Abstract
The role of natural versus acquired immunity to Leishmania aethiopica infection in humans is the focus of our studies. We found in previous studies that mononuclear cells from nonexposed healthy Swedish donors responded to Leishmania antigen stimulation by proliferation and gamma interferon production. The main cell type responding was CD3− CD16/56+ natural killer (NK) cells. These findings led us to suggest that the potential to produce a rapid, nonacquired NK cell response may be a protective phenotype. In order to test this hypothesis, an area in Ethiopia where Leishmania is endemic was selected, and peripheral blood mononuclear cells were obtained from individuals who had lived in the area most of their lives but had no evidence of past or present leishmaniasis. Their responses were compared with those of confirmed leishmaniasis patients from the same region with active lesions or cured leishmaniasis lesions. Cells from these donors were stimulated in vitro with L. aethiopica antigen. Responses were measured by proliferation, cytokine production, and phenotype analysis by fluorescence-activated cell sorting. The association of NRAMP1 alleles with the studied phenotype and susceptibility to L. aethiopica-induced leishmaniasis was also evaluated. The results show that Leishmania antigens can induce NK cell and CD8+-T-cell responses in vitro. This is clearly seen in proliferating cells from the cured (immune) individuals and the apparently protected controls from the area of endemicity. It contrasted with the reactivity of the patients, where some NK proliferation was coupled with enhanced CD4+-T-cell proliferation. We conclude from these observations that NK cells and CD8+ cells proliferating in response to Leishmania stimulation are involved in protection from and healing of (Ethiopian) cutaneous leishmaniasis; however, such mechanisms appear to be unrelated to the NRAMP1 host resistance gene.
Leishmania aethiopica causes cutaneous leishmaniasis in the highlands of Ethiopia (7). This is the only Leishmania sp. defined in this area of endemicity. In the most common form, the disease-characterizing skin lesion is localized (local cutaneous leishmaniasis [LCL]) and eventually self-heals within 3 months to 6 years, resulting in apparent solid protection against LCL. Healing of cutaneous lesions is associated with ulcer formation. Following healing of a clinically apparent leishmaniasis infection, the development of gamma interferon (IFN-γ)-producing T-cell clones is believed to render the individual resistant to leishmaniasis (15).
We have previously shown that peripheral blood mononuclear cells (PBMC) from nonexposed healthy donors are able to secrete IFN-γ and proliferate (at days 3 and 6, respectively) in response to L. aethiopica antigen in vitro (2). This response was stronger in some healthy donors than in LCL patients tested at the same time. When we further investigated the phenotype of the proliferating cells in the unexposed donors, we found a prominence of CD3− CD16/56+ natural killer (NK) cells. Furthermore, CD8+ cells represented a significant proportion of the responding CD3+ T cells (1). In contrast, CD4+-T-cell proliferation with subsequent IFN-γ production was the main feature in patients with cutaneous leishmaniasis. We had suggested that the ability to produce a rapid, nonacquired NK response in the unexposed individuals, if present in vivo, could influence the outcome of exposure or infection. Since in a given area of endemicity with known transmission of leishmaniasis only a portion of the exposed population shows disease expression, we postulated that the resistance phenotype may be associated with a differential induction of NK cell activation by Leishmania parasites. We have thus studied the immunologic profile of individuals apparently protected from leishmaniasis and have compared it with the profiles in individuals protected from reinfection after cure of leishmaniasis and in patients with ongoing disease. Since in the mouse, natural immunity to Leishmania donovani and other unrelated parasites of macrophages has been shown to be under control of the natural resistance macrophage protein gene Nramp1 (22), we evaluated a possible role of the human NRAMP1 homolog in cutaneous leishmaniasis by testing the association of NRAMP1 alleles with susceptibility to cutaneous leishmaniasis.
MATERIALS AND METHODS
Study site.
Sebeta, Ethiopia, is a small town located 25 km from the central part of Addis Ababa and is a known site of L. aethiopica transmission (23). Many of the patients attending the dermatology clinic of the All African Leprosy Rehabilitation and Training Centre live in Sebeta. The most recent survey of individuals of all ages in the Meta Abo area of Sebeta, in 1988, was by Negash (17a), who found a prevalence of active cutaneous leishmaniasis of 11.5 per 1,000, compared to 5.5 per 1,000 reported by Wilkins (23). The proportion of individuals, spanning all age groups, with characteristic scars consistent with past disease was 36 per 1,000.
Study groups.
The purpose of the study was explained to the inhabitants of the village of Sebeta, where leishmaniasis is endemic. We wished to enroll at least 25 individuals in each group, a number that was logistically feasible to work with. The enrollment process within each group was purely random and based on the participants volunteering to be part of the study. The 98 individuals of both sexes (53 male and 45 female) who agreed to participate in the study had lived in the area of endemicity for more than 10 years. They all underwent a physical examination. The individuals from the village were divided into three groups according to whether they had no history of previous or present leishmaniasis (referred to here as endemic controls; n = 26), had ongoing LCL lesions (patients; original n = 27), or had scars due to cured lesions (cured patients; original n = 27) which could be attributed to Leishmania infection. The age ranges in the participants from the village were similar (endemic controls, 13 to 65; patients, 4 to 51; cured patients, 10 to 60). For further comparison, healthy dwellers of the city of Addis Ababa (healthy Ethiopian controls; n = 18; age, 24 to 43), where leishmaniasis is not endemic, also participated. One non-Ethiopian donor, who was accidentally infected with L. aethiopica in the laboratory and did not develop clinical disease but converted to leishmanin skin test positivity, was also included in this study (referred to here as lab infected).
