Abstract
Salmonella is a significant foodborne pathogen that has a significant impact on public health, and different strains of multidrug resistance (MDR) have been identified in this genus. This study used a combination of phenotypic and genotypic approaches to identify distinct Salmonella species collected from poultry broiler and layer farms, and antibiotic sensitivity testing was performed on these species. A total of 56 Salmonella isolates were serotyped, and phenotypic antibiotic resistance was determined for each strain. The enterobacterial repetitive intergenic consensus polymerase chain reaction (ERIC-PCR) method was also used to provide a genotypic description, from which a dendrogram was constructed and the most likely phylogenetic relationships were applied. Salmonella isolates were detected in 20 (17%) out of 117 samples collected from small-scale broiler flocks. Salmonella isolates were classified as MDR strains after showing tolerance to 4 antibiotics, but no resistance to cloxacillin, streptomycin, vancomycin, or netilmicin was observed. From a genotypic perspective, these strains lack dfrD, parC, and blasfo-1 resistant genes, while harboring blactx-M, blaDHA-L, qnrA, qnrB, qnrS, gyrA, ermA, ermB, ermC, ermTR, mefA, msrA, tet A, tet B, tet L, tet M resistance genes. The genotyping results obtained with ERIC-PCR allowed isolates to be classified based on the source of recovery. It was determined that Salmonella strains displayed MDR, and many genes associated with them. Additionally, the ERIC-PCR procedure aided in the generation of clusters with biological significance. Extensive research on Salmonella serotypes is warranted, along with the implementation of long-term surveillance programs to monitor MDR Salmonella serotypes in avian-derived foods.
Key words: genotypic description, ERIC-PCR, multidrug-resistant strains, resistance genes, Salmonella
INTRODUCTION
Salmonella infections, one of the most common foodborne illnesses, continue to pose a global public health risk (Ye et al., 2011; Eguale et al., 2018; El-Saadony et al., 2022). In the 1950s, the World Health Organization (WHO) and the United Nations Food and Agriculture Organization (FAO) identified Salmonella as a serious zoonotic bacterium with financial consequences (Akinola et al., 2019; Marouf et al., 2022).
Salmonella enterica and Salmonella bongori are the 2 species that belong to the genus Salmonella, which is in the family Enterobacteriaceae (Shinohara et al., 2008). It involves more than 2,600 distinct serotypes, which can be distinguished from one another depending on the somatic (O) and flagellar (H) components of their genomes (Coburn et al., 2007; Eng et al., 2015).
Human infection is spread mostly through the consumption of contaminated bird eggs and meat products (Hald et al., 2016). In poultry farms, flocks of birds can become infected through either horizontal or vertical transmission (Antunes et al., 2016; El-Saadony et al., 2022). Droppings, litter, water, feed, equipment, other infected birds, other animals, rats, and afflicted farm workers all contributed to the horizontal spread of Salmonella among birds (Zamora-Sanabria and Alvarado, 2017; El-Saadony et al., 2022). Ovarian transmission or postlaying eggshell contamination are the primary vectors for vertical transfer from parents (Pande et al., 2016).
In general, several host-unrestricted S. enterica serovars that are frequently obtained from avian sources without exhibiting any clinical symptoms generally influence a wide variety of hosts and can also infect people (Gast, 2007).
Major public health concerns include the widespread application of antibiotics in the chicken industry and the subsequent rise of multidrug-resistant (MDR) Salmonella strains that can be spread from animals to humans via the food chain (Shang et al., 2018; Nabil et al., 2023). Several studies have indicated that MDR is prevalent among Salmonella serotypes isolated from animal feeds (Holt et al., 2007). Products derived from birds and other animals have also been related to the spread of MDR zoonotic bacteria (Zhao et al., 2006; Filho et al., 2023).
The MDR is an additional worldwide threat that can affect both humans and animals (Abd El-Hack et al., 2022a,b). Its main risk is the inability to completely cure patients infected with MDR bacteria, as well as the high risk of spreading MDR microorganisms (Roca et al., 2015). This resistance is linked to the irresponsible administration of antibacterial antibiotics, which includes both their application as growth promoters in the livestock industry and their use as therapeutic veterinary drugs (Zwe et al., 2018; Salem et al., 2023). This is a significant problem because the majority of MDR Salmonella infections were acquired through the consumption of contaminated food derived from animal sources. This exposes people to potential health risks and drives up the expense of human health care (Barreto et al., 2016).
Researchers are looking at alternative techniques, such as probiotics, prebiotics, and phytobiotics, which offer a broad spectrum of antimicrobial action, in order to combat the rise in the prevalence of multidrug-resistant organisms. These alternatives should be nontoxic, leave no residues in meat or eggs, be palatable to animals, maintain stability in the gut, promote the growth of beneficial bacteria, and eliminate harmful pathogens. Furthermore, these treatments will be assessed for their capacity to enhance feed efficiency and animal development while minimizing environmental impact. It is also important that these alternatives should not contribute to the development of antibiotic resistance in bacteria, particularly the beneficial gut microbiome.
During an epidemic, detection is essential for both the characterization of the bacteria and the diagnosis of the disease. On the other hand, subtyping, which can be accomplished through serotyping or DNA fingerprinting, is required for determining the source of the contamination (Tatavarthy, 2005). The enterobacterial repetitive intergenic consensus polymerase chain reaction (ERIC-PCR) assay has been used to recognize genetic diversity in enterobacteria. This assay has allowed for the isolation of clonal strains by recognizing particular chromosomal segments (ERIC sequences), and it has been proposed that this may be utilized in eco-epidemiological studies (Morton et al., 2003).
In comparison to the pulsed-field gel electrophoresis (PFGE) assay, the reliability and sensitivity of the ERIC assay, in addition to its ease of use, are generally regarded as its primary advantages (Dorneles et al., 2014). As a consequence of this, the objective of the current research was to conduct bacteriological isolation and molecular identification of MDR Salmonella strains on chicken farms that raise both broiler and layer chickens, with particular emphasis on S. enteritidis because of the severe zoonotic risk and human health hazard that it poses.
MATERIALS AND METHODS
Sample Collection
This protocol was performed by following the animal ethics guidelines and approved by Medical Research Ethics Committee of Mansoura University, Mansoura, Egypt with code number MU-ACUC (VM.R. 23.12.132).
In the yr 2022 and 2023, the current study was carried out in 6-broiler flocks (3 small scale and 3 large scale) and 6-layer flocks (3 home and 3 battery systems) in Sharqia governorate, Egypt. There was a total of 312 samples taken from broiler flocks. These samples included cloacal swabs (30 per small scale, and 50 per large scale), feed (3 per small scale, and 5 per large scale), drinking water (3 per small scale, and 5 per large scale), and litter (3 per small scale, and 5 per large scale).
There was also a total of 243 samples taken from layer flocks, including feed (2 per household, and 2 per battery system), drinking water (2 per household, and 2 per battery system), cage swabs (2 per household, and 2 per battery system), egg swabs (10 per household, and 20 per battery system), and cloacal swabs (5 per household, and 25 per battery system).
For the purposes of conducting microbiological examinations, each and every specimen obtained from the harvest was brought to the laboratory in sterile containers.
Bacterial Isolation and Biochemical Characterization
After the samples (25 g) were homogenized in sterile peptone buffer water (BPW, Lab M Limited, Lancashire, UK) they were inserted aseptically and pre-enriched at 37°C for 24 h (Arthur et al., 2004). Aliquots (10 mL) of Rappaport-Vasiliadis (RV) broth (Lab M) and 1 mL of BPW were aseptically combined and stored at 42°C for 24 h.
Loopfuls of the positive broths were then subcultured on xylose-lysine-desoxycholate (XLD) agar (Lab M) and maintained at 37°C for 24 h. After selecting the colonies, standard biochemical tests such as urea hydrolysis, production of H2S, production of indole, citrate test on Simmons citrate agar, lysine decarboxylation, methyl red test, and Voges-Proskauer test were performed.
Following the White-Kauffmann-Le Minor Scheme, representative Salmonella isolates have been identified through slide agglutination test with polyvalent and then monovalent antisera based on O and H antigens (Grimont and Weill, 2007).
The Antibiotic Resistance of S. enteritidis Isolates
The antibiograms of all known S. enteritidis strains obtained in the current study were evaluated using Mueller-Hinton agar (MHA, Lab M) and the disc diffusion method according to Clinical and Laboratory Standards Institute (CLSI, 2016) recommendations.
The following antibiotics, which are commonly used in human or veterinary medicine, were utilized in this study: penicillin G (10 IU), gentamicin (10 µg), amoxicillin (10 µg), streptomycin (10 µg), cefoxitin (30 µg), a member of the cephalosporin class, and cloxacillin (5 µg), a member of the β-lactam class, cefotaxime (30 µg), tetracycline (30 µg), a member of the tetracycline family, amikacin (30 µg), neomycin (30 µg), netilmicin, a member of the aminoglycoside family (30 µg), chloramphenicol (30 µg), ciprofloxacin (5 µg), norfloxacin (10 µg), vancomycin (30 µg) belongs to glycopeptides, nalidixic acid (30 µg) belongs to fluoroquinolones, sulfamethoxazole/trimethoprim (25 µg) belongs to sulfonamides, and erythromycin (15 µg) belongs to macrolides. All chemicals and media were acquired from Oxoid (Thermo Fisher Scientific, Wilmington, DE).
