Abstract
Abstract: Mycoparasites in Basidiomycota comprise a diverse group of fungi, both morphologically and phylogenetically. They interact with their hosts through either fusion-interaction or colacosome-interaction. Colacosomes are subcellular structures formed by the mycoparasite at the host–parasite interface, which penetrate the parasite and host cell walls. Previously, these structures were detected in 19 fungal species, usually by means of transmission electron microscopy. Most colacosome-forming species have been assigned to Microbotryomycetes (Pucciniomycotina, Basidiomycota), a highly diverse class, comprising saprobic yeasts, mycoparasites, and phytoparasites. In general, these myco- and phytoparasites are dimorphic organisms, with a parasitic filamentous morph and saprobic yeast morph. We investigated colacosome-forming mycoparasites based on fungarium material, freshly collected specimens, and cultures of yeast morphs. We characterised the micromorphology of filamentous morphs, the physiological characteristics of yeast morphs, and inferred phylogenetic relationships based on DNA sequence data from seven loci. We outline and employ an epifluorescence-based microscopic method to assess the presence and organisation of colacosomes. We describe five new species in the genus Colacogloea, the novel dimorphic mycoparasite Mycogloiocolax gerardii, and provide the first report of a sexual, mycoparasitic morph in Colacogloea philyla and in the genus Slooffia. We detected colacosomes in eight fungal species, which brings the total number of known colacosome-forming fungi to 27. Finally, we revealed three distinct types of colacosome organisation in Microbotryomycetes.
Taxonomic novelties and typifications: New family: Mycogloiocolacaeae Schoutteten & Yurkov; New genus: Mycogloiocolax Schoutteten & Rödel; New species: Colacogloea bettinae Schoutteten & Begerow, C. biconidiata Schoutteten, C. fennica Schoutteten & Miettinen, C. microspora Schoutteten, C. universitatis-gandavensis Schoutteten & Verbeken, Mycogloiocolax gerardii Schoutteten & Rödel; New combinations: Slooffia micra (Bourdot & Galzin) Schoutteten, Fellozyma cerberi (A.M. Yurkov et al.) Schoutteten & Yurkov, Fellozyma telluris (A.M. Yurkov et al.) Schoutteten & Yurkov; Epitypifications (basionyms): Achroomyces insignis Hauerslev, Platygloea micra Bourdot & Galzin, Platygloea peniophorae Bourdot & Galzin; Lectotypification (basionym): Platygloea peniophorae Bourdot & Galzin
Citation: Schoutteten N, Yurkov A, Leroux O, Haelewaters D, Van Der Straeten D, Miettinen O, Boekhout T, Begerow D, Verbeken A (2023). Diversity of colacosome-interacting mycoparasites expands the understanding of the evolution and ecology of Microbotryomycetes. Studies in Mycology 106: 41–94. doi: 10.3114/sim.2022.106.02
Keywords: Basidiomycota, epifluorescence microscopy, molecular phylogeny, new taxa, Transmission Electron Microscopy, Pucciniomycotina, systematics, yeasts
INTRODUCTION
Fungi are heterotrophic eukaryotes, relying on other living organisms or organic substrates to meet their nutritional needs (Willis 2018). Based on the specific nutrient substrate and type of interaction they engage in, fungi are generally assigned to the following ecological guilds: (i) saprotrophs decomposing dead organic material; (ii) mutualistic symbionts engaging in trophic interactions that are beneficial for both partners and (iii) parasites deriving nutrients from other living organisms. Recently, the scientific community started considering fungal ecological strategies rather as a continuum, in which fungal species have mixtures of ecological capabilities ranging from saprotrophic to symbiotic to parasitic (e.g., Selosse et al. 2018). Moreover, fungi with complex lifecycles may have changing ecological strategies when alternating the different stages of their life histories (Bandoni 1995, Boekhout et al. 2011, Begerow et al. 2017). Parasitic stages of fungi interact with a huge diversity of host organisms, comprising both prokaryotes as well as organisms in all major groups of eukaryotes: e.g., Amoebozoa, Alveolates, Heterokontae, Metazoa, Viridiplantae and Fungi (Begerow et al. 2017, 2018, Naranjo-Ortiz & Gabaldón 2019). Fungal species that engage in parasitic interactions with other fungi as host are denoted as mycoparasites (Kirk et al. 2008).
Mycoparasitism is phylogenetically widespread within the kingdom Fungi, and has been reported in eight phyla thus far. These are Rozellomycota, Blastocladiomycota, Zoopagomycota, Mortierellomycota, Kickxellomycota, Mucoromycota, Ascomycota, and Basidiomycota (Begerow et al. 2017, 2018, Naranjo-Ortiz & Gabaldón 2019). The prevalence of mycoparasitism in multiple early-diverging lineages has led to the hypothesis that this strategy arose early in fungal evolution, which is supported by 400 million-year-old year Devonian fossil data (Hass et al. 1994). Among Basidiomycota, roughly 200 species of mycoparasites are currently known, making up less than 0.5 % of the currently described species diversity (according to He et al. 2019). Although this number seems to be rather modest based on current knowledge, basidiomycetous mycoparasitic fungi exhibit a high level of phylogenetic, macro- and micromorphological, and ecological diversity.
Molecular phylogenies have revealed that mycoparasitism mainly occurs in two subphyla of Basidiomycota: Agaricomycotina and Pucciniomycotina (Fig. 1) (Weiß et al. 2004, Bauer et al. 2006, Begerow et al. 2017). In Agaricomycotina, the majority of mycoparasites are members of Tremellomycetes, whereas only few belong to Agaricomycetes, e.g., species of Asterophora, Pseudoboletus, and Squamanita (Redhead et al. 1994, Oberwinkler 2012, Weiß et al. 2014, Koch & Herr 2021, Caiafa & Smith 2022). In Pucciniomycotina, mycoparasitism is phylogenetically widespread, occurring in at least six out of ten currently recognised classes: Agaricostilbomycetes, Classiculomycetes, Cryptomycocolacomycetes, Cystobasidiomycetes, Microbotryo-mycetes, and Spiculogloeomycetes (Bauer et al. 2006, Aime et al. 2006, 2014, Oberwinkler 2017, Begerow et al. 2017, 2018). The occurrence of mycoparasitism in Tritirachiomycetes (Pucciniomycotina) was suggested by Aime et al. (2014), although no cellular interaction structures or specific mechanisms for nutrient transfer were reported (Beguin 2010).
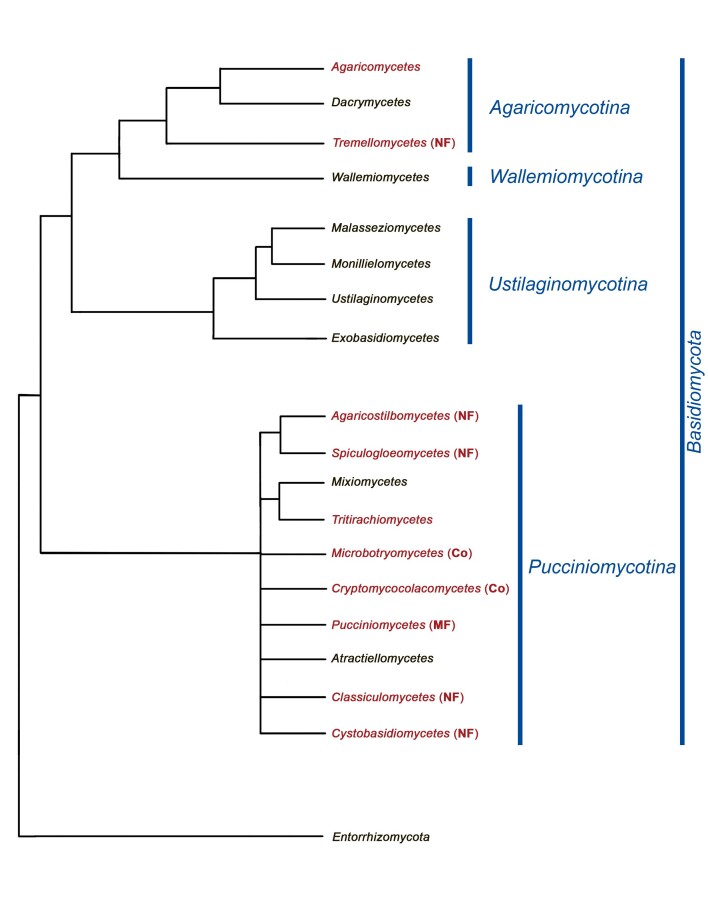
Fig. 1 .
Phylogram of Basidiomycota, interpretation based on of different previously published phylogenetic reconstructions of this phylum (Aime et al. 2006, Bauer et al. 2006, Schell et al. 2011, Wang et al. 2015a, Zhao et al. 2017, He et al. 2019). Names of classes indicated in red represent those comprising mycoparasitic species. Colacosome-interacting (Co) mycoparasites belong to Cryptomycocolacomycetes and Microbotryomycetes. Nanopore fusioninteracting (NF) mycoparasites belong to Agaricostilbomycetes, Classiculomycetes, Cystobasidiomycetes, Spiculogloeomycetes and Tremellomycetes. Micropore fusion-interacting (MF) mycoparasites belong to Pucciniomycetes
Basidiomycetous mycoparasites show remarkable variation in the production of basidiomata. Within Agaricomycetes, they typically produce mushroom-like basidiomata, whereas various Tremellomycetes normally produce gelatinous basidiomata. Moreover, many mycoparasites do not produce basidiomata, but grow in or between the tissues of their host. This characteristic growth type was referred to as intrahymenial growth by Oberwinkler (1964) and occurs in multiple genera of Tremellomycetes (e.g., Phragmoxenidium, Syzygospora, and Tremella) and Pucciniomycotina (Achroomyces, Colacogloea, Kryptastrina, Naohidea, Occultifur, Spiculogloea, and Zygogloea). However, not all intrahymenial species are mycoparasites, e.g., species in Tulasnella and Serendipita are regarded as species with saprobic and symbiotic capabilities (Weiß et al. 2016, Oberwinkler et al. 2017). Host species of basidiomycetous mycoparasites generally belong to Agaricomycetes, primarily corticioid fungi and jelly fungi, although some ascomycetous hosts are also known. Despite the hosts usually being widespread in nature, these mycoparasites are rarely reported. Due to their inconspicuousness, they are frequently overlooked and difficult to discern. Observations often happen accidentally, e.g., during microscopic investigation of the host fungus. This results in a limited availability of cultures and DNA sequence data for these mycoparasites, impeding their phylogenetic placement as well as their species delimitation (Kachalkin et al. 2019).
The majority of basidiomycetous mycoparasites in Pucciniomycotina and Tremellomycetes are characterised by dimorphic lifecycles. Generally, dimorphic fungi alternate between an ontogenetic haploid yeast stage, and an infectious dikaryotic hyphal stage (Brefeld 1888, Bandoni 1995, Boekhout et al. 2011, Begerow et al. 2017). These different stages of the lifecycle coincide with distinct types of growth, reproduction, karyological situation, and ecological strategies for nutrient acquisition (Begerow et al. 2017). Due to a certain degree of variation in these life histories, it is difficult to establish a uniform terminology that applies for all species. In literature considering dimorphic basidiomycetes, the two different stages are generally referred to as ‘yeast stage’ and ‘filamentous stage’. In this manuscript, we apply the terms ‘yeast morph’ and ‘filamentous morph’ to describe the different stages of the life cycle, based on how these stages can be observed and recognised. The yeast morph is a unicellular stage, characterised by budding of basidiospores. It is considered to be saprobic, and in most cases to represent the haploid stage. Following conjugation (mating) of compatible yeast cells, a dikaryotic hyphal stage is initiated, which generally leads to sexual reproduction. In the case of dimorphic mycoparasites, this stage has adaptations for host–parasite interaction and is here referred to as the filamentous morph. To complete the lifecycle, basidia develop from dikaryotic hyphae, in which meiosis takes place and eventually basidiospores are formed. In some species, mono- or dikaryotic conidia may be formed along with sexual structures. It is important to mention that not for all dimorphic species in Basidiomycota the entire lifecycle has been observed in natural or laboratory conditions. For example, many mycoparasites are only known from their filamentous morph. It is assumed that a yeast morph exists for these species, although it was never isolated in culture.
The functional interaction between a mycoparasite and its host fungus differs among various lineages of Basidiomycota. Two major interaction mechanisms have been described: (i) fusion-interaction and (ii) colacosome-interaction (Oberwinkler & Bauer 2018). A large variation at the ultrastructural level exists within each of these interaction types (Bauer 2004, Bauer et al. 2006, Oberwinkler & Bauer 2018).
The first interaction mechanism is the fusion-interaction. Most basidiomycetous mycoparasites interact with their host by means of haustoria, which are often referred to as ‘tremelloid haustoria’ or ‘nanopore fusion haustoria’ (Bauer 2004). Haustoria are produced by the parasite and can be recognised by light microscopy as structures with often three discernible regions: a swollen base, a tapered middle region and an apex. Haustoria either attach to host hyphae or invaginate host cells. Depending on the species, one or more nanopore channels, with a diameter of 14–19 nm, are formed at the contact interface of the haustorium apex and host hypha (Bauer 2004). These channels are formed by fusion of the host and parasite’s plasma membranes and establish cytoplasmic connection between host and parasite. This is in sharp contrast to basidiomycetous haustorial phytoparasites where no membrane fusion occurs and the cytoplasm of both interaction partners remains separated. As such, this phenomenon of cytoplasmic continuity between host and parasite is unique among fungal mycoparasites. Bauer (2004) hypothesised that cytoplasmic continuity facilitates nutrient transfer, but this remains to be investigated. The fusion-interaction is phylogenetically widespread in Tremellomycetes and Pucciniomycotina. Nevertheless, there is a large degree of difference in ultrastructure of these nanopore fusion haustoria among different lineages (Bauer 2004). The micropore fusion-interaction, in which fusion channels have a diameter of 1–2 µm, was so far only reported in Tuberculina species (Helicobasidiales) (Bauer et al. 2004, Lutz et al. 2004).
The second host–parasite interaction mechanism is the colacosome-interaction. Colacosomes are subcellular structures of 0.5–1 µm in diameter and are comprised of an electron-dense core surrounded by a membrane and an electron-transparent sheath (Kreger-van Rij & Veenhuis 1971b, Bauer & Oberwinkler 1991). They are formed in hyphae of the mycoparasite along the host–parasite interface (Fig. 2). Colacosomes, initially named lenticular bodies, were first reported from axenic cultures of Rhodosporidiobolus ruineniae, Rhodotorula toruloides, R. sphaerocarpa, and Sporobolomyces johnsonii (Kreger-van Rij & Veenhuis 1971b). These species, traditionally referred to as ‘red yeast’, are dimorphic fungi completing their lifecycle in culture, and colacosomes are formed along the contact surface of touching hyphae of the same species. Later, colacosomes were reported in hyphae of seven more dimorphic Microbotryomycetes growing in axenic culture (Table 1) (Kreger-van Rij & Veenhuis 1971a, De Hoog & Boekhout 1982, Boekhout et al. 1992, Sampaio et al. 2003). Bauer & Oberwinkler (1991) introduced the term ‘colacosomes’ when they discovered these structures for the first time along the host–parasite interface of the basidiomycetous mycoparasite Colacogloea effusa [as Platygloea peniophorae] and its host Peniophorella praetermissa. Since the term colacosomes has been in wider use than lenticular bodies, and several taxon names have their etymology based on this term, we prefer to adopt this term throughout the manuscript.
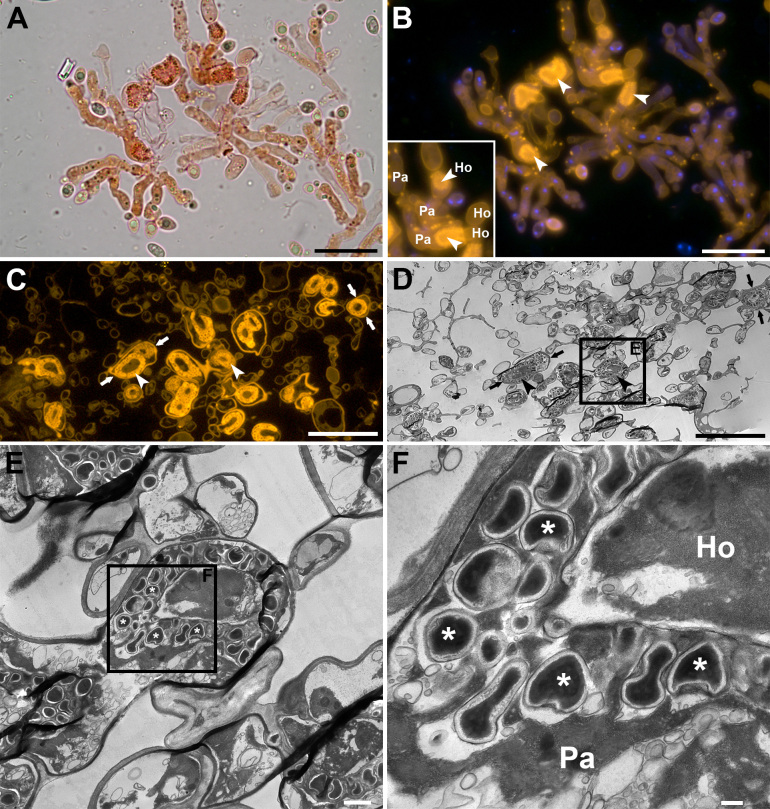
Fig. 2 .
Brightfield, epifluorescence and transmission electron microscopy (TEM) of Colacogloea Universitatis-gandavensis sp. nov. A, B. Whole-mount preparation, stained with Congo red and DAPI, visualised using brightfield (A) and epifluorescence (B) microscopy. Epifluorescence microscopy facilitates fast detection of colacosomes as they exhibit bright fluorescence signals. Inset shows the intricate host–parasite (Ho-Pa) interface. Arrowheads indicate regions of colacosome clustering. Note the occurrence of individual colacosomes in parasite tissue (bright spots). C, D. Serial sections of a Spurr-embedded sample, showing the same region. Corresponding structures are indicated with arrows. (C) Section stained with Congo red and visualised using epifluorescence microscopy. (D) Equivalent serial section of the same region as in (C), visualised using TEM. E, F. High-magnification details of colacosome clusters (arrowheads), composed of many individual colacosomes (asterisks), arranged in parasitic hyphae (Pa) along the host–parasite interface (Ho-Pa), showing their typical electron dense cores. Scale bars: A–D = 20 μm, E = 10 μm, F = 200 nm.
Table 1 .
Summary of species in which colacosomes have been detected, including data on the applied methodology for colacosome detection, organisation type of colacosomes, life cycle, host species, availability of cultures, and references.
| Species | Method colacosome detection | Colacosome organisation | Observed morphs | Sexual stage observed | Source of colacosome detection | Host species | Culture available | Selected references |
| Microbotryomycetes | ||||||||
| Atractocolax pulvinatus R. Kirschner, R. Bauer & Oberw. | TEM | Scattered in mycoparasite hyphae | Dimorphic | Yes | Axenic culture | Unknown, possibly member of Ascomycota | Yes | Kirschner et al. (1999) |
| Bannozyma yamatoana (Nakase, M. Suzuki & Itoh) Q.M. Wang, F.Y. Bai, M. Groenew. & Boekhout | TEM | n/d | Dimorphic | No | Axenic culture | Unknown - colacosomes formed in own mycelium | Yes | Boekhout et al. (1992) |
| Colacogloea bettinae Schoutteten & Begerow sp. nov. | Fluorescence microscopy | Vesicular gall-like cells | Dimorphic | Yes | Host basidiome | Peniophorella pubera (Fr.) P. Karst. | Yes | This publication |
| Colacogloea biconidiata Schoutteten sp. nov. | Fluorescence microscopy | Scattered in mycoparasite hyphae | Dimorphic | Yes | Host basidiome | Peniophorella praetermissa (P. Karst.) K.H. Larss. s.l. | Yes | This publication |
| Colacogloea effusa (J. Schröt.) V. Malysheva, Schoutteten & Spirin | TEM | Scattered in mycoparasite hyphae | Dimorphic | Yes | Host basidiome | Peniophorella praetermissa (P. Karst.) K.H. Larss. s.l. | Yes | Bauer & Oberwinkler (1991); This publication |
| Colacogloea fennica Schoutteten & Miettinen sp. nov. | Fluorescence microscopy | Scattered in mycoparasite hyphae | Dimorphic | Yes | Host basidiome | Peniophorella praetermissa (P. Karst.) K.H. Larss. s.l. | Yes | This publication |
| Colacogloea microspora Schoutteten sp. nov. | Fluorescence microscopy | Scattered in mycoparasite hyphae | Dimorphic | Yes | Host basidiome | Peniophorella praetermissa (P. Karst.) K.H. Larss. s.l. | Yes | This publication |
| Colacogloea papilionacea R. Kirschner & Oberw. | TEM | Coiling of mycoparasite hyphae | Dimorphic | Yes | Co-culture with host | Unknown, possibly member of Ascomycota | Yes | Kirschner & Oberwinkler (2000) |
| Colacogloea philyla (Van der Walt, Klift & D.B. Scott) Q.M. Wang, F.Y. Bai, M. Groenew. & Boekhout | Fluorescence microscopy | Scattered in mycoparasite hyphae | Dimorphic | Yes | Host basidiome | Peniophorella pubera (Fr.) P. Karst. | no | This publication |
| Colacogloea universitatis-gandavensis Schoutteten & Verbeken sp. nov. | Fluorescence microscopy | Vesicular gall-like cells | Only filamentous morph known | Yes | Host basidiome | Peniophorella praetermissa (P. Karst.) K.H. Larss. s.l. | no | This publication |
| Hyalopycnis hyalina Höhn. (syn. Heterogastridium pycnidioideum Oberw. & R. Bauer) | TEM | Vesicular gall-like cells | Only filamentous morph known | Yes | Axenic culture and host basidiome | Unknown, possibly member of Ascomycota | Yes | Bauer 2004 |
| Leucosporidium fellii Gim.-Jurado & Uden | TEM | n/d | Dimorphic | Yes | Axenic culture | Unknown - colacosomes formed in own mycelium | Yes | Sampaio et al. (2003) |
| Leucosporidium golubevii Gadanho, J.P. Samp. & R. Bauer | TEM | n/d | Dimorphic | Yes | Axenic culture | Unknown - colacosomes formed in own mycelium | Yes | Sampaio et al. (2003) |
| Leucosporidium intermedium (Nakase & M. Suzuki) M. Groenew. & Q.M. Wang | TEM | n/d | Dimorphic | Yes | Axenic culture | Unknown - colacosomes formed in own mycelium | Yes | Sampaio et al. (2003) |
| Leucosporidium scottii Fell, Statzell, I.L. Hunter & Phaff | TEM | n/d | Dimorphic | Yes | Axenic culture | Unknown - colacosomes formed in own mycelium | Yes | Kreger-van Rij & Veenhuis (1971a); Moore (1972) |
| Mycogloiocolax gerardii Schoutteten & Rödel sp. nov. | Fluorescence microscopy | Scattered in mycoparasite hyphae | Dimorphic | Yes | Host basidiome | Xenasmatella tulasnelloidea (Höhn. & Litsch.) Oberw. | Yes | This publication |
| Rhodosporidiobolus ruineniae (Holzschu, Tredick & Phaff) Q.M. Wang, F.Y. Bai, M. Groenew. & Boekhout | TEM | n/d | Dimorphic | Yes | Axenic culture | Unknown - colacosomes formed in own mycelium | Yes | Kreger-van Rij & Veenhuis (1971b); Moorey (1972) |
| Rhodotorula sphaerocarpa (S.Y. Newell & Fell) Q.M. Wang, F.Y. Bai, M. Groenew. & Boekhout | TEM | n/d | Dimorphic | Yes | Axenic culture | Unknown - colacosomes formed in own mycelium | Yes | Kreger-van Rij & Veenhuis (1971b); Moore (1972) |
| Rhodotorula toruloides (Banno) Q.M. Wang, F.Y. Bai, M. Groenew. & Boekhout | TEM | n/d | Dimorphic | Yes | Axenic culture | Unknown - colacosomes formed in own mycelium | Yes | Kreger-van Rij & Veenhuis (1971b); De Hoog & Boekhout (1982) |
| Slooffia micra (Bourdot & Galzin) Schoutteten comb. nov. | Fluorescence microscopy | Coiling of mycoparasite hyphae | Dimorphic | Yes | Host basidiome | Myxarium podlachicum (Bres.) Raitv. | Yes | This publication |
| Sporobolomyces johnsonii (Nyland) Q.M. Wang, F.Y. Bai, M. Groenew. & Boekhout | TEM | n/d | Dimorphic | Yes | Axenic culture | Unknown - colacosomes formed in own mycelium | Yes | Kreger-van Rij & Veenhuis (1971a); Moore (1972) |
| Sporobolomyces salmonicolor (B. Fisch. & Brebeck) Kluyver & C.B. Niel | TEM | n/d | Dimorphic | Yes | Axenic culture | Unknown - colacosomes formed in own mycelium | Yes | Moore (1972) |
| Cryptomycocolacomycetes | ||||||||
| Colacosiphon filiformis R. Kirschner, R. Bauer & Oberw. | TEM | Vesicular gall-like cells | Only filamentous morph known | Uncertain | Co-culture with host | Unknown, possibly member of Ascomycota | No | Kirschner et al. (2001) |
| Cryptomycocolax abnormis Oberw. & R. Bauer | TEM | Vesicular gall-like cells | Dimorphic | Yes | Host basidiome | Unknown, possibly member of Ascomycota | No | Oberwinkler & Bauer (1990) |
| Basidiomycota incertae sedis | ||||||||
| Colacogloea allantospora Ginns & Bandoni | Brightfield microsocpy | n/d | Unknown | Yes | Host basidiome | Tubulicrinis calothrix (Pat.) Donk | No | Bandoni et al.(2002) |
| Colacogloea bispora (Hauerslev) Oberw. & R. Bauer | TEM | Vesicular gall-like cells | Unknown | Yes | Host basidiome | Tubulicrinis angustus (D.P. Rogers & Weresub) Donk | No | Oberwinkler et al. (1999) |
| Krieglsteinera lasiosphaeriae Pouzar | TEM | Vesicular gall-like cells | Unknown | Yes | Host basidiome | Lasiosphaeria ovina (Pers.) Ces. & De Not. | No | Bauer (2004) |
Bauer & Oberwinkler (1991) studied the ultrastructure of colacosomes and provided a schematic hypothesis of their development, which remains largely hypothetical [figs 8–13 in Bauer & Oberwinkler (1991)]. During colacosome development, the plasmalemma of the mycoparasite invaginates internally, creating an entirely membrane-enclosed globular space. This enclosed compartment becomes filled with electron-dense components, and a secondary cell wall around the invagination is produced by the mycoparasite, visible as an electron-transparent sheath. Next, the electron-dense components extrude through a tubular projection, penetrating the outer cell wall of the parasite and eventually the cell wall of the host fungus.
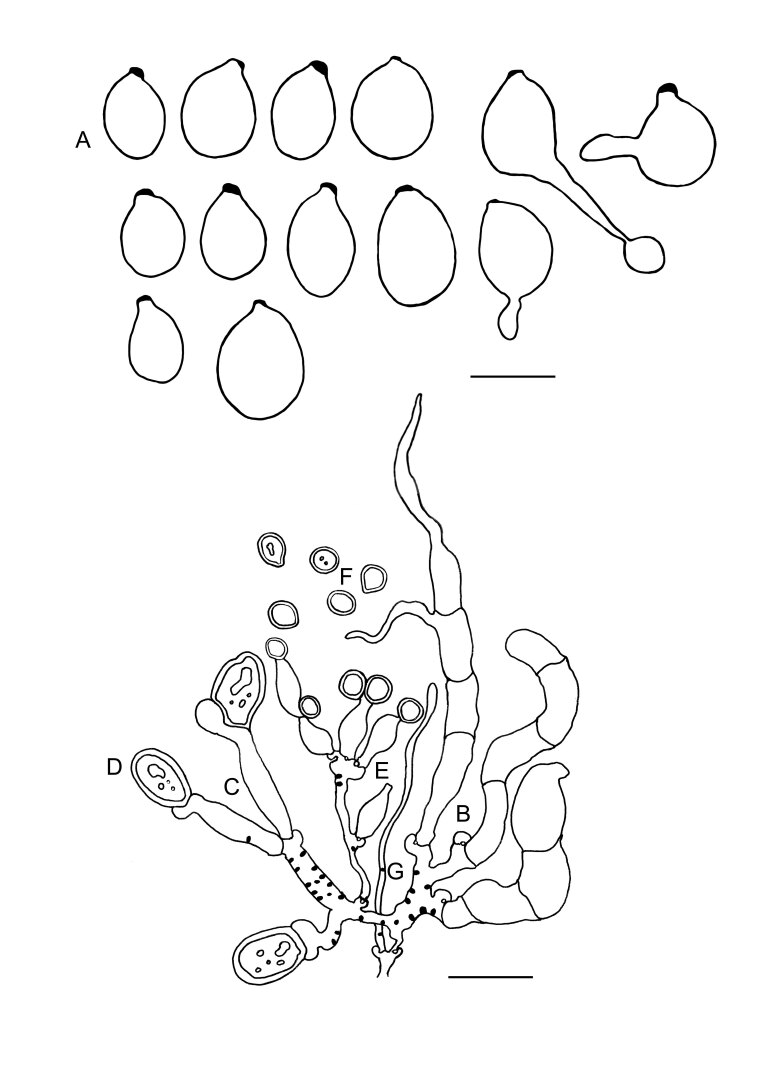
Fig. 8 .
