Abstract
The macrophage mannose receptor (MR) along with complement receptors mediates phagocytosis of the M. tuberculosis virulent strains Erdman and H37Rv. We have determined that the terminal mannosyl units of the M. tuberculosis surface lipoglycan, lipoarabinomannan (LAM), from the Erdman strain serve as ligands for the MR. The biology of the MR (receptor binding and trafficking) in response to phagocytic stimuli is not well characterized. This study analyzes the MR-dependent phagocytosis mediated by Erdman LAM presented on a 1-μm-diameter phagocytic particle. Erdman LAM microspheres exhibited a time- and dose-dependent rapid increase in attachment and internalization by human monocyte-derived macrophages (MDMs). In contrast, internalization of LAM microspheres by monocytes was minimal. Microsphere internalization by MDMs was visualized and quantitated by immunofluorescence and confocal and electron microscopy and resembled conventional phagocytosis. Phagocytosis of LAM microspheres by MDMs was energy, cytoskeleton, and calcium dependent and was mannan inhibitable. Trypsin treatment of MDMs at 37°C, which depleted surface and recycling intracellular pools of the MR, reduced the subsequent attachment of LAM microspheres. Trypsin treatment at 4°C allowed for subsequent recovery of LAM microsphere phagocytosis at 37°C by recycled MRs. Pretreatment of MDMs with cycloheximide influenced LAM microsphere phagocytosis to only a small extent, indicating that MR-dependent phagocytosis of the microspheres was occurring primarily by preformed recycled receptors. This study characterizes the requirements for macrophage phagocytosis of a LAM-coated particle mediated by the MR. This model will be useful in further characterization of the intracellular pathway taken by phagocytic particles coated with different LAM types in macrophages following ingestion.
Mycobacterium tuberculosis infects approximately one-third of the world’s population and is a major infectious cause of morbidity and mortality (14). M. tuberculosis is a facultative intracellular bacterium which is phagocytosed by monocytes and macrophages and multiplies within unique phagosomes that do not fuse with lysosomes (16). The process of phagocytosis represents the critical first step in the M. tuberculosis-phagocyte interaction, and this process involves microbial ligands and phagocyte surface receptors.
We have determined that whereas phagocytosis of two virulent strains, Erdman and H37Rv, and an attenuated strain, H37Ra, is mediated by complement protein C3 activation products on the bacterial surface and complement receptors (17), the virulent strains and H37Ra differ in that phagocytosis of the virulent strains is also mediated by the macrophage mannose receptor (MR) (15). Lipoarabinomannan (LAM), the major surface lipoglycan of M. tuberculosis, contains terminal mannose oligosaccharides (3). Recently, we have determined that the terminal mannose oligosaccharides from the Erdman strain serve as ligands for the MR (18).
The MR is expressed in abundance on monocyte-derived macrophages (MDMs) and tissue macrophages (including alveolar macrophages) but not monocytes (19, 22). This receptor recognizes terminal mannose and fucose glycoconjugates and has been found to play an important role in mediating the phagocytosis of intracellular pathogens (6, 8). Recent studies have proposed a role for the MR in granuloma formation and in antigen presentation (9, 10). The MR is implicated as playing a role in delivering LAM from the M. tuberculosis phagosome to late endosomes for loading onto CD1b molecules for LAM presentation to T cells (12). Binding of ligands to the MR on the surface of macrophages is calcium dependent (C-type lectin) (24). The extracellular portion of this receptor contains multiple carbohydrate-recognition domains which cooperate to enhance the avidity of ligand binding (28). Transfection of the MR cDNA into Cos-1 cells allows for endocytosis of mannose-rich glycoproteins and yeast (4). Detailed studies of the trafficking of the MR have focused primarily on pinocytosis by using mannosylated proteins such as mannose-bovine serum albumin (BSA) or horseradish peroxidase (23). These studies have shown that binding to the receptor is pH dependent, a characteristic postulated to allow for dissociation of these ligands within an acidified prelysosomal intracellular compartment (25). In addition to surface-expressed MR, macrophages contain a large intracellular pool of MRs (26). The receptor undergoes continual rapid recycling (5 to 10 min) to the cell surface (23). The intracellular compartment containing macroparticles (>1 μm in diameter) phagocytosed by human macrophages via the MR is less well characterized.
Here we analyze the requirements for MR-dependent phagocytosis mediated by Erdman LAM utilizing a 1-μm-diameter microsphere model that allows for the study of the specific interaction between LAM terminal mannose oligosaccharides and this receptor. We report that (i) phagocytosis of LAM microspheres is rapid and specific for macrophages; (ii) attachment and internalization of microspheres is temperature, actin cytoskeleton, and calcium dependent and is inhibitable by trypsin treatment of the macrophage; (iii) phagocytosis of LAM microspheres occurs by both surface-expressed MRs and preformed intracellular MRs that are recycled to the macrophage surface; and (iv) internalized LAM microspheres do not appear to interfere with this recycling process.
MATERIALS AND METHODS
Materials.
Mannan, dihydrocytochalasin B, and trypsin (Sigma, St. Louis, Mo.) and mannose-BSA (EY Laboratories, San Mateo, Calif.) were purchased. Purified Erdman LAM and rabbit polyclonal antibody to M. tuberculosis were provided by Patrick Brennan and colleagues.
Monocytes and macrophages.
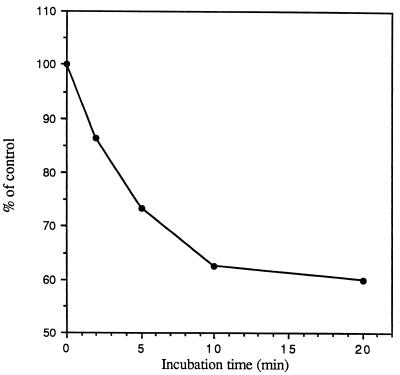
Mononuclear cells from single donors were isolated from heparinized blood on Ficoll-sodium diatrizoate (Pharmacia Fine Chemical, Piscataway, N.J.) gradients and then cultured in Teflon wells (Savillex Corp., Minnetonka, Minn.) for 1 day (monocytes) or for 5 to 7 days (MDMs) in RPMI containing 20% autologous serum (1.5 × 106 to 2.0 × 106 mononuclear cells/ml) at 37°C (15). On the day of each experiment, mononuclear cells were removed from Teflon wells and washed extensively, and the monocytes or MDMs were purified by adherence to Chromerge (Fisher Scientific Co.)-cleaned glass coverslips (2 h) in 24-well flat-bottom wells (Becton Dickinson, Lincoln Park, N.J.) in the presence of medium containing RPMI–20 mM HEPES and 10% autologous serum. Approximately 2.0 × 105 MDMs or monocytes were added to each well.
