Abstract
Background:
The diagnosis of small bowel diseases is challenging and device assisted enteroscopy (DAE) is a technique for visualizing the entire small bowel. DAE is considered as a safe procedure and the reported rate of adverse events associated with DAE in the literature is low.
Objective:
The present study tried to investigate the actual incidence of AP after DAE with a systematic review and meta-analysis of available relevant studies.
Methods:
Studies were searched through the PubMed, EMBASE, and Cochrane library databases. The following data were extracted from all eligible studies: author, country, publication year, publication type, study design, type of DAE used, route of DAE, number of patients with AP after DAE, and number of patients with hyperamylasemia after DAE.A random-effects model with RStudio version 4.2.0 was performed in all analyses. Heterogeneity was assessed using the I2 test. The risk of bias was assessed by the Newcastle-Ottawa Scale criteria and the publication bias was assessed by the Egger test.
Results:
Twenty three studies involving a total of 11145 patients were included in the analysis. The overall, pooled AP rate after DAE was 1% (95% CI:0-1%). There was significant heterogeneity among the studies (I2 = 65%; P < 0.01).The pooled AP rate was 1% (95% CI:0-2 %)in peroral route group. The pooled proportion of patients having hyperamylasemia after DAE was 29% (95% CI: 16-46%).Among the patients who had hyperamylasemia AP were identified in 2% (95% CI: 0-6%) of patients.
Conclusion:
The incidence of AP after DAE is about 1%. Hyperamylasemia is a common change in the patients undergoing DAE and only 2% of the patients with hyperamylasemia present with AP.
Keywords: acute pancreatitis, small bowel, enteroscopy, hyperamylasemia, complication
1. BACKGROUND
The diagnosis of small bowel diseases is challenging due to the complex configuration of small bowel. Device assisted enteroscopy (DAE) is a technique for visualizing the entire small bowel, including double balloon enteroscopy (DBE), single balloon enteroscopy (SBE) and spiral eneroscopy (SE). All three systems present comparable insertion depth, diagnostic and therapeutic yield and adverse event rate. The European Society of Gastrointestinal Endoscopy (ESGE) had published guidelines suggesting that the diagnostic yield and safety profile of DBE, SBE and SE were comparable and deemed all three modalities suitable for routine clinical use (1). DAE is mainly used for the investigation of patients with obscure gastrointestinal bleeding (OGIB) or iron deficiency anemia without definite causes after conventional upper endoscopy and colonoscopy.
DAE is considered as a safe procedure and the reported adverse event rate associated with DAE in the literatureis low (2-5). In a prospective multicenter survey from Germany including 2245 DBE procedures, the overall complication rate was 1.2% (6). Minor complications are more common, which include abdominal discomfort and minimal trauma to the intestinal mucosa. The most prevalent major complications are perforation, bleeding, acute pancreatitis (AP) and enteritis. The reported incidence of hyperamylasemia and pancreatitis after peroral SBE seems comparable to that after DBE (7). Abdominal pain and hyperamylasemia after DAE need not mean pancreatitis.It is usually easy to recognize severe pancreatitis,but the mild form may pose a problem. A lot of mild pancreatitis could be missed in patients with DAE performed on an outpatient basis because of inadequate follow-up. As the differentiation between clinically mild pancreatitis and hyperamylasemia with transient abdominal discomfort is difficult, it seems likely that an under estimate of post-DAE pancreatitis might have occurred. Understanding the potential endoscopic adverse events, their expected frequency, and the risk factors associated with their occurrence may help to minimize the incidence of adverse events.
2. OBJECTIVE
The present study tried to investigate the actual incidence of AP after DAE with a systematic review and meta-analysis of available relevant studies.
3. MATERIAL AND METHODS
Literature search
Relevant studies were identified by searching in the PubMed, EMBASE, and Cochrane Library databases from January 2001 to December 2021. Search items were listed as follows: “acute pancreatitis” or “pancreatitis” or “AP” or “SAP” and “device assisted enteroscopy” or “double balloon enteroscopy” or “single balloon enteroscopy” or “spiralenteroscopy”. The search was limited to studies in humans published in English. References of eligible articles and review articles were manually searched.
Selection of articles
The selection criteria were studies in (1) patients undergoing DAE including DBE, SBE and SE; (2) the AP rates after DAE were presented; (3) the number of patients was more than 10. Exclusion criteria were abstracts, reviews and meta-analyses, editorials, case reports, studies that did not report the A Prate after DAE. Each eligible article was reviewed in full text. Literature search was performed independently by 2 reviewers (SHT and ZYG) and thencross-checking search results was completed by the same reviewers. Two authors (SHT and ZYG) fulfilled study selection and data extraction and a third reviewer (SXD) was involved if there was any conflict.
Data extraction
The following data were extracted from all eligible studies: author, country, publication year, publication type, study design, type of DAE used, route of DAE, number of patients with AP after DAE, and number of patients with hyperamylasemia after DAE.
Definitions
AP is diagnosed on clinical, biochemical and radiological grounds according to revised Atlanta classification of AP (8).
AP after DAE: AP immediately after DAE; other than the instrumentation of the small bowel, no other cause of the AP could be identified in the patients.