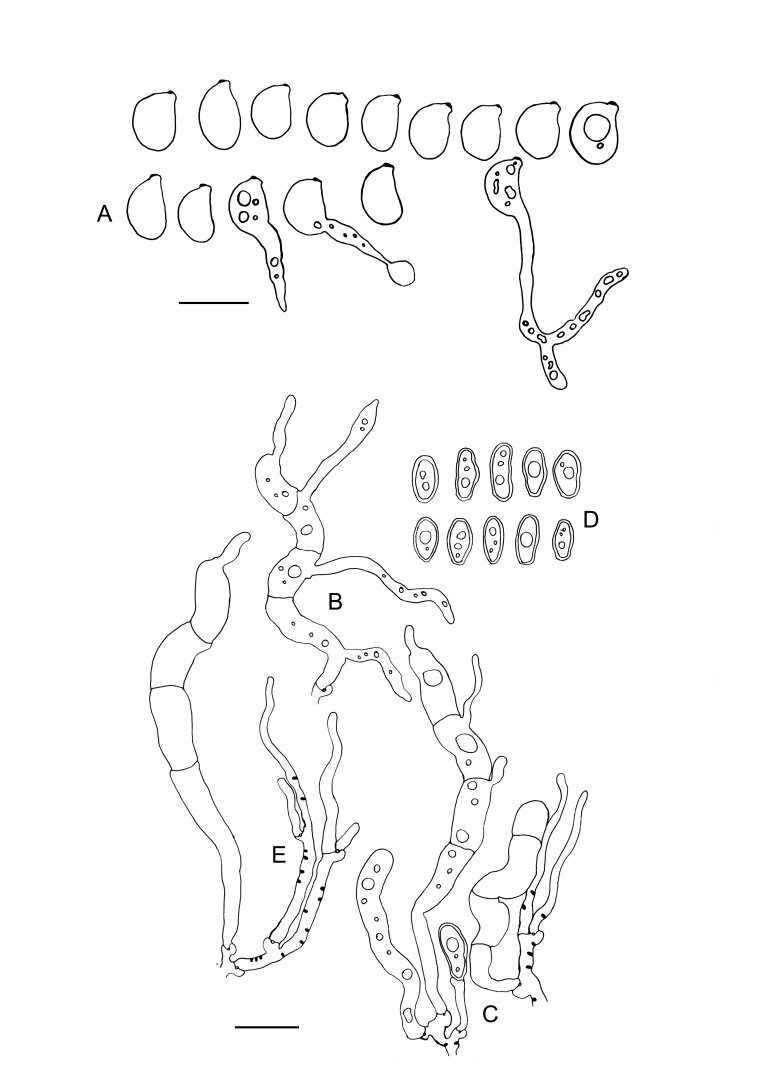
Colacogloea biconidiata sp. nov. (VS 12415) line drawings. A. Basidiospores and germinating basidiospores by hyphae and secondary spores. B. Cluster of basidia and basidioles. C. Type-1 conidiophores. D. Type-1 conidia with basal clamps. E. Type-2 conidiophores. F. Type-2 conidia. G. Hyphidium. Black dots represent colacosomes. Scale bars = 10 μm.
Fig. 13 .
Colacogloea fennica sp. nov. (OM 22483). A. Basidiome. B. Three-septate basidium with four sterigmata, note colacosomes in hyphae bearing the basidium but not in the basidium. C. Basidiole. D. Cluster of conidiophores and conidia, note colacosomes in hyphae but not in conidiophores. E. Conidia. F. Basidiospores. G, H. Host–parasite interface, Pa = parasite cell, Ho = host cell, arrowheads indicate some positions of colacosomes. Scale bars: A = 1 cm; B–G = 10 μm.
To date, the function of colacosomes remains unclear. Bauer & Oberwinkler (1991) provided the first hypothesis on the function of colacosomes, suggesting they are involved in the mycoparasitic interaction, possibly facilitating transfer of nutrients from host to parasite. Also a structural role was proposed, in which colacosomes can anchor parasite hyphae to host cells (Bauer & Oberwinkler 1991, Bauer 2004, Bauer et al. 2006, Begerow et al. 2017, Oberwinkler & Bauer 2018). Using X-ray diffraction, Kreger-van Rij & Veenhuis (1971b) determined that the electron-transparent sheath envelopping the colacosome is a chitin-rich structure. However, the biochemical composition of the electron-dense part of the colacosomes remains unknown.
Colacosomes have currently been reported from 19 fungal species, distributed over 11 genera in two classes of Pucciniomycotina: Cryptomycocolacomycetes and Microbotryomycetes (Table 1). For four species, i.e., Atractocolax pulvinatus, Colacogloea allantospora, C. bispora, and Krieglsteinera lasiosphaeriae, no living cultures and/or DNA sequence data are currently available, and their placement in Microbotryomycetes is tentative (Kirschner et al. 1999, Oberwinkler et al. 1999, Oberwinkler 2017). Filamentous morphs of colacosome-forming fungi which are associated with a host fungus are considered to represent a mycoparasitic stage. However, the ecology of fungi in which colacosomes were only observed in pure culture conditions is less clear, since no host–parasite interaction was observed. These species were often isolated as yeasts from a variety of substrates such as phylloplanes, soils, and (decaying) organic substrates, and are generally believed to be saprobes. However, because of their ability to produce colacosomes, these species are discussed to also have mycoparasitic capabilities (Sampaio et al. 2003, Boekhout et al. 2011, Begerow et al. 2017, 2018).
Most likely, the diversity of colacosome-forming mycoparasites is much broader than currently known, a statement for which at least three reasons can be put forward. A first argument is that for all currently known colacosome-forming species, only one or a few collections or isolates were investigated. This leaves room for unexplored diversity in species complexes and (pseudo-) cryptic diversity. Secondly, due to the rather recent discovery of colacosomes and the lack of specialised tools to visualise and detect them, it is likely that for various currently known fungicolous fungi the presence of colacosomes is yet to be assessed. Currently, more than 20 species assigned to the heterogenous morphogenera Achroomyces and Platygloea are presumed mycoparasites, for which no detailed information on the host–parasite interaction is available (Bandoni 1956, Oberwinkler et al. 1990a). Such mycoparasites, for which no haustoria have been observed, are potential colacosome-interacting species and should be investigated more carefully. Thirdly, many colacosome-forming fungi remain undescribed due to their inconspicuous nature. These species either have minute basidiomata, or only grow intrahymenially. It has also been noted that many yeasts in Cystobasidiomycetes and Microbotryomycetes are slow-growing fastidious or extremophilic species and are known from a few isolates only (Buzzini et al. 2018). Therefore, many groups in these two classes remain largely undersampled (Kachalkin et al. 2019).
Most studies that reported the presence of colacosomes in fungi made use of transmission electron microscopy (TEM) (Table 1). Sample preparation for TEM is a labour-intensive process requiring knowledge and equipment for embedding, sectioning, staining, and imaging (Oberwinkler & Bauer 2018). Therefore, it is currently challenging to perform a large-scale screening for the presence of colacosomes in fungal specimens. One study reported on the presumed presence of colacosomes based on Congo red stained samples visualised with brightfield microscopy (Bandoni et al. 2002). A reliable light microscopy-based method would be more efficient and accessible to detect the presence of colacosomes compared to TEM. Further, it could allow for a wide screening for colacosome-forming fungi towards improving our knowledge of the diversity of these mycoparasites.
In this paper, we aim to investigate the taxonomy and phylogenetic relationships of colacosome-forming mycoparasites. To do so, we developed an accessible and easy light microscopy-based method for colacosome detection, which we validated using correlative light microscopy and TEM. This helped us to find out how the colacosomes are organised along the host–parasite interface. Using this microscopy technique, freshly collected samples of mycoparasites from various host species were investigated for the presence of colacosomes. Positively assessed colacosome-interacting mycoparasites were isolated in pure culture. These samples were used for phenotypic characterisation of their filamentous- and yeast morphs, and DNA sequencing of seven genetic loci. To assess the phylogenetic relationships of these mycoparasites, we compiled an extensive dataset of Microbotryomycetes based on the seven loci commonly used in this class. We also generated DNA sequences of additional loci for certain species to obtain a better phylogenetic resolution (Table 3). This allowed to determine the phylogenetic diversity, -relationships, and -distribution of colacosome-forming mycoparasites, and to explore how they influence the current classification of Microbotryomycetes. We translated obtained results into a taxonomic arrangement of colacosome-forming mycoparasites, and an updated classification of Microbotryomycetes. Integration of these different types of information allows to formulate an evolutionary hypothesis on colacosome-interacting mycoparasites.
MATERIALS AND METHODS
Material examined
Samples of colacosome-forming fungi were collected from different places in Europe (Belgium, Denmark, Finland, France, Germany, Norway, The Netherlands) in recent years. Herbarium collections from C, GENT, H, LIP, and PC (sensu Thiers 2022) were investigated. Examined collections are listed under the species descriptions in the taxonomic part of this paper. Collections indicated with an asterisk (*) were isolated in pure culture and used for DNA sequencing. GenBank accession numbers are listed in Table 3. Specimens indicated with (°) were investigated using epifluorescence microscopy and/or TEM. Some additional ex-type yeast cultures were obtained from the fungal collection of the Leibniz Institute DSMZ – German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). These cultures were used to sequence additional loci for phylogenetic analysis.
Light microscopy and morphology
Whole-mount preparations from fresh and dried host basidiomes were mounted in a Congo red staining solution in ddH2O according to Clémençon (2009). In some cases, the Congo red staining solution was supplemented with DAPI (4’6-diamidino-2-phenylindole, with a final concentration of 1 µg/mL) for staining of nuclei. Some species were additionally studied using Cotton Blue staining solution (0.025 % w/v in Lactic acid). Specimens were investigated for micromorphological characters using phase-contrast optics (Leica DM 1000 Led), brightfield and epifluorescence microscopy using a Nikon Plan Fluor 100× objective with 1.3 numeric aperture on a Nikon Eclipse Ni-U microscope, using a TRITC (excitation: 543/22 nm; dichroic mirror 652 nm; emission: 593/40 nm) and/or DAPI filters (excitation: 387/11 nm; dichroic mirror 409 nm; emission: 447/60 nm). The presence of colacosomes was evaluated using epifluorescence microscopy of Congo red stained samples. Photographs of microscopic structures were taken with a Nikon DS-Fi3 camera and Nikon NIS-Elements software, including the Extended Depth of Field module. Pictures were edited and compiled in Photoshop CS6. The basidiospores and conidia represented in the composite plates are a compilation from different pictures. For each collection, at least 30 basidiospores and 15 basidia and conidia were measured. The measurements are presented following Parmasto & Parmasto (1987), with 5 % tails excluded and given in parentheses. The following abbreviations are used in the species descriptions: L – mean basidiospore length, W – mean basidiospore width, Q’ – L/W ratio, Q – mean L/W ratio, and n – number of measurements per specimens measured. The basidiospore length measurements include the apiculus since it is often impossible to unequivocally determine its exact border with the main spore body. Basidia were measured using Nikon software, by drawing a polygonal line from the basal clamp of the basidium, over the middle of each transversal septum, to the distal end of the top cell (not including the upper sterigma if inserted apically). Structure and terminology of morphological diagnoses follow Spirin et al. (2018) and Savchenko et al. (2021).
Correlative light and Transmission Electron Microscopy
The sample fixation protocol is based on Bauer et al. (2006), with slight modifications. Samples were fixed in 2 % v/v glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) at room temperature for 12 h in a rotating device. Following six 10 min incubations in 0.1 M sodium cacodylate buffer, samples were post fixed in 1 % v/v osmium tetroxide in cacodylate buffer for 1.5 h in darkness. Samples were dehydrated in acetone, using 15 min changes at 10, 20, 30, 50, 70, 95 % v/v and three times in 100 % acetone. Samples were infiltrated by Spurr’s resin in acetone using 15 min changes at 25, 50, 75 % v/v and three times in 100 % Spurr’s resin. Samples were polymerised overnight in Spurr’s resin at 60 °C. Serial sections were made to perform correlative light- and transmission electron microscopy. First, semi-thin sections of 300 nm thick, made using an ultramicrotome (UC6; Leica microsystems, Vienna) equipped with a diamond ultra-knife (DiATOME), were collected on polysine coated slides. Immediately after, ultrathin sections of 80 nm thick were made and collected on copper slot grids. Semi-thin sections were mounted in Congo red and viewed using an epifluorescence microscope equipped with a TRITC filter. Ultra-thin sections were stained for 27 min in 1 % uranyl acetate at 37 °C and 10 min in 3 % lead citrate at 20 °C. Grids were examined with a JEM-1010 TEM (Jeol Inc., Peabody, MA, USA) using a 60 keV electron beam. Images were recorded with a CCD side-mounted Veleta camera. Same areas were imaged.
Isolation procedure
Isolates of the different species were obtained by a spore drop method (Clémençon 2009) on MYP medium plates (0.4 g peptone 0.8 g yeast extract, 5.6 g malt extract and 16 g agar kobe-1 in 800 ml ddH2O). A small piece of infected host tissue was dissected and attached to the lid of a Petri plate. Plates were left at room temperature and the lid was rotated clockwise 1 h, 2 h, 4 h, 6 h, and 8 h after initial inoculation to allow sporulation on different places of the medium. Subsequently, the fungal sample was removed, and germinating spores were isolated on new MYP plates to obtain pure isolates. Cultures of all isolated collections were deposited at DSMZ.
Phenotypic characterisation of yeast morphs
Physiological tests were performed in liquid media according to the methods described in Kurtzman et al. (2011), in custom-made microplates (Nunc 96-Well Flat Bottom plate, Thermo Fisher Scientific) and tubes (Passer et al. 2019) using the same standard set of substrates. Tests were incubated at room temperature and controlled every 3–4 d until for in total 3 wk. Culture growth in microplates was measured on Varioskan LUX (Thermo Fisher Scientific) plate reader at 600 nm wavelength. Maximal growth temperature was determined on potato-dextrose agar (PDA, Difco BD) and micromorphological features were examined on PDA, CMA (DSMZ medium 191, https://mediadive.dsmz.de/medium/191), and YM agars (DSMZ medium 186, https://mediadive.dsmz.de/medium/186). A summary of the obtained results from the growth tests is given in Supplementary Table 1.
DNA extraction, PCR amplification, and sequencing
DNA from cultures was extracted using a CTAB-based protocol. From each culture, a loop of yeast cells was harvested and stored in 500 µL CTAB buffer. After addition of 0.3 % mercaptoethanol, the samples were homogenised in a thermoshaker at 65 °C and 600 rpm for 1.5 h. Subsequently, 500 µL chloroform-iso-amylalcohol was added and the samples were vortexed. Next, samples were centrifuged for 10 min at 12 000 rpm, after which the upper phase was transferred to another tube. After repeating this step one more time, 500 µL cold iso-propanol was added to the upper phase, samples were shaken and left at -20 °C for 20 min to precipitate the DNA. Subsequently, the samples were centrifuged at 12 000 rpm for 10 min at 4 °C and the pellet was washed twice with 70 ٪ EtOH. Finally, the DNA pellet was diluted in 50 µL Milli-Q water. PCR reactions were performed for the following seven loci: the small subunit (SSU), the internal transcribed spacers, including the 5.8S locus (ITS), and the large subunit (LSU) of the nuclear ribosomal DNA, the largest subunit of RNA polymerase II (RPB1), the second largest subunit of RNA polymerase II (RPB2), the translation elongation factor (TEF1-α) and mitochondrial cytochrome-b (CYT-B). Conditions for the amplification of seven genetic markers are given in Table 2. PCR products were purified using ThermoFisher FastAP Thermosensitive Alkaline Phosphatase and Exonuclease I (Thermo Fisher Scientific Inc., Massachusetts, USA). Purified products were sent to Macrogen (Amsterdam, The Netherlands) for Sanger sequencing using the same primers on an automated ABI 3730 XL capillary sequencer. Forward and reverse sequence reads were assembled into contigs in the BioloMICS software (BioAware SA NV, Hannut, Belgium). DNA extraction and amplification of Colacogloea universitatis-gandavensis sp. nov. was performed using a Multiple Displacement Amplification (MDA) procedure, using the Repli-g Whole Genome Amplification kit (QIAGEN, Hilden, Germany). Collection NS 20-022 was used for the dissection of two small pieces of parasite tissue (2 mm³ each) under a dissecting microscope. Subsequently, PCR reactions of the SSU, ITS, and LSU region were performed using conditions listed in Table 2. DSMZ cultures were cultivated on PDA (Difco BD) for 7 d at room temperature. Their DNA was isolated with the MasterPure Yeast DNA Purification Kit (Epicentre, San Diego, USA) following the manufacturer’s instructions. PCR products were purified with innuPREP PCRpure Kit (Analytik Jena, Jena, Germany) and sequenced on ABI 3500 XL capillary sequencer. Assembly and editing of sequence reads were performed with Sequencher v. 5.4.5 (Gene Codes Corporation, Michigan, USA).
Phylogenetic analyses
DNA sequences were downloaded from GenBank and are listed in Table 3. To compile the dataset, we used DNA sequence data from Wang et al. (2015a, b), which were complemented with sequence data of remaining taxa within Microbotryomycetes (Table 3). The phytoparasitic Microbotryales are represented by a limited set of taxa as in Wang et al. (2015a) and Li et al. (2020). To exclude possible contaminant sequences from public databases, we blasted all downloaded sequences against the NCBI nucleotide database. Contaminant sequences were removed from the dataset. Sequences of each region were aligned with the online version of MAFFT (Katoh et al. 2019) using the L-INS-i algorithm for the ITS dataset and the Iterative FFT-NS-i algorithm for the LSU, SSU, RPB1, RPB2, TEF1-α, and CYT-B datasets. Trailing ends of the alignments were trimmed and manually curated in MEGA v. 7 (Kumar et al. 2016). The ITS locus was partitioned in the ITS1, 5.8S and ITS2 regions. The ITS1 and ITS2 regions of the alignment were trimmed using TrimAL v. 1.1, with the following settings: 0.6 as gap threshold and 50 as minimum percentage of positions to conserve (Capella-Gutiérrez et al. 2009). Alignments of all regions were manually inspected and refined, and intronic regions manually removed. Final alignments are deposited in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S3032). ModelFinder as implemented in IQ-TREE v. 1.6.12 was used to infer the best model of evolution for each partition using the Akaike Information Criterion (AIC) (Kalyaanamoorthy et al. 2017). Maximum Likelihood analyses were performed using IQ-TREE v. 1.6.12 for single partitions and the concatenated dataset (Nguyen et al. 2015, Chernomor et al. 2016). The concatenated dataset was partitioned as follows: SSU, ITS1, 5.8S, ITS2, LSU, RPB1, RPB2, TEF1-α, and CYT-B. All analyses were performed using ultrafast bootstrapping procedure with 2 000 bootstrap replicates (Hoang et al. 2018).
Table 3 .
Summary of isolates and GenBank accession numbers of the seven genetic loci incorporated in the phylogenetic reconstruction. Accession numbers of sequences generated for this study are indicated in bold.
1 Acronyms of culture collections in alphabetic order: CBS, Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CGMCC, Chinese General Microbiological Culture Collection Center, Beijing, China; CRUB, Culture Collection of Yeasts from Centro Regional Universitario Bariloche, Bariloche, Argentinia; DSM, German Collection of Microorganisms and Cell Cultures GmbH, Braunschweig, Germany; EXF, Microbial Culture Collection Ex of the Infrastructural Centre Mycosmo, Ljubljana, Slovenia; JCM, Japan Collection of Microorganisms, RIKEN BioResource Center, Saitama, Japan; KBP Y, Yeast collection of the Lomonosov Moscow State University, Moscow, Russia; PYCC, Portuguese Yeast Culture Collection, Caparica, Portugal; TUB, Former Fungal Culture Collection of the university of Tübingen, now in laboratory of prof. D. Begerow, Hamburg, Germany. Other acronyms represent personal collections. T = ex-type strain or type specimen, ET = ex-epitype strain or epitype specimen, NT = ex-neotype strain or neotype specimen, PT = ex-paratype strain or paratype specimen.
2 Genetic loci are abbreviated as follows: partial small subunit (SSU), internal transcribed spacers including the 5.8S locus (ITS), and partial large subunit (LSU) of the nuclear ribosomal DNA, partial largest subunit of RNA polymerase II (RPB1), partial second largest subunit of RNA polymerase II (RPB2), partial translation elongation factor (TEF1-α) and partial mitochondrial cytochrome-b (CYT-B).
RESULTS
Epifluorescence-based colacosome visualisation
Because the assessment of the presence of colacosomes using TEM of fungal samples is a labour-intensive and time-consuming task, we developed a more efficient and affordable, light microscopy-based method for the detection of these structures. We compared the detectability of colacosomes in Congo red-stained samples of mycoparasites using brightfield and epifluorescence microscopy. Epifluorescence microscopy proved to be superior to brightfield imaging as colacosomes exhibit intense fluorescence signals and are visible as bright circular structures (compare Fig. 2A, B). As is evident from Fig. 2B, this approach allows to distinguish between host and parasite cells, as well as to detect individual and clustered colacosomes (Fig. 2B inset). Colacosomes are easy to distinguish due to the strong contrast between the bright signal emitted by the stained secondary cell wall enveloping them and the black background. To verify whether the bright signals originate from the colacosomes, we performed correlative light microscopy and TEM of Colacogloea universitatis-gandavensis sp. nov. (Fig. 2C, F). The host–parasite interface encompasses parasite gall-like cells enveloping host hyphae. Colacosomes are positioned in the parasite cells along the host–parasite interface. When the same area in the sections is imaged using epifluorescence microscopy and TEM (Fig. 2C, D), it becomes apparent that the bright fluorescent signals correspond to colacosomes. Contrary to the whole-mount prepared sample (Fig. 2A, B), individual colacosomes are visible as bright circles with a dark core in semithin sections (Fig. 2C). This core does not stain with Congo red and becomes visible because the 300 nm section is less thin than the diameter of colacosomes. Magnification of this area using TEM further shows the ultrastructure of individual colacosomes, which consist of an electron dense core surrounded by a membrane and a secondary cell wall (Fig. 2E, F). Colacosomes are also clearly visible using a 40× objective and could be detected and discriminated from other structures (data not shown). Although other chitin-containing structures such as thick-walled conidia also emit bright fluorescent signals, they cannot be mistaken for colacosomes due to their size, shape and/or organisation.
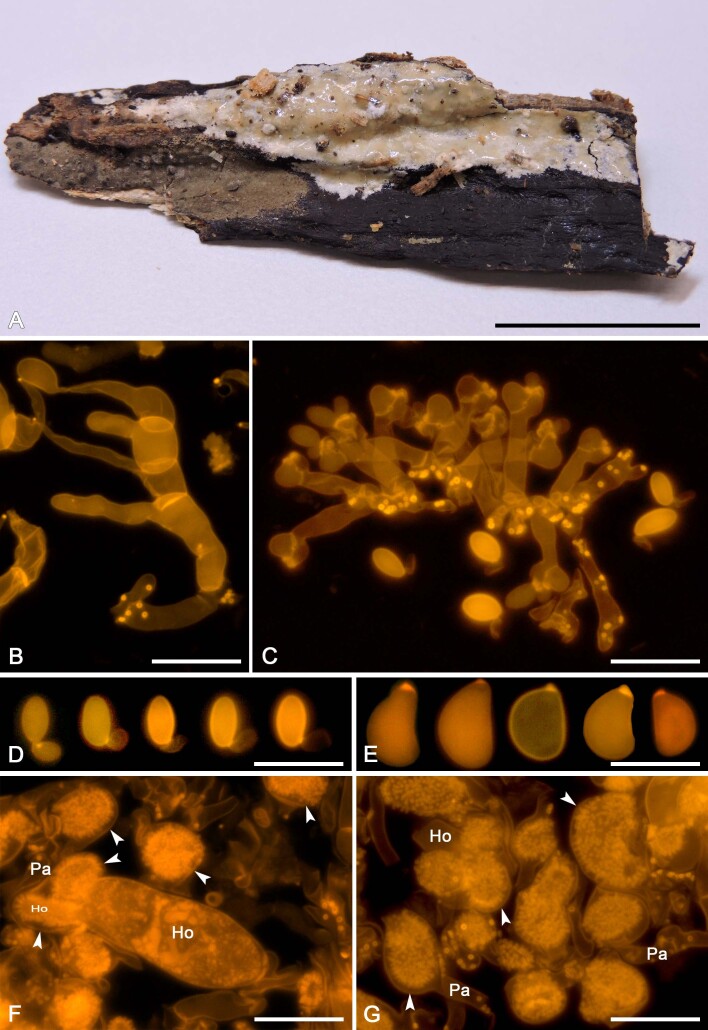
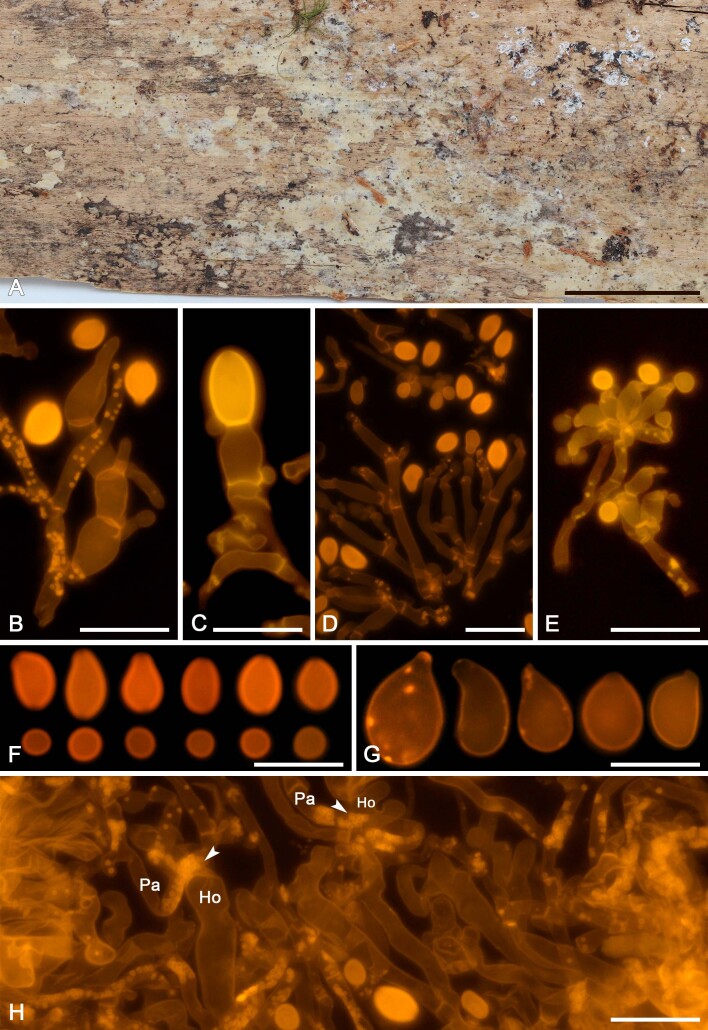
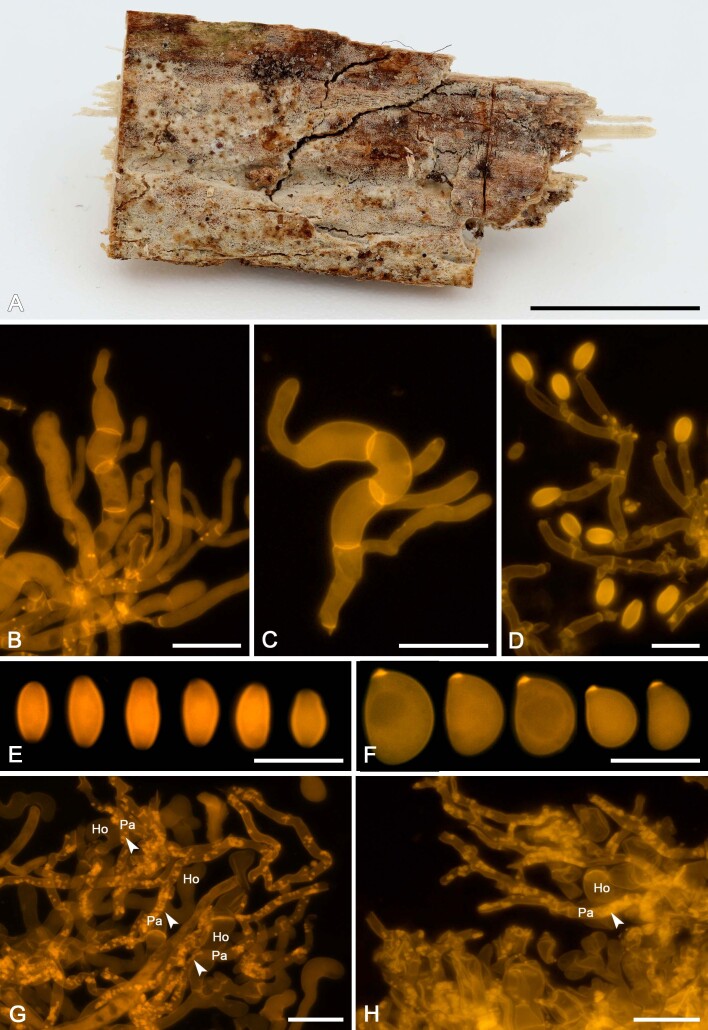
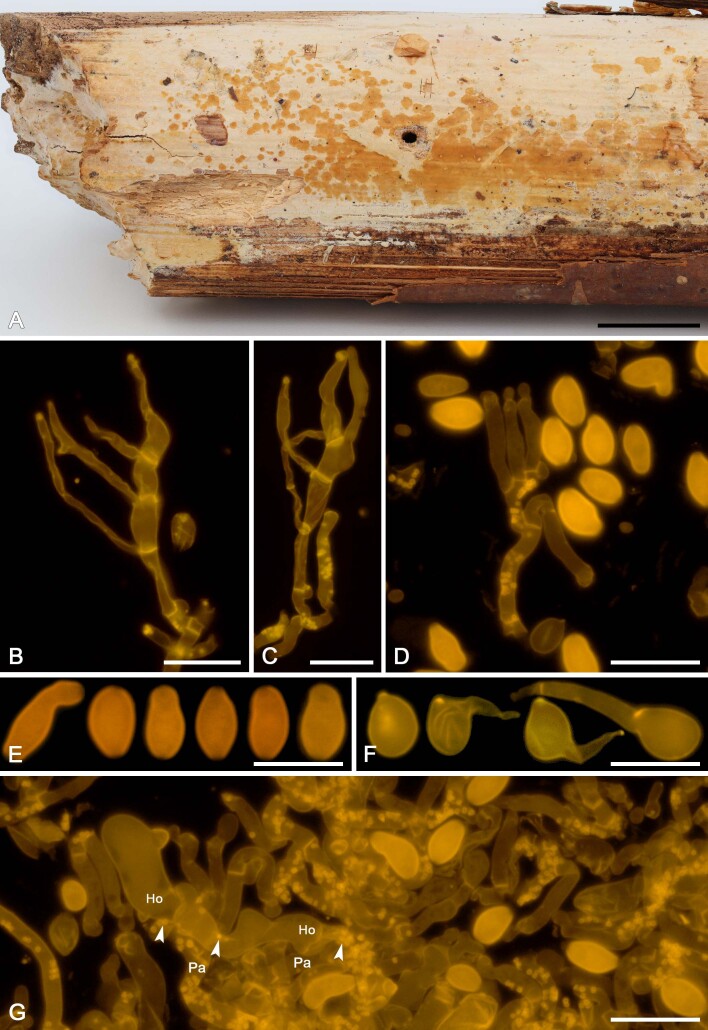
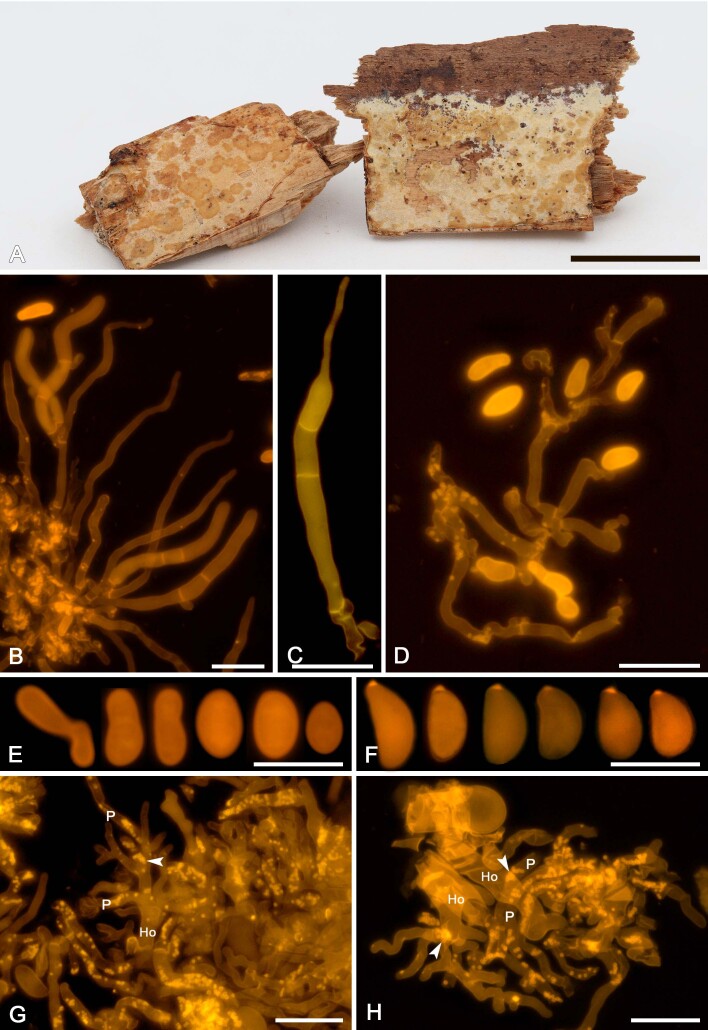
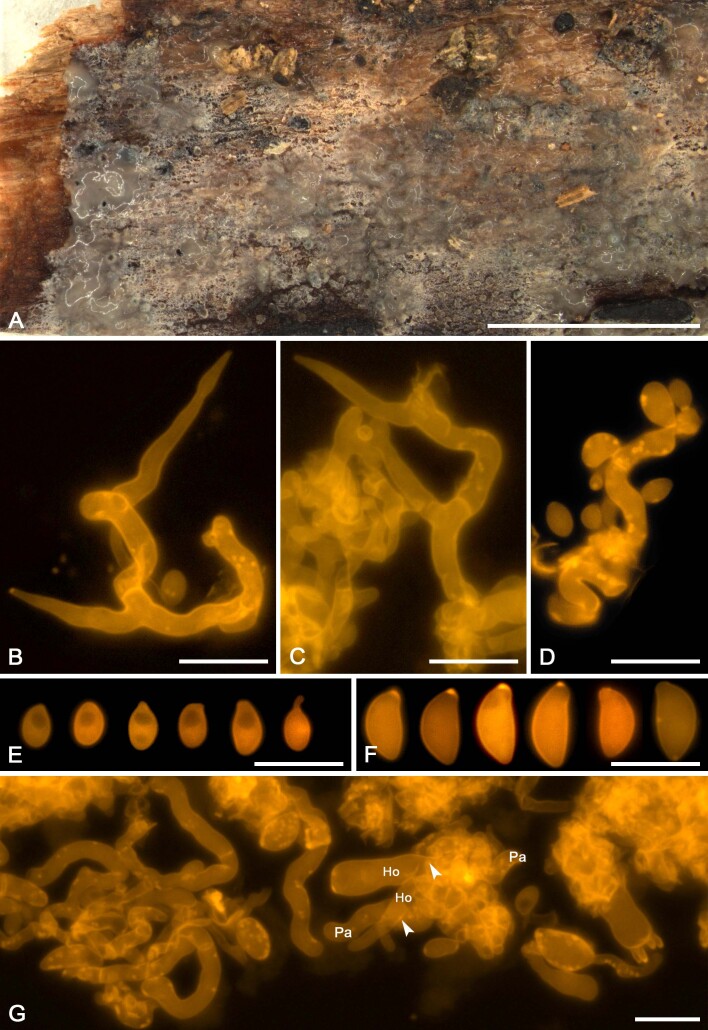
We applied this method to assess the presence of colacosomes in nine mycoparasitic species (Figs 5F, G, 7H, 9G, H, 11G, H, 13F, G, 15G, 17G, 19F, G, 21G). Samples for which the presence of colacosomes was positively assessed were isolated in pure culture and further studied for their phylogenetic relationships and phenotypic characteristics (i.e., micromorphology of the filamentous morph in the specimen, and characterisation of the yeast morph in axenic culture).