At least 25 ml of blood was obtained from each donor when possible. In some instances, however, the amount of blood obtained was too small to perform all of the analyses.
Diagnosis of past or present leishmaniasis.
Leishmania parasites were identified in smears or cultures of scrapings from active lesions as previously described (17). Sera were tested for the presence of anti-Leishmania antibodies by the direct agglutination test (DAT) or immunoflorescence. Skin reactivity to Leishmania major-derived leishmanin (Institute Pasteur, Tehran, Iran) was evaluated after 72 h (3).
Antigens and mitogens.
Freeze-thawed whole L. aethiopica promastigote antigen was prepared as previously described (14) and used at a final concentration of 1.25 × 106 promastigotes/ml (equivalent to 12.5 μg of protein/ml). This concentration has previously been shown to be optimal for the induction of a proliferative response (2). Purified protein derivative of tuberculin (PPD) (Statens Serum Institutet, Copenhagen, Denmark) and the T-cell mitogen phytohemagglutinin (PHA) (Murex, Hartford, United Kingdom) were used at 12.5 μg/ml.
Preparation and stimulation of mononuclear cells.
Mononuclear cells separated from defibrinated peripheral blood on a Ficoll gradient (6) were frozen in freezing medium (10% fetal calf serum plus 10% dimethyl sulfoxide in RPMI) and transported on dry ice to Stockholm, Sweden, where they were stored in liquid nitrogen until tested. Preliminary experiments performed before the start of the study to compare the proliferative responses and the assessments of the cellular phenotypes of fresh cells and frozen cells tested at two time points showed no appreciable differences. Upon thawing, cells were washed three times and eventually resuspended to the appropriate concentration in RPMI (Gibco BRL, Paisley, Scotland, United Kingdom) containing 2 mM l-glutamine, 100 U of penicillin (Gibco), and 100 μg of streptomycin (Gibco) per ml and supplemented with 10% heat inactivated normal Swedish AB serum. Cells were plated at 2 × 105/well in 96-well flat-bottom tissue culture plates (Nunclon; Nunc, Roskilde, Denmark), and each antigen or mitogen was tested in triplicate. Negative control cultures contained RPMI medium alone. Cells were cultured in 5% CO2 in air at 37°C for 6 days. During the last 18 h of culture, 1 μCi of tritiated thymidine (Amersham, Aylesbury, United Kingdom) per well was added. Cells were harvested onto a glass fiber filter mat (Wallac, Turku, Finland), and radioactivity was measured in a 1450 microbeta-counter (Wallac). Proliferation was measured as counts per minute and calculated as stimulation indices (SI) (counts per minute for cells in stimulated cultures/counts per minute for cells in unstimulated cultures) or as net counts per minute (counts per minute for antigen-stimulated cells minus counts per minute for unstimulated cultures).
Supernatants were harvested after 72 h of incubation from cells incubated as described above for cytokine analysis by commercial enzyme-linked immunosorbent assays (AMS Biotechnology, Stockholm, Sweden). The standard used in the interleukin-10 (IL-10) assay was a kind gift from Dynax (Palo Alto, Calif.), and measurements were expressed in units (107 U = 1 mg/ml). Enough cells were obtained to set up these assays for 24 endemic controls, 24 patients, 25 cured individuals, and 14 healthy Addis Ababa donors.
Phenotype analysis of proliferating cell types.
Mononuclear cells cultured for 6 days as described above were prepared for surface marker staining. Briefly, cells of triplicate wells (six wells when enough cells were available) from individual stimuli were pooled and washed once in phosphate-buffered saline (PBS) at 1,300 rpm for 10 min. Pellets were resuspended in PBS and dispensed at 100 μl/tube into tubes (Falcon) containing 10 μl (1:10 dilution) of combinations of either CD4 and CD8 (helper and cytotoxic), CD3 and CD16/56 (pan-T and NK cells), or control immunoglobulin G1 and immunoglobulin G2 antibodies, double stained with fluorescein isothiocyanate and phycoerythrin, respectively. After mixing the cell suspension was allowed to stand in the dark at room temperature for 20 min. The cells were then washed in 2 ml of PBS as described above. Pellets were resuspended in 300 μl of PBS and run on a FACScan (Becton Dickinson, Mountain View, Calif.). All antibodies were obtained from Becton Dickinson. Enough cells were obtained to fully carry out these fluorescence-activated cell sorter (FACS) analyses for 20 endemic controls, 18 patients, 20 cured individuals, and 9 healthy Addis Ababa donors.
Forward light scatter flow cytometry.
An enrichment of large blast cells after 6 days culture in response to antigenic stimulation is observed in responder mononuclear cells. As determined by the forward light scatter, smaller cells with the least light scatter, as seen in most unstimulated cultures, were gated in R1, and the area outside this region was gated in R2. In response to antigen stimulation, blast cells undergoing activation were scattered forwardmost, away from the R1 cell population into the R2 region (1).
The percent increase of responding cells was calculated for the R2 population as follows: [(percent large cells × percent large NK cells in stimulated culture) + 1]/[(percent large cells × percent large NK cells in unstimulated culture) + 1].
Genetic analysis.
The distributions of allelic variants for two NRAMP1 polymorphic markers [a microsatellite, (GT)n, in the promoter region and a biallelic polymorphism at nucleotide position 1465-85G/A in intron 13] in genomic DNA from 57 individuals were determined by PCR methods as previously described (13). For the purpose of genetic analysis, study groups were defined as equivalent to phenotypes. Association of single alleles with phenotypes was tested by standard chi-square analysis. In addition, the estimation of haplotype frequencies for each phenotype was derived by using iterative methods implemented in the EH program, which is part of the LINKAGE package (21). The same program was used to test for possible association between haplotypes and specific phenotypes or combinations of phenotypes.
