Abstract
Objective
This study aimed to examine rheumatoid arthritis (RA) risk associated with hormonal and reproductive factors in women from the large cohort of the UK Biobank.
Methods
Data on hormonal and reproductive factors in women were collected from a prospective cohort of 223 526 UK Biobank participants. The potential relationship between reproductive factors and RA risk was assessed using restricted cubic spline. Hazard ratios (HR) were estimated using Cox proportional hazard regressions.
Results
During a median follow-up of 12.39 years, 3313 women with RA were identified. Age at menarche >14 years was associated with a greater RA risk (HR 1.13, 95% CI 1.02 to 1.26) compared with menarche at 13. The multiple adjusted HR for RA in women with menopause at <45 years was 1.46. Reproductive years <33 increased the risk of RA (HR 1.39, 95% CI 1.21 to 1.59). Compared with those with 2 children, women with ≥4 children were associated with a higher risk of RA (HR 1.18, 95% CI 1.04 to 1.34). Women who had a hysterectomy (HR 1.40, 95% CI 1.25 to 1.56) or oophorectomy (HR 1.21, 95% CI 1.08 to 1.35) had a higher risk of RA than those without a hysterectomy or oophorectomy. Both hormone replacement therapy (HRT) use (HR 1.46, 95% CI 1.35 to 1.57) and HRT duration (HR 1.02, 95% CI 1.01 to 1.03) were associated with a higher risk of RA.
Conclusions
Some hormonal and reproductive factors were associated with a higher risk of RA. Hormonal and reproductive factors should be considered in risk assessment and formulating management plans in female patients with RA.
Keywords: rheumatoid arthritis, arthritis, epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Rheumatoid arthritis (RA), one of the most common autoimmune rheumatic diseases, can lead to irreversible joint damage and disability.
The prevalence of RA differs based on sex, with the occurrence frequency in women being 4–5 times higher than that in men under the age of 50 years, and twice that in men between the ages of 60 and 70. Furthermore, the disease progression and disease activities are adverse in women compared with men.
The available epidemiological data on the role of hormonal and reproductive factors in the pathogenesis of RA are inconsistent and uncertain.
WHAT THIS STUDY ADDS
There was a non-linear relationship between age of menarche, age of menopause and reproductive years and the risk of RA.
Age at menarche >14 years, ≥4 children, early menopause, reproductive years <33, hysterectomy and oophorectomy, and the use of the exogenous hormone hormone replacement therapy were observed to be associated with a higher risk of RA.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
In this study, we observed several hormonal and reproductive factors were associated with the risk of RA. When diagnosing and managing women with RA, hormonal and reproductive aspects should be carefully evaluated. In particular, women in later menarche or early menopause require additional attention. The findings of this study are significant and form a basis on which novel and target specific intervention measures to curb the risk of RA in women may be developed. Furthermore, future studies should investigate the involvement of female hormones in the pathophysiology of RA.
Introduction
Rheumatoid arthritis (RA), one of the most prevalent autoimmune rheumatic diseases, is a complicated multifactorial illness that can lead to joint inflammation and, in severe cases, irreversible joint damage and disability.1 Factors related to socioeconomic status, genetics, environmental exposures, and treatment have been linked to the onset and severity of the disease.2 RA can be severe enough to cause disability, and if untreated or poorly managed, it can have a far-reaching impact on a patient’s health.
There have been concerted efforts, by different scientists and stakeholders, to come up with knowledge and remedies appertaining to distinct variables of RA. Some studies have demonstrated that the prevalence of RA differs based on sex, with the occurrence frequency in women being 4–5 times higher than that of men under the age of 50 years, and twice that in men between the ages of 60 and 70.3 4 Furthermore, the disease progression and activities are adverse in women compared with men.5 Several studies have suggested the role of female hormonal and reproductive factors in the development of RA and the sex-based differences of the disease outcome.3 6 Female hormonal factors are basically reproductive traits associated with alterations in women’s lifetime levels of sex hormones, primarily progesterone and estrogens.
Female reproductive features, such as puberty, pregnancy, childbirth, menopause, breastfeeding, and exogenous exposure to hormone levels (hormone replacement therapy (HRT) or oral contraceptives) may influence the hormonal environment. For example, Jethwa et al reported reduced RA disease activity in pregnancy and a flare in the postpartum period, while Bengtsson et al reported peak incidence of the disease at menopause.7 8 These observations suggest that a decrease in the levels of female sex hormones, such as estrogens and progesterone, increase the risk of RA throughout menopause and after childbirth, but an increase in levels during pregnancy and breastfeeding are protective. Elsewhere, age at menarche and menopause, parity, breastfeeding,9–14 and exposure to oral contraceptives and HRT15 16 have been observed to have conflicting effects on the risk of RA.
While there is a wealth of literature linking hormonal and reproductive factors to an increased risk of RA, female-specific hormonal and reproductive factors and the gender differences in RA present a burgeoning research path that, to our understanding, has not been entirely explored. Thus, the present study examined the reproductive factors and exogenous hormone use in relation to the risk of RA in women from the large cohort of the UK Biobank.
Methods
Study design, data sources and population
The UK Biobank is a prospective population-based cohort that recruited over 500 000 women and men (aged 40 to 69 years) between 2006 and 2010. It is a large biomedical database and high-quality research resource.17 The UK Biobank established 22 assessment centres across the UK (England, Scotland, and Wales) with support from the Wellcome Trust and the UK government. The UK Biobank has been approved by the Northwest Multicenter Research Ethics Committee. During the assessment, participants completed a touchscreen questionnaire, face-to-face interviews, physical measures, and provided biological samples, as detailed elsewhere.17 18 This study was conducted under project number 80 827. In our study, we selected all female subjects and excluded subjects based on the following exclusion criteria: subjects who had reported RA at baseline, covariates, and the absence of major hormonal and reproductive factors.
