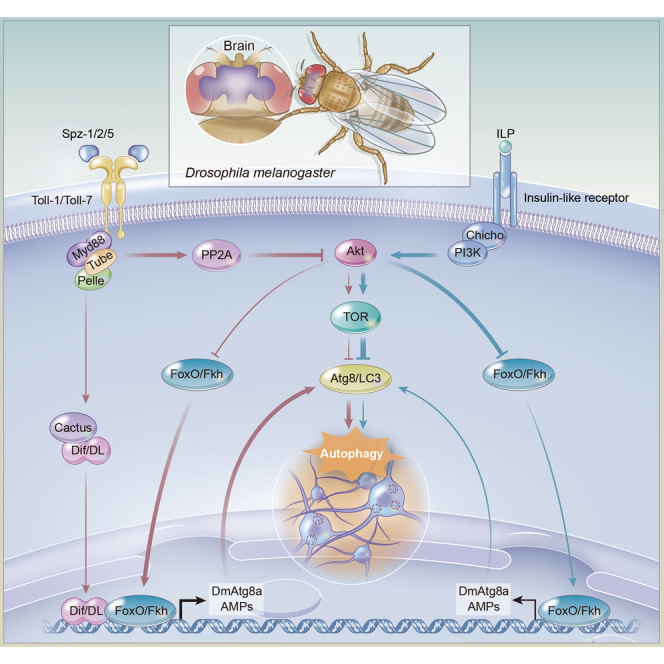
Summary
Macroautophagy/autophagy is a conserved process in eukaryotic cells to degrade and recycle damaged intracellular components. Higher level of autophagy in the brain has been observed, and autophagy dysfunction has an impact on neuronal health, but the molecular mechanism is unclear. In this study, we showed that overexpression of Toll-1 and Toll-7 receptors, as well as active Spätzle proteins in Drosophila S2 cells enhanced autophagy, and Toll-1/Toll-7 activated autophagy was dependent on Tube-Pelle-PP2A. Interestingly, Toll-1 but not Toll-7 mediated autophagy was dMyd88 dependent. Importantly, we observed that loss of functions in Toll-1 and Toll-7 receptors and PP2A activity in flies decreased autophagy level, resulting in the loss of dopamine (DA) neurons and reduced fly motion. Our results indicated that proper activation of Toll-1 and Toll-7 pathways and PP2A activity in the brain are necessary to sustain autophagy level for DA neuron survival.
Subject areas: Neuroscience, Cell biology
Graphical abstract
Highlights
-
•
Toll-1/Toll-7 signaling pathways can activate autophagy in Drosophila
-
•
Toll-1/Toll-7 mediated autophagy is Tube-Pelle-PP2A dependent
-
•
Toll-1/-7 receptors are required to maintain autophagy level in the Drosophila brain
-
•
Toll-1/-7 receptors are required to sustain dopamine neurons in Drosophila brain
Neuroscience; Cell biology
Introduction
Macroautophagy/autophagy is a conserved process in eukaryotic cells to degrade and recycle long-lived proteins, cellular macromolecules, and damaged organelles.1,2 In neurons which are long-lived and nondividing cells, autophagy plays an essential role in clearance of metabolic accumulations, misfolded proteins, and damaged organelles to maintain homeostasis.2,3,4 Studies in mice and Drosophila melanogaster showed that suppression of autophagy in the nervous system always causes accumulation of ubiquitinylated proteins and neural degeneration, revealing function of autophagy in continuous turnover of long-lived proteins to maintain nerve cell survival.5,6,7,8 Autophagy dysfunction in the nervous system is associated with neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS).4,5,6,9
Autophagy process is controlled by a cascade of kinases in response to starvation or environmental stress and involves many autophagy-related proteins (Atgs). Under nutrition-deprived conditions, autophagy is controlled by phosphatidylinositol 3-kinase (PI3K), Akt, and Tor (target of rapamycin), which are key kinases in the insulin-like signaling (ILS) pathway. When PI3K-Akt-Tor signaling is inhibited, Tor kinase cannot phosphorylate Atg1, resulting in binding of Atg1/ULK1 with Atg13 to initiate autophagy.3,5 Maintaining basic level autophagy is important for neurons, as dysregulated autophagy in the brain is associated with neurodegenerative diseases.7,8,10,11 In the brain of Parkinson’s disease patients, Toll-like receptor 2 (TLR2) expression is increased, and its expression correlates with the accumulation of pathological α-synuclein. Indeed, one study showed that activation of TLR2 negatively regulates neuronal autophagy and that synuclein lesions are rescued by antagonizing TLR2.12,13 However, the molecular mechanisms to maintain autophagy level in neurons are not well understood.
In D. melanogaster, the Toll-Spätzle (Toll-1-Spätzle-1) signaling pathway regulates dorsoventral patterning during embryogenesis, and it is also involved in defense against infection by gram-positive bacteria and fungi in larvae and adults.14 Studies showed that Drosophila Toll signaling pathway is important for brain development and plasticity.15,16,17 In immune response to pathogens, binding of Spätzle-1 (Spz-1) to Toll-1 receptor activates the downstream signaling, resulting in formation of the myeloid differentiation primary response 88 (Myd88)-Tube-Pelle complex, which phosphorylates NF-κB inhibitor Cactus, and degradation of Cactus releases NF-κB transcription factors Dif/Dorsal that translocate to the nucleus to regulate expression of target genes, such as antimicrobial peptide (AMP) genes.18,19,20
In Drosophila brains, Toll-1 and Spz-1 genes are highly expressed in different neuron cells,21 different Toll members are expressed in anatomical brain domains whereas Toll-2 regulates brain size and cell numbers via the downstream dMyd88/Wek/Yki pathway.15 Drosophila Spz-1, -2, and -5 belong to the neurotrophin (NT) superfamily,22 which are required for connectivity and synaptogenesis to promote neuronal survival.23,24,25 Toll-6 and Toll-7 are receptors for Spz-2 (Drosophila neurotrophin 1, DNT1) and Spz-5 (DNT2) to regulate neuronal survival, circuit connectivity, and structural synaptic plasticity; deprivation of Spz-2 and Spz-5 affects nervous system development.16,17,24,25,26,27,28
Our previous study showed that Drosophila Toll-1 and Toll-7 can bind to Spz-1/-2/-5 in vitro, and Toll-1-Spz and Toll-7-Spz complexes can stimulate the promoter activity of antifungal gene drosomycin.29 In this study, we like to know whether Toll-1 and Toll-7 signaling pathways are involved in autophagy in Drosophila. We first showed that overexpression of the intracellular domains (TIR-1 and TIR-7) of Toll-1 and Toll-7, full-length Toll-1 and Toll-7, active Spz-1, Spz-2 and Spz-5, as well as co-expression of Toll-1 and Toll-7 with active Spz proteins in Drosophila S2 cells increased the abundance of LC3-PE (phosphatidylethanolamine) (LC3-II), decreased phosphorylation level of Akt, and thus induced autophagy in S2 cells. Importantly, we showed that in the brain of Toll-1 and Toll-7 mutant flies (Toll-1/-7 loss of function mutants), autophagy level was decreased, resulting in the loss of dopamine (DA) neurons. Significantly, we showed that Toll-1 and Toll-7 mediated autophagy was dependent on the expression and activity of protein phosphatase 2 (PP2A), a key regulator of autophagy triggered by starvation,30 and loss of PP2A activity in Drosophila brain caused accumulation of P62 protein and decreased the number of DA neurons. Our collective results indicated that Toll-1 and Toll-7 pathways in Drosophila brain regulate and maintain autophagy homeostasis to prevent loss of DA neurons and different mechanisms are involved in Toll-1 and Toll-7 mediated autophagy.
Results
Overexpression of Toll, Spz, and Toll-Spz complex induces autophagy in S2 cells
In order to facilitate detection of autophagy process in cells, Drosophila S2 cell line stably expressing RFP-GFP tandem fluorescent-tagged LC3 protein was established, recombinant RFP-GFP-LC3 protein showed both green (GFP) and red (RFP) fluorescence in autophagosomes prior fusion with lysosomes, while green (GFP) fluorescence disappeared under lysosomal acidic condition in autolysosomes (after fusion of autophagosomes with lysosomes) and exhibited only red (RFP) fluorescence.31 When these S2 cells were cultured under starvation conditions, autophagosome (yellow puncta) and autolysosome (red puncta) were observed at 20 min and 6 h after starvation, respectively (Figure S1A), and the abundance of LC3-PE (LC3-II) was also enhanced (Figure S1B), indicating that the established S2 cell line can be used for autophagy study.
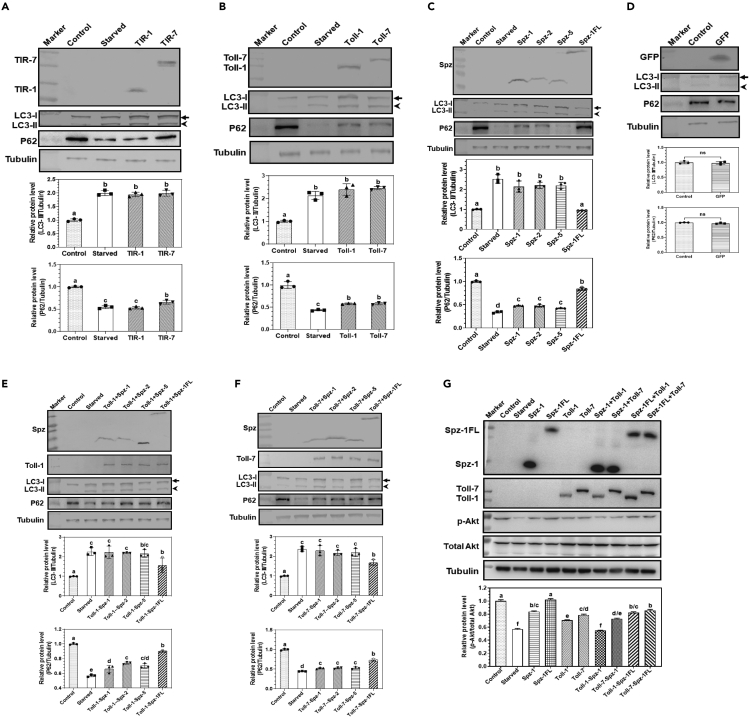
We previously showed that Drosophila Toll-1 and Toll-7 can bind to Spz-1, -2, and -5 to activate the promoter activity of an AMP gene drosomycin (Drs) in S2 cells,29 and it has been reported that Toll/TLR receptors can mediate autophagy under immune stimulations.32,33,34 To test whether expression of Tolls, Spz proteins or Toll-Spz complexes in S2 cells without immune challenge can induce autophagy, we first overexpressed the intracellular domains (TIR-1 and TIR-7) of Toll-1 and Toll-7, full-length Toll-1 and Toll-7, active Spz-1, Spz-2, and Spz-5 as well as full-length Spz-1 (Spz-1FL) in the established S2 cells which stably express RFP-GFP-LC3, and cell autophagy was determined by the abundance of LC3-II. Overexpression of TIR-1 and TIR-7 significantly enhanced the abundance of LC3-II to a level comparable to that of the starved cells (positive control) (Figure 1A). Similarly, overexpression of full-length Toll-1 and Toll-7 (Figure 1B), active Spz-1, Spz-2, and Spz-5 but not full-length Spz-1FL (Figure 1C), and co-expression of Toll-1 (Figure 1E) and Toll-7 (Figure 1F) with Spz-1/-2/-5 proteins also significantly increased the abundance of LC3-II, while overexpression of the control GFP protein did not have an effect on the level of LC3-II (Figure 1D). In Drosophila, refractory to sigma P (ref(2)P/CG10360) is a SQSTM1/P62 homolog,35 which is involved in autophagic clearance of protein bodies or sequestosomes containing ubiquitin and key components of the autophagy pathway. The level of P62 in most cases inversely correlates with autophagic flux; elevated autophagic flux decreases P62 level while inhibited flux increases its level.36,37 Thus, we also detected P62 protein level in the previously mentioned experiments, and the results showed that P62 protein level was decreased when LC3-II level was increased (Figures 1A–1F). In addition, the red autolysosome puncta were observed in the S2 cells co-expressing Toll-1 or Toll-7 with Spz-1, and endogenous Atg8a-II (Atg8a-PE) was also detected in the S2 cells co-expressing Toll-1 or Toll-7 with Spz-1 but not in the control S2 cells (Figure S1C). These results indicated that activation of Toll-1-Spz and Toll-7-Spz signaling pathways can induce autophagy in S2 cells.
