Abstract
Background
Commercially available irrigation solutions are used to reduce bacterial contamination and prevent surgical site infections. However, the effect of these solutions on the healing capacity of tissue has not been well-established. The purpose of this study was to investigate the effects of 5 commercially available irrigation solutions on host tissue in a murine model.
Methods
There were 5 treatment groups: bacitracin, Clorpactin, Irrisept, Prontosan, Bactisure, and normal saline control. The irrigation solutions were applied to the wound for 30 seconds or 1 minute, as per the manufacturer’s instructions, and then washed with normal saline. Mice were sacrificed at 3 days and 10 days. The tissue was examined histologically for inflammation, edema, granulation tissue formation, and re-epithelialization. Granulation tissue formation and re-epithelialization were surrogates for effective wound healing.
Results
All of the irrigation solutions had negative effects on host tissue in the acute phase. The inflammation and edema were improved in the later phase (10 days). Recovery and healing of the open wounds were observed for all groups at 10 days. The antiseptic irrigation solutions had similar cytotoxic effects on host tissue at 3 days and did not have delayed or compromised wound healing at 10 days when compared to normal saline control.
Conclusions
Single short-duration use of these commercially available antiseptic irrigation solutions appears to be safe in an uninfected wound. Data from this study will provide surgeons with useful information regarding the safety of using antiseptic wound irrigation solutions intraoperatively for prevention of surgical site infections.
Keywords: Antiseptics, Irrigation solutions, Open wound irrigation
Introduction
Surgical site infections (SSIs) are common but feared complications after orthopaedic surgery. Data suggest an incidence of 2.5% of all operations [1]. SSIs often necessitate additional surgeries creating increased morbidity to patients. This also becomes a financial burden to both the patient and the hospital for readmissions, increased lengths of stay, emergency department visits, outpatient visits, and use of ancillary services [2,3]. Bacterial contamination of the wound during surgery is a recognized and predictive cause of infection [[4], [5], [6]]. In 2017, the Centers for Disease Control and Prevention released updated guidelines for SSI prevention, including use of antiseptic irrigation solutions intraoperatively [7]. Antiseptics act in a broad-spectrum manner to destroy or inhibit the growth and development of microorganisms, unlike antibiotics which act in a more selective manner. Therefore, antiseptics are appealing over topical antibiotics as there is no concern for selective resistance. Many of the marketed antiseptic irrigation solutions also incorporate a surfactant, such as betaine and benzalkonium chloride, that acts as a detergent to help break down biofilm and other cellular exudate in addition to their cytotoxic active ingredients.
It has been reported that mechanical debridement of surgical wounds through saline irrigation can be effectively supported by antiseptic solutions in preventing SSIs [8,9]. This is paralleled by many in-vitro studies demonstrating the profound bactericidal effect of these solutions [10]. But, in addition to evidence that supports their anti-infective capabilities, these solutions have also demonstrated toxicity to host cells in vitro [[11], [12], [13], [14], [15]]. This toxicity could be detrimental to wound healing and even increase the rate of wound complications [16,17]. Meurs et. al. explored this bactericidal to cytotoxic relationship among 5 commercially available antiseptic irrigation solutions in vitro using fibroblasts and stromal progenitor cells [18]. Irrigation solutions were diluted until a minimal bactericidal concentration was established. All agents except polyhexanide were bactericidal and cytotoxic at commercially available levels. Diluted povidone iodine (1.3 g/L) was the only irrigant that was bactericidal at concentrations in which some regenerative cells remained viable. Toxicity in a cell culture environment may not precisely reflect cellular toxicity, tissue toxicity, or wound healing interference in an in vivo environment, due to the complexity of biological systems, including blood flow, immune response, and so on [19]. The effects of these solutions on the healing capacity of tissue in vivo have not been well established.
The purpose of this study was to investigate the cytotoxic effects of 5 commercially available antiseptic irrigation solutions on the healing capacity of full-thickness wounds in a murine model. We comparatively analyzed wounds in 2 distinct phases, acute and delayed. In the acute phase, we evaluated how each solution influenced the early inflammatory response in living, healthy tissue. In the delayed phase, we evaluated the capacity for tissue to initiate a healing response. Previously, we investigated the effect of irrigation solutions on osteoblast cytotoxicity and proliferation in vitro and witnessed morphologic changes with all irrigants and extreme detrimental effects of Irrisept [20]. In addition, we studied the effects of irrigation solutions on 3-dimensional sheets of fibroblasts in vitro and demonstrated severe cytotoxic effects of Irrisept and Bactisure [21]. Based on our previous in vitro research observations and the work of others, such as Meurs et al. [18], we formulated 2 hypotheses for this in vivo investigation: first, the antiseptic solutions would cause more inflammation in the host tissue acutely compared to normal saline and second, the groups treated with antiseptic irrigation solutions would show an impaired wound healing response at 10 days as measured by the degree of re-epithelialization. We believed that the study outcomes could help guide orthopaedic surgeons when assessing the safety of these solutions as tools to help prevent SSI and understand their impact on native tissues.
