Abstract
Background.
In women ≥ 70 years of age with T1N0 hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) breast cancer, breast surgery type and omission of axillary surgery or radiation therapy (RT) do not impact overall survival. Although frailty and life expectancy ideally factor into therapy decisions, their impact on therapy receipt is unclear. We sought to identify trends in and factors associated with locoregional therapy type by frailty and life expectancy.
Methods.
Women ≥ 70 years of age with T1N0 HR+/HER2− breast cancer diagnosed in the Surveillance, Epidemiology, and End Results (SEER)-Medicare database between 2010 and 2015 were stratified by validated claims-based frailty and life expectancy measures. Therapy trends over time by regimen intensity (‘high intensity’: lumpectomy + axillary surgery + RT, or mastectomy + axillary surgery; ‘moderate intensity’: lumpectomy + RT, lumpectomy + axillary surgery, or mastectomy only; or ‘low intensity’: lumpectomy only) were analyzed. Factors associated with therapy type were identified using generalized linear mixed models.
Results.
Of 16,188 women, 21.8% were frail, 22.2% had a life expectancy < 5 years, and only 12.3% fulfilled both criteria. In frail women with a life expectancy < 5 years, high-intensity regimens decreased significantly (48.8–31.2%; p < 0.001) over the study period, although in 2015, 30% still received a high-intensity regimen. In adjusted analyses, frailty and life expectancy < 5 years were not associated with breast surgery type but were associated with a lower likelihood of axillary surgery (frailty: odds ratio [OR] 0.86, 95% confidence interval [CI] 0.76–0.96; life expectancy < 5 years: OR 0.22, 95% CI 0.20–0.25). Life expectancy < 5 years was also associated with a lower likelihood of RT receipt in breast-conserving surgery patients (OR 0.30, 95% CI 0.27–0.34).
Conclusions.
Rates of high-intensity therapy are decreasing but overtreatment persists in this population. Continued efforts aimed at appropriate de-escalation of locoregional therapy are needed.
Keywords: Breast cancer, Geriatric oncology, Frailty, Life expectancy, Locoregional therapy, Surgery, Radiation
Over 30% of new breast cancer cases diagnosed annually in the United States are in women ≥ 70 years of age.1 Tailoring their therapy based not only on chronological age but also on physiologic age, is important. Overtreatment and undertreatment of older adults is defined by the intensity of cancer therapy relative to its associated benefits and harms,2 which, in an older population, requires clinicians to take geriatric-specific considerations, such as life expectancy and frailty, into account. While undertreatment of older adults with aggressive breast cancer has been a longstanding concern,1–5 attention to overtreatment of those with lower-risk breast cancers, such as early-stage hormone receptor-positive (HR+) disease, is growing.6–10
A range of locoregional treatment options are available to older adults with early-stage HR+ breast cancer, as randomized controlled trial (RCT) data demonstrate survival equivalency between mastectomy and breast-conserving therapy (BCT),11–13 axillary surgery (i.e. sentinel lymph node biopsy [SLNB] or axillary lymph node dissection [ALND]14–16 versus no axillary surgery, and radiation therapy (RT) versus no RT16,17 in women ≥ 70 years of age with clinical T1N0 HR+/human epidermal growth factor receptor 2-negative (HER2−) breast cancers. With respect to omission of RT or axillary surgery, there are slight trade-offs with small increases in locoregional recurrence but without detrimental survival outcomes. Ten-year follow-up from the Cancer and Leukemia Group B (CALGB)-9343 trial and PRIME II have demonstrated a decrease of approximately 7% in local recurrence with RT (PRIME II: 9.8% without RT, 0.9% with RT; CALGB: 10% without RT, 2% with RT), without any significant difference in overall survival.16,18 Similarly, omission of axillary surgery carries an approximately 3–6% risk of axillary recurrence without a decrement in survival.14–16 However, these risks are cumulative over a 10-year follow-up, highlighting a patient’s need to be fit enough and with a long enough life expectancy to potentially realize any potential benefit of RT or SLNB.
As such, women with early-stage HR+/HER2− breast cancer may reasonably opt for one of four possible lumpectomy-containing regimens (lumpectomy + axillary surgery + RT, lumpectomy + RT [without axillary surgery], lumpectomy + axillary surgery [without RT], or lumpectomy alone), or one of two mastectomy-containing regimens (mastectomy + axillary surgery or mastectomy alone, as post-mastectomy RT is not standard in women with this type of disease). Without a survival advantage to more aggressive therapy, the potential for overtreatment in women with early-stage HR+/HER2− breast cancer is clear, as regimens that would be considered standard of care in fit populations (i.e., lumpectomy + axillary surgery + RT and mastectomy + axillary surgery) may translate into overtreatment in a frail patient with limited life expectancy. However, the associations between life expectancy/frailty and locoregional treatment receipt in clinical practice and potential practice changes over time remain unclear. In order to better target future interventions optimizing treatment based on physiologic age and preferences, we sought to evaluate trends over time in locoregional therapy regimen receipt by life expectancy and frailty in women ≥ 70 years of age with cT1N0 HR+/HER2− breast cancer in the timeframe around landmark publications in this area, and the factors associated with receipt of different locoregional treatment regimens.
METHODS
Data Source
The Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute reports population-based data in areas representing 28% of the US population.19 Since 1991, SEER data have been linked with administrative Medicare data for individuals enrolled in fee-for-service. Given that this dataset (SEER-Medicare) is a limited dataset using previously collected data, it was deemed exempt for review by the Massachusetts General Brigham Institutional Review Board.
Patients
Women ≥ 70 years of age with clinical T1N0 HR+/HER2− breast cancer diagnosed from January 2010 to October 2015 were identified. As the data from RCTs considering de-escalation of locoregional therapy included only women, we chose to exclude men. Given that our claims-based frailty measure20 was originally described using International Classification of Diseases, Ninth Revision (ICD-9) codes, this timeframe was chosen to exclude the time after the change to ICD, Tenth Revision (ICD-10) coding. Women who were continuously enrolled in Medicare Parts A/B, and not a health maintenance organization, from 1 year prior to diagnosis through to 1 year after diagnosis were included. Those who were diagnosed at autopsy, those with unknown surgery type and unknown receptor status, those who were male, and those who underwent neoadjuvant RT or post-mastectomy RT or had previous history of breast cancer were excluded.
Variables
The stratifying variables of interest were frailty and life expectancy. Frailty was defined using the validated claims-based frailty indicator reported by Kim et al.20 This frailty index incorporates administrative codes for durable medical equipment claims, comorbid conditions, and health care facility use in the 12 months prior to the diagnosis. A binary variable was created to indicate the frailty status, with a score of ≥ 0.25 designating a patient as frail and < 0.25 deemed as not frail, as has been done in previous studies.21 Life expectancy was determined using the validated claims-based measure reported by Tan et al.22 This measure was based on patient sex, age, and comorbidities and can qualify patients as having a life expectancy of ≥ 5 years or < 5 years.