MDR isolates are resistant to at least 3 different classes of antibiotics (Hauser et al., 2011). Using the formula, a/b (where "a" is the number of antibiotics to which a strain was resistant and "b" is the total number of antibiotics to which the strain was subjected), we calculated the multiple antibiotic resistance index (MARI) for each S. enteritidis strain using data from Krumperman (1983).
In brief, a colony from each S. enteritidis isolate's on XLD plates was grown on MHA and maintained at 37°C overnight. Bacterial colonies were added to 0.9% NaCl to achieve a McFarland 0.5 with 1‒2 × 108 CFU/mL of the reference strain, Escherichia coli ATCC 25922.
Around 300 μL of the saline suspension was placed onto MHA plate and the antibiotic discs (Oxoid) were placed onto the MHA using an Oxoid multidisc applicator, and the plates were incubated at 37°C for 24 h. The size of the inhibited zone was determined using sliding calibers and interpreted using the European Committee on Antimicrobial Susceptibility Testing standard breakpoints (CLSI, 2016).
Genomic DNA Extraction and Purification
Following the protocol, the DNA was isolated from a 200 µL bacteria supernatant using a commercial kit QIAamp DNA Mini kit (Qiagen, Hilden, Germany), and it was then kept at 20°C for later use.
Molecular Identification of Genes Associated With Virulence and Pathogenicity
Because of the zoonotic and human health risks posed by S. enteritidis, virulence factors were detected using uni-plex PCR tests targeting the invA, hilC, ompF, and pefA genes (Table 1). Two hundred µL of bacterial supernatant was used to extract DNA using a commercial kit according to the manufacturer's instructions and stored at 20°C for future use. The DNA content was determined using the NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). Table 1 provides a summary of the primer sequences. For all PCR applications EmeraldAmp Max PCR Master Mix (Takara Bio Inc., Kusatsu, Shiga, Japan) was used.
Table 1.
Target genes and primers sequences for virulence and antibiotics resistant genes.
| Genes | Oligonucleotide primer sequences | References |
|---|---|---|
| invA | F: GTGAAATTATCGCCACGTTC GGGCAA R: TCATCGCACCGTC AAAGGAACC |
Oliveira et al. (2003) |
| hilC | F: GGACTTGTTGCCAGGGATG F: TGACCATTTGCG GGTGAG |
Yang et al. (2014) |
| ompF | F: CCTGGCAGCGGTGATCC R: TGGTGTAACCTAC GCCATC |
Tatavarthy and Cannons (2010) |
| pefA | F: TGT TTC CGG GCT TGT GCT R: CAG GGC ATT TGC TGATTC TTCC |
Murugkar et al. (2003) |
| blaCTX-M | F: SCSATGTGCAGYACCAGTAA R: ACCAGAAYVAG CGGBGC |
Ojdana et al. (2014) |
| blaDHA-1 | F: CCAGAATCACAATCGCCACC R: TATCAGCAGTGGCA GCCGT |
Guo et al. (2012) |
| blaSFO-1 | F: ATTCAGCAGCAACTGTCCG R: ACGCTTATCGCTG GGAAT |
Muratani et al. (2006) |
| qnrA, | F: GATAAAGTTTTTCAGCAAGAGG R: ATCCAGATCGGCAA AGGTTA |
Afzal et al. (2013) |
| qnrB | F: GATCGTGAAAGCCAGAAAGG R: ATGCCTGGTAGT TGTCC |
Gay et al. (2006) |
| qnrS | F: ACGACGACATTCGTCAACTGCAA R: TAAATTGGCACCC TGTAGGC |
|
| gyrA | F: GCCCTTCAATGCTGATGTCTTC R: TCTCCTCTGTGTCG CCTCTG |
Song et al. (2010) |
| parC | F: CTATGCGATGTCAGAGCTGG R: TAACAGCAGCTCG GCGTATT |
Afzal et al. (2013) |
| erm (A) | F: CTTCGATAGTTTATTAATATTAGT R: TCTAAAAAGCATGT AAAAGAA |
Morvan et al. (2010) |
| erm (B) | F: GAAAAGGTACTCAACCAAATA R: AGTAACGGTACTTAAA TTGTTTAC |
|
| erm (C) | F: TCAAAACATAATATAGATAAA 641 R: GCTAATATTGTTTAA ATCGTCAAT |
|
| erm (TR) | F: GAAGTTTAGCTTTCCTAA R: TTTCCACCATTAACA |
|
| msr (A) | F: GCAAATGGTGTAGGTAAGACAACT R: ATCATGTGATGTAAACAAAAT |
|
| mef (A) | F: AGTATCATTAATCACTAGTGC 345 R: TTCTTCTGGTACTAAAAGTGG |
|
| dfrD | F: AGAGTAATCGGCAAGGATAACG R: AATGGGCAATTTCACAATCC |
|
| tet (A) | F: TTGGCATTCTGCATTCACTC R: GTATAGCTTGCCGGAAGTCG |
Ma et al. (2007) |
| tet (B) | F: CTCAGTATTCCAAGCCTTTG R: CTAAGCACTTGTCTCCTGTT |
Afzal et al. (2013) |
| tet (L) | F: CCACCTGCGAGTACAAACTGG R: TCGGCAGTACTTAGCTGGTGA |
Morvan et al. (2010) |
| tet (M) | F: GTGGACAAAGGTACAACGAG R: CGGTAAAGTTCGTCACACAC |
Each PCR reaction had a final volume of 25 µL, which included 12.5 µL of the master mix, 1 µL of each primer at a concentration of 20 pmol, 4.5 µL of water, and 6 µL of DNA template. The PCR results were subjected to 1.5% agarose gel electrophoresis (AppliChem GmbH, Darmstadt, Germany).
The electropherograms were stained with ethidium bromide (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) and photographed under UV light using a gel documentation system (Alpha Innotech Corporation, San Leandro, CA). The positive control's size was recognized based on the positive bacteria S. enteritidis (ATCC 13076).
Molecular Identification of Antibiotic-Resistant Genes in S. enteritidis Strains
The following S. enteritidis antibiotic-resistance genes: blaCTX-M, blaDHA-1, and blaSFO-1 for extended-spectrum β-lactamases (ESBL); qnrA, qnrB, qnrS, gyrA, and parC for quinolones; erm (A), erm (B), erm (C), erm (TR), mef (A), and msr (A) for macrolides; dfrD for trimethoprim; and tet (A), tet (B), tet (L), tet (M) for tetracyclines were tested (Morvan et al., 2010).
The sequences of the primers are presented in Table 1. Two methods, PCR and electrophoresis, were used. Following the manufacturer's instructions, DNA was extracted from a 200 µL bacterial sample using QIAamp DNA Mini kit (Qiagen), and stored at 20°C for later use. EmeraldAmp Max HS PCR Master Mix (Takara) was employed in all PCR experiments.
Each PCR reaction had a final volume of 25 µL, which included 12.5 µL of the master mix, 1 µL of each primer (each containing 20 pmol), 4.5 µL of water, and 6 µL of DNA template. The Applied Biosystems 2720 thermal cycler (Applied Biosystems, Foster City, CA) was programmed with a selection of predefined parameters.
The amplified outputs were electrophoresed in 1.8% agarose gel (AppliChem GmbH) at an electric current of 1.5 V/cm of the agarose length and visualized with a Gel doc/UV trans-illuminator (Alpha Innotech). The positive control's size was recognized based on the positive bacteria S. enteritidis (ATCC 13076).
The following thermal cycles used to amplify all resistance genes and quinolone resistance-determining regions (QRDRs) were as follows: 1 cycle at 94°C for 5 min; 30 cycles of 94°C for 45 s, 57°C for 45 s, and extension at 72°C for 1 s; and a terminal extension at 72°C for 5 min. The intI3 gene was amplified using the same denaturation and annealing conditions, but the extension time was reduced to 45 s.
Genetic Diversity Analysis of Salmonella Isolates Using ERIC-PCR
The ERIC-PCR was performed in accordance with Fendri et al. (2013). Each PCR reaction utilized a 1× Dream TaqTM green buffer with MgCl2 (Thermo Fisher Scientific), 1 pmol of primers ERIC1R (5′-ATG TAA GCT CCT GGG GAT TCA C-3′), ERIC2 (5′-AAG TAA GTG ACT GGG GTG AGC G-3′) (Sigma-Aldrich), 5 mM of MgCl2 (Invitrogen Corporation, Waltham, MA), 240 µM of every deoxynucleotide triphosphate (dNTP) (Invitrogen Corporation), 25 µL of ultrapure water (Sigma-Aldrich), 2 U of Taq DNA polymerase (Thermo Fisher Scientific), and 5 µL of DNA template (about 60 ng).
The PCR conditions were as follows: 5 cycles of 3 min at 94°C, 1 min at 49°C, and 2 min at 72°C, followed by 35 cycles of 1 min at 94°C, 1 min at 56°C, and 2 min at 72°C, with a final extension of 5 min at 72°C. The amplified PCR isolates were electrophoresed and photographed under UV light (Alpha Innotech Corporation) after being placed on 1.5% agarose gels (Sigma-Aldrich).
Statistical Evaluation
The ERIC fingerprinting findings were converted into binary code based on whether each band was present or absent. An unweighted pair group method with an arithmetic average (UPGMA) and Ward's hierarchical clustering standard were used to generate the dendrogram. Clustering and dendrogram construction were demonstrated using IBM SPSS Statistics 23 (IBM Software, Chicago, IL) (Hunter, 1990). The online tool (https://planartcalc.com/1664/) compared similar indices (Jaccard/Tanimoto coefficient and number of intersecting elements) across all samples.