Figure 1.
Overexpression of Toll, Spz, and Toll-Spz complex in S2 cells induces autophagy
(A‒F) Detection of LC3-I, LC3-PE (LC3-II), and P62 proteins in S2 cells overexpressing Toll, Spz, and Toll-Spz complex by immunoblotting. S2 cells stably expressing RFP-GFP-LC3 were cultured in complete medium without treatment (control), or cultured in PBS instead of complete medium for 6 h (starved), or transfected with pMT-TIR-1-V5, pMT-TIR-7-V5 (A), pMT-Toll-1-V5, pMT-Toll-7-V5 (B), pMT-Spz-1-Flag, pMT-Spz-2-Flag, pMT-Spz-5-Flag, pMT-Spz-1FL-Flag (C), or pMT-GFP-V5 (D), or co-transfected with pMT-Toll-1-V5 or pMT-Toll-7-V5 with pMT-Spz-1-Flag, pMT-Spz-2-Flag, pMT-Spz-5-Flag, or pMT-Spz-1FL-Flag (E and F), then recombinant proteins were detected by mouse anti-V5 and mouse anti-Flag monoclonal antibodies, respectively. In these S2 cells, P62 protein was detected by anti-Ref(2)P antibody, LC3-I and LC3-II were detected by rabbit anti-LC3 polyclonal antibody, while tubulin was detected by mouse anti-tubulin monoclonal antibody.
(G) Detection of Akt protein in S2 cells overexpressing Toll-Spz complex by immunoblotting. S2 cells stably expressing RFP-GFP-LC3 were treated as described above, total Akt (t-Akt) and phosphorylated Akt (p-Akt) in cells were detected by rabbit anti-Akt and rabbit anti-phosphor-Akt (p-Akt) polyclonal antibodies, respectively. Protein bands from at least 3 membranes were scanned for each protein using ImageJ. Data were represented as means ± SEM. Significant difference was determined by one way ANOVA followed by a Tukey’s multiple comparison tests using GraphPad Prism, with different letters indicating significant difference (p < 0.05) and identical letters for non-significant (p > 0.05). Significant difference was also determined by the student’s t test, ns for non-significant.
To confirm that Toll-1-Spz-1 and Toll-7-Spz-1 complexes activate the autophagic process in S2 cells, the phosphorylation level of Akt, a key kinase in regulation of autophagy, was determined. The results showed that overexpression of full-length Toll-1 and Toll-7, active Spz-1 but not full-length Spz-1FL, and co-expression of Toll-1 and Toll-7 with active Spz-1 all decreased the phosphorylation level of Akt to certain extents in S2 cells, with co-expression of Toll-1-Spz-1 most significantly inhibited Akt phosphorylation to a level comparable to that of the starved cells (Figure 1G). Taken together, these results indicated that Toll-1-Spz and Toll-7-Spz complexes can activate the downstream signaling pathways to inhibit Akt phosphorylation, resulting in induced autophagy in S2 cells.
Loss of Toll-1 and Toll-7 functions decreases autophagy in Drosophila brain
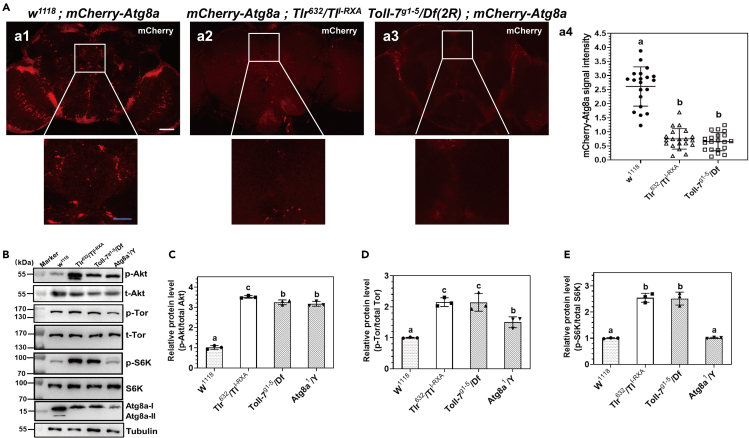
Drosophila Toll receptors are highly expressed in the adult brain, and they are involved in neuronal remodeling and plasticity.15,21 To explore whether Toll-1 and Toll-7 are associated with autophagy in Drosophila brain, pmcherry-Atg8a flies38 were used to determine the autophagy level in wild-type w1118, Toll-1 (TlI−RXA/Tlr632), and Toll-7 (Toll-7g1-5/Df(2R)) mutant flies (Toll-1/-7 loss of function mutants), respectively, and the autophagy level was detected by the red mcherry-Atg8a puncta. The results showed that in the control w1118 adult male flies (3 days old), mcherry-Atg8a puncta were detected in the brain, while in the TlI−RXA/Tlr632 and Toll-7g1-5/Df(2R) mutant flies, significantly less or almost no red puncta were observed (Figure 2A), and endogenous Atg8a-II (Atg8a-PE) was detected in w1118 flies but not in TlI−RXA/Tlr632 and Toll-7g1-5/Df(2R) mutant flies (Figure 2B), indicating that a certain level of autophagy is maintained in the brain of wild-type w1118 flies, and loss of Toll-1 or Toll-7 function in Drosophila brain decreases the autophagy level. Then the phosphorylation level of Akt and Tor kinases in fly brains were examined. Immunoblotting results showed that in the brains of w1118 flies, the phosphorylation levels of both Akt and Tor kinases in the brains of w1118 flies were significantly lower than in the brains of TlI−RXA/Tlr632, Toll-7g1-5/Df(2R), and Atg8a1/Y (UAS-P-element was inserted into the 5′-terminal region of the Atg8a gene, Atg8a mRNA level was significantly reduced resulting in autophagy dysfunction) mutant flies, while the phosphorylation level of S6K in the brains of TlI−RXA/Tlr632 and Toll-7g1-5/Df(2R) mutant flies was significantly higher than in w1118 and Atg8a1/Y flies (Figures 2B–2E). Together, these results indicated that expression of Toll-1 and Toll-7 receptors in fly brain can have an impact on the phosphorylation of Akt and Tor kinases and thus on autophagy in the brain.
Figure 2.
Toll-1 and Toll-7 receptors are required to maintain autophagy in Drosophila brain
(A) Detection of autophagy in Drosophila adult brain. Autophagy level in the brain of flies was determined by the formation of pmcherry-Atg8a puncta. Brains from different transgenic fly lines were dissected, and mCherry flourescence was examined by confocal microscope. The fluorescence intensity of mcherry-Atg8a puncta was quantified from at least 20 brains for each fly line by ImageJ (a4). Scale bar: 50 μm in a1 to a3, 20 μm in the amplified sections.
(B‒E) Detection of Akt, Tor, S6K, and Atg8a proteins in Drosophila adult brain. Akt, Tor, S6K, Atg8a, and tubulin protein levels in the brains of different transgenic flies were detected by immunoblotting (B). Relative protein level of p-Akt/total Akt (C), p-Tor/total Tor (D), or p-S6K/total S6K (E) was calculated by scanning 3 blots for each protein using ImageJ. Data were represented as means ± SEM. Significant difference was determined by one way ANOVA (see Figure 1 legend).
Loss of Toll-1 or Toll-7 function causes accumulation of P62 and ubiquitinated proteins in Drosophila brain
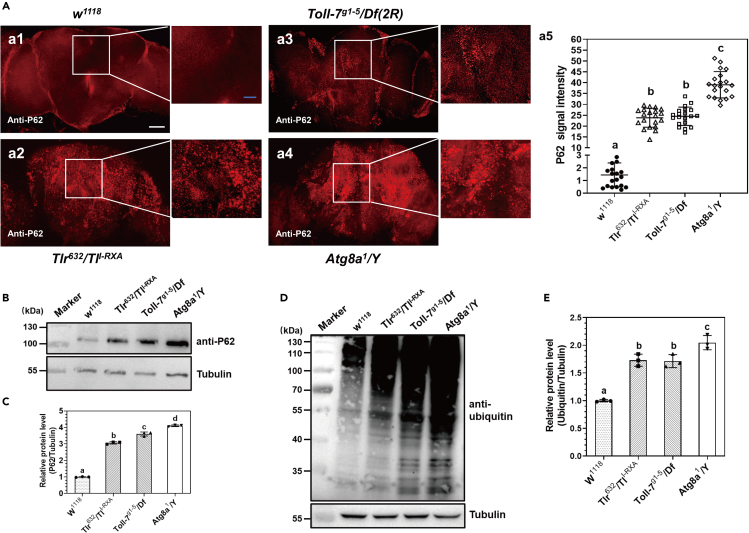
The P62 protein is degraded during autophagy process and thus its accumulation is associated with decrease in autophagy. We used anti-Ref(2)P antibody to detect P62 protein aggregates in fly brain. Immunofluorescence assays showed that P62 puncta were significantly more abundant in the brain of TlI−RXA/Tlr632, Toll-7g1-5/Df(2R) and Atg8a1/Y mutant flies than in w1118 flies (Figure 3A). Immunoblotting results showed that significantly more P62 protein accumulated in the brains of TlI−RXA/Tlr632, Toll-7g1-5/Df(2R), and Atg8a1/Y mutant flies than in w1118 flies (Figures 3B and 3C). Since accumulation of ubiquitinated proteins is also associated with reduced autophagy, we used anti-ubiquitin antibody to detect ubiquitinated proteins in fly brains, and the results showed that more ubiquitinated proteins were detected in the brains of TlI−RXA/Tlr632, Toll-7g1-5/Df(2R), and Atg8a1/Y mutant flies compared to w1118 flies (Figures 3D and 3E). These results indicated that loss of function in Toll-1 or Toll-7 decreased the level of autophagy in the brain, resulting in accumulation of P62 and ubiquitinated proteins, a phenotype similar to that of the Atg8a1/Y mutant flies.
Figure 3.
