their shared cardiometabolic criteria. PCOS is linked to MASLD through IR, SHBG, genetic factors, hepatokines, inflammation, gut microbiota, obesity, and other related factors. Clinicians should recognize the significance of these co-morbidities. For patients with PCOS or MASLD, regular monitoring of weight, glucose, and lipid metabolism is essential. Lifestyle adjustments are recommended as the first step. However, there is still lack of approved drugs for MASLD. Metabolism-related drugs such as metformin, GLP-1 RAs, DPP-4 inhibitors, and others should be used based on individual situations and symptoms. Nevertheless, these drugs and other options require further trials for use in treating MASLD. Our aim is to encourage the shift from defining NAFLD to MASLD, highlighting the connection with metabolism, and to raise awareness among obstetrician-gynecologists about the significance of metabolic influence in PCOS and MASLD. This will enable clinicians to better diagnose and treat patients with PCOS and MASLD on an individual basis. Clinicians currently lack understanding of the transition from NAFLD to MASLD and the connection between MASLD and PCOS. We need to further promote the concept of transition and highlight the role of metabolism in the development of MASLD and PCOS in order to improve disease diagnosis, treatment, and overall human health.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (nos. 82171685 and 81771574), and the Fund for Excellent Young Scholars of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (No. JYYQ004).
Author contributions
Y. L. and L.W. conceived the manuscript. Q.X. wrote and J. Z. revised the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Jie Zhang, Email: dr.zhangjie@qq.com.
Yan Lu, Email: luyan5011@shsmu.edu.cn.
Ling Wu, Email: wuling9hospital@126.com.
References
- 1.Fan J.G., Kim S.U., Wong V.W.S. New trends on obesity and NAFLD in Asia. J. Hepatol. 2017;67:862–873. doi: 10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z.M., Golabi P., de Avila L., Paik J.M., Srishord M., Fukui N., Qiu Y., Burns L., Afendy A., Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Riazi K., Azhari H., Charette J.H., Underwood F.E., King J.A., Afshar E.E., Swain M.G., Congly S.E., Kaplan G.G., Shaheen A.A. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet. Gastroenterol. Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- 4.Rinella M.E., Lazarus J.V., Ratziu V., Francque S.M., Sanyal A.J., Kanwal F., Romero D., Abdelmalek M.F., Anstee Q.M., Arab J.P., et al. A multi–society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023;79:1542–1556. doi: 10.1016/j.jhep.2023.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Bozdag G., Mumusoglu S., Zengin D., Karabulut E., Yildiz B.O. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. 2016;31:2841–2855. doi: 10.1093/humrep/dew218. [DOI] [PubMed] [Google Scholar]
- 6.Salva-Pastor N., López-Sánchez G.N., Chávez-Tapia N.C., Audifred-Salomón J.R., Niebla-Cárdenas D., Topete-Estrada R., Pereznuñez-Zamora H., Vidaltamayo-Ramírez R., Báez-Arellano M.E., Uribe M., Nuño-Lámbarri N. Polycystic ovary syndrome with feasible equivalence to overweight as a risk factor for non-alcoholic fatty liver disease development and severity in Mexican population. Ann. Hepatol. 2020;19:251–257. doi: 10.1016/j.aohep.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Kumarendran B., O’Reilly M.W., Manolopoulos K.N., Toulis K.A., Gokhale K.M., Sitch A.J., Wijeyaratne C.N., Coomarasamy A., Arlt W., Nirantharakumar K. Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarkar M., Terrault N., Duwaerts C.C., Tien P., Cedars M.I., Huddleston H. The Association of Hispanic Ethnicity with Nonalcoholic Fatty Liver Disease in Polycystic Ovary Syndrome. Curr. Opin. Gynecol. Obstet. 2018;1:24–33. [PMC free article] [PubMed] [Google Scholar]
- 9.Paschou S.A., Polyzos S.A., Anagnostis P., Goulis D.G., Kanaka-Gantenbein C., Lambrinoudaki I., Georgopoulos N.A., Vryonidou A. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Endocrine. 2020;67:1–8. doi: 10.1007/s12020-019-02085-7. [DOI] [PubMed] [Google Scholar]
- 10.Vassilatou E., Vassiliadi D.A., Salambasis K., Lazaridou H., Koutsomitopoulos N., Kelekis N., Kassanos D., Hadjidakis D., Dimitriadis G. Increased prevalence of polycystic ovary syndrome in premenopausal women with nonalcoholic fatty liver disease. Eur. J. Endocrinol. 2015;173:739–747. doi: 10.1530/EJE-15-0567. [DOI] [PubMed] [Google Scholar]
- 11.Liu D., Gao X., Pan X.F., Zhou T., Zhu C., Li F., Fan J.G., Targher G., Zhao J. The hepato-ovarian axis: genetic evidence for a causal association between non-alcoholic fatty liver disease and polycystic ovary syndrome. BMC Med. 2023;21:62. doi: 10.1186/s12916-023-02775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Targher G., Rossini M., Lonardo A. Evidence that non-alcoholic fatty liver disease and polycystic ovary syndrome are associated by necessity rather than chance: a novel hepato-ovarian axis? Endocrine. 2016;51:211–221. doi: 10.1007/s12020-015-0640-8. [DOI] [PubMed] [Google Scholar]
- 13.Hagström H., Vessby J., Ekstedt M., Shang Y. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J. Hepatol. 2023 doi: 10.1016/j.jhep.2023.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Song S.J., Lai J.C.T., Wong G.L.H., Wong V.W.S., Yip T.C.F. Can we use old NAFLD data under the new MASLD definition? J. Hepatol. 2023 doi: 10.1016/j.jhep.2023.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Diamanti-Kandarakis E., Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr. Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moghetti P., Tosi F. Insulin resistance and PCOS: chicken or egg? J. Endocrinol. Invest. 2021;44:233–244. doi: 10.1007/s40618-020-01351-0. [DOI] [PubMed] [Google Scholar]
- 18.Petersen M.C., Madiraju A.K., Gassaway B.M., Marcel M., Nasiri A.R., Butrico G., Marcucci M.J., Zhang D., Abulizi A., Zhang X.M., et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J. Clin. Invest. 2016;126:4361–4371. doi: 10.1172/JCI86013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan R.S., Bril F., Cusi K., Newsome P.N. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;70:711–724. doi: 10.1002/hep.30429. [DOI] [PubMed] [Google Scholar]