RESULTS
Prevalence of Salmonella Isolates in Examined Flocks
Salmonella isolates were detected in 20 (17%) out of 117 samples collected from small-scale broiler flocks. These positive samples included 10 from cloacal swabs, 4 from drinking water, 4 from feed, and 2 from litter. In contrast, only 12 (6%) out of 195 samples from large-scale commercial broiler flocks were positive for Salmonella. Among these positive samples, 8 were from cloacal swabs, 2 from drinking water, 1 from feed, and 1 from litter.
In contrast, in layers there were 18 positive samples (28.5%) out of 63 small-scale commercial flock samples (5 from cloacal swabs, 4 from drinking water, 1 from feed, 3 from cage swabs, and 5 from egg swabs), and 6 positive samples (3.3%) out of 180 large-scale commercial flock samples (5 from cloacal swabs and 1 from drinking water) (Table 2).
Table 2.
Prevalence of Salmonella isolates in the investigated flocks.
| Sample type | Number of broilers samples | Salmonella positive samples | Number of samples | Salmonella positive samples |
|---|---|---|---|---|
| Small-scale flocks | Large-scale commercial flocks | |||
| Cloacal swabs | 90 | 10 | 150 | 8 |
| Drinking water | 9 | 4 | 15 | 2 |
| Feed | 9 | 4 | 15 | 1 |
| Litter | 9 | 2 | 15 | 1 |
| Total | 117 | 20 | 195 | 12 |
| Layers | ||||
| Small-scale flocks | Large-scale commercial flocks | |||
| Cloacal swabs | 15 | 5 | 75 | 5 |
| Drinking water | 6 | 4 | 15 | 1 |
| Feed | 6 | 1 | 15 | 0 |
| Cages swabs | 6 | 3 | 15 | 0 |
| Egg swabs | 30 | 5 | 60 | 0 |
| Total | 63 | 18 | 180 | 6 |
Serotyping of Salmonella Isolates in Examined Flocks
Serotyping of Salmonella reveals 5 different serotypes of Salmonella (Salmonella enteritidis, Salmonella typhimurium, Salmonella Kentucky, Salmonella infantis, and Salmonella takoradi). There were 18 positive cloacal swabs from broiler flocks, including 6 isolates of S. enteritidis (33.3%) (4 from small scale and 2 from commercial farms), 2 isolates of S. typhimurium (11.1%) from small-scale farms but not detected in commercial farms, 6 isolates of S. Kentucky (33.3%) (2 isolates from small scale and 4 from commercial farms), 1 isolate of S. infantis (5.5%) from small scale farm, and 3 isolates of S. takoradi (16.6%) (1 from small-scale farms and 2 from commercial flocks) (Table 3).
Table 3.
Different Salmonella species isolated from examined flocks.
| Sample type | Number of Salmonella isolates |
Salmonella positive samples |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Salmonella enteritidis |
Salmonella typhimurium |
Salmonella kentucky |
Salmonella infantis |
Salmonella takoradi |
|||||||
| S | C | S | C | S | C | S | C | S | C | ||
| Broilers | |||||||||||
| Cloacal swabs | 18 | 4 | 2 | 2 | ‒ | 2 | 4 | 1 | ‒ | 1 | 2 |
| Drinking water | 6 | ‒ | 1 | 2 | ‒ | ‒ | ‒ | 1 | ‒ | 1 | 1 |
| Feed | 5 | 2 | ‒ | ‒ | ‒ | 2 | 1 | ‒ | ‒ | ‒ | ‒ |
| Litter | 3 | 1 | 1 | 1 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| Total | 32 | 7 | 4 | 5 | ‒ | 4 | 5 | 2 | ‒ | 2 | 3 |
| Layers | |||||||||||
| Cloacal swabs | 10 | 3 | 5 | ‒ | ‒ | ‒ | ‒ | 2 | ‒ | ‒ | ‒ |
| Drinking water | 5 | 1 | 1 | 2 | ‒ | ‒ | ‒ | 1 | ‒ | ‒ | ‒ |
| Feed | 1 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1 | ‒ | ‒ | ‒ |
| Cages swabs | 3 | ‒ | ‒ | 2 | ‒ | ‒ | ‒ | 1 | ‒ | ‒ | ‒ |
| Egg swabs | 5 | 1 | ‒ | 4 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| Total | 24 | 5 | 6 | 8 | ‒ | ‒ | ‒ | 5 | ‒ | ‒ | ‒ |
S = small-scale flocks; C = large-scale commercial flocks.
There were also 6 isolates obtained from drinking water: 1 isolate of S. enteriditis (16.6%) from a commercial flock, 2 isolates of S. typhimurium (33.3%) and 1 isolate of S. infantis (16.6%) from small-scale flocks, and 2 isolates of S. takoradi (33.3%) (1 from small scale and 2 from commercial farms). There were 5 Salmonella isolates from feed, with 2 (40%) being S. typhimurium and 3 (60%) being S. Kentucky (Table 3). Furthermore, 3 isolates were recovered from litter, with 2 isolates identified as S. enteritidis (66.6%) and 1 as S. typhimurium (33.3%) (Table 3).
However, there were 10 positive cloacal swabs in the layers, including 8 isolates of S. enteritidis (80%) and 2 S. infantis isolates (20%). There were also 5 isolates from drinking water, including 2 isolates of S. enteriditis (40%), 2 isolates of S. typhimurium (40%), and 1 isolate of S. infantis (20%) (Table 3). One S. infantis isolate was isolated from feed, and 3 isolates were isolated from cage swabs (2 S. typhimurium isolates, and 1 S. infantis isolate) (Table 3). In addition, there was 1 isolate of S. enteritidis (20%), and 4 isolates of S. typhimurium (80%) from egg swabs (Table 3).
Antibiotic Resistance of S. enteritidis Isolates
Table 4 displays the findings of an antibiotic sensitivity screen performed on 22 S. enteritidis isolates using 18 relevant antibiotics from 8 different classes, including beta-lactams, tetracyclines, phenicol's, quinolones, macrolides, sulfonamides, glycopeptides, and aminoglycosides. S. enteritidis was chosen solely for its significant zoonotic danger and human health concern.
Table 4.
Antimicrobial sensitivity test of isolated Salmonella enteritidis.
| Antibiotics | Number of resistant isolates |
|---|---|
| Penicillin G | 3 |
| Amoxicillin | 17 |
| Cefotaxime | 18 |
| Erythromycin | 15 |
| Cefoxitin | 21 |
| Tetracycline | 7 |
| Gentamicin | 13 |
| Amikacin | 16 |
| Neomycin | 22 |
| Chloramphenicol | 9 |
| Ciprofloxacin | 10 |
| Norfloxacin | 19 |
| Nalidixic acid | 21 |
| Sulfamethoxazole and trimethoprim (Bactrim) | 14 |
| Cloxacillin | 0 |
| Streptomycin | 0 |
| Netilmicin | 0 |
| Vancomycin | 0 |
S. enteritidis strains were found to be extremely resistant to neomycin (100%), nalidixic acid and cefoxitin (95.4%), norfloxacin (86.3%), cefotaxime (81.8%), amoxicillin (77.2%), amikacin (72.7%), and erythromycin (68.1%), chloramphenicol (40.9%), and tetracycline (31.8%) (Table 4). On the other hand, there was no evidence of resistance to cloxacillin, streptomycin, netilmicin, or vancomycin among the S. enteritidis strains (Table 4).
Antibiotic multiresistance patterns in S. enteritidis strains ranged from 1 to 10. All S. enteritidis isolates displayed a MARI to at least 5 of the 18 antibacterial antibiotics examined (Table 5). Their MARI ranged from 0.27 and 0.77. In 13.6% of the isolates, the highest MARI of 0.77 were recorded (Table 5). Furthermore, MARI of 0.72 were found in 18.1% of isolates and 0.5 in 27.2% (Table 5).
Table 5.
Antimicrobial resistant profiles of recovered Salmonella enteritidis.
| No. | Antibiotics | Number of antibiotic resistances | Number of isolates (Isolates ID) | Multiple antibiotic resistance index (MARI) |
|---|---|---|---|---|
| 1. | Penicillin G, amoxicillin, cefotaxime, erythromycin, cefoxitin, tetracycline, gentamicin, amikacin, neomycin, chloramphenicol, ciprofloxacin, norfloxacin, nalidixic acid, sulfamethoxazole/trimethoprim | 14 | 3 (1,9,15) | 0.77 |
| 2. | Amoxicillin, cefotaxime, erythromycin, cefoxitin, tetracycline, gentamicin, amikacin, neomycin, chloramphenicol, ciprofloxacin, norfloxacin, nalidixic acid, sulfamethoxazole/trimethoprim | 13 | 4 (3, 11,13, 17) | 0.72 |
| 3. | Amoxicillin, cefotaxime, erythromycin, cefoxitin, gentamicin, amikacin, neomycin, nalidixic acid, norfloxacin | 9 | 6 (7, 8,14, 16, 20, 21) | 0.5 |
| 4. | Cefotaxime, cefoxitin, amikacin, neomycin, ciprofloxacin, norfloxacin, nalidixic acid, sulfamethoxazole/trimethoprim | 8 | 1 (6) | 0.44 |
| 5. | Amoxicillin, cefotaxime, erythromycin, cefoxitin, neomycin, nalidixic acid, sulfamethoxazole/trimethoprim | 7 | 2 (2, 19) | 0.38 |
| 6. | Cefoxitin, amikacin, neomycin, ciprofloxacin, norfloxacin, nalidixic acid, sulfamethoxazole/trimethoprim | 7 | 1 (12) | 0.38 |
| 7. | Cefoxitin, neomycin, chloramphenicol, ciprofloxacin, norfloxacin, nalidixic acid, sulfamethoxazole/trimethoprim | 7 | 1 (18) | 0.38 |
| 8. | Cefoxitin, amikacin, neomycin, ciprofloxacin, nalidixic acid, sulfamethoxazole/trimethoprim | 6 | 1 (5) | 0.33 |
| 9. | Amoxicillin, cefotaxime, cefoxitin, neomycin, norfloxacin, nalidixic acid | 6 | 2 (4, 10) | 0.33 |
| 10. | Cefoxitin, neomycin, norfloxacin, nalidixic acid, sulfamethoxazole/trimethoprim | 5 | 1 (22) | 0.27 |
Virulence Genes
As shown in Table 6, all S. enteritidis strains were tested for virulent genes. All strains (100%) tested positive for invA, 45.45% tested positive for ompF, 36.3% tested positive for pefA, and 40.9% tested positive for the hilC gene (Figure 1).
