Abstract
The discovery of Mas-related G protein-coupled receptors (Mrgprs) has opened a compelling chapter in our understanding of immunity and sensory biology. This family of receptors, with their unique expression and diverse ligands, have emerged as key players in inflammatory states and hold the potential to alleviate human diseases. This review will focus on the members of this receptor family expressed on immune cells and how they govern immune and neuro-immune pathways underlying various physiological and pathological states. Immune cell-specific Mrgprs have been shown to control a variety of manifestations, including adverse drug reactions, inflammatory conditions, bacterial immunity, and the sensing of environmental exposures like allergens and irritants.
Introduction
The Mas-related G protein-coupled receptor (MRGPR) family of orphan G protein-coupled receptors (GPCRs) are a cornerstone in somatosensation.1 However, their role exceeds the domains of itch and pain, as these GPCRs substantially impact immune cell function. Mrgprs are expressed on various immune cell types, including mast cells, dendritic cells and neutrophils, highlighting their capacity to regulate a variety of immune responses. MRGPR stimulation can trigger different immune processes, leading to the release of pro-inflammatory cytokines, chemotaxis of immune cells, and modulation of immune cell activation states. One of the main identified mechanisms for MRGPRs is facilitating the crosstalk between sensory nerves and immune cells. Neuropeptides released from sensory nerves can activate MRGPRs on immune cells, leading to their recruitment and activation in response to allergens, irritants, and infections. Clinical trials of drugs inhibiting MRGPRs to treat and manage immune-related disorders are already underway, suggesting the possibility that several other MRGPRs with unknown immune-modulating functions could also serve as potential therapeutic targets.
Brief Historical Perspective
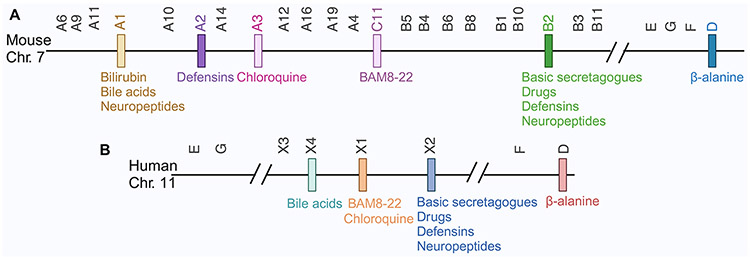
In 2001, via a subtractive cloning screen using RNA from dorsal root ganglion (DRG) neurons, Dong, Anderson, and colleagues discovered a previously unknown GPCR gene family.2 Following these findings, another group discovered these family members using rat neuronal tissue.3 As the genes in this family showed the highest homology (approximately 35%) to the MAS1 proto-oncogene, they were designated as the MAS-Related G-Protein Coupled Receptor (MRGPR). Based on sequence similarities, 3 subfamilies are clustered into Mrgpra, Mrgprb and Mrgprc, containing 50 members, of which only 22 have intact open reading frames (ORFs), and the rest are pseudogenes (Fig. 1A). Four human Mrgpr genes showed close homology (approximately 50%) to these 22 mouse Mrgprs. However, without apparent sequence homology needed to confirm the ortholog pair, these 4 human MRGPRs were initially coined as members of the ‘X’ cluster: MRGPRX1, MRGPRX2, MRGPRX3, and MRGPRX4.4 Further, 4 other Mrgprs with well-defined sequence homology in both mice and humans were identified, specifically, Mrgprd, Mrgpre, Mrgprf, and Mrgprg. In sum, 26 murine and 8 human MRGPRs with intact coding sequences constitute this receptor family (Fig. 1B).
Figure 1:
Structure of MRGPR receptor family in mice (A) and humans (B) and their ligands. Each bar represents a Mrgpr gene.
The Mrgpr family members show high structural similarity, having short (3-21 amino acid) N-termini and relatively conserved transmembrane and intracellular domains. The most variation among these family members resides in the extracellular environment, which imparts unique ligand binding potential.
The mast cell-MRGPRX2 axis in skin infection and inflammation
The first report of extra-neuronal expression of MRGPRs came in 2006 when Tatemoto et al. demonstrated that human mast cells express MRGPRX2.5 This discovery has led to a wealth of new research re-evaluating our understanding of what drives mast cell activation. Classically, mast cells are most known for their role in immunoglobulin E (IgE)-mediated allergic manifestations. In allergen-sensitized individuals, crosslinking of the high-affinity IgE receptor (FcεR1) by antigen-IgE complexes on mast cells drives degranulation, leading to the release of pre-formed mediators such as histamine, arachidonic acid metabolites, and cytokines.6 Although mast cells are known for their pre-formed content, they also synthesize lipid mediators and cytokines in response to IgE crosslinking.7 The secretory output of activated mast cells drives the hallmark features of type I hypersensitivity (hives, edema, bronchospasm, itching, etc). However, it had been well-established that mast cells can be triggered in an IgE-independent fashion, culminating in an inflammatory response similar to IgE-dependent activation. Before the discovery of IgE in 1967, and starting at least in the 1950s, reports were studying the effects of various mediators, including neuropeptides, on human mast cells and in later experiments beginning in the 1970s, had demonstrated that neuropeptides like substance P (SP) could drive human skin mast cell degranulation.8-10 Today, we know that these IgE-independent endogenous and exogenous mast cell activators, most of which are positively charged and are also collectively called basic secretagogues, form a diverse collection of synthetic and natural molecules like compound 48/80, FDA-approved drugs, neuropeptides, venoms, and toxins, now known to trigger mast cells via MRGPRX2.
In 2015, it was reported that Mrgprb2 was the mouse ortholog of human MRGPRX2 11, with similarly restricted expression to mast cells. Thus, MRGPRX2 and Mrgprb2 became a bona fide marker of mast cells. However, their expression is not equally found across different types of mast cells, highlighting the developmental dichotomy to the genesis of mast cells. The long-held belief that circulating bone marrow (BM) derived precursors give rise to tissue mast cells is now being replaced with the knowledge that in mice and humans, mast cells can originate from hematopoietic stem cells from the bone marrow or the yolk sac.12, 13 Mast cells with embryonic origins (i.e. yolk sac) tend to be enriched for granules containing tryptase, chymase, and carboxypeptidase (MC(TC)). They are found in connective tissues, fat, and sub-mucosa of the skin, in proximity to blood and lymphatic vessels, and neurons.14 MRGPRX2 is highly expressed in fat and skin mast cells15, and like in humans, Mrgprb2 is also majorly expressed by mouse connective tissue-associated MC(TC).16 Unlike yolk-sac-derived mast cells, mast cells that develop from the bone marrow are predominantly found in respiratory and gastrointestinal mucosal tissues and contain tryptase-loaded granules (MC(T)). In mice and humans, these express little Mrgprb2 16 or MRGPRX2 15, respectively. In line with the segregated expression of Mrgprb2/MRGPRX2 to connective tissue mast cells, engraftment of human fetal liver CD34+ hematopoietic stem cells into humanized mice resulted in the development of human mast cells in various organs where it was observed that only the skin engrafted mast cells expressed MRGPRX2, but not those in the lungs, spleen or liver.17 This may suggest the possibility that connective tissue-specific signals may support the expression of MRGPRX2.
Ligand discovery efforts using peptide and chemical screening libraries revealed that both Mrgprb2 and MRGPRX2 could be ligated by hundreds of compounds, including many endogenous secretagogues 18, which have been shown to trigger mast cell degranulation. These include substance P (SP), pituitary adenylate cyclase-activating polypeptide (PACAP), somatostatin (SST), cortistatin, the adrenomedullin-derived PAMP12 (also known as PAMP9-20), and anti-microbial peptides.5, 11, 18 In addition, Mrgprb2/MRGPRX2 were shown to bind various cationic FDA-approved drugs, including vancomycin, icatibant, ciprofloxacin, as well as several compounds derived from the opioid family like codeine and morphine.11, 19, 20 Engagement of this pathway resulted in mast cell degranulation and the development of drug-induced pseudo-allergic drug reaction in mice, suggesting that MRGPRX2 is may be responsible for adverse drug reactions like the red man syndrome characterized by itchy skin eruptions in response to vancomycin.21
Mast cells are a versatile component of innate immunity, and they essentially populate all organs. Mast cells are considered one of the most ancient immune cells originating nearly 500 million years ago from primitive test cells of urochordates, which are functionally and morphologically highly similar to mast cells, including their ability to degranulate and respond to compound 48/80 (C48/80).22 Like their ancestor test cells, which were thought to provide host protection against infections, Mrgpr-expressing mast cells are likely central to how we have evolved a rapid response to environmental triggers and pathogen exposures. This detection system can react to various host mediators released during infection, as well as molecules of microbial origin. One of the major anti-microbial pathways thought to be elicited during infection is the recognition of host-defense peptides by Mrgprb2/MRGPRX2 on mast cells.
