Abstract
Chronic spontaneous urticaria (CSU) is characterized by pruritus, urticaria and associated with substantial patient burden. Emerging clinical trial data suggest dupilumab, an anti-IL-4Rα biologic indicated for several Type-2 inflammatory diseases, may have clinical utility for CSU. Here we present real world clinical data evaluating dupilumab as add-on therapy for CSU. We queried our tertiary academic center electronic health record for all patients with an ICD-9/10 code for urticaria with a history of dupilumab use (1/1/2010-6/30/2022). Retrospective chart review was performed to confirm CSU diagnosis, dupilumab use, patient demographics, medical history, treatments, and outcomes. Data were evaluated using summary and descriptive statistics, paired t-test, and Fisher’s exact test. A total of 199 patients were identified: 39 had active CSU at time dupilumab initiation; six were excluded due to limited follow up. The most common indication for dupilumab prescription was atopic dermatitis (57.6%), followed by asthma (27.3%). Mean length of dupilumab therapy was 23 months. Following dupilumab, there was a significant reduction in number of patients on daily H1 antagonists (pre: 27 [81.8%]; post: 20 (60.1%); p=0.03), as well as total daily number of antihistamines (pre: 1.95±2.0; post: 0.13±0.2; p=0.01). For patients with moderate/severe vs. mild disease, there was greater improvement in disease control as assessed by physicians global impression of change (77% vs. 30%, p=0.02). In this real-world study, when used as add-on therapy for patients with CSU, dupilumab was associated with improved disease control and decreased H1 blocker use, suggesting a future for dupilumab as an approvedbiologic therapy for the treatment of CSU.
Chronic spontaneous urticaria (CSU) is a condition characterized by recurrent pruritic wheals, angioedema, or both lasting at least six weeks.1 Pathophysiology of CSU is multifactorial, but emerging evidence suggests an underlying role of Type 2 inflammation.2 Dupilumab, a humanized monoclonal antibody targeting IL-4Rα, is currently approved for multiple Type 2 inflammatory diseases including atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis, and is currently under investigation for treatment of CSU. However, published evidence supporting the use of dupilumab in patients with CSU is limited to a select number of case reports.3–5 Here, we report on outcomes in the largest described real-world cohort treated with dupilumab as add-on therapy for CSU in individuals with co-morbid atopic disease.
Participants were identified through electronic health record query of the Northwestern Medicine health system for all patients with ICD-9 (708.1/708.8/70.89) or ICD-10 (L50.1/L50.8/L50.9) codes for CSU diagnosis and history of dupilumab prescription. All patients had presence of comorbid allergic disease as primary indication for dupilumab. A manual chart review was performed to confirm active CSU diagnosis, dupilumab use, demographics, comorbidities, treatment outcomes, and adverse events. Outcomes were evaluated by physician global assessment of change and medication use pre- vs. post-dupilumab. Data were evaluated using summary and descriptive statistics, including paired t-test, Fisher’s exact test, and McNemar’s test for paired nominal data.
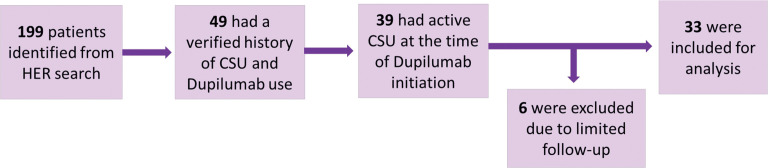
A total of 199 patients were identified, of which 49 had verified history of CSU and dupilumab use; 39 had active CSU at the time of dupilumab initiation. Six patients were excluded due to limited follow up resulting in a total of 33 patients included for this analysis (Figure 1). Patient demographics, comorbidities, and documented indications for dupilumab are summarized in Table 1. The mean±SD age was 46 ± 15.4 years, and the majority of patients were female (84.8%). Mean duration on dupilumab was 23 months. The most common primary indication for dupilumab approval was atopic dermatitis (57.6%) followed by asthma (27.3%). There was a significant reduction in number of patients on daily H1 antagonists (pre: 27 [81.8%]; post: 20 (60.1%); p=0.03), as well as total daily number of antihistamines (pre: 1.95±2.0; post: 0.13±0.2; p=0.01) following dupilumab (Table 2). Six patients pre-dupilumab were on omalizumab within 30 days of starting dupilumab (18.2%) vs. 3 (9.1%) post-dupilumab. Physician global impression of change was objectively assessed as disease control being worsened, unchanged or improved pre- vs. post-dupilumab (Table 2). Overall, two patients had worsened disease on dupilumab, 15 had unchanged control, and 16 had improvement of symptoms. For patients with moderate/severe (either ≥4x daily H1-antagonist or omalizumab use), vs. mild disease there was greater improvement in disease control (77% vs. 30%, p=0.02). Treatment-related adverse events were consistent with previously reported safety data (Table 2).6
FIGURE 1.
