Abstract
Introduction
Older adults are increasing in number in both developed and developing countries. However, as the world’s aging population grows, the burden of diseases among older people also increases. Despite this, co-occurring health problems due to physical inactivity in older adults have become a concern, and physical inactivity can be caused by different conditions. As a result, for older adults to have better health outcomes, early diagnosis of physical activity status and prevention of non-communicable diseases are crucial. There is a lack of data on the prevalence and associated factors of physical inactivity among older adults that is scarce in sub-Saharan Africa, particularly in the study area, Ethiopia. Therefore, this study aimed to assess the prevalence and associated factors of physical inactivity among older adults in Gondar town, Northwest Ethiopia, in 2022.
Methods
A community-based cross-sectional study was conducted from April 1st to June 30th, 2022, in Gondar town, Northwest Ethiopia. The data was collected by the Global Physical Activity Questionnaire, Volume 2 through face-to-face interviews among 838 participants. Data were entered into Epinfo version 7.1, then exported and analysed using the Statistical Package of Social Science version 25. A binary logistic regression model was performed to identify factors associated with physical inactivity. The result was considered statistically significant based on an adjusted odds ratio of 95% and a p-value less than 0.05.
Result
The prevalence of physical inactivity was 65.6% (95% CI: 62.1–68.9). Being female (AOR: 3.053, 95% CI:1.487–6.267), age group > = 80 (AOR: 4.082, 95% CI:1.234–13.497), primary school level (AOR: 3.020, 95% CI:1.433–6.367), no formal education (AOR: 8.573, 95% CI:2.843–25.847), unemployed (AOR: 10.273, 95% CI:5.628–18.753), and symptoms of depression (AOR: 7.152, 95% CI: 4.786–17.965) were significantly associated with physical inactivity.
Conclusion
Physical inactivity was relatively high among older adults in Gondar town. Being female, older age, having low levels of education, being unemployed, and having depression symptoms were associated with physical inactivity. We suggest promoting the health benefits of physical activity among females by reducing their burden, older age groups, and unemployed older adults, and avoiding depression among individuals.
Keywords: Physical inactivity, Older adults, Prevalence, Factors, Ethiopia
Background
The global population is increasing in both developed and developing nations, particularly with an increase in the number of older people [1]. However, as the world’s aging population grows, the burden of diseases among older people also increases, posing a problem for global healthcare system, particularly in under developing nations [2]. As people’s lifestyles change around the world, notably with regard to physical inactivity [3]. Older persons should exercise for more than 600 metabolic equivalents (METs) minutes per week, or at least 150 minutes per week of moderate-to-vigorous aerobic activity [4].
Physical inactivity is a serious public health concern worldwide, which is the fourth modifiable risk factor for non-communicable disease mortality and a significant contributor to disability [5]. The consequence of physical inactivity is 3.3 million deaths annually worldwide, of which 6–10% are caused by non-communicable illnesses [6, 7], and the majority of deaths (70%) are caused by physical inactivity in low- and middle-income countries [8]. Physical inactivity is expected to have annual expenditures on the worldwide health-care system of $538 billion [9]. In addition, research on the effects of physical inactivity revealed that it contributed to 10% of breast and colon cancers, 7% of type 2 diabetes mellitus, and 6% of coronary heart ailments [10]. However, the burden of physical inactivity was not well known in Ethiopia.
The prevalence of physical inactivity has increased, it is estimated that 31.1% of people worldwide are physically inactive [11]. Studies reported that the prevalence of physical inactivity among older adults ranged from 5 to 29% in Europe [12], 50.7% in Brazil [13], 63.1% in China [14], 29.8% in Malaysia [15], and 60.5% in South Africa [16]. Additionally, a report revealed that Ethiopia is seeing an increase in the number of people who aren’t physically active [17].
Moreover, different studies revealed that socio-demographic factors (sex, age, educational status, marital status, monthly incomes), body mass index, social support, smoking and alcohol consumption, depression, and history of NCDs were associated with physical inactivity [12, 15, 18–22].
Despite this, co-occurring health problems due to physical inactivity in older adults have become a concern, and the physical inactivity can be caused by different conditions. As a result, for older adults to have better health outcomes, early diagnosis of physical activity status and prevention of NCDs are crucial. Data on prevalence and associated factors of physical inactivity among older adults are scarce in sub-Saharan Africa, particularly in the study area, Ethiopia. Therefore, this study was aimed to assess the prevalence of physical inactivity and associated factors among older adults in Gondar town, Northwest Ethiopia, in 2022.