Other patient-level variables included age, race, ethnicity (non-Hispanic White, Black, Hispanic, other, and unknown), Charlson–Deyo Comorbidity Index (0, 1, 2, 3, ≥ 4),23 regional location of the patient’s home zip code (urban or rural), median income of the patient’s zip code (quartiles), SEER region (West, Northeast, Midwest, or South) and year of diagnosis. Disease characteristics included tumor grade (1, 2, 3), clinical tumor stage (T1a, T1b, or T1c), and tumor histology (invasive ductal carcinoma [IDC], invasive lobular carcinoma [ILC], or other).
Outcome Measures
The main measure of interest was the proportion of patients undergoing different locoregional therapy options, stratified by life expectancy and frailty status. Claims data were used to more robustly define a patient’s scope of surgery (i.e. scope of lymph node surgery) and RT receipt, which can be missing in the SEER files. Locoregional therapy regimen options included lumpectomy alone, lumpectomy + RT, lumpectomy + axillary surgery, lumpectomy + RT + axillary surgery, mastectomy only, and mastectomy + axillary surgery. Lumpectomy + axillary surgery + RT and mastectomy + axillary surgery were deemed ‘high intensity’ as they represented regimens in which there was no de-escalation of therapy. Lumpectomy + RT, lumpectomy + axillary surgery, and mastectomy alone were considered ‘moderate intensity’ therapy because there was omission of at least one possible treatment. Finally, lumpectomy alone was deemed ‘low intensity’ because there was omission of treatments not shown to improve survival. Patients who did not receive any locoregional therapy were labeled as having ‘no treatment’.
Statistical Analysis
The proportion of patients receiving low-, moderate-, and high-intensity therapy regimens was compared by year of diagnosis. These numbers were then stratified by frailty and life expectancy, grouping patients by four combinations (i.e., frail with life expectancy < 5 years, frail with life expectancy ≥ 5 years, not-frail with life expectancy < 5 years, and not-frail with life expectancy ≥ 5 years), to understand if trends by intensity of treatment regimen differed by frailty and life expectancy. Chi-square tests of proportions were run to determine significant differences by year. To evaluate each separate treatment decision comprising the intensity of regimen, generalized linear mixed models were used to identify a priori-selected patient-level and disease-level factors associated with each locoregional treatment decision. Separate models were run for each of the following decisions: lumpectomy versus mastectomy, axillary surgery versus no axillary surgery, and RT versus no RT among patients who underwent lumpectomy. All analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Of 16,188 women, 21.8% were frail, 22.2% had a life expectancy < 5 years, and 1987 (12.3%) fulfilled both criteria (Fig. 1a). The proportion of frail women increased with chronological age, with 15.7% of women aged 70–74 years and 34.8% of women aged ≥ 85 years qualifying as frail (p < 0.001) (Fig. 1b). There was a greater increase in the proportion of those with limited life expectancy (< 5 years) with age, with 71.6% of women ≥ 85 years compared with 5.2% of women aged 70–74 years having a life expectancy of < 5 years (p < 0.001). Other clinicopathologic characteristics by frailty and life expectancy status are detailed in Table 1. A higher proportion of Black women were frail compared with non-Hispanic White subjects (32.7 vs. 21.1%; p < 0.001), and had a < 5-year life expectancy (31.3 vs. 21.4%; p < 0.001).
FIG. 1.
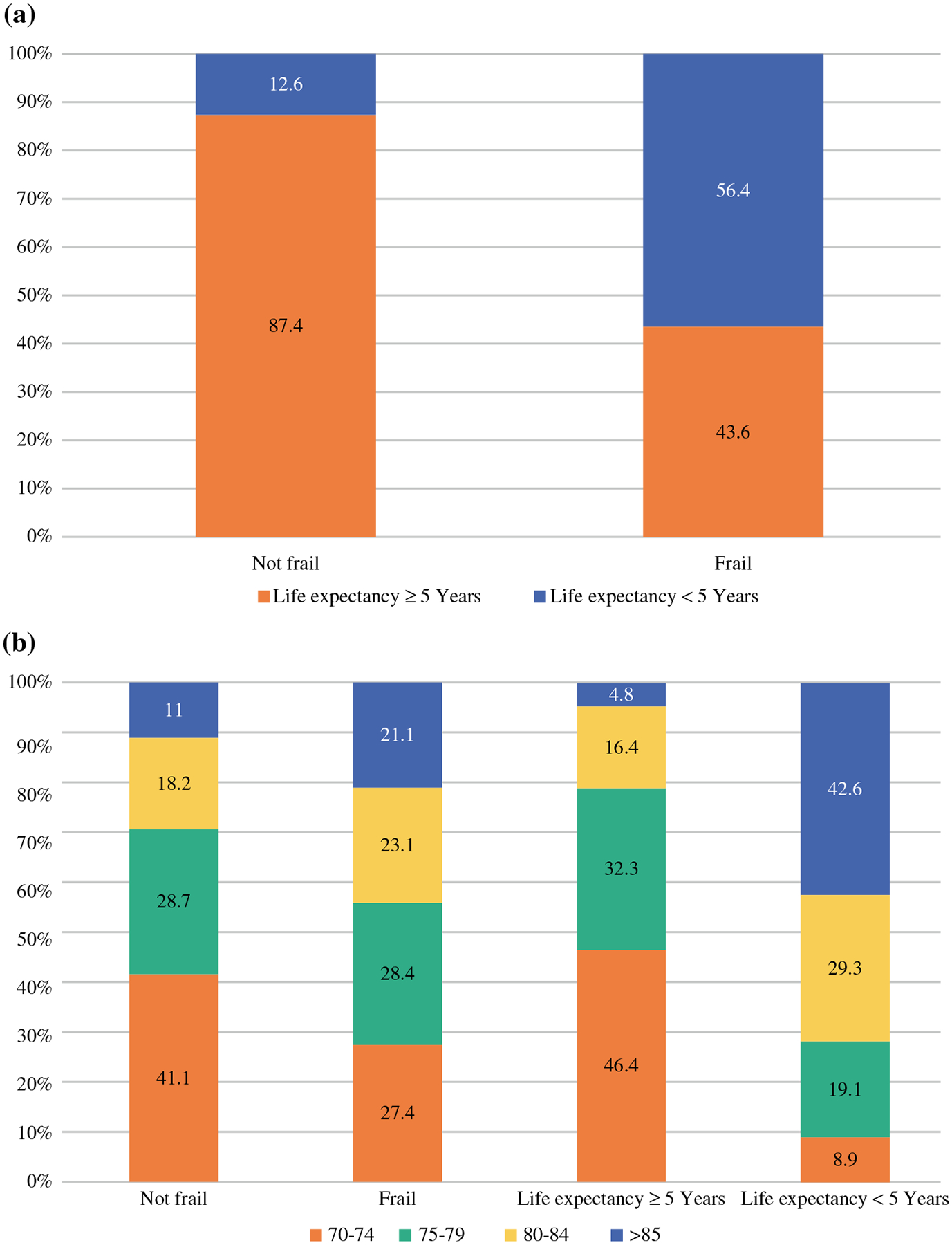
Study cohort showing a life expectancy by frailty status, and b age by frailty status and life expectancy
TABLE 1.
