Abstract
In Bordetella species, the BvgAS sensory transduction system mediates an alteration between the Bvg+ phase, characterized by expression of adhesins and toxins, and the Bvg− phase, characterized by the expression of motility and coregulated phenotypes in Bordetella bronchiseptica and by the expression of vrg loci in Bordetella pertussis. Since there is no known environmental or animal reservoir for B. pertussis, the causative agent of whooping cough, it has been assumed that this phenotypic alteration must occur within the human host during infection. Consistent with this hypothesis was the observation that a B. pertussis mutant, SK6, containing a TnphoA insertion mutation in a Bvg-repressed gene (vrg6) was defective for tracheal and lung colonization in a mouse model of respiratory infection (D. T. Beattie, R. Shahin, and J. Mekalanos, Infect. Immun. 60:571–577, 1992). This result was inconsistent, however, with the observation that a Bvg+ phase-locked B. bronchiseptica mutant was indistinguishable from the wild type in its ability to establish a persistent respiratory infection in rabbits and rats (P. A. Cotter and J. F. Miller, Infect. Immun. 62:3381–3390, 1994; B. J. Akerley, P. A. Cotter, and J. F. Miller, Cell 80:611–620, 1995). To directly address the role of Bvg-mediated signal transduction in B. pertussis pathogenesis, we constructed Bvg+ and Bvg− phase-locked mutants and compared them with the wild type for their ability to colonize the respiratory tracts of mice. Our results show that the Bvg+ phase of B. pertussis is necessary and sufficient for respiratory infection. By constructing a strain with a deletion in the bvgR regulatory locus, we also show that ectopic expression of Bvg− phase phenotypes decreases the efficiency of colonization, underscoring the importance of Bvg-mediated repression of gene expression in vivo. Finally, we show that the virulence defect present in strain SK6 cannot be attributed to the vrg6 mutation. These data contradict an in vivo role for the Bvg− phase of B. pertussis.
All of the known protein virulence factors expressed by Bordetella pertussis, the causative agent of whooping cough, are positively regulated by the BvgAS sensory transduction system (for reviews, see references 10, 32, and 33). When active, BvgAS also represses a class of genes (vrg genes) and outer membrane proteins (Vra proteins) of unknown function (16, 31). Hence, BvgAS mediates a phenotypic transition between the Bvg+ phase, characterized by the expression of adhesins and toxins, and the Bvg− phase, characterized by the expression of vrg genes and Vra proteins. Transition from the Bvg+ phase to the Bvg− phase has been termed phenotypic modulation. Since B. pertussis has no known environmental or animal reservoir, it has been assumed that phenotypic modulation must occur within the human host. Proposed roles for a switch to the Bvg− phase in vivo include evasion of antibodies directed primarily against Bvg+ phase factors, tempering of damage to host tissues as a result of decreased toxin expression, increased transmission as a result of decreased adhesin expression, and a requirement for Bvg− phase factors for the initial interaction with the host or for surviving within host cells (6, 9, 17, 20, 25). Experimental evidence that B. pertussis switches to the Bvg− phase in vivo is limited to the observation that a B. pertussis mutant containing a transposon insertion in a vrg locus (vrg6) was defective for virulence in a mouse model (6).
Studies with Bordetella bronchiseptica, a very closely related member of the Bordetella genus, contradict the hypothesis that Bordetella switches to the Bvg− phase in vivo. B. bronchiseptica causes respiratory infections in a wide range of nonhuman mammals, including dogs, pigs, rabbits, rats, and mice. It contains a BvgAS sensory transduction system with 96% amino acid identity to that of B. pertussis and expresses a nearly identical set of Bvg-activated adhesins and toxins (4, 13). Studies with Bvg+ and Bvg− phase-locked mutants showed that the Bvg+ phase of B. bronchiseptica is necessary and sufficient for respiratory infection, while the Bvg− phase is required for surviving nutrient limitation (1, 11). Moreover, failure to repress a Bvg− phase phenotype (motility) was detrimental to the development of infection, demonstrating the importance of Bvg-mediated repression of gene expression in vivo (1). These results led to the hypothesis that the role of BvgAS is to sense whether the organism is within or outside a mammalian host.
A possible explanation for these apparently contradictory results is that BvgAS plays different roles for these two species, sensing whether the organism is within or outside a mammalian host in the case of B. bronchiseptica and sensing specific niches within the host in the case of B. pertussis. In support of this hypothesis, the Bvg− phases of these organisms appear to differ dramatically (2, 6), and their BvgAS virulence control systems were recently shown to differ in their sensing capabilities (19). Alternatively, we have proposed that BvgAS may have originally evolved to serve the same purpose in both species, sensing whether the organism is within or outside its host, but that survival outside the host is no longer a significant part of the life cycle for B. pertussis (19). Here we report an experimental assessment of the role of BvgAS-mediated signal transduction in B. pertussis pathogenesis as a means of testing these opposing hypotheses. We constructed B. pertussis phase-locked and ectopic expression mutants and compared them with the wild type in a mouse model of respiratory infection. We also addressed the role of vrg6 expression during infection by using newly constructed vrg6 mutants and strains containing reporter fusions designed to detect vrg6 expression in vivo. Our results indicate that, like in B. bronchiseptica, the Bvg+ phase of B. pertussis is necessary and sufficient for respiratory infection and that ectopic expression of Bvg-repressed phenotypes under Bvg+ phase conditions is detrimental to the infection process. We also demonstrate that the virulence defect in the original vrg6 mutant, SK6, cannot be attributed to the vrg6 mutation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. pertussis strains are described in Table 1 and in the figure legends. B. pertussis SK6 and its parental strain, 18323, were kindly provided by D. Beattie and R. Shahin, respectively. B. pertussis strains were grown on BG agar (BBL, Becton Dickinson, Cockeysville, Md.) supplemented with 15% defibrinated sheep blood (Mission Labs, Rosemead, Calif.). Plates were incubated at 37°C for 72 to 96 h in loosely fitted screw-top jars to provide a moist environment. When mid-log-phase cells were needed, B. pertussis strains were grown in Stainer-Scholte (SS) medium (29) supplemented with 1 g of heptakis (15) per liter at 37°C with constant shaking. To grow B. pertussis under modulating (Bvg− phase) conditions, MgSO4 (20 mM) and nicotinic acid (5 mM) were added to the culture medium. When appropriate, antibiotics (Sigma Chemical Co., St. Louis, Mo.) were used at the following final concentrations: cephalexin, 10 μg/ml; gentamicin, 20 μg/ml; ampicillin, 30 μg/ml; kanamycin, 50 μg/ml; tetracycline, 10 μg/ml; streptomycin, 20 μg/ml; and chloramphenicol, 50 μg/ml. Escherichia coli strains were grown on Luria-Bertani (LB) agar or in Luria-Bertani broth (26) supplemented, when appropriate, with ampicillin (100 μg/ml) or gentamicin (20 μg/ml). E. coli DH5α (Stratagene, La Jolla, Calif.) was used in all of the cloning steps, and E. coli SM10 (28) was used to mobilize plasmids into B. pertussis.
