Abstract
Alcohol use disorder (AUD) most commonly presents as a polydrug disorder where greater than 85% are estimated to smoke. EtOH and nicotine (NIC) co-abuse or exposure results in unique neuroadaptations that are linked to behaviors that promote drug use. The current experiments aimed to identify neuroadaptations within the mesolimbic pathway produced by concurrent EtOH and NIC exposure. The experiments used four overall groups of male Wistar rats consisting of vehicle, EtOH or NIC alone, and EtOH+NIC. Drug exposure through direct infusion into the posterior ventral tegmental area (pVTA) stimulated release of glutamate and dopamine in the nucleus accumbens (NAc) shell, which was quantified through high-performance liquid chromatography (HPLC). Additionally, brain-derived neurotrophic factor (BDNF) protein levels were measured via enzyme-linked immunosorbent assay (ELISA). A second experiment investigated the effects of drug pretreatment within the pVTA on the reinforcing properties of EtOH within the NAc shell through intracranial self-administration (ICSA). The concluding experiment evaluated the effect of NAc shell pretreatment with BDNF on EtOH reward utilizing ICSA within that region. The data indicated that only EtOH+NIC administration into the pVTA simultaneously increased glutamate, dopamine, and BDNF in the NAc shell. Moreover, only pVTA pretreatment with EtOH+NIC enhanced the reinforcing properties of EtOH in the NAc shell. BDNF pretreatment in the NAc shell was also sufficient to enhance the reinforcing properties of EtOH in the NAc shell. The collected data suggest that concurrent EtOH+NIC exposure results in a distinct neurochemical response and neuroadaptations within the mesolimbic pathway that alter EtOH reward.
Keywords: Ethanol, Nicotine, BDNF, Dopamine, Glutamate, Mesolimbic Pathway
Introduction
Alcohol is the most commonly used drug of abuse worldwide and accounts for more than 5% of global deaths annually (WHO, 2018). Nearly 14% of the US population currently meets the diagnostic criteria for alcohol use disorder (AUD) which is associated with numerous adverse health, economic, and social consequences (Hasin and Grant 2015; Grant et al. 2015, 2017). Importantly, the majority of individuals with AUD have comorbid disorders involving other drugs of abuse. This includes an estimated 65 – 90% that also use tobacco products comprising the largest group of polysubstance abusers (Falk et al. 2006; Grant et al. 2015; Saha et al. 2018). The high comorbidity of alcohol and tobacco use is associated with more severe levels of dependency and increases the tendency to consume more of each drug (John et al. 2003; McKee et al. 2007). Additionally, concurrent use decreases the likelihood of initiating abstinence (Weinberger et al. 2013; Adams 2017). The propensity to consume alcohol and nicotine together creates an obstacle where each drug hinders successful cessation of the other.
Despite the high levels of alcohol (EtOH) and nicotine (NIC) comorbidity in humans, preclinical research often focuses on investigating the effects of individual drugs of abuse (Srisurapanont and Jarusuraisin 2005; Jorenby et al. 2006; Motschman et al. 2016). In order to develop successful therapeutics for AUD as a polydrug disorder, a better understanding of the mechanisms behind these associations is needed. It is necessary to examine the shared neurobiological mechanisms and subsequent effects on behavior by concurrent EtOH and NIC exposure, rather than individually. A growing body of research suggests the interactions between EtOH and NIC may stem from direct and indirect modulation of the mesocorticolimbic pathway as well as dysregulation of neurotransmitter regulatory systems (Van Skike et al. 2016). Previous research from our laboratory has demonstrated EtOH and NIC co-exposure consistently results in unique alterations to the mesocorticolimbic pathway that is not evident with either drug alone. For example, chronic self-administration of EtOH and NIC, but not equivalent amounts of EtOH or NIC alone, increases sensitivity to the reinforcing properties of NIC within the nucleus accumbens (NAc) shell. This chronic exposure also alters glutamate neurochemistry within the medial prefrontal cortex (Deehan et al. 2015). Tizabi and colleagues (2002, 2007) demonstrated that systemically administered EtOH and NIC generated an additive effect when measuring stimulated dopamine release in the NAc shell. EtOH and NIC was likewise found to interact within the posterior ventral tegmental area (pVTA) and produce synergistic effects on drug reward in the pVTA (Truitt et al. 2015). Furthermore, a single administration of EtOH and NIC directly into the pVTA results in numerous gene changes within the NAc shell which was not observed following comparable acute administration of EtOH or nicotine alone (Truitt et al. 2015). Specifically, acute intra-pVTA co-exposure to EtOH and NIC produced a robust upregulation of brain-derived neurotrophic factor (Bdnf) and a significant reduction in glial cell-derived neurotrophic factor (Gdnf) mRNA in the NAc shell (Truitt et al. 2015).
