Abstract
"Oncometabolite" 2-hydroxyglutarate (2-HG) is an aberrant metabolite found in tumor cells, exerting a pivotal influence on tumor progression. Recent studies have unveiled its impact on the proliferation, activation, and differentiation of anti-tumor T cells. Moreover, 2-HG regulates the function of innate immune components, including macrophages, dendritic cells, natural killer cells, and the complement system. Elevated levels of 2-HG hinder α-KG-dependent dioxygenases (α-KGDDs), contributing to tumorigenesis by disrupting epigenetic regulation, genome integrity, hypoxia-inducible factors (HIF) signaling, and cellular metabolism. The chiral molecular structure of 2-HG produces two enantiomers: D-2-HG and L-2-HG, each with distinct origins and biological functions. Efforts to inhibit D-2-HG and leverage the potential of L-2-HG have demonstrated efficacy in cancer immunotherapy. This review delves into the metabolism, biological functions, and impacts on the tumor immune microenvironment (TIME) of 2-HG, providing a comprehensive exploration of the intricate relationship between 2-HG and antitumor immunity. Additionally, we examine the potential clinical applications of targeted therapy for 2-HG, highlighting recent breakthroughs as well as the existing challenges.
Keywords: 2-Hydroxyglutarate, Isocitrate dehydrogenase, Tumor immune microenvironment, Oncometabolite, Therapeutic target
Highlights
-
•
D-2-HG interferes with a wide range of immune cells and even complement system to cause “cold tumor” characteristics.
-
•
L-2-HG provokes CD8+ T cells as well as induces memory T cells.
-
•
Small molecule inhibitors and therapeutic vaccines targeted 2-HG have potential in cancer treatment.
1. Introduction
The tumor immune microenvironment (TIME), is a dynamic and intricate system that comprises a complex network of immune cells, stromal cells, blood vessels, cytokines, and signaling pathways [1]. TIME plays a critical role in regulating tumor development and antitumor therapeutic responses [2]. In adaptive immunity, tumor-infiltrating lymphocytes (TILs), including CD3+, CD8+, and CD4+ T lymphocytes, as well as intra-tumoral FoxP3+ regulatory T lymphocytes (TReg), are predictable for the survival and treatment responses of cancer patients [3]. Tumor-infiltrating B lymphocytes are efficient in antibody production and immune memory, but the role of which in the context of anti-tumor immunity remains a topic of debate [4]. In innate immunity, natural killer (NK) cells participate in immune defense by directly attacking tumor cells [5]. Antigen-presenting cells (APCs), such as dendritic cells (DCs) and macrophages, facilitate the cross-presentation of tumor cell-related antigens to naive T cells and activate specific T cell-mediated anti-tumor response [6]. Tumor-associated macrophages (TAMs) can mediate processes such as angiogenesis, promote tumor invasion and metastasis, and contribute to the establishment of an immunosuppressive microenvironment [7]. Inflammatory factors, for example, interleukin-12 (IL-12) and interferon-α (IFN-α) have been identified as mediators of tumor regression, while IFN-γ secretion indicates effective antitumor activity [8]. Immune checkpoint programmed cell death ligand 1 (PD-L1) is expressed on the surface of malignant tumor cells, and its binding to programmed cell death protein 1 (PD-1) can inhibit T cell activity, playing a crucial role in tumor evasion of immune surveillance [9]. Cancer immunotherapy aims to alter the TIME by activating immune cells to enhance their inhibitory and cytotoxic effects on tumors [10]. Among these approaches, PD-1/PD-L1 inhibitors activate T cells by blocking immune checkpoints and have proved effective in a wide range of tumors [11]. Additionally, adoptive cell transfer (ACT) therapy utilizes engineered T cells, including chimeric antigen receptor (CAR)-T cells and T-cell receptor (TCR)-engineered T cells, to directly target and attack tumor cells [9]. In recent years, peptide-based therapeutic cancer vaccines have stimulated CD8 T cells or CD4 T helper cells to target Tumor-Associated Antigens (TAAs) or Tumor-Specific Antigens (TSAs), resulting in significant clinical benefits [12].
Growing evidence underscores the pivotal role of various metabolites as immune regulatory molecules, significantly modulating the balance between immune suppression and activation within the tumor [13]. For instance, lactate dehydrogenase A (LDHA)-mediated lactate accumulation in melanoma inhibits T cells and NK cells, fostering immune escape [14]. Methylglyoxal activates macrophages and lymphocytes to combat tumor cells [15]. Notably, the oncometabolite 2-hydroxyglutarate (2-HG) is reported to function as an immune signaling molecule, influencing both innate and adaptive immune components [16]. 2-HG is a low-abundance metabolite structurally akin to α-ketoglutarate (α-KG) [17,18]. It differs in having a hydroxyl group rather than a ketone group at the second carbon position, which creates a chiral center. Consequently, 2-HG manifests as two enantiomers: D(R)-2-HG and L(S)-2-HG [19]. Achieving prominence as an oncometabolite, 2-HG became associated with isocitrate dehydrogenase (IDH) mutations (mIDH) [20], prevalent in various tumors, including gliomas, acute myeloid leukemia (AML), cartilaginous tumors, and cholangiocarcinoma [[21], [22], [23]]. Therefore, the interplay between metabolic enzymes generated from mIDH and various malignancies has been studied. mIDH gains the capacity to prolifically generate D-2-HG from α-KG, which contributes to the main source of intracellular D-2-HG [24,25] On the other hand, L-2-HG has been observed to accumulate independently of mIDH in renal cell carcinoma (RCC) [26]. The biological function of 2-HG is quite complicated, chiefly through competitive inhibition of the α-KG-dependent dioxygenase superfamily (α-KGDDs) involved in epigenetic modification, metabolic regulation, hypoxia adaptation, and matrix formation [27]. Despite distinct origins, D-2-HG and L-2-HG play different roles in malignant transformation, tumor progression, antitumor immunity and therapeutic application [[28], [29], [30]]. However, scant attention has been devoted to this field in existing literature. This review aims to scrutinize the impact of 2-HG on antitumor immunity, exploring the underlying mechanisms by which 2-HG regulates immune components. Additionally, we will discuss the latest breakthroughs in 2-HG-based immunotherapy for malignancies.
2. 2-HG metabolism in physiological and tumor contexts
2.1. Origin of D-2-HG and IDH
The metabolic pathways of 2-HG are currently the subject of ongoing research. It is widely acknowledged that in typical cellular environments, 2-HG manifests as a metabolite of relatively low prevalence, originating from ancillary reactions of specific enzymatic activities. For instance, the enzyme hydroxyacid-oxoacid transhydrogenase (HOT) facilitates the conversion of γ-hydroxybutyrate (GHB) into succinic semialdehyde (SSA), concurrently effecting the reduction of α-KG to D-2-HG [31]. Moreover, D-2-HG is also generated as a secondary product by the enzyme phosphoglycerate dehydrogenase (PHGDH) [32]. Additionally, evidence indicates that D-2-HG serves as an intermediary compound in the metabolic processes involving 5-hydroxy-l-lysine and 5-aminolevulinic acid [33].
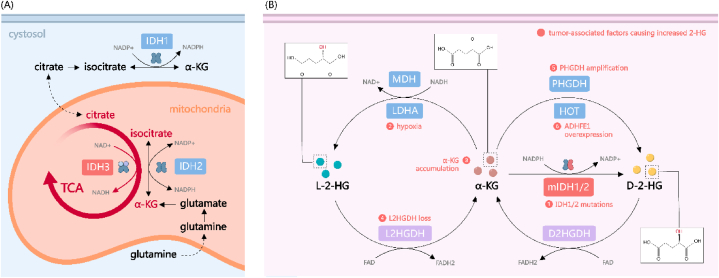
In their pivotal study, Dang and colleagues first established a connection between IDH mutations and the accumulation of D-2-HG [20]. Human cells express three distinct isoforms of IDH: IDH1 is localized in the cytosol and peroxisomes, whereas IDH2 and IDH3 are mitochondrial (Fig. 1A) [34]. The wild-type forms of IDH1 and IDH2 operate as homodimers, mediating the conversion of isocitrate to α-KG while concurrently reducing nicotinamide adenine dinucleotide phosphate (NADP+) to NADPH. In stark contrast, oncogenic mutations in IDH1 and IDH2 induce a neomorphic enzymatic activity, catalyzing the NADPH-consuming reduction of α-KG into D-2-HG, thereby significantly elevating intracellular D-2-HG levels (Fig. 1B) [34]. These cancer-associated mutations in IDH are exclusively missense mutations targeting arginine residues within the enzymes' active sites, predominantly manifesting as R132H in IDH1 and R172K or R140Q in IDH2 [21,22]. Notably, the heterozygous state of these IDH mutations appears to be a critical factor for elevated 2-HG production. This is evidenced by the observation that mIDH tumors undergoing loss of heterozygosity (LOH) exhibit reduced levels of D-2-HG [35]. In the cellular milieu of mIDH1/2 tumors, the majority of IDH enzyme complexes exist as heterodimers, comprising one wild-type and one mutant subunit [36]. This heterodimeric configuration markedly enhances the efficiency of D-2-HG synthesis, underlining the unique biochemical interplay in the pathogenesis of IDH-mutant cancers [37]. Regarding IDH3, this enzyme exhibits distinct functional and structural characteristics when compared to IDH1/2. Notably, a direct link between mutations in IDH3 and higher 2-HG levels remains unestablished. As a nicotinamide adenine dinucleotide (NAD+)-dependent enzyme, IDH3 is evolutionarily divergent from the other two isoforms and is structurally composed of a heterotetrameric assembly, including two α subunits, one β subunit, and one γ subunit [34]. Contrasting with IDH1 and IDH2, which catalyze the reversible conversion of isocitrate to α-KG, IDH3 uniquely mediates an irreversible reaction. IDH3 has been considered the main force driving the forward reaction of TCA cycle by supplying α-KG from the isocitrate pool [38]. Although emerging research has suggested that overexpression of IDH3A, the gene encoding the α subunit of IDH3, might contribute to certain oncogenic phenotypes in cancer cells, the underlying mechanisms are determined to be independent of 2-HG dynamics [[39], [40], [41]].
Fig. 1.
The metabolism of 2-HG in cancer cell. (A) The role of IDH in the TCA cycle under physiological conditions. (B) The generation of 2-HG under pathological conditions. Generally, 2-HG is transformed from its substrate α-KG. The heterogenous mutation of IDH1/2 represents the primary source of D(R)-2-HG. Additionally, PHGDH and HOT convert α-KG to D(R)-2-HG in a NADH-dependent manner. L(S)-2-HG is predominantly produced by LDHA and MDH under hypoxic conditions. Reversely, 2-HG is catabolized by 2HGDH to maintain a relatively low level. Abnormal accumulation of 2-HG promotes the tumor progression by inhibiting α-KGDDs, adapting to hypoxia and metabolism reprogramming. TCA: tricarboxylic acid; IDH: isocitrate dehydrogenase; NADP+: nicotinamide adenine dinucleotide phosphate; α-KG: α-ketoglutarate; 2-HG: 2-hydroxyglutarate; MDH: malate dehydrogenase; LDHA: lactate dehydrogenase A; 2HGDH: 2-HG dehydrogenase; HOT: hydroxyacid-oxoacid transhydrogenase; FAD: flavin adenine dinucleotide.
2.2. Origin of L-2-HG
As for the synthesis of L-2-HG, two primary sources have been identified (Fig. 1B). Malate dehydrogenase (MDH), which conventionally facilitates the conversion of l-malate to oxaloacetate within the TCA cycle, also possesses the capacity to reduce α-KG to L-2-HG, albeit with significantly reduced efficiency [42]. Additionally, LDHA exhibits a promiscuous activity, enabling it to convert α-KG to L-2-HG [43]. Both reactions necessitate the consumption of NADH and result in the generation of NAD+. In hypoxic conditions, an elevated level of 2-HG has been observed across various cell lines [43,44]. A possible explanation is that the production of 2-HG from α-KG by LDHA and MDH is favored by an acidic pH and increased NADH/NAD + ratio [44,45].
2.3. Cellular Clearance of 2-HG
D-2-HG and L-2-HG are maintained at low physiological levels through two distinct mitochondrial enzymes: D-2-HG dehydrogenase (D2HGDH) and L-2-HG dehydrogenase (L2HGDH), respectively (Fig. 1B). These two enzymes, utilizing flavin adenine dinucleotide (FAD) as an electron acceptor, catalyze the oxidation of 2-HG back to α-KG [34]. The rare neurodegenerative congenital disorder, 2-HG aciduria (2-HGA), is characterized by the pathological accumulation of 2-HG in body fluids and is closely associated with aberrations in these dehydrogenase enzymes [18]. 2-HGA is further classified into L-2-HG aciduria (L-2-HGA), D-2-HG aciduria (D-2-HGA), and the combined DL-2-HG aciduria (DL-2-HGA). Approximately half of the cases of D-2-HGA (type I D-2-HGA) and the vast majority of L-2-HGA cases are attributed to germline mutations in D2HGDH and L2HGDH, respectively, while IDH2 mutations account for the remaining D-2-HGA cases (type II D-2-HGA) [18]. In cases of combined DL-2-HGA, where both enantiomers accumulate, germline mutations in solute carrier family 25 member 1 (SLC25A1) have been identified [46]. It has been reported that in a Drosophila model, mutated forms of the Drosophila homolog of SLC25A1 impede citrate entry into the cytoplasm. This decrease in cytosolic citrate levels leads to enhanced glycolysis and consequent elevated lactate production, which in turn inhibits L-2-HG degradation by interfering with L2HGDH activity [47]. However, what results in D-2-HG accumulation in DL-2-HGA remains under investigation.
