Abstract
Phagocyte myeloperoxidase (MPO) is believed to be particularly important in defense against candida infection. We reported earlier that monocytes, rich in MPO, killed Candida albicans at a significantly higher rate and extent than did monocyte-derived macrophages, known to lack MPO, and that C. albicans is less resistant to MPO-dependent oxidants than less pathogenic Candida species. We hypothesized, therefore, that the capacity of macrophages to kill C. albicans might be improved in the presence of MPO. In this study, we evaluated the ability of recombinant human MPO (rhMPO) to augment the killing of C. albicans by resident macrophages and macrophages activated by recombinant human granulocyte-macrophage colony-stimulating factor. Addition of rhMPO (concentration range, 0.8 to 6.4 U/ml) to suspensions of resident and activated macrophages and opsonized C. albicans resulted in concentration-dependent and significant increases in candida killing. This enhancement was particularly pronounced with activated macrophages, whether C. albicans was opsonized or unopsonized and ingested through the macrophage mannose receptor. rhMPO did not affect the killing of C. albicans by monocytes, nor did it affect phagocytosis of opsonized or unopsonized C. albicans. These results indicate that exogenous rhMPO can augment the candidacidal capacity of both resident and activated macrophages, with a more profound effect on activated cells. We suggest that rhMPO may be effective in the treatment of invasive candidiasis.
In the phagocytosis-associated respiratory burst, phagocytic cells generate superoxide anion (O2−) and hydrogen peroxide (H2O2) by successive one-electron reductions of molecular oxygen (24). Two different pathways by which the microbicidal activity of H2O2 can be increased have been described. In the iron-mediated Fenton or Haber-Weiss reactions, H2O2 can react with iron or O2− to produce hydroxyl radical, one of the most reactive antimicrobial oxidants (5, 7, 13). H2O2 can also be used by myeloperoxidase (MPO), which catalyzes the peroxidation of chloride ion to form hypochlorous acid (HOCl), a potent microbicidal agent (9, 32). Reaction of HOCl with primary amines or other nitrogen-containing compounds results in the production of monochloramine, another powerful oxidizing agent generated by the MPO-H2O2-chloride system (6, 28).
MPO, a basic hemoprotein enzyme present in the primary granules of neutrophils and monocytes, plays a critical role in the fungicidal activity of these cells (12, 15). In vitro, phagocytes genetically deficient in MPO fail to kill Candida albicans, and patients with hereditary MPO deficiency have an increased susceptibility to invasive C. albicans infections (14, 20, 21, 31).
We found earlier that monocytes rich in MPO killed C. albicans to a significantly higher degree than did macrophages known to lack MPO, and that monocytes and MPO-dependent oxidants killed four less-pathogenic Candida species more effectively than they killed C. albicans (15). We have subsequently evaluated the ability of recombinant human MPO (rhMPO) to augment the candidacidal activity of resident and recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF)-treated macrophages. We report here that rhMPO increased the candidacidal capacity of macrophages, with a more profound effect on rhGM-CSF-activated cells.
MATERIALS AND METHODS
Monocytes.
Mixed mononuclear cells were isolated from heparinized (10 U/ml) venous blood of healthy adults with a gradient of lymphocyte separation medium (Organon Teknika, Durham, N.C.) (16). After centrifugation and washes in Krebs-Ringer phosphate buffer containing 0.2% glucose (KRPD; pH 7.34), the cell suspension contained monocytes, lymphocytes, and <0.2% contaminating granulocytes as determined by morphology and esterase staining done with an α-naphthyl acetate esterase diagnostic kit (Sigma Chemical, St. Louis, Mo.). Viability of monocytes before the experiments was >97% (trypan blue exclusion). The percentage of monocytes in fresh suspensions was between 18 and 32 as determined by Giemsa and esterase stainings.
Macrophages.
The washed suspension of mononuclear cells was resuspended in Dulbecco modified Eagle medium (Gibco, Grand Island, N.Y.) with 2 mM l-glutamine supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% heat-inactivated autologous serum and adjusted to a final concentration of 2.5 × 106 cells/ml (18). The cells were incubated in Teflon beakers (Savillex, Minnetonka, Minn.) at 37°C and 5% CO2 for 5 days. Viability of cultured cells remained >97%. The percentage of macrophages in cultured suspensions was between 14 and 24. Monocytes cultured for 5 days are referred to here as monocyte-derived macrophages or macrophages.