Cationic host-defense peptides also known as anti-microbial peptides (AMPs) are expressed across diverse species ranging from microorganisms to plants, amphibians, and mammals.23 These short (>50 amino acids), positively charged (at physiological pH) peptides from vertebrates can be categorized into 2 broad classes, defensins and cathelicidins.24 Defensins are broadly subdivided into 3 categories: alpha-defensins, beta (β)-defensin and θ-defensins; among these, beta-defensins are ubiquitous and found in all vertebrates.24 LL-37 is the only human cathelicidin.25 Both LL-37 26 and β-defensins 27 are ligands for MRGPRX2 and induce MRGPRX2-dependent mast cell degranulation.
Given their anatomical distribution at barrier surfaces, mast cells are known to be actively engaged by pathogens, including viruses, bacteria and fungi, where they generally provide protective immunity.28, 29 During infection, mast cells can be activated downstream of cell-surface receptors, like toll-like receptors (TLRs) and Fc-receptors (FcRs)30, and this leads to the secretion of a wide variety of mediators that are directly anti-microbial such as AMPs.31 A major mechanism by which mast cells are thought to mediate anti-microbial response is through increased recruitment and activation of other immune cells like neutrophils.32 In a model of localized dermonecrotic infection using an intradermal injection of S. aureus, mice with inducible deficiency in mast cells (Mcpt5Cre;iDTR) failed to clear bacteria efficiently when compared to mast cell sufficient animals.33 Also, injection of the Mrgprb2/MRGPRX2 agonist mastoparan, a peptide isolated from wasp venom, reduced skin bacterial burden, wound size, and scarring in mast cell-sufficient mice, but not when mast cells were depleted. Administration of mastoparan also induced mast cell-dependent recruitment of neutrophils, Langerhans cells and DCs in response to S. aureus. In line with this, the inactive mastoparan analog, mastoparan 17, was significantly less effective than mastoparan in reducing lesion size. However, several cationic amphiphilic peptides, such as mastoparan, not only activate mast cells via Mrgprb2/MRGPRX2 but can also kill bacteria directly by binding to and disrupting negatively charged bacterial membranes. Thus, Arifuzamman et al. 33 generated mastoparan analogs (Duke Mast F and MP-6I peptides) with uncoupled properties to separate the mast cell degranulating and direct anti-bacterial properties of mastoparan. The Duke Mast F peptide induced mast cell activation but had no anti-bacterial activity and was able to reduce S. aureus-induced lesion size. Conversely, the MP-6I peptide had no activity on mast cells but retained its anti-bacterial properties and showed no effect in reducing lesions. Thus, suggesting that sensing of endogenous anti-microbial peptides by Mrgprb2/MRGPRX2 is a key anti-microbial mechanism.
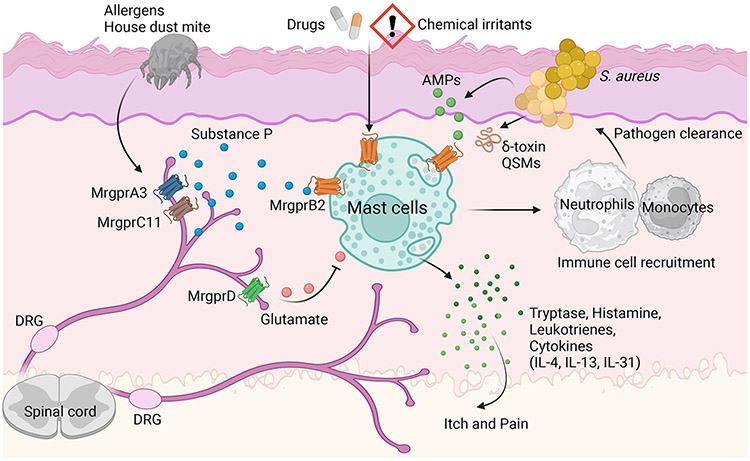
A recent study showed that Mrgprb2-mediated recognition of anti-microbial peptides is not the only mechanism by which mast cells protect the host. Bacterial gene expression is dictated by their cellular density in a phenomenon known as quorum sensing (QS). Both gram-negative and gram-positive bacteria synthesize QS molecules (QSM), which serve as messengers for communication within the bacterial colony.34 However, these can also be sensed by host immune cells. Various cationic QS peptides from gram-positive bacteria were shown to activate Mrgprb2 and MRGPRX2 and mediate host-pathogen clearance.35 Various QSM such as the competence-stimulating peptide (CSP) from Streptococcus pneumoniae, Entf from Enterococcus faecium, and Streptin-1 from S. pyogenes activated mast cells through Mrgprb2 and MRGPRX2, leading to mast cell degranulation. Further, CSP-deficient Streptococcus pneumoniae deficient could not activate mast cells. During infection, Mrgprb2−/− mice had elevated bacterial loads and reduced recruitment of neutrophils compared to controls. These findings are in line with others33, showing that an intact mast cell response facilitates the influx of immune cells, including neutrophils. This suggests that in addition to the ability of mast cells to directly produce AMPs 31, they coordinate the recruitment of other immune effectors to produce an anti-microbial response. Together, these reports support the role for an Mrgprb2/MRGPRX2-mast cell axis in controlling bacterial infections at barrier surfaces (Fig. 2).
Figure 2:
Mrgprb2 on mast cells can be activated by variety of endogenous and exogenous positively charged activators (e.g. neuropeptide substance P, drugs, chemical irritants, AMP, QSM, etc). The activation of Mrgprb2 on mast cells leads to the release of protease, cytokine, chemokines and recruitment of other immune cells. There are also mutual interactions between mast cells and sensory nerves in the skin leading to itch and pain.
Aberrant microbial activity is also thought to be pathogenic in rosacea, a chronic skin inflammatory condition with multifactorial etiology.36 Dysbiotic colonization in rosacea is believed to drive a heightened host defense response encompassing enhanced anti-microbial peptide generation and immune cell recruitment.37 Compared to normal skin, patients with rosacea harbor more mast cells.38 Consistent with this, studies in murine models have demonstrated the importance of mast cells in rosacea-like skin inflammation induced by intradermal injection of the cathelicidin LL-37. 38, 39 Moreover, in skin biopsies of patients with rosacea, more mast cells express MRGPRX2 than in normal subjects.40 Furthermore, LL-37-induced rosacea in mice was significantly reduced in mast cell deficient KitW-sh/W-sh mice and Mrgprb2−/− mice.40
The Mrgprb2/MRGPRX2-mast cell axis may go beyond responding to bacterial infections and could play a role in the host response to tick bites. A recent study showed that Mrgprb2 and MRGPRX2 can recognize tick (Ixodes persulcatus)-derived salivary defensin peptides (IPDef1, IPDef2).41 In vivo, IPDefs elicited scratching and vascular leakage, and in vitro stimulation of mouse peritoneal mast cells with recombinant IPDef induces the release of histamine, serotonin, and tryptase, although it is not clear whether this is dependent on Mrgprb2. Nonetheless, taken together this data is suggestive that the tick bite-mediated itch sensation may be dependent on the Mrgpr-mast cell system.
Mast cells and MRGPRs: the neuroimmune axis regulating dermatitis and itch
Sterile and non-sterile immunity is no longer the purview of only immune cells. In the last decade, the field of neuroimmunology has contributed to a more holistic view of our responses to infection, inflammation and somatosensation. We now know that neuronal signals are essential to condition an appropriate immune response. In many tissues, like the skin, subsets of immune populations, including mast cells, exist near sensory nerves and are receptive to neural outputs.