Methods
Study design and setting
A community-based cross-sectional study was conducted from April to June 2022. The study was conducted in Gondar town, Amhara regional state, Northwest Ethiopia. The city is located in the central Gondar zone, Amhara regional state, 748 km northwest of Addis Ababa, Ethiopia’s capital, and about 180 km from Bahir Dar, Amhara regional state’s capital. Gondar is one of the most ancient and largely populated cities in the country. It is at 12˚ 45˚ north latitude and 37˚ 45˚ east longitude, with an elevation of 6,998 feet above sea level. Gondar town has 25 kebeles (the smallest administrative units in Ethiopia). According to the Gondar Statistics Agency’s 2021/22 projection from 2007 population census data, the total population of Gondar town was estimated at 390,000, more than half of the population were women, and more than 9000 were older adults. Despite the population of the city increasing over time, it does not have well-designed and accessible infrastructure for physical activity, such as playgrounds, parks, sidewalks, bike lanes, and good public transportation systems that promote physical activities like walking and cycling, and as a result, it increases physical inactivity rates.
Source population, study population, inclusion and exclusion criteria
The source population was all older adults living in Gondar town. Older adults aged 60 years and older in selected kebeles during the study period were the study population. Older adults aged 60 years and older who were permanent residents (≥ 6 months) in the selected kebeles were included. However, older adults who were bedridden and unable to communicate during the data collection period were excluded.
Sample size determination and sampling procedure
The sample size was calculated by using the single population proportions formula, using the assumption of a 95% confidence interval, (α = 5%) level of significance, a marginal error of 5%, a 10% non-response rate, and a 45.5% prevalence of physical inactivity among adults [23].
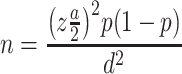 |
n = sample size.
α = (level of significance) = 5%,
Z = value of z statistic at 95% confidence level,
d = margin of error allowed = 5%,
P = 45.5%
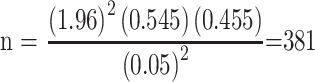 |
Finally, the final sample size was 838, including two design effects and 10% non-response rate.
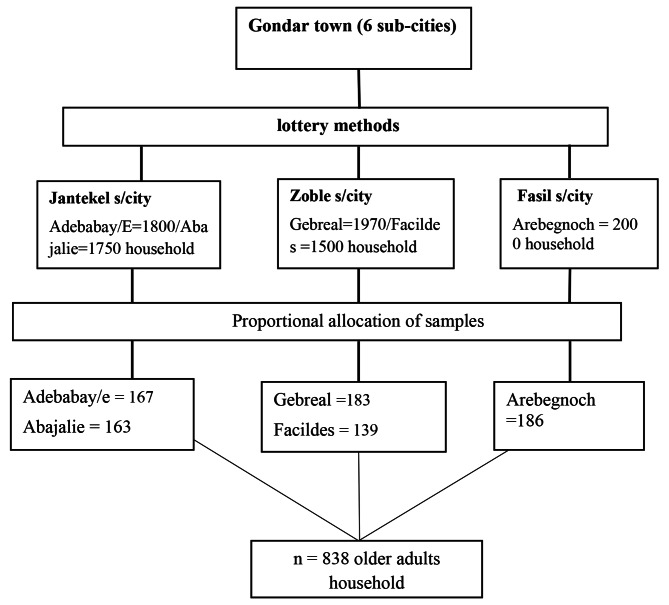
A multi-stage sampling technique was used to select the required sample. In the first step of sampling, three administrative areas (sub-cities), Jantekel, Zoble, and Facil were selected by using a simple random sampling technique from six sub-cities. In the second step of sampling, Adebabay Eyesus and Abajalie Kebeles from Jantekel sub-city, Gebreal and Facildes Kebeles from Zobel sub-city, and Arebegnoch from Facil sub-city were selected randomly. A total of 3,550 households in Abajalie and Adebabay Eyesus, 3,470 households in Gebreal and Facildes, and 2000 households in Arbegnoch Kebeles were found. Then, after proportional allocation to each selected kebeles, a systematic sampling technique was used to select households from each of the kebeles. The interval (𝐾) value was calculated by dividing the total household of the selected kebele by the total calculated sample size. The initial household was randomly selected by lottery method. Then other households were selected at every 11th interval. Whenever more than one eligible older adult was found in the same selected household, only one of them was chosen using the lottery method for interview. In case no eligible candidate was identified in a selected household or the selected household was closed even after revisit, the sampling process continued to the next household in a clockwise direction until getting an eligible person. See sampling procedure in Fig. 1.
Fig. 1.
Sampling procedure of older adults at Gondar town, Northwest Ethiopia, 2022
Operational definition
Older adults
are defined by the UN as a person aged 60 years or older [24].
Physical inactivity
Participants who achieved less than 600 MET-minutes per week total physical activity level were classified as “physically inactive” [25].
Depression
is measured by the Geriatrics Depression Scale, which has 15-item questions. A score from 0 to 4 is normal, and a score ≥ 5 suggests depression [26].
Social support
is assessed by the OSSS-3. The sum score ranges from 3 to 14, with scores of 3–8 poor social support, 9–11 moderate social support, and 12–14 strong social support [27].
Cigarette smoking
Those who smoke any tobacco products daily were considered tobacco users [28].