Preparation of Erdman and control polystyrene microspheres.
Erdman LAM microspheres were prepared as previously described (18). Briefly, 2.0 × 109 polybead polystyrene microspheres (catalog no. 07310; Polysciences, Inc., Warrington, Pa.), 1 μm in diameter, were washed two times in 0.05 M carbonate-bicarbonate buffer (pH 9.6) by centrifugation at 10,000 × g for 10 min in presiliconized polypropylene tubes (National Scientific Supply Company, Inc., San Rafael, Calif.). The microspheres were then incubated for 2 h at 37°C on an Adams Nutator (Clay Adams, Piscataway, N.J.) with 50 μg of purified Erdman LAM that had been suspended in 1 ml of carbonate-bicarbonate buffer or buffer alone (control). The microspheres were washed twice and then incubated in phosphate-buffered saline (PBS) containing 5% human serum albumin (HSA) for 1 h at 37°C to block nonspecific binding sites. The microspheres were washed in 0.5% HSA, resuspended in 0.5% HSA, and saved at 4°C until use (stable for approximately 2 weeks). The microspheres initially incubated in carbonate-bicarbonate buffer without LAM are designated HSA microspheres. The presence of LAM on LAM microspheres was detected by indirect immunofluorescence microscopy (18).
Assay for phagocytosis of Erdman LAM microspheres.
On the day of each experiment, MDMs or monocytes on coverslips in monolayer culture were washed extensively with RPMI and incubated with various amounts of Erdman LAM microspheres in RPMI–20 mM HEPES and 1 mg of HSA (Calbiochem Corp., La Jolla, Calif.) per ml (RHH) at 37 or 4°C in 5% CO2–95% air on a shaking platform at 100 rpm. After selected incubation times, in the presence or absence of inhibitors or after trypsin treatment of MDMs, phagocytes were washed in RPMI to remove nonadherent microspheres and fixed in 10% formalin for 10 min. The mean number of microspheres per phagocyte on each of duplicate coverslips was determined by counting a minimum of 100 consecutive phagocytes per coverslip by phase-contrast microscopy (18). In certain experiments to distinguish between attached and ingested microspheres after selected incubation periods, MDMs on coverslips were washed, fixed in 3.3% formalin for 10 min (to prevent permeabilization of the cell [11]), blocked with 2% BSA for 2 h at room temperature, washed three times in PBS without Ca2+ and Mg2+ (PD), incubated with rabbit polyclonal antibody against M. tuberculosis (1:100 dilution) overnight at 4°C, and incubated with fluorescein-conjugated goat anti-rabbit immunoglobulin G (Sigma) for 1 h at 4°C. The monolayers were then washed three times in PD, and coverslips were mounted on glass slides. The number of microspheres attached on the surface of MDMs on each of duplicate coverslips was determined by fluorescence microscopy. The total number of microspheres associated with MDMs was enumerated by phase-contrast microscopy as described above or in early experiments by indirect immunofluorescence microscopy as described above after methanol treatment (100% for 3 min) to both fix and permeabilize the cell (11). Since the results obtained by the two latter techniques were identical, phase-contrast microscopy was used for subsequent studies to determine total MDM-associated microspheres. In each experiment, the number of microspheres internalized in MDMs was calculated by subtracting the number of attached microspheres on the surface of MDMs (formalin fixation) from the total number of microspheres associated with MDMs (phase microscopy or methanol treatment).
To determine the effect of cycloheximide on cell association of LAM microspheres with MDMs, MDMs were incubated with 2 mg of mannan (to stimulate MR recycling activity) in the absence or presence of 10 μg of cycloheximide per ml for 60 min. MDMs were then incubated with 6 × 107 microspheres for 20 min at 37°C (in the absence or presence of 10 μg of cycloheximide per ml) and fixed in formalin, and the number of microspheres was enumerated.
EM and confocal microscopy.
In studies to verify whether Erdman LAM microspheres that attach to MDMs are ingested, the phagocytosis assay was performed as described above, and the results were analyzed by electron microscopy (EM) and confocal microscopy. For analysis by EM, MDMs were plated on glass coverslips, incubated with microspheres (2 × 107/well) for 10 or 60 min at 37°C, washed, and prepared for transmission EM as described (17). The location of microspheres, attached on the surface of or internalized in MDMs, was determined by examination of over 100 consecutive MDM cross sections that contained ≥1 microsphere for each coverslip preparation in each test group.
For analysis by confocal microscopy, MDMs were plated on glass coverslips, incubated with microspheres (2 × 107/well) for 10 or 60 min at 37°C, washed, and fixed in 10% formalin for 10 min. The location of microspheres, attached on the surface of MDMs or internalized in MDMs, was determined by examining serial cross sections of ≥10 individual MDMs containing ≥1 microsphere on glass coverslips for each test group with a confocal scanning laser microscope (MRC-600; Bio Red, Cambridge, Mass.).
Studies with 125I–mannose-BSA.
MDMs in monolayer culture were washed once with RPMI at 37°C and twice more with RPMI at 4°C. After incubation for 10 min at 4°C, monolayers were incubated with either RHH (control), RHH containing mannan (4 mg/ml), mannose-BSA (200 mg/ml), or Erdman LAM microspheres (6 × 107 per well) for 20 min at 4°C. After additional washing, the cells were incubated at 37°C for 5, 10, or 20 min. The cells were then cooled to 4°C for 10 min, and 1 mg of 125I–mannose-BSA was added (specific activity, 2.2 × 106 cpm/mg) for 30 min at 4°C. Glass coverslips were removed to new wells, and monolayers were washed six times with warm RPMI. Monolayers were lysed in 1% sodium dodecyl sulfate, and 125I counts were obtained with a gamma counter (Beckman Instruments, Irvine, Calif.).
RESULTS
General mechanism of phagocytosis mediated by Erdman LAM.