Hyperamylasemia after DAE: Increased serum amylase level of patients is found after DAE without abdominal pain and radiological pancreatic changes.
Risk of bias and publication bias analysis
The risk of bias was assessed by the Newcastle-Ottawa Scale (NOS) criteria for cohort studies. There are three major parts assessed: 1) Selection (Score 0-4); 2) Comparability (Score 0-2); and 3) Outcome (Score 0-3). The maximum score is 9. A score of 0-3, 4-6, and 7-9 represents low, moderate, and high quality, respectively.The publication bias was assessed by the Egger test.
Statistical analysis
A random-effects model with RStudio version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria)was performed in all analyses to generate a more conservative estimate. We pooled proportions with 95% confidence intervals (CIs), which are presented as forest plots. The heterogeneity between studies was estimated by the Cochran Q test and the I2 statistics. P<0.1 and I2>50% were considered to be significantly heterogeneous. Categorical variables are presented as absolute numbers and percentage. Statistically significant differences were evaluated using the Results were considered as significant at p<0.05.
4. RESULTS
Literature search results
Twenty three studies involving a total of 11145 patients were included in the analysis. All studies were published between 2006 and 2020 including 12 retrospective and 11 prospective studies. The results of the literature search are summarized in Figure 1. The characteristics of the 23 eligible studies are summarized in Table 1.
Figure 1. Study selection flow chart. Of a total of 272 studies only 23 studies met selection criteria.
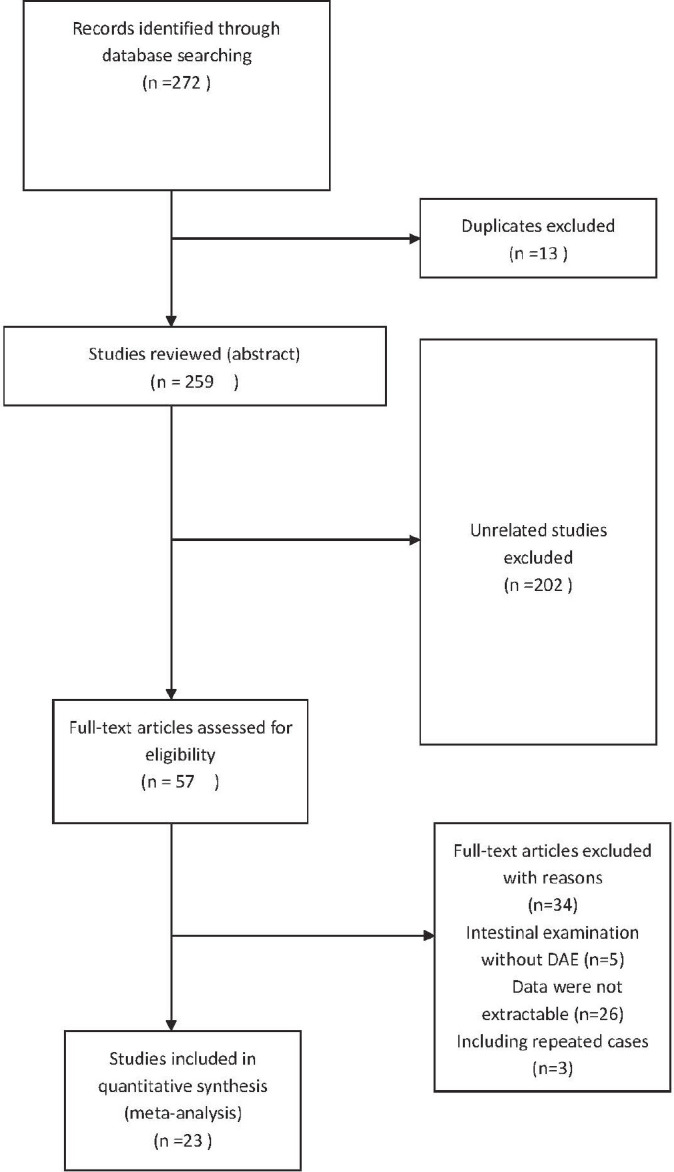
Table 1. Study characterisitcs.