Alcohol drinking
Those who consumed any type of alcohol with 2 or more bottles daily were considered alcohol users [29].
Body Mass Index (BMI)
According to the Centres for Disease Control and Prevention, BMI calculated as weight in kilograms divided by height in square meters and interpreted as underweight <(18.5), normal (18.5–24.9), overweight (25.0-29.9), and obese ≥(30.0) [30].
Data Collection instrument
The Global Physical Activity Questionnaire (GPAQ) version 2 was used to collect the data through face-to-face interviews [31]. During an interview, a GPAQ generic show card was displayed for the study participants to clarify types of physical activity. The reliability and validity of the Global Physical Activity Questionnaire (GPAQ) have been confirmed in many countries [32]. Questionnaires were answered by the older adult population about the frequency and duration of physical activity in the past 7 days to estimate the weekly time spent in PA (min/wk.). Which means, moderate MET minutes/week = 4.0 * moderate minutes * days, vigorous MET-minutes/week = 8.0 * vigorous activity minutes * days, and walking MET-minutes/week = 4 * walking minutes/cycling * days, if the combination of these < 600 MET-minutes/week total physical activity level is classified as “physically inactive according to GPAQ analysis guidelines.
Depression was assessed by geriatric depression scale, which is a self-reported scale. It is easy to administer, use, and freely available. It has 15-item questions. Older adults give response to what they feel within the past one week. It is scored as one point for each one selected. A score of 0 to 4 is normal and ≥ 5 suggest depression [26]. Social support was assessed by OSSS-3. The sum score ranges from 3 to 14. Scores of 3–8 poor social support, 9–11 moderate social support, and 12– 14 strong social support [27]. Moreover, weight and height were measured twice. The weight of the participants was measured using a light portable digital weighing machine (mini) to the nearest 0.1 kg. The scale was adjusted to a zero level between individual measurements, and height was measured by non-flexible inch tapes to the nearest 0.1 cm while respondents were standing in upright positions.
Data Quality Control
To control the quality of the data, the questionnaire was translated into the local language (Amharic) and back into English to maintain consistency by experts. A pre-test was done in Bahir Dar to check the questionnaire’s consistency and adaptability on 5% of the total sample size before the actual data collection was started. The data was collected by two health extension workers and two nurses. One nurse and one physiotherapist were supervisors. Two days of intense training were given to data collectors by the principal investigator (KB) and ES on how to approach study participants, how to use the questionnaire and guidelines, and data collection procedures. Furthermore, on each data collection day, the collected data were checked and reviewed for completeness by the main investigator.
Statistical analysis
The collected data were checked manually for the completeness of relevant information. And the data were checked, coded, and entered into EPI Info version 7 and exported to SPSS version 25 software. Descriptive statistics were done for the frequency of distribution, percentage, frequency, and median. Variables in the study are presented using tables and figures. A binary logistic regression model was performed to identify factors associated with physical inactivity. Assumptions for the binary logistic regression model were checked, like the Hosmer-Lemeshow goodness of fit test (0.41) and multicollinearity. In bivariate analysis, variables with a p-value less than 0.25 were entered into multivariable analysis to control for a confounding effect and to examine the association between different independent variables. The result was considered statistically significant in multivariable analysis on the basis of an adjusted odds ratio of 95% and p-value less than 0.05.
Results
Socio-demographic characteristics of study participants
Out of the total sample size of eight hundred thirty-eight study participants, only 764 were included in this study, with a response rate of 91.2%. Among the total participants, more than a third of fourth 577 (75.5%) were males, and the median age of participants was 66 with a range from 60 to 91 years old. The majority, 550 (72%) of participants were in the 60–69 age range. Four hundred eighty-seven 487 (63.7%) participants were orthodox religion followers, 134 (17.5%) of the participants were not formally educated, 507 (66.4%) were unemployed, and 523 (68.5%) of the participants were married. The details of socio-demographic characteristics are presented in Table 1.
Table 1.
Socio-demographic characteristics of older adults in Gondar town, North West Ethiopia, 2022 (N = 764)
| Variables | Categories | Frequency (N) | Percentage (%) |
|---|---|---|---|
| Sex | Male | 577 | 75.5 |
| Female | 187 | 24.5 | |
| Age | 60–69 | 550 | 72.0 |
| 70–79 | 126 | 16.5 | |
| >=80 | 88 | 11.5 | |
| Religion | Orthodox | 487 | 63.7 |
| Muslim | 196 | 25.4 | |
| Protestant | 59 | 7.7 | |
| Others | 24 | 3.1 | |
| Marital status | Married | 523 | 68.5 |
| Divorced | 44 | 5.7 | |
| Separated | 31 | 4.1 | |
| Windowed | 166 | 21.7 | |
| Occupation | Employed | 257 | 33.6 |
| Unemployed | 507 | 66.4 | |
| Monthly income (ETB) | > 5000 | 66 | 8.6 |
| 3001–5000 | 147 | 19.2 | |
| 1001–3000 | 418 | 54.7 | |
| <1000 | 133 | 17.4 | |
| Education level | College & above | 209 | 27.4 |
| Secondary school | 209 | 27.4 | |
| Primary school | 212 | 27.7 | |
| No formal educated | 134 | 17.5 |
ETB = Ethiopian Birr, Others = catholic, Adventist, seven-day followers
Behavioural and medical conditions of study participants
A total of four hundred fifty-nine participants (60.1%) reported having no prior history of non -communicable diseases (NCDs), 743 (97.3%) participants did not smoke, 757 (99.1%) participants said they did not drink alcohol, 303 (39.7%) participants had poor social support, and a total of 600 (78.5%) of the participants had normal body weight in detail see (Table 2).