TABLE 1.
Strains used in this study
| Species and strain | Characteristics | Source or reference |
|---|---|---|
| B. pertussis | ||
| 18323 (ATCC 9797) | Wild type, Sms | ATCC |
| SK6 | vrg6::TnphoA Smr; original mutant | 6 |
| NSK6 | vrg6::TnphoA Smr; 18323 transductant | This work |
| SC3 | bvgS-C3 Sms | This work |
| D6 | Δvrg6 Sms; in-frame deletion in vrg6 | This work |
| DR | ΔbvgR Sms; in-frame deletion in bvgR | This work |
| DS1 | bvgS::pGMT74 Sms; bvgS disruption | This work |
| SC3-SK6 | bvgS-C3 vrg6::TnphoA Smr; SC3 transductant | This work |
| DR-SK6 | ΔbvgR vrg6::TnphoA Smr; DR transductant | This work |
| DS1-SK6 | bvgS::pGMT74 vrg6::TnphoA Smr | This work |
| BP121 | vrg6::TnphoA′-′tnpR-res-tet-res-′vrg6 Smr | This work |
| BP147 | vrg6::TnphoA′-′tnpR-res-tet-res-vrg6 Smr | This work |
| NBP121 | vrg6::TnphoA′-′tnpR-res-tet-res-′vrg6 Smr; 18323 transductant | This work |
| NBP147 | vrg6::TnphoA′-′tnpR-res-tet-res-vrg6 Smr; 18323 transductant | This work |
| E. coli | ||
| DH5α | F−hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(argF-lac) U169 φ80dlacZΔM15 | Bethesda Research Laboratories |
| SM10 | RP4-2-TcMu Kmr | 28 |
| DM1187(pCLB1) | Colicin B-expressing strain | Theresa Finn |
Electroporation.
Cells were grown in SS medium to mid-exponential phase, chilled on ice for 10 min, and harvested by centrifugation (7,000 × g, 4°C). After two washes with ice-cold H2O, cells were resuspended in ice-cold H2O and the concentration was adjusted so that the optical density at 600 nm was 5.0. Forty microliters of this suspension was mixed with 1 μg of desalted plasmid DNA in a prechilled electroporation cuvette (Bio-Rad). After application of the electric pulse (25 μF, 2,500 V, 200 Ω; Bio-Rad Gene Pulser), cells were allowed to outgrow in 1 ml of SS medium for 60 min at 37°C, concentrated by centrifugation, and plated on selective BG-blood agar.
Conjugation and allelic exchange.
Matings were performed as previously described (19). To counterselect against the donor strain, mating products were plated on BG blood agar supplemented with a colicin B-enriched bacterial lysate (approximately 5 mg of total protein per ml), which was prepared from the colicin-producing E. coli strain DM1187(pCLB1) (a gift of Theresa Finn) as previously described (7). For allelic exchange, the sacBR-based system was used (1). Bacteria that had undergone a second recombination event resulting in loss of plasmid sequences were selected on BG-blood agar containing 10% sucrose.
Construction of B. pertussis phase-locked and deletion mutants.
A Bvg+ phase-locked derivative of strain 18323 was constructed as follows. Suicide plasmid pJM503, which contains a 2.3-kb SfiI fragment of ′bvgS′ containing the bvgS-C3 mutation (23), was electroporated into strain 18323, and cointegrates were selected on BG-blood agar supplemented with gentamicin. As expected, cointegrates grew as large, flat, nonhemolytic colonies characteristic of the Bvg− phase. Although pJM503 contains the rpsL gene, encoding streptomycin sensitivity (Sms), this selection was not used because the 18323 parental strain is Sms. Instead, two cointegrates were picked, grown without antibiotic selection, and plated on BG agar containing 40 mM nicotinic acid and 15 mM MgSO4 to screen phenotypically for colonies in which a second recombination event resulting in loss of plasmid sequences had occurred. Small, domed, hemolytic colonies on this medium, indicative of the Bvgc phenotype, were characterized further. These colonies were gentamicin sensitive (Gms), indicating that they had indeed lost plasmid pJM503. One was named SC3 and was used in all subsequent analyses.
A Bvg− derivative of 18323 was constructed by creating a disruption in bvgS by using plasmid pGMT74. pGMT74 is a suicide plasmid containing a 1.9-kb EcoRI-SnaBI internal bvgS fragment. Integration of this plasmid into strain 18323 disrupts bvgS, rendering the strain phenotypically Bvg− under all growth conditions. Integration of pGMT74 is not expected to have polar effects, as the gene 3′ to bvgAS, bvgR, is transcribed in the orientation opposite to that for bvgAS (reference 21 and our unpublished data). A Bvg− derivative of SK6 (DS1-SK6) was constructed by mobilization of pGMT74 into SK6.