| Author | Year | Country | Study type | Publication type | No. of cases | Enteroscope used in study | Peroral cases | Peranal cases |
|---|---|---|---|---|---|---|---|---|
| Honda et al(9) | 2006 | Japan | Retrospective | fulltext | 13 | DBE | 13 | 0 |
| Heine et al(10) | 2006 | Netherlands | Retrospective | fulltext | 275 | DBE | NA | NA |
| Mensink et al(11) | 2007 | Netherlands | Prospective | fulltext | 2362 | DBE | 1207 | 412 |
| Kopáčová et al(12) | 2007 | Czech | Prospective | fulltext | 35 | DBE | 35 | 0 |
| Gerson et al(13) | 2009 | USA | Retrospective | fulltext | 2413 | DBE | 1691 | 722 |
| Aktas et al(14) | 2009 | Netherlands | Prospective | fulltext | 135 | DBE | 135 | 0 |
| Pata et al(15) | 2010 | Turkey | Retrospective | fulltext | 56 | DBE | 48 | 8 |
| Aktas et al(7) | 2010 | Netherlands | Prospective | fulltext | 166 | SBE | 44 | 61 |
| Nishimura et al(16) | 2010 | Japan | Retrospective | fulltext | 92 | DBE | 25 | 10 |
| Zepeda-Gómez et al(17) | 2011 | Mexico | Prospective | fulltext | 92 | DBE | 92 | 0 |
| Möschler et al(6) | 2011 | Germany | Prospective | fulltext | 2245 | DBE | 1052 | 277 |
| Teshima et al(18) | 2012 | Netherlands | Prospective | fulltext | 32 | SE | 32 | 0 |
| Shibuya et al(19) | 2012 | Japan | Retrospective | fulltext | 202 | DBE | 202 | 0 |
| Itaba et al(20) | 2013 | Japan | Prospective | fulltext | 44 | DBE | 44 | 0 |
| Feng et al(21) | 2014 | China | Prospective | fulltext | 20 | DBE | 12 | 8 |
| Nakayama et al(22) | 2014 | Japan | Retrospective | fulltext | 538 | DBE | 295 | 243 |
| Tsujikawa et al(23) | 2015 | Japan | Retrospective | fulltext | 84 | SBE | 84 | 0 |
| Kopáčová et al(24) | 2016 | Czech | Prospective | fulltext | 117 | DBE | 117 | 0 |
| Matsushita et al(25) | 2016 | Japan | Retrospective | fulltext | 96 | DBE | 96 | 0 |
| Ivano et al(26) | 2017 | Brozil | Retrospective | fulltext | 65 | DBE | NA | NA |
| Hagiwara et al(27) | 2019 | Japan | Prospective | fulltext | 96 | DBE and SBE | 20 | 76 |
| Goenka et al(28) | 2020 | India | Retrospective | fulltext | 180 | SBE | 114 | 66 |
| Noujaim et al(29) | 2020 | USA | Retrospective | fulltext | 1787 | DBE, SBE and SE | 1514 | 270 |
Characteristics of study
In the 23 studies, a total of 11145 patients underwent DAE procedures. All studies were conducted between 2006 and 2020. There were 11 prospective studies and 12 retrospective studies. Eight studies were performed in Japan, followed by Netherland (5/23), Czech (2/23), USA (2/23), China (1/23), Germany (1/23), Mexico (1/23), Turkey (1/23), Brazil (1/23) and India (1/23). The number of patients ineach eligible study was more than 10 and the largest one included 2413 patients. DBE was the most common enteroscopy in the included studies which was used in 19 studies and SE was performed only in 2 studies. About 64% (6872/10805) of DAE were performed through peroral route. The results of the various outcomes of the individual studies are showed in Table 2.
Table 2. Outcomes of the individual studies.
| Author | No. of cases | Peroral cases | Peranal cases | AP after DAE | Hyperamylasemia after DAE |
|---|---|---|---|---|---|
| Honda et al(9) | 13 | 13 | 0 | 1(7.7%) | 6 |
| Heine et al(10) | 275 | NA | NA | 3(1.1%) | NA |
| Mensink et al(11) | 2362 | 1207 | 412 | 7(0.3%) | NA |
| Kopáčová et al(12) | 35 | 35 | 0 | 1(2.9%) | 18 |
| Gerson et al(13) | 2413 | 1691 | 722 | 6(0.2%) | NA |
| Aktas et al(14) | 135 | 135 | 0 | 1(0.7%) | 22 |
| Pata et al(15) | 56 | 48 | 8 | 6(10.7%) | NA |
| Aktas et al(7) | 166 | 44 | 61 | 0(0%) | 13 |
| Nishimura et al(16) | 92 | 25 | 10 | 0(0%) | 0 |
| Zepeda-Gómez et al(17) | 92 | 92 | 0 | 3(3.3%) | 36 |
| Möschler et al(6) | 2245 | 1052 | 277 | 4(0.2%) | NA |
| Teshima et al(18) | 32 | 32 | 0 | 0(0%) | 6 |
| Shibuya et al(19) | 202 | 202 | 0 | 0(0%) | 140 |
| Itaba et al(20) | 44 | 44 | 0 | 1(2.3%) | 14 |
| Feng et al(21) | 20 | 12 | 8 | 0(0%) | 9 |
| Nakayama et al(22) | 538 | 295 | 243 | 5(09%) | NA |
| Tsujikawa et al(23) | 84 | 84 | 0 | 3(3.6%) | NA |
| Kopáčová et al(24) | 117 | 117 | 0 | 0(0%) | NA |
| Matsushita et al(25) | 96 | 96 | 0 | 1(1.0%) | 38 |
| Ivano et al(26) | 65 | NA | NA | 1(1.5%) | NA |
| Hagiwara et al(27) | 96 | 20 | 76 | 1(1.0%) | NA |
| Goenka et al(28) | 180 | 114 | 66 | 2(1.1%) | NA |
| Noujaim et al(29) | 1787 | 1514 | 270 | 1(0.1%) | NA |
Risk of bias and publication bias analysis
The NOS score ranged from 4 to 8 points. Twenty studies were considered to be of moderate quality, and 3 were of high quality (12, 20, 25). Publication bias (Egger test): t=2.63; P=0.0155.