Our comprehension of the molecular pathways responsible for translating pruritic stimuli into the sensation of itch is still at an early stage. Itch can be categorized into two main origins: histaminergic and non-histaminergic.42 As the primary source of histamine, mast cells are crucial mediators of histaminergic-related acute itch. In sensitized allergic individuals, IgE-complex via crosslinking of high-affinity IgE receptors on mast cells can activate them, leading to histamine release.7 Histamine-induced itch involves the H1 and H4 histamine receptors on histaminergic nerves, this activates capsaicin-responsive nociceptors or TRPV1+ nociceptors, leading to the release of neuropeptides, like SP and calcitonin gene–related protein (CGRP).43 44 This so-called “neurogenic inflammation,” results in vasodilation in the surrounding tissue, plasma extravasation, and mast cell degranulation.42, 45 While histamine is an important mediator for both acute and chronic itch, non-histaminergic pathways are major contributors to different types of itch. For example, itch neurons can be activated by a variety of exogenous and endogenous nonhistaminergic pruritogens such as peptides, lipids, cytokines and proteases, etc.46 While histamine receptors are present in the 3 main classes of itch-sensing neurons in human and mouse, labeled as non-peptidergic nociceptor 1 (NP1), NP2, NP3 47, Mrgprs have selective expression. Single cell RNA sequencing of mouse DRG sensory neurons revealed that NP1, NP2, NP3 are specifically labeled by Mrgprd, Mrgpra3, and somatostatin (SST) expression, respectively.48 Consistently, human DRG single cell RNAseq study also demonstrated the existence of these three classes of itch-sensing neurons.47 In human DRG, MRGPRX1 is expressed in both NP1 and NP2 whereas MRGPRD is weakly expressed in NP1 and MRGPRX4 is specifically expressed in NP2 population. Like mice, SST is also a specific marker for human NP3 neurons.47
Mrgprb2 is an important mediator of non-histaminergic itch. Importantly, itch triggered by Mrgprb2-activated mast cells drives the release of different pruritogens, which results in the engagement of distinct itch sensory neurons compared to those initiated downstream of anti-IgE complexes. The Mrgprb2 agonist PAMP12 induced Mrgprb2-dependent scratching, and this was accompanied by the release of tryptase, but not histamine.49 On the other hand, itch induced by anti-IgE complexes or ovalbumin was not dependent on Mrgprb2 and induced histamine, but not tryptase release. The administration of Mrgprb2 agonists, PAMP12 and C48/80, in mice skin activates sensory neurons that partly overlap with those activated by substances like β-alanine, which triggers Mrgprd, chloroquine, which binds to Mrgpra3 and 5-hydroxytryptamine (5-HT).49 However, these neurons do not coincide with those sensitive to histamine and capsaicin. Conversely, neurons activated by anti-IgE complex administration overlap with those responsive to histamine and capsaicin but not those triggered by β-alanine, chloroquine, and 5-HT.49 This distinction highlights the complexity of itch pathways and the diversity of sensory neurons involved in different itch sensations. Mrgprb2 was also partially involved in driving itch and immune cell infiltration in different models of acute contact dermatitis (ACD) using dinitrochlorobenzene (DNCB), squaric acetyl dibutyl acid (SADBE) and oxazolone. Specifically, in these models, itch and immune cell influx was reduced by approximately 50% in Mrgprb2−/− mice as compared to controls.49 Itch responses not mediated by Mrgprb2 may be elicited downstream of histamine or other alternative pathways of itch possibly involving basophils, natural killer (NK) cells, IL-31, TSLP, or IL-33.50-53 Further, as Mrgprb2 agonists favors the release of tryptase, which is a known activator of protease-activated receptors (PARs), it remains a possibility that Mrgprb2-driven non-histaminergic itch involves PAR-mediated activation of sensory fibers.54, 55
Atopic dermatitis (AD) a.k.a. eczema and ACD manifest similar symptoms but have different causes: external vs. internal triggers, respectively.56 AD is a heterogeneous, chronic allergic inflammatory disease characterized by rashes, skin lesions and intense itch. While the complete etiology of AD is unknown, a dysregulated type 2 immune response with elevated IgE and barrier dysfunction is thought to underlie its pathogenesis.57, 58 Mast cells are elevated in the skin of patients with AD and they are thought to play a role in AD pathogenesis.59 Additionally, microbial dysbiosis is now appreciated as a potential driver of AD pathophysiology. Compared to non-AD patients, Staphylococcus aureus colonization is 20-30% in healthy individuals but reaches 30-100% in AD patients 60, and it can colonize lesional and non-lesional skin. This is thought to be driven in part by compromised barrier integrity. S. aureus produces a range of virulence factors, including proteases (aureolysin, serine protease, cysteine protease), phenol soluble modulins (PSMs) and superantigens (staphylococcal enterotoxin A, B, TSST-1) which can disrupt barrier function and engage inflammatory circuits driving AD.61
It is thought that dysregulated mast-cell-neuron circuits can drive AD. In a murine model of AD utilizing a mixture of house dust mite (HDM) and staphylococcal enterotoxin B (SEB), depletion of TRPV1+ nociceptors using the ultrapotent TRPV1 agonist, resiniferatoxin, attenuated HDM+SEB-induced skin pathology and as well as reducing eosinophil, and neutrophil recruitment.14 In this model, HDM is thought to trigger skin sensory neurons directly. Specifically, while SEB itself did not, HDM alone or in combination with SEB induced calcium influx in a capsaicin-sensitive subpopulation of DRG neurons in vitro and caused the release of SP in culture. SP is then thought to trigger Mrgprb2 on mast cells, leading to inflammation. Functionally, manifestations of experimental AD were almost entirely dependent on the SP-Mrgprb2 axis. Specifically, SP- or Mrgprb2-deficient animals had a significant reduction in skin pathology, inflammation and IgE production in response to allergen.14 Interestingly, HDM-induced activation of cultured DRG neurons is independent of MyD88 or the protease-activated receptor (PAR2) but requires the intrinsic proteolytic activity of HDM.14 Specifically, the cysteine, but not serine, protease activity of HDM was necessary for neuronal activation. In line with these findings, Der p 1, a cysteine protease and major allergen from house dust mite, was shown to activate MrgprC11, Mrgpra3 and the human ortholog MRGPRX162. This activation was entirely dependent on the protease activity of Derp 1. We thus envision an Mrgpr relay involving the direct activation of Mrgprs on sensory neurons, leading to the release of Mrgprb2-activating neuropeptides that induce mast cell degranulation (Fig. 2). On the other hand, the serine protease activity in sources of allergens is important in mediating IL-33 release.63 Moreover, endogenous serine proteases like mast cell tryptase and chymase potentiates IL-33 mediated ILC2 response.64 Aberrant IL-33 production is central in many allergic manifestations, including AD 65-67, where it is thought to play a pathogenic role.68, 69 Thus, it is reasonable to foresee that allergens can engage two different mechanisms: 1) via a cysteine protease-dependent nociceptor-mast cells module and 2) through a serine protease-induced IL-33 axis, both converging in driving the development of type 2 immunity in AD.
Lastly, it is important to appreciate the nuances in the murine models utilized to study dermatitis and the role of mast cells among these. Mast cells have been found to be largely dispensable in experimental models of AD using MC903 (calcipotriol, a vitamin D analog) 70 and acetone:ether-induced dry skin itch.65 In simpatico with these, the sensation of itch in MC903-induced AD and acetone:ether-induced dry skin, was independent of Mrgprb2.49 Perhaps, the contribution of mast-cell in AD can be appreciated in less reductive models of skin inflammation and likely better studied in approaches mimicking the more complex nature of the environmental triggers (allergen & microbial interaction) that drive AD in susceptible individuals.
While some mast-cell-neuron interactions can drive skin inflammation, others can establish a regulatory tone in the skin (Fig. 2). A group of skin sensory neurons, distinct from TRPV1+, express Mrgprd.71 These Mrgprd+ neurons could be maintained by Langerhans cells and promote homeostasis.72 During irritant-induced dermatitis or S. aureus-induced skin infection, the Mrgprd+ neurons were shown to protect against aberrant mast cell activation in response to chemical irritants via their glutamate release. Selective ablation of glutamate signaling on mast cells induces a hyperactive state. Administration of the Mrgprd ligand, β-alanine reduced mast cell activation in response to the Mrgprb2 agonist C48/80. In line with this, the depletion of Mrgprd+ neurons resulted in heightened SP release and hyperactivation of local mast cells via a Mrgprb2-dependent mechanism.72 Skin mast cells from Mrgprd+ neurons-depleted mice showed increased Mrgprb2 transcript and enhanced responses to C48/80. Despite the loss of skin homeostasis, the heightened mast cell activity in mice lacking Mrgprd+ neurons lead to improved control of S. aureus skin infection.72 Therefore, while SP-expressing TRPV1+ neurons activate the Mrgprb2-mast cell axis, a separate group of Mrgprd+ neurons can secrete glutamate to control this aberrant activation. We can envision that dysregulation of this balance could represent a novel disease pathway in susceptible individuals.
Mast cells, MRGPRs and pain
Unsurprisingly, mast cells have generated significant attention due to their pivotal role in inciting inflammation in response to diverse triggers. Yet, a compelling revelation is the newfound understanding that mast cells, primarily via Mrgprb2-dependent mechanisms, profoundly influence the sensory perception of cutaneous inflammatory pain. Traditionally, the conventional wisdom held that sensory neurons could elicit pain, in isolation from the participation of other cells. It was postulated that sensory neurons could directly sense bacterial products, such as formylated peptides 73 or endotoxin recognized through the TLR4 receptor 74 but also independent of TLR4, via the TPRA1 channel 75, thereby provoking pain during infections. Infection-induced pain was shown to occur independently of neutrophils and monocytes 73, and more largely, the role of these cells in driving pain in other models is also debated 76, 77. However, the contribution of mast cells remained to be explored. Emerging evidence has demonstrated the existence of neuro-mast cell mechanisms that underlie inflammation-induced pain, which may also be triggered in response to bacterial products. One model frequently employed to mimic inflammatory pain is the use of Complete Freund's Adjuvant (CFA), a suspension of inactivated mycobacterium. In this context, the work of Green et al. has demonstrated the critical role of Mrgprb2 in driving CFA-induced hyperalgesia.78 Furthermore, using a relevant model mimicking postoperative incision pain, it was observed that the depletion of mast cells expressing Mrgprb2 (Mrgprb2CreROSA2iDTR mice) resulted in a reduction in post-incision-induced thermal and mechanical hypersensitivity compared to controls. Like with CFA, this attenuation of hypersensitivity and pain was dependent on Mrgprb2, as supported by the diminished response observed in Mrgprb2−/− mice compared to controls. In addition to pain, the increased infiltration of immune cells, specifically neutrophils and monocytes, observed in the postoperative setting, as opposed to sham-treated animals, was entirely abolished in mice deficient in Mrgprb2. Mechanistically, SP-mediated activation of Mrgprb2, but not through its canonical receptor neurokinin-1 receptor (NK1R), could drive cell infiltration and the elicitation of pain. These observations emphasize the pivotal role of the mast cell-Mrgprb2 axis not only in driving immune responses in the skin but also in orchestrating the accompanying perception of pain.