Statistical analyses.
Group means were compared by using the Student t test. Chi-square tests (or Fisher’s exact test) and regression analyses were also used.
RESULTS
Prevalence of leishmaniasis.
The lifetime prevalence of 36 per 1,000 as defined by the presence of a characteristic scar, compared to a rate of 11.5 per 1,000 for active disease at a given time, appears to be low. This may be due to an underestimation of the presence of leishmaniasis-induced scars in the population, since small scars arising from relatively benign, inconspicuous leishmaniasis lesions may go unnoticed (5). However, this method of assessment of previous cutaneous leishmaniasis is well accepted (18), bearing in mind its limitations.
Previous exposure to other infection.
Serological assays were performed to evaluate the extent of previous parasitic and human immunodeficiency virus (HIV) infections in the groups (Table 1). The exposure to other parasites was essentially the same among the groups in Sebeta except for the presence of antischistosomal antibodies, which was most prevalent in the group with scars (P < 0.05 by chi-square analysis), and antibodies against Echinococcus, which was lower in the healthy control group than the individuals from the village (P < 0.01). However, after correction of the analysis by using the Bonferroni correction for multiple comparisons, no significant difference was found between the four groups (P = 0.08). The HIV-positive individuals (total of two [one patient and one endemic control]) were excluded from the rest of the study.
TABLE 1.
Serological analysis of samples from Sebeta to evaluate the extent of exposure to a number of potential parasites
| Virus or parasite | % Positive (no. positive/total no.):
|
||||
|---|---|---|---|---|---|
| Patients | Cured patients | Endemic controls | Healthy controls | Pa | |
| HIV | 4.0 (1/25) | 0.0 (0/27) | 5.9 (1/17) | 0.0 (0/18) | 0.503 (NSb) |
| Toxoplasma | 62.5 (15/24) | 74.1 (20/27) | 75.0 (9/12) | 56.3 (9/16) | 0.569 (NS) |
| Schistosoma | 0.0 (0/24) | 22.2 (6/27) | 0.0 (0/12) | 6.3 (1/16) | 0.022 |
| Plasmodium | 4.2 (1/24) | 3.7 (1/27) | 0.0 (0/12) | 0.0 (0/16) | 0.768 (NS) |
| Entamoeba | 29.2 (7/24) | 29.6 (8/27) | 16.7 (2/12) | 18.8 (3/16) | 0.731 (NS) |
| Giardia | 12.5 (3/24) | 3.7 (1/27) | 25.0 (3/12) | 12.5 (2/16) | 0.280 (NS) |
| Filaria | 33.3 (8/24) | 48.1 (13/27) | 58.3 (7/12) | 37.5 (6/16) | 0.465 (NS) |
| Echinococcus | 83.3 (20/24) | 85.3 (23/27) | 83.3 (10/12) | 43.8 (7/16) | 0.009 |
| Trichinella | 12.5 (3/24) | 18.5 (5/27) | 25.0 (3/12) | 12.5 (2/16) | 0.759 (NS) |
Groups were analyzed by chi-square analysis.
NS, not significant.
In those individuals for whom a stool or urine sample could be examined (the patient, endemic control, and scarred groups), the parasites that were identified were Ascaris (6 of 27 patients, 2 of 27 individuals with scars, and 4 of 26 endemic controls), Trichuris (1 patient and 2 scarred individuals), Giardia (1 patient), Entamoeba (1 patient), and Stronguloides (1 patient). Two patients and one scarred individual had both Ascaris and Trichuris, while one patient had both parasites as well as Giardia.
Physical features of participants.
There was no physical evidence of overt malnutrition in any of the participants from the village. However, their socioeconomic status, which was similar among the participants from the village, was appreciably lower than that of the healthy Addis Ababa controls.
Selection of bona fide groups for analyses.
Confirmation of previous and past leishmaniasis beyond the clinical criterion was difficult. Samples were taken from the clinically diagnosed LCL patients, and parasite identification by smear and culture was confirmed for 10 patients (37.0%). All participants from the village were skin tested after blood was taken. Twenty-four patients, 23 individuals with scars, and 23 endemic controls returned to have the skin test read. Nine of 24 clinically diagnosed LCL patients (37.5%) and 12 of 23 apparently cured patients (52.2%) had positive skin test responses to leishmanin (mean skin induration of greater than 5 mm). All 23 endemic controls tested were skin test negative. Quite surprisingly, only one patient, who was shown to have parasites in her lesions, among those whose skin tests were read, was positive in both assays. Low levels of skin test positivity with this skin test antigen in confirmed LCL patients have previously been reported by us (3), but this is the most reliable skin test antigen available which is prepared under good manufacturing practices. The number of individuals with active lesions and measurable antileishmanial antibody levels differed depending on the assay used (DAT, 14 of 24 patients; immunoflorescence, 5 of 24 patients). The antileishmanial antibody in those with scars showed a similar trend (DAT, 14 of 27; immunofluorescence, 2 of 27). These results reflect the difficulty in diagnosing LCL in Ethiopia.
Thus, of the original 27 clinically defined LCL patients, 20 were confirmed as active LCL patients (skin lesion plus positive for one laboratory examination for leishmaniasis). Of these, cells from one who was HIV seropositive were excluded. Thus, 19 individuals were considered bona fide patients. Since the presence of a characteristic scar could still be a consequence of other infections or trauma, a stricter criterion for selection of bona fide cured individuals, i.e., a positive skin test response, was used. With this criterion 12 of the 27 individuals with scars and an apparent history of healed LCL lesions were selected as bona fide cured patients.
