Abstract
Background
Systemic lupus erythematosus (SLE) is a complex systemic autoimmune disease characterized by the presence of numerous autoantibodies. The interaction of infectious agents (viruses, bacteria and parasites) and a genetically susceptible host may be a key mechanism for SLE. Toxoplasma gondii is a widespread intracellular parasite that has been implicated in the pathogenesis of autoimmune diseases. However, the relationship between T. gondii infection and the increased risk of SLE in Chinese populations remains unclear.
Methods
The seroprevalence of T. gondii infection was assessed in 1771 serum samples collected from Chinese individuals (908 healthy controls and 863 SLE patients) from different regions of China using an enzyme-linked immunosorbent assay. Serum autoantibodies and clinical information were obtained and analysed.
Results
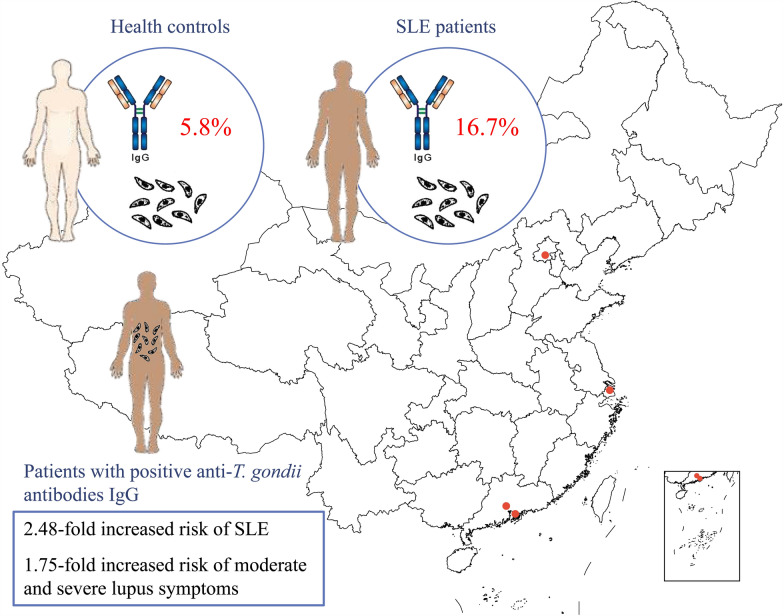
Our observations revealed a higher prevalence of anti-T. gondii antibodies (ATxA) immunoglobulin G (IgG) in serum samples from SLE patients (144/863, 16.7%) than in those from the healthy controls (53/917, 5.8%; P < 0.0001), indicating a 2.48-fold increased risk of SLE in the ATxA-IgG+ population, after adjustment for age and sex (95% confidence interval [CI] 1.70–3.62, P < 0.0001). ATxA-IgG+ SLE patients also showed a 1.75-fold higher risk of developing moderate and severe lupus symptoms (95% CI 1.14–2.70, P = 0.011) compared to ATxA-IgG− patients. Relative to ATxA-IgG− patients, ATxA-IgG+ patients were more likely to develop specific clinical symptoms, including discoid rash, oral ulcer, myalgia and alopecia. Seven antibodies, namely anti-ribosomal RNA protein (rRNP), anti-double stranded DNA (dsDNA), anti-cell membrane DNA (cmDNA), anti-scleroderma-70 (Scl-70), anti-cardiolipin (CL), anti-beta2-glycoprotein-I (B2GPI) and rheumatoid factor (RF), occurred more frequently in ATxA-IgG+ patients. When combined with anti-dsDNA and RF/anti-rRNP/anti-cmDNA/ESR, ATxA-IgG significantly increased the risk for severe lupus.
Conclusions
Our results suggest that ATxA-IgG may be a significant risk factor for SLE prevalence and severity in Chinese populations.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-024-06141-8.
Keywords: SLE, Toxoplasma gondii, Autoantibody, Risk factor
Background
Systemic lupus erythematosus (SLE) is a complex systemic autoimmune disease whose development is determined by both genetic predisposition and exposure to environmental factors such as UV light, drugs, psychological stress and infections [1–6]. This autoimmune disorder is characterized by the production of multiple autoantibodies, diverse clinical manifestations and the presence of anti-nuclear antibodies [7–9]. In patients with SLE, the polyreactive B cells produce a range of autoantibodies, including anti-double stranded DNA (dsDNA), anti-Smith (Sm), anti-ribonucleoprotein (RNP), anti-Ro, anti-La, anti-phospholipid and anti-nuclear antigen (ANA) antibodies [10]. Of these seven autoantibodies, the anti-Sm and anti-dsDNA antibodies are specific to SLE and have been reported to participate in immune complex formation and inflammatory damage to multiple end-organs, such as the kidney, skin and central nervous system [10, 11]. Researchers on SLE have also become interested in the platelet-mediated release of mitochondrial DNA, which is a additional source of nucleic acids, in SLE, such as rheumatoid factor (RF) [12, 13]. While the etiology and pathogenesis of SLE remain largely unknown, conventional assays for ANA, anti-dsDNA and other autoantibodies are usually performed for the diagnosis, monitoring and treatment of SLE [14, 15].