Treatment of macrophages with rhGM-CSF or rhIL-4.
rhGM-CSF (lot 89810, 5.9 × 106 U/mg) was generously provided by Ekke Liehl, Sandoz Forschungsinstitut, Vienna, Austria. Recombinant human interleukin-4 (rhIL-4) was purchased from Genzyme (Cambridge, Mass.; product code 2181-01). At concentrations indicated, rhGM-CSF or rhIL-4 was added to macrophages after 72 h of culture, and treatment was performed for an additional 48 h. Equivalent amounts of Dulbecco modified Eagle medium were added as a control.
MPO.
rhMPO was produced in transfected and amplified Chinese hamster ovary cells (19). The recombinant product was purified by ion-exchange chromatography. Briefly, the spent culture medium was passed through a Q-Sepharose fast flow column equilibrated with 20 mM phosphate buffer. The flowthrough fraction from the Q-Sepharose column was directly loaded onto a CM-Sepharose fast flow column at pH 7.5, and the rhMPO was eluted with a linear NaCl gradient (0 to 500 mM) in the same buffer. All of the peroxidase activity was eluted with a linear NaCl gradient (0 to 500 mM) in the same buffer. All of the peroxidase activity was eluted in one peak at 300 mM NaCl. Specific activity measurements and cytotoxicity in the presence of H2O2 and chloride ion were tested as described previously (19). Specific peroxidase activity of the rhMPO preparation used in this study was 143 U/mg, using o-phenylenediamine as the substrate (10). The rhMPO preparation was tested for endotoxin contamination by using the chromogenic Limulus amoebocyte lysate test (BioWhittaker, Walkersville, Md.). The rhMPO used in all studies contained less than 0.15 endotoxin units/mg.
C. albicans.
Stock cultures of C. albicans (ATCC 18804) were maintained on Sabouraud’s dextrose agar (Becton Dickinson, Cockeysville, Md.) at 4°C and transferred once a month by culturing overnight at 30°C (17). To prepare stationary-growth-phase yeast cells, C. albicans was inoculated into 100 ml of Sabouraud’s 2% dextrose broth (Difco Laboratories, Detroit, Mich.) and cultured for 2 to 3 days at 30°C with continuous rocking. The viability of C. albicans remained >97% as determined by the exclusion of 0.01% methylene blue (Fisher Scientific, Pittsburgh, Pa.).
MPO activity of mononuclear phagocytes.
The release of MPO from monocytes and macrophages was determined by incubation of 5 × 106 cells per ml and 5 × 106 candida yeast cells per ml at 37°C in the presence of 2.5% normal serum. After 60 min of incubation, aliquots of the mixture were removed and centrifuged at 1,200 × g, and MPO activity was determined in supernatants as described previously (2, 15). Release of MPO was expressed as the percentage of the total enzyme activity of Triton X-100-treated homogenates. The percentages of candida-induced MPO release by monocytes and macrophages after 60 min of incubation at 37°C were 25 ± 6 and 0.4 ± 0.3, respectively (means ± standard errors of the means [SEM]; n = 4 for both). In addition, macrophages were devoid of MPO activity by cytochemical staining (8). These results are consistent with the lack of MPO activity in monocyte-derived macrophages used in our assay system.
Preopsonization of C. albicans.
Normal pooled serum was prepared from five healthy adults and was stored in aliquots at −70°C. Preopsonization was performed by incubation of 5 × 106 candida cells/ml in the presence of 5% serum for 30 min at 37°C under rotation (4 rpm), followed by centrifugation and washes in KRPD at 4°C (16).
Killing assay.
