Abstract
Per- and polyfluoroalkyl substances (PFAS) are a broad class of synthetic chemicals; some are present in most humans in developed countries. Some studies suggest that certain PFAS may have immunotoxic effects in humans, which could put individuals with high levels of exposure at increased risk for infectious diseases such as COVID-19. We conducted a case-control study to examine the association between COVID-19 diagnosis and PFAS serum concentrations among employees and retirees from two 3 M facilities, one of which historically generated perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), and perfluorohexane sulfonic acid (PFHxS). Participants completed enrollment and follow-up study visits in the Spring of 2021. Participants were categorized as cases if they reported a COVID-19 diagnosis or became sick with at least one symptom of COVID-19 when someone else in their household was diagnosed, otherwise they were categorized as a control. COVID-19 diagnosis was modeled in relation to concentration of serum PFAS measured at enrollment after adjusting for covariates. The analytic sample comprised 573 individuals, 111 cases (19.4%) and 462 controls (80.6%). In adjusted models, the odds ratio of COVID-19 was 0.94 per interquartile range (14.3 ng/mL) increase in PFOS (95% confidence interval 0.85, 1.04). Results for PFOA, PFHxS, and perfluorononanoic acid (PFNA) were similar. Other PFAS present at lower concentrations were examined as categorical variables (above the limit of quantification [LOQ], yes vs. no [referent category]), and also showed no positive associations. In our study, which used individual-level data and included people with high occupational exposure, the serum concentrations of all PFAS examined were not associated with an increased odds ratio for COVID-19. At this point, the epidemiologic data supporting no association of COVID-19 occurrence with PFAS exposure are stronger than those suggesting a positive association.
Keywords: Perfluoroalkyl substances, Polyfluoroalkyl substances, PFAS, Vaccine, Coronavirus disease 2019, COVID-19
Introduction
Studies seeking to identify socio-demographic factors associated with incidence of coronavirus 2019 (COVID-19) have shown increased risk among individuals who are male, non-white, Hispanic, or lower-income, and decreased risk with older age [[1], [2], [3], [4], [5]]. Several comorbidities have also been associated with COVID-19 infection, including body mass index (BMI), cardiovascular disease, diabetes, chronic kidney disease, and chronic or infectious respiratory conditions, among others [4,[6], [7], [8], [9]]. Behaviors such as physical distancing, masking, and vaccination are important for disease prevention [4,10,11]. Research examining the characteristics associated with COVID-19 infection on an individual level in the general population has been relatively rare compared to the number of studies examining risk of severe infection, hospitalization, and mortality associated with COVID-19 [6].
Environmental exposures, such as air pollution, may also increase risk of COVID-19 [12]. Per- and polyfluoroalkyl substances (PFAS) are a group of man-made chemicals that are or were historically used in the manufacturing of industrial and consumer products such as stain resistant carpet, non-stick cookware, and fire-fighting foam [13]. Due to their chemical properties and widespread use, some PFAS are persistent in the environment and have long half-lives in humans [14]. While certain PFAS, including perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), and perfluorohexanesulphonic acid (PFHxS), are detectable in serum in most residents in the United States [15]; levels are declining [16]. Some studies suggest that certain PFAS may have immunotoxic effects in humans [17,18], such as reduced antibody levels, which could put individuals with high levels of exposure at increased risk for infectious diseases such as COVID-19. This suggested association led the Agency for Toxic Substances and Disease Registry to release a call for more research on PFAS exposure and COVID-19 [19].
The association between COVID-19 infection and PFAS has been examined in four previous studies [[20], [21], [22], [23]]. Two of the studies were ecologic [20,21], the third measured PFAS in urine on average two days after the onset of symptoms [22], and the fourth was a prospective cohort study [23]. The first three studies showed a positive association with exposure [[20], [21], [22]]; the prospective cohort study showed no association with risk [23]. Additionally, a study of COVID-19 disease severity found that serum perfluorobutanoic acid (PFBA) was associated with more severe disease, but not other PFAS [24]. In the Spring of 2021, we invited individuals from two manufacturing facilities, one of which historically generated PFAS, to participate in a longitudinal study to assess antibody responses to the COVID-19 vaccine and PFAS concentrations in serum. The longitudinal study showed a small inverse association between antibody response and PFAS, with confidence intervals that included zero [25]. Because of the frequency of reported COVID-19 diagnoses, these data provided an opportunity to investigate PFAS exposure and COVID-19 disease. Here we report the analysis of the 2021 data as a case-control study to examine the association between COVID-19 disease and PFAS serum concentrations in a population with a wide range of exposure.
Methods
This manuscript was written following STROBE guidelines for case-control studies [26].
Study population
As noted above, a longitudinal study was conducted in 2021 among a population comprising current and retired employees of 3 M facilities in Decatur, Alabama and Menomonie, Wisconsin [25]; these data were used to conduct a case-control study of COVID-19 disease and PFAS serum concentrations. These two locations were chosen for their historic PFAS production (Decatur) and non-PFAS production (Menomonie). The Decatur manufacturing site consists of two plants: a film plant and a chemical plant [27]. Until phase-out in the early 2000s, perfluorooctanesulfonyl fluoride (POSF) was produced at the chemical plant by electrochemical cell fluorination (ECF) and used in the batch production of a variety of perfluorinated amides, alcohols, acrylates (all of which have the potential to hydrolyze to PFOS) and PFAS polymers in protective and performance chemicals. PFHxS was similarly made and used in production of performance chemicals including fire suppression liquids. PFOA was occasionally manufactured by ECF for its use as a surfactant (e.g., ammonium perfluorooctanoate, APFO) but was also a by-product of POSF ECF production, as were other PFAS. PFAS had limited use at the Decatur film plant and Menomonie plant. Employees at the Decatur chemical plant continue to have elevated serum concentrations compared to the general population owing to the long serum elimination half-lives of PFOS (half-life ∼3 to 4 years), PFHxS (half-life ∼5 to 7 years), and PFOA (half-life ∼2 to 3 years) [[28], [29], [30]]. These serum concentrations, however, have markedly declined in Decatur chemical plant employees since 2000 [31].
Since the phase-out, POSF-based chemistries have been replaced with perfluorobutanesulfonyl fluoride (PBSF) as the base chemistry in the manufacturing of performance materials at the Decatur chemical plant. PBSF is also produced by ECF, but this particular process occurs elsewhere. PBSF is used in the formulations of a flame retardant additive, an acid mist suppressant, a curative used in fluoroelastomer production, and in perfluorinated amides and alcohols produced at the plant. PBSF and PBSF-derived materials have the potential to degrade to perfluorobutanesulfonate (PFBS), which has an average serum elimination half-life of approximately 30 days [32] and is rarely detected in the CDC National Health and Nutrition Examination Survey (NHANES) analyses of serum from the United States general population [16].