Toxoplasmosis is a parasitic infection caused by the obligate intracellular protozoan Toxoplasma gondii. This parasite can infect nearly all warm-blooded animals, including humans, and it has been reported that one third of all humans are infected, with large differences between countries (from 4% to 60%) [16–18]. The most common symptoms of toxoplasmosis in humans is lymphadenopathy, which may be associated with a sore throat, fever, fatigue, headache and muscle pain [19, 20]. In most immunocompetent individuals, T. gondii infection is asymptomatic. However, in immunocompromised individuals, patients undergoing immunosuppressive treatments or pregnant women, T. gondii infection can cause serious clinical symptoms and even death [16, 17, 21].
In humans, infection by parasites, viruses or bacteria frequently induces autoantibodies in the infected individual, which are most commonly associated with autoimmune disorders [22]. The authors of a previous study reported a correlation between Toxoplasma antibodies (ATxA) in patients with autoimmune diseases and serum anti-centromere antibodies, such as anti-cardiolipin (CL), anti-beta2-glycoprotein-I (B2GPI), complex anti-cardiolipin-beta2-glycoprotein-I complex (anti-CL-B2) antibodies, anti-gliadin, anti-phosphatidylethanolamine (PE), anti-prothrombin (PT) and anti-scleroderma-70 (anti-Scl-70) [18]. Among these, RF antibodies are typically associated with disease activity and inflammation in rheumatoid arthritis (RA) [23]. In up to 66% of patients with Sjögren syndrome (SS), the presence of ANA, RF, anti-Sjögren’s syndrome A (SSA) and anti-Sjögren’s syndrome B (SSB) antibodies can be detected years before symptom onset [24, 25]. Additionally, anti-nucleosome antigen (ANUA), anti-dsDNA, Sm and SSA antibodies are detected at a high frequency in SLE patients [26]. However, further studies are needed to investigate the link between T. gondii infection and autoantibodies in various autoimmune diseases.
Toxoplasma gondii has been previously reported to be associated with SLE, as high titres of Toxoplasma antibodies were found to be significantly more common in patients with SLE [27]. However, in European populations, anti-T. gondii antibodies immunoglobulin G (ATxA-IgG)-positive (ATxA-IgG+) individuals have been found to have a higher prevalence of RA but not SLE and SS [18]. Similarly, the prevalence of ATxA-IgG was significantly higher in arthritic patients than in healthy controls in eastern China [28]. However, the association between T. gondii infection and increased risk of SLE remains unknown in Chinese populations.
Given the high prevalence of T. gondii infection and its possible associations with SLE, we sought to evaluate the seroprevalence of ATxA-IgG in a large cohort of Chinese SLE patients, as well as the risk factors associated with T. gondii infection.
Methods
Patients and serum samples
Serum samples were collected from 1233 patients with various autoimmune diseases (AIDs) and 908 heathy controls from different regions of China (Beijing, Shanghai, Guangzhou and Shenzhen). The serum samples from the patients with AID included those from 863 SLE patients, 151 RA patients and 219 SS patients. Control serum samples were from healthy subjects of similar age and sex distribution as the patients (300 from northern China, 41 from middle China and 567 from southern China). All serum samples from patients were collected by a clinician following diagnosis. Samples were kept at − 80 °C until analysis.
Information on demographics, such as gender, age, area of residence and ethnicity, was obtained from the computerized inpatient case registry for all patients or was requested from the control individuals themselves. Clinical information, including clinical history, physical examination and laboratory results, was obtained for all clinical cases. All personal information was anonymized and treated as strictly confidential. The discriminant analysis (distinguishing clearly active vs mildly/nonactive disease) of SLE was assessed considering the modified Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) as the gold standard [29]. The study was approved by the local ethics committees and fulfilled the ethical guidelines of the most recent Declaration of Helsinki (1978, revised 2008).
Serological testing
Serum samples were analysed for the presence of anti-T. gondii IgG antibodies using commercially available enzyme-linked immunosorbent assays (ELISA) kits (Haitai Biotech, Inc., Zhuhai, China). Autoantibodies were assessed using the Bio-Plex 200 immunoassay multiplex array system (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s protocol. The autoantibodies included: anti-ANA, ANUA, RF, anti-ribosomal RNA protein (anti-rRNP), anti-dsDNA, anti-Sm, anti-Ro/SSA, anti-La/SSB, anti-Scl-70, anti-CL and anti-B2GPI.