AP rate after DAE
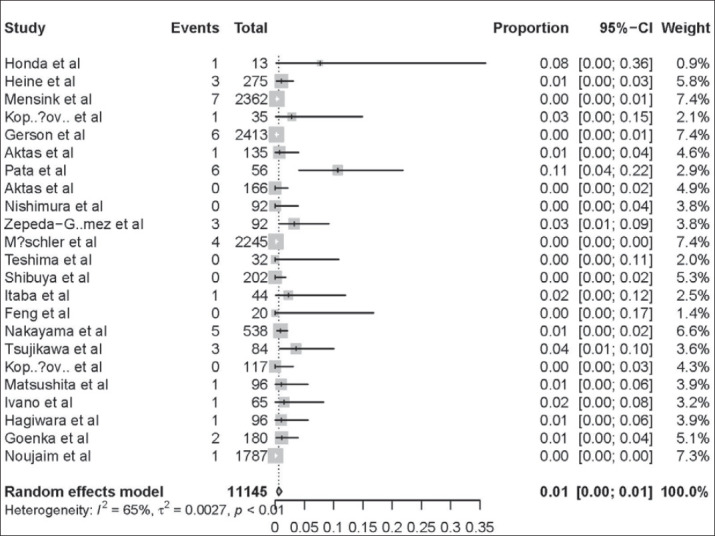
The AP rates of all included studies ranged from 0% to 11%. The overall, pooled AP rate after DAE was 1% (95% CI:0-1%) (Figure 2). There was significant heterogeneity among the studies (I2 = 65%; P < 0.01).
Figure 2. AP rate after DAE-approximately 1% AP in patients after DAE. Cases with APafter DAE were reported in 1% (95% CI:0–1%) of the 11145 patients in the 23 studies. There was significant heterogeneity among the studies (P<0.01).
AP rate after DAE through peroral route
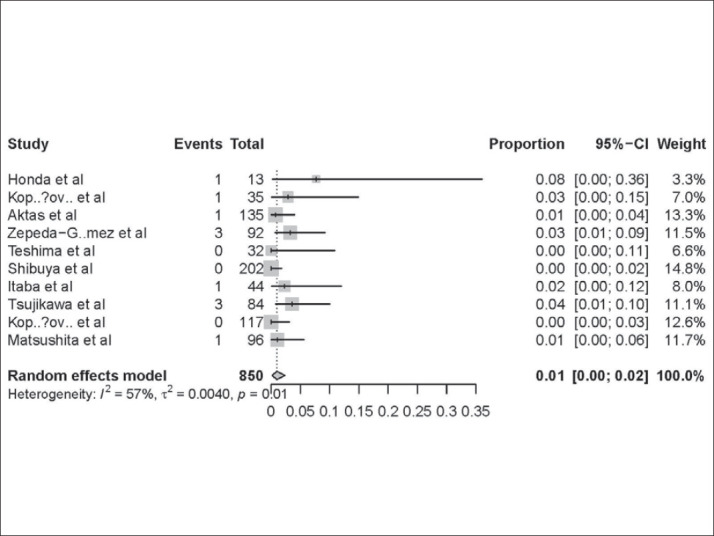
In 10 studies, all patients underwent only peroral DAE. As shown in Table 3, the AP ratesin peroral route group ranged from 0% to 8%. The pooled AP rate was 1% (95% CI:0-2 %) in this group. Significant heterogeneity was found among the studies (I2 = 57%; P =0.01) (Figure 3).
Table 3. AP incidence in patients after peroral DAE.
| Author | Year | N0. of cases | AP after DAE |
|---|---|---|---|
| Honda et al(9) | 2006 | 13 | 1(7.7%) |
| Kopáčová et al(12) | 2007 | 35 | 1(2.9%) |
| Aktas et al(14) | 2009 | 135 | 1(0.7%) |
| Zepeda-Gómez et al(17) | 2011 | 92 | 3(3.3%) |
| Teshima et al(18) | 2012 | 32 | 0(0%) |
| Shibuya et al(19) | 2012 | 202 | 0(0%) |
| Itaba et al(20) | 2013 | 44 | 1(2.3%) |
| Tsujikawa et al(23) | 2015 | 84 | 3(3.6%) |
| Kopáčová et al(24) | 2016 | 117 | 0(0%) |
| Matsushita et al(25) | 2016 | 96 | 1(1.0%) |
Figure 3. AP rate of patients in peroralDAE group-approximately 1% AP in patients undergoing peroral DAE. Forest plot shows that 1% (95% CI: 0–2%) of the patients who underwent peroral DAE experienced AP. There was evidence of heterogeneity among the studies (P=0.01).
