Abstract
Lichens are slow-growing associations of fungi and unicellular green algae or cyanobacteria. They are poikilohydric organisms whose lifestyle in many cases consists of alternating periods of desiccation, with low metabolic activity, and hydration, which induces increase in their metabolism. Lichens have apparently adapted to such extreme transitions between desiccation and rehydration, but the mechanisms that govern these adaptations are still poorly understood. In this study, the effect of rehydration on the production of reactive oxygen species and nitric oxide as well as low-molecular-weight antioxidants was investigated with the lichen Ramalina lacera. Rehydration of R. lacera resulted in the initiation of and a rapid increase in photosynthetic activity. Recovery of photosynthesis was accompanied by bursts of intracellular production of reactive oxygen species and nitric oxide. Laser-scanning confocal microscopy using dichlorofluorescein fluorescence revealed that formation of reactive oxygen species following rehydration was associated with both symbiotic partners of the lichen. The rate and extent of reactive oxygen species production were similar in the light and in the dark, suggesting a minor contribution of photosynthesis. Diaminofluorescein fluorescence, indicating nitric oxide formation, was detected only in fungal hyphae. Activities associated with rehydration did not have a deleterious effect on membrane integrity as assessed by measurement of electrolyte leakage, but water-soluble low-molecular-weight antioxidants decreased significantly.
Lichens are slow-growing associations of fungi (mycobionts) and photosynthetic partners (photobionts) that may be unicellular green algae or cyanobacteria. They occupy a vast range of habitats and substrates and produce unique biochemical compounds that have made them useful to humans as food, dyes, medicines, fine perfumes, and poisons (51). In addition, many lichen species are sensitive to air pollution, and this feature, along with their capability to accumulate mineral elements far above their need, makes them ideal bioindicators and biomonitors of air pollution (see, e.g., references 7, 19, and 52).
Metabolic activation of molecular oxygen frequently results in production of reactive oxygen species (ROS) (25). ROS are formed by normal metabolic activities such as respiration and photosynthesis, but their production is enhanced during stresses such as nutrient limitation, exposure to xenobiotics, or desiccation and rehydration. To evade the potential damaging effects of ROS, cells have evolved protection mechanisms, including antioxidant enzymes, such as superoxide dismutase and catalase, as well as low-molecular-weight antioxidants, such as glutathione, ascorbic acid, tocopherol, and carotenoids (25).
Nitric oxide (NO) is an intra- and intercellular signaling molecule involved in the regulation of diverse biochemical and physiological processes. In algae NO formation was reported to be involved in phototaxis (39) and stress responses (10, 41), and in fungi it is related to processes such as growth (40, 63) and formation of fruiting bodies (59).
Lichens are poikilohydric organisms, many of which spend most of their life in a dry, metabolically inactive state and are able to survive for prolonged periods when their thallus water content (WC) is at or below 10% of their dry weight (4, 53). In most organisms, desiccation is associated with production of ROS and associated deleterious effects (46). However, cycles of desiccation and rehydration are part of the natural life of many lichens, and apparently these organisms have adapted to cope with such changes. Desiccation stress in lichens imposed by exposure to dry air or to an osmotic stress shows features similar to reversible photoinhibition (9, 22). It is accompanied by total inactivation of photosynthetic gas exchange and loss of variable chlorophyll fluorescence (35, 55). Rewetting of the thalli with liquid water normally restores photosynthetic activity within minutes (12, 38). Moreover, many studies have shown that green algal lichens are able to regain photosynthetic activity by uptake of water from humid air (reviewed in reference 36). Most studies on desiccation and rehydration of lichens focused on photosynthetic activity and respiration (see, e.g., references 12 and 38), but there are only few reports on the involvement of ROS and antioxidant response in these organisms (3, 7, 13, 27-31, 43, 44, 58). Such studies are interesting in relation to the mechanism that governs the special adaptation of lichens to survive under extreme water conditions. Moreover, advancing our understanding of cellular changes that take place upon changes in the WC of the thalli is important, since application of desiccation and rehydration is a common practice in lichen research, as studies of their physiology and biochemistry have to be done, in many cases, when they are metabolically active in the hydrated state (13, 58).
There are some studies in which cellular levels of antioxidants were compared in freshly collected lichens and during wetting and drying cycles of lichens normally growing in moist, xeric, and extremely xeric microhabitats (8, 9, 28, 31, 43). The glutathione status was shown to vary between species differing in their desiccation tolerance in response to desiccation or rehydration, and the xanthophyll cycle was found to play an antioxidant function during desiccation of lichens (8, 9, 28, 29, 31). Cellular activities of the antioxidant enzymes ascorbate peroxidase, catalase, and superoxide dismutase as well as the auxiliary enzymes glutathione reductase and glucose-6-phosphate dehydrogenase were shown to increase, decrease, or remain unchanged in response to desiccation and rehydration, depending on the species and the experimental conditions.
Ramalina lacera (With.) J. R. Laund. is a moderately xeric epiphytic fruticose (shrub-like) lichen that grows in the Mediterranean areas of Israel on different shrubs and trees. Its thallus contains an ascomycetous fungus and a trebouxioid unicellular green alga. In its natural habitat it may encounter long days of desiccation and be rehydrated by dew during the night or by rain during the rainy season. This lichen has been used extensively in our laboratory in ecophysiological studies on the effect of air pollution on lichens (see, e.g., references 17 and 18).
The objective of this work was to study the effects of rehydration under controlled conditions on ROS-related parameters in naturally desiccated R. lacera. We hypothesized that the rapid initiation of metabolic activities as a result of rehydration is accompanied by production of ROS and consequently by alterations in the antioxidant status of the thallus. We showed that rehydration of R. lacera resulted in a rapid increase in photosynthesis. It was accompanied by a burst of intracellular production of ROS by both the photobiont and mycobiont, which was similar in the light and in the dark, as well as by alterations in low-molecular-weight antioxidant capacity. Rehydration of R. lacera was also accompanied by a burst of intracellular NO production in the fungal hyphae but not in the algae.
MATERIALS AND METHODS
Chemicals.
Phenylmethylsulfonyl fluoride, Triton X-100, Trizma base, leupeptin, 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), potassium persulfate, 2′,7′-dichlorofluorescein (DCF), 2′,7′-dichlorohydrofluorescin diacetate (DCFH-DA), pyrrolidinedithiocarbamate (PDTC), and 2-(4-carboxyphenyl)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide potassium salt (carboxy-PTIO) were purchased from Sigma (St. Louis, Mo.). 6-Hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (Trolox) was purchased from Aldrich (Steinheim, Germany). 4,5-Diaminofluorescein diacetate (DAF-2DA) and 4,5-diaminofluorescein (DAF-2T) were purchased from Alexis Biochemicals (Montreal, Canada).
Source and treatment of lichen material.
