Abstract
Background:
Malignant peritoneal mesothelioma (MPM) represents a rare clinical entity. The synchronous existence of MPM with other malignancies as colonic adenocarcinoma have been rarely reported. Its diagnosis and management are challenging given its complexity and rarity.
Objective:
Herein, we report a case of epithelioid subtype of MPM occurring synchronously with sigmoid colonic adenocarcinoma, along with review of the literature.
Case presentation:
An elderly female patient was referred as case of rectosigmoid mass. She reported history of abdominal pain, per-rectal bleeding, anorexia, and significant weight loss. Her computed-tomography scan of the abdomen revealed a fistulizing sigmoid mass and multiple enlarged lymphnodes with omental nodulation. The colonoscopy revealed a large fungating mass and the endoscopic biopsies were reported as colonic adenocarcinoma. The patient was scheduled laparoscopic low anterior resection. However, the diagnostic laparoscopy revealed several nodules disseminated all over the peritoneum, suggestive of peritoneal mesothelioma. Therefore, the decision was changed to create transverse colostomy after examination obtaining multiple biopsies from the omental and peritoneal nodules. The histopathological revealed MPM and the final diagnosis was sigmoid adenocarcinoma with synchronous MPM. The patient was started on palliative chemotherapy (capecitabine) without active management of MPM because of her general condition. She was followed up with a good clinical course.
Conclusion:
MPM is an overlooked entity with vague clinical presentation. Synchronous MPM with colorectal cancer is rare with only few published case reports. Its diagnosis is challenging, and its management should be tailored according to the patient. This case is the first reported case in Saudi Arabia and the Middle East.
Keywords: mesothelioma, colorectal cancer, sigmoid adenocarcinoma, asbestos, synchoronus malignancy
1. INTRODUCTION
Malignant mesothelioma represents a rare clinical entity, accounting for approximately 0.2% of all reported malignancies. Mesothelial cells of the pleural membrane are the preferred site of primary involvement for malignant mesothelioma. The peritoneal membrane, in particular, is a rare primary site of origin, constituting 5-10% of all malignant mesotheliomas (1). Several hypotheses have been proposed to explain the pathogenesis of the disease, with genetic mutations being the most recognized.
Viral infection, radiation and asbestos exposure have been implicated in etiopathogenesis. However, valid scientific evidence to point out the exact role of these factors in pathogenesis is still needs to be discovered (1-3). Mesothelial tumors of the peritoneum can be classified into diffuse and localized subtypes based on their macroscopic tissue involvement. In fact, a minority of cases present with a localized pattern, which contributes to the poor disease prognosis (1). The synchronous existence of malignant peritoneal mesothelioma (MPM) with other malignancies such as adenocarcinoma of the colon have been scarcely reported in English literature. Therefore, diagnosis and management of similar conditions remains challenging given its complexity and rarity (4).
Herein, we report a case of epithelioid subtype of MPM occurring synchronously with sigmoid colonic adenocarcinoma in an elderly female patient, along with comprehensive review of the literature of this rare co-occurrence. The current case was reported in accordance with SCARE guidelines (5).
2. CASE PRESENTATION
A 75-year-old female, known to have hypertension, gastroesophageal reflux disease, and recurrent urinary tract infections, was referred to the colorectal surgery outpatient clinic in a tertiary university institute as case of rectosigmoid mass for investigation. The patient reported one-month history of generalized abdominal pain, anorexia, weight loss of about 16 kilograms within two months and multiple episodes of per-rectal bleeding. Her family history was significant for malignancy as her father passed away after being diagnosed with brain tumour and her daughter had thyroid cancer. No clear history of asbestos exposure was reported by the patient. The initial colonoscopy in the referring hospital was aborted because of inability to pass beyond the obstructing mass.