- 20.Jahromi B.N., Borzou N., Parsanezhad M.E., Anvar Z., Ghaemmaghami P., Sabetian S. Associations of insulin resistance, sex hormone-binding globulin, triglyceride, and hormonal profiles in polycystic ovary syndrome: A cross-sectional study. Int. J. Reprod. Biomed. 2021;19:653–662. doi: 10.18502/ijrm.v19i7.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apridonidze T., Essah P.A., Iuorno M.J., Nestler J.E. Prevalence and Characteristics of the Metabolic Syndrome in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2005;90:1929–1935. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 22.Corbould A., Kim Y.B., Youngren J.F., Pender C., Kahn B.B., Lee A., Dunaif A. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am. J. Physiol. Endocrinol. Metab. 2005;288:E1047–E1054. doi: 10.1152/ajpendo.00361.2004. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y., Xu Y.N., Ye C.Y., Feng W.B., Zhou Q.T., Yang D.H., Wang M.W. GLP-1 mimetics as a potential therapy for nonalcoholic steatohepatitis. Acta Pharmacol. Sin. 2022;43:1156–1166. doi: 10.1038/s41401-021-00836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makri E., Goulas A., Polyzos S.A. Epidemiology, Pathogenesis, Diagnosis and Emerging Treatment of Nonalcoholic Fatty Liver Disease. Arch. Med. Res. 2021;52:25–37. doi: 10.1016/j.arcmed.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Liu J., Ayada I., Zhang X., Wang L., Li Y., Wen T., Ma Z., Bruno M.J., de Knegt R.J., Cao W., et al. Estimating Global Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in Overweight or Obese Adults. Clin. Gastroenterol. Hepatol. 2022;20:e573–e582. doi: 10.1016/j.cgh.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 26.Lim S.S., Davies M.J., Norman R.J., Moran L.J. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. Update. 2012;18:618–637. doi: 10.1093/humupd/dms030. [DOI] [PubMed] [Google Scholar]
- 27.Schwimmer J.B., Pardee P.E., Lavine J.E., Blumkin A.K., Cook S. Cardiovascular Risk Factors and the Metabolic Syndrome in Pediatric Nonalcoholic Fatty Liver Disease. Circulation. 2008;118:277–283. doi: 10.1161/CIRCULATIONAHA.107.739920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez Paris V., Solon-Biet S.M., Senior A.M., Edwards M.C., Desai R., Tedla N., Cox M.J., Ledger W.L., Gilchrist R.B., Simpson S.J., et al. Defining the impact of dietary macronutrient balance on PCOS traits. Nat. Commun. 2020;11:5262. doi: 10.1038/s41467-020-19003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robker R.L., Akison L.K., Bennett B.D., Thrupp P.N., Chura L.R., Russell D.L., Lane M., Norman R.J. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J. Clin. Endocrinol. Metab. 2009;94:1533–1540. doi: 10.1210/jc.2008-2648. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez Paris V., Bertoldo M.J. The Mechanism of Androgen Actions in PCOS Etiology. Med. Sci. 2019;7:E89. doi: 10.3390/medsci7090089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbano F., Chiarito M., Lattanzio C., Messa A., Ferrante M., Francavilla M., Mehmeti I., Lassandro G., Giordano P., Faienza M.F. Sex Hormone-Binding Globulin (SHBG) Reduction: The Alarm Bell for the Risk of Non-Alcoholic Fatty Liver Disease in Adolescents with Polycystic Ovary Syndrome. Children. 2022;9:1748. doi: 10.3390/children9111748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo J., Chen Q., Shen T., Wang X., Fang W., Wu X., Yuan Z., Chen G., Ling W., Chen Y. Association of sex hormone-binding globulin with nonalcoholic fatty liver disease in Chinese adults. Nutr. Metab. 2018;15:79. doi: 10.1186/s12986-018-0313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X., Xie J., Pang J., Zhang H., Chen X., Lin J., Li Q., Chen Q., Ma J., Xu X., et al. Serum SHBG Is Associated With the Development and Regression of Nonalcoholic Fatty Liver Disease: A Prospective Study. J. Clin. Endocrinol. Metab. 2020;105:dgz244. doi: 10.1210/clinem/dgz244. [DOI] [PubMed] [Google Scholar]
- 34.Hua X., Sun Y., Zhong Y., Feng W., Huang H., Wang W., Zhang T., Hu Y. Low serum sex hormone-binding globulin is associated with nonalcoholic fatty liver disease in type 2 diabetic patients. Clin. Endocrinol. 2014;80:877–883. doi: 10.1111/cen.12360. [DOI] [PubMed] [Google Scholar]
- 35.Feng C., Jin Z., Chi X., Zhang B., Wang X., Sun L., Fan J., Sun Q., Zhang X. SHBG expression is correlated with PI3K/AKT pathway activity in a cellular model of human insulin resistance. Gynecol. Endocrinol. 2018;34:567–573. doi: 10.1080/09513590.2017.1411474. [DOI] [PubMed] [Google Scholar]
- 36.Malini N.A., Roy George K. Evaluation of different ranges of LH:FSH ratios in polycystic ovarian syndrome (PCOS) - Clinical based case control study. Gen. Comp. Endocrinol. 2018;260:51–57. doi: 10.1016/j.ygcen.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Matias I., Gatta-Cherifi B., Cota D. Obesity and the Endocannabinoid System: Circulating Endocannabinoids and Obesity. Curr. Obes. Rep. 2012;1:229–235. [Google Scholar]
- 38.Jędrzejuk D., Laczmański L., Kuliczkowska J., Lenarcik A., Trzmiel-Bira A., Hirnle L., Dorobisz U., Milewicz A., Lwow F., Urbanovych A., Słoka N. Selected CNR1 polymorphisms and hyperandrogenemia as well as fat mass and fat distribution in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2015;31:36–39. doi: 10.3109/09513590.2014.946899. [DOI] [PubMed] [Google Scholar]