Table 2.
Behavioural and medical conditions of study participant among older adults in Gondar town, Northwest Ethiopia, 2022(N = 764)
| Variables | Categories | Frequency (N) | Percentage (%) |
|---|---|---|---|
| Having history of NCDs | No | 459 | 60.1 |
| Yes | 305 | 39.9 | |
| Cigarette smoking status | No | 743 | 97.3 |
| Yes | 21 | 2.7 | |
| Alcohol drink | No | 757 | 99.1 |
| Yes | 7 | 0.9 | |
| Social support | Strong social support | 170 | 22.3 |
| Moderate social support | 291 | 38.1 | |
| Poor social support | 303 | 39.7 | |
| Depression | No | 427 | 55.9 |
| Yes | 337 | 44.1 | |
| Body Mass Index | Normal weight | 600 | 78.5 |
| Under weight | 23 | 3.1 | |
| Over weight | 121 | 15.8 | |
| Obese | 20 | 2.6 |
NCD = Non communicable disease
Prevalence of physical inactivity among older adults
The overall prevalence of physical inactivity among older adults in Gondar town was 65.6% (95% CI: 62.1–68.9). The proportion of physical inactivity among significant variables was as follows: 162 (32%) participants were female, 80 (16%) participants were > = 80 years old, 185 (36.9%) participants completed primary education, 126 (25%) participants had no formal education, 448 (89.4%) older adults were unemployed, and 321 (64.1%) had depression symptoms.
Factors associated with physical inactivity among older adults
All variables were undertaken into bivariable analysis to identify the potential candidates for multivariable logistic regression. Thus, nine variables were found significant at p-value < 0.25 in a binary logistic regression analysis. The variables included age, sex, marital status, educational level, occupation, monthly income, body mass index, non-commendable disease, and depression. Then all nine variables were entered into multivariable logistic regression to identify statistically significant independent variables that are predictors of physical inactivity among older adults. Of all independent variables fitted into multivariable logistic regression analysis, only five variables (age, sex, educational level, occupation, and depression) were found to have a significant associated with physical inactivity among older adults. Regarding sex, the odds of likebeing female were about three times higher than those who were males (AOR: 3.053, 95% CI: 1.487–6.267). Older adults who were 80 years of age and older were 4.048 times more likely to be physically inactive (AOR: 4.082, 95% CI: 1.234–13.497) as compared to the 60–69-year-old age group. Unemployed participants had a 10.273 fold increased risk of developing physical inactivity (AOR: 10.273, 95% CI: 5.628–18.753) as compared to employed participants. Similarly, primary school level participants were 3.020 times more likely to be physically inactive (AOR: 3.020, 95% CI: 1.433–6.367) as compared to college and above levels. Older adults who had symptoms of depression were 7.152 times more likely to be physically inactive (AOR: 7.152, 95% CI: 4.786–17.965) as compared to those who had no symptoms of depression (See Table 3).
Table 3.