To study the requirements for phagocytosis mediated by Erdman LAM, we utilized a microsphere model in which the acyl moieties of LAM are bound to 1-μm-diameter hydrophobic microspheres, thus aggregating the lipoglycan on a phagocyte particle and preferentially exposing the glycan units in order to facilitate the study of events mediated by the LAM terminal mannose oligosaccharide-MR interaction (18).
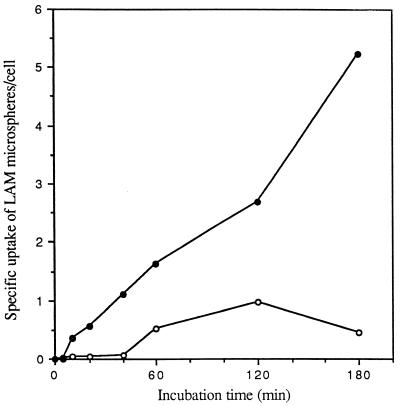
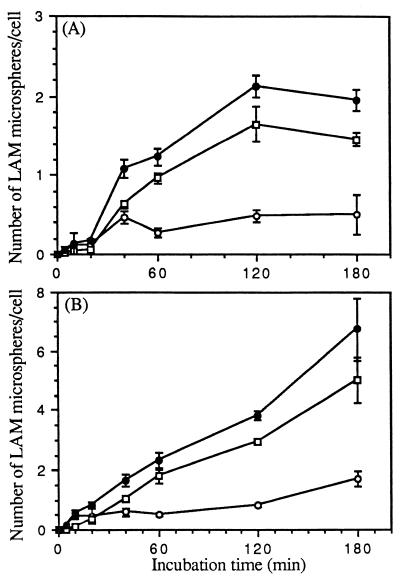
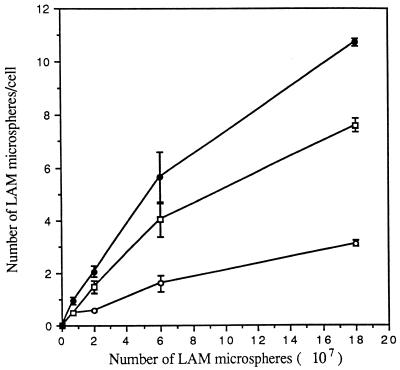
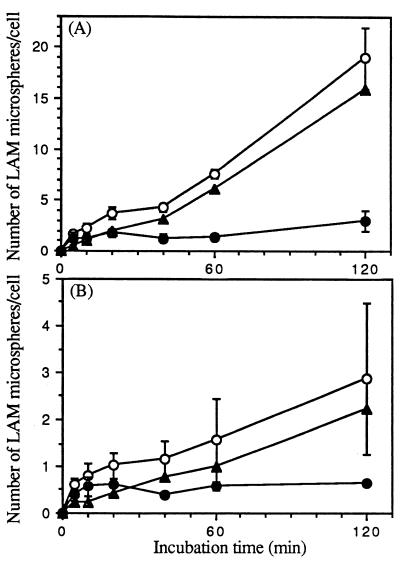
MR activity is dependent on cellular differentiation (i.e., activity is present on macrophages but not monocytes). To compare the relative uptakes of LAM microspheres by monocytes and macrophages, a time course study of microsphere uptake by these two cell types was performed. In each experiment, a single donor served as the source of both monocytes and MDMs. We prepared LAM and HSA microspheres, incubated them with monocytes or MDMs for 0 to 180 min, and assayed them for cell association of microspheres by phase microscopy. The specific microsphere association mediated by LAM was determined by subtracting the low level of saturable uptake of HSA microspheres from the uptake observed with LAM microspheres. As shown in Fig. 1, the cell association of LAM microspheres by MDMs was rapid and increased linearly over 180 min. In contrast, LAM microspheres demonstrated no association with monocytes for up to 40 min and minimal saturable uptake after that point. We next determined the kinetics of LAM microsphere internalization by monocytes and MDMs in a time course experiment by using the technique of macrophage fixation with or without permeabilization followed by indirect immunofluorescence microscopy (Fig. 2). The kinetics and extent of total cell association were similar to those shown in Fig. 1. For both monocytes and MDMs, ≥50% of the associated microspheres appeared to be intracellular by 10 to 20 min. By 60 min, the intracellular microspheres constituted ≥80% of the total microspheres in both cases. Figure 3 demonstrates that internalization of LAM microspheres by MDMs was dose dependent. The total number of microspheres associated with MDMs was variable from donor to donor in our experiments, but the pattern always remained the same. Taken together, these studies indicated that LAM microspheres were internalized by both monocytes and MDMs, but that the total cell association of LAM microspheres with monocytes was minimal compared to that observed with MDMs, consistent with uptake that is dependent on cell differentiation. The linear internalization of LAM microspheres by MDMs over 180 min suggested rapid recycling of the macrophage receptor responsible for internalization consistent with MR activity. Given the marked increase in internalization of LAM microspheres by MDMs, we elected to focus our efforts in subsequent experiments on this cell type.
FIG. 1.
Specificity of cell association of LAM microspheres with monocytes and MDMs. Monocytes and MDMs were incubated with 2 × 107 LAM- or HSA (control)-coated microspheres for the indicated time at 37°C. At the end of each incubation period, monocytes and MDMs were fixed with formalin. The total number of LAM or HSA microspheres associated with the cell was enumerated by phase microscopy. The specific uptake of LAM microspheres by monocytes (open circles) and MDMs (solid circles) was obtained by subtracting the total number of associated HSA microspheres from that of LAM microspheres at each point for each cell type. All points are means from two independent experiments.
FIG. 2.
Time course of cell association of microspheres with monocytes and MDMs. Monocytes (A) and MDMs (B) were incubated with 2 × 107 LAM microspheres for the indicated time at 37°C. At the end of each incubation period, monocytes and MDMs were washed and fixed with either 3.3% formalin (to visualize attached microspheres) or 100% methanol (to visualize all cell-associated microspheres). Microspheres associated with fixed monocytes and MDMs were visualized and enumerated by phase and indirect immunofluorescence microscopy with a primary polyclonal antibody against M. tuberculosis LAM and a fluorescein isothiocyanate-conjugated secondary antibody. The number of microspheres attached on the cell surface (open circles) or totally associated (solid circles) was measured. The number of internalized microspheres (open squares) was calculated. All points are means ± standard errors from two independent experiments.
FIG. 3.