Proliferative responses.
The proliferation to PHA and to PPD of the frozen cells used in the present study was within the range obtained when fresh cells from Ethiopian patients were tested at identical time points and under the same culture conditions (4). Thus, there was no evidence of transportation-related damage to the cells, including to their antigen-presenting capacity, as seen by the adequate induction of the PPD-driven responses.
No kinetics analyses were performed in these studies, since there were not enough cells for such analyses and since we have previously demonstrated that both patients and responder healthy controls showed similar kinetics when stimulated with L. aethiopica promastigote antigens under the conditions used here (2). Of the 24 clinically diagnosed LCL patients tested, only 10 had proliferative responses to L. aethiopica that were above an SI of 2 (maximum SI = 32). Of the 25 individuals with scars compatible with previous LCL disease, 14 had responses above an SI of 2 (maximum SI = 34) two donors from each of the two groups had too few cells for the assay to be adequately performed). Cells from 24 endemic controls were tested in culture, since cells from one individual in this group found to be HIV positive were discarded and one other donor had too few cells. Of the 24, 6 had proliferative responses (maximum SI = 8). The highest responses were in the group of cured individuals.
Evaluation of only the bona fide patients and controls showed that although proliferative responses were low in general, 4 of 17 of the patients tested (two had too few cells to be tested) (mean SI and standard deviation = 3.1 ± 5.4; range, 0.2 to 22.7) and 7 of 12 of the cured individuals (mean SI = 8.0 ± 10.6; range, 0.7 to 29.8), had proliferative responses (SI ≥ 2) to the Leishmania antigen preparation. Three of the 24 endemic controls (mean SI = 1.3 ± 1.0; range, 0.3 to 4.3) and 3 of 14 healthy controls (4 had too few cells) (mean SI = 1.8 ± 0.9; range, 1.0 to 4.1) showed proliferative responses to the Leishmania antigen preparation as measured by thymidine incorporation. The response to the mycobacterial antigen PPD was used as an antigen unrelated to Leishmania and as an evaluation of cross-reactivity to an organism to which the donors had previously been exposed. There was no significant correlation between the PPD- and L. aethiopica-induced proliferation in the individuals (Regression, r = 0.296 and P > 0.05) Table 2 shows the responses in L. aethiopica- and PPD-induced cultures. There was a large variation in the responses in the groups, and there was no significant difference between the groups.
TABLE 2.
Responses to L. aethiopica and PPD
| Donors | Responsea to:
|
|
|---|---|---|
| L. aethiopica | PPD | |
| Patients | 1,023 ± 1,919 (0–4,435) | 2,846 ± 3,103 (0–6,389) |
| Cured patients | 8,795 ± 17,387 (77–39,849) | 7,309 ± 4,779 (1600–13182) |
| Endemic controls | 747 ± 1,596 (0–5,496) | 11,458 ± 16,205 (0–45,447) |
| Healthy controls | 264 ± 266 (5–703) | 7,974 ± 6,111 (675–16396) |
Means ± standard deviations (ranges) of net counts per minute (counts per minute for antigen-stimulated cells − counts per minute for unstimulated cultures) in L. aethiopica- and PPD-induced cultures. There was no significant difference between the groups and no statistical correlation between the L. aethiopica response and the PPD response within the same individual.
The PPD and PHA responses of this selected group were within the range obtained in our previous studies (data for PHA not shown).
Relatedness of cellular immune responsiveness and antibody responses.
Data obtained in assays measuring cellular immune responses in vivo (skin testing) and in vitro (proliferation assay) and the antibody responses (as defined by DAT and immunoflorescence) were compared to evaluate the relatedness of the assays. There was no significant correlation (Table 3) between the antibody assay and the skin test response or a proliferative response index (as defined by an SI of ≥2).
TABLE 3.
Relatedness of cellular and antibody responses in donorsa
| Tests compared | Result for test A | No. with the following result for test B:
|
||
|---|---|---|---|---|
| Negative | Positive | Total | ||
| Antibody (A) and leishmanin skin test (B) | Negative | 23 | 9 | 32 |
| Positive | 14 | 13 | 27 | |
| Total | 37 | 22 | 59 | |
| Positive SI (A) and antibody (B) | Negative | 34 | 25 | 59 |
| Positive | 15 | 4 | 19 | |
| Total | 49 | 29 | 78 | |
| Positive SI (A) and leishmanin skin test (B) | Negative | 36 | 14 | 50 |
| Positive | 8 | 7 | 15 | |
| Total | 44 | 21 | 65 | |
There was no significant correlation between the antibody assay by DAT or immunofluorescence and the skin test response or a proliferative response index (as defined by an SI of ≥2).
Baseline total cell population in unstimulated cultures.
The percentages of NK, CD4+, and CD8+ cells in the total cell population, including large and small cells (R1 plus R2), were compared for the four groups (bona fide patients, cured individuals, endemic controls, and healthy Ethiopian controls) (Table 4). The percentages of CD4- and CD8-positive cells were similar in all of the groups. The individuals from the village tended to have a higher percentage of NK cells than the city Ethiopian controls, but the difference was statistically significant only for the cured patient group, where the highest mean percentage of NK cells was noted (P < 0.05 by Student’s t test).
TABLE 4.