Loss of functions in Toll-1 and Toll-7 receptors causes accumulation of P62 and ubiquitinated proteins in Drosophila brain
(A) Detection of P62 protein in Drosophila adult brain by immunostaining. Brains from the control flies (w1118), Toll-1 (TlI−RXA/Tlr632), Toll-7 (Toll-7g1-5/Df(2R)), and Atg8a1/Y mutant flies were dissected and fixed, P62 protein in the brain was detected by anti-Ref(2)P antibody followed by Alexa Fluor 568 labeled goat anti-rabbit IgG, and the confocal images were taken (a1-a4). Scale bar: 50 μm in a1 to a4, 20 μm in the amplified sections. P62 fluorescence intensity was quantified from at least 20 brains for each fly line by ImageJ (a5).
(B‒E) Detection of P62 and ubiquitinated proteins in Drosophila adult brain by immunoblotting. P62 protein (B) and ubiquitinated proteins (D) in the brains of different fly lines were detected by immunoblotting. Data were represented as means ± SEM. For determination of relative protein levels (C and E) and significant differences, see Figure 1 legend.
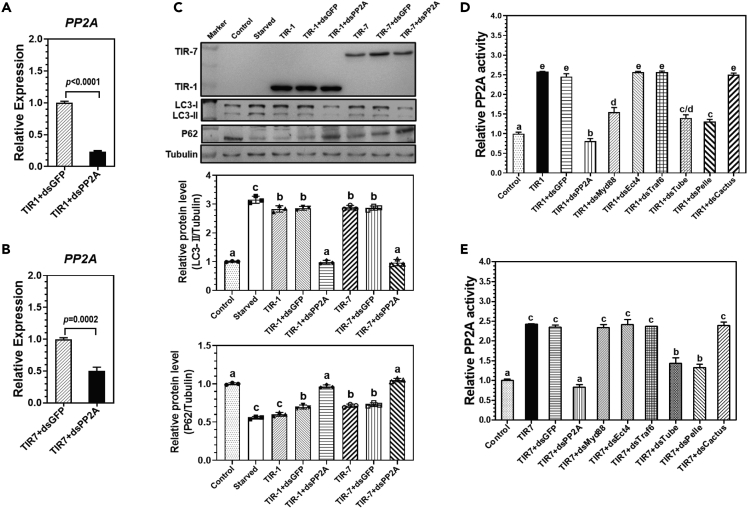
Toll-1 but not Toll-7 activated autophagy is dMyd88 dependent
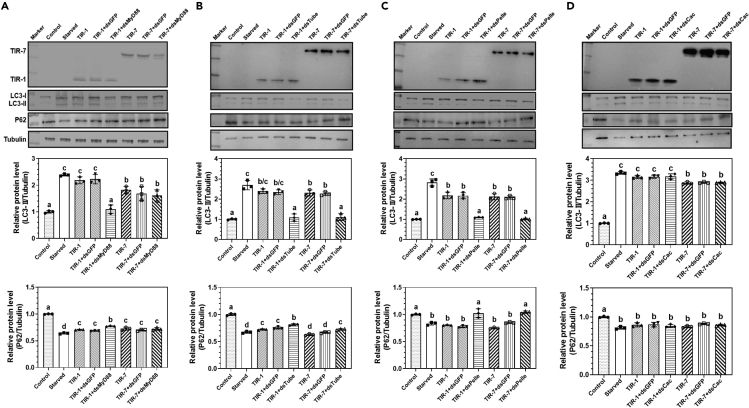
When Toll-1 receptor is activated by binding to Spz-1, intracellular adapter protein dMyd88 (Drosophila Myd88 homolog) binds to the TIR-1 domain and recruits Tube and Pelle to form “dMyd88-Tube-Pelle” complex, which in turn activates NF-κB transcription factors Dorsal/Dif to regulate expression of target genes such as AMP genes. To determine whether Toll-1 or Toll-7 mediated autophagy is dMyd88 dependent, RNAi experiments were performed to silence dMyd88 expression in S2 cells stably expressing TIR-1-V5 or TIR-7-V5. These S2 cells were then collected for real-time PCR analysis of expression of drosomycin (Drs, a target gene of the Toll pathway), diptericin (Dpt, a target gene of the IMD pathway), DmInR (insulin receptor), and DmAtg8a genes. The results showed that overexpression of TIR-1 and TIR-7 alone activated expression of Drs, DmAtg8a, and DmInR genes compared to the control cells without TIR overexpression (Figures S2C‒S2E). Transfection of dsRNA for dMyd88 in S2 cells stably expressing TIR-1-V5 or TIR-7-V5 significantly knocked down expression of dMyd88 compared to control dsGFP (Figures S2A and S2B). Interestingly, RNAi of dMyd88 suppressed TIR-1 but not TIR-7 activated expression of DmAtg8a and DmInR transcripts compared to the control cells; RNAi of dMyd88 suppressed both TIR-1 and TIR-7 activated expression of Drs (Figures S2C‒S2E). Neither overexpression of TIR-1 and TIR-7 nor RNAi of dMyd88 affected expression of Dpt mRNA (Figure S2F). Moreover, knockdown expression of dMyd88 in S2 cells significantly inhibited TIR-1 but not TIR-7 activated increase of LC3-II level and decrease of P62 level (Figure 4A). Together, these results indicated that Toll-1 but not Toll-7 activated autophagy is dMyd88 dependent.
Figure 4.
Toll-1 but not Toll-7 activated autophagy is dMyd88 dependent
(A‒D) Detection of LC3-I, LC3-II, and P62 proteins in S2 cells treated with dsRNAs for dMyd88, Tube, Pelle, and Cactus by immunoblotting. S2 cells stably expressing RFP-GFP-LC3 were cultured in complete medium without treatment (control), or cultured in PBS instead of complete medium for 6 h (starved), or co-transfected with pMT-TIR-1-V5 or pMT-TIR-7-V5 with dsRNA for GFP (dsGFP), dMyd88 (dsMyd88) (A), Tube (dsTube) (B), Pelle (dsPelle) (C), or Cactus (dsCactus) (D), expression of TIR-1-V5 and TIR-7-V5 was detected by immunoblotting with mouse anti-V5 monoclonal antibody, LC3-I and LC3-II in these S2 cells were detected by rabbit anti-LC3 polyclonal antibody, P62 protein was detected by anti-Ref(2)P antibody, while tubulin was detected by mouse anti-tubulin monoclonal antibody. Data were represented as means ± SEM. For determination of relative protein level and significant differences, see Figure 1 legend.
Both Toll-1 and Toll-7 activated autophagy is “Tube-Pelle-PP2A″ dependent
We next knocked down expression of Tube and Pelle kinases in the “dMyd88-Tube-Pelle” complex. The results showed that transfection of dsRNA for Tube or Pelle in TIR-1-V5 or TIR-7-V5 overexpressing S2 cells significantly knocked down expression of Tube and Pelle transcripts (Figures S3A, S3B, S4A, and S4B), and RNAi of both Tube and Pelle genes suppressed TIR-1 and TIR-7 activated expression of Drs, DmAtg8a, and DmInR transcripts (Figures S3C‒S3E and S4C‒S4E), and inhibited TIR-1 and TIR-7 activated increase of LC3-II level and decrease of P62 level (Figures 4B and 4C). RNAi of both Tube and Pelle genes in the TIR-1 and TIR-7 overexpressing S2 cells did not influence expression of Dpt (Figures S3F and S4F). RNAi of the Cactus gene, a negative regulator of NF-κB transcription factors, in the TIR-1 or TIR-7 overexpressing S2 cells significantly upregulated expression of Drs and Dpt but did not affect expression of DmAtg8a and DmInR or LC3-II and P62 protein levels (Figures 4D and S5). These results indicated that both Toll-1 and Toll-7 activated autophagy requires Tube and Pelle but is independent of Cactus.
It has been reported that some subunits of protein phosphatases are found in the Tube-Pelle complex,39 and PP2A (protein phosphatase 2A) plays a role in starvation-induced autophagy by dephosphorylation of Akt.30 To determine the relationship between PP2A and Toll-1/Toll-7 activated autophagy, RNAi experiments were also performed to knock down PP2A expression in S2 cells (Figures 5A and 5B). The results showed that RNAi of PP2A in the TIR-1 and TIR-7 overexpressing S2 cells significantly decreased LC3-II level and increased P62 level (Figure 5C), indicating that PP2A is involved in Toll-1 and Toll-7 induced autophagy. Then PP2A activity in S2 cells was also determined. Overexpression of both TIR-1 and TIR-7 significantly increased PP2A activity in S2 cells compared to the control cells, and knockdown expression of PP2A, Tube and Pelle, but not dEct4, dTraf-6 (two other adapter protein genes), or Cactus, in the TIR-1 or TIR-7 overexpressing S2 cells significantly decreased the PP2A activity, while RNAi of dMyd88 expression in S2 cells significantly inhibited TIR-1 but not TIR-7 activated PP2A activity (Figures 5D and 5E). Taken together, these results demonstrated that both Toll-1 and Toll-7 signaling pathways in S2 cells require dMyd88 adapter, Tube and Pelle kinases to regulate gene expression. While Toll-1 mediated autophagy is dMyd88 dependent, Toll-7 regulated autophagy is dMyd88 independent, and both Toll-1 and Toll-7 activated autophagy is “Tube-Pelle-PP2A″ dependent.
Figure 5.
Both Toll-1 and Toll-7 activated autophagy requires PP2A activity
(A and B) Expression of PP2A transcript in S2 cells after RNAi.
(C) Detection of LC3-I, LC3-II and P62 proteins in S2 cells after RNAi of PP2A by immunoblotting.
(D and E) Detection of PP2A activity in S2 cells treated with dsRNAs for several key genes in the Toll pathway. S2 cells stably expressing RFP-GFP-LC3 were cultured in complete medium without treatment (control), or cultured in PBS instead of complete medium for 6 h (starved), or co-transfected with pMT-TIR-1-V5 or pMT-TIR-7-V5 with dsRNA for GFP (dsGFP), PP2A (dsPP2A), dMyd88 (dsMyd88), Etc4 (dsEtc4), Traf6 (dsTraf6), Tube (dsTube), Pelle (dsPelle), or Cactus (dsCactus), RNAi efficiency of PP2A gene was determined by qRT-PCR (A, B), expression of TIR-1-V5 and TIR-7-V5 was detected by immunoblotting with mouse anti-V5 monoclonal antibody, LC3-I and LC3-II in these S2 cells were detected by rabbit anti-LC3 polyclonal antibody, P62 protein was detected by anti-Ref(2)P antibody, while tubulin was detected by mouse anti-tubulin monoclonal antibody (C). PP2A activity in these S2 cells was determined with the serine/threonine phosphatase assay system (D, E), and relative PP2A activity in the control samples was arbitrarily set as 1. Data were represented as means ± SEM. Significant difference was determined by the Student’s t test (A and B) and by one way ANOVA (C–E) (see Figure 1 legend).
