Abstract
Purpose
To investigate the value of apolipoprotein A1 (ApoA1), apolipoprotein B (ApoB), and ApoA1/B ratio in pathogenic diagnosis of chronic obstructive pulmonary disease (COPD) complicated by acute lower respiratory tract infection, assisting comprehensive disease assessment.
Patients and Methods
The study enrolled 171 COPD patients with acute lower respiratory tract infections, 35 COPD patients without acute lower respiratory tract infections, and 41 healthy controls. Correlation analysis and binary logistic regression were used to assess the roles of various factors in COPD with acute lower respiratory tract infections. Receiver operating characteristic (ROC) curves were plotted and area under curves (AUC) values were calculated to evaluate the predictive performance.
Results
Infections were the cause of alterations in ApoA1, ApoB and ApoA1/B index. In correlation analysis for pathogenic diagnosis of COPD complicated by acute lower respiratory infections, age, ApoA1, ApoA1/B ratio, lymphocyte count (LYMPH), neutrophil count (NEUT), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and endotoxin were significantly correlated. For predicting COPD complicated by acute lower respiratory tract bacterial infection, ApoA1 had the highest area under the ROC curve (AUC: 0.889), with sensitivity and specificity of 82.9% and 83.9%, respectively. The combination of NEUT and ApoA1 improved the prediction efficacy (AUC: 0.909; sensitivity/specificity: 85.1%/85.7%).
Conclusion
ApoA1, ApoB, and ApoA1/B ratio are good indicators for predicting pathogens in COPD complicated by acute lower respiratory tract infection, especially ApoA1 which has high predictive value.
Keywords: ApoA1, ApoB, ApoA1/B, COPD, Acute lower respiratory tract infection, pathogens
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable and treatable disease characterized by chronic inflammation of the airways and lung parenchyma, structural changes in the airways, ciliary dysfunction, and lung tissue destruction.1 The overall prevalence of COPD in adults aged 30 years and older globally is approximately 13.8%,2 making it the third leading cause of mortality worldwide.3,4 Patients are susceptible to acute lower respiratory tract infections due to declining lung function and weakened immunity.5,6 Common bacterial infections include Streptococcus pneumoniae, Haemophilus influenzae, and Pseudomonas aeruginosa.7 Common viral infections are often caused by influenza viruses, adenoviruses, and pneumoviruses.8 Currently, the diagnosis of COPD with acute lower respiratory tract infection mainly relies on symptoms, signs, sputum examination, blood tests, imaging, and pathogen detection. However, COPD patients may have compromised immune systems and disrupted generation and release of inflammatory markers like neutrophils during infection, making it difficult to accurately assess disease severity, which poses certain limitations for early diagnosis and evaluating severity.9,10
In the past, apolipoprotein A1 (ApoA1) and apolipoprotein B (ApoB) have been extensively studied in cardiovascular, metabolic and other diseases. Disease risk is negatively correlated with ApoA1 levels and positively correlated with ApoB levels.11 Their ratio is considered an important indicator of cardiovascular disease risk.12 With advances in scientific research, apolipoproteins have been found to play important roles in immune regulation and inflammatory responses.13–15 In addition to being structural components of lipoproteins, apolipoproteins also serve as ligands for cell surface receptors and cofactors for enzymes.16 It has been discovered that membrane cholesterol in host cells plays a vital role during microbial invasion, and interactions between serum lipids like low-density lipoprotein cholesterol (LDL) and high-density lipoprotein cholesterol (HDL) and lipid rafts in host cell membranes can alter microbe-host cell interactions, leading to more severe diseases.17–19 ApoA1 is the major constituent of HDL, while ApoB is one of the apolipoproteins of chylomicrons, very low-density lipoproteins (VLDL) and LDL. They possess important functions including acute phase response, complement activation, immune response, inflammatory response, protease inhibition, etc.20,21 Previous studies have shown that ApoA1 and ApoB change after inflammatory reactions occur and correlate with disease severity.22 However, COPD is a heterogeneous chronic disease interacting with various risk factors like metabolic disorders. Current research on the roles of ApoA1 and ApoB in COPD remains insufficient and unsystematic, requiring more clinical studies to validate their diagnostic value. Therefore, this study aims to investigate the relationships between ApoA1, ApoB, ApoA1/B and COPD patients with and without acute lower respiratory tract infections of different pathogens through retrospective analysis, hoping to provide broader perspectives for the comprehensive assessment of COPD patients.
