Abstract
Introduction
The extensive resources needed to train surgeons and maintain skill levels in low-income and middle-income countries (LMICs) are limited and confined to urban settings. Surgical education of remote/rural doctors is, therefore, paramount. Virtual reality (VR) has the potential to disseminate surgical knowledge and skill development at low costs. This study presents the outcomes of the first VR-enhanced surgical training course, ‘Global Virtual Reality in Medicine and Surgery’, developed through UK-Ugandan collaborations.
Methods
A mixed-method approach (survey and semistructured interviews) evaluated the clinical impact and barriers of VR-enhanced training. Course content focused on essential skills relevant to Uganda (general surgery, obstetrics, trauma); delivered through: (1) hands-on cadaveric training in Brighton (scholarships for LMIC doctors) filmed in 360°; (2) virtual training in Kampala (live-stream via low-cost headsets combined with smartphones) and (3) remote virtual training (live-stream via smartphone/laptop/headset).
Results
High numbers of scholarship applicants (n=130); registrants (Kampala n=80; remote n=1680); and attendees (Kampala n=79; remote n=556, 25 countries), demonstrates widespread appetite for VR-enhanced surgical education. Qualitative analysis identified three key themes: clinical education and skill development limitations in East Africa; the potential of VR to address some of these via 360° visualisation enabling a ‘knowing as seeing’ mechanism; unresolved challenges regarding accessibility and acceptability.
Conclusion
Outcomes from our first global VR-enhanced essential surgical training course demonstrating dissemination of surgical skills resources in an LMIC context where such opportunities are scarce. The benefits identified included environmental improvements, cross-cultural knowledge sharing, scalability and connectivity. Our process of programme design demonstrates that collaboration across high-income and LMICs is vital to provide locally relevant training. Our data add to growing evidence of extended reality technologies transforming surgery, although several barriers remain. We have successfully demonstrated that VR can be used to upscale postgraduate surgical education, affirming its potential in healthcare capacity building throughout Africa, Europe and beyond.
Keywords: Surgery, Global Health, Qualitative research, Diffusion of Innovation, Graduate medical education
WHAT IS ALREADY KNOWN ON THIS TOPIC
Training surgeons require extensive time, finances and specialist supervision. Virtual reality (VR) facilitates training resource dissemination to remote audiences.
In Uganda, the majority of surgical care is initially treated in remote centres, highlighting the need for surgical education of rural doctors.
WHAT THIS STUDY ADDS
Demonstrated feasibility of VR-enabled surgical training for remote low-income and middle-income country doctors, with appeal across diverse socioeconomic settings.
A 360° visualisation enhances learning by enabling multiple perspectives within procedure, increases learner connectivity and improves onward knowledge sharing.
Barriers to VR education include affordability, data connectivity limitations and need for breaks. Benefits include environmental, cross-cultural learning and scalability.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Evidence of local desire to incorporate VR into East African surgical training programmes; recommendation to embed VR within established education programmes, particularly where postgraduate opportunities are scarce.
Such projects require strong collaborative cross-cultural networks and bespoke context-specific content design.
Introduction
Training surgeons require extensive resources, including time, finances and specialist supervision. In many low-income and middle-income countries (LMICs), postgraduate surgical training opportunities are limited, while surgical disease burdens are high.1 For example, in Uganda, the estimated population suffering unmet surgical need is 3 680 000,2 despite only an estimated 230 surgeons. Furthermore, 80% of these work in urban areas, while the majority (75%) of the population live rurally.3 As a result, medical attention is initially sought at remote public health facilities,4 making surgical education of rural doctors a priority.
Virtual reality (VR) can be harnessed as a method of sharing surgical training resources with large, geographically dispersed audiences, using affordable and scalable equipment. VRiMS (Virtual Reality in Medicine and Surgery)5 is a UK-based organisation, which has delivered free-to-attend surgical training courses using VR and 360° recording, since 2020. These comprise:
Specialist surgeon faculty teaching surgical procedures using cadaveric specimens (at Anatomy Laboratory, Brighton and Sussex Medical School, BSMS).
Delegates attending in-person/hands-on training (surgical trainees, junior doctors and medical students, usually n=10–15 per course).
Content recorded with 360° video and multiple fixed camera angles.
Content live-streamed to remote delegates in 360° view with overlays from the fixed cameras allowing different angles.
Content accessed personal device availability, including high-specification VR headset, smartphone combined with low-cost VR headset or a smartphone/laptop alone (360° video).
VRiMS initially delivered five courses targeted at training needs of UK-based surgical trainees, and later adapted courses towards surgery training needs in LMICs. Using mixed-method evaluation, we report outcomes from the first Global VRIMS course, run-in partnership between UK and Ugandan academic and clinical groups.
Methods
Pilot study
A proposed single-day pilot (July 2021) encompassed life-saving ‘Bellwether’ procedures (emergency laparotomy and caesarean section). Teaching was delivered by faculty in-person (UK-based) and virtually (nine faculties in LMICs: Cameroon, Gabon, India, Kenya, Philippines, Uganda, Zimbabwe; two in the USA). The live-stream was advertised to doctors/medical students globally free-of-charge and delegates attended from 26 LMICs and 17 high-income countries (HICs). There was high user engagement (mean>140 min engagement per delegate) but there was minimal engagement with postcourse feedback.
