Abstract
Porphyromonas gingivalis is one of the pathogens associated with periodontal diseases, and its lipopolysaccharide (LPS) has been suggested as a possible virulence factor, acting by stimulation of host cells to secrete proinflammatory mediators. However, recent studies have shown that P. gingivalis LPS inhibited some components of the inflammatory response. The present study was designed to test the hypothesis that there are strain-dependent variations in the ability of P. gingivalis LPS to elicit the host inflammatory response. By using LPS preparations from two strains of P. gingivalis, W50 and A7346, the responses of mouse macrophages and human monocytes were evaluated by measuring the secretion of nitric oxide (NO) and tumor necrosis factor alpha (TNF-α). Both direct and indirect (priming) effects were investigated. LPS from Salmonella typhosa was used as a reference LPS. P. gingivalis A7436 LPS induced lower secreted levels of NO from the tested cells than S. typhosa LPS but induced similar levels of TNF-α. In contrast, LPS from P. gingivalis W50 did not induce NO or TNF-α secretion. Preincubation of macrophages with LPS from S. typhosa or P. gingivalis A7436 prior to stimulation with S. typhosa LPS upregulated NO secretion and downregulated TNF-α secretion, while preincubation with P. gingivalis W50 LPS enhanced both TNF-α and NO secretory responses. These results demonstrate that LPSs derived from different strains of P. gingivalis vary in their biological activities in vitro. The findings may have an impact on our understanding of the range of P. gingivalis virulence in vivo.
Periodontitis is a group of infectious diseases, characterized by an inflammatory reaction, which results in the destruction of the dental attachment apparatus. It is the major cause of tooth loss in adults over the age of 35. The primary etiologic factor for periodontal diseases is bacteria that accumulate in the gingival sulcus, among which Porphyromonas gingivalis is thought to be one major periodontal pathogen. Clinical studies have documented a strong association between the presence of P. gingivalis and periodontal destruction (41), and patients with periodontitis often exhibit high titers of antibodies against P. gingivalis (34). Furthermore, in vitro and in vivo studies have demonstrated that extracts of P. gingivalis have immunomodulatory and bone resorption activities (14, 16, 34). This microorganism possesses numerous virulence factors, which have been suggested to play an important role in periodontal diseases (16). These include the ability to invade tissues and to secrete various cysteine proteases, which degrade host tissues and cleave complement or other host-derived molecules. In addition, P. gingivalis is a gram-negative organism that synthesizes a lipopolysaccharide (LPS), which is unique because of its endotoxic activities (32, 33).
LPS is one major component of the outer membranes of gram-negative bacteria, and it is considered the major factor in the pathogenesis of septic shock (15, 19, 21, 22, 24, 28, 36, 44). In addition, LPS is also considered to be an important factor in the pathogenesis of periodontal diseases, and it has been shown to be adsorbed to root surfaces and gingival tissues in periodontal disease sites (2, 9, 10, 20, 26, 46). Among other activities, LPS is one of the most potent stimulators of macrophage secretory responses. When exposed to LPS, macrophages secrete a wide variety of proinflammatory mediators, such as tumor necrosis factor alpha (TNF-α), interleukin-1, and nitric oxide (NO). These mediators have been implicated in the pathogenesis of tissue destruction in periodontal disease (17, 30, 31, 42, 43), and the control of production of these cytokines has been investigated as a potential therapeutic approach.
Due to the unique structure of P. gingivalis LPS (4, 16), several laboratories have investigated the immunobiological responses induced by it. Several studies demonstrated that the endotoxic activity of P. gingivalis is very low compared to that of LPS isolated from enterobacteria (7, 32, 35). However, other reports suggested that P. gingivalis LPS is a potent inducer of various biological responses such as bone resorption, polyclonal B-cell activation, inhibition of bone formation, and fibroblast proliferation (1, 23, 25, 29). Other studies have investigated the effect of P. gingivalis LPS on monocyte and macrophage activation. Reduced secretion of TNF-α and prostaglandin E2 from macrophages stimulated by P. gingivalis LPS compared to those stimulated by standard LPS preparations from enterobacteria has been noted (7, 11, 33). However, we have demonstrated that P. gingivalis (A7436) LPS is a potent inducer of TNF-α secretion from human monocytes (39). Using an in vivo model of inflammation, one study demonstrated that P. gingivalis LPS is unable to induce the expression of adhesion molecules (35), while other studies demonstrated its ability to induce local tissue necrosis (3, 37). Taken together, it is reasonable to hypothesize that the potency of LPS preparations from P. gingivalis in inducing a biological response is dependent on the nature of the tested response, the strain of P. gingivalis used, and, possibly, the method of LPS preparation.
In an attempt to shed some light on the interactions between host cells and P. gingivalis LPS, the aim of the present study was to compare the abilities of LPS preparations isolated from two strains of P. gingivalis, W50 and A7436, to induce secretory response from monocytes and macrophages. Using NO and TNF-α secretion as our outcome variables, we measured LPS-induced secretion, priming, and tolerance. The results demonstrated that the response to P. gingivalis LPS is strain dependent and does not always mimic the classic responses to LPS of enterobacteria. These data suggest that the unique structure of P. gingivalis LPS results in discrete, and often profound, functional variations.
MATERIALS AND METHODS
LPS isolation.