Material and methods
Animal groups
This project was approved by the Institutional Animal Care and Use Committee (104-19). Sixty BALB C female mice, body weight 20-30 g, were used for study. There were 10 mice in each group, 5 at each time point (3 days and 10 days). Sample sizes were based on power analysis and previous reports from similar procedures. The mice were caged individually and allowed to feed ad libitum. The mice were randomly assigned to 6 groups (Table 1): bacitracin (33 IU/ml), Clorpactin (0.2% calcium hypochlorite; United-Guardian Inc., Hauppauge, NY), Irrisept (0.05% chlorhexidine Gluconate; Irrimax Corporation, Gainesville, FL), Bactisure (100 g/L ethanol, 59 g/L acetic acid, 30 g/L sodium acetate, 1.3 g/L benzalkonium chloride; Zimmer Biomet, Warsaw, IN), Prontosan (0.1% polyhexanide and 0.1% betaine; B. Braun Medical Inc., Bethlehem, PA), and control (0.9% normal saline).
Table 1.
Group characteristics.
| Group | Control | Bacitracin | Bactisure | Irrisept | Prontosan | Clorpactin | Total |
|---|---|---|---|---|---|---|---|
| Day 3 | 5 | 5 | 5 | 5 | 5 | 5 | 30 |
| Day 10 | 5 | 5 | 5 | 5 | 5 | 5 | 30 |
Operative procedure
Animals were acclimatized for 1 week prior to surgery. Mice were anesthetized using isoflurane (5% in oxygen) in an induction chamber for approximately 2 minutes followed by administration of ketamine (120 mg/kg) and xylazine (10 mg/kg) for extended anesthesia. Ketoprofen (5 mg/kg) was injected subcutaneously for preoperative analgesia. The designated surgical area was shaved and prepped with Betadine and 70% ethanol. Bilateral full-thickness excisional wounds were created with a 5-mm punch using the method of Dunn et al. [22], followed by splinting of the wound using sutured silicone rings (see Fig. 1) [23]. The precut silicone splint rings were glued to the skin then additionally secured using 6.0 Nylon sutures to prevent early wound closure. It is important to point out that wound healing is a very complex process which includes inflammation, cell proliferation and angiogenesis, re-epithelialization, and remodeling with reorganization of collagen. There is one major fundamental difference when comparing wound healing in mice to that of humans. Mice have an extensive subcutaneous striated muscle layer called the Panniculus Carnosus, which causes early wound contraction, which is not present in humans. Therefore, in order to more closely replicate the wound healing process in humans, early wound contraction was prevented. This also allows for a more ideal wound tissue collection for histologic analysis [22,23].
Figure 1.
Murine wound healing model. In this model, 2 full-thickness wounds were created on either side of the midline using a single 5-mm biopsy punch. Silicone splints were glued then sutured to the wound perimeter to prevent wound contraction, providing a model replicable to that of humans.
Initially, each mouse was to serve as its own control: right wound for treatment and left wound for control. However, concern was raised that the treatment solutions could leak under the skin and “contaminate” the contralateral control wound; therefore, the stand-alone control group was used for the control analysis.
According to prior group determination, each irrigation solution (100μl) was applied to the right side wound for 1 minute (bacitracin, Clorpactin, Irrisept, Prontosan, and normal saline) and for 30 seconds (Bactisure), as described in the respective manufacturer’s instructions. The wound was then dried and rinsed 3 times with sterile 0.9% normal saline (100μl). The control group was washed 3 times with sterile 0.9% saline and pat dried. Following surgery, the animals were placed in individual cages with a regular diet and once daily assessments made until the date of sacrifice (3 and 10 days). Ketoprofen was administered after surgery once daily for 1 week.
On sacrifice day 3 and day 10, the silicone splint was removed. Full excision of the tissue around and under the wound was performed sharply and the tissue fixed in 10% neutral buffered formalin and paraffin embedded for histology. Animals were removed from the remainder of the study and excluded from analysis for failure to thrive (weight loss ≥20%), infections unresponsive to treatment, and moribund state. Lost specimens were replaced with allotted replacements, such that final analysis was done with predetermined n values.
Histologic evaluation
Hematoxylin-and-eosin-stained slides were prepared from all 60 mice samples. The sections were sent to HistoWiz Inc. (Brooklyn, NY) for analysis, where they were blindly examined and graded by a veterinary pathologist. The grading system for observations of interest had been established and was similar to the Sessing Scale described by Ferrell [24] and techniques used by Gupta et al. [25]. In the acute/inflammatory phase (3 day) focus was placed on inflammation and edema. During the delayed term/healing phase (10 day) inflammation and edema were graded for comparison to the acute phase and granulation and re-epithelialization were graded to assess wound healing. The scoring system used for grading is seen in Figure 2.
Figure 2.
Histology grading scheme for inflammation, edema, granulation tissue formation, and re-epithelialization.