Fig. 5 .
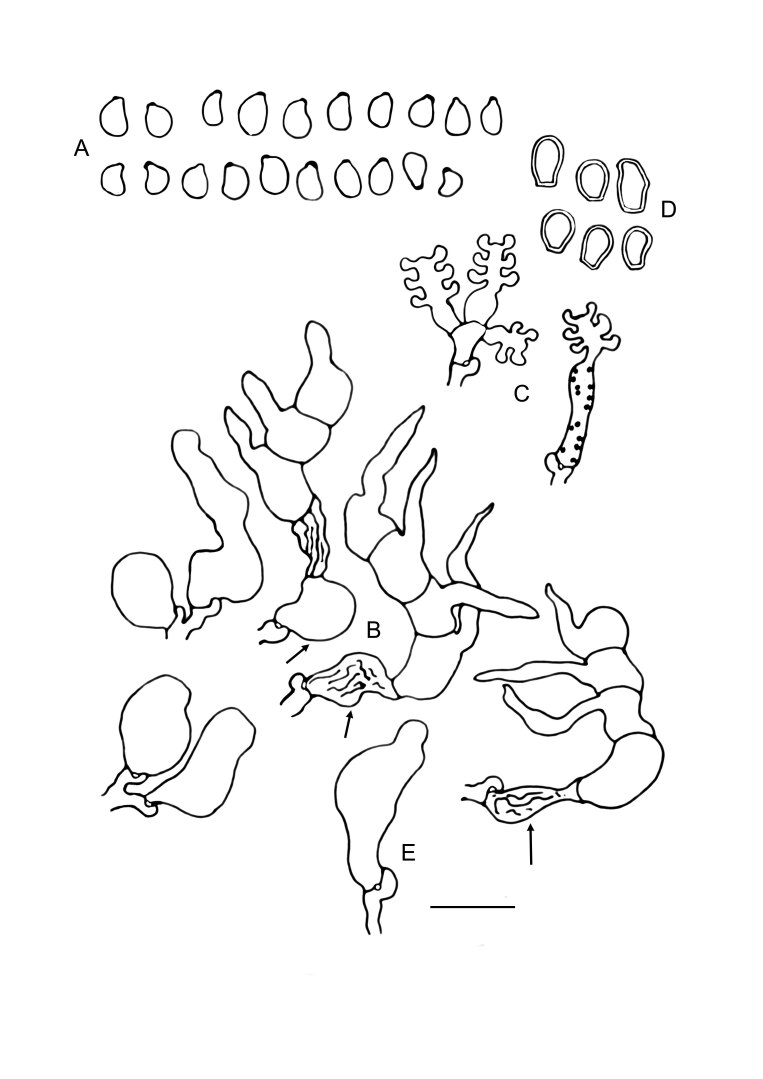
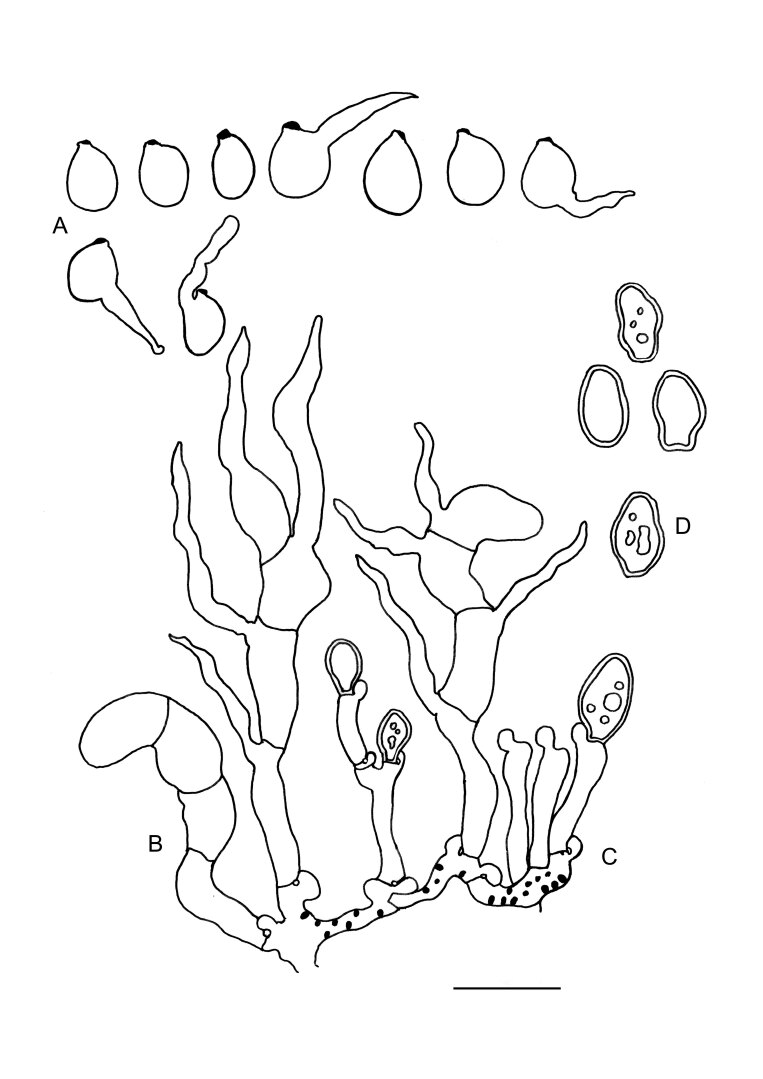
Slooffia micra comb. nov. (KH7222). A. Basidiome (VS 12419). B. Basidiole (left) and cluster of conidiophores (right), note colacosomes in hyphae and conidiophores. C. Three-septate basidium with three sterigmata, the first cell of the basidium and the probasidium are collapsed. D. Conidia. E. Basidiospores. F. Host–parasite interface, Pa = parasite hyphae, Ho = host hyphae, arrowheads indicate some positions of colacosomes. Scale bars = 10 μm.
Fig. 7 .
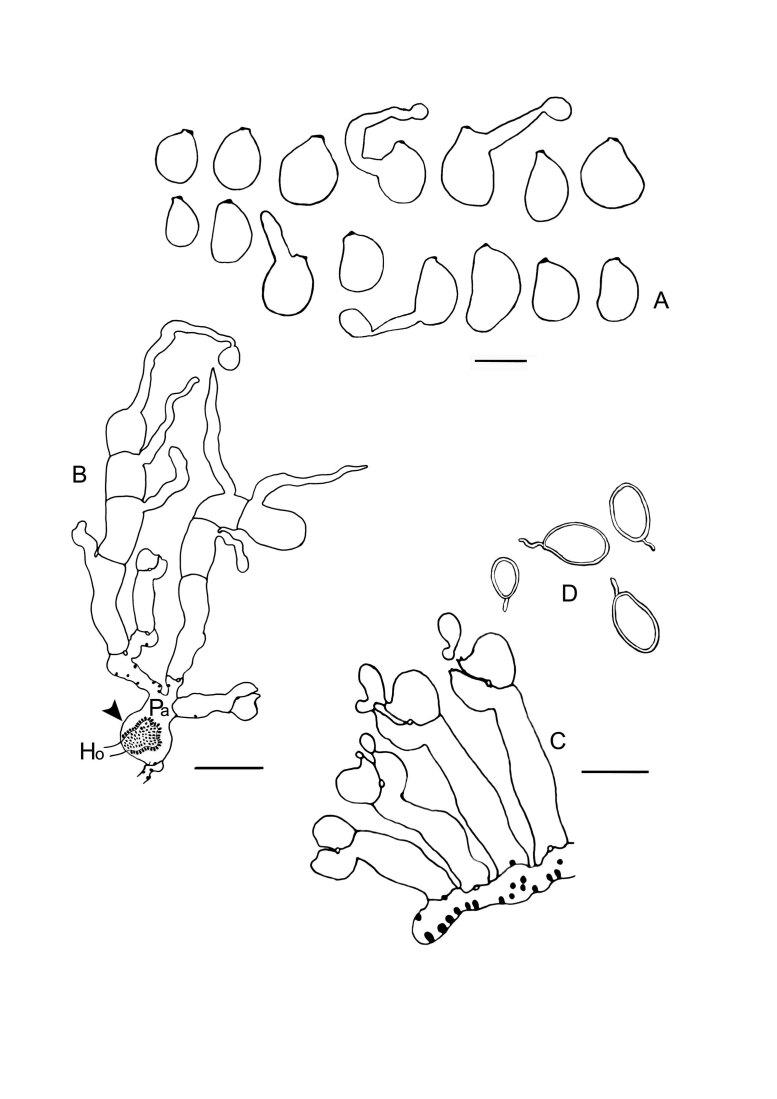
Colacogloea bettinae sp. nov. (NS 19-391). A. Basidiome. B. Three-septate basidium with four sterigmata, note one attached basidiospore. C. Cluster of conidiophores and conidia. D. Conidia. E. Basidiospores. F, G. Host–parasite interface, Pa = parasite cell, Ho = host cell, arrowheads indicate gall-like cells of the parasite enveloping host hyphae, colacosomes are formed along the contact interface within these galls. Scale bars: A = 1 cm; B–G = 10 μm.
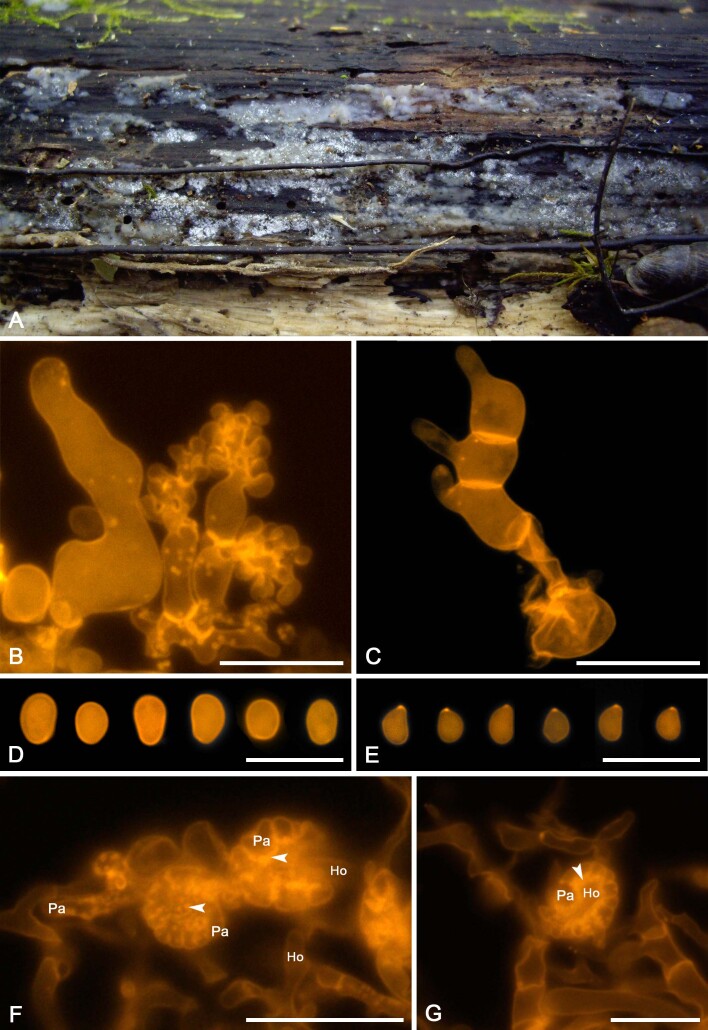
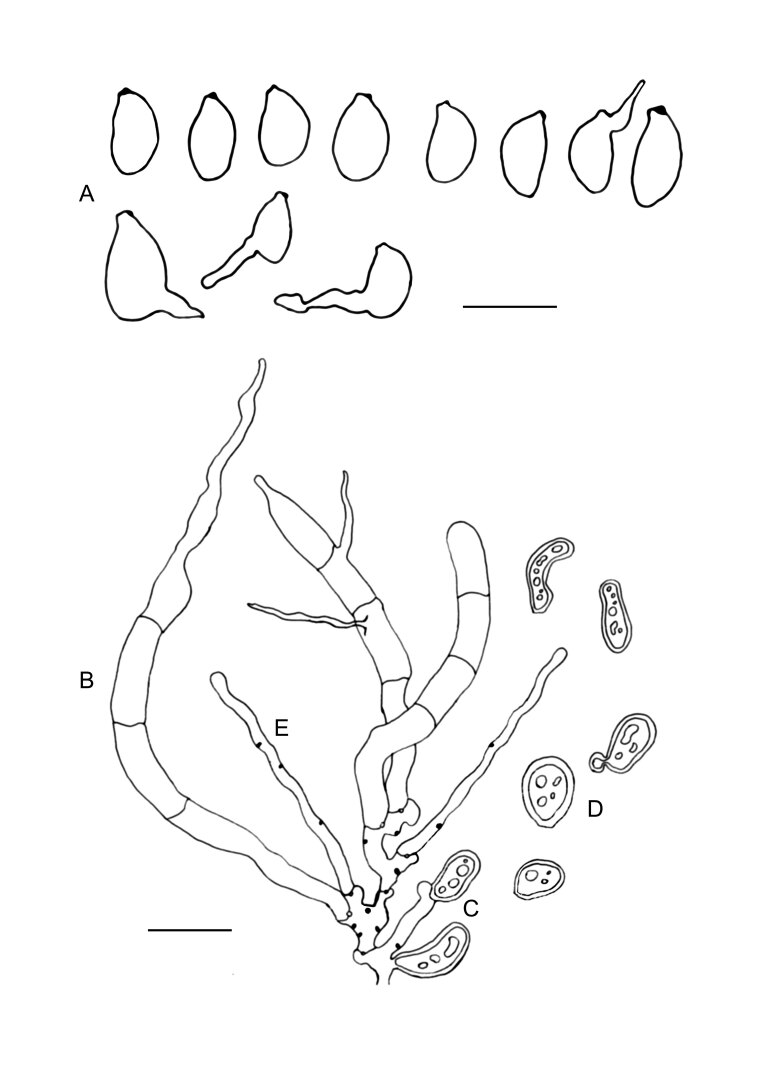
Fig. 9 .
Colacogloea biconidiata sp. nov. (VS 12415). A. Basidiome.. B. Three-septate basidium with four sterigmata, note hyphae with numerous colacosomes and three well stained conidia. C. Type-1 conidiophore and attached conidium with basal clamp. D. Cluster of type-1 conidiophores and conidia. E. Cluster of type-2 conidiophores. F. Upper row represent type-1 conidia, lower row represent type-2 conidia. G. Basidiospores. H. Host–parasite interface, Pa = parasite cell, Ho = host cell, arrowheads indicate some positions of colacosomes. Scale bars: A = 1 cm; B–G = 10 μm.
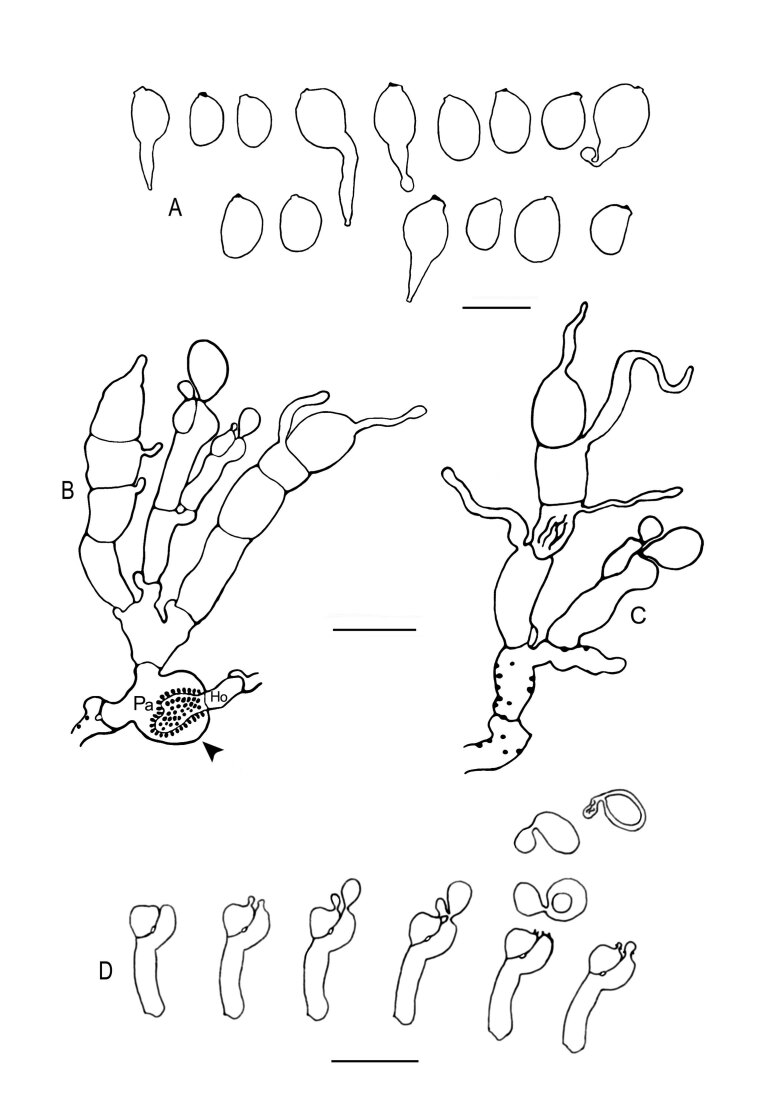
Fig. 11 .
Colacogloea effusa (NS 21-146). A. Basidiome. B. Cluster of basidium, basidiole and hyphidia. C. Three-septate basidium with four sterigmata. D. Conidiophores. E. Conidia. F. Basidiospores. G, H. Host–parasite interface, Pa = parasite cell, Ho = host cell, arrowheads indicate some positions of colacosomes. Scale bars: A = 1 cm; B–H = 10 μm.
Fig. 15 .
Colacogloea microspora sp. nov. (NS 20-141). A. Basidiome. B, C. Three-septate basidia with sterigmata, note colacosomes in hyphae. D. Cluster of conidiophores and conidia, note colacosomes in hyphae. E. Conidia. F. Basidiospores. G. Host–parasite interface, Pa = parasite cell, Ho = host cell, arrowheads indicate some positions of colacosomes. Scale bars: A = 1 cm; B–G = 10 μm.
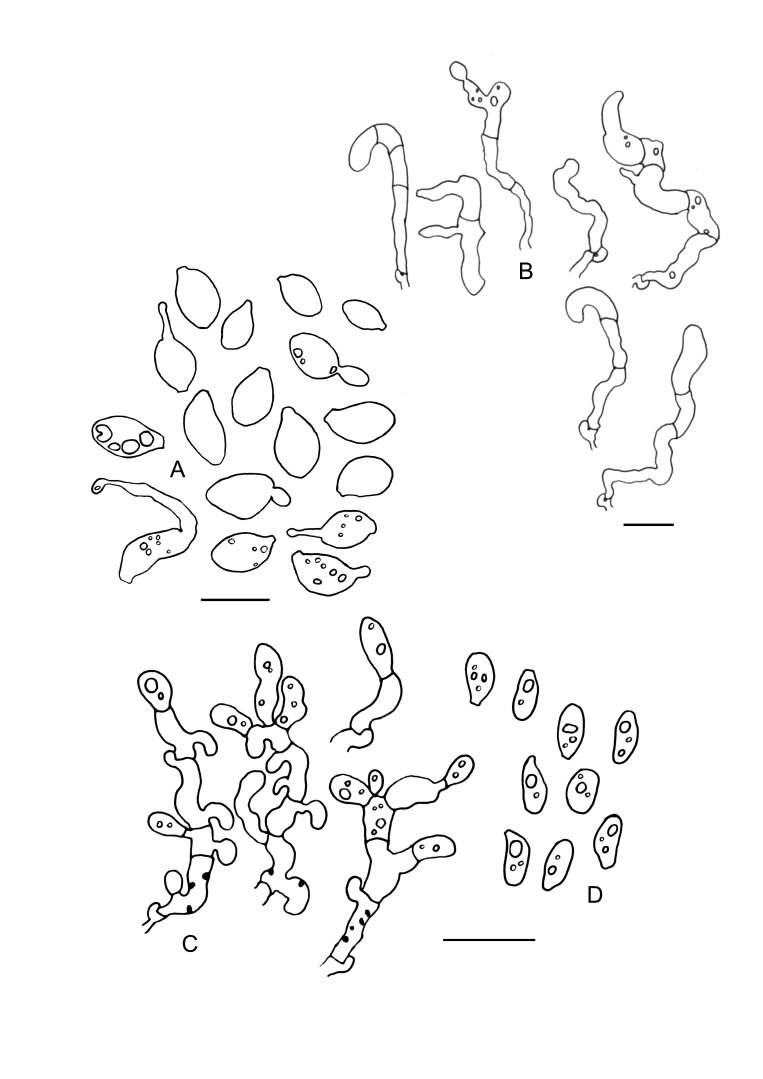
Fig. 17 .
Colacogloea philyla (MG 438). A. Basidiome. B. Cluster of three-septate basidia, basidioles and hyphidia. C. Two-septate basidium with apical sterigma. D. Cluster of conidiophores and conidia, note the colacosomes in the hyphae but not in the conidiophores. E. Conidia. F. Basidiospores. G, H. Host–parasite interface, Pa = parasite cell, Ho = host cell, arrowheads indicate some positions of colacosomes. Scale bars: A = 1 cm; B–H = 10 μm.
Fig. 19 .
Colacogloea universitatis-gandavensis sp. nov. (NS 21-013). A. Basidiome. B. Cluster of three-septate basidium with four sterigmata and conidiophores, note one attached basidiospore. C. Cluster of basidium, basidiole and conidiophores. D. Cluster of conidiophores, note the colacosomes in hyphae. E. Conidia. F. Basidiospores. G. Host–parasite interface, Pa = parasite cell, Ho = host cell, arrowheads indicate some gall-like cells of the parasite enveloping host hyphae, colacosomes are formed along the contact interface within these galls. Scale bars: A = 1 cm; B–G = 10 μm.
Fig. 21 .
Mycogloiocolax gerardii sp. nov. (TR 04096). A. Basidiome. B, C. One-septate basidia with two sterigmata. D. Conidiophore and conidia. E. Conidia. F. Basidiospores. G. Host–parasite interface, Pa = parasite hyphae, Ho = host hyphae, arrowheads indicate some positions of colacosomes. Scale bar: A = cm; B–G = 10 μm.
