Abstract
In September 2023, a patient in Italy who had never traveled abroad was referred for testing for suspected hepatic cystic echinococcosis. Lesions were incompatible with cystic echinococcosis; instead, autochthonous alveolar echinococcosis was confirmed. Alveolar echinococcosis can be fatal, and awareness must be raised of the infection’s expanding distribution.
Keywords: parasites, alveolar echinococcosis, Echinococcus multilocularis, Italy, autochthonous
The main human echinococcal infections are caused by Echinococcus granulosus sensu lato, which causes cystic echinococcosis (CE), and E. multilocularis, which causes alveolar echinococcosis (AE). The parasites have different life cycles and cause different diseases (1). E. granulosus s.l./CE is endemic worldwide in livestock-raising areas, including Italy, and accounts for most human echinococcal infections (2). The parasite is transmitted in a domestic cycle between dogs and livestock and causes generally benign disease in humans marked by the formation of well-defined fluid-filled cysts mostly in the liver (1,2). E. multilocularis/AE is endemic to the Northern Hemisphere and is transmitted in a sylvatic cycle between wild canids (e.g., foxes) and small rodents (e.g., voles) (2).
Humans become infected with the 2 pathogens by accidental ingestion of parasite eggs from material contaminated with infected definitive host feces. In Europe, North America, and Asia, expanding distribution has been observed in recent decades (2). In Europe, the historical endemic areas are Austria, France, Germany, and Switzerland, and that range has expanded to include Eastern and Northern Europe (3). In Italy, infected foxes have been reported over the past 20 years in the Trentino-Alto Adige region (4–7). Autochthonous animal transmission might occur in the area (8), and prevalence in foxes seems to be increasing (9). A 2017 survey conducted in the Liguria region first detected E. multilocularis in fecal samples of dogs and wolves, suggesting a southward expansion of the parasite (10) (Appendix Figure), as predicted by modeling (3). Surveillance of E. multilocularis in Europe is usually conducted voluntarily (11), and no structured surveillance program for animal infection in Italy occurs beside targeted surveillance through research projects.
We report a confirmed autochthonous human AE case in Italy. Ethics approval was not necessary because data were derived from routine clinical practice. The patient consented to the publication of this report.
The Study
In September 2023, a 55-year-old man in Italy was referred from his local hospital in Bolzano province, Trentino-Alto Adige region, to IRCCS Sacro Cuore Don Calabria Hospital, upon suspicion of CE. The patient was born and lived in Trentino-Alto Adige and had never traveled abroad; he worked in the tertiary sector and did not report contact with wild carnivores. He also did not report risk factors for E. granulosus infection.
The suspicion of CE was posed in June 2023, when he underwent abdominal ultrasound for a mild thrombocytopenia, revealing 5 recently developed small hepatic lesions (ultrasound results in 2016 were unremarkable). The lesions were described as septated and hypodense with no contrast enhancement and no calcifications on computed tomography performed in June 2023 (Figure, panels A–D), hypointense in T1-weighted magnetic resonance imaging (MRI), and hyperintense in T2-weighted MRI (Figure, panels E–H), with no diffusion restriction. No other lesions were present on total body imaging. Results of Echinococcus serologic testing using Western blot method were positive, but banding pattern was not reported.
Figure.