Statistical analysis
Statistical analysis was performed by using Microsoft Office Excel. Mean and standard deviations were calculated for each of the graded categories. Single-factor analysis of variance was used to compare the groups. Post hoc 2-tailed t-tests were then performed to compare each groups separately. Significance was set at P < .05.
Animal research guidelines
All standards for animal care and investigation established in the National Research Council’s Guide for the Care and Use of Laboratory Animals, The Federal Animal Welfare Act, and all policies established by Ascension-Providence Hospital Institutional Animal Care and Use Committee were followed for this study.
Results
Histologic grading demonstrations
The representative images of histologic grading scores for inflammation, edema, granulation tissue, and re-epithelialization are shown in Figure 3, Figure 4, Figure 5, Figure 6, respectively. These photos were taken from specimen slides at 3 days and 10 days and convey visualization of the grading scheme used by the pathologist.
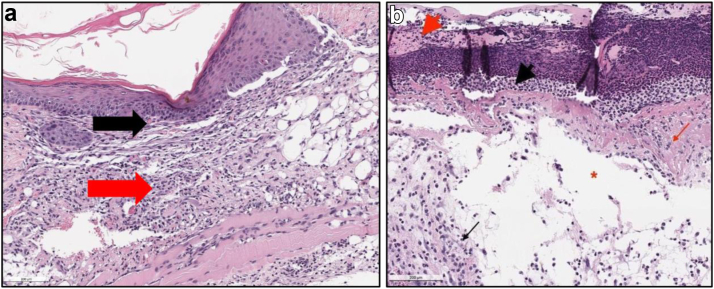
Figure 3.
Representative photos of H&E slides described by the pathologist demonstrating the inflammation grading scheme (0-4). (a) is showing a score of 1. Prontosan, Day 10, (10x). Mild inflammation (Score 1/4) in the dermis (black arrow) and subcutis (red arrow). The epithelium is intact. (b) is showing a score of 4. Clorpactin, Day 3, (10×). Numerous degenerative neutrophils (inflammation score 4/4) in the ulcerated area (black arrowhead) with inflammation in deep dermis (black arrow). Dermal collagen degeneration and necrosis (red arrow) and serocellular crusting of the ulcerated epithelium (red arrowhead). H&E, hematoxylin and eosin.
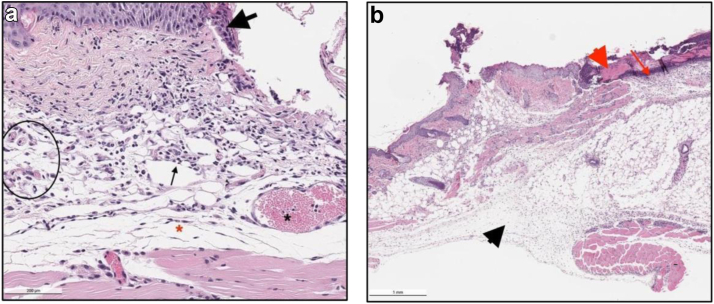
Figure 4.
Representative photos of H&E slides described by the pathologist demonstrating the edema grading scheme (0-4). (a) showing a score of 2. Bacitracin, Day 3, (10×). Moderate inflammation (score 2/4, black arrow), edema (score 2/4, red asterisk), small increased numbers of subcutaneous/deep dermal congested vessels (black circle), large, congested vessel (black asterisk) and degenerative and necrotic epithelium (black arrowhead), with epithelial hyperplasia and spongiosis (intracellular edema) left of black arrowhead. (b) showing a score of 4. Prontosan, Day 3, (2×). Severe inflammation consisting of numerous degenerative neutrophils in the dermis (red arrow) at the area of ulceration with serocellular crusting (red arrowhead) and severe edema (score 4/4 score, black arrowhead).
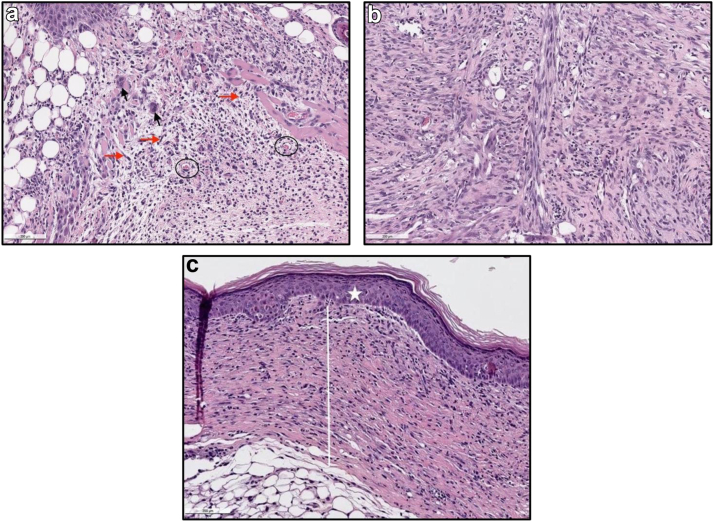
Figure 5.
