Abstract
We have previously shown that the addition of exogenous granulocyte-macrophage colony-stimulating factor (GM-CSF) to nonactivated mouse peritoneal macrophages (MPM) limits Trypanosoma cruzi infections in vitro (E. Olivares Fontt and B. Vray, Parasite Immunol. 17:135–141, 1995). Lower levels of infection were correlated with a higher level of production of tumor necrosis factor alpha (TNF-α) in the absence of nitric oxide (NO) release. These data suggested that GM-CSF and/or TNF-α might have a direct parasitocidal effect on T. cruzi trypomastigotes, independently of NO release. To address this question, T. cruzi trypomastigotes were treated with recombinant murine GM-CSF (rmGM-CSF), recombinant murine TNF-α (rmTNF-α), or both cytokines in a cell-free system. Treatment with rmGM-CSF but not rmTNF-α caused morphological changes in the parasites, and most became spherical after 7 h of incubation. Both cytokines exerted a cytolytic activity on the trypomastigotes, yet the trypanolytic activity of rmTNF-α was more effective than that of rmGM-CSF. Viable rmGM-CSF- and rmTNF-α-treated parasites were less able to infect MPM than untreated parasites, and this reduction in infectivity was greatest for rmGM-CSF. Treatments with both cytokines resulted in more lysis and almost complete inhibition of infection. The direct parasitocidal activity of rmTNF-α was inhibited by carbohydrates and monoclonal antibodies specific for the lectin-like domain of TNF-α. Collectively, these results suggest that cytokines such as GM-CSF and TNF-α may directly control the level of T. cruzi trypomastigotes at least in vitro and so could determine the outcome of infection in vivo.
Trypanosoma cruzi is a hemoflagellate protozoan parasite that infects humans and domestic and wild mammals. It is the etiological agent of Chagas’ disease, a major public health problem in South and Central America (34). Among other cells (fibroblasts, muscle cells, and nerve cells), macrophages are infected by T. cruzi trypomastigotes. Murine macrophages activated with gamma interferon (IFN-γ) control T. cruzi infection by producing nitric oxide (NO) (11, 25, 29, 37) increased by tumor necrosis factor alpha (TNF-α) (26), another cytokine which inhibits the intracytoplasmic multiplication of T. cruzi (3, 7).
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a multipotent cytokine released from various cell types, including macrophages (8, 15). GM-CSF induces the proliferation and differentiation of hematopoietic progenitor cells and stimulates the effector functions of macrophages (6, 13, 31). Recently, we have shown that high autocrine production of GM-CSF or an exogenous supply of GM-CSF can reduce T. cruzi infection by two pathways. First, IFN-γ-activated mouse peritoneal macrophages (MPM) treated with recombinant mouse GM-CSF (rmGM-CSF) limit infection by increasing the release of TNF-α and NO. Second, an exogenous supply of rmGM-CSF to nonactivated macrophages also limits T. cruzi infection and is associated with an enhanced production of TNF-α. Surprisingly, this control occurred in the absence of detectable NO production (28). Furthermore, we have shown that injection of T. cruzi-infected mice with neutralizing anti-GM-CSF monoclonal antibodies (MAbs) induced the early appearance of parasitemia and aggravated cumulative mortality. In contrast, rmGM-CSF caused sharp decreases in both parasitemia and cumulative mortality in T. cruzi-infected mice (27).
TNF-α exerts a cytostatic effect on Trypanosoma musculi (19) and has cytolytic activity against Trypanosoma brucei (22). The cytolytic effect of TNF-α is mediated by a lectin-like domain (TIP domain) situated at the upper side of the triangular pyramide shape of this cytokine. This TIP domain is involved in the lysis of T. brucei by TNF-α and is different from the mammalian TNF-α receptor binding site. Cytolysis by recombinant murine TNF-α (rmTNF-α) is inhibited both by N,N′-diacetylchitobiose (a disaccharide binding TIP) and by an anti-TNF-α TIP MAb directed against amino acids 99 to 115 of mouse TNF-α (23, 24), suggesting that TNF-α recognizes glycosylated molecules expressed on African trypanosomes.
Taken together, these observations suggested that GM-CSF and/or TNF-α might have a direct effect on T. cruzi trypomastigotes. The aim of this study was to test whether GM-CSF and TNF-α have a direct parasiticidal effect on trypomastigotes, the extracellular form of the parasite which invades host cells.
MATERIALS AND METHODS
Parasites.
T. cruzi (Tehuantepec strain) was maintained by weekly intraperitoneal inoculations in BALB/c mice. To obtain large amount of parasites, trypomastigotes (2.5 × 105/rat) were inoculated to 700-rad X-ray-irradiated F344 Fischer rats (Iffa Credo, Brussels, Belgium). Trypomastigotes were obtained from the blood (containing 10 U of heparin/ml) of infected rats by ion-exchange chromatography on DEAE-cellulose (Whatman DE52) equilibrated with phosphate-saline-glucose buffer at pH 7.4 (14, 20). Trypomastigotes were centrifuged (15 min, 1,800 × g, 4°C) and resuspended in endotoxin-free phosphate-buffered saline (PBS) (30, 38). T. cruzi epimastigotes were grown in GLSH medium (17, 38) at 28°C. Trypomastigotes and epimastigotes were resuspended in PBS supplemented with 1% d-glucose and 1% normal mouse serum (PBS-G).