Table 6.
Enterobacterial repetitive intergenic consensus polymerase chain reaction (ERIC-PCR), antimicrobial resistance, and virulence of Salmonella enteritidis recovered from broiler and layer chicken farms (n = 22).
| Farming type | Chicken type | Sample type | Isolate | ERIC-PCR Type | Antibiotics pattern | Virulence genes | Genes for antibiotic resistance |
|---|---|---|---|---|---|---|---|
| Small scale | Broilers | Cloacal swabs | 11 | A | 2 | invA, hilC, ompF, and pefA | blaCTX-M, qnrA, qnrS, gyrA, msr (A) |
| 22 | A | 10 | invA | blaCTX-M, gyrA, tet (A) | |||
| 19 | F | 5 | invA and hilC | blaCTX-M, qnrB, qnrS, gyrA | |||
| 18 | G | 7 | invA | blaDHA-1, tet (A), tet (B) | |||
| Feed | 1 | A | 1 | invA, hilC, ompF and pefA | blaCTX-M, qnrA, qnrB, qnrS, gyrA, erm (A), erm (B) and tet (A), tet (M), msr (A) | ||
| 3 | F | 2 | invA, hilC, ompF and pefA | blaCTX-M, qnrA, qnrS, gyrA, tet (A), tet (B) | |||
| Litter | 7 | F | 3 | invA, hilC, ompF and pefA | blaCTX-M, gyrA, tet (A), tet (B) | ||
| Layers | Cloacal swabs | 6 | E | 4 | invA | blaCTX-M, gyrA tet (A), tet (B) | |
| 8 | D | 3 | invA and hilC | blaCTX-M, qnrA, qnrB tet (A), tet (B), | |||
| 5 | E | 8 | invA | blaCTX-M & gyrA | |||
| Water | 15 | E | 1 | invA, hilC and ompF | blaCTX-M blaDHA-1, qnrB, qnrS, gyrA erm (B), erm (C), erm (TR) | ||
| Egg swabs | 9 | D | 1 | invA, hilC and ompF | blaCTX-M qnrB, qnrS, gyrA | ||
| Large scale | Broilers | Cloacal swabs | 10 | B | 9 | invA | gyrA and msr (A) tet (A), tet (B) |
| 12 | C | 6 | invA | blaCTX-M, msr (A) | |||
| Litter | 2 | I | 5 | invA and pefA | blaCTX-M, qnrA, qnrB gyrA tet (A), tet (B), tet (L) | ||
| Drinking water | 13 | B | 2 | invA, hilC and ompF | blaCTX-M, blaDHA-1, erm (B), erm (C), erm (TR), msr (A) | ||
| Layers | Cloacal swabs | 14 | K | 3 | invA and ompF | blaCTX-M, gyrA, erm (A), erm (C), erm (TR), msr (A) | |
| 17 | F | 2 | invA, ompF and pefA | blaCTX-M, gyrA, erm (B), erm (C), erm (TR) tet (A), tet (B) | |||
| 20 | F | 3 | invA | blaCTX-M, gyrA, erm (C), erm (C), erm (TR), msr (A) | |||
| 16 | H | 3 | invA, ompF and pefA | blaCTX-M, gyrA, erm (A), erm (C), erm (TR) tet (A), tet (B) | |||
| 21 | H | 3 | invA and pefA | gyrA), erm (B), mef (A), and msr (A) tet (A), tet (M) | |||
| Water | 4 | J | 9 | invA | blaCTX-M, tet (A), tet (B) |
Figure 1.
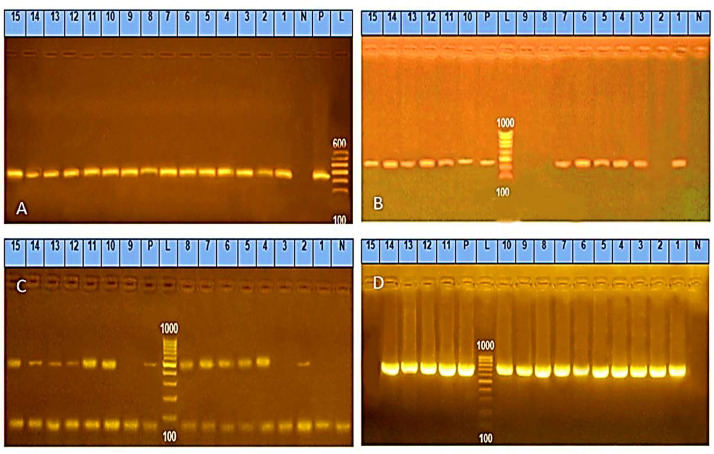
(A) Salmonella isolates' representative polymerase chain reaction (PCR) results were electrophoresed on an agarose gel to find the 284 bp invA gene. Lane L: DNA staircase controls are positive, negative, and samples in lanes (1‒15) were positive. (B) Salmonella isolates' representative PCR results were electrophoresed on agarose gels at 241 bp to find the hilC gene. DNA ladder (lane L), positive control (lane P), negative control (lane N). Lanes: 2, 8, and 9 were negative, all the samples from 1 to 15 were positive. (C) ompF gene was found in genomic DNA at 519 bp using representative agarose gel electrophoresis of PCR results for Salmonella isolates. DNA ladder (lane L), positive control (lane P), negative control (lane N), except for 1, 3, and 9, all the samples from 1 to 15 were positive. (D) Salmonella isolates' representative PCR results were electrophoresed on an agarose gel to find the 700 bp pefA gene. Lane L: DNA ladder, lanes 1 through 14 were positive samples, lane 15 was a negative sample, lanes P and N stood for positive and negative controls, respectively.
Antibiotic Resistance Genes
The PCR screening of antibacterial resistance genes in MDR S. enteritidis strains (Figure 2, Figure 3) revealed that all tested strains (n = 22) have at least 2 antibiotic resistance genes (Table 6). In particular, 19 (86.3%) strains carried the blaCTX-M gene, however only 3 (13.6%) S. enteritidis carried the blaDHA-1 resistance gene (Table 6).
Figure 2.
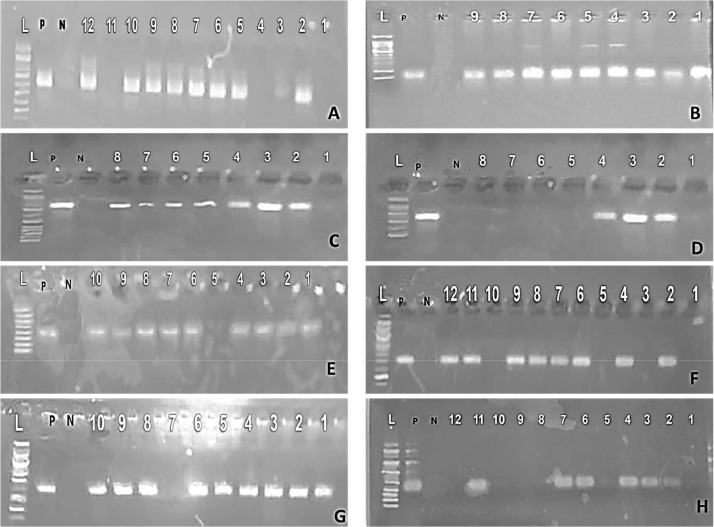
(A) Salmonella isolates' representative polymerase chain reaction results were electrophoresed on an agarose gel to find the 585 bp blaCTX-M gene. Lane P: positive control, lane N: negative control, lane L: ladder (100 bp), lanes 2, 5, 10, and 12 had positive samples, whereas lanes 1, 3, and 11 had negative samples. (B) Salmonella isolates' representative PCR results were electrophoresed on agarose gels at 190 bp to find the erm (TR) gene. P: positive control, N: negative control, and lanes: 1 to 9 were positive samples. Lane L: ladder (100 bp). (C) gyrA gene in genomic DNA at 805 bp using standard agarose gel electrophoresis of Salmonella isolates. Lane P: positive control, lane N: negative control, lane L: ladder (100 bp), and lanes (2–8) were positive samples, whereas lane 1 was negative samples. (D) Salmonella isolates' representative PCR results were electrophoresed on an agarose gel to find the 345 bp mefA gene. Ladder (100 bp) in Lane L, positive control (P), negative control (N), samples in lanes 2 to 4 were positive, while those in lanes 1 to 8 were negative. (E) msr (A) gene detection in genomic DNA at 401 bp using standard agarose gel electrophoresis of PCR results for Salmonella isolates. Lane P: positive control, lane N: negative control, lane L: ladder (100 bp), and the samples in lanes 1 to 4, 6 to 10 were positive, and L 5 was negative. (F) Detection of the qnrB gene at 469 bp in genomic DNA using standard agarose gel electrophoresis of PCR results for Salmonella isolates. Lane P: positive control, lane N: negative control, lane L: ladder (100 bp), lanes 1, 3, 5, 10 had negative samples, while lanes 2, 4, 6 to 9, 11, 12 had positive samples. (G) qnrS gene was found in genomic DNA at 417 bp using standard agarose gel electrophoresis of PCR results for Salmonella isolates. Lane P: positive control, Lane N: negative control, lane L: ladder (100 bp), except for lane 7, all of the samples in lanes from 1 to 10 were positive. (H) Salmonella isolates' electrophoresis of PCR results for tetA gene detection at 494 bp in genomic DNA. Lane P: positive control, lane N: negative control, lane L: DNA ladder (100 bp), lanes 2, 4, 6, and 11 had positive samples, whereas lanes 1, 8, 9, and 10 had negative samples.