Representative photos of H&E slides described by the pathologist demonstrating granulation tissue grading scheme (1-3). (a) showing score of 1. Clorpactin, Day 10, (10×). Immature granulation tissue (score 1/3): loose granulation tissue (macrophages black arrow, fibroblasts red arrows) with emerging vessels (black circles) and scattered inflammatory cells throughout the dermis and subcutis. (b) showing a score of 2. Bactisure, Day 10, (10×). Dermis with granulation tissue (score 2/3). Mature granulation tissue: fibroblasts and sparse extracellular matrix proteins forming layers and vessels running perpendicular. (c) showing a score of 3. Bacitracin, Day 10, (10×). Full thickness dermal scar formation (score 3/3, white line). Full re-epithelialization (score 4/4) of the epithelium (white star). H&E, hematoxylin and eosin.
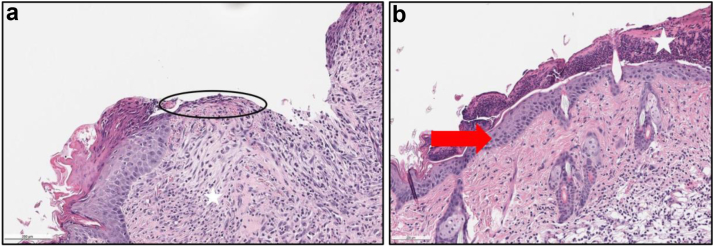
Figure 6.
Representative photos of H&E slides described by the pathologist demonstrating Re-epithelialization grading scheme (0-4). (a) showing a score of 1. Clorpactin, Day 10, (10×). Early re-epithelialization (score 1/4, circle), with migrating epithelial cells with unclosed ulcer. Early granulation tissue (1/4, white star). (b) showing a score of 4. Bactisure, Day 10, (10×). Re-epithelialization score (score 4/4, red arrow), thickened epithelium that is fully filled in with horizontal basement membrane. Overlying Scab (white star). H&E, hematoxylin and eosin.
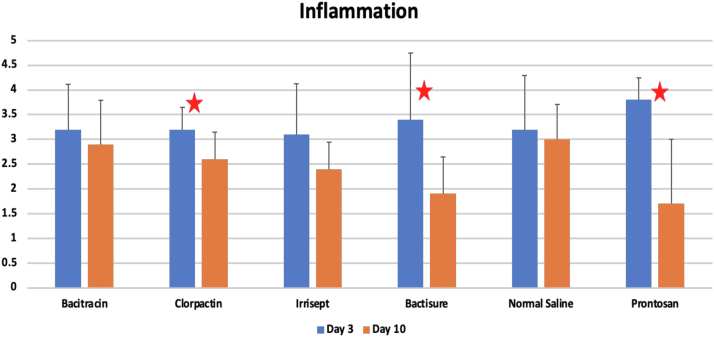
Inflammation scores
As shown in Figure 7, at 3 days, the inflammation scores were 3.2, 3.2, 3.1, 3.4, 3.8, and 3.2 for bacitracin, Clorpactin, Irrisept, Bactisure, Prontosan, and saline control, respectively. Although Bactisure and Prontosan showed higher inflammation scores than saline control, there were no statistical differences among groups (P = .86). At 10 days, the inflammation scores were 3.0, 2.6, 2.4, 1.9, 1.7, and 3.0 for bacitracin, Clorpactin, Irrisept, Bactisure, Prontosan, and saline control, respectively. There were no statistical differences among groups at 10 days (P = .11). However, a significant reduction of inflammation was observed in the Clorpactin, Bactisure, and Prontosan groups at 10 days as compared to 3 days (P < .05).
Figure 7.
Inflammation scores at 3 days and 10 days, respectively. Red stars denote statistical significance within groups between 3 days and 10 days (P < .05).
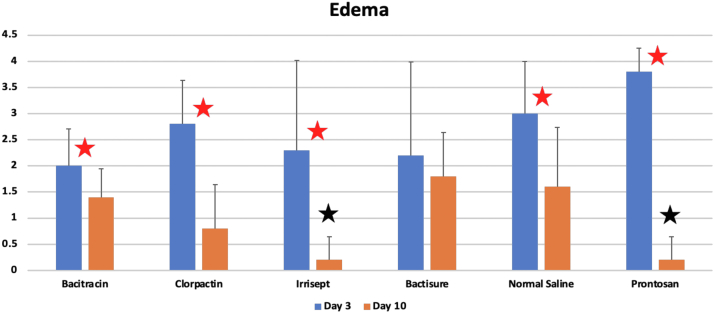
Edema scores
As shown in Figure 8, at 3 days, the edema scores were 2.0, 2.8, 2.3, 2.2, 3.8, and 3.0 for bacitracin, Clorpactin, Irrisept, Bactisure, Prontosan, and normal saline, respectively. There were no statistical differences among groups (P = .21), though Prontosan had a higher edema score than saline control. At 10 days, the edema scores were 1.4, 0.8, 0.2, 1.8, 0.2, and 1.6 for bacitracin, Clorpactin, Irrisept, Bactisure, Prontosan, and saline control, respectively. Analysis of variance testing showed there were statistical differences among groups at 10 days (P = .006). Post hoc analysis showed that there was less edema with Irrisept as compared to bacitracin (P = .01), Bactisure (P = .01), and normal saline (P = .03). Prontosan demonstrated significantly less edema at 10 days compared to bacitracin (P = .01), Bactisure (P = .01), and normal saline (P = .03). A significant reduction of edema was observed in the bacitracin, Clorpactin, Irrisept, normal saline, and Prontosan groups at 10 days as compared to that at 3 days (P < .05).