Statistical analysis
Statistical analysis was performed using SPSS v24.0 statistical software (SPSS IBM, Armonk, NY, USA. Student’s t-test or Chi-square test (χ2) was used to examine differences in the demographic characteristics and T. gondii infection status. Multivariate logistic regression models were used to adjust for potential confounders. Variables associated with T. gondii infection were identified by univariate analysis (P ≤ 0.05) and included in the multivariate logistic regression analysis. Odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) were calculated to identify independent risk factors for T. gondii infection. P < 0.05 was considered to indicate statistical significance. The interaction effects were determined on the additive scale, with three measures used to examine biological interaction: (i) attributable proportion due to interaction (AP); (ii) relative excess risk due to interaction (RERI); and (iii) synergy index (S), with S > 1 indicating synergetic effects and S < 1 indicating antagonistic effects [30, 31].
Results
Serum samples from 1233 AID patients (863 SLE patients, 151 RA patients and 219 SS patients) and 908 healthy controls from different regions of China were included in this study (Additional file 1: Table S1). We observed that the T. gondii infection rate was significantly higher in SLE patients (144/863, 16.7%) than in the healthy controls (53/908, 5.8%; P < 0.0001) and significantly higher in patients with SS (33/219, 15.1%; P < 0.0001) than in the healthy controls (Table 1). In contrast, the infection rate of T. gondii in the RA population (12/151, 8.0%, P = 0.319) was not significantly different from that in the healthy controls. Although an earlier study in European populations did not find a higher prevalence of ATxA in patients with SLE [18], we believed it worthwhile to evaluate the association between SLE and T. gondii infection in Chinese populations, given the large SLE cohort in our sample pool and their high seroprevalence of T. gondii.
Table 1.
Prevalence of anti-Toxoplasma gondii antibodies immunoglobulin G in serum samples of patients with different autoimmune diseases and healthy controls
| Study population | ATxA IgG+ | P valuea |
|---|---|---|
| Healthy controls | 53/908 (5.8%) | |
| Patients with an AID | ||
| Rheumatoid arthritis | 12/151 (8.0%) | 0.319 |
| Sjögren’s syndrome | 33/219 (15.1%) | < 0.0001* |
| Systemic lupus erythematosus | 144/863 (16.7%) | < 0.0001* |
AID Autoimmune disease, ATxA IgG+, anti-Toxoplasma gondii antibodies immunoglobulin G-positive, ATxA IgG− anti-Toxoplasma gondii antibodies immunoglobulin G-negative
*Statistically significant
aP value for the comparison with matched healthy controls
Risk factor analysis demonstrated a 2.48-fold higher risk of SLE in the ATxA-IgG+ patient population, after adjustment for age and sex (95% CI 1.70–3.62, P < 0.0001) (Table 2). Toxoplasms gondii infection was also significantly correlated with disease severity. In comparison with ATxA-IgG− SLE patients, ATxA-IgG+ SLE patients were 1.75-fold more likely to develop moderate and severe lupus symptoms (SLEDAI-2 K ≥ 10, 95% CI 1.14–2.70, P = 0.011) (Table 3). ATxA-IgG+ patients also showed higher frequencies of certain clinical symptoms, such as discoid rash, oral ulcer, myalgia and alopecia (Additional file 2: Table S2).
Table 2.
Higher risk of systemic lupus erythematosus in anti-Toxoplasma gondii antibodies immunoglobulin G population
| ATxA-IgG type | Healthy controls (N, %) | SLE patients (N, %) | Odds ratio (95% CI) | Odds ratio (95% CI) (age/sex adjusted) | P-valuea |
|---|---|---|---|---|---|
| ATxA-IgG− | 855 (94.2) | 719 (83.3) | Reference | Reference | |
| ATxA IgG+ | 53 (5.8) | 144 (16.7) | 3.23 (2.32–4.49) | 2.48 (1.70–3.62) | < 0.0001* |
ATxA IgG+, Anti-Toxoplasma gondii antibodies immunoglobulin G-positive, ATxA IgG− anti-Toxoplasma gondii antibodies immunoglobulin G-negative, CI confidence interval
*Statistically significant
aP value for the comparison with matched healthy controls
Table 3.
Associations of anti-T. gondii antibodies immunoglobulin G with disease severity, but not duration
| Disease activity and duration | Odds ratio | 95% CI | P valuea |
|---|---|---|---|
| Disease activity | |||
| Low activity (SLEDAI-2 K: < 10) | 1.0 | ||
| Moderate & Severe activity (SLEDAI-2 K: ≥ 10) | 1.75 | 1.14–2.70 | 0.011* |
| Disease duration | |||
| < 5 years | 1.0 | ||
| 5–10 years | 0.82 | 0.51–1.30 | 0.40 |
| ≥ 10 years | 1.57 | 0.85–2.90 | 0.150 |
CI Confidence interval, SLEDAI-2K Systemic Lupus Erythematosus Disease Activity Index 2000
*Statistically significant
aP value: adjusted for sex and age (≤ 40 and > 40 years)
Autoantibodies are considered hallmarks of SLE and are closely associated with disease progression [10]. To confirm whether ATxA-IgG increased the production of any autoantibodies related to lupus severity, we determined the presence or absence of autoantibodies present in the serum of SLE patients and compared the prevalence of these antibodies in ATxA-IgG+ and ATxA-IgG− patients. We found that ATxA-IgG+ patients were likely to produce more types of autoantibodies, such as anti-rRNP, anti-dsDNA, anti-cmDNA, anti-Scl-70, anti-CL, anti-B2GP1 and RF antibodies (Table 4). Among these autoantibodies, anti-rRNP, anti-dsDNA and anti-cmDNA, which are highly associated with SLE disease development, were significantly elevated in ATxA-IgG+ patients (P = 0.016, P = 0.011 and P = 0.00011, respectively; Table 4).