Biomarker values in serum and packed red blood cell samples were assessed at baseline for all UK Biobank participants. For the current study, if participants had data for a residual biomarker, very low levels of rheumatoid factor (RF) that were recorded as ‘missing’ in the original data were recoded conservatively as the square root of the minimal reported detectable value. With a threshold of 20 IU/mL, a new binary variable was constructed to divide RF levels into positive and negative groups (988646AR (beckmancoulter.com)).19
Measurement of hormonal and reproductive factors
Hormonal and reproductive factors were selected as exposure variables and the data provided at the participants’ self-assessment. The hormonal and reproductive factors studied in the present study include: age at menarche, pregnancy history, number of live children, menopause, age at menopause, reproductive years, history of hysterectomy, history of oophorectomy, contraceptive pill, duration of oral contraceptive pill use, HRT, and duration of HRT use (the field identifiers for all variables are listed in online supplemental table 1). For the purpose of this study, early menarche was defined as the first period occurring before the age of 12 years. Early menopause is the absence of menstrual periods permanently before the age of 45 years.20 21 Pregnancy history, reproductive years, duration of contraceptive pill use and duration of HRT use were defined according to the relative variables given in the UK Biobank. Pregnancy was determined by the number of children and whether there was a history of spontaneous abortion, stillbirth or termination of pregnancy. Reproductive years were taken as the time interval between the age at menarche and the age at menopause. The difference between the age of last use and the age of first use was used to determine the duration of oral contraceptive pills and HRT use.
rmdopen-2023-003338supp001.pdf (146.2KB, pdf)
Outcomes identification
The primary endpoint of this study was incident of RA. Subjects were determined to have RA by linking National Health Service (NHS) hospital admission records to the International Classification of Diseases, 10th revision (ICD-10) codes M05, M06 and M08 for RA. Each participant’s follow-up person-time was calculated from the date of initial assessment to the date of death, the first date of outcome diagnosis, the date of loss to follow-up, or the end of follow-up, whichever occurred first.
Covariate measures
The information obtained at the initial assessment visit was chosen as potential confounders of the association between hormonal and reproductive factors and risk of RA.22 23 Generally, the factors included: age at baseline, Townsend deprivation index, ethnicity, smoking status, alcohol drinker status, body mass index (BMI), total physical activity, vitamin D supplement, fracture history, diabetes, systolic blood pressure, and diastolic blood pressure. Information on ethnicity (white or other), smoking and alcohol drinker status (never, former, and current), vitamin D supplement (yes or no), history of fracture in the past 5 years (yes or no), and diabetes (yes or no) was collected from self-report questionnaires. The Townsend deprivation index was designed to measure the extent of socioeconomic deprivation. Higher values of this index indicated higher levels of socioeconomic deprivation.24 The total physical activity was divided into three mutually exclusive groups: low (<600 metabolic equivalents (MET)-min/week), moderate (600 to <3000 MET-min/week), and high (≥ 3000 MET-min/week).25 Its measurement was based on the revised International Physical Activity Questionnaire and included frequency and duration of walking on a typical day/week during the past 4 weeks (Field 864 and 874), moderate activity (Field 884 and 894) and vigorous activity (Field 904 and 914).26 The BMI was calculated by dividing the individual’s weight (kg), measured using the Paradigm BC-418 MA body composition analyser, by the square of the individual’s standing height (m). Blood pressure was measured at the baseline of the study using an Omron HEM-7015IT digital blood pressure monitor, as the average of the two measurements.
Statistical analysis
The variables used in this study were grouped based on the presence or absence of RA incidents. If the distribution of the variables conformed to a normal distribution, then the t-test was used; otherwise, the Wilcoxon rank-sum test was used. The χ2 test was used to compare categorical variables that were expressed as percentages (%). The earlier mentioned covariates were applied to fit two models. Model 1 was adjusted for age only, whereas model 2 was adjusted for age, ethnicity, Townsend deprivation index, smoking status, alcohol drinker status, BMI, vitamin D supplement, fracture history, diabetes, systolic blood pressure, and diastolic blood pressure. A dose-response relationship model fitted with a restricted cubic spline was used to determine the non-linear relationship between hormonal and reproductive factors and the probability of RA incidence in women. Univariate and multivariate Cox proportional hazard regression models were fitted to estimate hazard ratios (HR) and 95% confidence intervals (95% CI) for the hormonal and reproductive factors on RA outcomes, with change points as a reference. Furthermore, combination models for a series of hormonal and reproductive factors were constructed, adjusting for age at menarche, pregnancy or not, menopause or not, hysterectomy and/or oophorectomy, use of oral contraceptive pills and HRT, on the basis of model 2.
To evaluate the robustness of our findings, we performed a series of sensitivity analyses. First, participants who had the target outcome within 2 years of enrolment were removed and followed up. Second, participants who had undergone hysterectomy or oophorectomy were removed. Third, incomplete covariates in the data were re-filled for analysis using Multivariate Imputation by Chained Equations.
To examine the effect modifications by these characteristics, predefined subgroup analyses were conducted based on age group (≤60 and >60 years, grouped to produce approximately the same number of events in each group), social deprivation (determined using the Townsend deprivation index at or below vs above the national average (−1.42)), and BMI (≤25 kg/m2 and >25 kg/m2). The p value was obtained by fitting the interaction term between the predetermined subgroup and the exposure of interest.
All of the above analyses were performed using R 4.1.0 software, and p values <0.05 were considered statistically significant.
Results
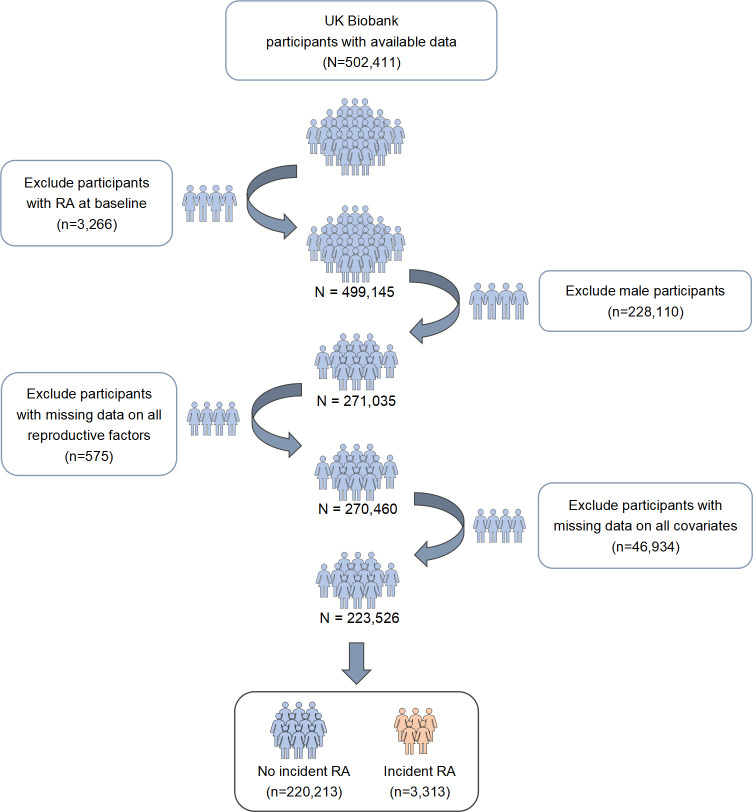
Figure 1 is a flow diagram showing the recruitment process of the participants. A total of 223 526 participants contributing 2 706 724 person-years at risk were included in the primary analysis. Of the total number of participants, 3313 (1.5%) individuals were first-ever diagnosed with RA during a median follow-up of 12.39 years. The baseline characteristics of the participants, based on whether RA occurred, are summarised in table 1. At the study baseline, the mean age of women and the mean age at menarche were 56 and 13 years, respectively. Approximately 85% of the participants reported having been pregnant at least once, and 43.9% reported having two children. For menopause, 72% of the women were post-menopausal; the mean age at menopause and reproductive years was 50 and 37 years, respectively. Meanwhile, a small number of women reported a history of hysterectomy (7%) and oophorectomy (8%), while 81.5% and 37.5% reported using oral contraceptive pills and HRT for a mean duration of 10 and 6 years, respectively.