Strain D6, containing an in-frame deletion in vrg6, was constructed as follows. An 818-bp EcoRI fragment containing vrg6 was amplified from the 18323 chromosome by PCR with oligonucleotides predicted to anneal at positions 1 (5′-GAATTCCGTCTGCTGAACCAGA-3′) and 792 (3′) (5′-GAATTCGCATAACGGCTGGTGGAAGG-3′) of the published sequence (6). The PCR product was digested with EcoRI and cloned into EcoRI-digested pUC19 to create pGMT42. To generate an in-frame deletion in vrg6, pGMT42 was digested with EcoRV and NcoI, filled in with Klenow fragment, and religated, resulting in the deletion of 0.2 kb of DNA corresponding to approximately 70% of vrg6. The deletion junction was confirmed to have occurred as intended by DNA sequence analysis. Sequences at the 5′ end of vrg6 reported to be essential for maintaining Bvg-dependent regulation were left intact to avoid polar effects on downstream genes. The 0.6-kb EcoRI fragment was then cloned into plasmid pEG25 (19), and the resulting plasmid, containing the sacBR cassette, was used to transfer the vrg6 deletion to the chromosome of strain 18323. PCR and Southern blot analyses confirmed that D6 was constructed as intended.
To construct an 18323 derivative containing an in-frame deletion in bvgR, a 2.5-kb BclI-EcoRV fragment containing the bvgR locus from B. pertussis 338 was cloned into FspI-BamHI-digested pACYC177. The resulting plasmid was digested with FspI and ScaI and religated, resulting in the deletion of 425 bp of DNA containing approximately 66% of bvgR. The deletion junction was confirmed to result in an in-frame deletion by DNA sequence analysis. The resulting ΔbvgR allele, contained on a 0.9-kb SalI-XhoI fragment, was then cloned into plasmid pEG25 (19) and used to transfer the ΔbvgR allele to the chromosome of strain 18323. Southern blot analysis confirmed that DR was constructed as intended.
Construction of B. pertussis strains containing resolvase reporters of vrg6 expression.
Strains BP121 and BP147, containing tnpR-res-tet-res cassettes for assessing vrg6 transcription, were constructed as follows. A plasmid derivative of pSS1129 containing a ′tnpR-res-tet-res-neo cassette (8) flanked by phoA sequences at the 5′ end and vrg6 3′ sequences at the 3′ end was used to transfer the ′tnpR-res-tet-res-neo cassette to the chromosome of SK6 by allelic exchange, resulting in the construction of BP121. In this strain, transcription of vrg6 drives expression of the promoterless ′tnpR. The tnpR gene product mediates site-specific recombination between the res sequences, resulting in the excision of the tet gene. vrg6 expression therefore results in the loss of tetracycline resistance (Tcr). Strain BP147 is identical to BP121 except that a complete copy of vrg6 was provided at the 3′ end of the construct such that this strain contains a wild-type copy of vrg6. Southern blots confirmed that BP121 and BP147 were constructed as intended.
Construction of B. pertussis strains by generalized transduction.
A Bordetella-specific bacteriophage capable of mediating generalized transduction was recently discovered in our laboratory (18a). This phage, designated BP3c, was used to transduce the vrg6-phoA fusion and linked sequences from B. pertussis SK6, BP121, and BP147 to the wild-type strain 18323 to create NSK6, NBP121, and NBP147, respectively. SK6, BP121, and BP147 are kanamycin resistant (Kmr) due to the Kmr gene contained on the TnphoA element. They are also Smr. The basis for their being Smr is unknown, as they are derivatives of 18323, which is Sms. Transductants were selected on BG-blood agar containing kanamycin. Unexpectedly, all transductants were found to be Smr, suggesting that the Kmr gene contained on TnphoA also confers Smr to B. pertussis. Southern blot analysis indicated that the genomic organizations of the vrg6-phoA regions were indistinguishable between SK6, BP121, and BP147 and NSK6, NBP121, and NBP147, respectively. This transduction protocol was also used to transfer the vrg6-phoA fusion from SK6 to SC3 and DR, creating SC3-SK6 and DR-SK6, respectively. Details of BP3c-mediated generalized transduction will be described elsewhere.
Western immunoblotting.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was performed by the method of Laemmli (18). B. pertussis whole-cell lysates solubilized in sample buffer (60 mM Tris, 2% SDS, 10% glycerol, 0.005% bromophenol blue, 0.1 M dithiothreitol, pH 6.8) were stacked in a 5% polyacrylamide gel and separated in a 4 to 12% acrylamide-bisacrylamide (29:1) linear gradient gel. Proteins were transferred to Immobilon-P membranes (Millipore Corp., Bedford, Mass.) and probed with a 1:2,500 dilution of serum from a patient recovering from whooping cough (a gift of James Cherry, UCLA Department of Pediatrics) or a 1:10 dilution of anti-VraB monoclonal antibody (a gift of Mark Peppler [31]). Sheep anti-human or sheep anti-mouse horseradish peroxidase-conjugated secondary antibodies (Amersham International, Little Chalfont, United Kingdom) were used at a dilution of 1:5,000. The immunocomplexes were detected by using an enhanced chemiluminescence assay (Amersham) according to the manufacturer’s directions.
Experimental animals.
Three-week-old, Bordetella-free, female BALB/cAnNCR mice obtained from Charles River Laboratories were used in this study. Inocula were prepared by growing B. pertussis strains on BG-blood agar for 3 days at 37°C and then suspending the harvested cells in sterile phosphate-buffered saline (PBS). Mice were inoculated intranasally with 50 μl of PBS containing 104 CFU while the animals were slightly anesthetized with halothane. At the indicated times postinoculation, mice were sacrificed by halothane inhalation, the chest cavity was opened, and blood was obtained by cardiac puncture. One centimeter of mid-trachea and the right lung lobes were removed aseptically, homogenized in PBS, diluted, and plated on BG-blood agar. The nasal cavity was opened, and the nasal septum and adjacent turbinates were removed, homogenized in PBS, diluted, and plated. Animal protocols were approved by the University of California, Los Angeles, Animal Research Committee (ARC protocol 94-043). Statistical significance was determined by using a paired t test (P ≤ 0.05).
Alkaline phosphatase activity assays.
Alkaline phosphatase activity was measured by a published method (22).