Few studies have investigated BDNF protein expression following exposure to EtOH and NIC within the mesolimbic pathway and how these changes may influence reward sensitivity. The NAc shell is well established as a critical brain region involved in motivated behaviors and reward (Sesack and Grace 2010). Preclinical models of EtOH and/or NIC associated drug-seeking and reward indicate the behavioral changes are potentially linked to altered neurotransmitter and BDNF levels in the NAc (Deehan et al. 2015; Truitt et al. 2015).
The overall hypothesis is that limited and concurrent exposure to EtOH and NIC results in unique neurochemical responses within the mesolimbic system that facilitate the development of neuroadaptations and promote future drug self-administration. The goal of the present study was to characterize alterations in neurochemistry and behavior that are specific to co-exposure of naïve animals to EtOH and NIC. The first experiment determined how acute intra-pVTA microinfusions of vehicle, EtOH, NIC, or EtOH+NIC stimulate dopamine and glutamate release within the NAc shell. Building on previous work, the second experiment determined alterations in BDNF protein levels of the NAc shell produced by acute or repeated intra-pVTA exposure to vehicle, EtOH, NIC, or EtOH+NIC as well as a temporal assessment of the observed effects. The biological consequence of concurrent exposure to EtOH+NIC was assessed by determining the effects of repeated intra-pVTA pretreatment with vehicle, EtOH, NIC, or EtOH+NIC on the reinforcing properties of EtOH in the NAc shell. The significant elevation in levels of BDNF was identified as a potential mediator for the enhancement of EtOH reward within the NAc shell following exposure to EtOH+NIC in the pVTA. Thus, exogenous BDNF was directly infused into the NAc shell to determine whether increased levels of BDNF were sufficient to enhance EtOH reward in that region.
Methods
Animals
Adult male Wistar rats (Hsd:WI, Research Resource Identifier [RRID]: RGD_737960, Harlan, Indianapolis, IN) were utilized in the current study. All animals were maintained in fully accredited facilities by the Association for the Assessment and Accreditation of Laboratory Animal Care. Research protocols were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee (Protocols 10761 and 11115) and followed the National Institutes of Health Guide for Care and Use of Laboratory Animals (NRCC. 2011). This study was not pre-registered.
Animals were received in groups of 16 around post-natal day (PND) 75 weighing 300–350 grams and arbitrarily paired in plastic shoebox cages for at least 2 weeks prior to use. A total of n = 351 animals were utilized in the current experiments with 85.5% (n = 300) included in the final analyses. Some animals were excluded for cannula placements outside the region of interest (ROI; n = 46) or technical complications (n = 5) during sample collection. All rats were kept on a 12-hour reverse light cycle with lights off at 0900. All experiments were carried out during the dark cycle. Food and water were available ad libitum to all animals throughout the experiment. Experiments were carried out following PND 90. Pretreatments and/or experimental days utilized 8 animals separated into 2 or 3 treatment groups (e.g. n = 8/experimental day composed of n = 2 controls, n = 3 of EtOH, and n = 3 of NIC). Experimental days were then repeated following identical procedures until a sufficient number of animals was reached for each treatment group. There was no specific randomization technique performed to allocate subjects in the following experiments. The number of animals in each group was based on previously published experiments demonstrating moderate to large effect sizes with similar methods utilizing Wistar rats (Rodd et al. 2004; Engleman et al. 2006, 2009; Hauser et al. 2014a). Immediately following all stereotaxic surgeries described herein, animals were administered 0.05 mg/kg carprofen subcutaneously via a short action preparation and 0.5% bupivicaine was applied locally to minimize pain according to the IACUC protocols. Additional injections of bupivicaine were administered as needed for 2–4 days post-surgery.