Table 4.
Autoantibody prevalence in serum from patients with systemic lupus erythematosus in relation to anti-Toxoplasma gondii antibodies immunoglobulin G
| Autoantibodiesa | All patients (n = 863) | ATxA-IgG+ SLE patients (n = 144) | ATxA-IgG− SLE patients (n = 719) | P valueb |
|---|---|---|---|---|
| ANA positive, n (%) | 669 (88.5%) | 107 (88.4%) | 562 (88.5%) | 0.981 |
| ANUA positive, n (%) | 417 (54.4%) | 76 (62.3%) | 341 (53.0%) | 0.057 |
| Anti-rRNP positive, n (%) | 246 (37.3%) | 51 (47.7%) | 195 (35.3%) | 0.016* |
| Anti-Ro/SSA positive, n (%) | 349 (46.7%) | 59 (49.6%) | 290 (46.2%) | 0.495 |
| Anti-La/SSB positive, n (%) | 87 (11.6%) | 14 (15.9%) | 73 (11.6%) | 0.960 |
| Anti-dsDNA positive, n (%) | 518 (65.4%) | 97 (75.2%) | 421 (63.5%) | 0.011* |
| Anti-cmDNA positive, n (%) | 33 (5.4%) | 13 (13.5%) | 20 (3.9%) | 0.00011* |
| Anti-Sm positive, n (%) | 142 (19.1%) | 29 (19.1%) | 113 (18.1%) | 0.113 |
| Anti-Scl-70, n (%) | 22 (4.4%) | 7 (9.5%) | 15 (3.5%) | 0.021* |
| Rheumatoid factor (RF), n (%) | 93 (12.9%) | 22 (19.0%) | 71 (11.8%) | 0.035* |
| Anti-CL, n (%) | 134 (19.2%) | 36 (32.4%) | 98 (16.7%) | 0.00011* |
| Anti-B2GPI, n (%) | 78 (11.1%) | 18 (16.8%) | 60 (10.1%) | 0.041* |
ATxA IgG+, Anti-Toxoplasma gondii antibodies immunoglobulin G-positive, ATxA IgG− anti-Toxoplasma gondii antibodies immunoglobulin G-negative, SLE systemic lupus erythematosus
*Statistically significant
aANA, Anti-nuclear antigen; ANUA, anti-nucleosome antigen; rRNP, ribosomal RNA protein, Ro/SSA, Ro/Sjögren’s syndrome A; La/SSB, La/Sjögren’s syndrome B; dsDNA, double-stranded DNA; cmDNA, cell membrane DNA; Sm, Smith; Scl-70, scleroderma-70; CL, cardiolipin; B2GPI, beta2-glycoprotein-I
bP value: adjusted for age(years) and sex
The interaction of T. gondii infection and serum autoantibody levels were also analysed. Based on the S index, there were eight significant synergetic interactions, including erythrocyte sedimentation rate (ESR), D-dimer, RF, anti-streptolysin O (ASO), anti-dsDNA, anti-cmDNA, anti-rRNP and anti-CL antibodies (Additional file 3: Table S3). When combined with anti-dsDNA and RF elements, ATxA-IgG increased the risk for severe lupus by 5.7-fold (OR=14.34 [95% CI 1.85–111.19, P = 0.011] vs OR=8.66 [95% CI 2.95–25.43, P < 0.0001]; Additional file 4: Table S4). Similarly, ATxA-IgG combined with anti-dsDNA and anti-rRNP increased the lupus severity risk by 1.92-fold (OR=9.00 [95% CI 3.27–29.95, P < 0.0001] vs OR=7.08 [95% CI 3.96–12.65, P < 0.0001]; Additional file 5: Table S5). Moreover, ATxA-IgG also significantly increased the lupus risk in combination with either anti-dsDNA and ESR (Additional file 6: Table S6) or anti-dsDNA and anti-cmDNA (Additional file 7: Table S7).
Discussion
Systemic lupus erythematosus is a complex systemic autoimmune disease with an unclear etiology. Previous studies have suggested a potential association between T. gondii infection and SLE [18, 27], but the association between T. gondii infection and increased risk of SLE remains unknown in Chinese populations. In this study, we sought to evaluate the seroprevalence of anti-T. gondii IgG in a large cohort of Chinese SLE patients and identify the risk factors associated with T. gondii infection.