Resolution of res-tet-res sequences.
To determine the resolution frequencies of res-tet-res sequences in NBP121 and NBP147 in vitro, NBP121 and NBP147 were grown on BG-blood agar with or without 5 mM nicotinic acid and 20 mM MgSO4 for 72 h at 37°C, and cells were harvested and plated on BG-blood agar without tetracycline and then replica plated onto BG-blood agar with and without tetracycline. Percent resolution was defined as number of Tcs colonies/total number of colonies. The percentage of Tcs colonies was also determined by individually patching colonies onto BG-blood agar with and without tetracycline. To determine the resolution frequency following in vivo growth, colonies recovered from the respiratory tracts of mice at 12 and 20 days postinoculation were plated onto BG-blood agar without tetracycline and then replica plated onto BG-blood agar with and without tetracycline. Individual colonies were also patched onto agar with and without tetracycline to confirm these data. For both the in vitro and in vivo assays, the inocula were prepared by growing cells on BG-blood agar containing tetracycline.
RESULTS AND DISCUSSION
Construction and in vitro characterization of phase-locked B. pertussis strains.
Strain 18323 (Table 1) is the American Type Culture Collection (ATCC) type strain for B. pertussis. Although it has been recognized for some time that this strain is not typical of clinical B. pertussis isolates (3, 14, 24), it was chosen for this study so that our results could be directly compared with those of Beattie et al., from which it was concluded that the Bvg− phase gene, vrg6, was required for virulence (6). 18323 is also the strain used in the intracerebral challenge test to assess the potencies of whole-cell pertussis vaccines, and it has been used extensively in an aerosol model of B. pertussis respiratory infection (27). 18323 is phenotypically wild type for Bvg. It forms small, domed, hemolytic colonies on BG-blood agar after 4 days of incubation at 37°C (nonmodulating or Bvg+ phase conditions) and large, flat, nonhemolytic colonies on BG-blood agar supplemented with 20 mM MgSO4 and 5 mM nicotinic acid (modulating or Bvg− phase conditions). When grown under nonmodulating conditions, 18323 expresses Bvg+ phase-specific antigens which can be detected by Western blotting with sera from children recovering from pertussis (Fig. 1A). It is interesting that the vast majority of antigens detected by using convalescent-phase sera are Bvg+ phase specific. When 18323 is grown under modulating conditions, these Bvg+ phase antigens are not expressed (Fig. 1A), and instead Bvg-repressed factors, including VraB (Fig. 1B) and vrg6 (Fig. 2), are expressed.
FIG. 1.
Western blot analysis of the antigenic profiles of wild-type and mutant B. pertussis strains. Whole-cell lysates of the indicated strains grown under nonmodulating (Bvg+ phase) conditions or modulating (Bvg− phase) conditions were separated on an SDS–4 to 12% gradient polyacrylamide gel, transferred to polyvinylidene difluoride, and probed with serum from a child recovering from pertussis (A) or anti-VraB antibody (B). The positions of the molecular weight markers (in thousands) are shown at the left. WT, wild type.
FIG. 2.
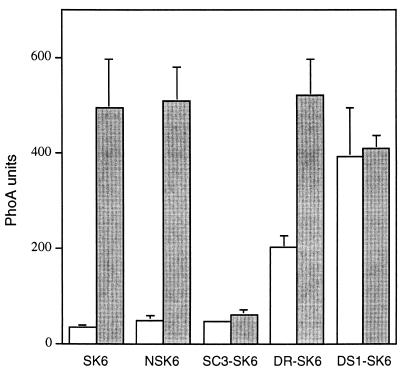
Effect of BvgAS and BvgR on vrg6 expression. Wild-type and mutant B. pertussis strains containing vrg6-phoA fusions were grown on BG-blood agar under nonmodulating (open bars) or modulating (shaded bars) conditions and suspended in assay buffer, and alkaline phosphatase activity was measured. Values are the means and standard errors for at least three independent assays performed in duplicate.
We constructed a Bvg+ phase-locked derivative of 18323 by transferring the bvgS-C3 mutation to the chromosome by allelic exchange (1, 23) (see Materials and Methods). The bvgS-C3 allele, a single nucleotide change resulting in an arginine-to-histidine substitution at amino acid position 570 in the linker region of BvgS, was originally isolated from and characterized for B. pertussis BP370 (23). bvgS-C3 also confers a Bvg constitutive (Bvgc) phenotype to B. bronchiseptica (11). Strain SC3 (18323 bvgS-C3 [Table 1]) formed small, domed, hemolytic colonies on BG-blood agar with or without the addition of 20 mM MgSO4 and 5 mM nicotinic acid. Its antigenic profile was similarly insensitive to modulating conditions; it constitutively expressed Bvg+ phase antigens recognized by convalescent-phase serum (Fig. 1A) as well as filamentous hemagglutinin, fimbriae, and pertactin (data not shown) and did not express Bvg-repressed factors such as VraB (Fig. 1B) or vrg6 (Fig. 2) even under modulating conditions.
To create a Bvg− phase-locked derivative of 18323, we disrupted the bvgS gene with plasmid pGMT74, a suicide plasmid containing an internal bvgS fragment (Table 1). DS1 (18323::pGMT74 [Table 1]) forms large, flat, nonhemolytic colonies under both modulating and nonmodulating conditions, never expresses Bvg+ phase factors (Fig. 1A), and constitutively expresses VraB (Fig. 1B) and vrg6 (Fig. 2).
The Bvg+ phase of B. pertussis 18323 is necessary and sufficient for respiratory infection in mice.