In addition to SP, PACAP, another neuropeptide ligand for MRGPRX2 5 was shown to mediate migraine pain.79, 80 Specifically, using Mrgprb2Cre;ROSA26tdTomato mice, it was shown that mouse meningeal mast cells expressed Mrgprb2. Further, in a migraine model, applying C48/80 and PACAP 1-38 directly onto the dura increased mechanical facial hypersensitivity which was significantly reduced in Mrgprib2−/− mice.79 To test the role of MRGPRX2 specifically in mast cells in vivo, transgenic mice harboring MRGPRX2 (ROSA26lsl-MRGPRX2) were crossed with Mrgprb2Cre mice, generating Mrgprb2MRGPRX2 animals. In order to circumvent the potentially confounding role of endogenous Mrgprb2 in mast cells, Mrgprb2MRGPRX2 mice were crossed to Mrgprb2−/− mice to generate Mrgprb2MRGPRX2;Mrgprb2−/− mice. This targeting strategy knocked in MRGPRX2 in approximately 70% of peritoneal and meningeal mast cells. Stimulation of these peritoneal mast cells with PACAP resulted in a calcium response in those cells cultured from Mrgprb2MRGPRX2;Mrgprbr2−/− animals; however this was not seen in control animals. Importantly, in vivo, dural application of PACAP resulted in migraine-like pain behavior in MRGPRX2-overpressing mice, significantly more than in control animals. While the precise mechanisms underlying how PACAP activates Mgprb2 and MRGPRX2 on mast cells to induce migraine pain remains an area of investigation, there is a possibility that their degranulation could result in the expansion of meningeal arteries. This assumption is rooted in the observation that PACAP has been demonstrated to induce arterial dilation in rats, and this process appears to be mast-cell dependent.81 Notably, efforts aimed at targeting the conventional receptors for SP (NK1R) and PACAP (PAC1R) 82, 83, have yielded limited success in mitigating pain, including migraine pain.
In line with the importance of mast cell and Mrgprb2 in pain perception, a recent study utilizing a mouse model of alcohol withdrawal-induced headache showed that MrgprB2 significantly impacts pain perception.84 Specifically, while WT and Mrgprb2−/− mice have similar intake and preference for alcohol over water, following alcohol withdrawal, Mrgprb2−/− mice had less pain (grimace score). They performed better in physical activity (open-field) tests. Using Pirt-GCaMP3 Ca2+ imaging, the authors demonstrated that following alcohol-withdrawal, a group of small-diameter (<20 mm) and medium-diameter (20-25 mm) trigeminal neurons are spontaneously activated. However, this is significantly reduced in Mrgprb2−/− mice. Ethanol consumption and withdrawal activate the Hypothalamic-Pituitary-Adrenal (HPA) axis. This is thought to lead to enhanced corticotropin-releasing factor (CRF) levels in the plasma and dura mater. CRF can then activate mast cells through MrgprB2, inducing the release of TNFα which could sensitize TRPV1 in sensory neurons and mediate alcohol withdrawal-induced pain.84 However, CRF has also been shown to have profound analgesic effects, in part through inducing endogenous opioids such as beta-endorphins.85 The role of CRF acting through its canonical receptors (CRFR1 and CRFR2) vs. MrgprB2 seems to be distinct. For clinical targeting, it will be necessary to further understand the interplay and usage of receptor subtypes by CRF in pain perception. Overall, MRGPRX2 may serve as an alternative receptor for these pain-inducing mediators (SP, PACAP, CRF), constituting a new and promising target for managing pain.
In the last decade, our understanding of MRGPR-expressing mast cells in an immuno-pathological context has grown (Fig. 2). However, yolk-sac-derived tissue-resident mast cells, akin to resident macrophages, have homeostatic functions, and the role of Mrgpr's in this context is a domain of novel exploration.12
Lastly, while the expression of Mrgprb2/MrgprX2 is thought to be largely restricted to mast cells, a few recent studies also indicate that other cell types, like sensory neurons, eosinophils, and basophils, can express MRGPRX2.86-88 The extent to which MRGPRX2 is expressed in these at baseline and during inflammation and its function on these cells remains to be studied.
Mrgprs in dendritic cells and neutrophils
Our current understanding is that, except for mast-cell exclusive Mrgprb2/MRGPRX2, all members of the Mrgpr family are expressed in DRG sensory neurons. However, new findings are highlighting that several Mrgprs are expressed by immune cells.
Mrgpra1 is the founding member of the Mrgpr family, which was initially described in 2001, where it was shown to be activated by a variety of neuropeptides, including neuropeptide FF (NPFF), SP, and SST.2 Mrgpra1 is known to be expressed by murine DRG.89 Moreover, using in vitro cultured mouse DRG, it has been suggested that SP-elicited itch could be driven by Mrgpra1 + DRG fibers.90 Though, the biology of Mrgpra1 extends beyond neurons. A recent report has shown that it is expressed in skin CD301b+ dendritic cells (DCs), promoting allergic inflammation. 91 In response to intradermal delivery of the protease papain, skin sensory neurons release SP, which acts on adjacent CD301b+ DCs through Mrgpra1 The SP-Mrgpra1 axis promoted the migration of CD301b+ DCs to local lymph nodes (LN), as Mrgpra1−/− CD301b+ DCs displayed significantly less migration than control CD301b+ DCs. LN recruitment of CD301b+ DCs promoted papain-induced Th2 responses and the development of skin allergy.91Although papain has been shown to directly activate human mast cells 92, papain-induced itch and numbers of CD301b+ DCs in the LN remained unaffected in mast cell-deficient (KitW-sh/W-sh ) mice.91 These data demonstrate that Mrgpra1 could function to enhance type 2 immunity in the skin in response to environmental triggers.
Other members of the Mrgpra subfamily, Mrgpra2a, and Mrgpra2b, are characterized by their expression in DRG neurons2 and neutrophils 93. While the function of DRG-expressed Mrgpr2a/2b remains to be explored, a recent study demonstrated that β-defensins, secreted by keratinocytes during skin infections, can engage Mrgpr2a/2b on neutrophils to promote anti-bacterial responses.94 Mice deficient in keratinocyte-derived defensins or deficient for Mrgpr2a/2b displayed impaired bacterial clearance compared to control animals in a S. aureus skin infection model. The stimulation of neutrophils with human beta-defensin-3 (hBD3), or its mouse homolog BD14, led to neutrophil degranulation, which was abrogated in neutrophils deficient for Mrgpr2a/2b (Mrgpra2 DKO). Further, at baseline, the bacterial species composition of skin differed considerably between control animals and mice deficient in either defensins or Mrgpr2a/2b. Given the importance of the microbiome in maintaining skin homeostasis, it is likely that this pathway could participate in the pathobiology of various cutaneous conditions by regulating the skin microbiome. Moreover, as several endogenous cationic antimicrobial peptides95 including β-defensins can ligate Mrgprb2/MRGPRX2 on mast cells, Mrgpra2a/2b on neutrophils, as well as other Mrgprs 95, we hypothesize that together, this form a united front to microbial aggressors.
Mrgprs structure and signaling
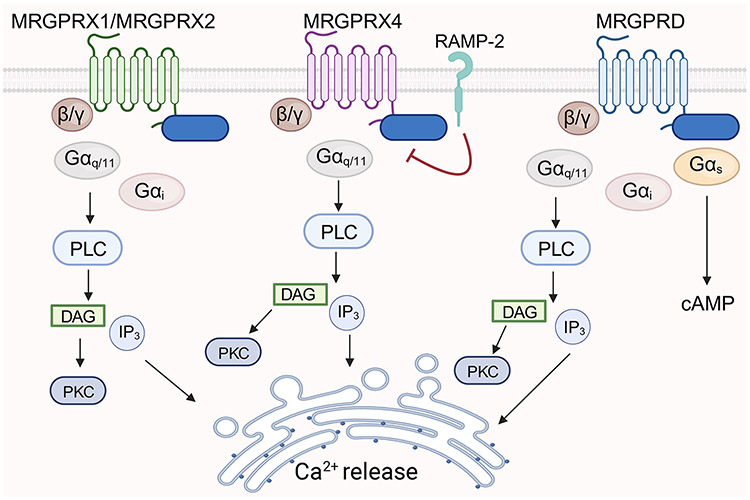
Canonically, GPCR signaling involves three heterotrimeric G proteins (Gα, Gβ, and Gγ), which associate with the receptor in an unstimulated state. Ligand binding leads to a conformational change in the GPCR, triggering binding of the Gα subunit to GTP, producing a downstream signaling cascade.96 The Gα subunit exists in 4 families: Gαs (s-stimulatory), Gαi (i-inhibitory), Gαq11 and Gβq12/13. Mrgprs can utilize all G-proteins (Gαs, Gαi, Gαq) (Fig 3). However, there is a preference for coupling to Gαq11, with little or no coupling with Gα12/13.97, 98 Gαq/11 signaling activates phospholipase C (PLC), which drives the formation of diacyl-glycerol (DAG) and inositol 1,4,5-triphosphate (IP3). IP3 activates calcium channels in the endoplasmic reticulum, leading to calcium release and DAG activates protein kinase C (PKC). Specifically, Gαs activation induces adenylyl cyclase activity, which results in cyclic AMP (cAMP) formation, leading to the activation of protein kinase A (PKA). Mrgprd is the only Mrgpr known to employ Gαs.97 Further, many Mrgprs employ the inhibitory Gαi pathway.99, 100 This pathway impedes adenylyl cyclase, suppressing cAMP production and consequently driving the inhibition of the stimulatory Gαs pathway. In addition, some Mrgprs, like MrgprA3, MrgprC11, and MRGPRX1, exhibit the utilization of Gαγ-signaling. Like various Gα signaling pathways, Gβγ can also activate PLC, PKC, and PKA. MRGPRX2 has been shown to activate to almost all G-coupled proteins but couples strongly to Gαq88, 98, as well as Gαi.98, 101
Figure 3:
Signaling mediators downstream of MRGPRs. MRGPRX1, MRGPRX2, MRGPRX4 and MRGPRD can mediate Gαq11 dependent pathways leading to phospholipase C (PLC) driven calcium responses. MRGPRX1, MRGPRX2 and MRGPRD can additionally engage Gαi and MRGPRD can recruit Gαs that drives cAMP production. In addition to G-proteins, β-arrestin signaling is engaged by MRGPRs and selectively other cell-surface receptor like RAMP-2 can inhibit MRGPRX4 expression and basal or agonist-induced activation.