Unadjusted proportions of patients ≥70 years of age with T1N0 HR+ breast cancer, by frailty status and life expectancy
| Frailty (total N = 16,188) | Life expectancy (total N = 16,188) | |||
|---|---|---|---|---|
| No (n = 12,664, 78.2%) | Yes (n = 3524, 21.8%) | ≥ 5 years (n = 12,603, 77.9%) | < 5 years (n = 3585, 22.2%) | |
| Patient characteristics | ||||
| Racea | ||||
| African-American/Black | 617 (4.9) | 300 (8.5) | 630 (5.0) | 287 (8.0) |
| Hispanic/Latinx White | 501 (4.0) | 184 (5.2) | 497 (3.9) | 188 (5.2) |
| Non-Hispanic/Latinx White | 10,812 (85.4) | 2899 (82.3) | 10,772 (85.5) | 2939 (82.0) |
| Other/unknown | 734 (5.8) | 141 (4.0) | 704 (5.6) | 171 (4.8) |
| Year of diagnosis | ||||
| 2010 | 2055 (16.2) | 548 (15.6) | 2025 (16.1) | 578 (16.1) |
| 2011 | 2161 (17.1) | 552 (15.7) | 2114 (16.8) | 599 (16.7) |
| 2012 | 2169 (17.1) | 615 (17.5) | 2138 (17.0) | 646 (18.0) |
| 2013 | 2220 (17.5) | 602 (17.1) | 2214 (17.6) | 608 (17.0) |
| 2014 | 2206 (17.4) | 642 (18.2) | 2243 (17.8) | 605 (16.9) |
| 2015 | 1853 (14.6) | 565 (16.0) | 1869 (14.8) | 549 (15.3) |
| Median incomea | ||||
| Quartile 1 (lowest) | 1536 (12.1) | 610 (17.3) | 1579 (12.5) | 567 (15.8) |
| Quartile 2 | 2484 (19.6) | 752 (21.3) | 2468 (19.6) | 768 (21.4) |
| Quartile 3 | 3426 (27.1) | 933 (26.5) | 3351 (26.6) | 1008 (28.1) |
| Quartile 4 (highest) | 5218 (41.2) | 1229 (34.9) | 5205 (41.3) | 1242 (34.6) |
| Charlson–Deyo Comorbidity Indexa | ||||
| 0 | 7045 (55.6) | 1105 (31.4) | 7103 (56.4) | 1047 (29.2) |
| 1 | 1777 (14.0) | 876 (24.9) | 1875 (14.9) | 778 (21.7) |
| 2 | 744 (5.9) | 588 (16.7) | 766 (6.1) | 566 (15.8) |
| 3 | 255 (2.0) | 332 (9.4) | 221 (1.8) | 366 (10.2) |
| ≥4 | 116 (0.9) | 437 (12.4) | 80 (0.6) | 473 (13.2) |
| Unknown | 2727 (21.5) | 186 (5.3) | 2558 (20.3) | 355 (9.9) |
| Urban/rural status | ||||
| Urban | 6422 (50.7) | 1758 (49.9) | 6288 (49.9) | 1892 (52.8) |
| Rural | 1251 (9.9) | 341 (9.7) | 1263 (10.0) | 329 (9.2) |
| Unknown | 4991 (39.4) | 1425 (40.4) | 5052 (40.1) | 1364 (38.1) |
| SEER regiona | ||||
| West | 5632 (44.5) | 1480 (42.0) | 5618 (44.6) | 1494 (41.7) |
| Northeast | 2564 (20.3) | 720 (20.4) | 2452 (19.5) | 832 (23.2) |
| Midwest | 1573 (12.4) | 408 (11.6) | 1536 (12.2) | 445 (12.4) |
| South | 2895 (22.9) | 916 (26.0) | 2997 (23.8) | 814 (22.7) |
| Disease characteristics | ||||
| Tumor grade | ||||
| 1 | 5110 (40.4) | 1382 (39.2) | 5138 (40.8) | 1354 (37.8) |
| 2 | 5993 (47.3) | 1658 (47.1) | 5931 (47.1) | 1720 (48.0) |
| 3 | 1193 (9.4) | 357 (10.1) | 1183 (9.4) | 367 (10.2) |
| Unknown | 368 (2.9) | 127 (3.6) | 351 (2.8) | 144 (4.0) |
| Tumor stagea | ||||
| T1a | 1690 (13.3) | 365 (10.4) | 1684 (13.4) | 371 (10.4) |
| T1b | 4564 (36.0) | 1182 (33.5) | 4600 (36.5) | 1146 (32.0) |
| T1c | 6410 (50.6) | 1977 (56.1) | 6319 (50.1) | 2046 (57.7) |
| Histology | ||||
| IDC | 9363 (73.9) | 2571 (73.0) | 9389 (74.5) | 2545 (71.0) |
| ILC | 1934 (15.3) | 542 (15.4) | 1926 (15.3) | 550 (15.3) |
| Other | 1367 (10.8) | 411 (11.7) | 1288 (10.2) | 490 (13.7) |
| Treatment characteristics | ||||
| No treatment | 490 (3.9) | 305 (8.7) | 400 (3.2) | 395 (11.0) |
| Low intensity | ||||
| Lumpectomy alone | 792 (6.3) | 434 (12.3) | 566 (4.5) | 660 (18.4) |
| Moderate intensity | ||||
| Mastectomy alone | 134 (1.1) | 68 (1.9) | 99 (0.8) | 103 (5.9) |
| Lumpectomy + axillary surgery | 2282 (18.0) | 692 (19.6) | 2247 (17.8) | 727 (20.3) |
| Lumpectomy + RT | 405 (3.2) | 185 (5.3) | 383 (3.0) | 207 (5.8) |
| High intensity | ||||
| Lumpectomy + axillary surgery + RT | 6097 (48.1) | 1162 (33.0) | 6392 (50.7) | 867 (24.2) |
| Mastectomy + axillary surgery | 2464 (19.5) | 678 (19.2) | 2516 (20.0) | 626 (17.5) |
p < 0.05 for differences within groups by frailty and life expectancy, respectively
HR+ hormone receptor-positive, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, RT radiation therapy, SEER Surveillance, Epidemiology, and End Results, other/unknown ethnicity patients coded as American Indian, Chinese, Japanese, Filipino, Hawaiian, Korean, Vietnamese, Laotian, Hmong, Kampuchean, Thai, Asian Indian, Pakistani, Micronesian, Chamorran, Guamanian, Polynesia, Tahitian, Samoan, Tongan, Melanesian, Fiji Islander, New Guinean, other Asian not otherwise specified, Pacific Islander not otherwise specified, other, or unknown in SEER
Overall, 10,401 (64.3%) women underwent high-intensity therapy (mastectomy + axillary surgery or lumpectomy + axillary surgery + RT), 3766 (23.3%) underwent moderate-intensity treatment (mastectomy alone, lumpectomy + axillary surgery, or lumpectomy + RT), 1226 (7.5%) underwent low-intensity locoregional treatment (lumpectomy alone), and 795 women did not receive any locoregional therapy (4.9%). Among those who were frail, 51.9% underwent high-intensity therapy, 27.0% underwent moderate-intensity therapy, 12.4% underwent low-intensity therapy, and 8.7% did not undergo locoregional treatment. Of those who had a life expectancy of < 5 years, 41.6% underwent high-intensity therapy, 28.9% underwent moderate-intensity therapy, 18.4% underwent low-intensity therapy, and 11.0% did not undergo locoregional therapy.