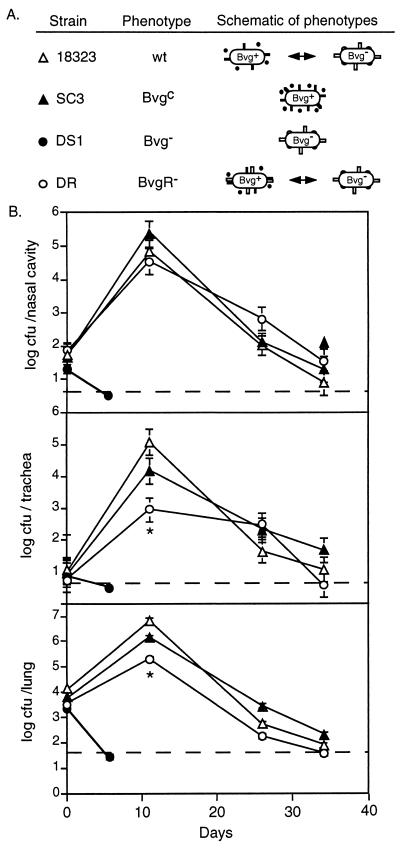
To determine if modulation to the Bvg− phase is important for B. pertussis respiratory infection, we compared isogenic wild-type and phase-locked strains in a mouse model. Groups of 3-week-old BALB/c mice were inoculated intranasally with 50 μl of PBS containing 5 × 104 CFU of either 18323 or its mutant derivatives. Animals were sacrificed at day 0 (to estimate the number of CFU delivered to each site in the respiratory tract) and days 11, 26, and 35 postinoculation. For both 18323 and SC3, the numbers of CFU recovered from the nasal cavity, trachea, and lungs were increased at day 11 compared to day 0 and then decreased at day 26 and further still at day 35 (Fig. 3). There was no significant difference between the number of CFU recovered from 18323- and SC3-inoculated mice at any site at any time point. In all cases, bacteria recovered from each site were phenotypically identical to the inoculum. In contrast, DS1 was not recovered from any site from any animal at day 6 postinoculation, confirming previous reports that Bvg− mutants are unable to colonize the respiratory tract (34, 35).
FIG. 3.
Time course of respiratory tract colonization by wild-type and mutant B. pertussis strains. (A) Schematic of strains used in this experiment. Bvg+ phase bacteria normally express adhesins (solid bars) and toxins (solid circles), while Bvg− phase bacteria express vrg6 (open bars) and other BvgR-regulated factors (open circles) and possibly other, non-BvgR-regulated vrg genes (not shown). wt, wild type. (B) Respiratory tract colonization. Mice were inoculated intranasally with 50 μl of PBS containing 5 × 104 CFU of the indicated strains, and the numbers of CFU present in the nasal cavity, trachea, and lungs were determined at the indicated times postinoculation. Each symbol represents the mean number of CFU recovered from three animals. Error bars represent ±1 standard error. Dashed lines indicate the lower limit of detection. ∗, P < 0.05.
Since strains 18323 and SC3 were indistinguishable in their ability to colonize the nasal cavity, trachea, and lungs, we conclude that the Bvg+ phase is necessary and sufficient for respiratory infection by B. pertussis. We cannot conclude from these data, however, that wild-type B. pertussis does not modulate to the Bvg− phase in vivo. To address this issue, we examined the antibody profile generated in response to B. pertussis infection. Sera from both 18323- and SC3-infected mice showed weak reactivity against Bvg+ phase antigens and antigens common to both the Bvg+ and Bvg− phase but showed no reactivity against Bvg− phase-specific antigens (data not shown). Similarly, sera from children recovering from pertussis contain high titers of antibody against Bvg+ phase factors and factors common to both phases but not Bvg− phase factors (Fig. 1A). These results indicate that either the transition to the Bvg− phase does not occur in vivo or Bvg− phase factors are nonantigenic. These results are exactly the same as those obtained with wild-type and phase-locked B. bronchiseptica strains in rabbits and rats (1, 11).
Bvg-mediated repression of gene expression is required for efficient tracheal colonization.
The bvgR locus is involved in Bvg+ phase repression of at least two vrg genes, vrg6 and vrg73 (21), as well as the VraB antigen (30). To further characterize the contribution of BvgR to regulation of vrg gene expression, we constructed a mutant derivative of strain 18323 containing an in-frame deletion in bvgR (see Materials and Methods) (Table 1). The colony morphologies displayed by DR (18323 ΔbvgR) under modulating and nonmodulating conditions were indistinguishable from those of 18323. Similarly, the ΔbvgR strain was unaltered in its ability to express Bvg+ phase antigens, as determined by Western blot analysis with convalescent-phase serum (Fig. 1A). VraB expression, however, was partially derepressed in the bvgR mutant grown under nonmodulating conditions (Fig. 1B), as was vrg6 expression as determined by measuring alkaline phosphatase activity in a strain containing a vrg6::TnphoA fusion (Fig. 2; also see below). These results are consistent with previous reports in which the involvement of BvgR in repression of vrg6 and VraB was first described (21, 30) and suggest that an additional form of Bvg-dependent negative regulation exists. Since deletion of bvgR results in the inappropriate Bvg+ phase expression of genes that are normally not expressed in this phase, the ΔbvgR strain can be classified as an ectopic expression mutant.
To assess the importance of BvgR-mediated repression of gene expression during infection, we compared the ΔbvgR strain with strain 18323 in our mouse model. The numbers of DR CFU recovered from the nasal cavity, trachea, and lungs at day 11 postinoculation were decreased compared to those for 18323. This moderate difference was statistically significant in the trachea and lungs (Fig. 3). Ectopic Bvg+ phase expression of at least one class of vrg genes therefore inhibits tracheal and lung colonization, demonstrating the importance of BvgR-mediated repression, and hence BvgAS-mediated repression, of gene expression in vivo. This result is analogous to our previous report showing that ectopic expression of motility in the Bvg+ phase of B. bronchiseptica is detrimental to the development of respiratory infection in rats (1).
Construction and in vitro characterization of vrg6 mutants.
B. pertussis SK6 is a derivative of strain 18323 containing a TnphoA insertion in the vrg6 locus (16). This strain is Smr and Kmr due to the transposon (see Materials and Methods). SK6 has been shown to be defective for tracheal and lung colonization in mice (6). To determine if the virulence defect of SK6 was in fact due to the vrg6 transposon insertion, we constructed two new mutants. First, we used a recently identified Bordetella-specific bacteriophage (18a) to transduce the vrg6::TnphoA mutation into wild-type 18323. The resulting strain, NSK6 (Table 1), is isogenic with 18323 except for the transposon insertion into vrg6. Like SK6, NSK6 is Smr and Kmr due to the transposon. To specifically examine vrg6 gene function in the absence of polar effects, we also constructed an 18323 derivative containing an in-frame deletion in vrg6 (Table 1) (see Materials and Methods). Like 18323, this strain (D6) is Sms.