EtOH and/or NIC microinjection-microdialysis and dialysate analysis
The microinjection-microdialysis procedure was implemented as previously described (Ding et al. 2009, Toalston et al. 2014; Deehan et al. 2018). Briefly, rats were stereotaxically implanted with two ipsilateral guide cannulas in the right hemisphere. A 22-guage microinjection cannula (Plastics One, Inc., Roanoke, VA) was aimed 1.0 mm above the pVTA with an 18-guage microdialysis cannula implanted 3.0 mm above the NAc shell while under isoflurane anesthesia. Coordinates for the pVTA and NAc shell were AP −5.6 mm, ML +2.1 mm, DV −8.2 mm and +1.4 mm, ML +2.2 mm, DV −5.3 mm, respectively (Paxinos and Watson, 1998). Cannulas were implanted at a 10° angle and inserted with sterile stylets while no experiments were being carried out to prevent blockage and infection. After surgery, rats were single housed in new shoebox cages and allowed one week of recovery. Rats were also habituated to the experimental housing and handled daily during this time.
Loop-style microdialysis probes were constructed as previously described with an active length of 2.0 mm and molecular weight cut-off of 13 kDa (Kohl et al. 1998; Engleman et al. 2000; Ding et al. 2009; Toalston et al. 2014). Rats were placed under isoflurane anesthesia and the microdialysis probes were inserted into the NAc shell by extending 3.0 mm below the guide cannula. The microinjection-microdialysis procedure was carried out the following day.
All experiments were carried out in awake freely moving animals. Subjects were placed in the experimental housing and the microdialysis probes were connected to a syringe pump to continuously perfuse the NAc shell with artificial cerebrospinal fluid (aCSF) at a rate of 1 μL/minute. Microdialysis aCSF was made up of 140.0 mM NaCl, 3.0 mM KCl, 1.2 mM CaCl2, 2.0 mM Na2HPO4 • 7H2O, and 1.0 mM MgCl2 with a pH 7.2 to 7.4. Samples were collected in 20-minute intervals beginning with five baseline samples that started following a 90-minute washout period.
Rats received challenge microinjections directly into the pVTA consisting of aCSF, EtOH only, NIC only or combinations of EtOH and NIC. Nicotine HCl (Sigma-Aldrich, St. Louis, MO) concentrations were calculated based on the salt. Ethyl alcohol (95%; McCormick Distilling Co., Weston, MO) was diluted to the desired concentration with aCSF. The behavioral effects of EtOH are commonly described based on blood alcohol concentration (BAC; Mello and Mendelson 1972; Spanagal 2009; Koob 2013). For example, the legal intoxication level in the United States was established at a BAC of 0.08% or 80 milligrams of EtOH per 100 milliliters of blood (i.e. 80 mg/dL or 80 mg% EtOH; Yadav and Velaga 2019). Pharmacologically relevant BACs range from 80–250 mg%, which is approximately 16–55 mM, in order to parallel levels observed in individuals with AUD (Bell et al. 2016; Harrison et al. 2017). Treatment groups are as follows: aCSF, 100 mg% EtOH, 150 mg% EtOH, 10 μM NIC, 50 μM NIC, 100 mg% EtOH+10 μM NIC, or 150 mg% EtOH+50 μM NIC. Drug concentrations were selected based on previous intracranial self-administration (ICSA) studies within the pVTA (Gatto et al. 1994; Rodd et al. 2004; Hauser et al. 2014a; Truitt et al. 2015). Lower drug doses represent “subthreshold” levels that are not readily self-administered. Higher doses considered “suprathreshold” have been consistently shown to be reinforcing and are self-administered at levels significantly greater than vehicle controls. Passive microinjections were carried out with an electrolytic microinfusion transducer (EMIT) system (Rodd-Henricks et al. 2000; Ding et al. 2009). Subjects underwent 30 pulse injections during a 10-minute period designed to match ICSA levels (Rodd-Henricks et al. 2000; Toalston et al. 2014). Pulse injections infused 100 nl over 5 seconds that was followed by a 15-second timeout for a total of 3 μl.