Cytokines and reagents.
rmGM-CSF was obtained by isolating the GM-CSF gene by PCR from the vector pCDNA I Amp-ORF GM-CSF, a generous gift of J. C. Renaud (Brussels, Belgium). It was cloned as a BglII-HindIII fragment in the bacterial expression vector pQE 30 (Qiagen GmbH, Hilden, Germany), giving a 5′ His6 tail. The resulting plasmid, pQE-moGMCSF, was transformed into Escherichia coli M15 cells. The expression of the recombinant protein was induced by the addition of 1 mM isopropyl-β-d-thiogalactoside (Immunosource, Zoersel, Belgium). After 3 h of incubation, most of the rmGM-CSF was present in the bacteria as inclusion bodies (4). The inclusion bodies were dissolved in lysis buffer (8 M urea, 0.1 M NaH2PO4, 0.01 M Tris [Sigma Chemical Co., St. Louis, Mo.] [pH 8.0]) and loaded onto a Ni2+-nitrilotriacetic acid column (Qiagen). Bound rmGM-CSF was renatured by washing the column for 60 min with renaturation buffer (250 mM NaCl, 0.1 M Tris [pH 8.0]). Renatured rmGM-CSF was eluted from the column with a linear pH gradient. The purity of the eluted protein was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). rmGM-CSF appeared on the gel as a discrete band of 16.5 kDa. The biological activity of the rmGM-CSF was assessed in a proliferation assay (incorporation of 3H-labeled thymidine) with the GM-CSF-dependent cell line NFS-60. rmGM-CSF was diluted in endotoxin-free PBS, aliquoted, and stored at −70°C until use. The endotoxin concentration was less than 15 pg/ml, as determined by the endotoxin test (Chromogenic, Mölndal, Sweden).
Recombinant murine IL-2 (rmIL-2) was used as the protein control and was produced in the same way as rmGM-CSF. Briefly, the mouse IL-2 gene was amplified from pRc-RSV-moIL-2, a generous gift of J. C. Renaud. By using PCR, a 5′ BamHI site and a 3′ KpnI site were added. The amplified product was sequenced and cloned in pQE 30 as a KpnI-BamHI fragment. This ligation resulted in the addition of a 5′ His6 tail. Plasmid pQE-moIL-2 was transformed into E. coli M15 cells. rmIL-2 was produced in the same way as rmGM-CSF. Purity was assessed by SDS-PAGE. rmIL-2 appeared on the gel as a single discrete band of 17 kDa. The biological activity of the rmIL-2 was assessed in a proliferation assay with the IL-2-dependent cell line CTLL-2. rmIL-2 was diluted in endotoxin-free PBS, aliquoted, and stored at −70°C until use. The endotoxin concentration was less than 15 pg/ml, as determined by the endotoxin test (Chromogenic).
TNF-α was obtained from Innogenetics (Ghent, Belgium). It was stored at −70°C until use in endotoxin-free PBS. The endotoxin concentration of the TNF-α solution was less than 15 pg/ml, as determined by the endotoxin test (Chromogenic).
Cytotoxic assay.
T. cruzi trypomastigotes (106/ml) were suspended in PBS-G; rmGM-CSF and rmTNF-α were added at the appropriate concentrations. The parasite suspensions were then incubated at 37°C in a 5% CO2 and water-saturated atmosphere for various time intervals. An aliquot (10 μl) was harvested every 30 min, and the number of parasites (motile and nonmotile trypomastigotes) was determined by counting in a Thoma’s chamber under a light microscope. The viability of T. cruzi trypomastigotes was evaluated with the MTT [3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide] test (16). Briefly, parasites were incubated with 25 μl of MTT (Sigma) for 4 h at 37°C; 100 μl of lysis buffer (20% [wt/vol] SDS in 50% N,N-dimethyl formamide) was added, and the parasites were incubated overnight at 37°C for color development. Optical density was then measured directly at 540 nm in an enzyme-linked immunosorbent assay reader (Titertek Multiscan MCC/340 MKII; ELAB, Helsinki, Finland). Parasites were fixed with methanol and stained with Giemsa stain. They were then observed under the light microscope to assess any morphological changes. Trypomastigotes were incubated with MPM (see below) to test their infectivity after cytokine treatment.
rmGM-CSF activity was neutralized by incubation for 1 h 30 with a neutralizing rat anti-GM-CSF MAb (MP1-22E9; immunoglobulin G2aκ; 10 μg/ml; Endogen, Boston, Mass.) or another neutralizing rat anti-GM-CSF MAb (MP1-31G6; immunoglobulin G1κ; 10 μg/ml; Endogen). Trypomastigotes were treated with isotype-matched MAb, PBS-G, or rmIL-2 (control protein).
rmTNF-α activity was inhibited by incubation for 1 h 30 either with N,N′-diacetylchitobiose (1 μg/ml, Sigma) (20, 31), an anti-TNF-α TIP MAb (10 μg/ml) (23, 24), or the anti-TNF-α MAb 1F3F3 (10 μg/ml) (21). Trypomastigotes were treated with an isotype-matched MAb (10 μg/ml) or PBS-G as a control.
Infection of MPM with T. cruzi.