- 39.Kuliczkowska Plaksej J., Laczmanski L., Milewicz A., Lenarcik-Kabza A., Trzmiel-Bira A., Zaleska-Dorobisz U., Lwow F., Hirnle L. Cannabinoid Receptor 1 Gene Polymorphisms and Nonalcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome and in Healthy Controls. Int. J. Endocrinol. 2014;2014 doi: 10.1155/2014/232975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dawson A.J., Kilpatrick E.S., Coady A.M., Elshewehy A.M.M., Dakroury Y., Ahmed L., Atkin S.L., Sathyapalan T. Endocannabinoid receptor blockade reduces alanine aminotransferase in polycystic ovary syndrome independent of weight loss. BMC Endocr. Disord. 2017;17:41. doi: 10.1186/s12902-017-0194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caruso V., Chen H., Morris M.J. Early hypothalamic FTO overexpression in response to maternal obesity--potential contribution to postweaning hyperphagia. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Church C., Moir L., McMurray F., Girard C., Banks G.T., Teboul L., Wells S., Brüning J.C., Nolan P.M., Ashcroft F.M., Cox R.D. Overexpression of Fto leads to increased food intake and results in obesity. Nat. Genet. 2010;42:1086–1092. doi: 10.1038/ng.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMurray F., Church C.D., Larder R., Nicholson G., Wells S., Teboul L., Tung Y.C.L., Rimmington D., Bosch F., Jimenez V., et al. Adult onset global loss of the fto gene alters body composition and metabolism in the mouse. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu A.L., Xie H.J., Xie H.Y., Liu J., Yin J., Hu J.S., Peng C.Y. Association between fat mass and obesity associated (FTO) gene rs9939609 A/T polymorphism and polycystic ovary syndrome: a systematic review and meta-analysis. BMC Med. Genet. 2017;18:89. doi: 10.1186/s12881-017-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan S., Scherag A., Janssen O.E., Hahn S., Lahner H., Dietz T., Scherag S., Grallert H., Vogel C.I.G., Kimmig R., et al. Large effects on body mass index and insulin resistance of fat mass and obesity associated gene (FTO) variants in patients with polycystic ovary syndrome (PCOS) BMC Med. Genet. 2010;11:12. doi: 10.1186/1471-2350-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wojciechowski P., Lipowska A., Rys P., Ewens K.G., Franks S., Tan S., Lerchbaum E., Vcelak J., Attaoua R., Straczkowski M., et al. Impact of FTO genotypes on BMI and weight in polycystic ovary syndrome: a systematic review and meta-analysis. Diabetologia. 2012;55:2636–2645. doi: 10.1007/s00125-012-2638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.BasuRay S., Smagris E., Cohen J.C., Hobbs H.H. The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation. Hepatology. 2017;66:1111–1124. doi: 10.1002/hep.29273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karamfilova V., Gateva A., Assyov Y., Alexiev A., Savov A., Yaneva N., Ivanova I., Ivanova-Boyanova R., Ivanova R., Vlahova Z., et al. PNPLA3 I148M Polymorphism in Patients with Nonalcoholic Fatty Liver Disease, Obesity and Prediabetes. J. Gastrointestin. Liver Dis. 2019;28:433–438. doi: 10.15403/jgld-506. [DOI] [PubMed] [Google Scholar]
- 49.Baselli G.A., Dongiovanni P., Rametta R., Meroni M., Pelusi S., Maggioni M., Badiali S., Pingitore P., Maurotti S., Montalcini T., et al. Liver transcriptomics highlights interleukin-32 as novel NAFLD-related cytokine and candidate biomarker. Gut. 2020;69:1855–1866. doi: 10.1136/gutjnl-2019-319226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pingitore P., Dongiovanni P., Motta B.M., Meroni M., Lepore S.M., Mancina R.M., Pelusi S., Russo C., Caddeo A., Rossi G., et al. PNPLA3 overexpression results in reduction of proteins predisposing to fibrosis. Hum. Mol. Genet. 2016;25:5212–5222. doi: 10.1093/hmg/ddw341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aly O., Zaki H.H., Herzalla M.R., Fathy A., Raafat N., Hafez M.M. Gene polymorphisms of Patatin-like phospholipase domain containing 3 (PNPLA3), adiponectin, leptin in diabetic obese patients. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cherubini A., Ostadreza M., Jamialahmadi O., Pelusi S., Rrapaj E., Casirati E., Passignani G., Norouziesfahani M., Sinopoli E., Baselli G., et al. Interaction between estrogen receptor-α and PNPLA3 p.I148M variant drives fatty liver disease susceptibility in women. Nat. Med. 2023;29:2643–2655. doi: 10.1038/s41591-023-02553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong B.S., Liu J., Zheng J., Ke W., Huang Z., Wan X., He X., Xiao H., Li Y. Angiopoietin-like protein 8/betatrophin correlates with hepatocellular lipid content independent of insulin resistance in non-alcoholic fatty liver disease patients. J. Diabetes Investig. 2018;9:952–958. doi: 10.1111/jdi.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fu Z., Berhane F., Fite A., Seyoum B., Abou-Samra A.B., Zhang R. Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Sci. Rep. 2014;4:5013. doi: 10.1038/srep05013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Izumi R., Kusakabe T., Noguchi M., Iwakura H., Tanaka T., Miyazawa T., Aotani D., Hosoda K., Kangawa K., Nakao K. CRISPR/Cas9-mediated Angptl8 knockout suppresses plasma triglyceride concentrations and adiposity in rats. J. Lipid Res. 2018;59:1575–1585. doi: 10.1194/jlr.M082099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H., Du L., Wu T., Yang G., Hu W., Wang H., Yang M., Liu D., Gu H.F., Zhu Z., et al. Circulating betatrophin is associated with insulin resistance in humans: cross-sectional and interventional studies in vivo and in vitro. Oncotarget. 2017;8:96604–96614. doi: 10.18632/oncotarget.21852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Talukdar S., Kharitonenkov A. FGF19 and FGF21: In NASH we trust. Mol. Metab. 2021;46 doi: 10.1016/j.molmet.2020.101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reinehr T., Woelfle J., Wunsch R., Roth C.L. Fibroblast growth factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: a longitudinal analysis. J. Clin. Endocrinol. Metab. 2012;97:2143–2150. doi: 10.1210/jc.2012-1221. [DOI] [PubMed] [Google Scholar]
- 59.Camporez J.P.G., Asrih M., Zhang D., Kahn M., Samuel V.T., Jurczak M.J., Jornayvaz F.R. Hepatic insulin resistance and increased hepatic glucose production in mice lacking Fgf21. J. Endocrinol. 2015;226:207–217. doi: 10.1530/JOE-15-0136. [DOI] [PubMed] [Google Scholar]
- 60.Coskun T., Bina H.A., Schneider M.A., Dunbar J.D., Hu C.C., Chen Y., Moller D.E., Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 61.Ramanjaneya M., Bensila M., Bettahi I., Jerobin J., Samra T.A., Aye M.M., Alkasem M., Siveen K.S., Sathyapalan T., Skarulis M., et al. Dynamic Changes in Circulating Endocrine FGF19 Subfamily and Fetuin-A in Response to Intralipid and Insulin Infusions in Healthy and PCOS Women. Front. Endocrinol. 2020;11 doi: 10.3389/fendo.2020.568500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sahin S.B., Ayaz T., Cure M.C., Sezgin H., Ural U.M., Balik G., Sahin F.K. Fibroblast growth factor 21 and its relation to metabolic parameters in women with polycystic ovary syndrome. Scand. J. Clin. Lab. Invest. 2014;74:465–469. doi: 10.3109/00365513.2014.900821. [DOI] [PubMed] [Google Scholar]