To our knowledge, this study is the first nationwide clinical study which involves an epidemiological investigation of T. gondii infection in Chinese patients with SLE. We measured ATxA-IgG in a large group of people from different geographic regions of China (Beijing, Shanghai, Guangzhou and Shenzhen). Our results show there was a higher prevalence of ATxA-IgG in our Chinese patients with SLE or SS than in the healthy Chinese controls. Similarly, high titres of Toxoplasma antibodies were significantly more common in SLE patients (n = 50) [27]. However, the authors of a study involving European populations reported a higher incidence of RS (27/35, 77%) but not SLE (54/169, 32%) in ATxA-IgG+ individuals [18]. In this same study, the frequency of ATxA-IgG in sera collected from Latin American patients with RA (55/152, 36%), SLE (42/120, 35%) and SS (33/82, 40%) was similar to that in the healthy controls [18]. A reasonable explanation for these contradictory findings is that individual susceptibility to SLE is highly associated with race, genetic lineage, lifestyle and environmental factors, which include socioeconomic status, dietary habits, exposure to environmental pollutants and infectious agents (either triggering or protective agents). The associations between ATxA-IgG and these factors are likely to contribute to the development of AID. The study [18] included a relatively small number of SLE patients (120 from Latin America and 169 from Europe), which may have limited the ability to fully capture the actual epidemiological mechanism of the disease.
Our data also report an association between ATxA-IgG and the level of serum autoantibodies or ESR, a clinical hallmark of rhupus patients. When combined with anti-dsDNA and RF/anti-rRNP/anti-cmDNA/ESR, ATxA-IgG significantly increased the risk for severe lupus. Anti-dsDNA antibodies, which are specific to SLE (70–80% positive rate), are included in the diagnostic criteria outlined by the American College of Rheumatology. They have been reported to participate in immune complex formation and inflammatory damage to multiple end organs, such as the kidney, skin and central nervous system [10, 11, 32]. RFs play a critical role in the differential diagnosis of polyarthritis and are frequently detected in patients with systemic autoimmune diseases, such as SLE (15–30% positive rate), mixed connective tissue disease, polymyositis and dermatomyositis [13]. Anti-rRNP antibodies are closely linked to severe thrombocytopenia and malar rash, which occur mostly in SLE patients [33, 34]. Anti-cmDNA antibodies could be an efficient diagnostic biomarker for SLE due to their high serum levels and diagnostic specificity, especially for SLE patients who test negative for anti-dsDNA or/and anti-Sm antibodies [35].
Thus, this study presents a new perspective for SLE disease management, as ATxA-IgG might be a significant risk factor for the prevalence and severity of SLE in the Chinese population. Moreover, T. gondii infection might produce a higher risk for developing moderate and severe lupus symptoms. However, more research is needed before ATxA-IgG testing can be performed as a routine clinical diagnostic test for SLE, similar to those carried out for autoantibodies.
In SLE disease management, diagnostic accuracy and the appropriate therapy are critical in the clinical setting and for research purposes [36]. Our study results were validated in the following ways. First, our T. gondii infection and SLE diagnoses were performed as accurately as possible. All T. gondii infection diagnoses were validated using anti-T. gondii IgG ELISA kits, which are commonly used for clinical diagnosis in China. Second, the collection of diagnostic and clinical data on SLE patients was performed by experienced clinicians, and all clinical data can be traced back to records in the hospital medical record system. Third, the sample and clinical data were collected from various geographic regions of China, effectively minimizing the impact of regional differences. Finally, we analysed clinical risk factor covariates using a multivariate regression model, which was highly effective in identifying the risk of each element.
However, there are some potential limitations in our study. First, our work did not explore the relationships among T. gondii infection, SLE treatment and disease prognosis. Second, we failed to accurately monitor the exact therapeutic regimen and disease prognosis of each patient, due to the diversity and complexity of patient disease progression and treatment. Third, our research did not look at the impact of anti-T. gondii immunoglobulin M (IgM) status on SLE disease progression and diagnosis. Thus, a larger and more comprehensive study is required to address this issue. Finally, there is a possibility that the autoantibodies in SLE patients can cross-react with T. gondii antigens. It is difficult to obtain a solid evidence, such as, for example, T. gondii gene PCR product from the samples, to exclude the possibility of cross-reaction. However, if the seroprevalence in SLE patients was due to a cross-reaction, a much higher serological positive rate—and the observed 16.7%—would be expected. Moreover, a cross-reaction usually results in a low antibody titre. In our study, the ATxA-IgG level was low (slightly above the cutoff point according to the ELISA kit) in 17 out of 144 (11.8%) ATxA-IgG+ SLE patients and in seven out of 53 (13.2%) of ATxA-IgG+ healthy controls. The similarity of these percentages indicate that both sets of patients have similar ATxA-IgG levels.
