Abstract
Heat-killed Borrelia burgdorferi spirochetes stimulate in vitro production of interleukin-10 (IL-10) at both mRNA and protein levels in peripheral blood mononuclear cells (PBMC) of uninfected rhesus monkeys. A concomitant down-modulation of IL-2 gene transcription was observed. Neither IL-4 nor gamma interferon gene expression was ostensibly affected by B. burgdorferi spirochetes. These phenomena were observed regardless of whether the stimulating spirochetes belonged to the B. burgdorferi sensu stricto, Borrelia afzelii, or Borrelia garinii genospecies, the three main species that cause Lyme disease. B. burgdorferi also induced production of IL-10 in uninfected human PBMC, indicating that this effect might play a role in human Lyme disease. Purified lipidated outer surface protein A (OspA), but not its unlipidated form, induced the production of high levels of IL-10 in uninfected human PBMC. Thus, the lipid moiety is essential in the induction of IL-10 in these PBMC. B. burgdorferi M297, a mutant strain that lacks the plasmid that encodes OspA and OspB, also induced IL-10 gene transcription in PBMC, indicating that this phenomenon is not causally linked exclusively to OspA and its lipid moiety. These results demonstrate that B. burgdorferi can stimulate the production of an antiinflammatory, immunosuppressive cytokine in naive cells and suggest that IL-10 may play a role both in avoidance by the spirochete of deleterious immune responses and in limiting the inflammation that the spirochete is able to induce.
Lyme borreliosis is a multisystem disease caused by infection with the tick-borne spirochete Borrelia burgdorferi. The spirochete can invade a variety of tissues (2) and has the ability to persist in them for a long time (32, 47). This persistence has been correlated with severe pathology and may be responsible for localized inflammation (2, 47). The association between tissue invasion and localized inflammation may be explained by the fact that the spirochete possesses potent cytokine-stimulatory properties. It has been shown that B. burgdorferi can induce the in vitro production of several proinflammatory cytokines such as interleukin-1 (IL-1) (23), IL-6 (18, 39, 40), and tumor necrosis factor alpha (9) as well as mediators of inflammation such as nitric oxide (29, 40) and superoxide (29). These responses are elicited by membrane lipoproteins, e.g., outer surface protein A (OspA) (27, 28, 39).
Many host factors, such as Borrelia-specific T cells, macrophages, and neutrophils, as well as their immune mediators, potentially contribute to the elimination of resident spirochetes from tissues. In mice, CD4+ T cells play an important role in controlling spirochete growth (22). Neutrophils and macrophages can phagocytize opsonized spirochetes and induce a metabolic burst (6, 29). Nitric oxide and reactive oxygen intermediates have not only been shown to mediate host inflammation (4) but may also contribute to spirochetal death (6, 29). Yet there must be a way for the spirochetes to circumvent harmful immune responses if they are to persist in the immunocompetent host.
Many investigators have noted that some Lyme disease patients appear to have down-regulated immune responses (38). Peripheral blood mononuclear cells (PBMC) from such patients failed to respond in vitro to B. burgdorferi antigens (24, 37). Other studies have shown that NK cell function is inhibited in patients with both early and chronic Lyme disease (8, 17) but is normal in patients who are convalescent (8). In one of these studies it was documented that the organisms themselves inhibited NK cell function (17). More recently, it has been reported that protein antigens of B. burgdorferi were able to inhibit antigen- and mitogen-induced lymphocyte proliferation and decrease IL-2 production (5). It is possible that, as a survival stratagem, the spirochete is able directly to effect down-regulation of deleterious immune responses by an as yet unidentified mechanism. Such a down-regulatory effect might also contribute to the limiting of inflammatory stimuli.
As we were investigating cytokine profiles in monkeys infected with B. burgdorferi, we observed that spirochetes induced in vitro the production of IL-10—a well-known antiinflammatory and immunosuppressive cytokine—in uninfected monkey PBMC. In view of the possible effect that the induction of IL-10 by spirochetes could have on the immune response and in the pathogenesis of Lyme disease, we further investigated this phenomenon. The present study demonstrates that B. burgdorferi is able to induce in vitro the production of IL-10, at both mRNA and protein levels, in uninfected human and rhesus monkey PBMC. This phenomenon is causally linked to the lipidation of spirochetal lipoproteins. The possible role of the induction of IL-10 by B. burgdorferi in the establishment of infection and disease pathogenesis is discussed.
MATERIALS AND METHODS
Bacterium and antigen preparation.
Two strains of B. burgdorferi sensu stricto (JD1 and M297) were used in this study. JD1 was from our stocks and had been obtained originally from the Centers for Disease Control and Prevention, Fort Collins, Colo. M297, a mutant strain that lacks the plasmid that encodes OspA and OspB (21), was obtained from Carrie Hughes (Georgetown University, Washington, D.C.). Borrelia afzelii ECM-1 and Borrelia garinii G-1 were obtained from Denee Thomas (University of Texas Health Science Center, San Antonio, Tex.). Spirochetes were grown in vitro in BSK-H medium (Sigma Chemical Co., St. Louis, Mo.) supplemented with 10% heat-inactivated young rabbit serum (Pel-Freez, Roger, Alaska), 7.5 μg of amphotericin per ml, 48 μg of rifampin per ml, and 192 μg of phosphomycin per ml, washed five times for 10 min each in sterile 0.01 M phosphate-buffered saline (PBS) (pH 7.2), heat killed by boiling for 10 min, aliquoted, and stored at −70°C until being used. Recombinant lipidated OspA (L-OspA) and unlipidated OspA (U-OspA) were obtained from John Dunn, Brookhaven National Laboratories, Brookhaven, N.Y. The recombinant ospA gene was from the B31 strain of B. burgdorferi sensu stricto. U-OspA was equivalent to mature OspA but lacked the cysteine in position 18 of the unprocessed protein (14).