- 63.Sujana C., Huth C., Zierer A., Meesters S., Sudduth-Klinger J., Koenig W., Herder C., Peters A., Thorand B. Association of fetuin-A with incident type 2 diabetes: results from the MONICA/KORA Augsburg study and a systematic meta-analysis. Eur. J. Endocrinol. 2018;178:389–398. doi: 10.1530/EJE-17-1053. [DOI] [PubMed] [Google Scholar]
- 64.Bayramoğlu E., Çetinkaya S., Özalkak S., Kurnaz E., Demirci G., Öztürk H.S., Savaş-Erdeve Ş., Aycan Z. Evaluation of the pathophysiological role of Fetuin A levels in adolescents with polycystic ovary syndrome. J. Pediatr. Endocrinol. Metab. 2021;34:911–916. doi: 10.1515/jpem-2020-0524. [DOI] [PubMed] [Google Scholar]
- 65.Pal D., Dasgupta S., Kundu R., Maitra S., Das G., Mukhopadhyay S., Ray S., Majumdar S.S., Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 2012;18:1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 66.Ou H.Y., Wu H.T., Hung H.C., Yang Y.C., Wu J.S., Chang C.J. Endoplasmic reticulum stress induces the expression of fetuin-A to develop insulin resistance. Endocrinology. 2012;153:2974–2984. doi: 10.1210/en.2011-2043. [DOI] [PubMed] [Google Scholar]
- 67.Díaz M., Gallego-Escuredo J.M., López-Bermejo A., de Zegher F., Villarroya F., Ibáñez L. Low-Dose Spironolactone-Pioglitazone-Metformin Normalizes Circulating Fetuin-A Concentrations in Adolescent Girls with Polycystic Ovary Syndrome. Int. J. Endocrinol. 2018;2018 doi: 10.1155/2018/4192940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qu H., Qiu Y., Wang Y., Liao Y., Zheng Y., Zheng H. Plasma fetuin-B concentrations are associated with insulin resistance and first-phase glucose-stimulated insulin secretion in individuals with different degrees of glucose tolerance. Diabetes Metab. 2018;44:488–492. doi: 10.1016/j.diabet.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Mokou M., Yang S., Zhan B., Geng S., Li K., Yang M., Yang G., Deng W., Liu H., Liu D., et al. Elevated Circulating Fetuin-B Levels Are Associated with Insulin Resistance and Reduced by GLP-1RA in Newly Diagnosed PCOS Women. Mediators Inflamm. 2020;2020 doi: 10.1155/2020/2483435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu L., Jiang Y., Steinle J.J. Inhibition of HMGB1 protects the retina from ischemia-reperfusion, as well as reduces insulin resistance proteins. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li L., Chen L., Hu L., Liu Y., Sun H.Y., Tang J., Hou Y.J., Chang Y.X., Tu Q.Q., Feng G.S., et al. Nuclear factor high-mobility group box1 mediating the activation of toll-like receptor 4 signaling in hepatocytes in the early stage of nonalcoholic fatty liver disease in mice. Hepatology. 2011;54:1620–1630. doi: 10.1002/hep.24552. [DOI] [PubMed] [Google Scholar]
- 72.Zhang C., Hu J., Wang W., Sun Y., Sun K. HMGB1-induced aberrant autophagy contributes to insulin resistance in granulosa cells in PCOS. FASEB J. 2020;34:9563–9574. doi: 10.1096/fj.202000605RR. [DOI] [PubMed] [Google Scholar]
- 73.Sun X., Tang D. HMGB1-dependent and -independent autophagy. Autophagy. 2014;10:1873–1876. doi: 10.4161/auto.32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baumeier C., Schlüter L., Saussenthaler S., Laeger T., Rödiger M., Alaze S.A., Fritsche L., Häring H.U., Stefan N., Fritsche A., et al. Elevated hepatic DPP4 activity promotes insulin resistance and non-alcoholic fatty liver disease. Mol. Metab. 2017;6:1254–1263. doi: 10.1016/j.molmet.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdalla M.A., Deshmukh H., Atkin S., Sathyapalan T. The potential role of incretin-based therapies for polycystic ovary syndrome: a narrative review of the current evidence. Ther. Adv. Endocrinol. Metab. 2021;12 doi: 10.1177/2042018821989238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi H.Y., Hwang S.Y., Lee C.H., Hong H.C., Yang S.J., Yoo H.J., Seo J.A., Kim S.G., Kim N.H., Baik S.H., et al. Increased selenoprotein p levels in subjects with visceral obesity and nonalcoholic Fatty liver disease. Diabetes Metab. J. 2013;37:63–71. doi: 10.4093/dmj.2013.37.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mita Y., Nakayama K., Inari S., Nishito Y., Yoshioka Y., Sakai N., Sotani K., Nagamura T., Kuzuhara Y., Inagaki K., et al. Selenoprotein P-neutralizing antibodies improve insulin secretion and glucose sensitivity in type 2 diabetes mouse models. Nat. Commun. 2017;8:1658. doi: 10.1038/s41467-017-01863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bilson J., Scorletti E., Bindels L.B., Afolabi P.R., Targher G., Calder P.C., Sethi J.K., Byrne C.D. Growth differentiation factor-15 and the association between type 2 diabetes and liver fibrosis in NAFLD. Nutr. Diabetes. 2021;11:32. doi: 10.1038/s41387-021-00170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y., Chen C., Chen J., Sang T., Peng H., Lin X., Zhao Q., Chen S., Eling T., Wang X. Overexpression of NAG-1/GDF15 prevents hepatic steatosis through inhibiting oxidative stress-mediated dsDNA release and AIM2 inflammasome activation. Redox Biol. 2022;52 doi: 10.1016/j.redox.2022.102322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma Y., Zheng L., Wang Y., Gao Y., Xu Y. Arachidonic Acid in Follicular Fluid of PCOS Induces Oxidative Stress in a Human Ovarian Granulosa Tumor Cell Line (KGN) and Upregulates GDF15 Expression as a Response. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.865748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anstee Q.M., Reeves H.L., Kotsiliti E., Govaere O., Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019;16:411–428. doi: 10.1038/s41575-019-0145-7. [DOI] [PubMed] [Google Scholar]