LPS was extracted from P. gingivalis A7436 and W50 by a hot phenol-water method and further purified by cesium chloride isopyknic density gradient centrifugation as described by Morrison and Leive (27). The fractions with a density of 1.42 to 1.52 gm/cm3, containing peak endotoxin activity as determined by the Limulus amebocyte clotting assay, were pooled, dialyzed extensively, and lyophilized. Yields of LPS preparations were similar for both strains of P. gingivalis (4 to 5 mg from 500 ml of original culture). A stock solution of 1 mg of LPS per ml was prepared in phosphate-buffered saline (PBS) (pH 7.4), divided into aliquots, and kept at −20°C. Prior to each experiment, LPS solutions were sonicated for 10 s to disperse the LPS aggregates. Protein contamination of LPS preparations was determined routinely on overloaded sodium dodecyl sulfate (SDS)-polyacrylamide gels stained with Coomassie blue and silver nitrate. All LPS lots were negative for Coomassie blue staining, indicating the purity of the preparation.
Salmonella typhosa LPS and Escherichia coli 055:B5 LPS (phenol extracts) were purchased from Sigma (St. Louis, Mo.) and further purified by cesium chloride isopyknic density gradient as described above.
LPS preparations were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) as described before (5) with 15% (wt/vol) acrylamide containing 0.2% (wt/vol) SDS. LPS bands were visualized by the silver stain kit method based on periodate oxidation as described previously (5).
Preparation of mouse inflammatory macrophages.
Female BALB/c mice, 10, 12, or 7 weeks old (Charles River, Wilmington, Mass.), were injected intraperitoneally with 1.5 ml of 3% sterile thioglycolate broth (Difco, Detroit, Mich.). Four days after injection, macrophages were harvested from the peritoneal cavities of the mice by washing with 7 ml of PBS followed by aspiration of the wash from the peritoneal cavity. Macrophages were pooled, washed twice, and counted with a hemocytometer. Cell viability was verified by the trypan blue exclusion technique. Macrophages were suspended in RPMI 1640 medium supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM l-glutamine, and 5% fetal calf serum (C-RPMI) (Life Technologies Inc., Gaithersburg, Md.). Cells were plated in 24-well culture plates at a concentration of 106 per well and incubated for 60 min at 37°C with 5% CO2. Nonadherent cells were removed by aspiration and three successive washes with PBS. Stimulation of the cells by LPS was performed in C-RPMI. No LPS-binding protein was added. At the end of the stimulation period, cell culture supernatants were collected and either assayed immediately or kept at −70°C until analyzed. All tissue culture materials used were of endotoxin-free grade.
Isolation of human peripheral blood monocytes.
Fresh monocytes were isolated from the blood of healthy donors by the adherence method as described previously (38, 40). Briefly, heparinized peripheral blood was fractionated with Mono-Poly resolving medium (Flow Laboratories, McLean, Va.). The mononuclear cell fraction was collected, and the cells were washed three times. Following resuspension in C- RPMI medium, the cells were plated in 24-well culture plates at a concentration of 4 × 106 per well and incubated for 90 min at 37°C with 5% CO2. Nonadherent cells were removed by aspiration and three successive washes with PBS. Stimulation of the cells by LPS was performed in C-RPMI medium. No LPS-binding protein was added. At the end of the stimulation period, cell culture supernatants were collected and kept at −70°C until analyzed.
Secretion of NO.
The secretion of NO by the cells was evaluated by measuring NO2− accumulation in the culture supernatants (8). Briefly, 100 μl of Gries reagent (1% sulfanilamide, 0.1% naphthylethylene diamine dihydrochloride, 2.5% H3PO4) (Sigma) was added to 100 μl of cell supernatants in a 96-well plate. The colorimetric reaction was allowed to proceed for 10 min at room temperature, and the optical density (OD) at 550 nm was measured in a Vmax microplate reader (Molecular Devices, Palo Alto, Calif.). The measured OD values were converted to concentrations from a standard curve established from serial dilutions of NaNO2 (Sigma) in culture medium. All samples were assayed in triplicates.
NOS activity assay.
Nitric oxide synthase (NOS) activity was determined by measuring the conversion of [3H]arginine to [3H]citrulline with an assay system from Stratagene (La Jolla, Calif.). Mouse macrophages were cultured for 18 h, washed with cold PBS, and scraped off the plates with a sterile rubber policeman into PBS containing 0.1 mM EDTA and 0.1 mM phenylmethylsulfonyl fluoride. Cells were pelleted by a 10-min centrifugation at 4°C, and the pellets were kept frozen at −20°C until assayed. Cell pellets were homogenized in 25 mM Tris-HCl (pH 7.4) containing 1 mM EDTA and 1 mM EGTA, and the protein concentration was determined by the Bradford protein assay (Bio-Rad, Richmond, Calif.). For the NOS activity assay, equivalents of 5 μg of protein from the cell homogenates were mixed with a solution containing 25 mM Tris-HCl (pH 7.4), 3 μM tetrahydrobiopterin, 1 μM flavin adenine dinucleotide, 1 μM flavin adenine mononucleotide, 1 mM NADPH, and 10 μCi of [3H]arginine and further incubated for 30 min at room temperature. Positive controls included rat cerebellum extract and CaCl2, while negative controls included the NOS inhibitor N-nitro-l-argingine-methylester. The reaction was stopped by the addition of 8 volumes of 50 mM HEPES (pH 5.5)–5 mM EDTA. The reaction mixtures were then transferred into spin columns containing an equilibrated resin, which bound the positively charged arginine, and centrifuged at 10,000 × g for 30 s. The neutrally charged citrulline at pH 5.5 did not adhere to the resin, and NOS activity was quantified by counting the radioactivity in the collected eluate.
TNF-α determination.