Isolation of PBMC.
Preservative-free heparinized blood was obtained from uninfected 3- to 4-year-old Chinese male rhesus monkeys (Macaca mulatta) and healthy human donors who had never been exposed to B. burgdorferi. Blood was centrifuged at 450 × g for 15 min to obtain buffy-coat cells. Cells were resuspended in RPMI 1640 medium, and the PBMC were isolated by density gradient centrifugation with Ficoll-Hypaque (Sigma). Cells were washed three times for 10 min each in cold medium, and final suspensions were made in RPMI 1640 supplemented with 25 mM HEPES buffer, 2 mM l-glutamine, 10% heat-inactivated fetal bovine serum (Hyclone, Logan, Utah), 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 0.25 μg of fungizone per ml (supplemented medium). Viability of cells (greater than 95%) was determined by trypan blue exclusion.
Stimulation of cytokine production.
PBMC (3 × 106/ml) were cultured in supplemented medium in round-bottom polypropylene tubes (Sarstedt, Nümbrecht, Germany) in the presence or absence of heat-killed spirochetes (106 to 107 spirochetes/ml), L-OspA or U-OspA (1 μg/ml), concanavalin A (ConA) (8 μg/ml), phytohemagglutinin (PHA) (10 μg/ml), or lipopolysaccharide (LPS) from Escherichia coli O26:B6 (1 μg/ml) (ConA, PHA, and LPS were from Sigma). Cultures were incubated at 37°C in a humidified atmosphere (5% CO2 and 95% air) for 24 h (for RNA expression) or 48 h (for cytokine production). Where indicated, cultures also contained 50 μg of polymyxin B sulfate (Sigma) per ml. The concentration of polymyxin B used was in a range that has been reported not to be toxic to human cells (25, 41). The viability of the cells cultured in the presence of polymyxin B was greater than 95% after 48 h of culture. At the end of the culture, the cells were centrifuged at 400 × g at 4°C and processed immediately for RNA extraction. The supernatants were aliquoted and stored at −70°C until being assayed for IL-10.
Detection of cytokine mRNA by semiquantitative RT-PCR.
Total RNA was isolated with 4 M guanidinium isothiocyanate and precipitated with isopropanol (43). Air-dried RNA pellets from PBMC stimulated in vitro with different antigen or mitogen preparations (approximately 1 μg of RNA) were resuspended in 30 μl of reverse transcription (RT) mixture (10 mM Tris-HCl [pH 9.0], 5 mM MgCl2, 50 mM KCl, 25 μM dithiothreitol, 0.1% Triton X-100, 5 μM random hexamers, 1 mM deoxynucleoside triphosphates, 20 U of RNasin [Promega, Madison, Wis.], and 100 units of murine leukemia virus reverse transcriptase [Gibco BRL, Grand Island, N.Y.]). RT was allowed to proceed for 10 min at room temperature and then for 1 h at 42°C, after which it was heat inactivated at 95°C for 5 min. One-fifth of that mixture was then used as a template for specific amplification of each cytokine. PCR was performed with primers and probes that cross-hybridize between human and rhesus monkey cytokine genes according to a protocol previously described (43). Primer and probe sequences for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), IL-2, IL-4, IL-10, and gamma interferon (IFN-γ) have been published elsewhere (3, 42, 43). An optimal number of PCR cycles was determined initially by using a variable number of cycles to identify a linear range of amplification for each cytokine transcript. Thirty cycles (IL-2 and IL-10), 35 cycles (IL-4 and IFN-γ), or 25 cycles (GAPDH) of denaturation (95°C), annealing (60°C), and elongation (72°C) were performed in a Perkin-Elmer model 480 thermocycler (The Perkin-Elmer Corp., Norwalk, Conn.). Positive and negative controls were included in each assay to confirm that only cDNA PCR products were detected and that none of the reagents were contaminated with cDNA or PCR products. PCR products were separated on agarose gels, Southern transferred to a Nytran nylon membrane (Schleicher & Schuell, Keene, N.H.), probed with digoxigenin-labelled oligonucleotide probes, and visualized with a chemiluminescence detection system (Genius kit; Boehringer Mannheim, Indianapolis, Ind.). All cytokine amplicon levels were normalized with respect to the amount of mRNA encoding GAPDH, the product of a housekeeping gene, detected in the same sample. The chemiluminescent signals were quantified with 1D Image Analysis Software (Kodak Digital Science, Eastman Kodak Co., Rochester, N.Y.). The results are expressed in terms of fold increase over the mRNA levels of PBMC cultured in the absence of antigen or mitogen.
IL-10 ELISA.
A sandwich enzyme-linked immunosorbent assay (ELISA) was employed to detect secreted IL-10. Monoclonal antibody JDS3-9D7 (PharMingen, San Diego, Calif.) was used as the capture antibody, and biotin-conjugated monoclonal antibody JDS3-12G8 (PharMingen) was used as the detection antibody. The capture antibody was diluted in 0.1 M Na2HPO4, pH 9.0, to a concentration of 4 μg/ml. ELISA plates (catalog no. 3590; Costar, Cambridge, Mass.) were coated with 50 μl of that solution and incubated overnight at 4°C. Plates were blocked with 200 μl of 1% bovine serum albumin (BSA)–PBS per well for 30 min at room temperature. After three washes with PBS–0.05% Tween 20, duplicate samples (culture supernatants) and standards were incubated overnight at 4°C in the antibody-coated wells. Dilutions of recombinant human IL-10 (PharMingen) in supplemented medium were used for the standard curve. After four washes with PBS–0.05% Tween 20, 100 μl of detection antibody per well diluted to 1 μg/ml in 1% BSA–PBS–0.05% Tween 20 was added and the mixture was incubated for 1 h at room temperature. Wells were washed six times with PBS–0.05% Tween 20, and 100 μl of streptavidin-horseradish peroxidase (Vector Laboratories Inc., Burlingame, Calif.) diluted 1/350 in 1% BSA–PBS–0.05% Tween 20 was added. Plates were incubated for 30 min at room temperature and then washed eight times with PBS–0.05% Tween 20. One hundred microliters of 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) · H2O2 substrate (Sigma) was added, and the mixture was incubated at room temperature for 30 min. Absorbance at 405 nm was determined with a microtest plate spectrophotometer, model SLT. Spectra II and the concentration of the samples were determined with soft2000 4.02 software Tecan US, SLT Lab Instruments, Research Triangle Park, N.C.