Conclusions
In summary, our study provides new epidemiological evidence on the important role of T. gondii infection in SLE disease and further insights into these interactions in SLE etiology. Our results also provide additional evidence that T. gondii infection should be considered in SLE disease, especially in Chinese patients.
Supplementary Information
Additional file 1: Table S1. The number of serum samples of patients with different autoimmune diseases and healthy controls.
Additional file 2: Table S2. The demographics and disease characteristics of SLE patients.
Additional file 3: Table S3. The anti-T. gondii antibodies IgG combined with biochemical indexes/autoantibodies for the prediction of SLE.
Additional file 4: Table S4. Risk factors for disease severity (analysis with 3 factors): anti-T. gondii antibodies IgG, anti-dsDNA and RF.
Additional file 5: Table S5. Risk factors for disease severity (analysis with 3 factors): anti-T. gondii antibodies IgG, anti-dsDNA and anti-rRNP.
Additional file 6: Table S6. Risk factors for disease severity (analysis with 3 factors): anti-T. gondii antibodies IgG, anti-dsDNA and ESR.
Additional file 7: Table S7. Risk factors for disease severity (analysis with 3 factors): anti-T. gondii antibodies IgG, anti-dsDNA and anti-cmDNA.
Acknowledgements
Not applicable.
Abbreviations
- AIDs
Autoimmune diseases
- ANA
Anti-nuclear antigen
- Anti-CL-B2
Complex anti-cardiolipin-Beta2-glycoprotein-I antibodies
- ANUA
Anti-nucleosome antigen
- ATxA
Anti-Toxoplasma antibodies
- ATxA-IgG
Anti-Toxoplasma gondii antibodies immunoglobulin G
- ATxA-IgG+
Anti-Toxoplasma gondii antibodies immunoglobulin G-positive
- ATxA-IgG−
Anti-Toxoplasma gondii antibodies immunoglobulin G-negative
- B2GPI
Beta2-glycoprotein-I
- CI
Confidence interval
- CL
Cardiolipin
- cmDNA
Cell membrane DNA
- dsDNA
Double-stranded DNA
- ELISA
Enzyme-linked immunosorbent assay
- ESR
Erythrocyte sedimentation rate
- HFF
Human foreskin fibroblast
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- PE
Phosphatidylethanolamine
- PT
Anti-prothrombin
- RA
Rheumatoid arthritis
- RF
Rheumatoid factor
- RNP
Ribonucleoprotein
- rRNP
Ribosomal RNA protein
- Scl-70
Scleroderma-70
- SLE
Systemic lupus erythematosus
- Sm
Smith
- SS
Sjögren syndrome
- SSA
Sjögren’s syndrome A
- SSB
Sjögren’s syndrome B
Author contributions
ZNY, HWY, ZGL and WGX designed and supervised the project. ZZL, ZWL, WYY and MMX performed the experiments. XLS, XZZ, MJZ, YJT, QWW, JZ, QLP, GCW, PJZ, EWS, NS and ZGL recruited patients and provided clinical information. ZWL, CXJ, GY, ZXZ, ZHW and XJC analysed data. ZZL, ZWL, HWY and ZNY wrote the manuscript with support from all other co-authors. All authors revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant 32200079, 32070121 and 32141004), the ‘111 project’ (B16021), Guangzhou Key Laboratory for Germ-free Animals and Microbiota Application (202201020381), 2023 Zhuhai Social Development Science and Technology Plan Project (2320004000047), Key Projects of the Clinical Research Improvement Plan of Zhuhai People’s Hospital (202201020381) and the internal science funding of the Qingyuan People’s Hospital.
Availability of data and materials
All data and materials of the experiments described here are included in this published article and its additional files.
Declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Medical Ethical Committee of the Peking University People’s Hospital. Written informed consent to participate in this study was provided by the participants or by the participants’ legal guardian.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhongzhen Li and Zhiwei Lei contributed equally to this work.
Zhanguo Li, Hengwen Yang and Zhinan Yin jointly supervised this work.
Contributor Information
Weiguo Xu, Email: xwg315@163.com.
Hengwen Yang, Email: hengwenyang@jnu.edu.cn.
Zhinan Yin, Email: tzhinan@jnu.edu.cn.