MPM were harvested from mice by washing their peritoneal cavities with chilled Hanks’ balanced salt solution without Ca2+ and Mg2+ (pH 7.4) (GIBCO, Grand Island, N.Y.). They were allowed to adhere (105 cells/well) in eight-chamber LabTek slides (Nunc, Roskilde, Denmark) for 2 h at 37°C in a 5% CO2 atmosphere. They were cultured in RMPI 1640 medium supplemented with HEPES (25 mM), glutamine (2 mM), fetal calf serum (10%; mycoplasma free and endotoxin concentration less than 27.5 pg/ml), penicillin (100 IU/ml), and streptomycin (100 μg/ml) and incubated at 37°C in a 5% CO2 atmosphere. Nonadherent cells were removed by washing with prewarmed RPMI 1640.
Trypomastigotes (106 parasites/ml) were incubated with rmGM-CSF and/or rmTNF-α for 7 h and washed in PBS. Cytokine-treated trypomastigotes (300 μl) were then added to MPM. After 16 h, the cultures were washed to remove free parasites and cells were incubated for 48 h. Cells were fixed with methanol and stained with Giemsa stain. The percentage of infected MPM was recorded after examination under a light microscope of at least 200 cells per well.
RESULTS
rmGM-CSF alters the morphotype and impairs the infectivity of T. cruzi trypomastigotes.
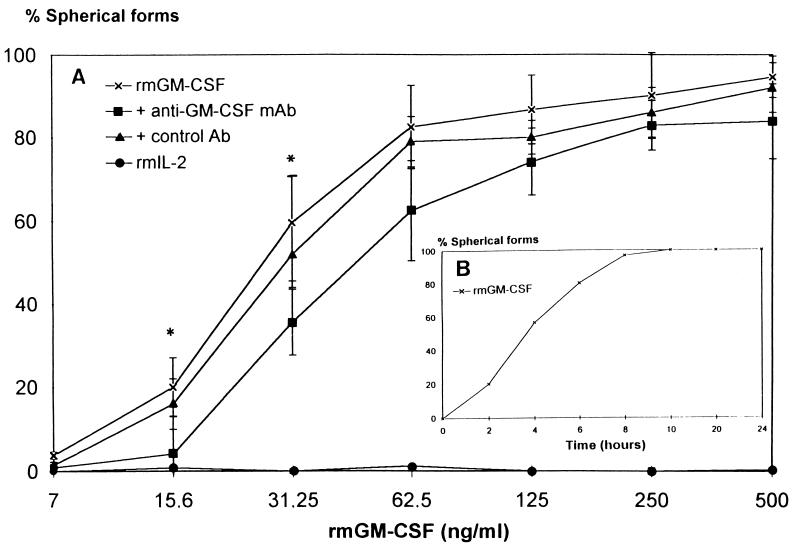
Suspensions of T. cruzi trypomastigotes were treated for 7 h with graded concentrations of rmGM-CSF, and morphological changes (transitions from slender to spherical forms) were monitored under a light microscope. rmGM-CSF induced the spherical morphotype in a concentration-dependent manner (Fig. 1A). Most of the trypomastigotes (82% ± 10% [mean ± standard error of the mean {SEM}]) changed morphotype and became spherical following treatment with 62.5 ng of rmGM-CSF per ml, whereas only 17% ± 5% remained slender. The formation of spherical forms was inhibited by an anti-GM-CSF MAb (MP1-22E9) at low concentrations of rmGM-CSF (15.6 and 31.25 pg/ml) only. Another neutralizing anti-GM-CSF MAb (MP1-31G6) was tested, but it did not inhibit the formation of spherical forms induced by rmGM-CSF treatment (data not shown). Morphotype changes were already evident after 3 h of incubation with rmGM-CSF (62.5 ng/ml) (Fig. 1B). Most (92% ± 20%) of the spherical trypomastigotes were viable as determined with the MTT test (16). No morphological changes or impaired motility was observed with rmIL-2-treated trypomastigotes. Similar experiments with T. cruzi epimastigotes showed that rmGM-CSF treatment did not affect the morphotype of this insect-specific form (data not shown).
FIG. 1.
Effect of rmGM-CSF on the morphotype of T. cruzi trypomastigotes. (A) T. cruzi trypomastigotes were incubated with rmGM-CSF, or with a mixture of rmGM-CSF and an anti-GM-CSF MAb, a control MAb, or rmIL-2 for 7 h, and the percentage of spherical forms was counted. There were no spherical forms of trypomastigotes when parasites were treated with PBS-G, even after 24 h of incubation (not shown). Data are means ± SEM of three independent experiments performed in duplicate. ∗, P < 0.05 compared with trypomastigotes treated with rmGM-CSF alone (Mann-Whitney U test). (B) Kinetics of the morphotype change caused by rmGM-CSF determined by incubating T. cruzi trypomastigotes with rmGM-CSF (62.5 ng/ml) at 37°C for various time periods.