Materials and Methods
Study Design and Population
This was a retrospective, single-center study. The study population consisted of COPD patients admitted to the First Affiliated Hospital of Nanchang University from January 2021 to June 2023. Clinical data (including demographics, symptoms, laboratory tests, imaging, lung function, etc.) were comprehensively evaluated. Finally, 171 COPD patients with acute lower respiratory tract infections (viral infections: 42 cases, bacterial infections: 67 cases, combined bacterial and viral infections: 62 cases), 35 COPD patients without acute lower respiratory tract infections, and 41 healthy controls who underwent physical examinations in the same period and were diagnosed with no other diseases were included. Patients who met the criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 202323 and had complete clinical data were eligible. Patients aged <18 years or with conditions other than COPD that could affect ApoA1 and ApoB levels were excluded, such as combined with other pulmonary diseases, infections of other sites, metabolic diseases, hypertension, cardiovascular diseases, malignant tumors, hematological system diseases, autoimmune diseases, etc. Prior to study design, we understood that there were no significant statistical differences in laboratory test results between COPD patients with acute exacerbations caused by noninfectious factors and stable COPD patients. The main differences were in significantly worsened clinical symptoms. Therefore, our COPD patients without acute lower respiratory tract infections included both stable COPD patients and AECOPD caused by other noninfectious factors. This study was approved and permitted by the Ethics Committee of the First Affiliated Hospital of Nanchang University.
Clinical Parameters and Laboratory Results
The general clinical data of enrolled subjects included symptoms, age, gender, Body Mass Index (BMI), smoking history, etc. The recorded clinical indexes included lymphocyte count (LYMPH), neutrophil count (NEUT), ApoA1, ApoB, ApoA1/B, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), Procalcitonin (PCT), pathogenic respiratory microbes, echocardiography, chest CT imaging findings, and lung function. Blood samples for testing were collected within 24 hours of admission under fasting conditions. Pathogen diagnosis for acute lower respiratory tract infections was based on polymerase chain reaction (PCR) and bacterial culture of lower respiratory tract specimens obtained within 24 hours of admission, excluding the possibility of colonizers. Lung function tests were performed at the pulmonary function laboratory of The First Affiliated Hospital of Nanchang University. Standardized lung function testing procedures were utilized to ensure reliability of the collected data. Testing items included pulmonary function tests, bronchial provocation tests, and fractional exhaled nitric oxide measurements (FeNO). Results were calculated based on percent predicted values derived from subjects’ age, height, and BMI. For AECOPD patients enrolled in this study caused by infectious or non-infectious factors, lung function measurements were conducted after clinical stabilization to ensure accuracy of GOLD classification of lung function.
Reference ranges for related laboratory parameters: Normal reference ranges according to The First Affiliated Hospital of Nanchang University standards: NEUT: (1.8–6.3) x109/L, LYMPH: (1.1–3.2) x109/L, CRP: (0–8) mg/L, ESR: ≤15mm/h, PCT: (0–0.046) ng/mL, Endotoxin: (0–0.1) EU/mL, ApoA1: (1.07–1.77) g/L, ApoB: (0.60–1.38) g/L.
Statistical Analysis
IBM SPSS version 27.0 and GraphPad Prism version 9.00 were used for statistical analysis. Data were presented as number (n), percentage (%), and mean ± standard deviation (SD). Normally distributed measurement data were tested by Shapiro–Wilk test. One-way analysis of variance (One-way ANOVA) was used to examine differences in variables between different groups, followed by paired t-tests for comparisons between each two groups. Non-normally distributed measurement data were expressed as M (P25, P75). Comparisons between groups were performed using Wilcoxon Mann–Whitney test. Inter-group differences were tested by χ2 test or independent sample t-test. If severely skewed, data was log-transformed. P < 0.05 was considered statistically significant. Spearman correlation analysis and Chi-square tests were used to assess the relationships between acute lower respiratory tract infection in COPD and factors including ApoA1, ApoB. After adjusting for baseline confounding factors, binary Logistic regression analyses were conducted to evaluate the roles of various factors in COPD with acute lower respiratory tract infection. All P values were two-tailed, and P < 0.05 was considered statistically significant. Finally, receiver operating characteristic (ROC) curves were generated to evaluate the diagnostic value of these factors for COPD with acute lower respiratory tract infection. Areas under curves (AUC) were calculated, and optimal cut-off points with maximum sensitivity and specificity were selected as threshold values to validate diagnostic performance and predictive value.