References
- 1.Cooper GS, Parks CG. Occupational and environmental exposures as risk factors for systemic lupus erythematosus. Curr Rheumatol Rep. 2004;6:367–374. doi: 10.1007/s11926-004-0011-6. [DOI] [PubMed] [Google Scholar]
- 2.James JA, Neas BR, Moser KL, Hall T, Bruner GR, Sestak AL, et al. Systemic lupus erythematosus in adults is associated with previous Epstein-Barr virus exposure. Arthritis Rheum. 2001;44:1122–1126. doi: 10.1002/1529-0131(200105)44:5<1122::AID-ANR193>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.Song H, Fang F, Tomasson G, Arnberg FK, Mataix-Cols D, de la Fernandez Cruz L, et al. Association of stress-related disorders with subsequent autoimmune disease. JAMA. 2018;319:2388–400. doi: 10.1001/jama.2018.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowe W, Allsopp PJ, Watson GE, Magee PJ, Strain JJ, Armstrong DJ, et al. Mercury as an environmental stimulus in the development of autoimmunity—a systematic review. Autoimmun Rev. 2017;16:72–80. doi: 10.1016/j.autrev.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Nelson P, Rylance P, Roden D, Trela M, Tugnet N. Viruses as potential pathogenic agents in systemic lupus erythematosus. Lupus. 2014;23:596–605. doi: 10.1177/0961203314531637. [DOI] [PubMed] [Google Scholar]
- 6.Rigante D, Mazzoni MB, Esposito S. The cryptic interplay between systemic lupus erythematosus and infections. Autoimmun Rev. 2014;13:96–102. doi: 10.1016/j.autrev.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 8.Fava A, Petri M. Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun. 2019;96:1–13. doi: 10.1016/j.jaut.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blomberg J, Nived O, Pipkorn R, Bengtsson A, Erlinge D, Sturfelt G. Increased antiretroviral antibody reactivity in sera from a defined population of patients with systemic lupus erythematosus. Correlation with autoantibodies and clinical manifestations. Arthritis Rheum. 1994;37:57–66. doi: 10.1002/art.1780370109. [DOI] [PubMed] [Google Scholar]
- 10.Lou HT, Ling GS, Cao XT. Autoantibodies in systemic lupus erythematosus: From immunopathology to therapeutic target. J Autoimmun. 2022;132:102861. doi: 10.1016/j.jaut.2022.102861. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Xia Y. Anti-double stranded DNA antibodies: origin, pathogenicity, and targeted therapies. Front Immunol. 2019;10:1667. 10.3389/fimmu.2019.01667. [DOI] [PMC free article] [PubMed]
- 12.Mustelin T, Lood C, Giltiay NV. Sources of pathogenic nucleic acids in systemic lupus erythematosus. Front Immunol. 2019;10:1028. doi: 10.3389/fimmu.2019.01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linge P, Fortin PR, Lood C, Bengtsson AA, Boilard E. The non-haemostatic role of platelets in systemic lupus erythematosus. Nat Rev Rheumatol. 2018;14:195–213. doi: 10.1038/nrrheum.2018.38. [DOI] [PubMed] [Google Scholar]
- 14.Leuchten N, Hoyer A, Brinks R, Schoels M, Schneider M, Smolen J, et al. Performance of antinuclear antibodies for classifying systemic lupus erythematosus: a systematic literature review and meta-regression of diagnostic data. Arthritis Care Res. 2018;70:428–438. doi: 10.1002/acr.23292. [DOI] [PubMed] [Google Scholar]
- 15.Selvananda S, Kan SL. Performance of the 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus in a multiethnic Malaysian cohort. Int J Rheum Dis. 2022;25:131–139. doi: 10.1111/1756-185X.14269. [DOI] [PubMed] [Google Scholar]
- 16.Munoz M, Liesenfeld O, Heimesaat MM. Immunology of Toxoplasma gondii. Immunol Rev. 2011;240:269–285. doi: 10.1111/j.1600-065X.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 17.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 18.Shapira Y, Agmon-Levin N, Selmi C, Petrikova J, Barzilai O, Ram M, et al. Prevalence of anti-Toxoplasma antibodies in patients with autoimmune diseases. J Autoimmun. 2012;39:112–116. doi: 10.1016/j.jaut.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Hammadi SA, Al-Anbari AJK, Al-Alosi BM. Toxoplasma lymphadenopathy: a comparative diagnostic assessment of clinical, serological and histopathological findings. Iran J Otorhinolaryngol. 2023;35:157–163. doi: 10.22038/IJORL.2023.64479.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canadian Centre for Occupational Health and Safety. Diseases, disorders and injuries. Toxoplasmosis. 2023. https://www.ccohs.ca/oshanswers/diseases/toxoplasmosis.html?=undefined&wbdisable=true. Accessed 26 Dec 2023.