Factors associated with physical inactivity among older adults in Gondar town, Northwest Ethiopia, 2022 (N = 764)
| Variables | Categories | Physical inactivity | COR (95%CI) | AOR (95%CI) | |
|---|---|---|---|---|---|
| No | Yes | ||||
| Sex | Male | 238 | 339 | 1 | 1 |
| Female | 25 | 162 | 4.549(2.893–7.153) | 3.053(1.487–6.267) ** | |
| Age | 60–69 | 238 | 312 | 1 | 1 |
| 70–79 | 17 | 109 | 4.891(2.855–8.378) | 1.093(0.393–3.043) | |
| >=80 | 8 | 80 | 7.628(3.617–16.086) | 4.082(1.234–13.497) * | |
| Marital status | Married | 239 | 284 | 1 | 1 |
| Divorced | 4 | 40 | 8.415(2.968–23.860) | 2.308(0.465–11.463) | |
| Separated | 5 | 26 | 4.376(1.655–11.572) | 2.617(0.537–12.755) | |
| Windowed | 15 | 151 | 8.472(4.850-14.798) | 1.501(0.585–3.842) | |
| Education level | >=College | 133 | 76 | 1 | 1 |
| Secondary | 95 | 114 | 2.100(1.419–3.107) | 1.086(0.558–2.114) | |
| Primary | 27 | 185 | 11.991(7.328–19.620) | 3.020(1.433–6.367) ** | |
| No formal educates | 8 | 126 | 27.563(12.785–59.420) | 8.573(2.843–25.847) ** | |
| Occupation | Employed | 204 | 53 | 1 | 1 |
| Unemployed | 59 | 448 | 29.227(19.470-43.872) | 10.273(5.628–18.753)** | |
| Monthly Income | > 5000 | 52 | 14 | 1 | 1 |
| 3001–5000 | 98 | 49 | 1.857(0.938-3.675) | 0.600(0.215–1.672) | |
| 1001–3000 | 104 | 314 | 11.214(5.970-21.065) | 0.839(0.302–2.327) | |
| < 1000 | 9 | 124 | 51.175(20.852-125.595) | 1.564(0.356–6.865) | |
| Body Mass Index | Normal | 222 | 378 | 1 | 1 |
| Under wt. | 6 | 17 | 2.664(0.647–4.283) | 0.518(0.103–2.593) | |
| Over wt. | 26 | 95 | 2.146(1.349–3.414) | 1.170(0.508–2.697) | |
| Obese | 9 | 11 | 0.718(0.293-1.759) | 0.529(0.110–2.553) | |
| NCDs | No | 212 | 247 | 1 | 1 |
| Yes | 51 | 254 | 4.275(3.005–6.080) | 1.416(0.750–2.673) | |
| Depression | No | 247 | 180 | 1 | 1 |
| Yes | 16 | 321 | 27.530(12.776–58.410) | 7.152(4.786–17.965) ** | |
* Statistically significant at p < 0.05, **highly statistically significant at p < 0.01, 1 = constant, COR = crude odd ratio, AOR = adjusted odd ratio, CI = confidence interval, NCD = non communicable disease, wt. =weight
Discussion
This study aimed to determine the prevalence of physical inactivity and its associated factors among older adults in Gondar town, Northwest Ethiopia. The overall prevalence of physical inactivity among the older population in Gondar town, northwest Ethiopia, was 65.6% (95% CI: 62.1–68.9), and age, sex, educational level, occupation, and depression were variables significantly associated with physical inactivity among older adults. The current study finding was consistent with the prevalence of physical inactivity among middle-aged and older adults reported from China (63.1%) [14]. This similarity might be due to the pandemic occurrence of physical inactivity [33]. However, the findings of the current study were lower compared with the study conducted in Brazil (70.1%) [18]. These differences might be related to the lower cutoff criteria of the tool used by the Brazilian study, which is defined as “physical inactivity” as engaging in regular activities that take up fewer than 60 min per week. Nonetheless, the current study’s cut-off points were higher, with regular activities being defined as those that last fewer than 150 min each week.
In addition, the finding of the current study were higher than the study done among adults in Harar town, Ethiopia (45.5%) [23]. This could be due to the fact that the study populations are different, where the study in Harari used adult participants in the age range of 18–64 years old. Evidence also showed that as age increases, physical inactivity increases, resulting in rapid declines in physical activity level, mobility, and functional independence [34]. The present study finding were also higher than studies done in Brazil (50.7%) [13], Malaysia (48.8%) [20], China (24.1%), India (22.0%), and South Africa (50.9%) [35]. This discrepancy might be due to a different physical activity measurement tool and the study participant’s age. For example, the participants’ ages in China, India, and South Africa studies were ≥ 50 years old. Evidence also supported that physical inactivity increased with increasing age, 25.4% among adults aged 50–64 years, 26.9% among those aged 65–74 years, and 35.3% among those aged ≥ 75 years in the USA [36]. An increase in age leads to a decline in physical activity which is attributed loss of muscle mass, physical strength, endurance, flexibility, living a sedentary life, and a loss of functional health [37]. In addition, our study finding is also higher than studies in Malaysia and Brazil due to the short version of the International Physical Activity Questionnaire (IPAQ) to assess physical activity levels where many simple activities were considered as physical activity.
According to the findings of the present study, females were more likely to be physically inactive than males. The finding is supported by the studies done in Ireland [19], China in Shenzhen [14], Malaysia [20], and the USA [36]. The reasons could be due to the fact that females have been affected by various factors, including family and societal roles, physical and psychological issues, and life conditions between men and women [38]. In addition, elderly women are not willing to do physical activity as they are engaged in excess family care, caring for children, and are prone to disease, marriage, and the fear of falling [39]. Thus, women in the current study setting are the main caregivers and home managers in the family, which tends to restrict their participation in physical activities.
The results of this study also showed that people aged 80 and older were four times more likely to be physically inactive than people aged 60 to 69 years. This study is supported by the studies done in Brazil [18] and Malaysia [15]. Evidence also showed increased inactivity in older individuals due to poor health and psychosocial factors such as lack of family support, loneliness, fear of physical injury during exercise, and lack of interest [40]. Another reason could be that the majority of the older age group in this study were retired, which leads to sedentary physical activities.