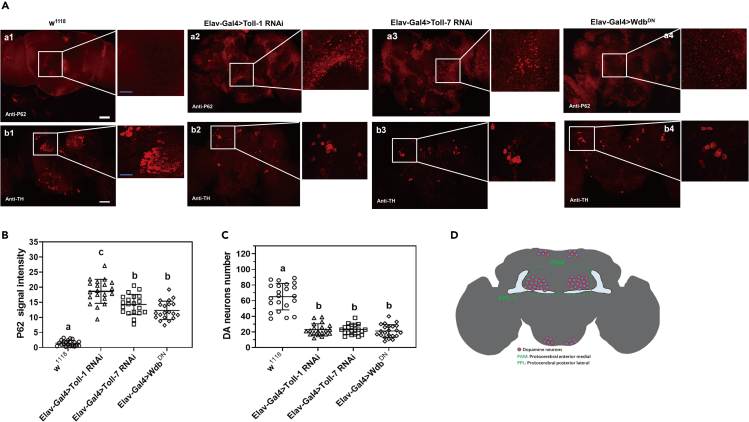
Toll-1/-7 receptors and PP2A activity are required for autophagy and DA neuron survival in Drosophila brain
To confirm that Toll-1 and Toll-7 also mediate autophagy in Drosophila, we used elav-Gal4 to drive Toll-1 and Toll-7 RNAi as well as constitutively expression of an inactive PP2A subunit (WdbDN) in flies, then accumulation of P62 protein and the number of dopamine (DA) neurons in the brain were examined. In the brain of w1118 flies, almost no P62 accumulation was detected (Figure 6A, a1), while in the brains of Toll-1 and Toll-7 RNAi as well as WdbDN overexpression flies, P62 puncta were distributed and detected (Figures 6A, a2-a4 and 6B).
Figure 6.
PP2A activity and expression of Toll-1 and Toll-7 receptors are required for autophagy and DA neuron survival in Drosophila brain
(A‒C) Detection of P62 protein and DA neurons in Drosophila adult brain.
(D) Schematic diagram of the PAM region in Drosophila adult brain. Brains from the control flies (w1118), Elav-Gal4 driven Toll-1 and Toll-7 RNAi flies, and WdbDN flies which constitutively express an inactive PP2A subunit were dissected and fixed, P62 protein in the brain was detected with anti-Ref(2)P antibody (A, a1-a4) and DA neurons were labeled with anti-TH antibody (A, b1-b4), then confocal images were taken. Scale bar: 50 μm in a1 to a4 and b1 to b4, 20 μm in the amplified sections. P62 fluorescence intensity was quantified (B) and DA neurons were counted (C) in the PAM region (D) of at least 20 brains for each fly line by ImageJ. Data were represented as means ± SEM. Significant difference was determined by one way ANOVA (see Figure 1 legend).
DA neurons play a role in addictive behavior, arousal and sleep, learning and memory, locomotor activity, and aggression in Drosophila, and autophagy dysfunction is associated with the number of DA neurons and neurodegenerative diseases in different animal model.5,6,7,8,40,41,42,43,44 Using anti-TH antibody to label DA neurons in the brain, we showed that the number of TH-positive DA neurons (distribution in PAM region, Figure 6D) was significantly less in the Toll-1 and Toll-7 RNAi and WdbDN overexpression flies than in w1118 flies (Figures 6A, b1-b4 and 6C). These results indicated that expression of Toll-1 and Toll-7 and PP2A activity are associated with autophagy level and DA neuron survival.
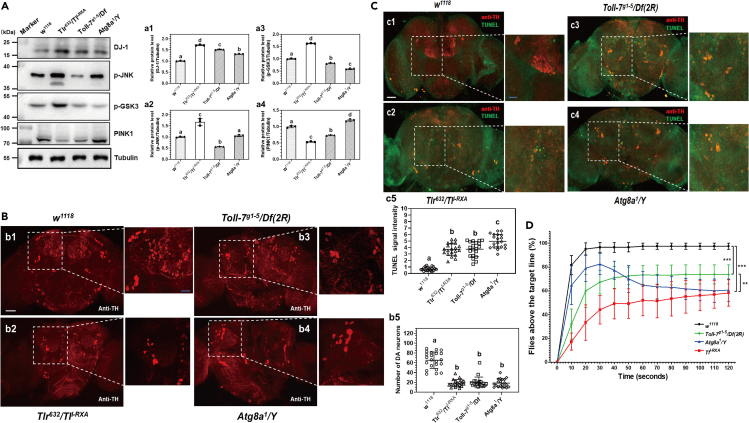
Toll-1 and Toll-7 regulate DA neuron survival in Drosophila brain and control fly climbing ability
DJ-1 (Parkinson disease protein 7, DJ-1/PARK7) is a multifunctional protein involved in multiple enzymatic activities and in multilayered regulation of protein synthesis. Abnormal level of DJ-1 can lead to widespread alterations of enzymatic activities under pathologic conditions.45 PINK1 (PTEN (phosphatase and tensin homolog)-induced kinase-1) is a mitochondrial serine/threonine protein kinase involved in mitochondrial control and dynamics. Loss of PINK1 results in mitochondrial dysfunction which is associated with Parkinson's disease.46 GSK-3 (Glycogen synthase kinase-3) regulates the production of Aβ peptides and the microtubule-associated protein tau, which is associated with neuronal death. Phosphorylation of tau Ser396 by GSK-3 destabilizes microtubules in AD.47 The c-Jun N-terminal kinases (JNKs) are multifunctional molecules which, on one hand, regulate various processes in brain development, repairing, and memory formation; on the other hand, JNKs are potent effectors of neuronal death and neuroinflammation.48 We then examined expression of these key homologous proteins associated with neurodegenerative diseases in the brains of adult flies.
The results showed that the abundance of DJ-1 protein was enhanced in the brains of TlI−RXA/Tlr632, Toll-7g1-5/Df(2R), and Atg8a1/Y mutant flies, and PINK1 abundance was significantly decreased in the brains of TlI−RXA/Tlr632 and Toll-7g1-5/Df(2R) mutant flies, the level of phosphor-JNK was increased in the TlI−RXA/Tlr632 mutant flies but decreased in the Toll-7g1-5/Df(2R) mutant flies, and phosphor-GSK3 level was significantly increased in the TlI−RXA/Tlr632 and decreased in Toll-7g1-5/Df(2R) and Atg8a1/Y mutant flies (Figure 7A).
Figure 7.
Toll-1 and Toll-7 regulate DA neuron survival in Drosophila brain and control fly climbing ability
(A) Detection of DJ-1, JNK, GSK3, and PINK1 proteins in Drosophila adult brain by immunoblotting. DJ-1, phosphorylated JNK (p-JNK), phosphorylated GSK3 (p-GSK3), and PINK1 proteins in the brains of the control flies (w1118), Toll-1 (TlI−RXA/Tlr632), Toll-7 (Toll-7g1-5/Df(2R)), and Atg8a1/Y mutant flies were detected by immunoblotting, tubulin was detected as a loading control.
(B and C) Detection of DA neurons and apoptosis in Drosophila adult brain. Brains from different fly lines were dissected, DA neurons in the brain were labeled with anti-TH antibody. Brains from these flies were also stained with TUNEL and anti-TH antibody (C). DA neurons number was counted (B, b5) and TUNEL fluorescence intensity was quantified (C, c5) from at least 20 brains for each fly line by ImageJ. Scale bar: 50 μm in a1 to a4, 20 μm in the amplified sections.
(D) Locomotor ability of Drosophila adult flies. The w1118 flies, Toll-1, Toll-7, and Atg8a1/Y mutant flies were used for climbing assays to determine fly locomotor ability. The percentage of flies passing the threshold line is represented every 10 s over the duration of the assay. For each genotype, 10 biological replicates, each with 20 flies (a total of 200 flies), were used for the assays. Data were represented as means ± SEM. Significant difference was determined by one way ANOVA (a1–a4, b5, c5) (see Figure 1 legend) and by the Student’s t test (D, indicated by ∗ p < 0.05, ∗∗ p < 0.01, and ∗∗∗ p < 0.001).
To further confirm the role of Toll-1 and Toll-7 receptors in the autophagy process and in neuron survival, the numbers of DA neurons in the brains of Toll-1 and Toll-7 mutant flies were examined. Significantly fewer numbers of TH-positive DA neurons were observed in the brains of TlI−RXA/Tlr632, Toll-7g1-5/Df(2R), and Atg8a1/Y mutant flies compared to w1118 flies (Figure 7B), a result consistent with that of Toll-1 and Toll-7 RNAi lines. We also performed TUNEL staining in fly brains, and the results showed that TUNEL signal was increased in the brains of TlI−RXA/Tlr632, Toll-7g1-5/Df(2R), and Atg8a1/Y mutant flies compared to w1118 flies (Figure 7C), indicating that more neuronal cells died in the brains of mutant flies. Since Toll-1 and Toll-7 can bind to Spz-1/-2/-5, and binding of Spz to Toll receptors activates the Toll pathway, we also examined the numbers of DA neurons in Spz-1 (Spz-12/Df(3R), and Spz-14/Df(3R)), Spz-5 (Spz-5AW/Df(3L), and Spz-5e03444/Df(3L)) mutant flies (loss of function Spz-1 and Spz-5 mutants). The numbers of DA neurons in the brains of Spz-1 mutant lines were not significantly different from those in the w1118 and Df(3L)/+ control flies, but DA neuron numbers were significantly fewer in the brains of Spz-5 mutant flies than in the two control flies (Figure S6).
Since DA neurons are related to locomotor ability, thus fly climbing assays were performed. The results showed that almost 100% of w1118 flies passed the target line in 2 min, and about 70% of Toll-7g1-5/Df(2R), 55% of TlI−RXA, and 55% of Atg8a1/Y mutant flies passed the target line (Figure 7D), indicating that the climbing ability in Toll-1, Toll-7, and Atg8a mutant flies was significantly impaired. Taken together, these results indicated that proper expression of Toll-1 and Toll-7 as well as PP2A activity are required for maintaining a certain level of autophagy and the number of DA neurons in the Drosophila brain.
Discussion
Drosophila Toll family receptors not only play a vital role in innate immunity but also regulate embryonic development, brain size, cell numbers, neuron survival, and brain plasticity.16,17,26 In this study, we showed that in Drosophila S2 cells, activation of Toll-1 and Toll-7 signaling pathways enhanced autophagy, and that in Drosophila adult brain, expression of Toll-1 and Toll-7 and PP2A activity were required to maintain a certain level of autophagy in the brain for DA neuron survival. Significantly, we found that the “Tube-Pelle-PP2A″ axis is downstream of the Toll-1 and Toll-7 receptors and is necessary to sustain autophagy.
Activation of Toll-1 signaling stimulates formation of the “dMyd88-Tube-Pelle” complex, which then activates NF-κB transcription factors Dif/Dorsal to regulate expression of immune genes such as Drs.49 Tube and Pelle kinases may also regulate the activity of protein phosphatases and kinases that participate in the autophagy process. We showed that Toll-1 and Toll-7 activated expression of Drs in S2 cells was dMyd88 dependent, and Toll-1 but not Toll-7 mediated autophagy process was dMyd88 dependent, while both the Toll-1 and Toll-7 regulated autophagy required Tube and Pelle kinases. Thus, Toll-1 and Toll-7 receptors require the adapter dMyd88 to recruit Tube and Pelle for activation of immune genes, while Toll-1 also needs the “dMy88-Tube-Pelle” complex to activate autophagy through PP2A, Toll-7 may recruit Tube and Pelle through its intracellular TIR domain or other adapter proteins (but not dMyd88) to alter PP2A activity in regulation of autophagy. Since TIR-7 (∼39 kDa) is larger than TIR-1 (∼27 kDa), the extra polypeptide in TIR-7 may facilitate direct interaction of TIR-7 with Tube and Pelle without adapter proteins. We also noticed that Toll-1 but not Toll-7 activated expression of DmAtg8a and DmInR genes is dMyd88 dependent, thus, expression of autophagy-related genes mediated by Toll-7 is different from that by Toll-1. Our results indicated that Toll-1 and Toll-7 signaling pathways may regulate autophagy in two ways: regulating expression of autophagy-related genes and altering the activity of protein phosphatases and kinases involved in autophagy.