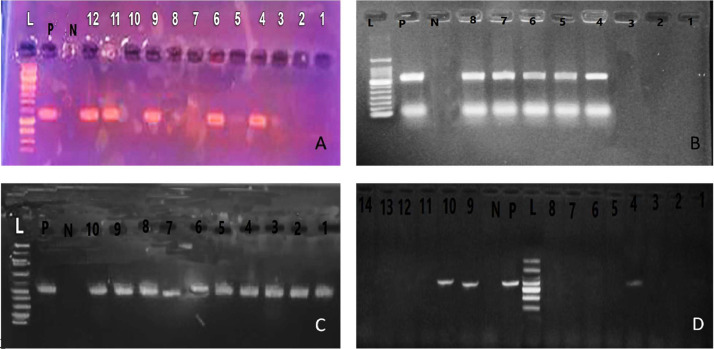
Figure 3.
Representative agarose gel electrophoresis of polymerase chain reaction (PCR) products for (A): Salmonella isolates to detect tet (B) gene in genomic DNA at 416 bp. Lane L: DNA ladder (100 bp), P: positive control, N: negative control, lanes: 4, 6, 9, 11, and 12 were positive samples and lanes 1 to 3, 5, 7, 8, and 10 were negative, (B): Salmonella isolates to detect tet (L) gene in genomic DNA at 739 bp. Lane L: DNA ladder (100 bp), P: positive control, N: negative control, lanes: 4 to 8 were positive samples, and lanes 1 to 3 were negative, (C): Salmonella isolates to detect qnr A gene in genomic DNA at 543 bp. Lane L: DNA ladder (100 bp), P: positive control, N: negative control and lanes: 1 to 10 were positive samples, (D): Salmonella isolates to detect tet M gene in genomic DNA at 405 bp. Lane L: DNA ladder (100 bp), P: positive control, N: negative control and Lanes: 1 to 14 were negative samples except 4, 9, and 10 were positive.
On the other hand, none of the strains possessed the blaSFO-1 gene. The findings of the genotyping showed the existence of quinolone resistance genes. The qnrS gene was found in 6 (27.2%) strains, the qnrA gene was found in 5 (22.7%) strains, and the qnrB gene was found in 6 (27.2%) (Table 6). The gyrA gene was found in 17 strains (77.2%).
In comparison, none of the strains included the parC gene (Table 6). Macrolide resistance genes were found in the following strains: erm (B) (22.7%, 5/22), erm (C) (27.2%, 6/22), and msr (A) (36.3%, 8/22), erm (A) (13.6%, 3/22), erm (TR) (27.2%, 6/22), and mef (A) (4.5%, 1/22) (Table 6).
Furthermore, none of the strains possessed trimethoprim dfrD gene. The tet(M) gene, which is a factor in ribosome protection and resistance to tetracycline, was found in 9% (2/22) of the strains that were investigated. There was evidence of the tet(A), tet(B), and tet (cl) genes in 54.5% (12/22) of the samples, 45.4% (10/22) of the samples, and 4.5% (1/22) of the samples, respectively (Table 6).
Genetic Diversity Analysis of Salmonella Isolates Using ERIC-PCR
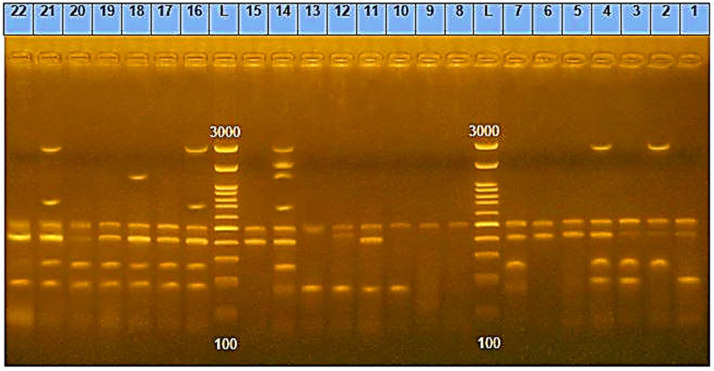
Following ERIC-PCR, DNA fragments from 22 strains generated 1 to 8 bands on an electrophoretic profile with diameters ranging from 163 to 3,074 bp (Figure 4). When the banding patterns were compared, 11 different ERIC profiles (A-k) were observed (Figure 4).
Figure 4.
Patterns of enterobacterial repetitive intergenic consensus polymerase chain reaction (ERIC-PCR) on agarose gel electrophoresis. Lanes 1 to 22 include the strains of 1 to 22 and lane L indicates DNA ladder 100 bp.
Table 6 shows that the most prevalent ERIC type was ERIC F (22.7%, 5), followed by ERIC A (13.6%, 3), ERIC E (13.6%, 3), ERIC B (9%, 2), ERIC D (9%, 2), ERIC H (9%, 2), ERIC C (4.5%, 1), ERIC G (4.5%, 1), ERIC I (4.5%, 1), ERIC J (4.5%, 1), and ERIC K (4.5%, 1).
In broilers, ERIC A and F were prevalent in cloacal swab and feed isolates, while ERIC B was common in cloacal swab and drinking water isolates, indicating that the main source of contamination was contaminated feed and water with Salmonella isolates. In addition, ERIC E was frequently found in the isolates of cloacal swabs and drinking water samples.
DISCUSSION
Microbial MDR is a threat to public health on a global scale (El-Saadony et al., 2023; Marouf et al., 2023). As a result, the current study aimed to examine the prevalence of Salmonella species in chicken flocks, as well as antibiotic tolerance and the discovery of resistant gene clusters in individual isolates of S. enteritidis due to the zoonotic and human health hazards of S. enteritidis.
The current study found that the prevalence of Salmonella species was higher in small-scale broiler farms (17%) than in commercial broiler farms (6%), and higher in small-scale layer flocks (28.5%) than in large scale (3.3%). These findings were consistent with other studies that found a lower frequency of Salmonella ranging from 8 to 15.5% (Osman, 2017; Abd El-Tawab et al., 2019; Awad et al., 2020; Tawfik et al., 2022). Other studies, on the other hand, reported a greater prevalence of Salmonella, ranging from 34 to 73% (Ziyate et al., 2016; Fagbamila et al., 2017; Djeffal et al., 2018; Shalaby et al., 2022).
The current study found that the prevalence of Salmonella species in cloacal swabs taken from broilers was highest for S. enteritis, followed by S. kentucky, S. typhimurium, S. takoradi, and lastly S. infantis. S. enteritis was the most prevalent species. While only 3 serotypes were identified with high frequency in cloacal swabs and egg swabs in layer flocks. S. enteritidis was the most common, followed by S. typhimurium, and finally S. infantis.
According to the findings of the present study, the prevalence of Salmonella contamination in poultry farms located in Sharqia governorate in Egypt is substantially higher than previously thought, which significantly increases the risk of human illness. These findings corresponded with earlier research that found S. enteritidis to be more prevalent (Ziyate et al., 2016; Osman, 2017; Shalaby et al., 2022) but contrasted with some reports that found S. kentucky to be more prevalent (Djeffal et al., 2018; Abd El-Tawab et al., 2019).
When compared to other samples taken from the farm, the prevalence of Salmonella was substantially higher in cloacal swabs. These findings agree with those found in studies carried out by Islam et al. (2016), Karim et al. (2017), and Alam et al. (2020). This variation in isolation percentages, as well as variations in isolation percentages among different Salmonella serotypes across studies, may be attributed to the different localities in which the samples were collected, time of sampling, hygienic as well as management control practices in the investigated farms, vaccination and medication programs that have been implemented in the investigated farms, breeds, age, and intensity of reared birds, and the isolation and laboratory conditions.
As has been widely reported by Abd El-Hack et al. (2022c), MDR is a major threat to human health, the MARI in the current study ranged from 0.27 to 0.77 for the Salmonella serovars studied. These findings are consistent with those found by Zhao et al. (2017) and Kim (2021), but some researchers have found a MARI as high as 0.91 (Siddique et al., 2021).
According to El-Saadony et al. (2022), different species of Salmonella include many virulent genes, which contribute to their pathogenicity and increase the likelihood of infections occurring in humans. The invA gene was found in all of the isolates that were examined, and its prevalence indicated that it had the highest occurrence among virulence genes. These findings are consistent with those of earlier research, which found a prevalence of 100% for the invA gene (Ramatla et al., 1994; Khaltabadi Farahani et al., 2018; Bahramianfard et al., 2021).