The release of TNF-α by mouse macrophages was quantified with a commercial ELISA kit (BioSource, Camarillo, Calif.). TNF-α release by human monocytes was quantified by an ELISA as previously described (40) with slight modifications. Ninety-six-well ELISA plates (Maxisorp; Nunc, Naperville, Ill.) were coated with mouse anti-human TNF-α monoclonal antibody (R&D Systems, Minneapolis, Minn.) in coating buffer (carbonate-bicarbonate buffer, pH 9.6) by overnight incubation at 4°C. The wells were blocked overnight (4°C) with 2% bovine serum albumin in coating buffer, and samples were added. After overnight incubation (4°C), goat anti-TNF-α polyclonal antibody (R&D Systems) was added, followed by donkey anti-goat antibody–horseradish peroxidase conjugate (Sigma). o-Phenylenediamine was used as the substrate. The reaction was stopped by addition of 4 N sulfuric acid, and the OD was measured by using a Vmax microplate reader (Molecular Devices) at 490 to 600 nm. Samples with OD values outside the standard range were assayed again at an appropriate dilution.
Statistical analysis.
The significance of the differences between the different LPS preparations were analyzed by one-way analysis of variance (ANOVA). When ANOVA showed significant differences between the treatments, a multiple comparison procedure (Student-Newman-Keuls method) was used to compare group pairs for significant differences. Comparisons between dose and kinetic responses of the different LPS preparations were evaluated by using repeated-measurement ANOVA.
RESULTS
SDS-PAGE analysis of LPS preparations.
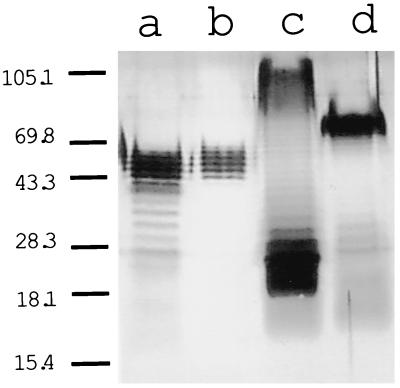
In order to assess structural characteristics of the different LPS preparations used in the present study (from P. gingivalis A7436 and W50, S. typhosa, and E. coli 055:B5), SDS-PAGE analyses were performed (Fig. 1). All LPS preparations presented a stepladder pattern, corresponding to various polysaccharide chains lengths anchored to the core lipid A and characteristic of a smooth-type LPS. S. typhosa LPS was different from the other three, with most of the material concentrated in the low-molecular-weight range (Fig. 1, lane c). This means that S. typhosa LPS contains higher levels of core components of the LPS and molecules with lower numbers of polysaccharide units. Moreover, the majority of the S. typhosa LPS molecules had long chains of polysaccharides (80 to 110 kDa), although a wide range could be detected (30 to 110 kDa). LPS molecules isolated from E. coli 055:B5 also exhibited a stepladder pattern, with the major bands in the 70-kDa range (Fig. 1, lane d). LPS molecules prepared from both strains of P. gingivalis had the majority of their bands in at the 40- to 60-kDa range (Fig. 1, lanes a and b). The pattern of the LPS preparation from P. gingivalis A7436 resembled the pattern of E. coli LPS (Fig. 1, lanes a and d). P. gingivalis W50 showed a higher percentage of the high-molecular-weight molecules than P. gingivalis A7436, suggesting an elevated fraction of polysaccharide-containing molecules.
FIG. 1.
SDS-PAGE analysis of LPSs from P. gingivalis A7436 (lane a), P. gingivalis W50 (lane b), S. typhosa (lane c), and E. coli 055:B5 (lane d). Positions of molecular mass standards (in kilodaltons) are indicated on the left.
NO release and NOS activity in LPS-stimulated mouse macrophages.
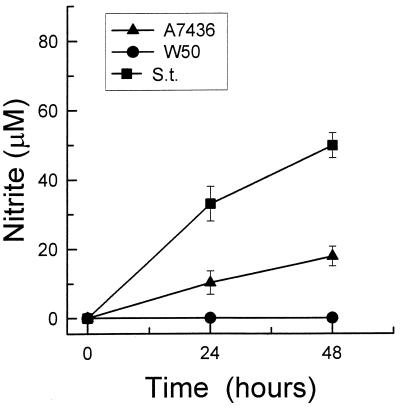
Unstimulated macrophages did not secrete NO into the culture medium during 48 h of incubation (data not shown). Stimulation with 1 μg of S. typhosa LPS per ml caused a marked elevation in NO secretion, which continued to rise over 48 h of incubation (Fig. 2). LPS from P. gingivalis A7436 (1 μg/ml) also induced NO secretion by mouse macrophages, but it was approximately threefold less active than S. typhosa LPS. In contrast, stimulation with LPS isolated from P. gingivalis W50 did not induce any NO secretion into the medium (Fig. 2).
FIG. 2.
Nitrite levels in culture supernatants of macrophages stimulated with LPS isolated from P. gingivalis A7436 or W50 or S. typhosa (S.t.). Thioglycolate-elicited macrophages were cultured with 1 μg of LPS per ml for 24 or 48 h. Culture supernatants were harvested, and nitrite levels were determined by using Gries reagent. Data is presented as means ± standard deviations for four replicates.