Uganda was selected as the partner country based on strong clinical and academic connections, and previous effective utilisation of VR and 360° videos in healthcare training in Uganda.6 Two venues in Kampala were secured: UCU (Ugandan Christian University) and ACE (African Centre of Excellence in Bioinformatics and Data Science) at Infectious Diseases Institute, Makerere University. Significant thought was given regarding the composition of the research team, including local and international researchers, skill development and gender balance. Full details can be found in the author reflexivity statement (online supplemental file 1).
bmjoq-2023-002477supp001.pdf (47.9KB, pdf)
A Ugandan-based pilot was carried out with medical students using recorded VRiMS content (March 2022) and loaning cardboard headsets used in combination with personal smartphones. Fifty-four delegates attended and their feedback validated the appropriateness of the venues and highlighted some issues with data connectivity, which were addressed.
Content development
Development of bespoke course content was based on the ‘Essential Surgery Package’ WHO list of procedures for general surgical, obstetric and trauma care.7 This was further refined through discussions with Ugandan-based specialist surgeons, trainees and rural/remote general practitioners; sourced through the extensive surgical network of GASOC (the Global Anaesthesia, Surgery and Obstetrics Collaborative8).
VR content was built by capturing 360° footage from the perspective of an assistant standing at the operating table. In addition, to allow the overlay of several perspectives, simultaneous recordings were made using standard cameras fixed at different points. The live-stream, therefore, displayed the 360° view of the dissection room, with overlay of different standard fixed views displayed as squares in a surround view, as though simulating screens hanging around the operating table (figure 1).
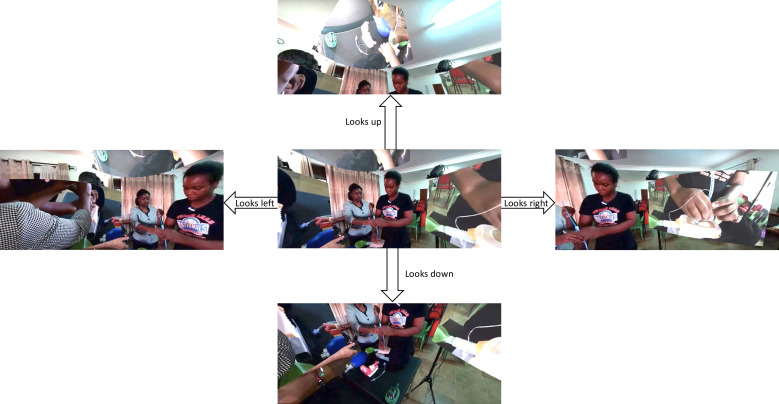
Figure 1.
Example of the VR interface visualised by virtual delegates viewing the live-streamed 360° content. This example is taken from a VRiMS module using mannequins to demonstrate cricothyroidotomy (images of cadaveric specimens cannot be published according to the Human Tissues Act). The upper, lower, left and right screenshots represent how the view changes if the viewer looks up, down, left or right, respectively (by moving their head if wearing a VR headset or by clicking and dragging the screen if using a standard laptop/smartphone view). Overlays of different camera angles can be clearly seen in the upper, left right images. Published with faculty members’ knowledge and consent. VR, virtual reality; VRiMs, Virtual Reality in Medicine and Surgery.
Delegate recruitment
Preregistration was required, and no delegate paid course fees. Advertisement to doctors/surgeons and medical students was through word-of-mouth; a national television feature-piece (Uganda); social media; and special-interest email groups. Dissemination was facilitated by GASOC and local Ugandan partners: UCU; ACE; Mengo Hospital; Association of Surgeons of Uganda; College of Surgeons of Eastern Central and Southern Africa.
Three groups of course delegates were enlisted:
In-person hands-on cadaveric training, Brighton (10 places). Scholarships (£1500) were offered to subsidise flights and accommodation, funded by Saving Faces.9 Eligibility criteria included: working as a doctor in an LMIC (defined by the Organisation for Economic Co-operation and Development10); English language proficiency; age <40 years (to recruit those earlier in career development). Selection by a panel of UK and Ugandan surgeons was based on submission of personal statement, CV, surgical logbook, professional recommendation letter and a valid passport. To maximise impact, priority was of applicants serving large/remote/rural populations, teaching other healthcare workers and (for this iteration of the course) working in East Africa.
In-person virtual training, Kampala (up to 30 places per day). Required to bring their own smartphone, charger and earphones; provided with low-cost cardboard VR headsets (courtesy of Medical Realities11).
Remote virtual training (unlimited capacity). Remote attendance was available from any global location with the internet, using either a 360° VR headset, a smartphone or laptop.
Course delivery
The course lasted 4 days (April 2022). Brighton faculty was recruited from previous courses, with the addition of two Ugandan specialist surgeons (general surgery and obstetrics/gynaecology) from Mengo Hospital (reimbursed for travel/accommodation costs) to ensure relevance to local resource availability and surgical techniques. Kampala faculty included information technology/VR specialists (ACE) providing technical support/trouble-shooting; specialist surgeons (Mengo Hospital) answering clinical questions and facilitating virtual dialogue between Kampala and Brighton; and medical students supporting logistical co-ordination.
UK-based delegates received practical ‘hands-on’ training, which was filmed and live-streamed for remote delegates. They also had access to VR live-streaming of the content (each had a 15 min session using Oculus Quest 2 or cardboard headset). Kampala-based delegates viewed the live-stream via low-cost cardboard headsets and had access to two Oculus headsets; available for 15 min sessions. Remote attendance was accessed via GoToWebinar and YouTube platforms.
Outcomes assessment
Survey
Demographics were collected at registration. Subjective qualitative and quantitative data were collected via ‘precourse’ and ‘postcourse’ surveys (Google Forms), including questions related to VR (influence on course attendance, ease of access and potential use in local clinical practice). As our pilots identified barriers with postcourse feedback engagement, surveys were circulated at the start and end of each day while delegates were physically present. The only exception was in-person UK delegates who completed postcourse surveys 5 months after the course, to facilitate analysis of longer-term impact on clinical practice. We anticipated this group would be likely to respond later due to extensive in-person interactions with the research team.
