Summary
Background
Mpox recently infected more than 87,000 people, raising concerns about our preparedness against this emerging viral pathogen. Licensed and approved for mpox, the JYNNEOS vaccine has fewer side effects than previous smallpox vaccines and demonstrates immunogenicity against monkeypox in animal models. This study aims to inform human immune responses to JYNNEOS vaccination compared to mpox-induced immunity.
Methods
PBMCs and sera were obtained from JYNNEOS recipients and mpox-infected people. We examined the polyclonal serum (ELISA), single B cell (heavy chain gene and transcriptome data) antibody repertoires and T cells responses (AIM and ICS assays) induced by the JYNNEOS vaccine compared to mpox infection.
Findings
Gene-level plasmablast and antibody responses were negligible and JYNNEOS vaccinee sera displayed moderate binding to recombinant orthopoxviral proteins and native proteins from the 2022 monkeypox outbreak strain. In contrast, recent mpox infection (within 20–102 days) induced robust serum antibody responses to A29L, A35R, E8L, A30L, A27L, A33R, B18R, and to native monkeypox proteins from a viral isolate obtained during the 2022 outbreak. JYNNEOS vaccine recipients presented robust orthopoxviral CD4 and CD8 T cell responses.
Interpretation
Infection with mpox results in robust B and T cell responses, while immunization with JYNNEOS elicited more robust T cell responses. These data can help inform vaccine design and policies for preventing mpox in humans.
Funding
Research reported in this publication was supported, in part, by NCI/NIH (U54CA267776 to CHC), NIAID/NIH (U19AI168631 to VS), and contract number 75N93019C00065 (AS). In addition, institutional funds from the Icahn School of Medicine partially supported this research.
Introduction
Mpox is a zoonotic infection caused by an orthopoxvirus (OPXV). This viral genus includes smallpox, which has dramatically affected humanity for centuries. As we progressed toward smallpox eradication in the 1980s, monkeypox virus (which causes mpox) became the most concerning OPXV for humans, causing multiple outbreaks in Central and West Africa since 1970, and its first outbreak outside Africa (in the US) in 2003. The 2022 global mpox outbreak infected > 87,000 people, providing an unprecedented opportunity to study human immune responses to mpox outside Africa. While the World Health Organization reported that disease incidence has decreased by 90% compared to the outbreak’s peak in July 2022, mpox transmission continues in Africa and Latin America.
The recently implemented US mpox vaccination strategy made two smallpox vaccines available to healthcare workers and individuals 18 years of age and older: ACAM2000 (a second-generation, live-replicating vaccinia strain) and JYNNEOS (a third generation, live non-replicating modified Ankara strain of vaccinia). Licensed in the US in 2019, JYNNEOS (also known as Imvanex and Imvamune) was designed to elicit fewer side effects than ACAM2000.
A majority of mpox cases during the 2022 outbreak were observed in gay, bisexual, and other men who have sex with men, with few cases observed in women or children (1). To date, robust data demonstrating JYNNEOS protection has only been shown in animal models (2). Initial clinical trial data demonstrated that JYNNEOS efficacy in humans could reach 78% (3), 87% (4), or 86% (5). Unpublished data also demonstrate a decrease in the incidence of mpox after JYNNEOS vaccination (0.010 incidence rate ratio) (1). However, most published studies did not account for behavioral differences between vaccinated and unvaccinated individuals, which could affect the efficacy data.
Human smallpox vaccination responses are highly mediated by antibodies (6,7) which can remain elevated for years (7), but it is unclear how JYNNEOS shapes the human antibody repertoire or how efficiently the antibodies of vaccinated or convalescent participants bind to proteins from the strain of monkeypox virus causing the 2022 outbreak.
Given the prolonged antibody responses of older smallpox vaccines and high sequence homology among common orthopoxviruses (OPXVs) (8), JYNNEOS was expected to elicit neutralizing immune responses against the 2022 outbreak strain. However, initial data suggests low humoral immunogenicity and virus neutralization activity (9,10).
We aim to examine the human antibody repertoire induced by JYNNEOS in US adult vaccinees at the B cell, T cell, and polyclonal antibody serum levels. This is the first report of a single-cell human antibody repertoire induced by mpox vaccination and the first systematic examination of T cell activation after JYNNEOS vaccination.
Methods
Participant demographic and sample collection
Protocols for clinical specimen collection from convalescent and post-vaccination individuals by the Personalized Virology Initiative were reviewed and approved by the Mount Sinai Hospital Institutional Review Board (IRB-16–16772, IRB-16–00791). All participants provided written informed consent before specimens and clinical information was collected. Permissions to store and share biospecimen were also obtained from all participants. All specimens were coded before processing and analysis. Whole blood was collected through venipuncture into serum separator tubes and ethylenediaminetetraacetic acid tubes. Peripheral blood mononuclear cell (PBMC) isolation was performed by density gradient centrifugation using SepMate tubes (STEMCELL Technologies, Cambridge, MA, USA). Serum and plasma samples were stored at −80 °C and PBMC samples were cryo-preserved in liquid nitrogen until analysis.
Samples were collected from 16 participants. Ten were vaccinated with JYNNEOS and six were diagnosed with mpox infection (table 1). None of the participants had prior orthopox exposure. Blood samples were collected from seven vaccinees before vaccination to serve as a baseline. Vaccinee samples were collected on average 19 days post-dose one (range: 6–33 days) and 18 days post-dose two (range: 5–40 days). Two vaccinees chose to only receive one dose of JYNNEOS. Mpox convalescent samples were collected on average 55 days post-infection (range: 20–102 days; (table 1)). Five of the six convalescent individuals were HIV positive with well-controlled viral replication and CD4+ T cells above 200mm/ml. All the mpox-infected participants experienced mild mpox disease, which was self-limiting and did not require antiviral treatment or hospitalization. None of the vaccinees enrolled self-reported bloodborne pathogens. We did not collect information on co-morbidities.
Table 1.