Autophagy in neurons is dependent on classical Atg1/ULK1-Atg13 and Beclin1/Atg6-class III phosphatidylinositol 3-kinase (PtdIns3K)/Vps34 complexes.2,3 We showed that in fly brain, loss of functions in Toll-1 and Toll-7 receptors increased the phosphorylation level of Akt and Tor kinases which may impair the autophagy process in the brain, a result consistent with previous studies that Akt phosphorylation is decreased in the Toll10b mutant flies in which the Toll-1 signaling is constitutively activated.50,51 DmInR was previously reported as a target gene of FoxO in Drosophila.52 When insulin signal was insufficient, FoxO was activated to upregulate DmInR gene expression and phosphorylation level of Akt was decreased. We showed that DmInR gene expression was upregulated in Toll-1/-7 overexpressing S2 cells, while total Akt protein (t-Akt) level did not change significantly (Figure S7A), suggesting that phosphorylation level of Akt was decreased. But total Akt level was decreased in Toll-1, Toll-7 and Atg8a mutant flies compared to w1118 flies (Figure S7B). This may be due to more t-Akt was phosphorylated to p-Akt in the mutant flies. In Toll-1 and Toll-7 mutant flies, accumulation of P62 and ubiquitinated proteins increased, resulting in reduced number of DA neurons in the brain and decreased locomotor activity of flies, which were in line with Atg8a mutant flies, indicating that Toll-1 and Toll-7 mediated autophagy influences the brain function of flies. Our results are in accordance with those that Toll-1 and Toll-7 receptors are highly expressed in neuron cells21 and that higher level of autophagy is observed in neurons than in other cell types,6,10 as expression of Toll family receptors is required to sustain the autophagy process for clearance of any protein aggregates in neurons to maintain neuronal functions. We also showed that activation of Toll-1 and Toll-7 signaling pathways enhanced PP2A activity in the Tube-Pelle dependent manner, a result in agreement with that PP2A subunits (PP2A-29B and Mts) are detected in the Tube-Pelle complex.39 PP2A plays a role in cell cycle, self-renewal of neural stem cells, and autophagy by regulating the activity of many kinases associated with cell signaling pathways such as Wnt, PI3K-Akt and MAPK pathways.53,54 PP2A complex plays an essential role in regulation of autophagy. PP2A-A/wdb/C complex acts upstream of dTOR, whereas PP2A-A/B’/C complex functions as a target of dTOR and may regulate elongation of autophagosomes and their subsequent fusion with lysosomes.30 Thus, Toll-1 and Toll-7 signaling pathways associated with PP2A activity in the brain are necessary to sustain autophagy in neurons, consistent with previous studies that Toll receptors are involved in deciding cell fate.16,17
Triggering of Toll signaling requires binding of Spz proteins to Toll receptors.12 We showed that unlike in the Toll-1 and Toll-7 mutant flies, decrease in the numbers of DA neurons was not signficant in the Spz-1 but was significant in Spz-5 mutant flies, suggesting that Spz-5 may be a ligand for Toll-1 and Toll-7 receptors in the brain. It is also possible that Toll-1 and Toll-7 bind to multiple Spz ligands in the brain, and the overall effect of Toll-1 and Toll-7 signaling pathways determines the autophagy process and DA neuron survival.
Autophagy dysfunction can result in accumulation of P62 and ubiquitinated proteins and is always associated with loss of neurons in neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease.5,6,7,55,56 PP2A is a key molecule in self-renewal of Drosophila neural stem cells, and PP2A dysfunction has an impact on asymmetric cell division that results in hyperproliferation of neuroblasts.53 Phosphorylation of JNK (p-JNK) and GSK3 (p-GSK3), the two kinases with important roles in apoptosis, cell cycle progression, cell renewal, differentiation, stem cell biology, and survival,57 was inhibited by PP2A activity, and loss of PP2A activity caused hyperactivity of p-JNK and p-GSK3, resulting in a phenotype resembling brain tumors.53,54 We showed that in the brains of Toll-1 mutant flies, p-JNK and p-GSK3 levels were increased, which may result from decrease in Toll-1-mediated PP2A activity, and the number of DA neurons was decreased. It has been reported that activation of Toll-7 signaling enhances phosphorylation of JNK through Ect-4,16,26 thus in the brains of Toll-7 mutant flies, loss of function in Toll-7 receptor may result in decreased p-JNK level. In conclusion, our results indicated that Toll-1 and Toll-7 receptors regulate the autophagy level through PP2A in the brain to sustain the numbers of dopamine neurons and maintain neuronal functions.
Limitations of the study
DA neurons are associated with multiple behaviors in flies. We only detected locomotor ability using climbing assay in Toll-1/-7 mutant flies and are not sure whether loss of functions in Toll-1/-7 receptors also has an impact on other behaviors, such as learning, memory, and aggression. In addition, we used young flies (3-day adults) to study the functions of Toll-1/-7 receptors in maintaining autophagy level in the brain to sustain the numbers of dopamine neurons, we do not know whether, with aging, more dopamine neurons would be lost in old Toll-1/-7 mutant flies. Finally, we did not perform rescue experiments in Toll-1/-7 mutants by artificially inducing autophagy either through rapamycin treatment or Atg1 overexpression, thus a direct link between Toll signaling, autophagy induction, and neuron survival and function needs to be determined in future study.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| V5-tag | Sigma-Aldrich | Cat#V-8012; RRID: AB_261888 |
| FLAG tag | Sigma-Aldrich | Cat#F-1804; RRID: AB_262044 |
| Tubulin | Sigma-Aldrich | Cat#T9026; RRID: AB_477593 |
| LC3 | Sigma-Aldrich | Cat#L7543; RRID: AB_796155 |
| phosphor-Tor | Cell Signaling Technology | Cat#5536; RRID: AB_10691552 |
| phosphor-Akt | Cell Signaling Technology | Cat#4054; RRID: AB_331414 |
| Akt | Cell Signaling Technology | Cat#9272; RRID: AB_329827 |
| Dj-1 | Cell Signaling Technology | Cat#5933; RRID: AB_11179085 |
| phosphor-JNK | Cell Signaling Technology | Cat#9251; RRID: AB_331659 |
| PINK-1 | Cell Signaling Technology | Cat#6946; RRID: AB_11179069 |
| phosphor-GSK3 | Cell Signaling Technology | Cat#9316; RRID: AB_659836 |
| Ref(2)P (p62) | Abcam | Cat#ab178440; RRID: AB_2938801 |
| Atg8 | Abcam | Cat#ab109364; RRID: AB_10861928 |
| Ubiquitin | PTM biolabs | Cat#PTM-1106; RRID: N/A |
| Tyrosine hydroxylase | Novus Biologicals | Cat#NB300-109; RRID: AB_10077691 |
| HRP-conjugated goat anti-rabbit IgG | Beyotime | Cat#A0208; RRID: AB_2892644 |
| HRP-conjugated goat anti-mouse IgG | Beyotime | Cat#A0216; RRID: AB_2860575 |
| Goat anti-rabbit IgG, Alexa Fluor 568 | Invitrogen | Cat#A-11011; RRID: AB_143157 |
| Experimental models: Cell lines | ||
| D. melanogaster Schneider 2 (S2) cells | This study | N/A |
| RFP-GFP-LC3-S2 cells | This study | N/A |
| Experimental models: Organisms/strains | ||
| w1118 | Storage in lab | N/A |
| Tlr632/TM6B | Storage in lab | N/A |
| TlI−RXA/TM6B | Storage in lab | N/A |
| Toll-7g1-5/Cyo | Storage in lab | N/A |
| w; pmCherryAtg8a | Lab of Neal Silverman | N/A |
| Atg8a1 | Bloomington Drosophila Stock Center | BDSC:10107 |
| Spz-12/TM1 | Bloomington Drosophila Stock Center | BDSC:3115 |
| Spz-14/TM3, Sb1 | Bloomington Drosophila Stock Center | BDSC:30915 |
| w1118; PBac{RB}spz5e03444 | Bloomington Drosophila Stock Center | BDSC:18155 |
| Spz-5AW18/TM6B, Tb1 | Bloomington Drosophila Stock Center | BDSC:64069 |
| Elav-Gal4/Cyo | Bloomington Drosophila Stock Center | BDSC:8765 |
| Df(2R)BSC22/SM6a | Bloomington Drosophila Stock Center | BDSC:6647 |
| Df(3L)Exel6092/TM6BlacZ | Bloomington Drosophila Stock Center | BDSC:7571 |
| Df(3R)ro80b, st1e1/TM3, Sb1 | Bloomington Drosophila Stock Center | BDSC:2198 |
| UAS-Toll-1 RNAi | Tsinghua Fly Center | THU4003 |
| UAS-Toll-7 RNAi | Tsinghua Fly Center | THU3507 |
| UAS-wdbDN | Lab of Sheng Li | BDSC:53708 |
| Bc/Cyo; MKRS/TM6B | Lab of Sheng Li | N/A |
| Toll-1 mutant | Genotype: Tlr632/TlI−RXA | N/A |
| Toll-7 mutant | Genotype: Toll-7g1-5/Df(2R)BSC22 | N/A |
| Spz-1 mutant | Genotype: Spz-12/Df(3R)ro80b | N/A |
| Spz-1 mutant | Genotype: Spz-14/Df(3R)ro80b | N/A |
| Spz-5 mutant | Genotype: Spz5AW/Df(3L)Exel6092 | N/A |
| Spz-5 mutant | Genotype: Spz5e03444/Df(3L)Exel6092 | N/A |
| Atg8a mutant | Genotype: Atg8a1/Y | N/A |
| Oligonucleotides | ||
| Primers used for double-stranded RNA (dsRNA) synthesis, see Table S1 | This study | N/A |
| Primers used for quantitative real-time PCR, see Table S2 | This study | N/A |
| Recombinant DNA | ||
| pMT/BiP/V5-His A | Invitrogen | Cat#V413020 |
| pAc5.1/V5-His A | Invitrogen | Cat#V4110-20 |
| pMT-Toll-1FL | Storage in lab | N/A |
| pMT-Toll-7FL | Storage in lab | N/A |
| pMT-TIR-1 | Storage in lab | N/A |
| pMT-TIR-7 | Storage in lab | N/A |
| pMT-Spz-1 | Storage in lab | N/A |
| pMT-Spz-2 | Storage in lab | N/A |
| pMT-Spz-5 | Storage in lab | N/A |
| pMT-Spz-1FL | This study | N/A |
| pAc5.1-RFP-GFP-LC3 | This study | N/A |
Resource availability
Lead contact
Further information and requests for resources, reagents and source data should be directed to and will be fulfilled by the lead contact, Xiao-Qiang Yu (xqyu@m.scnu.edu.cn). In key resources table section labeled with “this study”, which means resources generated and stored in Professor Xiao-Qiang Yu lab, School of Life Sciences, South China Normal University, Guangzhou 510631, China.