Semistructured interviews
Qualitative data were collected through 14 in-depth semistructured interviews. All in-person UK delegates were interviewed (n=10) face to face on the final course day. Remote delegates (n=4) were interviewed virtually (Zoom) following the course (April–July 2022). Interviewees received information sheets describing the scope and purposes of the project and signed a consent form. Thematic guides were used; interviews were recorded and transcribed verbatim; and data were analysed thematically using NVivo.12 The analysis process was iterative, and themes emerged inductively from a coding process, while being also informed by theory.
The BSMS Anatomy Laboratory dissection lab is licensed under The Human Tissue Act 2004, regulated by the Human Tissue Authority (HTA), and holds both a HTA Anatomy Licence and a Public Display Licence.
Results
All averages reported are stated as means, and all figures are rounded to one decimal space.
Overview of VR training programme
The consensus of topics included in the course as per WHO guidance and input from Ugandan clinicians/surgeons are listed in table 1. Most topics included a short lecture (10 min) then practical demonstrations and delegate repetition using cadavers (one male and one female, to facilitate obstetrics/gynaecology). Manikins from Limbs and Things were occasionally required, that is, unable/unethical to use cadaver with gravid uterus for obstetric emergencies. The full programme can be found in online supplemental file 2.
Table 1.
Course content
| Topic | Delivery method | Time allocated |
| Access to essential surgical care | L | 40 min |
| Trauma care: epidemiology and principles of management | L | 40 min |
| Resuscitation and stabilisation: airway | L, C, M | 1 hour 30 min |
| Resuscitation and stabilisation: breathing and circulation, including vascular access | L, C | 3 hours 30 min |
| Wound and burn management | L, C | 2 hours |
| Managing abdominal trauma | L, C | 2 hours |
| Essential abdominal surgery and emergencies | L, C | 3 hours 30 min |
| Principles of fracture management | L, C | 2 hours |
| Managing limb trauma | L, C | 2 hours |
| Managing head trauma | L, C | 3 hours 30 min |
| Managing complications of pregnancy | L, C, M | 2 hours |
| Postpartum haemorrhage and emergency obstetrics/gynaecology | L, C | 3 hours |
Topics covered in the course including method of delivery and time allocated.
C, practical Cadaveric procedural demonstrations; L, lecture; M, practical manikin demonstrations.
bmjoq-2023-002477supp002.pdf (3.5MB, pdf)
Mode of access
In-person delegates chose ‘hands-on’ training for all practical sessions, other than their allocated 15 min VR experience. Kampala-based delegates preferentially used low-cost carboard headsets coupled with smartphones, interspersed with short periods of using smartphone/laptop alone. The two Oculus headsets were in use throughout every course day due to high popularity, and a limitation identified was the need to pause their use for regular recharging. This was not the case with smartphones as they could be plugged in during use.
Delegate demographics
For delegates using VR in Kampala, 92.4% had no prior experience of VR (two exposed through gaming, others unspecified). Regarding remote delegates there was a discrepancy between feedback form completion (n=617), and attendees recorded on the digital platform (n=556). This suggests that some attendees shared devices (and submitted separate feedback forms), a phenomenon also observed in a few cases in the Kampala group. This usage type was not anticipated and was, therefore, not explicitly captured in the postcourse feedback, however, the data captured implies that the majority were solo device users.
The geographical workplace distribution of the remote group included 25 different nations: Bangladesh, Brazil, Cameroon, Egypt, Ethiopia, Ghana, India, Ireland, Kenya, Lesotho, Malawi, Namibia, Nigeria, Pakistan, Papua New Guinea, Rwanda, Somalia, South Sudan, Spain, Sudan, Tanzania, Uganda*, UK*, Zambia and Zimbabwe (stars indicate the two nations with largest number of attendees, table 2).
Table 2.
The demographic data of each delegate group collected from registration forms, precourse and postcourse surveys
| Delegate type (and number of postcourse survey responses) | In person, Brighton (n=10) | Kampala (n=115) | Remote (n=724) |
| Preregistration | 130 scholarship applications | 80 | 1680 |
| Attendees | 9 attended full course (10 selected, 1 attended in Kampala due to a visa issue) |
79 over 4 days (43% attended multiple days and 57% a single day) |
556 over 4 days (recorded on the digital platform) Mean 139 per day (range 106–210) |
| Mean age | 31 years (range 26–38) | 26 (range 21–41) | Not recorded |
| Male:female ratio | 6:4 | 71:44 | Not recorded |
| Geographical distribution of workplace | 60% Uganda 20% Kenya 10% Tanzania 10% Rwanda 90% reported working in a rural/remote setting |
100% Uganda | 80.7% Africa (of which 83.7% Uganda) 11% Europe (of which 92.6% UK) 7.8% Asia 0.3% South America 0.2% No response |
| Clinical expertise | 100% doctors (worked for a mean of 4 years since graduation, range 1–9) | 63.3% doctors (including interns, medical officers, surgical residents and obstetricians) 36.7% medical students |
Included doctors of various levels and medical students (data from registration, specific numbers not captured in postcourse survey) |
| Engagement with feedback | 100% compliance with feedback and interviews | 115 daily postcourse surveys completed (137 total with 22 duplicates discarded) |
617 daily postcourse surveys completed (724 total with 107 duplicates discarded) |
Survey data
There were high survey response rates across the three different types of course delegates: in-person UK hands-on (n=9), in-person with VR live-stream in Uganda (n=115) and remote/virtual (n=617).