Partcipant demographics and immunization routes of JYNNEOS vaccine recipients and mpox- convalescent participants
| Sample ID | Sex | Age | Race | Orthopoxviral exposure |
Days between doses | IR 1st Dose | IR 2nd Dose |
|---|---|---|---|---|---|---|---|
|
| |||||||
| V1 | M | 21–30 | C | JYNNEOS | 59 | Subcutaneous | Intradermal |
| V2 | M | 31–40 | C | JYNNEOS | 54 | Subcutaneous | Intradermal |
| V3 | M | 41–50 | C | JYNNEOS | - | Subcutaneous | - |
| V4 | M | 41–50 | H | JYNNEOS | 28 | Subcutaneous | Subcutaneous |
| V5 | M | 31–40 | H | JYNNEOS | 30 | Subcutaneous | Intradermal |
| V6 | M | 41–50 | C | JYNNEOS | 57 | Subcutaneous | Intradermal |
| V7 | M | 31–40 | C | JYNNEOS | 45 | Subcutaneous | Intradermal |
| V8 | M | 41–50 | C | JYNNEOS | 45 | Subcutaneous | Intradermal |
| V9 | M | 41–50 | C | JYNNEOS | - | Intradermal | - |
| V10 | M | 31–40 | C | JYNNEOS | 28 | Subcutaneous | Subcutaneous |
| C1 | M | 41–50 | AA | Mpox Infection | - | - | - |
| C2 | F | 31–40 | AA | Mpox Infection | - | - | - |
| C3 | M | 31–40 | C | Mpox Infection | - | - | - |
| C4 | M | 41–50 | C | Mpox Infection | - | - | - |
| C5 | M | 31–40 | C | Mpox Infection | - | - | - |
| C6 | M | 51–60 | H | Mpox Infection | - | - | - |
IR= immunization route. C = Caucasian, H = Hispanic, AA= African American.
B cell detection and sorting by Flow cytometry
Cryopreserved PBMCs from JYNNEOS recipients were thawed in Roswell Park Memorial Institute RPMI 1640 Medium w/ L-glutamine and 25 mM HEPES (Corning, Corning, NY, USA, 10–041-CV) supplemented with 10% fetal bovine serum (FBS) and 500 U of Benzonase Nuclease HC (MilliporeSigma, Burlington, MA, USA, 71206–25KUN) at 37°C. Cells were washed with cold FACS buffer (phosphate-buffered saline (PBS; Thermo Fisher Scientific, Waltham, MA, USA, 10010023) with 2% FBS), resuspended for counting, then aliquoted into 96-well plates (Corning 96-well Clear Polystyrene Microplates, Corning, #3788) and stained for plasmablasts (table 2) defined as (Dump-(CD3, CD14, CD16, CD56), CD19+ IgD−, CD20-CD38hi, and CD71+) (appendix p 6) in BD Horizon Brilliant Stain Buffer (BD Biosciences, San Jose, CA, USA, 566349) for 30 min at 4°C. Cells were then washed with cold FACS buffer and dead cells were detected using the LIVE/DEAD Fixable Blue Dead Cell Stain Kit (Thermo Fisher Scientific, L34962) in Dulbecco’s PBS with calcium and magnesium (Thermo Fisher Scientific, 14040133) at 4°C for 15 min. Cells were washed and resuspended in FACS buffer, then sorted into Eppendorf™ DNA LoBind Microcentrifuge Tubes (Thermo Fisher Scientific, 022431021) containing 12 μL of PBS with 0.05% bovine serum albumin (BSA) and directly loaded into a 10x Genomics Chromium Next GEM Chip K (10x Genomics, Pleasanton, CA, USA, PN-2000182) for analysis.
Table 2.
Antibodies used for flow cytometry and cell sorting
| Antibody | Vendor | Dilution |
|---|---|---|
|
| ||
| IgD BUV737 (IA6–2) | BD Horizon | 1:200 |
| CD20 BV510 (2H7) | BioLegend | 1:100 |
| CD38 BV650 (HB-7) | BioLegend | 1:200 |
| CD27 BB515 (M-T271) | BD Horizon | 1:200 |
| CD3 PerCP (SK7) | BioLegend | 1:100 |
| CD14 PerCP (63D3) | BioLegend | 1:200 |
| CD16 PerCP (3G8) | BioLegend | 1:200 |
| CD56 PerCP (HCD56) | BioLegend | 1:200 |
| CD71 PE/Dazzel 594 (CY1G4) | BioLegend | 1:200 |
Enzyme-linked immunosorbent assays (ELISAs)
Serum antibody titers were quantified via ELISA using the recombinant proteins listed above and lysates derived from mpox infected HRT-18 cells. Following lysis in 1% SDS solution and freezing at −8oC, the SDS was removed using the SDS-out SDS Precipitation Kit (Thermo Fisher Scientific, 20308) according to the manufacturer’s instructions. ELISAs were performed in MultiScreen® 96-well ELISA high binding plates (Millipore, MSEHNFX40). Plates were coated with 50 μL of 2 μg/mL recombinant protein, except for A30L and E8L, which were coated at 1 μg/mL. Inactivated SDS-free mpox cell lysates were coated at 5 μg/mL. All plates were coated for 1 hour in a 37°C incubator, then blocked for 1 hour at room temperature in PBS supplemented with 0.1% Tween 20 (PBST; Thermo Fisher Scientific ref. J61419.K3) and 3% milk powder (AmericanBio, Canton, MA, USA, AB1010901000). Heat-inactivated serum samples were serially diluted 1:3 from a starting dilution of 1:40 in PBST containing 1% milk powder. The ELISA for E8L was tested in a separate laboratory (Wadsworth Center, New York State Department of Health). Serum dilutions were incubated on plates for 2 hours at room temperature and then were washed three times with PBST using a BioTek 405 TS microplate washer (Agilent). After washing, 100 μL of Anti-Human IgG (Fab specific)–Peroxidase secondary antibody (Sigma-Aldrich, St. Louis, MO, USA, A0293, RRID: AB_257875, diluted 1:3000) was added and incubated for one hour. After washing, 100 μL of SIGMAFAST™ OPD (Sigma-Aldrich, P9187) was added and incubated for 10 minutes. The reaction was then stopped by adding 50 μL of 3 M hydrochloric acid (Thermo Fisher Scientific, S25856). The optical density at 490 nm of each plate was measured using a BioTek Synergy H1 Multi-Mode Reader (Agilent). The area under the curve (AUC) for each plate was calculated, plotted, and analyzed using GraphPad Prism 9 software (GraphPad Software, San Diego, CA, USA). ELISA data analysis consisted of a non-matching/pairing nonparametric Kruskal-Wallis test followed by Dunn’s multiple comparison test by comparing the mean rank of each cohort with the mean rank of every other cohort. The limit of detection (LOD) was defined as the mean plus three standard deviations from pre-vaccination participants.