Figure 8.
Edema scores at 3 days and 10 days, respectively. Red stars denote statistical significance within groups between 3 days and 10 days (P < .05). Black stars denote statistical significance between groups at 10 days.
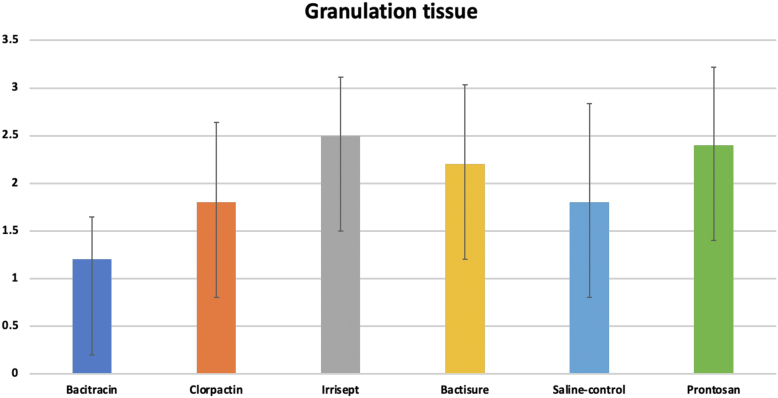
Granulation scores
As shown in Figure 9, at 10 days, the granulation scores were 1.2, 1.8, 2.5, 2.2, 2.4, and 1.8 for bacitracin, Clorpactin, Irrisept, Bactisure, Prontosan, and normal saline, respectively. There were no statistical differences among groups (P = .14), though bacitracin showed lower granulation scores than saline control.
Figure 9.
Granulation tissue scores at 10 days.
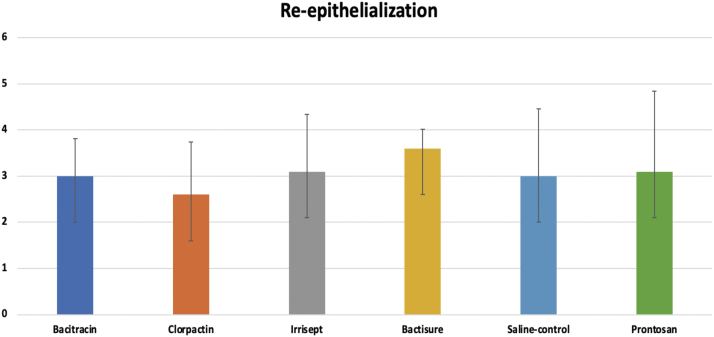
Re-epithelialization scores
As shown in Figure 10, at 10 days, the re-epithelialization scores were 3.0, 2.6, 3.1, 3.6, 3.1, and 3.0 for bacitracin, Clorpactin, Irrisept, Bactisure, Prontosan, and saline control, respectively. The re-epithelization was similar for the groups of antiseptic solution as compared to the saline control (P = .88), though Clorpactin showed lower re-epithelialization scores than saline control.
Figure 10.
Re-epithelialization scores at 10 days.
Discussion
SSIs are a significant contributor to morbidity and mortality in orthopaedic surgery. It is common to try to reduce the risk of infection of wounds with thorough irrigation prior to wound closure [14]. Antiseptic irrigation solutions are frequently used for this task in an attempt to actively kill bacteria [26,27]. The benefits of antiseptic adjunct use are well-established and include broad-spectrum antimicrobial and antibiofilm activity, without acquired bacterial resistance [28]. It has been demonstrated that a 1-minute exposure of 0.05% chlorhexidine gluconate produces a >5-log reduction against selective healthcare-associated pathogens and reduces microbial adherence to the surface of implantable biomedical devices [10]. Additionally, polyhexamethylene biguanide (PHMB) was shown to be effective against methicillin-resistant staphylococcus aureus 3-day and 6-day biofilms in porcine models [29]. Although these solutions decrease bacterial load, there is concern that the very properties that make them attractive for potential prevention of infection may also be harmful to host tissue and wound healing [14]. In vitro cell culture models have demonstrated that PHMB has a time-dependent cytotoxic effect on keratinocytes, osteoblasts, and fibroblasts [30]. Previously, in our laboratory, we found that bacitracin, Clorpactin, and Irrisept all caused damage to osteoblasts in vitro. There was some reversal of the deleterious effect once bacitracin and Clorpactin were removed but complete annihilation of all cells with Irrisept [20]. The literature is lacking in vivo studies that investigate the effect of antiseptic irrigation solutions on host tissue, particularly for single short duration applications as used for surgical site sterilization. Therefore, there is ongoing debate over the most appropriate use of these potent chemicals [11,12].