Figure 1.
Flow diagram showing the recruitment process of participants. RA, rheumatoid arthritis.
Table 1.
Baseline characteristics of study participants in the UK Biobank
| Characteristics | Total participants (n=223 526) |
No incident RA (n=220 213) | Incident RA (n=3313) | P value |
| Age at baseline, years (mean±SD) | 56.2±8.02 | 56.2±8.02 | 59.3±7.20 | <0.001 |
| Townsend deprivation index (mean±SD) | -1.41±2.98 | -1.42±2.98 | -0.94±3.15 | <0.001 |
| Ethnicity, n (%) | <0.001 | |||
| White | 211 513 (94.6) | 208 425 (94.6) | 3088 (93.2) | |
| Other | 12 013 (5.4) | 11 788 (5.4) | 225 (6.8) | |
| Smoking status, n (%) | ||||
| Never smoker | 134 016 (60.0) | 132 305 (60.0) | 1711 (51.7) | <0.001 |
| Former smoker | 70 741 (31.6) | 69 514 (31.6) | 1227 (37.0) | |
| Current smoker | 18 769 (8.4) | 18 394 (8.4) | 375 (11.3) | |
| Alcohol drinker status, n (%) | <0.001 | |||
| Never drinker | 12 197 (5.5) | 11 901 (5.4) | 292 (8.8) | |
| Former drinker | 7557 (3.4) | 7348 (3.3) | 209 (6.3) | |
| Current drinker | 203 776 (91.1) | 200 964 (91.3) | 2812 (84.9) | |
| BMI, kg/m2 (mean±SD) | 26.9±5.01 | 26.8±4.99 | 28.4±5.70 | <0.001 |
| Physical activity, MET-min/week, n (%) | <0.001 | |||
| Low (<600) | 44 581 (19.9) | 43 794 (19.9) | 787 (23.8) | |
| Moderate (≥600, <3000) | 116 859 (52.3) | 115 272 (52.3) | 1587 (47.9) | |
| High (≥3000) | 62 086 (27.8) | 61 147 (27.8) | 939 (28.3) | |
| Vitamin D supplement, n (%) | 0.036 | |||
| No | 175 222 (78.4) | 172 575 (78.4) | 2647 (79.9) | |
| Yes | 48 304 (21.6) | 47 638 (21.6) | 666 (20.1) | |
| Fracture history, n (%) | <0.001 | |||
| No | 200 847 (89.9) | 197 979 (89.9) | 2868 (86.6) | |
| Yes | 22 679 (10.1) | 22 234 (10.1) | 445 (13.4) | |
| Diabetes, n (%) | <0.001 | |||
| No | 215 709 (96.5) | 212 614 (96.5) | 3095 (93.4) | |
| Yes | 7817 (3.5) | 7659 (3.5) | 218 (6.6) | |
| Blood pressure | ||||
| SBP, mm Hg (mean±SD) | 137.0±20.25 | 137.0±20.25 | 140.0±19.85 | <0.001 |
| DBP, mm Hg (mean±SD) | 80.0±10.54 | 80.0±10.54 | 81.0±10.42 | 0.006 |
| Rheumatoid factor status, n (%) | <0.001 | |||
| RF-negative | 202 037 (90.4) | 199 580 (90.6) | 2457 (74.2) | |
| RF-positive Missing |
7060 (3.2) 14 429 (6.4) |
6589 (3.0) 14 044 (6.4) |
471 (14.2) 385 (11.6) |
|
| Age at menarche, years (mean±SD) | 123.0±1.62 | 13.0±1.61 | 13.0±1.74 | 0.823 |
| Ever been pregnant, n (%) | <0.001 | |||
| No | 33 204 (14.8) | 32 787 (14.9) | 417 (12.6) | |
| Yes | 189 479 (84.8) | 186 592 (84.7) | 2887 (87.1) | |
| Missing | 843 (0.4) | 834 (0.4) | 9 (0.3) | |
| Number of children, n (%) | <0.001 | |||
| None | 42 427 (19.0) | 41 894 (19.0) | 533 (16.1) | |
| 1 | 29 814 (13.3) | 29 353 (13.3) | 461 (13.9) | |
| 2 | 98 159 (43.9) | 96 762 (43.9) | 1397 (42.2) | |
| 3 | 39 249 (17.6) | 38 635 (17.6) | 614 (18.5) | |
| ≥4 | 13 732 (6.1) | 13 426 (6.1) | 306 (9.2) | |
| Missing | 145 (0.1) | 143 (0.1) | 2 (0.1) | |
| Menopause, n (%) | <0.001 | |||
| No | 62 312 (27.9) | 61 831 (28.1) | 481 (14.5) | |
| Yes | 160 991 (72.0) | 158 164 (71.8) | 2827 (85.3) | |
| Missing | 223 (0.1) | 218 (0.1) | 5 (0.2) | |
| Age at menopause, years (mean±SD) | 50.0±5.08 | 50.0±5.07 | 50.0±5.95 | <0.001 |
| Reproductive years, years (mean±SD) | 37.0±5.32 | 37.0±5.30 | 36.0±6.09 | <0.001 |
| History of hysterectomy, n (%) | <0.001 | |||
| No | 182 785 (81.8) | 180 447 (81.9) | 2338 (70.5) | |
| Yes | 15 896 (7.1) | 15 500 (7.1) | 396 (12.0) | |
| Missing | 24 845 (11.1) | 24 266 (11.0) | 579 (17.5) | |
| History of oophorectomy, n (%) | <0.001 | |||
| No | 203 279 (90.9) | 200 418 (91.0) | 2861 (86.4) | |
| Yes | 17 368 (7.8) | 17 003 (7.7) | 365 (11.0) | |
| Missing | 2879 (1.3) | 2792 (1.3) | 87 (2.6) | |
| Ever used oral contraceptive pills, n (%) | <0.001 | |||
| No | 40 867 (18.3) | 40 100 (18.2) | 767 (23.2) | |
| Yes | 182 152 (81.5) | 179 616 (81.6) | 2536 (76.5) | |
| Missing | 507 (0.2) | 497 (0.2) | 10 (0.3) | |
| Duration of oral contraceptive pill use, years (mean±SD) | 10.4±7.68 | 10.4±7.68 | 9.8±7.65 | <0.001 |
| Ever used HRT, n (%) | <0.001 | |||
| No | 139 012 (62.2) | 137 479 (62.4) | 1533 (46.3) | |
| Yes | 83 911 (37.5) | 82 145 (37.3) | 1766 (53.3) | |
| Missing | 603 (0.3) | 589 (0.3) | 14 (0.4) | |
| Duration of HRT use, years (mean±SD) | 6.3±5.28 | 6.3±5.26 | 7.1±5.88 | <0.001 |
BMI, body mass index; DBP, diastolic blood pressure; HRT, hormone replacement therapy; MET, metabolic equivalents; RA, rheumatoid arthritis; RF, rheumatoid factor; SBP, systolic blood pressure.