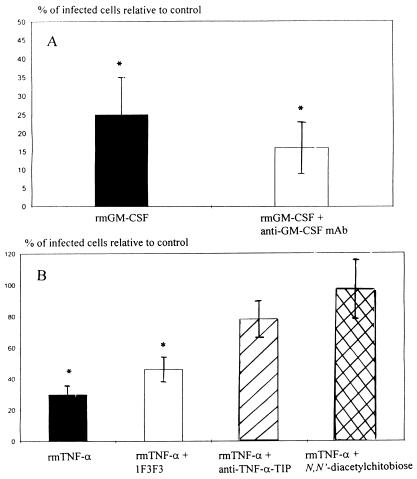
We tested whether the rmGM-CSF-mediated morphological changes affected the ability of T. cruzi trypomastigotes to infect MPM by comparing the infection of MPM by rmGM-CSF-treated trypomastigotes with a PBS control. The percentage of infected MPM (the index 100 corresponds to 60% ± 6% infected MPM) was sharply reduced (a 75% ± 10% reduction of infectivity was recorded) when trypomastigotes were incubated for 7 h with rmGM-CSF (62.5 ng/ml) (Fig. 2A). This proportion was reduced to a lesser extent (84% ± 7%) when parasites were treated with a mixture of rmGM-CSF and anti-GM-CSF MAb MP1-22E9 (Fig. 2A). Collectively, the data indicate that rmGM-CSF affects both the morphology and the infectivity of T. cruzi trypomastigotes.
FIG. 2.
Ability of rmGM-CSF- or rmTNF-α-treated T. cruzi trypomastigotes to infect MPM. (A) T. cruzi trypomastigotes were treated either with rmGM-CSF (62.5 ng/ml) or with a combination of rmGM-CSF (62.5 ng/ml) and anti-GM-CSF MAb MP1-22E9 (10 μg/ml). The parasites were incubated for 7 h, washed, and incubated with MPM for 16 h. Free parasites were removed by washing, the incubation was continued for 48 h, and the percentage of infected MPM was determined. The percentage of infected MPM is given relative to the percentage of MPM infected with untreated trypomastigotes as a control (the index 100 corresponds to 60% ± 6% infected MPM). Data are means ± SEM of three independent experiments performed in duplicate. ∗, P < 0.05 compared to MPM infected with PBS-G-treated trypomastigotes infecting MPM (Mann-Whitney U test). (B) T. cruzi trypomastigotes were treated either with rmTNF-α alone, with rmTNF-α in combination with either an anti-TNF-α MAb (1F3F3) or an anti-TNF-α TIP MAb, or with N,N′-diacetylchitobiose. The percentage of infected cells is presented as relative to macrophages infected with untreated trypomastigotes (control; 60% ± 6% of the MPM were infected by untreated trypomastigotes). Data are means ± SEM of three experiments performed in duplicate. ∗, P < 0.05 compared to data obtained with PBS-G-treated trypomatigotes infecting MPM (Mann-Whitney U test).
rmTNF-α exerts a cytolytic activity on T. cruzi trypomastigotes and reduces their infectivity.
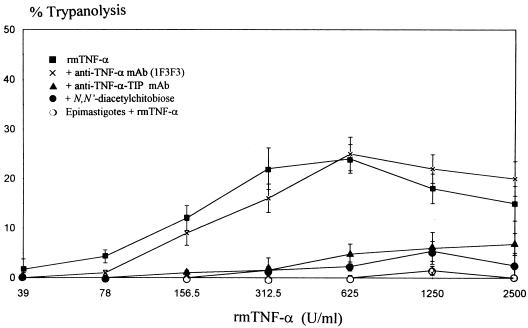
Incubation of T. cruzi trypomastigotes with rmTNF-α for 7 h caused lysis of the parasites in a concentration-dependent manner (Fig. 3). The nonlysed parasites remained slender, and there was no transition in morphotype (i.e., spherical forms). The cytolysis by rmTNF-α was inhibited by both N,N′-diacetylchitobiose (a disaccharide binding TIP) and an anti-TNF-α TIP MAb directed against the TIP sequence of mouse TNF-α (23, 24). This finding shows that the lectin-like domain of TNF-α is involved in this activity. However, an anti-TNF-α MAb (1F3F3), reported to neutralize the cytotoxic effect of TNF-α on mammalian cell lines (21), did not significantly inhibit TNF-α-mediated trypanolysis. No cytolysis was observed when epimastigotes were incubated with rmTNF-α under the same conditions (Fig. 3).
FIG. 3.
Cytolytic effect of rmTNF-α on T. cruzi trypomastigotes. T. cruzi trypomastigotes were treated with rmTNF-α or with rmTNF-α preincubated with either anti-TNF-α MAb 1F3F3, an anti-TNF-α TIP MAb, (▴), or N,N′-diacetylchitobiose. T. cruzi epimastigotes were not lysed by rmTNF-α. Data are means ± SEM of three independent experiments performed in triplicate.
When MPM were incubated with rmTNF-α-treated trypomastigotes, a 70% ± 6% reduction in infectivity was recorded (Fig. 2B). The capacity of rmTNF-α to reduce the infectivity of the parasites was not affected by prior incubation of the cytokine with MAb 1F3F3. In contrast, prior incubation of rmTNF-α with an anti-TNF-α TIP MAb or N,N′-diacetylchitobiose significantly lowered the capacity of rmTNF-α to reduce the infectivity of trypomastigotes (Fig. 2B). Thus, rmTNF-α lyses T. cruzi trypomastigotes and reduces their ability to infect MPM.
Combined activities of rmGM-CSF and rmTNF-α on T. cruzi trypomastigotes.