Statistics.
ELISA results were logarithmically transformed and analyzed with Student’s paired t test. Significance was assessed at a probability level of 0.05.
RESULTS
Cytokine mRNA expression of rhesus monkey PBMC that were stimulated with B. burgdorferi.
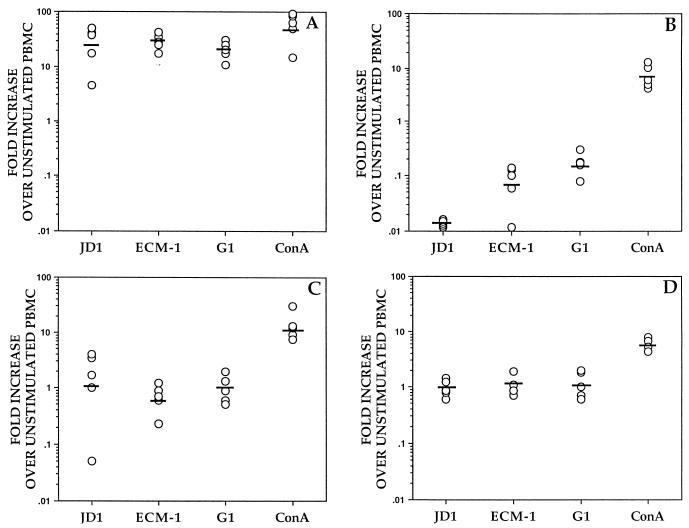
The ability of B. burgdorferi to induce mRNA transcription of the IL-2, IL-4, IL-10, and IFN-γ cytokine genes in rhesus monkey PBMC was evaluated in vitro. For that purpose, PBMC from uninfected rhesus monkeys were cultured for 24 h in the absence or presence of whole heat-killed spirochetes of the B. burgdorferi sensu stricto strain JD1 or ConA. Cytokine gene expression was assessed by semiquantitative RT-PCR. Compared to unstimulated cells, a marked up-regulation of the expression of the IL-10 gene in JD1-stimulated PBMC was observed in all animals (geometric mean fold increase [GMFI] of 22.9) (Fig. 1A). In contrast, there was a concomitant down-regulation of IL-2 gene expression when cells were stimulated with JD1 (GMFI of 0.014) (Fig. 1B). JD1 did not ostensibly affect the expression of IL-4 (GMFI of 0.92) or IFN-γ (GMFI of 1) genes in normal cells (Fig. 1C and D, respectively).
FIG. 1.
Cytokine mRNA expression induced by B. burgdorferi in PBMC from uninfected rhesus monkeys. PBMC (3 × 106/ml) from uninfected rhesus monkeys were stimulated with heat-killed spirochetes of the B. burgdorferi sensu stricto strain JD1, B. afzelii ECM-1, or B. garinii G-1 (107 spirochetes/ml) or with ConA (8 μg/ml) for 24 h. The induced mRNA levels of IL-10 (A), IL-2 (B), IFN-γ (C), and IL-4 (D) were determined by RT-PCR. Responses are shown as fold increases over unstimulated PBMC. Each point represents the response of PBMC from an individual monkey, and the horizontal lines indicate geometric means. All values were normalized with respect to GAPDH mRNA levels.
We further asked whether spirochetes of B. afzelii and B. garinii, the other major genospecies that cause Lyme disease, would also have the capacity to induce the expression of the IL-10 gene in vitro. PBMC from rhesus monkeys were incubated with either whole heat-killed spirochetes of B. afzelii ECM-1 or B. garinii G1. RT-PCR results showed that ECM-1 and G1 strains induced a prominent up-regulation of IL-10 gene expression (GMFI of 28.7 and 20, respectively) with a concomitant down-regulation of IL-2 gene expression (GMFI of 0.065 and 0.16, respectively) (Fig. 1A and B). As with B. burgdorferi sensu stricto spirochetes, incubation of PBMC with B. afzelii or B. garinii spirochetes did not substantially modify the expression of the IL-4 (GMFI of 1.18 and 1.08, respectively) and IFN-γ (GMFI of 0.63 and 0.93, respectively) genes (Fig. 1C and D). Thus, spirochetes of the three main genospecies that cause Lyme disease up-regulate IL-10 gene transcription in uninfected monkey cells. Stimulation of PBMC with the mitogen ConA induced high levels of mRNA expression of all of the cytokines investigated (Fig. 1), indicating that under appropriate conditions rhesus monkey PBMC are able to transcribe the cytokine genes investigated.
The induction of IL-10 gene transcription was a function of the number of spirochetes present in the culture. For cells from three different monkeys, a marked upregulation of IL-10 gene expression was detected in cultures containing between 106 and 107 spirochetes/ml (spirochete/cell ratios of 0.3 to 3 spirochete/PBMC). Induction of the IL-10 gene in cultures containing 105 spirochetes/ml or fewer dropped dramatically, to the levels of unstimulated cells (data not shown). Thus, in all experiments either 106 or 107 spirochetes/ml were used to stimulate PBMC.
Production of IL-10 in culture supernatants of rhesus monkey PBMC that were stimulated with B. burgdorferi.