Baseline cell population sizes
| Donors | Mean ± SD (range) % of totala
|
||
|---|---|---|---|
| NK cells | CD4+ cells | CD8+ cells | |
| Patients | 2.48 ± 2.01 (0.4–5.4) | 37.85 ± 17.28 (12.0–60.0) | 37.0 ± 9.44 (28.0–53.0) |
| Cured individuals | 5.38 ± 4.84b (0.8–11.0) | 51.2 ± 8.14 (40.0–59.0) | 33.6 ± 6.07 (25.0–41.0) |
| Endemic controls | 4.27 ± 4.49 (0.3–20.0) | 43.19 ± 14.61 (18.0–63.0) | 36.56 ± 11.30 (24.0–63.0) |
| Healthy controls | 1.39 ± 1.25 (0.4–5.4) | 45.89 ± 17.44 (28.0–69.0) | 32.89 ± 11.46 (20.0–50.0) |
Both large and small cells in unstimulated cultures.
P < 0.05 compared to healthy lethiopian controls.
FACS analyses of L. aethiopica-stimulated cells in bona fide donors.
In order to have an idea of how a known Leishmania infection can influence the proliferating cell types during infection and after cure, when apparent resistance to reinfection is achieved, we compared FACS analyses of the cells of the bona fide patients and cured individuals. Of the 13 samples from bona fide patients for which FACS analyses could be performed, in only 8 was the full complement of the analysis achieved. Of the nine bona fide cured individuals analyzed, the complete FACS data are available for six. In agreement with previous studies, L. aethiopica induced significant CD4+-cell proliferation as seen by the percentage of large cells (R2) in this population after stimulation (Table 5) (mean ± standard deviation for patients, 42.1 ± 17.9; range, 14.9 to 72.7) (for cured patients, 28.6 ± 9.9; range, 19.7 to 47.1). In addition the percentage of large CD8+ cells in stimulated cultures from the cured group was similar to the CD4+ level (23.3 ± 7.8; range, 14.9 to 34.5), while the percentage of CD8+ cells in patients was on average significantly lower than the CD4 levels (18.3 ± 11.9; range, 2.0 to 32.3) (P < 0.05 by Student’s t test). The percentage of large NK cells in L. aethiopica-stimulated cultures ranged from 1.8 to 43.5% (mean, 15 ± 15.5) in bona fide cured individuals and from 0 to 18.0% (mean, 6.1 ± 7.0) in bona fide patients. There were no significant differences in the percentages of the proliferating large cells (of any of the cell phenotypes) between the patient and cured groups.
TABLE 5.
Mononuclear cells expressing the CD4+ and CD8+ phenotypes after stimulation by L. aethiopica antigen
| Cells | Mean ± SD (range) % ina:
|
||
|---|---|---|---|
| Patients | Cured patients | Lab-infected individual | |
| CD4+ | 42.1 ± 17.9 (14.9–72.7) | 28.6 ± 9.9 (19.7–47.1) | 47.7 |
| CD8+ | 18.3 ± 11.9 (2.0–32.3) | 23.3 ± 7.8 (14.9–34.5) | 27 |
| CD16/56+ | 6.1 ± 7.0 (0.0–18.0) | 15.0 ± 15.5 (1.8–43.5) | 16.6 |
The percentages of large CD8+ cells in L. aethiopica-stimulated cultures from patients were on average significantly lower (P < 0.05 by Student’s t test) than the CD4+-cell levels, while there was no significant difference between the percentages of CD8+ and CD4+ cells in the cured group.
Proportional increase in T cells and NK cells following in vitro stimulation with L. aethiopica in bona fide donors.
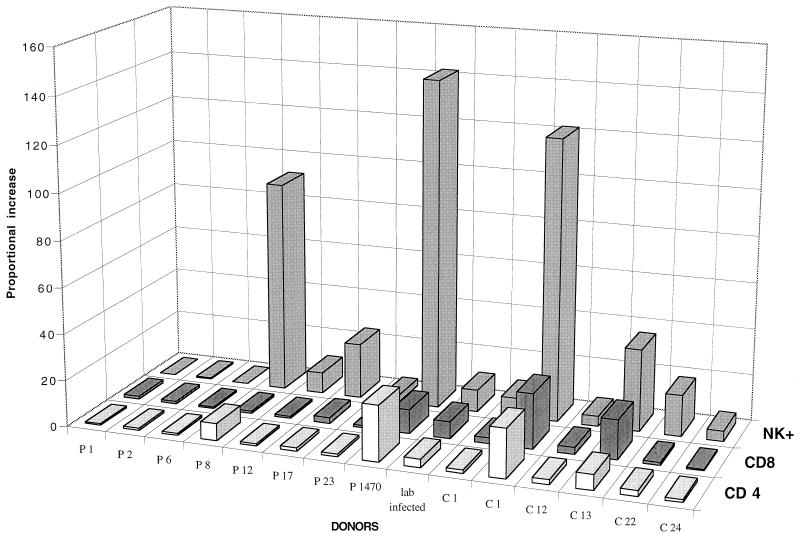
Since evaluation of the percentage of cells does not take into account the actual number of proliferating cells, we decided to evaluate the proportional increase in the percentage of responding cells as defined in Materials and Methods, i.e., after 6 days culture in response to antigenic stimulation. A greater-than-twofold proportional increase in CD4+ cells induced to proliferate in response to L. aethiopica stimulation was observed in six of six cured individuals and two of eight bona fide patients with ongoing disease (Fig. 1). Since the percentage of CD4+ cells started out at a high level in most of the individuals tested, the proportional increases was not as dramatic. However, the CD8+-cell proliferation, which was sometimes substantial and greater than the CD4+ increase, was seen especially after cure of infection, in four of six cured individuals. There was an even larger proportional increase in CD16/CD56+ NK cells than in CD8+ cells, and this was again seen especially in the cured group (all tested) but also in five of eight of the patients (Fig. 1). There was no significant difference in the mean proportional increase in NK, CD4, or CD8 cells after L. aethiopiaca stimulation between the cured and patient groups (Student’s t test).