R. lacera was collected from the HaZorea forest (Ramot Menashe, northeast Israel), where it grows on twigs of carob trees (Ceratonia siliqua L.), and was frozen promptly in liquid nitrogen and stored at −80°C. In control experiments we compared all of the parameters tested in this work by running the experiments with freshly collected thalli as well as frozen thalli that were collected at the same time, and we found no effect of freezing (not shown). Prior to the experiments, lichen samples were weighed and defrosted for 30 min at room temperature. The lichen samples were immersed in deionized water for 5 min, wiped to remove excess water, and placed inside a growth chamber (25°C, 97% relative humidity [RH], 73 μmol of photons m−2 s−1) for various periods of time. At the end of each treatment, the lichen samples were frozen promptly in liquid nitrogen and stored at −80°C. To maintain high relative humidity inside the growth chamber, ambient air (55% RH, 23°C) was passed through the chamber at a constant rate of 1,063 ml min−1 over distilled water. This air stream also served to maintain a constant temperature and fresh atmosphere inside the growth chamber and to prevent water condensation on its inner walls and glass cover.
Determination of WC in lichen thalli.
The WC in the lichen thalli was determined by weighing four lichen samples (∼1 g) from each treatment and subtracting their dry weights, which were obtained by drying at 95°C overnight. Water content was expressed in percent as a difference between wet and dry weight relative to dry weight (36, 38).
Assessment of photosynthesis.
Photosynthesis was assessed by chlorophyll fluorescence measurements with a pulse-modulated fluorescence meter (Diving PAM; Walz, Effeltrich, Germany). The lichens were rehydrated by immersion in distilled water for 5 min. Subsequently, the thalli were wiped, fixed onto “light leaf” clips, and placed inside the growth chamber (light intensity of 73 μmol of photons m−2 s−1). A flash of white light (12,000 μmol of photons m−2 s−1) was administered every 5 min through an optical fiber at a 45° angle relative to the thallus surface. The apparent electron transport rate (ETR) through photosystem II (PSII) was calculated as the effective quantum yield of PSII in the light (ΔF/Fm′) multiplied by the incident irradiance in units of PARI (quantum flux density of photosynthetically active radiation [micromoles of photons meter−2 second−1]) (20, 54), with the assumption that irradiance is fully absorbed by the thalli, where ΔF is the fluorescence yield reached during the saturation pulse (Fm′) minus the fluorescence yield shortly before triggering of the saturation pulse (Ft).
Assessment of in vivo ROS and NO production.
In vivo ROS and NO production in the lichen thalli was estimated by using the probe DCFH-DA (11) and fluorophore 4,5-diaminofluorescin diacetate DAF-2DA (26), respectively, by imaging with a laser-scanning confocal microscope and, quantitatively, by estimating fluorescence in crude extracts. DCFH-DA is a nonpolar, nonfluorescent compound that readily diffuses across membranes. Within the cell, it is hydrolyzed by esterases to the polar, nonfluorescent, membrane-impermeable derivative DCFH. DCFH is rapidly oxidized by ROS to the highly fluorescent DCF (2). DAF-2DA is a nonfluorescent compound that can permeate readily into the cells, where it is hydrolyzed by intracellular esterases to generate DAF-2, which interacts with NO to form the fluorescent triazole derivative DAF-2T.
(i) Laser-scanning confocal microscopy.
A laser-scanning confocal microscope (LSM 510; Carl Zeiss, Jena, Germany) with an air-cooled, argon-ion laser as the excitation source at 488 nm was used to view the sites of ROS and NO production. For assessment of ROS, samples of 0.5 g of thalli were immersed in 20 ml of 10 μM DCFH-DA in water for 5 min, wiped to remove excess solution, and placed inside the growth chamber (25°C, 97% RH, 73 μmol of photons m−2 s−1). At the end of the incubation period, the thalli were rinsed briefly with deionized water to remove any solution from the surface of the thalli, wiped to remove excess water, and frozen promptly in liquid nitrogen. Samples treated with water alone served as controls. Images were obtained with a C-Apochromat 40×/1.2W objective lens. The beam splitter was set at 570 nm. DCF was detected in the green channel through a 505- to 550-nm band-pass filter. Chlorophyll was detected in the red channel through a 585-nm long-pass filter. The laser intensity was identical for all experiments and was set at 5%. Data were collected by a computer attached to the instrument, stored on the hard drive, processed with a Zeiss LSM Image Browser, and transferred to Adobe Photoshop 5.0 for preparation of figures. For in vivo imaging of NO formation in R. lacera, 1-cm2 thallus pieces were placed on microscope slides. One hundred microliters of 10 μM DAF-2DA in deionized water was applied to the thallus fragments. Using the parameters mentioned above for DCF, consecutive images of a specific area on each sample were obtained immediately after application of DAF-2DA and every 3 min, for a total of 30 min.
(ii) Quantitative fluorescence measurements.
The kinetics of intracellular formation of ROS and NO, using fluorescence of DCF and DAF-2T, was monitored in rehydrated lichens. Samples of 0.5 or 0.1 g of thalli were immersed for 5 min in 20 ml of 10 μM DCFH-DA or DAF-2DA in water, respectively; wiped to remove excess solution; and placed inside the growth chamber in light or darkness. At the end of the incubation period, the thalli were rinsed briefly with deionized water to remove any solution from their surfaces, wiped to remove excess water, and frozen promptly in liquid nitrogen. Samples treated with water alone served as controls. Each sample was ground with liquid nitrogen by using a mortar and pestle and suspended in 2.5 ml of 40 mM Tris (pH 6.8) containing 0.1% Triton X-100. The slurry was passed twice through a French pressure cell and centrifuged at 3000 × g at 4°C for 30 min, and the resulting supernatant was centrifuged at 226,000 × g at 4°C for 1 h. Fluorescence in the latter supernatant was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm (FL500 microplate fluorescence reader; Bio-Tek Instruments, Winooski, Vt.). Fluorescence levels were corrected by subtracting the fluorescence of untreated lichen extracts and quantified by using a standard curve prepared with DCF or DAF-2T diluted in the same buffer as the samples. Fluorescence of DCF was linear in the range of 0 to 5 μM, and fluorescence of DAF-2T was linear up to 100 nM, and the detected fluorescence in our samples was within this range.
Assessment of the integrity of cell membranes.
Impairment of membrane integrity was used as an index of cellular damage. It was assessed by immersing the thalli in water and measuring the electrical conductivity, which expresses electrolyte leakage (17, 48). Thalli were rinsed briefly with double-distilled water at a temperature of 20°C to eliminate dust, leaf debris, insects, etc., and allowed to dry at room temperature. Photosynthesis was not induced due to this brief rinsing. Samples of 1 g were immersed in a beaker containing 100 ml of double-distilled water stirred with magnetic stirrer. A nylon mesh was placed inside the beaker in order to separate one part containing the thalli and a second part in which conductivity was measured. To avoid physical damage to the thalli due to stirring that could result in release of intracellular ions into the water, the stirrer was placed inside the beaker with avoidance of any direct contact with the thalli during the experiment. The electrical conductivity of the water was measured every 2 min for a period of 30 min with an electrical conductivity meter (TH-2400; El-Hamma Instruments, Mevo Hamma, Israel). Electrical conductivity values were corrected by subtracting the values for water alone.