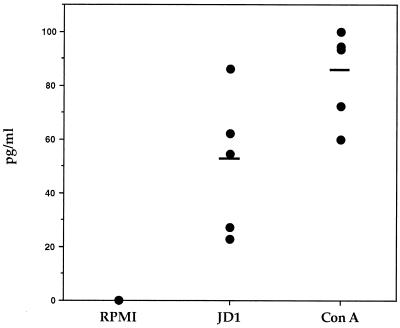
We further investigated the capacity of JD1 to induce secretion of IL-10 from uninfected monkey cells. PBMC from normal monkeys were cultured for 48 h in the absence or presence of JD1 or ConA. Production of IL-10 in the culture supernatants was assessed by ELISA. IL-10 was significantly elevated in culture supernatants from PBMC that were stimulated with JD1 or ConA compared with the supernatants of unstimulated cells (P values of <0.01 and 0.005, respectively) (Fig. 2). The amount of IL-10 secreted in response to JD1 was comparable to that induced by ConA in three of the five uninfected monkeys tested. The background levels of IL-10 in the supernatants from unstimulated cells were below the sensitivity level of the assay (15 pg/ml).
FIG. 2.
IL-10 production induced by B. burgdorferi in PBMC from uninfected rhesus monkeys. PBMC (3 × 106/ml) from uninfected rhesus monkeys were incubated with supplemented medium (RPMI), heat-killed spirochetes of the JD1 strain (106 spirochetes/ml), or ConA (8 μg/ml) for 48 h. IL-10 in the supernatant was determined by ELISA. Values are means of duplicate determinations. Each point represents the concentration of IL-10 in the culture supernatant of PBMC from an individual monkey, and the horizontal lines indicate arithmetic means. The lower limit of detection of the ELISA was 15 pg/ml.
B. burgdorferi spirochetes induce IL-10 transcription and production in human PBMC.
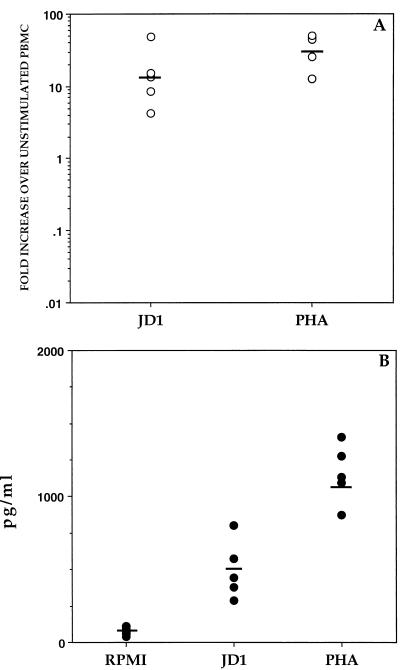
As a first step toward determining whether the induction of IL-10 by B. burgdorferi might play a role in human Lyme disease, we investigated whether JD1 could also induce the production of IL-10 in human cells. PBMC from healthy human donors were cultured in the presence or absence of JD1 or PHA, and IL-10 gene expression was assessed by RT-PCR. As observed with monkey PBMC, when human cells were cultured with JD1 there was a marked up-regulation in expression of the IL-10 gene (GMFI of 13) (Fig. 3A). As with monkey cells, transcription of the IL-2 gene was down-regulated and that of the IL-4 and IFN-γ genes was not substantially modified by JD1 (data not shown). Furthermore, a significant amount of IL-10 was produced in culture supernatants of human PBMC that were stimulated with either JD1 or PHA, compared with the unstimulated PBMC (P values of <0.005 and 0.0001, respectively) (Fig. 3B).
FIG. 3.
IL-10 production induced by B. burgdorferi in PBMC from healthy human donors. PBMC (3 × 106/ml) from each individual were incubated with supplemented medium (RPMI), heat-killed JD1 (106 spirochetes/ml), or PHA (10 μg/ml). Levels of IL-10 mRNA were determined by RT-PCR after 24 h of culture (A), or IL-10 production in the supernatant was determined by ELISA after 48 h of culture (B). Data points of the RT-PCR and ELISA are presented as described in the legends to Fig. 1 and 2, respectively.
The lipid moiety of spirochetal lipoproteins is essential in IL-10 production.
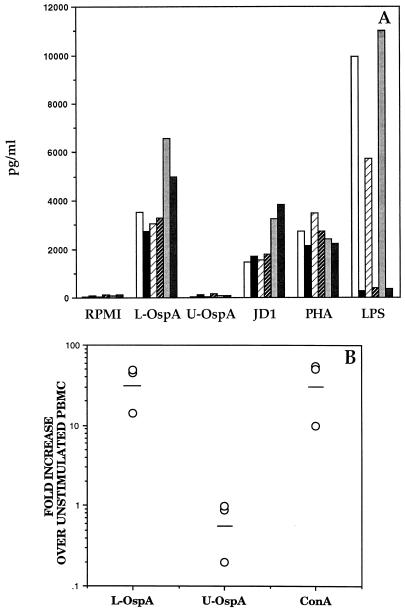
Using OspA as a model of a spirochetal lipoprotein, we investigated the capacity of B. burgdorferi lipoproteins to up-regulate the production of IL-10 in normal cells. PBMC from three different human donors were incubated with L-OspA or U-OspA and, after 48 h of culture, production of IL-10 was evaluated in the culture supernatants by ELISA. Cells from all three donors produced significant levels of IL-10 when stimulated with L-OspA (P < 0.005). In contrast, U-OspA did not induce an amount of IL-10 higher than that produced by RPMI (background) (Fig. 4A). To ensure that the IL-10 production was not due to E. coli LPS that might have been copurified with the recombinant OspA, cultures were incubated with or without polymyxin B (50 μg/ml), a specific inhibitor of the activity of LPS (31). As additional controls, cells were incubated with PHA, JD1, or LPS. These controls made it possible to assess the specificity of the inhibitory effect of polymyxin B as well as the integrity of the cells used in the experiment. Polymyxin B at a concentration of 50 μg/ml inhibited the activity of 1 μg of LPS/ml by 90 to 95%. However, it had no significant effect on the L-OspA-stimulated production of IL-10 (P > 0.05). JD1 or PHA also induced high levels of IL-10 in PBMC cultures, but these levels were not significantly affected by the addition of polymyxin B (P > 0.05) (Fig. 4A). The ability of L-OspA to induce IL-10 gene expression in normal rhesus monkey PBMC was investigated by RT-PCR. When PBMC from three different uninfected rhesus monkeys were stimulated with L-OspA, there was a marked up-regulation of IL-10 gene expression relative to that of unstimulated cells (GMFI of 31.4). ConA induced a similar level of transcription (Fig. 4B). In contrast, U-OspA failed to induce transcription of IL-10 in PBMC from these animals (GMFI of 0.56) (Fig. 4B). These results showed that the lipid modification of OspA is crucial for the induction of IL-10 in normal cells.