From 2010 to 2015, the proportion of women in the entire cohort undergoing high-intensity treatment decreased (71–49.2%; p < 0.001) (Fig. 2a), with a larger decrease in the lumpectomy + axillary surgery + RT group (49.1–33.5%; p < 0.001) compared with the mastectomy + axillary surgery group (21.9–15.7%; p < 0.001). Moderate-intensity treatment regimens significantly increased from 18.1 to 36.3% (p < 0.001), driven by increases in lumpectomy + axillary surgery (12.7–32.7%; p < 0.001). Rates of lumpectomy + RT (3.6–2.6%; p = 0.015) and mastectomy only (1.8–1.0%; p = 0.002) statistically significantly declined over the study period. Low-intensity treatment increased (6.5–9.0%; p < 0.001) and omission of locoregional therapy (no treatment) stayed stable (4.4–5.5%; p = 0.30).
FIG. 2.
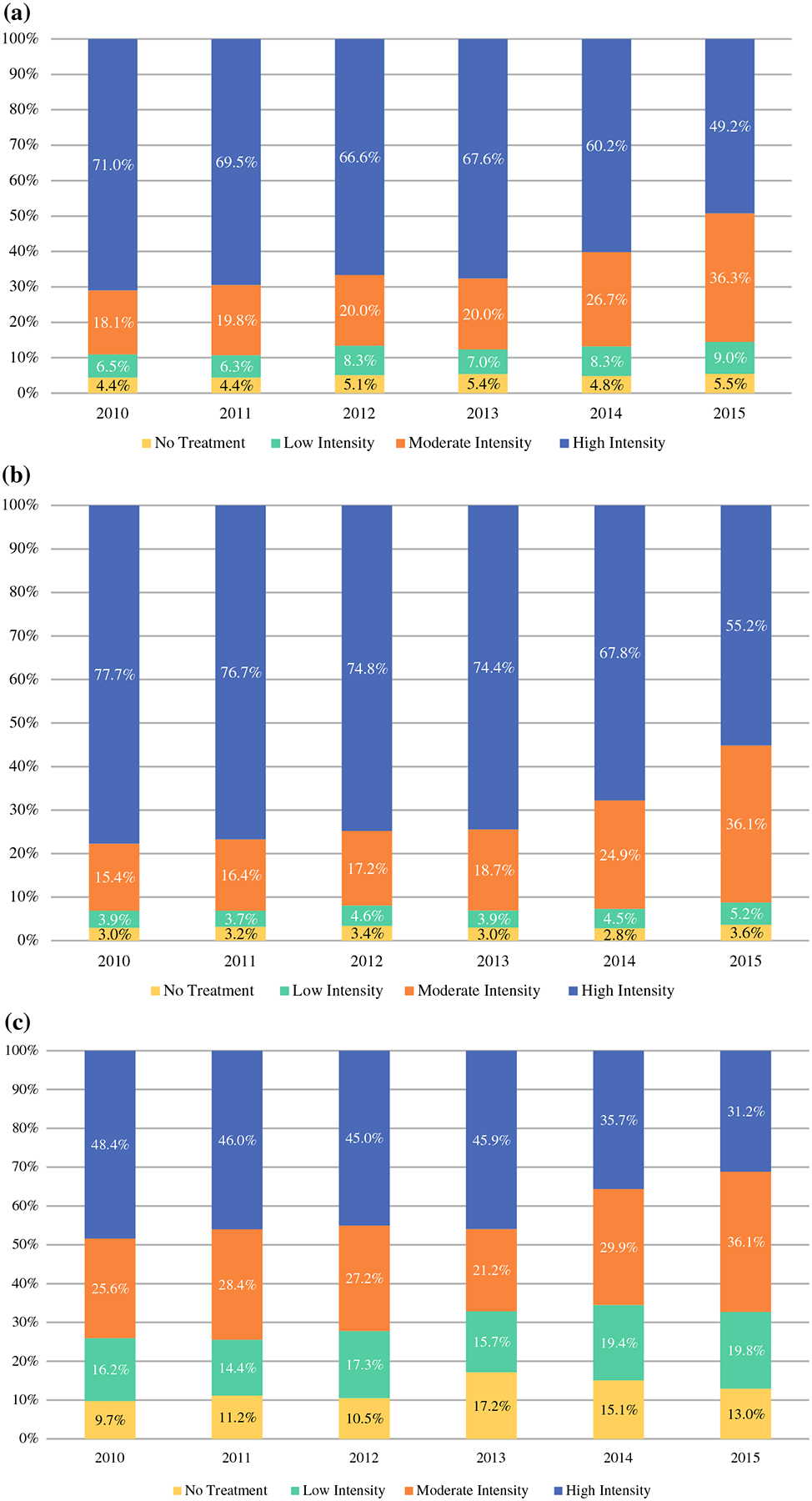
Locoregional therapy trends over time. a Locoregional therapy trends over time (whole cohort). Trends from 2010 to 2015 in women ≥ 70 years of age with T1N0 HR+ disease, by therapy intensity: high intensity: lumpectomy, axillary surgery and RT, or mastectomy and axillary surgery; moderate intensity: lumpectomy and RT, lumpectomy and axillary surgery, or mastectomy only; low intensity: lumpectomy only. b Locoregional therapy trends over time in non-frail women with a ≥ 5-year life expectancy. Trends from 2010 to 2015 in women ≥ 70 years of age with T1N0 HR+ disease who are robust and have a ≥ 5-year life expectancy, by therapy intensity: high intensity: lumpectomy, axillary surgery and RT, or mastectomy and axillary surgery; moderate intensity: lumpectomy and RT, lumpectomy and axillary surgery, or mastectomy only; low intensity: lumpectomy only. c Locoregional therapy trends over time in frail women with a < 50-year life expectancy. Trends from 2010 to 2015 in women ≥ 70 years of age with T1N0 HR+ disease who are frail and have a < 5-year life expectancy, by therapy intensity: high intensity: lumpectomy, axillary surgery and RT, or mastectomy and axillary surgery; moderate intensity: lumpectomy and RT, lumpectomy and axillary surgery, or mastectomy only; low intensity: lumpectomy only. HR+ hormone receptor-positive, RT radiation therapy
Trends were largely paralleled in subpopulations stratified by frailty status and life expectancy (Fig. 2a, b, c). In non-frail women with a life expectancy ≥ 5 years, high-intensity regimens overall made up the majority of locoregional treatments offered, but also decreased over time (lumpectomy + axillary surgery + RT: 55.7–39.3%, p < 0.001; mastectomy + axillary surgery: 22.0–15.9%, p < 0.001) (Fig. 1b). Moderate-intensity regimens increased significantly over time, being driven by a significant increase in lumpectomy + axillary surgery (11.9–33.0%; p < 0.001), while lumpectomy + RT (2.5–2.3%; p = 0.56) and mastectomy only (1.0–0.8%; p = 0.2) did not change significantly. The low-intensity regimen of lumpectomy increased only slightly but did not reach statistical significance (3.9–5.2%; p = 0.06).
In frail women with a life expectancy < 5 years, high-intensity regimens decreased significantly (overall: 48.4–31.2%, p < 0.001; lumpectomy + axillary surgery + RT: 27.5–17.3%, p < 0.001; mastectomy + axillary surgery: 20.8–13.9%, p = 0.02) over the study period. Low-intensity treatment increased slightly from 16.2 to 19.8% (p = 0.08) but did reach statistical significance, and, similar to the overall cohort and to non-frail women with life expectancies over 5 years, moderate-intensity regimens increased (25.6–36.1%; p = 0.02), driven by increases in lumpectomy + axillary surgery (14.6–30.6%; p < 0.001). However, in 2015, 31.2% of frail women with a life expectancy < 5 years still received a high-intensity regimen.
On adjusted analysis, both frailty and life expectancy < 5 years were found to be significantly associated with a lower likelihood of axillary surgery receipt (Table 2). However, there was no significant association between frailty or life expectancy and receipt of mastectomy compared with lumpectomy. Other patient characteristics significantly associated with intensity of locoregional therapy included race, ethnicity, and year of diagnosis. Black women were more likely than non-Hispanic White women to undergo more intensive locoregional therapy. The end of the study period (2015), compared with 2010, was associated with lower odds of receipt of mastectomy and RT in breast-conserving surgery patients, but not of axillary surgery. Disease characteristics were also associated with locoregional therapy type. Grade 2 (vs. grade 1) and grade 3 (vs. grade 1) tumors were associated with more intense locoregional therapy, as was T1c disease.