In addition to G-protein mediated signaling, ligand binding to GPCR also induces recruitment of adaptor β-arrestin proteins.102 Initially characterized for their role in GPCR desensitization and downregulation, β-arrestins can also initiate signaling events, resulting in unique biological outcomes.103 GPCR agonists, termed balanced agonists, can trigger both calcium and β-arrestin pathways. However, some GPCR agonists can selectively trigger G proteins or β-arrestins and are known respectively as G protein-biased and β-arrestin-biased agonists. Like other GPCRs, signaling downstream of Mrgprs can be different depending on the nature of the agonist. Research from Ali and his team has shed light on the activation of MRGPRX2, revealing two distinct activation models that depend on the ligand involved. For example, AG-30/5C, icatibant, codeine, C48/80 and the antimicrobial peptide LL-37 induced calcium mobilization and degranulation through MRGPRX2 via a G protein-dependent pathway; their impact on β-arrestin recruitment varies significantly. Notably, unlike AG-30/5C and LL-37, codeine and C48/80 induce MRGPRX2 internalization through β-arrestin20, 26, 104, which may function to restrain hyperactivation of MRGPRX2.105 Downstream of these pathways, MRGPRX2-mediated degranulation in response to C48/80 and substance P requires ERK and PI3K in primary human mast cells.106, 107 Similarly, C48/80- and LL-37-induced chemokines also appear to rely on ERK activation in the LAD2 human mast cell line.108 Recently, a non-synonymous polymorphism (N62S) in MRGPRX2 was identified in ulcerative colitis (UC) patients.109 The serine (S) allele was shown to protect against UC. In response to various MRGPRX2 ligands, including PAMP12, the 62S variant was shown to induce significantly more β-arrestin recruitment and ERK phosphorylation but less inositol trisphosphate accumulation than the wildtype asparagine (N) allele (62N). Adrenomedullin, the PAMP12 precursor, is increased in inflamed UC tissue and it is speculated that this could drive pathology through the wildtype MRGPRX2. However, patients harboring the N62S variant, possibly due to increased β-arrestin-mediated MRGPRX2 desensitization, could be protected from aberrant activation by endogenous ligands.
Stimulation through FcεRI and MRGPRX2 are fundamentally distinct modes of mast cell activation, however they both appear to converge to the downstream activation of lysyl-tRNA synthetase (LysRS), a component of the translation machinery and a regulator of the transcription factor MITF (Microphthalmia associated-transcription factor). MITF is a well-known driver of melanocyte differentiation, but it also has a key role in driving mast cell identity. In response to FcεRI or MRGPRX2-dependent (via substance P) stimulation, the LysRS-MITF pathway drives genes that characterize mast cell identity and function like Kit and Mmcp6 as well as promote degranulation.107, 110-112 Although there may only be a partial signaling overlap between these two-mast cell activating modalities since blockade of this pathway only partially impaired MRGPRX2 activation.107 Further, MRGPRX4 has been shown to associate with receptor activating-modifying protein-2 (RAMP-2), but not other members of the RAMP family.113 RAMP-2 reduces the surface expression of MRGPRX4 (Fig. 3), as well as inhibits basal and agonist-induced signaling driven by the MRGPRX4 ligands nateglinide114 and bile acids.115, 116
Although the various MRGPRs may use similar downstream signaling effectors, we know that MRGPRs exhibit responsiveness to a wide range of structurally distinct agonists, encompassing both small molecules and large peptides. This suggested the presence of distinct extracellular ligand-binding sites within these GPCRs. This was confirmed by CryoEM 3D structural studies of MRGPRX1, MRGPRX2, MRGPRX4 and MRGPRD that have demonstrated diverse residue composition and charge distribution in their extracellular binding sites.98, 100, 117-119 This is well illustrated when comparing MRGPRX2 and MRGPRX4. The primary binding pocket between these two MRGPRs was found to be quite different. The negatively charged binding site of MRGPRX2 explains why many of its agonists are positively charged. Conversely, MRGPRX4 does not have a negatively charged binding pocket and has several basic residues that confer a positive charge and a preference for negatively charged ligands like bile acids. Lastly, due to the lack of selective agonists for MRGPRX3, MRGPRE, MRGPRF and MRGPRG, the downstream signaling effectors to these are unknown.
Targeting Mrgprs in the clinic
Various clinical trials to block MRGPRs are underway (Table 1). MRGPRX4 is being targeted as it drives itch in response to pathological levels of bile acids and heme metabolites115, 116, as seen in patients with chronic liver and kidney diseases. Also, the blockade of MRGPRX2 is being studied for skin diseases where mast cell dysregulation is thought to play a pathological role. Here, we will discuss the human data supporting these studies.
Table 1:
Drug development targeting MRGPRs
| Target | Company | Disease | Phase | Comment |
|---|---|---|---|---|
| MRGPRX2 | Escient Pharmaceuticals | AD and CU | Phase 1 completed Phase 2 ongoing for CU (NCT6077773) Phase 2 ongoing for AD (NCT06144424) |
Safety trials found to be safe and well-tolerated |
| MRGPRX2 | Evommune | Urticaria Interstitial Cystitis | Preclinical | N.A. |
| MRGPRX4 | Escient Pharmaceuticals | Cholestatic/Uremic Pruritus | Phase 1 completed (NCT04510090) Phase 2 ongoing (NCT05525520) |
Safety trials found to be safe and well-tolerated |
In AD patients, nerve fibers show positivity for substance P are elevated as compared to controls.120 Further, another study showed upregulation of TAC1 (the gene encoding SP) in itchy skin, that positively correlated with itch intensity in AD patients.121 Also, the number of mast cells and production of PAMP12 by keratinocytes was enhanced in patients with ACD (n=5) compared to controls (n=5).49 Urticaria, another common and heterogeneous skin condition characterized by itching, hives, and inflammation, is also thought to be driven by aberrant mast cell activity. 122 While acute urticaria (≤ 6 weeks) has identifiable triggers (food, drugs), chronic urticaria or CU (> 6 weeks-1 year) can be both inducible and spontaneous with unknown etiology.123 Degranulation of mast cells releases inflammatory mediators, including histamine, that result in sensory nerve activation, vasodilatation, plasma extravasation, and cellular recruitment, and these sequelae underlie the development of key features of urticaria like hives, itch, and angioedema.122 In a small cohort of CU patients, staining of skin sections showed that in severe CU patients (n=9), the total number of mast cells was not increased compared to nonatopic controls (n=13). However, the frequency of MRGPRX2+ mast cells was higher (47.0% ± 6.9%) as compared to controls (21.6% ± 7.8%).124 Further, eosinophils, which also accumulate in CU patients, are thought to play a role via the production of major basic protein and eosinophil peroxidase, which were shown to activate mast cells to release histamine in an MRGPRX2-dependent manner in cultured skin mast cells.124 Further, in a cohort of mild CU patients (n=10), it was shown that the skin test response, as measured by the mean wheal diameter to two MRGPRX2 activating drugs, atracurium and icatibant, but not histamine, was significantly higher compared to controls (n=10).125 These 2 studies, albeit with a small number of patients, show promise for targeting MRGPRX2 in CU patients. In addition to having greater sensitivities to drugs known to activate MRGPRX2, some CU patients show elevated levels of the MRGPRX2 agonist substance P.126, 127
While Mrgprb2 and MRGPRX2 expression in mouse and human mast cells respectively shows a dichotomy between connective tissue and mucosal sites15, there exists a broad diversity of mast cell states in humans, as recently shown.16, 128 Therefore, the relative expression of MRGPRX2 in a continuum of mast cell states may be varied. This complexity could dictate the efficacy of the MRGPRX2 antagonists currently in the clinic.