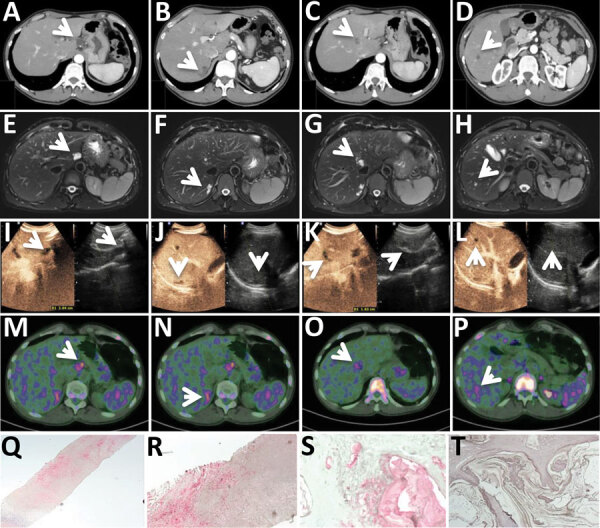
Diagnostic tests for patient in Italy with confirmed autochthonous case of human alveolar echinococcosis, 2023; white arrows indicate lesions. A–D) Contrast-enhanced computed tomography arterial phase. E–H) T2-weighted magnetic resonance imaging. I–L) Ultrasonography and contrast-enhanced ultrasonography. M–P) 18F-FDG-PET scan delayed acquisition (4 hours). Q–T) Em2 immunohistochemistry indicating small particles of Echinococcus multilocularis (spems) stained in red in patient’s sample (original magnification ×2.5 [Q] and ×20 [R]); positive alveolar echinococcosis sample control (original magnification ×20 [S]); Em2 negative control (cystic echinococcosis case, negative laminated layer; original magnification ×20) (T).
We excluded the diagnosis of CE on the basis of the lesions’ morphology on ultrasound, which did not show any CE pathognomonic or compatible features. We observed 5 lesions: 1 with 2.3 cm diameter in hepatic segment I, 1 of 0.9 cm in VI, 2 subcapsular of 2.7 cm and 0.5 cm in VII, and 1 of 1.6 cm in segment VIII (adjacent to the median hepatic vein). The lesions were hypoechoic with irregular margins, particularly the lesion in segment I, which had fine and tightly packed septations (Figure, panels I–L). The lesions were not enhancing on contrast-enhanced ultrasound (Figure, panels I–L). Serologic testing using the Echinococcus Western Blot IgG (LDBIO Diagnostics, https://ldbiodiagnostics.com) was positive for Echinococcus spp., showing genus-specific 7 kDa and 26–28 kDa bands, not assignable specifically to a species. Results of an 18F-FDG-PET scan showed light hypermetabolism in delayed (4-hour) acquisitions (Figure, panels M–P). Taken together, those results made the lesions highly indicative of AE.
We performed a biopsy of the only accessible lesion, located in segment VI, and submitted the specimen to the German Reference Laboratory for Tropical Parasites at the Bernhard-Nocht Institute for Tropical Medicine (Hamburg, Germany). A serum sample also submitted for further serologic testing showed low antibody titers against crude antigen preparations of E. multilocularis and E. granulosus (1:40–1:80 in indirect hemagglutination [negative <1:20] and 30–40 arbitrary units in ELISA [negative <20]). Results of Em18-ELISA (12) were negative. Histology revealed necrotic granuloma and fibrosis without PAS-positive structures. Results of PCR testing targeting cestode cytochrome oxidase and Echinococcus-specific 12S rDNA (13) were negative. In contrast, immunohistochemistry with the monoclonal antibody Em2G11 (14) stained positive for small particles of E. multilocularis (spems) (Figure, panels Q–R). Spems consist of outer laminated layer of Em2 antigen in close proximity to AE lesions and thus confirmed the diagnosis of AE, defined by the WHO Informal Working Group on Echinococcosis (WHO-IWGE) as the presence of clinical-epidemiologic factors plus compatible imaging findings plus seropositivity for echinococcosis plus compatible histopathology (15). The laboratory uses Em2G11-IHC regularly for suspected AE. The immunohistochemistry has been extensively validated and is also used by other laboratories; CE lesions and other cestode lesions stain negatively (14), whereas lesions by neotropical E. vogeli stain faintly (Appendix reference 16).
Staging according to the WHO-IWGE (1,15) was P2N0M0 (i.e., central lesions with proximal vascular and/or biliary involvement of 1 lobe, no regional involvement, no metastasis). We adopted a conservative management approach because removing the lesions would require major surgery and because the results of Em18 serologic testing and 18F-FDG-PET scan suggested low-viability parasites (1,13). At the time of publication, the patient was receiving albendazole (400 mg 2×/d) with fat-containing meals and tolerating the medication well. Follow-up with contrast-enhanced ultrasound and serologic testing was scheduled every 6 months, MRI in 1 year, and 18F-FDG-PET scan in 2 years (1).