Preparation of cell extracts and assessment of water-soluble low-molecular-weight antioxidants.
For assessment of water-soluble low-molecular-weight antioxidants, thalli were ground with liquid nitrogen by using a mortar and pestle and suspended in 0.1 M sodium phosphate buffer (pH 7.4) containing 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin ml−1, 1.5 mM EDTA, and 0.1% Triton X-100. The slurry was passed twice through a French pressure cell and centrifuged at 226,000 × g at 4°C for 1 h, and the supernatant was collected, frozen promptly in liquid nitrogen, and stored at −80°C. The protein concentration was determined by Bradford's method (5) with bovine serum albumin as a standard. Total water-soluble low-molecular-weight antioxidants were assessed by the Trolox equivalent antioxidant capacity (TEAC) assay with ABTS (50), and TEAC values were normalized to protein.
Statistical analysis.
The results were evaluated with SPSS software version 10. One-way analysis of variance (ANOVA) and Tukey honestly significantly different (HSD) tests were applied to determine the significance (P < 0.05) of the differences between the treatments.
RESULTS
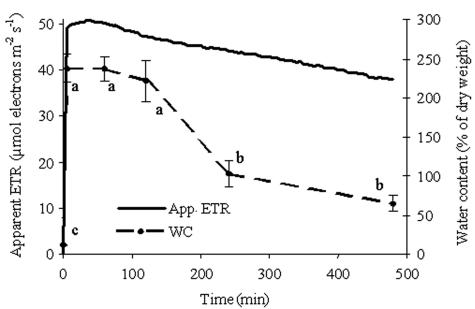
To monitor the resuscitation of R. lacera under our experimental conditions of rehydration and to adjust the conditions inside the growth chamber in order to set up the experimental design, we monitored the changes in photosynthetic activity and WC in lichen thalli. Dry thalli did not exhibit photosynthetic activity, as assessed by measurement of apparent ETR, whereas rehydration of the thalli and incubation at 97% relative humidity resulted in a rapid increase in this activity that reached a peak within a few minutes and subsequently started to decline slowly (Fig. 1). The apparent ETR reached 49 μmol of electrons m−2 s−1 after 5 min of immersion in water, which was 96% of its maximum value. This activity increased to its highest value during the following 35 min and then declined slowly, reaching a value of 38 μmol of electrons m−2 s−1 after 8 h. These changes in photosynthetic activity followed a pattern similar to that of the WC of the thalli (Fig. 1). At the termination of 5 min of immersion in water, the WC of the thalli increased from 11% in the dry state to 237% (P < 0.001), and it retained this value up to 60 min after immersion. Subsequently it started to decline, reaching 65% at the end of the experiment. Photosynthesis increased concurrently with an increase in WC of the thalli up to a WC of 90 to 100%, and it remained at the same value up to a WC of 237% (not shown). Based on these results as well as additional experiments conducted with different immersion times, incubation times, and RH conditions in the growth chamber (not shown), we have found that 5 min of immersion followed by 8 h of incubation at 97% RH were the optimal experimental conditions that resulted in the highest photosynthetic activity which lasted the longest.
FIG. 1.
Effective quantum yield of PSII expressed as apparent (App.) ETR and WC in untreated and rehydrated R. lacera placed in the growth chamber. Lichen samples were immersed in deionized water for 5 min., wiped to remove excess water, and placed inside a growth chamber (25°C, 97% RH, 73 μmol of photons m−2 s−1). Measurements were taken every 5 min with a pulse amplitude modulation fluorometer. Results for the apparent ETR are expressed as the means from four independent experiments. Results for WC are expressed as means ± standard deviations (n = 4). The values for treatments indicated by different letters differ significantly by one-way ANOVA and the Tukey HSD test (P < 0.001; F ratio = 139.063).
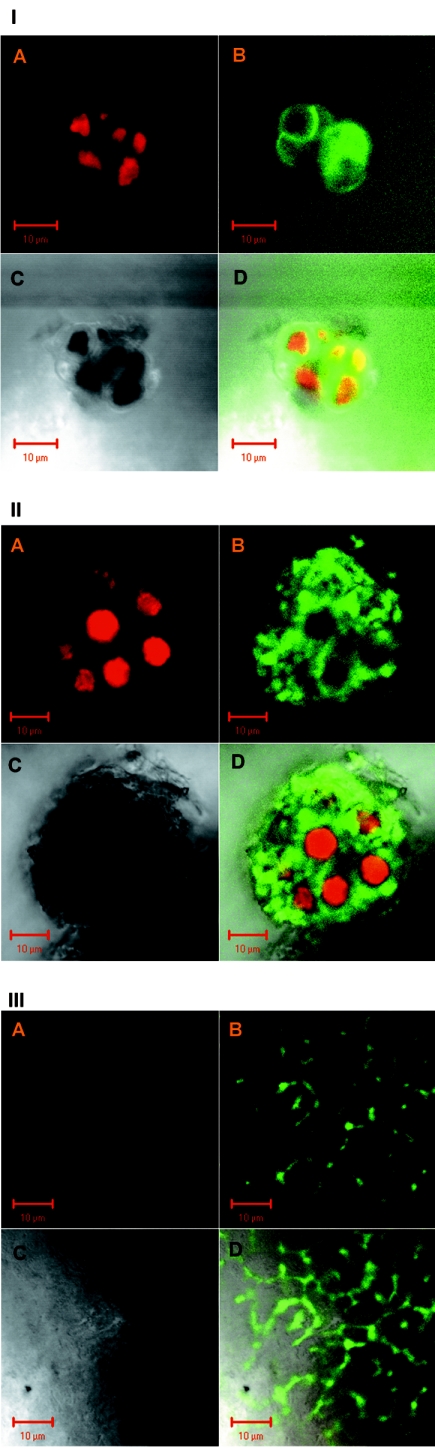
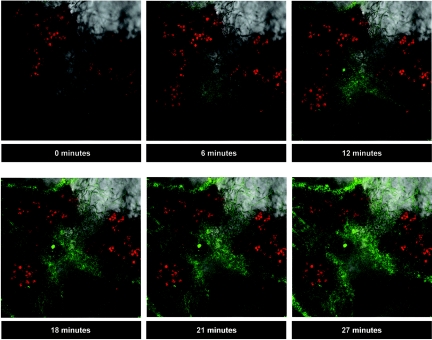
ROS were produced intracellularly upon rehydration, by both the fungi and the algae, as was visualized by laser-scanning confocal microscopy with the probe DCFH-DA (11) (Fig. 2). DCF fluorescence was detected inside algal cells at the end of 5 min of rehydration (Fig. 2I) and increased in intensity as seen at the end of 2 h incubation in the growth chamber (Fig. 2II). High-intensity DCF fluorescence was also detected inside fungal hyphae surrounding algal cells (Fig. 2II) or independently of their presence, as seen in an image of the lichen's cortex, where no algal cells are present (Fig. 2III).