Combined activation-induced marker (AIM)/intracellular cytokine staining (ICS) assays to detect antigen-specific T cell responses
We investigated orthopox-specific T cell responses using previously described peptide pools (11). The OPXV peptides were based on experimentally defined CD4 and CD8 epitopes from IEDB (www.IEDB.org). Peptides were synthesized as crude materials (TC Peptide Lab, San Diego, CA, USA), pooled into OPXV CD4 and OPXV CD8 mega pools, and sequentially lyophilized (11). To assess OPXV-specific T cell responses, PBMCs (appendix p 21) were cultured with the OPXV-specific peptide mega pools (1 μg/mL). An equimolar amount of dimethyl sulfoxide (DMSO) was added to duplicate wells as a negative control, and phytohemagglutinin-L (1 μg/mL, Millipore, Saint Louis, MO, USA 431784) was used as a positive control. Stimulated cells were incubated with CD40 (1:133, Miltenyi Biotec, Gaithersburg, MD, USA, 130–094-133) and CXCR5 BV650 (1:100, BD Biosciences, 740528) antibodies at 37°C in 5% CO2 for 26 hours. During the last 4 hours of incubation, a combination of Golgi-Plug containing brefeldin A, Golgi-Stop containing monensin (1:1000, both BD Biosciences), and CD137 APC antibodies (1:100, BioLegend, San Diego, CA, USA, 309810) was added. Membrane surface staining was performed for 30 minutes at 4°C with eBioscience™ Fixable Viability Dye eFluor™ 506 (1:1,000, Thermo Fisher Scientific, 65–0866-14) and the following antibodies: CD3 BUV805 (1:50, BD Biosciences, 612895), CD8 BUV496 (1:50, BD Biosciences, 612942), CD4 BUV395 (2:100, BD Biosciences, 564724), CD14 V500 (1:50, BD Biosciences, 561391), CD19 V500 (1:50, BD Biosciences, 561121), CD69 BV605 (4:100, BioLegend, 310938), CD137 APC (1:50, BioLegend, 309810), OX40 PE-Cy7 (1:50, BioLegend, 350012), CXCR5 BV650 (2:100, BD Biosciences, 740528), and CD154 (CD40 Ligand) Monoclonal Antibody (24–31), APC-eFluor™ 780, eBioscience (5:100, Thermo Fisher Scientific, 47–1548-42). Cells were then fixed with 4% paraformaldehyde (Sigma-Aldrich), permeabilized with saponin buffer (0.5% saponin (Sigma-Aldrich), 1% BSA, and 0.1% sodium azide), and blocked for 15 minutes with 10% human serum (Gemini Bio-Products, Sacramento, CA, USA) in saponin buffer. Intracellular staining was performed for 30 minutes at room temperature with the following antibodies: TNFɑ-PE (1:500, Thermo Fisher Scientific, 12–7349-82), IFNγ FITC (1:50, Thermo Fisher Scientific, 11–7319-82), IL4 BV711 (1:50, BD Biosciences, 564112), IL10 PE-Dazzle594 (1:100, BioLegend, 506812), and granzyme B Alexa 700 (1:100, BD Biosciences, 560213). Samples were run on a ZE5 Cell Analyzer (Bio-Rad, Hercules, CA, USA) and were analyzed with FlowJo 10.8.1 (Tree Star Inc., Ashland, OR, USA). Limit of detection (twice the upper 95% confidence interval of the geometric mean) and limit of sensitivity (LOS, twice the standard deviation from the median) (11) calculations were based on the DMSO-only conditions for AIM and ICS, as previously described (11). Responses were considered positive with a Stimulation Index (SI) > 2 and AIM LOS values of > 0.02 and > 0.04 for CD4 and CD8, respectively. Likewise, for ICS, a SI > 2 was considered in combination of a LOS > 0.01 for both CD4 and CD8. For AIM and ICS data graphed on a log10 scale, a value of 0 was graphed as 0.01.
Vaccine samples were collected before and after vaccination. Comparisons were performed using a paired non-parametric comparison (Wilcoxon). Comparisons pre- or post-vaccination with samples collected from convalescent participants were performed using an unpaired non-parametric Mann-Whitney test.
Results
B cell activation by immunization leads to its differentiation into short-lived, antibody-secreting plasmablasts. Neither one nor two doses of JYNNEOS significantly increased the frequency of circulating plasmablasts obtained 6–9 days after vaccination (appendix p 6). Single-cell sequencing of plasmablasts demonstrated that the number of variable heavy chain (VH) sequences (appendix p 7, 20) or their diversity, as defined by three different criteria (figure 1A), were not altered after vaccination or between the first and second dose. Two doses did not significantly alter the frequency of VH mutations (figure 1B), suggesting that at least shortly after each immunization, JYNNEOS vaccination might not increase the number of heavy chain mutations during antigen-mediated germinal center response. Similarly, the length of complementarity-determining region 3 (CDR3) in the immunoglobulin heavy chain gene, an indicator of antigen binding specificity after vaccination, was unaltered after JYNNEOS immunization (figure 1C) as well physicochemical properties of CDR3 (appendix p 8). In pre-vaccinated (unvaccinated, and uninfected) participants, the antibody repertoire of naïve B cells mainly comprised IgM sequences (appendix p 7), as expected. Compared to pre-vaccination, IgA levels were increased after one dose, accompanied by decreased IgM levels (appendix p 7). Two doses of JYNNEOS did not alter immunoglobulin isotype frequencies compared to pre-vaccination (appendix p 7). Similarly, one or two doses of JYNNEOS did not affect heavy chain mutation frequencies in the IgM, IgG, and IgA isotypes (figure 1D, appendix p 7). Compared to pre-vaccination, one or two doses of JYNNEOS did not significantly alter immunoglobulin heavy chain variable (IGHV) gene families (appendix p 7). Although usage of IGHV1–2, IGHV1–46, IGHV4–39, and IGHV4–59 seemed slightly increased in two participants after the first dose (appendix p 9), these increases were not significant.
Figure 1. Antibody repertoires of single B cells after one and two doses of JYNNEOS.