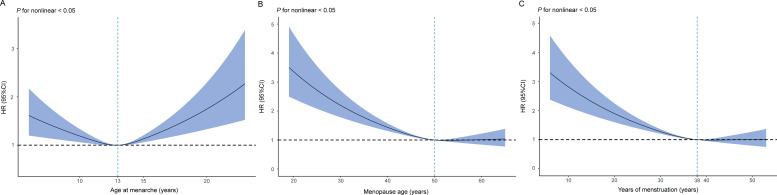
Age at menarche
The association between age at menarche and RA was U-shaped (table 2, figure 2A). Using women who had their menarche at 13 years as a reference, both early menarche (HR 1.19, 95% CI 1.07 to 1.32, p<0.001) and age at menarche >14 (HR 1.17, 95% CI 1.05 to 1.30, p=0.004) were associated with the occurrence of RA. However, the effect of early menarche on RA was weakened (HR 1.09, 95% CI 0.98 to 1.21, p=0.109), and age at menarche >14 still increased the risk of RA (HR 1.13, 95% CI 1.02 to 1.26, p=0.025), after adjusting for potential confounding factors.
Table 2.
Associations between hormonal and reproductive factors and the risk of rheumatoid arthritis
| Characteristics | Total population (n=223 526) |
Number incident cases (n=3313) |
Model 1 | Model 2 | ||
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age at menarche, years | ||||||
| <12 | 43 140 | 720 | 1.19 (1.07 to 1.32) | <0.001 | 1.09 (0.98 to 1.21) | 0.109 |
| 12 | 41 288 | 563 | 0.98 (0.88 to 1.10) | 0.755 | 0.96 (0.86 to 1.07) | 0.434 |
| 13 | 53 582 | 735 | Reference | Reference | ||
| 14 | 43 078 | 613 | 1.01 (0.91 to 1.12) | 0.873 | 1.00 (0.90 to 1.12) | 0.941 |
| >14 | 36 431 | 595 | 1.17 (1.05 to 1.30) | 0.004 | 1.13 (1.02 to 1.26) | 0.025 |
| Ever been pregnant | ||||||
| No | 33 204 | 417 | Reference | Reference | ||
| Yes | 189 479 | 2887 | 1.06 (0.96 to 1.18) | 0.261 | 1.04 (0.94 to 1.16) | 0.412 |
| Number of children | ||||||
| None | 42 427 | 533 | 1.04 (0.94 to 1.14) | 0.507 | 0.99 (0.89 to 1.10) | 0.840 |
| 1 | 29 814 | 461 | 1.19 (1.07 to 1.32) | <0.001 | 1.10 (0.99 to 1.23) | 0.066 |
| 2 | 98 159 | 1397 | Reference | Reference | ||
| 3 | 39 249 | 614 | 1.06 (0.96 to 1.17) | 0.224 | 1.00 (0.91 to 1.10) | 0.969 |
| ≥4 | 13 732 | 306 | 1.46 (1.29 to 1.66) | <0.001 | 1.18 (1.04 to 1.34) | 0.010 |
| Each child | 223 381 | 3311 | 1.05 (1.02 to 1.08) | <0.001 | 1.02 (0.99 to 1.05) | 0.186 |
| Menopause | ||||||
| No | 62 312 | 481 | Reference | Reference | ||
| Yes | 160 991 | 2827 | 1.24 (1.08 to 1.41) | 0.002 | 1.19 (1.05 to 1.36) | 0.008 |
| Age at menopause, years | ||||||
| <45 | 16 359 | 384 | 1.62 (1.42 to 1.86) | <0.001 | 1.46 (1.27 to 1.67) | <0.001 |
| 45–49 | 30 401 | 491 | 1.13 (0.99 to 1.28) | 0.065 | 1.08 (0.95 to 1.22) | 0.266 |
| 50–51 | 29 574 | 442 | Reference | Reference | ||
| 52–54 | 31 441 | 445 | 0.93 (0.82 to 1.06) | 0.287 | 0.94 (0.82 to 1.07) | 0.363 |
| >54 | 19 402 | 321 | 1.00 (0.86 to 1.15) | 0.983 | 0.99 (0.86 to 1.14) | 0.876 |
| Reproductive years | ||||||
| <33 | 22 078 | 495 | 1.53 (1.33 to 1.75) | <0.001 | 1.39 (1.21 to 1.59) | <0.001 |
| 33–35 | 18 962 | 297 | 1.08 (0.92 to 1.26) | 0.354 | 1.03 (0.88 to 1.20) | 0.696 |
| 36–37 | 21 136 | 310 | 0.98 (0.84 to 1.14) | 0.818 | 0.98 (0.84 to 1.14) | 0.769 |
| 38–39 | 23 513 | 355 | Reference | Reference | ||
| 40–42 | 25 320 | 372 | 0.94 (0.82 to 1.09) | 0.444 | 0.95 (0.82 to 1.10) | 0.472 |
| >42 | 13 638 | 221 | 0.98 (0.83 to 1.16) | 0.802 | 0.95 (0.81 to 1.13) | 0.586 |
| History of hysterectomy | ||||||
| No | 182 785 | 2338 | Reference | Reference | ||
| Yes | 15 896 | 396 | 1.51 (1.35 to 1.68) | <0.001 | 1.40 (1.25 to 1.56) | <0.001 |
| History of oophorectomy | ||||||
| No | 203 279 | 2861 | Reference | Reference | ||
| Yes | 17 368 | 365 | 1.28 (1.15 to 1.43) | <0.001 | 1.21 (1.08 to 1.35) | <0.001 |
| Ever used oral contraceptive pills | ||||||
| No | 40 867 | 767 | Reference | Reference | ||
| Yes | 182 152 | 2536 | 0.93 (0.86 to 1.02) | 0.106 | 0.99 (0.91 to 1.07) | 0.770 |
| Duration of oral contraceptive pill use, per year | 159 658 | 2195 | 1.00 (0.99 to 1.01) | 0.708 | 1.00 (1.00 to 1.01) | 0.793 |
| Ever used HRT | ||||||
| No | 139 012 | 1533 | Reference | Reference | ||
| Yes | 83 911 | 1766 | 1.46 (1.35 to 1.57) | <0.001 | 1.46 (1.35 to 1.57) | <0.001 |
| Duration of HRT use, per year | 61 660 | 1255 | 1.02 (1.01 to 1.03) | <0.001 | 1.02 (1.01 to 1.03) | <0.001 |
Model 1: adjusted for age.