Following the microinjection challenge, six 20-minute samples of dialysate samples were also collected into tubes containing five μl of 0.1 N perchloric acid. Samples were frozen on dry ice and stored at −80 °C until analysis for dopamine and glutamate content with high performance liquid chromatography (HPLC). The current microdialysis study utilized 57 male Wistar rats. Subjects were arbitrarily assigned to one of seven microinjection conditions with n = 7–10/group for dopamine analysis and n = 6–9/group for glutamate analysis.
Dopamine content was determined as described previously with a reversed-phase HPLC system and electrochemical detection (Engleman et al. 2000, 2006; Ding et al. 2009; Toalston et al. 2014). Dialysate samples were loaded into a 10 μl loop and injected onto an analytical column (Hypersil BDS C18, 150 mm x 2.1 mm, 3 μm, Thermo Fisher Scientific, Waltham, MA). Mobile phase was made up of 0.1 mM EDTA, 8 mM KCl, 50 mM phosphoric acid, 100 mg/L OSA, and 10% MeOH with a pH of 6.0. Detection occurred with a glassy-carbon electrode and an amperometric detector. The oxidation potential was set at 350 mV with a sensitivity of 100 pA/V (Decade II EC Detector, Antec Scientific, Netherlands). Dopaminergic signal analysis was resolved with ChromePerfect chromatography data system (Justice Innovations, Inc., Palo Alto, CA).
Extracellular glutamate concentrations were also determined using a reversed-phase HPLC system with electrochemical detection as described previously (Donzanti and Yamamoto 1988; Ding et al. 2012, 2013; Deehan et al. 2015). Precolumn glutamate derivatization was carried out with o-phthalaldehyde and performed using an ESA Model 542 autosampler (ESA, Inc., Chelmsford, MA). Mobile phase was composed of 35% MeOH and 100 mM Na2HPO4 • 7H2O with a pH of 6.75. Samples were injected onto a Hypersil ODS C18 column (150 mm x 2.1 mm, 3 μm, Thermo Fisher Scientific, Waltham, MA). Separation and detection of glutamate was done with an amperometric detector (BAS LC-4C, Bioanalytical Systems, Inc., West Lafayette, IN) with the oxidation potential of 550 mV and sensitivity of 0.2 μA. Glutamatergic signal analysis was again determined with ChromePerfect data system (Justice Innovations, Inc., Palo Alto, CA).