The mini-TnphoA element in SK6 inserted into vrg6 such that a vrg6-phoA translational fusion was created, allowing alkaline phosphatase activity to serve as an indicator of vrg6 expression (16). In both SK6 and NSK6, alkaline phosphatase activity was about 10-fold higher when the strains were grown under modulating conditions compared to nonmodulating conditions (Fig. 2), consistent with previous reports (5, 16). To confirm the roles of BvgAS and BvgR in vrg6 repression, we transduced the vrg6::TnphoA allele into SC3, the Bvg+ phase-locked strain, and DR, the ΔbvgR strain, creating SC3-SK6 and DR-SK6, respectively. Integration of pGMT74 into NSK6 created DS1-NSK6, a Bvg− mutant containing the vrg6-phoA fusion. vrg6-phoA expression was constitutively low in SC3-SK6 and high in DS1-NSK6 regardless of growth conditions (Fig. 2), confirming that BvgAS mediates repression, either directly or indirectly, of vrg6 gene expression under Bvg+ phase conditions. vrg6-phoA expression was partially derepressed in DR-SK6 grown under nonmodulating conditions compared to in DS1-NSK6 and compared to in DR-SK6 grown under modulating conditions. This result confirms that of Merkel and Stibitz (21) and suggests that while BvgR plays a role in BvgAS-mediated repression of vrg6, it may not account for full repression of vrg6 under nonmodulating conditions.
vrg6 is not required for respiratory infection.
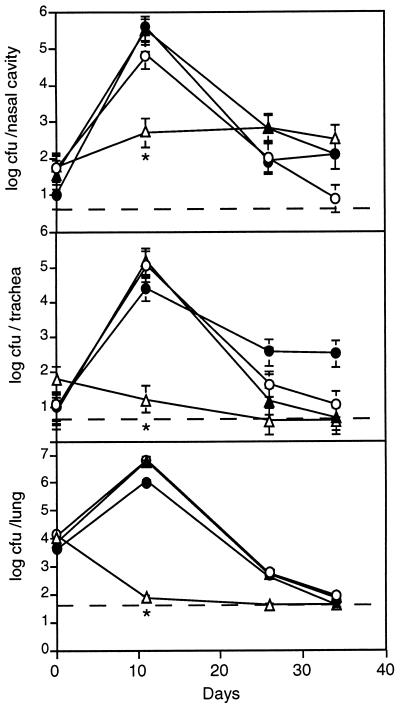
We compared the various vrg6 mutant strains with strain 18323 in our murine model of respiratory infection. Consistent with the results of Beattie et al. (6), recovery of SK6 from all sites in the respiratory tract was dramatically reduced compared to that of 18323 at day 11 postinoculation, and SK6 was not recovered from the trachea or lungs on day 26 or 35 postinoculation (Fig. 4). In contrast, the numbers of CFU of NSK6 and D6 were statistically indistinguishable from those of 18323 at all sites and time points (Fig. 4). These results demonstrate first and foremost that vrg6 is not required for respiratory infection by B. pertussis. Since polar effects present in SK6 would also be present in NSK6, the colonization defect of SK6 is also not due to polar effects on genes downstream of vrg6. Additionally, since NSK6, which is Smr, is not defective for colonization, the colonization defect of SK6 is not due to the fact that it is Smr. Taken together, these results indicate that the SK6 virulence defect observed here and by Beattie et al. (6) must be due to a mutation in a locus other than vrg6. In vitro comparison of SK6 with 18323 revealed no obvious differences in colony morphology or antigenic profile, suggesting that SK6 may contain a mutation in a previously unrecognized virulence factor. Whatever the mutation, it has a profound effect on virulence.
FIG. 4.
Time course of respiratory tract colonization by the wild-type strain and vrg6 mutants. Mice were inoculated intranasally with 50 μl of PBS containing 5 × 104 CFU of strain 18323 (○), SK6 (▵), NSK6 (▴), or D6 (•) (Table 1), and the numbers of CFU present in the nasal cavity, trachea, and lungs were determined at the indicated times postinoculation. Each symbol represents the mean number of CFU recovered from three animals. Error bars represent ±1 standard error. Dashed lines indicate the lower limit of detection. ∗, P < 0.05.
vrg6 expression is not induced in vivo.
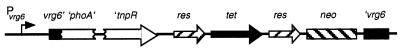
Comparison of SK6, NSK6, and D6 with 18323 indicates that vrg6 expression is not required during infection in our murine model. These results do not, however, rule out the possibility that vrg6 expression is induced in vivo. To investigate this possibility, we constructed strains containing recombinase gene fusions as reporters of vrg6 expression (Fig. 5). This reporter system is based on site-specific recombination in response to tnpR (resolvase) gene expression (8). NBP121 and NBP147 are SK6 derivatives in which a promoterless resolvase gene (′tnpR) is inserted downstream of vrg6::phoA followed by res-tet-res sequences. The res sequences are the targets of the resolvase, and tet confers Tcr. Expression of vrg6 results in expression of the resolvase, excision of the res-tet-res cassette, and conversion of the strain from Tcr to Tcs. Approximately 7% of the colonies tested following 4 days of growth on BG-blood agar at 37°C (Bvg+ phase conditions) were Tcs, while almost 90% of the colonies tested following growth on BG-blood agar containing 5 mM nicotinic acid and 20 mM MgSO4 (Bvg− phase conditions) were Tcs (Table 2). Since the levels of vrg6 expression in NSK6 grown on BG-blood agar in the absence and presence of 5 mM nicotinic acid and 20 mM MgSO4 are similar to those in SC3-SK6 and DS1-SK6, respectively (Fig. 2), we conclude that 7 and 90% resolutions reflect the levels of vrg6 expression under nonmodulating and modulating conditions, respectively. To determine if vrg6 expression is induced in vivo, we inoculated groups of 3-week-old BALB/c mice with 5 × 104 CFU of NBP121 and determined the ratio of Tcs to Tcr CFU recovered from the nasal cavity, trachea, and lungs on days 12 and 20 postinoculation. An average of 17% of all colonies recovered from all sites in the respiratory tract on any day were Tcs (Table 2). These results indicate that vrg6 expression is low in vivo, only slightly greater than levels observed following in vitro growth under nonmodulating conditions, suggesting that modulation to the Bvg− phase may not occur in vivo.