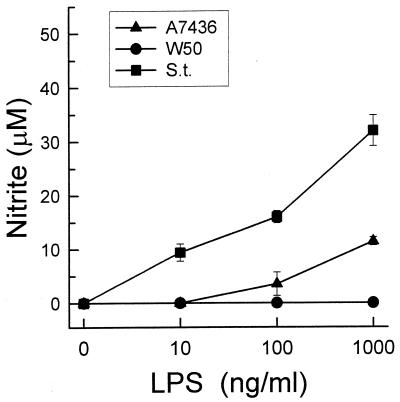
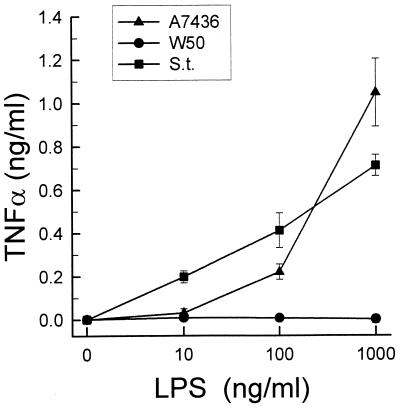
The effect of LPSs from P. gingivalis A7436 and S. typhosa on the macrophages was dose dependent (Fig. 3). However, the stimulatory effect of P. gingivalis A7436 LPS was seen only at doses higher than 10 ng/ml. The concentration of LPS required to trigger the production of approximately 10 nM NO was 100 times higher for P. gingivalis A7436 LPS than for S. typhosa LPS. Again, concentrations of P. gingivalis W50 LPS ranging from 10 ng/ml to 10 μg/ml induced no NO production.
FIG. 3.
Dose-dependent release of nitrite by LPS-stimulated macrophages. Thioglycolate-elicited macrophages were cultured with increasing doses of LPS, isolated from P. gingivalis A7436 or W50 or S. typhosa (S.t.), for 24 h. Culture supernatants were harvested, and nitrite levels were determined by using Gries reagent. Data is presented as means ± standard deviations for four replicates.
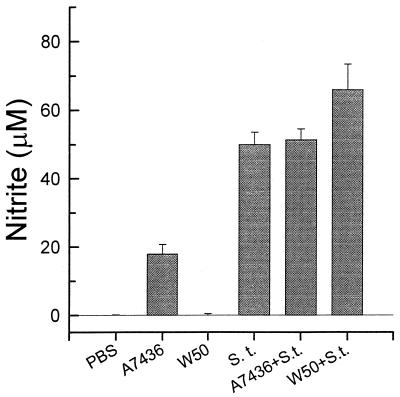
Since the ability of P. gingivalis LPS to inhibit stimulation by enterobacterial LPS had been described previously (7), we tested the possibility that combinations of P. gingivalis W50 and A7436 LPSs would modify the response of mouse macrophages to S. typhosa LPS (Fig. 4). In these experiments, mouse macrophages were incubated for 48 h with 1 μg of S. typhosa LPS in the presence or in the absence of 1 μg of LPS from P. gingivalis W50 or A7436 per ml. P. gingivalis A7436 LPS did not alter the response of the macrophages to S. typhosa LPS. However, the presence of P. gingivalis W50 LPS enhanced the macrophage response to S. typhosa LPS by approximately 30% (P < 0.05).
FIG. 4.
Nitrite secretion by macrophages stimulated with S. typhosa (S.t.) LPS (1 μg/ml). Thioglycolate-elicited macrophages were cultured with medium alone, S. typhosa LPS, S. typhosa LPS plus P. gingivalis A7436 LPS, or S. typhosa LPS plus P. gingivalis W50 LPS for 24 h. Culture supernatants were harvested, and nitrite levels were determined by using Gries reagent. Data is presented as means ± standard deviations for four replicates.
We evaluated the effects of the different LPS preparations on the activation of NOS in mouse macrophages (Fig. 5). The results were positively correlated with the NO secretion results. P. gingivalis A7436 LPS (1 μg/ml) triggered approximately 50% of the NOS activity induced by the same concentration of S. typhosa LPS. NOS activity in P. gingivalis W50 LPS-stimulated macrophages did not exceed background levels.
FIG. 5.
Activation of NOS by LPS of S. typhosa (S.t.), P. gingivalis (Pg) A7436, or P. gingivalis W50. Macrophages were cultured in the presence or absence (Non) of LPS (1 μg/ml) for 18 h. Cells were harvested, and cell lysates were analyzed for NOS activity as described in Materials and Methods. Results are for pooled samples from six different wells.
TNF-α release from LPS-stimulated mouse macrophages and human monocytes.
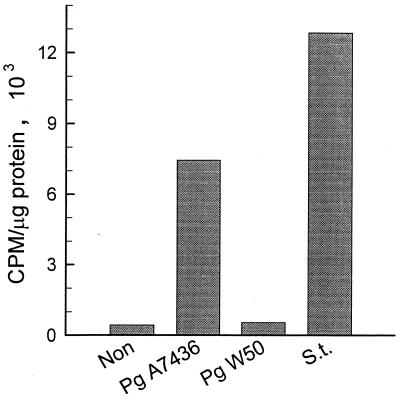
Concentrations of P. gingivalis W50 LPS ranging from 10 ng/ml to 10 μg/ml did not induce TNF-α secretion from mouse macrophages. In contrast, both P. gingivalis A7436 LPS and S. typhosa LPS triggered TNF-α release in a dose-dependent manner. The kinetics (data not shown) and the magnitudes of TNF-α release induced by these two LPS preparations were almost identical, with no significant differences between the two responses (Fig. 6).
FIG. 6.
Dose-dependent secretion of TNF-α by LPS-stimulated macrophages. Macrophages were cultured with increasing doses of S. typhosa (S.t.), P. gingivalis A7436, or P. gingivalis W50 LPS for 24 h. Culture supernatants were harvested, and TNF-α levels were determined by ELISA. Data is presented as means ± standard deviations for four replicates.