Equal volumes of preopsonized candida suspension (107/ml) and mononuclear phagocytic cell suspension (concentration, 107/ml) were mixed in sterile polypropylene tubes and incubated at 37°C under rotation (4 rpm) (15). At various time points, 0.1-ml aliquots of the incubation mixture were removed and diluted in 0.9 ml of ice-cold water. Phagocytic cells were disrupted by freezing in liquid nitrogen and thawing in a water bath (37°C). This treatment did not influence viability of C. albicans as checked by methylene blue staining and colony count. The percentage of yeast cells that had been killed was determined by colony counting (15). Before serial dilutions of the candida suspensions were made for plating, cells were vortexed vigorously for 10 s.
Measurement of O2− release.
The release of O2− from macrophages was quantitated as the superoxide dismutase-inhibitable reduction of ferricytochrome c (type III; Sigma) (17). Preopsonized candida cells (5 × 106) were added to macrophages (5 × 106) in KRPD buffer with 80 μM cytochrome c, with or without 50 μg of superoxide dismutase per ml (17). Reaction volume was 1.5 ml. Incubation was at 37°C with rotation (4 rpm).
Expression of data.
Results are expressed as means ± SEM; n refers to the number of experiments, each done in duplicate or triplicate. Statistical significance was determined by Student’s t test.
RESULTS
Effect of rhMPO on killing of C. albicans by resident macrophages.
We incubated macrophages and serum-opsonized C. albicans in the presence or absence of rhMPO and studied macrophage candidacidal activity (Table 1). Preincubation of rhMPO with macrophages or addition of rhMPO to the phagocytic mixture at the time of initiation of incubation resulted in a dose-related and significant increase in the killing of C. albicans, a plateau being achieved at a concentration of 3.2 U of rhMPO per ml. Equivalent results were achieved by preincubating macrophages with rhMPO for 30 min and then centrifuging and washing the cells before adding C. albicans (Table 1). These data suggest that the rhMPO effect was achieved primarily through binding to and internalization by macrophages, as previously shown for a U937 macrophage-like cell line that lacks mannose receptor (29). (rhMPO binds specifically to these cells with a Kd of 2.2 × 10−7 M, the number of exposed binding sites per cell being 340,000 [29]. rhMPO accumulates intracellularly, and the intracellular content remains constant, with 2.6 ± 0.5 pmol/mg of cell proteins becoming pronase resistant [29].)
TABLE 1.
Effect of rhMPO on killing of C. albicans by resident macrophages
| Addition of rhMPOa | rhMPO concn (U/ml) | C. albicans killedb (% of inoculum) |
|---|---|---|
| None | 20 ± 6 | |
| Concomitant | 0.8 | 22 ± 6 |
| 1.6 | 28 ± 9 | |
| 3.2 | 36 ± 6* | |
| Preincubation | 3.2 | 32 ± 7* |
| Concomitant | 6.4 | 35 ± 8* |
| Preincubation | 6.4 | 33 ± 7* |
In concomitant conditions, MPO was added immediately before addition of C. albicans. In other experiments, as indicated, macrophages were preincubated with the concentration of rhMPO shown for 30 min at 37°C under rotation (4 rpm) and then centrifuged and washed twice with buffer.
Incubation of macrophages (5 × 106/ml) and preopsonized C. albicans (5 × 106/ml) was performed under slow rotation (4 rpm) for 60 min. Data represent means ± SEM (n > 5). *, P < 0.05 compared with buffer control.
rhMPO had no effect on phagocytosis of preopsonized C. albicans by macrophages (81% ± 8 and 78% ± 9% ingestion of C. albicans in the presence and absence of 3.2 U of rhMPO per ml, respectively, after 60 min; n = 4). rhMPO itself did not exert a stimulatory effect on O2− release by macrophages. Addition of rhMPO to macrophages did not initiate O2− release over an incubation period of 60 min (5 ± 4 and 4 ± 2 nmol of O2− released by macrophages in the absence and presence, respectively, of 3.2 U of rhMPO per ml). When catalase (0.2 μM; Sigma) was present in the reaction mixture, the amount of O2− released by macrophages remained negligible (data not shown).
Effects of rhGM-CSF treatment on metabolic and functional activities of macrophages.