In addition, the current study finding showed that those who have no formal education and were in primary school were significantly associated with physical inactivity compared to college and those above educational levels. This study’s findings were consistent with the research studies in Brazil [18] and Italy [38]. The reason could be that people with a higher level of education might have a better understanding of the benefits of adequate physical activity and adopt a healthy lifestyle [41].
This study finding also revealed that physical inactivity among unemployed participants were ten times higher compared with employed participants. The finding is also supported by the studies done in Malaysia [15] and low and middle-income countries [35]. These might be unemployed participants who are less likely to perform the recommended level of physical activity that leads to a more sedentary and inactive lifestyle as a result of low socioeconomic status and poor access to resources for participation in physical activity [42, 43].
Furthermore, the present study finding showed that older adults with depression were more likely to be physically inactive as compared to non-depressed older adults. These findings were consistent with the studies done in Italy [38] and Brazil [44]. The possible reason could be that depressed older adults might have diminished interest, pleasure, lack of energy, psychomotor retardation or decreased cognitive function, and lifestyle factors [45].
Strength and limitation of the study
Our study’s strength was that we assessed physical inactivity using the GPAQ, which has been validated in low- and middle-income countries, to measure physical inactivity and that we adhered to the GPAQ analysis protocol. In addition, we used a large sample size. However, the main limitations of this study were that it did not assess cultural and environmental factors for physical activity, and the data were based on self-reports, which were subjected to recall bias.
Conclusion
Physical inactivity was relatively high among older adults in Gondar town. Being female, older age, having low levels of education, being unemployed, and having depression symptoms were associated with physical inactivity. We suggest promoting the health benefits of physical activity among females by reducing their burden, older age groups, and unemployed older adults and avoiding depression among individuals.
Acknowledgements
First, we would like to express our gratitude to the University of Gondar for providing their ethical permission and financial support. We would also like to say thanks to the Gondar town administration for giving us the necessary information. Finally, we would like to extend our heartfelt thanks to our data collectors and study participants for their civility and cooperation throughout the data collection process.
Abbreviations
- AOR
Adjusted Odd Ratio
- BMI
Body Mass Index
- CI
Confidence Interval
- CM
Centimeter
- COR
Crude Odd Ratio
- ETB
Ethiopian Birr
- GDS
Geriatrics Depression Scale
- GPAQ
Global Physical Activity Questionnaire
- KG
Kilogram
- MET
Metabolic Equivalents of Task
- NCDs
Non-Communicable Diseases
- OSSS
Oslo Social Support Scale
- PA
Physical Activity
- SPSS
Statistical Package of Social Science
Author contributions
KBS was involved primarily in the initial idea conception, wrote a proposal, lead the data collection activity, analysed the data, and drafted the manuscript. ESY, AZ, MMY, KBS, GJB, MA, AKK, and MG were also responsible for critically appraising the proposal, conducting data analysis and writing the first draft. MDT and YA critically revised the manuscript and prepared Fig. 1; Table 3. In addition, the final version of the manuscript was also approved by all of the authors.
Funding
This study received no financial support from any government, private, or non-profit organization.
Data availability
The manuscript contains all of the data that is crucial to our findings. Requests for additional information on the dataset and questions about data sharing will be treated in accordance with a reasonable request to uogbelay@gmail.com.
Declarations
Ethical consideration
This study was conducted in accordance with the Helsinki Declaration. Ethical clearance was obtained from the Research and Ethical Review Committee of the School of Medicine at the University of Gondar. Moreover, a support letter was obtained from the Department Physiotherapy, School of Medicine, University of Gondar. The purposes and importance of the study were explained to the participants, and written informed consent was obtained from each participant. All participants’ privacy and confidentiality were kept confidential at all levels.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.He W, Goodkind D, Kowal PR. An aging world: 2015. United States Census Bureau Washington, DC; 2016.
- 2.Prince MJ, Wu F, Guo Y, Robledo LMG, O’Donnell M, Sullivan R, et al. The burden of disease in older people and implications for health policy and practice. The Lancet. 2015;385(9967):549–62. doi: 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- 3.Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23(3):471–87. doi: 10.1123/japa.2014-0164. [DOI] [PubMed] [Google Scholar]
- 4.Sparling PB, Howard BJ, Dunstan DW, Owen N. Recommendations for physical activity in older adults. BMJ. 2015;350. [DOI] [PubMed]
- 5.Haileamlak A. Physical inactivity: the major risk factor for non-communicable diseases. Ethiop J Health Sci. 2019;29(1):810. doi: 10.4314/ejhs.v29i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organization WH. Global health risks: mortality and burden of disease attributable to selected major risks. World Health Organization; 2009.