In-person UK delegates indicated high impact on clinical work 5 months after the course. All delegates reported using knowledge/skills learnt at least once a month or more frequently (10% every day, 60% most days, 10% fortnightly, 20% monthly). Every delivery method had >60% of delegates indicate a positive impact on their clinical work (100% ‘practical sessions’; 60% ‘lectures’; 60% ‘VR learning’). In terms of application, all delegates reported improvement in their operative procedures and 80% in patient assessment. All delegates reported use of knowledge/skills learnt to teaching others (60% frequently; 30% occasionally, 10% rarely).
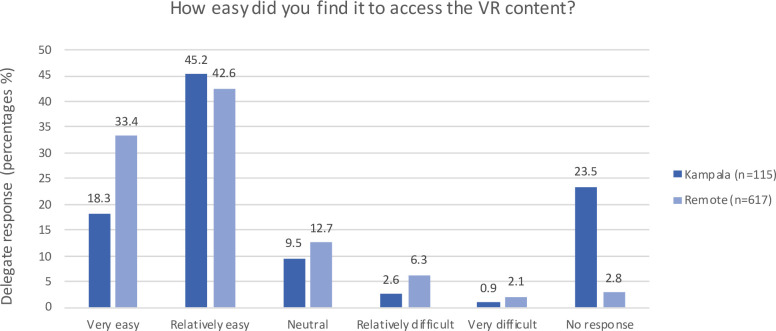
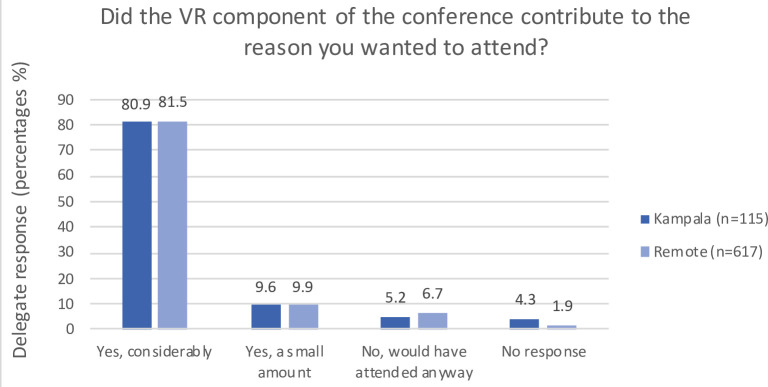
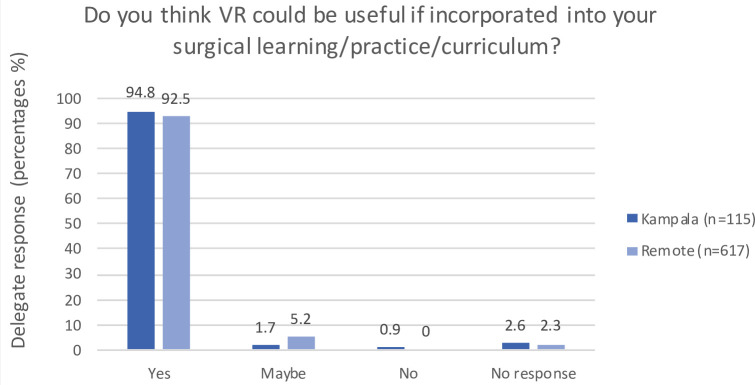
The responses of delegates who experienced the live-stream (in Kampala or remotely) are illustrated in figures 2–4. The majority reported that VR content access was relatively or very easy; the VR aspect of the course contributed to attending; and they felt VR has application in local surgical learning, practice and curriculum. The free-text sections of the survey highlighted the main barrier to access was poor internet connectivity.
Figure 2.
Postcourse survey responses of delegates who used the live-stream regarding ease of VR use. VR, virtual reality.
Figure 3.
Postcourse survey responses of delegates who used the live-stream regarding attractiveness of VR to attend the course. VR, virtual reality.
Figure 4.
Postcourse survey responses of delegates who used the live-stream regarding relevance of VR in personal learning, surgical practice and local training. VR, virtual reality.
Semistructured interviews
Our interviews with participants led to identification of three main themes: the context of the study in regard to the problem of clinical education and skill development in East Africa; the ways VR could address aspects of this through its embedded ‘knowing as seeing’ mechanism in terms of 360° visualisation; and outstanding challenges VR poses in surgical education in terms of accessibility and acceptability. It is worth noting that interviews were carried out in English, and although English proficiency was a requirement for in person delegates in Brighton, English was not the first language for 90%.
Clinical education and skills development in East Africa
Interviewees described scarce postgraduate training opportunities in their home countries, with many reporting that they perform surgical procedures based on knowledge acquired during bachelor studies. Following graduation, professional development is seen as an individual endeavour that is not necessarily supported by medical institutions. Educational costs fall to individuals, resulting in personal financial sacrifice and reduced motivation to keep skills up to date.
I still rely on my knowledge of medicine at a bachelor’s level to actually do a little surgical work (P12)
…for every doctor, it’s a requirement to complete a number of CPD [continuing professional development] points for our licenses to be renewed. You have to attend different seminars or trainings. But then, it’s a bit of a challenge, because most of those seminars, you have to pay for. So, if you can't afford to pay, it’s a real problem. You might not be able to complete the required CPD points at the end of the year (P2).
Interviewees reported other challenges to clinical education delivery in contexts such as East Africa, including prioritisation of private consultations (for income) over teaching, patients’ reluctance to be consulted by medical students, and congestion of operating theatres compromising patient safety. Clinicians, therefore, graduate with limited procedural experience, which VR training opportunities could potentially help address.