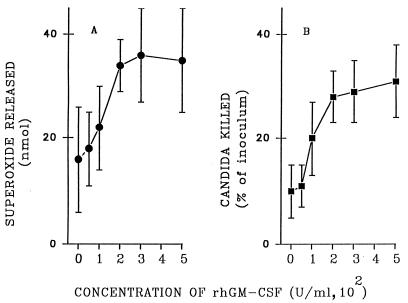
To study the effect of MPO with activated macrophages, we exposed monocytes cultured for 3 days to rhGM-CSF for an additional 2 days. In Fig. 1, the extents of O2− release (Fig. 1A) and candidacidal activity (Fig. 1B) by macrophages stimulated with preopsonized C. albicans are shown as functions of the concentration of rhGM-CSF present during preincubation for 48 h. Maximal augmentation of both activities was achieved with 200 U of rhGM-CSF per ml, with minimal or no further increase being achieved by treatment of macrophages with 300 or 500 U of rhGM-CSF per ml.
FIG. 1.
Effect of rhGM-CSF on the candida-stimulated release of O2− and killing of C. albicans by macrophages. After 72 h in culture, macrophages were incubated for 48 h, with medium alone or medium containing increasing concentrations of rhGM-CSF, as shown on the horizontal axis. For the superoxide assay (A), macrophages were incubated for 60 min with an equal number of preopsonized (5% serum) candida cells. Killing of candida cells (B) was measured in suspensions of macrophages and C. albicans (5 × 106/ml for each) after 60 min of incubation. Data represent means ± SEM (n = 6).
Effect of rhMPO on killing of C. albicans by rhGM-CSF-treated macrophages.
We used 200 U of rhGM-CSF per ml, which achieved maximal activation, to study the effect of rhMPO on C. albicans killing by activated cells. Macrophages were incubated with preopsonized C. albicans in the presence of various concentrations of rhMPO (Table 2). In these experiments, rhMPO was added before C. albicans at the beginning of the incubation. In the absence of rhMPO, killing of C. albicans by rhGM-CSF-treated macrophages was significantly higher than that by resident cells (P < 0.05).
TABLE 2.
Effect of rhMPO on killing of C. albicans by rhGM-CSF-treated macrophages
| Treatment of macrophagesa | rhMPO concn (U/ml) | C. albicans killedb (% of inoculum) |
|---|---|---|
| None | 17 ± 8 | |
| rhGM-CSF | 29 ± 5 | |
| 0.4 | 33 ± 7 | |
| 0.8 | 44 ± 6* | |
| 1.6 | 54 ± 4** | |
| 3.2 | 59 ± 7** | |
| 6.4 | 57 ± 6** |
After 72 h in culture, macrophages were preincubated with buffer or 200 U of rhGM-CSF per ml for another 48 h.
Incubation of macrophages (5 × 106/ml) and preopsonized C. albicans (5 × 106/ml) was performed under slow rotation (4 rpm) for 60 min. Data represent means ± SEM (n = 6). Statistical significance was determined by comparing the killing of C. albicans by rhGM-CSF-treated macrophages in the absence and in the presence of rhMPO. *, P < 0.05; **, P < 0.01.
The effect of rhMPO on the capacity of rhGM-CSF-activated macrophages to kill C. albicans was dose dependent, and the presence of 0.8 U or more of rhMPO per ml resulted in significant increases in the killing capacity of macrophages (Table 2).
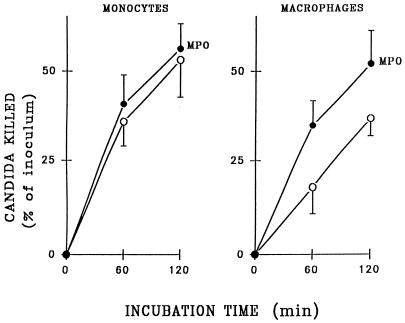
We also compared the effects of rhMPO on the candidacidal activity of monocytes, which contain MPO, and macrophages, which do not. As shown in Fig. 2, the killing capacity of monocytes was not affected by addition of 3.2 U of rhMPO per ml. In contrast, macrophages killed C. albicans at a significantly higher rate and extent in the presence of 3.2 U of rhMPO per ml under the same experimental conditions (P < 0.01 after both 60 and 120 min of incubation).
FIG. 2.