- 7.Lee I-M, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. The Lancet. 2012;380(9838):219–29. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kankeu HT, Saksena P, Xu K, Evans DB. The financial burden from non-communicable diseases in low-and middle-income countries: a literature review. Health Res Policy Syst. 2013;11(1):1–12. doi: 10.1186/1478-4505-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding D, Kolbe-Alexander T, Nguyen B, Katzmarzyk PT, Pratt M, Lawson KD. The economic burden of physical inactivity: a systematic review and critical appraisal. Br J Sports Med. 2017;51(19):1392–409. doi: 10.1136/bjsports-2016-097385. [DOI] [PubMed] [Google Scholar]
- 10.Lee I-M, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. The Lancet. 2012;380(9838):219–29. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. The Lancet. 2012;380(9838):247–57. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 12.Gomes M, Figueiredo D, Teixeira L, Poveda V, Paul C, Santos-Silva A, et al. Physical inactivity among older adults across Europe based on the SHARE database. Age Ageing. 2017;46(1):71–7. doi: 10.1093/ageing/afw165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gobbi S, Sebastiao E, Papini CB, Nakamura PM, Valdanha Netto A, Gobbi LTB et al. Physical inactivity and related barriers: a study in a community dwelling of older brazilians. Journal of aging research. 2012;2012. [DOI] [PMC free article] [PubMed]
- 14.Zhou Y, Wu J, Zhang S, Yan S, He L, Mkandawire N, et al. Prevalence and risk factors of physical inactivity among middle-aged and older Chinese in Shenzhen: a cross-sectional study. BMJ open. 2018;8(10):e019775. doi: 10.1136/bmjopen-2017-019775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan YY, Lim KK, Omar MA, Mohd Yusoff MF, Sooryanarayana R, Ahmad NA, et al. Prevalence and factors associated with physical inactivity among older adults in Malaysia: a cross-sectional study. Geriatr Gerontol Int. 2020;20:49–56. doi: 10.1111/ggi.13977. [DOI] [PubMed] [Google Scholar]
- 16.Peltzer K, Phaswana-Mafuya N. Physical inactivity and associated factors in older adults in South Africa: geriatrics. Afr J Phys Health Educ Recreation Dance. 2012;18(3):447–60. [Google Scholar]
- 17.EPHI F. WHO, Ethiopia STEPS. Report on risk factors for chronic non-communicable diseases and Prevalence of selected NCDs. Ethiopian Public Health Institute, Addis Ababa, Ethiopia; 2016.
- 18.Ribeiro AQ, Salgado SML, Gomes IS, Fogal AS, Martinho KO, Almeida LFF, et al. Prevalence and factors associated with physical inactivity among the elderly: a population-based study. Revista Brasileira De Geriatria E Gerontologia. 2016;19:483–93. doi: 10.1590/1809-98232016019.150047. [DOI] [Google Scholar]
- 19.Murtagh EM, Murphy MH, Murphy NM, Woods C, Nevill AM, Lane A. Prevalence and correlates of physical inactivity in community-dwelling older adults in Ireland. PLoS ONE. 2015;10(2):e0118293. doi: 10.1371/journal.pone.0118293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan YY, Sooryanarayana R, Kasim NM, Lim KK, Cheong SM, Kee CC, et al. Prevalence and correlates of physical inactivity among older adults in Malaysia: findings from the National Health and Morbidity Survey (NHMS) 2015. Arch Gerontol Geriatr. 2019;81:74–83. doi: 10.1016/j.archger.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Marques A, Santos T, Martins J, Matos MGD, Valeiro MG. The association between physical activity and chronic diseases in European adults. Eur J Sport Sci. 2018;18(1):140–9. doi: 10.1080/17461391.2017.1400109. [DOI] [PubMed] [Google Scholar]
- 22.de Sousa BA, da Silva FC, Ribeiro ÍL, de Lima DB, da Silva R. Sociodemographic, anthropometric, functional and psychosocial factors associated with physical activity in older adults. Ageing Int. 2022;47(4):617–29. doi: 10.1007/s12126-021-09450-w. [DOI] [Google Scholar]
- 23.Abdeta C, Teklemariam Z, Seyoum B. Prevalence of physical inactivity and associated factors among adults in Harar town, Eastern Ethiopia. Baltic J Health Phys Activity. 2018;10(2):8. doi: 10.29359/BJHPA.10.2.08. [DOI] [Google Scholar]
- 24.refworld. Vulnerability of older people in Ethiopia the Case of Oromia, Amhara and SNNP Regional States. HelpAge International in Ethiopia; 2013.
- 25.Organization WH. Global physical activity questionnaire (GPAQ) analysis guide. Geneva: World Health Organization. 2012:1–22.