FIG. 5.
Use of a resolvase reporter fusion to determine vrg6 expression in vivo. A ′tnpR-res-tet-res-neo cassette was inserted into the TnphoA present in SK6. tnpR expression is under the control of the vrg6 promoter, and therefore excision of res-tet-res sequences reflects vrg6 expression. neo contains a Kmr gene.
TABLE 2.
Resolution of res-tet-res sequences following growth in vitro and in vivoa
| Strain or site | % Tcs colonies (mean ± SE) after growth:
|
|||
|---|---|---|---|---|
| In vitrob
|
In vivoc
|
|||
| Bvg+ phase conditions | Bvg− phase conditions | Day 12 | Day 20 | |
| NBP121 | 7.4 ± 4.5 | 89.6 ± 5.2 | ||
| NBP147 | 7.6 ± 3.4 | 86.9 ± 4.4 | ||
| Nasal cavity | 14 ± 6.4 | 16 ± 5.8 | ||
| Trachea | 17 ± 4.3 | 18 ± 7.2 | ||
| Lungs | 19 ± 5.5 | 16 ± 6.7 | ||
See Fig. 5.
Strains were grown on BG-blood agar with or without the addition of 5 mM nicotinic acid and 5 mM MgSO4 for 72 h. Cells were then harvested and plated, and the percentage of Tcs resolvants was determined.
Mice were inoculated with NBP121 grown on plates containing tetracycline. At 12 and 20 days postinoculation, the percentages of Tcs colonies recovered from the nose, trachea, and lungs were determined.
Implications.
We have shown, by using phase-locked and ectopic expression mutants, that the Bvg+ phase of B. pertussis is necessary and sufficient for respiratory tract colonization and that inappropriate expression of Bvg− phase factors in the Bvg+ phase decreases colonization efficiency. We further demonstrated that vrg6 is not required for virulence. These data repudiate an in vivo role for the Bvg− phase of B. pertussis and suggest that BvgAS may in fact perform the same function(s) in B. pertussis and B. bronchiseptica. If this suggestion is true, how do we account for differences in Bvg− phase phenotypes and for the fact that B. pertussis is thought to be incapable of surviving outside its human host? We recently reported the discovery of Bvg intermediate (Bvgi) phase antigens in B. bronchiseptica and put forth the hypothesis that the Bvgi phase may be important for aerosol transmission while the Bvg− phase may be important for transmission via an environmental reservoir (12). Characterization of the Bvgi phase of B. pertussis has revealed cross-reacting Bvgi phase antigens, and sera from children recovering from pertussis contain antibodies that recognize some of these antigens (19a). These data suggest that the Bvgi phase of B. pertussis is expressed in vivo. We propose, therefore, that BvgAS may function to distinguish sites within and outside the mammalian respiratory tract in both B. pertussis and B. bronchiseptica. Since B. pertussis appears to be confined to transmission by the aerosol route, the role of BvgAS in this species may be primarily to mediate the transition between the Bvg+ and Bvgi phases, allowing B. pertussis to alternate between a virulent phase and a transmission-competent phase. In B. bronchiseptica, the role of BvgAS may be extended to include the transition to the Bvg− phase, which may allow this organism to survive in environmental reservoirs. We are currently characterizing Bvgi and Bvg− phase phenotypes in B. pertussis and B. bronchiseptica and their potential roles in transmission to test these hypotheses.
ACKNOWLEDGMENTS
We thank the late Roberta Shahin, David Beattie, Theresa Finn, James Cherry, and Mark Peppler for strains and members of our laboratory for helpful discussions and comments on the manuscript. Special thanks go to Mingsiun Liu for discovery and characterization of the Bordetella-specific bacteriophage that was essential to our analysis.
We are supported by grants from the NIH (AI38417 to J.F.M. and AI26289 to J.J.M.) and postdoctoral fellowships (a Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation Fellowship [DRG-1371] to B.J.A., Gobierno de Navarra and HOECHST-Sociedad Espanola de Enfermedades Infecciosas y Microbiologia Clinica fellowships and a postdoctoral fellowship of Universidad de Navarra [PIUNA] to G.M.T., and a European Society for Pediatric Infectious Diseases [ESPID] Fellowship Award to U.H.).
Footnotes
This paper is dedicated to the memory of Roberta Shahin.