Phylogenetic reconstruction
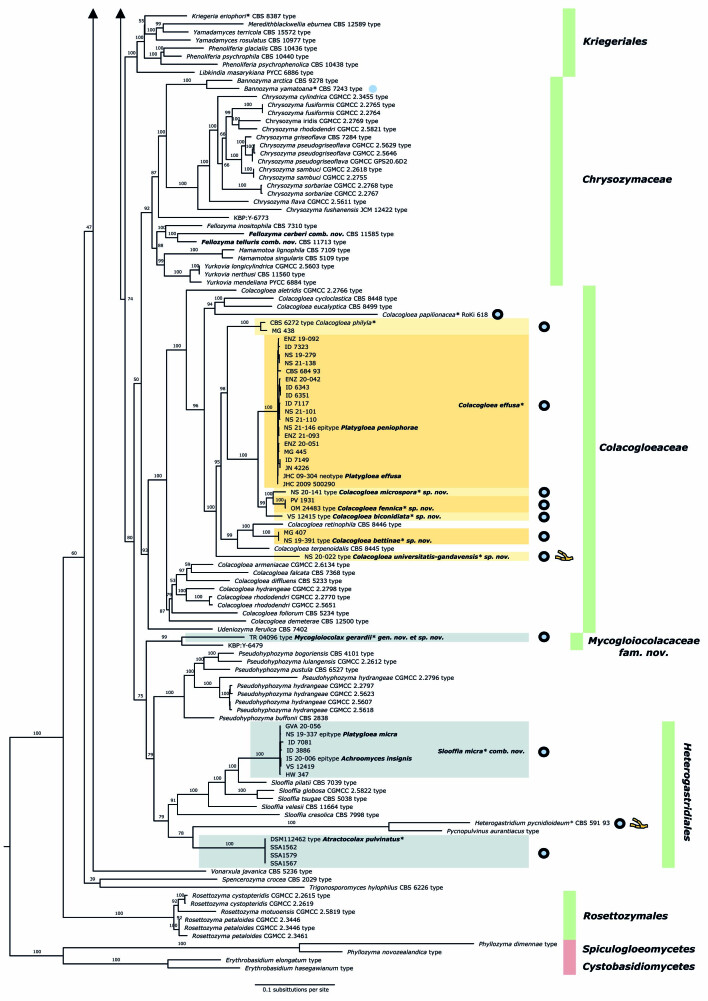
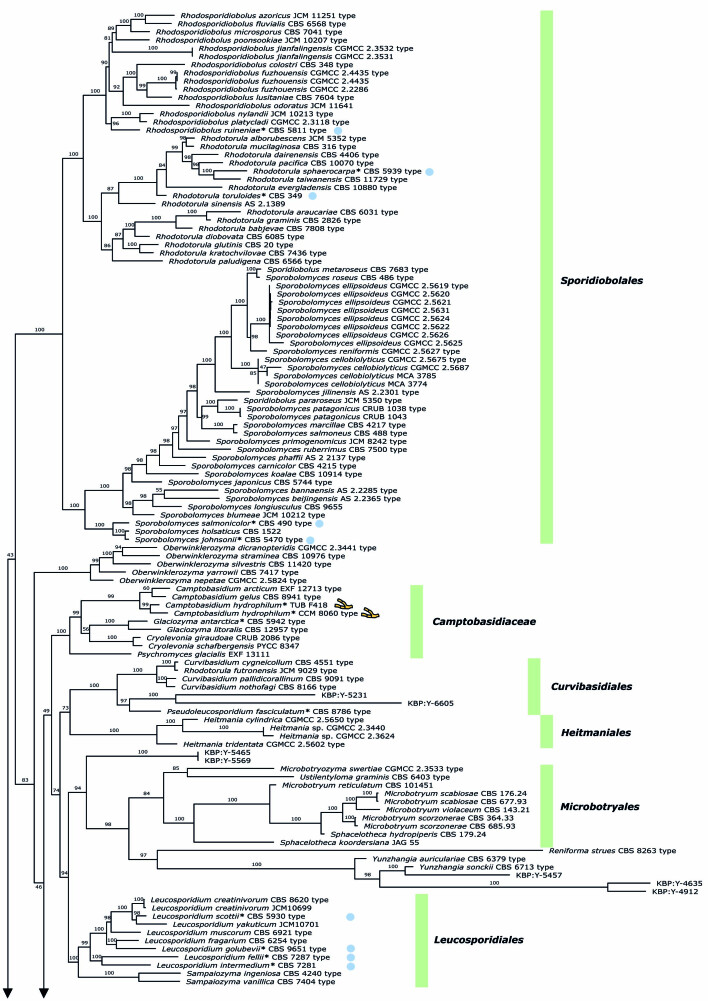
To visualise the placement of colacosome-forming species in the Microbotryomycetes and their evolutionary relationships, we performed a phylogenetic analysis using the commonly used seven loci, incorporating a broad representation of all known lineages within this class. The final dataset included 238 isolates and 5 855 characters, of which 2 815 were parsimony-informative and 2 281 were invariant. A summary of the partitions, number of sequences, number of parsimony-informative- and constant sites, and selected models is presented in Table 4. The full partition model AIC score is 381 436.460 (LnL = -190 078,230 df:640). Figure 3 shows the retrieved tree topology. This seven-locus ML tree is used as basis for clade recognition, an updated classification of Microbotryomycetes, and one of the criteria used for species delimitation.
Fig. 3 .
Phylogenetic relationships of colacosome-forming species in Microbotryomycetes based on a seven-locus ML tree inference. Species names in bold indicate taxonomic novelties. Species which were explicitly investigated for the presence of colacosomes are indicated with a * symbol behind the species name. Species for which the presence of colacosomes was positively assessed are indicated by blue-filled circles. Blue circles with black outline indicate species which have been isolated as a mycoparasite and for which an interaction with a host was reported. Blue circles without outline indicate species which were only reported to form colacosomes in pure culture. Species for which currently only a filamentous morph was observed are indicated by a branching hyphae icon, for all other species in the tree, at least a yeast morph is known. Clades investigated in detail in this study are indicated with boxes. Boxes in yellow tones represent the Colacogloea effusa complex. Green vertical lines represent the highest described taxon available for species in the tree (family or order). Numbers on branches indicate ultrafast bootstrap values. Cystobasidiomycetes and Spiculogloeomycetes are used as outgroup.
All currently described families and orders of Microbotryomycetes are recovered as monophyletic clades, with support values given in Table 5. Deeper nodes, depicting the relationships among higher taxa, are not resolved. Clades containing isolates and specimens that were newly sequenced are indicated in boxes in the phylogenetic reconstruction (Fig. 3). The inclusion of colacosome-forming mycoparasites allows to recognise several new phylogenetic lineages in Microbotryomycetes. A first new lineage comprises four isolates of Atractocolax pulvinatus, which forms a distinct lineage within Microbotryomycetes. A second new lineage comprises the clade of Mycogloiocolax gerardii sp. nov. and the currently undescribed yeast isolate KBP Y-6479, for which the family Mycogloiocolacaceae fam. nov. is proposed (see taxonomy section). Within the genus Colacogloea, five new lineages can be recognised, each representing a new species in the Colacogloea effusa species complex (see taxonomy section). A separate ML phylogenetic reconstruction of the genus Colacogloea based on the three rDNA loci SSU, ITS and LSU (results not shown) rendered the same topology as retrieved in our seven-locus class-wide reconstruction. Seven isolates identified as Platygloea micra cluster within the genus Slooffia with high support. These isolates are clearly conspecific, but are distant from the other described species within the genus, prompting a recombination (see taxonomy section).
Taxonomy
Based on the combined results from comparison of micromorphological characters of filamentous morphs, assimilation growth essays of yeast morphs, and the seven-locus phylogenetic reconstruction, we draw the following taxonomic conclusions as outlined below.
Order Heterogastridiales Oberw. & R. Bauer, Mycologia 82: 57. 1990.
Slooffia Q.M. Wang et al., Stud. Mycol. 81: 186. 2015. emend.
Generic description: Genus of dimorphic fungi. Basidiomata are absent. Filamentous morph develops intrahymenial in the host, sometimes producing a whitish layer overgrowing the host basidiome. Hyphal system monomytic, hyaline, thin-walled, smooth, clamped at all septa. Hyphidia absent. Cystidia absent. Basidia cylindrical to slightly clavate, often strongly curved to a 90° angle, transversally septate, mature basidia four-celled, clamped, thin-walled, originating from a distinct probasidium which collapses after maturation of the basidium. Sterigmata originating laterally or apically from basidial cells, rarely bifurcating. Basidiospores of irregular shape, smooth, hyaline, inamyloid. Germination of basidiospores occurs by either hyphae, budding or secondary spore production. Conidiophores stalked, basally clamped, with apically numerous appendages. Conidia irregularly shaped, thick-walled, cyanophilous.
Habitat, substrate, and ecology: Slooffia species have been isolated as yeasts from soils, litter, insect faeces and basidiomata of Myxarium podlachicum. Yeast morphs are presumed to have a saprobic ecology. A filamentous morph has only been observed for Slooffia micra comb. nov., which represents a colacosome-interacting mycoparasitic stage, developing intrahymenially in the host Myxarium podlachicum.
Distribution: Slooffia species have been recorded from various European countries, Brazil, India and the USA (Hamamoto et al. 2011, Sampaio 2011, Bezerra et al. 2013, Buzzini et al. 2017).
Type: Slooffia tsugae (Phaff & Carmo Souza) Q.M. Wang, F.Y. Bai, M. Groenew. & Boekhout
Slooffia micra (Bourdot & Galzin) Schoutteten, comb. nov. MycoBank MB 848660. Figs 4, 5.
Fig. 4 .
Slooffia micra comb. nov. (KH7222) line drawings. A. Basidiospores. B. Basidia. C. Cluster of conidiophores. D. Conidia. E. Basidioles, arrows indicate probasidia. Black dots represent colacosomes. Scale bar = 10 μm.
Basionym: Platygloea micra Bourdot & Galzin, Bull. Trimestriel Soc. Mycol. France 39: 261. 1924.
Typus: France, Aveyron, on rotten wood of Populus, 23 Oct. 1915, A. Galzin (holotype, PC Bourdot 19438°). As only one specimen of this species is available in the collection of Bourdot and Galzin in the Paris herbarium (PC), this specimen is to be interpreted as the holotype, although it was not mentioned as such by the original authors (ICNafp Art. 9.1). Belgium, Prov. West-Vlaanderen, Ieper, Palingbeek, on piece of wood of an unidentified deciduous tree, growing in the hymenium of Myxarium podlachicum, 16 Oct. 2019, M. Detollenaere (epitype GENT NS 19-337*°, designated here, MycoBank MBT 10013261, culture ex-epitype DSM 112421).
Synonym: Achroomyces insignis Hauerslev, Mycotaxon 49: 218. 1993.
Typus: Denmark, Zealand, Copenhaguen, Hareskoven, on decorticated branch of an unidentified tree, growing in the hymenium of Myxarium podlachicum, 21 Sep. 1991, K. Hauerslev (holotype, C C19753 = KH7222°). The Netherlands, Prov. Groningen, Tjuchem, Huisweersterbos, on decorticated branch of an unidentified deciduous tree, growing in the hymenium of Myxarium podlachicum, 14 Feb. 2020, I. Somhorst (epitype GENT IS 20-006*°, designated here, MycoBank MBT 10013262, culture ex-epitype DSM 112423).
Description of filamentous morph: Intrahymenial, often invisible but sometimes producing a whitish pruinose layer on the host species. Monomitic; hyphae hyaline, thin-walled, smooth, clamped at all septa, 1.1–2.7 µm in diam. Hyphidia absent. Cystidia absent. Probasidia variable in shape, often pyriform, thin walled, collapsing after maturation of the basidium, 8.1–17.8 × 2.3–8.6 µm. Basidia narrowly clavate, often strongly curved, (21.6–)22.2–29.8(–31.4) × (4.5–)4.8–6.4 µm (n=20/1), transversally septate, mature basidia four-celled, often somewhat constricted at each septum, clamped at the base, thin-walled. Sterigmata simple or more rarely bifurcate, up to 18 µm long. Basidiospores of irregular shape, ellipsoid-angular to drop- or comma-shaped, (2.9–)3.0–4.5(–4.8) × 1.5–2.9(–3.0), L = 3.69, W = 2.19, Q’ = (1.35–)1.36–2.21(–2.25), Q = 1.72 (n = 30/1), germinating by hyphae, budding or secondary spores. Conidiophores stalked, stalk often somewhat widened, basally clamped, with numerous apical appendages (where conidia are formed), (9.1–)10.5–22.3(–30.6) × (1.4–)2.0–3.9(–4.3) µm. Conidia irregularly shaped, ellipsoid to curved, often with one flattened side, thick-walled (wall up to 1 µm), cyanophilous, (4.0–) 4.1–5.7(–5.8) × (2.8–)3.1–3.9(–4.3) µm. Colacosomes scattered, no vesicular gall-like cells observed.
Description of yeast morph: After growth on YM agar plates for 1 mo at 22 °C, the streak culture is white to cream-coloured, glistening, mucoid and smooth. The margin is entire. Cells are subglobose to ovoid, occurring singly or in pairs, and proliferating by polar budding. Good growth on D-glucose, L-sorbose, D-glucosamine, D-arabinose, sucrose (delayed), a,a-trehalose, me a-D-glucoside, cellobiose, raffinose, melezitose, ribitol, D-glucitol, D-mannitol, 5-keto-D-gluconate, D-gluconate, and D-glucuronate. Weak growth on maltose, lactose, glycerol, L-arabinitol, galactitol, ethanol, D-glucarate, and L-tartaric acid. No growth on D-galactose, D-ribose, D-xylose, L-arabinose, L-rhamnose, salicin, melibiose, inulin, starch, erythritol, myo-inositol, D-galacturonate, DL-lactate, succinate, citrate, D-tartaric acid, and L-malic acid. No growth in the presence of 5 %, 8 %, and 10 % NaCl. No growth on MEA with 50 % and 60 % glucose. No starch-like substance is produced. Urea hydrolysis and the Diazonium blue B reaction is positive. Maximum growth temperature: 35 °C.
Habitat and distribution: Growing in the hymenium of Myxarium podlachicum (= M. subhyalinum), for further synonymy see Spirin et al. (2019). This species has been recorded from various European countries: Belgium, Denmark, Germany, France, Norway and The Netherlands.
Materials examined: Denmark, Zealand, Enghave Skov ved Dragsholm, on decorticated branch of Fraxinus, growing in the hymenium of Myxarium podlachicum, 28 Jun. 2009, J. Heilmann-Clausen, JHC 09-049 (H, duplicate in GENT). Belgium, Prov. Antwerpen, Mechelen, Kauwdaalbos, on fallen log op Populus, growing in the hymenium of Myxarium podlachicum, 28 Feb. 2020, G. Van Autgaerden, GVA 20-056* (GENT). Netherlands, Prov. Utrecht, Houten, Nieuw Wulven, on piece of wood of an unidentified deciduous tree, growing in the hymenium of Myxarium podlachicum, 8 Mar. 2019, I. Nannenga-Bruggeman, ID 3883* (GENT); Prov. Utrecht, Zeist-West, De Brink, on piece of wood of an unidentified deciduous tree, growing in the hymenium of Myxarium podlachicum, 2 Oct. 2020, I. Nannenga-Bruggeman, ID 7081* (GENT); Prov. Gelderland, Ruurlo, Morsdijk, on fallen decorticated branch of Alnus, growing in the hymenium of Myxarium podlachicum, 27 Jul. 2020, H. Wassink, HW 347* (GENT). Norway, Hedmark, Stange, Rotlia, rotten stem of Corylus avellana, growing on Myxarium podlachicum, 26 Sep. 2018, V. Spirin, VS 12419* (O, H).
Notes: Colacosomes in this species are formed in mycoparasite hyphae in places where physical contact with other hyphae occurs (mostly host hyphae). Colacosomes can also be found in conidiophores and probasidia. In certain places at the host–parasite interface, hyphae of the mycoparasite coil around hyphae of the host, resulting in rosette-like structures when viewed in epifluorescence microscopy. In these structures, colacosomes are formed abundantly at the contact surface (see Fig. 5F, G). Colacosomes have also been observed attaching to hyphae of the mycoparasite, which may be interpreted as self-parasitism. During fluorescence microscopical investigation of the holotypes of A. insignis (1991) and P. micra (1915), colacosomes could easily be observed. This may indicate a high durability of these structures.
Microbotryomycetes incertae sedis
Family Chrysozymaceae Q.M. Wang et al., Stud. Mycol. 81: 183. 2015.
Fellozyma cerberi (A.M. Yurkov et al.) Schoutteten & Yurkov, comb. nov. MycoBank MB 848664.
Basionym: Hamamotoa cerberi A.M. Yurkov et al., Mycol. Prog. 15: 854. 2016.
Fellozyma telluris (A.M. Yurkov et al.) Schoutteten & Yurkov, comb. nov. MycoBank MB 848665.
Basionym: Hamamotoa telluris A.M. Yurkov et al., Mycol. Prog. 15: 855. 2016.
Family Colacogloeaceae Q.M. Wang et al., Stud. Mycol. 81: 182. 2015.
Colacogloea Oberw. & Bandoni, Canad. J. Bot. 68: 2532. 1991. emend.
Generic description: Genus of dimorphic fungi. Basidiomata pulvinate or absent. Filamentous morphs mostly develop intrahymenially in the hymenium of their host species, producing a yellow to orange, slimy to arid layer overgrowing the host basidiome. Hyphal system monomytic, hyaline, thin-walled, smooth, clamped at all septa. Hyphidia present in some species. Cystidia absent. Basidia cylindrical to clavate, straight to sinuous to curved in some species, transversally septate, two- to four-celled, clamped at basal cell, thin-walled, without distinct probasidium. Sterigmata originating laterally or apically from basidial cells. Basidiospores ellipsoid to curved, smooth, hyaline, thin-walled, often with a prominent apiculus. Germination of basidiospores either occurs by hyphae, budding or secondary spore production. Conidia present in most species, usually thick-walled and cyanophilous, globose, ellipsoid to ovoid or irregularly shaped, monokaryotic or dikaryotic, zygoconidia present in some species. Yeast colonies are usually cream-coloured, mucoid to butyrous. Yeast cells proliferate by polar budding, no ballistoconidia are formed. Major CoQ system Q-10.
Habitat, substrate, and ecology: Filamentous morphs of Colacogloea species which have been observed to engage in mycoparasitic interactions were mainly isolated from the hymenia of corticioid fungi, especially from the genera Peniophorella and Tubulicrinis. Colacogloea papilionacea was isolated from bark beetle galleries of Pinus sylvestris and is characterised by a dikaryotic yeast morph. Colacogloea species of which currently only the yeast morph has been observed were isolated from marine and terrestrial environments, including soils and phylloplanes. Yeast morphs are presumed to have a saprobic ecology.
Distribution: Colacogloea species have been recorded from various countries, including Austria, Belgium, Brazil, Canada, China, Denmark, Finland, France, Germany, India, Italy, Japan, The Netherlands, Norway, Poland, Portugal, Russian Federation, Spain, Sweden, Switzerland, and the Unites States of America (Sampaio et al. 2011, Bezerra et al. 2013, Buzzini et al. 2017, Menolli & Sánchez-García 2020).
Type: Colacogloea effusa (J. Schröt.) V. Malysheva et al.
Colacogloea bettinae Schoutteten & Begerow, sp. nov. MycoBank MB 848655. Figs 6, 7.
Fig. 6 .
Colacogloea bettinae sp. nov. (NS 19-391) line drawings. A. Basidiospores and germinating basidiospores with secondary spores. B. Cluster of basidia and conidiophores. C. Cluster of conidiophores, showing subsequent stages of conidiogenesis. Each conidiophore consists of two conidiogenous cells. Each cell produces a conidium, of which one grows larger than the other. Subsequently the two daughter conidia fuse, and the cellular content of the smaller conidium is transferred to the larger conidium, after which the zygoconidium is abscised. The cell wall of the smeller conidium remains attached to the larger conidium. D. Conidia. E. Gall-like cell of the parasite (Pa) enveloping a host hyphae (Ho). Black dots represent colacosomes. Note the different distribution of colacosomes in the gall-like cell and the hyphae. Scale bars = 10 μm.
Etymology: Named after Bettina Greschner-Aschenbrenner, who conducted an extensive study of the Colacogloea effusa species complex for her master dissertation (Diplomarbeit) at the former Lehrstuhl für Spezielle Botanik und Mykologie (University of Tübingen), supervised by the late dr. Robert Bauer and prof. Franz Oberwinkler.
Typus: Netherlands, Prov. Gelderland, Veluwe region, Brummen, Leusveld, on a decorticated branch of an unidentified deciduous tree, growing in the hymenium of Peniophorella pubera, 15 Nov. 2019, N. Schoutteten (holotype GENT NS 19-391*°, culture ex-type DSM 112418).
Description of filamentous morph: Intrahymenial, producing a whitish to yellowish slimy layer on the hymenial surface of the host basidiome. Monomitic; hyphae hyaline, thin-walled, smooth, clamped at all septa, 2.7–4.4 µm in diam. Hyphidia absent. Cystidia absent. Basidia tubular-clavate, sinuous to strongly curved, (25.5–)31–50(–51) × 4.6–7.2(–7.4) µm (n = 20/1), transversally septate, four-celled when mature, clamped at the base, thin-walled, often arranged in clusters of 2–5. Sterigmata up to 46 µm long. Basidiospores ellipsoid, with ventral side often flattened to concave, (6.7–)6.8–8.8(–9.0) × 4–5.9(–6.7) µm, L = 7.60 µm, W = 4.96 µm, Q’ = (1.1–)1.2–1.8, Q = 1.54 (n = 60/2), often with prominent apiculus up to 1.8 × 1.2 µm, germinating by hyphae, budding or secondary spore production. Conidiophores comprised of two cells being separated by a septum, each cell apically giving rise to a conidium, basally clamped, often arranged in clusters, intermixed with basidia, 12.5–24.5 × 3–4.8 µm. Mature conidia ellipsoid to ovoid, more rarely subfusiform, sometimes asymmetrical or becoming oblong, rarely with a small lateral outgrowth, always with an appendage (cell wall remnant of the smaller twin-conidium), thick-walled (wall up to 1 µm), cyanophilous, dikaryotic, 6–8.1(–8.3) × (2.9–)3.0–4.6(–4.8) µm (n = 30). Colacosomes arranged both scattered in parasite hyphae and in vesicular gall-like cells produced by this species.
Description of yeast morph: After growth on YM agar plates for 1 mo at 22 °C, the streak culture is white to cream-coloured, glistening, mucoid and smooth. The margin is entire. Cells are subglobose to ovoid, occurring singly or in pairs, and proliferating by polar budding. Good growth on D-glucose, D-glucosamine, D-ribose, D-arabinose, sucrose, me a-D-glucoside, glycerol, ribitol, D-glucitol, D-mannitol, 5-keto-D-gluconate, D-gluconate, and succinate. Weak growth on L-sorbose, D-xylose, L-arabinose, L-rhamnose, lactose, raffinose, galactitol, ethanol, D-glucarate, and L-tartaric acid. No growth on D-galactose, maltose, a,a-trehalose, cellobiose, salicin, melibiose, melezitose, inulin, starch, erythritol, L-arabinitol, myo-inositol, D-glucuronate, D-galacturonate, DL-lactate, citrate, D-tartaric acid, and L-malic acid. Growth in the presence of 5 % and 8 % but not 10 % NaCl. Weak growth on MEA with 50 % and 60 % glucose. No starch-like substance is produced. Urea hydrolysis and the Diazonium blue B reaction is positive. Maximum growth temperature: 35 °C.
Habitat and distribution: This species has up to now only been found in the Netherlands, in mixed forests, always associated with the host species Peniophorella pubera.
Materials examined: The Netherlands, Drenthe, Gasteren, Gasterensche Holt, on a rotten branch of an unidentified deciduous tree, growing in the hymenium of Peniophorella pubera, 5 Sep. 2020, R. Enzlin, ENZ 20-043 (GENT); Gelderland, Bronckhorst, Hekenbroek, Hoog Keppel, on a fallen branch of an unidentified tree, growing in the hymenium of Peniophorella pubera, 19 Jul. 2020, M. Gotink, MG 407* (GENT).
Notes: This is one of the two Colacogloea species described in this study which agrees with the morphotype illustrated by Martin (1940) (see also C. universitatis-gandavensis sp. nov. and in discussion). Conidiogenesis in this species is of the same type as in C. universitatis-gandavensis, where more elaborate observations are provided. The colacosome organisation is similar to the one observed in C. universitatis-gandavensis. Colacosomes are mainly arranged in vesicular gall-like cells produced by the mycoparasite. To a lesser extent, colacosomes are also scattered in mycoparasite hyphae. The cell wall of these vesicular gall-like cells invaginates at places where a host hypha makes physical contact. The latter continues to grow into the invagination. As a result, the host hypha is surrounded by the gall-like cell of the mycoparasite. Along the contact surface, colacosomes are formed in the gall-like cell at regular distance from each other.
Colacogloea biconidiata Schoutteten, sp. nov. MycoBank MB 848656. Figs 8, 9.
Etymology: Referring to two different types of conidia in this species.
Typus: Norway, Hedmark, Gitvola, on decorticated branch of Picea abies, growing in the hymenium of Peniophorella praetermissa s.l., 26 Sep. 2018, V. Spirin (holotype O VS12415*°, isotype GENT GENTFT00143, culture ex-type DSM 112405).
Description of filamentous morph: Intrahymenial, producing a yellow to orange, gelatinous layer on the host, remaining visible as yellow or orange patches when dried. Monomitic; hyphae hyaline, thin-walled, smooth, clamped at all septa, 1.3–4.5 µm in diam. Hyphidia present, simple, 1–2 µm in diam. Cystidia absent. Basidia tubular-clavate, straight to sinuous or slightly curved, (31.1–)31.8–50.2(–50.6) × 4.1–5.3(–6.9) µm (n = 17/1), transversally septate, four-celled when mature, clamped at the base, thin-walled. Sterigmata up to 54 µm long. Basidiospores ellipsoid to broadly ellipsoid, 6.7–12.2(–12.5) × 4.4–8.8(–10.2) µm, L = 8.06, W = 5.22, Q’ = 1.2–1.8(–1.9), Q = 1.49 (n = 67/1), with distinct apiculus up to 2.5 × 2.3 µm, germinating by hyphae, budding or secondary spores. Conidia of two types: (1) irregularly shaped - ellipsoid, subfusiform to oblong or barrel-shaped, sometimes angular, thick-walled (wall up to 1.2 µm), strongly cyanophilous, 6.1–13.2(–15.4) × 3.2–7.1(–7.2) (n = 20/1); (2) predominantly (sub)globose, thick-walled, cyanophilous, (3.1–)3.5–4.5(–4.6) × (2.7–)2.8–3.7(–3.8) (n = 20/1). Colacosomes scattered, no vesicular gall-like cells observed.
Description of yeast morph: After growth on YM agar plates for 1 mo at 22 °C, the streak culture is white to cream-coloured, glistening, mucoid and smooth. The margin is entire. Cells are subglobose to ovoid, occurring singly or in pairs, and proliferating by polar budding. Growth on D-glucose, D-glucosamine, D-ribose, D-arabinose, me a-D-glucoside, glycerol, D-glucitol, D-gluconate, succinate and L-malic acid. Weak growth on maltose (delayed), salicin, inulin, galactitol, and D-tartaric acid. No growth on D-galactose, L-sorbose, D-xylose, L-arabinose, L-rhamnose, sucrose, a,a-trehalose, cellobiose, melibiose, lactose, raffinose, melezitose, starch, erythritol, ribitol, L-arabinitol, D-mannitol, myo-inositol, 5-keto-D-gluconate, D-glucuronate, D-galacturonate, DL-lactate, citrate, ethanol, D-glucarate, and L-tartaric acid. Growth in the presence of 5 % but not 8 % and 10 % NaCl. Weak growth on MEA with 50 % but not 60 % glucose. No starch-like substance is produced. Urea hydrolysis and the Diazonium blue B reaction is positive. Maximum growth temperature: 35 °C.
Habitat and distribution: Currently only known from the type location in Norway, where it was collected in in a subalpine grazing area, on coniferous wood.
Material examined: This species is only known from the type collection.
Notes: This is the only species in the genus currently known to produce two types of conidia, produced by two distinct types of conidiophores. The colacosomes of this species occur scattered throughout the mycoparasite hyphae, more densely arranged in the places of physical contact between host and parasite cells. Interestingly, this mycoparasite seems to induce additional branching of host hyphae, probably to increase the contact surface where colacosomes can be formed.
Colacogloea effusa (J. Schröt.) V. Malysheva et al., Mycol. Prog. 20: 414. 2021. Figs 10, 11.
Fig. 10 .
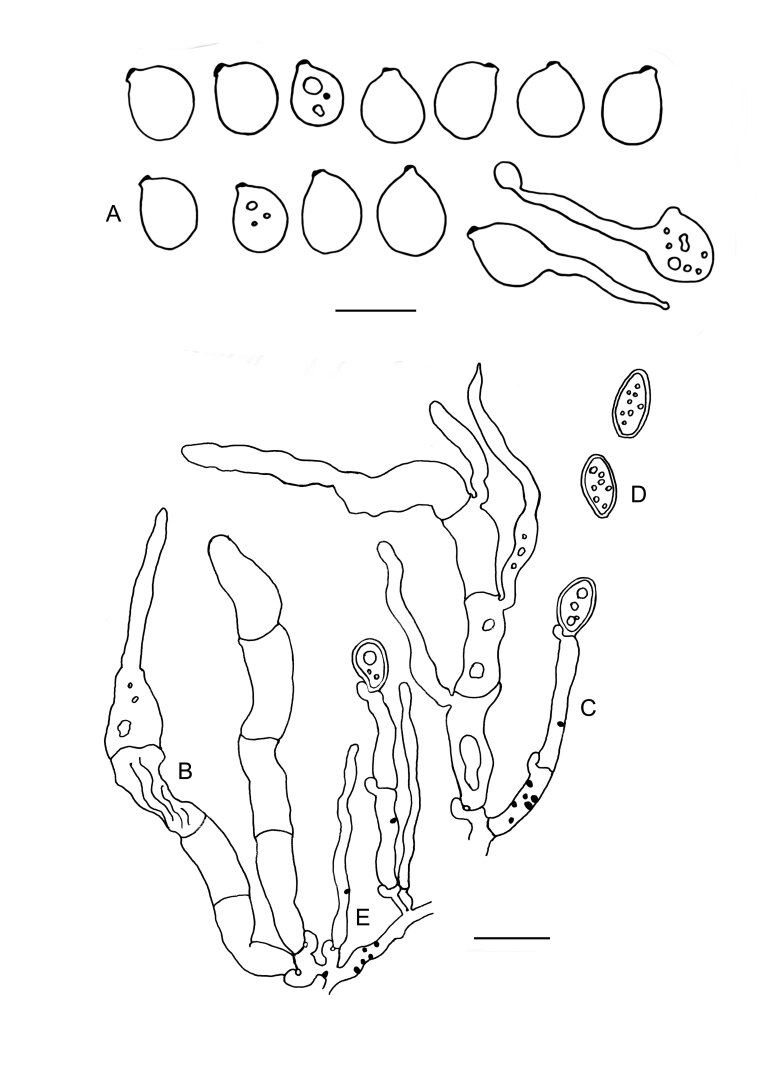
Colacogloea effusa (NS 21-146) line drawings. A. Basidiospores and germinating basidiospores by hyphae and secondary spores. B. Basidia. C. Conidiophore. D. Conidia. E. Hyphidia. Black dots represent colacosomes. Scale bars = 10 μm.
Basionym: Platygloea effusa J. Schröt. in Cohn, Kryptogamen Flora von Schlesien 3(1): 384. 1889.
Typus: Denmark, Midtjylland: Norddjurs, Løvenholm Skov, on rotten deciduous wood, 26 Aug. 2009, J. Heilmann-Clausen (neotype C JHC 09–304, isoneotype GENT GENTFT00145).
Synonyms: Colacogloea peniophorae (Bourdot & Galzin) Oberw. & Bandoni, Canad. J. Bot. 68: 2532. 1991.