Individuals were eligible to participate if they were at least 18 years of age and an employee or retiree from the Decatur facility or an employee from the Menomonie facility; no other inclusion or exclusion criteria were applied. The study included an enrollment and a follow-up visit. Enrollment visits took place starting April 19, 2021 at the Decatur facility and May 4, 2021 at the Menomonie facility. Follow-up visits were conducted at each site 5–6 weeks later; the final visit was June 17, 2021. At each study visit, participants provided a blood sample and completed a self-administered health questionnaire. All questionnaire responses were reviewed by the study staff to ensure completion before the end of the visit. Serum was aliquoted from each blood sample and stored at or below −20 °C and shipped on dry ice via overnight delivery to the laboratory.
This study was reviewed and approved by the WCG Institutional Review Board. All participants provided written informed consent at the time of enrollment and were notified of their individual test results.
Information about COVID-19 diagnosis
As part of the enrollment and follow-up questionnaires, participants were asked, “Have you ever been diagnosed with COVID-19?”, with No and Yes response options. At the Decatur site, employed participants were additionally asked to bring documentation of the results of any positive COVID-19 test. Those who reported no COVID-19 diagnosis were further asked if anyone in their household had been diagnosed with COVID-19, and if so, if the participant became sick around the same time. Participants were categorized as cases if they reported at enrollment or follow-up that they had been diagnosed with COVID-19 or became sick with at least one symptom of COVID-19 when someone else in their household was diagnosed, otherwise they were categorized as a control (see supplementary material section I for further details).
We asked participants who reported having COVID-19 about the symptoms they had when they were sick. From the symptoms reported, we created a dichotomous disease severity variable based on the United States National Institutes of Health (NIH) severity of COVID-19 illness classification: asymptomatic – no symptoms; mild illness – any signs or symptoms reported other than shortness of breath; moderate-to-severe illness – shortness of breath reported [33].
Serum PFAS analysis
Serum PFAS concentration was assessed by the 3 M Strategic Toxicology Laboratory (St. Paul, MN) using solid phase extraction (SPE) and quantitation by liquid chromatography-tandem mass spectrometry (LC-MS\MS) modified from a previously reported method [34]. Thirteen PFAS compounds were measured: PFOS, PFOA, PFHxS, PFBA, PFBS, perfluoropentanoic acid [PFPeA], perfluorohexanoic acid [PFHxA], perfluoroheptanoic acid [PFHpA], perfluorononanoic acid [PFNA], perfluorodecanoic acid [PFDA], 2-(N-methyl-perfluorooctane sulfonamido) acetic acid [MeFOSAA], 2-(N-Ethyl-perfluorooctane sulfonamido acetic acid [EtFOSAA], and perfluorooctane sulfonamide [PFOSA]. All PFAS were quantified in ng/mL, with a lower limit of quantification (LOQ) of 0.1 ng/mL. PFAS with values below the LOQ were re-expressed as the LOQ divided by the square root of 2.
Spiked quality control (QC) samples were prepared at two concentration levels for a low calibration range (0.1–50 ng/mL) and high calibration range (1.0–250 ng/mL) and were analyzed (n = 6 each level) prior to the analysis of all samples to characterize the QC and to evaluate intra-day precision. The spiked QC were subsequently analyzed in duplicate in each sample batch to monitor the inter-batch precision during the analyses. Depending on the calibration range, concentration level, and analyte the between-batch coefficient of variation ranged from 1.8 to 12.1%. Further information is presented in supplementary material section II.
Covariates
Additional data collected via the health questionnaires were examined as covariates. Date of birth was used to calculate years of age at enrollment; for descriptive purposes age was further categorized as: <45 years, 45 to <65 years, 65+ years. Gender response options included female, male, and other; no participants reported their gender as “other”. Questions regarding race and ethnicity were used to create a race/ethnicity variable with 3 categories: non-Hispanic White, non-Hispanic Black, and other. Self-reported height and weight were used to calculate body mass index (BMI, kg/m2), categorized for descriptive purposes as: normal weight (<25), overweight (25 to <30), and obese (30+). Individuals who had smoked fewer than 100 cigarettes in their lifetime were considered never smokers, while those who had smoked at least 100 cigarettes were divided into former smokers, smoke some days, or smoke every day. The site of current or former employment was categorized as: Decatur Film, Decatur Chemical/Both (included chemical plant only and those who had worked at both the chemical plant and the film plant), and Menomonie. Participants provided their current residential zip code, which was used to identify county of residence.
Statistical analyses
Participants who reported a COVID-19 diagnosis date that occurred after their first vaccination date were excluded from these analyses, as well as those who were missing data on PFAS, COVID-19 diagnosis, or covariates. Median and interquartile range (IQR) were used to describe continuous variables, while count and percentage were used to describe categorical variables. Descriptive statistics, including PFAS distributions, were calculated for the overall sample and stratified by disease status. Self-reported COVID-19 diagnosis was validated against the results of a positive lab test for a subset of participants who reported a positive test and were currently employed at the Decatur site (n = 57).
Logistic regression models were used to assess the association of being a case with serum PFAS. PFAS that had ≥75% of values above the LOQ were examined in models as continuous variables; those with >10% but <75% of values above the LOQ were examined as categorical variables (above the LOQ, yes/no). PFAS examined as continuous variables were re-expressed by dividing raw values by the interquartile range (IQR). A directed acyclic graph [35] (DAG, Fig. S8.1) was used to identify the minimal adjustment set of confounding factors: age, gender, race/ethnicity, site, BMI, and smoking. Various covariate structures (e.g., interactions between adjustment factors) and non-linear representation of continuous variables were considered; percent change in estimates were utilized to identify the most parsimonious models that adequately adjusted for confounding factors in the minimal adjustment set. For each PFAS, bivariate associations were first examined, followed by multivariable models controlling for the confounding factors.
As a sensitivity analysis, results of multivariable models were compared to mixed effect models with site removed from the covariate structure and county of residence included as a random effect. Multivariable models were also used for two stratified analyses: site (Decatur, Menomonie) and employment status (currently employed, retired). For the site-stratified analyses, PFAS were examined as continuous variables expressed as ng/mL due to differences in the range of serum concentrations at each site. The employment-status stratified analyses did not adjust for smoking due to few current smokers among retirees. We also examined coefficients for PFAS-age interactions. In the subset of participants currently employed at the Decatur site, unadjusted and adjusted models were also run after using cases who had a validated COVID-19 diagnosis.
At 3 M facilities, including those studied here, a mask mandate was in effect from July 23, 2020, until April 2022 (our field work was completed by June 2021). The type of mask mandated was an N95 or equivalent for tasks for which social distancing (> 6 ft. separation between employees) could not be achieved. Employees could remove the mask to eat although social distancing had to be maintained. The number of COVID-19 cases reported by our participants to have occurred before the mask mandate was in place, July 23, 2020, was 11 (2 in Menomonie, 9 in Decatur). As a sensitivity analysis, we repeated the analyses after excluding these 11 participants.