Hyperamylasemia rate after DAE
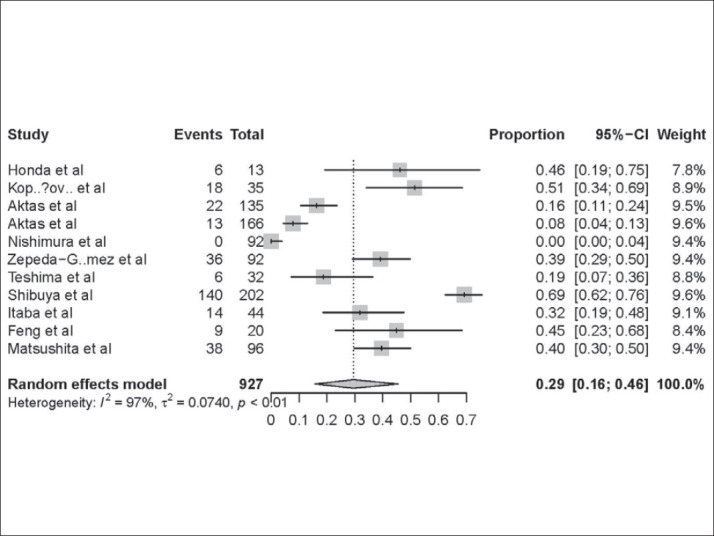
Eleven studies provided the data of patients who had hyperamylasemia after DAE (Table 4). The pooled proportion of patients having hyperamylasemia after DAE was 29% (95% CI: 16-46%) (Figure 4). Among the patients who hadhyperamylasemia AP was identified in 2% (95% CI: 0-6%) of patients.
Table 4. Hyperamylasemia incidence after DAE.
| Author | Year | No. of cases | Hyperamylasemia after DAE |
|---|---|---|---|
| Honda et al(9) | 2006 | 13 | 6(46.2%) |
| Kopáčová et al(12) | 2007 | 35 | 18(51.4%) |
| Aktas et al(14) | 2009 | 135 | 22(16.3%) |
| Aktas et al(7) | 2010 | 166 | 13(7.8%) |
| Nishimura et al(16) | 2010 | 92 | 0(0%) |
| Zepeda-Gómez et al(17) | 2011 | 92 | 36(39.1%) |
| Teshima et al(18) | 2012 | 32 | 6(18.8%) |
| Shibuya et al(19) | 2012 | 202 | 140(69.3%) |
| Itaba et al(20) | 2013 | 44 | 14(31.8%) |
| Feng et al(21) | 2014 | 20 | 9(45%) |
| Matsushita et al(25) | 2016 | 96 | 38(39.6%) |
Figure 4. Hyperamylasemia rate of patients undergoing DAE -approximately 29% Hyperamylasemia in patients undergoing DAE. Forest plot shows that 29% (95% CI: 16–46%) of the patients who underwent DAEpresented with hyperamylasemia. There was evidence of heterogeneity among the studies (P<0.01).
5. DISCUSSION
Besides choledocholithiasis and alcohol, many other factors, such as iatrogenic factors, may lead to the development of AP (30). Hyperamylasemia may occur after endoscopic retrograde cholangiopancreatography (ERCP), which is predictive of the post-ERCP pancreatitis (PEP), the most common complication of ERCP (31). AP may be a major complication not only of ERCP but also of DAE.A study showed that after peroral DBE patients were more likely to have hyperamylasemia than peranal route and hyperamylasemia appeared to be associated with subsequent AP. (9). The actual incidence of AP after DAE may be under estimated because mild pancreatitis cases may not be recognized due to slight symptoms (32). The rate of AP after DAE has been reported to be between 0.3% and 1% (10, 33, 11, 22, 6, 32). Our study showed that the AP rates of all included studies ranged from 0% to 11% and the pooled AP rate after DAE was 1% (95% CI:0-1%). This result is similar with previous reports. AP after DAE is usually not severe and most patients resolved with supportive care, although there were few cases of necrotizing pancreatitis and death have been reported (32).
DAE can be inserted in two opposite ways: peroral or peranal route. The choice of the insertion way depends on the possible location of the lesion. In the cases where the location of the lesion is unpredictable the peroral route is preferred, since the procedure through peranal route is more complex and has higher failure rates . Some studies suggested that the risk of AP after DBE through peroral routemay be greater compared with peranal way. DBE is manipulated by alternately inflating and deflating two balloons that are attached to the enteroscope end and the overtube. One hypothesis suggested that an inflated balloon in the duodenum might cause reflux of duodenal fluids into the pancreatic duct (34). An animal model study demonstrated that the inflated balloon could cause duodenal papillary damage and increased biochemical markers associated with pancreatitis (35). When an inflated balloon is in the duodenal bulb and the other distal to the papilla, intraluminal hypertension in the occluded duodenal segmentwill develop, which may lead to the development of AP. As for SBE, an advantage is the prevention of hypertension in duodenum because of avoiding occlusion of a duodenum segment. But the tip of SBE is often angulated when the endoscope is pulled back and this hooking might lead to papillary damage and AP. Based on above mentioned theories, a possible preventive strategy is inflating the DBE balloons after the overtube has been passed distal to the ligament of Treitz (14). Pata et al have described that amylase levels after DBE are negatively correlated to the depth at which the balloons are first inflated (15). The inflation of the balloons after the duodenal papilla may diminish the iatrogenic effects on the pancreas. Some endoscopists prefer to inflate the balloons after passing the ligament of Treitz (36). However, in our study the incidence of AP (1%) after peroral route DAE did not significantly increase compared with overall incidence of AP after DAE through mixed routes. This result indicated that there were various mechanisms of AP after DAE except duodenal or papillary damage by peroral route. It has been postulated that stretching of the small bowel or mesentery, mechanical stress on the pancreas by the endoscope, torsion of the pancreatic body and ischemia of the pancreas during DAE could induce AP after DAE (7). A case of AP after peranal SBE was reported and the author attributed the AP to the inflated balloon in the colon pressing against the pancreas (37). After enteroscopy in the porcine model, pancreatic necrotic foci are produced and this can be related to the possible ischemic etiopathogenesis of AP after DAE (38).Histological injuries found in this study are more likely related to an ischemic process in the vascular supply to the pancreas. The continuous pressure of the small intestine and the mesentery during the push and pull maneuvers could compromise the vascular supply to the pancreas, resulting in an increase in the pancreatic enzymes. Radiological studies showed that AP after DAE was mostly localized to the pancreas body and/or tail (25). Some investigators suspected that AP after DAE may occur as a result of traumatic injury and/or ischemia on the pancreas body and/or tail (39-41). And they pointed out that clockwise insertion is a significant risk factor of pancreatic hyperamylasemia and AP after DAE. Thus,shorter DAE time, fewer passes, and cautious insertion may be useful in reducing pancreatic injury.