TABLE 2.
Adjusted logistic regression predicting more intense locoregional therapy type in patients ≥70 years of age with T1N0 HR+ breast cancer
| Breast surgerya | Axillary surgery | RT in BCS patients | |
|---|---|---|---|
| Frailty | |||
| No | Ref | Ref | Ref |
| Yes | 1.05 [0.95–1.17] | 0.86 [0.76–0.96] | 0.92 [0.82–1.02] |
| Life expectancy, years | |||
| ≥ 5 | Ref | Ref | Ref |
| < 5 | 1.03 [0.93–1.15] | 0.22 [0.20–0.25] | 0.30 [0.27–0.34] |
| Race | |||
| African- American/Black | 1.22 [1.03–1.44] | 1.31 [1.04–1.66] | 1.24 [1.02–1.50] |
| Hispanic/Latinx White | 1.12 [0.99–1.45] | 1.19 [0.93–1.53] | 1.09 [0.89–1.33] |
| Non-Hispanic/Latinx White | Ref | Ref | Ref |
| Other | 1.67 [1.41–1.99] | 1.08 [0.85–1.37] | 1.27 [1.04–1.55] |
| Unknown | 1.27 [0.71–2.26] | 0.91 [0.46–1.78] | 0.79 [0.45–1.38] |
| Year of diagnosis | |||
| 2010 | Ref | Ref | Ref |
| 2011 | 0.84 [0.74–0.96] | 0.99 [0.84–1.18] | 0.96 [0.83–1.12] |
| 2012 | 0.91 [0.79–1.03] | 0.83 [0.70–0.98] | 0.80 [0.69–0.92] |
| 2013 | 0.86 [0.74–1.01] | 0.98 [0.81–1.20] | 0.78 [0.66–0.92] |
| 2014 | 0.76 [0.64–0.90] | 0.81 [0.66–1.00] | 0.52 [0.44–0.61] |
| 2015 | 0.64 [0.54–0.77] | 0.87 [0.71–1.08] | 0.29 [0.24–0.34] |
| Median income | |||
| Quartile 1 (lowest) | Ref | Ref | Ref |
| Quartile 2 | 0.92 [0.81–1.05] | 0.94 [0.79–1.13] | 1.06 [0.92–1.23] |
| Quartile 3 | 0.77 [0.68–0.88] | 1.04 [0.88–1.24] | 1.09 [0.95–1.26] |
| 4 (highest) | 0.69 [0.60–0.78] | 0.94 [0.79–1.12] | 1.22 [1.06–1.40] |
| Urban/rural status | |||
| Urban | Ref | Ref | Ref |
| Rural | 1.33 [1.16–1.51] | 0.99 [0.83–1.18] | 0.93 [0.80–1.08] |
| Unknown | 1.09 [0.96–1.24] | 1.11 [0.94–1.30] | 1.02 [0.89–1.15] |
| SEER region | |||
| Northeast | Ref | Ref | Ref |
| Midwest | 1.66 [1.42–1.95] | 1.87 [1.54–2.23] | 1.05 [0.90–1.24] |
| South | 2.11 [1.83–2.43] | 1.80 [1.53–2.13] | 0.80 [0.70–0.92] |
| West | 1.37 [1.20–1.55] | 1.48 [1.29–1.70] | 0.67 [0.60–0.76] |
| Tumor grade | |||
| 1 | Ref | Ref | Ref |
| 2 | 1.24 [1.13–1.35] | 1.19 [1.07–1.33] | 1.17 [1.01–1.28] |
| 3 | 1.53 [1.33–1.76] | 1.54 [1.27–1.88] | 1.72 [1.46–2.02] |
| Unknown | 1.61 [1.27–2.04] | 1.06 [0.79–1.42] | 1.10 [0.85–1.43] |
| Tumor stage | |||
| T1a | Ref | Ref | Ref |
| T1b | 0.80 [0.70–0.92] | 1.44 [1.24–1.67] | 1.14 [1.01–1.29] |
| T1c | 1.18 [1.04–1.34] | 1.47 [1.27–1.70] | 1.31 [1.15–1.48] |
| Histology | |||
| IDC | Ref | Ref | Ref |
| ILC | 1.34 [1.21–1.49] | 1.09 [0.95–1.26] | 1.07 [0.95–1.20] |
| Other | 1.03 [0.90–1.18] | 0.81 [0.70–0.94] | 0.85 [0.74–0.96] |
Bolded values denote statistical significance at p < 0.05
Odds of undergoing mastectomy
BCS breast-conserving surgery, HR+ hormone receptor-positive, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, RT radiation therapy, SEER Surveillance, Epidemiology, and End Results, other/unknown ethnicity patients coded as American Indian, Chinese, Japanese, Filipino, Hawaiian, Korean, Vietnamese, Laotian, Hmong, Kampuchean, Thai, Asian Indian, Pakistani, Micronesian, Chamorran, Guamanian, Polynesia, Tahitian, Samoan, Tongan, Melanesian, Fiji Islander, New Guinean, other Asian not otherwise specified, Pacific Islander not otherwise specified, other, or unknown in SEER
DISCUSSION
In this cohort of older patients with T1N0 HR+/HER2− breast cancer, the rates of high-intensity locoregional therapy declined by 21.8% over the study years. These high-intensity regimens were instead mostly replaced by moderate-intensity regimens (in which there was omission of one element of locoregional therapy, be it RT or axillary surgery), although low-intensity treatment rates did increase, albeit to a lesser extent, by 2.5%. Omission of locoregional therapy increased only 1.1% throughout 2010–2015. The overall trend of a statistically significant decline in high-intensity therapy with increases in moderate-intensity regimens held true across subgroups defined by frailty and life expectancy status. On adjusted models for each treatment decision faced by a given patient, we showed that frailty and life expectancy were significantly associated with axillary and RT receipt, but not with type of breast surgery received.