A quantitative bias analysis was conducted to assess change in estimate from unmeasured confounding due to socioeconomic status (SES) [36]. We conducted a record-level simple bias analysis informed by data [27,[37], [38], [39]], using a script written in R. The target estimand was an unbiased estimate of the association between PFOS and COVID-19 diagnosis [40], with the final result being the percent difference in the biased and unbiased odds ratios. The Decatur employees had occupational exposure to PFAS, while the Menomonie employees had background exposure similar to the general United States population. Because the sources of exposures for these two groups are theoretically associated with SES differently, our bias analysis was conducted separately for Decatur and Menomonie. Information on the association between income and COVID-19 was available from the literature, and data on the association between income and serum PFOS was obtained for the Decatur population, therefore we used income as a proxy for SES. Further information is provided in supplementary material section III. Example R code for the bias analysis conducted for the Menomonie site is provided in supplementary material section IV.
A second quantitative bias analysis was done to evaluate whether longer COVID-19 illness among more highly exposed cases and their resulting non-participation might have biased the results; this analysis is described in supplementary material section V. In a third quantitative bias analysis (supplementary material section VI), we used a structural approach to adjust for potential selection bias due to nondifferential nonparticipation (Hernan et al., 2004; Hernan & Robins, 2020). This analysis used information on the population of Decatur 3 M employees as of April 2021. We focused on this population as eligible to participate because we had information on plant (surrogate measure of exposure), COVID-19 diagnosis, age, gender, and participation. For retirees and the Menomonie employees we had less information about the eligible population; in Menomonie, because they were not occupationally exposed, we did not believe that selection bias was a concern.
Bayesian kernel machine regression (BKMR) was used to examine the joint association of the four PFAS (PFOS, PFHxS, PFOA, PFNA) modeled as continuous variables as an exposure mixture, which allowed for non-linear and non-additive associations between the mixture and outcome of interest [41]. BKMR was implemented using a fully adjusted probit model with 4 chains and 35,000 iterations per chain. The exposure-response function was characterized as an overall association between the mixture of PFAS and odds of COVID-19 disease. Interactions among the four PFAS were also explored.
In addition to the above analyses of COVID-19 occurrence, we assessed COVID-19 disease severity in relation to PFAS. A complete description of the analysis and its results are presented in the supplementary material section VII.
Analyses were conducted in R 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria) using RStudio 2022.02.2 + 485 (RStudio PBC, Boston, MA) and Stata 17.0 (StataCorp, College Station, TX).
Results
Of the 2338 employees and retirees invited to participate, 586 (25%) enrolled in the study (as described below, the quantitative bias analysis addressing the potential effect of selection bias indicated that the low participation rate was unlikely to have an important effect on the results). After excluding participants who reported a COVID-19 diagnosis that occurred after vaccination (n = 3), had missing PFAS values (n = 9), or had missing covariates (n = 1), the analytic sample comprised 573 individuals. Of these, 462 were categorized as controls (80.6%) and 111 as cases (19.4%, Table 1); 95% of cases reported a COVID-19 diagnosis, while 5% reported becoming sick when someone else in their household was diagnosed. The majority of participants were from the Decatur site, and the median age was 52 years. A higher percentage of cases were from the Decatur plant, and cases were slightly younger than controls. The proportion of the participants that was currently employed was 100% in Menomonie (by design) and 71% in Decatur (Table S9.1). The highest median PFAS concentration was for PFOS, followed by PFHxS, PFOA, and PFNA; these four compounds were above the LOQ for >90% of participants (Table 2). PFBS, MeFOSAA, PFHpA, and PFDA were above the LOQ for >10% but <75% of participants; PFHxA was not above the LOQ in any participants. Distributions of PFAS were similar when stratified by case-control status (Table S9.2). Based on serum concentrations of PFAS, the higher exposure in Decatur was obvious, as was the higher exposure in Decatur among those who had worked in the chemical plant (Table S9.3). The somewhat elevated PFAS concentrations among those who worked in the Decatur film plant may have been due to residence in an area where the municipal water supply was contaminated with PFAS [42]. Proof of a positive laboratory test, used to test the validity of self-reported COVID-19, was available for 87% (n = 48) of the participants in the analytic sample who were currently employed at the Decatur site and self-reported being diagnosed with COVID-19 (n = 55).
Table 1.
Descriptive characteristics of the study sample.
|
Cases n = 111 (19.4%) |
Controls n = 462 (80.6%) |
Total n = 573 |
|
|---|---|---|---|
| Age in years, median (IQR) | 49.8 (23.3) | 51.7 (19.7) | 51.5 (20.0) |
| Gender, n (%) | |||
| Male | 82 (73.9) | 341 (73.8) | 423 (73.8) |
| Female | 29 (26.1) | 121 (26.2) | 150 (26.2) |
| Race/Ethnicity, n (%) | |||
| Non-Hispanic White | 91 (82.0) | 387 (83.8) | 478 (83.4) |
| Non-Hispanic Black | 11 (9.9) | 32 (6.9) | 43 (7.5) |
| Other | 9 (8.1) | 43 (9.3) | 52 (9.1) |
| BMI in kg/m2, median (IQR) | 30.0 (7.3) | 28.2 (6.5) | 28.6 (6.3) |
| Smoking, n(%) | |||
| Never | 73 (65.8) | 304 (65.8) | 377 (65.8) |
| Former | 29 (26.1) | 133 (28.8) | 162 (28.3) |
| Some Days | 8 (7.2) | 10 (2.2) | 18 (3.1) |
| Every Day | 1 (0.9) | 15 (3.3) | 16 (2.8) |
| Site, n (%) | |||
| Decatur Chemical/Botha | 44 (39.6) | 163 (35.3) | 207 (36.1) |
| Decatur Film | 34 (30.6) | 105 (22.7) | 139 (24.3) |
| Menomonie | 33 (29.7) | 194 (42.0) | 227 (39.6) |
Decatur Chemical/Both includes individuals who reported working at the Chemical plant and both the Chemical and Film plants
Table 2.
Percentiles of serum concentrations of per- and polyfluoroalkyl substances (PFAS), ng/mL (n = 573).