Effect of rhMPO on killing of C. albicans by mononuclear phagocytes. Killing of 5 × 106 preopsonized C. albicans cells was measured in suspensions of monocytes or resident macrophages (5 × 106/ml for each). Results are compared for phagocytes treated with 3.2 U of rhMPO (filled circles) per ml or untreated (open circles). Data represent means ± SEM (n = 6).
Effect of rhMPO on killing of C. albicans ingested through the mannose receptor.
Macrophages ingest unopsonized C. albicans primarily if not exclusively through the macrophage mannose receptor (16, 17). In contrast to phagocytosis of opsonized C. albicans by nonactivated macrophages, ingestion of unopsonized yeasts initiates much less release of superoxide by these cells (15). Therefore, it was of interest to evaluate whether rhMPO could augment resident and rhGM-CSF-activated macrophage candidacidal activity through the mannose receptor in an opsonin-free assay system.
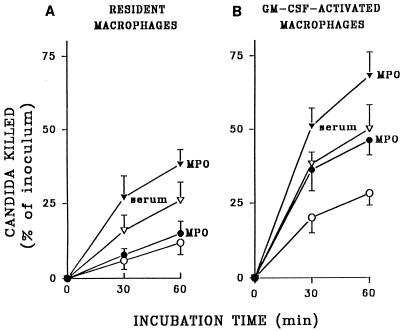
As shown in Fig. 3A, no augmentation of killing of unopsonized fungi by resident macrophages could be achieved by addition of 3.2 U of rhMPO per ml. Macrophages activated with 200 U of rhGM-CSF per ml killed two to three times more unopsonized C. albicans than did untreated cells at the two time points studied (Fig. 3B). Contrary to results with resident cells, the addition of rhMPO resulted in significant (P < 0.01) increases in killing of unopsonized C. albicans by activated macrophages (Fig. 3B). The rate and degree of killing of opsonized candida by both resident and activated macrophages were significantly increased by addition of rhMPO (Fig. 3; P < 0.05).
FIG. 3.
Killing of serum-opsonized (triangles) and unopsonized (circles) C. albicans by macrophages with 3.2 U of rhMPO per ml (filled symbols) or an equivalent volume of buffer (open symbols). Results are compared for macrophages treated with rhGM-CSF (200 U/ml) (B) or untreated (A). Data represent means ± SEM (n = 5).
Effect of rhMPO on phagocytosis of unopsonized C. albicans by resident, rhIL-4-treated, and rhGM-CSF-activated macrophages.
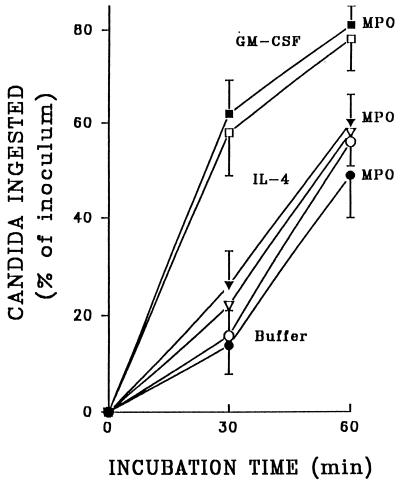
We explored the possibility that rhMPO can interfere with phagocytosis of C. albicans through the macrophage mannose receptor, since this receptor can bind neutrophil-derived MPO (25, 26). As shown in Fig. 4, there was no inhibition of phagocytosis when rhMPO was present throughout the 60-min incubation of unopsonized C. albicans with resident or rhGM-CSF-activated macrophages or macrophages treated with rhIL-4, which has been shown to increase the expression of mannose receptors on mouse peritoneal macrophages (27) as well as on monocyte-derived macrophages from humans (4, 14a). The slight but insignificant increase in the extent of phagocytosis achieved with 2,000 pg of rhIL-4 per ml compared with buffer (Fig. 4) was not further increased by treatment of cells with 3,000 or 5,000 pg of this cytokine per ml (n = 4 [not shown]).
FIG. 4.