The ompF gene was identified in 45.5% of Salmonella isolates during this study. The outer membrane porin (ompF) that is found in gram-negative bacteria allows substrates to pass through the membrane and favors porin over a nonspecific cation (Nikaido, 2003). Elkenany et al. (2019) found the ompF gene in 20% of Salmonella strains, although a prior study by Tatavarthy and Cannons (2010) found it in all Salmonella strains. While Ulaya (2013) found no evidence of the hilC gene in any of their isolates, we found it in 40.9% of our samples.
The MDR procedures were developed in order to ascertain whether or not each S. enteritidis sample included in this investigation had at least 1 antibiotic resistance gene. The data showed that cefotaxime-resistant bacteria had a significantly higher frequency of the blaCTX-M gene, which is responsible for the production of CTX-M -lactamases (86.3%), in comparison to the gene that codes for DHA-type-lactamases (13.6%); nevertheless, there was an absolute absence of the blasFO-1 gene. In quinolone-resistant bacteria, plasmid-mediated quinolone resistance genes (qnrS, qnrA, qnrB, and gyr A) were found (Cui et al., 2019).
Tetracycline-resistant genes were found in our strains, with tet A being the most common followed by tet B (Adesiji et al., 2014; McDermott et al., 2016; Das et al., 2022). The dendrograms that were constructed based on the isolation patterns revealed clusters that had a significant degree of variation. According to Campioni et al. (2014), the increasing prevalence of MDR Salmonella may cause Salmonella to transform into super bacterium. Based on the results of our investigation, Eric-PCR was used to investigate the likelihood of various sources of Salmonella species infection at poultry farms.
According to the results of a recent study (Gosling et al., 2022), water, feed, and litter are all major contributors to Salmonella infection on chicken farms and pose a significant threat to human health. Because of this, we need to pay attention to this group of MDR genes, which is typically overlooked, as well as the health issues that they present in relation to food safety. In addition, there is a pressing need for additional research into the characteristics of Salmonella transmission as well as the genes related with antibiotic resistance. Control methods such as checking, hygiene measures, medications, and vaccines should be applied to limit the infection of chickens and eggs in order to lower the risk that they provide to public health. This will help reduce the overall risk to public health. Producers of eggs should be certified in food safety considering the growing awareness among customers of the importance of correct egg storage, cooking, and handling.
Finally, the issue of antibiotic resistance has resulted in far-reaching outcomes in human health and wellbeing due to the increased health care costs and productivity loss, and high proclivity toward acquiring other serious illnesses (Akinola et al., 2019; Filho et al., 2023). A major issue with antibiotic resistance is that antibiotic-resistant clones of several major pathogens, including Salmonella, have been increasingly isolated from the food supply, including food animals, poultry, retail meat products, fresh produce, and seafood (Marouf et al., 2022). All major resistance determinants, including those that confer resistance to β-lactams, extended spectrum β-lactams, fluoroquinolones, aminoglycosides, tetracyclines, and chloramphenicol, have been identified in various Salmonella serovars isolated from the food supply. It has become increasingly clear that antibiotic resistance will remain a significant hurdle to tackle in the near future (Marouf et al., 2022).
In order to address this problem, the United States Food and Drug Administration (FDA) has issued a final rule to phase out the use of antibiotics in agricultural production, reducing the prevalence of clinically relevant antibiotics in food animals and poultry, and requiring veterinary oversight of antibiotic use for therapeutic purposes. In response to the problem, many options, including probiotics, prebiotics, phytobiotics, and others, are being tried against drug-resistant microorganisms (Abd El-Hack et al., 2022a,b). This is possible since these therapies offer a broad range of antibacterial action. Ideally, the alternatives should not be harmful and should not cause residue buildup in meat or eggs (Abd El-Hack et al., 2022a). It should be palatable to animals, stable in the gut, augment beneficial flora, and inactivate harmful microorganisms. Furthermore, these interventions will be evaluated for better feed efficiency and growth while minimizing environmental impact (Abd El-Hack et al., 2022b). Most importantly, they should not cause antibiotic resistance in microorganisms, including healthy gut microflora (Salem et al., 2023).
Although studies targeting multiple serovars of Salmonella with these interventions are increasing, most of the studies are at their preliminary stages, warranting additional research to address significant gaps in the knowledge before recommending their use for improving preharvest and postharvest food safety. It will be a significant task to characterize, optimize, and scale-up these interventions to the level of potency and safety that antibiotics were providing in the past several decades.
CONCLUSIONS
The widespread presence of Salmonella in chicken farms poses a significant public health threat. The majority of S. enteritidis strains identified in this study exhibit MDR and harbor a number of associated genes. The ERIC-PCR method was successfully employed to generate biologically meaningful clusters of Salmonella strains. These findings underscore the need for intensified epidemiological investigations into Salmonella infections and further research aimed at elucidating the mechanisms underlying the emergence of MDR. Additionally, exploration of natural and safe antibiotic alternatives is warranted to address the MDR crisis among various bacterial pathogens, with a particular focus on Salmonella.
Acknowledgments
ACKNOWLEDGMENTS
The authors gratefully acknowledge Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R31), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Authors are grateful to the deanship of King Khalid University for supporting this work under the grant number (R.G.P2 161-44). This research was funded by the Abu Dhabi Award for Research Excellence, Department of Education and Knowledge (Grant #: 21S105) to K. A. El-Tarabily.
DISCLOSURES
The authors declare no conflict of interest in the present study.
Author Contributions
Conceptualization, M. M. E., Y. F. H. E.-B., A. H. E.-B., H. A. D., K. A. E.-T., and M. H. E. K., formal Analysis, A. S., F. A. A.-S., A. A., H. M. S., and W. A. A., investigation, M. M. E., Y. F. H. E.-B., A. H. E.-B., H. A. D., K. A. E.-T., and M. H. E. K., data curation, A. S., F. A. A.-S., A. A., H. M. S., and W. A. A., writing original draft preparation M. M. E., Y. F. H. E.-B., A. H. E.-B., H. A. D., K. A. E.-T., and M. H. E. K. writing final manuscript and editing, A. S., F. A. A.-S., A. A., H. M. S., W. A. A., K. A. E.-T., and M. H. E. K, visualization and Methodology M. M. E., Y. F. H. E.-B., A. H. E.-B., H. A. D., M. H. E. K., A. S., F. A. A.-S., A. A., H. M. S., and W. A. A. All authors have read and agreed to the published version of the manuscript.
REFERENCES
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., Gado A.R., Nader M.M., Saad A.M., El-Tahan A.M., Taha A.E., Salem H.M., El-Tarabily K.A. Hot red pepper powder as a safe alternative to antibiotics in organic poultry feed: an updated review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., Youssef G.B.A., Taha A.E., Soliman S.M., Ahmed A.E., El-Kott A.F., Al Syaad K.M., Swelum A.A. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Salem H.M., Khafaga A.F., Soliman S.M., El-Saadony M.T. Impacts of polyphenols on laying hens' productivity and egg quality: a review. J. Anim. Physiol. Anim. Nutr. 2022;107:928–947. doi: 10.1111/jpn.13758. [DOI] [PubMed] [Google Scholar]
- Abd El-Tawab A., Abdelbaset E., Hegazy A.E., Abd-Elmonem R. Bacteriological and molecular studies on Salmonella species isolated from poultry farms. Benha Vet. Med. J. 2019;36:280–293. [Google Scholar]
- Adesiji Y.O., Deekshit V.K., Karunasagar I. Antimicrobial-resistant genes associated with Salmonella spp. isolated from human, poultry, and seafood sources. Food Sci. Nutr. 2014;2:436–442. doi: 10.1002/fsn3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal A., Sarwar Y., Ali A., Maqbool A., Salman M., Habeeb M.A., Haque A. Molecular evaluation of drug resistance in clinical isolates of Salmonella enterica serovar Typhi from Pakistan. J. Infect. Dev. Ctries. 2013;12:929–940. doi: 10.3855/jidc.3154. [DOI] [PubMed] [Google Scholar]