- 82.Escobar-Morreale H.F., Luque-Ramírez M., González F. CIRCULATING INFLAMMATORY MARKERS IN POLYCYSTIC OVARY SYNDROME: A SYSTEMATIC REVIEW AND META-ANALYSIS. Fertil. Steril. 2011;95:1048–1058.e1-2. doi: 10.1016/j.fertnstert.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Phelan N., O’Connor A., Kyaw Tun T., Correia N., Boran G., Roche H.M., Gibney J. Leucocytosis in women with polycystic ovary syndrome (PCOS) is incompletely explained by obesity and insulin resistance. Clin. Endocrinol. 2013;78:107–113. doi: 10.1111/j.1365-2265.2012.04454.x. [DOI] [PubMed] [Google Scholar]
- 84.Ehses J.A., Böni-Schnetzler M., Faulenbach M., Donath M.Y. Macrophages, cytokines and beta-cell death in Type 2 diabetes. Biochem. Soc. Trans. 2008;36(Pt 3):340–342. doi: 10.1042/BST0360340. [DOI] [PubMed] [Google Scholar]
- 85.Rudnicka E., Suchta K., Grymowicz M., Calik-Ksepka A., Smolarczyk K., Duszewska A.M., Smolarczyk R., Meczekalski B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021;22:3789. doi: 10.3390/ijms22073789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cai D., Yuan M., Frantz D.F., Melendez P.A., Hansen L., Lee J., Shoelson S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shoelson S.E., Lee J., Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int. J. Obes. Relat. Metab. Disord. 2003;27:S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 88.Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 89.Kaden-Volynets V., Basic M., Neumann U., Pretz D., Rings A., Bleich A., Bischoff S.C. Lack of liver steatosis in germ-free mice following hypercaloric diets. Eur. J. Nutr. 2019;58:1933–1945. doi: 10.1007/s00394-018-1748-4. [DOI] [PubMed] [Google Scholar]
- 90.Martinez-Guryn K., Hubert N., Frazier K., Urlass S., Musch M.W., Ojeda P., Pierre J.F., Miyoshi J., Sontag T.J., Cham C.M., et al. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe. 2018;23:458–469.e5. doi: 10.1016/j.chom.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lindheim L., Bashir M., Münzker J., Trummer C., Zachhuber V., Leber B., Horvath A., Pieber T.R., Gorkiewicz G., Stadlbauer V., Obermayer-Pietsch B. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Torres P.J., Ho B.S., Arroyo P., Sau L., Chen A., Kelley S.T., Thackray V.G. Exposure to a Healthy Gut Microbiome Protects Against Reproductive and Metabolic Dysregulation in a PCOS Mouse Model. Endocrinology. 2019;160:1193–1204. doi: 10.1210/en.2019-00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Q., Xing W., Wang Q., Tang Z., Wang Y., Gao W. Gut microbiota–mitochondrial inter-talk in non-alcoholic fatty liver disease. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.934113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chiu C.C., Ching Y.H., Li Y.P., Liu J.Y., Huang Y.T., Huang Y.W., Yang S.S., Huang W.C., Chuang H.L. Nonalcoholic Fatty Liver Disease Is Exacerbated in High-Fat Diet-Fed Gnotobiotic Mice by Colonization with the Gut Microbiota from Patients with Nonalcoholic Steatohepatitis. Nutrients. 2017;9:E1220. doi: 10.3390/nu9111220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang R., Li H., Yang X., Xue X., Deng L., Shen J., Zhang M., Zhao L., Zhang C. Genetically Obese Human Gut Microbiota Induces Liver Steatosis in Germ-Free Mice Fed on Normal Diet. Front. Microbiol. 2018;9:1602. doi: 10.3389/fmicb.2018.01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qi X., Yun C., Sun L., Xia J., Wu Q., Wang Y., Wang L., Zhang Y., Liang X., Wang L., et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019;25:1225–1233. doi: 10.1038/s41591-019-0509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., Burcelin R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet–Induced Obesity and Diabetes in Mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 98.Kawano Y., Edwards M., Huang Y., Bilate A.M., Araujo L.P., Tanoue T., Atarashi K., Ladinsky M.S., Reiner S.L., Wang H.H., et al. Microbiota imbalance induced by dietary sugar disrupts immune-mediated protection from metabolic syndrome. Cell. 2022;185:3501–3519.e20. doi: 10.1016/j.cell.2022.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zeng X., Xie Y.J., Liu Y.T., Long S.L., Mo Z.C. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin. Chim. Acta. 2020;502:214–221. doi: 10.1016/j.cca.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 100.McCartney C.R., Campbell R.E. Abnormal GnRH Pulsatility in Polycystic Ovary Syndrome: Recent Insights. Curr. Opin. Endocr. Metab. Res. 2020;12:78–84. doi: 10.1016/j.coemr.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu F., Liu R., Cao X. Hyperandrogenism stimulates inflammation and promote apoptosis of cumulus cells. Cell. Mol. Biol. 2017;63:64–68. doi: 10.14715/cmb/2017.63.10.10. [DOI] [PubMed] [Google Scholar]
- 102.Jones H., Sprung V.S., Pugh C.J.A., Daousi C., Irwin A., Aziz N., Adams V.L., Thomas E.L., Bell J.D., Kemp G.J., Cuthbertson D.J. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2012;97:3709–3716. doi: 10.1210/jc.2012-1382. [DOI] [PubMed] [Google Scholar]
- 103.Song X., Shen Q., Fan L., Yu Q., Jia X., Sun Y., Bai W., Kang J. Dehydroepiandrosterone-induced activation of mTORC1 and inhibition of autophagy contribute to skeletal muscle insulin resistance in a mouse model of polycystic ovary syndrome. Oncotarget. 2018;9:11905–11921. doi: 10.18632/oncotarget.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seidu T., McWhorter P., Myer J., Alamgir R., Eregha N., Bogle D., Lofton T., Ecelbarger C., Andrisse S. DHT causes liver steatosis via transcriptional regulation of SCAP in normal weight female mice. J. Endocrinol. 2021;250:49–65. doi: 10.1530/JOE-21-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jensen T., Abdelmalek M.F., Sullivan S., Nadeau K.J., Green M., Roncal C., Nakagawa T., Kuwabara M., Sato Y., Kang D.H., et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018;68:1063–1075. doi: 10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hu Y., Wen S., Yuan D., Peng L., Zeng R., Yang Z., Liu Q., Xu L., Kang D. The association between the environmental endocrine disruptor bisphenol A and polycystic ovary syndrome: a systematic review and meta-analysis. Gynecol. Endocrinol. 2018;34:370–377. doi: 10.1080/09513590.2017.1405931. [DOI] [PubMed] [Google Scholar]