Hyperamylasemia is frequently observed after DAE and an incidence of up to 51% has been reported. But most patients with hyperamylasemia do not progress to AP (17). The incidence of hyperamylasemia after DAE in our study was 29% and only 2% of the patients with hyperamylasemia presented with AP. Damage to the intestinal mucosa by local strain and fiction during DAE may increase intestinal permeability and amylase absorption, which lead to the development of hyperamylasemiawithout severe pancreatic injury (14, 21). Hyperamylasemia may be related to papilla damage or pancreatic ischemia caused by DAE procedure, but this is not the main cause of hyperamylasemia after DAE because of the low incidence of AP among patients with hyperamylasemia. Factors predicting pancreatic hyperamylasemia were elderly patients, deeper insertion and clockwise insertion (23) and ulinastatin does not prevent hyperamylasemia following peroral DBE (20).On the basis of frequent hyperamylasemia after DAE, the factors affecting the outcome of the latent hyperamylasemia and the onset of AP need to be determined.
There are some limitations in the present study. The heterogeneity of the studies was significant. Much of the literature mixes patients undergoing DAE through peroral and peranal routes.These patients should be analyzed separately because they may have different incidence of AP.Most of the included studies adopted DBE, but three studies only used SBE and one study only used SE, respectively, which may influence the analyzing results.Risk factors for AP after DAE may include sex, age, previous abdominal surgery, duration of DAE, panenteroscopy, indication or endoscopic finding, type of endoscope, number of push-and-pull procedures, diagnostic or therapeutic procedure, and initial learning curve for DAE (32). However, the included studies did not report these details and we cannot perform systematic review and meta-analysis to estimate relevant risk factors.
6. CONCLUSION
In conclusion, the incidence of AP after DAE is about 1% and all patients undergoing DAE should be warned of the risk of AP prior to the exam. Hyperamylasemia is a common change after DAE which does not necessarily mean AP after DAE.
Author contributions:
(I) Conception and design: Xiao-Dong Shao, (II) Administrative support: Xiao-Dong Shao, (III) Provision of study materials or patients: Ye Tian, Cheng-Kun Li, Le Wang and Xiao-Dong Shao, (IV) Collection and assembly of data: Hao-Tian Shao, Yong-Guo Zhang, Le Wang and Xiao-Dong Shao, (V) Data analysis and interpretation: Hao-Tian Shao, Yong-Guo Zhang, Le Wang and Xiao-Dong Shao, (VI) Manuscript writing: Hao-Tian Shao, Yong-Guo Zhang, Le Wang, Ye Tian and Xiao-Dong Shao, (VII) Final approval of manuscript: Hao-Tian Shao, Yong-Guo Zhang, Le Wang, Ye Tian, Cheng-Kun Li and Xiao-Dong Shao
Conflict of interest:
The authors declare that research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding:
There is no any funding for the systematic review and meta-analysis.