Back in Uganda, in the past, our patients were referred, to act as a teaching specimen during the course of treatment. But now with the increasing level of literacy many, are now opting out. Like 'oh, I don't want students around me'. In the future, we are anticipating that our students may not have a good hands-on as it had been before. So this kind of technology is likely to bridge this gap. (P1)
Participants highlighted that their training environments generally put more focus on volume and exposure to a breadth of procedures as compared with depth of knowledge. They felt this sometimes results in learn suboptimal operative approaches, which may affect overall quality of training. In particular, a lack of mentorship and postgraduate training opportunities was identified and participants felt this can lead to the passing on of poor technique and clinical errors.
…the kind of training we go through is evaluated on usually quantity of the work you have done, and not really the quality… you might be able to actually do a lot of procedures and serve a number of patients, but there is also a high likelihood that you may actually be doing the wrong thing because you didn't get a proper training. But then unfortunately given that the facility where I also work, we get an opportunity to train others, so keep on passing the wrong thing to a generation (P6)
Knowing as seeing: 360° visualisation
Research participants reflected that their experience of 360° video offers ability to learn procedures from a number of angles compared with conventional physical observation in theatre in a fixed position. The option of changing angle in 360° views removes obstacles restricting views, provides a more holistic view, thus facilitating knowledge acquisition. They also felt 360° recordings gave a dynamic perspective, with freedom to move the camera, compared with the conventional static view. This identifies a liberating aspect of the technology, allowing different views and giving participants an ‘eye around’ (P4). Thus, the technology, ironically, made interviewees feel more like a person with flexibility to move around, compared with being confined in one position.
…when you’re observing you have to be in that specific angle you’re present in theatre, but you can only focus on the only one thing that that you can see…the 360° technology gives me the ability to twist my camera or my view wherever I am in to see every procedure at any angle that I intend to. (P.12).
It kind of makes you feel like you are a person, like you are able to move and wiggle, and see what is happening over there. (P4)
These aspects were identified as particularly important when learning new and essential surgical skills. An interviewee from Kenya described for example how only a handful of students have a direct view in procedural learning. So some participants felt this technology was better than learning in person, as it virtually got them round the table, in front row seats.
…let’s say we have a class of 300 people. Only less than ten people are going to see how such a thing is done very clearly. The rest are going to have to imagine it or ask from the guys who are in front to actually demonstrate it for them. With 360, you get the experience that you are one of the people staring right at the table or the cadaver when the dissection is happening and better than that, you get multiple views (P10).
One interviewee compared the power of 360° technology to the multiple views orthopaedic imaging offers, enabling holistic understanding of operative anatomy.
From orthopaedics point of view when you fix a fracture, even when you have an x-ray you’ll have different views. Anterior and posterior view lateral view and depending on the fracture in the region, you may have different views. So, if you don’t see your fracture from different angles, you don’t always know whether you did it right or not. You may assume that you corrected the fracture and when you come out it’s a mess, so you have to see every direction (P13).
Participants felt the ability to see from many different viewpoints improves learning experience and enhances clinical outcomes.
I believe it gives you an opportunity to have a better experience and actually also better learning and that means that you can teach better, so you can work better as a surgeon (P6).
Not all clinical procedures though can be learnt through enhanced visualisation. An interviewee expressed that the idiosyncrasy and complexity of some procedures require learning with hands-on experience in real life cases.
‘Procedures like laparotomy is not easy to learn just by seeing. Each case comes in different. It’s not as straightforward as you see it in the cadaver demonstration, where it’s just clean cut. No spillage. Everything is just right there.’ (P2)
Interviewees argued that they gained surgical skills and knowledge which they can immediately apply in practice and teach peers. In this way, learning effects are multiplied, increasing the capacity-building potential of the VR programme.
I want to transfer the knowledge I got here and teach my colleagues the actual way of doing it, because in medicine you can learn to do things the wrong way for the rest of your life, which is very dangerous. So, you need to do the right thing (P5).
Intubation, for example, is a skill that I learnt, I think I got it quite well. I am comfortable doing that right now (P10).
The potential for a library of accessible videorecorded procedures was discussed as a knowledge base that could support doctors in LMIC and boost their confidence. This was identified as particularly important for refreshing knowledge regarding clinical procedures doctors encounter in their countries or are not typically trained for. The quote below highlights the significance of refreshing surgical memory in a timely fashion and the way VR can achieve this through the instant knowledge base it creates.
Sometimes when you're in the hospital you get a case, and at that particular moment you can't remember very well the technique. For example, tracheostomy… I wouldn’t get so many cases, right? But if I do get one, and I feel like maybe my confidence at that particular point is… like, I remember, but I'm not quite sure the chronology of the steps. If you have a headset then you can just quickly go in and look at it, and be like, oh yes, this is what you're supposed to do. And you get out, you're confident and you're ready to tackle it (P3).
The collection of knowledge brought in the, let me say like a knowledge bank, which is very nice for people in the medical field. There are new changes always with the research, that are brought together, so if we can access all these it’s really quite important (P7).
Accessibility and acceptability
Participants identified barriers to accessibility of VR for clinical training in LMICs, with affordability as the most prominent. This included acquiring the necessary equipment, including smartphones and headsets, and costs involved in ensuring continued internet connectivity. This is a significant barrier especially in rural areas due to limited infrastructure. Participants reported that most medical students own a smartphone, however the data costs are high.
Number one is internet connection. Power outages. Not everyone has electricity in their home or in their place… where I am coming from, I have to buy data. It’s not free Wi-Fi. So, it will be an expensive adventure, having to put on data to watch a video (P4).