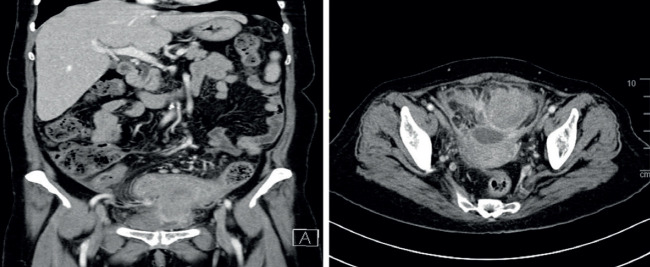
In our institute, the patient was investigated. All laboratory parameters were unremarkable including inflammatory markers. Her computed-tomography (CT) scan of the abdomen revealed a fistulizing sigmoid mass extended to the urinary bladder and the left adnexa with anterior cul-de-sac collection. As well, multiple enlarged lymph nodes were noted along the inferior mesenteric artery with omental nodulation (Figure 1). The patient underwent a second colonoscopy in our institution which revealed a large fungating mass. Endoscopic biopsies were obtained and sent for histopathological examination. Microscopic evaluation of the sigmoid colon biopsies using H&E stain revealed predominant neoplastic proliferation of colonic glands arranged in tubular and cribriform/back-to-back patterns (figure-2A). The cell lining exhibits hyperchromatic nuclei with visible nuclei, loss of nuclear polarity, and amphophilic cytoplasm. There are scattered mitotic figures situated in the colonic crypts. The lamina propria is mildly inflamed with mixed chronic and acute inflammatory cells. The overall morphology is that of moderately differentiated colonic adenocarcinoma, grade 2 (figure-2B).
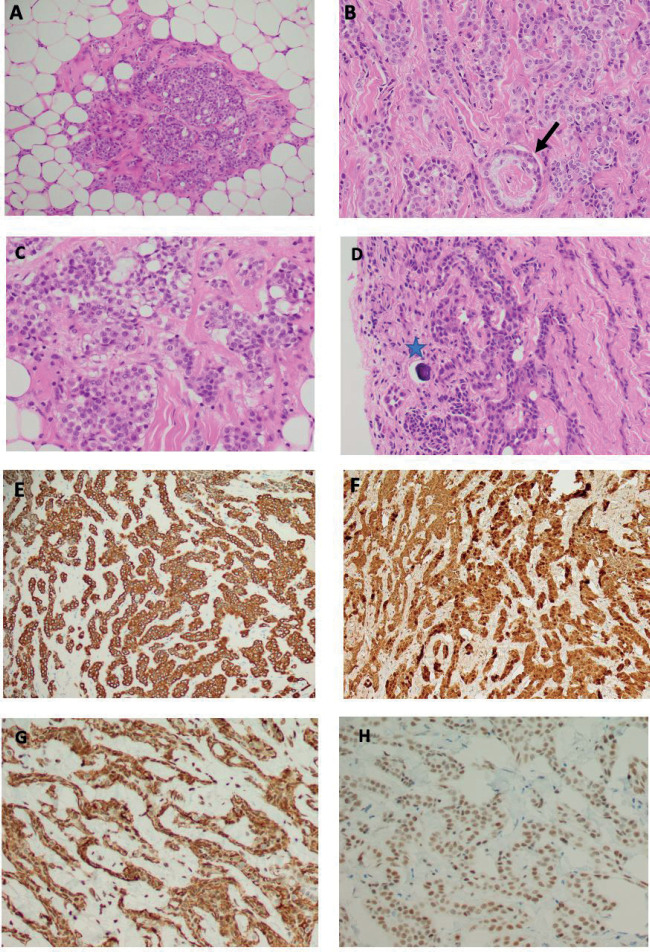
After multidisciplinary discussion, the plan was to proceed with laparoscopic low anterior resection. Intraoperatively, the patient underwent a routine double J- stent insertion, as per our protocol. However, the diagnostic laparoscopy revealed several nodules disseminated all over the peritoneum, suggestive of peritoneal mesothelioma rather than metastatic peritoneal nodules from the sigmoid adenocarcinoma. Therefore, the decision was made intraoperatively to perform transverse colostomy only after obtaining multiple biopsies from the omental and peritoneal nodules. The histopathological examination (H&E stain) of the peritoneal nodules revealed malignant mesothelioma of the predominant epithelioid subtype. The specimen typically consisted of fibrofatty tissue infiltrated by neoplastic cells arranged in solid nests, cords, trabeculae, and rare papillae and acini (figures-3 A and B). The cells displayed epithelioid morphology with amphophilic cytoplasm, round nuclei, vesicular chromatin, and visible nucleoli (figures-3 C and D). There are also occasional psammomatous calcifications (figure-3 D). By immunohistochemical studies, the tumor cells are diffusely and strongly reactive to pan-cytokeratin (figure-3 E), CK7, and calretinin (figure-3 F). In addition, podoplanin/D2-40 and WT-1 (nuclear staining) are diffusely positive in the tumor cells (Figures-3 G and H, respectively). However, CK20, PAX-8, and CDX2 are immunonegative. Thus, the overall pathologic and immunoprofile of this tumor is in keeping with mesothelial cell origin (malignant peritoneal mesothelioma, epithelioid subtype) rather than metastatic colonic adenocarcinoma and the patient was diagnosed as sigmoid adenocarcinoma with synchronous malignant peritoneal mesothelioma.