- 107.Feisa S.V., Chopei I.V. Subclinical hypothyroidism in patients with non-alcoholic fatty liver disease at the background of carbohydrate metabolism disorders. Wiad. Lek. 2018;71(2 pt 1):261–264. [PubMed] [Google Scholar]
- 108.Singla R., Gupta Y., Khemani M., Aggarwal S. Thyroid disorders and polycystic ovary syndrome: An emerging relationship. Indian J. Endocrinol. Metab. 2015;19:25–29. doi: 10.4103/2230-8210.146860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao H., Lu X., Huang H., Ji H., Zhang L., Su Z. Thyroid-stimulating hormone level is negatively associated with fertilization rate in patients with polycystic ovary syndrome undergoing in vitro fertilization. Int. J. Gynaecol. Obstet. 2021;155:138–145. doi: 10.1002/ijgo.13581. [DOI] [PubMed] [Google Scholar]
- 110.Kim D., Vazquez-Montesino L.M., Escober J.A., Fernandes C.T., Cholankeril G., Loomba R., Harrison S.A., Younossi Z.M., Ahmed A. Low Thyroid Function in Nonalcoholic Fatty Liver Disease Is an Independent Predictor of All-Cause and Cardiovascular Mortality. Am. J. Gastroenterol. 2020;115:1496–1504. doi: 10.14309/ajg.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 111.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L., Friedman S.L., Diago M., Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367–378.e5. doi: 10.1053/j.gastro.2015.04.005. quiz e14-15. [DOI] [PubMed] [Google Scholar]
- 112.Li C., Xing C., Zhang J., Zhao H., Shi W., He B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J. Transl. Med. 2021;19:148. doi: 10.1186/s12967-021-02817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kazemi M., Hadi A., Pierson R.A., Lujan M.E., Zello G.A., Chilibeck P.D. Effects of Dietary Glycemic Index and Glycemic Load on Cardiometabolic and Reproductive Profiles in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Adv. Nutr. 2021;12:161–178. doi: 10.1093/advances/nmaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kort J.D., Winget C., Kim S.H., Lathi R.B. A retrospective cohort study to evaluate the impact of meaningful weight loss on fertility outcomes in an overweight population with infertility. Fertil. Steril. 2014;101:1400–1403. doi: 10.1016/j.fertnstert.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 115.Mirabelli M., Chiefari E., Arcidiacono B., Corigliano D.M., Brunetti F.S., Maggisano V., Russo D., Foti D.P., Brunetti A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients. 2020;12 doi: 10.3390/nu12041066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hakimi O., Cameron L.C. Effect of Exercise on Ovulation: A Systematic Review. Sports Med. 2017;47:1555–1567. doi: 10.1007/s40279-016-0669-8. [DOI] [PubMed] [Google Scholar]
- 117.Diniz T.A., de Lima Junior E.A., Teixeira A.A., Biondo L.A., da Rocha L.A.F., Valadão I.C., Silveira L.S., Cabral-Santos C., de Souza C.O., Rosa Neto J.C. Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-α signaling in obese mice. Life Sci. 2021;266 doi: 10.1016/j.lfs.2020.118868. [DOI] [PubMed] [Google Scholar]
- 118.Ezpeleta M., Gabel K., Cienfuegos S., Kalam F., Lin S., Pavlou V., Song Z., Haus J.M., Koppe S., Alexandria S.J., et al. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: A randomized controlled trial. Cell Metab. 2023;35:56–70.e3. doi: 10.1016/j.cmet.2022.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cao W., Wang K., Liang C., Su Y., Liu S., Li J., Qing H., Zeng Z., Dai L., Song J.L. Dietary tea seed saponin combined with aerobic exercise attenuated lipid metabolism and oxidative stress in mice fed a high-fat diet (HFD) J. Food Biochem. 2022;46:e14461. doi: 10.1111/jfbc.14461. [DOI] [PubMed] [Google Scholar]
- 120.He L., Sabet A., Djedjos S., Miller R., Sun X., Hussain M.A., Radovick S., Wondisford F.E. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang C.S., Li M., Ma T., Zong Y., Cui J., Feng J.W., Wu Y.Q., Lin S.Y., Lin S.C. Metformin Activates AMPK through the Lysosomal Pathway. Cell Metab. 2016;24:521–522. doi: 10.1016/j.cmet.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 122.Howell J.J., Hellberg K., Turner M., Talbott G., Kolar M.J., Ross D.S., Hoxhaj G., Saghatelian A., Shaw R.J., Manning B.D. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017;25:463–471. doi: 10.1016/j.cmet.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li Y., Xu S., Mihaylova M.M., Zheng B., Hou X., Jiang B., Park O., Luo Z., Lefai E., Shyy J.Y.J., et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kristensen J.M., Treebak J.T., Schjerling P., Goodyear L., Wojtaszewski J.F.P. Two weeks of metformin treatment induces AMPK-dependent enhancement of insulin-stimulated glucose uptake in mouse soleus muscle. Am. J. Physiol. Endocrinol. Metab. 2014;306:E1099–E1109. doi: 10.1152/ajpendo.00417.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y., An H., Liu T., Qin C., Sesaki H., Guo S., Radovick S., Hussain M., Maheshwari A., Wondisford F.E., et al. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep. 2019;29:1511–1523.e5. doi: 10.1016/j.celrep.2019.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van Syoc E., Weaver E., Rogers C.J., Silverman J.D., Ramachandran R., Ganda E. Metformin modulates the gut microbiome in broiler breeder hens. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu Y., Tu M., Huang Y., Liu Y., Zhang D. Association of Metformin With Pregnancy Outcomes in Women With Polycystic Ovarian Syndrome Undergoing In Vitro Fertilization: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jorquera G., Echiburú B., Crisosto N., Sotomayor-Zárate R., Maliqueo M., Cruz G. Metformin during Pregnancy: Effects on Offspring Development and Metabolic Function. Front. Pharmacol. 2020;11:653. doi: 10.3389/fphar.2020.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Song Y.M., Lee Y.h., Kim J.W., Ham D.S., Kang E.S., Cha B.S., Lee H.C., Lee B.W. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy. 2015;11:46–59. doi: 10.4161/15548627.2014.984271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pinyopornpanish K., Leerapun A., Pinyopornpanish K., Chattipakorn N. Effects of Metformin on Hepatic Steatosis in Adults with Nonalcoholic Fatty Liver Disease and Diabetes: Insights from the Cellular to Patient Levels. Gut Liver. 2021;15:827–840. doi: 10.5009/gnl20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Oliveira S., Houseright R.A., Graves A.L., Golenberg N., Korte B.G., Miskolci V., Huttenlocher A. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. J. Hepatol. 2019;70:710–721. doi: 10.1016/j.jhep.2018.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Y., Wang H., Xiao H. Metformin Actions on the Liver: Protection Mechanisms Emerging in Hepatocytes and Immune Cells against NASH-Related HCC. Int. J. Mol. Sci. 2021;22:5016. doi: 10.3390/ijms22095016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Siamashvili M., Davis S.N. Update on the effects of GLP-1 receptor agonists for the treatment of polycystic ovary syndrome. Expert Rev. Clin. Pharmacol. 2021;14:1081–1089. doi: 10.1080/17512433.2021.1933433. [DOI] [PubMed] [Google Scholar]