Results
Characteristics of the Subjects
A total of 247 subjects were enrolled, including 41 healthy controls (17%), 35 COPD patients without acute lower respiratory tract infections (14%), and 171 COPD patients with acute lower respiratory tract infections. Among the COPD with infections group, there were 42 viral infections (17%), 67 bacterial infections (27%), and 62 mixed bacterial and viral infections (25%). The COPD complicated by acute lower respiratory tract infection group was characterized by higher lung function grading, higher prevalence of cor pulmonale, and more respiratory failure cases (Table 1).
Table 1.
General Clinical Characteristics of All Subjects
| Characteristics | Control (N=41) | COPD Without Acute Lower Respiratory Tract Infection (N=35) | COPD Complicated by Acute Lower Respiratory Tract Infection (N=171) | ||
|---|---|---|---|---|---|
| Viral (N=42) | Bacterial (N=67) | Viral + Bacterial (N=62) | |||
| Age (years) | 64.12±6.81 | 68.71±13.43 | 67.07±13.02 | 73.96±9.42 | 70.92±10.08 |
| Male | 29 (70.7%) | 21 (60.0%) | 31 (73.8%) | 56 (83.6%) | 48 (77.4%) |
| Smoker | 29 (70.7%) | 20 (57.1%) | 28 (66.7%) | 51 (76.1%) | 43 (69.4%) |
| BMI (kg/m2) | 21.22±1.68 | 20.36±2.82 | 20.09±3.18 | 19.73±3.41 | 20.08±3.81 |
| GOLD classification | |||||
| I–II | – | 19 (54.3%) | 17 (40.5%) | 26 (38.8%) | 23 (37.1%) |
| III–IV | – | 16 (45.7%) | 25 (59.5%) | 41 (61.2%) | 39 (62.9%) |
| Pulmonary hypertension | – | 20 (57.1%) | 23 (54.8%) | 43 (64.2%) | 41 (66.1%) |
| Cor pulmonale | – | 9(25.7%) | 14 (33.3%) | 26 (38.8%) | 26 (41.9%) |
| Respiratory failure | |||||
| Type I | – | 0(0%) | 3(7.1%) | 16 (23.9%) | 21 (33.9%) |
| Type II | – | 7(20%) | 8(19.0%) | 23 (34.3%) | 25 (40.3%) |
Note: Data are presented as mean ± standard deviation or n (%).
Abbreviation: BMI, Body Mass Index.
First, we compared the ApoA1, ApoB, and ApoA1/B parameters among the healthy control, COPD without acute lower respiratory tract infection, and COPD with acute lower respiratory tract infection groups. The results showed that there were no statistically significant differences between the healthy control group and the COPD without infection group (P>0.05). However, the COPD with infection group differed significantly compared to the other two groups (P<0.01) (Table 2).
Table 2.
Comparison of ApoA1, ApoB, and ApoA1/B Ratio Between Control Group and Experiment Group
| Indices | Control (N=41) | COPD Without Acute Lower Respiratory Tract Infection (N=35) | COPD Complicated by Acute Lower Respiratory Tract Infection (N=171) | Pa | Pb | Pc |
|---|---|---|---|---|---|---|
| ApoA1 (g/L) | 1.32±0.14 | 1.30±0.24 | 0.94±0.25 | 0.69 | *** | *** |
| ApoB (g/L) | 0.80±0.12 | 0.80±0.18 | 0.68±0.19 | 0.81 | *** | *** |
| ApoA1/ B | 1.61±0.32 | 1.67±0.43 | 1.47±0.50 | 0.66 | ** | ** |
Notes: Pa: Healthy controls vs COPD patients without acute lower respiratory tract infection; Pb: Healthy controls vs COPD patients with acute lower respiratory tract infection; Pc: COPD patients without acute lower respiratory tract infection vs COPD patients with acute lower respiratory tract infection. ***P<0.001; **P<0.01.
Abbreviations: ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoA1/ B, apolipoprotein A1/apolipoprotein B.