FIG. 2.
Laser-scanning confocal microscopy images of R. lacera rehydrated for 5 min in the presence of DCFH-DA and incubated in the growth chamber. Samples of 0.5 g of thalli were immersed in 20 ml of 10 μM DCFH-DA in water for 5 min, and placed in the growth chamber (25°C, 97% RH) in light (73 μmol of photons m−2 s−1). (A) Red channel, showing chlorophyll fluorescence in chloroplasts of the algal cells; (B) green channel, showing DCF fluorescence; (C) natural image; (D) A, B, and C combined. Bars, 10 μm. (I) DCF fluo-rescence within algal cells after 15 min in the growth chamber. (II) High-level DCF fluorescence within algal cells and fungal hyphae after 2 h in the growth chamber. (III) DCF fluorescence after 2 h in the growth chamber within a network of cortical fungal hyphae, where no algal cells are present.
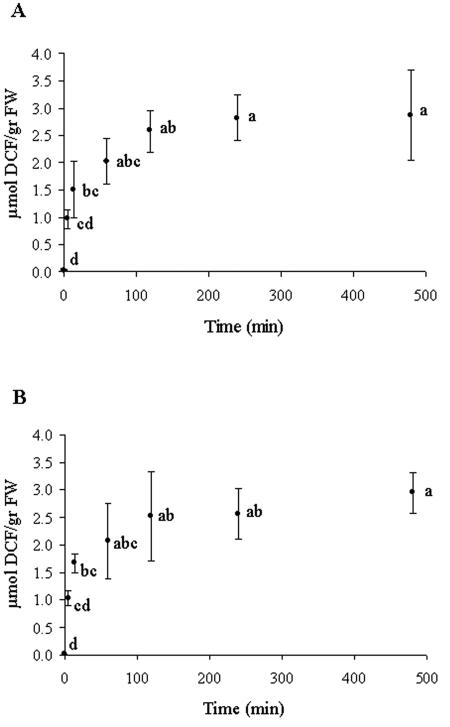
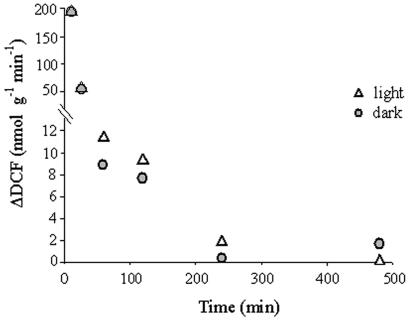
ROS production was assessed quantitatively by using the probe DCFH-DA. ROS production was found to have an appearance of an oxidative burst that was similar in the light and in the dark (Fig. 3). It reached 34% of the maximum accumulated levels of 2.87 and 2.95 μmol of DCF/g (fresh weight) in the light and in the dark, respectively, at the end of 5 min of immersion in water and reached 50% within 15 min. (P < 0.001) (Fig. 3), but the rate declined continuously until the end of the experiment (Fig. 4). The DCF formation rate at the end of 15 min and 1 h of incubation in the growth chamber declined to 28 to 31% and 4.5 to 5.7% of the rate at the end of 5 min of rehydration, respectively, and reached less than 1% of that rate at the end of 4 h. Addition of a 10 μM concentration of the antioxidant PDTC (24) to the double-distilled water during rehydration resulted in lower (65%) fluorescence of DCF (not shown), indicating that this reaction was due to interaction with ROS.
FIG. 3.
Relative fluorescence of DCF in crude extracts of untreated and rehydrated R. lacera. Samples of 0.5 g of thalli were immersed in 20 ml of 10 μM DCFH-DA in water for 5 min and placed in the growth chamber (25°C, 97% RH) in light (73 μmol of photons m−2 s−1) (A) or darkness (B). Results are expressed as means ± standard deviations (n = 4). The values for treatments indicated by different letters differ significantly by one-way ANOVA and the Tukey HSD test. (A) P < 0.001; F ratio = 15.505. (B) P < 0.001; F ratio = 17.536.
FIG. 4.
Calculated rates of DCF formation in rehydrated R. lacera. Samples of 0.5 g of thalli were immersed in 20 ml of 10 μM DCFH-DA in water for 5 min and placed in the growth chamber (25°C, 97% RH) in light (73 μmol of photons m−2 s−1) or dark. Results are expressed as means of four repeats. ΔDFC was normalized to the fresh weight of the sample.
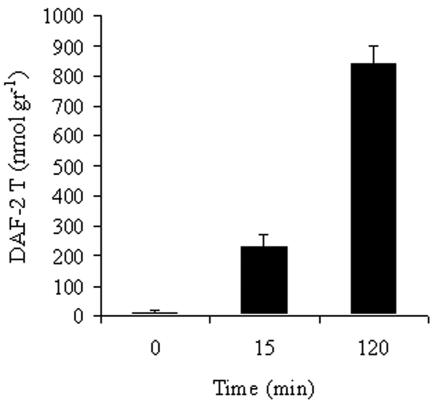
Rehydration of the thalli resulted in intracellular production of nitric oxide (NO), as visualized with the NO-sensitive fluorophore DAF-2DA (27) Using laser-scanning confocal microscopy, NO was detected in the fungal hyphae but not in the algal cells (Fig. 5). Quantitative estimation of NO production with the same probe showed that NO production lasted at least 2 h and reached an accumulated level of 842 nmol of DAF-2T/g (fresh weight) (Fig. 6). Addition of 150 μM carboxy-PTIO, a scavenger of nitric oxide (14, 16), to the double-distilled water during rehydration resulted in lower (68%) fluorescence of DAF-2T (not shown), indicating that fluorescence of DAF-2T is specific for NO and is not a general response to free radicals.
FIG. 5.
In vivo imaging of the kinetics of nitric oxide production in R. lacera upon rehydration, using the fluorescent probe DAF-2DA in conjunction with laser-scanning confocal microscopy. Red channel, chlorophyll fluorescence in chloroplasts of the algal cells; green channel, DAF-2T fluorescence. Thallus pieces (1 cm2) were placed on microscope slides. One hundred microliters of 10 μM DAF-2DA in deionized water was applied to the thallus fragments. Scale: 1 cm = 55 μm. DAF-2T fluorescence is localized within fungal hyphae.
FIG. 6.
Relative fluorescence of DAF-2T in crude extracts of untreated and rehydrated R. lacera. Samples of 0.5 g of thalli were immersed in 20 ml of 10 μM DAF-2DA in water for 5 min and placed in the growth chamber (25°C, 97% RH) in light (73 μmol of photons m−2 s−1). Results are expressed as means ± standard deviations (n = 4). DAF-2T was normalized to the fresh weight of the sample.
