Abstract
We studied 50 patients with invasive nocardiosis treated during 2004–2023 in intensive care centers in France and Belgium. Most (65%) died in the intensive care unit or in the year after admission. Nocardia infections should be included in the differential diagnoses for patients in the intensive care setting.
Keywords: Nocardia, immunocompromised, intensive care, bacteria, France, Belgium
Nocardia is a ubiquitous, filamentous, gram-positive bacillus present in soil and decaying plants (1), affecting immunocompromised patients by way of inhalation, with a risk of secondary dissemination. Invasive Nocardia infections are mainly observed in patients who have undergone organ transplantation (incidence 0.2%) and hematopoietic stem cell transplantation (incidence 1.7%). Infections also occur in persons with primary immunodeficiency, solid cancer, or autoimmune disease. Other previously identified risk factors include use of long-term steroids and calcineurin inhibitors (2–4). Pulmonary involvement constitutes the most common manifestation of Nocardia infection, which can potentially lead to secondary dissemination, particularly in immunocompromised populations; the central nervous system is a common site, and many cases involving asymptomatic manifestations (5).
Blood cultures are positive in 10%–20% of cases involving Nocardia infection, and lung PCR can indicate colonization, requiring such tests as bronchoalveolar lavage and abscess needle aspiration. Nocardia species are typically resistant to common antibiotics, which contribute to the complexity of diagnosing and managing disseminated infections (6,7). The mortality rate associated with Nocardia infection is substantial; 16%–40% of patients die within the first year of diagnosis, and outcomes depend largely on the underlying disease (6–8). We explored the risk factors, characteristics, and prognosis of patients with invasive nocardiosis in the context of the intensive care setting.
The Study
We conducted a retrospective, multicenter study of patients with invasive nocardiosis admitted to 22 intensive care units (ICUs) from the Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique (Grrr-OH) during 2004–2023 in France and Belgium. We established inclusion criteria as unplanned ICU medical admission, age >18 years, and a documented invasive nocardiosis diagnosis (before or during ICU stay). We excluded cases of suspected nocardiosis without microbiological documentation or those with a lack of medical chart data.
Documented nocardiosis was determined by a positive culture for Nocardia species or a Nocardia PCR-based assay coupled with organ involvement. Disseminated nocardiosis was characterized by the infection affecting >2 noncontiguous sites; bacteremia constituted dissemination if 1 organ was involved. Organ failures were identified based on the Sepsis-related Organ Failure Assessment score.
We performed a comparison between patients admitted to the ICU for Nocardia infection and patients enrolled in the HIGH multicenter clinical trial (9), which included immunocompromised patients admitted to the ICU for acute respiratory failure and compared the effect of high-flow nasal oxygen versus standard oxygen on 28-day mortality. We excluded diagnoses of Pneumocystis infection, acute pulmonary edema, and specific lung lesions, which we assumed could be easily distinguished from nocardiosis.
We present continuous data as median (interquartile range) and categorical data as numbers and percentages. We compared characteristics between our cohort and data from the HIGH clinical trial by using a Wilcoxon rank-sum test (continuous variables) or Fisher exact test (categorical variables). We used only variables that were statistically significant (p<0.05) in univariate analysis in multivariate analysis and conducted an assessment of collinearity. We performed 2-sided statistical analyses by using R statistical software version 2023.03.0+386 (The R Foundation for Statistical Computing, https://www.r-project.org).
In total, we studied 50 patients with invasive nocardiosis who were admitted to the ICU. The median age was 59 (47–67) years; 39 (78%) were men, and 11 (22%) were women. We took into account such details as patient demographics, concurrent diseases, and immunosuppressive therapies (Table 1). Almost all patients (46 [92%]) were immunocompromised; the primary causes were solid organ transplantation (18 [36%]), systemic autoimmune diseases (12 [24%]), and hematologic malignancies (10 [20%]). Steroid therapy was administered to most patients (34 [68%]). Low-dose trimethoprim/sulfamethoxazole prophylaxis was given to 12 (24%) patients.
Table 1. Baseline characteristics of patients in study of critically ill patients with visceral Nocardia infection, France and Belgium, 2004–2023*.