We found in Table 2 that infection was the cause of changes in ApoA1, ApoB, and ApoA1/B levels. Next, we compared the levels of various indexes between COPD groups with different pathogenic microorganisms causing the acute lower respiratory tract infections. The results showed that there were no statistically significant differences in ApoB between the viral infection group and the bacterial infection group (P>0.05). However, the differences in ApoA1, ApoA1/B, NEUT, LYMPH, CRP, ESR, and endotoxin were statistically significant (P<0.01). Comparisons between the viral infection group and the mixed viral-bacterial infection group showed statistically significant differences in all the studied indexes (P<0.05). There were no statistically significant differences in the comparisons between the bacterial infection group and the mixed viral-bacterial infection group (P>0.05) (Table 3).
Table 3.
Comparison of Indices Between Groups in COPD Patients with Acute Lower Respiratory Tract Infection
| Indices | Viral (N=42) | Bacterial (N=67) | Viral + Bacterial (N=62) | Pa | Pb | Pc |
|---|---|---|---|---|---|---|
| ApoA1 (g/L) | 1.14±0.21 | 0.89±0.21 | 0.84±0.23 | *** | *** | 0.14 |
| ApoB (g/L) | 0.73±0.20 | 0.68±0.19 | 0.64±0.17 | 0.19 | * | 0.33 |
| ApoA1/B | 1.68±0.52 | 1.41±0.47 | 1.38±0.48 | ** | ** | 0.60 |
| NEUT (*10^9/L) | 3.54 (3.00, 4.72) | 6.23 (4.46, 9.41) | 6.21 (4.37, 9.61) | *** | *** | 0.93 |
| LYMPH (*10^9/L) | 1.42 (0.86, 1.86) | 0.91 (0.69, 1.29) | 0.78 (0.56, 1.38) | *** | *** | 0.54 |
| CRP (mg/L) | 3.19 (1.11, 8.34) | 20.20 (4.05, 88.32) | 45.40 (9.24, 80.38) | *** | *** | 0.29 |
| ESR (mm/h) | 6.0 (4.75, 10.00) | 19.0 (9.0, 50.0) | 14.0 (5.0, 32.5) | *** | *** | 0.13 |
| Endotoxin (EU/mL) | 0.01 (0.01, 0.01) | 0.01 (0.01, 0.06) | 0.01 (0.01, 0.02) | *** | *** | 0.05 |
Notes: Pa: Viral group vs Bacterial group; Pb: Viral group vs Viral + Bacterial group; Pc: Bacterial group vs Viral + Bacterial group. ***P<0.001; **P<0.01; *P<0.05.
Abbreviations: ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoA1/ B, apolipoprotein A1/apolipoprotein B. LYMPH, lymphocyte; NEUT, neutrophil; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
Table 3 indicates that there were statistically significant differences in the indexes between the viral infection group and the bacterial infection group in our study data. Therefore, we performed correlation analysis. The results showed that gender, smoking history, BMI, and ApoB were not significantly correlated with pathogen diagnosis in COPD complicated by acute lower respiratory tract infections. However, age, ApoA1, ApoA1/B, LYMPH, NEUT, CRP, ESR, and endotoxin were significantly correlated with pathogen diagnosis. Among them, ApoA1, ApoA1/B, and LYMPH were negatively correlated, while NEUT, CRP, ESR, endotoxin, and age were positively correlated (Table 4).
Table 4.
Correlation of Various Factors with COPD Complicated by Acute Lower Respiratory Bacterial Infection
| Indices | Value | P |
|---|---|---|
| Gender | χ2=1.530 | 0.216 |
| Age (years) | r=0.238# | * |
| Smoker | χ2=1.156 | 0.282 |
| BMI(kg/m2) | r=−0.067 | 0.492 |
| NEUT (*10^9/L) | r=0.508## | *** |
| LYMPH (*10^9/L) | r=−0.346## | *** |
| CRP (mg/L) | r=0.484## | *** |
| ESR (mm/h) | r=0.446## | *** |
| Endotoxin (EU/mL) | r=0.385## | *** |
| ApoA1 (g/L) | r=−0.498## | *** |
| ApoB (g/L) | r=−0.086 | 0.374 |
| ApoA1/B | r=−0.255## | ** |
Notes: χ2: Chi-square value from Chi-square test; r: #Correlation is significant at the 0.05 level (two-tailed); ##Correlation is significant at the 0.01 level (two-tailed). ***P<0.001; **P<0.01; *P<0.05.