REFERENCES
- 1.Akerley B J, Cotter P A, Miller J F. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell. 1995;80:611–620. doi: 10.1016/0092-8674(95)90515-4. [DOI] [PubMed] [Google Scholar]
- 2.Akerley B J, Monack D M, Falkow S, Miller J F. The bvgAS locus negatively controls motility and synthesis of flagella in Bordetella bronchiseptica. J Bacteriol. 1992;174:980–990. doi: 10.1128/jb.174.3.980-990.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arico B, Rappuoli R. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J Bacteriol. 1987;169:2847–2853. doi: 10.1128/jb.169.6.2847-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arico B, Scarlato V, Monack D M, Falkow S, Rappuoli R. Structural and genetic analysis of the bvg locus in Bordetella species. Mol Microbiol. 1991;5:2481–2491. doi: 10.1111/j.1365-2958.1991.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 5.Beattie D T, Knapp S, Mekalanos J J. Evidence that modulation requires sequences downstream of the promoters of two vir-repressed genes of Bordetella pertussis. J Bacteriol. 1990;172:6997–7004. doi: 10.1128/jb.172.12.6997-7004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beattie D T, Shahin R, Mekalanos J J. A vir-repressed gene of Bordetella pertussis is required for virulence. Infect Immun. 1992;60:571–577. doi: 10.1128/iai.60.2.571-577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullock J O, Armstrong S K, Shear J L, Lies D P, McIntosh M A. Formation of ion channels by colicin B in planar lipid bilayers. J Membr Biol. 1990;114:79–95. doi: 10.1007/BF01869387. . (Erratum, 116:185.) [DOI] [PubMed] [Google Scholar]
- 8.Camilli A, Beattie D T, Mekalanos J J. Use of genetic recombination as a reporter of gene expression. Proc Natl Acad Sci USA. 1994;91:2634–2638. doi: 10.1073/pnas.91.7.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coote J G. Antigenic switching and pathogenicity: environmental effects on virulence gene expression in Bordetella pertussis. J Gen Microbiol. 1991;137:2493–2503. doi: 10.1099/00221287-137-11-2493. [DOI] [PubMed] [Google Scholar]
- 10.Cotter P A, Miller J F. BvgAS dependent phenotypic modulation of Bordetella species. In: Rappuoli R, Scarlato V, Aroci B, editors. Signal transduction and bacterial virulence. R. G. Austin, Tex: Landes; 1995. pp. 21–42. [Google Scholar]
- 11.Cotter P A, Miller J F. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun. 1994;62:3381–3390. doi: 10.1128/iai.62.8.3381-3390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotter P A, Miller J F. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol Microbiol. 1997;24:671–685. doi: 10.1046/j.1365-2958.1997.3821741.x. [DOI] [PubMed] [Google Scholar]
- 13.Goodnow R A. Biology of Bordetella bronchiseptica. Microbiol Rev. 1980;44:722–738. doi: 10.1128/mr.44.4.722-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross R, Arico B, Rappuoli R. Families of bacterial signal-transducing proteins. Mol Microbiol. 1989;3:1661–1667. doi: 10.1111/j.1365-2958.1989.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 15.Imaizumi A, Suzuki Y, Ono S, Sato H, Sato Y. Heptakis (2,6-O-dimethyl)beta-cyclodextrin: a novel growth stimulant for Bordetella pertussis phase I. J Clin Microbiol. 1983;17:781–786. doi: 10.1128/jcm.17.5.781-786.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knapp S, Mekalanos J J. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J Bacteriol. 1988;170:5059–5066. doi: 10.1128/jb.170.11.5059-5066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacey B W. Antigenic modulation of Bordetella pertussis. J Hyg. 1960;58:57–93. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18a.Liu, M., and J. F. Miller. Unpublished data.
- 19.Martinez de Tejada G, Miller J F, Cotter P A. Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica. Mol Microbiol. 1996;22:895–908. doi: 10.1046/j.1365-2958.1996.01538.x. [DOI] [PubMed] [Google Scholar]
- 19a.Martinez de Tejada, G., J. F. Miller, and P. A. Cotter. Unpublished data.
- 20.Masure H R. Modulation of adenylate cyclase toxin production as Bordetella pertussis enters human macrophages. Proc Natl Acad Sci USA. 1992;89:6521–6525. doi: 10.1073/pnas.89.14.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merkel T J, Stibitz S. Identification of a locus required for the regulation of bvg-repressed genes in Bordetella pertussis. J Bacteriol. 1995;177:2727–2736. doi: 10.1128/jb.177.10.2727-2736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michaelis S, Inouye H, Oliver D, Beckwith J. Mutations that alter the signal sequence of alkaline phosphatase in Escherichia coli. J Bacteriol. 1983;154:366–374. doi: 10.1128/jb.154.1.366-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller J F, Johnson S A, Black W J, Beattie D T, Mekalanos J J, Falkow S. Constitutive sensory transduction mutations in the Bordetella pertussis bvgS gene. J Bacteriol. 1992;174:970–979. doi: 10.1128/jb.174.3.970-979.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musser J M, Hewlett E L, Peppler M S, Selander R K. Genetic diversity and relationships in populations of Bordetella spp. J Bacteriol. 1986;166:230–237. doi: 10.1128/jb.166.1.230-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson A, Duggleby C J, Gorringe A R, Livey I. Antigenic variation in Bordetella pertussis. In: Birkbeck T H, editor. Antigenic variation in infectious diseases. Oxford, United Kingdom: IRL Press; 1986. pp. 147–161. [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Shahin R D, Cowell J L. Mouse respiratory infection models for pertussis. Methods Enzymol. 1994;235:47–58. doi: 10.1016/0076-6879(94)35130-9. [DOI] [PubMed] [Google Scholar]
- 28.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 29.Stiner D W, Scholte M J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 30.Stenson T H, Merkel T J, Peppler M S. Characterization of mini-Tn5 chromosomal mutants of Bordetella pertussis which have altered expression of vir-repressed antigens. 1996. p. 96. , abstr. B-168. In Abstracts of the 96th General Meeting of the American Society for Microbiology 1996, American Society for Microbiology, Washington, D.C. [Google Scholar]
- 31.Stenson T H, Peppler M S. Identification of two Bvg-repressed surface proteins of Bordetella pertussis. Infect Immun. 1995;63:3780–3789. doi: 10.1128/iai.63.10.3780-3789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stibitz S, Miller J F. Coordinate regulation of virulence in Bordetella pertussis mediated by the vir (bvg) locus. In: Miller V L, Kaper J B, Portnoy D A, Isberg R R, editors. The molecular biology of bacterial pathogenesis. Washington, D.C: ASM Press; 1994. pp. 407–422. [Google Scholar]
- 33.Uhl M A, Miller J F. Bordetella pertussis BvgAS virulence control system. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: ASM Press; 1995. pp. 333–349. [Google Scholar]
- 34.Weiss A A, Goodwin M S. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect Immun. 1989;57:3757–3764. doi: 10.1128/iai.57.12.3757-3764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss A A, Hewlett E L, Myers G A, Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983;42:33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]