Dose dependence of attachment and ingestion of LAM microspheres by MDMs. MDMs were incubated with the indicated number of LAM microspheres for 60 min at 37°C and then fixed with 3.3% formalin. Fixed MDMs were visualized as in Fig. 2, except that the number of total cell-associated microspheres was visualized and enumerated by phase microscopy instead of fluorescence microscopy. The number of microspheres attached to the cell (open circles) or totally associated (solid circles) was measured. The number of internalized microspheres (open squares) was calculated. All points are means ± standard errors from two independent experiments.
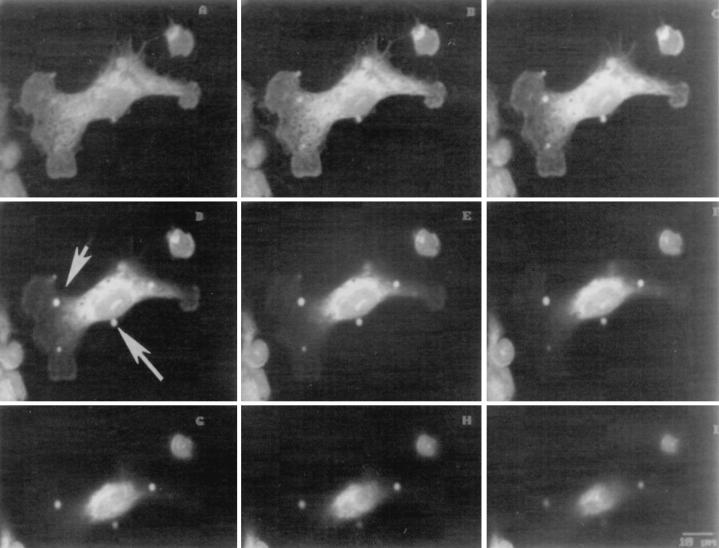
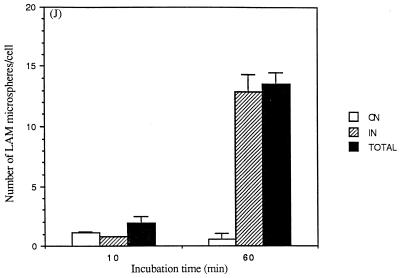
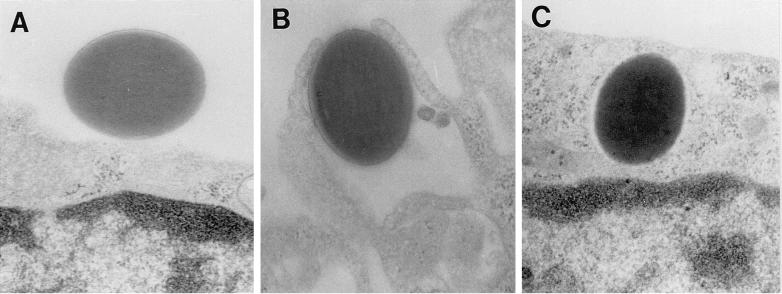
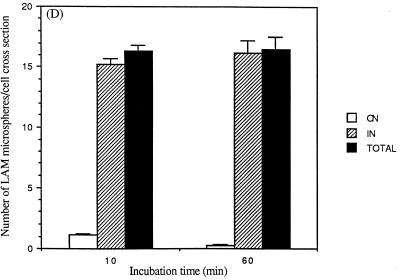
Studies that assess internalization of macroparticles by using techniques that involve fixation with or without permeabilization followed by immunofluorescence microscopy are potentially limited by the completeness of permeabilization and by the resolution of light microscopy. To further examine the kinetics and extent of internalization as well as the mechanism of internalization, we performed experiments with confocal microscopy (Fig. 4) and transmission EM (Fig. 5). Similar to the results obtained by immunofluorescence microscopy (Fig. 2), confocal microscopy experiments revealed that the number of microspheres associated with and internalized by MDMs increased markedly over 60 min and that 50% of the associated microspheres were internalized by 10 min (Fig. 4J). EM experiments revealed that LAM microspheres are internalized by a conventional-appearing form of phagocytosis (Fig. 5A to C), similar to that observed for intact M. tuberculosis bacilli (17). Although the EM studies showed that the overall pattern of internalization of LAM microspheres was similar to the results obtained by light microscopy techniques, >95% of microspheres were internalized by 10 min as quantitated by EM (Fig. 5D). We interpreted these data to indicate that the EM technique was more sensitive in evaluating microspheres that had just completed the process of phagocytosis with recent closure of the macrophage pseudopod leaflets.
FIG. 4.
Confocal microscopy to examine internalization of LAM microspheres. Glass slides prepared for the experiments shown in Fig. 1 to 3 were examined by confocal microscopy. Serial sections of a single cell (A to I) were taken from the base of the cell to the apical surface. In panel D, the small arrow shows an internalized microsphere and the large arrow shows an attached microsphere (×1,000). Results are quantitated in panel J (for MDMs containing ≥1 microsphere). The number of microspheres attached (ON) and internalized (IN) and the total number of microspheres (TOTAL) were determined.
FIG. 5.
EM to examine internalization of LAM microspheres. MDMs on glass coverslips were incubated with LAM microspheres as in Fig. 1 for 60 min. MDMs were then fixed, prepared, and sectioned for analysis by transmission EM. (A) LAM microsphere attached to the cell surface (×40,000). (B) LAM microsphere in the process of phagocytosis (×40,000). (C) LAM microsphere within a phagosome (×40,000). Results are quantitated in panel D (for MDM cross sections containing ≥1 microsphere). The number of microspheres attached (ON) and internalized (IN) and the total number of microspheres (TOTAL) were determined.