Platygloea peniophorae Bourdot & Galzin, Bull. Trimestriel Soc. Mycol. France 25: 17. 1909.
Typus: France, Allier, Saint-Priest, 10 Aug. 1908, H. Bourdot (lectotype PC Bourdot 5945, designated here, MycoBank MBT 10013259). Ibid., Saint-Bonnet de Tronçais, Forêt de Tronçais, réserve de Futaine Colbert, 16 Nov. 2021, N. Schoutteten (epitype GENT NS 21-146*°, designated here, MycoBank MBT 10013260, culture ex-epitype DSM 113583).
Description of filamentous morph: Intrahymenial, first visible as yellowish to orange, slimy patches or pustules on the host species, later fusing together and forming opalescent or yellowish, crustaceous basidiomes with tuberculate hymenial surface, darkening to reddish or brownish and remaining well-visible after drying. Monomitic; hyphae hyaline, often guttulate, thin-walled, smooth, clamped at all septa, 1.8–2.6 µm in diam. Hyphidia simple or occasionally branched, 1.2–2.5 µm in diam. Cystidia absent. Basidia narrowly tubular-clavate, straight to curved, sometimes slightly sinuous, (33.5–)42.2–64.1(–70.8) × (4.4–)4.6–5.6 µm (n = 40/2), transversally septate, four-celled when mature, clamped at the base, thin-walled, without distinct probasidium. Sterigmata up to 48 µm long. Basidiospores ellipsoid to reniform, (6.7–) 6.9–10.6(–11) × (4.5–)4.7–7.3(–8) µm, L = 8.33 µm, W = 5.81 µm, Q’ = (1.0–)1.2–1.7, Q = 1.4–1.7 (n = 80/2), with prominent apiculus up to 2 µm, germinating by hyphae, budding or secondary spores. Conidia ellipsoid, ovoid to subfusiform, often asymmetric, sometimes angular, mostly guttulate, thick-walled (up to 1 µm), strongly cyanophilous, basally clamped, (5.7–)6.5–8.7(–8.9) × (3.1–)3.2–4(–4.1) µm. Colacosomes scattered, no vesicular gall-like cells observed.
Description of yeast morph: After growth on YM agar plates for 1 mo at 22 °C, the streak culture is white to cream-coloured, glistening, mucoid and smooth. The margin is entire. Cells are subglobose to ovoid, occurring singly or in pairs, and proliferating by polar budding. Growth on D-glucose, D-ribose, D-arabinose, me a-D-glucoside, glycerol, ribitol, D-glucitol, D-mannitol, D-gluconate, succinate, and D-glucarate. No growth on D-galactose, L-sorbose, D-glucosamine, D-xylose, L-arabinose, L-rhamnose, sucrose, maltose, a,a-trehalose, cellobiose, salicin, melibiose, lactose, raffinose, melezitose, inulin, starch, erythritol, L-arabinitol, galactitol, myo-inositol, 5-keto-D-gluconate, D-glucuronate, D-galacturonate, DL-lactate, citrate, ethanol, L-malic acid, L-tartaric acid, and D-tartaric acid. Growth in the presence of 5 % and 8 % but not 10 % NaCl. Weak growth on MEA with 50 % and 60 % glucose. No starch-like substance is produced. Urea hydrolysis and the Diazonium blue B reaction is positive. Maximum growth temperature: 35 °C.
Habitat and distribution: Colacogloea effusa is presumably the most common species in the C. effusa species complex, with records from most European countries. Most specimens we collected, isolated and sequenced belong to this species. On a global scale, this species has been reported from various continents: Africa, Asia, Europe, North America, and South America (most of them under the name Colacogloea peniophorae). However, since most of these observations have been identified based on micromorphological characteristics only, it may well be that a substantial part of them belongs to other species within this species complex. It is also possible that previous reports of C. effusa actually comprise yet undescribed species, which may be especially true for specimens reported outside our sampling area. All our C. effusa collections come from deciduous wood substrates in temperate forests in Europe.
Materials examined: Finland, Varsinaissuomi, Raasepori, Framnäs, on an unidentified deciduous tree, growing in the basidiome of Peniophorella praetermissa s.l., 21 Nov. 2019, J. Pennanen, JN 4226* (H). France, Departement Allier, St. Bonnet de Tronçais, Tour de l’étang, on an unidentified deciduous tree, growing in the basidiome of Peniophorella praetermissa s.l., 15 Nov. 2021, N. Schoutteten, NS 21-138* (GENT); Departement Allier, St. Bonnet de Tronçais, Réserve de Nantigny, on an unidentified deciduous tree, growing in the basidiome of Peniophorella praetermissa s.l., 14 Nov. 2021, N. Schoutteten, NS 21-128* (GENT). Italy, Piedmont: Alessandria, Voltaggio, Capanne di Marcarolo Nat. Regional Park, on a fallen branch of an unidentified tree, growing in the basidiome of Peniophorella praetermissa s.l., 16 Oct. 2019, N. Schoutteten, NS 19-279* (GENT). Netherlands, Prov. Utrecht, Zeist, Beerschoten, on decorticated piece of deciduous wood, growing in the basidiome of Peniophorella praetermissa s.l., 10 Oct. 2019, I. Nannenga-Bruggeman, ID 6351* (GENT); ibid. ID 6343* (GENT); Prov. Utrecht, Nieuw Wulven, Iepenbos, on fallen branch of a deciduous tree, growing in the basidiome of Peniophorella praetermissa s.l., 13 Oct. 2020, I. Nannenga-Bruggeman, ID 7117* (GENT); Prov. Utrecht, Zeist, Overrijnwijck, on a fallen branch of an unidentified deciduous tree, growing in the basidiome of Peniophorella praetermissa s.l., 5 Nov. 2020, I. Nannenga-Bruggeman, ID 7149* (GENT); Prov. Utrecht, De Bilt, Sandwijck, on a fallen branch of an unidentified deciduous tree, growing in the basidiome of Peniophorella praetermissa s.l., 10 Dec. 2020, I. Nannenga-Bruggeman, ID 7323* (GENT); Prov. Gelderland, Barchem, Beekvliet, on fallen branch of Alnus, growing in the basidiome of Peniophorella praetermissa s.l., 11 Jul. 2020, M. Gotink, MG 445* (GENT); Prov. Groningen, Kolham, Uiterdijken, paddenstoelenreservaat, on fallen branch of Picea, growing in the basidiome of Peniophorella praetermissa s.l., 23 Nov. 2019, R. Enzlin, ENZ 19-092* (GENT); Prov. Groningen, Weende, Lieftinghsbroek, on a fallen branch of an unidentified deciduous tree, growing in the basidiome of Peniophorella praetermissa s.l., 30 Oct. 2020, R. Enzlin, ENZ 20-051* (GENT); Prov. Drenthe, Gasteren, Gasterensche Holt, on a fallen branch of an unidentified deciduous tree, growing in the basidiome of Peniophorella praetermissa s.l., 5 Sep. 2020, R. Enzlin, ENZ 20-042* (GENT). Prov. Zeeland, Oosterland, De Maire, on a fallen branch of an unidentified deciduous tree, growing in the basidiome of Peniophorella praetermissa s.l., 6 Nov. 2021, N. Schoutteten, NS 21-110* (GENT).
Notes: The colacosomes of this species occur scattered in the mycoparasite hyphae, more densely arranged in the places of physical contact between host and parasite cells. No proliferation of host hyphae has been observed.
Colacogloea fennica Schoutteten & Miettinen, sp. nov. MycoBank MB 848657. Figs 12, 13.
Fig. 12 .
Colacogloea fennica sp. nov. (OM 22483) line drawings. A. Basidiospores and germinating basidiospores by secondary spores. B. Basidium and basidiole. C. Conidiophore and basidium. D. Conidia, note the clamp at the base of conidia. E. Hyphidium. Black dots represent colacosomes. Scale bar = 10 μm.
Etymology: Referring to the country where the holotype of this species was collected.
Typus: Finland, Helsinki, Koskela, on fallen log of Pinus sylvestris, growing in the hymenium of Peniophorella praetermissa s.l., 3 Dec. 2020, O. Miettinen (holotype GENT OM 24483*°, isotype H 6014790, culture ex-type DSM 112417).
Description of filamentous morph: Intrahymenial, producing yellow to orange, slimy layer on the host species, remaining visible as yellow to orange warts when dried. Monomitic; hyphae hyaline, thin-walled, smooth, clamped at all septa, 2.2–4.2 µm in diam. Hyphidia present, simple or occasionally branched, 1–2 µm in diam. Cystidia absent. Basidia narrowly tubular-clavate, straight to curved or sinuous, (50.7–)52.1–73.0(–73.2) × (4.7–)5.3–6.9(–7.0) µm (n = 20/1), transversally septate, four-celled when mature, clamped at the base, thin-walled. Sterigmata up to 46 µm long. Basidiospores ellipsoid to broadly ellipsoid, more rarely subglobose, guttulate, (6.7–)6.8–10.5 × (5.2–)5.3–8.2(–8.8) µm, L = 8.56 µm, W = 6.91 µm, Q’ = (1.0–)1.1–1.5(–1.6), Q = 1.27 (n = 81/3), with prominent apiculus up to 1.2 × 1 µm, germinating by hyphae, budding or secondary spores. Conidia fusiform to amygdaliform, rarely oblong or asymmetric, mostly guttulate, thick-walled (walls up to 1 µm), strongly cyanophilous, basally clamped, 7.2–10.8(–13) × 3.5–5.0(–5.2) µm. Colacosomes scattered, no vesicular gall-like cells observed.
Description of yeast morph: After growth on YM agar plates for 1 mo at 22 °C, the streak culture is white to cream-coloured, glistening, mucoid and smooth. The margin is entire. Cells are subglobose to ovoid, occurring singly or in pairs, and proliferating by polar budding. Growth on D-glucose, D-glucosamine, D-ribose, D-arabinose, me a-D-glucoside, glycerol, D-mannitol, D-glucitol, and D-gluconate. Weak growth on erythritol, galactitol, and D-tartaric acid. No growth on D-galactose, L-sorbose, sucrose, maltose, cellobiose, a,a-trehalose, melibiose, raffinose, melezitose, inulin, starch, D-xylose, L-arabinose, L-rhamnose, ribitol, salicin, lactose, L-arabinitol, myo-inositol, 5-keto-D-gluconate, D-glucuronate, D-galacturonate, DL-lactate, succinate, citrate, ethanol, D-glucarate, L-tartaric acid, and L-malic acid. Growth in the presence of 5 % but not 8 % and 10 % NaCl. Growth on MEA with 50 % but not 60 % glucose. No starch-like substance is produced. Urea hydrolysis and the Diazonium blue B reaction is positive. Maximum growth temperature: 35 °C.
Habitat and distribution: Growing on coniferous wood, currently found only on Pinus sylvestris. Up to now only known from Finland, where it was collected in mixed forests and parks.
Materials examined: Finland, Helsinki, Koskela, on fallen log of Pinus sylvestris, growing in the hymenium of Peniophorella praetermissa s.l., 26 May 2020, O. Miettinen, OM 23714 (= H 6200175); ibid. 1 Sep. 2021, N. Schoutteten, NS 21-014* (GENT); Helsinki, Lehtisaari, on fallen branch of Pinus sylvestris, growing in the hymenium of Peniophorella praetermissa s.l., 15 Oct. 2008, H. Kotiranta, Kotiranta 22473 (= H 6073961).
Notes: The colacosomes occur scattered in the mycoparasite hyphae, more densely arranged in the places of physical contact between host and parasite cells. No proliferation of host hyphae has been observed.
Colacogloea microspora Schoutteten, sp. nov. MycoBank MB 848658. Figs 14, 15.
Fig. 14 .
Colacogloea microspora sp. nov. (NS 20-141) line drawings. A. Basidiospores and germinating basidiospores by hyphae and secondary spores. B. Two basidia and basidiole. C. Conidiophore. D. Conidia. Black dots represent colacosomes. Scale bar = 10 μm.
Etymology: Referring to the small size of the basidiospores of this species compared to other representatives of the Colacogloea effusa species complex.
Typus: Belgium, Flanders, Vlaams-Brabant, Asse, domain of Hoeve Heierveld, on fallen branch of a deciduous tree (probably Corylus avellana), growing in the hymenium of Peniophorella praetermissa s.l., 27 Oct. 2020, N. Schoutteten (holotype GENT NS 20-141*°, culture ex-type DSM 112413).
Description of filamentous morph: Intrahymenial, producing a yellow to orange, slimy to arid layer on the host species, remaining visible as yellow to orange warts after drying. Monomitic; hyphae hyaline, thin-walled, smooth, clamped at all septa, 2.4–3.9 µm in diam. Hyphidia absent. Cystidia absent. Basidia tubular-clavate, straight to sinuous to curved, (26.0–)27.6–43.7(–44.3) × (3.3–)4.2–5.4(–5.5) µm (n=18/1), transversally septate, four-celled when mature, clamped at the base, thin-walled, often arranged in clusters of 3–5 and appearing as scattered groups, quickly collapsing after reaching maturity. Sterigmata up to 22 µm long. Basidiospores ellipsoid to broadly ellipsoid to subglobose, (5.1–)5.2–8.0(–8.2) × (3.0–)3.8–5.3 µm, L = 6.66 µm, W = 4.53 µm, Q’ = 1.1–1.8(2.1), Q = 1.48 (n = 48/1), germinating by hyphae, budding or secondary spores; apiculus occasionally eccentric, up to 1 µm. Conidia variable, fusiform to angular, often widened in the middle, sometimes oblong, slightly curved or slightly asymmetric, occasionally with a small basal outgrowth, mostly guttulate, thick-walled (walls up to 1.2 µm), strongly cyanophilous, basally clamped, (6.9–)7.2–11.1(–12.7) × (3.1–)3.6–4.8(–5.3) µm. Colacosomes scattered, no vesicular gall-like cells observed.
Description of yeast morph: After growth on YM agar plates for 1 mo at 22 °C, the streak culture is white to cream-coloured, glistening, mucoid and smooth. The margin is entire. Cells are subglobose to ovoid, occurring singly or in pairs, and proliferating by polar budding. Growth on D-glucose, D-glucosamine, D-ribose, D-arabinose, me a-D-glucoside, melezitose, glycerol, ribitol, D-glucitol, D-mannitol, galactitol, D-gluconate, D-glucarate, and L-tartaric acid. Weak growth on L-sorbose, sucrose, erythritol, and D-tartaric acid. No growth on D-galactose, D-xylose, L-arabinose, L-rhamnose, maltose, a,a-trehalose, cellobiose, salicin, melibiose, lactose, raffinose, inulin, starch, L-arabinitol, myo-inositol, 5-keto-D-gluconate, D-glucuronate, D-galacturonate, DL-lactate, succinate, citrate, ethanol, and L-malic acid. Growth in the presence of 5 % but not 8 % and 10 % NaCl. Growth on MEA with 50 % but not 60 % glucose. No starch-like substance is produced. Urea hydrolysis and the Diazonium blue B reaction is positive. Maximum growth temperature: 35 °C.
Habitat and distribution: Up to now only found in Belgium, in a private forest-like garden, growing on a fallen, partly decorticated branch of a deciduous tree species, probably Coryllus avellana.
Material examined: This species is only known from the type collection.
Notes: The yellow patches on the host hymenium mostly comprise conidial tissue. Basidia and basidiospores are to be found in adjacent regions which macroscopically do not seem to be infected. The colacosomes of this species occur scattered in the mycoparasite hyphae, more densely arranged in the places of physical contact between host and parasite cells. No proliferation of host hyphae has been observed.
Colacogloea philyla (Van der Walt et al.) Q.M. Wang et al., Stud. Mycol. 81: 183. 2015. Figs 16, 17.
Fig. 16 .
Colacogloea philyla (MG 438) line drawings. A. Basidiospores and germinating basidiospores by hyphae and secondary spores. B. Basidium. C. Conidiophore. D. Conidia. E. Hyphidium. Black dots represent colacosomes. Scale bars = 10 μm.
Basionym: Torulopsis philyla Van der Walt et al., Antonie van Leeuwenhoek 37: 464. 1971.
Description of filamentous morph: Intrahymenial, producing a yellow to orange slimy layer on the host species, remaining visible as yellow to orange warts after drying. Monomitic; hyphae hyaline, thin-walled, smooth, clamped at all septa, 1.8–2.5 µm in diam. Hyphidia present, simple or occasionally branched, 1–2 µm in diam. Cystidia absent. Basidia narrowly tubular-clavate, straight to curved, (31.0–)39.0–56.2(–65.0) × (3.0–)3.2–4.5 µm (n = 20/1), transversally septate, four-celled when mature, clamped at the base, thin-walled. Sterigmata up to 39 µm long. Basidiospores subfusiform, often somewhat curved, guttulate, (6.8–)6.9–9.5(–10.2) × (3.3–)3.4–5.1 µm, L = 8.31 µm, W = 4.27 µm, Q’ = (1.6–)1.7–2.4(–2.7), Q = 1.96 (n = 30/1), with prominent apiculus up to 2 µm, germinating by hyphae, budding or secondary spores. Conidia highly variable in shape, ellipsoid, ovoid, subfusiform, elongated, angular, often asymmetric with variable outgrowths, mostly guttulate, thick-walled (wall up to 1 µm), strongly cyanophilous, basally clamped, (6.3–) 6.6–9.8(–10.6) × 3–4.9(–5.1) µm. Colacosomes scattered, no vesicular gall-like cells observed.
Habitat and distribution: Currently, the filamentous morph of Colacogloea philyla has only been observed in one collection from a conifer forest in the Netherlands, and is described and illustrated in this study. The ex-type strain of C. philyla was isolated as a yeast obtained from beetle galleries in Harpephyllum caffru (Anacardiaceae) in South Africa. Other yeast strains of this species were isolated from decaying wood in South Africa and Portugal (Sampaio 2011). Since the host Peniophorella pubera is a geographically widespread species, it is likely that the dikaryotic mycoparasitic stage can also be found around the localities where C. philyla was isolated as a yeast. Blast results of the ITS region in GenBank indicate this species was also isolated from mangrove sediments in India.
Material examined: Netherlands, Prov. Flevoland, Zeewolde, Horsterwold, Stille Kern, on a decorticated Picea branch, growing in the hymenium of Peniophorella pubera, 3 Oct. 2020, M. Gotink, MG 438*° (GENT).
Notes: This is one of two species recovered from the host Peniophorella pubera. The colacosomes of this species occur scattered in the mycoparasite hyphae, more densely arranged in the places of physical contact between host and parasite cells. Similar to observations in C. biconidiata sp. nov., this mycoparasite seems to induce additional branching of host hyphae, increasing the availability of contact surface where colacosomes can be formed.
Colacogloea universitatis-gandavensis Schoutteten & Verbeken, sp. nov. MycoBank MB 848659. Figs 2, 18, 19.
Fig. 18 .
Colacogloea universitatis-gandavensis sp. nov. (H6086094) line drawings. A. Basidiospores and germinating basidiospores by secondary spores. B. Cluster of basidia and conidiophores. C. Conidiophore. D. Conidiophores showing subsequent stages of conidiogenesis and conidia. E. Gall-like cell of the parasite (Pa) enveloping a host hyphae (Ho). Black dots represent colacosomes. Scale bars = 10 μm.
Etymology: The holotype is found on one of the campuses of Ghent University and we name the species after the university to acknowledge and stimulate the efforts for the Biodiversity plan, which is an official policy plan approved by the Board of Governors in 2020 and aims to realize a net gain in biodiversity on UGent campuses by 2030.
Typus: Belgium, Prov. Oost-Vlaanderen, Gontrode, Aelmoeseneiebos, on a log of an unidentified deciduous tree, growing in the hymenium of Peniophorella praetermissa s.l., 18 Sep. 2021, N. Schoutteten (holotype GENT NS 21-013°).
Description of filamentous morph: Intrahymenial, producing a yellow to orange, slimy to arid layer on the host species, rarely making small emergences (< 0.5 mm long) on the host hymenium. Monomitic; hyphae hyaline, thin-walled, smooth, clamped at all septa, 1.8–4.8 µm in diam. Hyphidia absent. Cystidia absent. Basidia tubular-clavate, straight to curved or sinuous, (25.5–)26–36(–37.5) × 5–6.5(–7) µm (n = 17/1), transversally septate and, often somewhat constricted at each septum, four-celled when mature, clamped at the base, thin-walled, often arranged in clusters of 2–5. Sterigmata up to 38 µm long. Basidiospores ellipsoid to broadly ellipsoid, (6.7–)6.8–9.8(–10.8) × (4.2–)4.5–7.2(–7.5) µm, L = 7.83 µm, W = 5.22 µm, Q’ = 1.2–1.8, Q = 1.51 (n = 34/1), with prominent apiculus up to 1 µm, germinating by hyphae or secondary spores, budding by secondary spores. Conidiophores are comprised of two cells being separated by a septum, each cell apically giving rise to a conidium, basally clamped, often arranged in clusters, intermixed with basidia, 11.5–28.5 × 3–5 µm. Mature conidia ellipsoid to ovoid, more rarely subfusiform, sometimes asymmetrical or becoming oblong, rarely with a small side outgrowth, mature conidia bearing an appendage (cell wall remnant of the smaller twin-conidium), thick-walled (walls up to 1 µm), cyanophilous, dikaryotic, (5.0–) 5.2–7.9(–8.8) × (3.2–)3.3–4.9(–5.1). Colacosomes arranged both scattered in parasite hyphae and in gall-like cells produced by this species.
Habitat and distribution: Collections of this species have been found in deciduous and mixed forests in Western, Central and Northern Europe (Belgium, Finland, Switzerland and The Netherlands). This species may also be present in North America, as morphologically similar collections have been reported by Martin (1940) and Bandoni (1973). Whether these North-American collections are truly conspecific with C. universitatis-gandavensis sp. nov. or rather represents a closely related species remains to be investigated.
Materials examined: Belgium, Prov. Oost-Vlaanderen, Aalst, Osbroek, on fallen branch of an unidentified deciduous tree species, growing in the hymenium of Peniophorella praetermissa s.l., 05 Sep. 2020, N. Schoutteten, NS 20-022*° (GENT). Finland, Varsinaissuomi, Turku, Ruissalo, on fallen branch of Quercus robur, growing in the hymenium of Peniophorella praetermissa s.l., 9 Sep. 1937, M. Laurila, H 6086094. Netherlands, Zeeland, Schelphoek, on a decorticated branch of an unidentified deciduous tree, growing in the hymenium of Peniophorella praetermissa s.l., 5 Nov. 2021, B. Miedema, Miedema 2021014 (GENT); Zeeland, nature reserve De Schotsman, on a decorticated branch of an unidentified deciduous tree, growing in the hymenium of Peniophorella praetermissa s.l., 7 Nov. 2021, H. Wassink, NS 21-112 (GENT). Switzerland, Ticino region, Sementina, Boschetti, on a decorticated branch of an unidentified tree, growing in the hymenium of Peniophorella praetermissa s.l., 13 Oct. 2019, N. Schoutteten, NS 19-119 (GENT).
Notes: This is the second species that we propose which is similar to the morphotype illustrated by Martin (1940) (see also C. bettinae sp. nov. and discussion). Basidia have only been observed in three out of six investigated specimens. The conidial state is always the most prominent, with basidia occurring in clusters with conidiophores. Unfortunately, no cultures could be obtained of this species.
Conidiogenesis in this species is a remarkable process with conidiophores consisting of two distinct cells (Fig. 17D). These two cells are separated by a septum, which is characterised by a simple septal pore (Greschner-Aschenbrenner 1997). One of these cells comprises the ‘stalk’ of the conidiophore and an apical abscission site where the conidium is produced (conidiophore cell 1). The second cell is much smaller and has a similar apical abscission site (conidiophore cell 2). Each cell of the conidiophore produces a conidium at the apical abscission site. The conidium produced by conidiophore cell 1 grows remarkably larger than the conidium produced by conidiophore cell 2. At this stage, each daughter conidium is monokaryotic. At a certain moment, the two daughter conidia fuse forming a zygoconidium. Greschner-Aschenbrenner (1997) showed that the zygoconidium is abscised shortly after formation, leaving a scar at the conidiophore which can only be seen by TEM. During the short-lived zygoconidium stage, the cytoplasm (including the nucleus) of the smaller conidium is transferred to the larger conidium. Following this transfer the larger conidium becomes dikaryotic. The remnants of the smaller conidium, i.e., the empty, collapsed cell wall remains attached to the cell wall of the larger conidium. The same type of conidiogenesis occurs in C. bettinae sp. nov.
Colacosomes are mainly arranged in vesicular gall-like cells produced by the mycoparasite. To a lesser extent, colacosomes also scattered in mycoparasite hyphae. The cell wall of these gall-like structures invaginates at places where a host hypha makes physical contact. The latter continues to grow into the invagination where it becomes surrounded by the cell wall of the mycoparasite. Colacosomes are formed at regular distances along the contact surface within these galls.
Identification key based on filamentous morphs to the species within the Colacogloea effusa species complex
1a Growing on Peniophorella pubera 2
1b Growing on Peniophorella praetermissa s.l. 3
2a Conidia of the mycoparasite of irregular shape, no appendage present. Basidiospores subfusiform.
Colacosomes scattered Colacogloea philyla
2b Conidia of the mycoparasite of type of regular shape, generally with appendage of remaining cell wall. Ventral side of basidiospores flattened to concave. Colacosomes arranged in gall-like cells Colacogloea bettinae
3a Conidia of the mycoparasite of regular shape, generally with appendage of the remaining cell wall. Basidiospores (broadly) ellipsoid. Colacosomes arranged in gall-like cells Colacogloea universitatis-gandavensis
3b Conidia without appendage. Colacosomes scattered 4
4a Two types of conidia and conidiophores present. Basidiospores large, up to 12.5 µm in length Colacogloea biconidiata
4b Only one type of conidia. Basidiospores not exceeding 11 µm in length 5
5a Basidiospores small, most spores ≤ 8µm in length, (5.1–)5.2–8.0(–8.2) × (3.0–)3.8–5.3 µm Colacogloea microspora
5b Basidiospores larger, often ≥ 8 µm in length, up to 11 µm 6
6a Basidiospores ellipsoid to reniform, Q > 1.4 Colacogloea effusa
6b Basidiospores ellipsoid to broadly ellipsoid, Q < 1.4 Colacogloea fennica
Family Mycogloiocolacaceae Schoutteten & Yurkov, fam. nov. MycoBank MB 848661.
Description: Member of Microbotryomycetes. This family is mainly circumscribed by the phylogenetic analysis based on seven loci, in which it forms a well-supported lineage. The family includes species with a dimorphic life cycle. Filamentous morphs are mycoparasitic, only develop in presence of the host, and are characterised by transversally septate basidia and the presence of colacosomes. The diagnosis and nomenclature of the family Mycogloiocolacaceae are based on the genus Mycogloiocolax gen. nov.
Type genus: Mycogloiocolax Schoutteten & Rödel
Mycogloiocolax Schoutteten & Rödel, gen. nov. MycoBank MB 848662.
Etymology: The name is based on a similar etymology used for other genera of colacosome-interacting mycoparasites. Gloios refers to the slimy layer produced by the mycoparasite Mycogloiocolax gerardii when growing in its host. Colax refers to the parasitic nature of this species.
Type: Mycogloiocolax gerardii Schoutteten & Rödel
Generic description: Genus of dimorphic fungi. Basidiomata absent. Filamentous morph develops intrahymenial in the host, visible in fresh condition as a hyaline gelatinous, slimy layer overgrowing the host basidiome, turning to a thin, almost invisible, gelatinous layer in dry condition. Monomitic; hyphae hyaline, thin-walled, smooth, clamped at all septa. Hyphidia absent. Cystidia absent. Basidia cylindrical to tubular-clavate, often curved, sinuous or winding, transversally septate, mature basidia two-, rarely three- or four-celled, basally clamped, thin-walled. Basidiospores fusiform to amygdaliform, with suprahilar depression, asymmetric, smooth, hyaline, inamyloid, with a prominent apiculus, germinating by hyphae, budding or secondary spores. Conidia ellipsoid to subfusiform, thin-walled. Colacosomes scattered, no vesicular gall-like cells observed.
Mycogloiocolax gerardii Schoutteten & Rödel, sp. nov. MycoBank MB 848663. Figs 20, 21.
Fig. 20 .
Mycogloiocolax gerardii sp. nov. (TR 04096) line drawings. A. Basidiospores and germinating basidiospores by budding, hyphae and secondary spores. B. Basidia and basidioles. C. Conidiophores. D. Conidia. Black dots represent colacosomes. Scale bars = 10 μm.
Etymology: Named after the French amateur mycologist Gérard Trichies, who has made large efforts in documenting and illustrating the diversity of heterobasidiomycetes in France.
Typus: Germany, Saxony, near Mölbis (51°11’16.2”N 12°30’28.9”E), growing in the basidiome of Xenasmatella tulasnelloidea (Höhn. & Litsch.) Oberw., 22 Oct. 2020, T. Rödel (holotype GENT TR 04096*°, culture ex-type DSM 112426).
Description of filamentous morph: Intrahymenial, visible in fresh condition as a hyaline gelatinous, slimy layer overgrowing the host basidiome, turning to a thin, almost invisible, gelatinous layer in dry condition. Monomitic; hyphae hyaline, thin-walled, smooth, clamped at all septa, 1.2–3.3 µm in diam. Hyphidia absent. Cystidia absent. Basidia cylindrical to tubular-clavate, often curved, sinuous or winding, (28.3–)31.8–34.9(–37.8) × (3.0-)3.2-4.7(4.9) µm (n = 20/2), transversally septate, mature basidia two-, rarely three- or four-celled, basally clamped, thin-walled. Sterigmata up to 27 µm long. Basidiospores fusiform to amygdaliform, with suprahilar depression, asymmetric, smooth, hyaline, inamyloid, (5.8–)6.0–10.1(–10.2) × (2.4–)3.6–5.8(–6.0) µm, L = 8.20, W =4.72, Q’ = (1.2–)1.3–2.1(–4.1), Q = 1.79 (n = 31/1), with a prominent apiculus, germinating by hyphae, budding or secondary spores. Conidia ellipsoid to subfusiform, thin-walled, (3.6–)4.0–5.2(–5.8) × 2.0–2.6(–2.8) µm. Colacosomes scattered, no vesicular gall-like cells observed.