EtOH and/or NIC microinjections, tissue preparation, and BDNF ELISA
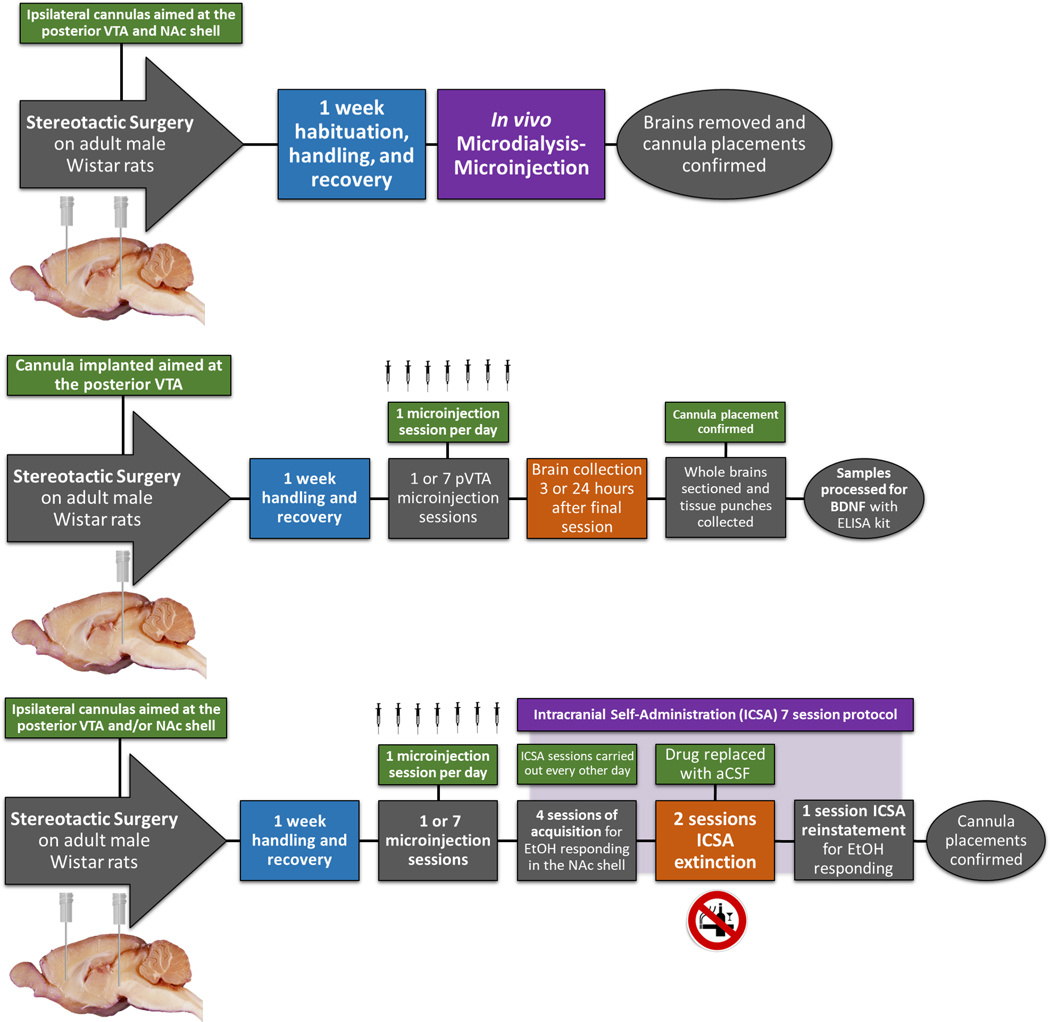
A separate cohort of male Wistar rats were stereotaxically implanted with a guide cannula aimed at the pVTA. The same passive microinjection protocol and EMIT units outlined in the microinjection-microdialysis procedure were used to administer aCSF, EtOH only, NIC only or combinations of EtOH and NIC directly to the pVTA (n = 77). Additionally, rats either underwent a single (acute) microinjection session or one microinjection session every day (repeated) for seven consecutive days with n = 4–8/group (Fig. 1, Top Panel). Single session treatment groups include aCSF, 100 mg% EtOH, 150 mg% EtOH, 10 μM NIC, 50 μM NIC, 100 mg% EtOH+10 μM NIC, or 150 mg% EtOH+50 μM NIC. Repeated microinjection session groups consisted of the same treatments. Following pVTA pretreatment, rats were returned to home-cage for 3 or 24 hours.
Figure 1.
Illustration of experimental timelines (n = 351). Top panel: Experimental timeline (n = 57) which investigated the effect of 1 (acute) intra-pVTA microinjection sessions on NAc shell dopamine (n = 7–10 animals/group) and glutamate (n = 6–10 animals/group) levels. Treatment groups included aCSF control, 100 mg% EtOH, 150 mg% EtOH, 10 μM NIC, 50 μM NIC, 100 mg% EtOH+10 μM NIC, or 150 mg% EtOH+50 μM NIC. Middle panel: Experimental timeline which investigated the effect of 1 (acute) or 7 (repeated) intra-pVTA microinjection sessions on NAc shell BDNF protein levels (n = 77). Treatment groups included aCSF control, 100 mg% EtOH, 150 mg% EtOH, 10 μM NIC, 50 μM NIC, 100 mg% EtOH+10 μM NIC, or 150 mg% EtOH+50 μM NIC (n = 4–8 animals/group). Bottom panel: Experimental timeline (n = 166) to determine the effects of repeated microinjection sessions of aCSF control, 100 mg% EtOH, 10 μM NIC, 100 mg% EtOH+10 μM NIC, or 0.125 μg BDNF on EtOH reward in the NAc shell (n = 4–8 animals/group). Some animals were excluded for cannula placements outside the region of interest (n = 46) or technical complications (n = 5) during sample collection.