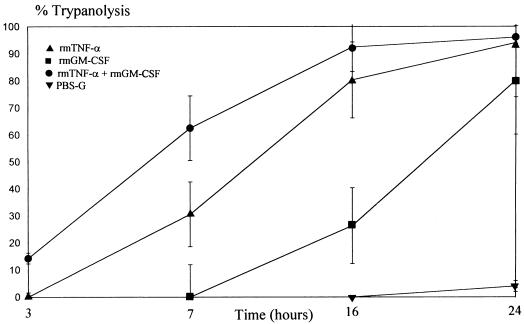
As rmGM-CSF and rmTNF-α had direct but different activities against T. cruzi trypomastigotes, we investigated effects on the parasite of the two cytokines. Incubation of trypomastigotes with rmGM-CSF (62.5 ng/ml) and rmTNF-α (2,500 U/ml) for 7 h resulted in a higher level of parasite lysis than with PBS-incubated trypomastigotes (P < 0.05, Mann-Whitney U test) (Table 1). Lysis of the trypomastigotes by rmTNF-α and rmGM-CSF was inhibited by the anti-TNF-α-TIP MAb or N,N′-diacetylchitobiose (Table 1), whereas an anti-GM-CSF MAb (MP1-22E9) did not affect this activity (data not shown). The percentage of spherical forms was drastically reduced after rmGM-CSF–rmTNF-α treatment, suggesting that these spherical forms were more susceptible to the lytic activity of the two cytokines. Treatment with rmTNF-α and rmGM-CSF together resulted in even lower ability of the cytokine-treated trypomastigotes to infect MPM (P < 0.05, Mann-Whitney U test). This additional reduction presumably reflects additive effects of rmTNF-α and rmGM-CSF on infectivity, the 50% reduction in the number of viable parasites, or both. As rmGM-CSF and rmTNF-α together lyse more parasites than rmTNF-α alone, we analyzed the kinetics of T. cruzi lysis by rmTNF-α and/or rmGM-CSF over 24 h. After 3 h of incubation, a low yet significant lysis was recorded with rmGM-CSF- and rmTNF-α-treated parasites only (Fig. 4). After 7 h of incubation, the parasites were lysed by rmTNF-α alone but there was still significantly more lysis with rmTNF-α and rmGM-CSF together (P < 0.05, Mann-Whitney U test). Incubating the parasites with rmTNF-α alone or with rmTNF-α and rmGM-CSF together for 24 h resulted in maximal lysis (94% ± 11% or 96% ± 6%, respectively). Interestingly, after 16 h of incubation with rmGM-CSF alone, lysis of the trypomastigotes started as well, and most of the parasites (80% ± 20%) were lysed by rmGM-CSF after 24 h.
TABLE 1.
Combined activities of rmGM-CSF and rmTNF-α on T. cruzi trypomastigotesa
| Cytokine treatment of trypomastigotes | Lysis (%) | Spherical forms (%) | Slender forms (%) | Infectivity (%)b |
|---|---|---|---|---|
| rmGM-CSF | 0 ± 0 | 80 ± 12 | 20 ± 5 | 4.2 ± 2 |
| rmTNF-α | 30 ± 5 | 0 ± 0 | 70 ± 15 | 22.8 ± 5 |
| rmGM-CSF + rmTNF-α | 50 ± 9 | 35 ± 8 | 14 ± 3 | 1.8 ± 1.5 |
| rmGM-CSF + rmTNF-α + anti-TNF-α-TIP | 5 ± 2 | 65 ± 6 | 30 ± 10 | 12 ± 4 |
| rmGM-CSF + rmTNF-α + N,N′-diacetylchitobiose | 10 ± 3 | 72 ± 12 | 18 ± 7 | 8 ± 6 |
T. cruzi trypomastigotes were incubated with rmGM-CSF (62.5 ng/ml), rmTNF-α (2,500 U/ml), or a combination of both cytokines. In some experiments, the combination of rmGM-CSF and rmTNF-α was first incubated with either an anti-TNF-α TIP MAb or N,N′-diacetylchitobiose. The trypomastigotes were washed after 7 h and the lysis, morphotypes and infectivities of the trypomastigotes were evaluated as described in Materials and Methods. As a control, MPM were incubated with untreated trypomastigotes (the index 100 corresponds to 60% ± 6% infected MPM). Data are means ± SEM of three independent experiments performed in duplicate.
For all values, P < 0.05 compared to MPM infected with untreated trypomastigotes (Mann-Whitney U test).
FIG. 4.
Cytolytic activity of rmGM-CSF and rmTNF-α on T. cruzi trypomastigotes. T. cruzi trypomastigotes were incubated with rmTNF-α (2,500 U), rmGM-CSF (62.5 ng/ml), a combination of both cytokines, or PBS-G. Lysis of the trypomastigotes was determined at different time intervals ranging from 3 h to 24 h of incubation. Data are means ± SEM of three independent experiments performed in duplicate.
DISCUSSION
We have previously reported that the addition of exogenous GM-CSF to nonactivated MPM substantially reduces T. cruzi infection in vitro (28). This reduction is correlated with higher levels of TNF-α production and occurs in the absence of detectable NO production. Thus, NO is not solely involved in the control of T. cruzi infections, at least in vitro. Furthermore, neutralization of endogenous GM-CSF with a neutralizing MAb aggravates whereas injection of rmGM-CSF decreases both parasitemia and cumulative mortality of T. cruzi-infected mice (27). These observations raised the possibility of a combined, direct effect of GM-CSF and TNF-α on T. cruzi trypomastigotes. Indeed, besides exerting pleiotropic effects on mammalian cells (18, 36), these two cytokines have been shown to interact directly with parasites; for instance, GM-CSF acts as a growth factor for promastigotes of Leishmania mexicana amazonensis (5), and TNF-α was reported to stimulate the growth of Schistosoma mansoni (1).