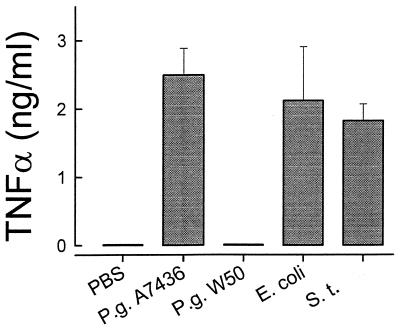
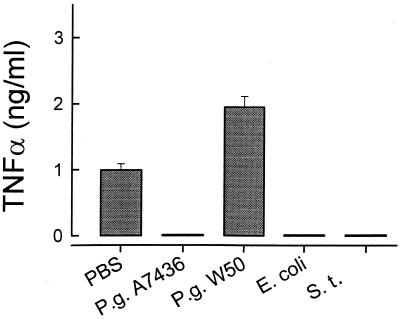
Similarly to the mouse macrophages, resting human monocytes did not secrete TNF-α (Fig. 7). LPSs derived from P. gingivalis A7436, S. typhosa, and E. coli (1 μg/ml) triggered similar levels of TNF-α secretion, with no statistical differences between the three (Fig. 7). Again, P. gingivalis W50 LPS behaved differently and triggered no TNF-α release from human monocytes (Fig. 7).
FIG. 7.
Secretion of TNF-α by LPS-stimulated human monocytes. Human monocytes were cultured with S. typhosa (S.t.), E. coli, P. gingivalis (P.g.) A7436, or P. gingivalis W50 LPS (1 μg/ml) for 24 h. Culture supernatants were harvested, and TNF-α levels were determined by ELISA. Data is presented as means ± standard deviations for four replicates.
Effect of preexposure of macrophages and monocytes to LPS on the LPS-induced response.
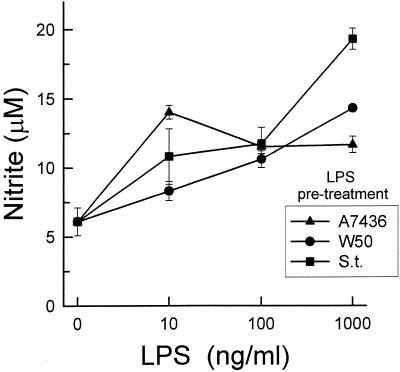
It has been reported that preexposure of macrophages to LPS reduced the cytokine response to subsequent LPS challenge and simultaneously primed the cell for an enhanced NO response to LPS (18, 47). We compared the P. gingivalis A7436 and W50 LPS priming and desensitization effects on macrophage responsiveness to a second stimulus, i.e., S. typhosa LPS (1 μg/ml) for additional 24 h. When macrophages were preexposed to S. typhosa LPS (10 ng/ml to 1 μg/ml) for 24 hs, followed by stimulation with the same LPS (1 μg/ml), NO secretion was elevated two- to fourfold (Fig. 8). P. gingivalis A7436 and W50 LPSs also primed NO secretion. No significant differences were found between the three LPS preparations in regard to their priming effects.
FIG. 8.
Effect of pretreatment of macrophages with different LPSs on their LPS-induced nitrite production. Macrophages were preexposed to increasing doses of S. typhosa (S.t.), P. gingivalis A7436, or P. gingivalis W50 LPS for 24 h. Cultures were washed, and fresh S. typhosa LPS (1 μg/ml) was added. After an additional 24 h, culture supernatants were harvested and nitrite levels were determined by using Gries reagent. Data is presented as means ± standard deviations for four replicates.
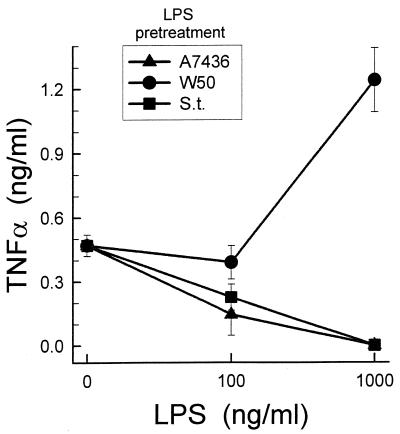
As expected, preexposure of macrophages to S. typhosa LPS induced desensitization or tolerance to a second stimulus, as determined by TNF-α secretion (Fig. 9). Preincubation with 1 μg of LPS per ml completely abolished the TNF-α response to the secondary LPS challenge (Fig. 9). Preexposure to P. gingivalis A7436 LPS induced the same effect. In contrast, preexposure to 1 μg of P. gingivalis W50 LPS per ml primed the mouse macrophages to the second stimulus, and the response was enhanced threefold.
FIG. 9.
Effect of pretreatment of macrophages with different LPS preparations on their LPS-induced TNF-α production. Macrophages were preexposed to increasing doses of S. typhosa (S.t.), P. gingivalis A7436, or P. gingivalis W50 LPS for 24 h. Culture wells were washed, and fresh S. typhosa LPS (1 μg/ml) was added. After an additional 24 h, culture supernatants were harvested and TNF-α levels were determined by ELISA. Data is presented as means ± standard deviations for four replicates.
Similar results were obtained when TNF-α release from human monocytes was examined (Fig. 10). While preexposure to P. gingivalis A7436, S. typhosa, or E. coli LPS (1 μg/ml) eliminated any TNF-α secretion resulting from a second stimulus, preexposure to P. gingivalis W50 LPS enhanced the response to the second stimulus twofold.
FIG. 10.
Effect of pretreatment of human monocytes with different LPS preparations on their LPS-induced TNF-α production. Human monocytes were preexposed to medium alone, S. typhosa (S.t.), P. gingivalis (P.g.) A7436, or P. gingivalis W50 LPS (1 μg/ml) for 24 h. Cultures were washed, and fresh S. typhosa LPS (1 μg/ml) was added. After an additional 24 h, culture supernatants were harvested and TNF-α levels were determined by ELISA. Data is presented as means ± standard deviations for four replicates.