The purpose of this study was to histologically compare the effects of 5 commercially available antiseptic irrigation solutions on healthy skin using a full-thickness open-wound murine model. There were 2 phases of interest: acute and delayed. First, we examined the acute inflammatory phase. This served as a surrogate for the severity of the cytotoxic load on the tissue. This was followed by the healing phase, which reflected the tissue’s ability to mount a regenerative response after single exposure to the various irrigants. In the acute phase, all groups showed substantial inflammation at 3 days (Score >3, moderate to marked), this was, however, comparable to the normal saline control. Each group also demonstrated some decrease in inflammation by the 10-day mark; however, this improvement was only significant in the Clorpactin, Bactisure, and Prontosan groups. All solutions caused considerable edema (score ≥2, moderate) at 3 days, but again all showed significant improvement at 10 days, except for the Bactisure group. With regard to granulation tissue, all groups laid down immature granulation tissue (score ≥1) at 10 days. However, we did not find differences in granulation tissue between groups of antiseptic irrigation solutions and normal saline control at 10 days. All groups at 10 days showed complete closure of the wound (score ≥2, re-epithelialization: full closure of ulcer), and many groups also showed thickening of the regenerated epithelium into the dermis (re-epithelialization grade 3). No groups were found to be inferior to normal saline in terms of re-epithelialization potential.
These results demonstrated that all the irrigation solutions had a negative effect on the host tissue in the acute phase, and all showed potential for recovery as they were able to effectively heal the open wound. The antiseptic irrigation solutions had no greater cytotoxic effect on host tissue when compared to normal saline alone. Likewise, wound healing was not delayed or compromised when treated with any antiseptic irrigation solution when compared to normal saline alone. This illustrates the important regeneration potential of living wounds that must be considered when discussing the effect of antiseptics. In vivo models such as the murine model used in this study, described by Dunn et al [22], consist of a complex environment of wound exudate, extracellular proteins, immune cells, proteases, and natural nutrition through blood and lymphatic flow. Presence of organic compounds (eg, proteins) alone have been shown to provide a protective effect against antimicrobial compounds [31]. Also, skin wound healing is a highly complex and 3-dimensionally organized process, including many cell-cell signal pathways and epidermal stem cells, behaving substantially different than cells in a monolayer on culture medium [19,32]. Duration of exposure and concentration of antiseptic solution must also be considered when discussing the effects of irrigation solutions. Bolten et al. [33] showed that daily treatments of full-thickness skin guinea pig wounds using antiseptic solutions, including chlorhexidine (0.1%) and bacitracin (200/ml), caused delayed wound healing at 7 days. Saatman et al. [34] showed that full-thickness incisions and abrasions treated with 4% and 0.5% chlorhexidine at the time of surgery and once daily thereafter resulted in delayed wound healing at 6 days. The prolonged repetitive exposures would be more relevant to chronic open wound management rather than single short duration application followed by saline rinse as used intraoperatively in SSI prevention and studied herein. Muller and Kramer [35] showed a concentration dependent toxicity of fibroblasts and keratinocytes when exposed to benzalkonium chloride, chlorhexidine, and PHMB. Therefore, it is important to point out that commercially available Irrisept as used in this experiment contains a lower concentration of chlorhexidine (0.05%), as compared to concentrations used by Boltman and Saatman.
There were limitations to this study. The most obvious are the inherent limitations of animal research. Smaller sample sizes were required for Institutional Animal Care and Use Committee approval, which could have potentially led to underpowering. While the mouse model is certainly more practical and economic for this in vivo preclinical investigation, there are fundamental differences in the biology and wound healing of murine compared to humans. However, efforts were made to mitigate these differences using the methods described previously, including splinting of wounds [22,23]. Future studies using larger animals would better ensure transferability of these results to human tissue in the clinical setting. Finally, the sensitivity of the grading systems used for inflammation, edema, granulation, and epithelialization scoring is a limitation. The grading systems attempt to quantify the more qualitative characteristics of histologic slides for statistical analysis. However, given the small samples sizes and small number of categories in each grading system, the ability to detect a difference is a limitation.
Conclusions
Despite our previous in vitro data illustrating, the extreme cytotoxic effect of these irrigation solutions and other studies suggesting that prolonged exposure leads to impaired wound healing, our data suggest that single short duration use of these commercially available antiseptic irrigation solutions was no different than normal saline in vivo. All the irrigants showed inflammation in the acute phase that was reversible by 10 days and did not inhibit wound healing. These findings did not however address the efficacy in the face of infection and/or cost benefit issues. The key finding was that they appear safe in the noninfected setting if one chooses to use them clinically.