The direct effects of rmGM-CSF and rmTNF-α were thus tested separately on trypomastigotes. Our results indicate that the two cytokines affect T. cruzi trypomastigotes in different ways. (i) rmGM-CSF rapidly changes the morphotype of the trypomastigotes and strongly reduces their ability to infect MPM, although most of them remain alive. rmGM-CSF also lyses trypomastigotes but only after longer incubation periods (16 h). (ii) rmTNF-α has also a cytolytic effect on T. cruzi trypomastigotes, and lysis occurs after short incubation periods (7 h). Furthermore, TNF-α reduces the ability of trypomastigotes to infect MPM without affecting their morphology. The antiparasite action of both cytokines is specific for the infective form of the parasite because the vector form (epimastigotes) was completely resistant to the parasitocidal activities of rmGM-CSF and rmTNF-α.
Incubating the parasites in a medium containing rmGM-CSF cause rapidly the morphological changes of slender forms into amastigote-like forms which are noninfectious. Such changes also occur in medium devoid of cytokines. However, this transformation requires long incubation periods (24 to 48 h), and these amastigote-like forms are infectious (2). Incubation of T. cruzi trypomastigotes with rmIL-2 had no effect on the parasite, showing that rmGM-CSF had a specific activity because the two cytokines were prepared by the same procedure.
The cytolysis of T. cruzi by TNF-α is similar to the trypanolytic activity of this cytokine on African trypanosomes such as T. brucei. This trypanolytic activity is mediated by the lectin-like domain of TNF-α and not by other TNF-α domains that bind to physiological TNF-α receptors on mammalian cells (22, 24). The trypanolytic activity of TNF-α against T. cruzi also involves the lectin-like domain of TNF-α because this activity was sharply reduced after incubation with N,N′-diacetylchitobiose (33) and an anti-TNF-α TIP MAb (22) but not by the neutralizing anti-TNF-α MAb 1F3F3 (21). Although TNF-α had only a weak trypanolytic activity against T. cruzi trypomastigotes (30% ± 5% lysis after 7 h of incubation), its effect on parasite infectivity was significant (77% ± 5% reduction). This activity was further lowered upon preincubation of the cytokine with N,N′-diacetylchitobiose and the anti-TNF-α TIP MAb (23) but not with the anti-TNF-α MAb 1F3F3 (21). The data suggest that glycosylated molecules may act as receptors or ligands for TNF-α. Lectins such as concanavalin A have been reported to have cytolytic activity against T. cruzi trypomastigotes (9); furthermore, T. cruzi trypomastigotes derived from the mammalian host have more and distinct concanavalin A receptors of various types than noninfective epimastigotes (10). Thus, the lectin-like domain of TNF-α may be specific for glycosylated moieties that are selectively produced on T. cruzi trypomastigotes.
It is not clear which GM-CSF domain is implicated in the observed activity. At the optimum concentration (i.e., 62.5 ng/ml), one of the two neutralizing anti-GM-CSF MAbs (MP1-22E9) tested reduced marginally the biological activities of GM-CSF against T. cruzi trypomastigotes. Thus, GM-CSF may also interact with T. cruzi via a lectin-like domain that is not recognized by the currently available anti-GM-CSF MAb.
The different activities of the two cytokines against T. cruzi trypomastigotes may reflect differences in the acceptor molecules for TNF-α and GM-CSF on the parasite and/or different mechanisms underlying cytotoxicity, morphotype transitions, and reduced infectivity. Indeed, the trypomastigotes used in this study were harvested from infected rats. Thus, this population probably contains parasites at various stages of development, and there may be some heterogeneity in the putative cytokine receptors on the parasite membrane. Treatment of parasites with a combination of both rmGM-CSF and rmTNF-α resulted in a higher percentage of lysed parasites than with rmTNF-α alone (Table 1). The rmGM-CSF-induced morphotype may be more susceptible to lysis by TNF-α.
The individual and combined activities of GM-CSF and TNF-α were recorded in vitro and may not reflect their activities in vivo. However, parasitemia and cumulative mortality are lowered by injecting rmGM-CSF (500 pg per mouse every 2 days) into T. cruzi-infected mice (27). If the cumulative effect of these repeated injections and the synthesis of endogenous GM-CSF are taken into account, concentrations in vivo are probably similar to those used in our in vitro experiments. Similarly, the concentrations of rmTNF-α used in our experiments (39 to 2,500 U/ml) may be also physiologically relevant. Indeed, it has been reported that T. cruzi infection causes a large increase in TNF-α production in mice and that TNF-α concentrations reach 3,200 to 6,400 U/ml in the serum of T. cruzi-infected mice (35). In addition, transgenic mice producing high levels of soluble TNF-α receptors, which neutralize the effects of TNF-α in vivo, are highly susceptible to T. cruzi infections (32).