Model 2: adjusted for age, Townsend deprivation index, ethnicity, smoking status, alcohol drinker status, body mass index, physical activity, vitamin D supplement, fracture history, diabetes, systolic blood pressure, and diastolic blood pressure.
HRT, hormone replacement therapy.
Figure 2.
Multiple adjusted restricted cubic splines showing hazard ratios (HR) for the risk of rheumatoid arthritis (RA) associated with reproductive factors. The HR for RA with the corresponding 95% CI as a function of reproductive factors from Cox proportional hazard regression models are adjusted for age, Townsend deprivation index, ethnicity, smoking status, alcohol drinker status, body mass index, physical activity, vitamin D supplement, fracture history, diabetes, systolic blood pressure, and diastolic blood pressure. (A) Restricted cubic spline plot with multiple adjusted HR (95% CI) for RA associated with age at menarche. (B) Restricted cubic spline plot with multiple adjusted HR (95% CI) for RA associated with age at first birth. (C) Restricted cubic spline plot with multiple adjusted HR (95% CI) for RA associated with reproductive years.
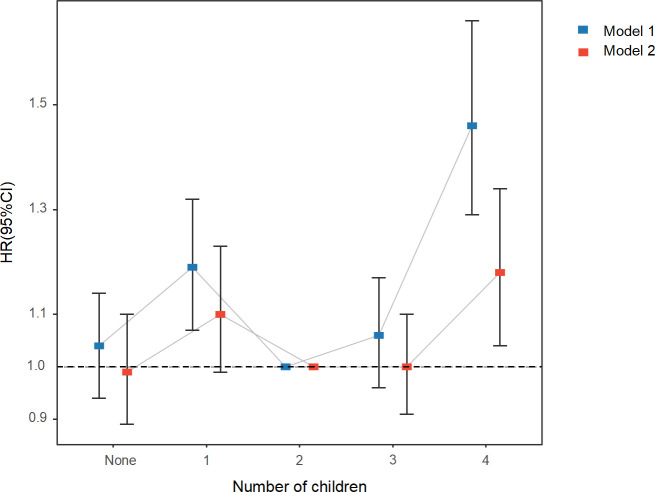
Pregnant and number of children
The risk of RA between women who had ever been pregnant and those who had never been pregnant was not statistically significant (HR 1.04, 95% CI 0.94 to 1.16, p=0.412) (table 2). However, the association between the number of children and RA was roughly U-shaped compared with women with two children (figure 3). Those who had four or more children were associated with a higher risk of RA (HR 1.18, 95% CI 1.04 to 1.34, p=0.010) (table 2).
Figure 3.
Adjusted hazard ratios for rheumatoid arthritis associated with the number of children. Model 1: adjusted for age. Model 2: adjusted for age, Townsend deprivation index, ethnicity, smoking status, alcohol drinker status, body mass index, physical activity, vitamin D supplement, fracture history, diabetes, systolic blood pressure, and diastolic blood pressure.
Menopause-related factors
An older age at menopause and longer reproductive years were found to be L-shaped associated with RA risk (table 2, figure 2B,C). Postmenopausal women showed a greater risk of RA (HR 1.19, 95% CI 1.05 to 1.36, p=0.008). The multiple-adjusted HR for RA in women who had menopause before the age of 45 years was 1.46 (95% CI 1.27 to 1.67, p<0.001) compared with women who had menopause at age 50–51 years. Furthermore, reproductive years were connected to the ages of menarche and menopause. Later menarche and early menopause were both risk factors for RA, while reproductive years <33 was associated with an increased risk of RA (HR 1.39, 95% CI 1.21 to 1.59, p<0.001). The multiple-adjusted HR for RA in women reporting a history of hysterectomy or oophorectomy were, respectively, 1.40 (95% CI 1.25 to 1.56, p<0.001) and 1.21 (95% CI 1.08 to 1.35, p<0.001) compared with women who had never had a hysterectomy or oophorectomy (table 2).
Exogenous hormone use
Results of the exogenous hormone use showed that there was no clear evidence that the use of oral contraceptive pills (HR 0.99, 95% CI 0.91 to 1.07, p=0.770) and the duration of use (HR 1.00, 95% CI 1.00 to 1.01, p=0.793) were associated with the risk of developing RA (table 2). On the other hand, it was revealed that HRT duration was associated with a higher risk of RA per year (HR 1.02, 95% CI 1.01 to 1.03, p<0.001). The adjusted HR for women who had used HRT was 1.46 (95% CI 1.35 to 1.57, p<0.001) compared to that of women who had never used HRT.