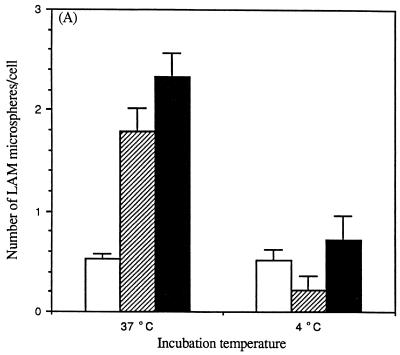
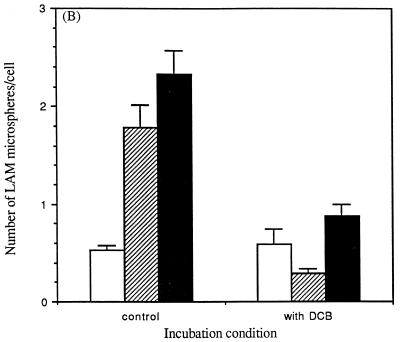
Phagocytosis is distinguished from pinocytosis by its strict dependence on temperature and the actin cytoskeleton (30). Consistent with receptor-mediated phagocytosis of microspheres mediated by LAM, the total cell association of microspheres was reduced 70% at 4°C compared to 37°C (Fig. 6A). Also, the percentage of internalized LAM microspheres by MDMs was markedly reduced at 4°C (29%) compared to that observed at 37°C (77%). In similar fashion, pretreatment of MDMs with the fungal metabolite dihydrocytochalasin B to block actin polymerization markedly reduced both the total cell association with MDMs and the proportion of microspheres that were internalized (Fig. 6B).
FIG. 6.
Temperature dependence and the role of the actin cytoskeleton in the cell association of LAM microspheres with MDMs. (A) MDMs were incubated with LAM microspheres for 60 min at 37 or 4°C. (B) MDMs were treated with dihydrocytochalasin B (5 μg/ml) for 30 min at 37°C and then incubated with LAM microspheres for 60 min at 37°C. At the end of each incubation period, MDMs were fixed with 3.3% formalin. Fixed MDMs were enumerated as described in the legend to Fig. 3. The number of microspheres attached to the cell (open bars) or totally associated (solid bars) was measured. The number of internalized microspheres (striped bars) was calculated. All points are means ± standard errors from two independent experiments.
Evidence for MR involvement in the phagocytosis of LAM microspheres.
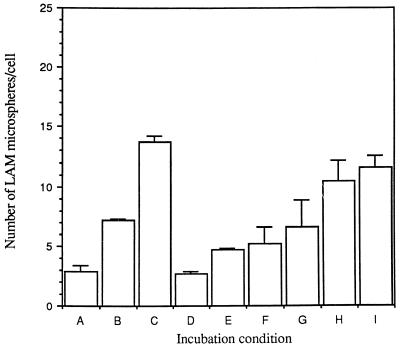
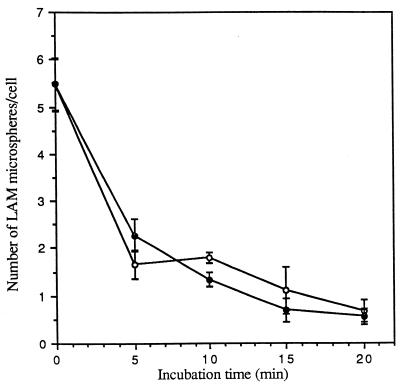
MR activity is markedly reduced in the presence of competing multivalent ligands such as mannan and has been found to reduce the binding of LAM microspheres to MDMs. To further examine the specificity, time course, and temperature requirements for LAM microsphere uptake by MDMs, time course experiments were performed in the presence and absence of mannan at 37 and 4°C (Fig. 7). These experiments revealed mannan-inhibitable cell association of microspheres and thus were consistent with MR-dependent phagocytosis of LAM microspheres. At 37°C in the absence of mannan, a time-dependent linear increase in cell association was seen (Fig. 7A). In contrast, at 4°C in the absence of mannan, near-maximal binding was seen by 10 min (Fig. 7B). These results were consistent with the idea that at 4°C, microspheres attached to the surface of MDMs in a saturable manner and that at 37°C, efficient internalization of microspheres occurred with rapid recycling of receptors. MR activity is calcium dependent and trypsin susceptible (23). Consistent with this, the uptake of LAM microspheres was markedly reduced in Ca2+-free RPMI or 1 mM EDTA and was restored with the addition of 1 to 2 mM Ca2+ (Fig. 8). Trypsin and EGTA were effective in removing attached microspheres (4°C) from the cell surface (Fig. 9).
FIG. 7.
Time course of mannan-inhibitable cell association of LAM microspheres with MDMs. MDMs were incubated in the absence or presence of 4 mg of mannan per ml at 37 or 4°C and then incubated with 6 × 107 microspheres for the indicated time at 37°C (A) or 4°C (B) and fixed in formalin. The number of total cell-associated microspheres was enumerated by phase-contrast microscopy. The specific uptake of microspheres (solid triangles) was obtained by subtracting the number of microspheres in the presence of mannan (solid circles) from that of microspheres in the absence of mannan (open circles). All points are means ± standard errors from two independent experiments.
FIG. 8.
Calcium dependence of cell association of LAM microspheres with MDMs. MDMs were incubated in calcium-free RPMI in the absence or presence of 1 mM EDTA with increasing concentrations of calcium for 30 min at 37°C. MDMs were then incubated with 6 × 107 microspheres for 60 min at 37°C, washed, and fixed in formalin. The number of total cell-associated microspheres was enumerated by phase-contrast microscopy. (A) Ca2+-free medium. (B) Ca2+-free medium plus 1 mM Ca2+. (C) Ca2+-free medium plus 2 mM Ca2+. (D) EDTA (1 mM). (E) EDTA (1 mM) plus 0.125 mM Ca2+. (F) EDTA (1 mM) plus 0.25 mM Ca2+. (G) EDTA (1 mM) plus 0.5 mM Ca2+. (H) EDTA (1 mM) plus 1 mM Ca2+. (I) EDTA (1 mM) plus 2 mM Ca2+. All points are means ± standard errors from two independent experiments.
FIG. 9.
Effects of trypsin and EGTA on release of LAM microspheres attached to MDMs. MDMs were incubated with 6 × 107 microspheres for 60 min at 4°C, washed, and then incubated in medium containing 0.01% trypsin (open circles) or 10 mM EGTA (solid circles) for the indicated time at 4°C, washed, and fixed in formalin. The number of total cell-associated microspheres was enumerated by phase-contrast microscopy. All points are means ± standard errors from two independent experiments.
Evidence that MRs recycled from the macrophage interior mediate phagocytosis of LAM microspheres.