Abbreviations: BMI, Body Mass Index; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoA1/ B, apolipoprotein A1/apolipoprotein B; LYMPH, lymphocyte; NEUT, neutrophil; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
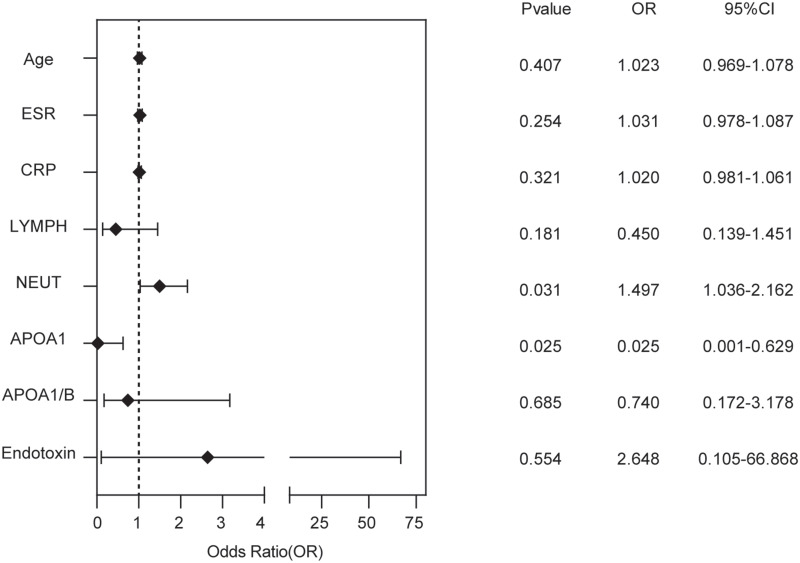
Next, we included the factors that were significantly correlated with pathogen diagnosis in COPD complicated by acute lower respiratory tract infections in a binary logistic regression analysis. The results showed that NEUT and APOA1 were independent predictive factors for pathogen diagnosis (Figure 1).
Figure 1.
Binary logistic regression analysis of COPD patients with acute lower respiratory tract infection.
Abbreviations: ApoA1, apolipoprotein A1; ApoA1/ B, apolipoprotein A1/apolipoprotein B; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; LYMPH, lymphocyte; NEUT, neutrophil.
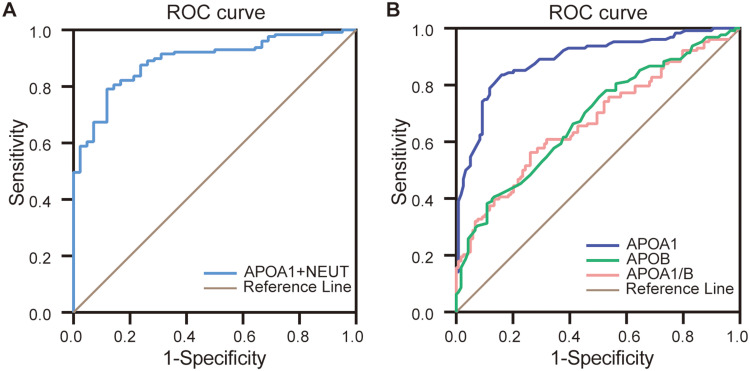
Based on Figure 1, we plotted the ROC curve for predicted probabilities, which showed that the AUC of NEUT and ApoA1 combined in diagnosing COPD complicated by acute lower respiratory bacterial infections was 0.909, with a sensitivity of 85.1% and specificity of 85.7% (Figure 2A). According to the serum levels of ApoA1, ApoB, and ApoA1/B, we plotted ROC curves for diagnosing COPD complicated by acute lower respiratory bacterial infections in the whole dataset (Figure 2B). The results showed that when the ApoA1 cutoff was 1.055 g/L, the AUC for diagnosing COPD complicated by acute lower respiratory bacterial infections was 0.889, with a sensitivity of 82.9% and specificity of 83.9%. When the ApoB cutoff was 0.585 g/L, the AUC was 0.677, with a sensitivity of 38% and specificity of 89%. When the ApoA1/B cutoff was 1.331 g/L, the AUC was 0.677, with a sensitivity of 56.6% and specificity of 74.6% for diagnosing COPD with acute lower respiratory bacterial infections.