FIG. 4.
(A) IL-10 production induced by OspA in PBMC from healthy human donors. PBMC (3 × 106/ml) from each of three donors were incubated with supplemented medium (RPMI), L-OspA or U-OspA (1 μg/ml), heat-killed JD1 (107 spirochetes/ml), LPS (1 μg/ml), or PHA (10 μg/ml) for 48 h. Each pair of bars (open and solid, light and dark hatched, and light and dark stippled) corresponds to one donor. Samples were incubated without (open, light-hatched, and light-stippled bars) or with (solid, dark-hatched, and dark-stippled bars) polymyxin B (50 μg/ml). IL-10 in the supernatant was determined by ELISA. Values are means of duplicate determinations. (B) IL-10 mRNA expression induced by OspA in PBMC from uninfected rhesus monkeys. PBMC (3 × 106/ml) from uninfected rhesus monkeys were stimulated with L-OspA (1 μg/ml), U-OspA (1 μg/ml), or ConA (8 μg/ml) for 24 h. The induced mRNA levels of IL-10 were determined by RT-PCR. Responses are shown as fold increases over unstimulated PBMC. Each point represents the response of PBMC from an individual monkey, and the horizontal lines indicate geometric means. All values were normalized with respect to GAPDH mRNA levels.
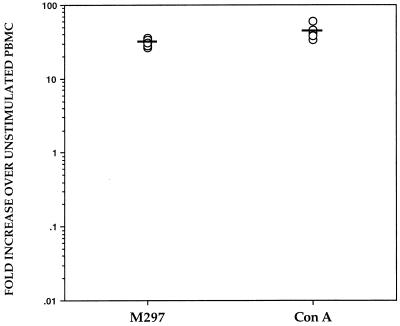
We next investigated whether other lipoproteins of B. burgdorferi could induce the production of IL-10 from normal PBMC or if this phenomenon is an exclusive effect of OspA and its lipid moiety. Monkey PBMC were incubated with whole heat-killed spirochetes of B. burgdorferi M297, a mutant strain that lacks the plasmid encoding OspA and OspB (21), and expression of the IL-10 gene was assessed by RT-PCR. The M297 spirochetes caused an up-regulation in expression of the IL-10 gene (GMFI of 30.6) (Fig. 5), suggesting that lipoproteins other than OspA (or OspB) can induce the same phenomenon that we had observed with wild-type spirochetes.
FIG. 5.
IL-10 mRNA expression induced by B. burgdorferi M297 in PBMC from uninfected rhesus monkeys. PBMC (3 × 106/ml) from uninfected rhesus monkeys were stimulated with heat-killed spirochetes of the mutant strain M297 (106 spirochetes/ml) or ConA (8 μg/ml) for 24 h. The induced mRNA levels of IL-10 were determined by RT-PCR. Responses are shown as fold increases over unstimulated PBMC for each monkey. Each point represents the response of PBMC from an individual monkey, and the horizontal lines indicate geometric means. All values were normalized with respect to GAPDH mRNA levels.
DISCUSSION
A vexing enigma of Lyme borreliosis is the long-term persistence of the infecting spirochete (32, 47) despite a vigorous and specific immune response (1, 30). A panoply of defensive resources may be available to the spirochete, including antigenic variation (46, 49), limited exposure of antigenic targets (7), seclusion into immune privileged sites (26), and local and/or systemic suppression of harmful immune responses.
In this paper we present evidence demonstrating that B. burgdorferi can induce in vitro the production of IL-10, a potent immunosuppressive and antiinflammatory cytokine, in uninfected rhesus monkey and human mononuclear cells. Cells from uninfected rhesus monkeys exhibited a marked up-regulation of expression of the IL-10 gene when cultured in vitro with one representative strain of each of the three main species of the B. burgdorferi sensu lato complex. Together with the up-regulation in IL-10 gene transcription was an associated protein synthesis and release of the IL-10 molecule. Concomitantly, spirochetes induced a marked down-regulation in expression of the IL-2 gene. In contrast, none of the strains examined affected ostensibly the expression of IL-4 and IFN-γ genes. The up-regulation of IL-10 was not a phenomenon unique to rhesus monkey cells. B. burgdorferi also induced production of IL-10, at both the mRNA and protein levels, in PBMC of human donors with no evidence of previous exposure to B. burgdorferi. It is noteworthy that the amount of IL-10 produced by human cells stimulated with JD1 was higher than that generated by monkey PBMC stimulated with the same concentration of antigen. The difference in the production of IL-10 between human and monkey cells may reflect the inability of the anti-human IL-10 antibody used in the ELISA to bind with the same affinity to the monkey IL-10 molecule, or it could be due to inherent differences in the production of IL-10 between human and monkey cells. The finding that both monkey and human normal cells produce IL-10 in response to B. burgdorferi suggests that IL-10 might be implicated in human Lyme disease. The parallels between rhesus monkeys and humans further underscore the usefulness of the monkey model developed in our laboratory (33, 36) for the study of immune mechanisms in human Lyme borreliosis.