- Akinola S.A., Mwanza M., Ateba C.N. Occurrence, genetic diversities and antibiotic resistance profiles of Salmonella serovars isolated from chickens. Infect. Drug Resist. 2019;12:3327–3342. doi: 10.2147/IDR.S217421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S.B., Mahmud M., Akter R., Hasan M., Sobur A., Nazir K.N.H., Noreddin A., Rahman T., El Zowalaty M.E., Rahman M. Molecular detection of multidrug resistant Salmonella species isolated from broiler farm in Bangladesh. Pathogens. 2020;9:201. doi: 10.3390/pathogens9030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes P., Mourão J., Campos J., Peixe L. Salmonellosis: the role of poultry meat. Clin. Microbiol. Infect. 2016;22:110–121. doi: 10.1016/j.cmi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Arthur T.M., Bosilevac J.M., Nou X., Shackelford S.D., Wheeler T.L., Kent M.P., Jaroni D., Pauling B., Allen D.M., Koohmaraie M. Escherichia coli O157 prevalence and enumeration of aerobic bacteria, Enterobacteriaceae, and Escherichia coli O157 at various steps in commercial beef processing plants. J. Food Prot. 2004;67:658–665. doi: 10.4315/0362-028x-67.4.658. [DOI] [PubMed] [Google Scholar]
- Awad E.A., Najaa M., Zulaikha Z.A., Zulkifli I., Soleimani A.F. Effects of heat stress on growth performance, selected physiological and immunological parameters, caecal microflora, and meat quality in two broiler strains. Asian-Australas. J. Anim. Sci. 2020;33:778. doi: 10.5713/ajas.19.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahramianfard H., Derakhshandeh A., Naziri Z., Farahani R.K. Prevalence, virulence factor and antimicrobial resistance analysis of Salmonella enteritidis from poultry and egg samples in Iran. BMC Vet. Res. 2021;17:196. doi: 10.1186/s12917-021-02900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto M., Castillo-Ruiz M., Retamal P. Salmonella enterica: a review or the trilogy agent, host and environment and its importance in Chile. Rev. Chile. Infectol. 2016;33:547–557. doi: 10.4067/S0716-10182016000500010. [DOI] [PubMed] [Google Scholar]
- Campioni F., Zoldan M.M., Falcão J.P. Characterization of Salmonella enteritidis strains isolated from poultry and farm environments in Brazil. Epidemiol. Infect. 2014;142:1403–1410. doi: 10.1017/S0950268814000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI . Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2016. Performance Standards for Antimicrobial Susceptibility Testing. M100S, 26th ed. Volume 36 number 1. pp. 251. [Google Scholar]
- Coburn B., Grassl G.A., Finlay B. Salmonella, the host and disease: a brief review. Immunol. Cell Biol. 2007;85:112–118. doi: 10.1038/sj.icb.7100007. [DOI] [PubMed] [Google Scholar]
- Cui M., Zhang P., Li J., Sun C., Song L., Zhang C., Zhao Q., Wu C. Prevalence and characterization of fluoroquinolone resistant Salmonella isolated from an integrated broiler chicken supply chain. Front. Microbiol. 2019;10:1865. doi: 10.3389/fmicb.2019.01865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T., Rana E.A., Dutta A., Bostami M.B., Rahman M., Deb P., Nath C., Barua H., Biswas P.K. Antimicrobial resistance profiling and burden of resistance genes in zoonotic Salmonella isolated from broiler chicken. Vet. Med. Sci. 2022;8:237–244. doi: 10.1002/vms3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeffal S., Mamache B., Elgroud R., Hireche S., Bouaziz O. Prevalence and risk factors for Salmonella spp. contamination in broiler chicken farms and slaughterhouses in the northeast of Algeria. Vet. World. 2018;11:1102–1108. doi: 10.14202/vetworld.2018.1102-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorneles E.M., Santana J.A., Ribeiro D., Dorella F.A., Guimarães A.S., Moawad M.S., Selim S.A., Garaldi A.L.M., Miyoshi A., Ribeiro M.G., Gouveia M.G., Azevedo V., Heinemann M.B., Lage A.P. Evaluation of ERIC-PCR as genotyping method for Corynebacterium pseudotuberculosis isolates. PLoS One. 2014;9:e98758. doi: 10.1371/journal.pone.0098758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguale T. Non-typhoidal Salmonella serovars in poultry farms in central Ethiopia: prevalence and antimicrobial resistance. BMC Vet. Res. 2018;14:217–218. doi: 10.1186/s12917-018-1539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkenany R., Elsayed M.M., Zakaria A.I., El-Sayed S.A., Rizk M.A. Antimicrobial resistance profiles and virulence genotyping of Salmonella enterica serovars recovered from broiler chickens and chicken carcasses in Egypt. BMC Vet. Res. 2019;15:124. doi: 10.1186/s12917-019-1867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Yang T., Salem H.M., Korma S.A., Ahmed A.E., Mosa W.F.A., Abd El-Mageed T.A., Selim S., Al Jaouni S.K., Zaghloul R.A., Abd El-Hack M.E., El-Tarabily K.A., Ibrahim S.A. Avian campylobacteriosis, prevalence, sources, hazards, antibiotic resistance, poultry meat contamination and control measures: a comprehensive review. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Salem H.M., El-Tahan A.M., Abd El-Mageed T.A., Soliman S.M., Khafaga A.F., Swelum A.A., Ahmed A.E., Alshammari F.A., Abd El-Hack M.E. The control of poultry salmonellosis using organic agents: an updated overview. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng S.K., Pusparajah P., Mutalib N.S.A., Ser H.L., Chan K.G., Lee L.H. Salmonella: a review on pathogenesis epidemiology and antibiotic resistance. Front. Life Sci. 2015;8:284–293. [Google Scholar]
- Fagbamila I.O., Barco L., Mancin M., Kwaga J., Ngulukun S.S., Zavagnin P., Lettini A.A., Lorenzetto M., Abdu P.A., Kabir J., Umoh J., Ricci A., Muhammad M. Salmonella serovars and their distribution in Nigerian commercial chicken layer farms. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendri I., Ben Hassena A., Grosset N., Barkallah M., Khannous L., Chuat V., Gautier M., Gdoura R. Genetic diversity of food-isolated Salmonella strains through pulsed field gel electrophoresis (PFGE) and enterobacterial repetitive intergenic consensus (ERIC-PCR) PLoS One. 2013;8:e81315. doi: 10.1371/journal.pone.0081315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filho R.A.C.P., Ferreira J.C., Galetti R., Kanashiro A.M.I., Berchieri A., Jr., Darini A.L.D.C. The rise of multidrug resistant Salmonella isolates in healthy chickens in Brazil by successful establishment of plasmid IncHI2A carrying several antibiotic resistance genes. Braz. J. Microbiol. 2023;54:469–474. doi: 10.1007/s42770-022-00893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gast R.K. Serotype-specific and serotype-independent strategies for preharvest control of food-borne Salmonella in poultry. Avian Dis. 2007;51:817–828. doi: 10.1637/8090-081807.1. [DOI] [PubMed] [Google Scholar]
- Gay K., Robicsek A., Strahilevitz J., Park C.H., Jacoby G., Barrett T.J., Medalla F., Chiller T.M., Hooper D.C. Plasmid-mediated quinolone resistance in non-Typhi serotypes of Salmonella enterica. Clin. Infect. Dis. 2006;43:297–304. doi: 10.1086/505397. [DOI] [PubMed] [Google Scholar]
- Gosling R., Oastler C., Nichols C., Jackson G., Wales A.D., Davies R.H. Investigations into Salmonella contamination in feed mills producing rations for the broiler industry in Great Britain. Vet. Sci. 2022;9:307. doi: 10.3390/vetsci9070307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont P.A., Weill F.X. World Health Organization, Institut Pasteur; Paris Cedex, France: 2007. (Antigenic Formulae of the Salmonella Serovars. WHO collaborating center for reference and research on Salmonella). 9:1–166. [Google Scholar]
- Guo Q., Wang P., Ma Y., Yang Y., Ye X., Wang M. Co-production of SFO-1 and DHA-1 β-lactamases and 16S rRNA methylase ArmA in clinical isolates of Klebsiella pneumoniae. J. Antimicrob. Chemother. 2012;67:2361–2366. doi: 10.1093/jac/dks244. [DOI] [PubMed] [Google Scholar]
- Hald T., Aspinall W., Devleesschauwer B., Cooke R., Corrigan T., Havelaar A.H., Gibb H.J., Torgerson P.R., Kirk M.D., Angulo F.J., Lake R.J., Speybroeck N., Hoffmann S. World Health Organization estimates of the relative contributions of food to the burden of disease due to selected foodborne hazards: a structured expert elicitation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser E., Hebner F., Tietze E., Helmuth R., Junker E., Prager R., Schroeter A., Rabsch W., Fruth A., Malorny B. Diversity of Salmonella enterica serovar Derby isolated from pig, pork and humans in Germany. Int. J. Food. Microbiol. 2011;151:141–149. doi: 10.1016/j.ijfoodmicro.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Holt K.E., Thomson N.R., Wain J., Phan M.D., Nair S., Hasan R., Bhutta Z.A., Quail M.A., Norbertczak H., Walker D., Dougan G., Parkhill J. Multidrug-resistant Salmonella enterica serovar Paratyphi A harbors IncHI1 plasmids similar to those found in serovar Typhi. J. Bacteriol. 2007;189:4257–4264. doi: 10.1128/JB.00232-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P.R. Reproducibility and indices of discriminatory power of microbial typing methods. J. Clin. Microbiol. 1990;28:1903–1905. doi: 10.1128/jcm.28.9.1903-1905.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.J., Mahbub-E-Elahi A.T.M., Ahmed T., Hasan M.K. Isolation and identification of Salmonella spp. from broiler and their antibiogram study in Sylhet, Bangladesh. J. Appl. Biol. Biotechnol. 2016;4:046–051. [Google Scholar]
- Karim M.R., Giasuddin M., Samad M.A., Mahmud M.S., Islam M.R., Rahman M.H., Yousuf M.A. Prevalence of Salmonella spp. in poultry and poultry products in Dhaka, Bangladesh. Int. J. Anim. Biol. 2017;3:18–22. [Google Scholar]
- Khaltabadi Farahani R., Ehsani P., Ebrahimi-Rad M., Khaledi A. Molecular detection, virulence genes, biofilm formation, and antibiotic resistance of Salmonella enterica serotype enteritidis isolated from poultry and clinical samples. Jundishapur. J. Microbiol. 2018;11:e69504. [Google Scholar]