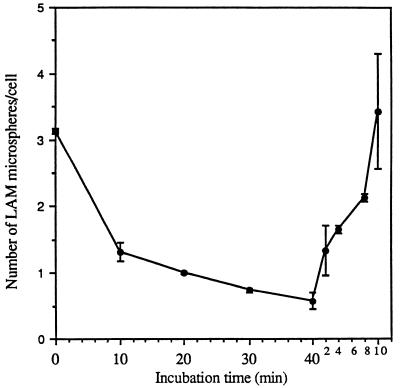
At any given time, the MDM contains a large internal pool of MRs that recycle to the cell surface. Figure 10 shows that incubation of MDMs with trypsin at 37°C for short periods of time resulted in a significant reduction in the subsequent attachment of LAM microspheres, consistent with a depletion of both surface-expressed and newly expressed MRs from the cell interior. Trypsin treatment of MDMs at 4°C removes only surface-expressed MR activity. Such treatment allowed for a rapid recovery of LAM microsphere uptake by subsequent warming of the cell to 37°C in the absence of trypsin (expression from intracellular pools) (Fig. 11).
FIG. 10.
Effect of treatment of MDMs with trypsin on the subsequent attachment of LAM microspheres. MDMs were incubated in the absence (control) or presence of 0.01% trypsin for the indicated times at 37°C and then incubated with 6 × 107 microspheres for 20 min at 4°C, washed, and fixed in formalin. The condition chosen for trypsin treatment allowed the monolayer to remain intact. The number of total cell-associated microspheres was enumerated by phase-contrast microscopy, and the percentage of control was calculated for each time point (n = 2).
FIG. 11.
Removal of surface-expressed MR activity by trypsin treatment at 4°C and recovery of activity by warm up of the cell at 37°C. MDMs were incubated in the presence of 0.01% trypsin for the indicated time at 4°C (0 to 40 min, left side of the figure) and then incubated with 6 × 107 microspheres for 60 min at 4°C and fixed in formalin, and the number of microspheres was enumerated. For the recovery assay (right side of the figure), MDMs were incubated in the presence of 0.01% trypsin for 40 min at 4°C, warmed for the indicated time (0 to 10 min) at 37°C, and then incubated with 6 × 107 microspheres for 60 min at 4°C and fixed in formalin, and the number of microspheres was enumerated. All points are means ± standard errors from two independent experiments.
To determine whether the phagocytosis of microspheres is dependent upon newly synthesized MRs, MDMs were incubated in the absence or presence of cycloheximide for 60 min at 37°C prior to addition of LAM microspheres. Trichloroacetic acid precipitation of [35S]methionine-incorporated proteins confirmed >60% inhibition of new protein synthesis with cycloheximide (data not shown). The presence of cycloheximide reduced the phagocytosis of microspheres by only 21% (0.92 ± 0.28 microspheres/cell in the absence of cycloheximide to 0.73 ± 0.45 microspheres/cell in the presence of cycloheximide; mean ± standard error; n = 2). Thus, cycloheximide had only a small effect on the phagocytosis of microspheres, supporting the notion that the phagocytosis of LAM microspheres was dependent primarily upon recycling of preformed MRs in MDMs rather than on newly synthesized MRs. Although our studies provided evidence that recycled MRs in MDMs are competent to mediate phagocytosis, it remained possible that phagocytosis of LAM microspheres alters the kinetics of subsequent MR recycling as reported for Leishmania spp. (1). Therefore, we evaluated the influence of ingested LAM microspheres on the subsequent binding of 125I–mannose-BSA to determine if the binding was reduced. MDMs were incubated with mannose-BSA, mannan, or LAM microspheres at 4°C, washed extensively, warmed up at 37°C for short periods of time to synchronize endocytic events, and then incubated with 125I–mannose-BSA to assess binding. The phagocytosis of LAM microspheres did not alter the subsequent binding of 125I–mannose-BSA compared to that of MDMs that had been preincubated with mannan or mannose-BSA (data not shown), suggesting that the phagocytic events mediated by LAM did not alter the subsequent recycling of the receptor.
DISCUSSION
M. tuberculosis and other pathogenic mycobacteria enter mononuclear phagocytes by receptor-mediated phagocytosis, resist destruction, and multiply inside the cell with a unique phagosome that does not fuse with lysosomes (16). The molecular mechanisms that allow for this series of events to occur are just beginning to be uncovered and are critical to our understanding of disease pathogenesis. Phagocytosis of M. tuberculosis by the cell is a multireceptor-mediated event. The terminal mannosyl units of LAM from the virulent Erdman strain of M. tuberculosis bind to the macrophage MR, which, along with complement receptors, mediates phagocytosis of the bacterium. LAM is considered one important virulence determinant for M. tuberculosis, based on its ability to modulate several phagocyte responses such as cell signalling, cytokine release, and oxidant generation (2). The macrophage MR plays an important role in both the pinocytosis of molecules terminating in mannose and in the phagocytosis of macromolecules. Detailed studies of the biology of the MR (receptor binding and intracellular trafficking) have relied mainly on the use of labeled mannosylated proteins which are taken up by MR-dependent pinocytosis. Receptor-mediated phagocytosis of particles of >1 μm in diameter (such as a bacterium) differs fundamentally from pinocytosis in its strict dependence on energy (e.g., temperature) and the cytoskeleton. The details of MR binding to phagocytic particles and its subsequent intracellular trafficking are less well elucidated. Here we characterize the uptake of microspheres coated with LAM (a model of phagocytosis mediated by LAM) in mononuclear phagocytes in order to enhance both our knowledge of MR function and our understanding of the early mycobacterium-mononuclear phagocyte interaction. Our studies demonstrate that Erdman LAM microsphere binding to the MR allows for efficient phagocytosis of microspheres which is energy, calcium, and cytoskeleton dependent. Furthermore, we present evidence that MR recycling enhances the phagocytosis of microspheres and that ingested LAM microspheres do not appear to alter subsequent recycling of the receptor.
Our studies demonstrate that LAM microspheres exhibit a time- and dose-dependent rapid increase in phagocyte attachment and internalization that is specific for macrophages, consistent with the known MR expression on this cell type. Monocytes have a minimal capacity to phagocytose LAM microspheres when assessed relative to control microspheres. The low level of uptake by monocytes seen in Fig. 1 and 2 is saturable, whereas the phagocytosis of LAM microspheres by macrophages over this time period is linear. Although the receptor for LAM microsphere uptake by monocytes is unknown, the kinetics of uptake and the finding of saturability at 37°C argue against involvement of the MR. In contrast, the linear uptake of microspheres over time by macrophages at 37°C along with the fact that the total number of microspheres associated with macrophages greatly exceeds the attachment of microspheres by these cells at 4°C are both consistent with MR activity, specifically that this receptor rapidly recycles from a large intracellular pool.