FIG. 1.
Proportional increases in the percentages of proliferating CD4+, CD8+, and CD3− CD16/56+ NK cells following in vitro stimulation by L. aethiopica antigens of mononuclear cells from bona fide patients (P1 to P1470) and cured individuals (C1 to C24). The absolute percentages of cell phenotypes in the groups are given in Table 3.
There was no statistical correlation between the proportional increase induced by PPD and L. aethiopica antigen (Regression; r = 0.208 for NK cells, 0.111 for CD4+ cells, and 0.228 for CD8+ cells).
Large NK cells in unstimulated cultures.
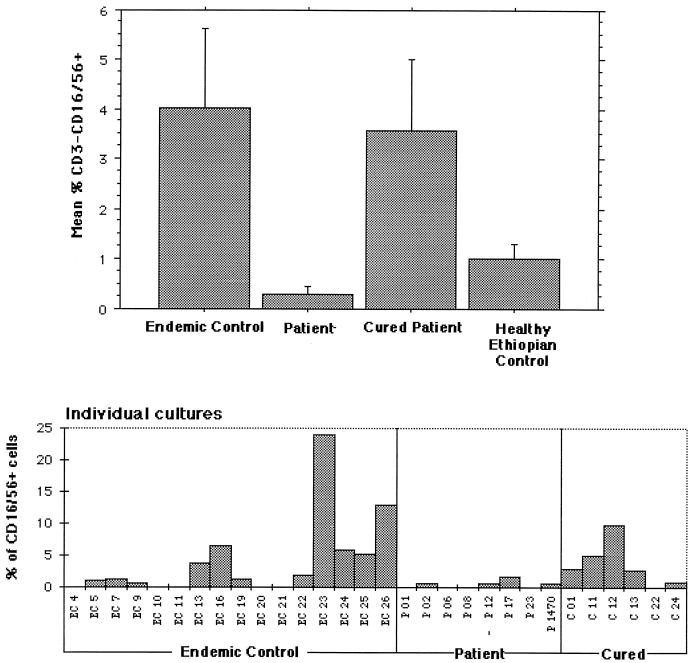
Analyses of unstimulated cultures showed a proportion (≥1%) of large NK cells in cultures of 4 of 6 cured individuals, 10 of 16 endemic controls, and 1 of 8 bona fide patients with ongoing infection. The mean percentage of such large NK cells in unstimulated cultures from the cured individuals was not different from the percentage in the endemic control group but was significantly different from that in the patients with ongoing disease (P < 0.05 by Student’s t test) (Fig. 2). There was an apparent difference in the percentage of these cells between the endemic controls and the active leishmaniasis patient group, but this difference did not reach significance due to the large variance within the endemic control group.
FIG. 2.
Individual and mean percentages (± standard error) of large NK cells in unstimulated 6-day cultures.
Cytokines.
The sensitivities of the IFN-γ and IL-10 assays were 27 pg/ml and 9.8 U, respectively. The levels of L. aethiopica-induced IFN-γ were highest in the supernatants isolated from cultures of PBMC of bona fide cured patients (mean ± standard error of the mean = 1,389 ± 898 pg/ml); the endemic controls showed the next highest, although appreciably lower, levels (mean ± standard error of the mean = 231 ± 60 pg/ml), and the levels in patients were also low (168 ± 117 pg/ml). The levels of PHA-induced IFN-γ were comparable in all the groups (mean = 3,326 to 4,249 pg/ml), as was the PPD-induced response (1,056 to 1,311 pg/ml), except in the patient group, where the mean PPD-induced IFN-γ level was 213 ± 498 pg/ml. There were no statistically significant differences (by Student’s t test) in IFN-γ production between the groups in response to any of the stimuli.
IL-10 secretion was induced by PHA and PPD but was not a feature of L. aethiopica stimulation, where measurable levels were only observed in the cured patient group.
NRAMP1 genotyping.
A total of 57 individuals were genotyped for two NRAMP1 polymorphisms. Allele frequencies did not differ significantly from those determined previously for two random Caucasian and Asian populations (13). There was no significant association of NRAMP1 alleles with any of the groups tested (data not shown). Furthermore, NRAMP1 haplotypes which were derived from the observed allele frequencies by maximum-likelihood estimates (21) were not found to be associated with cutaneous leishmaniasis (P > 0.2). These results are consistent with our inability to detect NRAMP1 expression by purified unstimulated NK cells from healthy Swedish individuals (data not shown).
DISCUSSION
We have previously shown that NK cells were induced to proliferate in a portion of unexposed healthy donors (1), which formed the basis for the present study, in which we wished to test whether these cells indeed constitute the protective phenotype in the individuals without disease in the village where leishmaniasis is endemic. The study groups in Sebeta were comparable in age, sex, socioeconomic status, and potential exposure to infected-sandfly bites. The serological analyses of previous infection with other parasites showed similar distributions in the different groups. The only apparent differences were observed in an overrepresentation of antischistosomal antibodies in the individuals with scars and lower antiechinococcus antibodies in the healthy Addis Ababa donors. However, when the data were analyzed together, there was no significant difference between the groups. The endemic controls had no distinguishing features in terms of social or nutritional status. It is evident that most of the participants in the study were previously exposed to other parasites. While cellular reactivities to other parasite antigens could not be compared because of the scarcity of cells, the response to PPD was tested. There was no significant correlation between the PPD and L. aethiopica responses in terms of the SIs and the proportional increases in the different cell phenotypes.