Sensitivity analysis
In the combination model, a total of 190 574 women developed 2622 cases of RA. Hormonal and reproductive factors were not significantly different in the composite model compared with models that included these factors alone, with only ovariectomy reporting an opposite result (online supplemental table 2).
Similar results were observed after removing participants who had a target outcome within 2 years of enrolment (online supplemental table 3). After women who had undergone hysterectomy or oophorectomy were excluded from the analysis, the associations between RA risk and age at menarche, number of children, reproductive years, HRT, and HRT duration were similar to the primary findings (online supplemental table 4). After filling in the missing data for the covariates, the results of the multiple adjustment were consistent with the main results of the analyses described above (online supplemental table 5).
Subgroup analysis
The association between early menarche, early menopause, history of hysterectomy or oophorectomy, use of oral contraceptive pills and HRT, and RA showed little heterogeneity in the subgroup. However, pregnancy and the number of children were heterogeneous in subgroups of those aged ≤60 years, those with a high Townsend deprivation index, and overweight or obese people. These were associated with a high risk of developing RA (online supplemental table 6).
Discussion
The high incidence of RA in women has been linked to reproductive hormones involved in the disease mechanism.27 This has prompted researchers to focus on the reproductive events that are closely related to estrogenic changes. In the present study, we examined the risk of RA cases associated with hormonal and reproductive factors in women. We relied on data from a prospective cohort study in the UK Biobank. The findings of this large population-based cohort study showed that age at menarche >14 years, ≥4 children, menopause, early menopause, reproductive years <33, history of hysterectomy or oophorectomy, and HRT use all increased the risk of RA to differing degrees.
As opposed to the current study, that showed no significant association between pregnancy and RA risks, previous epidemiological investigations found that multiple pregnancies increased the risk of developing RA in women of childbearing age.10 12 Pregnancy induces significant changes in endogenous oestrogen levels,28 and oestrogen can be neuroprotective or neurotoxic, depending on the concentration of oestrogen.29 Additionally, there are physiological changes in muscles and joints, changes in hormonal state, and a normal increase in weight during pregnancy.30 The results of the subgroup analysis indicated that the association between RA and pregnancy was not altered by overweight and obesity in the BMI. These results contradict previous studies that reported a high prevalence of RA in the overweight or obese population31 and pointed to the complexity of the relationship between pregnancy and RA. Briefly explained, motherhood comes with an increased workload at home. Having children over time32 may influence the complexity of the relationship between pregnancy and RA. As a result, having more than four children considerably increases the likelihood of developing RA in overweight and obese women, adding to their physical burden. Another plausible explanation may be related to the additional expenses and responsibilities. The high number of children may lead to financial hardship, which may have a more detrimental effect on mothers of lower socioeconomic status.33 34
Age at menarche >14 years, menopause, early menopause, and reproductive years <33 were all associated with a higher risk of RA incident. Results of two case-control studies suggest that early menarche is a protective factor for RA.16 35 Another cohort analysis based on the Nurses' Health Study showed that age at menarche ≤10 years was associated with an increased risk of RA.36 We discovered a possible U-shaped association between menarche age and the risk of RA. To some extent, both early menarche and age at menarche >14 years increased the risk of RA. In contrast, age at menarche >14 years increased the risk of RA incidence more than early menarche. Perhaps there is heterogeneity in the results of these studies because of the different definitions of early menarche. Both the age at menopause and reproductive years were negatively log-linearly associated with the risk of RA, and some stability was verified in sensitivity analyses and subgroup analyses. Other studies on the effects of menopause on the risk of RA have produced generally consistent results.7 22 35 37 38 It has been revealed that menopause is associated with a rapid decline in circulating estrogens.39 Oestrogen is a complex immune system regulator that inhibits helper T cell 1 (TH1) and TH17 cells through oestrogen receptor-α, and has pro-inflammatory effects on B cells and anti-inflammatory effects on T cells.40 41 Additionally, oestrogen seems to support regulatory T cells and TH2 cell-associated cytokine production, such as interleukin 6 (IL-6), IL-1β, and tumour necrosis factor α. However, low endogenous oestrogen concentrations may increase the risk of developing autoimmune rheumatic diseases mainly driven by T cells. Decreased oestrogen levels after menopause lead to chronic activation of the immune system, altering cytokines and immune cell profiles, directly or indirectly affecting the phenotype of fibroblast-like synoviocytes, osteoblasts or osteoclasts, and thereby damaging the skeletal system.3
Surgically-induced menopause (hysterectomy and oophorectomy) is an artificially premature cessation of endogenous hormone production and alteration of oestrogen levels.42 The current analysis supports the conclusion that both hysterectomy and oophorectomy increase the risk of RA. Exogenous hormone exposure includes oral contraceptives and HRT use. The current study found no risk effect of oral contraceptives on RA. This is consistent with a meta-analysis on cohort studies that reported no dose-response association between the length of oral contraceptive use and the risk of RA.43 However, previous studies have demonstrated that early oral contraceptive exposure significantly reduced the risk of developing RA.15 44 While our findings support HRT as a risk factor for RA, other studies have found that HRT has little effect on RA development.45 46 Paradoxically, HRT is the predominant treatment for the relief of menopausal symptoms.47 Therefore, the use of HRT may modify the circulating levels of other endogenous hormones, which may adversely affect joint health.
This study has several strengths. We used a well-characterised, large population-based cohort with an adequate sample size. More comprehensive information on hormonal and reproductive factors was collected prospectively, minimising bias associated with retrospective study design. The linkage with NHS hospital admission data provides strong validation of RA cases. We analysed as many hormonal and reproductive factors as possible, and also explored the non-linear relationship between reproductive factors and RA to provide more complete evidence on the factors affecting RA from a reproductive perspective. However, certain limitations remain. First, the UK Biobank population is a cohort predominantly comprising relatively healthy and affluent people of white ethnic background, so it is unlikely to produce reliable estimates of either the prevalence of female reproductive factors or the risk of RA in the UK population at large. Second, the acquirement of some demographic and clinical information was based on individuals who self-reported, which might be subject to possible measurement error. Third, residual effects from other unadjusted confounders might still exist despite adjusting as much as possible for strongly influential confounders. Fourth, because using the date of the initial assessment as the start of follow-up may be affected by left-truncated bias,48 conclusions need to be drawn with caution. Furthermore, the UK Biobank database did not provide the RF levels during the diagnostic process, thus we could not analyse the associations of hormonal and reproductive factors with RF.