Funding
This study was funded in full by the Ascension Providence Hospital Department of Orthopedic Surgery and Research.
Conflicts of interest
David C. Markel reports being a consultant for Stryker and Smith-Nephew; received royalties from Smith-Nephew; being a reviewer/editorial for The Journal of Bone and Joint Surgery, Journal of Arthroplasty, Clinical Orthopedics and Related Research, Arthroplasty Today; having stock in Arboretum Ventures; and is a part of boards for CORE (Center for Orthopedic Research and Education), MOS (Michigan Orthopedic Society), and MARCQI (Michigan Arthroplasty Registry Collaborative Quality Initiative). All other authors declare no potential conflicts of interest.
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2023.101300.
Appendix A. Supplementary data
References
- 1.Al-Mulhim F.A., Baragbah M.A., Sadat-Ali M., Alomran A.S., Azam M.Q. Prevalence of surgical site infection in orthopedic surgery: a 5-year analysis. Int Surg. 2014;99:264–268. doi: 10.9738/INTSURG-D-13-00251.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fry D.E. The economic costs of surgical site infection. Surg Infect (Larchmt) 2002;3(Suppl 1):S37–S43. doi: 10.1089/sur.2002.3.s1-37. [DOI] [PubMed] [Google Scholar]
- 3.Urban J.A. Cost analysis of surgical site infections. Surg Infect (Larchmt) 2006;7(Suppl 1):S19–S22. doi: 10.1089/sur.2006.7.s1-19. [DOI] [PubMed] [Google Scholar]
- 4.Lidwell O.M. Clean air at operation and subsequent sepsis in the joint. Clin Orthop Relat Res. 1986:91–102. [PubMed] [Google Scholar]
- 5.Lidwell O.M., Lowbury E.J., Whyte W., Blowers R., Stanley S.J., Lowe D. Airborne contamination of wounds in joint replacement operations: the relationship to sepsis rates. J Hosp Infect. 1983;4:111–131. doi: 10.1016/0195-6701(83)90041-5. [DOI] [PubMed] [Google Scholar]
- 6.Cruse P.J., Foord R. The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds. Surg Clin North Am. 1980;60:27–40. doi: 10.1016/s0039-6109(16)42031-1. [DOI] [PubMed] [Google Scholar]
- 7.Berríos-Torres S.I., Umscheid C.A., Bratzler D.W., Leas B., Stone E.C., Kelz R.R., et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152:784–791. doi: 10.1001/jamasurg.2017.0904. [DOI] [PubMed] [Google Scholar]
- 8.Cheng M.T., Chang M.C., Wang S.T., Yu W.K., Liu C.L., Chen T.H. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine (Phila Pa 1976) 2005;30:1689–1693. doi: 10.1097/01.brs.0000171907.60775.85. [DOI] [PubMed] [Google Scholar]
- 9.Brown N.M., Cipriano C.A., Moric M., Sporer S.M., Della Valle C.J. Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27:27–30. doi: 10.1016/j.arth.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Edmiston C.E., Jr., Bruden B., Rucinski M.C., Henen C., Graham M.B., Lewis B.L. Reducing the risk of surgical site infections: does chlorhexidine gluconate provide a risk reduction benefit? Am J Infect Control. 2013;41(5 Suppl):S49–S55. doi: 10.1016/j.ajic.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Atiyeh B.S., Dibo S.A., Hayek S. Wound cleansing, topical antiseptics and wound healing. Int Wound J. 2009;6:420–430. doi: 10.1111/j.1742-481X.2009.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drosou A., Falabella A., Kirsner R.S. Antiseptics on wounds: an area of controversy. Wounds (King of Prussia, Pa.) 2003;15:149–166. [Google Scholar]
- 13.Hirsch T., Seipp H.M., Jacobsen F., Goertz O., Steinau H.U., Steinstraesser L. Antiseptics in surgery. Eplasty. 2010;10:e39. [PMC free article] [PubMed] [Google Scholar]
- 14.Anglen J.O. Wound irrigation in musculoskeletal injury. J Am Acad Orthop Surg. 2001;9:219–226. doi: 10.5435/00124635-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch T., Jacobsen F., Rittig A., Goertz O., Niederbichler A., Steinau H.U., et al. Vergleichende in-vitro-Studie zur zytotoxizität klinisch eingesetzter Antiseptika [A comparative in vitro study of cell toxicity of clinically used antiseptics] Hautarzt. 2009;60:984–991. doi: 10.1007/s00105-009-1842-x. [DOI] [PubMed] [Google Scholar]
- 16.Högele A.M., Neu J. Wundverschluss nach wundspülung mit octenisept® ohne abflussmöglichkeit [Wound closure after irrigation with octenisept® without possibility for drainage] Unfallchirurg. 2011;114:70–72. doi: 10.1007/s00113-010-1942-1. [DOI] [PubMed] [Google Scholar]