With no survival benefit seen with RT or axillary surgery in RCT data, current guidelines and recommendations urge consideration of omission of certain locoregional therapies in older women with early-stage HR+/HER2− breast cancer.24–26 However, the absence of specific, prescriptive wording of these statements leaves physicians to determine the best candidates for omission, which can lead to anxiety regarding appropriate patient selection.27 Incorporating objective indicators of physiologic age should help to guide tailored cancer therapy,28 and there are clinical programs pushing to incorporate geriatric assessment in oncology29–33 and surgical clinics.34–36 Previous data would suggest that use of such tools in practice is still low27 but our multivariate model did demonstrate associations between the presence of frailty and limited life expectancy and lower likelihood of axillary surgery receipt, and between limited life expectancy and a lower likelihood of RT receipt. However, our analysis is unable to assess if physicians were using clinical tools to objectively assess older adults or simply using the ‘eyeball’ test.
That only 56% of women who are frail have < 5 years life expectancy speaks to the fact that these are two different constructs, despite the fact that both of our validated claims-based measures used did incorporate patient comorbidities.20,22 The literature regarding the significance of frailty with respect to the risk of adverse outcomes in low-risk surgery and radiation is relatively limited37 and thus it may not be expected that frailty would necessarily be associated with RT receipt. It may be that limited life expectancy is more relevant to RT treatment decisions as relatively long follow-up times are necessary to see the modest local benefits in this patient population.16,18
There may be several factors contributing to the lack of association between frailty and limited life expectancy with breast surgery type on multivariable analysis. First, although all patients had stage I disease, breast conservation candidacy rests on factors other than tumor stage; multifocality, multicentrality of disease, or presence of extensive associated ductal carcinoma in situ (DCIS) may preclude breast conservation. Second, breast surgery type is a prototypical preference-sensitive decision, and patients, despite possibly being frail and having limited life expectancy, may be opting for mastectomy for any number of reasons, such as the anxiety/worry regarding recurrence38–41 or wish to avoid RT (or wish to avoid the modest increase in local recurrence with omission of RT).42–44 Third, clinician perception of the relative operative stress of a lumpectomy versus mastectomy may vary. In a study rating operative stress by Delphi consensus, lumpectomy and mastectomy were classified as having the same stress level.45 The fact that frailty and limited life expectancy were significantly associated with a lower likelihood of axillary surgery is encouraging, demonstrating that surgeons may be taking these factors into consideration. Even if formal geriatric screening or assessment is not being done clinically, clinician judgment may be adequate to appropriately de-escalate axillary surgery.
Overtreatment is a concept that can be difficult to define.46 However, given the reductions in local recurrence seen in RCTs, it is reasonable to suggest that overtreatment is manifest in the 30% of women who were frail and who had a life expectancy of <5 years and received maximum locoregional therapy at the end of the study period. The perceived operative and treatment risks associated with breast surgery and RT may be low but are not negligible. Locoregional complication rates (including infection, seroma, hematoma, breast edema, skin toxicities, wound complications, and paresthesias) in past SEER-Medicare analysis, have recorded overall complication rates of 37.6% in women receiving lumpectomy + RT.47 The burdens of daily travel associated with RT48,49 and increased cost of care47,50 are also not to be ignored. In addition, there has been some suggestion from a recent observational study that frailty is associated with increased local complication rates.51 As physicians become more comfortable discussing omission of locoregional therapy, patients in turn may be more comfortable with less therapy. However, it remains to be determined exactly how much of this overtreatment may be driven by patient preference, and if nuances in patient/physician communication may have an effect on patients’ treatment decisions.