Identification of Study Participants
TABLE 1.
Patient Demographics and Documented Indication for Dupilumab (n=33)
| VARIABLES | FREQUENCY (%) |
|---|---|
| Age (yrs), mean ± SD | 46 ± 15.4 |
| Body Mass Index, mean ± SD | 28.8 ± 6.8 |
| Sex | |
| Female | 28 (84.8%) |
| Race | |
| White | 18 (54.5%) |
| Black | 5 (15.2%) |
| Asian | 4 (12.1%) |
| Other/declined/not specified | 6 (18.2%) |
| Comorbidities | |
| Angioedema | 7 (21.2%) |
| AD | 21 (63.6%) |
| Asthma | 20 (60.6%) |
| CRSwNP | 3 (9.1%) |
| EoE | 2 (6.1%) |
| Documented Indication for Dupilumab | |
| AD (moderate-severe) | 19 (57.6%) |
| Asthma (moderate-severe, persistent) | 9 (27.3%) |
| CRSwNP | 1 (3%) |
| Asthma and CRSwNP | 2 (6.1%) |
| EoE | 2 (6.1) |
AD: atopic dermatitis, CRSwNP: chronic rhinosinusitis with nasal polyps, EoE: eosinophilic esophagitis
TABLE 2.
Dupilumab Treatment Outcomes and Safety
| MEDICATION USE PRE- AND POST-DUPILUMAB | |||
|---|---|---|---|
| MEDICATION USE | PRE-DUPILUMAB | POST-DUPILUMAB | P-VALUE |
| Daily H1 antagonist | 27 (81.8%) | 20 (60.1%) | 0.03 |
| Total # of daily antihistamines, mean±SD | 1.95 ± 2.0 | 0.13 ± 0.20 | 0.01 |
| Daily oral corticosteroids | 2 (6%) | 1 (3%) | 0.32 |
| LTRA | 11 (33.3%) | 9 (27.3%) | 0.8 |
| H2 antagonist | 5 (15.2%) | 3 (9.1%) | 0.32 |
| Cyclosporine | 1 (3%) | 1 (3%) | 1.0 |
| Omalizumab* | 6 (18.2%) | 3 (9.1%) | 0.18 |
| PHYSICIAN GLOBAL IMPRESSION OF CHANGE | |||
| ASSESSMENT | MILD (N=20) | MODERATE/ SEVERE (N=13) | P-VALUE |
| Worse | 1 (5.0%) | 1 (7.9%) | 0.02 |
| Unchanged | 13 (65.0%) | 2 (15.4%) | 0.02 |
| Improved | 6 (30%) | 10 (77%) | 0.02 |
| TREATMENT EMERGENT ADVERSE EVENTS | |||
| ADVERSE EVENT (n=33) | FREQUENCY (%) | ||
| None | 26 (78.8%) | ||
| Anaphylaxis | 1 (3%) | ||
| Arthralgias | 3 (9.1%) | ||
| Conjunctivitis | 2 (6.1%) | ||
| CSU recurrence on Dupilumab | 1 (3%) | ||
| Injection Site Redness/ Irritation | 0 (0%) | ||
*Within 30 days of starting Dupilumab. LTRA: leukotriene receptor antagonist
Previous case reports suggested clinical utility for dupilumab in the treatment of refractory CSU.3–5 In our study, dupilumab was associated with a significant reduction in daily antihistamine use, and a trend towards decreased omalizumab use. In addition, patients with more severe disease activity demonstrated improved disease control following dupilumab therapy. CSU is a condition with significant morbidity and often co-occurs with other atopic conditions.7 Dysregulation of CD4+ Type 2 helper T cells and increased levels of IL-4 and IL-13 are observed in CSU patients, indicating a possible role for Type 2 inflammation-targeting therapies.2 Further, it is increasingly recognized that IL-4 and IL-13 are involved in the neuromodulation of itch signals, as evidenced by dupilumab’s clinical utility in other Type 2 inflammatory skin conditions.6 Multiple treatments targeting Type 2 signaling pathways (e.g., IL-4/IL-13, IL-5, siglec-8, c-KIT, BTK) are currently under investigation for use in CSU.8 A Phase 3 clinical trial for dupilumab in CSU (LIBERTY-CSU CUPID Study A) showed improved itch and urticaria activity scores relative to placebo in patients who had previously had an inadequate response to oral antihistamines.9 However, the subsequent Phase 3 Study B (which enrolled patients who had failed omalizumab therapy) was halted after interim analysis showed that the primary endpoint would not be achieved, but reportedly did show relative improvement in itch and urticaria activity scores. On the basis of these clinical trials, dupilumab was submitted to the FDA for consideration of approval for CSU in March of 2023 and is currently under review. Omalizumab, a humanized monoclonal antibody targeting unbound IgE, is currently approved by the FDA for CSU, allergic asthma, and chronic rhinosinusitis with nasal polyps, while dupilumab is approved for a broader array of Type 2 inflammatory conditions including the pruritic skin conditions such as atopic dermatitis and prurigo nodularis. Our data adds to the limited body of evidence, suggesting that that for patients with CSU and comorbid atopic conditions not treated by omalizumab, add-on therapy with dupilumab warrant further investigation. Furthermore, dupilumab and omalizumab act on distinct pathways and could potentially serve a synergistic function for select patients. Promising data across clinical trial programs for a variety of targets in CSU suggests that patient endotyping and comorbidities may eventually inform treatment selection.8
Limitations of this study include single center, retrospective analysis. ICD codes were used to screen for patients with CSU, and it is possible some patients were miscoded or not coded for CSU diagnosis, thereby leading us to miss some patients for study inclusion. All patients had comorbid allergic disease, and our data may not be generalizable to other populations. The chart data were limited by lack of patient reported outcomes or other objective measures of disease activity.