2.4. Transport of 2-HG
It is noteworthy that D-2-HG possesses the capability to be secreted and subsequently absorbed by adjacent cells through dicarboxylate transporters, including solute carrier family 13 member 3 (SLC13A3) [48]. This phenomenon suggests that 2-HG exerts an influence not solely on the tumor cells over-producing it, but also holds the potential to modulate the tumor microenvironment.
2.5. Diverse mechanisms of 2-HG elevation in tumors
Although the prevailing notion is that mIDH induces elevated D-2-HG in a variety of tumors, it's important to note that high levels of 2-HG are also detected in numerous tumors with wild-type IDH (wt-IDH). This points to the existence of additional factors that contribute to the increased levels of 2-HG. In fact, several mechanisms independent of mIDH, leading to an increase in both D-2-HG and L-2-HG in tumors, have been reported [49].
Hypoxia, a common condition within tumor microenvironments, facilitates the conversion of α-KG to L-2-HG, partially through the activation of LDHA and MDH [50]. Concurrently, a distinguishing characteristic of tumor metabolism is the increased uptake of glutamine, coupled with intensified glutaminolysis within the mitochondria, mediated by glutaminase 1 (GLS1) [51]. This metabolic shift leads to a heightened accumulation of glutamine-derived α-KG, which can subsequently serve as the substrate for the synthesis of both D-2-HG and L-2-HG [49,52]. In RCC, a noted reduction in L2HGDH expression correlates with the consequential accumulation of L-2-HG [53]. PHGDH amplification is linked to increased D-2-HG in breast cancers without IDH mutations [32]. In a subset of estrogen receptor-negative breast cancers, the accumulation of D-2-HG may be linked to the activation of the Myc signaling pathway, which in turn, via alterations in iron metabolism, upregulates the expression of alcohol dehydrogenase iron containing 1 (ADHFE1) gene, which encodes HOT enzyme [54].
3. General role of 2-HG in cancer
3.1. 2-HG's influence on DNA methylation
2-HG has been widely reported as an oncometabolite involved in tumor progression, the primary mechanism of 2-HG involves interference with α-KGDDs. 2-HG, a mild antagonist of α-KG, effectively inhibits various α-KG-dependent dioxygenases in tumors when its concentration (3–35 mM) surpasses their IC50 values, as exemplified by jumonji domain-containing 2C (JMJD2C) at 79 ± 7 μM and hypoxia-inducible factors (HIF) at 1500 ± 400 μM [[55], [56], [57]]. These α-KGDDs are associated with DNA methylation, as a hypermethylated state with low levels of 5-hydroxymethylcytosine (5hmC) was observed in mIDH glioma and AML [[58], [59], [60]]. This phenotype has been attributed to D-2-HG-mediated inhibition of the ten-eleven translocation (TET) family of methylcytosine hydroxylases, which promote DNA demethylation via conversion of 5-methylcytosine (5 mC) into 5hmC [61]. Similarly, elevated L-2-HG has also been reported to inhibit TET and lead to hypermethylation in RCC [53]. The precise molecular mechanisms through which inhibition of TET enzymes contributes to tumorigenesis remain inadequately elucidated. It is currently believed that TET inactivation could induce failure in DNA demethylation and thus transcriptional silencing of tumor suppressor genes or genes indispensable for differentiation [62]. Either accumulation of D-2-HG or loss of TET2 alone is sufficient to block hematopoietic differentiation and induce cytokine-independence, suggesting that D-2-HG-mediated inhibition of TET2 may be a driving force in some cases of AML [27,60,63].
3.2. 2-HG and histone modification
DNA hypermethylation alone is insufficient to account for the full spectrum of epigenetic effects exerted by 2-HG [64]. D-2-HG also interferes with histone modifications by impeding the activity of a superfamily of α-KG-dependent Jumonji C domain-containing histone lysine demethylases (KDMs). KDMs, often mutated or underexpressed in various cancers, exhibit complex and context-dependent roles in tumorigenesis [65]. D-2-HG treatment has been reported to result in differentiation defects through mechanisms related to altered histone modifications, such as enrichment of histone 3 Lys-9 trimethylation (H3K9me3), near differentiation-related genes [[66], [67], [68], [69]]. In Saccharomyces cerevisiae, 2-HG inhibits Rph1, the yeast homolog of human KDM4 demethylases, resulting in extensive changes in gene expression, particularly the down-regulation of genes already silenced through histone modification [70]. A recent study showed that D-2-HG inhibits KDM5 and further contributes to cellular transformation in mIDH AML and glioma, providing strong evidence that the mIDH/D-2-HG axis induces tumorigenesis through aberrant histone methylation by inhibiting KDMs [64].
3.3. 2-HG and genomic integrity
2-HG also participates in tumorigenesis by adversely affecting the maintenance of genomic integrity [71]. Both D-2-HG and L-2-HG inhibit α-KG-dependent alkB homolog (ALKBH) DNA repair enzymes, which could contribute to an increased mutation rate and thus exacerbate tumor progression [72]. Notably, this also leads to cells with mIDH becoming more susceptible to alkylating agents [73]. D-2-HG was also found to impair homology-dependent repair (HDR), giving rise to a “BRCAness” phenotype and poly-ADP-ribose polymerase (PARP) inhibitors sensitivity [74]. The exact mechanism by which D-2-HG inhibits HDR remains elusive. Research has shown that D-2-HG reduces ataxia telangiectasia mutated (ATM) expression, a critical DNA damage sensor [75]. More recent evidence suggests that 2-HG-mediated inhibition of KDM4B leads to abnormal histone 3 Lys-9 (H3K9) hypermethylation at DNA break sites. This hypermethylation obscures the H3K9me3 signal vital for recruiting lysine acetyltransferases 5 (KAT5) and ATM, two key proximal HDR factors [76].
3.4. 2-HG's effect on HIF signaling
2-HG also affects the HIF signaling pathway, which plays a pleiotropic role in tumorigenesis and regulates the metabolic cross-talk in the tumor microenvironment [77,78]. Considerable debate persists regarding the differential impacts of the two enantiomers of 2-HG on HIF. Early studies suggested that both D-2-HG and L-2-HG resulted in intracellular HIF-1α accumulation by inhibiting HIF prolyl hydroxylase (PHD), an α-KGDD that hydroxylates HIF-1α for further degradation [61,79]. These observations align with the role of L-2-HG in facilitating cellular adaptation to hypoxia [44,80,81]. However, subsequent studies have yielded contrasting views. In mIDH glioma samples from The Cancer Genome Atlas (TCGA) database, a marked diminution in the expression of HIF-1α target genes was observed, coupled with an inhibition of downstream biological functions, such as angiogenesis [82]. D-2-HG was reported to stimulate PHD activity as a co-substrate through enzyme-catalyzed oxidation to α-KG or non-enzymatic mechanisms, triggering HIF-1α destabilization [[83], [84], [85]]. PHD knockdown was shown to inhibit the proliferation of mIDH1 astrocytes and erythroleukemic cell lines, suggesting that HIF-1α may function as a tumor suppressor in mIDH tumors [63,83].
3.5. Metabolic changes induced by 2-HG
The accumulation of 2-HG can significantly alter the metabolic landscape of cells [[86], [87], [88], [89]]. These metabolic changes could confer a variety of vulnerabilities to cells. D-2-HG accumulation sensitizes cells to oxidative stress, attributed partly to the consumption of reducing equivalents during D-2-HG production [88]. Further, 2-HG inhibits α-KG-dependent branched-chain aminotransferase (BCAT), resulting in reduced glutamate and glutathione levels, which, coupled with suppressed alternate glutathione synthesis routes, amplifies oxidative stress vulnerability [90,91]. mIDH1 exacerbates this by lowering NAD + levels via nicotinate phosphoribosyltransferase (NAPRT) downregulation, increasing sensitivity to NAD + depletion [92]. Additionally, 2-HG boosts cell dependence on anti-apoptotic proteins. D-2-HG-mediated inhibition of mitochondrial cytochrome c oxidase (COX) reduces the apoptosis threshold, inducing Bcl-2 dependency in mIDH AML and making mIDH1 gliomas susceptible to Bcl-xL inhibition [93,94]. A recent study revealed that impaired de novo lipid synthesis in mIDH1 AML and solid tumors creates a dependency on acetyl-CoA carboxylase 1 (ACC1), a lipid synthesis enzyme, with pharmacological inhibition of ACC1 proving effective against mIDH1 cancers [95]. In summary, 2-HG-induced metabolic alterations render cells more dependent on specific pathways, offering potential metabolic targets. This could complement current clinical use of mutation-specific 2-HG inhibitors [29].
3.6. Broader implications of 2-HG in tumorigenesis
Additional mechanisms through which 2-HG contributes to tumorigenesis have been elucidated. The involvement of D-2-HG in promoting immature collagen formation and defects in the basement membrane might play a crucial role in glioblastoma development [96]. Moreover, the potential for 2-HG to be excreted by tumor cells and subsequently absorbed by adjacent cells renders its influence on the tumor microenvironment a subject of considerable scientific interest. Particularly striking is the effect of 2-HG on the immune components within tumors, a topic that will be examined in further detail in the next section of the text [16,97]. 2-HG also exerts effects on non-immune cells within the tumor microenvironment. Recent evidence suggests that D-2-HG uptake by vascular endothelial cells may facilitate tumor angiogenesis in mIDH1 solid tumors [98]. Additionally, 2-HG originating from tumors can be released into the circulatory system, acting as a highly sensitive and specific biomarker for detecting IDH1/2 mutations, or exerting systemic effects [99]. For example, research has shown that 2-HG plays a pivotal role in inducing skeletal muscle atrophy during cancer cachexia. This effect is observable in vitro, manifested as changes in myotube diameter, and is further validated in vivo, where mutations in IDH1 in CT26 cancer models are associated with muscle loss [100]. However, a cytotoxic effect on cancer cells was also observed in glioma and AML, as thoroughly discussed in a previous review [101]. In glioma, IDH mutation indicates a favorable prognosis by inhibiting ATP synthase/mammalian target of rapamycin (mTOR) activation and fat mass- and obesity-associated protein (FTO)/m6A/MYC/CCAAT enhancer binding protein alpha (CEBPA) axis. And FTO-suppression, in turn, results in decreased expression of PD-L1/2 and the leukocyte Ig-like receptor subfamily B (LILRB), suggesting a counteraction of D-2-HG induced immune evasion [[102], [103], [104]]. The multifaceted roles of 2-HG in cancer remain a fertile area for ongoing research.
4. The role of 2-HG in tumor immune microenvironment
Various strategies aim to enhance antitumor immunity by targeting immune cells [105,106]. The accumulation of specific metabolites, such as lactate and succinate, in the tumor microenvironment may have an impact on immune components, thus potentially disrupting anti-tumor immunity [107]. Oncometabolite 2-HG produced in tumor cells can also be secreted into the extracellular fluid and efficiently taken by immune cells [108]. Apart from that, 2-HG was first defined by Tyrakis et al. as an immunometabolite [109]. The authors showed that L-2-HG accumulated within CD8+ T cells and functioned to modulate post-transcriptional modifications and metabolic alteration as a result of TCR mediated T cell activation. Besides, after lipopolysaccharides (LPS) stimulus, 2-HG is the second most abundant metabolite in bone marrow-derived macrophages (BMDMs) [110]. The rise of intracellular amount of stereoisomer in immune cells had been discussed in detail by Iosifina P Foskolou [111]. Given that more and more studies are devoted to discovering the influence of 2-HG on multiple components of the tumor microenvironment, especially TIME [97,98], we will give a comprehensive illustration on the role of 2-HG in the context of acquired immunity (Fig. 2) and innate immunity.
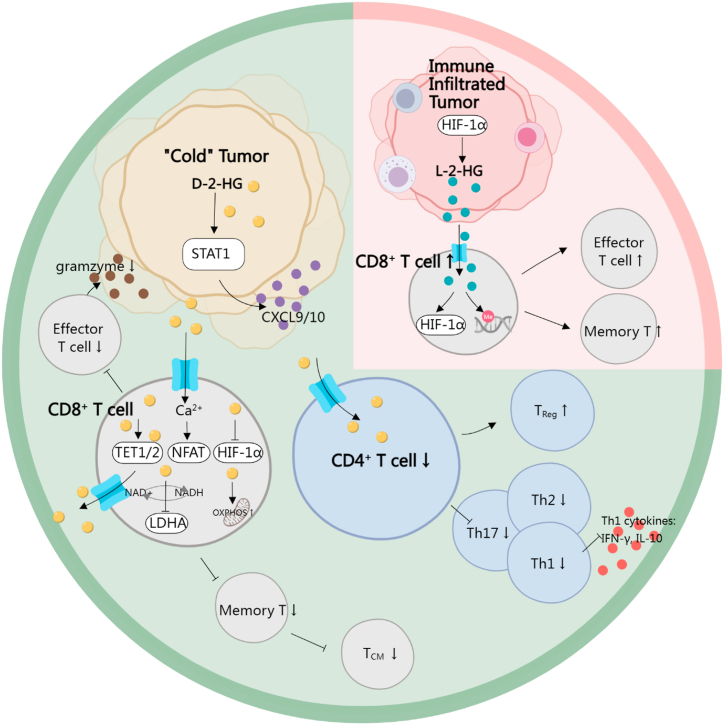
Fig. 2.