Conclusions
AE is a complex disease with a high fatality rate (0%–25% 10-year survival) if untreated (1). It primarily affects the liver and is characterized by infiltrating, metastatic, tumor-like behavior (1). Unlike CE, AE lesions have no pathognomonic signs on imaging, and the differential diagnosis is mainly tumors (1). Even in AE-endemic areas, misdiagnosis and consequent incorrect treatment occurs frequently (1; Appendix reference 17).
Curative treatment options include surgery and albendazole if radical resection is achievable, or albendazole alone indefinitely in other cases (1). Treatment interruption can be envisaged in selected cases when serologic testing and 18F-FDG-PET scans become negative (1). In this case, the Western blot banding pattern, low antibody concentrations against crude parasite antigens, negativity of Em18 ELISA, and faint hypermetabolism on 18F-FDG-PET scan indicate low parasite viability (1,13). PCR on bioptic material was negative, explained by the absence of cell-containing larval structures on histology; however, E. multilocularis–specific immunochemistry was positive, confirming the diagnosis (1,13).
A report from 1928 mentioned 2 human AE cases identified in South Tyrol in 1906 and 1922 (2), but reports of human confirmed AE in Italy are otherwise lacking; a 2019 research review identified no reports from this country (12). Italian Hospital Discharge Records reports cases labeled as AE according to International Classification of Diseases, 9th Edition (Appendix reference 18). From the analysis of cases that we could examine (Appendix reference 19), those cases seem to be CE with multiloculated cyst morphology (CE2 and CE3b stages according to WHO-IWGE), erroneously recorded as E. multilocularis (1).
An expanding area of endemicity of E. multilocularis in Europe has been observed and predicted by modeling (3). Because of the high lethality of this disease if misdiagnosed and mistreated, physicians, especially in Italy’s alpine regions, must be informed about this infection and its possibility even in patients who have never lived in or traveled to known endemic areas.
Additional information about a confirmed autochthonous case of human alveolar echinococcosis, Italy, 2023
Acknowledgments
We thank Andrea Angheben, Paola Rodari, Maria Luca D’Errico, and the infectious-tropical diseases ward at IRCCS Sacro Cuore Don Calabria hospital in Negrar for clinical management during the patient’s hospitalization; Stefano Tais for support with sample management; Ansgar Deibel for the discussion on the case diagnosis and management; and Dora Buonfrate for critically reviewing the manuscript.
Funding was provided through Italian Ministry of Health “Fondi Ricerca Corrente –L2” to IRCCS Sacro Cuore Don Calabria hospital, Negrar di Valpolicella, Verona, Italy.
F.T., M.D., E.O., and T.D. performed and interpreted imaging and laboratory tests; F.T., N.R., B.G., and F.G. clinically managed the patient. F.T., D.T., and G.B. wrote the manuscript. All authors reviewed and approved the published version of the manuscript.
Biography
Dr. Tamarozzi is a senior research physician and cohead of the WHO Collaborating Centre on Strongyloidiasis and other NTDs at the Department of Infectious-Tropical Diseases and Microbiology, IRCCS Sacro Cuore Don Calabria Hospital, in Negrar di Valpolicella, Verona, Italy, as well as a member of the steering committee of the WHO Informal Working Group on Echinococcosis. Her main field of work is the laboratory- and imaging-based diagnosis of neglected helminthic infections, in particular echinococcal infections.