Concluding remarks
So far, we know that Mrgprs are expressed by sensory neurons, mast cells, neutrophils, and dendritic cells, where they regulate their function in various scenarios, including infection, allergy, and somatosensation. Several essential aspects of their biology remain unknown: 1) what drives the expression of Mrgprs on immune cells in a restricted manner? Is their surface expression modulated during inflammation or by disease-defining modifiers like environmental exposures, nutritional states, biome composition? 2) The genes encoding Mgrprs are highly polymorphic, and the function of these mutations on immune cell function and disease is largely unknown, 3) What is the role of immune-expressed Mrgprs in developing immune cell lineages and 4) It is important to appreciate that Mrgprs can function independently of their ligands, as they exhibit elevated basal activity.129 What is the functional consequence of this basal activity?
We anticipate that our knowledge of the immune functions of Mrgprs is not only going to expand but will also lead to the design of new clinical trials and the development of novel future therapeutics.
The Mas-related G protein-coupled receptor (Mrgpr) family of orphan GPRCs are crucial for somatosensation, but more recently they have been shown to substantially impact immune cell function. Gour and Dong review the roles of Mrgprs expressed on immune cells and how they govern immune and neuro-immune pathways underlying various physiological and pathological states.
Acknowledgement
We thank Dr. Stephane Lajoie (JHU) for input, discussions and corrections. This work was supported by the Howard Hughes Medical Institute, and NIH 2R37NS054791 to X.D. Illustrations were made using BioRender.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
X.D. is the scientific founder and consultant of Escient Pharmaceuticals., a pharmaceutical company developing drugs targeting Mrgprs. Dong lab is collaborating with GlaxoSmithKline (GSK) on Mrgpr-related project. N.G has served as consultant for Escient Pharmaceuticals. X.D. and N.G. are inventors of patent applications on Mrgpr-related works.
References:
- 1.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139(7):1353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106(5):619–32. [DOI] [PubMed] [Google Scholar]
- 3.Lembo PM, Grazzini E, Groblewski T, O'Donnell D, Roy MO, Zhang J, et al. Proenkephalin A gene products activate a new family of sensory neuron--specific GPCRs. Nat Neurosci. 2002;5(3):201–9. [DOI] [PubMed] [Google Scholar]
- 4.Solinski HJ, Gudermann T, Breit A. Pharmacology and signaling of MAS-related G protein-coupled receptors. Pharmacol Rev. 2014;66(3):570–97. [DOI] [PubMed] [Google Scholar]
- 5.Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. 2006;349(4):1322–8. [DOI] [PubMed] [Google Scholar]
- 6.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112(4):946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagermark O, Hokfelt T, Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1978;71(4):233–5. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs BF, Wierecky J, Welker P, Henz BM, Wolff HH, Grabbe J. Human skin mast cells rapidly release preformed and newly generated TNF-alpha and IL-8 following stimulation with anti-IgE and other secretagogues. Exp Dermatol. 2001;10(5):312–20. [DOI] [PubMed] [Google Scholar]
- 10.Caulfield JP, el-Lati S, Thomas G, Church MK. Dissociated human foreskin mast cells degranulate in response to anti-IgE and substance P. Lab Invest. 1990;63(4):502–10. [PubMed] [Google Scholar]
- 11.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519(7542):237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentek R, Ghigo C, Hoeffel G, Bulle MJ, Msallam R, Gautier G, et al. Hemogenic Endothelial Fate Mapping Reveals Dual Developmental Origin of Mast Cells. Immunity. 2018;48(6):1160–71 e5. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Liu S, Xu J, Zhang X, Han D, Liu J, et al. Adult Connective Tissue-Resident Mast Cells Originate from Late Erythro-Myeloid Progenitors. Immunity. 2018;49(4):640–53 e5. [DOI] [PubMed] [Google Scholar]
- 14.Serhan N, Basso L, Sibilano R, Petitfils C, Meixiong J, Bonnart C, et al. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat Immunol. 2019;20(11):1435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plum T, Wang X, Rettel M, Krijgsveld J, Feyerabend TB, Rodewald HR. Human Mast Cell Proteome Reveals Unique Lineage, Putative Functions, and Structural Basis for Cell Ablation. Immunity. 2020;52(2):404–16 e5. [DOI] [PubMed] [Google Scholar]
- 16.Tauber M, Basso L, Martin J, Bostan L, Pinto MM, Thierry GR, et al. Landscape of mast cell populations across organs in mice and humans. J Exp Med. 2023;220(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mencarelli A, Gunawan M, Yong KSM, Bist P, Tan WWS, Tan SY, et al. A humanized mouse model to study mast cells mediated cutaneous adverse drug reactions. J Leukoc Biol. 2020;107(5):797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al Hamwi G, Riedel YK, Clemens S, Namasivayam V, Thimm D, Muller CE. MAS-related G protein-coupled receptors X (MRGPRX): Orphan GPCRs with potential as targets for future drugs. Pharmacol Ther. 2022;238:108259. [DOI] [PubMed] [Google Scholar]
- 19.Lansu K, Karpiak J, Liu J, Huang XP, McCorvy JD, Kroeze WK, et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol. 2017;13(5):529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babina M, Wang Z, Roy S, Guhl S, Franke K, Artuc M, et al. MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through beta-Arrestin and Lack of Correlation with the FcepsilonRI Pathway. J Invest Dermatol. 2021;141(5):1286–96 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azimi E, Reddy VB, Lerner EA. Brief communication: MRGPRX2, atopic dermatitis and red man syndrome. Itch (Phila). 2017;2(1):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong GW, Zhuo L, Kimata K, Lam BK, Satoh N, Stevens RL. Ancient origin of mast cells. Biochem Biophys Res Commun. 2014;451(2):314–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown KL, Hancock RE. Cationic host defense (antimicrobial) peptides. Curr Opin Immunol. 2006;18(1):24–30. [DOI] [PubMed] [Google Scholar]
- 24.Mookherjee N, Anderson MA, Haagsman HP, Davidson DJ. Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov. 2020;19(5):311–32. [DOI] [PubMed] [Google Scholar]
- 25.Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758(9):1408–25. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian H, Gupta K, Guo Q, Price R, Ali H. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization. J Biol Chem. 2011;286(52):44739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian H, Gupta K, Lee D, Bayir AK, Ahn H, Ali H. beta-Defensins activate human mast cells via Mas-related gene X2. J Immunol. 2013;191(1):345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piliponsky AM, Romani L. The contribution of mast cells to bacterial and fungal infection immunity. Immunol Rev. 2018;282(1):188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall JS, Portales-Cervantes L, Leong E. Mast Cell Responses to Viruses and Pathogen Products. Int J Mol Sci. 2019;20(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krystel-Whittemore M, Dileepan KN, Wood JG. Mast Cell: A Multi-Functional Master Cell. Front Immunol. 2015;6:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170(5):2274–8. [DOI] [PubMed] [Google Scholar]
- 32.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10(6):440–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arifuzzaman M, Mobley YR, Choi HW, Bist P, Salinas CA, Brown ZD, et al. MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci Adv. 2019;5(1):eaav0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rutherford ST, Bassler BL. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med. 2012;2(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pundir P, Liu R, Vasavda C, Serhan N, Limjunyawong N, Yee R, et al. A Connective Tissue Mast-Cell-Specific Receptor Detects Bacterial Quorum-Sensing Molecules and Mediates Antibacterial Immunity. Cell Host Microbe. 2019;26(1):114–22 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rainer BM, Kang S, Chien AL. Rosacea: Epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9(1):e1361574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daou H, Paradiso M, Hennessy K, Seminario-Vidal L. Rosacea and the Microbiome: A Systematic Review. Dermatol Ther (Heidelb). 2021;11(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muto Y, Wang Z, Vanderberghe M, Two A, Gallo RL, Di Nardo A. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J Invest Dermatol. 2014;134(11):2728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aroni K, Tsagroni E, Kavantzas N, Patsouris E, Ioannidis E. A study of the pathogenesis of rosacea: how angiogenesis and mast cells may participate in a complex multifactorial process. Arch Dermatol Res. 2008;300(3):125–31. [DOI] [PubMed] [Google Scholar]