In conclusion, this large prospective study among 223 526 women in the UK Biobank indicates that several hormonal and reproductive factors were associated with the risk of RA. When diagnosing and managing women with RA, hormonal and reproductive aspects should be carefully evaluated. In particular, women in later menarche or early menopause require additional attention. The findings of this study are significant and form a basis on which novel and target-specific intervention measures to curb the risk of RA in women may be developed. Furthermore, future studies should investigate the involvement of female hormones in the pathophysiology of RA.
Acknowledgments
This research was conducted using the UK Biobank study under Application Number 80827. We want to thank all UK Biobank participants and the management team for their participation and assistance.
Footnotes
L-QJ and R-DZ contributed equally.
Contributors: All authors were involved in drafting the article or revising it critically for important intellectual contact, and all authors approved the final version to be published. H-FP and JN had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. H-FP accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. L-QJ and R-DZ contributed to the acquisition, analysis, interpretation of the data and wrote the manuscript. H-AM, H-YZ, HT and TT contributed to the analysis and interpretation of the data. Y-SH, C-NZ, Z-XG, FY and PW contributed to the statistical expertise. JN contributed to the conception and design.
Funding: This study was funded by grants from the National Natural Science Foundation of China (82273710), Anhui Provincial Natural Science Foundation (2108085Y26, 2108085QH361) and Research Fund of Anhui Institute of Translational Medicine (2021zhyx-B04).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. The UK Biobank data that support the findings of this study are available from the UK Biobank (www.ukbiobank.ac.uk), subject to approval by UK Biobank.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. The UK Biobank has been approved by the Northwest Multicenter Research Ethics Committee (16/NW/0274). All participants gave written informed consent. Participants gave informed consent to participate in the study before taking part.
References
- 1.Smith MH, Berman JR. What is rheumatoid arthritis? JAMA 2022;327:1194. 10.1001/jama.2022.0786 [DOI] [PubMed] [Google Scholar]
- 2.Cush JJ. Rheumatoid arthritis: early diagnosis and treatment. Med Clin North Am 2021;105:355–65. 10.1016/j.mcna.2020.10.006 [DOI] [PubMed] [Google Scholar]
- 3.Alpízar-Rodríguez D, Pluchino N, Canny G, et al. The role of female hormonal factors in the development of rheumatoid arthritis. Rheumatology (Oxford) 2017;56:1254–63. 10.1093/rheumatology/kew318 [DOI] [PubMed] [Google Scholar]
- 4.van Vollenhoven RF. Sex differences in rheumatoid arthritis: more than meets the eye. BMC Med 2009;7:12. 10.1186/1741-7015-7-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokka T, Toloza S, Cutolo M, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther 2009;11:R7. 10.1186/ar2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raine C, Giles I. What is the impact of sex hormones on the pathogenesis of rheumatoid arthritis? Front Med (Lausanne) 2022;9:909879. 10.3389/fmed.2022.909879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bengtsson C, Malspeis S, Orellana C, et al. Association between menopausal factors and the risk of seronegative and seropositive rheumatoid arthritis: results from the nurses' health studies. Arthritis Care Res (Hoboken) 2017;69:1676–84. 10.1002/acr.23194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jethwa H, Lam S, Smith C, et al. Does rheumatoid arthritis really improve during pregnancy? A systematic review and metaanalysis. J Rheumatol 2019;46:245–50. 10.3899/jrheum.180226 [DOI] [PubMed] [Google Scholar]
- 9.Pikwer M, Bergström U, Nilsson J-A, et al. Breast feeding, but not use of oral contraceptives, is associated with a reduced risk of rheumatoid arthritis. Ann Rheum Dis 2009;68:526–30. 10.1136/ard.2007.084707 [DOI] [PubMed] [Google Scholar]
- 10.Peschken CA, Robinson DB, Hitchon CA, et al. Pregnancy and the risk of rheumatoid arthritis in a highly predisposed North American native population. J Rheumatol 2012;39:2253–60. 10.3899/jrheum.120269 [DOI] [PubMed] [Google Scholar]
- 11.Adab P, Jiang CQ, Rankin E, et al. Breastfeeding practice, oral contraceptive use and risk of rheumatoid arthritis among Chinese women: the Guangzhou Biobank cohort study. Rheumatology (Oxford) 2014;53:860–6. 10.1093/rheumatology/ket456 [DOI] [PubMed] [Google Scholar]
- 12.Orellana C, Wedrén S, Källberg H, et al. Parity and the risk of developing rheumatoid arthritis: results from the Swedish epidemiological investigation of rheumatoid arthritis study. Ann Rheum Dis 2014;73:752–5. 10.1136/annrheumdis-2013-203567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orellana C, Saevarsdottir S, Klareskog L, et al. Oral contraceptives, breastfeeding and the risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis 2017;76:1845–52. 10.1136/annrheumdis-2017-211620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eun Y, Jeon KH, Han K, et al. Menopausal factors and risk of seropositive rheumatoid arthritis in postmenopausal women: a nationwide cohort study of 1.36 million women. Sci Rep 2020;10:20793. 10.1038/s41598-020-77841-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doran MF, Crowson CS, O’Fallon WM, et al. The effect of oral contraceptives and estrogen replacement therapy on the risk of rheumatoid arthritis: a population based study. J Rheumatol 2004;31:207–13. [PubMed] [Google Scholar]
- 16.Pedersen M, Jacobsen S, Klarlund M, et al. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res Ther 2006;8:R133. 10.1186/ar2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer LJ. UK Biobank: bank on it. Lancet 2007;369:1980–2. 10.1016/S0140-6736(07)60924-6 [DOI] [PubMed] [Google Scholar]