| Baseline characteristics | Nocardiosis cases, n = 50 |
|---|---|
| Age, y, median (IQR) |
59 (47–67) |
| Sex | |
| M | 39 (78) |
| F |
11 (22) |
| Charlson score, median (IQR) | 4 (3–7) |
| Cardiovascular risk factors, median (IQR) |
2 (1–3) |
| Immunosuppression | 46 (92) |
| Corticosteroids at admission | 34 (68) |
| 5–10 mg/d | 5 (10) |
| >10 mg/d | 29 (58) |
| Tacrolimus treatment | 16 (32) |
| Mycophenolate mofetil treatment | 12 (24) |
| Other conventional immunosuppressive drugs† |
5 (10) |
| Organ transplantation | 18 (36) |
| Kidney | 12 (24) |
| Heart | 4 (8) |
| Liver | 1 (2) |
| Lung |
1 (2) |
| Systemic autoimmune disease‡ |
12 (24) |
| Hematologic malignancies | 10 20) |
| Aggressive B cell lymphoma | 6 (12) |
| Acute lymphoid leukemia | 3 (6) |
| Acute myeloid leukemia |
1 (2) |
| No. lymphocytes/mm3, median (IQR) | 552 (287–1,210) |
| Gamma globulin, g/L, median (IQR) | 6 (4–10) |
| Trimethoprim/sulfamethoxazole prophylaxis | 12 (24) |
*Values are no. (%) except as indicated. IQR, interquartile range. †Azathioprine, n = 4 (8%); methotrexate, n = 1 (2%). ‡Connective tissue disease, n = 2; glomerulonephritis, n = 1; periarteritis nodos, n = 1; bullous pemphigoid, n = 1; Evans syndrome, n = 1; IgA vasculitis, n = 1; type 1 diabetes, n = 1; myasthenia gravis, n = 1; sarcoidosis, n = 1; chronic inflammatory demyelinating polyradiculoneuropathy, n = 1; inflammatory bowel disease, n = 1.
We noted disseminated infection in almost half of the patients (48%); the most frequently involved organs were lungs (98%), central nervous system (47%), and skin (20%) (Table 2). At admission to intensive care, 33 (66%) patients had acute respiratory distress and 19 (38%) experienced coma (defined by a Glasgow Coma Scale score ≤8) or septic shock. Overall, 45 (90%) patients exhibited >1 organ failure; the most common were respiratory failure (33 [66%]) and multiorgan dysfunction (25 [50%]). Computed tomography scans revealed alveolar consolidations in 43 (86%) patients and cavitated nodules in 26 (52%) patients. Magnetic resonance imaging of the brain in 23 (46%) patients revealed multiple lesions in 14 (61%) patients and brain herniation in 6 (26%) patients.
Table 2. Patient clinical and radiologic findings from the intensive care unit in study of critically ill patients with visceral Nocardia infection, France and Belgium, 2004–2023*.
| Findings | Nocardiosis cases, n = 50 |
|---|---|
| Clinical features | |
| Chronic cough† | 36 (72) |
| No. previous antibacterial therapy lines | 2 (0–3) |
| Fever | 29 (58) |
| Co-infection | 22 (44) |
| Fungal‡ | 11 (22) |
| Bacterial§ | 8 (16) |
| Viral¶ | 5 (10) |
| Lung involvement | 49 (98) |
| Oxygen therapy at admission | 32 (64) |
| Oxygen flow, L/min, median (IQR) | 8 (4–15) |
| Respiratory rate, L/min, median (IQR) | 30 (25–36) |
| Hemoptysis | 8 (16) |
| Neurologic involvement | 24 (48) |
| Confusion | 21 (42) |
| Coma | 16 (32) |
| Motor deficit | 13 (26) |
| Cranial nerve lesions | 10 (20) |
| Meningitis | 8 (16) |
| Epilepsy | 6 (12) |
| Glasgow score, median (IQR) | 13 (12–14) |
| Skin/muscle abscess | 10 (20) |
| Disseminated infection | 24 (48) |
| Organ failures | 45 (90) |
| Multiorgan | 25 (50) |
| Respiratory | 33 (66) |
| Including acute respiratory distress syndrome | 3 (8) |
| Acute kidney injury | 11 (22) |
| Hemodynamic | 17 (34) |
| Neurologic | 19 (38) |
| Hepatic | 4 (8) |
| Sequential organ failure assessment score, median (IQR) |
5 (3–7) |
| Imaging findings | |
| Computed tomography scan | |
| Lung consolidation | 43 (86) |
| Lung nodules with cavitation | 26 (52) |
| Pleural effusion | 15 (30) |
| Interstitial syndrome | 8 (16) |
| Alveolar hemorrhage | 6 (12) |
| Lung lobes involved | |
| 1 lobe | 16 (32) |
| Multilobe | 16 (32) |
| Bilateral | 18 (36) |
| Brain magnetic resonance imaging, n = 23 | |
| Single lesion | 9 (39) |
| Multiple lesions | 14 (61) |
| >10 mm | 17 (74) |
| <10 mm | 6 (26) |
| Brain herniation | 6 (26) |
| Ventriculitis |
2 (9) |
| Diagnostics methods | |
| Bronchoalveolar lavage analysis | 42 (84) |
| Diagnostic yield, n = 42 | 25 (60) |
| Computed tomography–scan targeted biopsy | 18 (36) |
| Blood culture positivity | 7 (14) |
| Nocardia PCR-based assay positivity | 26 (52) |
| Nocardia culture positivity | 24 (48) |
| Diagnosis made in intensive care unit | 23 (46) |
*Values are no. (%) except as indicated. IQR, interquartile range. †Chronic cough is defined as a cough persisting for >8 weeks. ‡Fungal infections (n = 11) comprised 8 invasive Aspergillus sp. infections, 2 Pneumocystis jirovecii infections, and 1 case of cutaneous candidosis. §Comprised 7 gram-negative bacillus co-infections and 1 methicillin-resistant Staphylococcus aureus co-infection. ¶Virus infections (n = 5) comprised 3 influenza infections (including 1 H1N1 co-infection) and 2 respiratory syncytial virus infections.