In a rural setting the network is not really stable. The internet keeps cutting…, it may be expensive for the people in the rural areas to be able to afford the headsets (P8).
When considering accessibility regarding ease of usage, most participants reported the required technology is easy to setup. However, they reported fatiguability in a virtual environment for long periods, and reported the need to take breaks.
…these headsets, with time, when you use them… for a lot of time, they are becoming uncomfortable. Because you lose contact with your environment.
So you will need, a little pause and get oriented in your environment
So you cannot be in these headsets, say for 3 hours non-stop (P1).
Participants also highlighted the potential for VR-enhanced training to increase opportunities for socialisation, networking, peer-learning and access at a time of convenience. They compared this to conventional clinical lectures being rare, with scarce opportunities for dialogue and scheduled at specific often inconvenient times.
In a traditional way a lecture is always one off, you cannot consult, because that space is not created, but now in this virtual reality, you are always in a group. You can sit in a group even though somebody’s somewhere else, you can have discussions and you can still discuss whatever you are seeing, in real time. Learning is always when you have people around you. And two, traditional way of learning is at specific times, this is at any time…, at any moment, anywhere. So learning is continuous, regardless of space, regardless of time, regardless of distance (P5).
Regarding acceptability, interviewees saw potential for generational differences causing resistance, with newer doctors being more accepting of technology. They felt the older generation of doctors might be suspicious or feel threatened, particularly academics with responsibility to deliver medical expertise/teaching.
The lecturers, can you imagine, they'd be like 'no, that’s what we used to do, and how we used to… and now you want to tell us technology is going to do that for us. So that may be a challenge. There might be a bit of a resistance in that area. Especially with the older generation’ (P3).
‘In our environment, people fear to lose jobs, so they may think new technology is coming to deprive them of their employment (P1).
Discussion
The findings of our mixed-method study illustrate the importance of establishing HIC and LMIC collaborative networks to create content bespoke to the recipients’ local environment and culture. The high numbers and wide geographical distribution of scholarship applicants, registrations and attendees demonstrate an appetite for VR in postgraduate surgical education, with relevance to a diversity of economic and cultural settings. The highest geographical representation (Africa and Europe, specifically Uganda and the UK) was unsurprising based on the advertising focus. The precourse survey results demonstrate that the use of VR in the conference was a significant contributor to the appeal of this educational activity for the majority of attendees, despite relatively low prior exposure to VR. The postcourse surveys demonstrate the technology’s intuitive use and strong appeal to embedding VR into locally established educational programmes.
The semistructured interview analysis highlights challenges to clinical training in East Africa, and the potential of VR to address some of these. Many are consistent with those described in the literature,13 raising the broader question of whether surgical training in LMICs is adequate. The Lancet Commission on Global Surgery reported a significant shortage of trained surgeons, anaesthetists and obstetricians in LMICs, and often inadequate quality of surgical training programmes.1 Contributing factors are well known, including limited resources/infrastructure, inconsistency in training programmes, lack of postgraduate opportunities and limited access to advanced technologies/simulation. There is a growing evidence that simulation-based training should be incorporated into training programmes to improve surgical education14 while potentially useful exchanges between LMIC/HIC surgical trainees often neglect the learning needs of LMIC trainees as educational goals poorly matched to local clinical needs.15 Our study aimed to address these issues by designing the programme based on both WHO guidance and input from generalists and specialists in Uganda to ensure high-yield content for this setting.
The interviews revealed several advantages of VRiMS, including the visual angles, increased connectivity between learners and the feeling of being in the room with the specimen and trainer (particularly relevant rurally when isolated from colleagues/supervisors). Users felt VR-enhanced training improves onward knowledge/skill sharing, affirming the capacity-building potential of such programmes and will be furthered in future Global VRiMS work. These findings support the idea that VRiMS can offer high quality surgical training to a large and diverse audience, by using affordable headsets to disseminate carefully curated resources to remote and rural areas. The delegates’ reports that simulation creates a safe learning environment are consistent with current literature, including recommendation to increase surgical simulation opportunities in LMICs.16 The main barriers identified were affordability and internet instability, need for VR breaks, reduced accuracy compared with hands-on learning, and resistance from existing educators threated by perception of technology changing education delivery. These findings can inform how to effectively embed VR as an adjunct to conventional methods, allowing a shift towards technological hybridisation in established training programmes, while ensuring VR does not replace real-life clinical experiences. For example, VR modules of operative procedures could be included at medical school prior to or following cadaveric dissection and theatre observations. This would ensure surgical application of anatomical learning, and enable supervisors to record detailed operative explanations in a virtual environment where their focus can be directed towards training. VR modules could also be introduced into trainee programmes to provide breaks from service provision, practice of knowledge application without clinical risk and connections with remote supervisors in specialties unavailable locally.
The ideal surgical training route of prolonged one-to-one supervision in a high-quality clinical centre with adequate supporting educational resources is undisputed; however, this is currently unachievable in most LMICs, therefore VRiMS provides a pragmatic solution to improving current training structures. A large-scale solution to the connectivity barriers might be building extended reality (XR) hubs throughout LMICs providing stable internet and low-cost headset use, allowing rural clinicians to travel to for training days.
Beyond the interview analysis, several other identified benefits include:
Environmental: reduced international travel compared with traditional cross-continental courses.
Cross-cultural clinical knowledge sharing: hybridisation of local (Uganda) and remote (UK) faculty allowed unique clinical discussions between delegates and faculty, demonstrating invaluable bidirectional learning regarding differences in pathophysiology, solutions and equipment availability.
Scalability: low-cost headsets combined with ubiquitous smartphones.