Although it may be fair to label high-intensity therapy in frail women with a limited life expectancy as overtreatment, undertreatment is more difficult to define in this population. As the trial data supporting the safety of lower-intensity therapy in this population did not include geriatric-specific data that allow for speculation on physiologic age, it is difficult to suggest that a robust woman with a > 5-year life expectancy was being undertreated by lumpectomy only, especially since older women who participate in trials tend to be healthier than those who refuse to participate.52 If endocrine therapy is taken, the data support no compromise in survival and some women are likely to accept the trade-off in single-digit increases in locoregional recurrence risk for less-intense therapy.16,18,53 The importance of informed, frank conversations in this arena are paramount, as these decisions are preference-sensitive.
There are a number of limitations in our study. First, analyses using SEER-Medicare are subject to possible coding errors in data collection. Second, as we did not have Medicare Part D data, we did not have data regarding endocrine therapy, which is an important therapeutic consideration in this patient population. Third, while we used validated claims-based frailty and life expectancy measures, the determination of overtreatment and undertreatment may rest on other patient factors, such as patient preference and conversational dynamics between patient and physician. Finally, certain data (e.g. extent of DCIS, multicentric nature of disease) that can influence the extent of locoregional therapy are not available in this dataset.
CONCLUSION
We illustrate how geriatric-specific considerations such as frailty and life expectancy may play a role in locoregional treatment decision making in older women with early-stage HR+/HER2− breast cancer, and that overtreatment persists in frail women with limited life expectancy. Further work is needed to decrease the rates of overtreatment and to also understand how to use currently existing objective measures of physiological age to appropriately tailor physician treatment recommendations and conversations.
ACKNOWLEDGMENT
This research was funded by the American Society of Clinical Oncology Conquer Cancer Foundation (Young Investigator Award in Geriatric Oncology). Mara A. Schonberg’s effort was supported by NIH/NIA K24 (5K24AG071906). Christina A. Minami reports research support (to the institution) from the American Society of Clinical Oncology Conquer Cancer Foundation (Young Investigator Award, 2020–2021), American College of Surgeons (Faculty Research Fellowship, 2020–2022), and a Grant for Early Medical/Surgical Specialists’ Transition to Aging Research (GEMSSTAR). Elizabeth A. Mittendorf acknowledges support as the Rob and Karen Hale Distinguished Chair in Surgical Oncology. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; Information Management Services (IMS), Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database. This project included data from the California Cancer Registry. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC’s) National Program of Cancer Registries, under cooperative agreement 1NU58DP007156; the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the authors and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the CDC, or their contractors and subcontractors.
FUNDING
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
DISCLOSURE
Tari A. King reports speaker honoraria and compensated service on the Scientific Advisory Board of Exact Sciences. Elizabeth A. Mittendorf reports compensated service on Scientific Advisory Boards for Astra Zeneca, BioNTech and Merck; uncompensated service on Steering Committees for Bristol Myers Squibb and Roche/Genentech; speakers honoraria and travel support from Merck Sharp & Dohme; and institutional research support from Roche/Genentech (via an SU2C grant) and Gilead. She also reports research funding from Susan Komen for the Cure for which she serves as a Scientific Advisor, and uncompensated participation as a member of the American Society of Clinical Oncology Board of Directors. Christina A. Minami, Ginger Jin, Rachel A. Freedman, and Mara A. Schonberg report no disclosures or conflicts of interest.
Footnotes
This paper was previously presented as a poster at the American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, USA, 3–8 June 2022 (https://ascopubs.org/doi/abs/10.1200/JCO.2022.40.16_suppl.12047).
DATA AVAILABILITY
These data will not be shared as this dataset is widely used/publicly available.
REFERENCES
- 1.Schonberg MA, Marcantonio ER, Li D, Silliman RA, Ngo L, McCarthy EP. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol. 2010;28(12):2038–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith BD, Jiang J, McLaughlin SS, et al. Improvement in breast cancer outcomes over time: are older women missing out? J Clin Oncol. 2011;29(35):4647–53. [DOI] [PubMed] [Google Scholar]
- 3.Van Leeuwen BL, Rosenkranz KM, Feng LL, et al. The effect of under-treatment of breast cancer in women 80 years of age and older. Crit Rev Oncol Hematol. 2011;79(3):315–20. [DOI] [PubMed] [Google Scholar]
- 4.Owusu C, Buist DS, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26(4):549–55. [DOI] [PubMed] [Google Scholar]
- 5.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26(4):556–62. [DOI] [PubMed] [Google Scholar]
- 6.Ojala K, Meretoja TJ, Mattson J, Leidenius MHK. Surgical treatment and prognosis of breast cancer in elderly—a population-based study. Eur J Surg Oncol. 2019;45(6):956–62. [DOI] [PubMed] [Google Scholar]
- 7.Derks MG, Bastiaannet E, van de Water W, et al. Impact of age on breast cancer mortality and competing causes of death at 10 years follow-up in the adjuvant TEAM trial. Eur J Cancer. 2018;99:1–8. [DOI] [PubMed] [Google Scholar]
- 8.Shumway DA, Griffith KA, Sabel MS, et al. Surgeon and radiation oncologist views on omission of adjuvant radiotherapy for older women with early-stage breast cancer. Ann Surg Oncol. 2017;24(12):3518–26. [DOI] [PubMed] [Google Scholar]
- 9.Katz SJ, Jagsi R, Morrow M. Reducing overtreatment of cancer with precision medicine: just what the doctor ordered. JAMA. 2018;319(11):1091–2. [DOI] [PubMed] [Google Scholar]
- 10.Katz SJ, Morrow M. Addressing overtreatment in breast cancer: the doctors’ dilemma. Cancer. 2013;119(20):3584–8. [DOI] [PubMed] [Google Scholar]
- 11.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92(14):1143–50. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41. [DOI] [PubMed] [Google Scholar]
- 13.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–32. [DOI] [PubMed] [Google Scholar]
- 14.Martelli G, Boracchi P, Ardoino I, et al. Axillary dissection versus no axillary dissection in older patients with T1N0 breast cancer: 15-year results of a randomized controlled trial. Ann Surg. 2012;256(6):920–4. [DOI] [PubMed] [Google Scholar]
- 15.Rudenstam CM, Zahrieh D, Forbes JF, et al. Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: first results of International Breast Cancer Study Group Trial 10–93. J Clin Oncol. 2006;24(3):337–44. [DOI] [PubMed] [Google Scholar]
- 16.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266–73. [DOI] [PubMed] [Google Scholar]
- 18.Kunkler IH, Williams LJ, Jack WJL, Cameron DA, Dixon JM. Breast-conserving surgery with or without irradiation in early breast cancer. N Engl J Med. 2023;388(7):585–94. [DOI] [PubMed] [Google Scholar]
- 19.NCI: SEER-Medicare data fact sheet. Available at https://healthcaredelivery.cancer.gov/seermedicare/overview/. Accessed 3 May 2019.