| Percentile |
|||||||
|---|---|---|---|---|---|---|---|
| % > LOQ | 5 | 25 | 50 | 75 | 95 | Maximum Value | |
| PFOS | 99.5 | 1.20 | 3.41 | 7.37 | 17.70 | 102.00 | 432.00 |
| PFOA | 99.0 | 0.37 | 0.91 | 1.66 | 5.00 | 31.70 | 139.00 |
| PFHxS | 99.5 | 0.29 | 1.06 | 2.17 | 5.65 | 45.90 | 430.50 |
| PFNA | 93.9 | 0.07 | 0.23 | 0.35 | 0.55 | 1.13 | 7.18 |
| PFBS | 46.2 | 0.07 | 0.07 | 0.07 | 0.24 | 22.90 | 2680.00 |
| PFOSA | 0.2 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.12 |
| MeFOSAA | 38.9 | 0.07 | 0.07 | 0.07 | 0.19 | 1.08 | 6.60 |
| EtFOSAA | 5.8 | 0.07 | 0.07 | 0.07 | 0.07 | 0.12 | 1.76 |
| PFBA | 9.1 | 0.07 | 0.07 | 0.07 | 0.07 | 0.15 | 4.99 |
| PFPeA | 5.1 | 0.07 | 0.07 | 0.07 | 0.07 | 0.10 | 1.06 |
| PFHpA | 10.6 | 0.07 | 0.07 | 0.07 | 0.07 | 0.16 | 1.37 |
| PFDA | 64.7 | 0.07 | 0.07 | 0.14 | 0.23 | 0.45 | 1.22 |
| PFHxA | 0.0 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
Note: Limit of quantification (LOQ) = 0.1, values <LOQ re-expressed as LOQ/√2 = 0.07
PFOS = perfluoroalkyl substances; PFOA = perfluorooctanoic acid; PFHxS = perfluorohexanesulfonic acid; PFNA = perfluorononanoic acid; PFBS = perfluorobutanesulfonic acid; PFOSA = perfluorooctane sulfonamide; MeFOSAA = 2-(N-methyl-perfluorooctane sulfonamido) acetic acid; EtFOSAA = 2-(N-Ethyl-perfluorooctane sulfonamido acetic acid; PFBA = perfluorobutanoic acid; PFPeA = perfluoropentanoic acid; PFHpA = perfluoroheptanoic acid; PFDA = perfluorodecanoic acid; PFHxA = perfluorohexanoic acid
Unadjusted and adjusted associations between PFAS and COVID-19 diagnosis are shown in Table 3. In adjusted models, the odds ratio (OR) of COVID-19 was 0.94 per IQR (14.29 ng/mL) increase in PFOS (95% confidence interval [CI] 0.85, 1.04). PFOA, PFHxS, and PFNA showed similar associations. PFBS, MeFOSAA, and PFHpA, examined as categorical variables (above the LOQ, yes vs. no [referent category]), showed adjusted ORs that were further below one, with wider confidence intervals. Individuals who had a PFDA value above the LOQ had the lowest OR of COVID-19 (0.60; 95% CI 0.38, 0.93). No difference in estimates was seen when comparing the results of the multivariable model to a mixed effects model with county of residence as a random effect (data not shown). In analyses stratified by site (Table S9.4), for PFAS expressed as ng/mL, all ORs were ≤ 1. For PFAS expressed as >LOQ versus not, the ORs for all but MeFOSAA at Decatur were < 1, but in all instances the confidence intervals included 1. In analyses stratified by employment status (Table S9.5), for PFAS expressed as IQR difference, the ORs were generally close to one. For PFAS expressed as >LOQ versus not, the ORs varied, but where the OR was >1, the confidence intervals included 1. Coefficients for PFAS-age interactions were not statistically significant at the p < 0.05 level. Results of models with the subset of validated cases compared to controls were comparable to those from other models (Table S9.6).
Table 3.
Odds ratios of COVID-19 diagnosis per IQRa or > LOQb difference in per-and polyfluoroalkyl substances (PFAS) and 95% confidence interval (n = 573).
| Unadjusted |
Adjustedc |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Continuous (IQRa) | ||||
| PFOS | 0.97 | 0.89, 1.05 | 0.94 | 0.85, 1.04 |
| PFOA | 0.99 | 0.94, 1.05 | 0.98 | 0.92, 1.04 |
| PFHxS | 0.98 | 0.93, 1.03 | 0.98 | 0.93, 1.03 |
| PFNA | 0.96 | 0.84, 1.11 | 0.97 | 0.83, 1.13 |
| Categorical (>LOQb) | ||||
| PFBS | 1.08 | 0.71, 1.63 | 0.68 | 0.40, 1.17 |
| MeFOSAA | 0.99 | 0.65, 1.52 | 0.93 | 0.56, 1.53 |
| PFHpA | 1.02 | 0.52, 1.99 | 0.81 | 0.39, 1.67 |
| PFDA | 0.57 | 0.37, 0.86 | 0.60 | 0.38, 0.93 |
Per Interquartile range (IQR) difference (ng/ml); PFOS IQR = 14.29; PFOA IQR = 4.09; PFHxS IQR = 4.59; PFNA IQR = 0.32
> Limit of Quantification (LOQ) vs. ≤ LOQ (referent)
Adjusted for age, gender, race/ethnicity, smoking, BMI, and site
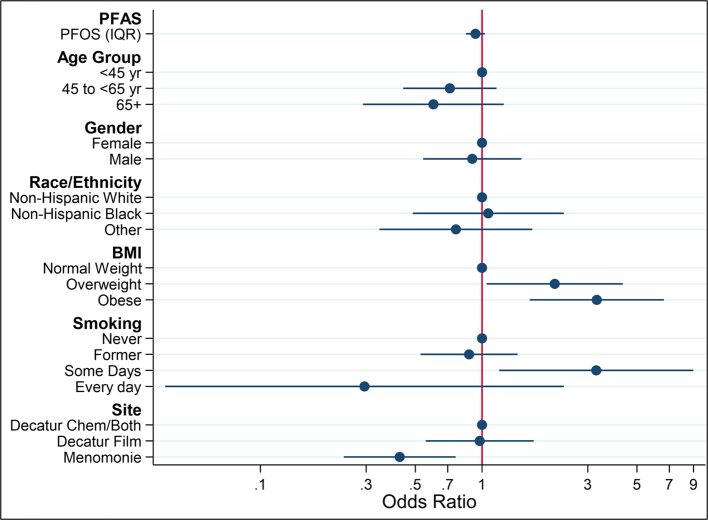
As shown in Fig. 1, in fully adjusted models with PFOS as the exposure, compared to individuals <45 years of age, those in the two older age groups had lower odds of COVID-19 (45 to <65 years OR = 0.72, 95% CI 0.44, 1.16; 65+ OR = 0.60, 95% CI 0.29, 1.25). As compared to normal weight individuals, overweight and obese individuals had a higher odds of COVID-19 (overweight OR = 2.13, 95% CI 1.05, 4.32; obese OR = 3.29, 95% CI 1.64, 6.61). Individuals who smoked some days had higher odds of COVID-19 as compared to never smokers (OR = 3.28, 95% CI 1.19, 8.98), while individuals who smoked every day had lower odds of COVID-19 (OR = 0.29, 95% CI 0.04, 2.34). The OR of having COVID-19 was lower in Menomonie than in Decatur.
Fig. 1.
Odds ratios and 95% confidence intervals for COVID-19 diagnosis according to covariate level (n = 573). PFOS per interquartile range (IQR, 14.29 ng/mL) difference.
Results of the quantitative bias analysis of unmeasured confounding showed that in the Decatur sample the mean unbiased estimate, expressed as an OR, of COVID-19 per IQR difference in PFOS was 0.940 (see supplementary material section III). The percent difference between the biased and unbiased estimate was −1.16%, meaning that the unbiased estimate of the association between PFOS and COVID-19 was 1.16% smaller than the income-unadjusted (biased) estimate of 0.951. In the Menomonie sample the mean unbiased (OR) estimate of COVID-19 per ng/mL change in PFOS was 0.703. The percent difference between the biased and unbiased estimate was 0.86%, meaning that the unbiased estimate of the association between PFAS and COVID-19 was 0.86% larger than the income-unadjusted (biased) estimate of 0.697. The quantitative bias analysis of the potential effect of non-participation among more highly exposed cases with longer COVID-19 illness indicated that such non-participation would not have had an appreciable effect on our results due to the low prevalence of COVID-19 at the time of enrollment (supplementary material section V). In a sensitivity analysis where we repeated the analysis in Table 3 after excluding the 11 cases whose COVID-19 occurred before the 3 M mask mandate, the results remained essentially the same (results not shown).