REFERENCES
- 1.Rondonotti E, Spada C, Adler S, et al. Small- bowel capsuleendoscopy and device-assisted enteroscopy for diagnosis andtreatment of small-bowel disorders: European Society ofGastrointestinal Endoscopy (ESGE) technical review. Endosc. 2018;50:423–446. doi: 10.1055/a-0576-0566. [DOI] [PubMed] [Google Scholar]
- 2.Pennazio M, Venezia L, Valdivia PC, et al. Device-assisted enteroscopy: An update on techniques, clinical indications and safety. Dig Liver Dis. 2019;51:934–943. doi: 10.1016/j.dld.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Riccioni ME, Urgesi R, Cianci R, et al. Current status of device-assisted enteroscopy: technical matters, indication, limits and complications. World J Gastrointest Endosc. 2012;4(10):453–461. doi: 10.4253/wjge.v4.i10.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider M, Hollerich J, Beyna T. Device-assisted enteroscopy: a review of available techniques and upcoming new techniques. World J Gastroenterol, 2019;25(27):3538–3545. doi: 10.3748/wjg.v25.i27.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nehme F, Goyal H, Perisetti, et al. The evolution of device-assisted enteroscopy: from sonde enteroscopy to motorized spiral enteroscopy. Front Med. 2021;8:792668. doi: 10.3389/fmed.2021.792668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moschler O, May A, Muller MK, et al. Complications in and performance of doubleballoonenteroscopy (DBE): results from a large prospective DBE database in Germany. Endosc. 2011;43:484–489. doi: 10.1055/s-0030-1256249. [DOI] [PubMed] [Google Scholar]
- 7.Aktas H, et al. Complications of SBE: prospective evaluation of 166 procedures. Endosc. 2010;42:365–368. doi: 10.1055/s-0029-1243931. [DOI] [PubMed] [Google Scholar]
- 8.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 9.Honda K, Itaba S, Mizutani T, et al. An increase in the serum amylase level in patients after peroral double-balloon enteroscopy: an association with the development of pancreatitis. Endosc. 2006;38(10):1040–1043. doi: 10.1055/s-2006-944831. [DOI] [PubMed] [Google Scholar]
- 10.Heine GD, Hadithi M, Groenen MJ, et al. Double-balloon enteroscopy: indications, diagnostic yield, and complications in a series of 275 patients with suspected small-bowel disease. Endosc. 2006;38:42–48. doi: 10.1055/s-2005-921188. [DOI] [PubMed] [Google Scholar]
- 11.Mensink PB, Haringsma J, Kucharzik T, et al. Complications of double balloon enteroscopy: a multicenter survey. Endosc. 2007;39:613–615. doi: 10.1055/s-2007-966444. [DOI] [PubMed] [Google Scholar]
- 12.Kopacova M, Rejchrt S, Tacheci I, et al. Hyperamylasemia of uncertain significance associated with oral double-balloon enteroscopy. Gastrointest Endosc. 2007;66:1133–1138. doi: 10.1016/j.gie.2007.03.1085. [DOI] [PubMed] [Google Scholar]
- 13.Gerson LB, Tokar J, Chiorean M, et al. Complications associated with double balloon enteroscopy at nine US centers. Clinic Gastroenterol Hepatol. 2009;7:1177–1182. doi: 10.1016/j.cgh.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Aktas H, Mensink PB, Haringsma J, et al. Low incidence of hyperamylasemia after proximal double-balloon enteroscopy: has the insertion technique improved? Endosc. 2009;41:670–673. doi: 10.1055/s-0029-1214976. [DOI] [PubMed] [Google Scholar]
- 15.Pata C, Akyuz U, Erzin Y, et al. Post-procedure elevated amylase and lipase levels after double-balloon enteroscopy: relations with the double-balloon technique. Dig Dis Sci. 2010;55:1982–1988. doi: 10.1007/s10620-009-0956-4. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura N, Yamamoto H, Yano T, et al. Safety and efficacy of double-balloon enteroscopy in pediatric patients. Gastrointest Endosc. 2010;71:287–294. doi: 10.1016/j.gie.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Zepeda-Gomez S, Barreto-Zuniga R, Ponce-de-Leon S, et al. Risk of hyperamylasemia and acute pancreatitis after double-balloon enteroscopy: a prospective study. Endosc, 2011;43:766–770. doi: 10.1055/s-0030-1256473. [DOI] [PubMed] [Google Scholar]
- 18.Teshima CW, Aktas H, Kuipers EJ, et al. Hyperamylasemia and pancreatitis following spiral enteroscopy. Can J Gastroenterol. 2012;26(9):603–606. doi: 10.1155/2012/696187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibuya T, Osada T, Nomura O, et al. The origin of hyperamylasemia associated with peroral double-balloon endoscopy. J Clin Gastroenterol. 2012;46(10):888–889. doi: 10.1097/MCG.0b013e3182634f35. [DOI] [PubMed] [Google Scholar]
- 20.Itaba S, Nakamura K, Aso A, et al. Prospective, randomized, double-blind, placebo-controlled trial of ulinastatin for prevention of hyperenzymemia after double balloon endoscopy via the antegrade approach. Dig Endosc. 2013;25(4):421–427. doi: 10.1111/den.12014. [DOI] [PubMed] [Google Scholar]