The impact of 2-HG on the adaptive immune system within TIME. D-2-HG generally contributes to the "cold tumor" characteristic by interfering with the proliferation and differentiation of CD8+ T cells. This interference occurs through the inhibition of Ca2+-dependent NFAT-mediated T cell activation, TET1/2-mediated DNA methylation, HIF-1α, LDHA, and the orchestration of glucose metabolism towards OXPHOS. Consequently, the cytotoxic effect and immune memory of CD8+ T cells are impaired. CD4+ T cells are also inhibited, resulting in a higher population of TReg cells and fewer effective cytokines released from mature CD4+ cells. In contrast, L-2-HG induces an immune-infiltrated status by upregulating the proliferation and differentiation of effector and memory T cells. 2-HG: 2-hydroxyglutarate; TIME: tumor immune microenvironment; TET: ten-eleven translocation; HIF: hypoxia-inducible factors; LDHA: lactate dehydrogenase A; OXPHOS: oxidative phosphorylation; TReg: regulatory T lymphocytes; STAT1: Signal transducer and activator of transcription 1.
4.1. Regulation of acquired immunity
Reduced tumor infiltration T cells are detected in mIDH tumors. The inhibition of T cell function is an important mechanism leading to “cold tumor” characteristics in tumors, especially glioma [112]. IDH1R132H mice model is used to investigate the function of high 2-HG concentration in vivo as involving the injection of a plasmid encoding human IDH1R132H cDNA into astrocytes. Patient sample assessment, along with both in vitro and in vivo studies, supports the notion that D-2-HG limits the activation, proliferation, migration, and cytotoxic effects of CD8+ T cells [113,114]. Additionally, it has also been reported that the proportion of central memory cells (TCM) is significantly, impairing tumor-related immune memory [115]. CD4+ CD25+ FOX3+ TReg cells are recognized as immunosuppressive cells, and the T helper 17 (Th17)/TReg ratio is an important factor in determining immune balance [116]. In Martin Böttcher's study, D-2-HG was found to suppress Th17 cell formation while positively affecting CD25 CD127+ low FoxP3 naturally occurring-like TReg cells. However, D-2-HG did not significantly impact T cell proliferation, senescence, or IFN-γ secretion following activation by allogeneic DCs. It also did not affect memory T-cell formation [117]. Lingjun Zhang et al. observed an augmentation in differentiation of TReg cells by D-2-HG through a DC-independent pathway in mIDH glioma. This phenomenon was confirmed by co-culturing OVA-specific CD4+ or CD8 T+ cells and DCs with OVA protein. However, their conclusions differ from the aforementioned study, as they declare that the overall proliferation and activation level of Th1 and TReg cells, as well as Th1 cytokines (IFN-γ and IL-10) were inhibited by D-2-HG [113]. Unfortunately, both studies lacked in vivo verification of their conclusions.
The exploration of the molecular mechanisms through which tumor cells employ metabolites to inhibit T cells is of considerable importance. High D-2-HG in mIDH C57BL/6 mice induces immune tolerance, evidenced by the downregulation of cytotoxic T lymphocyte-associated cytokines, including C-X-C motif chemokine ligand 9/10 (CXCL9/10), by signal transducer and activator of transcription 1 (STAT1) in the tumor microenvironment [118]. On the other hand, exogenously administered 2-HG directly impacts T cell function. Firstly, D-2-HG shifts glucose catabolism towards glycolysis through a HIF-independent mechanism. Non-competitive inhibition of LDHA in T cells by D-2-HG leads to decreased NAD+/NADH ratio, membrane hyperpolarization induced mitochondrial respiration, and reactive oxygen species (ROS) generation. This process is reversible upon removal of D-2-HG. LDHA inhibitors mimic the effects of D-2-HG, hindering cytokine and granzyme release, retarding CD8+ T cell proliferation, and impairing cytotoxicity [114]. However, L-2-HG does not effectively inhibit LDHA, or alter glycolysis, prompting speculation on whether D-2-HG inhibits LDHA activity at an allosteric site mimicking other metabolites [119]. On the contrary, Martin Böttcher stated that D-2-HG altered T cell differentiation by destabilizing HIF-α and activating the mTOR signaling pathway. This leads to increased glucose uptake and a shift towards aerobic oxidative phosphorylation (OXPHOS) during metabolic reprogramming [117]. Another potential mechanism arises from D-2-HG inhibiting the adenosine 5′-triphosphate (ATP)-dependent T cell receptor signaling pathway, downregulating calcium signaling. Consequently, nuclear factor of activated T Cells (NFAT)-mediated T cell activation is disrupted due to its calcium dependence, leading to the disturbance of polyamine biosynthesis [48]. PD-1 on T cells and PD-L1 on tumor cells are important immune checkpoints to maintain balance in immune response [120]. PD-L1 expression is lower in mIDH gliomas than in IDH-wt gliomas [121]. Furthermore, both PD-1 and PD-L1 are downregulated upon D-2-HG stimulation [48,115]. This is likely due to differential methylation levels in gene promoters, suggesting that D-2-HG may have relevance in immune checkpoint inhibitor treatment [33].
However, the l-enantiomer of 2-HG seems to have an opposite function of enhancing immune killing. At first, overexpression of L2HGDH in CD8+ T cells was used to investigate the decrease of L-2-HG in immune-mediated ischemic brain injury. L-2-HG was found to significantly accumulate after ischemic stress and exacerbated cognitive dysfunction in Rag 1−/− mice by promoting the proliferation and differentiation of CD8+ T lymphocytes into effector T lymphocytes [122]. In tumor tissues, HIF-1α is a major stimulus to synthesize multiple metabolites in CD8+ T cells, among which L-2-HG ranks at the top level. Nevertheless, the differentiation of CD8+ T cells promoted by L-2-HG would be HIF-1α-independent. H3K27me3 levels are closely related to the differentiation of CD8+ T cells [123]. With L-2-HG treatment, TCM (CD62LHighCD44High) and naïve (CD62LHighCD44Low) CD8+ T cells exhibit higher H3K27me3 level at the transcription start site (TSS) of CD62L in vivo, compared to effector T cells (CD62LLowCD44Low). L-2-HG increased the global level of H3K27me3, and a higher 5mC/5hmC ratio in genome stabilized CD62L expression, which serves as another driving force to shape anti-tumor immune responses [109]. Besides, glutarate, one molecular structurally similar to L-2-HG, can enzymatically inhibit α-KGDDs to regulate CD8+ T cell metabolism and anti-tumor cytotoxicity. Impaired α-KGDDs-dependent activities are seen in CD8+ T cells, leading to increased TCM populations during the differentiation and activation of CD8+ T cells. As a product of amino acid catabolism, glutarate directly decreased the catalytic activity of the pyruvate dehydrogenase complex (PDHc) E2 subunit via glutarylation, and increased basal extracellular acidification rate (ECAR) [124]. Major histocompatibility complex class I (MHC I)-restricted ovalbumin (OVA) specific T cell receptor (TCR) transgenic mice (OT-1) are commonly used as a source of CD8+ T cells that specifically target OVA [125]. It is the most widely used mice model to track the antitumor function of CD8+ T cell populations. Transgenic OT-1 CD8+ T cells increased in both tumor and peripheral blood, exhibiting higher cytotoxicity against B16F10-OVA cancer cells after glutarate treatment. Loss of function of the enzyme glutaryl-CoA dehydrogenase (GCDH) could result in excessive glutarate. In another mice model bearing with HER2+ SKOV3 tumors, HER2 CAR T_shGCDH cells were more efficient at killing tumor cells [124]. Two in vivo studies collectively showed that the analog of L-2-HG could enhance antitumor immunity.
4.2. 2-HG modulates innate immune components
4.2.1. 2-HG shapes macrophage phenotype
The roles of macrophages in the tumor microenvironment present bewildering complexity. It's mainly reflected in the identification of a growing number of macrophage subtypes that span a spectrum of alternative phenotypes [126]. In response to diverse signals, macrophages can be driven into various states with specialized functions. Advances in immunometabolism have highlighted the tight link between metabolic processes and the regulation of macrophage biology [127]. Intermediates from the tricarboxylic acid (TCA) cycle, such as succinate, fumarate, and itaconate, have been discovered to possess “non-metabolic” signaling functions in macrophages [16]. In recent years, evidence has continuously emerged for the role of 2-HG in modulating macrophage phenotypes, whether in the context of general inflammation or tumors (Fig. 3A).
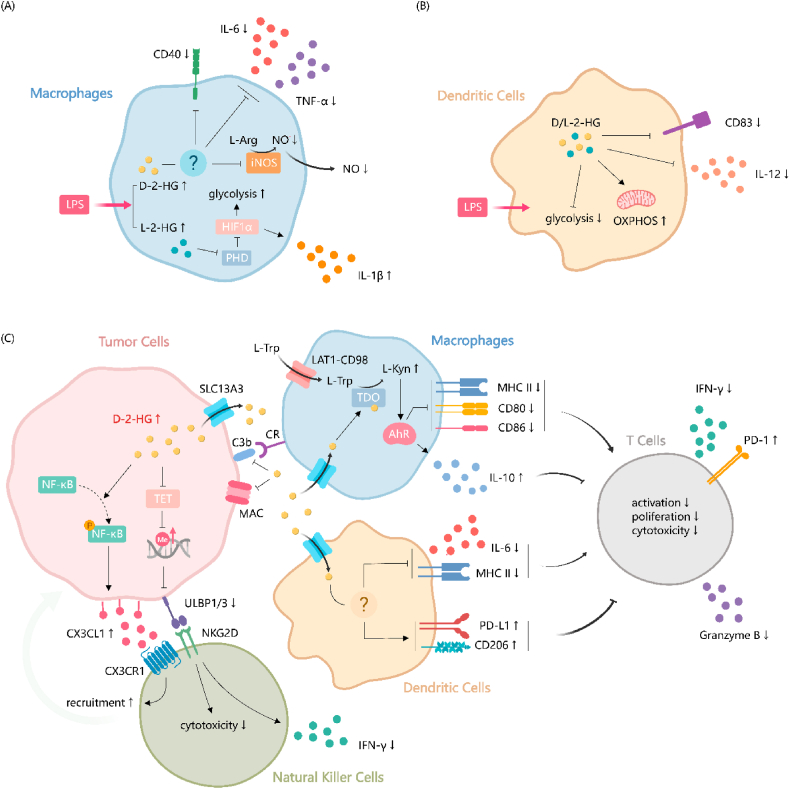
Fig. 3.
| Roles of 2-HG in modulating innate immune components. (A) LPS stimulation results in an increase of both D-2-HG and L-2-HG within macrophages. (B) Treatment with either D-2-HG or L-2-HG elicits a consistent response in LPS-activated DCs, characterized by diminished glycolysis, augmented OXPHOS, reduced secretion of the pro-inflammatory cytokine IL12, and downregulation of the dendritic cell maturation marker CD83. (C) Within tumor cells, D-2-HG recruits NK cells through CX3CL1- CX3CR1 interaction. Concurrently, D-2-HG leads to a diminution in the cytotoxic capabilities and a decrease in IFN-γ production in NK cells by constraining the expression of NKG2D receptor ligands ULBP1/3. In tumor-associated macrophages, D-2-HG activates TDO/L-kyn/AhR and suppresses the expression of pro-inflammatory molecules such as MHCII, CD86, and CD80, while simultaneously upregulating the secretion of the anti-inflammatory cytokine IL10. D-2-HG induces a tolerogenic phenotype in DCs. It upregulates the expression of immunosuppressive mediators such as PD-L1 and CD206 and reduces the expression of IL-6 and MHCII. Additionally, D-2-HG adversely impacts the complement system, characterized by less MAC formation and C3b deposition on tumor cell surfaces. Impaired the functions of macrophages and DCs indirectly inhibits T cell activity by. AhR, aryl hydrocarbon receptor; CR, complement receptor; CX3CL1, C-X3-C motif chemokine ligand 1; CX3CR1, C-X3-C motif chemokine receptor 1; D-2-HG, D-2-hydroxyglutarate; DC, dendritic cell; HIF1α, hypoxia-inducible factor 1-alpha; IFN-γ, interferon-gamma; IL-1β, interleukin-1 beta; IL-6, interleukin-6; IL-10; interleukin-10; IL-12, interleukin-12; iNOS, inducible nitric oxide synthase; L-kyn, l-kynurenine; L-trp, l-tryptophan; LAT1, L-type amino acid transporter 1; LPS, Lipopolysaccharide; MAC, membrane attack complex; MHCII, major histocompatibility complex class II; F-κB, nuclear factor kappa B; NK, natural killer; NKG2D, natural killer group 2D; NO, nitric oxide; OXPHOS, oxidative phosphorylation; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; PHD, prolyl hydroxylase; SLC13A3, solute carrier family 13 member 3; TDO, tryptophan 2,3-dioxygenase; TET, ten-eleven translocation; TNF-α, tumor necrosis factor-alpha; ULBP1/3, UL16-binding protein 1/3.