- 134.Patel Chavez C., Cusi K., Kadiyala S. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists for the Management of NAFLD. J. Clin. Endocrinol. Metab. 2022;107:29–38. doi: 10.1210/clinem/dgab578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cena H., Chiovato L., Nappi R.E. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J. Clin. Endocrinol. Metab. 2020;105:e2695–e2709. doi: 10.1210/clinem/dgaa285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Taher J., Baker C.L., Cuizon C., Masoudpour H., Zhang R., Farr S., Naples M., Bourdon C., Pausova Z., Adeli K. GLP-1 receptor agonism ameliorates hepatic VLDL overproduction and de novo lipogenesis in insulin resistance. Mol. Metab. 2014;3:823–833. doi: 10.1016/j.molmet.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cusi K. Incretin-Based Therapies for the Management of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes. Hepatology. 2019;69:2318–2322. doi: 10.1002/hep.30670. [DOI] [PubMed] [Google Scholar]
- 138.Jensterle M., Kocjan T., Kravos N.A., Pfeifer M., Janez A. Short-term intervention with liraglutide improved eating behavior in obese women with polycystic ovary syndrome. Endocr. Res. 2015;40:133–138. doi: 10.3109/07435800.2014.966385. [DOI] [PubMed] [Google Scholar]
- 139.Newsome P.N., Buchholtz K., Cusi K., Linder M., Okanoue T., Ratziu V., Sanyal A.J., Sejling A.S., Harrison S.A., NN9931-4296 Investigators A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021;384:1113–1124. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 140.Moon J.S., Hong J.H., Jung Y.J., Ferrannini E., Nauck M.A., Lim S. SGLT-2 inhibitors and GLP-1 receptor agonists in metabolic dysfunction-associated fatty liver disease. Trends Endocrinol. Metab. 2022;33:424–442. doi: 10.1016/j.tem.2022.03.005. [DOI] [PubMed] [Google Scholar]
- 141.Ferjan S., Janez A., Jensterle M. DPP4 INHIBITOR SITAGLIPTIN AS A POTENTIAL TREATMENT OPTION IN METFORMIN-INTOLERANT OBESE WOMEN WITH POLYCYSTIC OVARY SYNDROME: A PILOT RANDOMIZED STUDY. Endocr. Pract. 2018;24:69–77. doi: 10.4158/EP-2017-0027. [DOI] [PubMed] [Google Scholar]
- 142.Devin J.K., Nian H., Celedonio J.E., Wright P., Brown N.J. Sitagliptin Decreases Visceral Fat and Blood Glucose in Women With Polycystic Ovarian Syndrome. J. Clin. Endocrinol. Metab. 2020;105:136–151. doi: 10.1210/clinem/dgz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Okuyama T., Shirakawa J., Tajima K., Ino Y., Vethe H., Togashi Y., Kyohara M., Inoue R., Miyashita D., Li J., et al. Linagliptin Ameliorates Hepatic Steatosis via Non-Canonical Mechanisms in Mice Treated with a Dual Inhibitor of Insulin Receptor and IGF-1 Receptor. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21217815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kabel A.M., Al-Shehri A.H., Al-Talhi R.A., Abd Elmaaboud M.A. The promising effect of linagliptin and/or indole-3-carbinol on experimentally-induced polycystic ovarian syndrome. Chem. Biol. Interact. 2017;273:190–199. doi: 10.1016/j.cbi.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 145.Vanden Berghe W., Vermeulen L., Delerive P., De Bosscher K., Staels B., Haegeman G. A paradigm for gene regulation: inflammation, NF-kappaB and PPAR. Adv. Exp. Med. Biol. 2003;544:181–196. doi: 10.1007/978-1-4419-9072-3_22. [DOI] [PubMed] [Google Scholar]
- 146.Li Y., Wang C., Lu J., Huang K., Han Y., Chen J., Yang Y., Liu B. PPAR δ inhibition protects against palmitic acid-LPS induced lipidosis and injury in cultured hepatocyte L02 cell. Int. J. Med. Sci. 2019;16:1593–1603. doi: 10.7150/ijms.37677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Consoli A., Formoso G. Do thiazolidinediones still have a role in treatment of type 2 diabetes mellitus? Diabetes Obes. Metab. 2013;15:967–977. doi: 10.1111/dom.12101. [DOI] [PubMed] [Google Scholar]
- 148.Tanaka A., Komukai S., Shibata Y., Yokoi H., Iwasaki Y., Kawasaki T., Horiuchi K., Nakao K., Ueno T., Nakashima H., et al. Effect of pioglitazone on cardiometabolic profiles and safety in patients with type 2 diabetes undergoing percutaneous coronary artery intervention: a prospective, multicenter, randomized trial. Heart Ves. 2018;33:965–977. doi: 10.1007/s00380-018-1143-3. [DOI] [PubMed] [Google Scholar]
- 149.Cusi K. A diabetologist’s perspective of non-alcoholic steatohepatitis (NASH): Knowledge gaps and future directions. Liver Int. 2020;40:82–88. doi: 10.1111/liv.14350. [DOI] [PubMed] [Google Scholar]
- 150.Silva-Veiga F.M., Miranda C.S., Vasques-Monteiro I.M.L., Souza-Tavares H., Martins F.F., Daleprane J.B., Souza-Mello V. Peroxisome proliferator-activated receptor-alpha activation and dipeptidyl peptidase-4 inhibition target dysbiosis to treat fatty liver in obese mice. World J. Gastroenterol. 2022;28:1814–1829. doi: 10.3748/wjg.v28.i17.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hurren K.M., Dunham M.W. Understanding the impact of commonly utilized, non-insulin, glucose-lowering drugs on body weight in patients with type 2 diabetes. Expert Opin. Pharmacother. 2018;19:1087–1095. doi: 10.1080/14656566.2018.1494727. [DOI] [PubMed] [Google Scholar]
- 152.Du Q., Yang S., Wang Y.J., Wu B., Zhao Y.Y., Fan B. Effects of thiazolidinediones on polycystic ovary syndrome: a meta-analysis of randomized placebo-controlled trials. Adv. Ther. 2012;29:763–774. doi: 10.1007/s12325-012-0044-6. [DOI] [PubMed] [Google Scholar]