Materials availability
This study did not create new unique reagents. Further information and requests for resources and reagents listed in key resources table should be directed to the lead contact.
Data and code availability
-
•
The dataset is publicly available as of the date of publication.
-
•
This study did not report the original code.
-
•
Any additional information required to reanalyze the data reported in this study is available from the lead contact upon request.
Experimental model and study participant details
Fly stocks and cells
Wild-type w1118, Toll-1 mutant (Tlr632/TlI−RXA), Toll-7 mutant (Toll-7g1-5/Df(2R)BSC22) and Toll-7g1-5/Cyo flies used in this study were maintained in the laboratory,29 other Drosophila lines were purchased either from Bloomington Drosophila Stock Center or Tsinghua Fly Center, all Drosophila lines used in this study are listed in key resources table. In the Atg8a1/Y line (w1118 P{EP}Atg8aEP362, Stock number 10107), due to a UAS-P-element located in the 5′ terminal region of the Atg8a gene, Atg8a mRNA level was significantly reduced. Since Atg8a gene is located in X chromosome, all Atg8a1/Y flies used in the expreriment were males. The pmcherry-Atg8a lines (in which pmcherry-Atg8a gene is located in either chromosome II or III) were gifts from Professor Neal Silverman (Department of Medicine, University of Massachusetts Medical School), and in these lines, about 2-kb promoter region of Atg8a (CG32672) gene was inserted into the upstream of mCherry-Atg8a and cloned into pCaSpeR4 vector for Drosophila transformation to generate the transgenic lines as described.38 UAS-wdbDN (UAS-PP2ADN, Stock number 53708) and Bc/Cyo; MKRS/TM6B flies were gifts from Professor Sheng Li (School of Life Sciences, South China Normal University). Tlr632/TM6B, TlI−RXA/TM6B, and pmcherry-Atg8a flies (in which pmcherry-Atg8a gene is located in chromosome II) were crossed with Bc/Cyo; MKRS/TM6B flies, respectively, to generate Bc/Cyo; Tlr632/TM6B, Bc/Cyo; TlI−RXA/TM6B, and pmcherry-Atg8a/Cyo; MKRS/TM6B flies. Then Bc/Cyo; Tlr632/TM6B or Bc/Cyo; TlI−RXA/TM6B flies were crossed with pmcherry-Atg8a/Cyo; MKRS/TM6B flies to generate pmcherry-Atg8a/Cyo; Tlr632/TM6B or pmcherry-Atg8a/Cyo; TlI−RXA/TM6B flies. These two fly lines were crossed to generate pmcherry-Atg8a; Tlr632/TlI−RXA line, in which autophagy was detected by formation of mcherry-Atg8a puncta under loss of Toll-1 function. Same crossing strategy was designed to generate Toll-7g1-5/Df(2R)BSC22; pmcherry-Atg8a line by using Toll-7g1-5/Cyo, Df(2R)BSC22/SM6a, pmcherry-Atg8a (in which pmcherry-Atg8a gene is located in chromosome III), and Bc/Cyo; MKRS/TM6B flies. Spz-1 mutant flies were generated by crossing Spz-12/TM1 or Spz-14/TM3, Sb1 flies with Df(3R)ro80b, st1e1/TM3, Sb1 flies to obtain Spz-12/Df(3R)ro80b and Spz-14/Df(3R)ro80b lines which are Spz-1 loss of function. Spz-5 mutant flies were generated by crossing w1118; PBac{RB}Spz5e03444 or Spz-5AW18/TM6B, Tb1 flies with Df(3L)Exel6092/TM6BlacZ to obtain Spz5e03444/Df(3L)Exel6092 and Spz5AW/Df(3L)Exel6092 lines which are Spz-5 loss of function. All the mutant Drosophila lines generated and used in this study are listed in key resources table. All flies were cultured on standard corn-meal diet at 25°C under 12 h light/dark cycle. All mutant flies were used at the same age and sex with w1118 flies (3 days old, male).
D. melanogaster Schneider 2 (S2) cells were originally purchased from American Type Culture Collection (ATCC, www.atcc.org) and maintained at 25°C in SF-X medium (Hyclone, SH30610.02) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen, 10082063).
Method details
Construction of recombinant protein expression vectors
For assays conducted in S2 cells, cDNAs encoding full-length Toll-1, Toll-7 and their TIR domains (TIR-1, TIR-7) were PCR amplified and then cloned in-frame into pMT/BiP/V5-His A vector (Invitrogen, V413020) which uses the MT (Metallothionein) promoter and has a C-terminal V5 tag, and cDNAs encoding mature active spätzles (Spz-1, Spz-2 and Spz-5) and full-length Spz-1 (Spz-1FL) were also PCR amplified and cloned into a modified pMT/Bip A vector that has an N-terminal FLAG tag as described previously.29
To detect autophagy flux in S2 cells, recombinant plasmid pAc5.1-RFP-GFP-LC3 was constructed. The cDNA containing RFP-GFP-LC3 was purchased from Hanbio Company (HB-LP210), cDNA sequences encoding red fluorescence protein (RFP) and green fluorescence protein (GFP) were fused with LC3 (MAP1LC3B, Homo sapiens microtubule-associated protein 1 light chain 3 beta). Then the cDNA was amplified by PCR with forward primer (5′-ATA TGA TAT CAT GGT GAG CAA GGG CGA GGA GGA T-3′) and reverse primer (5′-ATA TGC GGC CGC TTA CAC TGA CAA TTT CAT CCC GAA CGT-3′) using the following conditions: 98°C for 5 min, 35 cycles of 94°C for 30 s, 58°C for 30 s, 72°C for 90 s, and a final extension at 72°C for 10 min. The PCR product was then cloned in-frame into pAc5.1/V5-His A (Invitrogen, V4110-20) vector with actin5C promoter in Eco RV and Not I restriction enzyme sites. The recombinant construct was Sanger sequenced (Sangon Biotech, China) to confirm the sequence identity.
Transient transfection and establishment of stable S2 cell lines
Transient transfection in S2 cells was performed as described previously58 for expression of recombinant GFP, Toll-1, Toll-7, TIR-1, TIR-7, Spz-1, Spz-2, Spz-5, and Spz-1FL (full-length Spz-1). In brief, S2 cells (3×106 cells/well) were seeded overnight in SF-X medium with 5% FBS to ∼70% confluence, and then transfected with recombinant expression vector (pMT-GFP-V5, pMT-TIR-1-V5, pMT-TIR-7-V5, pMT-Toll-1-V5, pMT-Toll-7-V5, pMT-Spz-1-Flag, pMT-Spz-2-Flag, pMT-Spz-5-Flag, or pMT-Spz-1FL-Flag) using Effectene Transfection reagent (Qiagen, 301427) according to the manufacturer’s instructions. After 12 h transfection, copper sulfate was directly added to the culture medium to a final concentration of 500 μM, and the cells were incubated for 60 h. Cell culture media and S2 cells were collected, and cells were lyzed with NP-40 cell lysis buffer. Cell culture medium and cell lysate samples were analyzed for protein expression by immunoblotting analysis.
S2 cell lines stably expressing RFP-GFP-LC3, TIR-1-V5 and TIR-7-V5 were established following previously established protocol29 using Effectene Transfection reagent. Briefly, S2 cells (0.5–1×106 cells/well) were plated in 30-mm cell culture dishes and cells were grown over night to 70–80% confluency prior to transfection. Then, pCoBlast (Invitrogen, K5120-01) was co-transfected with recombinant pAc5.1-RFP-GFP-LC3, pMT-TIR-1-V5 and pMT-TIR-7-V5 vectors, respectively (pCoBlast: expression vector = 1:20) in S2 cells for 48 h, old cell culture medium was removed and replaced with complete growth medium containing 25 μg/mL of Blasticidine S hydrochloride (Sigma-Aldrich, 15205) and cells were cultured for 72 h. This culture process was repeated to select resistant S2 cells. Resistant colonies appeared about 1–2 weeks later. S2 cells which were resistant to Blasticidine S hydrochloride were selected and expression of recombinant proteins in S2 cells were detected by immunoblotting analysis. These S2 cells were selected for passage and used for the following experiments.
Immunoblotting analysis
Protein samples used for immunoblotting analysis of recombinant proteins were prepared from transiently transfected S2 cells or S2 cells stably expressing recombinant proteins described above. To detect protein abundance in fly heads, fly heads (at least 20 individuals per genotype line) were homogenized in RIPA lysis buffer containing protease inhibitors on ice. The homogenates were centrifuged for 10 min at 10,000 g at 4°C and the supernatants were collected for analysis. Total protein concentrations were determined using the BCA Protein Assay Kit (Beyotime, China). Equal amounts of total proteins (50 μg) were loaded to 10%, 12% or 15% SDS-PAGE, proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad), and the membrane was probed with one of the following primary antibodies: anti-V5-tag, anti-Flag-tag or anti-tubulin mouse monoclonal antibody, anti-LC3 rabbit polyclonal antibody (Sigma), anti-phosphor-Tor rabbit monoclonal antibody (Cell Signaling Technology, 5536), anti-Tor rabbit polyclonal antibody (ABclonal, A11354), anti-phosphor-S6K rabbit polyclonal antibody (ABclonal, AP0564), anti-S6K rabbit polyclonal antibody (ABclonal, A2190), anti-phosphor-Akt rabbit polyclonal antibody (Phospho-Drosophila Akt S505, Cell Signaling Technology, 4054), anti-Akt rabbit polyclonal antibody (Cell Signaling Technology, 9272), anti-DJ-1 rabbit monoclonal antibody (Cell Signaling Technology, 5933), anti-phosphor-JNK rabbit polyclonal antibody (Cell Signaling Technology, 9251), anti-PINK-1 rabbit monoclonal antibody (Cell Signaling Technology, 6946), anti-phosphor-GSK3 rabbit monoclonal antibody (Cell Signaling Technology, 9316), anti-Ref(2)P (P62) rabbit polyclonal antibody (abcam, ab178440), anti-Atg8 rabbit polyclonal antibody (abcam, ab109364), or anti-ubiquitin rabbit monoclonal antibody (PTM biolabs, PTM-1106). The secondary antibody used for detection of P62, LC3, ubiquitin, DJ-1, phosphor-JNK, PINK1, phosphor-GSK3, Akt and phospho-Akt was HRP (horseradish peroxidase)-conjugated goat anti-rabbit IgG (Beyotime, A0208), and secondary antibody used for detection of V5-tag, FLAG-tag and tublin was HRP-conjugated goat anti-mouse IgG (Beyotime, A0216). All primary antibodies but anti-tubulin (1:1000), anti-tubulin (1:3000) and secondary antibodies (1:3000) were diluted in 5% dry skim milk prepared in Tris-buffered saline (TBS) containing 0.05% Tween 20 (TBS-T). Detection was performed using ECL reagent (Bio-Rad). Protein bands (LC3-I, LC3-II, p-Akt, total Akt, and tubulin) were scanned using ImageJ (at least 3 membranes for each protein), relative protein level was calculated by first dividing the gray value of p-Akt band by the gray value of total Akt band, and then dividing this value by the gray value of tubulin band, or by first dividing the gray value of p-Tor band (or p-S6K band) by the gray value of total Tor band (or total S6K band), and then dividing this value by the gray value of tubulin band, or by dividing the gray value of P62 or LC3-II band by the gray value of tubulin band. Relative protein level in the control samples was arbitrarily set as 1.