In conclusion, dupilumab as add-on therapy for CSU reduces the need for daily antihistamine use and improves clinical outcomes in patients with comorbid atopic disease. Additional prospective studies and randomized, controlled trials are needed to best determine which CSU patients may be the best candidates for dupilumab.
Contributor Information
Elizabeth Kudlaty, Dr. Kudlaty is with the Division of Allergy and Immunology and the Department of Medicine at Northwestern University Feinberg School of Medicine in Chicago, Illinois..
Phoebe Newell, Dr. Newell is with the Northwestern University Feinberg School of Medicine in Chicago, Illinois..
Raj Chovatiya, Dr. Chovatiya is with Department of Dermatology at the Northwestern University Feinberg School of Medicine in Chicago, Illinois..
REFERENCES
- Bernstein JAMD, Lang DMMD, Khan DAMD et al. The diagnosis and management of acute and chronic urticaria: 2014 update. Journal of allergy and clinical immunology. 2014;133(5):1270–1277.e66. doi: 10.1016/j.jaci.2014.02.036. [DOI] [PubMed] [Google Scholar]
- Zhou B, Li J, Liu R et al. The Role of Crosstalk of Immune Cells in Pathogenesis of Chronic Spontaneous Urticaria. Frontiers in immunology. 2022;13:879754. doi: 10.3389/fimmu.2022.879754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Simpson RS. Dupilumab as a novel therapy for difficult to treat chronic spontaneous urticaria. The journal of allergy and clinical immunology in practice (Cambridge, MA). 2019;7(5):1659–1661.e1. doi: 10.1016/j.jaip.2018.11.018. [DOI] [PubMed] [Google Scholar]
- Zhu C, Fok JS, Lin L et al. Complete response to dupilumab in a patient with chronic spontaneous urticaria who did not tolerate omalizumab. JAAD Case Reports. 2023;32:109–112. doi: 10.1016/j.jdcr.2022.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abadeh A, Lee JK. Long-term follow-up of patients treated with dupilumab for chronic spontaneous urticaria: A case report. SAGE Open Medical Case Reports. 2022;10:2050313X211177. doi: 10.1177/2050313X221117702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck LA, Deleuran M, Bissonnette R et al. Dupilumab Provides Acceptable Safety and Sustained Efficacy for up to 4 Years in an Open-Label Study of Adults with Moderate-to-Severe Atopic Dermatitis. American Journal of Clinical Dermatology. 2022;23(3):393–408. doi: 10.1007/s40257-022-00685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfar MN, Kibsgaard L, Thomsen SF et al. Risk of comorbidities in patients diagnosed with chronic urticaria: A nationwide registry-study. World Allergy Organization Journal. 2020;13(1):100097. doi: 10.1016/j.waojou.2019.100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casale TB. Novel biologics for treatment of chronic spontaneous urticaria. Journal of allergy and clinical immunology. 2022;150(6):1256–1259. doi: 10.1016/j.jaci.2022.06.027. [DOI] [PubMed] [Google Scholar]
- Maurer M, Casale T, Saini S et al. Dupilumab Significantly Reduces Itch and Hives in Patients With Chronic Spontaneous Urticaria: Results From a Phase 3 Trial (LIBERTY-CSU CUPID Study A). Journal of Allergy and Clinical Immunology. 2022;149(2):AB312. [Google Scholar]