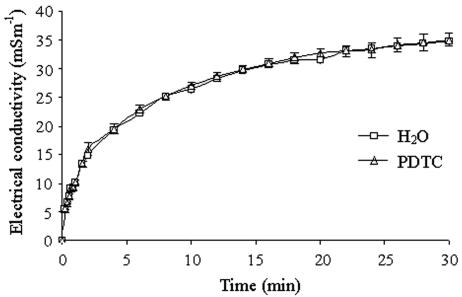
To check whether rehydration of the lichen thalli resulted in membrane damage, we assayed electrolyte leakage. Figure 7 shows that immersing the lichen in water resulted in a high initial rate of increase in the electrical conductivity, reaching 43% of the apparent maximal electrical conductivity value after 2 min, and a subsequent lower rate, reaching 56, 76, and 90% of the maximal value after 4, 10, and 20 min, respectively (P < 0.001). The rate decreased gradually until the end of this experiment after 30 min. To check whether this damage was due to ROS produced during rehydration, we added the antioxidant PDTC. The addition of 10 μM PDTC, which was found to inhibit ROS formation by 35%, did not affect electrical conductivity values (Fig. 7).
FIG. 7.
Electrolyte leakage from rehydrated R. lacera measured as the electrical conductivity (mSm−1) of double-distilled water with or without 10 μM PDTC, in which lichen samples were immersed. Results are expressed as means ± standard deviations (n = 3).
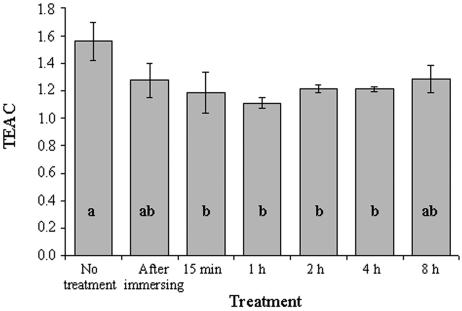
Rehydration of R. lacera resulted in a decrease in the water-soluble low-molecular-weight antioxidant capacity of the thalli, as estimated by the TEAC method (Fig. 8) (50). It decreased 18 and 24% (P = 0.004) compared with the dry-state values within, respectively, 5 and 15 min of rehydration. TEAC values remained at this low level up to the end of the experiment at 8 h.
FIG. 8.
TEAC values of total water-soluble low-molecular-weight antioxidants in untreated and rehydrated R. lacera. Lichen samples were immersed in deionized water for 5 min., wiped to remove excess water, and placed inside a growth chamber (25°C, 97% RH, 73 μmol of photons m−2 s−1) for the indicated durations. Results are expressed as means ± standard deviations (n = 4) and normalized to protein. The values for treatments indicated by different letters differ significantly by one-way ANOVA and the Tukey HSD test (P = 0.004; F ratio = 6.196).
DISCUSSION
In this paper we present findings on the effects of rehydration on the lichen R. lacera. We showed that treatment with water of thalli that were collected from their natural habitat in a dry state and their maintenance at 97% RH caused a rapid increase in photosynthesis. This was accompanied by a burst of intracellular production of ROS by both symbionts, the formation of nitric oxide by the fungus, and a decrease in water-soluble low-molecular-weight antioxidants. These potential deleterious activities did not cause measurable membrane damage.
There are various reports in the literature on the effects of rehydration on lichens. Most of these studies reported changes in photosynthetic activity and respiration (see, e.g., references 12 and 38), while only few publications dealt with ROS-related parameters (3, 7, 13, 27-31, 43, 44, 58). Furthermore, in our experiments we used thalli in their naturally dried state, while in other studies the thalli were pretreated by at least one cycle of exposure to liquid water or water vapor, which was preceded or followed by desiccation, prior to the actual experiment. Based on findings by others and those reported here, baseline values of the various parameters may have been altered by these pretreatments, which may have affected the results and the conclusions.
Measurement of chlorophyll fluorescence is a convenient tool for estimation of photosynthetic activity (32). It allows analysis of the status of the photosynthetic apparatus of lichens in situ (36) and was previously used to assess the vitality of lichens (17, 18). In the present study, we used this technique to monitor resuscitation of R. lacera upon rehydration, in order to obtain a time course for the metabolic changes that take place during rehydration. We showed that rehydration of R. lacera and incubation at 97% RH resulted in a fast resumption of photosynthetic activity, reaching 96% of the maximal apparent ETR values within 5 min, with complete induction after 40 min. The kinetics of this response is similar to that found for other lichens that contain Trebouxia photobionts, such as Cetraria islandica (47) and Hypogymnia physodes (23), suggesting that the time scale for resuscitation of 10 to 40 min is common among this type of lichen. We also found that the levels of apparent ETR in R. lacera following rehydration increased with increasing WC up to 100%, and there was no further increase at WCs of up to 237%. The water content of lichens, as well as photosynthesis as a function of WC, varies greatly between species and depends on the experimental conditions. In some lichen species, maximum values of chlorophyll fluorescence may show little change at a WC of above 56% (62). However, in general, net photosynthesis in lichens increases with increasing WC, until maximal rates are reached, followed by no further change (21, 34, 37, 61) or by a depression (21, 37) with additional increase of the WC. These variations may be related to the morphology of the lichens, which is determined by the nature of the symbionts.
We found that rehydration of R. lacera resulted in intracellular production of ROS, the rate of which was significant in the first 30 min and then declined to a very low level. This activity was similar in the light and in the dark, suggesting a minor contribution of photosynthetic processes to the total production of these oxygen metabolites. It was shown (60) that when dry lichen thalli are rehydrated, three distinct stages are apparent: a large and rapid nonmetabolic release of CO2, which lasts for a few minutes, followed by high rate of “resaturation respiration,” which gradually declines to a constant steady-state rate. The duration and amplitude of these activities vary among species. In species that, like R. lacera, contain Trebouxia sp. photobionts (C. islandica, H. physodes, Lasallia pustulata, and Platismatia glauca) the initial respiration rate was always 1.5 to 3 times higher than that in the final steady state that was reached within 3 to 7 h after immersion in water (60). It was proposed that the initially high respiration rate is the result of uncoupling of the mitochondrial electron transport chain or of a burst in respirable substrates related to drought damage of membranes. We propose that ROS are generated in R. lacera as a result of these transient imbalanced cellular activities during resuscitation. This “rearrangement” in metabolism may result in uncontrolled dehydrogenation of substrates, leading to excessive production of reducing equivalents. Some of these reduced molecules may react with molecular oxygen, giving rise to ROS by auto-oxidation or via the uncoupled mitochondrial electron transport chain. Our findings on the initial kinetics of resumption of photosynthesis and ROS production in R. lacera suggest a cause-and-effect relationship between these activities. The onset of photosynthesis induces metabolic activities (12, 47), which supply reduced substrates for both partners of the lichen symbiosis, and oxidation of these substrates is the source of ROS. After photosynthesis reaches steady-state rates, cellular metabolism stabilizes and ROS production declines to that of normal metabolic activity. Our finding that light did not enhance ROS production may be explained simply by the fact that algal cells comprise only 10 to 20% of the thalli. However, it is also possible that reducing equivalents generated by photosynthetic electron transport are utilized by the chloroplasts for carbon fixation before they interact with O2.