Connectivity: increasing network coverage facilitates expansion of internet-based educational resources to remote/rural settings.
Our work demonstrates that with careful consideration and collaboration, the challenges to remote digital training (ie, cost, scalability and technological ability of users) are not insurmountable, even in resource-limited settings. One approach is development of a specialised restreaming platform providing ongoing 360° surgical training resources bespoke to contextual needs.
The project identified a host of practical challenges resulting from embedding cross-continental travel into an educational programme, which will be invaluable to similar programmes. For example, selection of successful scholarship candidates required local expertise due to context-specific application details. This was overcome using panel of surgeons from a mixture of LMICs and HICs adhering to carefully compiled selection criteria. Regarding scholar travel logistics there were visa delays due to a mandatory East African passport upgrade; time limitations between scholar selection and course delivery reduced flight affordability; lack of flying experience requiring logistical support; workplace leave was not always usual employee practice locally; and transfer of scholarship funds was difficult due to limited bank account usage and high intercontinental bank transfer fees (overcome by using reliable ‘mobile money’ transfers systems, ubiquitous in East Africa). These understandable logistical challenges highlight the multitude of barriers that most doctors in LMICs face when trying to access in-person conferences abroad, highlighting an even greater need to use technologies such as VR to facilitate inclusive and easily accessible remote access options for postgraduate learning.
The limitations of this study include lack of a control group for comparison; lack of formal accreditation and quality assessment being primarily reliant on delegate feedback. To address these, we plan to conduct a future study with comparison between VR users and a control group using conventional methods, to allow evidence-based conclusions into the impact of VR in surgical education. We also plan to gather data on how delegates perform newly learnt skills in clinical settings following the training programme. One option would be to assess this through supervisor assessment of a particular procedure before and after the course. Other drawbacks include current need to gather trainees/doctors who work remotely to a location with adequate data connectivity, and lack of integration with hand-on clinical work. We hope that ongoing upgrading of mobile connectivity across the country will address the first; and we plan to address the second by working with local surgical colleges, universities and hospitals to embed VRiMS training into existing clinical teaching programmes. This study nevertheless serves as a proof-of-concept event highlighting the interest and feasibility of VR, and will inform ongoing efforts towards a sustainable model of regular VR course delivery involving evaluation and accreditation appropriate to local surgical structures. To this end, we are building links to design and deliver VR courses with surgical education groups in several settings including Kenya, India, Zambia and Cameroon. A logical next step is also to apply this technology to live operations, enhancing its value for trainees. The technology to record and disseminate 360° visualisation of live operations requires minimal adaptation, however, it carries significant ethical, consent and data protection considerations which we are currently exploring. Other ethical considerations include ensuring VR training is not considered sufficient learning to perform operations, rather an adjunct to thorough medical training. For this reason, our course delegates had to be students and doctors already embedded in accredited medical education. Furthermore, video access was restricted to delegates during the courses, with future intentions of making them accessible via a secure smartphone app to ensure controlled and verified usage. This will allow sustainable ongoing training, while safeguarding access to registered doctors and medical students. Despite taking all possible measures, we recognise a potential risk with any surgical education tool (including textbooks) necessitating reliance on local health authorities to oversee healthcare provider’s practices.
Conclusion
The qualitative and quantitative outcomes of our first VR training course for emergency and essential surgery in global surgery are very encouraging. The programme design process demonstrates the importance of collaboration between clinicians, educators, academics and technologists across HIC and LMICs to enable scalable content delivery relevant to local contexts. There is a strong appetite for VR surgical training and incorporation into established local educational programmes, to address shortcoming in clinical education. VRiMS has thus committed to further events, initially planned in Kenya, to build on the lessons learnt.
The qualitative analysis identified a number of key themes which can inform VR-enhanced global surgical education courses, including positive aspects, barriers and future potentials. Our initiative adds to a growing body of evidence that videoconferencing and next generation technology are transforming surgery 17, with education being the priority area. Overall, we demonstrate successful upscaling of remote VR-enhanced postgraduate surgical training. This highlights enormous potential of XR technologies for capacity building and healthcare strengthening in Africa, Europe and beyond.
Acknowledgments
Thanks to BSMS (Brighton and Sussex Medical School) dissection lab, for providing cadaveric specimens for the pilot and main course, and anatomical support, as well as hosting.Thanks to ACE at IDI (African Centre of Excellence in Bioinformatics and Data Science at the Infectious Diseases Institute, Makerere University and UCU (Uganda Christian University, School of Medicine) for providing venues in Kampala, inclusive of data, free-of-charge for 4 days, as well as technical support (and VR headset provision by ACE). In particular thanks to technical faculty: Grace Kebirungi, Patricia Nabisubi, Henry Mutegeki.Thanks to Medical Realities for providing low-cost cardboard VR headsets and covered shipping costs for the pilot and main event (100×headsets delivered to Kampala).Thanks to partnership with GASOC (the Global Anaesthesia, Surgery and Obstetrics Collaborative, UK-based) who provided contacts with surgeons and doctors throughout Uganda, and other countries to act as faculty and consultants for course design. Thanks to partnership with Mengo Hospital and FOMHUK (Friends of Mengo Hospital UK, a registered UK Charity) for providing Ugandan surgical faculty. Thanks to all our partners for advertising efforts, including BSMS, ACE, UCU, GASOC, Mengo Hospital; ASOU (Association of Surgeons of Uganda); COSECSA (College of Surgeons of Eastern Central and Southern Africa) and NTV Uganda. Thanks to all surgical faculty involved in teaching on the pilot and main course (UK, Uganda and virtual/remote, names listed in Supplementary File Course Programme). Thanks to VRiMS course faculty captured in screenshot figure 1: Lionelle Tchokam and Maria Kansenga Moonga
Thanks to all course attendees, and their respective hospitals allowing study leave for delegates to attend. Thanks to Nicecreation for 360° videography and live-streaming. Thanks for equipment provision by VRiMS, Limbs and Things, BSMS, and surgical faculty.We acknowledge the use of the ChatGPT language model (3.5), developed by OpenAI, for minor corrections/writing support during the preparation of this manuscript. Presentation of content related to the courses presented in this article have been made at:
ASOU (Association of Surgeons of Uganda) AGM (Annual General Meeting), Kampala, Uganda, March 2023
SARS (Surgical Research Society) Annual Meeting 2022, UK: Oral presentation and abstract in Oxford Academic (focus on pilot course)
ASiT (Associations of Surgeons in Training) Conference 2023, UK: poster (focus on global use of VR)
UK Defence Conference, 2022, UK: oral presentation and abstract in BMJ Military Health (focus on neurosurgery component).