Carbohydrate residues exposed at the surface of bacteria and parasites may bind many soluble factors with various effects. The binding of soluble cytokines to the parasite surface may be a major pathway for leukocyte recognition and activation. Cytokines bound to the surface carbohydrates of parasites may produce opsonin-like signals mediating attachment and phagocytosis by effector cells and may also trigger leukocyte cytotoxicity (12). This activity may be similar to the direct lysis, mediated by anti-T. cruzi antibodies, which is inhibited by carbohydrates such as melibiose (9). High levels of T. cruzi parasites in hosts with neutralized GM-CSF or TNF-α may be due to an impaired cytokine-dependent immunoprotective response such as NO release. Alternatively, a direct parasitocidal activity of TNF-α and GM-CSF on trypomastigotes could lead to lower infectivity in vivo even in the absence of NO release.
ACKNOWLEDGMENTS
We thank M. Goldman (ULB, Brussels, Belgium) for critical reading of the manuscript and J. C. Renaud (UCL, Brussels, Belgium) for the gift of pCDNA I Amp-ORF GM-CSF and pRc-RSV-moIL-2. Endotoxin contamination was assessed by J. Duchateau and M. H. Collet (Fondation Reine Elisabeth, Brussels, Belgium). The valuable technical assistance of V. Vercruysse and the help of I. Mazza in preparing the manuscript are greatly appreciated. O. Parkes edited the English text.
E.O.F. was the recipient of a grant from Agence Générale de Coopération au Développement. P.D.B. was supported by the Belgian National Fund for Scientific Research (G. 0325.95) and the Flemish Government (Vlaams Actieprogramma Biotechnologie). This work was supported by a grant from Action de Recherche Concertée, ULB, 1991, 1994, and 1995.
REFERENCES
- 1.Amiri P, Locksley R M, Parslow T G, Sadick M, Ritter D, McKerrow J H. Tumor necrosis factor-α restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992;356:604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- 2.Andrews N W, Hong K-S, Robbins E S, Nussenzweig V. Stage-specific surface antigens expressed during the morphogenesis of vertebrate forms of Trypanosoma cruzi. Exp Parasitol. 1987;64:474–484. doi: 10.1016/0014-4894(87)90062-2. [DOI] [PubMed] [Google Scholar]
- 3.Black C M, Israeliski D M, Suzuki Y, Remington J S. Effect of recombinant tumor necrosis factor on acute infection in mice with Toxoplasma gondii or Trypanosoma cruzi. Immunology. 1989;68:570–574. [PMC free article] [PubMed] [Google Scholar]
- 4.Buchner J, Pastan I, Brinkmann U. A method for increasing the yield of properly folded recombinant fusion proteins: single-chain immunotoxins from renaturation of bacterial inclusion bodies. Anal Biochem. 1992;205:263–270. doi: 10.1016/0003-2697(92)90433-8. [DOI] [PubMed] [Google Scholar]
- 5.Charlab R, Blaineau C, Schechtman D, Barcinsky M A. Granulocyte-macrophage colony-stimulating factor is a growth-factor for promastigotes of Leishmania mexicana amazonensis. J Protozool. 1990;37:352–357. doi: 10.1111/j.1550-7408.1990.tb01157.x. [DOI] [PubMed] [Google Scholar]
- 6.Denis M. Tumor necrosis factor and granulocyte macrophage colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium. J Leukocyte Biol. 1991;49:380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- 7.De Titto E, Catteral J R, Remington J S. Activity of recombinant tumor necrosis factor-α on Toxoplasma gondii and Trypanosoma cruzi. J Immunol. 1986;137:1342–1345. [PubMed] [Google Scholar]
- 8.Gasson J C. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991;77:1131–1145. [PubMed] [Google Scholar]
- 9.Gazzinelli R T, Pereira M E S, Romanha A, Gazzinelli G, Brener Z. Direct lysis of Trypanosoma cruzi: a novel effector mechanism of protection mediated by human anti-gal antibodies. Parasite Immunol. 1991;13:345–356. doi: 10.1111/j.1365-3024.1991.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 10.Gazzinelli R T, Romanha A J, Fontes G, Chiari E, Gazzinelli G, Brener Z. Distribution of carbohydrates recognized by the lectins Euonymus europeaus and concanavalin A in monoxenic and heteroxenic trypanosomatids. J Protozool. 1991;38:320–325. doi: 10.1111/j.1550-7408.1991.tb01366.x. [DOI] [PubMed] [Google Scholar]
- 11.Gazzinelli R T, Oswald I P, Hieny S, James S L, Sher A. The microbicidal activity of interferon-γ-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-β. Eur J Immunol. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 12.George A J T. Surface-bound cytokines a possible effector mechanism in bacterial immunity. Immunol Today. 1994;15:89–90. doi: 10.1016/0167-5699(94)90139-2. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 13.Grabstein K H, Urdal L, Tushiski R J, Price V L, Mochizuki D Y, Cantrell M A, Gillis S, Conlon P J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986;232:506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- 14.Gutteridge W E, Cover B, Gaborak M. Isolation of blood and intracellular forms of Trypanosoma cruzi from rats and other rodents and preliminary studies of their metabolism. Parasitology. 1978;76:159–176. doi: 10.1017/s0031182000047740. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton J A. Colony stimulating factors, cytokines and monocyte-macrophages—some controversies. Immunol Today. 1993;14:18–24. doi: 10.1016/0167-5699(93)90319-G. [DOI] [PubMed] [Google Scholar]