- 21.Feng N, Dai J, L u H, et al. Hyperamylasemia is associated with increased intestinal permeability in patients undergoing diagnostic oral double-balloon enteroscopy. World J Gastroenterol. 2014;20(2):539–545. doi: 10.3748/wjg.v20.i2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama S, Tominaga K, Obayashi T, et al. The prevalence of adverse events associated with double-balloon enteroscopy from a single-center dataset in Japan. Dig Liver Dis. 2014;46:706–709. doi: 10.1016/j.dld.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Tsujikawa T, Bamba S, Inatomi O, et al. Factors affecting pancreatic hyperamylasemia in patients undergoing peroral single-balloon enteroscopy. Dig Endosc. 2015;27(6):674–678. doi: 10.1111/den.12449. [DOI] [PubMed] [Google Scholar]
- 24.Kopacova M, Bures J, Rejchrt S, et al. Risk factors of acute pancreatitis in oral double balloon enteroscopy. Acta Medica. 2016;59(3):84–90. doi: 10.14712/18059694.2016.95. [DOI] [PubMed] [Google Scholar]
- 25.Matsushita M, Shimatani M, Okazaki K, et al. Clockwise insertion: A risk factor of pancreatic hyperamylasemia and acute pancreatitis after peroral balloon-assisted enteroscopy. Dig Endosc. 2016;28:481–485. doi: 10.1111/den.12614. [DOI] [PubMed] [Google Scholar]
- 26.Ivano FH, Villela IR, Miranda LF, et al. Analysis of double balloon enteroscopy: indications, findings, therapeutic and complicaitons. Arq Bras Cir Dig. 2017;30(2):83–87. doi: 10.1590/0102-6720201700020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagiwara S, Kudo T, Kakuta F, et al. Clinical safety and utility of pediatric balloon assisted enteroscopy:a multicenter prospective study in Japan. J Pediatr Gastroenterol Nutr. 2019;68(3):306–310. doi: 10.1097/MPG.0000000000002181. [DOI] [PubMed] [Google Scholar]
- 28.Goenka MK, Afzalpurkar S, Rai VK, et al. Single-balloon enteroscopy in management of small-bowel disorders. Indian J Gastroenterol. 2020;39(6):550–556. doi: 10.1007/s12664-020-01088-x. [DOI] [PubMed] [Google Scholar]
- 29.Noujaim MG, Parish A, Raines D, et al. Use, yield, and risk of device-assisted enteroscopy in the United States. J Clin Gastroenterol. 2021;55(9):792–797. doi: 10.1097/MCG.0000000000001426. [DOI] [PubMed] [Google Scholar]
- 30.Levy MJ, Geenen JE. Idiopathic acute recurrent pancreatitis. Am J Gastroenterol. 2001;96:2540–2555. doi: 10.1111/j.1572-0241.2001.04098.x. [DOI] [PubMed] [Google Scholar]
- 31.Thomas PR, Sengupta S. Prediction of pancreatitis following endoscopic retrograde cholangiopancreatography by the 4h post procedure amylase level. J Gastroenterol Hepatol. 2001;16:923–926. doi: 10.1046/j.1440-1746.2001.02547.x. [DOI] [PubMed] [Google Scholar]
- 32.Kopacova M, Tacheci I, Rejchrt S, et al. Double balloon enteroscopy and acute pancreatitis. World J Gastroenterol. 2010;16(19):2331–2340. doi: 10.3748/wjg.v16.i19.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xin L, Liao Z, Jiang YP, et al. Indications, detectability, positive findings, total enteroscopy, and complications of diagnostic double-balloon endoscopy: a systematic review of data over the first decade of use. GastrointestEndosc. 2011;74(3):563–570. doi: 10.1016/j.gie.2011.03.1239. [DOI] [PubMed] [Google Scholar]
- 34.Groenen MJ, Moreels TG, Orlent H, et al. Acute Pancreatitis after Double−Balloon Enteroscopy: an old pathogenetic theory revisited as a result of using a new endoscopic tool. Endosc. 2006;38:82–85. doi: 10.1055/s-2005-921179. [DOI] [PubMed] [Google Scholar]
- 35.Latorre R, López-Albors O, Soria F, et al. Effect of the manipulation of the duodenal papilla during double balloon enteroscopy. World J Gastroenterol. 2016;22(17):4330–4337. doi: 10.3748/wjg.v22.i17.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia Q, Quan L, Yang XN, et al. Comparison of integrated Chinese and Western medicine with and without somatostatin supplement in the treatment of severe acute pancreatitis. World J Gastroenterol. 2005;11(7):1073–1076. doi: 10.3748/wjg.v11.i7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yip WM, Lok KH, Lai L, et al. Acute pancreatitis:rare complication of retrograde single-balloon enteroscopy. Endosc. 2009;41:E324. doi: 10.1055/s-0029-1215002. [DOI] [PubMed] [Google Scholar]
- 38.Soria F, Pérez-Cuadrado E, López-Albors O, et al. Ischemic etiopathogenesis as thepossible origin of post-double balloon enteroscopypancreatitis.A porcine model study. Rev EspEnferm Dig. 2015;107:17–22. [PubMed] [Google Scholar]
- 39.Matsushita M, Shimatani M, Uchida K, et al. Mechanism of acute pancreatitis after peroral double-balloon enteroscopy. Endosc. 2007;39:480. doi: 10.1055/s-2007-966258. [DOI] [PubMed] [Google Scholar]
- 40.Matsushita M, Shimatani M, Uchida K, et al. Safer endoscopic therapy of small-bowel diseases during double-balloon enteroscopy. Endosc. 2007;39:1107. doi: 10.1055/s-2007-966975. [DOI] [PubMed] [Google Scholar]
- 41.Matsushita M, Shimatani M, Uchida K, et al. Association of hyperamylasemia and longer duration of peroral double-balloon enteroscopy: present and future. Gastrointest Endosc. 2008;68:811. doi: 10.1016/j.gie.2008.02.082. [DOI] [PubMed] [Google Scholar]