It has been previously reported that B. burgdorferi lipoproteins—OspA being the most studied—can induce production of several proinflammatory cytokines (27, 28, 35, 39); this attribute is linked to the lipid moiety (35, 44). The present study showed that transcription of the IL-10 gene is induced both in uninfected monkey and in uninfected human PBMC when these cells are incubated with lipidated OspA but not in the presence of the unlipidated molecule. High levels of the IL-10 cytokine were induced by lipidated OspA in uninfected human PBMC. This effect was dose dependent (16). Polymyxin B treatment did not abrogate this IL-10 production, regardless of whether the latter was induced by L-OspA or heat-killed spirochetes. Since the same JD1 preparation was used throughout, the result reported in Fig. 4A indicates that in all previous experiments induction of the IL-10 gene was also specifically caused by B. burgdorferi. Lipidation of the OspA molecule is thus required for production of IL-10 in PBMC from uninfected donors.
It has been shown that several lipoproteins from B. burgdorferi, Treponema pallidum, and E. coli, with different polypeptide sequences, can stimulate cellular proliferation as well as inflammatory cytokine production in normal cells (19, 20, 28, 35, 45). Moreover, Radolf and colleagues (35) have shown that lipohexapeptides have the same biological activity as the entire lipoprotein. The lipid modification of B. burgdorferi lipoproteins is likely to be identical in all molecules, suggesting that all spirochetal lipoproteins have the potential ability to induce IL-10 production. Indeed, spirochetes of B. burgdorferi M297, a mutant strain that lacks the plasmid encoding OspA and OspB, also induced IL-10 gene transcription in uninfected monkey PBMC. This indicates that production of IL-10 by PBMC is not strictly linked to OspA and its lipid moiety but may be elicited by other lipoproteins. It follows that despite the down-regulation of OspA expression during transmission to the mammalian host (11), spirochetes might conceivably down-modulate an early and damaging immune response by inducing local or systemic IL-10 production with lipoproteins such as OspC and BmpA.
The finding that B. burgdorferi lipoproteins up-regulate production of IL-10 may shed some light on the observation that cells from Lyme disease patients often fail to respond to B. burgdorferi antigens (24, 37). IL-10 has been shown to down-modulate the proliferation of T-helper subsets by selectively inhibiting the production of Th1 cytokines such as IL-2 and IFN-γ (15) as well as by suppressing the expression of class II major histocompatibility complex molecules (12) and accessory molecules such as CD80 and CD86 (13). T-cell lines derived from Lyme disease patients have been shown to produce IL-10 together with IFN-γ (34). In a recent study, it was demonstrated that IL-10 can be concomitantly produced with Th1 cytokines when synovial fluid mononuclear cells from infected patients are incubated with B. burgdorferi (48). The same study showed that this endogenously produced IL-10 also can inhibit both Borrelia-specific lymphocyte proliferation and tumor necrosis factor alpha and IFN-γ production. In the rhesus monkey model, we have observed that PBMC from monkeys chronically infected with B. burgdorferi display a down-modulation of the antigen-specific T-cell response (10). The same cells showed an elevated level of B. burgdorferi-induced IL-10, with diminished production of IL-2 mRNA (16); this may suggest a possible role for IL-10 in the T-cell hyporesponsiveness of these animals.
Tai and colleagues have shown that B. burgdorferi and its lipoprotein OspA possess a B-cell mitogenic and cytokine-stimulatory activity in human cells (39). While this may appear to contradict our findings, their results showed that the proliferative response of the B-enriched cell population was stronger than that of unfractionated PBMC, possibly because of the production of inhibitory cytokines such as IL-10 by other cells of the PBMC population. Studies are currently being conducted in our laboratory to determine which cell types produce IL-10 when stimulated with B. burgdorferi spirochetes.
Recently, a model which postulates that at least three factors contribute to the development of cytokine-mediated inflammation in Lyme disease has been proposed (28). These factors are invasion of and persistence of spirochetes in tissues, interaction of stimulatory lipoproteins with effector cells, and regulation of this effect by modulatory cytokines produced in the microenvironment of the invaded tissues. In view of our results, IL-10 may be one such modulatory cytokine. It may be that the pathologic fate of Lyme disease, in particular the transient and recurrent nature of the disease’s inflammatory episodes, depends on a delicate balance between pro- and antiinflammatory responses that are elicited by spirochetal lipoproteins.
ACKNOWLEDGMENTS
This work was supported by grant U50/CCU606604 from the Centers for Disease Control and Prevention and by grant RR00164 from the National Center for Research Resources, National Institutes of Health.
We thank Barbara Lasater for skillful technical assistance and Christie Trew for excellent secretarial help. We also thank François Villinger (Emory University) for advice and cytokine reagents and John Dunn (Brookhaven National Laboratory) and Carrie Hughes (Georgetown University) for purified recombinant OspA and B. burgdorferi M297, respectively. G.H.G. is a postdoctoral fellow of CONICET (Argentina).