Upon activation of macrophages with LPS, a significant elevation in the intracellular abundance of both enantiomers of 2-HG has been observed [128,129]. The enzymes HOT and D2HGDH are likely contributors to the LPS-induced elevation of D-2-HG in macrophages. HOT was found to be induced by LPS, while D2HGDH expression was downregulated during LPS stimulation [110]. D-2-HG was shown to dampen inflammatory responses in both mouse and human macrophages. Pre-treatment with D-2-HG suppressed LPS-induced secretion of key inflammatory mediators such as IL-6, tumor necrosis factor-alpha (TNF-α), and nitric oxide (NO), along with reduced expression of inflammatory markers like CD40 and inducible nitric oxide synthase (iNOS) [110]. These effects were observed across different types of macrophages, including BMDMs and human monocyte-derived macrophages (HMDMs), indicating a broad anti-inflammatory role for D-2-HG. Mice pre-treated with D-2-HG exhibited reduced levels of circulating and peritoneal TNFα, and increased levels of the anti-inflammatory cytokine IL-10 following LPS challenge [110]. However, the exact mechanisms of 2-HG's immunosuppressive effects in macrophages remain unclear. D-2-HG treatment did not affect nuclear factor kappa B (NF-κB) activation, and additional α-KG cannot rescue IL-6 and NO secretion, suggesting that the anti-inflammatory effects of D-2-HG are at least partially independent of its role as a competitive inhibitor of α-KGDDs [110]. It is noteworthy that, just as the scenario observed in T cells, D-2-HG and L-2-HG seem to act differently in macrophages [129]. L-2-HG, on the contrary, was shown to promote the expression of the proinflammatory cytokine IL-1β and induce an inflammatory, highly glycolytic metabolic state in macrophages. These changes are mediated through the inhibition of PHDs by L-2-HG, leading to increased stability of HIF-1α [129]. Currently, research on the effects of 2-HG on TAMs is primarily conducted in gliomas. Brain TAMs can originate either from tissue-resident microglia (MG) or MDMs recruited from the peripheral circulation [130]. Glioma tumor immune microenvironment is characterized by excess infiltration of TAMs, while the presence of T cells is considerably lower, especially in tumors with IDH mutations [131]. This observation reinforces the concept that gliomas are immunologically “cold” tumors.
Friedrich and colleagues provide new insights into this field by illustrating how 2-HG impacts the functional capabilities of TAMs and further induces immunological impairment in high-grade gliomas [132]. Using single-cell RNA-sequencing and mass cytometry techniques, they revealed that within mIDH gliomas, both MG and MDMs demonstrate an immunosuppressive phenotype. TAMs from mIDH high-grade gliomas possess the ability to suppress the production of IFN-γ by T cells, and concurrently, to promote increased expression of the exhaustion marker PD-1 in T cells [132]. They also elucidated a novel, α-KGDD-inhibition-independent mechanism, through which 2-HG regulates macrophage function. Upon uptake by macrophages, 2-HG serves as an allosteric activator for tryptophan-2,3-dioxygenase (TDO), thereby facilitating the transformation of intracellular l-tryptophan (L-Trp) into the aryl-hydrocarbon receptor (AhR) ligand l-kynurenine (L-Kyn) [133]. AhR, an ancestral gene product, operates as a cellular sensor for the chemical microenvironment within cells and is acknowledged for its pivotal role in directing macrophage polarization [134]. Under elevated levels of l-kynurenine in macrophages, AhR actively induces the immunosuppressive expression of cytokine IL-10 while simultaneously diminishing the synthesis of proinflammatory effector molecules such as MHC-II and co-stimulatory molecules CD86 and CD80 [132]. As previously mentioned, TAMs are highly plastic cells. This plasticity, coupled with their pivotal roles in the crosstalk between malignant cells and tumor-infiltrating T cells, positions TAMs as a viable target for TME-directed therapies across various cancer types [135]. The findings of Friedrich and colleagues uncover the metabolic vulnerability of TAMs in mIDH gliomas conferred by tumor-derived 2-HG. Incorporating an AhR inhibitor into the anti-PD-1 therapeutic resulted in an increased survival duration in mice bearing mIDH tumors, whereas no such improvement was observed in mice with wild-type tumors [136].
4.2.2. 2-HG induces tolerogenic phenotypes in dendritic cells
DCs distinguished as the most efficacious class of APCs, epitomize a crucial element in triggering the adaptive immune response. Their preeminence is primarily attributed to their unparalleled ability to present a broad array of peptides, derived from proteins synthesized in other cells, thereby facilitating a robust immune reaction [137]. Analogous to macrophages in their lineage, DCs are constituents of the myeloid cell compartment and also exhibit significant plasticity, allowing them to differentiate into diverse phenotypes based on environmental cues. This adaptability is crucial in various immunological contexts, including tumor immunity [138]. Notably, DCs infiltrating tumors have been shown to activate T helper cells, thus initiating antitumor responses in solid tumors [139]. This capability highlights the potential of DCs as therapeutic targets in cancer treatment. In DCs stimulated by LPS, treatment with D-2-HG and L-2-HG has been observed to attenuate the expression of CD83, a marker indicative of DC maturation (Fig. 3B). Administration of both D-2-HG and L-2-HG also markedly diminishes the secretion of IL-12 by DCs, exhibiting a dose-dependent effect [140].
Based on previous transcriptomic and proteomic profiling of high-grade gliomas, Friedrich and colleagues further conducted pseudotime trajectory analyses within the antigen-presenting cell compartment [141]. This investigation elucidated a distinctive block in the differentiation pathway from monocytes to DCs that was specifically inherent to mIDH tumors. DCs educated by mIDH exhibit an immature cellular phenotype, characterized by diminished expression levels of MHC and co-stimulatory molecules, such as CD74, H2-Aa, and CD86 [142]. While IL-4 and IL-13 have been identified as agents capable of counteracting the suppressive influence of tumor cells on DC differentiation, their concentrations were found to be lower in the microenvironment of mIDH gliomas [143]. In co-culture experiments, both CD4+ T cells and CD8+ T cells exhibited a notable reduction in proliferation when interacting with tumor-infiltrating DCs from mIDH-tumor-bearing mice [142]. It is reasonable to speculate that IDH mutations modulate DCs through D-2-HG and this has been further validated. Upon exposure to D-2-HG, human monocyte-derived DCs exhibited a tolerogenic phenotype, characterized by a marked decrease in the expression of IL-6 and MHC-II, as well as moderate upregulation of two immunosuppressive mediators, the mannose receptor (CD206) and PD-L1. Ultimately, when co-cultured with D-2-HG-treated murine DCs, T cell activation and IFN-γ secretion were inversely correlated with the escalating exposure of DCs to D-2-HG, exhibiting a dose-dependent reduction [142].
However, there is still a dearth of research and reliable conclusions concerning the specific molecular mechanisms by which 2-HG impacts dendritic cell biology. In DCs activated by LPS, treatment with 2-HG did not alter the crucial signaling pathways that are central to cytokine production induced by LPS, such as the NF-κB, Akt, and p38 pathways [140]. An indicative insight is derived from the observation of 2-HG-induced metabolic reprogramming within DCs. LPS normally promotes glycolysis while reducing respiration in DCs [144]. However, this effect is modified in the presence of 2-HG, which bolsters mitochondrial activity and slightly diminishes glycolysis [140]. This observation is consistent with the previously described metabolic phenotype of human tolerogenic DCs, characterized by augmented OXPHOS and reduced glycolytic activity [145]. Notably, blocking mitochondrial respiration by oligomycin results in elevated IL-12 levels in the presence of 2-HG [140]. Critical questions that remain to be explored include the mechanism by which 2-HG enhances OXPHOS in DCs, the role of OXPHOS in inducing an immunosuppressive phenotype in DC, and whether targeting OXPHOS with agents such as metformin can reverse the immunosuppression in 2-HG-rich tumors and potentiate the efficacy of immune checkpoint inhibitors.
4.2.3. 2-HG alters NK Cell dynamics and resistance
NK cells are vital components of the innate immune system and play a pivotal role in orchestrating the adaptive immune responses involved in the early defense against virus-infected and transformed cells [139]. The display of stress-induced ligands on the surface of cancer cells can be detected by an NK cell receptor known as NK group 2D (NKG2D), resulting in strong activation of the NK cell's cytotoxic response. The ligands recognized by NKG2D, collectively known as NKG2D ligands (NKG2DLs), encompass a range of molecules such as MHC class I–related chains A and B (MICA and MICB) and members of the UL16-binding protein (ULBP) family [139].
Analysis based on open-access cancer genome data and patient-derived tumor samples indicates higher infiltration of NK cells in gliomas harboring the IDH1R132H mutation (Fig. 3C) [146]. Elevated C-X3-C motif chemokine ligand 1 (CX3CL1) expression in IDH1R132H gliomas and subsequently enhanced CX3CL1/C-X3-C motif chemokine receptor 1 (CX3CR1) chemotaxis may play a pivotal role in the recruitment of NK cells. This upregulation of CX3CL1 is attributed to the conversion of α-KG to D-2-HG by the IDH1 mutant and the resultant phosphorylation of NF-κB [146].
However, an increase in the number of NK cells does not necessarily imply an enhancement of their mediated immune surveillance. Zhang et al. elucidated that mIDH glioma cells develop resistance to NK cells via the epigenetic downregulation of NKG2D ligands, notably ULBP1 and ULBP3. D-2-HG alone is capable of inducing NK cell resistance in IDH wild-type astrocytes and glioma stem cells, underscoring the direct involvement of 2-HG in this resistance mechanism [147]. Moreover, their study identified that in vitro, application of low-dose DNA-demethylating agent 5-aza-2ʹdeoxycytodine (decitabine, DAC) effectively restores the expression of these critical NKG2D ligands, thereby re-sensitizing mIDH gliomas to NK cell-mediated cytotoxicity [147]. The same research team further investigated the effects of DAC in vivo by employing mIDH xenograft models within athymic nude mice. The results indicated a notable upregulation of NKG2D ligand expression in response to DAC treatment, concomitant with a marked inhibition of tumor proliferation. Notably, this inhibition was observed to be dependent on the presence of NK cells, as the effect was abolished when NK cells were depleted [122].
4.2.4. 2-HG inhibits complement system activation
In the intricate landscape of the innate immune system, the complement system emerges as a pivotal player. Its activation, orchestrated through the classical, alternative, or lectin pathways, culminates in the formation of the membrane attack complex (MAC). This complex ingeniously constructs transmembrane pores, leading to the lysis of targeted cells [148]. However, research has unveiled that D-2-HG adeptly modulates this immune response by inhibiting the complement system's activation pathways, specifically the classical and alternative pathways, in a dose-dependent fashion (Fig. 3C). This modulation notably diminishes complement-mediated cellular damage [113]. D-2-HG could impede the formation of MAC and thereby attenuate the subsequent complement-mediated cellular damage through interventions at multiple stages of the complement activation cascade. In the classical pathway, D-2-HG significantly hampers the assembly of C5 convertase. The alternative pathway is not immune to D-2-HG's influence either, as it impedes the assembly of both C3 and C5 convertases. Furthermore, D-2-HG extends its effect to the activity of preassembled C3 and C5 convertases in the classical pathway, albeit in a more modest capacity [113]. The complement system's role extends beyond cell lysis to facilitating phagocytosis, a process where C3b/iC3b fragments deposited on target cells tag them for destruction. These fragments bind to complement receptors on phagocytes, signaling the engulfment of the tagged cells [148]. Here again, D-2-HG emerges as a critical regulator, significantly curtailing the deposition of C3b/iC3b on cells, leading to impaired "opsonization" process and thus lower efficiency of complement-mediated phagocytosis [113].
5. Effects of D-2-HG-related therapies on immune status
Immune therapy has entered the stage of history in cancer treatment [149,150]. In view of the regulatory effect of 2-HG on TIME, reducing 2-HG or inducing IDH mutation epitopes may improve the anti-tumor immune effect in high 2-HG tumors. We have compiled a list of ongoing and completed clinical trials on 2-HG-related therapies.
Chimeric antigen receptor (CAR)-modified T cell (CAR-T) therapy has been a successful clinical trial for highly refractory hematologic malignancies [151]. CAR-T therapy aimed to eliminate tumor cells by harnessing the T cells from host immune system. These immune cells were genetically modified with the antigen-specific sequence to directly identify or activate the immune system to kill tumor cells [152]. However, immune tolerance and T cell exhaustion were two problems limiting potential CAR-T therapy [153]. As high D-2-HG could impair the cytotoxicity of CD19+ CAR-T cells, D2HGDH-modified CAR-T cells were produced to carry different phenotypic features as D2HGDH catalyzed endogenous 2-HG into 2-oxoglutarate. D2HGDH overexpression CAR-T cells exhibited a significant increase in TCM differentiation and cytokine secretion. The immunosuppressive effect of excessively high D-2-HG in the medium was reversed by D2HGDH over-expression CAR-T cells. In vivo assays were conducted on NALM6 cancer cells with mIDH1, demonstrating D2HGDH's efficiency in reducing serum 2-HG and improving overall survival in mice [154]. Another scientific research found that endogenously elevated glutarate, a metabolite structurally similar to L-2-HG, in HER2+ CAR-T cells increased CD8+ T cytotoxicity against HER2+ SKOV3 ovarian cancer cells [124]. Iosifina P. Foskolou et al. discovered the positive effect of L-2-HG in memory CD8+ T cells during CAR-T therapy. After donor CAR-T cells were generated from precursors, L-2-HG treatment prolonged the duration and increased the proportion of memory T cells in vitro. L-2-HG-treated CAR-T cells showed an enhanced antitumor activity in NSG mice bearing CD19+ Raji cells. This study showed the promise of L-2-HG treated CD19-CAR-T cells in addressing immune cell depletion and provided basis for phase 1 clinical trial in B-cell malignancies [155].