Most (62%) patients received dual therapy or triple therapy, including aminoglycosides (10 [20%]), most commonly trimethoprim/sulfamethoxazole (80%) and carbapenem (51%) (Appendix Table 1). Upon admission to the intensive care unit, 32 patients (63%) required oxygen support, and 19 (38%) required mechanical ventilation. The ICU mortality rate was 22%, and the all-cause mortality rate at 1 year was 44%. In multivariable analysis, factors significantly associated with 1-year mortality included vasopressor use, fungal coinfection, and neurologic involvement (Appendix Table 2).
We compared cases of Nocardia infection against cases of other immunocompromised pneumonia in patients admitted to the ICU (2,11) (Figure; Appendix Table 3). Patients with Nocardia infection were younger and had a higher prevalence of autoimmune diseases and solid organ transplants. Lung consolidation (86% vs. 27%; p = 0.001) and cavitated nodules (52% vs. 1%; p = 0.001) were significantly more frequent. Upon admission to the ICU, patients with nocardiosis were rated as more severe on the Sepsis-related Organ Failure Assessment and Glasgow Coma Scale compared with patients with other immunocompromised pneumonia, but there was no significant difference in ICU mortality (22% vs. 32%; p = 0.184).
Figure.
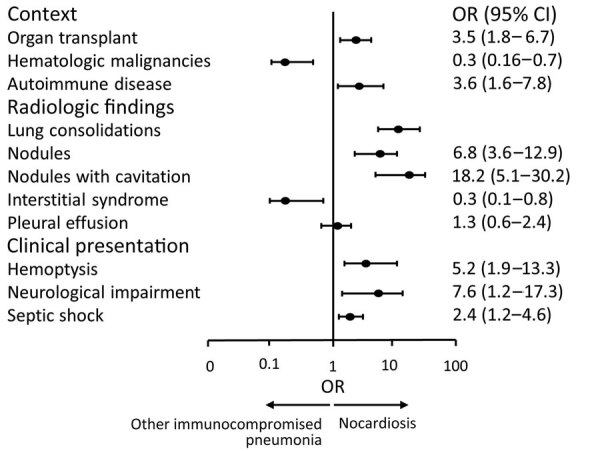
Comparison between nocardiosis and other immunocompromised pneumonia in a study of patients admitted to intensive care units in France and Belgium during 2004–2023. Other immunocompromised pneumonia data extracted from the HIGH clinical trial (9). OR, odds ratio.
Conclusions
Based on the findings for our study population, critically ill patients with nocardiosis exhibit frequent and severe pulmonary and neurologic involvement; 44% of patients die (22 of 50) and 14% (7 of 50) experience disability at the 1-year mark. Several cohorts have documented Nocardia infections within diverse immunocompromised populations, reporting mortality rates of 16%–40% (3,4). In our analysis, we conducted a comparative assessment with other pneumonia cases in immunocompromised patients (9) to elucidate situations warranting consideration of Nocardia infection. Cellular immunosuppression appears to be necessary for the development of a severe Nocardia infection, which is consistent with previous studies (4,6,10), particularly among organ transplant recipients, patients with systemic autoimmune diseases, and those with hematologic malignancies. Co-infections, particularly fungal ones, were reported as an independent prognostic factor for mortality in this population (11) and could partially explain this initial severity. Such findings highlight the burden of immunosuppression and the need for vigilance in assessing concurrent infections in this population. Two recent studies suggest that trimethoprim/sulfamethoxazole could be protective against Nocardia infections (11,12). Because invasive Nocardia infections are rare, results of our study may lack statistical power, and significant prognostic or distinctive factors might have gone unnoticed. However, we believe the inclusion of patients from 22 ICUs, with few cases missing data, provides a relevant overview of nocardiosis in critically ill patients.
In summary, in this study of critically ill patients with nocardiosis, we observed high mortality rates, posing a diagnostic challenge for critical care practitioners. Our findings emphasize the need for a heightened level of vigilance in monitoring patients for Nocardia infection in the intensive care setting, especially among immunocompromised patients who exhibit pulmonary nodules and neurologic involvement.