- 21.Demar M, Ajzenberg D, Maubon D, Djossou F, Panchoe D, Punwasi W, et al. Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clin Infect Dis. 2007;45:e88–95. doi: 10.1086/521246. [DOI] [PubMed] [Google Scholar]
- 22.Rivera-Correa J, Rodriguez A. Divergent roles of antiself antibodies during infection. Trends Immunol. 2018;39:515–522. doi: 10.1016/j.it.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pertsinidou E, Saevarsdottir S, Manivel VA, Klareskog L, Alfredsson L, Mathsson-Alm L, et al. In early rheumatoid arthritis, anticitrullinated peptide antibodies associate with low number of affected joints and rheumatoid factor associates with systemic inflammation. Ann Rheum Dis. 2023 doi: 10.1136/ard-2023-224728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Zhu T, Wu D, Zhang L. Sjögren’s syndrome initially presented as thrombotic thrombocytopenic purpura in a male patient: a case report and literature review. Clin Rheumatol. 2017;37:1421–1426. doi: 10.1007/s10067-017-3912-2. [DOI] [PubMed] [Google Scholar]
- 25.Jonsson R, Theander E, Sjöström B, Brokstad K, Henriksson G. Autoantibodies present before symptom onset in primary Sjogren Syndrome. JAMA. 2013;310:1854–1855. doi: 10.1001/jama.2013.278448. [DOI] [PubMed] [Google Scholar]
- 26.Wang WC, Gao HM. The combined detection of autoantibody characteristics in systemic lupus erythematosus. Am J Transl Res. 2021;13:7242–7248. [PMC free article] [PubMed] [Google Scholar]
- 27.Wilcox MH, Powell RJ, Pugh SF, Balfour AH. Toxoplasmosis and systemic lupus erythematosus. Ann Rheum Dis. 1990;49:254. doi: 10.1136/ard.49.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian AL, Gu YL, Zhou N, Cong W, Li GX, Elsheikha HM, et al. Seroprevalence of Toxoplasma gondii infection in arthritis patients in eastern China. Infect Dis Poverty. 2017;6:153. doi: 10.1186/s40249-017-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.América GU, Luis MV, McGwin Gerald, Martha LS, Jr, John DR, Graciela SA. The systemic lupus activity measure-revised, the Mexican Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), and a modified SLEDAI-2K are adequate instruments to measure disease activity in systemic lupus erythematosus. J Rheumatol. 2004;31:1934. [PubMed] [Google Scholar]
- 30.Tian X, Wang C, Qiao X, Liu N, Dong L, Butler M, et al. Association between pain and frailty among Chinese community-dwelling older adults: depression as a mediator and its interaction with pain. Pain. 2018;159:306–313. doi: 10.1097/j.pain.0000000000001105. [DOI] [PubMed] [Google Scholar]
- 31.Rothman KJ. The estimation of synergy or antagonism. Am J Epidemiol. 1976;103:506–511. doi: 10.1093/oxfordjournals.aje.a112252. [DOI] [PubMed] [Google Scholar]
- 32.Shapira Y, Agmon-Levin N, Shoenfeld Y. Geoepidemiology of autoimmune rheumatic diseases. Nat Rev Rheumatol. 2010;6:468–476. doi: 10.1038/nrrheum.2010.86. [DOI] [PubMed] [Google Scholar]
- 33.Zhou X-j, Jiang N, Li M, Zhang M, Xu J, Jiang L, et al. Chinese SLE Treatment and research group (CSTAR) registry: clinical significance of thrombocytopenia in Chinese patients with systemic lupus erythematosus. PLoS ONE. 2019;14:e0225516. doi: 10.1371/journal.pone.0225516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Leng X, Li Z, Ye Z, Li C, Li X, et al. Chinese SLE treatment and research group registry: III. Association of autoantibodies with clinical manifestations in Chinese patients with systemic lupus erythematosus. J Immunol Res. 2014;2014:1–8. doi: 10.1155/2014/342693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ru J-l, Zhao Y, Xie X-x, Che G-z, Cheng C-f, Zhao H-m, et al. Clinical applications of the indirect immunofluorescence assay for detection of anticell membrane-associated DNA antibodies in juvenile systemic lupus erythematosus. Pediatr Res. 2014;77:376–80. doi: 10.1038/pr.2014.182. [DOI] [PubMed] [Google Scholar]
- 36.Thong B, Olsen NJ. Systemic lupus erythematosus diagnosis and management. Rheumatology. 2017;56:i3–i13. doi: 10.1093/rheumatology/kew401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The number of serum samples of patients with different autoimmune diseases and healthy controls.
Additional file 2: Table S2. The demographics and disease characteristics of SLE patients.
Additional file 3: Table S3. The anti-T. gondii antibodies IgG combined with biochemical indexes/autoantibodies for the prediction of SLE.
Additional file 4: Table S4. Risk factors for disease severity (analysis with 3 factors): anti-T. gondii antibodies IgG, anti-dsDNA and RF.
Additional file 5: Table S5. Risk factors for disease severity (analysis with 3 factors): anti-T. gondii antibodies IgG, anti-dsDNA and anti-rRNP.
Additional file 6: Table S6. Risk factors for disease severity (analysis with 3 factors): anti-T. gondii antibodies IgG, anti-dsDNA and ESR.
Additional file 7: Table S7. Risk factors for disease severity (analysis with 3 factors): anti-T. gondii antibodies IgG, anti-dsDNA and anti-cmDNA.
Data Availability Statement
All data and materials of the experiments described here are included in this published article and its additional files.