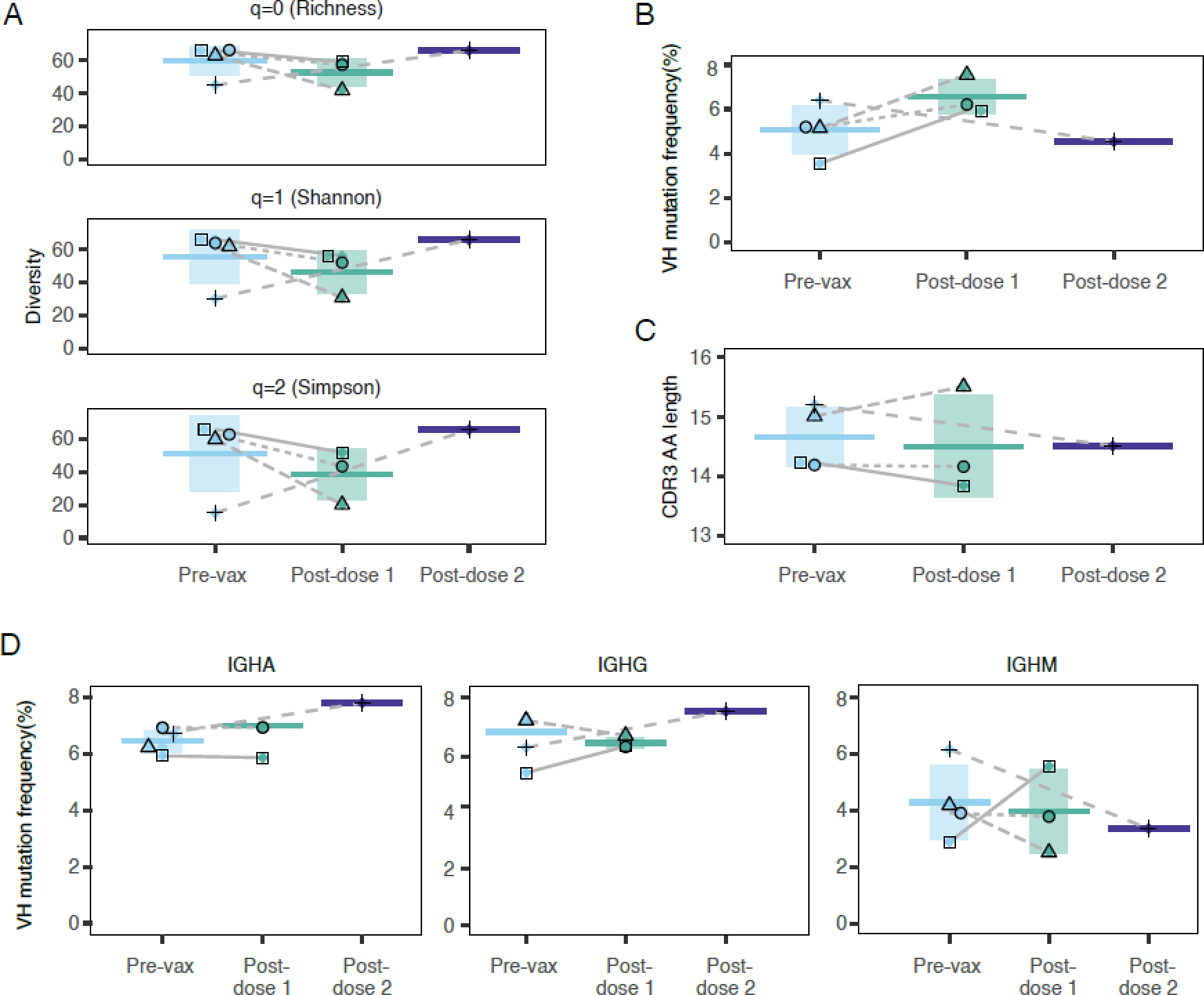
(A) Heavy chain variable (VH) sequence diversity was measured using three different parameters. Plasmablasts were collected 6–9 days after one or two doses of JYNNEOS. Total B cells (CD19+) from the same participants were collected before immunization and sorted for single-cell sequencing.
(B) Frequency of VH mutations.
(C) CDR3 amino acid (AA) lengths.
(D) Frequencies of heavy chain gene mutations according to immunoglobulin isotype. Participants are shown by unique symbols.
Since no changes in plasmablast immunoglobulin gene expression were observed after one or two doses, we sought to characterize global gene expression in plasmablasts. After quality control analyses (appendix p 10, 11), we found that in pre-vaccination CD19+ B cells, there was a differential cluster (appendix p 12) in which we identified transcripts commonly expressed in B cells, such as BANK1, BACH2, and ARHGAP24 (appendix p 12). Given the high levels of ribosomal proteins expressed in total B cells, we re-analyzed the dataset after removing these reads; however, the gene expression profiles remained comparable after their removal (appendix p 13). Compared to pre-vaccination, plasmablasts obtained from the paired participant post-dose one expressed high levels of CD74 (appendix p 13), which is expressed in antigen-experienced cells (12) and was part of a gene cluster (cluster 1) that expanded after vaccination (appendix p13). This change was expected since we analyzed plasmablasts, which are a different cell type than the total B cell population obtained pre-vaccination. However, and more importantly, the differentially expressed genes found after dose 1 remained similar in dose 2 (appendix p 13), suggesting that a second dose of JYNNEOS did not alter plasmablast gene expression.
We also analyzed antibodies secreted into circulation, evaluating vaccine responses up to two months post-immunization. For that, we quantified serum IgG responses and compared them with post-infection responses. Initially, we expressed the monkeypox virus proteins A29L, A35R, A30L and E8L (strain Zaire-96-I-16), known antibody targets (13) that can induce B cell immunogenicity (10,14–17). After 18–60 days post-second dose of JYNNEOS, only one participants out of seven presented an IgG response to A29L (figure 2A, appendix p 14). E8L and A30L-specific IgG levels were, respectively, increased after second dose in six and five, participants out of seven (figure 2C and D), while A35R IgG response was also considered positive in five out of seven participants, although lower than those elicited by infection (figure 2B). For most of the antigens analyzed, IgG levels in the serum of vaccinees were low shortly after the first or second doses (appendix p 15).
Figure 2. Serum antibody responses to recombinant monkeypox and vaccinia proteins, and lysates from mpox-infected cells.