- 26.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clinical gerontology: Routledge; 2014. pp. 165–73. [Google Scholar]
- 27.Kocalevent R-D, Berg L, Beutel ME, Hinz A, Zenger M, Härter M, et al. Social support in the general population: standardization of the Oslo social support scale (OSSS-3) BMC Psychol. 2018;6(1):1–8. doi: 10.1186/s40359-018-0249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright C. Standardized methods for the regulation of cigarette-smoke constituents. TRAC Trends Anal Chem. 2015;66:118–27. doi: 10.1016/j.trac.2014.11.011. [DOI] [Google Scholar]
- 29.Van Bui T, Blizzard CL, Luong KN, Van Truong NL, Tran BQ, Otahal P, et al. Alcohol consumption in Vietnam, and the use of ‘standard drinks’ to measure alcohol intake. Alcohol Alcohol. 2016;51(2):186–95. doi: 10.1093/alcalc/agv082. [DOI] [PubMed] [Google Scholar]
- 30.Adult Body. Mass Index or BMI. CDC. 2021.
- 31.Armstrong T, Bull F. Development of the world health organization global physical activity questionnaire (GPAQ) J Public Health. 2006;14(2):66–70. doi: 10.1007/s10389-006-0024-x. [DOI] [Google Scholar]
- 32.Chu AH, Ng SH, Koh D, Müller-Riemenschneider F. Reliability and validity of the self-and interviewer-administered versions of the global physical activity questionnaire (GPAQ) PLoS ONE. 2015;10(9):e0136944. doi: 10.1371/journal.pone.0136944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohl HW, Craig CL, Lambert EV, Inoue S, Alkandari JR, Leetongin G, et al. The pandemic of physical inactivity: global action for public health. The Lancet. 2012;380(9838):294–305. doi: 10.1016/S0140-6736(12)60898-8. [DOI] [PubMed] [Google Scholar]
- 34.Hong S. The prevalence and determinants of physical activity among Korean older adults and its implications for public health. J Aging Sci. 2016;4(3):3–7. [Google Scholar]
- 35.Koyanagi A, Stubbs B, Smith L, Gardner B, Vancampfort D. Correlates of physical activity among community-dwelling adults aged 50 or over in six low-and middle-income countries. PLoS ONE. 2017;12(10):e0186992. doi: 10.1371/journal.pone.0186992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson KB, Carlson SA, Gunn JP, Galuska DA, O’Connor A, Greenlund KJ, et al. Physical inactivity among adults aged 50 years and older—United States, 2014. Morb Mortal Wkly Rep. 2016;65(36):954–8. doi: 10.15585/mmwr.mm6536a3. [DOI] [PubMed] [Google Scholar]
- 37.Suryadinata RV, Wirjatmadi B, Adriani M, Lorensia A. Effect of age and weight on physical activity. J Public Health Res. 2020;9(2):jphr. doi: 10.4081/jphr.2020.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giuli C, Papa R, Mocchegiani E, Marcellini F. Predictors of participation in physical activity for community-dwelling elderly italians. Arch Gerontol Geriatr. 2012;54(1):50–4. doi: 10.1016/j.archger.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Krug RR, Lopes MA, Mazo GZ. Barriers and facilitators for the practice of physical activity in old and physically inactive women. Revista Brasileira De Medicina do Esporte. 2015;21:57–64. doi: 10.1590/1517-86922015210101673. [DOI] [Google Scholar]
- 40.Moschny A, Platen P, Klaaßen-Mielke R, Trampisch U, Hinrichs T. Barriers to physical activity in older adults in Germany: a cross-sectional study. Int J Behav Nutr Phys Activity. 2011;8(1):1–10. doi: 10.1186/1479-5868-8-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, While AE, Hicks A. Physical activity among older people living alone in Shanghai, China. Health Educ J. 2015;74(2):156–67. doi: 10.1177/0017896914523943. [DOI] [Google Scholar]
- 42.Ying C, Kuay LK, Huey TC, Hock LK, Abd Hamid HA, Omar MA, et al. Prevalence and factors associated with physical inactivity among Malaysian adults. Southeast Asian J Trop Med Public Health. 2014;45(2):467. [PubMed] [Google Scholar]
- 43.Eime RM, Charity MJ, Harvey JT, Payne WR. Participation in sport and physical activity: associations with socio-economic status and geographical remoteness. BMC Public Health. 2015;15(1):1–12. doi: 10.1186/s12889-015-1796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souza AM, Fillenbaum GG, Blay SL. Prevalence and correlates of physical inactivity among older adults in Rio Grande do sul, Brazil. PLoS ONE. 2015;10(2):e0117060. doi: 10.1371/journal.pone.0117060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wassink-Vossen S, Collard RM, Voshaar RCO, Comijs HC, de Vocht HM, Naarding P. Physical (in) activity and depression in older people. J Affect Disord. 2014;161:65–72. doi: 10.1016/j.jad.2014.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The manuscript contains all of the data that is crucial to our findings. Requests for additional information on the dataset and questions about data sharing will be treated in accordance with a reasonable request to uogbelay@gmail.com.