IDH1 inhibitor has been proven to effectively reduce 2-HG level to delay cell growth and promote cell differentiation in glioma cells (Table 1). For example, vorasidenib (AG-881), a dual inhibitor of IDH1 and IDH2 reduces 2-HG levels in glioma cells. The tumor microenvironment has also been modified, with an increase in proinflammatory IFN-γ-related gene expression and the number of CD4+ and CD8+ tumor-infiltrating T-cells. The application of AG-881 promotes histone H3K9me3 demethylation and induces tumor cell differentiation, thereby inhibiting the progression of mIDH glioma. Studies showed a combination of AG-881 and PD-1 blocking antibody had the best survival in mice model when compared with monotherapy [128]. AG-881 has successfully entered Phase III trial and has the potential to be the first approved targeted therapy for mIDH1/2 low-grade glioma (NCT04164901). Besides, IDH1R132H inhibitor AGI-5198 alone or in combination with radiotherapy and temozolomide (standard treatment) can induce immunogenic cell death and prolong mIDH1R132H/mATRX/mTP53 patient survival [115]. Oral pan-mutant IDH1 inhibitor, BAY1436032, successfully increased the overall survival in S/MAR-IDH1R132H glioma-bearing C57BL/6J mice [48]. Due to PD-L1 expression in high 2-HG tumors, inhibitor combined with standard treatment and anti-PD-L1 immune checkpoint inhibitor was another alternative approach. To date, inhibitors against mIDH/2 malignancies, such as ivosidenib (AG-120), enasidenib (AG-221) and olutasidenib (FT-2102), have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with recurrent or refractory AML (R/R AML). These inhibitors are also enrolled in clinical trials like cholangiocarcinoma, chondrosarcoma, glioma, and other advanced hematologic malignancies.
Table 1.
2-HG-targeted therapies.
| Agent | Target | FDA-approved indications | Trial | Phase | Participants | Enrollment | Status | Reference |
|---|---|---|---|---|---|---|---|---|
| FDA-approved agents | ||||||||
| Ivosidenib (AG-120) | mIDH1 | mIDH1 AML in patients ≥75 years old or not suitable for intensive chemotherapy Adult patients with relapsed or refractory mIDH1 myelodysplastic syndromes (MDS) |
NCT02074839 | 1 | Advanced hematologic malignancies with an IDH1 mutation | 291 (estimated) | Recruiting | [156] |
| In combination with azacitidine for mIDH1 AML in patients ≥75 years old or not suitable for chemotherapy | NCT03173248 | 3 | Previously intreated AML with an IDH1 mutation | 146 | Active, not recruiting | [157] | ||
| Adult patients with previously treated, locally advanced, or metastatic mIDH1 cholangiocarcinoma | NCT02989857 | 3 | Previously treated nonresectable or metastatic cholangiocarcinoma with an IDH1 Mutation | 187 | Completed | [158] | ||
| Enasidenib (AG-221) | mIDH2 | Adult patients with relapsed or refractory mIDH2 AML | NCT01915498 | 1/2 | Advanced hematologic malignancies with an IDH2 mutation | 345 | Active, not recruiting | [159] |
| Olutasidenib (FT-2102) | mIDH1 | Adult patients with relapsed or refractory mIDH1 AML | NCT02719574 | 1/2 | AML or MDS with an IDH1 mutation | 336 | Active, not recruiting | [160] |
| Agents not yet approved by the FDA | ||||||||
| Vorasidenib (AG-881) | mIDH1/2 | / | NCT04164901 | 3 | Residual or recurrent grade 2 glioma with an IDH1/2 mutation who have undergone surgery as their only treatment | 331 | Active, not recruiting | [161] |
| NCT03343197 | 1 | Recurrent, non-enhancing, IDH1 mutant, low-grade glioma for patients who require surgery | 49 | Active, not recruiting | [162] | |||
| Safusidenib (AB-218) | mIDH1 | / | NCT05577416 | 0 | mIDH1 low-grade glioma who have not received prior radiation or chemotherapy and are planned to undergo surgical resection | 10 (estimated) | Recruiting | / |
| NCT05814536 | 1 | Advanced mIDH1 cholangiocarcinoma and other solid tumors who have failed at least one prior therapy in the advanced stage | 63 (estimated) | Recruiting | / | |||
| NCT05303519 | 2 | Recurrent or progressive histologically confirmed mIDH1 WHO grade 2/3 glioma | 95 (estimated) | Recruiting | / | |||
| IDH305 | mIDH1 | / | NCT02381886 | 1 | Advanced malignancies that harbor IDH1R132 mutations | 166 | Active, not recruiting | [163] |
| LY3410738 | mIDH1/2 | / | NCT04603001 | 1 | Advanced hematologic malignancies with IDH1 or IDH2 mutations | 260 (estimated) | Active, not recruiting | / |
| BAY1436032 | mIDH1 | / | NCT03127735 | 1 | mIDH1R132X advanced AML | 27 | Completed | [164] |
| NCT02746081 | 1 | mIDH1R132X advanced solid tumors | 81 | Active, not recruiting | [165] | |||
Tumor with low mutation burden has insufficient neoantigen to benefit from immune therapies such as checkpoint inhibitors [166]. However, peptide-based vaccine therapy may help to inspire anti-tumor immunity [167]. An in vivo study showed glioma-associated antigens (GAAs) vaccination, improved the survival of mice injected IDHR132H gliomas combined with IDH-C35, a mIDH1 inhibitor, by restoring T cell infiltration. However, prophylactic vaccination alone had no remarkable effect on the therapeutic effect regardless of the mutation status [118]. Vaccination therapy targeting 2-HG is generally referred to as a therapeutic IDH1R132H peptide vaccine. IDH1R132H mutations form neoepitopes with a consistently high degree of penetrance on the cell membrane [168,169]. After vaccination with peptide vaccines, mutant peptides can be presented to MHC Ⅱ in the context of human leukocyte antigens (HLA)-DR presentation, which provides the possibility for glioma immune vaccine treatment. Peptide vaccines stimulate CD4+ Th1 cells to activate cytotoxic effects, which would also exert anti-tumor effects on pre-existing mIDH1R132H tumors [168,170]. In the HLA-A2/HLA-DR1-syngeneic IDH1R132H glioma model, mice vaccinated with HLA-DR1-restricted IDH1R132H peptide or tumor-associated HLA-A2-restricted peptides successfully prolonged the survival, but only in combination with IDH inhibition therapy [128]. However, two opposing effects could be generated by 2-HG-based vaccination: antigen recognition and 2-HG exposure. 2-HG produced by mIDH might incapacitate antitumor immunity caused by mIDH vaccination. In the double IDH1R132H/D252G-vaccinated C57BL/6 mice who produced less D-2-HG than IDH1R132H, were able to produce Th1 effector cytokine, which demonstrated antigenic function of IDH1R132H-peptide vaccination is efficient to altering TIME, rather than relying on the enzymatic function of 2-HG [48]. The enzymatic function might explain why pharmacologic inhibition and IDH1R132H-peptide-vaccinated therapy alleviate intratumoral immune suppression.
6. Conclusion
2-Hydroxyglutarate (2-HG) was initially identified in metabolism-related disorders and quickly recognized as an oncometabolite and immunometabolite [109]. The two enantiomers, D-2-HG and L-2-HG, may exert distinct effects on the components of the adaptive and innate immune systems within the tumor immune microenvironment (TIME) [50]. For example, in gliomas and hematologic malignancies, the proliferation and activation of T cells, NK cells, macrophages, and DCs, as well as the complement system, are constrained under a high D-2-HG burden [114]. Conversely, L-2-HG differs from D-2-HG in promoting T cell proliferation and differentiation [122]. However, since the role of humoral immunity in the anti-tumor immune response remains unclear, detailed reports on the regulation of B cells by 2-HG are currently lacking.
The 2-HG-based strategy has demonstrated effectiveness in enhancing cancer immunotherapy and patient prognosis. Currently, treatments related to 2-HG in anticancer therapy primarily involve three aspects. One involves small molecule inhibitors targeting different IDH mutation states aimed at reducing intracellular D-2-HG levels in tumor cells and exploring the feasibility of combined immunotherapies, such as PD-1/PD-L1 inhibitors [128]. Three IDH inhibitors, namely ivosidenib (AG-120), enasidenib (AG-221), and olutasidenib (FT-2102), are currently FDA-approved and available worldwide. In 2022, ivosidenib became the first and currently the only IDH inhibitor approved in China for R/R AML with IDH1 mutations. Numerous next-generation IDH inhibitors are undergoing clinical trials in the advanced or recurrent tumors, many of which have shown promising clinical efficacy. Additionally, peptide vaccines are utilized to induce the expression of IDH mutant epitopes, present TAAs, thereby activating anti-tumor immunity within the body. However, the suppression of D-2-HG on the TIME has become a significant factor limiting the efficacy of IDH1R132H peptide vaccines [48]. Notably, both therapies rely on the mutation status of IDH in tumor patients. Another approach involves genetic modification to reduce D-2-HG or increase L-2-HG expression levels within CAR-T cells, thereby enhancing cytotoxic T cell anti-tumor capabilities and immune memory [155]. Although CAR-T therapy is still in its early stages and has not yet entered the clinical trial phase, we cannot ignore the potential of 2-HG as a candidate therapeutic target and molecular targeted therapy. Besides, by acknowledging the challenges and clinical potentials associated with 2-HG inhibitors and 2-HG-related peptide vaccination, we hope that more precise and effective immunotherapy methods will be developed to improve the survival rates and quality of life for cancer patients.
Funding information
This study was supported by the Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX22_0682).
Data availability statement
Data associated with the study has not been deposited into a publicly available repository and no additional data was used for the research described in the review article.
Ethics statement
Not applicable. Approval by an ethics committee was not needed for this study because no applicants/patients were enrolled for this review article.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Mengyuan Cai: Writing – review & editing, Writing – original draft. Jianyi Zhao: Writing – review & editing, Writing – original draft. Qiang Ding: Validation, Project administration. Jifu Wei: Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
All figures are created with Medpeer (www.medpeer.cn).
Contributor Information
Qiang Ding, Email: dingqiang@njmu.edu.cn.
Jifu Wei, Email: weijifu@njmu.edu.cn.