A subsequent tumor board discussion in a specialized oncology center was conducted, for which the patient was started on palliative chemotherapy in the form of reduced-dose capecitabine (Xeloda) for the sigmoid adenocarcinoma and a consensus was made to avoid active management of the peritoneal mesothelioma because of her age and general condition. She was followed up in the oncology out-patient clinic for nine month and she was tolerating the chemotherapy with a good clinical course.
3. DISCUSSION
Colorectal cancer is the third most common cancer affecting both genders and the 5th leading cause of cancer related deaths worldwide (6). Malignant mesothelioma on the other hand is a rare disease with approximately 3,300 new cases of malignant mesotheliomas diagnosed every year in the USA (7). The incidence of mesothelioma in Kingdom of Saudi Arabia is still unclear, which is attributed to the rarity of the disease and under-recognition of cases. In Saudi Arabia, Khan et al. published the first case of malignant mesothelioma in 1992. In King Faisal Specialist Hospital and Research Center, ten cases of malignant mesothelioma were reported as a single institution experience. In 2000, the first case of MPM in Saudi Arabia was reported in a case of exudative ascites that was found to be malignant peritoneal mesothelioma(7, 8).
Mesothelioma generally arises from the mesothelial cells of pleura, peritoneum, pericardium, and tunica vaginalis. Histologically, peritoneal mesothelioma can be differentiated into diffuse malignant peritoneal mesothelioma, deciduoid peritoneal mesothelioma, well-differentiated papillary mesothelioma of the peritoneum, multicystic mesothelioma, and adenomatoid tumor (9). Malignant peritoneal mesothelioma has three histological subtypes: epithelioid, sarcomatoid, and biphasic type. Epithelioid subtype is considered the most common type with better prognosis compared to the sarcomatoid (8).
In general, malignant mesothelioma may be attributed to asbestos exposure. Other reported associations were with radiation, diffuse lymphocytic lymphoma, simian virus, and chronic peritonitis. While colon adenocarcinoma has been linked to tranformation from adenomatous polyps, familial adenomatous polyposis, dietary factors, smoking, family history of colon cancer, inflammatory bowel disease, and environmental factors (9).
Synchronous occurrence of malignant peritoneal mesothelioma and colorectal adenocarcinoma is very rare. We performed a comprehensive review of literature using the terms of “mesothelioma”, “peritoneal mesothelioma”, and/or “malignant mesothelioma”, combined with the terms of “colorectal cancer”, “colon cancer”, “rectal cancer”, “colorectal malignancy”, “colon” and/or “rectal cancer” in the title and abstract of MEDLINE (via PubMed), Web of Science and Google Scholar databases (Table 1). Only publications or abstracts written in English language were included. Jatzko et al. reported the first case in 1997 for a patient diagnosed with malignant peritoneal mesothelioma and rectal carcinoma (10). In a study conducted by Attanoos et al. in 2003 a series of 500 cases of malignant mesothelioma in asbestos exposed patients underwent post-mortem examination to identify concomitant malignancies. One of these cases showed concomitant malignant peritoneal mesothelioma and colorectal adenocarcinoma (11). In 2014, Xie et al. reported another case of synchronous colonic adenocarcinoma and malignant peritoneal mesothelioma (4). Serio et al. reported four cases of synchronous and metachronous development of colorectal adenocarcinoma and mesothelioma in asbestos-exposed patients. Two of these cases had peritoneal mesothelioma while the other two had pleural mesothelioma (12).