Figure 2.
ROC curve of COPD complicated by acute lower respiratory tract bacterial infection. (A) ROC curve of NEUT and ApoA1 combined diagnosis of COPD with acute lower respiratory tract bacterial infection. (B) ROC curves of ApoA1, ApoB, and ApoA1/B respectively diagnosing COPD with acute lower respiratory tract bacterial infection in the whole data set.
Abbreviations: ApoA1, apolipoprotein A1; ApoA1/ B, apolipoprotein A1/apolipoprotein B; NEUT, neutrophil.
Discussion
COPD is a heterogeneous disease, and the pathogenesis is characterized by persistent respiratory symptoms and airflow limitation due to abnormalities involving the airway and lung parenchyma. The abnormal production and release of inflammatory mediators is one of the important factors leading to inflammatory responses and fibrosis in lung tissues.1 Our studies have shown that in recent years, it has been discovered that ApoA1, in addition to its basic role in regulating lipids, can also have an impact on the immune system, including anti-endotoxin, antibacterial, and regulatory effects on neutrophils.24 Currently, there is no evidence showing ApoB has direct immune regulatory functions, and the exact mechanisms still need further study. Previous studies have shown that ApoA1 and ApoA/B ratios may be slightly lower in patients than healthy people, while ApoB levels are relatively higher.25 However, our study found that there were no significant differences in ApoA1, ApoB, and ApoA1/B between COPD patients with and without acute lower respiratory tract infections and healthy controls. The inconsistent research results may be related to factors like sample size, patients’ clinical characteristics, and study design. Because the specific extent of these changes may differ depending on the patient’s condition, age, smoking history, other diseases, etc.
Our research found that ApoA1, ApoB, and ApoA1/B are predictive factors for COPD complicated by acute lower respiratory tract infections, with significant differences compared to the healthy group and the COPD without acute lower respiratory tract infection group. In the subgroup analysis of COPD complicated by acute lower respiratory tract infections, there was no statistically significant difference in the comparison of ApoB between the virus-only group and bacteria-only group, while the comparisons of ApoA1 and ApoA1/B showed significant differences. The comparisons of all three indicators between the virus-only group and mixed virus-bacteria group showed statistical significance, while there were no statistically significant differences in the three comparisons between the bacteria-only group and mixed virus-bacteria group. This is because during the acute phase of infection, ApoA1 is used to inhibit the phagocytosis, digestive function and active oxygen production of neutrophils, and influence the inflammatory response and apoptosis of neutrophils through multiple pathways, such as activating the AMPK pathway and regulating NF-κB, MAPK and other signaling pathways.26,27 During the acute infection period, the body will produce and release large amounts of cytokines, including tumor necrosis factor (TNF) and interleukin-6 (IL-6), etc. These high levels of cytokines can lead to liver damage, which in turn affects the synthesis of methyl pentanediol Acyl coenzyme A, and eventually results in decreased synthesis of ApoA1 and ApoB.28 In addition, COPD patients may also have lipid metabolism and gastrointestinal dysfunction, which can also be aggravated by eating to increase respiratory load and lead to decreased synthesis and release of VLDL and LDL due to diaphragm descent, and may also be due to the use of antibiotics and methylxanthines during treatment, leading to a low state of ApoB levels.1,29 In the comparison between the virus-only group and bacteria-only group, there was no statistical significance in ApoB, while there was statistical significance in the virus-only group versus the mixed virus-bacteria group. We can find the answer to this point in the subsequent correlation analysis between various factors and pathogen diagnosis prediction of COPD complicated by acute lower respiratory tract infection: no significant correlation was observed between ApoB and pathogen prediction. This may be related to the fact that this is a single center study with a small sample size, which may lead to statistical errors. In addition, due to the role of ApoB in detecting COPD complicated by acute lower respiratory tract infections, the diagnostic efficacy of the ApoA1/B ratio is reduced and does not have significant clinical implications like in diagnosing cardiovascular diseases.