- 40.Roy S, Alkanfari I, Chaki S, Ali H. Role of MrgprB2 in Rosacea-Like Inflammation in Mice: Modulation by beta-Arrestin 2. J Invest Dermatol. 2022;142(11):2988–97 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Yang H, Han Y, Yin S, Shen B, Wu Y, et al. Tick peptides evoke itch by activating MrgprC11/MRGPRX1 to sensitize TRPV1 in pruriceptors. J Allergy Clin Immunol. 2021;147(6):2236–48 e16. [DOI] [PubMed] [Google Scholar]
- 42.Yosipovitch G, Rosen JD, Hashimoto T. Itch: From mechanism to (novel) therapeutic approaches. J Allergy Clin Immunol. 2018;142(5):1375–90. [DOI] [PubMed] [Google Scholar]
- 43.Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27(9):2331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosa AC, Fantozzi R. The role of histamine in neurogenic inflammation. Br J Pharmacol. 2013;170(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmelz M, Petersen LJ. Neurogenic inflammation in human and rodent skin. News Physiol Sci. 2001;16:33–7. [DOI] [PubMed] [Google Scholar]
- 46.Garibyan L, Rheingold CG, Lerner EA. Understanding the pathophysiology of itch. Dermatol Ther. 2013;26(2):84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu H, Usoskin D, Nagi SS, Hu Y, Kupari J, Bouchatta O, et al. Single-Soma Deep RNA Sequencing of Human Dorsal Root Ganglion Neurons Reveals Novel Molecular and Cellular Mechanisms Underlying Somatosensation. bioRxiv. 2023:2023.03.17.533207. [Google Scholar]
- 48.Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18(1):145–53. [DOI] [PubMed] [Google Scholar]
- 49.Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, et al. Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-histaminergic Itch. Immunity. 2019;50(5):1163–71 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang F, Trier AM, Li F, Kim S, Chen Z, Chai JN, et al. A basophil-neuronal axis promotes itch. Cell. 2021;184(2):422–40 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mack MR, Brestoff JR, Berrien-Elliott MM, Trier AM, Yang TB, McCullen M, et al. Blood natural killer cell deficiency reveals an immunotherapy strategy for atopic dermatitis. Sci Transl Med. 2020;12(532). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaplan DH, Igyarto BZ, Gaspari AA. Early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol. 2012;12(2):114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neis MM, Peters B, Dreuw A, Wenzel J, Bieber T, Mauch C, et al. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol. 2006;118(4):930–7. [DOI] [PubMed] [Google Scholar]
- 54.Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23(15):6176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kempkes C, Buddenkotte J, Cevikbas F, Buhl T, Steinhoff M. Role of PAR-2 in Neuroimmune Communication and Itch. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Frontiers in Neuroscience. Boca Raton (FL)2014. [PubMed] [Google Scholar]
- 56.Owen JL, Vakharia PP, Silverberg JI. The Role and Diagnosis of Allergic Contact Dermatitis in Patients with Atopic Dermatitis. Am J Clin Dermatol. 2018;19(3):293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomsen SF. Atopic dermatitis: natural history, diagnosis, and treatment. ISRN Allergy. 2014;2014:354250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Dermatitis. J Clin Cell Immunol. 2011;2(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y. Mast cells in atopic dermatitis. Curr Opin Immunol. 2009;21(6):666–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paller AS, Kong HH, Seed P, Naik S, Scharschmidt TC, Gallo RL, et al. The microbiome in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ogonowska P, Gilaberte Y, Baranska-Rybak W, Nakonieczna J. Colonization With Staphylococcus aureus in Atopic Dermatitis Patients: Attempts to Reveal the Unknown. Front Microbiol. 2020;11:567090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reddy VB, Lerner EA. Activation of mas-related G-protein-coupled receptors by the house dust mite cysteine protease Der p1 provides a new mechanism linking allergy and inflammation. J Biol Chem. 2017;292(42):17399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snelgrove RJ, Gregory LG, Peiro T, Akthar S, Campbell GA, Walker SA, et al. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134(3):583–92 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lefrancais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, et al. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci U S A. 2014;111(43):15502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trier AM, Mack MR, Fredman A, Tamari M, Ver Heul AM, Zhao Y, et al. IL-33 signaling in sensory neurons promotes dry skin itch. J Allergy Clin Immunol. 2022;149(4): 1473–80 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang G, Lehtimaki S, et al. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol. 2012;132(5):1392–400. [DOI] [PubMed] [Google Scholar]
- 67.Nakamura N, Tamagawa-Mineoka R, Yasuike R, Masuda K, Matsunaka H, Murakami Y, et al. Stratum corneum interleukin-33 expressions correlate with the degree of lichenification and pruritus in atopic dermatitis lesions. Clin Immunol. 2019;201:1–3. [DOI] [PubMed] [Google Scholar]
- 68.Li C, Maillet I, Mackowiak C, Viala C, Di Padova F, Li M, et al. Experimental atopic dermatitis depends on IL-33R signaling via MyD88 in dendritic cells. Cell Death Dis. 2017;8(4):e2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen YL, Gutowska-Owsiak D, Hardman CS, Westmoreland M, MacKenzie T, Cifuentes L, et al. Proof-of-concept clinical trial of etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci Transl Med. 2019;11(515). [DOI] [PubMed] [Google Scholar]
- 70.Keith YH, Egawa G, Honda T, Kabashima K. Mast cells in type 2 skin inflammation: Maintenance and function. Eur J Immunol. 2023;53(8):e2250359. [DOI] [PubMed] [Google Scholar]
- 71.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106(22):9075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang S, Edwards TN, Chaudhri VK, Wu J, Cohen JA, Hirai T, et al. Nonpeptidergic neurons suppress mast cells via glutamate to maintain skin homeostasis. Cell. 2021;184(8):2151–66 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501 (7465):52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin JJ, Du Y, Cai WK, Kuang R, Chang T, Zhang Z, et al. Toll-like receptor 4 signaling in neurons of trigeminal ganglion contributes to nociception induced by acute pulpitis in rats. Sci Rep. 2015;5:12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 2014;5:3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Segelcke D, Pradier B, Reichl S, Schafer LC, Pogatzki-Zahn EM. Investigating the Role of Ly6G(+) Neutrophils in Incisional and Inflammatory Pain by Multidimensional Pain-Related Behavioral Assessments: Bridging the Translational Gap. Front Pain Res (Lausanne). 2021;2:735838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sahbaie P, Li X, Shi X, Clark JD. Roles of Gr-1+ leukocytes in postincisional nociceptive sensitization and inflammation. Anesthesiology. 2012;117(3):602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. A Mast-Cell-Specific Receptor Mediates Neurogenic Inflammation and Pain. Neuron. 2019;101(3):412–20 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sbei S, Moncrief T, Limjunyawong N, Zeng Y, Green DP. PACAP activates MRGPRX2 on meningeal mast cells to drive migraine-like pain. Sci Rep. 2023;13(1):12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schytz HW, Birk S, Wienecke T, Kruuse C, Olesen J, Ashina M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain. 2009;132(Pt 1):16–25. [DOI] [PubMed] [Google Scholar]
- 81.Bhatt DK, Gupta S, Olesen J, Jansen-Olesen I. PACAP-38 infusion causes sustained vasodilation of the middle meningeal artery in the rat: possible involvement of mast cells. Cephalalgia. 2014;34(11):877–86. [DOI] [PubMed] [Google Scholar]
- 82.Navratilova E, Porreca F. Substance P and Inflammatory Pain: Getting It Wrong and Right Simultaneously. Neuron. 2019;101(3):353–5. [DOI] [PubMed] [Google Scholar]
- 83.Ashina M, Dolezil D, Bonner JH, Zhou L, Klatt J, Picard H, et al. A phase 2, randomized, double-blind, placebo-controlled trial of AMG 301, a pituitary adenylate cyclase-activating polypeptide PAC1 receptor monoclonal antibody for migraine prevention. Cephalalgia. 2021;41(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Son H, Zhang Y, Shannonhouse J, Ishida H, Gomez R, Kim YS. Mast-cell-specific receptor mediates alcohol-withdrawal-associated headache in male mice. Neuron. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lariviere WR, Melzack R. The role of corticotropin-releasing factor in pain and analgesia. Pain. 2000;84(1):1–12. [DOI] [PubMed] [Google Scholar]
- 86.Wedi B, Gehring M, Kapp A. The pseudoallergen receptor MRGPRX2 on peripheral blood basophils and eosinophils: Expression and function. Allergy. 2020;75(9):2229–42. [DOI] [PubMed] [Google Scholar]
- 87.Toscano A, Elst J, Van Gasse AL, Beyens M, van der Poorten ML, Bridts CH, et al. Mas-related G protein-coupled receptor MRGPRX2 in human basophils: Expression and functional studies. Front Immunol. 2022;13:1026304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Robas N, Mead E, Fidock M. MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J Biol Chem. 2003;278(45):44400–4. [DOI] [PubMed] [Google Scholar]