- 19.McQueenie R, Nicholl BI, Jani BD, et al. Patterns of multimorbidity and their effects on adverse outcomes in rheumatoid arthritis: a study of 5658 UK Biobank participants. BMJ Open 2020;10:e038829. 10.1136/bmjopen-2020-038829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu D, Chung H-F, Dobson AJ, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health 2019;4:e553–64. 10.1016/S2468-2667(19)30155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong J, Harris K, Peters SAE, et al. Reproductive factors and the risk of incident dementia: a cohort study of UK Biobank participants. PLoS Med 2022;19:e1003955. 10.1371/journal.pmed.1003955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salliot C, Nguyen Y, Gusto G, et al. Female hormonal exposures and risk of rheumatoid arthritis in the French E3N-EPIC cohort study. Rheumatology (Oxford) 2021;60:4790–800. 10.1093/rheumatology/keab101 [DOI] [PubMed] [Google Scholar]
- 23.Mazzucca CB, Scotti L, Cappellano G, et al. Nutrition and rheumatoid arthritis onset: a prospective analysis using the UK Biobank. Nutrients 2022;14:1554. 10.3390/nu14081554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Townsend P. Deprivation and ill health. Nursing (Lond) 1991;4:11–5. [PubMed] [Google Scholar]
- 25.Chudasama YV, Khunti KK, Zaccardi F, et al. Physical activity, multimorbidity, and life expectancy: a UK Biobank longitudinal study. BMC Med 2019;17:108. 10.1186/s12916-019-1339-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 27.Gerosa M, De Angelis V, Riboldi P, et al. Rheumatoid arthritis: a female challenge. Womens Health (Lond) 2008;4:195–201. 10.2217/17455057.4.2.195 [DOI] [PubMed] [Google Scholar]
- 28.Tulchinsky D, Hobel CJ, Yeager E, et al. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. Am J Obstet Gynecol 1972;112:1095–100. 10.1016/0002-9378(72)90185-8 [DOI] [PubMed] [Google Scholar]
- 29.Liu JH. Does estrogen provide "neuroprotection" for postmenopausal women? Menopause 2019;26:1361–2. 10.1097/GME.0000000000001459 [DOI] [PubMed] [Google Scholar]
- 30.Borg-Stein J, Dugan SA. Musculoskeletal disorders of pregnancy, delivery and postpartum. Phys Med Rehabil Clin N Am 2007;18:459–76, 10.1016/j.pmr.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 31.Onuora S. Rheumatoid arthritis: obesity skews markers of inflammation. Nat Rev Rheumatol 2017;13:323. 10.1038/nrrheum.2017.62 [DOI] [PubMed] [Google Scholar]
- 32.Bliddal M, Pottegård A, Kirkegaard H, et al. Association of pre-pregnancy body mass index, pregnancy-related weight changes, and parity with the risk of developing degenerative musculoskeletal conditions. Arthritis Rheumatol 2016;68:1156–64. 10.1002/art.39565 [DOI] [PubMed] [Google Scholar]
- 33.Bengtsson C, Nordmark B, Klareskog L, et al. Socioeconomic status and the risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis 2005;64:1588–94. 10.1136/ard.2004.031666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massardo L, Pons-Estel BA, Wojdyla D, et al. Early rheumatoid arthritis in Latin America: low socioeconomic status related to high disease activity at baseline. Arthritis Care Res (Hoboken) 2012;64:1135–43. 10.1002/acr.21680 [DOI] [PubMed] [Google Scholar]
- 35.Pikwer M, Bergström U, Nilsson J-Å, et al. Early menopause is an independent predictor of rheumatoid arthritis. Ann Rheum Dis 2012;71:378–81. 10.1136/ard.2011.200059 [DOI] [PubMed] [Google Scholar]
- 36.Karlson EW, Mandl LA, Hankinson SE, et al. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the nurses' health study. Arthritis Rheum 2004;50:3458–67. 10.1002/art.20621 [DOI] [PubMed] [Google Scholar]
- 37.Alpizar-Rodriguez D, Mueller RB, Möller B, et al. Female hormonal factors and the development of anti-citrullinated protein antibodies in women at risk of rheumatoid arthritis. Rheumatology (Oxford) 2017;56:1579–85. 10.1093/rheumatology/kex239 [DOI] [PubMed] [Google Scholar]
- 38.Park EH, Kang EH, Lee YJ, et al. Impact of early age at menopause on disease outcomes in postmenopausal women with rheumatoid arthritis: a large observational cohort study of Korean patients with rheumatoid arthritis. RMD Open 2023;9:e002722. 10.1136/rmdopen-2022-002722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burger HG, Dudley EC, Hopper JL, et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab 1999;84:4025–30. 10.1210/jcem.84.11.6158 [DOI] [PubMed] [Google Scholar]
- 40.Pfeilschifter J, Köditz R, Pfohl M, et al. Changes in proinflammatory cytokine activity after menopause. Endocr Rev 2002;23:90–119. 10.1210/edrv.23.1.0456 [DOI] [PubMed] [Google Scholar]
- 41.Cutolo M, Gotelli E. Complex role of oestrogens in the risk and severity of rheumatoid arthritis in menopause. RMD Open 2023;9:e003176. 10.1136/rmdopen-2023-003176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause 2007;14:572–9. 10.1097/gme.0b013e31803df49c [DOI] [PubMed] [Google Scholar]
- 43.Chen Q, Jin Z, Xiang C, et al. Absence of protective effect of oral contraceptive use on the development of rheumatoid arthritis: a meta-analysis of observational studies. Int J Rheum Dis 2014;17:725–37. 10.1111/1756-185X.12413 [DOI] [PubMed] [Google Scholar]
- 44.Brennan P, Bankhead C, Silman A, et al. Oral contraceptives and rheumatoid arthritis: results from a primary care-based incident case-control study. Semin Arthritis Rheum 1997;26:817–23. 10.1016/s0049-0172(97)80025-x [DOI] [PubMed] [Google Scholar]
- 45.Merlino LA, Cerhan JR, Criswell LA, et al. Estrogen and other female reproductive risk factors are not strongly associated with the development of rheumatoid arthritis in elderly women. Semin Arthritis Rheum 2003;33:72–82. 10.1016/s0049-0172(03)00084-2 [DOI] [PubMed] [Google Scholar]
- 46.Koepsell TD, Dugowson CE, Nelson JL, et al. Non-contraceptive hormones and the risk of rheumatoid arthritis in menopausal women. Int J Epidemiol 1994;23:1248–55. 10.1093/ije/23.6.1248 [DOI] [PubMed] [Google Scholar]
- 47.Lobo RA. Hormone-replacement therapy: current thinking. Nat Rev Endocrinol 2017;13:220–31. 10.1038/nrendo.2016.164 [DOI] [PubMed] [Google Scholar]
- 48.Cain KC, Harlow SD, Little RJ, et al. Bias due to left truncation and left censoring in longitudinal studies of developmental and disease processes. Am J Epidemiol 2011;173:1078–84. 10.1093/aje/kwq481 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003338supp001.pdf (146.2KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The UK Biobank data that support the findings of this study are available from the UK Biobank (www.ukbiobank.ac.uk), subject to approval by UK Biobank.