Phagocytosis of unopsonized C. albicans by resident macrophages (circles) and macrophages that were treated with rhIL-4 (2,000 pg/ml; triangles) or rhGM-CSF (200 U/ml; squares). Results are compared for macrophages in the presence of rhMPO (3.2 U/ml; filled symbols) or in the absence of MPO (open symbols). Data represent means ± SEM (n = 5).
In contrast to the effect of rhMPO, purified mannan exerted an inhibitory effect on the uptake of unopsonized C. albicans by rhGM-CSF-treated macrophages (84% ± 9% and 32% ± 10% ingestion of C. albicans in the absence and presence, respectively, of 5 mg of Saccharomyces cerevisiae mannan per ml [16, 17] [60-min incubation, n = 4]). Similar inhibition of ingestion of unopsonized candida by resident and rhIL-4-treated macrophages could be detected in the presence of 5 mg of mannan per ml (data not shown).
DISCUSSION
The prognosis of severe candidal infections in the immunocompromised host remains poor, and improved treatment is needed (1, 3). Previous studies have reported that macrophage-activating agents, including gamma interferon and colony-stimulating factors, can improve the ability of macrophages to kill C. albicans in vitro (17, 18, 30). rhMPO appears to be another human protein with the ability to augment the candidacidal function of macrophages, phagocytic cells that lack MPO.
In this study, we used C. albicans, the most common fungal pathogen (1, 3, 22) and the most common microorganism isolated from blood cultures from four university-affiliated hospitals (22), to investigate the effect of rhMPO on the fungicidal capacity of human macrophages. C. albicans provides an ideal phagocytic target for such study by virtue of its requirement for MPO to be efficiently killed by phagocytic cells (15, 20) and the role of macrophages in clearing blood-borne infection (23).
The results of these studies indicate that exogenous rhMPO can modulate macrophage candidacidal function in vitro. rhMPO had no effect on the respiratory burst itself (O2− release) or on phagocytosis and presumably enhanced C. albicans killing by increasing HOCl and monochloramine formation by macrophages. The extent of the rhMPO enhancement of C. albicans killing was higher in association with activated macrophages, which have an accelerated respiratory burst, than with resident cells, and this effect occurred at lower concentrations of rhMPO. These findings support the concept that human macrophage candidacidal activity depends on products of oxidative metabolism (13, 15, 17, 18).
Modulation of macrophage candidacidal function by MPO might occur in vivo at sites of inflammation. Experimental data suggest that granulocyte-derived MPO can be taken up by macrophages at a site of infection, resulting in augmentation of macrophage-mediated cytotoxicity (11). However, in patients with congenital neutropenia or secondary neutropenia with depletion of granulocyte reserves in the bone marrow due to hematologic disease or cytotoxic therapy, granulocyte-derived MPO could not be sufficiently provided locally. Under these circumstances, exogenous MPO may be beneficial to enhance nonspecific defense against Candida species, which are particularly troublesome pathogens in patients with neutropenia (3).
We reported earlier that phagocytosis of unopsonized candida by human macrophages occurred primarily through the mannose receptor (16) and that macrophage activation by gamma interferon resulted in greatly increased uptake and killing of C. albicans in spite of 80% downregulation of mannose receptor numbers (17). Data presented in this report indicate that macrophage activation by rhGM-CSF also enhances the functional activity of the macrophage mannose receptor. That ingestion of candida cells by activated macrophages was mediated primarily by the mannose receptor was shown here by inhibition of uptake of C. albicans by yeast mannan.
These results reveal that rhMPO augments the killing of C. albicans when yeast cells are ingested by activated macrophages. However, the presence of rhMPO in the reaction mixture had no effect on the mannose receptor-mediated phagocytosis of C. albicans (Fig. 4). These data suggest that although the mannose receptor may be involved, it is not the only way for MPO to enter macrophages. Pertinent to this point, we reported recently that rhMPO binds to and is internalized by the macrophage-like U937 cells, which are deficient in mannose receptors, with a Kd of 2.2 × 10−7 M, and that the binding property of rhMPO is governed by its highly cationic nature (29).