DISCUSSION
In this study, we evaluated the biological activities of P. gingivalis LPSs from two strains, A7436 and W50. The abilities of these LPSs to induce direct activation of mouse inflammatory macrophages and human peripheral blood monocytes were investigated. While peripheral blood monocytes are immature cells, thioglycolate-elicited cells are mature macrophages that have been primed in vivo. We quantified the cellular response by the release of NO and TNF-α, both of which are considered important inflammatory mediators. The results demonstrated that both human monocytes and mouse macrophages were affected differently by different LPS preparations, in terms of NO and TNF-α release. S. typhosa LPS was the most potent inducer of NO release. Macrophages stimulated with 1 μg of S. typhosa LPS per ml secreted two- to threefold-higher NO levels than did those stimulated with a similar concentration of P. gingivalis A7436 LPS, moreover, the concentration required to trigger the production of approximately 10 nM NO was 100 times higher for P. gingivalis A7436 LPS than for S. typhosa LPS. P. gingivalis A7436 was not able to antagonize or synergize the activity of S. typhosa LPS. For TNF-α secretion, no differences were noted between LPS preparations from S. typhosa, E. coli, and P. gingivalis A7436. P. gingivalis W50 LPS induced no response (neither NO nor TNF-α secretion) at the tested concentrations. The fact that LPS extracted from P. gingivalis A7436 is a potent inducer of macrophage TNF-α secretion is in agreement with our earlier findings (39). This LPS was also found to induce TNF-α-dependent lesions in vivo (3, 37). However, results from this study show that there are clearly strain differences in terms of LPS biological activity and differences in biological activities within the same strain. For instance, P. gingivalis A7436 LPS was a more potent inducer of TNF-α release than of NO synthesis, compared to S. typhosa LPS. The present report not only emphasizes the earlier observation that LPSs isolated from some strains of P. gingivalis are potent inducers of TNF-α production but also suggests differences in virulence between strains. In contrast to P. gingivalis A7436 LPS, LPS extracted from P. gingivalis W50 and tested in the same manner did not trigger any NO or TNF-α secretion or NOS activity. Previous studies have suggested that some strains of P. gingivalis are weak inducers of inflammation (7, 35). Results from the present study are not in contradiction with the current literature, but they introduce the concept of strain variability in terms of biological activity measured in vitro. Furthermore, it is important to emphasize that P. gingivalis A7436 is a clinical isolate from human aggressive periodontal disease, whereas the source of P. gingivalis W50 is unclear. The biological activity of P. gingivalis A7436 has been further characterized in other models, and this strain was found to be extremely virulent (6, 12, 13).
Since incubation of macrophages with different LPSs induced a variety of responses, it was of interest to examine the influence of preincubation with each LPS on a second challenge with a reference LPS. LPS pretreatment is known to increase secondary NO responses and decrease secondary TNF-α responses (endotoxin tolerance). The data presented here demonstrate that LPSs from S. typhosa, P. gingivalis A7436, and E. coli elicited the classic LPS response, reducing the secondary TNF-α response and increasing the secondary NO response. In contrast, P. gingivalis W50 LPS primed mouse macrophages for both NO and TNF-α release and primed human monocytes for TNF-α secretion. This priming effect suggests that P. gingivalis W50 LPS has a mode of action different from that of other LPSs. However, P. gingivalis W50 LPS does not directly activate monocytes and macrophages.
In conclusion, LPS from P. gingivalis was found to affect the secretory response of cells from the monocyte/macrophage lineage. The activity of LPS from P. gingivalis A7436 was found to be comparable to that of enterobacterial LPS, but the activity of LPS from P. gingivalis W50 was not. The differences in activity might play a role in periodontal disease pathogenesis. Although the presence of a periodontal pathogen such as P. gingivalis is a prerequisite for periodontal destruction, the destruction of the periodontal tissues is dependent on the host response (45). Some strains of P. gingivalis might colonize the gingival pocket but not initiate tissue destruction. However, they can prime the immune response for a second challenge, presumably leading to an episode of tissue destruction. These aspects of host-parasite interactions emphasize the complexity of the periodontal environment and the delicate balance between health and disease.
ACKNOWLEDGMENTS
We acknowledge the highly skilled technical assistance of Barbara Gordon and Martha Warbington.
This work was supported in part by Public Health Service grants DE06436 and NIH DE-10709 and a grant from The Israeli Ministry of Health.