Description of yeast morph: After growth on YM agar plates for 1 mo at 22 °C, the streak culture is white to cream-coloured, glistening, mucoid and smooth. The margin is entire. Cells are subglobose to ovoid, occurring singly or in pairs, and proliferating by polar budding. Good growth on D-glucose, D-arabinose, cellobiose, inulin, starch, glycerol, ribitol, D-glucitol, D-mannitol, galactitol, D-glucarate, D-tartaric acid, and L-malic acid. Weak growth on L-sorbose, D-glucosamine, D-ribose, L-arabinose, L-rhamnose, me a-D-glucoside, salicin, raffinose, erythritol, D-gluconate, and succinate. No growth on D-xylose, sucrose, maltose, a,a-trehalose, melibiose, lactose, melezitose, L-arabinitol, myo-inositol, 5-keto-D-gluconate, D-glucuronate, D-galacturonate, DL-lactate, citrate, ethanol, and L-tartaric acid. Weak growth in the presence of 5 % and 8 % but not 10 % NaCl. Growth on MEA with 50 % but not with 60 % glucose. No starch-like substance is produced. Urea hydrolysis and the Diazonium blue B reaction is positive. Maximum growth temperature: 35 °C.
Habitat and distribution: This species is an intrahymenial mycoparasite of the corticioid fungus Xenasmatella tulasnelloidea. Only three collections of this mycoparasite have currently been reported, from Denmark, France, and Germany. Based on the distribution of the host fungus, it is likely that this mycoparasite has a wider distribution than currently known and may be expected in various other (European) countries.
Materials examined: Denmark, Tadre Mølle, growing on the basidiome of Xenasmatella tulasnelloidea, 05 Jan. 2013, T. Læssøe, DMS-495673° = GENTFT00154 (GENT). France, Moselle, Neufchef, growing on the basidiome of Xenasmatella tulasnelloidea (as Phlebiella tulasnelloidea), 25 Jun. 2004, G. Trichies, GT 04098° (LIP).
Notes: This species has been reported and illustrated for the first time by Gerard Trichiès (2006), based on a collection from 2004 growing on Xenasmatella tulasnelloidea. Although the author realised that the specimen most likely represented an undescribed species, he decided not to describe it due to the limited set of micromorphological characters available for species delimitation. So far, it is the only mycoparasite reported from this host species. Colacosomes of Mycogloiocolax gerardii sp. nov. are formed in mycoparasite hyphae in places where physical contact with other hyphae occurs. Colacosomes can also be found in conidiophores, basidia and (germinating) basidiospores (Fig. 21D, G).
Updated classification of Microbotryomycetes
Below we provide an updated classification of the currently described genera in Microbotryomycetes, including colacosome-forming species.
Genera accepted in Curvibasidiales:
Curvibasidium Samp. & Golubev Pseudoleucosporidium V. de García et al.
Genus accepted in Heitmaniales:
Heitmania X.Z. Liu et al.
Genera accepted in Heterogastridiales:
Atractocolax R. Kirschner et al.Hyalopycnis Höhn (syn. Heterogastridium Oberw. & R. Bauer)Pycnopulvinus Toome & Aime Slooffia Q.M. Wang et al.
Genera accepted in Kriegeriales:
Kriegeria Bres.Yamadamyces Q.M. Wang et al.Meredithblackwellia Toome & Aime Phenoliferia Q.M. Wang et al.Libkindia Mašínová, A. Pontes et al.
Genera accepted in Leucosporidiales:
Leucosporidium Fell et al.Sampaiozyma Q.M. Wang et al.
Genera accepted in Sporidiobolales:
Sporobolomyces Kluyver & C.B. Niel Rhodosporidiobolus Q.M. Wang et al. Rhodotorula F.C. Harrison
Genus accepted in Rosettozymales:
Rosettozyma Q.M. Wang & F.Y. Bai
Genera accepted in Camptobasidiaceae:
Camptobasidium Marvanová & Suberkr.Glaciozyma Turchetti et al.Cryolevonia A. Pontes et al.Psychromyces L. Perini & Zalar
Genera accepted in Chrysozymaceae:
Chrysozyma Q.M. Wang et al.Bannozyma Q.M. Wang et al.Fellozyma Q.M. Wang et al.Hamamotoa Q.M. Wang et al.Yurkovia Mašínová et al.
Genera accepted in Colacogloeaceae:
Colacogloea Oberw. & Bandoni Udeniozyma Q.M. Wang et al.
Genus accepted in Mycogloiocolacaceae fam. nov.:
Mycogloiocolax Schoutteten & Rödel gen. nov.
Microbotryomycetes incertae sedis:
Oberwinklerozyma Q.M. Wang et al.Pseudohyphozyma Q.M. Wang et al.Reniforma Pore & Sorenson Spencerozyma Q.M. Wang et al.Trigonosporomyces Q.M. Wang et al.Vonarxula Q.M. Wang et al.Yunzhangia Q.M. Wang et al.
DISCUSSION
In order to formulate an evolutionary hypothesis on colacosome-interacting mycoparasites in Microbotryomycetes, we organised the discussion in three major sections. In the first section, we focus on the proposed method for epifluorescence-based colacosome visualisation. The second section deals with three different aspects of the phylogenetic reconstruction, discussing (A) general aspects of the Microbotryomycetes phylogenetic reconstruction, (B) specific clades comprising colacosome-forming species and clades for which we sequenced additional loci, and (C) the phylogenetic distribution of colacosome-forming species. In the third section we discuss our results on the diversity, ecology and morphology of the four mycoparasitic genera investigated in this study: Atractocolax, Colacogloea, Mycogloiocolax gen. nov. and Slooffia.
Epifluorescence-based colacosome visualisation
The detection of colacosomes in fungi is an indispensable step in order to understand the species diversity that form these structures, as well as the evolution of the colacosome-interaction. Most previous reports of colacosomes were solely based on TEM imaging of fungal samples, derived either from (co-)cultures or directly from fresh basidiomata (Table 1). We describe an epifluorescence-based method to easily detect colacosomes and infer their organisation. This is in contrast to Oberwinkler & Bauer (2018) who stressed the necessity of TEM for the detection of colacosomes. The sample preparation encompasses conventional Congo red staining of whole-mount preparations (Clémençon 2009). We showed that epifluorescence microscopy is a more suitable method to detect colacosomes because it provides more contrast compared to traditional brightfield imaging. Colacosomes are clearly visible as they emit intense fluorescent signals in the red part of the spectrum upon illumination with green fluorescent light. Congo red strongly stains the secondary cell wall surrounding the colacosomes (Fig. 2C), which was shown by Kreger-van Rij & Veenhuis (1971b) to be a chitin-rich structure. Congo red has a strong affinity for chitin and polysaccharides (Matsuoka et al. 1995). Additionally, host- and parasite cell walls emit fluorescence signals that are strong enough to discriminate host and parasite hyphae and to determine the organisation of colacosomes. If nuclei need to be visualised, DAPI can be added to the Congo red staining solution, and DAPI emission can be observed using an appropriate UV filter set (Fig. 2B). In contrast to TEM, fluorescence microscopy is more accessible to researchers. Our approach requires a TRITC filter, which is one of the standard filter sets in most epifluorescence microscopes. Combined with the easy sample preparation, this method will allow more researchers to easily screen for the presence of colacosomes in fungal samples, and enlarge the list of species known to be capable of forming these structures.
Phylogenetic reconstruction
A. General Microbotryomycetes phylogeny
Our analysis of Microbotryomycetes included 33 isolates derived from colacosome-interacting mycoparasites belonging to the genera Atractocolax, Colacogloea, Mycogloiocolax gen. nov. and Slooffia. To obtain a better phylogenetic resolution, newly generated DNA sequences of ribosomal and/or protein-coding loci of Colacogloea demeterae, Glaciozyma litorale, Hamamotoa cerberi, Hamamotoa telluris, Libkindia masarykiana, Slooffia velesii, and Yurkovia mendeliana were analysed in the current study.
Our seven-locus ML phylogenetic reconstruction of the class Microbotryomycetes (Fig. 3) follows previous studies (Wang et al. 2015a, Li et al. 2020, Perini et al. 2021) and includes almost all currently described species from this group, except for the order Microbotryales, that is represented by ten isolates from four genera, similar to Wang et al. (2015a, b) and Li et al. (2020). Most previously described higher taxa within Microbotryomycetes are resolved as strongly supported monophyletic clades in our analysis (Table 5).
Table 5 .
Summary of support values for higher taxa in Microbotryomycetes recovered in the seven-locus ML phylogenetic reconstruction.
| Taxon | Ultrafast Bootstrap value | Reference |
| Camptobasidiaceae | 100 | Moore (1996) |
| Chrysozymaceae | 92 | Wang et al. (2015b) |
| Colacogloeaceae | 100 | Wang et al. (2015b) |
| Curvibasidiales | 100 | Doweld (2014) |
| Heitmaniales | 100 | Li et al. (2020) |
| Heterogastridiales | 79 | Oberwinkler et al. (1990b) |
| Kriegeriales | 100 | Toome et al. (2013) |
| Leucosporidiales | 100 | Sampaio et al. (2003) |
| Microbotryales | 84 | Bauer et al. (1997) |
| Rosettozymales | 100 | Li et al. (2020) |
| Sporidiobolales | 100 | Doweld (2001) |
Our analysis reveals five distinct clades that were not yet assigned to higher taxa: 1) the lineage of the genus Oberwinklerozyma; 2) the lineage of the genera Reniforma and Yunzhangia, along with presently unclassified yeast isolates KBP Y-5457, KBP Y-4635 and KBP Y-4912; 3) the genus Pseudohyphozyma; 4) the genus Slooffia; and 5) the genus Atractocolax. The supported clustering of Reniforma and Yunzhangia as sister to Microbotryales was also observed in the seven-locus phylogenetic reconstruction of Li et al. (2020), whereas Wang et al. (2015a, b) found these two genera in a cluster with Heterogastridium. As in Li et al. (2020), the order Rosettozymales is found here to be sister to all other Microbotryomycetes, although with low support in both analyses. As observed in various previous studies, the phylogenetic relationships of the monotypic genera Reniforma, Spencerozyma, Trigonosporomyces, and Vonarxula remain unresolved, and these representatives are generally placed on long branches in phylogenetic ML analyses (Wang et al. 2015b, Li et al. 2020). Long branches can be the result of fast-evolving genetic regions and/or taxon sampling error (Prasanna et al. 2019, Galindo et al. 2021). Improved sampling will potentially lead to a more robust phylogenetic placement for these taxa.
Our phylogenetic analysis (Fig. 3) includes the recently described genera Cryolevonia and Psychromyces and revises the placement of the genera Libkindia, and Yurkovia, and the composition of the order Kriegeriales. The order Kriegeriales was erected by Toome & Aime (2013) based on phylogenetic analyses incorporating the three nuclear ribosomal loci SSU, ITS and LSU to accommodate the families Camptobasidiaceae and Kriegeriaceae. In our analysis, Kriegeriales is monophyletic and strongly supported, but only accommodates the family Kriegeriaceae, with the genera Kriegeria, Libkindia, Meredithblackwellia, Phenolipheria and Yamadamyces. Similar to findings by Wang et al. (2015a), Mašínová et al. (2017), de Garcia et al. (2020), Li et al. (2020) and Perini et al. (2021), our analysis reveals Camptobasidiaceae as a separate monophyletic, strongly supported lineage including the genera Camptobasidium, Glaciozyma, Cryolevonia, and Psychromyces. The inclusion of Camptobasidiaceae in Kriegeriales seems to be an artefact retrieved in analyses based on phylogenetic reconstructions that only incorporate ribosomal DNA sequence data (Toome & Aime 2013, Wang et al. 2015a, Li et al. 2020). When nuclear (and mitochondrial) protein coding genes are incorporated in the analyses, both families are retrieved as separate monophyletic lineages (Li et al. 2020, Perini et al. 2021). However, due to the lack of support for deeper nodes, a possible sister relationship of the families cannot be ruled out.
Interestingly, the family Kriegeriaceae has not always been recovered as monophyletic by several authors (Wang et al. 2015b, Li et al. 2020, Pontes et al. 2020, Perini et al. 2021). On one hand, these analyses were sensitive to taxon sampling. On the other hand, we found that the published DNA sequences of the protein-coding genes RPB1, RPB2, TEF-1α and mitochondrial CYT-B for the type strains of Kriegeria eriophori (CBS 8387) and Libkindia masarykiana (PYCC 6886) and Yurkovia mendeliana (PYCC 6884) were in fact derived from Candida (Ascomycota) contaminations. Furthermore, for the ex-type strain of Meredithblackwellia eburnea only SSU, ITS and LSU rDNA sequences are available in public databases. It may be expected that accurate DNA sequences of the aforementioned protein-coding genes of these species will lead to more consistent phylogenetic reconstructions of Microbotryomycetes, and the family Kriegeriaceae and order Kriegeriales in particular. In our analyses, the genus Yamadamyces is polyphyletic (Fig. 3), and it could be argued that both Yamadamyces species should be placed in Meredithblackwellia. A more robust dataset could also reveal whether the two yeast genera Meredithblackwellia and Yamadamyces should be merged with the dimorphic genus Kriegeria. These genera share a unique morphology of budding yeast morphs, forming rosettes (Oberwinkler 2017). However, due to the lack of protein-coding DNA sequence data for multiple loci, we refrain from such taxonomic conclusions here.
B. Discussion of specific clades
Chrysozymaceae — This family was proposed by Wang et al. (2015b), to accommodate the genera Bannozyma, Chrysozyma, Fellozyma, and Hamamotoa which formed a strongly supported clade in seven-locus phylogenetic reconstructions (Wang et al. 2015a, b). In our phylogenetic reconstruction, this family is recovered as monophyletic, with an ultrafast bootstrap (UFB) support value of 92 (Fig. 3). Interestingly, in the analysis of Li et al. (2020), this family proved to be polyphyletic in a constrained LSU ML analyses, whereas in the seven-locus ML analysis this family was found strongly supported and monophyletic. Perini et al. (2021) found this family to be monophyletic and strongly supported based on a seven-locus phylogenetic ML reconstruction (including SSU, 5.8S, LSU, RPB1, RPB2, EF1-α and CYT-B). In our analysis, three strongly supported clades can be recognised within this family: 1) the lineage with the genera Bannozyma and Chrysozyma, 2) the lineage comprising Fellozyma, Hamamotoa cerberi and H. telluris, and 3) the lineage comprising the genus Yurkovia, Hamamotoa lignophila and H. singularis. Our analyses indicate the genus Hamamotoa to be polyphyletic, based on which we propose Fellozyma cerberi comb. nov. and Fellozyma telluris comb. nov. These two species were described by Yurkov et al. (2016) using a LSU-based analysis. Even though the statistical support for the placement was low in their analysis (NJ: 53 %), the authors justified their placement to Hamamotoa by their high sequence similarity (99 %) to H. singularis (Yurkov et al. 2016). The polyphyly of Hamamotoa was also detected by Kachalkin (2022) based on a combined ITS-LSU analysis. Our study additionally highlights the fact that LSU phylogenies have strong limitations compared to multi-locus analyses. Comparing overall distances in the type genus of the family, Chrysozyma, and in the genera Fellozyma, Hamamotoa and Yurkovia (Fig. 3), we cannot exclude merging the three latter genera into a single genus in the future.
Colacogloeaceae — This family comprises the genera Colacogloea and Udeniozyma, and is retrieved as a monophyletic clade in our phylogenetic reconstruction (Fig. 3). The monotypic genus Udeniozyma is recovered as sister to the genus Colacogloea with strong bootstrap support. The same sister relationship was also recovered by Wang et al. (2015a, b) and Li et al. (2020). Similar to the pattern observed in the LSU-based phylogenetic ML reconstruction of Wang et al. (2021), the genus Colacogloea is composed of two distinct subclades. Subclade 1 comprises species recovered as yeast morphs from soils and phylloplanes, and no conjugation or filamentous morphs have been reported for them so far. Subclade 2 mainly comprises dimorphic species of which the filamentous morph represents a mycoparasitic stage and engages in colacosome-interaction. Subclade 2 includes the genus type Colacogloea effusa, and a few species isolated as yeasts from plant substrates for which the filamentous morph was not yet reported. Most likely all Colacogloea species are dimorphic colacosome-interacting mycoparasites. However, for many species the mycoparasitic stage remains to be discovered. This is especially true for Colacogloea retinophila and C. terpenoidalis, which are nested in subclade 2 and whose closest relatives are known to have a mycoparasitic stage. Colacogloea philyla, another member of subclade 2, was originally isolated as a yeast from bark beetle galleries. Crossing experiments using the available strains failed to induce dikaryotisation, and a filamentous morph was until now never reported (Sampaio 2011). We discovered that the filamentous morph of C. philyla is a mycoparasite developing in the hymenium of Peniophorella pubera. Whether the genus Colacogloea should be split in two based on these subclades remains to be seen, but we argue that this phylogenetic pattern alone, combined with insufficient knowledge on the biology and ecology of these species provide insufficient ground to make such decision.
Heterogastridiales — This order was established by Oberwinkler et al. (1990) along with the family Heterogastridiaceae to accommodate Hyalopycnis blepharistoma, a filamentous fungus originally described as asexually reproducing. Oberwinkler et al. (1990) observed the sexual stage of this fungus, for which they proposed the name Heterogastridium pycnidioideum, which serves as nomenclatural basis for the family and the order. Contrary to Aime et al. (2018), we advocate for the protection of the name Heterogastridium over Hyalopycnis. In our analysis, this species clusters with Pycnopulvinus aurantiacus. These two species produce pycnidioid and stilboid structures respectively, and both are presumed mycoparasites. Various colacosome-forming fungi from the genera Atractocolax, Colacogloea and Krieglsteinera have been tentatively assigned to this order based on micromorphological similarities and the presence of colacosomes, but these relationships have never been tested for phylogenetic support. We show that the genera Atractocolax and Slooffia can be assigned to Heterogastridiales (Fig. 3). On the contrary, the genus Colacogloea forms a well-supported and distinct clade in our analysis. It is possible that other, yet to be sequenced, Colacogloea species or Krieglsteinera lasiosphaeriae belong to Heterogastridiales. For the time being, we prefer to treat those species for which no genetic data is available as Microbotryomycetes incertae sedis.
C. Phylogenetic distribution of colacosome-forming species
Prior to this study, the presence of colacosomes was reported from 17 species from nine genera in Microbotryomycetes (Table 1). We provide evidence for the presence of colacosomes in eight more species belonging to three genera in this class, resulting in at least 25 species and 11 genera of Microbotryomycetes from which colacosomes are reported. Phylogenetically, colacosome-forming species are widely distributed within this class, and are currently reported from six lineages: the families Chrysozymaceae, Colacogloeaceae, and Mycogloiocolacaceae fam. nov., and orders Heterogastridiales, Leucosporidiales, and Sporidiobolales. The clade with most colacosome-forming taxa is Colacogloeaceae (eight species), followed by Sporidiobolales (five species) and Leucosporidiales (four species). Within Colacogloeaceae, colacosome-forming species are restricted to the so-called subclade 2 of the genus Colacogloea (sensu Wang et al. 2021), which contains all currently known mycoparasites within the genus. As outlined below, reconstructing the evolution of the capability of colacosome formation in Microbotryomycetes remains difficult for two major reasons, being firstly, an insufficient screening for these structures, and secondly, a poor phylogenetic resolution of the deeper nodes of this class.
As shown in Fig. 3, only few species of Microbotryomycetes have been subjected to adequate screening for the presence of colacosomes. We believe that the vast majority of Microbotryomycetes are dimorphic fungi, although for many species only the haploid stage or yeast morph is known. It should be considered that colacosomes are generally produced only in the dikaryotic stage of the lifecycle of these organisms, which requires conjugation of cells from compatible strains and it might be complicated to obtain in culture under laboratory conditions due to the lack of a compatible strain. Since only one or a few strains are available for the majority of Microbotryomycetes, compatible strains for crossing experiments are rarely available. Also, for some species, colacosome forming may require the presence of a suitable host. Consequently, the situation in which for a certain species the dikaryotic stage was not observed, or colacosomes have not been observed, should not be interpreted as the proved inability of that respective species to produce colacosomes. Due to these reasons, proving the inability of a species to produce colacosomes is very difficult, and a certain degree of ambiguity will often remain. As a good example, Sampaio et al. (2003) applied such crossing experiments in a number of Microbotryomycetes. The authors assessed the presence of colacosomes based on TEM investigation of yeast- and filamentous morphs, and reported four species which were devoid of colacosomes, i.e., Camptobasidium hydrophylum, Kriegeria eriophori, Glaciozyma antarctica, and Pseudoleucosporidium fasciculatum.
The second reason is that the absence of strongly supported deeper nodes in phylogenetic reconstructions of Microbotryomycetes does not allow to infer relationships between the different clades and, thus, predict the presence of colacosomes in ancestors and any random species within Microbotryomycetes. One distinct pattern that was already reported is the absence of colacosomes in the two phytoparasitic lineages within Microbotryomycetes, namely the genus Kriegeria and the order comprising anther smuts, Microbotryales (Sampaio et al. 2003, Bauer 2004, Bauer et al. 2006). Weiß et al. (2004) already suggested that phytoparasitic lineages in Microbotryomycetes, i.e., Microbotryales and Kriegeria, most likely evolved from colacosome-interacting mycoparasitic ancestors. This hypothesis is strongly supported by our data, given the wide phylogenetic distribution of the colacosome-interaction and mycoparasitic taxa in our reconstruction of Microbotryomycetes.
Mycoparasitic genera in Microbotryomycetes
The genus Colacogloea
The genus Platygloea comprises a heterogenous group of fungi that only shares the character of transversally septate basidia (Schröter 1887, Bandoni 1956). Bourdot & Galzin (1909) described Platygloea peniophorae as a mycoparasite of the corticioid fungi Peniophorella praetermissa and Peniophorella pubera. Following the discovery of colacosomes in Platygloea peniophorae by Bauer and Oberwinkler (1991), the genus Colacogloea was introduced for this species (Oberwinkler et al. 1990a). The authors argued that the combined occurrence of simple septal pores, colacosomes, and yeast budding of basidiospores is sufficient to separate Pl. peniophorae from Platygloea disciformis, which is considered the type species of the genus Platygloea (syn. Achroomyces). Following the introduction of the genus Colacogloea, three more filamentous mycoparasites were assigned to the genus, based on the presence of colacosomes: C. allantospora, C. bispora, and C. papilionacea (Oberwinkler et al. 1999, Kirschner & Oberwinkler 2000, Bandoni et al. 2002). More recently, Wang et al. (2015a, b) assigned several yeast species from the genera Rhodotorula and Sporobolomyces to the genus Colacogloea based on phylogenetic reconstructions. These species were isolated as yeasts from various substrates, mostly plants and soils, but little is known about their ecology. Although it may be assumed these species have dimorphic lifecycles, only the yeast morph was observed for these species, and the presence of colacosomes has not been assessed (Wang et al. 2015b, Yurkov et al. 2016, Li et al. 2021, Wang et al. 2021).
The instatement of the genus Colacogloea to accommodate the mycoparasite Platygloea peniophorae resulted in the name Colacogloea peniophorae, which was assigned as generic type (Oberwinkler et al. 1990a). Recently, Malysheva et al. (2021) provided evidence for the synonymy of Platygloea effusa and C. peniophorae, and proposed the name Colacogloea effusa as valid name for this taxon. Platygloea effusa was originally interpreted as a resupinate saprobic species with transversally septate basidia growing on rotten stumps, and was not recognised as a mycoparasite at that time (Schröter 1887). Malysheva et al. (2021) assigned a neotype for Platygloea effusa, but the authors did not provide typification of Platygloea peniophorae. Here, we selected a lectotype from the herbarium of Bourdot (PC) which was used for the original description of Platygloea peniophorae, and assigned an epitype to support the lectotype. The selected epitype was recently collected in the same area of the lectotype, and its yeast morph was isolated in pure culture allowing molecular characterisation.
Literature research shows that the mycoparasite Pl. peniophorae has been reported and documented from Europe and North America (Bourdot & Maire 1920, Bourdot & Galzin 1928, Pilat 1957, Bandoni 1973). As outlined below, a certain degree of morphological variation has been observed, and two distinct morphotypes can be recognised.
In their seminal work Hyménomycètes de France, Bourdot & Galzin (1928) mentioned some degree of variation in macro- and micromorphological characters between studied collections of Platygloea peniophorae and assigned them to different forms of the same species (see also Bourdot & Maire 1920, Bourdot 1932). The authors also reported two different host species for this mycoparasite, but never suggested this taxon may comprise different species. The morphotype illustrated by Bourdot & Galzin (1928) is here referred to as the ‘non-gall-like morphotype’.
Martin (1940) reported a strongly deviating collection from this typical Platygloea peniophorae. He illustrated an American collection of Pl. peniophorae (fig. 5 in Martin 1940), which produced vesicular gall-like cells and thick-walled oval conidia with attached remnants – here referred to as the ‘gall-like morphotype’ (Martin 1940). These characters described from Martins’ collection are highly reminiscent of those that we observed in Colacogloea bettinae sp. nov. and C. universitatis-gandavensis sp. nov. (see Figs 6 and 18). Interestingly, Martin (1940) noted that these gall-like cells become filled with ‘oval bodies’ in some cases ‘surrounding a central columella-like stalk’. These oval bodies can now be interpreted as colacosomes, surrounding an invaginating host hyphae in the gall-like cell of the mycoparasite. As such, Martin (1940) was the first to provide an illustrated report of colacosomes. Based on morphological studies of Canadian and European collections, Bandoni (1973) illustrated these two distinct morphotypes of Pl. peniophorae and argued they may constitute two different species, though he neither mentioned nor illustrated the gall-like cells. According to his insights, specimens of the gall-like morphotype are restricted to North America, whereas those of the non-gall-like morphotype occurred in both Europe and North-America. When Oberwinkler et al. (1990) proposed the genus Colacogloea, the authors investigated and illustrated the typical non-gall-like morphotype of Pl. peniophorae. They briefly mentioned the existence of the gall-like morphotype illustrated by Martin (1940) and suggested the two forms may comprise two different species. This idea was later supported by Greschner-Aschenbrenner (1997) based on detailed comparison of micromorphological and ultrastructural characters. However, her taxonomic conclusions were never formally published. We name one of our species after her, since she made the most in-depth comparative study of these two aforementioned morphotypes (see C. bettinae sp. nov.).
For our study of the Colacogloea effusa species complex, we studied freshly sampled European collections from Belgium, France, The Netherlands, Finland and Norway. Our collections were found on two different host species: Peniophorella praetermissa and Pe. pubera. Among our collections, we not only found the typical non-gall-like morphotype, but also the gall-like morphotype as illustrated by Martin (1940), which until now was believed to be restricted to North America (Bandoni 1973).A polyphasic approach combining micromorphological analyses, yeast morph characterisation, and a multilocus phylogenetic reconstruction allow us to recognise seven different species within the Colacogloea effusa species complex. Most of our sequenced collections are assigned to C. effusa (syn. Platygloea peniophorae), which is a parasite of Pe. praetermissa. On the same host species, four other colacosome-forming mycoparasites can be recognised. Of them, C. biconidiata sp. nov., C. fennica sp. nov., and C. microspora sp. nov. resemble the non-gall-like morphotype, whereas C. universitatis-gandavensis sp. nov. resembles the gall-like morphotype. On the host Pe. pubera, two distinct mycoparasitic species were found. The first, C. philyla, constitutes the non-gall-like morphotype, whereas C. bettinae sp. nov. constitutes the gall-like morphotype. These seven species within the C. effusa complex can be separated based on a combination of characteristics such as yeast morph physiology, host species, shape and dimensions of spores, basidia, and conidia. Hallenberg et al. (2007) showed that Pe. praetermissa, one the two host species of the C. effusa species complex, constitutes a species complex itself. It is possible that the mycoparasites might be strictly host specific, although there currently is not enough data available to test this hypothesis.
Two major differences can be recognised between species of the gall-like (Colacogloea bettinae sp. nov. and C. universitatis-gandavensis sp. nov.) and the non-gall-like morphotypes (C. biconidiata sp. nov., C. effusa, C. fennica sp. nov., C. microspora sp. nov., and C. philyla).
The first difference concerns the conidiogenesis and the shape of conidia. In the gall-like morphotype, conidia are born on distinct, stalked conidiophores which consist of two apical lids, each giving rise to a daughter conidium (Figs 6C, 18D). In a later stage, these two daughter conidia merge, forming a zygoconidium. The content, incl. the nucleus, of one daughter conidium is transferred to the other daughter conidium. In the non-gall-like morphotype, conidia are born singly, with a basal clamp connection, and formed on conidiophores which can be interpreted as terminal hyphae (Figs 8C, 10C, 12C, 14C, 16C). In the gall-like morphotype, conidia are characterised by a regular, oval shape and the presence of an appendage (= cell wall remnants of the second daughter conidium of which the content was transferred) (Figs 6D, 18D). In species of the non-gall-like morphotype, conidia have a more irregular shape, and do not have such an appendage (Figs 8D, 10D, 12D, 14D, 16D). To date, zygoconidia have been reported from three different Colacogloea species: C. bettinae sp. nov., C. papilionacea and C. universitatis-gandavensis sp. nov. (Kirschner & Oberwinkler 2000). This character is considered rare among Basidiomycota, with only few other genera sharing this character: Papiliotrema, Trimorphomyces, Syzygospora (Tremellomycetes), and Zygogloea (Basidiomycota incertae sedis) (Oberwinkler & Bandoni 1983, Roberts 1994, Weiß et al. 2014).
The second difference is the organisation of colacosomes in the hyphae of the mycoparasite. In the gall-like morphotype, most colacosomes are positioned at regular distances in vesicular gall-like cells along the host–parasite interface (Figs 6E, 18E). To a lesser extent, colacosomes also occur scattered in hyphae, conidiophores, or rarely, other elements of the mycoparasite. In the non-gall-like morphotype, no vesicular gall-like cells are present and colacosomes occur scattered in hyphae of the mycoparasite, with highest concentrations at those places where physical contact with host hyphae is established. In all the species that have been investigated by TEM, colacosomes were also observed in mycoparasite hyphae making contact with other hyphae of the same individual. This phenomenon was also observed in other colacosome-forming species and self-parasitism was put forward as an explanation for this phenomenon (Greschner-Aschenbrenner 1997, van der Klei et al. 2011). Both the scattered colacosome organisation and the organisation of colacosomes in vesicular gall-like cells have been observed in other mycoparasites in Microbotryomycetes and Cryptomycocolacomycetes (Table 1). Regarding the vesicular gall-like cells, an interesting question on whether the mycoparasite attracts a host hypha to grow into the invagination of the gall-like cell, or the gall-like cell actively overgrows a host hypha after which colacosomes are formed at the contact interface, remains unanswered.