The results of the studies reported here clearly suggest that exogenous rhMPO augments the candidacidal capacity of both resident and cytokine-activated macrophages in vitro. Recent animal studies have shown partial protection against rickettsiosis of mice treated with rhMPO (29). Administration of exogenous rhMPO to mice infected with Cowdria ruminantium increased survival significantly, and rhMPO conjugated with the Fc domain of human immunoglobulin G1 was more effective than rhMPO alone (29). These data suggest that the effect of rhMPO shown here in vitro might also be achieved by in vivo administration. To avoid the interaction of rhMPO with any strongly negatively charged surface, the delivery of rhMPO should be targeted to macrophages by using liposomes, microparticles, or nanoparticles. Taken together, these encouraging in vitro observations and animal studies strongly support the need for further research into the possible clinical application of rhMPO, particularly for the treatment of neutropenic patients with disseminated candidiasis.
ACKNOWLEDGMENTS
This work was supported by grants from the European Commission (PECO no. CIPD CT 940303), the Hungarian Research Fund (OTKA 17100), and the Hungarian Ministry of Welfare (ETT 340/96) to L. Maródi, by the March of Dimes Birth Defects Foundation and a grant from the National Institutes of Health (AI 24782) to R. B. Johnston, Jr., and by a grant from the Belgian National Fund for Scientific Research (FNRS 1.5.020.97F) to N. Moguilevsky.
We thank J. A. Mahoney and S. Gordon for helpful discussions.
REFERENCES
- 1.Beck-Sague C M, Jarvis W R. National Nosocomial Infections Surveillance System: secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 2.Bentwood B J, Henson P M. The sequential release of granule constituents from human neutrophils. J Immunol. 1980;124:855–859. [PubMed] [Google Scholar]
- 3.Bodey G P. Disseminated candidiasis in neutropenic patients. J Infect Dis. 1997;1:S2–S11. [Google Scholar]
- 4.deFife K M, Jenney C R, McNally A K, Colton E, Anderson J M. Interleukin-13 induces human monocyte/macrophage fusion and macrophage mannose receptor expression. J Immunol. 1997;158:3385–3390. [PubMed] [Google Scholar]
- 5.Fridovich I. The biology of oxygen radicals: the superoxide radical is an agent of oxygen toxicity; superoxide dismutases provide an important defense. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 6.Grisham M B, Jefferson M M, Melton D M, Thomas E L. Chlorination of endogenous amines by isolated neutrophils: ammonia-dependent bactericidal, cytotoxic, and cytolytic activities of the chloramines. J Biol Chem. 1984;259:10404–10413. [PubMed] [Google Scholar]
- 7.Johnston R B, Jr, Keele B B, Jr, Misra H P, Lehmeyer J E, Webb L S, Baehner R L, Rajagopalan K V. The role of superoxide anion generation in phagocytic bactericidal activity: studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975;55:1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplow L S. Simplified myeloperoxidase stain using benzidine dihydrochloride. Blood. 1965;26:215–219. [PubMed] [Google Scholar]
- 9.Klebanoff S J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980;93:480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- 10.Krawisz J E, Sharon P, Stenson W F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- 11.Lefkowitz S S, Gelderman M P, Lefkowitz D L, Moguilevsky N, Bollen A. Phagocytosis and intracellular killing of Candida albicans by macrophages exposed to myeloperoxidase. J Infect Dis. 1996;173:1202–1207. doi: 10.1093/infdis/173.5.1202. [DOI] [PubMed] [Google Scholar]
- 12.Lehrer R I. Antifungal effects of peroxidase systems. J Bacteriol. 1969;99:361–378. doi: 10.1128/jb.99.2.361-365.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitz S M, Diamond R D. Killing of Aspergillus fumigatus spores and Candida albicans yeast phase by the iron-hydrogen peroxide-iodide cytotoxic system: comparison with the myeloperoxidase-hydrogen peroxide-halide system. Infect Immun. 1984;43:1100–1102. doi: 10.1128/iai.43.3.1100-1102.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludviksson B R, Thorarensen O, Gudnason T, Halldorsson S. Candida albicans meningitis in a child with myeloperoxidase deficiency. Pediatr Infect Dis J. 1993;12:162–164. doi: 10.1097/00006454-199302000-00015. [DOI] [PubMed] [Google Scholar]
- 14a.Mahoney, J. A., and S. Gordon. Personal communication.