In our analyses of COVID-19 disease severity in relation to PFAS (Table S7.2), for PFAS expressed as IQR difference, the ORs were slightly greater than one but the confidence intervals were wide. For PFAS expressed as > LOQ vs. not, the ORs varied but the confidence intervals were especially wide. The results adjusted for potential selection bias using a structural approach (OR per IQR of PFOS, 0.95 [95% CI 0.83–1.18], supplementary material table S6.2) were essentially the same as in the main analysis (Table 3). The model of participation indicated that plant (surrogate of exposure), COVID-19 diagnosis, and their interaction were weakly related to participation, and the estimates had wide confidence intervals.
Fitting the BKMR mixture model to the data for PFOS, PFOA, PFHxS, and PFNA indicated that as the percentiles of joint exposure to the overall mixture increased, the OR of COVID-19 was essentially unaffected (Fig. S8.2a). Furthermore, there was no indication of interaction among the four PFAS (Fig. S8.2b).
Discussion
In this study we examined the association of serum concentration of PFAS with odds of COVID-19 in a sample of individuals with a wide range of exposure. In our study data, we found that in fully adjusted models there was a small inverse association per IQR difference in PFOS, PFOA, PFHxS, and PFNA, with confidence intervals that included one. For the PFAS present at lower concentrations (PFBS, MeFOSAA, PFHpA) the associations were further below one but less precise. Individuals with PFDA levels above the LOQ had a lower odds ratio of COVID-19 diagnosis, an association that was statistically significant, possibly due to multiple testing.
By the Spring of 2021 the cumulative incidence of COVID-19 in the communities where the 3 M facilities were was about 10% in both Decatur and Menomonie [43,44]. The cumulative incidence of COVID-19 among participants in our study was 23% in Decatur and 15% in Menomonie, as reflected by the lower adjusted odds of COVID-19 for Menomonie compared with Decatur (Fig. 1). Because the different disease frequency by location in the study population was present after adjusting for serum concentration of PFAS, it was likely due to local factors other than PFAS exposure.
Three out of the four previous studies examining the occurrence of COVID-19 in relation to PFAS exposure suggested a positive association. In an ecologic study, COVID-19 incidence was compared between a community with high PFAS exposure and one without [20]. The incidence was higher in the PFAS-exposed community (standardized incidence ratio 1.19; 95% CI 1.12, 1.27). The authors of the study acknowledged that ecological studies are prone to bias [20]. One other ecologic study examined associations between PFAS and COVID-19 mortality and reported an adjusted rate ratio of 1.60 (90% credibility interval = 0.94, 2.51) for the PFAS-exposed communities [21]. In a case-control study with PFAS measured in urine obtained after disease onset in cases, urine PFOS and PFOA concentrations were higher in cases [22]. The proximal tubule cells in the kidney are an important site for reabsorption of PFAS from the glomerular filtrate, and their apical borders are rich in angiotensin converting enzyme 2 [45,46], which facilitates entry of SARS-COV2 into cells. Patients with COVID-19 have been shown to have renal dysfunction [47]; the higher urine PFAS concentrations among cases in the referenced study may have been due to decreased reabsorption in the nephron. The prospective cohort study included data for 154 participants, of whom 41 were seropositive for COVID-19 [23]. The authors of the cohort study, which showed no association, noted that the concentration of PFAS was relatively low in their participants. Associations between PFAS and COVID-19 have also been examined more broadly. Grandjean and colleagues examined the association between PFAS exposure and severity of COVID-19 in a Danish sample and found no association with the four PFAS we studied in detail [24]. In our case group, few participants had severe disease, thus our power to detect an association with severe disease was low.
Certain PFAS have been shown to alter selected aspects of immune function in animal experiments [14,48,49]. While no molecular mechanism of action accounting for the immunotoxic effects of PFAS has been identified, a mode of action for such toxicity is supported and biologically plausible [14,50]. For example, certain PFAS in animals alter cell signaling involving B-cell/plasma cells and immune cell function, and they diminish antibody response [49]; in humans they have been associated with decreased antibody response to vaccines [51]. Furthermore, PFAS are known to bind with many nuclear receptors, and these receptors are involved in activation of immune cells [[52], [53], [54]]. However, whether clinical infectious disease outcomes are related to serum concentration of PFAS is not clear, because of inconsistent findings [[55], [56], [57], [58], [59], [60]].
Comparing the results of the present study to previous research on COVID-19 risk factors in U.S. adults, the associations seen here are somewhat consistent. While age has been associated with increased disease severity, several studies have shown an inverse association with infection [1,4,5,61], as seen here. BMI has been shown to be directly associated with COVID-19 disease [8,9], as observed in our data. Smoking has generally shown an inverse association with COVID-19 [8,62,63], which is partially consistent with our findings of an inverse association among every day smokers.
Although our sample size was small for a case-control study, a strength of this study is the relatively wide distribution of serum concentrations of PFOS, PFOA, and PFHxS, which increased statistical power to detect an association. We used high-quality biomarkers of exposure. The long half-life of the PFAS we focused on [28,29,59] meant that exposure during a window of susceptibility, if any, was likely captured by the exposure measure, recognizing that the study addressed adult exposure only. In the total sample, COVID-19 diagnosis was based on self-report, rather than through laboratory testing or evidence of a positive test, which may lead to misclassification bias. However, the comparable results in the subsample of participants with validated diagnosis mitigated concerns. Some potential confounders, most notably socioeconomic status, were not measured in this study; inability to control for these may have resulted in biased estimates. However, results of the quantitative bias analysis indicated that adjusting for socioeconomic status would have produced a negligible change in estimates.
Exposure to PFAS and other potentially immunotoxic agents in the Decatur facility was and still is much lower in the film plant as compared with the chemical plant. In the chemical plant, the workers with high past exposure to PFOA, PFOS, or PFHxS, for which 3 M phased-out of the manufacturing of products with these chemistries, assumed the handling of new or replacement chemistries. These new chemistries varied in their level of exposure to employees containing potentially immunotoxic agents such as acrylates. The correlation of past exposure to PFOA, PFOS, or PFHxS, or recent exposure to short-chain PFAS, with other potentially immunotoxic agents in Decatur was likely positive and moderate at most. Furthermore, exposure to other potentially immunotoxic agents, when present, was at a low level. Uncontrolled confounding of COVID-19-PFAS associations by other potentially immunotoxic agents, if it occurred, would have been likely to result in more positive associations to be observed than were in fact present.