Viral infections can affect the immune system and metabolism of the human body, and these effects may cause changes in indicators such as ApoA1, ApoB and ApoA1/B. After viral infection, the immune system releases inflammatory mediators such as cytokines and leukocytes to fight the virus. These inflammatory mediators may affect lipid metabolism and transport, leading to changes in ApoA1 and ApoB levels.30 These changes may vary in different populations and viral strains. Therefore, when interpreting the test results of indicators such as ApoA1, ApoB and ApoA1/B, comprehensive consideration should be given to the patient’s condition and related factors. In addition, in our study, the LYMPH results in COPD patients with acute lower respiratory tract infections were at a low level, more pronounced in bacterial infections, suggesting that the immune system function of these patients has been damaged, which can explain their susceptibility to bacterial infections.
In summary, this study shows that ApoA1, ApoB and ApoA1/B can serve as predictors of pathogen diagnosis for COPD complicated by acute lower respiratory tract infections, especially ApoA1 has the highest diagnostic value. It should be noted that in diagnostic applications, they still need to be comprehensively evaluated in combination with other indicators (such as inflammatory indicators, imaging examinations, etc.). In addition, it should be emphasized that currently there are no truly effective anti-endotoxin drugs, but ApoA1 is considered a drug with potential antibacterial and immunomodulatory effects.
Conclusion
ApoA1, ApoB, and ApoA1/B ratio are good indicators for predicting pathogens in COPD complicated by acute lower respiratory tract infection, especially ApoA1 which has high predictive value.
Funding Statement
This study was supported by Science and Technology Research Project of Education Department of Jiangxi Province (No.GJJ190021).
Ethics Approval and Consent to Participate
The study is in line with the Declaration of Helsinki. This study was approved and permitted by the Ethics Committee of The First Hospital of Nanchang City (No.KY2023044). It complies with ethical norms and laws and regulations to protect patients’ privacy and rights. The principle of strict confidentiality of data is adhered to, and there was no leakage of patients’ personal information during the research process. Due to the retrospective nature of the study, the requirement to obtain written informed consent was waived.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Labaki WW, Rosenberg SR. Chronic Obstructive Pulmonary Disease. Ann Intern Med. 2020;173(3):Itc17–itc32. doi: 10.7326/AITC202008040 [DOI] [PubMed] [Google Scholar]
- 2.Soriano JB, Abajobir AA, Abate KH; Global, regional. and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. doi: 10.1016/S2213-2600(17)30293-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev. 2009;18(114):213–221. doi: 10.1183/09059180.00003609 [DOI] [PubMed] [Google Scholar]
- 4.López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23. doi: 10.1111/resp.12660 [DOI] [PubMed] [Google Scholar]
- 5.Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6(6):421–430. doi: 10.1016/S2213-2600(18)30103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelgrim CE, van Ark I, van Berkum RE, et al. Effects of a nutritional intervention on impaired behavior and cognitive function in an emphysematous murine model of COPD with endotoxin-induced lung inflammation. Front Nutr. 2022;9:1010989. doi: 10.3389/fnut.2022.1010989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh B, Gaike AH, Pyasi K, et al. Bacterial load and defective monocyte-derived macrophage bacterial phagocytosis in biomass smoke-related COPD. Eur Respir J. 2019;53(2):1702273. doi: 10.1183/13993003.02273-2017 [DOI] [PubMed] [Google Scholar]
- 8.Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37–42. doi: 10.1136/thorax.58.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27. doi: 10.1016/j.jaci.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 10.El-Gazzar AG, Kamel MH, Elbahnasy OKM, El-Naggar ME. Prognostic value of platelet and neutrophil to lymphocyte ratio in COPD patients. Expert Rev Respir Med. 2020;14(1):111–116. doi: 10.1080/17476348.2019.1675517 [DOI] [PubMed] [Google Scholar]
- 11.Mehta A, Shapiro MD. Apolipoproteins in vascular biology and atherosclerotic disease. Nat Rev Cardiol. 2022;19(3):168–179. doi: 10.1038/s41569-021-00613-5 [DOI] [PubMed] [Google Scholar]