Table 1. Summary of the literature review of cases with synchronous mesothelioma and colorectal cancer, including our case. * no documented asbestos exposure.
| Author | Year | Gender | Age | Risk factors | Management | Outcome |
|---|---|---|---|---|---|---|
| Jatzko et al. | 1997 | Female | 55 | Asbestos exposure | Low anterior resection with omentectomy and peritonectomy | Survived more than 6 months |
| Attanoos et al. | 2003 | Male | 75 | Asbestos exposure | N/A | Identified post-mortem |
| Xia et al. | 2014 | Male | 61 | Tobacco smoking Working in construction* |
Chemotherapy | Survived for 56 months |
| Serior et al. | 2022 | Male | 71 | Exposure to asbestos | Surgical resection | Survived 42 months |
| Male | 89 | Exposure to asbestos. Family history of colon cancer, bone tumor and pancreatic cancer |
Surgical resection | Survived 5 months | ||
| Our case | 2022 | Female | 75 | Family history of brain & thyroid malignancy | Transverse loop colostomy and palliative chemotherapy | Survived more than 6 months |
Clinical presentation of malignant peritoneal mesothelioma is vague with nonspecific abdominal pain, bloating, distention or feeling of abdominal mass. As the disease progresses, patients may develop early satiety, weight loss, and fatigue indicating advanced disease (9). Patients may exhibit signs and symptoms of paraneoplastic syndromes including thrombocytosis, hypoglycemia, deep vein thrombosis, paraneoplastic hepatopathy, and wasting syndrome (6).
Diagnosis of malignant peritoneal mesothelioma can be suggested by history of exposure to asbestos and the presence of diffuse peritoneal thickening in absence of other organs primary tumors. CT is the most commonly used imaging modality in such cases. Common findings in CT scan include solid, heterogenous, soft tissue mass with irregular margins as well as peritoneal and mesenteric thickening. Other imaging modalities are less commonly utilized such as magnetic resonance imaging (MRI) and positron emission tomography (PET-CT) (13). In some cases diagnosis is established by diagnostic laparoscopy or laparotomy with multiple biopsies. Confirming the diagnosis can be made by having at least 2 positive markers of mesothelioma panel and 2 negative carcinoma markers, as seen in our case (14).
The mainstay management of MPM is a combination of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (15). Some reports studied the use of perioperative chemotherapy in MPM have not shown any benefit for disease treatment or even increasing the overall survival (16). Five years overall survival is reported to reach up to 45-50% in patients younger than 60 years, epithelioid type, and near complete cytoreductive surgery (17).
In our case, diagnosis of malignant peritoneal mesothelioma was overlooked initially considering the absence of asbestos exposure and the presence of confirmed colonic adenocarcinoma. Clinical presentation and radiological findings were attributed to colon cancer and peritoneal involvement was considered to be metastatic from the primary colon cancer. Diagnosis was suspected during the diagnostic laparoscopy by the extensive disseminated peritoneal nodules and was postoperatively confirmed by histopathological evidence of malignant peritoneal mesothelioma. After thorough discussion in the multidisciplinary tumor board, the patient general condition was not favorable for active management of MPM in the form of cytoreductive surgery. Based on our literature review, we report the first case in Saudi Arabia and the Middle East and the sixth case worldwide of synchronous malignant peritoneal mesothelioma with colorectal cancer (Table 1).
4. CONCLUSION:
Malignant peritoneal mesothelioma is an overlooked clinical entity with vague non-specific clinical presentation. Synchronous malignant peritoneal mesothelioma with colorectal cancer is rare with only few published case reports. This case is the first case to report such entity in Saudi Arabia and the Middle East. Its diagnosis is challenging as the radiological findings may be misinterpreted. Its management should be tailored according to the general condition.
Author’s contribution:
The author was involved on all steps of preparation this article including final proofreading.
Conflicts of Interest:
The author have no conflicts of interest to disclose.
Financial support and sponsorship:
No specific funding was received in relation to this study.