- 17.Hülsemann W., Habenicht R. Schwere nebenwirkungen nach octenisept-spülung von perforations wunden im kindesalter [Severe side effects after octenisept irrigation of penetrating wounds in children] Handchir Mikrochir Plast Chir. 2009;41:277–282. doi: 10.1055/s-0029-1238282. [DOI] [PubMed] [Google Scholar]
- 18.van Meurs S.J., Gawlitta D., Heemstra K.A., Poolman R.W., Vogely H.C., Kruyt M.C. Selection of an Optimal antiseptic solution for intraoperative irrigation. J Bone Joint Surg Am. 2014;96:285–291. doi: 10.2106/JBJS.M.00313. [DOI] [PubMed] [Google Scholar]
- 19.Ward W.G., Corey R.M. To wash or not to wash: that is the question: commentary on an article by S.J. van Meurs, MD, et al.: “Selection of an optimal antiseptic solution for intraoperative irrigation. An in vitro study”. J Bone Joint Surg Am. 2014;96:e34. doi: 10.2106/JBJS.M.01439. [DOI] [PubMed] [Google Scholar]
- 20.Markel J.F., Bou-Akl T., Dietz P., Afsari A.M. The effect of different irrigation solutions on the cytotoxicity and recovery potential of human osteoblast cells in vitro. Arthroplast Today. 2021;7:120–125. doi: 10.1016/j.artd.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sosnoski D., Dietz P., Bou-Akl T., Ren W.P., Markel D. Irrigation solutions negatively affect the viability and function of human fibroblasts: an in vitro study. Biomed Hub. 2022;7:165–172. doi: 10.1159/000527110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn L., Prosser H.C., Tan J.T., Vanags L.Z., Ng M.K., Bursill C.A. Murine model of wound healing. J Vis Exp. 2013 doi: 10.3791/50265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreira C.F., Cassini-Vieira P., Silva M.F., Barcelos L.S. Skin wound healing model- excisional wounding and assessment of lesion area. Bio-Protocol. 2015;5 [Google Scholar]
- 24.Ferrell B.A. The sessing scale for measurement of pressure ulcer healing. Adv Wound Care. 1997;10:78–80. [PubMed] [Google Scholar]
- 25.Gupta A., Kumar P. Assessment of the histological state of the healing wound. Plast Aesthet Res. 2015;2:239–242. [Google Scholar]
- 26.Falagas M.E., Vergidis P.I. Irrigation with antibiotic-containing solutions for the prevention and treatment of infections. Clin Microbiol Infect. 2005;11:862–867. doi: 10.1111/j.1469-0691.2005.01201.x. [DOI] [PubMed] [Google Scholar]
- 27.George J., Klika A.K., Higuera C.A. Use of chlorhexidine preparations in total joint arthroplasty. J Bone Jt Infect. 2017;2:15–22. doi: 10.7150/jbji.16934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alves P.J., Barreto R.T., Barrois B.M., Gryson L.G., Meaume S., Monstrey S.J. Update on the role of antiseptics in the management of chronic wounds with critical colonisation and/or biofilm. Int Wound J. 2021;18:342–358. doi: 10.1111/iwj.13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis S.C., Harding A., Gil J., Parajon F., Valdes J., Solis M., et al. Effectiveness of a polyhexanide irrigation solution on methicillin-resistant staphylococcus aureus biofilms in a porcine wound model. Int Wound J. 2017;14:937–944. doi: 10.1111/iwj.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yabes J.M., White B.K., Murray C.K., Sanchez C.J., Mende K., Beckius M.L., et al. In vitro activity of manuka honey and polyhexamethylene biguanide on filamentous fungi and toxicity to human cell lines. Med Mycol. 2017;55:334–343. doi: 10.1093/mmy/myw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitten F.A., Werner H.P., Kramer A. A standardized test to assess the impact of different organic challenges on the antimicrobial activity of antiseptics. J Hosp Infect. 2003 Oct;55:108–115. doi: 10.1016/s0195-6701(03)00260-3. [DOI] [PubMed] [Google Scholar]
- 32.Yang R., Liu F., Wang J., Chen X., Xie J., Xiong K. Epidermal stem cells in wound healing and their clinical applications. Stem Cell Res Ther. 2019;10:229. doi: 10.1186/s13287-019-1312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolton L., Oleniacz W., Constantine B., et al. In: Maibach H., Lowe I., editors. vol. 2. Karger; Basel: 1985. Repair and antibacterial effects of topical antiseptic agents in vivo; pp. 145–158. (Models in Dermatology). [Google Scholar]
- 34.Saatman R., Carlton W.W., Hubben K., Streett C.S., Tuckosh J.R., DeBaecke P.J. A wound healing study of chlorhexidine digluconate in Guinea pigs. Fundam Appl Toxicol. 1986;6:1–6. doi: 10.1016/0272-0590(86)90258-7. [DOI] [PubMed] [Google Scholar]
- 35.Müller G., Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother. 2008;61:1281–1287. doi: 10.1093/jac/dkn125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.