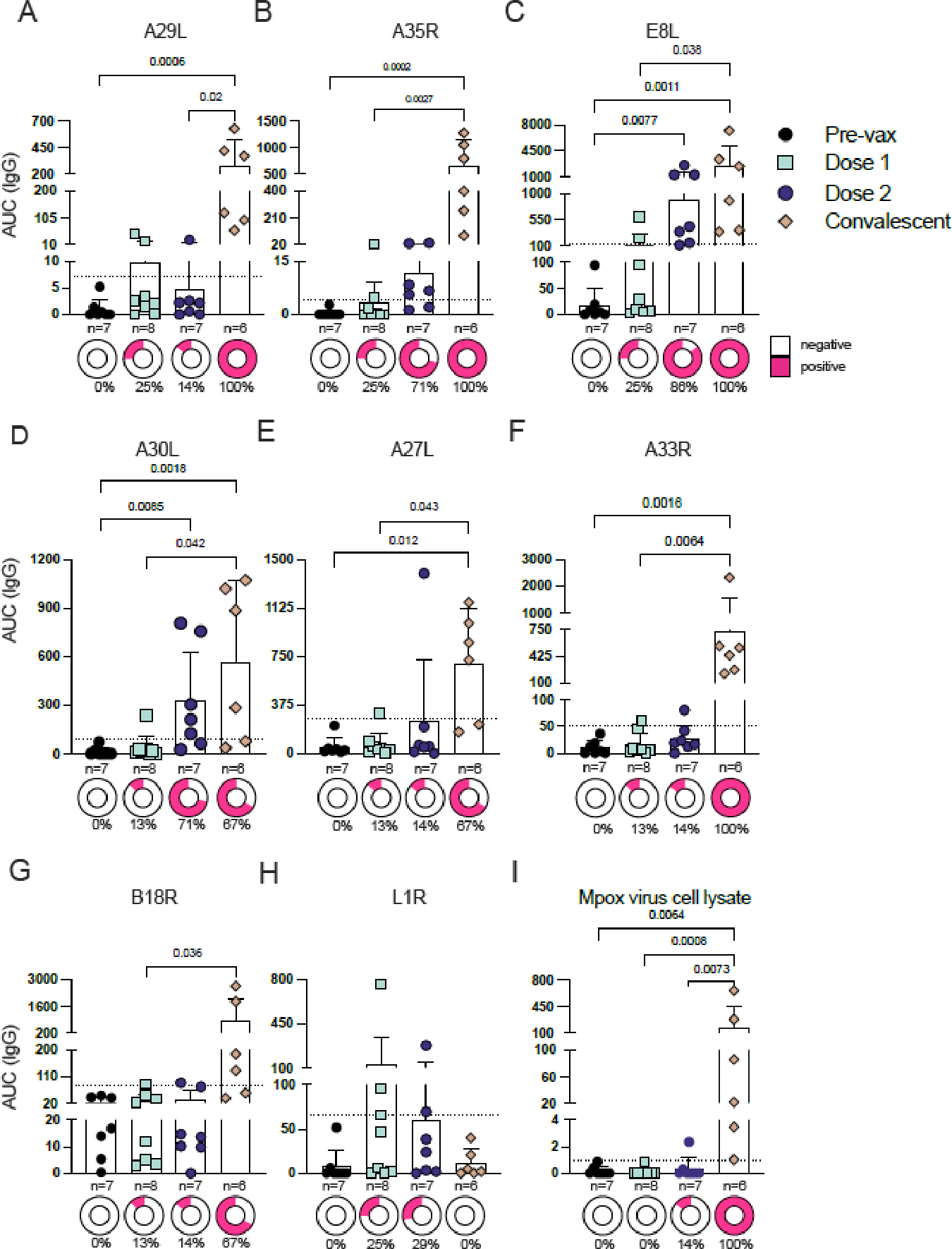
(A–D) Serum IgG responses to recombinant monkeypox proteins A29L (A), A35R (B), E8L (C), and A30L (D) at pre-vaccination and 18–60 days post-immunizations or 45–102 days post-infection. AUC, area under the curve. (E–H) Serum IgG responses to recombinant vaccinia proteins A27L (E), A33R (F), B18R (G), and L1R (H) at pre- and post-vaccination or post-mpox infection.
(I) Serum IgG responses of pre- and post-vaccination or post-mpox infection to a lysate from mpox-infected cells.
Mean with 95% CI are depicted. Dashed lines represent the LOD. Positivity was defined as the mean +3 standard deviations from pre-vaccination participants. p values are listed above the data points. The percentages below the donut graphs describe the percentage of positivity. Comparisons were performed using the Kruskal-Wallis test followed by Dunn’s multiple comparisons test. Appendix p 14 depicts the same data presented as error plots. Appendix p 15 shows additional IgG data from an earlier time points post-immunization.
Since the JYNNEOS vaccine was designed with vaccinia, and given that the high conservation of OPXVs results in the production of cross-reactive antibodies upon vaccination (8), we next sought to assess serum antibody responses to vaccinia proteins previously identified as targets of human neutralizing antibodies (13). One or two doses of JYNNEOS did not elicit robust IgG responses to the four proteins we tested (A27L, A33R, B18R, and L1R). Infection, however, generated robust serum IgG responses against A27L, A33R, and B18R (figure 2E–G). Mpox infection did not generate antibodies against L1R, and only two participants were responsive after their first or second vaccination dose (figure 2H).
Finally, we isolated monkeypox virus from an infected participant’s lesion during the 2022 outbreak and developed an ELISA to assess IgG responses to lysates from mpox-infected cells. After two doses of JYNNEOS, only one of the vaccinees mounted antibody responses to mpox proteins in the lysate, while five out of six convalescent participants elicited robust antibody responses, and the other participant presented antibody titers slightly above the limit of detection (figure 2I).
Taken together, our results demonstrate that—unlike previous generations of smallpox vaccines—JYNNEOS does not induce a robust humoral response to OPXVs. Therefore, we next investigated how JYNNEOS vaccination induces T cell immunity in humans compared to mpox infection. We measured the antigen-specific CD4+ and CD8+ T cell responses to JYNNEOS immunization or mpox infection using a previously reported (11) pool of VACV peptides (figure 3, appendix p 16). T cell responses were measured using a combined AIM/ICS assay (see appendix p 17 for the gating strategy). CD4+ T cells showed pre-existing immunity before vaccination, with a higher frequency of positivity and increased reactivity after two doses, and a response comparable to post mpox-infected samples (figure 3A–B). Circulating T follicular helper (cTFH) cell levels were significantly increased post-vaccination but not after mpox infection (figure 3E). CD8+ T cells displayed little to no evidence of pre-existing immunity but robustly increased in response to two doses of JYNNEOS compared to pre-vaccination, and after mpox infection, (p= 0.15 for AIM assay (figure 3C), p= 0.49 for ICS assay (figure 3D)).
Figure 3. Human CD4+ and CD8+ T cell responses to JYNNEOS immunization or mpox infection.