Immunofluorescence and confocal microscopy
Whole brains were collected from 3-day old adult flies. Briefly, brains were dissected in PBS, fixed in PBS containing 4% formaldehyde for 1 h, washed with 1% PBT (100 μL Triton X-100 in 10 mL PBS) three times (each for 5 min), and incubated with 5% bovine serum albumin (BSA) in PBS to block nonspecific binding. Then, the brains were incubated with primary antibody (1:200 of anti-tyrosine hydroxylase (anti-TH) rabbit polyclonal antibody (Novus Biologicals, NB300-109), or 1:400 of anti-Ref(2)P in PBT) for two nights at 4°C, washed with PBT four times (each for 10 min), incubated with secondary antibody (Alexa Fluor 568 goat anti-rabbit IgG, 1:400 in PBT) at 4°C overnight, and washed with PBT three times (each for 10 min). Finally, the brains were mounted in antifading mounting medium with DAPI and observed under a confocal laser scanning microscope (Olympus Microsystems FV3000, CA, USA). The magnification used to scan whole brain was 20×, and the amplified section was 40×. At least 20 brains were used for counting DA neurons number, or for quantification of fluorescence signal intensity by ImageJ.
In S2 cells stably expressing RFP-GFP-LC3, the fluorescence of both RFP (red) and GFP (green) were detected in the autophagosomes, and thus yellow puncta were observed, while in the acidified autolysosomes, the green fluorescence of GFP was quenched, therefore only red puncta (the fluorescence of RFP) were observed.31 To detect autophagy flux, S2 cells stably expressing RFP-GFP-LC3 were transfected with pMT-Toll-1-V5 or pMT-Toll-7-V5 using Effectene Transfection reagent as described above. After transfection for 12 h, copper sulfate was directly added to the culture medium to a final concentration of 500 μM to induce expression of Toll-1 or Toll-7 for 24 h, then Spz-1 conditioned medium was added and the cells were incubated for 36 h (500 μL conditioned medium in 2 mL of complete medium). Cells were starved by culturing in PBS instead of complete culture medium for 20 min or 6 h. The cells were collected and fixed with 4% paraformaldehyde, permeabilized with 1% Triton X-100, blocked with 5% BSA blocking solution, and then mounted in antifading mounting medium with DAPI. All images were acquired using a confocal microscope (Olympus Microsystems FV3000).
TUNEL staining
Whole brains were collected from 3-day old adult flies. The brain was fixed in 4% formaldehyde for 1 h at room temperature, washed with 1% PBT (100 μL Triton X-100 in 10 mL PBS) three times (each for 5 min), TUNEL (TdT-mediated dUTP Nick-End Labeling) staining was performed using the One Step TUNEL Apoptosis Assay Kit by Beyotime Company (C1088).
Fly climbing assay
The fly climbing assay was performance following a described method59 with 1-week old adult flies. The climbing ability was described as the percentage (%) of total number of flies that were able to climb 17.5 cm in 120 s over total number of flies in each assayed group. The percentage of flies which passed the threshold line is represented every 10 s over duration of the assay. For each genotype, 10 biological replicates (each with 20 flies, a total of 200 flies) were performed.
RNAi in S2 cells
For RNA interference (RNAi) experiments in S2 cells, DNA templates used for double-stranded RNA (dsRNA) synthesis were amplified by PCR using a pair of primers (Table S1), with T7 RNA polymerase-binding site (5′-GGATCCTAATACGACTCACTATAGG-3′) attached to the 5′-end of each primer, and dsRNA was synthesized using T7 RiboMAX Express RNAi kit (Promega, P1700) according to the manufacturer’s instructions. GFP dsRNA was used as a control. S2 cells were seeded overnight in SF-X medium with 5% FBS to ∼70% confluence in six-well plate, and then transfected with dsRNA (10 μg per well) using Effectene Transfection reagent as described previusly.58 After transfection for 72 h, cells were collected for qRT-PCR and immunoblotting analyses.
Quantitative real-time PCR
Total RNA was extracted from S2 cells (at least 1 × 106 cells) by TRIzol (TakaRa, 9108) according to the manufacturer’s instructions, resuspended in 50 μL of nuclease-free water, and RNA concentration was detemined by Nanodrop UV-Vis spectrophotometer (Thermo scientific, ND-1000).
Total RNA (2–3 μg) from each sample was used for cDNA synthesis using reverse transcriptase (RTase) (Promega, USA) and oligo dT18 primer (Intergrated DNA Technologies, USA) at 42°C for 60 min in a 25-μL reaction volume. Real-time PCR was performed using an Applied Biosystems with an SYBR Green qPCR mix (Roche, USA) in 20-μL reactions containing 10 μL SYBR premix, 4 μL H2O, 1 μL each of forward and reverse primer (10 pmol/μL), 2 μL of cDNA template (1:20 diluted in deionized H2O), using the following program: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 1 min and the dissociation curve analysis. Three biological replicates and three technical replicates were performed. Relative gene expression was determined by the 2−ΔΔCt method60 using rp49 as a reference gene. Primers used for qRT-PCR experiments are listed in Table S2. The qRT-PCR experiment was in compliance with the criteria of the MIQE guidelines.61
PP2A activity assay
PP2A activity in S2 cells was determined using the serine/threonine phosphatase assay system (Promega, V2460) according to the manufacturer’s instruction. Briefly, S2 cells were collected and lysed in phosphatase lysis buffer (20 mM HEPES (pH 7.4), 10% glycerol, 0.1%NP-40, 30 mM β-mercaptoethanol, 1 mM EGTA), and phosphatase activity was measured using a PP2A-specific buffer (50 mM imidazole (pH 7.2), 0.2 mM EGTA, 0.03% β-mercaptoethanol, 0.1 mg/mL BSA). Free phosphate generated from a synthetic phosphothreonine peptide (RRA(pT)VA) specifically removed by PP2A was quantified by measuring molybdate/malachite green/phosphate complex at 600 nm as previously described.62
Quantification and statistical analysis
Experiments were repeated with three independent biological samples (or three independent cell cultures), three replicates of each sample were analyzed for each experiment, data were represented as means ± SEM, and a typical set of data was used to make figures using the GraphPad Prism (GraphPad, CA). Significance of difference was determined by one way ANOVA followed by a Tukey’s multiple comparison tests using GraphPad Prism, and identical letters are not significant difference (p > 0.05) while different letters (a, b, c, d, etc.) indicate significant difference (p < 0.05). Significant difference was also determined by the Student’s t test and indicated by ∗ p < 0.05, ∗∗ p < 0.01, and ∗∗∗ p < 0.001, ns for non-significant.
Acknowledgments
We thank Professor Neal Silverman, Professor Sheng Li, Bloomington Drosophila Stock Center, and Tsinghua Fly Center for supplying fly stocks. This study was supported by the National Natural Science Foundation of China (No. 31970474 and 32272606) (Xiao-Qiang Yu and YuZhen Lu).
Author contributions
J.Z. participated in experimental design, performed most experiments, analyzed the data, and wrote the manuscript; T.T. and L.W. helped prepare samples and performed fly cross experiment; R.Z., X.D., and W.Y. provided tools for analysis of protein expression level and helped analyze relative PP2A activity; X.X., F.J., and Y.C. contributed to the conception of the study; Y.L. and X.Q.Y. participated in study conception and experimental design, performed data analysis, supervised the study, and wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: January 4, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.108795.
Contributor Information
Yuzhen Lu, Email: luyuzhen2015@outlook.com.
Xiao-Qiang Yu, Email: xqyu@m.scnu.edu.cn.
Supplemental information
References
- 1.Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24:9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Z., Klionsky D.J. Eaten alive: a history of macroautophagy. Nat. Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakatogawa H., Ichimura Y., Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130:165–178. doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Wirawan E., Vanden Berghe T., Lippens S., Agostinis P., Vandenabeele P. Autophagy: for better or for worse. Cell Res. 2012;22:43–61. doi: 10.1038/cr.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo F., Liu X., Cai H., Le W. Autophagy in neurodegenerative diseases: pathogenesis and therapy. Brain Pathol. 2018;28:3–13. doi: 10.1111/bpa.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stavoe A.K.H., Holzbaur E.L.F. Autophagy in Neurons. Annu. Rev. Cell Dev. Biol. 2019;35:477–500. doi: 10.1146/annurev-cellbio-100818-125242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juhász G., Erdi B., Sass M., Neufeld T.P. Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Genes Dev. 2007;21:3061–3066. doi: 10.1101/gad.1600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komatsu M., Waguri S., Chiba T., Murata S., Iwata J.i., Tanida I., Ueno T., Koike M., Uchiyama Y., Kominami E., Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 9.La Barbera L., Vedele F., Nobili A., Krashia P., Spoleti E., Latagliata E.C., Cutuli D., Cauzzi E., Marino R., Viscomi M.T., et al. Nilotinib restores memory function by preventing dopaminergic neuron degeneration in a mouse model of Alzheimer's Disease. Prog. Neurobiol. 2021;202:102031. doi: 10.1016/j.pneurobio.2021.102031. [DOI] [PubMed] [Google Scholar]
- 10.Maday S., Holzbaur E.L.F. Compartment-Specific Regulation of Autophagy in Primary Neurons. J. Neurosci. 2016;36:5933–5945. doi: 10.1523/JNEUROSCI.4401-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizushima N., Yamamoto A., Matsui M., Yoshimori T., Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemaitre B., Hoffmann J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 13.Dzamko N., Gysbers A., Perera G., Bahar A., Shankar A., Gao J., Fu Y., Halliday G.M. Toll-like receptor 2 is increased in neurons in Parkinson's disease brain and may contribute to alpha-synuclein pathology. Acta Neuropathol. 2017;133:303–319. doi: 10.1007/s00401-016-1648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim C., Rockenstein E., Spencer B., Kim H.K., Adame A., Trejo M., Stafa K., Lee H.J., Lee S.J., Masliah E. Antagonizing Neuronal Toll-like Receptor 2 Prevents Synucleinopathy by Activating Autophagy. Cell Rep. 2015;13:771–782. doi: 10.1016/j.celrep.2015.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G., Forero M.G., Wentzell J.S., Durmus I., Wolf R., Anthoney N.C., Parker M., Jiang R., Hasenauer J., Strausfeld N.J., et al. A Toll-receptor map underlies structural brain plasticity. Elife. 2020;9 doi: 10.7554/eLife.52743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foldi I., Anthoney N., Harrison N., Gangloff M., Verstak B., Nallasivan M.P., AlAhmed S., Zhu B., Phizacklea M., Losada-Perez M., et al. Three-tier regulation of cell number plasticity by neurotrophins and Tolls in Drosophila. J. Cell Biol. 2017;216:1421–1438. doi: 10.1083/jcb.201607098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIlroy G., Foldi I., Aurikko J., Wentzell J.S., Lim M.A., Fenton J.C., Gay N.J., Hidalgo A. Toll-6 and Toll-7 function as neurotrophin receptors in the Drosophila melanogaster CNS. Nat. Neurosci. 2013;16:1248–1256. doi: 10.1038/nn.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kingsolver M.B., Huang Z., Hardy R.W. Insect Antiviral Innate Immunity: Pathways, Effectors, and Connections. J. Mol. Biol. 2013;425:4921–4936. doi: 10.1016/j.jmb.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tikhe C.V., Dimopoulos G. Mosquito antiviral immune pathways. Dev. Comp. Immunol. 2021;116 doi: 10.1016/j.dci.2020.103964. [DOI] [PubMed] [Google Scholar]