- 20.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castillo-Angeles M, Cooper Z, Jarman MP, Sturgeon D, Salim A, Havens JM. Association of frailty with morbidity and mortality in emergency general surgery by procedural risk level. JAMA Surg. 2021;156(1):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan A, Kuo YF, Goodwin JS. Predicting life expectancy for community-dwelling older adults from Medicare claims data. Am J Epidemiol. 2013;178(6):974–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–90. [DOI] [PubMed] [Google Scholar]
- 24.Choosing Wisely. Available at: https://abimfoundation.org/what-we-do/choosing-wisely. Accessed 17 Oct 2019.
- 25.National Comprehensive Cancer Network (NCCN) Guidelines. Breast Cancer. Version 2.2023 Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 22 Feb 2023.
- 26.Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 2012;13(4):e148–60. [DOI] [PubMed] [Google Scholar]
- 27.Minami CA, Bryan AF, Freedman RA, et al. Assessment of oncologists’ perspectives on omission of sentinel lymph node biopsy in women 70 years and older with early-stage hormone receptor-positive breast cancer. JAMA Netw Open. 2022;5(8):e2228524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raza S, Rudolph JL, Mujahid N, et al. Assessment of frailty and risk of chemotherapy toxicity at a geriatric-oncology multidisciplinary clinic. R I Med J. 2023;106(4):13–8. [PubMed] [Google Scholar]
- 30.Barthelemy P, Heitz D, Mathelin C, et al. Adjuvant chemotherapy in elderly patients with early breast cancer. Impact of age and comprehensive geriatric assessment on tumor board proposals. Crit Rev Oncol Hematol. 2011;79(2):196–204. [DOI] [PubMed] [Google Scholar]
- 31.Clough-Gorr KM, Stuck AE, Thwin SS, Silliman RA. Older breast cancer survivors: geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow-up. J Clin Oncol. 2010;28(3):380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orum M, Gregersen M, Jensen K, Meldgaard P, Damsgaard EMS. Frailty status but not age predicts complications in elderly cancer patients: a follow-up study. Acta Oncol. 2018;57(11):1458–66. [DOI] [PubMed] [Google Scholar]
- 33.Nishijima TF, Shimokawa M, Komoda M, et al. Survival in older Japanese adults with advanced cancer before and after implementation of a geriatric oncology service. JCO Oncol Pract. 2023. 10.1200/OP.22.00842. [DOI] [PubMed] [Google Scholar]
- 34.Ma M, Peters XD, Zhang LM, et al. Multi-site implementation of an American College of surgeons geriatric surgery quality improvement initiative. J Am Coll Surg. 2023;237(2):171–81. [DOI] [PubMed] [Google Scholar]
- 35.Sharon CE, Strohl C, Saur NM. Frailty assessment and prehabilitation as part of a perioperative evaluation and planning (PREP) program for patients undergoing colorectal surgery. Clin Colon Rectal Surg. 2023;36(3):184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deeb AL, Garrity M, Cooper L, Frain LN, Jaklitsch MT, DuMontier C. Implementing 4-meter gait speed as a routine vital sign in a thoracic surgery clinic. J Geriatr Oncol. 2023;14(4):101481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minami CA, Cooper Z. The frailty syndrome: a critical issue in geriatric oncology. Crit Care Clin. 2021;37(1):151–74. [DOI] [PubMed] [Google Scholar]
- 38.Ballinger RS, Mayer KF, Lawrence G, Fallowfield L. Patients’ decision-making in a UK specialist centre with high mastectomy rates. Breast. 2008;17(6):574–9. [DOI] [PubMed] [Google Scholar]
- 39.Dicks E, Roome R, Chafe J, et al. Factors influencing surgical treatment decisions for breast cancer: a qualitative exploration of surgeon and patient perspectives. Curr Oncol. 2019;26(2):e216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gollop SJ, Kyle SM, Fancourt MW, Gilkison WT, Mosquera DA. Why Taranaki women choose to have a mastectomy when suitable for breast conservation treatment. ANZ J Surg. 2009;79(9):604–9. [DOI] [PubMed] [Google Scholar]
- 41.Hamelinck VC, Bastiaannet E, Pieterse AH, et al. A prospective comparison of younger and older patients’ preferences for breast-conserving surgery versus mastectomy in early breast cancer. J Geriatr Oncol. 2018;9(2):170–3. [DOI] [PubMed] [Google Scholar]
- 42.Collins ED, Moore CP, Clay KF, et al. Can women with early-stage breast cancer make an informed decision for mastectomy? J Clin Oncol. 2009;27(4):519–25. [DOI] [PubMed] [Google Scholar]
- 43.Greenup RA, Rushing C, Fish L, et al. Financial costs and burden related to decisions for breast cancer surgery. J Oncol Pract. 2019;15(8):e666–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirby RM, Basit A, Manimaran N. Patient choice significantly affects mastectomy rates in the treatment of breast cancer. Int Semin Surg Oncol. 2008;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shinall MC Jr, Arya S, Youk A, et al. Association of preoperative patient frailty and operative stress with postoperative mortality. JAMA Surg. 2020;155(1):e194620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DuMontier C, Loh KP, Bain PA, et al. Defining undertreatment and overtreatment in older adults with cancer: a scoping literature review. J Clin Oncol. 2020;38(22):2558–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith BD, Jiang J, Shih YC, et al. Cost and complications of local therapies for early-stage breast cancer. J Natl Cancer Inst. 2016;109(1):djw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goyal S, Chandwani S, Haffty BG, Demissie K. Effect of travel distance and time to radiotherapy on likelihood of receiving mastectomy. Ann Surg Oncol. 2015;22(4):1095–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onega T, Cook A, Kirlin B, et al. The influence of travel time on breast cancer characteristics, receipt of primary therapy, and surveillance mammography. Breast Cancer Res Treat. 2011;129(1):269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bredbeck BC, Baskin AS, Wang T, et al. Incremental spending associated with low-value treatments in older women with breast cancer. Ann Surg Oncol. 2022;29(2):1051–9. [DOI] [PubMed] [Google Scholar]
- 51.Morgan JL, George J, Holmes G, et al. Breast cancer surgery in older women: outcomes of the Bridging Age Gap in Breast Cancer study. Br J Surg. 2020;107(11):1468–79. [DOI] [PubMed] [Google Scholar]
- 52.Sedrak MS, Freedman RA, Cohen HJ, et al. Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA Cancer J Clin. 2021;71(1):78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hind D, Wyld L, Beverley CB, Reed MW. Surgery versus primary endocrine therapy for operable primary breast cancer in elderly women (70 years plus). Cochrane Database Syst Rev. 2006;25(1):CD004272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
These data will not be shared as this dataset is widely used/publicly available.