References
- 1.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim S., Kim A., Shin J.Y., Seo J.S. The tumor immune microenvironmental analysis of 2,033 transcriptomes across 7 cancer types. Sci. Rep. 2020;10:9536. doi: 10.1038/s41598-020-66449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brummel K., Eerkens A.L., de Bruyn M., Nijman H.W. Tumour-infiltrating lymphocytes: from prognosis to treatment selection. Br. J. Cancer. 2023;128:451–458. doi: 10.1038/s41416-022-02119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S.S., Liu W., Ly D., Xu H., Qu L., Zhang L. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell. Mol. Immunol. 2019;16:6–18. doi: 10.1038/s41423-018-0027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laskowski T.J., Biederstadt A., Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer. 2022;22:557–575. doi: 10.1038/s41568-022-00491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Prete A., Salvi V., Soriani A., Laffranchi M., Sozio F., Bosisio D., et al. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell. Mol. Immunol. 2023;20:432–447. doi: 10.1038/s41423-023-00990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng N., Bai X., Shu Y., Ahmad O., Shen P. Targeting tumor-associated macrophages as an antitumor strategy. Biochem. Pharmacol. 2021;183 doi: 10.1016/j.bcp.2020.114354. [DOI] [PubMed] [Google Scholar]
- 8.Hotz C., Wagenaar T.R., Gieseke F., Bangari D.S., Callahan M., Cao H., et al. Local delivery of mRNA-encoded cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abc7804. [DOI] [PubMed] [Google Scholar]
- 9.Liu J., Chen Z., Li Y., Zhao W., Wu J., Zhang Z. PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.731798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020;17:807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X., Huang Y., Yang X. The complex role of PD-L1 in antitumor immunity: a recent update. Cell. Mol. Immunol. 2021;18:2067–2068. doi: 10.1038/s41423-021-00702-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W., Tang H., Li L., Wang X., Yu Z., Li J. Peptide-based therapeutic cancer vaccine: current trends in clinical application. Cell Prolif. 2021;54 doi: 10.1111/cpr.13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye L., Jiang Y., Zhang M. Crosstalk between glucose metabolism, lactate production and immune response modulation. Cytokine Growth Factor Rev. 2022;68:81–92. doi: 10.1016/j.cytogfr.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Brand A., Singer K., Koehl G.E., Kolitzus M., Schoenhammer G., Thiel A., et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24:657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharyya N., Pal A., Patra S., Haldar A.K., Roy S., Ray M. Activation of macrophages and lymphocytes by methylglyoxal against tumor cells in the host. Int. Immunopharm. 2008;8:1503–1512. doi: 10.1016/j.intimp.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Ryan D.G., Murphy M.P., Frezza C., Prag H.A., Chouchani E.T., O'Neill L.A., et al. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat. Metab. 2019;1:16–33. doi: 10.1038/s42255-018-0014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker S.J., Encarnación-Rosado J., Hollinshead K.E.R., Hollinshead D.M., Ash L.J., Rossi J.A.K., et al. Spontaneous hydrolysis and spurious metabolic properties of α-ketoglutarate esters. Nat. Commun. 2021;12:4905. doi: 10.1038/s41467-021-25228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kranendijk M., Struys E.A., Salomons G.S., Van der Knaap M.S., Jakobs C. Progress in understanding 2-hydroxyglutaric acidurias. J. Inherit. Metab. Dis. 2012;35:571–587. doi: 10.1007/s10545-012-9462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Q.Y., Xiong J., Huang W., Ma Q., Ci W., Feng Y.Q., et al. Sensitive determination of onco-metabolites of D- and L-2-hydroxyglutarate enantiomers by chiral derivatization combined with liquid chromatography/mass spectrometry analysis. Sci. Rep. 2015;5 doi: 10.1038/srep15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dang L., White D.W., Gross S., Bennett B.D., Bittinger M.A., Driggers E.M., et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan H., Parsons D.W., Jin G., McLendon R., Rasheed B.A., Yuan W., et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amary M.F., Bacsi K., Maggiani F., Damato S., Halai D., Berisha F., et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J. Pathol. 2011;224:334–343. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 23.Wang P., Dong Q., Zhang C., Kuan P.F., Liu Y., Jeck W.R., et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene. 2013;32:3091–3100. doi: 10.1038/onc.2012.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward P.S., Patel J., Wise D.R., Abdel-Wahab O., Bennett B.D., Coller H.A., et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang F., Jha A., Meuter L., Pacak K., Yang C. Identification of isocitrate dehydrogenase 2 (IDH2) mutation in carotid body paraganglioma. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.731096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shelar S., Shim E.H., Brinkley G.J., Kundu A., Carobbio F., Poston T., et al. Biochemical and epigenetic insights into L-2-hydroxyglutarate, a potential therapeutic target in renal cancer. Clin. Cancer Res. 2018;24:6433–6446. doi: 10.1158/1078-0432.Ccr-18-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Losman J.A., Koivunen P., Kaelin W.G., Jr. 2-Oxoglutarate-dependent dioxygenases in cancer. Nat. Rev. Cancer. 2020;20:710–726. doi: 10.1038/s41568-020-00303-3. [DOI] [PubMed] [Google Scholar]
- 28.Pirozzi C.J., Yan H. The implications of IDH mutations for cancer development and therapy. Nat. Rev. Clin. Oncol. 2021;18:645–661. doi: 10.1038/s41571-021-00521-0. [DOI] [PubMed] [Google Scholar]
- 29.Miller J.J. Targeting IDH-mutant glioma. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2022;19:1724–1732. doi: 10.1007/s13311-022-01238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran A.N., Lai A., Li S., Pope W.B., Teixeira S., Harris R.J., et al. Increased sensitivity to radiochemotherapy in IDH1 mutant glioblastoma as demonstrated by serial quantitative MR volumetry. Neuro Oncol. 2014;16:414–420. doi: 10.1093/neuonc/not198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Struys E.A., Verhoeven N.M., Ten Brink H.J., Wickenhagen W.V., Gibson K.M., Jakobs C. Kinetic characterization of human hydroxyacid-oxoacid transhydrogenase: relevance to D-2-hydroxyglutaric and gamma-hydroxybutyric acidurias. J. Inherit. Metab. Dis. 2005;28:921–930. doi: 10.1007/s10545-005-0114-x. [DOI] [PubMed] [Google Scholar]
- 32.Fan J., Teng X., Liu L., Mattaini K.R., Looper R.E., Vander Heiden M.G., et al. Human phosphoglycerate dehydrogenase produces the oncometabolite D-2-hydroxyglutarate. ACS Chem. Biol. 2015;10:510–516. doi: 10.1021/cb500683c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalmers R.A., Lawson A.M., Watts R.W., Tavill A.S., Kamerling J.P., Hey E., et al. D-2-hydroxyglutaric aciduria: case report and biochemical studies. J. Inherit. Metab. Dis. 1980;3:11–15. doi: 10.1007/bf02312516. [DOI] [PubMed] [Google Scholar]
- 34.Dang L., Su S.M. Isocitrate dehydrogenase mutation and (R)-2-Hydroxyglutarate: from basic discovery to therapeutics development. Annu. Rev. Biochem. 2017;86:305–331. doi: 10.1146/annurev-biochem-061516-044732. [DOI] [PubMed] [Google Scholar]
- 35.Luchman H.A., Chesnelong C., Cairncross J.G., Weiss S. Spontaneous loss of heterozygosity leading to homozygous R132H in a patient-derived IDH1 mutant cell line. Neuro Oncol. 2013;15:979–980. doi: 10.1093/neuonc/not064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pietrak B., Zhao H., Qi H., Quinn C., Gao E., Boyer J.G., et al. A tale of two subunits: how the neomorphic R132H IDH1 mutation enhances production of αHG. Biochemistry. 2011;50:4804–4812. doi: 10.1021/bi200499m. [DOI] [PubMed] [Google Scholar]
- 37.Ward P.S., Lu C., Cross J.R., Abdel-Wahab O., Levine R.L., Schwartz G.K., et al. The potential for isocitrate dehydrogenase mutations to produce 2-hydroxyglutarate depends on allele specificity and subcellular compartmentalization. J. Biol. Chem. 2013;288:3804–3815. doi: 10.1074/jbc.M112.435495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sazanov L.A., Jackson J.B. Proton-translocating transhydrogenase and NAD- and NADP-linked isocitrate dehydrogenases operate in a substrate cycle which contributes to fine regulation of the tricarboxylic acid cycle activity in mitochondria. FEBS Lett. 1994;344:109–116. doi: 10.1016/0014-5793(94)00370-x. [DOI] [PubMed] [Google Scholar]
- 39.Zeng L., Morinibu A., Kobayashi M., Zhu Y., Wang X., Goto Y., et al. Aberrant IDH3α expression promotes malignant tumor growth by inducing HIF-1-mediated metabolic reprogramming and angiogenesis. Oncogene. 2015;34:4758–4766. doi: 10.1038/onc.2014.411. [DOI] [PubMed] [Google Scholar]
- 40.Du B., Sun T., Li X., Diao Y., Li Y. Effect of IDH3a on glucose uptake in lung adenocarcinoma: a pilot study based on [(18) F]FDG. Cancer Med. 2019;8:5341–5351. doi: 10.1002/cam4.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X., Qiao Y., Ting X., Si W. Isocitrate dehydrogenase 3A, a rate-limiting enzyme of the TCA cycle, promotes hepatocellular carcinoma migration and invasion through regulation of MTA1, a core component of the NuRD complex. Am. J. Cancer Res. 2020;10:3212–3229. [PMC free article] [PubMed] [Google Scholar]
- 42.Duran M., Kamerling J.P., Bakker H.D., van Gennip A.H., Wadman S.K. L-2-Hydroxyglutaric aciduria: an inborn error of metabolism? J. Inherit. Metab. Dis. 1980;3:109–112. doi: 10.1007/bf02312543. [DOI] [PubMed] [Google Scholar]
- 43.Intlekofer A.M., Dematteo R.G., Venneti S., Finley L.W., Lu C., Judkins A.R., et al. Hypoxia induces production of L-2-hydroxyglutarate. Cell Metabol. 2015;22:304–311. doi: 10.1016/j.cmet.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oldham W.M., Clish C.B., Yang Y., Loscalzo J. Hypoxia-mediated increases in L-2-hydroxyglutarate coordinate the metabolic response to reductive stress. Cell Metabol. 2015;22:291–303. doi: 10.1016/j.cmet.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Intlekofer A.M., Wang B., Liu H., Shah H., Carmona-Fontaine C., Rustenburg A.S., et al. L-2-Hydroxyglutarate production arises from noncanonical enzyme function at acidic pH. Nat. Chem. Biol. 2017;13:494–500. doi: 10.1038/nchembio.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nota B., Struys E.A., Pop A., Jansen E.E., Fernandez Ojeda M.R., Kanhai W.A., et al. Deficiency in SLC25A1, encoding the mitochondrial citrate carrier, causes combined D-2- and L-2-hydroxyglutaric aciduria. Am. J. Hum. Genet. 2013;92:627–631. doi: 10.1016/j.ajhg.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H., Hurlburt A.J., Tennessen J.M. A Drosophila model of combined D-2- and L-2-hydroxyglutaric aciduria reveals a mechanism linking mitochondrial citrate export with oncometabolite accumulation. Disease models & mechanisms. 2018;11 doi: 10.1242/dmm.035337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bunse L., Pusch S., Bunse T., Sahm F., Sanghvi K., Friedrich M., et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med. 2018;24:1192–1203. doi: 10.1038/s41591-018-0095-6. [DOI] [PubMed] [Google Scholar]
- 49.Peng S., Chen H., Chen L., Yang G., Liu J., Cheng X., et al. Beyond isocitrate dehydrogenase mutations: emerging mechanisms for the accumulation of the oncometabolite 2-hydroxyglutarate. Chem. Res. Toxicol. 2022;35:115–124. doi: 10.1021/acs.chemrestox.1c00254. [DOI] [PubMed] [Google Scholar]
- 50.Gupta V.K., Sharma N.S., Durden B., Garrido V.T., Kesh K., Edwards D., et al. Hypoxia-driven oncometabolite L-2HG maintains stemness-differentiation balance and facilitates immune evasion in pancreatic cancer. Cancer Res. 2021;81:4001–4013. doi: 10.1158/0008-5472.can-20-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pavlova N.N., Zhu J., Thompson C.B. The hallmarks of cancer metabolism: still emerging. Cell Metabol. 2022;34:355–377. doi: 10.1016/j.cmet.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nadtochiy S.M., Schafer X., Fu D., Nehrke K., Munger J., Brookes P.S. Acidic pH is a metabolic switch for 2-hydroxyglutarate generation and signaling. J. Biol. Chem. 2016;291:20188–20197. doi: 10.1074/jbc.M116.738799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shenoy N., Bhagat T.D., Cheville J., Lohse C., Bhattacharyya S., Tischer A., et al. Ascorbic acid-induced TET activation mitigates adverse hydroxymethylcytosine loss in renal cell carcinoma. J. Clin. Invest. 2019;129:1612–1625. doi: 10.1172/jci98747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mishra P., Tang W., Putluri V., Dorsey T.H., Jin F., Wang F., et al. ADHFE1 is a breast cancer oncogene and induces metabolic reprogramming. J. Clin. Invest. 2018;128:323–340. doi: 10.1172/jci93815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chowdhury R., Yeoh K.K., Tian Y.M., Hillringhaus L., Bagg E.A., Rose N.R., et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011;12:463–469. doi: 10.1038/embor.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu W., Yang H., Liu Y., Yang Y., Wang P., Kim S.H., et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dang L., White D.W., Gross S., Bennett B.D., Bittinger M.A., Driggers E.M., et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2010;465:966. doi: 10.1038/nature09132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turcan S., Rohle D., Goenka A., Walsh L.A., Fang F., Yilmaz E., et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farshidfar F., Zheng S., Gingras M.C., Newton Y., Shih J., Robertson A.G., et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;19:2878–2880. doi: 10.1016/j.celrep.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Figueroa M.E., Abdel-Wahab O., Lu C., Ward P.S., Patel J., Shih A., et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu W., Yang H., Liu Y., Yang Y., Wang P., Kim S.H., et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bray J.K., Dawlaty M.M., Verma A., Maitra A. Roles and regulations of TET enzymes in solid tumors. Trends in cancer. 2021;7:635–646. doi: 10.1016/j.trecan.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 63.Losman J.A., Looper R.E., Koivunen P., Lee S., Schneider R.K., McMahon C., et al. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science (New York, N.Y.) 2013;339:1621–1625. doi: 10.1126/science.1231677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gunn K., Myllykoski M., Cao J.Z., Ahmed M., Huang B., Rouaisnel B., et al. (R)-2-Hydroxyglutarate inhibits KDM5 histone lysine demethylases to drive transformation in IDH-mutant cancers. Cancer Discov. 2023;13:1478–1497. doi: 10.1158/2159-8290.cd-22-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arifuzzaman S., Khatun M.R., Khatun R. Emerging of lysine demethylases (KDMs): from pathophysiological insights to novel therapeutic opportunities. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2020;129 doi: 10.1016/j.biopha.2020.110392. [DOI] [PubMed] [Google Scholar]