Footnotes
Twitter: @Hplease2
Contributors: Idea conception: HP, WB, JaD; UK Pilot Course conceptualisation and delivery: HP, WB, JoD, MK, MFB, JaD; Uganda Pilot Course conceptualisation and delivery: HP, MN, HL, NBR, CT, DJ; Programme development: (1) Clinical input: HP, KN, WB, HL, NBR, JoD, CT, MK, MFB, JaD, (2) Technical input: HP, KN, MN, DJ, JaD; Scholarship design and selection HP, KN, HL, NBR, MFB, JaD; UK-based aspect of course delivery: KN, WB, HL, NBR, JoD, MFB, DP; Uganda-based aspect of course delivery HP, MN, HL, NBR, CT, DJ; Funding sourcing: JaD; Course interviews and qualitative analysis: DP. Initial core writing group comprised first and senior authors: HP, DJ, MFB, JaD. Writing/drafting/revising/final approval of manuscript: all authors. Guarantor (responsibe for overall content): JaD. Corresponding author: HP.
Funding: Saving Faces (The Facial Surgery Research Foundation, a registered UK Charity) provided funding for scholarships (10×£1500) to successful scholars to subsidise flights from East Africa and accommodation in Brighton to attend the course. No award/grant number (charitable donation). VRiMS (Virtual Reality in Medicine and Surgery), UK provided funding for food/refreshments, a publicity banner and several other overhead costs required for the Kampala-based course. No award/grant number (charitable donation).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. The participants of this study did not give consent for their data to be shared publicly, so the empirical data are not available due to confidentiality reasons.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and ethical approval was provided by the Social Sciences & Arts, Cross Schools Research Ethics Committee, University of Sussex (reference number ER/DP254/7). Participants gave informed consent to participate in the study before taking part.
References
- 1.Meara JG, Leather AJM, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet 2015;386:569–624. 10.1016/S0140-6736(15)60160-X [DOI] [PubMed] [Google Scholar]
- 2.Tran TM, Fuller AT, Butler EK, et al. Burden of Surgical Conditions in Uganda: A Cross-sectional Nationwide Household Survey. Ann Surg 2017;266:389–99. 10.1097/SLA.0000000000001970 [DOI] [PubMed] [Google Scholar]
- 3.World Bank . Rural population (% of total population) - Uganda. n.d. Available: https://data.worldbank.org/indicator/SP.RUR.TOTL.ZS?locations=UG80
- 4.Ministry of Health . Annual health sector performance report; 2016.
- 5.Available: https://www.vrims.tv/
- 6.Buyego P, Katwesigye E, Kebirungi G, et al. Feasibility of virtual reality based training for optimising COVID-19 case handling in Uganda. BMC Med Educ 2022;22:274. 10.1186/s12909-022-03294-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mock CN, Donkor P, Gawande A. Disease control priorities, third edition (volume 1): essential surgery. In: Essential surgery: key messages. Disease control priorities: essential surgery. Washington: World Bank, 2015: 8. 10.1596/978-1-4648-0346-8 [DOI] [PubMed] [Google Scholar]
- 8.Available: www.gasoc.co.uk
- 9.Available: www.savingfaces.co.uk
- 10.Available: https://wellcome.ac.uk/funding/guidance/low-and-middle-income-countries
- 11.Available: https://www.medicalrealities.com
- 12.Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology 2006;3:77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 13.Talib Z, Narayan L, Harrod T. Postgraduate Medical Education in Sub-Saharan Africa: A Scoping Review Spanning 26 Years and Lessons Learned. J Grad Med Educ 2019;11(4 Suppl):34–46. 10.4300/JGME-D-19-00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rickard J. Systematic Review of Postgraduate Surgical Education in Low- and Middle-Income Countries. World J Surg 2016;40:1324–35. 10.1007/s00268-016-3445-x [DOI] [PubMed] [Google Scholar]
- 15.Greive-Price T, Mistry H, Baird R. North-South surgical training partnerships: a systematic review. Can J Surg 2020;63:E551–61. 10.1503/cjs.008219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasan OH, Ayaz A, Khan M, et al. The need for simulation in surgical education in developing countries. The wind of change. Review Article The Journal of the Pakistan Medical Association 2019;69:S62. [PubMed] [Google Scholar]
- 17.Best J. From Ukraine to remote robotics: how videoconferencing and next generation technology are transforming surgery. BMJ 2022;377:1078. 10.1136/bmj.o1078 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjoq-2023-002477supp001.pdf (47.9KB, pdf)
bmjoq-2023-002477supp002.pdf (3.5MB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The participants of this study did not give consent for their data to be shared publicly, so the empirical data are not available due to confidentiality reasons.