- 153.Xu Y., Wu Y., Huang Q. Comparison of the effect between pioglitazone and metformin in treating patients with PCOS:a meta-analysis. Arch. Gynecol. Obstet. 2017;296:661–677. doi: 10.1007/s00404-017-4480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Staels B., Rubenstrunk A., Noel B., Rigou G., Delataille P., Millatt L.J., Baron M., Lucas A., Tailleux A., Hum D.W., et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–1952. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]
- 155.Westerouen Van Meeteren M.J., Drenth J.P.H., Tjwa E.T.T.L. Elafibranor: a potential drug for the treatment of nonalcoholic steatohepatitis (NASH) Expert Opin. Investig. Drugs. 2020;29:117–123. doi: 10.1080/13543784.2020.1668375. [DOI] [PubMed] [Google Scholar]
- 156.Kuchay M.S., Krishan S., Mishra S.K., Farooqui K.J., Singh M.K., Wasir J.S., Bansal B., Kaur P., Jevalikar G., Gill H.K., et al. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial) Diabetes Care. 2018;41:1801–1808. doi: 10.2337/dc18-0165. [DOI] [PubMed] [Google Scholar]
- 157.Nakano S., Katsuno K., Isaji M., Nagasawa T., Buehrer B., Walker S., Wilkison W.O., Cheatham B. Remogliflozin Etabonate Improves Fatty Liver Disease in Diet-Induced Obese Male Mice. J. Clin. Exp. Hepatol. 2015;5:190–198. doi: 10.1016/j.jceh.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rieg T., Masuda T., Gerasimova M., Mayoux E., Platt K., Powell D.R., Thomson S.C., Koepsell H., Vallon V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am. J. Physiol. Renal Physiol. 2014;306:F188–F193. doi: 10.1152/ajprenal.00518.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Javed Z., Papageorgiou M., Deshmukh H., Rigby A.S., Qamar U., Abbas J., Khan A.Y., Kilpatrick E.S., Atkin S.L., Sathyapalan T. Effects of empagliflozin on metabolic parameters in polycystic ovary syndrome: A randomized controlled study. Clin. Endocrinol. 2019;90:805–813. doi: 10.1111/cen.13968. [DOI] [PubMed] [Google Scholar]
- 160.Yaribeygi H., Maleki M., Butler A.E., Jamialahmadi T., Sahebkar A. New insights into cellular links between sodium-glucose cotransporter-2 inhibitors and ketogenesis. J. Cell. Biochem. 2022;123:1879–1890. doi: 10.1002/jcb.30327. [DOI] [PubMed] [Google Scholar]
- 161.Cussons A.J., Watts G.F., Mori T.A., Stuckey B.G.A. Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: a randomized controlled trial employing proton magnetic resonance spectroscopy. J. Clin. Endocrinol. Metab. 2009;94:3842–3848. doi: 10.1210/jc.2009-0870. [DOI] [PubMed] [Google Scholar]
- 162.Nagashimada M., Ota T. Role of vitamin E in nonalcoholic fatty liver disease. IUBMB Life. 2019;71:516–522. doi: 10.1002/iub.1991. [DOI] [PubMed] [Google Scholar]
- 163.Rahmani E., Samimi M., Ebrahimi F.A., Foroozanfard F., Ahmadi S., Rahimi M., Jamilian M., Aghadavod E., Bahmani F., Taghizadeh M., et al. The effects of omega-3 fatty acids and vitamin E co-supplementation on gene expression of lipoprotein(a) and oxidized low-density lipoprotein, lipid profiles and biomarkers of oxidative stress in patients with polycystic ovary syndrome. Mol. Cell. Endocrinol. 2017;439:247–255. doi: 10.1016/j.mce.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 164.Ebrahimi F.A., Samimi M., Foroozanfard F., Jamilian M., Akbari H., Rahmani E., Ahmadi S., Taghizadeh M., Memarzadeh M.R., Asemi Z. The Effects of Omega-3 Fatty Acids and Vitamin E Co-Supplementation on Indices of Insulin Resistance and Hormonal Parameters in Patients with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Exp. Clin. Endocrinol. Diabetes. 2017;125:353–359. doi: 10.1055/s-0042-117773. [DOI] [PubMed] [Google Scholar]
- 165.Borzoei A., Rafraf M., Asghari-Jafarabadi M. Cinnamon improves metabolic factors without detectable effects on adiponectin in women with polycystic ovary syndrome. Asia Pac. J. Clin. Nutr. 2018;27:556–563. doi: 10.6133/apjcn.062017.13. [DOI] [PubMed] [Google Scholar]
- 166.Javed Z., Papageorgiou M., Deshmukh H., Kilpatrick E.S., Mann V., Corless L., Abouda G., Rigby A.S., Atkin S.L., Sathyapalan T. A Randomized, Controlled Trial of Vitamin D Supplementation on Cardiovascular Risk Factors, Hormones, and Liver Markers in Women with Polycystic Ovary Syndrome. Nutrients. 2019;11:E188. doi: 10.3390/nu11010188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li Y.J., Han Y., He B. Effects of bariatric surgery on obese polycystic ovary syndrome: a systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2019;15:942–950. doi: 10.1016/j.soard.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 168.Lassailly G., Caiazzo R., Ntandja-Wandji L.C., Gnemmi V., Baud G., Verkindt H., Ningarhari M., Louvet A., Leteurtre E., Raverdy V., et al. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology. 2020;159:1290–1301.e5. doi: 10.1053/j.gastro.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 169.Głuszyńska P., Lemancewicz D., Dzięcioł J.B., Razak Hady H. Non-Alcoholic Fatty Liver Disease (NAFLD) and Bariatric/Metabolic Surgery as Its Treatment Option: A Review. J. Clin. Med. 2021;10:5721. doi: 10.3390/jcm10245721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xia Y., Ren M., Yang J., Cai C., Cheng W., Zhou X., Lu D., Ji F. Gut microbiome and microbial metabolites in NAFLD and after bariatric surgery: Correlation and causality. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.1003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Athyros V.G., Boutari C., Stavropoulos K., Anagnostis P., Imprialos K.P., Doumas M., Karagiannis A. Statins: An Under-Appreciated Asset for the Prevention and the Treatment of NAFLD or NASH and the Related Cardiovascular Risk. Curr. Vasc. Pharmacol. 2018;16:246–253. doi: 10.2174/1570161115666170621082910. [DOI] [PubMed] [Google Scholar]
- 172.El Sobky S.A., Aboud N.K., El Assaly N.M., Fawzy I.O., El-Ekiaby N., Abdelaziz A.I. Regulation of lipid droplet (LD) formation in hepatocytes via regulation of SREBP1c by non-coding RNAs. Front. Med. 2022;9 doi: 10.3389/fmed.2022.903856. [DOI] [PMC free article] [PubMed] [Google Scholar]