- 66.Lu C., Ward P.S., Kapoor G.S., Rohle D., Turcan S., Abdel-Wahab O., et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saha S.K., Parachoniak C.A., Ghanta K.S., Fitamant J., Ross K.N., Najem M.S., et al. Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature. 2014;513:110–114. doi: 10.1038/nature13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin Y., Elalaf H., Watanabe M., Tamaki S., Hineno S., Matsunaga K., et al. Mutant IDH1 dysregulates the differentiation of mesenchymal stem cells in association with gene-specific histone modifications to cartilage- and bone-related genes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schvartzman J.M., Reuter V.P., Koche R.P., Thompson C.B. 2-hydroxyglutarate inhibits MyoD-mediated differentiation by preventing H3K9 demethylation. Proc. Natl. Acad. Sci. U.S.A. 2019;116:12851–12856. doi: 10.1073/pnas.1817662116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gavriil M., Proietto M., Paczia N., Ginolhac A., Halder R., Valceschini E., et al. 2-Hydroxyglutarate modulates histone methylation at specific loci and alters gene expression via Rph1 inhibition. Life Sci. Alliance. 2024;7 doi: 10.26508/lsa.202302333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi D.D., Anand S., Abdullah K.G., McBrayer S.K. DNA damage in IDH-mutant gliomas: mechanisms and clinical implications. Journal of neuro-oncology. 2023;162:515–523. doi: 10.1007/s11060-022-04172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen F., Bian K., Tang Q., Fedeles B.I., Singh V., Humulock Z.T., et al. Oncometabolites d- and l-2-hydroxyglutarate inhibit the AlkB family DNA repair enzymes under physiological conditions. Chem. Res. Toxicol. 2017;30:1102–1110. doi: 10.1021/acs.chemrestox.7b00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang P., Wu J., Ma S., Zhang L., Yao J., Hoadley K.A., et al. Oncometabolite D-2-hydroxyglutarate inhibits ALKBH DNA repair enzymes and sensitizes IDH mutant cells to alkylating agents. Cell Rep. 2015;13:2353–2361. doi: 10.1016/j.celrep.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sulkowski P.L., Corso C.D., Robinson N.D., Scanlon S.E., Purshouse K.R., Bai H., et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Inoue S., Li W.Y., Tseng A., Beerman I., Elia A.J., Bendall S.C., et al. Mutant IDH1 downregulates ATM and alters DNA repair and sensitivity to DNA damage independent of TET2. Cancer Cell. 2016;30:337–348. doi: 10.1016/j.ccell.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sulkowski P.L., Oeck S., Dow J., Economos N.G., Mirfakhraie L., Liu Y., et al. Oncometabolites suppress DNA repair by disrupting local chromatin signalling. Nature. 2020;582:586–591. doi: 10.1038/s41586-020-2363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Missiaen R., Lesner N.P., Simon M.C. HIF: a master regulator of nutrient availability and metabolic cross-talk in the tumor microenvironment. The EMBO journal. 2023;42 doi: 10.15252/embj.2022112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer. 2016;138:1058–1066. doi: 10.1002/ijc.29519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Semenza G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annual review of pathology. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 80.Xiao W., Loscalzo J. Metabolic responses to reductive stress. Antioxidants Redox Signal. 2020;32:1330–1347. doi: 10.1089/ars.2019.7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He H., Mulhern R.M., Oldham W.M., Xiao W., Lin Y.D., Liao R., et al. L-2-Hydroxyglutarate protects against cardiac injury via metabolic remodeling. Circ. Res. 2022;131:562–579. doi: 10.1161/circresaha.122.321227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kickingereder P., Sahm F., Radbruch A., Wick W., Heiland S., Deimling A., et al. IDH mutation status is associated with a distinct hypoxia/angiogenesis transcriptome signature which is non-invasively predictable with rCBV imaging in human glioma. Sci. Rep. 2015;5 doi: 10.1038/srep16238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koivunen P., Lee S., Duncan C.G., Lopez G., Lu G., Ramkissoon S., et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012;483:484–488. doi: 10.1038/nature10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tarhonskaya H., Rydzik A.M., Leung I.K., Loik N.D., Chan M.C., Kawamura A., et al. Non-enzymatic chemistry enables 2-hydroxyglutarate-mediated activation of 2-oxoglutarate oxygenases. Nat. Commun. 2014;5:3423. doi: 10.1038/ncomms4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Böttcher M., Renner K., Berger R., Mentz K., Thomas S., Cardenas-Conejo Z.E., et al. D-2-hydroxyglutarate interferes with HIF-1α stability skewing T-cell metabolism towards oxidative phosphorylation and impairing Th17 polarization. OncoImmunology. 2018;7 doi: 10.1080/2162402x.2018.1445454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chesnelong C., Chaumeil M.M., Blough M.D., Al-Najjar M., Stechishin O.D., Chan J.A., et al. Lactate dehydrogenase A silencing in IDH mutant gliomas. Neuro Oncol. 2014;16:686–695. doi: 10.1093/neuonc/not243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Viswanath P., Radoul M., Izquierdo-Garcia J.L., Luchman H.A., Gregory Cairncross J., Pieper R.O., et al. Mutant IDH1 gliomas downregulate phosphocholine and phosphoethanolamine synthesis in a 2-hydroxyglutarate-dependent manner. Cancer Metabol. 2018;6:3. doi: 10.1186/s40170-018-0178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gelman S.J., Naser F., Mahieu N.G., McKenzie L.D., Dunn G.P., Chheda M.G., et al. Consumption of NADPH for 2-HG synthesis increases pentose phosphate pathway flux and sensitizes cells to oxidative stress. Cell Rep. 2018;22:512–522. doi: 10.1016/j.celrep.2017.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tabata S., Kojima Y., Sakamoto T., Igarashi K., Umetsu K., Ishikawa T., et al. L-2 hydroxyglutaric acid rewires amino acid metabolism in colorectal cancer via the mTOR-ATF4 axis. Oncogene. 2023;42:1294–1307. doi: 10.1038/s41388-023-02632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McBrayer S.K., Mayers J.R., DiNatale G.J., Shi D.D., Khanal J., Chakraborty A.A., et al. Transaminase inhibition by 2-hydroxyglutarate impairs glutamate biosynthesis and redox homeostasis in glioma. Cell. 2018;175:101–116. doi: 10.1016/j.cell.2018.08.038. e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu D., Liu Y., Zhou Y., Ruiz-Rodado V., Larion M., Xu G., et al. Triptolide suppresses IDH1-mutated malignancy via Nrf2-driven glutathione metabolism. Proc. Natl. Acad. Sci. U.S.A. 2020;117:9964–9972. doi: 10.1073/pnas.1913633117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tateishi K., Wakimoto H., Iafrate A.J., Tanaka S., Loebel F., Lelic N., et al. Extreme vulnerability of IDH1 mutant cancers to NAD+ depletion. Cancer Cell. 2015;28:773–784. doi: 10.1016/j.ccell.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chan S.M., Thomas D., Corces-Zimmerman M.R., Xavy S., Rastogi S., Hong W.J., et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat. Med. 2015;21:178–184. doi: 10.1038/nm.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karpel-Massler G., Ishida C.T., Bianchetti E., Zhang Y., Shu C., Tsujiuchi T., et al. Induction of synthetic lethality in IDH1-mutated gliomas through inhibition of Bcl-xL. Nat. Commun. 2017;8:1067. doi: 10.1038/s41467-017-00984-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thomas D., Wu M., Nakauchi Y., Zheng M., Thompson-Peach C.A.L., Lim K., et al. Dysregulated lipid synthesis by oncogenic IDH1 mutation is a targetable synthetic lethal vulnerability. Cancer Discov. 2023;13:496–515. doi: 10.1158/2159-8290.cd-21-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sasaki M., Knobbe C.B., Itsumi M., Elia A.J., Harris I.S., Chio, et al. D-2-hydroxyglutarate produced by mutant IDH1 perturbs collagen maturation and basement membrane function. Genes & development. 2012;26:2038–2049. doi: 10.1101/gad.198200.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nathan J.A. Metabolite-driven antitumor immunity. Science (New York, N.Y.) 2022;377:1488–1489. doi: 10.1126/science.ade3697. [DOI] [PubMed] [Google Scholar]
- 98.Wang X., Chen Z., Xu J., Tang S., An N., Jiang L., et al. SLC1A1-mediated cellular and mitochondrial influx of R-2-hydroxyglutarate in vascular endothelial cells promotes tumor angiogenesis in IDH1-mutant solid tumors. Cell Res. 2022;32:638–658. doi: 10.1038/s41422-022-00650-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee C.L., O'Kane G.M., Mason W.P., Zhang W.J., Spiliopoulou P., Hansen A.R., et al. Circulating oncometabolite 2-hydroxyglutarate (2HG) as a potential biomarker for isocitrate dehydrogenase (IDH1/2) mutant cholangiocarcinoma. Mol. Cancer Therapeut. 2023 doi: 10.1158/1535-7163.mct-23-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu X., Hao J., Zhang H., Chi M., Wang Y., Huang J., et al. Oncometabolite D-2-hydroxyglutarate-dependent metabolic reprogramming induces skeletal muscle atrophy during cancer cachexia. Commun. Biol. 2023;6:977. doi: 10.1038/s42003-023-05366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Du X., Hu H. The roles of 2-hydroxyglutarate. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.651317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fu X., Chin R.M., Vergnes L., Hwang H., Deng G., Xing Y., et al. 2-Hydroxyglutarate inhibits ATP synthase and mTOR signaling. Cell Metab. 2015;22:508–515. doi: 10.1016/j.cmet.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Su R., Dong L., Li C., Nachtergaele S., Wunderlich M., Qing Y., et al. R-2HG exhibits anti-tumor activity by targeting FTO/m(6)A/MYC/CEBPA signaling. Cell. 2018;172:90–105. doi: 10.1016/j.cell.2017.11.031. e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Su R., Dong L., Li Y., Gao M., Han L., Wunderlich M., et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020;38:79–96.e11. doi: 10.1016/j.ccell.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu Q., Liu Z., Gao Z., Luo Y., Li F., Yang C., et al. KLF5 inhibition potentiates anti-PD1 efficacy by enhancing CD8(+) T-cell-dependent antitumor immunity. Theranostics. 2023;13:1381–1400. doi: 10.7150/thno.82182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang W., Chen J.J., Xing R., Zeng Y.C. Combination therapy: future directions of immunotherapy in small cell lung cancer. Transl Oncol. 2021;14 doi: 10.1016/j.tranon.2020.100889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Elia I., Rowe J.H., Johnson S., Joshi S., Notarangelo G., Kurmi K., et al. Tumor cells dictate anti-tumor immune responses by altering pyruvate utilization and succinate signaling in CD8(+) T cells. Cell Metab. 2022;34:1137–1150. doi: 10.1016/j.cmet.2022.06.008. e1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fathi A.T., Nahed B.V., Wander S.A., Iafrate A.J., Borger D.R., Hu R., et al. Elevation of urinary 2-hydroxyglutarate in IDH-mutant glioma. Oncol. 2016;21:214–219. doi: 10.1634/theoncologist.2015-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tyrakis P.A., Palazon A., Macias D., Lee K.L., Phan A.T., Velica P., et al. S-2-hydroxyglutarate regulates CD8(+) T-lymphocyte fate. Nature. 2016;540:236–241. doi: 10.1038/nature20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Goede K.E., Harber K.J., Gorki F.S., Verberk S.G.S., Groh L.A., Keuning E.D., et al. d-2-Hydroxyglutarate is an anti-inflammatory immunometabolite that accumulates in macrophages after TLR4 activation. Biochim. Biophys. Acta, Mol. Basis Dis. 2022;1868 doi: 10.1016/j.bbadis.2022.166427. [DOI] [PubMed] [Google Scholar]
- 111.Foskolou I.P., Bunse L., Van den Bossche J. 2-hydroxyglutarate rides the cancer-immunity cycle. Curr. Opin. Biotechnol. 2023;83 doi: 10.1016/j.copbio.2023.102976. [DOI] [PubMed] [Google Scholar]