REFERENCES
- 1.Aleo J J. Stimulation of macromolecular synthesis by endotoxin-treated 3T6 fibroblasts. Experimentia. 1980;36:546–547. doi: 10.1007/BF01965790. [DOI] [PubMed] [Google Scholar]
- 2.Aleo J J, DeRenzis F A, Farber P A, Varboncoeur A P. The presence and the biologic activity of cementum bound endotoxin. J Periodontol. 1974;45:672–675. doi: 10.1902/jop.1974.45.9.672. [DOI] [PubMed] [Google Scholar]
- 3.Amar S, Van Dyke T E, Eugster H P, Schultze N, Koebel P, Bluethmann H. Tumor necrosis factor (TNF)-induced cutaneous necrosis is mediated by TNF receptor 1. J Inflammation. 1996;47:180–189. [PubMed] [Google Scholar]
- 4.Bramanti T E, Wong G G, Weinstraub S T, Holt S C. Chemical characterization and biologic properties of lipopolysaccharides from Bacteroides gingivalis strains W50, W83 and ATCC 33277. Oral Microbiol Immunol. 1989;4:183–192. doi: 10.1111/j.1399-302x.1989.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 5.Champagne C M, Holt S C, Van Dyke T E, Gordon B J, Shapira L. Lipopolysaccharide isolated from Porphyromonas gingivalis grown in hemin-limited conditions has a reduced capacity for human neutrophil priming. Oral Microbiol Immunol. 1996;5:319–325. doi: 10.1111/j.1399-302x.1996.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 6.Collins J G, Windley H W, Arnold R R, Offenbacher S. Effects of Porphyromonas gingivalis infection on inflammatory mediator response and pregnancy outcome in hamsters. Infect Immun. 1994;62:4356–4361. doi: 10.1128/iai.62.10.4356-4361.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darveau R P, Cunningham M D, Bailey T, Seachord C, Ratkliffe K, Bainbridge B, Dietsch M, Page R C, Aruffo A. Ability of bacteria associated with chronic inflammatory disease to stimulate E-selectin expression and promote neutrophil adhesion. Infect Immun. 1995;63:1311–1317. doi: 10.1128/iai.63.4.1311-1317.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 9.Fine D H, Mendieta C, Barnett M L, Furgany D, Naini A, Vincent J W. Endotoxin levels in periodontally healthy and diseased sites: correlation with levels of Gram-negative bacteria. J Periodontol. 1992;63:897–901. doi: 10.1902/jop.1992.63.11.897. [DOI] [PubMed] [Google Scholar]
- 10.Fine D H, Morris M L, Tabak L, Cole J D. Preliminary characterization of material eluted from roots of periodontally diseased teeth. J Periodontal Res. 1980;15:10–19. doi: 10.1111/j.1600-0765.1980.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 11.Garrison S W, Holt S C, Nichols F C. Lipopolysaccharide stimulated PG-E2 release from human monocytes. Comparison of lipopolysaccharides prepared from suspected periodontal pathogens. J Periodontol. 1988;59:684–687. doi: 10.1902/jop.1988.59.10.684. [DOI] [PubMed] [Google Scholar]
- 12.Genco C A, Arko R J. Animal chamber models for study of host-parasite interactions. Methods Enzymol. 1994;235:120–140. doi: 10.1016/0076-6879(94)35136-8. [DOI] [PubMed] [Google Scholar]
- 13.Genco C A, Cutler C W, Kapczyski D, Maloney K, Arnold R R. A novel mouse model to study the virulence of and the host response to Porphyromonas (Bacteroides) gingivalis. Infect Immun. 1991;59:1255–1263. doi: 10.1128/iai.59.4.1255-1263.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Getka T P, Alexander D C, Parker W B, Miller G A. Immunomodulatory and superantigen activities of bacteria associated with adult periodontitis. J Periodontol. 1996;67:909–917. doi: 10.1902/jop.1996.67.9.909. [DOI] [PubMed] [Google Scholar]
- 15.Hesse D G, Tracey K J, Fong Y, Manogue K R, Palladino M A, Cerami A, Shires G T, Lowry S F. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988;166:147–153. [PubMed] [Google Scholar]
- 16.Holt S C, Bramanti T E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2:177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- 17.Honig J, Rordorf-Adam C, Siegmund C, Wiedemann W, Erard F. Increased interleukin-1 beta concentration in gingival tissue from periodontitis patients. J Periodontal Res. 1989;24:362–367. doi: 10.1111/j.1600-0765.1989.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnston C A, Greisman S E. Mechanism of endotoxin tolerance. In: Hinshaw L B, editor. Pathophysiology of endotoxin. Amsterdam, The Netherlands: Elsevier Science Publishers; 1985. pp. 369–401. [Google Scholar]
- 19.Klosterhalfen B, Horstmann-Jungemann K, Vogel P, Flohe S, Offner F, Kirkpatrick C J, Heinrich P C. Time course of various inflammatory mediators during recurrent endotoxemia. Biochem Pharmacol. 1992;43:2103–2109. doi: 10.1016/0006-2952(92)90167-h. [DOI] [PubMed] [Google Scholar]
- 20.Maidwell-Smith M, Wilson M, Keiser J B. Lipopolysaccharide (endotoxin) from individual periodontally involved teeth. J Clin Periodontol. 1987;14:453–456. doi: 10.1111/j.1600-051x.1987.tb02250.x. [DOI] [PubMed] [Google Scholar]
- 21.Martich G D, Boujoukos A J, Suffredini A F. Response of man to endotoxin. Immunobiology. 1993;187:403–416. doi: 10.1016/S0171-2985(11)80353-0. [DOI] [PubMed] [Google Scholar]
- 22.Martich G D, Danner R L, Ceska M, Suffredini A F. Detection of interleukin 8 and tumor necrosis factor in normal humans after intravenous endotoxin: the effect of antiinflammatory agents. J Exp Med. 1991;173:1021–1024. doi: 10.1084/jem.173.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayrand D, Holt S C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988;52:134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michie H R, Manogue K R, Spriggs D R, Revhaug A, O’Dwyer S, Dinarello C A, Cerami A, Wolf S M, Wilmore D W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988;318:1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- 25.Millar S J, Goldstein E G, Levine M J, Hausmann E. Modulation of bone metabolism by two chemically distinct lipopolysaccharide fractions from Bacteroides gingivalis. Infect Immun. 1986;51:302–306. doi: 10.1128/iai.51.1.302-306.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore J, Wilson M, Kieser J B. The distribution of bacterial lipopolysaccharide (endotoxin) in relation to periodontally involved root surfaces. J Clin Periodontol. 1986;13:748–751. doi: 10.1111/j.1600-051x.1986.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 27.Morrison D C, Leive L. Fractions of lipopolysaccharide from Escherichia coli 0111:B4 prepared by two extraction procedures. J Biol Chem. 1975;250:2911–2919. [PubMed] [Google Scholar]