REFERENCES
- 1.Barbour A G, Burgdorfer W, Grunwaldt E, Steere A C. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. J Clin Invest. 1983;72:504–515. doi: 10.1172/JCI110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 3.Benveniste O, Vaslin B, Villinger F, LeGrand R, Ansari A A, Dormont D. Cytokine mRNA levels in unmanipulated (ex vivo) and in vitro stimulated monkey PBMCs using a semi-quantitative RT-PCR and high sensitivity fluorescence-based detection strategy. Cytokine. 1996;8:32–41. doi: 10.1006/cyto.1996.0005. [DOI] [PubMed] [Google Scholar]
- 4.Brown C, Reiner S L. Prevention of experimental Lyme arthritis by blocking nitric oxide induction with aminoguanidine. FASEB J. 1996;10:A1345. [Google Scholar]
- 5.Chiao J W, Pavia C, Riley M, Altmann-Lasekan W, Abolhassani M, Liegner K, Mittelman A. Antigens of the Lyme disease spirochete Borrelia burgdorferi inhibit antigen or mitogen-induced lymphocyte proliferation. FEMS Immunol Med Microbiol. 1994;8:151–155. doi: 10.1111/j.1574-695X.1994.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 6.Cinco M, Murgia R, Perticarari S, Presani G. Simultaneous measurement by flow cytometry of phagocytosis and metabolic burst induced in phagocytic cells in whole blood by Borrelia burgdorferi. FEMS Microbiol Lett. 1994;122:187–193. doi: 10.1111/j.1574-6968.1994.tb07163.x. [DOI] [PubMed] [Google Scholar]
- 7.Cox D L, Akins D R, Bourell K W, Lahdenne P, Norgard M V, Radolf J D. Limited surface exposure of Borrelia burgdorferi outer surface lipoproteins. Proc Natl Acad Sci USA. 1996;93:7973–7978. doi: 10.1073/pnas.93.15.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dattwyler R J, Thomas J A, Benach J L, Golightly M G. Cellular immune responses in Lyme disease. Zentbl Bakteriol Mikrobiol Hyg A. 1986;263:151–159. doi: 10.1016/s0176-6724(86)80118-3. [DOI] [PubMed] [Google Scholar]
- 9.Defosse D L, Johnson R C. In vitro and in vivo induction of tumor necrosis factor alpha by Borrelia burgdorferi. Infect Immun. 1992;60:1109–1113. doi: 10.1128/iai.60.3.1109-1113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennis, V. A., M. K. Aydintug, B. L. Lasater, A. L. Alvarez, and M. T. Philipp. Antigen-specific immune unresponsiveness of peripheral blood mononuclear cells during infection with Borrelia burgdorferi: a longitudinal study in the rhesus monkey. Submitted for publication.
- 11.de Silva A M, Telford III S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeWaal Malefyt R, Haanen J, Spits H, Roncolo M G, TeVelde A, Fidgor C, Johnson K, Kastelein R, Yssel H, DeVries J E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding L, Linsley P S, Huang L Y, Germain R N, Shevach E M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the upregulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 14.Dunn J J, Lade B N, Barbour A G. Outer surface protein A (OspA) from the Lyme disease spirochete, Borrelia burgdorferi: high level expression and purification of a soluble recombinant form of OspA. Protein Expr Purif. 1990;1:159–168. doi: 10.1016/1046-5928(90)90011-m. [DOI] [PubMed] [Google Scholar]
- 15.Fiorentino D F, Zlotnik A, Vieira P, Mosmann T R, Howard M, Moore K W, O’Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 16.Giambartolomei, G. H., V. A. Dennis, B. L. Lasater, and M. T. Philipp. Unpublished data.
- 17.Golightly M, Thomas J, Volkman D, Dattwyler R. Modulation of natural killer cell activity by Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:103–109. doi: 10.1111/j.1749-6632.1988.tb31843.x. [DOI] [PubMed] [Google Scholar]
- 18.Habicht G S, Katona L I, Benach J L. Cytokines and the pathogenesis of neuroborreliosis: Borrelia burgdorferi induces glioma cells to secrete interleukin-6. J Infect Dis. 1991;164:568–574. doi: 10.1093/infdis/164.3.568. [DOI] [PubMed] [Google Scholar]
- 19.Hauschildt S, Hoffmann P, Beuscher H U, Dufhues G, Heinrich P, Wiesmuller K H, Jung G, Bessler W G. Activation of bone marrow-derived mouse macrophages by bacterial lipopeptide: cytokine production, phagocytosis and Ia expression. Eur J Immunol. 1990;20:63–68. doi: 10.1002/eji.1830200110. [DOI] [PubMed] [Google Scholar]
- 20.Honarvar N, Schaible U E, Galanos C, Wallich R, Simon M M. A 14,000 MW lipoprotein and a glycolipid-like structure of Borrelia burgdorferi induce proliferation and immunoglobulin production in mouse B cells at high frequencies. Immunology. 1994;82:389–396. [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes C A, Engstrom S M, Coleman L A, Kodner C B, Johnson R C. Protective immunity is induced by a Borrelia burgdorferi mutant that lacks OspA and OspB. Infect Immun. 1993;61:5115–5122. doi: 10.1128/iai.61.12.5115-5122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keane-Myers A, Nickell S P. T cell subset-dependent modulation of immunity to Borrelia burgdorferi infection in mice. J Immunol. 1995;154:1770–1776. [PubMed] [Google Scholar]
- 23.Kenefick K B, Lim L C L, Alder J D, Schmitz J L, Czuprynski C J, Schell R F. Induction of interleukin-1 release by high- and low-passage isolates of Borrelia burgdorferi. J Infect Dis. 1993;167:1086–1092. doi: 10.1093/infdis/167.5.1086. [DOI] [PubMed] [Google Scholar]