The genus Mycogloiocolax gen. nov.
In this study, we erect the genus Mycogloiocolax gen. nov. and family Mycogloiocolacaceae fam. nov. for a clade comprising isolates of two distinct species. The first isolate represents Mycogloiocolax gerardii sp. nov., a dimorphic fungus, of which the filamentous morph represents a colacosome-interacting mycoparasitic stage. This stage develops intrahymenially in the basidiome of the corticioid fungus Xenasmatella tulasnelloidea and is visible as a gelatinous, hyaline layer overgrowing the host in humid conditions. Its yeast morph was isolated in pure culture. Currently, this is the only known mycoparasite of Xenasmatella species. This species was reported for the first time by Trichies (2006), who illustrated his collection with drawings and a description, but did not decide to formally publish it as a new species. Due to the limited set of available morphological characteristics, he assigned his collection tentatively to the genus Achroomyces, which is a heterogenous gathering of Basidiomycota with transversally septate basidia. In his description, he mentions the basidia as strictly bisporic. We observed only bisporic basidia with sterigmata, although we also observed some basidioles with two and three septa, but never with outgrowing sterigmata in these cases.
The second isolate in this clade represents the yeast strain KBP Y-6479 (DSM 110867), representing a currently undescribed species. It was derived as an endophyte from a lichen thallus of Cladonia rangiferina, collected near Pokachi town, Tyumen region, Russia. We know this species only from this single strain, and its ecology and distribution patterns remain largely unknown (Dr. Aleksey Kachalkin, pers. comm.). Physiologically, strain KBP Y-6479 differs very markedly from Mycogloiocolax gerardii sp. nov., and has a maximum growth temperature below 30 °C (Supplementary Table 1).
The genus Slooffia
The genus Slooffia was erected by Wang et al. (2015b) to accommodate yeast species comprising the Sporobolomyces tsugae clade. All these species were isolated as yeasts from different natural environments and substrates, including phylloplanes, dead organic material and soils. Only one or a few number of isolates are available for the currently known Slooffia species, and no filamentous morphs were reported in literature so far. The yeast morphs are considered to be saprobic (Sampaio 2011, Wang et al. 2015b, Begerow et al. 2018). Slooffia micra comb. nov. represents the first species in the genus for which the filamentous morph is observed in natural conditions, representing a colacosome-interacting mycoparasitic stage. It can be assumed that filamentous morphs of other Slooffia species exist, and it is possible that they also engage in mycoparasitism, although this remains to be investigated. As outlined below, the filamentous morph of S. micra has previously been illustrated and formally described under two names by different authors.
Bourdot & Galzin (1924) described Platygloea micra as a heterobasidiomycete with transversally septate basidia, growing on rotten Populus wood. The authors did not mention the presence of a second fungal species or a possible mycoparasitic interaction in the description of their collection. Reinvestigation of the only specimen of this species in the herbarium of Bourdot (PC), showed the presence of hyphae, longitudinally septate basidia and basidiospores of the host species Myxarium podlachicium, although the state of these structures was degraded. The gelatinous context of the host basidiome was probably misinterpreted by Bourdot & Galzin (1924, 1928) as being part of Pl. micra. We easily detected the presence of colasosomes in hyphae of the mycoparasite by epifluorescence microscopy (result not shown). Hauerslev (1993) described Achroomyces insignis as an intrahymenial mycoparasite of Myxarium podlachicium, although he did not observe interaction structures. However, investigation of the holotype by epifluorescence microscopy clearly demonstrated the presence of colacosomes (Fig. 5). Interestingly, Hauerslev (1993) interpreted the conidia as chlamydospores (= thick-walled spores which are formed directly on hyphae), and did not mention the presence of the typical conidiophores (Fig. 5B). Comparing the type specimens of both species for their ecological and micromorphological characteristics, we conclude that Platygloea micra and A. insignis are conspecific. We select epitypes for both names and ex-type cultures are available for both types. The host–parasite interface of Slooffia micra comb. nov. is characterised by hyphae of the mycoparasite coiling around host hyphae. This results in rosette-like structures when imaged with epifluorescence microscopy (Fig. 5F, G). At the contact interface, colacosomes are densely arranged. To a lesser extent, colacosomes also occur scattered in hyphae of this species. The coiling of mycoparasite hyphae around host hyphae is also known from Colacogloea papilionacea (compare fig. 1 in Kirschner & Oberwinkler (2000) with Fig. 5F, G in this publication).
We investigated two other presumed mycoparasitic species of Myxarium spp. which have been published previously. Platygloea abdita Bandoni was described from the USA (Bandoni 1959), and Cystobasidium sebaceum G.W. Martin was described from Colombia (Martin 1939). Since no recent collections or cultures of these species are available, DNA sequence data is not available. Epifluorescence microscopy imaging of Pl. abdita and Cystobasidium sebaceum showed that both species are colacosome-interacting mycoparasites of Myxarium spp. (N. Schoutteten, results not shown). Differences in micromorphological characters suggest that these species are not conspecific with Slooffia micra comb. nov. (results not shown). Since both mycoparasites share the colacosome-interaction, the same type of conidiophores, and the same host genus as S. micra, it is possible that they belong to the genus Slooffia. However, such conclusions can only be made when DNA sequences are available and support is provided by phylogenetic reconstructions.
The genus Atractocolax
The genus Atractocolax was erected by Kirschner et al. (2001) to accommodate A. pulvinatus, a peculiar dimorphic mycoparasite, isolated from bark beetle galleries in decaying logs of coniferous tree species in Germany and Switzerland. This species develops pulvinate basidiomes and has transversally septate basidia. Passively released spores accumulate in slimy droplets extruding from the basidiome, which may be an adaptation to insect dispersal (Kirschner et al. 1999). Although the authors succeeded in isolating the species in axenic culture, DNA sequence data was never generated and its classification in Microbotryomycetes was tentative. In our seven-locus ML phylogenetic reconstruction (Fig. 3), A. pulvinatus is recovered as sister lineage to the clade of Hyalopycnis and Pycnopulvinus, and thus we propose to include this species in the order Heterogastridiales.
It is unclear whether the samples for TEM were derived from mixed or axenic cultures, but the published pictures only show colacosomes interacting with hyphae of the mycoparasite itself (Kirschner 1999, Oberwinkler & Bauer 2018). The host range of A. pulvinatus is not known, but it is assumed that the host is an ophiostomatoid ascomycete, a group of fungi frequently co-occurring on the same substrate (Kirschner et al. 1999). To our knowledge, the species was never recollected in its sexual morph after the original publication. A search for similar sequences in public sequence databases revealed three yeasts from a study screening for xylose-fermenting yeasts in the gut microbiome of the wood-feeding termite Reticulitermes chinensis (Ali et al. 2017). These isolates were originally identified as Hamamotoa lignophila. Our results show that these three isolates are conspecific with A. pulvinatus. By extrapolating physiological results of Ali et al. (2017), we conclude that A. pulvinatus is capable of fermenting D-xylose and producing ethanol. Anaerobic conversion of carbohydrates, fermentation, is rare among Basidiomycota species, and is limited to slow glucose fermentation by, e.g., Rhynchogastrema glucofermentans, Filobasidium capsuligenum, and species of the genera Mrakia and Phaffia (Tremellomycetes) (Fell 2011, Fell & Johnson 2011, Kwon-Chung 2011). Touchette et al. (2022) recently reported fermentation of glucose for one other species in Microbotryomycetes, i.e., Rhodotorula frigidialcoholis, suggesting that production of ethanol could be a yet little studied adaptation to life at low temperatures. This character should be carefully studied in the future for other representatives of this class, especially those isolated from cold habitats.
CONCLUSIONS
The present study demonstrates the diversity of colacosome-forming fungi in Microbotryomycetes and shows the utility of Congo red staining combined with epifluorescence microscopy for easy colacosome detection. Freshly collected and cultivated colacosome-forming mycoparasites allowed analyses of micromorphology, yeast morph characterisation, and generation of nucleotide sequence data. Based on our results, the total number of fungi in which colacosomes have been detected increases to 27. We reveal three distinct types of colacosome organisation in Microbotryomycetes, being a scattered occurrence, hyphae of the mycoparasite coiled around host hyphae, and vesicular gall-like cells of the mycoparasite surrounding host hyphae. We show that the colacosome-forming fungus Atractocolax pulvinatus is a member of Microbotryomycetes, related to Slooffia and other members of Heterogastridiales. Platygloea micra is identified as a colacosome-interacting mycoparasite of Myxarium podlachicum. This species is combined in the genus Slooffia, hitherto only known from yeast morphs, and thus it represents the first species for which a filamentous morph is reported, representing a mycoparasitic stage. In the genus Colacogloea, five new species are described in the C. effusa species complex, and the first report of the filamentous morph of C. philyla is presented as a colacosome-interacting mycoparasite. The family Mycogloiocolacaceae fam. nov. is proposed for the newly described Mycogloiocolax gerardii sp. nov., a colacosome-interacting mycoparasite of Xenasmatella tulasnelloidea. Sequences obtained from these mycoparasites, derived from yeast cultures and filamentous morphs, allowed us to produce a robust phylogeny of Microbotryomycetes, resolving several problematic taxa. Within Microbotryomycetes, colacosomes occur in the families Chrysozymaceae, Colacogloeaceae, and Mycogloiocolacaceae fam. nov. and the orders Heterogastridiales, Leucosporidiales, and Sporidiobolales. A mycoparasitic strategy is likely for species that were found to only produce colacosomes in pure culture, although their host range remains to be determined. These combined results improve our understanding of the diversity and ecology of Microbotryomycetes. Further field sampling and careful analyses of mycoparasites and lichenicolous fungi, including their cultivation, will be key to further resolving evolutionary relationships in this class of fungi.
DECLARATION ON CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Table 2 .
PCR conditions and primers used for DNA amplification.
| Locus | Initial denaturation | Number of cycles | Denaturation | Annealing | Elongation | Final extension | Primers | Sense | Sequence (5’ → 3’) | Reference |
| ITS | 95 °C for 5 min | 35 | 95 °C for 30 s | 55 °C for 45 s | 72 °C for 45 s | 72 °C for 5 min | ITS1-F | Forward | CTTGGTCATTTAGAGGAAGTAA | Gardes & Bruns (1993) |
| ITS4 | Reverse | TCCTCCGCTTATTGATATGC | White et al. (1990) | |||||||
| LSU | 94 °C for 1 min | 35 | 94 °C for 1 min | 52 °C for 45 s | 72 °C for 45 s | 72 °C for 5 min | NL1 | Forward | GCATATCAATAAGCGGAGGAAAAG | O’Donnel (1993) |
| NL4 | Reverse | GGTCCGTGTTTCAAGACGG | O’Donnel (1993) | |||||||
| 95 °C for 5 min | 35 | 94 °C for 1 min | 55 °C for 45 s | 72 °C for 45 s | 72 °C for 5 min | LR0R | Forward | GTACCCGCTGAAGTTAAGC | Cubeta et al. (1991) | |
| LR5 | Reverse | ATCCTGAGGGAAACTTC | Vilgalys & Hester (1990) | |||||||
| SSU | 95 °C for 5 min | 35 | 95 °C for 35 s | 55 °C for 45 s | 72 °C for 1 min | 72 °C for 5 min | NS1 | Forward | GTAGTCATATGCTTGTCTC | White et al. (1990) |
| NS4 | Reverse | CTTCCGTCAATTCCTTTAAG | White et al. (1990) | |||||||
| RPB1 | 94 °C for 4 min | 36 | 94 °C for 1 min | 52 °C for 1 min | 72 °C for 1 min | 72 °C for 8 min | RPB1-Af | Forward | GARTGYCCDGGDCAYTTYGG | Matheny et al. (2002) |
| RPB1-Cr | Reverse | CCNGCDATNTCRTTRTCCATRTA | Matheny et al. (2002) | |||||||
| RPB2 | 94 °C for 4 min | 36 | 94 °C for 1 min | 52 °C for 1 min | 72 °C for 1 min | 72 °C for 8 min | fRPB2-5F | Forward | GAYGAYMGWGATCAYTTYGG | Liu et al. (1999) |
| fRPB2-7cR | Reverse | CCCATRGCTTGYTTRCCCAT | Matheny (2005) | |||||||
| TEF-1 α | 95 °C for 5 min | 35 | 94 °C for 1 min | 55 °C for 45 s | 72 °C for 45 s | 72 °C for 5 min | EF1-983F | Forward | GCYCCYGGHCAYCGTGAYTTYAT | Rehner & Buckley (2005) |
| EF1-2218R | Reverse | ATGAACCRACRGRACRGTYTG | Rehner & Buckley (2005) | |||||||
| EF1-1567R | Reverse | ACHGTRCCRATACCACCRATCTT | Rehner & Buckley (2005) | |||||||
| CYT-B | 94 °C for 4 min | 36 | 94 °C for 1 min | 46 °C for 1 min | 72 °C for 1 min | 72 °C for 5 min | E1M4 | Forward | TGRGGWGCWACWGTTATTACTA | Biswas et al. (2001) |
| E2mr3 | Reverse | GGWATAGCACGTARAAYWGCRTA | Biswas et al. (2001) |
Table 4 .
Summary of the seven genetic loci included in the phylogenetic ML analysis, with for every partition the number of incorporated sequences, total number of sites, number of parsimony informative sites, number of invariable sites, and the selected model of nucleotide substitution as selected by ModelFinder.
| Partition | Locus | Sequences | Sites | Informative sites | Invariable sites | Model |
| 1 | SSU | 192 | 1 848 | 408 | 1 108 | TIM+F+R8 |
| 2 | ITS1 | 226 | 153 | 146 | 3 | GTR+F+R5 |
| 3 | 5.8S | 226 | 168 | 117 | 45 | K2P+R7 |
| 4 | ITS2 | 226 | 207 | 185 | 12 | TVM+F+R5 |
| 5 | LSU | 229 | 646 | 313 | 277 | GTR+F+R10 |
| 6 | RPB1 | 174 | 616 | 447 | 107 | GTR+F+R6 |
| 7 | RPB2 | 192 | 866 | 476 | 297 | SYM+R4 |
| 8 | TEF1- α | 196 | 950 | 469 | 347 | GTR+F+R10 |
| 9 | CYT-B | 163 | 401 | 254 | 85 | TVM+F+R5 |
| Concatenated | 9 loci | 238 | 5 855 | 2 815 | 2 281 | — |
Acknowledgments
Research Foundation Flanders (FWO) supported this study through a PhD Fellowship Fundamental Research to Nathan Schoutteten (11E0420N) and a Junior Postdoctoral Fellowship to Danny Haelewaters (1206620N). The work of Otto Miettinen was supported by a project grant from the Academy of Finland (grant No. 315927). We are grateful to Myriam Claeys of the TEM Expertise Centrum of Ghent University. Dr Aleksey Kachalkin and Dr Vera Malysheva are gratefully acknowledged for providing DNA sequences and metadata of specific isolates, as well as providing comments on the manuscript. Dr Viacheslav Spirin is gratefully acknowledged for providing specimens, aid in microscopic examination of specimens, outlining the species descriptions, and advise on nomenclatural aspects. Sincere acknowledgements go to the Belgian and Dutch contributors of the Phragmoproject (https://www.mycologen.nl/onderzoek/systematiek-taxonomie/phragmo-project/): Roeland Enzlin, Martin Gotink, Ida Nannenga-Bruggeman, Hermien Wassink†, Inge Somhorst, Greet Van Autgaerden, Marc Detollenaere, and Bregtje Miedema; and to Thomas Rödel, who provided valuable collections for this research. Peter Roberts is gratefully acknowledged for providing comments on the group of Platygloea micra. Juliana Leshchenko is greatfully acknowledged for editing figure 1. We are grateful to Geoffrey Kibby for editing drawings and proofreading of the manuscript. Ruben De Lange is gratefully acknowledged for proofreading the manuscript. We acknowledge the fungaria of C, H, LIP, and PC for providing loans or access to specimens for investigation. Nadine Masuch (DSMZ) is acknowledged for assistance in the lab.
Supplementary Material
Table S1.
Physiological and biochemical characteristics of Atractocolax, Colacogloea, Mycogloiocolax and Slooffia species.
| Characteristic | Fermentation of glucose | Glucose | Galactose | L-Sorbose | Sucrose | Maltose | Cellobiose | Trehalose | Lactose | Melibiose | Raffinose | Melezitose | Inulin | Soluble Starch | D-Xylose | L-Arabinose | D-Arabinose | D-Ribose | L-Rhamnose | D-Glucosamine | N-Acetyl-D-glucosamine | Methanol | Ethanol | Glycerol | Erythritol | Ribitol | Galactitol | D-Mannitol | D-Glucitol | Methyl-α-D-glucoside | Salicin | D-Gluconate | DL-Lactate | Succinate | Citrate | myo-Inositol | Hexadecane | Ammonium sulfate | Potassium nitrate | Sodium nitrite | L-lysine | Ethylamine | Cadaverine | Growth in vitamin-free medium | Starch like compounds formation | Growth with 50% glucose | Hydrolysis of urea | Diazonium Blue B reaction | Major ubiquinone | 22 ? | 25 ? | 30? | 32 ? | 35 ? | 37 ? |
| Atractocolax | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atractocolax pulvinatus | n/a | + | w | w | + | - | - | - | + | - | + | - | - | - | - | w | + | + | - | + | n/a | n/a | - | w | - | + | + | + | + | + | w | w | n/a | w | - | - | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | - | - | + | + | n/a | + | + | + | + | - | - |
| Colacogloea | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| C. aletridis | - | + | - | - | + | w | - | + | - | - | - | + | - | - | - | - | - | - | - | - | n/a | - | + | - | - | l | - | w | w | w | - | n/a | - | - | - | - | - | + | + | + | + | + | + | + | - | - | + | + | n/a | + | + | + | - | - | - |
| C. bettinae sp. nov. | n/a | + | - | w | + | - | - | - | w | - | w | - | - | - | w | w | + | + | w | + | n/a | n/a | w | + | - | + | w | + | + | + | - | + | - | + | - | - | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | - | w | + | + | n/a | + | + | + | + | + | - |
| C. biconidiata sp. nov. | n/a | + | - | - | - | dw | - | - | - | - | - | - | w | - | - | - | + | + | - | + | n/a | n/a | - | + | - | - | w | - | + | + | w | + | - | + | - | - | n/a | n/a | + | + | n/a | n/a | n/a | n/a | - | w | + | + | n/a | + | + | + | + | + | - |
| C. cycloclastica | - | + | - | - | - | - | - | + | - | - | - | - | - | - | v | - | - | - | - | v | n/a | - | + | v | - | + | - | + | + | - | - | - | + | + | + | - | + | n/a | - | - | n | n | n | + | - | n/a | n/a | + | 10 | + | + | + | n/a | v | - |
| C. demeterae | - | + | + | - | + | n/a | n/a | + | -,w | n/a | + | + | - | - | + | + | + | -,w | n/a | - | n/a | n/a | - | w | n/a | w | n/a | + | + | n/a | n/a | n/a | + | + | + | + | n/a | n/a | + | + | + | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | + | n/a | n/a | n/a | n/a |
| C. diffluens | - | + | + | + | + | + | - | + | - | - | + | + | - | - | + | - | + | + | - | + | n/a | - | + | + | + | + | + | + | + | - | + | + | - | + | + | - | n/a | n/a | + | + | n/a | n/a | n/a | + | - | n/a | n/a | + | 10 | + | + | - | - | - | - |
| C. effusa | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | v | - | - | + | n/a | - | v | - | - | - | - | + | + | - | - | - | v | + | v | - | n/a | n/a | - | - | n | n | n | + | - | w | n/a | + | 10 | + | + | + | n/a | - | - |
| C. eucalyptica | - | + | - | - | + | + | - | + | - | - | - | + | - | - | + | - | - | - | - | + | n/a | - | + | - | - | - | - | + | + | w | - | - | - | + | + | - | + | n/a | - | - | w | + | - | + | - | - | + | n/a | n/a | n/a | + | n/a | n/a | n/a | - |
| C. falcata | - | + | + | - | - | - | + | + | - | - | - | - | - | - | + | - | - | - | - | + | n/a | - | + | + | - | - | - | + | + | - | + | + | - | + | + | - | n/a | n/a | + | + | - | + | - | - | - | - | + | + | 10 | + | + | - | n/a | - | - |
| C. fennica sp. nov. | n/a | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | - | + | n/a | n/a | - | + | w | - | w | + | + | + | - | + | - | - | - | - | n/a | n/a | + | + | n/a | n/a | - | - | - | - | + | + | n/a | + | + | + | + | + | - |
| C. foliorum | - | + | - | - | - | - | + | + | - | - | - | - | - | - | + | + | + | + | - | + | n/a | - | + | + | - | + | - | + | + | - | - | + | + | + | + | - | n/a | n/a | + | + | n | n | n | + | - | n/a | n/a | + | 10 | + | + | - | n/a | - | - |
| C. hydrangeae | - | + | - | - | + | + | - | + | - | - | - | l | - | - | - | - | - | - | - | + | n/a | - | + | - | - | l | - | + | + | - | + | n/a | - | - | - | - | - | + | + | + | + | + | - | w | - | - | + | + | n/a | + | + | - | - | - | - |
| C. microspora sp. nov. | n/a | + | - | w | w | - | - | - | - | - | - | v | - | - | - | - | + | + | - | + | n/a | n/a | - | + | w | + | + | + | + | + | - | + | - | - | - | - | n/a | n/a | + | + | n/a | n/a | n/a | n/a | - | + | + | + | n/a | + | + | + | + | + | - |
| C. papilionacea | - | + | + | - | - | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | n/a | - | + | + | - | - | - | + | - | - | - | - | - | + | - | - | n/a | n/a | - | - | n | n | n | - | - | n/a | n/a | + | n | + | + | + | n/a | - | - |
| C. philyla | - | + | - | - | - | - | - | + | - | - | - | - | - | - | + | - | + | - | - | + | n/a | - | + | v | - | + | - | + | + | - | - | + | v | + | + | - | n/a | n/a | - | - | n | n | n | + | - | n/a | n/a | + | 10 | + | + | + | n/a | - | - |
| C. retinophila | - | + | - | - | - | - | - | + | - | - | - | - | - | - | + | - | + | - | - | + | n/a | - | + | v | - | + | - | + | + | - | - | + | + | + | + | - | + | n/a | - | - | n | n | n | + | - | n/a | n/a | + | 10 | + | + | + | n/a | + | + |
| C. rhododendri | - | + | v | - | + | + | v | + | - | - | - | + | v | - | - | - | - | - | - | w | w | - | + | l | - | v | - | + | v | - | - | + | - | - | - | - | - | + | w | v | + | + | v | + | - | - | + | + | n/a | + | + | - | - | - | - |
| C. subericola | - | + | - | - | + | - | - | + | - | - | - | - | - | - | + | - | + | - | - | + | + | - | + | - | - | + | - | + | + | - | - | + | - | + | w | - | - | n/a | + | + | + | - | n/a | + | - | - | + | n/a | n/a | n/a | + | + | n/a | n/a | - |
| C. terpenoidalis | - | + | - | - | + | + | - | + | - | - | - | + | - | - | + | - | + | - | - | + | n/a | - | + | + | - | + | - | + | + | + | - | + | + | + | + | - | n/a | n/a | - | - | n | n | n | + | - | n/a | n/a | + | 10 | + | + | + | n/a | + | + |
| Mycogloiocolax gen. nov. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| M. gerardii sp. nov. | n/a | + | n/a | w | - | - | + | - | - | - | w | - | + | + | - | w | + | w | w | w | n/a | n/a | - | + | w | + | + | + | + | w | w | w | - | w | - | - | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | - | + | + | + | n/a | + | + | + | + | + | - |
| KBP:Y-6479 | - | + | - | - | + | - | w | + | - | - | - | - | - | - | - | - | - | - | - | - | n/a | - | + | + | - | - | - | + | w | - | w | n/a | - | - | - | - | n/a | + | - | n/a | - | n/a | - | + | - | - | + | + | n/a | + | - | - | - | - | - |
| Slooffia | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S. cresolica | - | + | + | - | + | + | + | + | - | - | - | + | - | - | + | - | + | + | - | + | n | - | + | + | - | + | - | + | + | + | - | + | + | + | + | - | n/a | n/a | + | + | n/a | n/a | n/a | + | - | n/a | n/a | + | 10 | + | + | - | n/a | - | - |
| S. globosa | - | + | - | - | + | + | + | + | w | - | - | lw | - | - | - | - | - | - | - | - | - | - | + | + | - | - | - | + | - | lw | - | w | - | - | - | - | - | + | + | - | w | + | lw | - | - | - | + | + | n/a | + | + | - | - | - | - |
| S. micra comb. nov. | n/a | + | - | + | l | w | + | + | w | - | + | + | - | - | - | - | + | - | - | + | n/a | n/a | w | w | - | + | w | + | + | + | - | + | - | - | - | - | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | - | - | + | + | n/a | + | + | + | + | + | - |
| S. pilati | - | + | - | + | + | + | + | + | + | - | - | + | - | - | + | - | + | - | - | - | n/a | - | + | + | - | + | - | + | + | + | - | + | + | + | + | - | n/a | n/a | + | + | n/a | n/a | n/a | + | - | n/a | n/a | + | n/a | + | + | - | - | - | - |
| S. tsugae | - | + | - | + | + | + | + | + | + | - | - | + | - | - | + | - | v | - | - | - | n/a | - | + | + | - | v | - | + | + | v | + | + | + | + | + | - | n/a | n/a | + | + | v | + | - | - | - | - | + | + | 10 | + | + | - | - | - | - |
| S. velesii | - | + | + | + | + | + | + | + | + | + | + | - | n/a | + | + | + | w | - | - | n/a | n/a | + | + | n/a | w | n/a | + | + | n/a | n/a | n/a | + | + | + | + | n/a | n/a | + | + | + | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | + | n/a | n/a | n/a | n/a |
Abbreviations: +, positive; -, negative; l, latent; w, weak; lw, latent and weak; v, variable; n/a, not available.
Species names in bold are novel characterized species from this study.