- 112.Palmer D.C., Guittard G.C., Franco Z., Crompton J.G., Eil R.L., Patel S.J., et al. Cish actively silences TCR signaling in CD8+ T cells to maintain tumor tolerance. J. Exp. Med. 2015;212:2095–2113. doi: 10.1084/jem.20150304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang L., Sorensen M.D., Kristensen B.W., Reifenberger G., McIntyre T.M., Lin F. D-2-Hydroxyglutarate is an intercellular mediator in IDH-mutant gliomas inhibiting complement and T cells. Clin. Cancer Res. 2018;24:5381–5391. doi: 10.1158/1078-0432.CCR-17-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Notarangelo G., Spinelli J.B., Perez E.M., Baker G.J., Kurmi K., Elia I., et al. Oncometabolite d-2HG alters T cell metabolism to impair CD8(+) T cell function. Science. 2022;377:1519–1529. doi: 10.1126/science.abj5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kadiyala P., Carney S.V., Gauss J.C., Garcia-Fabiani M.B., Haase S., Alghamri M.S., et al. Inhibition of 2-hydroxyglutarate elicits metabolic reprogramming and mutant IDH1 glioma immunity in mice. J. Clin. Invest. 2021:131. doi: 10.1172/JCI139542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bantug G.R., Hess C. The burgeoning world of immunometabolites: T(h)17 cells take center stage. Cell Metab. 2017;26:588–590. doi: 10.1016/j.cmet.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 117.Bottcher M., Renner K., Berger R., Mentz K., Thomas S., Cardenas-Conejo Z.E., et al. D-2-hydroxyglutarate interferes with HIF-1 alpha stability skewing T-cell metabolism towards oxidative phosphorylation and impairing Th17 polarization. OncoImmunology. 2018;7 doi: 10.1080/2162402X.2018.1445454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kohanbash G., Carrera D.A., Shrivastav S., Ahn B.J., Jahan N., Mazor T., et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J. Clin. Invest. 2017;127:1425–1437. doi: 10.1172/JCI90644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schvartzman J.M., Intlekofer A.M. D-2HG comes out of its shell: metabolic effects on the immune environment. Mol Cell. 2022;82:4407–4409. doi: 10.1016/j.molcel.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 120.He Y., Yu H., Dai S., He M., Ma L., Xu Z., et al. Immune checkpoint inhibitors break whose heart? Perspectives from cardio-immuno-oncology. Genes Dis. 2024;11:807–818. doi: 10.1016/j.gendis.2023.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berghoff A.S., Kiesel B., Widhalm G., Wilhelm D., Rajky O., Kurscheid S., et al. Correlation of immune phenotype with IDH mutation in diffuse glioma. Neuro Oncol. 2017;19:1460–1468. doi: 10.1093/neuonc/nox054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang F., Niu M., Guo K., Ma Y., Fu Q., Liu Y., et al. The immunometabolite S-2-hydroxyglutarate exacerbates perioperative ischemic brain injury and cognitive dysfunction by enhancing CD8(+) T lymphocyte-mediated neurotoxicity. J. Neuroinflammation. 2022;19:176. doi: 10.1186/s12974-022-02537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Russ B.E., Olshanksy M., Smallwood H.S., Li J., Denton A.E., Prier J.E., et al. Distinct epigenetic signatures delineate transcriptional programs during virus-specific CD8(+) T cell differentiation. Immunity. 2014;41:853–865. doi: 10.1016/j.immuni.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Minogue E., Cunha P.P., Wadsworth B.J., Grice G.L., Sah-Teli S.K., Hughes R., et al. Glutarate regulates T cell metabolism and anti-tumour immunity. Nat. Metab. 2023;5:1747–1764. doi: 10.1038/s42255-023-00855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wright K.O., Murray D.A., Crispe N.I., Pierce R.H. Quantitative PCR for detection of the OT-1 transgene. BMC Immunol. 2005;6:20. doi: 10.1186/1471-2172-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kloosterman D.J., Akkari L. Macrophages at the interface of the co-evolving cancer ecosystem. Cell. 2023;186:1627–1651. doi: 10.1016/j.cell.2023.02.020. [DOI] [PubMed] [Google Scholar]
- 127.Galván-Peña S., O'Neill L.A. Metabolic reprograming in macrophage polarization. Front. Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chuntova P., Yamamichi A., Chen T., Narayanaswamy R., Ronseaux S., Hudson C., et al. Inhibition of D-2HG leads to upregulation of a proinflammatory gene signature in a novel HLA-A2/HLA-DR1 transgenic mouse model of IDH1R132H-expressing glioma. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2022-004644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Williams N.C., Ryan D.G., Costa A.S.H., Mills E.L., Jedrychowski M.P., Cloonan S.M., et al. Signaling metabolite L-2-hydroxyglutarate activates the transcription factor HIF-1α in lipopolysaccharide-activated macrophages. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2021.101501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Z., Feng X., Herting C.J., Garcia V.A., Nie K., Pong W.W., et al. Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res. 2017;77:2266–2278. doi: 10.1158/0008-5472.can-16-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Klemm F., Maas R.R., Bowman R.L., Kornete M., Soukup K., Nassiri S., et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell. 2020;181:1643–1660. doi: 10.1016/j.cell.2020.05.007. e1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Friedrich M., Sankowski R., Bunse L., Kilian M., Green E., Ramallo Guevara C., et al. Tryptophan metabolism drives dynamic immunosuppressive myeloid states in IDH-mutant gliomas. Nat Cancer. 2021;2:723–740. doi: 10.1038/s43018-021-00201-z. [DOI] [PubMed] [Google Scholar]
- 133.Halaby M.J., McGaha T.L. 2-HG modulates glioma macrophages via Trp metabolism. Nat Cancer. 2021;2:677–679. doi: 10.1038/s43018-021-00231-7. [DOI] [PubMed] [Google Scholar]
- 134.Rakateli L., Huchzermeier R., van der Vorst E.P.C., AhR P.X.R., Car From xenobiotic receptors to metabolic sensors. Cells. 2023;12 doi: 10.3390/cells12232752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van Dierendonck X., de Goede K.E., Van den Bossche J. IDH-mutant brain tumors hit the achilles' heel of macrophages with R-2-hydroxyglutarate. Trends Cancer. 2021;7:666–667. doi: 10.1016/j.trecan.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 137.Boltjes A., van Wijk F. Human dendritic cell functional specialization in steady-state and inflammation. Front. Immunol. 2014;5:131. doi: 10.3389/fimmu.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goswami S., Anandhan S., Raychaudhuri D., Sharma P. Myeloid cell-targeted therapies for solid tumours. Nat. Rev. Immunol. 2023;23:106–120. doi: 10.1038/s41577-022-00737-w. [DOI] [PubMed] [Google Scholar]
- 139.Yi M., Li T., Niu M., Mei Q., Zhao B., Chu Q., et al. Exploiting innate immunity for cancer immunotherapy. Mol. Cancer. 2023;22:187. doi: 10.1186/s12943-023-01885-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ugele I., Cardenas-Conejo Z.E., Hammon K., Wehrstein M., Bruss C., Peter K., et al. D-2-Hydroxyglutarate and L-2-hydroxyglutarate inhibit IL-12 secretion by human monocyte-derived dendritic cells. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Prins R.M., Everson R.G. Commentary on "Dysfunctional dendritic cells limit antigen-specific T cell response in glioma". Neuro Oncol. 2023;25:277–278. doi: 10.1093/neuonc/noac256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Friedrich M., Hahn M., Michel J., Sankowski R., Kilian M., Kehl N., et al. Dysfunctional dendritic cells limit antigen-specific T cell response in glioma. Neuro Oncol. 2023;25:263–276. doi: 10.1093/neuonc/noac138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Menetrier-Caux C., Thomachot M.C., Alberti L., Montmain G., Blay J.Y. IL-4 prevents the blockade of dendritic cell differentiation induced by tumor cells. Cancer Res. 2001;61:3096–3104. [PubMed] [Google Scholar]
- 144.Krawczyk C.M., Holowka T., Sun J., Blagih J., Amiel E., DeBerardinis R.J., et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Malinarich F., Duan K., Hamid R.A., Bijin A., Lin W.X., Poidinger M., et al. High mitochondrial respiration and glycolytic capacity represent a metabolic phenotype of human tolerogenic dendritic cells. J. Immunol. 2015;194:5174–5186. doi: 10.4049/jimmunol.1303316. [DOI] [PubMed] [Google Scholar]
- 146.Ren F., Zhao Q., Huang L., Zheng Y., Li L., He Q., et al. The R132H mutation in IDH1 promotes the recruitment of NK cells through CX3CL1/CX3CR1 chemotaxis and is correlated with a better prognosis in gliomas. Immunol. Cell Biol. 2019;97:457–469. doi: 10.1111/imcb.12225. [DOI] [PubMed] [Google Scholar]
- 147.Zhang X., Rao A., Sette P., Deibert C., Pomerantz A., Kim W.J., et al. IDH mutant gliomas escape natural killer cell immune surveillance by downregulation of NKG2D ligand expression. Neuro Oncol. 2016;18:1402–1412. doi: 10.1093/neuonc/now061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhu S., Fu Y., Zhu B., Zhang B., Wang J. Pneumonitis induced by immune checkpoint inhibitors: from clinical data to translational investigation. Front. Oncol. 2020;10:1785. doi: 10.3389/fonc.2020.01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dong Q., Tong M., Yu X., Wang L., Ao J., Guan D., et al. Carbohydrate strengthens the immunotherapeutic effect of small-molecule PD-L1 inhibitors. J. Med. Chem. 2023;66:7179–7204. doi: 10.1021/acs.jmedchem.2c01347. [DOI] [PubMed] [Google Scholar]
- 151.Efficace F., Vignetti M. Quality of life and CAR-T cell therapy in children, adolescents, and young adults with haematological malignancies. Lancet Oncol. 2019;20:1625–1626. doi: 10.1016/S1470-2045(19)30641-2. [DOI] [PubMed] [Google Scholar]
- 152.Du M.Y., Zhang Y.Q., Liao D.Y., Xie W., Xiong W., Mei H., et al. [Long-term follow-up of humanized and murine CD19 CAR-T-cell therapy for B-cell acute lymphoblastic leukemia] Zhonghua Xue Ye Xue Za Zhi. 2023;44:793–799. doi: 10.3760/cma.j.issn.0253-2727.2023.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Murad J.P., Tilakawardane D., Park A.K., Lopez L.S., Young C.A., Gibson J., et al. Pre-conditioning modifies the TME to enhance solid tumor CAR T cell efficacy and endogenous protective immunity. Mol. Ther. 2021;29:2335–2349. doi: 10.1016/j.ymthe.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang Q., Hao J., Chi M., Wang Y., Li J., Huang J., et al. D2HGDH-mediated D2HG catabolism enhances the anti-tumor activities of CAR-T cells in an immunosuppressive microenvironment. Mol. Ther. 2022;30:1188–1200. doi: 10.1016/j.ymthe.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Foskolou I.P., Barbieri L., Vernet A., Bargiela D., Cunha P.P., Velica P., et al. The S enantiomer of 2-hydroxyglutarate increases central memory CD8 populations and improves CAR-T therapy outcome. Blood Adv. 2020;4:4483–4493. doi: 10.1182/bloodadvances.2020002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.DiNardo C.D., Stein E.M., de Botton S., Roboz G.J., Altman J.K., Mims A.S., et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N. Engl. J. Med. 2018;378:2386–2398. doi: 10.1056/NEJMoa1716984. [DOI] [PubMed] [Google Scholar]
- 157.Montesinos P., Recher C., Vives S., Zarzycka E., Wang J., Bertani G., et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N. Engl. J. Med. 2022;386:1519–1531. doi: 10.1056/NEJMoa2117344. [DOI] [PubMed] [Google Scholar]
- 158.Zhu A.X., Macarulla T., Javle M.M., Kelley R.K., Lubner S.J., Adeva J., et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol. 2021;7:1669–1677. doi: 10.1001/jamaoncol.2021.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stein E.M., DiNardo C.D., Pollyea D.A., Fathi A.T., Roboz G.J., Altman J.K., et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722–731. doi: 10.1182/blood-2017-04-779405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Watts J.M., Baer M.R., Yang J., Prebet T., Lee S., Schiller G.J., et al. Olutasidenib alone or with azacitidine in IDH1-mutated acute myeloid leukaemia and myelodysplastic syndrome: phase 1 results of a phase 1/2 trial. The Lancet. Haematology. 2023;10:e46–e58. doi: 10.1016/s2352-3026(22)00292-7. [DOI] [PubMed] [Google Scholar]
- 161.Mellinghoff I.K., van den Bent M.J., Blumenthal D.T., Touat M., Peters K.B., Clarke J., et al. Vorasidenib in IDH1- or IDH2-mutant low-grade glioma. N. Engl. J. Med. 2023;389:589–601. doi: 10.1056/NEJMoa2304194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mellinghoff I.K., Lu M., Wen P.Y., Taylor J.W., Maher E.A., Arrillaga-Romany I., et al. Vorasidenib and ivosidenib in IDH1-mutant low-grade glioma: a randomized, perioperative phase 1 trial. Nat. Med. 2023;29:615–622. doi: 10.1038/s41591-022-02141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.DiNardo C.D., Hochhaus A., Frattini M.G., Yee K., Zander T., Krämer A., et al. A phase 1 study of IDH305 in patients with IDH1(R132)-mutant acute myeloid leukemia or myelodysplastic syndrome. J. Cancer Res. Clin. Oncol. 2023;149:1145–1158. doi: 10.1007/s00432-022-03983-6. [DOI] [PubMed] [Google Scholar]
- 164.Heuser M., Palmisiano N., Mantzaris I., Mims A., DiNardo C., Silverman L.R., et al. Safety and efficacy of BAY1436032 in IDH1-mutant AML: phase I study results. Leukemia. 2020;34:2903–2913. doi: 10.1038/s41375-020-0996-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wick A., Bähr O., Schuler M., Rohrberg K., Chawla S.P., Janku F., et al. Phase I assessment of safety and therapeutic activity of BAY1436032 in patients with IDH1-mutant solid tumors. Clin. Cancer Res. : an official journal of the American Association for Cancer Research. 2021;27:2723–2733. doi: 10.1158/1078-0432.ccr-20-4256. [DOI] [PubMed] [Google Scholar]
- 166.Dutoit V., Migliorini D., Dietrich P.Y., Walker P.R. Immunotherapy of malignant tumors in the brain: how different from other sites? Front. Oncol. 2016;6:256. doi: 10.3389/fonc.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lucca L.E., Hafler D.A. Resisting fatal attraction: a glioma oncometabolite prevents CD8+ T cell recruitment. J. Clin. Invest. 2017;127:1218–1220. doi: 10.1172/JCI93565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schumacher T., Bunse L., Pusch S., Sahm F., Wiestler B., Quandt J., et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512:324–327. doi: 10.1038/nature13387. [DOI] [PubMed] [Google Scholar]
- 169.Capper D., Weissert S., Balss J., Habel A., Meyer J., Jager D., et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2010;20:245–254. doi: 10.1111/j.1750-3639.2009.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pellegatta S., Valletta L., Corbetta C., Patane M., Zucca I., Riccardi Sirtori F., et al. Effective immuno-targeting of the IDH1 mutation R132H in a murine model of intracranial glioma. Acta Neuropathol Commun. 2015;3:4. doi: 10.1186/s40478-014-0180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with the study has not been deposited into a publicly available repository and no additional data was used for the research described in the review article.