Footnotes
Suggested citation for this article: Tamarozzi F, Ronzoni N, Degani M, Oliboni E, Tappe D, Gruener B, et al. Confirmed autochthonous case of human alveolar echinococcosis, Italy, 2023. Emerg Infect Dis. 2024 Feb [date cited]. https://doi.org/10.3201/eid3002.231527
References
- 1.Kern P, Menezes da Silva A, Akhan O, Müllhaupt B, Vizcaychipi KA, Budke C, et al. The echinococcoses: diagnosis, clinical management and burden of disease. Adv Parasitol. 2017;96:259–369. 10.1016/bs.apar.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 2.Deplazes P, Rinaldi L, Alvarez Rojas CA, Torgerson PR, Harandi MF, Romig T, et al. Global distribution of alveolar and cystic echinococcosis. Adv Parasitol. 2017;95:315–493. 10.1016/bs.apar.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 3.Cenni L, Simoncini A, Massetti L, Rizzoli A, Hauffe HC, Massolo A. Current and future distribution of a parasite with complex life cycle under global change scenarios: Echinococcus multilocularis in Europe. Glob Change Biol. 2023;29:2436–49. 10.1111/gcb.16616 [DOI] [PubMed] [Google Scholar]
- 4.Manfredi MT, Genchi C, Deplazes R, Trevisiol K, Fraquelli C. Echinococcus multilocularis infection in red foxes in italy. Vet Rec. 2002;150:757. 10.1136/vr.150.24.757 [DOI] [PubMed] [Google Scholar]
- 5.Casulli A, Manfredi MT, La Rosa G, Di Cerbo AR, Dinkel A, Romig T, et al. Echinococcus multilocularis in red foxes (Vulpes vulpes) of the Italian Alpine region: is there a focus of autochthonous transmission? Int J Parasitol. 2005;35:1079–83. 10.1016/j.ijpara.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 6.Citterio CV, Obber F, Trevisiol K, Dellamaria D, Celva R, Bregoli M, et al. Echinococcus multilocularis and other cestodes in red foxes (Vulpes vulpes) of northeast Italy, 2012-2018. Parasit Vectors. 2021;14:29. 10.1186/s13071-020-04520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celva R, Crestanello B, Obber F, Dellamaria D, Trevisiol K, Bregoli M, et al. Assessing red fox (Vulpes vulpes) demographics to monitor wildlife diseases: a spotlight on Echinococcus multilocularis. Pathogens. 2022;12:60. 10.3390/pathogens12010060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casulli A, Bart JM, Knapp J, La Rosa G, Dusher G, Gottstein B, et al. Multi-locus microsatellite analysis supports the hypothesis of an autochthonous focus of Echinococcus multilocularis in northern Italy. Int J Parasitol. 2009;39:837–42. 10.1016/j.ijpara.2008.12.001 [DOI] [PubMed] [Google Scholar]
- 9.Obber F, Celva R, Da Rold G, Trevisiol K, Ravagnan S, Danesi P, et al. A highly endemic area of Echinococcus multilocularis identified through a comparative re-assessment of prevalence in the red fox (Vulpes vulpes), Alto Adige (Italy: 2019-2020). PLoS One. 2022;17:e0268045. 10.1371/journal.pone.0268045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massolo A, Valli D, Wassermann M, Cavallero S, D’Amelio S, Meriggi A, et al. Unexpected Echinococcus multilocularis infections in shepherd dogs and wolves in south-western Italian Alps: A new endemic area? Int J Parasitol Parasites Wildl. 2018;7:309–16. 10.1016/j.ijppaw.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023;21:e8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumann S, Shi R, Liu W, Bao H, Schmidberger J, Kratzer W, et al. ; interdisciplinary Echinococcosis Working Group Ulm. Worldwide literature on epidemiology of human alveolar echinococcosis: a systematic review of research published in the twenty-first century. Infection. 2019;47:703–27. 10.1007/s15010-019-01325-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siles-Lucas M, Casulli A, Conraths FJ, Müller N. Laboratory diagnosis of Echinococcus spp. in human patients and infected animals. Adv Parasitol. 2017;96:159–257. 10.1016/bs.apar.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 14.Barth TF, Herrmann TS, Tappe D, Stark L, Grüner B, Buttenschoen K, et al. Sensitive and specific immunohistochemical diagnosis of human alveolar echinococcosis with the monoclonal antibody Em2G11. PLoS Negl Trop Dis. 2012;6:e1877. 10.1371/journal.pntd.0001877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1–16. 10.1016/j.actatropica.2009.11.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information about a confirmed autochthonous case of human alveolar echinococcosis, Italy, 2023