To the best of our knowledge, there are only two previous publications on production of ROS upon rehydration of lichens (3, 44). Minibayeva and Beckett (44) reported that some lichen species in the suborder Peltigerineae, but not others, such as members of the genus Ramalina, responded to rehydration in the light by producing a burst of extracellular superoxide. They proposed that it functions in defense against pathogenic fungi and bacteria (3, 44). At this stage we cannot rule out extracellular production of ROS by R. lacera, because in our experimental system we used the probe DCFH-DA, which does not interact with ROS unless the diacetate group is removed, which requires the enzymatic action of an esterase. Since, as far as we know, there are no extracellular esterases in lichens, this probe cannot be used for extracellular detection of ROS. A major obstacle in lichen research at the biochemical and molecular levels is the ability to distinguish between the fungus and the alga. Although there are a few techniques for cultivation of free-living symbionts, the separated fungi and the algae were shown to differ significantly from their lichenized counterparts in morphology, reproductive strategies, cellular and subcellular structures, cytochemical properties, secondary metabolites, and phenolics and fatty acids (6, 15, 45). Thus, the ideal experimental system should enable investigation of each of the symbionts while in the lichenized state. Such experimental systems may involve noninvasive techniques, for example, the use of fluorescent probes in conjunction with confocal microscopy. Unlike in other studies, by using the confocal microscope, we were able to show both the organismal and the cellular origins of ROS in a lichen.
We showed that rehydration of R. lacera caused a burst of NO production that was detected only in the fungal hyphae. NO, which was detected in most eukaryotes, exhibits multiple physiological functions, mainly in signal transduction and regulatory pathways (33), but to the best of our knowledge this is the first report on NO production by a lichen. In “free-living” fungi, the involvement of NO was related to processes such as growth (40, 63) and formation of fruiting bodies (59). In R. lacera, NO may serve similar functions or similar regulatory roles.
In this study we assessed electrolyte leakage from the thalli as an estimation of membrane damage (17, 48) due to the action of ROS. We found significant electrolyte leakage upon rehydration, but 35% inhibition of ROS formation in the presence of an antioxidant did not change its rate or extent. This suggests that the transient production of ROS did not inflict apparent membrane damage, which may reflect a protection mechanism that enables this lichen to survive continuous cycles of desiccation and rehydration. The high initial rate of electrolyte leakage can be the result of membrane damage that occurred during desiccation prior to rehydration, while the later slow release of electrolytes may be due to the simple effect of immersion and hypo-osmotic shock induced by deionized water (1). There are some studies with higher plants and with mosses that documented increased membrane permeability due to water deficit conditions in conjunction with an increase in ROS production (42, 49, 56). Alternatively, our findings may also indicate that the 65% of the ROS that escaped inhibition by the antioxidant were enough to cause transient membrane damage. To the best of our knowledge, there are no reports in the literature on the possible damaging effects of rehydration on lichen membranes.
Rehydration of R. lacera caused a fast decrease in water-soluble, low-molecular-weight antioxidants. This suggests that in desiccated thalli their concentration was high relative to that in the rehydrated state and that it decreased due to production of ROS upon rehydration, as indicated by the correlation between the kinetics of ROS production and the decrease in antioxidant capacity. Previous studies with lichens and resurrection plants focused on glutathione and ascorbic acid as the major water-soluble low-molecular-weight antioxidants. Those studies showed that both glutathione and ascorbic acid increased, decreased, or did not change, depending on the desiccation tolerance of the organism as well as the mode and duration of the treatment. For example, desiccation of the lichens Pseudevernia furfuracea, Lobaria pulmonaria, and Peltigera polydactyla, which differ in their desiccation tolerances, caused a decrease in reduced glutathione (GSH) and an increase in oxidized glutathione, which according to the authors was probably due to the oxidation of GSH by ROS generated during desiccation (30). Following long-term desiccation of P. furfuracea, a desiccation-tolerant lichen, GSH increased rapidly when the lichen was rehydrated in liquid water or water vapor. L. pulmonaria, an intermediate desiccation-tolerant lichen, regenerated initial concentrations of GSH only when rehydrated in liquid water, while in P. polydactyla, neither method of rehydration reestablished the initial GSH pool (27). In our system, similar to the case for other lichens (27-31), ascorbate and glutathione probably contribute significantly to water-soluble antioxidant capacity. The difference between our studies and others and among the others may be explained, as mentioned above, by the choice of species and the variability in the methodologies, particularly in the various pretreatments of the thalli in the laboratory or in performing the rehydration experiments.
In summary, this publication is the first to report that initiation of metabolic activities by rehydration of the lichen R. lacera is accompanied by bursts of intracellular ROS and NO production. ROS were produced by both symbionts, but the contribution of algal photosynthesis was negligible, and NO was generated only by the fungus. These activities did not cause membrane damage, but resulted in a decrease in low-molecular-weight antioxidants.
Acknowledgments
This research was supported by grant 2001238 from the United States-Israel Binational Science Foundation (BSF), Jerusalem, Israel.