REFERENCES
- 1.Takehara Y, Endo S, Mori Y, Nakahara K, Takayanagi D, Shimada S, et al. Malignant peritoneal mesothelioma with lymph node metastasis that originated in the transverse colon. World Journal of Surgical Oncology. 2014;12(1):112. doi: 10.1186/1477-7819-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang Y, Mei Z, Cao H, Li S, Xu H, Qiu H, et al. Meningeal metastasis of a malignant peritoneal mesothelioma: A case report and literature review. Cancer Biology & Therapy. 2019;20(12):1409–15. doi: 10.1080/15384047.2019.1647053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed IA, Tipu S, Ishtiaq S. Malignant mesothelioma. Pakistan Journal of Medical Sciences. 2013;29(6) doi: 10.12669/pjms.296.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie W, Green LK, Patel RA, Lai S. A case of unsuspected peritoneal mesothelioma occurring with colonic adenocarcinoma masquerading as peritoneal metastases. Case Reports in Pathology. 2014;2014:1–6. doi: 10.1155/2014/838506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agha RA, Franchi T, Sohrabi C, Mathew G, Kerwan A, Thoma A, et al. The scare 2020 guideline: Updating consensus surgical case report (SCARE) guidelines. International Journal of Surgery. 2020;84:226–30. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Robalino Gonzaga ES, Guzman Rojas P, Vanar V., editors. The Global Cancer Observatory–All Rights Reserved–March, 2021. Malignant Peritoneal Mesothelioma. Mimicking Recurrent Diverticulitis. Cureus. 2019 Jan 17;11(1):e3906. doi: 10.7759/cureus.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan J, Dossing M, Curley W, Akhtar M, Ali MA. Malignant mesothelioma: King Faisal Specialist Hospital and Research Centre experience. Ann Saudi Med. 1992 Jan;12(1):47–51. doi: 10.5144/0256-4947.1992.47. [DOI] [PubMed] [Google Scholar]
- 8.Fukunaga T, Somatomo Y, Kamiyama J, Kasanami T. Malignant Peritoneal Mesothelioma. [2022 Sep .23];Chonnam Med J. 2022 Sep;58(3):133–134. doi: 10.4068/cmj.2022.58.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan R, Alexander R. Nonpleural mesotheliomas: mesothelioma of the peritoneum, tunica vaginalis, and pericardium. Hematol Oncol Clin North Am. 2005 Dec;19(6):1067–87. vi. doi: 10.11016/j.hoc.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Jatzko GR, Jester J. Simultaneous occurrence of a rectal carcinoma and a diffuse well differentiated papillary mesothelioma of the peritoneum. Int J Colorectal Dis. 1997;12(6):326–8. doi: 10.1007/s003840050117. [DOI] [PubMed] [Google Scholar]
- 11.Attanoos RL, Thomas DH, Gibbs AR. Synchronous diffuse malignant mesothelioma and carcinomas in asbestos-exposed individuals. Histopathology. 2003 Oct;43(4):387–92. doi: 10.1046/j.1365-2559.2003.01685.x. [DOI] [PubMed] [Google Scholar]
- 12.Serio G, Pezzuto F, Fortarezza F, Marzullo A, Delfino MC, d’Amati A, Romano DE, Maniglio S, Caporusso C, Lettini T, Cavone D, Vimercati L. Mesothelioma and Colorectal Cancer: Report of Four Cases with Synchronous and Metachronous Presentation. Int J Mol Sci. 2022 Feb 27;23(5):2630. doi: 10.3390/ijms23052630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregory SN, Sarvestani AL, Blakely AM. Malignant peritoneal mesothelioma literature review: past, present, and future. [2022 Jun 30];Dig Med Res. 2022 Jun;5:29. doi: 10.21037/dmr-22-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdul Razzak S, Awan F, Ahmed S. Malignant peritoneal mesothelioma-a diagnostic challenge. J Surg Case Rep. 2022 Dec 7;2022(12):rjac555. doi: 10.1093/jscr/rjac555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander H, Richard, Li Claire Yue, et al. Current management and future opportunities .11 for peritoneal metastases: peritoneal mesothelioma. Annals of surgical oncology. 25(2018):2159–2164. doi: 10.1245/s10434-018-6337-5. [DOI] [PubMed] [Google Scholar]
- 16.Deraco M, Baratti D, Hutanu I, et al. The role of perioperative systemic chemotherapy in .13 diffuse malignant peritoneal mesothelioma patients treated with cytoreductive surgery and .hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2013;20:1093–1100. doi: 10.1245/s10434-012-2845-x. [DOI] [PubMed] [Google Scholar]
- 17.Alexander HR Jr, Li CY, Kennedy TJ. Current Management and Future Opportunities for Peritoneal Metastases: Peritoneal Mesothelioma. [2018 Feb 8];Ann Surg Oncol. 2018 Aug;25(8):2159–2164. doi: 10.1245/s10434-018-6337-5. [DOI] [PubMed] [Google Scholar]