At the Decatur facility, like all 3 M facilities, during the pandemic current employees underwent a temperature check at the beginning of each workday and those with an elevated temperature underwent testing for COVID-19. Furthermore, employees were told to not enter the facility if they had any COVID-19 symptoms (fever, cough, difficulty breathing, new loss of taste or smell, sore throat) or were in close contact with a person with COVID-19 in the past two weeks; employees had to acknowledge that they did not have any of these symptoms or close contact before being allowed to enter. In addition, employees provided 3 M with results of COVID-19 tests done by local providers outside the facility. 3 M instituted a COVID-19 paid pandemic leave policy in March 2020. According to the 3 M records, among all current Decatur employees, 23% had COVID-19 by April 22nd, 2021. Among the current Decatur employees who participated in the study, 24% reported having had COVID-19 by April 22nd, 2021. Thus, for the large proportion of the target population for which we have data on cumulative incidence of COVID-19, we have evidence that COVID-19 status had little or no relation to participation, making selection bias unlikely. Because 3 M screened employees at both facilities for elevated temperature and obtained results of COVID-19 tests, current employees' COVID-19 history was already known to 3 M before the study questionnaires were completed. The questionnaires were self-administered with no influence from the study staff regarding specific responses to questions. The staff only checked questionnaires for completeness and any missing responses were reconciled with the study participant.
Infectious disease epidemiology generally considers interaction between individuals in the population studied [64]. While the 3 M mask mandate would have minimized risk from interaction at work, we had no information about viral exposure outside work. In our study, however, interaction among individuals outside work was unlikely to be an important confounding factor. The unusually high exposure to PFAS in our study population began long before such interaction. Such interaction would not be a pre-exposure covariate, and thus not a confounder [65].
When we designed our longitudinal study of antibody response in relation to PFAS serum concentration, we did not anticipate utilizing it for this case-control study. As a consequence, we did not measure antibody concentrations that reflect naturally-acquired immunity rather than immunization [66], which would have allowed us to identify those who had had asymptomatic COVID-19. About 40% of COVID-19 cases are asymptomatic [67]. Thus, our results address only risk of symptomatic COVID-19 in relation to PFAS. A similar limitation occurred in three of the other studies on this topic [[20], [21], [22]]. The one study using a cohort design, however, did measure antibodies allowing detection of previous asymptomatic disease, and, as noted above, they also found no positive association of COVID-19 occurrence and PFAS serum concentrations [23].
The adjustment for potential selection bias had little effect on the results (supplementary material section VI). The assumptions underlying the structural bias approach, described in supplementary material section VI, appeared to be met. Hernan and Robins state that a large selection bias requires a strong association between participation and both exposure and the outcome ([68], (p112)). Although we relied on a surrogate measure of exposure (plant) when implementing the approach, the outcome was especially well measured. In the population of Decatur employees, we found that study participation was not much associated with plant, COVID-19 diagnosis, or their interaction. Thus, a large selection bias was unlikely. While it's possible that knowledge of exposure influenced the decision to participate, we have no reason to suspect that more-exposed cases would be less likely to enroll in the study.
Conclusions
In our study, which used individual-level data and included people with high occupational exposure, the serum concentrations of all PFAS examined were not associated with an increased odds ratio for COVID-19. At this point, the epidemiologic data supporting no association of COVID-19 occurrence with PFAS exposure are stronger than those suggesting a positive association.
CRediT authorship contribution statement
Anna K. Porter: Writing – review & editing, Writing – original draft, Software, Methodology, Formal analysis. Sarah E. Kleinschmidt: Writing – review & editing, Writing – original draft, Supervision, Project administration, Investigation, Conceptualization. Kara L. Andres: Writing – review & editing, Investigation. Courtney N. Reusch: Writing – review & editing, Investigation. Ryan M. Krisko: Writing – review & editing, Investigation. Oyebode A. Taiwo: Writing – review & editing, Supervision. Geary W. Olsen: Writing – review & editing, Supervision, Investigation, Conceptualization. Matthew P. Longnecker: Writing – review & editing, Writing – original draft, Supervision, Methodology, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
AKP and MPL are employees of Ramboll. Their involvement in this project was funded through a contract between 3 M and Ramboll, an international science and engineering company that provided salary compensation to these authors. None of these authors are currently engaged to testify as experts on behalf of the sponsors in litigation related to the compounds discussed in this manuscript.
SEK, KLA, CNR, RMK, OAT, and GWO are employees of 3 M. 3 M, the company that funded this research, previously manufactured perfluorooctyl and perfluorohexyl chemistries in manufacturing.
The final version of this manuscript was negotiated between the employees of Ramboll and 3 M. The authors retained sole control of the manuscript content and the findings, and statements in this paper are those of the authors and not those of the authors' employers.
Acknowledgements
We thank the following individuals for their contribution to the study: Cathy Simpson, Rosemary Berger, Mary Jane Mitchell, Sandi Cook, Bryan Topp, Kari Kilbride, Jamie Hantsbarger, Tracy Renn, Barbara Letourneau, Amy Donlonc, Lynn Kosterman, Jeremy Zitzow and Alan Eveland. In addition, we thank the 3 M workers and retirees who participated in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gloepi.2024.100137.
Contributor Information
Anna K. Porter, Email: anna@annaporter.me.
Sarah E. Kleinschmidt, Email: sekleinschmidt@mmm.com.
Kara L. Andres, Email: klandres@mmm.com.
Courtney N. Reusch, Email: cnnyberg@mmm.com.
Ryan M. Krisko, Email: rmkrisko@mmm.com.
Oyebode A. Taiwo, Email: oataiwo@mmm.com.
Geary W. Olsen, Email: gwolsen@mmm.com.
Matthew P. Longnecker, Email: mlongnecker@ramboll.com.