Our results show that NK cells and CD8+ cells, both strong inducers of IFN-γ, are also capable of proliferating in response to L. aethiopica stimulation. These responses were frequently seen in individuals who had cured their infection, including the lab-infected individual, who consistently showed high percentages of CD16/56+ cells and CD8+ cells in response to Leishmania stimulation in vitro. Proliferation of CD4+ cells in response to leishmanial antigen stimulation is a feature of patients with ongoing Leishmania infection as well as those cured of the disease. The CD4+ cells are believed to be producers of IFN-γ, to be effector cells to control the infection in the patients (15, 19, 20), and to be the memory cells sustaining the solid resistance to new disease expression, associated with cure from this infection, in the cured individuals. These Leishmania-specific T cells expand as a result of the infection and circulate in the peripheral blood of patients recovered from cutaneous leishmaniasis (11). Paradoxically, measurable levels of the down-regulating cytokine IL-10 were evident only in L. aethiopica-stimulated culture supernatants of cured individuals.
The largest proportional increase of cell types induced to proliferate in response to L. aethiopica stimulation was of NK cells, followed by CD8+ cells, a feature most prominent in the cured patients. A study in which cells from patients with South American cutaneous leishmaniasis were monitored before and after treatment showed that patients with active lesions had high percentages of CD4+ cells before therapy and that Leishmania-reactive CD8+ cells were activated in PBMC of these same patients only after cure and apparent protection (8). The induction of CD8+ cells in individuals immunized against American tegumentary leishmaniasis with a vaccine made of whole antigens from killed promastigotes of five American dermotropic Leishmania strains has been reported (16). Thus, the CD8+-cell induction could be associated with the protective mechanisms against subsequent infection, a conclusion that is compatible with our findings, since this cell type was prominent in the cured patients.
NK cell induction and activation may be influenced by macrophage factors such as IL-12; thus, macrophage function could be related to NK cell induction. In this human form of leishmaniasis that we have been studying, the mechanisms related to this apparently protective NK cell effect appear to be unrelated to the NRAMP1 host resistance gene, since we failed to detect NRAMP1 expression by human NK cells (data not shown), nor was there a significant association of NRAMP1 polymorphisms with clinically defined disease. However, our sample size was small, and only a very strong association of NRAMP1 alleles with cutaneous leishmaniasis would have been detectable in our study. Finally, there was no preferential association of NRAMP1 alleles with the phenotype of a proportional increase of proliferating NK cells. Taken together, these results demonstrate that NK cell-mediated protection from LCL is largely independent of the NRAMP1 alleles tested in our study.
Two mechanisms of elimination of Leishmania parasites in cutaneous lesions have been suggested (10), i.e., through a lytic process within activated macrophages and through a necrotizing process taking place at the site of the lesion via the destruction of the parasitized macrophages, presumably via the involvement of cytotoxic cells. It has also been shown by the same group that CD45RO+ memory T cells form a substantial component of the inflammatory infiltrate of cutaneous leishmaniasis lesions (9). If present in the lesion, the increased proliferation of cytotoxic CD8+ T cells and NK cells that we see in our study group could contribute to the ulcer formation which seem to be a prerequisite for the healing of L. aethiopica lesions. Even in the absence of a cytotoxic potential (which has not been addressed in this study), the ability of these cells to induce IFN-γ could still make them important in the control of the spread of the infection. Our results show that large NK cells are present at low levels in the unstimulated cultures of the cured individuals but only in one of the patients with ongoing disease tested. In contrast, the majority of the endemic control group tested (63%) had these large NK cells in unstimulated cultures. Assays designed to assess the function of cells from the various donors would give a deeper insight into the role of NK cells in this situation. NK cells have been implicated to exist as preactivated cytotoxic cells capable of mediating their effector function without the need for prior activation or immunization, which makes them an important component of innate immunity (12). The biological significance of our observation, however, is yet to be ascertained.
Our findings are consistent with a role of NK cells in innate immunity and suggest that the presence of this low background level of large NK cells is an important aspect of the protective mechanisms that operate in the leishmaniasis-resistant endemic controls and is established following cure from the disease. It is noteworthy that we have not detected a background of NK cells in the healthy Swedish donors reported to have the capacity to induce NK proliferation in response to L. aethiopica antigens (1). We conclude from these observations that NK cells and CD8+ cells proliferating in response to Leishmania antigen stimulation are involved in protection from and healing of (Ethiopian) cutaneous leishmaniasis.
ACKNOWLEDGMENTS
This study was supported by funds from The Swedish Medical Research Council (MFR) and the Swedish International Development Agency (SIDA/SAREC).
Our thanks go to Mathieu Cellier for his help in the NRAMP expression studies and to Ellen Buschman for helpful suggestions in writing the manuscript.