- 15.Maródi L, Forehand J R, Johnston R B., Jr Mechanisms of host defense against Candida species. II. Biochemical basis for killing of Candida by mononuclear phagocytes. J Immunol. 1991;146:2790–2794. [PubMed] [Google Scholar]
- 16.Maródi L, Korchak H M, Johnston R B., Jr Mechanisms of host defense against Candida species. I. Phagocytosis by monocytes and monocyte-derived macrophages. J Immunol. 1991;146:2783–2789. [PubMed] [Google Scholar]
- 17.Maródi L, Schreiber S, Anderson D, MacDermott R P, Korchak H M, Johnston R B., Jr Enhancement of macrophage candidacidal activity by IFN-γ: increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. J Clin Invest. 1993;91:2596–2601. doi: 10.1172/JCI116498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maródi L, Káposzta R, Campbell D E, Polin R A, Csongor J, Johnston R B., Jr Candidacidal mechanisms in the human neonate: impaired IFN-γ activation in newborn infants. J Immunol. 1994;153:5643–5649. [PubMed] [Google Scholar]
- 19.Moguilevsky N, Garcia-Quintana L, Jacquet A, Tournay C, Fabry L, Pirard R, Bollen A. Structural and biological properties of human recombinant myeloperoxidase produced by Chinese hamster ovary cell lines. Eur J Biochem. 1991;197:605–614. doi: 10.1111/j.1432-1033.1991.tb15950.x. [DOI] [PubMed] [Google Scholar]
- 20.Nauseef W M. Myeloperoxidase deficiency. Hematol Oncol Clin North Am. 1988;2:135–147. [PubMed] [Google Scholar]
- 21.Okuda T, Yasuoka T, Oka N. Myeloperoxidase deficiency as a predisposing factor for deep mucocutaneous candidiasis: a case report. J Oral Maxillofac Surg. 1991;49:183–186. doi: 10.1016/0278-2391(91)90108-x. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller M A. Epidemiology and control of fungal infections. Clin Infect Dis. 1994;19:S8–S13. doi: 10.1093/clinids/19.supplement_1.s8. [DOI] [PubMed] [Google Scholar]
- 23.Quian Q, Jutila M A, Rooijen N V, Cutler J E. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J Immunol. 1994;152:5000–5008. [PubMed] [Google Scholar]
- 24.Segal A W, Abo A. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem Sci. 1993;18:43–47. doi: 10.1016/0968-0004(93)90051-n. [DOI] [PubMed] [Google Scholar]
- 25.Shepherd V L, Hoidal J R. Clearance of neutrophil-derived myeloperoxidase by the macrophage mannose receptor. Am J Respir Cell Mol Biol. 1990;2:335–340. doi: 10.1165/ajrcmb/2.4.335. [DOI] [PubMed] [Google Scholar]
- 26.Stahl P D, Rodman J S, Miller M J, Schlesinger P H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by macrophages. Proc Natl Acad Sci USA. 1978;75:1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas E L. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia coli. Infect Immun. 1979;23:522–531. doi: 10.1128/iai.23.2.522-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tournay C, Courtoy P J, Maródi L, Totté P, Werenne J, Jacquet A, Garcia-Quintana L, Bollen A, Moguilevsky N. Uptake of recombinant myeloperoxidase, free or fused to Fcγ, by macrophages enhances killing activity toward microorganisms. DNA Cell Biol. 1996;15:617–624. doi: 10.1089/dna.1996.15.617. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Friedman H, Djeu J Y. Enhancement of human monocyte function against Candida albicans by the colony-stimulating factors (CSF): IL-3, granulocyte-macrophage CSF, and macrophage CSF. J Immunol. 1989;143:671–677. [PubMed] [Google Scholar]
- 31.Weber M I, Abela A, Repintigny L, Garel L, Lapointe N. Myeloperoxidase deficiency with extensive candidal osteomyelitis of the base of the skull. Pediatrics. 1987;80:876–879. [PubMed] [Google Scholar]
- 32.Weiss S J, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils: evidence for hypochlorous acid generation. J Clin Invest. 1982;70:598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]