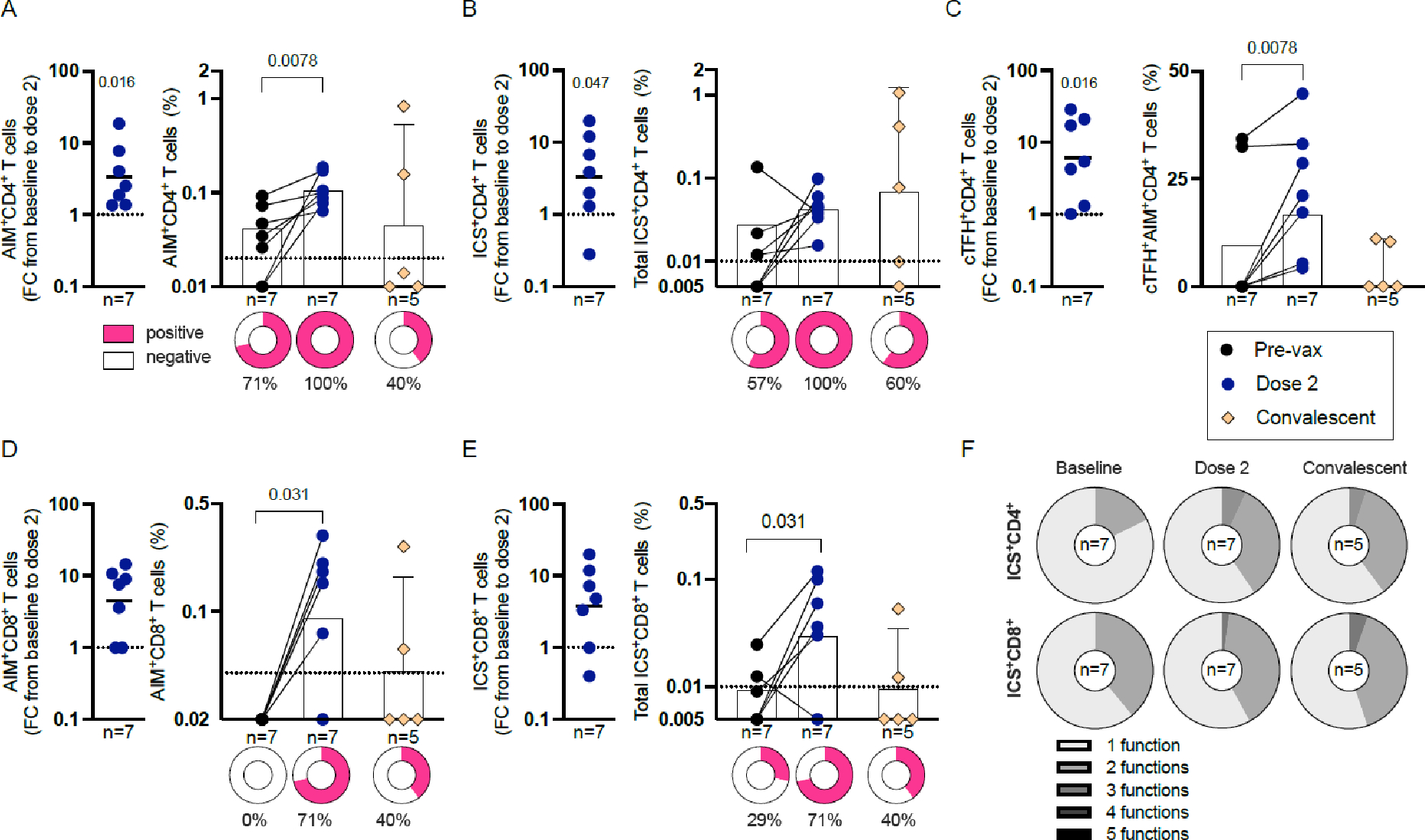
(A–B) CD4 T cell responses to the OPXV peptide pool 9–114 days after vaccination and the magnitudes of their AIM (A) or ICS (B) responses pre- and post-vaccination or 20–130 days post-mpox infection. FC, fold change.
(C-D) CD8 T cell responses to the OPXV peptide pool after vaccination and the magnitudes of their AIM (C) or ICS (D) responses pre- and post-vaccination or post-mpox infection.
(E) OPXV-specific CD4 cTFH cell responses to the OPXV peptide pool after vaccination and AIM magnitudes pre- and post-vaccination or post-mpox infection.
(F) Functionality of OPXV-specific CD40L+ CD4 and CD69+ CD8 cytokine responses pre- and post-vaccination or post-mpox infection.
(A-D) Geometric mean is depicted and error bars represent 95% CI. (E) Mean is depicted and error bars represent 95% CI. Dashed lines represent the LOS. Positivity was defined as twice the standard deviation from the median. All fold change (FC) graphs depict the geometric mean. The significance of the FC was assessed by Wilcoxon signed-rank test. Pre-vaccination and post-dose two values were compared by Wilcoxon matched pairs signed-rank test. Pre-vaccination and convalescent samples were compared by one-tailed Mann-Whitney test. Dose two and convalescent were compared by two-tailed Mann-Whitney test. p values are listed above the data points. The donut graphs represent the the percentage of positivity. The percentages below the donut graphs describe the positive participants. Appendix p 16 shows the same data presented as error plots. Appendix p 17 shows the gating strategy for the T cell assay and the individual cytokine responses.
Despite the absence of pre-existing immunity, CD8+ T cell responses presented similar FC increases to CD4+ T cells in response to vaccination (figure 3A–D). The T cell responses involved the production of multiple cytokines, revealing a trend toward increased polyfunctionality post-vaccination or -infection (figure 3F), including the presence of mixed T helper type (Th)1/(Th)2 cell phenotypes (appendix p 17). Finally, no significant correlations were observed between T cell and antibody responses (appendix p 18) and the lower cTFH responses observed in convalescent samples were not correlated with the differences in the time following exposure between infected and vaccinated participants (appendix p 19). Antibody responses to vaccination were also not associated with the days post-immunization, suggesting that although these samples were collected closer to exposure than the convalescent samples (appendix p 18), this difference is likely not responsible for the superior antibody responses mounted against infection.
Discussion
The first-generation Dryvax vaccine prevented smallpox but caused severe side effects, leading to the development of second- (ACAM2000) and third- (JYNNEOS) generation vaccines. JYNNEOS is considered safe, immunogenic, and protective against smallpox in humans. In a Cynomolgus macaque model of mpox infection, two doses of JYNNEOS were shown to reduce viral burden compared to one dose or to unvaccinated animals (2). JYNNEOS was authorized by the FDA for mpox prevention and administered in humans during the 2022 outbreak. However, the immunogenicity and efficacy of a third-generation smallpox vaccine have not been thoroughly evaluated for prevention against mpox. To gain insight into JYNNEOS’ immunogenicity and mpox protection, we evaluated various metrics of adaptive responses in US adults after one and two JYNNEOS vaccine doses. A strength of this study is that we used paired samples to evaluate B cell responses at the cellular, single-cell, and serum levels combined with cellular antigen-specific T cell analyses.