That LAM microspheres are internalized by macrophages via receptor-mediated phagocytosis was demonstrated in several experiments. First, the level of cell association of LAM microspheres with macrophages and the percentage of internalized microspheres were highly temperature dependent. Second, both the total cell association of LAM microspheres with macrophages and the percentage of internalized microspheres were reduced by pretreatment of macrophages with dihydrocytochalasin B. Third, immunofluorescence microscopy studies in which cells were fixed with or without permeabilization demonstrated efficient ingestion by 10 to 20 min. Finally, EM experiments demonstrated that LAM microspheres enter macrophages by a conventional-appearing form of receptor-mediated phagocytosis in which phagocyte pseudopods move circumferentially around the microsphere until they fuse at the distal tip. This type of phagocytosis mirrors that seen with M. tuberculosis bacilli (17). The EM experiments demonstrated that >95% of microspheres are phagocytosed by 10 min. These results generally patterned those seen by the light microscopy techniques but demonstrated more rapid ingestion. We believe that the EM technique is more sensitive for assessing newly ingested microspheres.
Specific involvement of the MR in the phagocytosis of LAM microspheres is supported by several types of experiments. First, phagocytosis was dependent on cellular differentiation from monocytes to macrophages, consistent with the known increase in MR expression. Second, the level of uptake was markedly inhibited in the presence of the competing multivalent ligand mannan. Third, phagocytosis was both calcium dependent and trypsin susceptible. Incubation of macrophages with trypsin at 37°C for short periods of time resulted in a significant reduction in the subsequent attachment of LAM microspheres. Pretreatment of the macrophages with trypsin at 4°C, which removes only surface-expressed MR activity, allowed for rapid recovery of MR-dependent phagocytosis upon warming of the cell at 37°C for short periods of time. This is consistent with the known large intracellular pool of MRs that can recycle to the cell surface. Finally, experiments in which protein synthesis was reduced by cycloheximide indicated that the majority of phagocytosis of LAM microspheres occurs by preformed MRs that recycle from the cell interior rather than by newly synthesized MRs. A previous study showed that infection of mouse peritoneal macrophages with Leishmania donovani resulted in a decrease in MR activity on the cell surface which may have an impact on the effector function(s) of infected cells (1). Our results show that prior ingestion of LAM microspheres by macrophages does not alter the subsequent binding of 125I–mannose-BSA relative to cells pretreated with mannosylated molecules. These results do not exclude the possibility that the phagocytosis of viable bacteria may result in a different effect.
Our results differ somewhat from those of recent studies which have evaluated the interaction between free LAM and mammalian cells, indicating that the way in which LAM is presented to the host cell is critical in these types of studies. During active disease, LAM may be released as a free lipoglycan in various tissue sites. Host cell binding of LAM in solution, a condition in which all domains of the lipoglycan are exposed to the phagocyte, involves the acylated glycosylphosphatidylinositol anchor of the molecule and phagocyte CD14 as well as potentially several portions of the LAM molecule (7, 13, 27, 29). For example, using biotinylated mannose-capped LAM from Mycobacterium bovis BCG for studies of LAM-mammalian cell interactions, Venisse et al. provide evidence for a temperature- and calcium-dependent, trypsin-susceptible interaction with several host types, including murine granulocytes, splenocytes, and, to a lesser degree, lymphocytes (29). The uptake of LAM was particularly intense for granulocytes and was enhanced in heat-inactivated serum. Since granulocytes do not express the MR, the authors speculated that the mannose binding protein may be involved in this process. Others have shown that the anchor of LAM integrates into the glycosylphosphatidylinositol-rich domains of mammalian cell membranes without internalization (7).
The aforementioned study, done with biotinylated LAM, did not specifically assess the efficiency of internalization of LAM. The specificity of our results for macrophages relative to monocytes, combined with microscopy studies that demonstrate efficient internalization of microspheres mediated by LAM, provides evidence that LAM adhered to a phagocytic particle in which the acyl moieties of LAM bind to the hydrophobic surface of microspheres preferentially exposing the glycan units, which allows for a more direct assessment of the carbohydrate-lectin interaction between LAM terminal mannosyl units and the macrophage MR. This orientation of LAM may more closely approximate that found on an intact bacillus.
Recent studies demonstrating that phagocytes can present LAM in the context of human CD1b molecules on antigen-presenting cells for T cells have provided evidence that the MR may play an important role in this process (12, 20). In the recent study by Prigozy et al., uptake of free LAM by human peripheral blood mononuclear cells treated with granulocyte-macrophage colony-stimulating factor and interleukin 4 was found to be MR dependent (12). Confocal microscopy and EM studies demonstrated that the majority of the intracellular MRs in these cells were located in either early endosomes or, to a lesser degree, in the major histocompatibility complex class II compartment (MIIC compartment), where peptides are loaded into class II molecules. In contrast, CD1b molecules localized primarily to lysosomes and, to a lesser degree, to the MIIC compartment. LAM localized to late endosomes, MIICs, and lysosomes. Thus, the MR, LAM, and CD1b were all colocalized to the MIIC compartment, indicating that the MR could potentially deliver LAM to late endosomes for loading onto CD1b for presentation to T cells. The authors hypothesize that the pathway of MR-mediated uptake leading to antigen presentation by CD1b could be important in the immune response to a variety of pathogens.
The study of phagocytosis and the earliest intracellular vesicle fusion events associated with an intact bacillus such as M. tuberculosis is complex and does not easily allow one to establish a specific mechanism for the observations made. One goal of our studies is to determine whether a major M. tuberculosis surface molecule such as LAM, a potential virulence determinant, can itself modulate the early phagocytic pathway. In this regard, M. tuberculosis cord factor and sulfatides have been reported to influence vesicle fusion events (5, 21). Our EM studies demonstrate that the LAM microsphere resides within a membrane-bound vesicle or phagosome in the macrophage. Our preliminary immuno-EM studies suggest that LAM is shed from the microsphere shortly after phagocytosis has occurred and thus may resemble observations made in EM studies with intact bacilli (31). Using our model, we are currently investigating the intracellular trafficking pathway mediated by LAM types that differ in their interaction with the MR.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (AI33004) and the Department of Veterans Affairs.