- 24.Krause A, Burmester G R, Rensing A, Schoerner C, Schaible U E, Simon M M, Herzer P, Kramer M D, Wallich R. Cellular immune reactivity to recombinant OspA and flagellin from Borrelia burgdorferi in patients with Lyme borreliosis. J Clin Invest. 1992;90:1077–1084. doi: 10.1172/JCI115923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee E H, Rikihisa Y. Absence of tumor necrosis factor alpha, interleukin-6 (IL-6), and granulocyte-macrophage colony-stimulating factor expression but presence of IL-1β, IL-8, and IL-10 expression in human monocytes exposed to viable or killed Ehrlichia chaffeensis. Infect Immun. 1996;64:4211–4219. doi: 10.1128/iai.64.10.4211-4219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma Y, Sturrok A, Weis J J. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect Immun. 1991;59:671–678. doi: 10.1128/iai.59.2.671-678.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y, Seiler K P, Tai K, Yang L, Woods M, Weis J J. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect Immun. 1994;62:3663–3671. doi: 10.1128/iai.62.9.3663-3671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Y, Weis J J. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect Immun. 1993;61:3843–3853. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Modolell M, Schaible U E, Rittig M, Simon M M. Killing of Borrelia burgdorferi by macrophages is dependent on oxygen radicals and nitric oxide and can be enhanced by antibodies to outer surface proteins of the spirochete. Immunol Lett. 1994;40:139–146. doi: 10.1016/0165-2478(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 30.Moffat C M, Sigal L H, Steere A C, Freeman D A, Dwyer J M. Cellular immune findings in Lyme disease: correlation with serum IgM and disease activity. Am J Med. 1984;77:625–632. doi: 10.1016/0002-9343(84)90352-8. [DOI] [PubMed] [Google Scholar]
- 31.Morrison D C, Jacobs D M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976;13:813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 32.Nocton J J, Dressler F, Rutledge B J, Rys P N, Persing D H, Steere A C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330:229–234. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 33.Philipp M T, Aydintug M K, Bohm R P, Jr, Cogswell F B, Dennis V A, Lanners H N, Lowrie R C, Jr, Roberts E D, Conway M D, Karaçorlu M, Peyman G A, Gubler D J, Johnson B J B, Piesman J, Gu Y. Early and early disseminated phases of Lyme disease in the rhesus monkey: a model for infection in humans. Infect Immun. 1993;61:3047–3059. doi: 10.1128/iai.61.7.3047-3059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pohl-Koppe A, Balachov K, Logigian E L, Steere A C, Hafler D A. The immune response to Borrelia burgdorferi (BB) is characterized by T-cell lines secreting both IFN-γ and IL-10 in patients with Lyme disease. FASEB J. 1996;10:A1184. [Google Scholar]
- 35.Radolf J D, Arndt L L, Akins D R, Curetty L L, Levi M E, Shen Y, Davis L S, Norgard M V. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J Immunol. 1995;154:2866–2877. [PubMed] [Google Scholar]
- 36.Roberts E D, Bohm R P, Jr, Cogswell F B, Lanners H N, Lowrie R C, Jr, Povinelli L, Piesman J, Philipp M T. Chronic Lyme disease in the rhesus monkey. Lab Investig. 1995;72:146–160. [PubMed] [Google Scholar]
- 37.Sigal L H, Steere A C, Freeman D H, Dwyer J M. Proliferative responses of mononuclear cells in Lyme disease. Reactivity to Borrelia burgdorferi antigens is greater in joint fluid than in blood. Arthritis Rheum. 1986;29:761–769. doi: 10.1002/art.1780290609. [DOI] [PubMed] [Google Scholar]
- 38.Sigal L H. Lyme disease, 1988. Immunologic manifestation and possible immunopathogenetic mechanisms. Semin Arthritis Rheum. 1989;18:153–167. doi: 10.1016/0049-0172(89)90058-9. [DOI] [PubMed] [Google Scholar]
- 39.Tai K-F, Ma Y, Weis J J. Normal human B lymphocytes and mononuclear cells respond to the mitogenic and cytokine-stimulatory activities of Borrelia burgdorferi and its lipoprotein OspA. Infect Immun. 1994;62:520–528. doi: 10.1128/iai.62.2.520-528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatro J B, Romero L I, Beasley D, Steere A C, Reichlin S. Borrelia burgdorferi and Escherichia coli lipopolysaccharides induce nitric oxide and interleukin-6 production in cultured rat brain cells. J Infect Dis. 1994;169:1014–1022. doi: 10.1093/infdis/169.5.1014. [DOI] [PubMed] [Google Scholar]
- 41.Vecchiarelli A, Retini C, Monari C, Tascini C, Bistoni F, Kozel T R. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin-10 secretion by human monocytes. Infect Immun. 1996;64:2846–2849. doi: 10.1128/iai.64.7.2846-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villinger F, Hunt D, Mayne A, Vuchetich M, Findley H, Ansari A A. Qualitative and quantitative studies of cytokines synthesized and secreted by non-human primate peripheral blood mononuclear cells. Cytokine. 1993;5:469–479. doi: 10.1016/1043-4666(93)90038-7. [DOI] [PubMed] [Google Scholar]
- 43.Villinger F, Brar S S, Mayne A, Chikkala N, Ansari A A. Comparative sequence analysis of cytokine genes from human and nonhuman primates. J Immunol. 1995;155:3946–3954. [PubMed] [Google Scholar]
- 44.Weis J J, Ma Y, Erdile L F. Biological activities of native and recombinant Borrelia burgdorferi outer surface protein A: dependence on lipid modification. Infect Immun. 1994;62:4632–4636. doi: 10.1128/iai.62.10.4632-4636.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitmire W M, Garon C F. Induction of B-cell mitogenesis by outer surface protein C of Borrelia burgdorferi. 1994. J Spirochetal Tick-Borne Dis. 1994;1:64–68. [Google Scholar]
- 46.Wilske B, Busch U, Fingerle V, Jauris-Heipke S, Preac Mursic V, Rossler D, Will G. Immunological and molecular variability of OspA and OspC. Implications for Borrelia vaccine development. Infection. 1996;24:208–212. doi: 10.1007/BF01713341. [DOI] [PubMed] [Google Scholar]
- 47.Yang L, Weis J H, Eichwald E, Kolbert C P, Persing D H, Weis J J. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin Z, Braun J, Neure L, Wu P, Eggens U, Krause A, Kamradt T, Sieper J. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheum. 1997;40:69–79. doi: 10.1002/art.1780400111. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J R, Hardham J M, Barbour A G, Norris S J. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]