Vaccines that are highly mediated by antibodies usually induce mutations in antibody genes that increase their binding and functional activity. Also, VH gene usage usually changes after human immunization but did not significantly increase or decrease in the six gene families we analyzed. Additionally, gene expression data revealed no significant changes after vaccination, although only one individual was analyzed at each time point (same participant for pre-vaccination and post-dose 1). We observed no gene expression changes between post-dose one and two, confirming that JYNNEOS did not significantly impact human B cell responses.
OPXV-elicited antibodies have significantly better cross-neutralizing potential compared to those targeting other viruses, such as HIV and influenza virus, due to the a) high sequence homology between the surface proteins of VACV, monkeypox virus, smallpox virus, and cowpox virus (89–100% similarity) and b) the broad neutralization effects of OPXV antibodies, which can target multiple viral surface proteins concomitantly (6). Therefore, we evaluated antibody secretion by plasmablasts, with the idea that if serum antibodies offered direct protection against the virus in circulation, we could combine this knowledge with the single-cell data to better characterize humoral responses to JYNNEOS in humans.
JYNNEOS induced moderate serum antibody responses to monkeypox and vaccinia proteins. The strong IgG responses to the same OPXV proteins by sera from participants 20–102 days post-mpox infection illustrate the vaccine’s limited antibody response. Convalescent participants presented a range of IgG responses against the proteins we analyzed, consistent with studies showing that different viral loads and clinical determinants dictate the response to monkeypox virus infection. A recent ELISA-based study reported vaccine-specific IgG peaks in the serum of JYNNEOS-vaccinated participants after 42 days (18). Additionally, another recent study reported lower A35R- and H3L-IgG titers in sera from JYNNEOS recipients compared to convalescent participants (10). The reference strains of VACV and monkeypox virus share > 90% identity. The 2022 monkeypox virus strain has unique mutations compared to the reference strain, including A35R mutations at positions A67 and A88, which were exclusively found during the 2022 outbreak (13). Whether these mutations can impact serum IgG binding to the recombinant monkeypox virus protein remains to be investigated. Finally, we confirmed increased levels of A35R-IgG as a serum marker of human mpox infection (10), given the low levels of A35R-IgG in the serum after first and second doses of JYNNEOS.
Our data establish that the B cell immunogenicity of JYNNEOS vaccination is low up to two months post-vaccination. Thus, what will mediate JYNNEOS’ protective effects in humans, if not B cells and antibodies?
We tested T cell responses against peptide pools designed based on VACV antigens. Two doses of JYNNEOS elicited substantial CD8+, CD4+, and cTFH T cell responses with similar fold increases. We also noted the presence of pre-existing CD4+, but not CD8+, T cell immunity, as previously reported for Dryvax vaccination (11). JYNNEOS vaccination and mpox infection produced similar magnitudes of reactivity for CD4+ and CD8+ T cells; however, after infection, the frequency of the T cell responses trended lower after infection than after vaccination, consistent with a previous study showing that mpox infection specifically decreased antiviral-specific T cell reactivity (19). Intriguingly, we also observed significantly lower cTFH T cell induction after infection versus vaccination. Finally, the quality of the vaccinia-specific T cells indicates the presence of a Th1/Th2 mixed phenotype, as previously reported for smallpox vaccination (20) but not, until now, in the context of monkeypox virus infection. Indeed, a previous study has shown a Th1 phenotype in the context of mpox infection (21) but did not measure Th2 cytokines. Our current study has a more comprehensive assessment of the cytokine patterns and provides additional insights into the functionality of those cells that warrants further investigation. Our study was not designed to address particularities across different vaccinia virus-based vaccination platforms. Accordingly, whether the reduced antibody activity seen in response to JYNNEOS is related to inferior neutralization activity compared to Dryvax (22) needs further assessment. Additionally, although previous reports demonstrated that the antigens recognized by T cells are highly correlated with those recognized by antibody responses (15), JYNNEOS’ T cell-mediated protection might be due to epitopes elicited only in response to vaccinia-based vaccines and not mpox infection as the two proteomes share 61–67% of sequence conservation (11). Alternatively, genes lost in the newly designed MVA version versus Dryvax (23) may account for a fraction of T cell responses not induced in JYNNEOS vaccination. Future studies should address, more in-depth, the antigens recognized by T cell responses.
Taken together, our results indicate that JYNNEOS, compared to infection, elicits moderate B cell and antibody responses in humans. However, vaccination induces a robust T cell response that can recognize monkeypox virus and VACV peptides. This data provides insight into the protective mechanisms of a third-generation OPXV vaccine, which can be used to inform vaccine design and clinical data assessment during future OPXV outbreaks.
Limitations of the study
Our study has a reduced number of participants; when we started these experiments, JYNNEOS vaccines were not readily available to the community in NYC. We did not assess the roles of other immune cells (e.g., innate immune cells) in mediating mpox protection. The gene expression data was assessed using one participant per time point; however, in our opinion, this does not reduce the importance of the data, which was used for descriptive purposes, with no statistical comparisons performed between the different groups. Our study did not include virus neutralization assays; thus, we cannot assess whether the low humoral response is directly associated with a low viral neutralization. Convalescent samples were collected from 20–102 days post-infection, which can be considered a variable range. However, we did not find a correlation between the time of OPXV exposure and IgG titers or T cell level. We did not perform additional readouts to confirm the serology data, such as immunofluorescence.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number U54CA267776. We thank the Sequencing Core Facility at the La Jolla Institute for Immunology for sequencing the cDNA libraries. The NovaSeq 6000 was acquired through the Shared Instrumentation Grant Program (S10; 6000 S10OD025052). Substantive, stylistic, and copy editing of the manuscript and supplemental materials were provided by Dr. Nicole St. Denis at High-Fidelity Science Communications (www.hifiscicomm.ca). This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. 75N93021C00065 to A.S. WTL was funded by the US Centers for Disease Control and Prevention (CDC).
Footnotes
Declaration of Interests
Florian Krammer has consulted for Curevac, Seqirus and Merck and is currently consulting for Pfizer, Third Rock Ventures, Avimex and GSK. He is named on several patents regarding influenza virus and SARS-CoV-2 virus vaccines, influenza virus therapeutics and SARS-CoV-2 serological tests. Some of these technologies have been licensed to commercial entities and Dr. Krammer is receiving royalties from these entities. Dr. Krammer is also an advisory board member of Castlevax, a spin-off company formed by the Icahn School of Medicine at Mount Sinai to develop SARS-CoV-2 vaccines. The Krammer laboratory has received funding for research projects from Pfizer, GSK, and Dynavax and three of Dr. Krammer’s mentees have recently joined Moderna.