We thank members of the Central Microscopy Research Facility at the University of Iowa for assistance and Deb Nollen for editorial assistance. We also thank Patrick Brennan and colleagues for providing purified Erdman LAM and rabbit polyclonal antibody to M. tuberculosis (NIH sponsorship N01-AI 25147).
REFERENCES
- 1.Basu N, Sett R, Das P K. Down-regulation of mannose receptors on macrophages after infection with Leishmania donovani. Biochem J. 1991;277:451–456. doi: 10.1042/bj2770451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan P J, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee D, Lowell K, Rivoire B, McNeil M R, Brennan P J. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannosyl residues in some strains. J Biol Chem. 1992;267:6234–6239. [PubMed] [Google Scholar]
- 4.Ezekowitz R A B, Sastry K, Bailly P, Warner A. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med. 1990;172:1785–1794. doi: 10.1084/jem.172.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goren M B, Hart P D, Young M R, Armstrong J A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1976;73:2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horwitz M A. Intracellular parasitism. Curr Opin Immunol. 1988;1:41–46. doi: 10.1016/0952-7915(88)90049-0. [DOI] [PubMed] [Google Scholar]
- 7.Ilangumaran S, Arni S, Poincelet M, Theler J-M, Brennan P J, Din N, Hoessli D C. Integration of mycobacterial lipoarabinomannans into glycosylphosphatidylinositol-rich domains of lymphomonocytic cell plasma membranes. J Immunol. 1995;155:1334–1342. [PubMed] [Google Scholar]
- 8.Kahn S, Wleklinski M, Aruffo A, Farr A, Coder D, Kahn M. Trypanosoma cruzi amastigote adhesion to macrophages is facilitated by the mannose receptor. J Exp Med. 1995;182:1243–1258. doi: 10.1084/jem.182.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koning F. Enhancement of HLA class II restricted antigen presentation by mannose-receptor-mediated uptake. Biochem Soc Trans. 1997;25:664–665. doi: 10.1042/bst0250664. [DOI] [PubMed] [Google Scholar]
- 10.McNally A K, DeFife K M, Anderson J M. Interleukin-4-induced macrophage fusion is prevented by inhibitors of mannose receptor activity. Am J Pathol. 1996;149:975–985. [PMC free article] [PubMed] [Google Scholar]
- 11.Noel G J, Mosser D M, Edelson P J. Role of complement in mouse macrophage binding of Haemophilus influenzae type b. J Clin Invest. 1990;85:208–218. doi: 10.1172/JCI114414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prigozy T I, Sieling P A, Clemens D, Stewart P L, Behar S M, Porcelli S A, Brenner M B, Modlin R L, Kronenberg M. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity. 1997;6:187–197. doi: 10.1016/s1074-7613(00)80425-2. [DOI] [PubMed] [Google Scholar]
- 13.Pugin J, Heumann D, Tomasz A, Kravchenko V V, Akamatsu Y, Nishijima M, Glauser M P, Tobias P S, Ulevitch R J. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 14.Raviglione M C, Snider D E, Jr, Kochi A. Global epidemiology of tuberculosis: morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 15.Schlesinger L S. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 16.Schlesinger L S. Role of mononuclear phagocytes in M.-tuberculosis pathogenesis. J Invest Med. 1996;44:312–323. [PubMed] [Google Scholar]
- 17.Schlesinger L S, Bellinger-Kawahara C G, Payne N R, Horwitz M A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 18.Schlesinger L S, Hull S R, Kaufman T M. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- 19.Shepherd V L, Campbell E J, Senior R M, Stahl P D. Characterization of the mannose/fucose receptor on human mononuclear phagocytes. J Reticuloendothel Soc. 1982;32:423–431. [PubMed] [Google Scholar]
- 20.Sieling P A, Chatterjee D, Porcelli S A, Prigozy T I, Mazzaccaro R J, Soriano T, Bloom B R, Brenner M B, Kronenberg M, Brennan P J, Modlin R L. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 21.Spargo B J, Crowe L M, Ioneda T, Beaman B L, Crowe J H. Cord factor (a,a-trehalose 6,6′-dimycolate) inhibits fusion between phospholipid vesicles. Proc Natl Acad Sci USA. 1991;88:737–740. doi: 10.1073/pnas.88.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Speert D P, Silverstein S C. Phagocytosis of unopsonized zymosan by human monocyte-derived macrophages: maturation and inhibition by mannan. J Leukoc Biol. 1985;38:655–658. doi: 10.1002/jlb.38.5.655. [DOI] [PubMed] [Google Scholar]
- 23.Stahl P, Schlesinger P H, Sigardson E, Rodman J S, Lee Y C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980;19:207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- 24.Stahl P D. The macrophage mannose receptor: current status. Am J Respir Cell Mol Biol. 1990;2:317–318. doi: 10.1165/ajrcmb/2.4.317. [DOI] [PubMed] [Google Scholar]
- 25.Stahl P D, Rodman J S, Miller M J, Schlesinger P H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci USA. 1978;75:1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stahl P D, Wileman T E, Diment S, Shepherd V L. Mannose-specific oligosaccharide recognition by mononuclear phagocytes. Biol Cell. 1984;51:215–218. doi: 10.1111/j.1768-322x.1984.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 27.Stokes R W, Speert D P. Lipoarabinomannan inhibits nonopsonic binding of Mycobacterium tuberculosis to murine macrophages. J Immunol. 1995;155:1361–1369. [PubMed] [Google Scholar]
- 28.Taylor M E, Drickamer K. Structural requirements for high affinity binding of complex ligands by the macrophage mannose receptor. J Biol Chem. 1993;268:399–404. [PubMed] [Google Scholar]
- 29.Venisse A, Fournié J-J, Puzo G. Mannosylated lipoarabinomannan interacts with phagocytes. Eur J Biochem. 1995;231:440–447. doi: 10.1111/j.1432-1033.1995.tb20717.x. [DOI] [PubMed] [Google Scholar]
- 30.Wright S D. Receptors for complement and the biology of phagocytosis. In: Gallin J I, Goldstein I M, Snyderman R, editors. Inflammation: basic principles and clinical correlates. New York, N.Y: Raven Press, Ltd.; 1992. pp. 477–495. [Google Scholar]
- 31.Xu S, Cooper A, Sturgill-Koszycki S, Van Heyningen T, Chatterjee D, Orme I, Allen P, Russell D G. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994;153:2568–2578. [PubMed] [Google Scholar]