- Kim T., Kim G., Son J., Lai V.D., Mo I., Jang H. Prevalence, biosecurity factor, and antimicrobial susceptibility analysis of Salmonella species isolated from commercial duck farms in Korea. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumperman P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983;46:165–170. doi: 10.1128/aem.46.1.165-170.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M., Wang H., Yu Y., Zhang D., Liu S. Detection of antimicrobial resistance genes of pathogenic Salmonella from swine with DNA microarray. J. Vet. Diagn. Invest. 2007;19:161–167. doi: 10.1177/104063870701900204. [DOI] [PubMed] [Google Scholar]
- Marouf S., Ibrahim H.M., El-Naggar M.S., Swelum A.A., Alqhtani A.H., El-Saadony M.T., El-Tarabily K.A., Salem H.M. Inactivated pentavalent vaccine against mycoplasmosis and salmonellosis for chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marouf S., Li X., Salem H.M., Ahmed Z.S., Nader Z.S.S.M., Shaalan M., Awad F.H., Zhou H., Cheang T. Molecular detection of multidrug resistant Pseudomonas aeruginosa of different avian sources with pathogenicity testing and in vitro evaluation of antibacterial efficacy of silver nanoparticles against multidrug resistant P. aeruginosa. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott P.F., Tyson G.H., Kabera C., Chen Y., Li C., Folster J.P., Ayers J.P., Lam C., Tate H.P., Zhao S. Whole-genome sequencing for detecting antimicrobial resistance in nontyphoidal Salmonella. Antimicrob. Agents Chemother. 2016;60:5515–5520. doi: 10.1128/AAC.01030-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton C.O., Mauchline T.H., Kerry R., Hirsch P.R. PCR-based DNA fingerprinting indicates host-related genetic variation in the nematophagous fungus Pochonia chlamydosporia. Mycol. Res. 2003;107:198–205. doi: 10.1017/s0953756203007251. [DOI] [PubMed] [Google Scholar]
- Morvan A., Moubareck C., Leclercq A., Hervé-Bazin M., Bremont S., Lecuit M., Courvalin P., Le Monnier A. Antimicrobial resistance of Listeria monocytogenes strains isolated from humans in France. Antimicrob. Agents Chemother. 2010;54:2728–2731. doi: 10.1128/AAC.01557-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratani T., Kobayashi T., Matsumoto T. Emergence and prevalence of β-lactamase-producing Klebsiella pneumoniae resistant to cephems in Japan. Int. J. Antimicrob. Agents. 2006;27:491–499. doi: 10.1016/j.ijantimicag.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Murugkar H.V., Rahman H., Dutta P.K. Distribution of virulence genes in Salmonella serovars isolated from man and animals. Indian J. Med. Res. 2003;117:66–70. [PubMed] [Google Scholar]
- Nabil N.M., Tawakol M.M., Samir A., Hassan H.M., Yonis A.E., Reda R.M., Elsayed M.M. Synergistic influence of probiotic and florfenicol on embryonic viability, performance, and multidrug-resistant Salmonella enteritidis in broiler chickens. Sci. Rep. 2023;13:9644. doi: 10.1038/s41598-023-36238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojdana D., Sacha P., Wieczorek P., Czaban S., Michalska A., Jaworowska J., Jurczak A., Poniatowski B., Tryniszewska E. The occurrence of blaCTX-M, blaSHV, and blaTEM genes in extended-spectrum β-lactamase-positive strains of Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis in Poland. Intern. J. Antibiot. 2014;2014:7. [Google Scholar]
- Oliveira S.D.D., Rodenbusch C.R., Michael G.B., Cardoso M., Canal C.W., Brandelli A. Detection of virulence genes in Salmonella enteritidis isolates from different sources. Braz. J. Microbiol. 2003;34:123–124. [Google Scholar]
- Osman N., Waheed D. Virulence associated genes and antibiotic resistance profiles in Salmonella species isolated from chickens. Int. J. Poult. Sci. 2017;16:303–309. [Google Scholar]
- Pande V.V., Devon R.L., Sharma P., McWhorter A.R., Chousalkar K.K. Study of Salmonella Typhimurium infection in laying hens. Front. Microbiol. 2016;7:203. doi: 10.3389/fmicb.2016.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramatla T.A., Mphuthi N., Ramaili T., Taioe M.O., Thekisoe O.M.M., Syakalima M. Molecular detection of virulence genes in Salmonella spp. isolated from chicken faeces in Mafikeng, South Africa. J. S. Afr. Vet. Assoc. 2020;91:1994. doi: 10.4102/jsava.v91i0.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca I., Akova M., Baquero F., Carlet J., Cavaleri M., Coenen S., Cohen J., Findlay D., Gyssens I., Heur O.E., Kahlmeter G., Kruse H., Laxminarayan R., Liébana E., López-Cerero L., MacGowan A., Martins M., Rodríguez-Baño J., Rolain J.M., Segovia C., Sigauque B., Tacconelli E., Wellington E., Vila J. The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect. 2015;6:22–29. doi: 10.1016/j.nmni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Saad A.M., Soliman S.M., Selim S., Mosa W.F., Ahmed A.E., Al Jaouni S.K., Almuhayawi M.S., Abd El-Hack M.E., El-Tarabily K.A., El-Saadony M.T. Ameliorative avian gut environment and bird productivity through the application of safe antibiotics alternatives: a comprehensive review. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby A., Ismail M.M., El-Sharkawy H. Isolation, identification, and genetic characterization of antibiotic resistance of Salmonella species isolated from chicken farms. J. Trop. Med. 2022;2022 doi: 10.1155/2022/6065831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang K., Wei B., Kang M. Distribution and dissemination of antimicrobial-resistant Salmonella in broiler farms with or without enrofloxacin use. BMC Vet. Res. 2018;14:257. doi: 10.1186/s12917-018-1590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara N.K.S., Barros V.B.D., Jimenez S.M.C., Machado E.D.C.L., Dutra R.A.F., Lima Filho J.L.D. Salmonella spp., importante agente patogênico veiculado em alimentos. Ciênc. Saúde Colet. 2008;13:1675–1683. doi: 10.1590/s1413-81232008000500031. [DOI] [PubMed] [Google Scholar]
- Siddique A., Azim S., Ali A., Andleeb S., Ahsan A., Imran M., Rahman A. Antimicrobial resistance profiling of biofilm forming non typhoidal Salmonella enterica isolates from poultry and its associated food products from Pakistan. Antibiotics. 2021;10:785. doi: 10.3390/antibiotics10070785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Roumagnac P., Weill F.X., Wain J., Dolecek C., Mazzoni C.J., Holt K.E., Achtman M. A multiplex single nucleotide polymorphism typing assay for detecting mutations that result in decreased fluoroquinolone susceptibility in Salmonella enterica serovars Typhi and Paratyphi A. J. Antimicrob. Chemother. 2010;65:1631–1641. doi: 10.1093/jac/dkq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatavarthy A. Ph.D. Dissertation; University of South Florida, Tampa, FL, USA: 2005. Molecular subtyping and antibiotic resistance analysis of Salmonella species. pp. 224. [Google Scholar]
- Tatavarthy A., Cannons A. Real-time PCR detection of Salmonella species using a novel target: the outer membrane porin F gene (ompF) Lett. Appl. Microbiol. 2010;50:645–652. doi: 10.1111/j.1472-765X.2010.02848.x. [DOI] [PubMed] [Google Scholar]
- Tawfik R.G., Gawish M.F., Abotaleb M.M., Nada H.S., Morsy K., Abumandour M.M., Torky H. Genetic relationship between Salmonella isolates recovered from calves and broilers chickens in Kafr El-Sheikh city using ERIC PCR. Animals. 2022;12:3428. doi: 10.3390/ani12233428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulaya W.D. M.Sc. Dissertation; University of Zambia, Lusaka, Zambia: 2013. Determination of virulence factors in Salmonella isolates of human, poultry and dog origin in Lusaka district, Zambia. pp. 98. [Google Scholar]
- Yang X., Brisbin J., Yu H., Wang Q., Yin F., Zhang Y., Sabour P., Sharif S., Gong J. Selected lactic acid-producing bacterial isolates with the capacity to reduce Salmonella translocation and virulence gene expression in chickens. PLoS One. 2014;9:e93022. doi: 10.1371/journal.pone.0093022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Wu Q., Zhang J., Lu J., Lin L. Isolation of Salmonella from meat samples and characterization by enterobacterial repetitive intergenic consensus-polymerase chain reaction and antibiotics test. Foodborne Pathog. Dis. 2011;8:935–937. doi: 10.1089/fpd.2010.0799. [DOI] [PubMed] [Google Scholar]
- Zamora-Sanabria R., Alvarado A.M. Current Topics in Salmonella and Salmonellosis. InTechOpen Limited; London, United Kingdom: 2017. Preharvest Salmonella risk contamination and the control strategies. In: M. Mares (ed) pp. 193–213. [Google Scholar]
- Zhao S., McDermott P.F., Friedman S., Qaiyumi S., Abbott J., Kiessling C., Ayers S., Singh R., Hubert S., Sofos J., White D.G. Characterization of antimicrobial-resistant Salmonella isolated from imported foods. J. Food Prot. 2006;69:500–507. doi: 10.4315/0362-028x-69.3.500. [DOI] [PubMed] [Google Scholar]
- Zhao X., Yang J., Zhang B., Sun S., Chang W. Characterization of integrons and resistance genes in Salmonella isolates from farm animals in Shandong Province, China. Front. Microbiol. 2017;8:1300. doi: 10.3389/fmicb.2017.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyate N., Karraouan B., Kadiri A., Darkaoui S., Soulaymani A., Bouchrif B. Prevalence and antimicrobial resistance of Salmonella isolates in Moroccan laying hens farms. J. Appl. Poult. Res. 2016;25:539–546. [Google Scholar]
- Zwe Y.H., Tang V.C.Y., Aung K.T., Gutiérrez R.A., Ng L.C., Yuk H.G. Prevalence, sequence types, antibiotic resistance and, gyrA mutations of Salmonella isolated from retail fresh chicken meat in Singapore. Food Control. 2018;90:233–240. [Google Scholar]