- 20.Ferrandon D., Imler J.L., Hetru C., Hoffmann J.A. The systemic immune response: sensing and signaling during bacterial and fungal infections. Nat. Rev. Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- 21.Shmueli A., Shalit T., Okun E., Shohat-Ophir G. The Toll Pathway in the Central Nervous System of Flies and Mammals. NeuroMolecular Med. 2018;20:419–436. doi: 10.1007/s12017-018-8515-9. [DOI] [PubMed] [Google Scholar]
- 22.Weber A.N.R., Gangloff M., Moncrieffe M.C., Hyvert Y., Imler J.L., Gay N.J. Role of the Spatzle Pro-domain in the generation of an active toll receptor ligand. J. Biol. Chem. 2007;282:13522–13531. doi: 10.1074/jbc.M700068200. [DOI] [PubMed] [Google Scholar]
- 23.Ballard S.L., Miller D.L., Ganetzky B. Retrograde neurotrophin signaling through Tollo regulates synaptic growth in Drosophila. J. Cell Biol. 2014;204:1157–1172. doi: 10.1083/jcb.201308115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutcliffe B., Forero M.G., Zhu B., Robinson I.M., Hidalgo A. Neuron-type specific functions of DNT1, DNT2 and Spz at the Drosophila neuromuscular junction. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu B., Pennack J.A., McQuilton P., Forero M.G., Mizuguchi K., Sutcliffe B., Gu C.J., Fenton J.C., Hidalgo A. Drosophila neurotrophins reveal a common mechanism for nervous system formation. PLoS Biol. 2008;6:e284. doi: 10.1371/journal.pbio.0060284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin C.N., Nechipurenko I.V., Liu N., Broihier H.T. A Toll receptor-FoxO pathway represses Pavarotti/MKLP1 to promote microtubule dynamics in motoneurons. J. Cell Biol. 2016;214:459–474. doi: 10.1083/jcb.201601014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward A., Hong W., Favaloro V., Luo L. Toll receptors instruct axon and dendrite targeting and participate in synaptic partner matching in a Drosophila olfactory circuit. Neuron. 2015;85:1013–1028. doi: 10.1016/j.neuron.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber A.N.R., Tauszig-Delamasure S., Hoffmann J.A., Lelièvre E., Gascan H., Ray K.P., Morse M.A., Imler J.L., Gay N.J. Binding of the Drosophila cytokine Spätzle to Toll is direct and establishes signaling. Nat. Immunol. 2003;4:794–800. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- 29.Chowdhury M., Li C.F., He Z., Lu Y., Liu X.S., Wang Y.F., Ip Y.T., Strand M.R., Yu X.Q. Toll family members bind multiple Spätzle proteins and activate antimicrobial peptide gene expression in Drosophila. J. Biol. Chem. 2019;294:10172–10181. doi: 10.1074/jbc.RA118.006804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bánréti Á., Lukácsovich T., Csikós G., Erdélyi M., Sass M. PP2A regulates autophagy in two alternative ways in Drosophila. Autophagy. 2012;8:623–636. doi: 10.4161/auto.19081. [DOI] [PubMed] [Google Scholar]
- 31.Kimura S., Noda T., Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 32.Moy R.H., Gold B., Molleston J.M., Schad V., Yanger K., Salzano M.V., Yagi Y., Fitzgerald K.A., Stanger B.Z., Soldan S.S., Cherry S. Antiviral autophagy restrictsRift Valley fever virus infection and is conserved from flies to mammals. Immunity. 2014;40:51–65. doi: 10.1016/j.immuni.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delgado M.A., Elmaoued R.A., Davis A.S., Kyei G., Deretic V. Toll-like receptors control autophagy. The EMBO journal. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yano T., Kurata S. Induction of autophagy via innate bacterial recognition. Autophagy. 2008;4:958–960. doi: 10.4161/auto.6802. [DOI] [PubMed] [Google Scholar]
- 35.Bartlett B.J., Isakson P., Lewerenz J., Sanchez H., Kotzebue R.W., Cumming R.C., Harris G.L., Nezis I.P., Schubert D.R., Simonsen A., Finley K.D. p62, Ref(2)P and ubiquitinated proteins are conserved markers of neuronal aging, aggregate formation and progressive autophagic defects. Autophagy. 2011;7:572–583. doi: 10.4161/auto.7.6.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagy P., Varga Á., Kovács A.L., Takáts S., Juhász G. How and why to study autophagy in: It's more than just a garbage chute. Methods (San Diego, Calif.) 2015;75:151–161. doi: 10.1016/j.ymeth.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lőrincz P., Mauvezin C., Juhász G. Exploring Autophagy in Drosophila. Cells-Basel. 2017;6:22. doi: 10.3390/cells6030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denton D., Chang T.K., Nicolson S., Shravage B., Simin R., Baehrecke E.H., Kumar S. Relationship between growth arrest and autophagy in midgut programmed cell death in Drosophila. Cell Death Differ. 2012;19:1299–1307. doi: 10.1038/cdd.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanoh H., Kato H., Suda Y., Hori A., Kurata S., Kuraishi T. Dual comprehensive approach to decipher the Drosophila Toll pathway, ex vivo RNAi screenings and immunoprecipitation-mass spectrometry. Biochem. Biophys. Res. Commun. 2019;508:332–337. doi: 10.1016/j.bbrc.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Alekseyenko O.V., Chan Y.B., Li R., Kravitz E.A. Single dopaminergic neurons that modulate aggression in. Proc. Natl. Acad. Sci. USA. 2013;110:6151–6156. doi: 10.1073/pnas.1303446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjordal M., Arquier N., Kniazeff J., Pin J.P., Léopold P. Sensing of Amino Acids in a Dopaminergic Circuitry Promotes Rejection of an Incomplete Diet in. Cell. 2014;156:510–521. doi: 10.1016/j.cell.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 42.Riemensperger T., Issa A.R., Pech U., Coulom H., Nguyễn M.V., Cassar M., Jacquet M., Fiala A., Birman S. A Single Dopamine Pathway Underlies Progressive Locomotor Deficits in a Model of Parkinson Disease. Cell Rep. 2013;5:952–960. doi: 10.1016/j.celrep.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 43.Waddell S. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 2010;33:457–464. doi: 10.1016/j.tins.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waddell S. Reinforcement signalling in; dopamine does it all after all. Curr. Opin. Neurobiol. 2013;23:324–329. doi: 10.1016/j.conb.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang M., Chen S. DJ-1 in neurodegenerative diseases: Pathogenesis and clinical application. Prog Neurobiol. 2021;204 doi: 10.1016/j.pneurobio.2021.102114. [DOI] [PubMed] [Google Scholar]
- 46.Kim Y., Park J., Kim S., Song S., Kwon S.K., Lee S.H., Kitada T., Kim J.M., Chung J. PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem. Biophys. Res. Commun. 2008;377:975–980. doi: 10.1016/j.bbrc.2008.10.104. [DOI] [PubMed] [Google Scholar]
- 47.Johnson G.V.W., Stoothoff W.H. Tau phosphorylation in neuronal cell function and dysfunction. J. Cell Sci. 2004;117:5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- 48.Haeusgen W., Boehm R., Zhao Y., Herdegen T., Waetzig V. Specific activities of individual c-Jun N-terminal kinases in the brain. Neuroscience. 2009;161:951–959. doi: 10.1016/j.neuroscience.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 49.Zhong X., Xu X.X., Yi H.Y., Lin C., Yu X.Q. A Toll-Spätzle pathway in the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 2012;42:514–524. doi: 10.1016/j.ibmb.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DiAngelo J.R., Bland M.L., Bambina S., Cherry S., Birnbaum M.J. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc. Natl. Acad. Sci. USA. 2009;106:20853–20858. doi: 10.1073/pnas.0906749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roth S.W., Bitterman M.D., Birnbaum M.J., Bland M.L. Innate Immune Signaling in Drosophila Blocks Insulin Signaling by Uncoupling PI(3,4,5)P(3) Production and Akt Activation. Cell Rep. 2018;22:2550–2556. doi: 10.1016/j.celrep.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puig O., Marr M.T., Ruhf M.L., Tjian R. Control of cell number by FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 2003;17:2006–2020. doi: 10.1101/gad.1098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C., Chang K.C., Somers G., Virshup D., Ang B.T., Tang C., Yu F., Wang H. Protein phosphatase 2A regulates self-renewal of Drosophila neural stem cells. Development (Cambridge, England) 2009;136:2287–2296. doi: 10.1242/dev.035758. [DOI] [PubMed] [Google Scholar]
- 54.Wlodarchak N., Xing Y. PP2A as a master regulator of the cell cycle. Crit. Rev. Biochem. Mol. Biol. 2016;51:162–184. doi: 10.3109/10409238.2016.1143913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shin W.H., Park J.H., Chung K.C. The central regulator p62 between ubiquitin proteasome system and autophagy and its role in the mitophagy and Parkinson's disease. BMB Rep. 2020;53:56–63. doi: 10.5483/BMBRep.2020.53.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klein M.O., Battagello D.S., Cardoso A.R., Hauser D.N., Bittencourt J.C., Correa R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019;39:31–59. doi: 10.1007/s10571-018-0632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCubrey J.A., Rakus D., Gizak A., Steelman L.S., Abrams S.L., Lertpiriyapong K., Fitzgerald T.L., Yang L.V., Montalto G., Cervello M., et al. Effects of mutations in Wnt/β-catenin, hedgehog, Notch and PI3K pathways on GSK-3 activity-Diverse effects on cell growth, metabolism and cancer. Biochim. Biophys. Acta. 2016;1863:2942–2976. doi: 10.1016/j.bbamcr.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J., Yang W., Xu J., Yang W., Li Q., Zhong Y., Cao Y., Yu X.Q., Deng X. Regulation of antimicrobial peptide genes via insulin-like signaling pathway in the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 2018;103:12–21. doi: 10.1016/j.ibmb.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Madabattula S.T., Strautman J.C., Bysice A.M., O’Sullivan J.A., Androschuk A., Rosenfelt C., Doucet K., Rouleau G., Bolduc F. Quantitative analysis of climbing defects in a Drosophila model of neurodegenerative disorders. J. Vis. Exp. 2015;100 doi: 10.3791/52741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 61.Bustin S.A., Beaulieu J.F., Huggett J., Jaggi R., Kibenge F.S.B., Olsvik P.A., Penning L.C., Toegel S. MIQE précis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol. Biol. 2010;11:74. doi: 10.1186/1471-2199-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim K.Y., Baek A., Hwang J.E., Choi Y.A., Jeong J., Lee M.S., Cho D.H., Lim J.S., Kim K.I., Yang Y. Adiponectin-activated AMPK stimulates dephosphorylation of AKT through protein phosphatase 2A activation. Cancer Res. 2009;69:4018–4026. doi: 10.1158/0008-5472.CAN-08-2641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The dataset is publicly available as of the date of publication.
-
•
This study did not report the original code.
-
•
Any additional information required to reanalyze the data reported in this study is available from the lead contact upon request.