Appendix A. Supplementary data
Supplementary material
References
- 1.Anderson J.L., May H.T., Knight S., et al. Association of sociodemographic factors and blood group type with risk of COVID-19 in a US population. JAMA Netw Open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niedzwiedz C.L., O’Donnell C.A., Jani B.D., et al. Ethnic and socioeconomic differences in SARS-CoV-2 infection: prospective cohort study using UK Biobank. BMC Med. 2020;18:160. doi: 10.1186/s12916-020-01640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vahidy F.S., Nicolas J.C., Meeks J.R., et al. Racial and ethnic disparities in SARS-CoV-2 pandemic: analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2020-039849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu K.H.H., Hornsby W.E., Klunder B., et al. Exposure and risk factors for COVID-19 and the impact of staying home on Michigan residents. PloS One. 2021;16(2) doi: 10.1371/journal.pone.0246447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morlock R., Morlock A., Downen M., Shah S.N. COVID-19 prevalence and predictors in United States adults during peak stay-at-home orders. PloS One. 2021;16(1) doi: 10.1371/journal.pone.0245586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKeigue P.M., Weir A., Bishop J., et al. Rapid epidemiological analysis of comorbidities and treatments as risk factors for COVID-19 in Scotland (REACT-SCOT): a population-based case-control study. PLoS Med. 2020;17(10) doi: 10.1371/journal.pmed.1003374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman J., Ballin M., Nordström A., Nordström P. Risk factors for COVID-19 diagnosis, hospitalization, and subsequent all-cause mortality in Sweden: a nationwide study. Eur J Epidemiol. 2021;36(3):287–298. doi: 10.1007/s10654-021-00732-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu T., Mack J.A., Salvatore M., et al. Characteristics associated with racial/ethnic disparities in COVID-19 outcomes in an academic health care system. JAMA Netw Open. 2020;3(10) doi: 10.1001/jamanetworkopen.2020.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karmakar M., Lantz P.M., Tipirneni R. Association of social and demographic factors with COVID-19 incidence and death rates in the US. JAMA Netw Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.36462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet Lond Engl. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor C.A. Severity of disease among adults hospitalized with laboratory-confirmed COVID-19 before and during the period of SARS-CoV-2 B.1.617.2 (Delta) predominance — COVID-NET, 14 states, January–august 2021. MMWR Morb Mortal Wkly Rep. 2021:70. doi: 10.15585/mmwr.mm7043e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zang S.T., Luan J., Li L., et al. Ambient air pollution and COVID-19 risk: evidence from 35 observational studies. Environ Res. 2022;204 doi: 10.1016/j.envres.2021.112065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck R.C., Franklin J., Berger U., et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011;7(4):513–541. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agency for Toxic Substances and Disease Registry (ATSDR) U.S. Department of Health and Human Services; 2021. Toxicological profile for Perfluoroalkyls.https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf [PubMed] [Google Scholar]
- 15.Evich M.G., Davis M.J.B., McCord J.P., et al. Per- and polyfluoroalkyl substances in the environment. Science. 2022;375(6580) doi: 10.1126/science.abg9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention . Updated Tables. U.S. Department of Health and Human Services; Centers for Disease Control and Prevention: 2021. Fourth National Report on human exposure to environmental chemicals.https://www.cdc.gov/exposurereport/ [Google Scholar]
- 17.National Toxicology Program (NTP) U.S. Department of Health and Human Services; 2016. Monograph on immunotoxicity associated with exposure to perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS)https://ntp.niehs.nih.gov/ntp/ohat/pfoa_pfos/pfoa_pfosmonograph_508.pdf [Google Scholar]
- 18.Lopez-Espinosa M.J., Carrizosa C., Luster M.I., et al. Perfluoroalkyl substances and immune cell counts in adults from the mid-Ohio Valley (USA) Environ Int. 2021;156 doi: 10.1016/j.envint.2021.106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agency for Toxic Substances and Disease Registry (ATSDR) June 24, 2020. Per- and Polyfluoroalkyl Substances (PFAS) and Your Health. Accessed April 5, 2022. https://www.atsdr.cdc.gov/pfas/health-effects/index.html. [Google Scholar]
- 20.Nielsen C., Jöud A. Susceptibility to COVID-19 after high exposure to perfluoroalkyl substances from contaminated drinking water: an ecological study from Ronneby, Sweden. Int J Environ Res Public Health. 2021;18(20):10702. doi: 10.3390/ijerph182010702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catelan D., Biggeri A., Russo F., et al. Exposure to perfluoroalkyl substances and mortality for COVID-19: a spatial ecological analysis in the veneto region (Italy) Int J Environ Res Public Health. 2021;18(5):2734. doi: 10.3390/ijerph18052734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji J., Song L., Wang J., et al. Association between urinary per- and poly-fluoroalkyl substances and COVID-19 susceptibility. Environ Int. 2021;153 doi: 10.1016/j.envint.2021.106524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pumarega J., Gasull M., Koponen J., et al. Prepandemic personal concentrations of per- and polyfluoroalkyl substances (PFAS) and other pollutants: specific and combined effects on the incidence of COVID-19 disease and SARS-CoV-2 infection. Environ Res. 2023;237 doi: 10.1016/j.envres.2023.116965. [DOI] [PubMed] [Google Scholar]
- 24.Grandjean P., Timmermann C.A.G., Kruse M., et al. Severity of COVID-19 at elevated exposure to perfluorinated alkylates. Meliker J, ed. PloS One. 2020;15(12) doi: 10.1371/journal.pone.0244815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter A.K., Kleinschmidt S.E., Andres K.L., et al. Antibody response to COVID-19 vaccines among workers with a wide range of exposure to per-and polyfluoroalkyl substances. Environ Int. September 2022:107537. doi: 10.1016/j.envint.2022.107537. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)Statement: guidelines for reporting observational studies. March 6, 2023. Accessed September 6, 2023. https://www.equator-network.org/reporting-guidelines/strobe/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsen G.W., Logan P.W., Hansen K.J., et al. An occupational exposure assessment of a Perfluorooctanesulfonyl fluoride production site: biomonitoring. AIHA J. 2003;64(5):651–659. doi: 10.1080/15428110308984859. [DOI] [PubMed] [Google Scholar]
- 28.Li Y., Fletcher T., Mucs D., et al. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med. 2018;75(1):46–51. doi: 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen G.W., Burris J.M., Ehresman D.J., et al. Half-life of serum elimination of Perfluorooctanesulfonate, Perfluorohexanesulfonate, and Perfluorooctanoate in retired Fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Y., Fletcher T., Pineda D., et al. Serum half-lives for short- and long-chain perfluoroalkyl acids after ceasing exposure from drinking water contaminated by firefighting foam. Environ Health Perspect. 2020;128(7) doi: 10.1289/EHP6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen G.W., Andres K.L., Kleinschmidt S.E. Presented at: SETAC north American focused topic meeting - environmental risk assessment of per- and Polyfluoroalkyl substances (PFAS) August 12, 2019. Decline in serum PFAS levels among 3M Decatur employees and retirees. Durham, NC. [Google Scholar]
- 32.Olsen G.W., Chang S.C., Noker P.E., et al. A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicology. 2009;256(1–2):65–74. doi: 10.1016/j.tox.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 33.National Institutes of Health . September 26, 2022. Clinical Spectrum of SARS-CoV-2 Infection. COVID-19 Treatment Guidelines. Accessed October 28, 2022. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ [Google Scholar]
- 34.Ehresman D.J., Froehlich J.W., Olsen G.W., Chang S.C., Butenhoff J.L. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ Res. 2007;103(2):176–184. doi: 10.1016/j.envres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Ankan A., Wortel I.M.N., Textor J. Testing graphical causal models using the R package “dagitty”. Curr Protoc. 2021;1(2) doi: 10.1002/cpz1.45. [DOI] [PubMed] [Google Scholar]
- 36.Fox M.P., MacLehose R.F., Lash T.L. Springer International Publishing; 2021. Applying quantitative Bias analysis to epidemiologic data. [Google Scholar]
- 37.Lin P.I.D., Cardenas A., Hauser R., et al. Temporal trends of concentrations of per- and polyfluoroalkyl substances among adults with overweight and obesity in the United States: results from the diabetes prevention program and NHANES. Environ Int. 2021;157 doi: 10.1016/j.envint.2021.106789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allan-Blitz L.T., Goldbeck C., Hertlein F., Turner I., Klausner J.D. Association of lower socioeconomic status and SARS-CoV-2 positivity in Los Angeles, California. J Prev Med Public Health. 2021;54(3):161–165. doi: 10.3961/jpmph.21.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. NHANES 2017–2018 Overview. Accessed July 21, 2022. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overview.aspx?BeginYear=2017.