The other authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing
Sequencing data were deposited at NCBI under the accession number PRJNA956545. Additional deidentified data may be made available to investigators whose proposed use of the data has been approved by the Icahn School of Medicine Ethics Review Board upon request to the corresponding authors.
References
- 1.Zucker J CROI 2023: EPIDEMIOLOGY, DIAGNOSIS, AND MANAGEMENT OF MPOX. In 2023. [cited 2023 May 16]. p. 1–16. Available from: https://www.iasusa.org/wp-content/uploads/2023/04/30-3-zucker-v1.pdf [PMC free article] [PubMed]
- 2.Hatch GJ, Graham VA, Bewley KR, Tree JA, Dennis M, Taylor I, et al. Assessment of the Protective Effect of Imvamune and Acam2000 Vaccines against Aerosolized Monkeypox Virus in Cynomolgus Macaques. J Virol. 2013. Jul 15;87(14):7805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertran M, Andrews N, Davison C, Dugbazah B, Boateng J, Lunt R, et al. Effectiveness of one dose of MVA–BN smallpox vaccine against mpox in England using the case-coverage method: an observational study. Lancet Infect Dis [Internet]. 2023. Mar 13 [cited 2023 Apr 20];0(0). Available from: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(23)00057-9/fulltext [DOI] [PubMed] [Google Scholar]
- 4.Preliminary JYNNEOS Vaccine Effectiveness Estimates Against Medically Attended Mpox Disease in the U.S., August 15, 2022 – October 29, 2022 | Mpox| Poxvirus | CDC; [Internet]. 2023. [cited 2023 Apr 20]. Available from: https://www.cdc.gov/poxvirus/mpox/cases-data/JYNNEOS-vaccine-effectiveness.html [Google Scholar]
- 5.Wolff Sagy Y, Zucker R, Hammerman A, Markovits H, Arieh NG, Abu Ahmad W, et al. Real-world effectiveness of a single dose of mpox vaccine in males. Nat Med. 2023. Mar;29(3):748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mr B, Mm M, Hp S, K S, J H, Dh D, et al. Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine. J Virol [Internet]. 2008. Apr [cited 2023 Apr 20];82(7). Available from: https://pubmed.ncbi.nlm.nih.gov/18234801/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006. Jun;211:320–37. [DOI] [PubMed] [Google Scholar]
- 8.Gilchuk I, Gilchuk P, Sapparapu G, Lampley R, Singh V, Kose N, et al. Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections. Cell. 2016. Oct 20;167(3):684–694.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaeck LM, Lamers MM, Verstrepen BE, Bestebroer TM, van Royen ME, Götz H, et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat Med. 2023. Jan;29(1):270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yefet R, Friedel N, Tamir H, Polonsky K, Mor M, Cherry-Mimran L, et al. Monkeypox infection elicits strong antibody and B cell response against A35R and H3L antigens. iScience. 2023. Feb 17;26(2):105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grifoni A, Zhang Y, Tarke A, Sidney J, Rubiro P, Reina-Campos M, et al. Defining antigen targets to dissect vaccinia virus and monkeypox virus-specific T cell responses in humans. Cell Host Microbe. 2022. Dec 14;30(12):1662–1670.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barclay AN, Brown MH, Law SKA, McKnight AJ, Tomlinson MG, van der Merwe PA, editors. Index. In: The Leucocyte Antigen FactsBook (Second Edition) [Internet]. San Diego: Academic Press; 1997. [cited 2023 Apr 20]. p. 596–613. (Factsbook). Available from: https://www.sciencedirect.com/science/article/pii/B9780120781850506375 [Google Scholar]
- 13.Ahmed SF, Sohail MS, Quadeer AA, McKay MR. Vaccinia-Virus-Based Vaccines Are Expected to Elicit Highly Cross-Reactive Immunity to the 2022 Monkeypox Virus. Viruses. 2022. Sep 3;14(9):1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roper RL. Characterization of the Vaccinia Virus A35R Protein and Its Role in Virulence. J Virol. 2006. Jan;80(1):306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franceschi V, Parker S, Jacca S, Crump RW, Doronin K, Hembrador E, et al. BoHV-4-Based Vector Single Heterologous Antigen Delivery Protects STAT1(−/−) Mice from Monkeypoxvirus Lethal Challenge. PLoS Negl Trop Dis. 2015. Jun 18;9(6):e0003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fantini J, Chahinian H, Yahi N. A Vaccine Strategy Based on the Identification of an Annular Ganglioside Binding Motif in Monkeypox Virus Protein E8L. Viruses. 2022. Nov 16;14(11):2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matho MH, Maybeno M, Benhnia MREI, Becker D, Meng X, Xiang Y, et al. Structural and biochemical characterization of the vaccinia virus envelope protein D8 and its recognition by the antibody LA5. J Virol. 2012. Aug;86(15):8050–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Priyamvada L, Carson WC, Ortega E, Navarra T, Tran S, Smith TG, et al. Serological responses to the MVA-based JYNNEOS monkeypox vaccine in a cohort of participants from the Democratic Republic of Congo. Vaccine. 2022. Nov 28;40(50):7321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammarlund E, Dasgupta A, Pinilla C, Norori P, Früh K, Slifka MK. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc Natl Acad Sci U S A. 2008. Sep 23;105(38):14567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson EJ, Lai L, Wrammert J, Kabbani S, Xu Y, Priyamvada L, et al. Plasmablast, Memory B Cell, CD4+ T Cell, and Circulating Follicular Helper T Cell Responses to a Non-Replicating Modified Vaccinia Ankara Vaccine. Vaccines. 2020. Feb 6;8(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrati C, Cossarizza A, Mazzotta V, Grassi G, Casetti R, De Biasi S, et al. Immunological signature in human cases of monkeypox infection in 2022 outbreak: an observational study. Lancet Infect Dis. 2023. Mar;23(3):320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nigam P, Earl PL, Americo JL, Sharma S, Wyatt LS, Edghill-Spano Y, et al. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology. 2007. Sep 15;366(1):73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volz A, Sutter G. Modified Vaccinia Virus Ankara: History, Value in Basic Research, and Current Perspectives for Vaccine Development. Adv Virus Res. 2017;97:187–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data were deposited at NCBI under the accession number PRJNA956545. Additional deidentified data may be made available to investigators whose proposed use of the data has been approved by the Icahn School of Medicine Ethics Review Board upon request to the corresponding authors.