- 12.Deng F, Li D, Lei L, et al. Association between apolipoprotein B/A1 ratio and coronary plaque vulnerability in patients with atherosclerotic cardiovascular disease: an intravascular optical coherence tomography study. Cardiovasc Diabetol. 2021;20(1):188. doi: 10.1186/s12933-021-01381-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolska A, Reimund M, Sviridov DO, Amar MJ, Remaley AT. Apolipoprotein Mimetic Peptides: potential New Therapies for Cardiovascular Diseases. Cells. 2021;10(3):597. doi: 10.3390/cells10030597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aladjem F, Gofman JW, Lieberman M. Immunochemical studies on human plasma lipoproteins. J Exp Med. 1957;105(1):49–67. doi: 10.1084/jem.105.1.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alaupovic P. Apolipoprotein composition as the basis for classifying plasma lipoproteins. Characterization of ApoA- and ApoB-containing lipoprotein families. Prog Lipid Res. 1991;30(2–3):105–138. doi: 10.1016/0163-7827(91)90008-S [DOI] [PubMed] [Google Scholar]
- 16.Sacks FM. The apolipoprotein story. Atheroscler Suppl. 2006;7(4):23–27. doi: 10.1016/j.atherosclerosissup.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 17.Paukner K, Králová Lesná I, Poledne R. Cholesterol in the Cell Membrane-An Emerging Player in Atherogenesis. Int J Mol Sci. 2022;23(1):533. doi: 10.3390/ijms23010533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothblat GH, Mahlberg FH, Johnson WJ, Phillips MC. Apolipoproteins, membrane cholesterol domains, and the regulation of cholesterol efflux. J Lipid Res. 1992;33(8):1091–1097. doi: 10.1016/S0022-2275(20)40761-8 [DOI] [PubMed] [Google Scholar]
- 19.Kumar GA, Jafurulla M, Chattopadhyay A. The membrane as the gatekeeper of infection: cholesterol in host-pathogen interaction. Chem Phys Lipids. 2016;199:179–185. doi: 10.1016/j.chemphyslip.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 20.Kotlyarov S. High-Density Lipoproteins: a Role in Inflammation in COPD. Int J Mol Sci. 2022;23(15):8128. doi: 10.3390/ijms23158128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito H, Lund-Katz S, Phillips MC. Contributions of domain structure and lipid interaction to the functionality of exchangeable human apolipoproteins. Prog Lipid Res. 2004;43(4):350–380. doi: 10.1016/j.plipres.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez Reguero JJ, Iglesias Cubero G, Vazquez M, et al. Variation in plasma lipid and lipoprotein concentrations in community-acquired pneumonia a six-month prospective study. Eur J Clin Chem Clin Biochem. 1996;34(3):245–249. doi: 10.1515/cclm.1996.34.3.245 [DOI] [PubMed] [Google Scholar]
- 23.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2023 Report. Global Initiative Chronic Obstructive Lung Dis. 2023. [Google Scholar]
- 24.van der Vorst EPC. High-Density Lipoproteins and Apolipoprotein A1. Subcell Biochem. 2020;94:399–420. [DOI] [PubMed] [Google Scholar]
- 25.Franceschini G. Apolipoprotein function in health and disease: insights from natural mutations. Eur J Clin Invest. 1996;26(9):733–746. doi: 10.1046/j.1365-2362.1996.2120536.x [DOI] [PubMed] [Google Scholar]
- 26.Georgila K, Vyrla D, Drakos E. Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer. Cancers. 2019;11(8):1097. doi: 10.3390/cancers11081097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris G, Gevezova M, Sarafian V, Maes M. Redox regulation of the immune response. Cell Mol Immunol. 2022;19(10):1079–1101. doi: 10.1038/s41423-022-00902-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan L, Han X, Li W, Ren D, Yang X. Isoorientin Prevents Hyperlipidemia and Liver Injury by Regulating Lipid Metabolism, Antioxidant Capability, and Inflammatory Cytokine Release in High-Fructose-Fed Mice. J Agric Food Chem. 2016;64(13):2682–2689. doi: 10.1021/acs.jafc.6b00290 [DOI] [PubMed] [Google Scholar]
- 29.Alves-Bezerra M, Cohen DE. Triglyceride Metabolism in the Liver. Compr Physiol. 2017;8(1):1–8. doi: 10.1002/cphy.c170012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Begue F, Chemello K, Veeren B, et al. Plasma Apolipoprotein Concentrations Are Highly Altered in Severe Intensive Care Unit COVID-19 Patients: preliminary Results from the LIPICOR Cohort Study. Int J Mol Sci. 2023;24(5):4605. doi: 10.3390/ijms24054605 [DOI] [PMC free article] [PubMed] [Google Scholar]