REFERENCES
- 1.Bajji, M., J. M. Kinet, and S. Lutts. 2002. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 36:61-70. [Google Scholar]
- 2.Bass, D. A., J. W. Parce, L. R. Dechatelet, P. Szejda, M. C. Seeds, and M. Thomas. 1983. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J. Immunol. 130:1910-1917. [PubMed] [Google Scholar]
- 3.Beckett, R. P., F. V. Minibayeva, N. N. Vylegzhanina, and T. Tolpysheva. 2003. High rates of extracellular superoxide production by lichens in the suborder Peltigerineae correlate with indices of high metabolic activity. Plant Cell Environ. 26:1827-1837. [Google Scholar]
- 4.Bewley, J. D. 1979. Physiological aspects of desiccation tolerance. Annu. Rev. Plant Physiol. 30:195-238. [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Bubrick, P. 1988. Effects of symbiosis on the photobiont, p. 133-144. In M. Galun (ed.), Handbook of lichenology, vol. II. CRC Press, Boca Raton, Fla. [Google Scholar]
- 7.Calatayud, A., V. I. Deltoro, A. Abadia, J. Abadia, and E. Barreno. 1999. Effects of ascorbate feeding on chlorophyll fluorescence and xanthophyll cycle components in the lichen Parmelia quercina (Willd.) Vainio exposed to atmospheric pollutants. Physiol. Plant 105:679-684. [Google Scholar]
- 8.Calatayud, A., V. I. Deltoro, E. Barreno, and S. del Valle-Tascon. 1997. Changes in in vivo chlorophyll fluorescence quenching in lichen thalli as a function of water content and suggestion of zeaxanthin-associated photoprotection. Physiol. Plant. 101:93-102. [Google Scholar]
- 9.Chakir, S., and M. Jensen. 1999. How does Lobaria pulmonaria regulate photosystem II during progressive desiccation and osmotic water stress? A chlorophyll fluorescence study at room temperature and at 77 K. Physiol. Plant. 105:257-265. [Google Scholar]
- 10.Chen, K., H. Feng, M. Zhang, and X. Wang. 2003. Nitric oxide alleviates oxidative damage in the green alga Chlorella pyrenoidosa caused by UV-B radiation. Folia Microbiol. 48:389-393. [DOI] [PubMed] [Google Scholar]
- 11.Collen, J., and I. R. Davison. 1997. In vivo measurement of active oxygen production in the brown alga Fucus evanescens using 2′,7′-dichlorohydrofluorescein diacetate. J. Phycol. 33:643-648. [Google Scholar]
- 12.Coxson, D. S. 1988. Recovery of net photosynthesis and dark respiration on rehydration of the lichen Cladina mitis, and the influence of prior exposure to sulphur dioxide while desiccated. New Phytol. 108:483-487. [Google Scholar]
- 13.Deltoro, V. I., C. Gimeno, A. Calatayud, and E. Barreno. 1999. Effects of SO2 fumigations on photosynthetic CO2 gas exchange, chlorophyll a fluorescence emission and antioxidant enzymes in the lichens Evernia prunastri and Ramalina farinacea. Physiol. Plant 105:648-654. [Google Scholar]
- 14.Foissner, I., D. Wendehenne, C. Langebartels, and J. Durner. 2000. In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J. 23:817-824. [DOI] [PubMed] [Google Scholar]
- 15.Galun, M. 1988. Effects of symbiosis on the mycobiont, p. 145-151. In M. Galun (ed.), Handbook of lichenology, vol. II. CRC Press, Boca Raton, Fla. [Google Scholar]
- 16.Garces, H., D. Durzan, and M. C. Pedroso. 2001. Mechanical stress elicits nitric oxide formation and DNA fragmentation in Arabidopsis thaliana. Ann. Bot. 87:567-574. [Google Scholar]
- 17.Garty, J., L. Weissman, O. Tamir, S. Beer, Y. Cohen, A. Karnieli, and L. Orlovsky. 2000. Comparison of five physiological parameters to assess the vitality of the lichen Ramalina lacera exposed to air pollution. Physiol. Plant 109:410-418. [Google Scholar]
- 18.Garty, J., O. Tamir, I. Hassid, A. Eshel, Y. Cohen, A. Karnieli, and L. Orlovsky. 2001. Photosynthesis, chlorophyll integrity, and spectral reflectance in lichens exposed to air pollution. J. Environ. Qual. 30:884-893. [DOI] [PubMed] [Google Scholar]
- 19.Garty, J., Y. Cohen, and N. Kloog. 1998. Airborne elements, cell membranes, and chlorophyll in transplanted lichens. J. Environ. Qual. 27:973-979. [Google Scholar]
- 20.Genty, B., J. M. Briantais, and N. R. Baker. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990:87-92. [Google Scholar]
- 21.Green, T. G. A., and O. L. Lange. 1995. Photosynthesis in poikilohydric plants—a comparison of lichens and bryophytes, p. 319-341. In E. D. Schulze and M. M. Caldwell (ed.), Ecophysiology of photosynthesis. Springer, Berlin, Germany.
- 22.Jensen, M., and G. B. Feige. 1991. Quantum efficiency and chlorophyll fluorescence in the lichens Hypogymnia physodes and Parmelia sulcata. Symbiosis 11:179-191. [Google Scholar]
- 23.Jensen, M., S. Chakir, and G. B. Feige. 1999. Osmotic and atmospheric dehydration effects in the lichens Hypogymnia physodes, Lobaria pulmonaria and Peltigera aphtosa: an in vivo study of chlorophyll fluorescence induction. Photosynthetica 37:393-404. [Google Scholar]
- 24.Kobayashi, D., K. Kondo, N. Uehara, S. Otokozawa, N. Tsuji, A. Yagihashi, and N. Watanabe. 2002. Endogenous reactive oxygen species is an important mediator of miconazole antifungal effect. Antimicrob. Agent. Chemother. 46:3113-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohen, R., and A. Nyska. 2002. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 30:620-650. [DOI] [PubMed] [Google Scholar]
- 26.Kojima, H., N. Nakatsubo, K. Kikuchi, S. Kawahara, Y. Kirino, H. Nagoshi, Y. Hirata, and T. Nagano. 1998. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal. Chem. 70:2446-2453. [DOI] [PubMed] [Google Scholar]
- 27.Kranner, I. 2002. Glutathione status correlates with different degrees of desiccation tolerance in three lichens. New Phytol. 154:451-460. [DOI] [PubMed] [Google Scholar]
- 28.Kranner, I., and D. Grill. 1994. Rapid changes of the glutathione status and the enzymes involved in the reduction of glutathione-disulfide during the initial stage of wetting of lichens. Crypt. Bot. 4:203-206. [Google Scholar]
- 29.Kranner, I., and D. Grill. 1997. The glutathione status during recovery of desiccated lichens: is desiccation tolerance correlated with a high reducing capacity for glutathione disulphide?, p. 249-252. In W. J. Cram, L. J. De Kok, I. Stulen, C. Brunold, and H. Rennenberg (ed.), Sulphur metabolism in higher plants. Backhuys Publishers, Leiden, The Netherlands.
- 30.Kranner, I., and D. Grill. 1997. Desiccation of lichens: changes in the glutathione status, p. 253-255. In W. J. Cram, L. J. De Kok, I. Stulen, C. Brunold, and H. Rennenberg (ed.), Sulphur metabolism in higher plants. Backhuys Publishers, Leiden, The Netherlands.