- 89.Meixiong J, Vasavda C, Green D, Zheng Q, Qi L, Kwatra SG, et al. Identification of a bilirubin receptor that may mediate a component of cholestatic itch. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Azimi E, Reddy VB, Pereira PJS, Talbot S, Woolf CJ, Lerner EA. Substance P activates Mas-related G protein-coupled receptors to induce itch. J Allergy Clin Immunol. 2017;140(2):447–53 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perner C, Flayer CH, Zhu X, Aderhold PA, Dewan ZNA, Voisin T, et al. Substance P Release by Sensory Neurons Triggers Dendritic Cell Migration and Initiates the Type-2 Immune Response to Allergens. Immunity. 2020;53(5):1063–77 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seaf M, Ben-Zimra M, Mankuta D, Dayan N, Levi-Schaffer F. Papain Activates Human Mast Cells to Release Proinflammatory Mediators via its Enzymatic Activity. J Invest Dermatol. 2016;136(7):1523–5. [DOI] [PubMed] [Google Scholar]
- 93.Ericson JA, Duffau P, Yasuda K, Ortiz-Lopez A, Rothamel K, Rifkin IR, et al. Gene expression during the generation and activation of mouse neutrophils: implication of novel functional and regulatory pathways. PLoS One. 2014;9(10):e108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dong X, Limjunyawong N, Sypek EI, Wang G, Ortines RV, Youn C, et al. Keratinocyte-derived defensins activate neutrophil-specific receptors Mrgpra2a/b to prevent skin dysbiosis and bacterial infection. Immunity. 2022;55(9):1645–62 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tseng PY, Hoon MA. Specific beta-Defensins Stimulate Pruritus through Activation of Sensory Neurons. J Invest Dermatol. 2022;142(3 Pt A):594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weis WI, Kobilka BK. The Molecular Basis of G Protein-Coupled Receptor Activation. Annu Rev Biochem. 2018;87:897–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Steele HR, Han L. The signaling pathway and polymorphisms of Mrgprs. Neurosci Lett. 2021;744:135562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cao C, Kang HJ, Singh I, Chen H, Zhang C, Ye W, et al. Structure, function and pharmacology of human itch GPCRs. Nature. 2021;600(7887):170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ogasawara H, Noguchi M. Therapeutic Potential of MRGPRX2 Inhibitors on Mast Cells. Cells. 2021;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo L, Zhang Y, Fang G, Tie L, Zhuang Y, Xue C, et al. Ligand recognition and G protein coupling of the human itch receptor MRGPRX1. Nat Commun. 2023;14(1):5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chompunud Na Ayudhya C, Roy S, Alkanfari I, Ganguly A, Ali H. Identification of Gain and Loss of Function Missense Variants in MRGPRX2's Transmembrane and Intracellular Domains for Mast Cell Activation by Substance P. Int J Mol Sci. 2019;20(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gurevich VV, Gurevich EV. GPCR Signaling Regulation: The Role of GRKs and Arrestins. Front Pharmacol. 2019;10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jean-Charles PY, Kaur S, Shenoy SK. G Protein-Coupled Receptor Signaling Through beta-Arrestin-Dependent Mechanisms. J Cardiovasc Pharmacol. 2017;70(3):142–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roy S, Ganguly A, Haque M, Ali H. Angiogenic Host Defense Peptide AG-30/5C and Bradykinin B(2) Receptor Antagonist Icatibant Are G Protein Biased Agonists for MRGPRX2 in Mast Cells. J Immunol. 2019;202(4):1229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Z, Li Z, Bal G, Franke K, Zuberbier T, Babina M. beta-arrestin-1 and beta-arrestin-2 Restrain MRGPRX2-Triggered Degranulation and ERK1/2 Activation in Human Skin Mast Cells. Front Allergy. 2022;3:930233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Z, Franke K, Bal G, Li Z, Zuberbier T, Babina M. MRGPRX2-Mediated Degranulation of Human Skin Mast Cells Requires the Operation of G(alphai), G(alphaq), Ca++ Channels, ERK1/2 and PI3K-Interconnection between Early and Late Signaling. Cells. 2022;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guo Y, Olle L, Proano-Perez E, Aparicio C, Guerrero M, Munoz-Cano R, et al. MRGPRX2 signaling involves the Lysyl-tRNA synthetase and MITF pathway. Front Immunol. 2023;14:1154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Callahan BN, Kammala AK, Syed M, Yang C, Occhiuto CJ, Nellutla R, et al. Osthole, a Natural Plant Derivative Inhibits MRGPRX2 Induced Mast Cell Responses. Front Immunol. 2020;11:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen E, Chuang LS, Giri M, Villaverde N, Hsu NY, Sabic K, et al. Inflamed Ulcerative Colitis Regions Associated With MRGPRX2-Mediated Mast Cell Degranulation and Cell Activation Modules, Defining a New Therapeutic Target. Gastroenterology. 2021;160(5):1709–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee YN, Nechushtan H, Figov N, Razin E. The function of lysyl-tRNA synthetase and Ap4A as signaling regulators of MITF activity in FcepsilonRI-activated mast cells. Immunity. 2004;20(2):145–51. [DOI] [PubMed] [Google Scholar]
- 111.Ofir-Birin Y, Fang P, Bennett SP, Zhang HM, Wang J, Rachmin I, et al. Structural switch of lysyl-tRNA synthetase between translation and transcription. Mol Cell. 2013;49(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Proano-Perez E, Olle L, Guo Y, Aparicio C, Guerrero M, Munoz-Cano R, et al. MITF Downregulation Induces Death in Human Mast Cell Leukemia Cells and Impairs IgE-Dependent Degranulation. Int J Mol Sci. 2023;24(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kotliar IB, Ceraudo E, Kemelmakher-Liben K, Oren DA, Lorenzen E, Dodig-Crnkovic T, et al. Itch receptor MRGPRX4 interacts with the receptor activity-modifying proteins. J Biol Chem. 2023;299(5):104664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kroeze WK, Sassano MF, Huang XP, Lansu K, McCorvy JD, Giguere PM, et al. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol. 2015;22(5):362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yu H, Zhao T, Liu S, Wu Q, Johnson O, Wu Z, et al. MRGPRX4 is a bile acid receptor for human cholestatic itch. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meixiong J, Vasavda C, Snyder SH, Dong X. MRGPRX4 is a G protein-coupled receptor activated by bile acids that may contribute to cholestatic pruritus. Proc Natl Acad Sci U S A. 2019;116(21):10525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Suzuki S, Iida M, Hiroaki Y, Tanaka K, Kawamoto A, Kato T, et al. Structural insight into the activation mechanism of MrgD with heterotrimeric Gi-protein revealed by cryo-EM. Commun Biol. 2022;5(1):707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu Y, Cao C, Huang XP, Gumpper RH, Rachman MM, Shih SL, et al. Ligand recognition and allosteric modulation of the human MRGPRX1 receptor. Nat Chem Biol. 2023;19(4):416–22. [DOI] [PubMed] [Google Scholar]
- 119.Yang F, Guo L, Li Y, Wang G, Wang J, Zhang C, et al. Structure, function and pharmacology of human itch receptor complexes. Nature. 2021;600(7887): 164–9. [DOI] [PubMed] [Google Scholar]
- 120.Jarvikallio A, Harvima IT, Naukkarinen A. Mast cells, nerves and neuropeptides in atopic dermatitis and nummular eczema. Arch Dermatol Res. 2003;295(1):2–7. [DOI] [PubMed] [Google Scholar]
- 121.Nattkemper LA, Tey HL, Valdes-Rodriguez R, Lee H, Mollanazar NK, Albornoz C, et al. The Genetics of Chronic Itch: Gene Expression in the Skin of Patients with Atopic Dermatitis and Psoriasis with Severe Itch. J Invest Dermatol. 2018;138(6):1311–7. [DOI] [PubMed] [Google Scholar]
- 122.Kolkhir P, Gimenez-Arnau AM, Kulthanan K, Peter J, Metz M, Maurer M. Urticaria. Nat Rev Dis Primers. 2022;8(1):61. [DOI] [PubMed] [Google Scholar]
- 123.Radonjic-Hoesli S, Hofmeier KS, Micaletto S, Schmid-Grendelmeier P, Bircher A, Simon D. Urticaria and Angioedema: an Update on Classification and Pathogenesis. Clin Rev Allergy Immunol. 2018;54(1):88–101. [DOI] [PubMed] [Google Scholar]
- 124.Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol. 2014;134(3):622–33 e9. [DOI] [PubMed] [Google Scholar]
- 125.Shtessel M, Limjunyawong N, Oliver ET, Chichester K, Gao L, Dong X, et al. MRGPRX2 Activation Causes Increased Skin Reactivity in Patients with Chronic Spontaneous Urticaria. J Invest Dermatol. 2021;141(3):678–81 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tedeschi A, Lorini M, Asero R. No evidence of increased serum substance P levels in chronic urticaria patients with and without demonstrable circulating vasoactive factors. Clin Exp Dermatol. 2005;30(2):171–5. [DOI] [PubMed] [Google Scholar]
- 127.Metz M, Krull C, Hawro T, Saluja R, Groffik A, Stanger C, et al. Substance P is upregulated in the serum of patients with chronic spontaneous urticaria. J Invest Dermatol. 2014;134(11):2833–6. [DOI] [PubMed] [Google Scholar]
- 128.Dwyer DF, Ordovas-Montanes J, Allon SJ, Buchheit KM, Vukovic M, Derakhshan T, et al. Human airway mast cells proliferate and acquire distinct inflammation-driven phenotypes during type 2 inflammation. Sci Immunol. 2021;6(56). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zeghal M, Laroche G, Freitas JD, Wang R, Giguere PM. Profiling of basal and ligand-dependent GPCR activities by means of a polyvalent cell-based high-throughput platform. Nat Commun. 2023;14(1):3684. [DOI] [PMC free article] [PubMed] [Google Scholar]