Additional information regarding critically ill patients with visceral Nocardia infection, France and Belgium, 2004–2023.
Biography
This study received approval from the ethics committee of the “Société de Réanimation de langue Française” (reference 22–055). Due to the retrospective nature of the study, patient consent was waived in accordance with French law.
Dr. Khellaf has worked in the medical intensive care unit at Saint-Louis Hospital during his residency. Research interests include medical intensive care for immunocompromised patients, systemic diseases, and opportunistic infections.
Footnotes
Suggested citation for this article: Khellaf L, Lemiale V, Decavèle M, Pineton de Chambrum M, Beurton A, Kamel T, et al. Critically ill patients with visceral Nocardia infection, France and Belgium, 2004–2023. Emerg Infect Dis. 2024 Feb [date cited]. https://doi.org/10.3201/eid3002.231440
References
- 1.Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev. 1994;7:213–64. 10.1128/CMR.7.2.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coussement J, Lebeaux D, van Delden C, Guillot H, Freund R, Marbus S, et al. ; European Study Group for Nocardia in Solid Organ Transplantation. European Study Group for Nocardia in Solid Organ Transplantation. Nocardia infection in solid organ transplant recipients: a multicenter European case-control study. Clin Infect Dis. 2016;63:338–45. 10.1093/cid/ciw241 [DOI] [PubMed] [Google Scholar]
- 3.Coussement J, Lebeaux D, Rouzaud C, Lortholary O. Nocardia infections in solid organ and hematopoietic stem cell transplant recipients. Curr Opin Infect Dis. 2017;30:545–51. 10.1097/QCO.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 4.Yagishita M, Tsuboi H, Tabuchi D, Sugita T, Nishiyama T, Okamoto S, et al. Clinical features and prognosis of nocardiosis in patients with connective tissue diseases. Mod Rheumatol. 2021;31:636–42. 10.1080/14397595.2020.1823070 [DOI] [PubMed] [Google Scholar]
- 5.Averbuch D, De Greef J, Duréault A, Wendel L, Tridello G, Lebeaux D, et al. ; European Study Group for Nocardia in Hematopoietic Cell Transplantation. Nocardia Infections in Hematopoietic Cell Transplant Recipients: A Multicenter International Retrospective Study of the Infectious Diseases Working Party of the European Society for Blood and Marrow Transplantation. Clin Infect Dis. 2022;75:88–97. 10.1093/cid/ciab866 [DOI] [PubMed] [Google Scholar]
- 6.Rouzaud C, Rodriguez-Nava V, Catherinot E, Méchaï F, Bergeron E, Farfour E, et al. Clinical assessment of a Nocardia PCR-based assay for diagnosis of nocardiosis. J Clin Microbiol. 2018;56:e00002–00018. 10.1128/JCM.00002-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebeaux D, Bergeron E, Berthet J, Djadi-Prat J, Mouniée D, Boiron P, et al. Antibiotic susceptibility testing and species identification of Nocardia isolates: a retrospective analysis of data from a French expert laboratory, 2010-2015. Clin Microbiol Infect. 2019;25:489–95. 10.1016/j.cmi.2018.06.013 [DOI] [PubMed] [Google Scholar]
- 8.Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ Jr. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259–82. 10.1128/CMR.19.2.259-282.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azoulay E, Lemiale V, Mokart D, Nseir S, Argaud L, Pène F, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA. 2018;320:2099–107. 10.1001/jama.2018.14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebeaux D, Freund R, van Delden C, Guillot H, Marbus SD, Matignon M, et al. ; European Study Group for Nocardia in Solid Organ Transplantation; European Study Group for Nocardia in Solid Organ Transplantation. European Study Group for Nocardia in Solid Organ Transplantation. Outcome and treatment of nocardiosis after solid organ transplantation: new insights from a European study. Clin Infect Dis. 2017;64:1396–405. 10.1093/cid/cix124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yetmar ZA, Thoendel MJ, Bosch W, Seville MT, Hogan WJ, Beam E. Risk factors and outcomes of nocardiosis in hematopoietic stem cell transplantation recipients. Transplant Cell Ther. 2023;29:206.e1–7. 10.1016/j.jtct.2022.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passerini M, Nayfeh T, Yetmar ZA, Coussement J, Goodlet KJ, Lebeaux D, et al. Trimethoprim-sulfamethoxazole significantly reduces the risk of nocardiosis in solid organ transplant recipients: systematic review and individual patient data meta-analysis. Clin Microbiol Infect. Oct 19:S1198-743X(23)00500-1 [Epub ahead of print]. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information regarding critically ill patients with visceral Nocardia infection, France and Belgium, 2004–2023.