- 31.Kranner, I., M. Zorn, B. Turk, S. Wornik, R. P. Beckett, and F. Batic. 2003. Biochemical traits of lichens differing in relative desiccation tolerance. New Phytol. 160:167-176. [DOI] [PubMed] [Google Scholar]
- 32.Krause, G. H., and E. Weis. 1984. Chlorophyll fluorescence as a tool in plant physiology. II. Interpretation of fluorescence signals. Photosynth. Res. 5:139-157. [DOI] [PubMed] [Google Scholar]
- 33.Landar, A., and V. M. Darley-Usmar. 2003. Nitric oxide and cell signaling; modulation of redox tone and protein modification. Amino Acids 25:313-321. [DOI] [PubMed] [Google Scholar]
- 34.Lange, O. L. 1969. Experimentell-okologische untersuchungen an flechten der Negev-Wuste. I. CO2-Gas-wechsel von Ramalina maciformis (Del.) Bory. Unter Kontrollierten Bedingungen im Laboratorium. Flora 158:324-359. [Google Scholar]
- 35.Lange, O. L., and J. D. Tenhunen. 1982. Water relations and photosynthesis of desert lichens. J. Hattori Bot. Lab. 53:309-313. [Google Scholar]
- 36.Lange, O. L., T. G. A. Green, and U. Heber. 2001. Hydration-dependent photosynthetic production of lichens: what do laboratory studies tell us about field performance? J. Exp. Bot. 52:2033-2042. [DOI] [PubMed] [Google Scholar]
- 37.Lange, O. L., T. G. A. Green, and H. Reichenberger. 1999. The response of lichen photosynthesis to external CO2 concentration and its interaction with thallus water-status. J. Plant Physiol. 154:157-166. [Google Scholar]
- 38.Lange, O. L., W. Bilger, S. Rimke, and U. Schreiber. 1989. Chlorophyll fluorescence of lichens containing green and blue-green algae during hydration by water vapor uptake and by addition of liquid water. Bot. Acta 102:306-313. [Google Scholar]
- 39.Lobysheva, I. I., A. F. Vanin, O. A. Sineshchekov, and E. G. Govorunova. 1996. Phototaxis in Chlamydomonas reinhardtii is modulated by nitric oxide. Biofizika 41:538-541. [Google Scholar]
- 40.Maier, J., R. Hecker, P. Rockel, and H. Ninnemann. 2001. Role of nitric oxide synthase in the light-induced development of sporangiophores in Phycomyces blakesleeanus. Plant Physiol. 126:1323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mallick, N., F. H. Mohn, C. J. Soeder, and J. U. Grobbelaar. 2002. Ameliorative role of nitric oxide on H2O2 toxicity to a chlorophycean alga Scenedesmus obliquus. J. Gen. Appl. Microbiol. 48:1-7. [DOI] [PubMed] [Google Scholar]
- 42.Mayaba, N., F. Minibayeva, and R. P. Beckett. 2002. An oxidative burst of hydrogen peroxide during rehydration following desiccation in the moss Atrichum androgynum. New Phytol. 155:275-284. [Google Scholar]
- 43.Mayaba, N., and R. P. Beckett. 2001. The effect of desiccation on the activities of antioxidant enzymes in lichens from habitats of contrasting water status. Symbiosis 31:113-121. [Google Scholar]
- 44.Minibayeva, F., and R. P. Beckett. 2001. High rates of extracellular superoxide production in bryophytes and lichens, and an oxidative burst in response to rehydration following desiccation. New Phytol. 152:333-341. [Google Scholar]
- 45.Molina, M. C., A. Crespo, C. Vicente, and J. A. Elix. 2003. Differences in the composition of phenolics and fatty acids of cultured mycobiont and thallus of Physconia distorta. Plant Physiol. Biochem. 41:175-180. [Google Scholar]
- 46.Oliver, M. J., and J. D. Bewley. 1997. Desiccation-tolerance of plant tissues: a mechanistic overview. Hort. Rev. 18:171-213. [Google Scholar]
- 47.Palmqvist, K. 2000. Carbon economy in lichens. New Phytol. 148:11-36. [DOI] [PubMed] [Google Scholar]
- 48.Pearson, L. C., and G. A. Rodgers. 1982. Air pollution damage to cell membranes in lichens. III. Field experiments. Phyton 22:329-337. [Google Scholar]
- 49.Quartacci, M. F., F. Navari Izzo. 1992. Water-stress and free-radical mediated changes in sunflower seedlings. J. Plant Physiol. 139:621-625. [Google Scholar]
- 50.Re, R., N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, and C. Rice-Evans. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 26:1231-1237. [DOI] [PubMed] [Google Scholar]
- 51.Richardson, D. H. S. 1988. Medicinal and other economic aspects of lichens, p. 93-108. In M. Galun (ed.), Handbook of lichenology, vol. III. CRC Press, Boca Raton, Fla. [Google Scholar]
- 52.Rose, C. I., and D. L. Hawksworth. 1981. Lichen recolonization in London's cleaner air. Nature 289:289-292. [Google Scholar]
- 53.Rundel, P. W. 1988. Water relations, p. 17-36. In M. Galun (ed.), Handbook of lichenology, vol. II. CRC Press, Boca Raton, Fla. [Google Scholar]
- 54.Schroeter, B., L. G. Sancho, and F. Valladares. 1999. In situ comparison of daily photosynthetic activity patterns of saxicolous lichens and mosses in Sierra de Guadarrama, central Spain. Bryologist 102:623-633. [Google Scholar]
- 55.Schroeter, B., P. Jacobsen, and L. Kappen. 1991. Thallus moisture and microclimatic control of CO2 exchange of Peltigera aphthosa (L.) Willd. on Disko Island (West Greenland). Symbiosis 11:131-146. [Google Scholar]
- 56.Seel, W., G. Hendry, N. Atherton, and J. Lee. 1991. Radical formation and accumulation in vivo, in desiccation tolerant and intolerant mosses. Free Rad. Res. Commun. 15:133-141. [DOI] [PubMed] [Google Scholar]
- 57.Sgherri, C. L. M., B. Loggini, S. Puliga, and F. Navari-Izzo. 1994. Antioxidant system in Sporobolus stapfianus: changes in response to desiccation and rehydration. Phytochemistry 35:561-565. [Google Scholar]
- 58.Silberstein, L., B. Z. Siegel, S. M. Siegel, A. Mukhtar, and M. Galun. 1996. Comparative studies on Xanthoria parietina, a pollution-resistant lichen, and Ramalina duriaei, a sensitive species. II. Evaluation of possible air pollution-protection mechanisms. Lichenologist 28:367-383. [Google Scholar]
- 59.Song, N. K., C. S. Jeong, and H. S. Choi. 2000. Identification of nitric oxide synthase in Flammulina velutipes. Mycologia 92:1027-1032. [Google Scholar]
- 60.Sundberg, B., A. Ekblad, T. Nasholm, and K. Palmqvist. 1999. Lichen respiration in relation to active time, temperature, nitrogen and ergosterol concentrations. Funct. Ecol. 13:119-125. [Google Scholar]
- 61.Sundberg, B., K. Palmqvist, P. A. Esseen, and K. E. Renhorn. 1997. Growth and vitality of epiphytic lichens. II. Modelling of carbon gain using field and laboratory data. Oecologia 109:10-18. [DOI] [PubMed] [Google Scholar]
- 62.Tuba, Z., Z. Csintalan, and M. C. F. Proctor. 1996. Photosynthetic responses of a moss, Tortula ruralis, ssp. ruralis, and the lichens Cladonia convoluta and C. furcata to water deficit and short periods of desiccation, and their ecophysiological significance: a baseline study at present-day CO2 concentrations. New Phytol. 133:353-361. [DOI] [PubMed] [Google Scholar]
- 63.Wilken, M., and B. Huchzermeyer. 1999. Suppression of mycelia formation by NO produced endogenously in Candida tropicalis. Eur. J. Cell Biol. 78:209-213. [DOI] [PubMed] [Google Scholar]