REFERENCES
- Aime MC, Castlebury LA, Abbasi M. et al. (2018). Competing sexual and asexual generic names in Pucciniomycotina and Ustilaginomycotina (Basidiomycota) and recommendations for use. IMA Fungus 9: 75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aime MC, Matheny PB, Henk DA. et al. (2006).An overview of the higher-level classification of Pucciniomycotina based on combined analyses of nuclear large and small subunit rDNA sequences. Mycologia 98: 896–905. [DOI] [PubMed] [Google Scholar]
- Aime MC, Toome M, McLaughlin DJ. (2014). Pucciniomycotina. In: The Mycota VII. Systematics and evolution. Part A. (McLaughlin DJ, Spatafora JW eds). Springer, Germany: 271–294. [Google Scholar]
- Ali SS, Wu J, Xie R. et al. (2017). Screening and characterizing of xylanolytic and xylose-fermenting yeasts isolated from the wood-feeding termite, Reticulitermes chinensis. PLoS One 12: e0181141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandoni RJ. (1956). A preliminary survey of the genus Platygloea. Mycologia 48: 821–840. [Google Scholar]
- Bandoni RJ. (1959). An undescribed species of Platygloea from Iowa. Mycologia 51: 94–96. [Google Scholar]
- Bandoni RJ. (1973). Epistolae mycologicae II. Species of Platygloea from British Columbia. Syesis 6: 229–232. [Google Scholar]
- Bandoni RJ. (1995). Dimorphic Heterobasidiomycetes: taxonomy and parasitism. Studies in Mycology 38: 13–28. [Google Scholar]
- Bandoni RJ, Krug J, Ginns J. (2002). On some Colacogloea species from Canada. Czech Mycology 54: 31–43. [Google Scholar]
- Bauer R. (2004). Basidiomycetous interfungal cellular interactions–a synopsis. In: Frontiers in basidiomycote mycology. (Agerer R, Piepenbring M, Blanz P, eds). IHW-Verlag, Germany: 325–337. [Google Scholar]
- Bauer R, Begerow D, Sampaio JP. et al. (2006). The simple-septate basidiomycetes: a synopsis. Mycological Progress 5: 41–66. [Google Scholar]
- Bauer R, Lutz M, Oberwinkler F. (2004). Tuberculina-rusts: a unique basidiomycetous interfungal cellular interaction with horizontal nuclear transfer. Mycologia 96: 960–967. [PubMed] [Google Scholar]
- Bauer R, Oberwinkler F. (1991). The Colacosomes: New Structures at the Host-parasite Interface of a Mycoparasitic Basidiomycete. Botanica Acta 104: 53–57. [Google Scholar]
- Bauer R, Oberwinkler F, Vánky K. (1997). Ultrastructural markers and systematics in smut fungi and allied taxa. Canadian Journal of Botany 75: 1273–1314. [Google Scholar]
- Begerow D, Kemler M, Feige A. et al. (2017). Parasitism in yeasts. In: Yeasts in natural ecosystems: Ecology. (Buzzini P, Lachance MA, Yurkov A, eds). Springer Cham, Switserland:179–210. [Google Scholar]
- Begerow D, McTaggart A, Agerer R. (2018). 1/3 Basidiomycota and Entorrhizomycota In: A. Engler’s Syllabus der Pflanzenfamilien (Frey W, ed). Bontraeger Science Publishers, Germany. [Google Scholar]
- Beguin H. (2010). Tritirachium egenum, a thiamine-and siderophore-auxotrophic fungal species isolated from a Penicillium rugulosum. FEMS Microbiology Ecology 74: 165–173. [DOI] [PubMed] [Google Scholar]
- Bezerra JDP, Santos MGS, Barbosa RN. et al. (2013). Fungal endophytes from cactus Cereus jamacaru in Brazilian tropical dry forest: a first study. Symbiosis 60: 53–63. [Google Scholar]
- Biswas SK, Yokoyama K, Nishimura K. et al. (2001). Molecular phylogenetics of the genus Rhodotorula and related basidiomycetous yeasts inferred from the mitochondrial cytochrome b gene. International Journal of Systematic and Evolutionary Microbiology 51: 1191–1199. [DOI] [PubMed] [Google Scholar]
- Boekhout T, Fonseca A, Sampaio JP. et al. (2011). Discussion of teleomorphic and anamorphic basidiomycetous yeasts. In: The yeasts (Kurtzman CP, Fell JW, Boekhout T, eds). Elsevier, Germany: 1339–1372. [Google Scholar]
- Boekhout T, Yamada Y, Weijman A, et al. (1992). The significance of coenzyme Q, carbohydrate composition and septal ultrastructure for the taxonomy of ballistoconidia-forming yeasts and fungi. Systematic and Applied Microbiology 15: 1–10. [Google Scholar]
- Bourdot H. (1932). Hyménomycètes nouveaux ou peu connus. Bulletin Trimestriel de la Société Mycologique de France 48: 204–232. [Google Scholar]
- Bourdot H, Galzin A. (1909). Hyménomycètes de France. I Hetérobasidiés.Bulletin Trimestriel de la Société Mycologique de France 25: 14–36. [Google Scholar]
- Bourdot H, Galzin A. (1924). Heterobasidiae nondum descriptae. Bulletin Trimestriel de la Société Mycologique de France 39: 261–266. [Google Scholar]
- Bourdot H, Galzin A. (1928). Hyménomycètes de France. Hétérobasidiés — homobasidiés gymnocarpes. P. Lechevalier, published under the auspices of the Société Mycologique de France, France. [Google Scholar]
- Bourdot H, Maire L. (1920). Notes critiques sur quelques Hymenomycètes nouveaux ou peu connus. Bulletin Trimestriel de la Société Mycologique de France 36: 69–85. [Google Scholar]
- Brefeld O. (1888). Basidiomyceten II. Protobasidiomyceten. Untersuchungen aus den Gesammtgebiet der Mykologie 7. Arthur Felix Verlag, Germany. [Google Scholar]
- Buzzini P, Lachance MA, Yurkov A. (Eds). (2017). Yeasts in natural ecosystems: diversity. Springer Cham, Switserland. [Google Scholar]
- Buzzini P, Turchetti B, Yurkov A. (2018). Extremophilic yeasts: the toughest yeasts around? Yeast 35: 487–497. [DOI] [PubMed] [Google Scholar]
- Caiafa MV, Smith ME. (2022). Polyphyly, asexual reproduction and dual trophic mode in Buchwaldoboletus. Fungal Ecology 56: 101141. [Google Scholar]
- Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. (2009). trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25: 1972–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomor O, Von Haeseler A, Minh BQ. (2016). Terrace aware data structure for phylogenomic inference from supermatrices. Systematic Biology 65: 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clémençon H. (2009). Methods for Working with Macrofungi. Laboratory Cultivation and Preparation of Larger Fungi for Light Microscopy. IHW-Verlag, Germany. [Google Scholar]
- Cubeta MA, Echandi E, Abernethy T, et al. (1991). Characterization of anastomosis groups of binucleate Rhizoctonia species using restriction analysis of an amplified ribosomal RNA gene. Phytopathology 81: 1395–1400. [Google Scholar]
- De Garcia V, Trochine A, Uetake J, et al. (2020). Novel yeast taxa from the cold: description of Cryolevonia giraudoae sp. nov. and Camptobasidium gelus sp. nov. International Journal of Systematic and Evolutionary Microbiology 70: 3711–3717. [DOI] [PubMed] [Google Scholar]
- De Hoog GS, Boekhout T. (1982). Teliospores, teliospore-mimics and chlamydospores. Studies in Mycology 22: 15–22. [Google Scholar]
- Doweld A. (2001). Prosyllabus Tracheophytorum: Tentamen Systematis Plantarum Vascularium Tracheophy. Geos, Russian Federation. [Google Scholar]
- Doweld A. (2014). Nomenclatural novelties. Index Fungorum 95. [Google Scholar]
- Fell JW. (2011). Mrakia Y. Yamada & Komagata (1987). In: The Yeasts (Kurtzman CP, Fell JW, Boekhout T, eds). Elsevier, Germany: 1503–1510. [Google Scholar]
- Fell JW, Johnson EA. (2011). Phaffia MW Miller, Yoneyama & Soneda (1976). In: The Yeasts (Kurtzman CP, Fell JW, Boekhout T, eds). Elsevier, Germany: 1853–1855. [Google Scholar]
- Gardes M, Bruns TD. (1993). ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Galindo LJ, López-García P, Torruella G, et al. (2021). Phylogenomics of a new fungal phylum reveals multiple waves of reductive evolution across Holomycota. Nature communications 12: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greschner-Aschenbrenner B. (1997). Vergleichende morphologische und ultrastrukturelle Untersuchungen an Colacogloea–Arten. Diplomarbeit, Lehrstühl Spezieller Botanik und Mykologie, Universität Tübingen, Germany. [Google Scholar]
- Hallenberg N, Nilsson RH, Antonelli A, et al. (2007). The Peniophorella praetermissa species complex (Basidiomycota). Mycological Research 111: 1366–1376. [DOI] [PubMed] [Google Scholar]
- Hamamoto M, Boekhout T, Nakase T. (2011). Sporobolomyces Kluyver & van Niel (1924). In: The yeasts (Kurtzman CP, Fell JW, Boekhout T, eds). Elsevier, Germany: 1929–1990. [Google Scholar]
- Hauerslev K. (1993). New tremellaceous fungi from Denmark. Mycotaxon 49: 217–233. [Google Scholar]
- Hass H, Taylor TN, Remy W. (1994). Fungi from the Lower Devonian Rhynie chert: Mycoparasitism. American Journal of Botany 81: 29–37. [PubMed] [Google Scholar]
- He MQ, Zhao RL, Hyde KD, et al. (2019). Notes, outline and divergence times of Basidiomycota. Fungal Diversity 99: 105–367. [Google Scholar]
- Hoang DT, Chernomor O, von Haeseler A, et al. (2018). UFBoot2: Improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachalkin AV. (2014). Yeasts of the White Sea intertidal zone and description of Glaciozyma litorale sp. nov. Antonie van Leeuwenhoek 105: 1073–1083. [DOI] [PubMed] [Google Scholar]
- Kachalkin AV. (2022). Phylogeny of Rhodotorula pinalis and its reclassification as Fellozyma pinalis comb. nov. Microbiology 91: 417–420. [Google Scholar]
- Kachalkin AV, Turchetti B, Inácio J, et al. (2019). Rare and undersampled dimorphic basidiomycetes. Mycological Progress 18: 945–971. [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF, et al. (2017). ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nature Methods 14: 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. (2019). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, et al. (2008). Dictionary of the Fungi. 10th ed. CABI Europe, UK. [Google Scholar]
- Kirschner R, Bauer R, Oberwinkler F. (1999). Atractocolax, a new heterobasidiomycetous genus based on a species vectored by conifericolous bark beetles. Mycologia 91: 538–543. [Google Scholar]
- Kirschner R, Bauer R, Oberwinkler F. (2001). Colacosiphon: a new genus described for a mycoparasitic fungus. Mycologia 93: 634–644. [Google Scholar]
- Kirschner R, Oberwinkler F. (2000). A new species of Colacogloea with zygoconidia. Sydowia 52: 195–203. [Google Scholar]
- Kreger-van Rij NJW, Veenhuis M. (1971a). A comparative study of the cell wall structure of basidiomycetous and related yeasts. Microbiology 68: 87–95. [Google Scholar]
- Kreger-van Rij NJW, Veenhuis M. (1971b). Some features of yeasts of the genus Sporidiobolus observed by electron microscopy. Antonie van Leeuwenhoek 37: 253–255. [DOI] [PubMed] [Google Scholar]
- Koch RA, Herr JR. (2021). Transcriptomics Reveals the Putative Mycoparasitic Strategy of the Mushroom Entoloma abortivum on Species of the Mushroom Genus Armillaria. Msystems 6: e00544-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman CP, Fell JW, Boekhout T, et al. (2011). Methods for isolation, phenotypic characterization and maintenance of yeasts. In: The Yeasts (Kurtzman CP, Fell JW, Boekhout T, eds). Elsevier, Germany: 87–110. [Google Scholar]
- Kwon-Chung KJ. (2011). Filobasidium Olive (1968). In: The Yeasts (Kurtzman CP, Fell JW, Boekhout T, eds). Elsevier, Germany: 1457–1465. [Google Scholar]
- Li AH, Yuan FX, Groenewald M, et al. (2020). Diversity and phylogeny of basidiomycetous yeasts from plant leaves and soil: proposal of two new orders, three new families, eight new genera and one hundred and seven new species. Studies in Mycology 96: 17–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Whelen S, Hall BD. (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Lutz M, Bauer R, Begerow D, et al. (2004). Tuberculina: rust relatives attack rusts Mycologia 96: 614–626. [PubMed] [Google Scholar]
- Malysheva V, Schoutteten N, Verbeken A, et al. (2021). Identity and typification of Achroomyces effusus (Pucciniomycotina, Basidiomycota). Mycological Progress 20: 413–417. [Google Scholar]
- Martin GW. (1939). New or noteworthy fungi from Panama and Colombia. Mycologia 31: 507–518. [Google Scholar]
- Martin GW. (1940). Some heterobasidiomycetes from eastern Canada. Mycologia 32: 683–695. [Google Scholar]
- Mašínová T, Pontes A, Carvalho C, et al. (2017). Libkindia masarykiana gen. et sp. nov., Yurkovia mendeliana gen. et sp. nov. and Leucosporidium krtinense fa sp. nov., isolated from temperate forest soils. International Journal of Systematic and Evolutionary Microbiology 67: 902–908. [DOI] [PubMed] [Google Scholar]
- Matheny PB. (2005). Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences. Inocybe; Agaricales. Molecular Phylogenetics and Evolution 35: 1–20. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Liu YJ, Ammirati JF, et al. (2002). Using RPB1 sequences to improve phylogenetic inference among mushrooms. Inocybe, Agaricales. American Journal of Botany 89: 688–698. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Yang HC, Homma T, et al. (1995). Use of Congo red as a microscopic fluorescence indicator of hyphal growth. Applied Microbiology and Biotechnology 43: 102–108. [Google Scholar]
- Menolli N, Jr, Sánchez-García M. (2020). Brazilian fungal diversity represented by DNA markers generated over 20 years. Brazilian Journal of Microbiology 51: 729–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RT. (1972). Ustomycota, a new division of higher fungi. Antonie van Leeuwenhoek 38: 567–584. [Google Scholar]
- Moore RT. (1996). An inventory of the phylum Ustomycota. Mycotaxon 59: 1–31. [Google Scholar]
- Naranjo-Ortiz MA, Gabaldón T. (2019). Fungal evolution: major ecological adaptations and evolutionary transitions. Biological Reviews 94: 1443–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LT, Schmidt HA, von Haeseler A, et al. (2015). IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberwinkler F. (1964). Intrahymeniale Heterobasidiomyceten. Frucht-körperlose Sebacina-Sippen und ihre systematische Stellung. Nova Hedwigia 7: 489–499. [Google Scholar]
- Oberwinkler F. (2012). Evolutionary trends in Basidiomycota. Stapfia 96: 45–104. [Google Scholar]
- Oberwinkler F. (2017). Yeasts in Pucciniomycotina. Mycological Progress 16: 831–856. [Google Scholar]
- Oberwinkler F, Bandoni RJ. (1983). Trimorphomyces: a new genus in the Tremellaceae. Systematic and Applied Microbiology 4: 105–113. [DOI] [PubMed] [Google Scholar]
- Oberwinkler F, Bauer R. (1990). Cryptomycocolax: a new mycoparasitic heterobasidiomycete. Mycologia 82: 671–692. [Google Scholar]
- Oberwinkler F, Bauer R. (2018). Ultrastructure in basidiomycetes–requirement for function. In: Biodiversity and Ecology of Fungi, Lichens, and Mosses. (Blanz P, ed). Austrian Academy of Sciences Press, Austria: 381–418. [Google Scholar]
- Oberwinkler F, Bauer R, Bandoni RJ. (1990a). Colacogloea - A new genus in the auricularioid Heterobasidiomycetes. Canadian Journal of Botany 68: 2531–2536. [Google Scholar]
- Oberwinkler F, Bauer R, Bandoni RJ. (1990b). Heterogastridiales: a new order of Basidiomycetes. Mycologia 82: 48–58. [Google Scholar]
- Oberwinkler F, Bauer R, Tschen J. (1999). The mycoparasitism of Platygloea bispora. Kew Bulletin 54: 763–769. [Google Scholar]
- Oberwinkler F, Cruz D, Suárez JP. (2017). Biogeography and ecology of Tulasnellaceae. In: Biogeography of mycorrhizal symbiosis. (Tederso L, ed). Springer International Publishing AG, Switserland: 237–271. [Google Scholar]
- O’donnell K. (1993). Fusarium and its near relatives. In: The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics (Reynolds DR, Taylor JW, eds). CAB International, Great Britain: 225–233. [Google Scholar]
- Parmasto E, Parmasto I. (1987). Variation in basidiospores in the Hymenomycetes and its significance to their taxonomy. Bibliotheca Mycologica 115: 1–168. [Google Scholar]
- Passer AR, Coelho MA, Billmyre RB, et al. (2019). Genetic and genomic analyses reveal boundaries between species closely related to Cryptococcus pathogens. Mbio 103 e00764-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini L, Andrejašič K, Gostinčar C, et al. (2021). Greenland and Svalbard glaciers host unknown basidiomycetes: the yeast Camptobasidium arcticum sp. nov. and the dimorphic Psychromyces glacialis gen. and sp. nov. International Journal of Systematic and Evolutionary Microbiology 71: 004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilat A. (1957). Übersicht der europäischen Auriculariales und Tremellales unter besonderer Berücksichtung der Tschechoslowakischen Arten. Acta Musei Nationalis Pragae 13: 115–210. [Google Scholar]
- Pontes A, Ruethi J, Frey B. et al. (2020). Cryolevonia gen. nov. and Cryolevonia schafbergensis sp. nov., a cryophilic yeast from ancient permafrost and melted sea ice. International Journal of Systematic and Evolutionary Microbiology 70: 2334–2338. [DOI] [PubMed] [Google Scholar]
- Prasanna AN, Gerber D, Kijpornyongpan T, et al. (2019). Model choice, missing data, and taxon sampling impact phylogenomic inference of deep Basidiomycota relationships. >Systematic Biology 69: 17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redhead SA, Ammirati JF, Walker GR, et al. (1994). Squamanita contortipes, the Rosetta Stone of a mycoparasitic agaric genus. Canadian Journal of Botany 72: 1812–1824. [Google Scholar]
- Rehner SA, Buckley E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. [DOI] [PubMed] [Google Scholar]
- Roberts P. (1994). Zygogloea gemellipara: an auricularioid parasite of Myxarium nucleatum. Mycotaxon 52: 241–246. [Google Scholar]
- Sampaio JP. (2011). Rhodotorula Harrison (1928). In: The yeasts (Kurtzman CP, Fell JW, Boekhout T, eds).Elsevier, Germany: 1873–1927. [Google Scholar]
- Sampaio JP, Gadanho M, Bauer R, et al. (2003). Taxonomic studies in the Microbotryomycetidae: Leucosporidium golubevii sp. nov., Leucosporidiella gen. nov. and the new orders Leucosporidiales and Sporidiobolales. Mycological Progress 2: 53–68. [Google Scholar]
- Sampaio JP, Kirschner R, Oberwinkler F. (2011). Colacogloea Oberwinkler & Bandoni (1990). In: The yeasts (Kurtzman CP, Fell JW, Boekhout T, eds). Elsevier, Germany: 1403–1408. [Google Scholar]
- Savchenko A, Zamora JC, Shirouzu T. et al. (2021). Revision of Cerinomyces (Dacrymycetes, Basidiomycota) with notes on morphologically and historically related taxa. Studies in Mycology 99: 1–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröter J. (1887). Die Pilze Schlesiens. In: Kryptogamen Flora von Schlesien 3. (Cohn, FJ ed). J. V. Kern’s Verlag, Germany: 1–814. [Google Scholar]
- Selosse MA, Schneider-Maunoury L, Martos F. (2018). Time to re-think fungal ecology? Fungal ecological niches are often prejudged. New Phytologist 217: 968–972. [DOI] [PubMed] [Google Scholar]
- Spirin V, Malysheva V, Roberts P, et al. (2019). A convolute diversity of the Auriculariales (Agaricomycetes, Basidiomycota) with sphaeropedunculate basidia. Nordic Journal of Botany 37: 1–26. [Google Scholar]
- Spirin V, Malysheva V, Trichies G, et al. (2018). A preliminary overview of the corticioid Atractiellomycetes (Pucciniomycotina, Basidiomycetes). Fungal Systematics & Evolution 2: 311–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiers B. (2022). Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Accessed on 01 October 2022. [Google Scholar]
- Toome M, Aime MC. (2014). Pycnopulvinus aurantiacus gen. et sp. nov., a new sporocarp-forming member of Pucciniomycotina. MycoKeys 8: 43–50. [Google Scholar]
- Toome M, Roberson RW, Aime MC. (2013). Meredithblackwellia eburnea gen. et sp. nov., Kriegeriaceae fam. nov. and >Kriegeriales ord. nov. – toward resolving higher-level classification in Microbotryomycetes. Mycologia 105: 486–495. [DOI] [PubMed] [Google Scholar]
- Touchette D, Altshuler I, Gostinčar C, et al. (2022). Novel Antarctic yeast adapts to cold by switching energy metabolism and increasing small RNA synthesis. The ISME Journal 16: 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichies G. (2006). Hétérobasidiomycètes inusuels ou nouveaux découverts en France. Bulletin de la Société Mycologique de France 122: 29–60. [Google Scholar]
- Van Der Klei I, Veenhuis M, Brul S, et al. (2011). Cytology, cell walls and septa: a summary of yeast cell biology from a phylogenetic perspective. In: The yeasts – A taxonomic study, Vol. 1,5th edition (Kurtzman CP, Fell JW, Boekhout T, eds). Elsevier, Germany: 111–128. [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GS, Sun Y, Wang QM. (2021). Colacogloea armeniacae sp. nov., a novel pucciniomycetous yeast species isolated from apricots. Mycoscience 62: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QM, Groenewald M, Takashima M, et al. (2015a). Phylogeny of yeasts and related filamentous fungi within Pucciniomycotina determined from multigene sequence analyses. Studies in Mycology, 81: 27–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QM, Yurkov AM, Göker M, et al. (2015b). Phylogenetic classification of yeasts and related taxa within Pucciniomycotina. Studies in Mycology 81: 149–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiß M, Bauer R, Begerow D. (2004). Spotlights on heterobasidiomycetes. In: Frontiers in basidiomycote mycology. (Agerer R, Piepenbring M, Blanz P, eds). IHW-Verlag, Germany: 7–48. [Google Scholar]
- Weiß M, Bauer R, Sampaio JP, et al. (2014). Tremellomycetes and Related Groups. In: The Mycota VII. Systematics and evolution. Part A. (McLaughlin DJ, Spatafora JW, eds). Springer, Germany: 331–355. [Google Scholar]
- Weiß M, Waller F, Zuccaro A, et al. (2016). Sebacinales – one thousand and one interactions with land plants. New Phytologist 211: 20–40. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee SJWT, et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications. (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press Inc., USA: 315–322. [Google Scholar]
- Willis KJ. (ed.) (2018). State of the Worlds Fungi 2018. Report. Royal Botanic Gardens, Kew, Great Britain: 1–92. [Google Scholar]
- Yurkov AM, Wehde T, Federici J. et al. (2016). Yeast diversity and species recovery rates from beech forest soils. Mycological Progress 15: 845–859. [Google Scholar]
- Zhao RL, Li GJ, Sánchez-Ramírez S, et al. (2017). A six-gene phylogenetic overview of Basidiomycota and allied phyla with estimated divergence times of higher taxa and a phyloproteomics perspective. Fungal Diversity 84: 43–74. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Physiological and biochemical characteristics of Atractocolax, Colacogloea, Mycogloiocolax and Slooffia species.
| Characteristic | Fermentation of glucose | Glucose | Galactose | L-Sorbose | Sucrose | Maltose | Cellobiose | Trehalose | Lactose | Melibiose | Raffinose | Melezitose | Inulin | Soluble Starch | D-Xylose | L-Arabinose | D-Arabinose | D-Ribose | L-Rhamnose | D-Glucosamine | N-Acetyl-D-glucosamine | Methanol | Ethanol | Glycerol | Erythritol | Ribitol | Galactitol | D-Mannitol | D-Glucitol | Methyl-α-D-glucoside | Salicin | D-Gluconate | DL-Lactate | Succinate | Citrate | myo-Inositol | Hexadecane | Ammonium sulfate | Potassium nitrate | Sodium nitrite | L-lysine | Ethylamine | Cadaverine | Growth in vitamin-free medium | Starch like compounds formation | Growth with 50% glucose | Hydrolysis of urea | Diazonium Blue B reaction | Major ubiquinone | 22 ? | 25 ? | 30? | 32 ? | 35 ? | 37 ? |
| Atractocolax | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atractocolax pulvinatus | n/a | + | w | w | + | - | - | - | + | - | + | - | - | - | - | w | + | + | - | + | n/a | n/a | - | w | - | + | + | + | + | + | w | w | n/a | w | - | - | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | - | - | + | + | n/a | + | + | + | + | - | - |
| Colacogloea | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| C. aletridis | - | + | - | - | + | w | - | + | - | - | - | + | - | - | - | - | - | - | - | - | n/a | - | + | - | - | l | - | w | w | w | - | n/a | - | - | - | - | - | + | + | + | + | + | + | + | - | - | + | + | n/a | + | + | + | - | - | - |
| C. bettinae sp. nov. | n/a | + | - | w | + | - | - | - | w | - | w | - | - | - | w | w | + | + | w | + | n/a | n/a | w | + | - | + | w | + | + | + | - | + | - | + | - | - | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | - | w | + | + | n/a | + | + | + | + | + | - |
| C. biconidiata sp. nov. | n/a | + | - | - | - | dw | - | - | - | - | - | - | w | - | - | - | + | + | - | + | n/a | n/a | - | + | - | - | w | - | + | + | w | + | - | + | - | - | n/a | n/a | + | + | n/a | n/a | n/a | n/a | - | w | + | + | n/a | + | + | + | + | + | - |
| C. cycloclastica | - | + | - | - | - | - | - | + | - | - | - | - | - | - | v | - | - | - | - | v | n/a | - | + | v | - | + | - | + | + | - | - | - | + | + | + | - | + | n/a | - | - | n | n | n | + | - | n/a | n/a | + | 10 | + | + | + | n/a | v | - |
| C. demeterae | - | + | + | - | + | n/a | n/a | + | -,w | n/a | + | + | - | - | + | + | + | -,w | n/a | - | n/a | n/a | - | w | n/a | w | n/a | + | + | n/a | n/a | n/a | + | + | + | + | n/a | n/a | + | + | + | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | + | n/a | n/a | n/a | n/a |
| C. diffluens | - | + | + | + | + | + | - | + | - | - | + | + | - | - | + | - | + | + | - | + | n/a | - | + | + | + | + | + | + | + | - | + | + | - | + | + | - | n/a | n/a | + | + | n/a | n/a | n/a | + | - | n/a | n/a | + | 10 | + | + | - | - | - | - |
| C. effusa | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | v | - | - | + | n/a | - | v | - | - | - | - | + | + | - | - | - | v | + | v | - | n/a | n/a | - | - | n | n | n | + | - | w | n/a | + | 10 | + | + | + | n/a | - | - |
| C. eucalyptica | - | + | - | - | + | + | - | + | - | - | - | + | - | - | + | - | - | - | - | + | n/a | - | + | - | - | - | - | + | + | w | - | - | - | + | + | - | + | n/a | - | - | w | + | - | + | - | - | + | n/a | n/a | n/a | + | n/a | n/a | n/a | - |
| C. falcata | - | + | + | - | - | - | + | + | - | - | - | - | - | - | + | - | - | - | - | + | n/a | - | + | + | - | - | - | + | + | - | + | + | - | + | + | - | n/a | n/a | + | + | - | + | - | - | - | - | + | + | 10 | + | + | - | n/a | - | - |
| C. fennica sp. nov. | n/a | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | - | + | n/a | n/a | - | + | w | - | w | + | + | + | - | + | - | - | - | - | n/a | n/a | + | + | n/a | n/a | - | - | - | - | + | + | n/a | + | + | + | + | + | - |
| C. foliorum | - | + | - | - | - | - | + | + | - | - | - | - | - | - | + | + | + | + | - | + | n/a | - | + | + | - | + | - | + | + | - | - | + | + | + | + | - | n/a | n/a | + | + | n | n | n | + | - | n/a | n/a | + | 10 | + | + | - | n/a | - | - |
| C. hydrangeae | - | + | - | - | + | + | - | + | - | - | - | l | - | - | - | - | - | - | - | + | n/a | - | + | - | - | l | - | + | + | - | + | n/a | - | - | - | - | - | + | + | + | + | + | - | w | - | - | + | + | n/a | + | + | - | - | - | - |
| C. microspora sp. nov. | n/a | + | - | w | w | - | - | - | - | - | - | v | - | - | - | - | + | + | - | + | n/a | n/a | - | + | w | + | + | + | + | + | - | + | - | - | - | - | n/a | n/a | + | + | n/a | n/a | n/a | n/a | - | + | + | + | n/a | + | + | + | + | + | - |
| C. papilionacea | - | + | + | - | - | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | n/a | - | + | + | - | - | - | + | - | - | - | - | - | + | - | - | n/a | n/a | - | - | n | n | n | - | - | n/a | n/a | + | n | + | + | + | n/a | - | - |
| C. philyla | - | + | - | - | - | - | - | + | - | - | - | - | - | - | + | - | + | - | - | + | n/a | - | + | v | - | + | - | + | + | - | - | + | v | + | + | - | n/a | n/a | - | - | n | n | n | + | - | n/a | n/a | + | 10 | + | + | + | n/a | - | - |
| C. retinophila | - | + | - | - | - | - | - | + | - | - | - | - | - | - | + | - | + | - | - | + | n/a | - | + | v | - | + | - | + | + | - | - | + | + | + | + | - | + | n/a | - | - | n | n | n | + | - | n/a | n/a | + | 10 | + | + | + | n/a | + | + |
| C. rhododendri | - | + | v | - | + | + | v | + | - | - | - | + | v | - | - | - | - | - | - | w | w | - | + | l | - | v | - | + | v | - | - | + | - | - | - | - | - | + | w | v | + | + | v | + | - | - | + | + | n/a | + | + | - | - | - | - |
| C. subericola | - | + | - | - | + | - | - | + | - | - | - | - | - | - | + | - | + | - | - | + | + | - | + | - | - | + | - | + | + | - | - | + | - | + | w | - | - | n/a | + | + | + | - | n/a | + | - | - | + | n/a | n/a | n/a | + | + | n/a | n/a | - |
| C. terpenoidalis | - | + | - | - | + | + | - | + | - | - | - | + | - | - | + | - | + | - | - | + | n/a | - | + | + | - | + | - | + | + | + | - | + | + | + | + | - | n/a | n/a | - | - | n | n | n | + | - | n/a | n/a | + | 10 | + | + | + | n/a | + | + |
| Mycogloiocolax gen. nov. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| M. gerardii sp. nov. | n/a | + | n/a | w | - | - | + | - | - | - | w | - | + | + | - | w | + | w | w | w | n/a | n/a | - | + | w | + | + | + | + | w | w | w | - | w | - | - | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | - | + | + | + | n/a | + | + | + | + | + | - |
| KBP:Y-6479 | - | + | - | - | + | - | w | + | - | - | - | - | - | - | - | - | - | - | - | - | n/a | - | + | + | - | - | - | + | w | - | w | n/a | - | - | - | - | n/a | + | - | n/a | - | n/a | - | + | - | - | + | + | n/a | + | - | - | - | - | - |
| Slooffia | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S. cresolica | - | + | + | - | + | + | + | + | - | - | - | + | - | - | + | - | + | + | - | + | n | - | + | + | - | + | - | + | + | + | - | + | + | + | + | - | n/a | n/a | + | + | n/a | n/a | n/a | + | - | n/a | n/a | + | 10 | + | + | - | n/a | - | - |
| S. globosa | - | + | - | - | + | + | + | + | w | - | - | lw | - | - | - | - | - | - | - | - | - | - | + | + | - | - | - | + | - | lw | - | w | - | - | - | - | - | + | + | - | w | + | lw | - | - | - | + | + | n/a | + | + | - | - | - | - |
| S. micra comb. nov. | n/a | + | - | + | l | w | + | + | w | - | + | + | - | - | - | - | + | - | - | + | n/a | n/a | w | w | - | + | w | + | + | + | - | + | - | - | - | - | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | - | - | + | + | n/a | + | + | + | + | + | - |
| S. pilati | - | + | - | + | + | + | + | + | + | - | - | + | - | - | + | - | + | - | - | - | n/a | - | + | + | - | + | - | + | + | + | - | + | + | + | + | - | n/a | n/a | + | + | n/a | n/a | n/a | + | - | n/a | n/a | + | n/a | + | + | - | - | - | - |
| S. tsugae | - | + | - | + | + | + | + | + | + | - | - | + | - | - | + | - | v | - | - | - | n/a | - | + | + | - | v | - | + | + | v | + | + | + | + | + | - | n/a | n/a | + | + | v | + | - | - | - | - | + | + | 10 | + | + | - | - | - | - |
| S. velesii | - | + | + | + | + | + | + | + | + | + | + | - | n/a | + | + | + | w | - | - | n/a | n/a | + | + | n/a | w | n/a | + | + | n/a | n/a | n/a | + | + | + | + | n/a | n/a | + | + | + | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | + | n/a | n/a | n/a | n/a |
Abbreviations: +, positive; -, negative; l, latent; w, weak; lw, latent and weak; v, variable; n/a, not available.
Species names in bold are novel characterized species from this study.