- 28.Mozes T, Ben-Efraim S, Tak C J, Heiligers J P, Saxena P R, Bonta I L. Serum levels of tumor necrosis factor determine the fatal or non-fatal course of endotoxic shock. Immunol Lett. 1991;27:157–162. doi: 10.1016/0165-2478(91)90144-y. [DOI] [PubMed] [Google Scholar]
- 29.Mundy G R. Inflammatory mediators and the destruction of bone. J Periodontal Res. 1991;26:213–217. doi: 10.1111/j.1600-0765.1991.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 30.Offenbacher S, Odle B M, Van Dyke T E. The use of cervicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J Periodontol Res. 1986;21:101–112. doi: 10.1111/j.1600-0765.1986.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 31.Offenbacher S, Soskolne W A, Collins J G. Prostaglandins and other eicosanoids in gingival crevicular fluid as a markers of periodontal disease susceptibility and activity. In: Johnson N W, editor. Risk markers for oral diseases: periodontal diseases. Cambridge, United Kingdom: Cambridge University Press; 1991. pp. 313–337. [Google Scholar]
- 32.Ogawa T. Immunobiological properties of chemically defined lipid A from lipopolysaccharide of Porphyromonas gingivalis. Eur J Biochem. 1994;219:737–742. doi: 10.1111/j.1432-1033.1994.tb18552.x. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa T, Uchida H, Amino K. Immunobiological activities of chemically defined lipid A from lipopolysaccharides of Porphyromonas gingivalis. Microbiology. 1994;140:1209–1216. doi: 10.1099/13500872-140-5-1209. [DOI] [PubMed] [Google Scholar]
- 34.Okuda K, Saito T, Hirai K, Harano K, Kato T. Role of antibodies in periodontopathic bacterial infection. In: Genco R, Hamada S, Lehner T, McGhee J, Mergenhagen S, editors. Molecular pathogenesis of periodontal disease. Washington, D.C: ASM Press; 1994. pp. 257–265. [Google Scholar]
- 35.Reife R A, Shapiro R A, Bamber B A, Berry B B, Mick G E, Darveau R P. Porphyromonas gingivalis lipopolysaccharide is poorly recognized by molecular components of innate host defense in a mouse model of early inflammation. Infect Immun. 1995;63:4686–4694. doi: 10.1128/iai.63.12.4686-4694.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Remick D G, Strieter R M, Eskandari M K, Nguyen D T, Genord M A, Raiford C L, Kunkel S L. Role of tumor necrosis factor alpha in lipopolysaccharide-induced pathologic alterations. Am J Pathol. 1990;136:49–60. [PMC free article] [PubMed] [Google Scholar]
- 37.Shapira L, Soskolne W A, Houri Y, Barak V, Halabi A, Stabholz A. Protection against endotoxic shock and lipopolysaccharide-induced local inflammation by tetracycline: correlation with inhibition of cytokine secretion. Infect Immun. 1996;64:825–828. doi: 10.1128/iai.64.3.825-828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shapira L, Soskolne W A, Sela M N, Offenbacher S, Barak V. The secretion of PGE2, IL-1 beta, IL-6 and TNF alpha by adherent mononuclear cells from early-onset periodontitis patients. J Periodontol. 1994;65:139–146. doi: 10.1902/jop.1994.65.2.139. [DOI] [PubMed] [Google Scholar]
- 39.Shapira L, Takashiba S, Amar S, Van Dyke T E. Porphyromonas gingivalis lipopolysaccharide stimulation of human monocytes: dependence on serum and CD14 receptor. Oral Microbiol Immunol. 1994;9:112–117. doi: 10.1111/j.1399-302x.1994.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 40.Shapira L, Takashiba S, Champagne C, Amar S, Van Dyke T E. Involvement of protein kinase C and protein tyrosine kinase in lipopolysaccharide-induced TNF alpha and IL-1 beta production by human monocytes. J Immunol. 1994;153:1818–1824. [PubMed] [Google Scholar]
- 41.Socransky S S, Haffajee A D. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 42.Stashenko P, Fujiyoshi P, Obernesser M N, Prostak L, Haffajee A D, Socransky S S. Levels of interleukin-1 beta in tissue from sites of active periodontal disease. J Clin Periodontol. 1991;18:548–554. doi: 10.1111/j.1600-051x.1991.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 43.Stashenko P, Jandinski J J, Fujiyioshi P, Rynar J, Socranski S S. Tissue levels of bone resorptive cytokines in periodontal disease. J Periodontol. 1991;62:504–509. doi: 10.1902/jop.1991.62.8.504. [DOI] [PubMed] [Google Scholar]
- 44.Suffredini A F, Fromm R E, Parker M M, Brenner M, Kovacs J A, Wesley R A, Parrillo J E. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989;321:280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- 45.Van Dyke T E, Lester M A, Shapira L. The role of host response in periodontal disease progression: implications for future treatment strategies. J Periodontol. 1993;64:792–806. doi: 10.1902/jop.1993.64.8s.792. [DOI] [PubMed] [Google Scholar]
- 46.Wilson M, Moore J, Kieser J B. Identity of limulus amoebocyte lysate-active root surface materials from periodontally involved teeth. J Clin Periodontol. 1986;13:743–747. doi: 10.1111/j.1600-051x.1986.tb00876.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Morrison D C. Lipopolysaccharide-induced selective priming effects on tumor necrosis factor alpha and nitric oxide production in mouse peritoneal macrophages. J Exp Med. 1993;177:511–516. doi: 10.1084/jem.177.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]