- 40.Morris T.P., White I.R., Crowther M.J. Using simulation studies to evaluate statistical methods. Stat Med. 2019;38(11):2074–2102. doi: 10.1002/sim.8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bobb J.F., Claus Henn B., Valeri L., Coull B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health. 2018;17(1):67. doi: 10.1186/s12940-018-0413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Worley R.R., Moore S.M., Tierney B.C., et al. Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ Int. 2017;106:135–143. doi: 10.1016/j.envint.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong E., Ratcliff J., Goyea T.D., et al. The Johns Hopkins University Center for systems science and engineering COVID-19 dashboard: data collection process, challenges faced, and lessons learned. Lancet Infect Dis. 2022;22(12):e370–e376. doi: 10.1016/S1473-3099(22)00434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monteil V., Kwon H., Prado P., et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su H., Yang M., Wan C., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Legrand M., Bell S., Forni L., et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021;17(11):751–764. doi: 10.1038/s41581-021-00452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corsini E., Luebke R.W., Germolec D.R., DeWitt J.C. Perfluorinated compounds: emerging POPs with potential immunotoxicity. Toxicol Lett. 2014;230(2):263–270. doi: 10.1016/j.toxlet.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeWitt J.C., Williams W.C., Creech N.J., Luebke R.W. Suppression of antigen-specific antibody responses in mice exposed to perfluorooctanoic acid: role of PPAR α and T- and B-cell targeting. J Immunotoxicol. 2016;13(1):38–45. doi: 10.3109/1547691X.2014.996682. [DOI] [PubMed] [Google Scholar]
- 50.DeWitt J.C., Blossom S.J., Schaider L.A. Exposure to per-fluoroalkyl and polyfluoroalkyl substances leads to immunotoxicity: epidemiological and toxicological evidence. J Expo Sci Environ Epidemiol. 2019;29(2):148–156. doi: 10.1038/s41370-018-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crawford L., Halperin S.A., Dzierlenga M.W., et al. Systematic review and meta-analysis of epidemiologic data on vaccine response in relation to exposure to five principal perfluoroalkyl substances. Environ Int. 2023;172 doi: 10.1016/j.envint.2023.107734. [DOI] [PubMed] [Google Scholar]
- 52.Beggs K.M., McGreal S.R., McCarthy A., et al. The role of hepatocyte nuclear factor 4-alpha in perfluorooctanoic acid- and perfluorooctanesulfonic acid-induced hepatocellular dysfunction. Toxicol Appl Pharmacol. 2016;304:18–29. doi: 10.1016/j.taap.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiss M., Nagy L. In: Encyclopedia of immunobiology. Elsevier; 2016. Nuclear receptors in immune function; pp. 146–156. [DOI] [Google Scholar]
- 54.Rosen M.B., Das K.P., Rooney J., Abbott B., Lau C., Corton J.C. PPARα-independent transcriptional targets of perfluoroalkyl acids revealed by transcript profiling. Toxicology. 2017;387:95–107. doi: 10.1016/j.tox.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ait Bamai Y., Goudarzi H., Araki A., et al. Effect of prenatal exposure to per- and polyfluoroalkyl substances on childhood allergies and common infectious diseases in children up to age 7 years: the Hokkaido study on environment and children’s health. Environ Int. 2020;143 doi: 10.1016/j.envint.2020.105979. [DOI] [PubMed] [Google Scholar]
- 56.Chang E.T., Adami H.O., Boffetta P., Wedner H.J., Mandel J.S. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and immunological health conditions in humans. Crit Rev Toxicol. 2016;46(4):279–331. doi: 10.3109/10408444.2015.1122573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Impinen A., Nygaard U.C., Lødrup Carlsen K.C., et al. Prenatal exposure to perfluoralkyl substances (PFASs) associated with respiratory tract infections but not allergy- and asthma-related health outcomes in childhood. Environ Res. 2018;160:518–523. doi: 10.1016/j.envres.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 58.Impinen A., Longnecker M.P., Nygaard U.C., et al. Maternal levels of perfluoroalkyl substances (PFASs) during pregnancy and childhood allergy and asthma related outcomes and infections in the Norwegian mother and child (MoBa) cohort. Environ Int. 2019;124:462–472. doi: 10.1016/j.envint.2018.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kvalem H.E., Nygaard U.C., Lødrup Carlsen K.C., Carlsen K.H., Haug L.S., Granum B. Perfluoroalkyl substances, airways infections, allergy and asthma related health outcomes – implications of gender, exposure period and study design. Environ Int. 2020;134 doi: 10.1016/j.envint.2019.105259. [DOI] [PubMed] [Google Scholar]
- 60.Manzano-Salgado C.B., Casas M., Lopez-Espinosa M.J., et al. Variability of perfluoroalkyl substance concentrations in pregnant women by socio-demographic and dietary factors in a Spanish birth cohort. Environ Int. 2016;92-93:357–365. doi: 10.1016/j.envint.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Hong D., Lee S., Choi Y.J., et al. The age-standardized incidence, mortality, and case fatality rates of COVID-19 in 79 countries: a cross-sectional comparison and their correlations with associated factors. Epidemiol Health. 2021;43 doi: 10.4178/epih.e2021061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trübner F., Steigert L., Echterdiek F., et al. Predictors of COVID-19 in an outpatient fever clinic. PloS One. 2021;16(7) doi: 10.1371/journal.pone.0254990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao X., Ni W., Zhu S., et al. Per- and polyfluoroalkyl substances exposure during pregnancy and adverse pregnancy and birth outcomes: a systematic review and meta-analysis. Environ Res. 2021;201 doi: 10.1016/j.envres.2021.111632. [DOI] [PubMed] [Google Scholar]
- 64.Straif-Bourgeois S., Ratard R., Kretzschmar M. In: Handbook of epidemiology. Ahrens W., Pigeot I., editors. Springer; New York: 2014. Infectious disease epidemiology; pp. 2041–2119. [DOI] [Google Scholar]
- 65.VanderWeele T.J., Shpitser I. On the definition of a confounder. Ann Stat. 2013;41(1) doi: 10.1214/12-AOS1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pérez-Olmeda M., Saugar J.M., Fernández-García A., et al. Evolution of antibodies against SARS-CoV-2 over seven months: experience of the nationwide seroprevalence ENE-COVID study in Spain. J Clin Virol. 2022;149 doi: 10.1016/j.jcv.2022.105130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma Q., Liu J., Liu Q., et al. Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and Meta-analysis. JAMA Netw Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.37257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hernan M.A., Robins J.M. Chapman & Hall/CRC; 2020. Causal inference: What if.https://www.hsph.harvard.edu/miguel-hernan/causal-inference-book/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material