- 16.Hansen M B, Nielsen E S, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell/growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 17.Jadin J B, Pierreu G. Un milieu de culture pour trypanosomatides. Ann Soc Belge Med Trop. 1960;40:903–906. [PubMed] [Google Scholar]
- 18.Jones T C. The effects of rhGM-CSF on macrophage function. Eur J Cancer. 1993;29A:S10–S13. doi: 10.1016/0959-8049(93)90625-p. [DOI] [PubMed] [Google Scholar]
- 19.Kongshavn P A, Ghadirian E. Enhancing and suppressive effects of tumour necrosis factor/cachectin on growth of Trypanosoma musculi. Parasite Immunol. 1991;10:581–588. doi: 10.1111/j.1365-3024.1988.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 20.Lanham S M, Godfrey D G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970;28:521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- 21.Lucas R, Heirwegh K, Neirynck A, Remels L, Van Heuverswyn H, de Baetselier P. Generation and characterization of a rat anti rTNF-α monoclonal antibody. Immunology. 1990;71:218–223. [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas R, Magez S, De Leys R, Fransen L, Scheerlinck J-P, Rampelberg M, Sablon E, de Baetselier P. Mapping the lectin-like activity of tumor necrosis factor. Science. 1994;263:814–817. doi: 10.1126/science.8303299. [DOI] [PubMed] [Google Scholar]
- 23.Lucas R, Magez S, De Leys R, de Baetsellier P. Cytokines: multifunctional pathogen-specific mammalian lectins? In: Van Driessche E, Fischer J, Beeckmans S, Bøg-Hanssen T C, editors. Lectins: biology, biochemistry, clinical biochemistry. Hellerup, Denmark: Textop; 1994. pp. 244–249. [Google Scholar]
- 24.Magez S, Geuskens M, Beschin A, del Favero H, Verschueren H, Lucas R, Pays E, de Baetselier P. Specific uptake of tumor necrosis factor-α is involved in growth control of Trypanosoma brucei. J Cell Biol. 1997;137:715–727. doi: 10.1083/jcb.137.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metz G, Carlier Y, Vray B. Trypanosoma cruzi upregulates nitric oxide release by IFN-γ-preactivated macrophages, limiting cell infection independently of the respiratory burst. Parasite Immunol. 1993;15:693–699. doi: 10.1111/j.1365-3024.1993.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 26.Munoz-Fernandez M A, Fernandez M A, Fresno M. Synergism between tumor necrosis factor-α and interferon-γ on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanisms. Eur J Immunol. 1992;22:301–307. doi: 10.1002/eji.1830220203. [DOI] [PubMed] [Google Scholar]
- 27.Olivares Fontt E, Heirman C, Thielemans K, Vray B. Granulocyte-macrophage colony stimulating factor: involvement in control of Trypanosoma cruzi infection in mice. Infect Immun. 1996;64:3429–3434. doi: 10.1128/iai.64.8.3429-3434.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivares Fontt E, Vray B. Relationship between granulocyte macrophage-colony stimulating factor, tumor necrosis factor-α and Trypanosoma cruzi infection in murine macrophages. Parasite Immunol. 1995;17:135–141. doi: 10.1111/j.1365-3024.1995.tb01015.x. [DOI] [PubMed] [Google Scholar]
- 29.Petray P, Rottenberg M, Grinstein S, Orn A. Release of nitric oxide during the experimental infection with Trypanosoma cruzi. Parasite Immunol. 1994;16:193–199. doi: 10.1111/j.1365-3024.1994.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 30.Plasman N, Guillet J G, Vray B. Impaired protein catabolism in Trypanosoma cruzi-infected macrophages: possible involvement in antigen presentation. Immunology. 1995;86:636–645. [PMC free article] [PubMed] [Google Scholar]
- 31.Reed S G, Grabstein K H, Pihl D, Morrissey P. Recombinant granulocyte-macrophage colony-stimulating factor restores deficient responses in mice with chronic Trypanosoma cruzi infections. J Immunol. 1990;145:1564–1570. [PubMed] [Google Scholar]
- 32.Santos Lima E C, Garcia I, Vicentelli M-H, Vassalli P, Minoprio P. Evidence for a protective role of tumor necrosis factor in the acute phase of Trypanosoma cruzi infection in mice. Infect Immun. 1997;65:457–465. doi: 10.1128/iai.65.2.457-465.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherblom A P, Decker J M, Muchmores A V. The lectin-like interaction between recombinant tumor necrosis factor and uromodulin. J Biol Chem. 1988;263:5418–5424. [PubMed] [Google Scholar]
- 34.Tanowitz H B, Simon D, Morris S A, Weiss L M, Wittner M. Chagas’ disease. Clin Microbiol Rev. 1992;5:404–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarleton R L. Tumour necrosis factor (cachectin) production during experimental Chagas’ disease. Clin Exp Immunol. 1988;73:186–190. [PMC free article] [PubMed] [Google Scholar]
- 36.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 37.Vespa G N R, Cunha F Q, Silva J S. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177–5182. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vray B, de Baetselier P, Ouaissi A, Carlier Y. Trypanosoma cruzi but not Trypanosoma brucei fails to induce a chemiluminescent signal in a macrophage hybridoma cell line. Infect Immun. 1991;59:3303–3308. doi: 10.1128/iai.59.9.3303-3308.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]