REFERENCES
- 1.Akuffo H, Maasho K, Howe R. Natural and acquired resistance to Leishmania: cellular activation of Leishmania aethiopica of mononuclear cells from unexposed individuals is through the stimulation of NK cells. Clin Exp Immunol. 1993;94:516–521. doi: 10.1111/j.1365-2249.1993.tb08227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akuffo H O, Britton S F F. Contribution of non-Leishmania-specific immunity to resistance to Leishmania infection in humans. Clin Exp Immunol. 1992;87:58–64. doi: 10.1111/j.1365-2249.1992.tb06413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akuffo H O, Darce M, Maasho K, Yamane Berhan T. In vivo evaluation of immune responses in leishmaniasis: the use of cross-species leishmanin preparations for skin testing. Am J Trop Med Hyg. 1995;53:16–22. [PubMed] [Google Scholar]
- 4.Akuffo H O, Fehniger T E, Britton S. Differential recognition of Leishmania aethiopica antigens by lymphocytes from patients with local and diffuse cutaneous leishmaniasis. J Immunol. 1988;141:2461–2466. [PubMed] [Google Scholar]
- 5.Ashford R W, Rioux J, Jalouk L, Khiami A, Dye C. Evidence for a long-term increase in the incidence of Leishmania tropica in Aleppo, Syria. Trans R Soc Trop Med Hyg. 1993;87:247–249. doi: 10.1016/0035-9203(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 6.Bøyum, A. 1969. Separation of lymphocytes from blood and bone marrow. Scand. J. Clin. Lab. Invest. 21(Suppl.):77–89.
- 7.Bray R S, Ashford R W, Bray M A. The parasite causing cutaneous leishmaniasis in Ethiopia. Trans R Soc Trop Med Hyg. 1973;67:345–348. doi: 10.1016/0035-9203(73)90111-9. [DOI] [PubMed] [Google Scholar]
- 8.Coutinho S, Oliveira M, Da-Cruz A, De Luca P, Mendonca S, Bertho A, Soong L, McMahon-Pratt D. T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure. Exp Parasitol. 1996;84:144–55. doi: 10.1006/expr.1996.0100. [DOI] [PubMed] [Google Scholar]
- 9.el Hassan A M, Gaafar A, Theander T G. Antigen-presenting cells in human cutaneous leishmaniasis due to Leishmania major. Clin Exp Immunol. 1994;99:445–453. doi: 10.1111/j.1365-2249.1995.tb05571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaafar A, el Kadaro A Y, Theander T G, Permin H, Ismail A, Kharazmi A, el Hassan A M. The pathology of cutaneous leishmaniasis due to Leishmania major in Sudan. Am J Trop Med Hyg. 1995;52:438–442. doi: 10.4269/ajtmh.1995.52.438. [DOI] [PubMed] [Google Scholar]
- 11.Kemp M, Hey A S, Kurtzhals J A, Christensen C B, Gaafar A, Mustafa M D, Kordofani A A, Ismail A, Kharazmi A, Theander T G. Dichotomy of the human T cell response to Leishmania antigens. I. Th1-like response to Leishmania major promastigote antigens in individuals recovered from cutaneous leishmaniasis. Clin Exp Immunol. 1994;96:410–5. doi: 10.1111/j.1365-2249.1994.tb06043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kos F J, Engleman E G. Immune regulation: a critical link between NK cells and CTLs. Immunol Today. 1996;17:174–176. doi: 10.1016/0167-5699(96)80616-5. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Fujiwara T M, Buu N T, Sanchez F O, Cellier M, Paradis A J, Frappier D, Skamene E, Gros P, Morgan K, Schurr E. Identification of polymorphisms and sequence variants in the human homologue of the mouse natural resistance-associated macrophage protein gene. Am J Hum Genet. 1995;56:845–853. [PMC free article] [PubMed] [Google Scholar]
- 14.Maasho K, Akuffo H O. Cells from healthy non-exposed individuals produce cytokines to selected fractions of Leishmania promastigotes. Scand J Immunol. 1992;36:179–184. doi: 10.1111/j.1365-3083.1992.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 15.Melby P C, Sacks D L. Identification of antigens recognized by T cells in human leishmaniasis: analysis of T-cell clones by immunoblotting. Infect Immun. 1989;57:2971–2976. doi: 10.1128/iai.57.10.2971-2976.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendonca S C, De Luca P M, Mayrink W, Restom T G, Conceicao-Silva F, Da-Cruz A M, Bertho A L, Da Costa C A, Genaro O, Toledo V P, et al. Characterization of human T lymphocyte-mediated immune responses induced by a vaccine against American tegumentary leishmaniasis. Am J Trop Med Hyg. 1995;53:195–201. doi: 10.4269/ajtmh.1995.53.195. [DOI] [PubMed] [Google Scholar]
- 17.Mengistu G, Akuffo H O, Fehniger T E, Yohannese N, Nilsen R. Comparision of different parasitological and immunological methods in the diagnosis of leishmaniasis in Ethiopia. Trans R Soc Trop Med. 1992;86:154–157. doi: 10.1016/0035-9203(92)90548-q. [DOI] [PubMed] [Google Scholar]
- 17a.Negash W. Present status of cutaneous leishmaniasis, hyraxes and Phlebotomus Longipes in Meta-Abo, Sebeta. M.Sc. thesis. Addis Ababa, Ethiopia: Addis Ababa University; 1988. [Google Scholar]
- 18.Sang D K, Njeru W K, Ashford R W. A zoonotic focus of cutaneous leishmaniasis due to Leishmania tropica at Utut, Rift Valley Province, Kenya. Trans R Soc Trop Med Hyg. 1994;88:35–37. doi: 10.1016/0035-9203(94)90486-3. [DOI] [PubMed] [Google Scholar]
- 19.Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 20.Scott P, Pearce E, Cheever A W, Coffman L R, Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 21.Terwilliger J D, Ott J. Handbook of human genetic linkage. Baltimore, Md: Johns Hopkins University Press; 1994. [Google Scholar]
- 22.Vidal S M, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for bcg. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 23.Wilkins H A. Studies on leishmaniasis in Ethiopia. VI. Incidence rates of cutaneous leishmaniasis at Meta Abo. Ann Trop Med Parasitol. 1972;66:457–466. [PubMed] [Google Scholar]