At the assigned time points, rats were deeply anesthetized with isoflurane and rapidly decapitated. Brains were removed immediately and flash frozen in isopentane on dry ice. Brains were stored at −80 °C until ready for analysis. Serial coronal sections at a thickness of 300 μm were collected with a freezing microtome. Micro-punches containing the ipsilateral NAc shell were obtained using a 1 mm diameter Harris micro-punch (Electron Microscopy Sciences, Hatfield, PA) as previously described (McBride et al. 2009; Ding et al. 2013). The tissue was immediately homogenized in 150 μl of ice-cold N-PER lysis buffer with Halt Protease inhibitor cocktail (Thermo Scientific, Waltham, MA). Samples were incubated at 4 °C for 20 minutes on a nutator followed by centrifugation at 10,000 x g for 20 minutes. Supernatants protein content was measured with the Pierce BCA Protein Assay Kit (Thermo Scientific, Waltham, MA). Protein concentrations were determined with 10 μl of lysate and 200 μl of working reagent on a microplate reader (Bio-Rad, Hercules, CA). Samples were analyzed in duplicate and absorbance values averaged. The levels of BDNF in the NAc shell was determined with a rat enzyme-linked immunosorbent assay (ELISA) kit for BDNF from RayBiotech, Inc. (Catalog # ELR-BDNF-2, Norcross, GA) according to the manufacturer’s guidelines. ELISA samples were run in duplicate and absorbance values normalized to total protein content.
EtOH and/or NIC microinjections and ICSA
Another cohort of male Wistar rats were stereotaxically implanted with microinjection cannulas placed ipsilaterally 1.0 mm dorsal to the pVTA as well as the NAc shell. The NAc shell coordinates for this experiment were different than those of the microdialysis study with +1.4 mm, ML +2.2 mm, DV −7.3 mm relative to bregma (Paxinos and Watson, 1998). The EMIT units and passive microinjection protocol were again utilized to administer aCSF, EtOH only, NIC only, or EtOH and NIC. Subjects were arbitrarily assigned to undergo one or seven microinjection sessions for aCSF, 100 mg% EtOH, 10 μM NIC, or 100 mg% EtOH+10 μM (n = 105). Following the final pretreatment session, rats were maintained in home-cage for 2 days prior to initiating ICSA testing for EtOH reward in the NAc shell (Fig. 1, Bottom Panel).
Standard two-lever operant chambers contained within sound attenuating boxes were used in all ICSA experiments as described previously (Rodd et al. 2004; Engleman et al. 2009; Hauser et al. 2014a; Toalston et al. 2014; Deehan et al. 2015; Truitt et al. 2015). The EMIT system was used to control microinfusions of drug or vehicle. On testing days, subjects had stylets removed and an airtight tank fitted with an injection cannula containing the designated infusate tightened onto the NAc shell guide cannula extending 1.0 mm past the end. A single, non-contingent administration of infusate was delivered during insertion of the injector to prime the system and avoid trapping air. ICSA test sessions occurred every other day and lasted 4 hours. The position of the active lever and inactive lever were counterbalanced between subjects but remained the same for individual rats. Throughout the 4-hour session, depression of the active lever, set to a fixed ratio-1 (FR1) schedule of reinforcement, delivered a 100 nl infusion over the course of 5 seconds followed by a 5 second timeout. During this period, responses on the active lever did not generate additional infusions but were recorded. Responses on the inactive lever were always recorded but did not cause an infusion. Subjects were arbitrarily assigned aCSF, 75 mg% EtOH, or 125 mg% EtOH to self-administer into the NAc shell with n = 6–10/pretreatment/EtOH concentration. Subjects received their respective doses of aCSF or EtOH during acquisition sessions 1 through 4. All subjects then received only aCSF during extinction sessions 5 and 6. The original concentration was then made available during session 7 for reinstatement. The vehicle control group received only infusions of aCSF for all seven sessions.
BDNF microinjections and ICSA
A final cohort of male Wistar rats were stereotaxically implanted with a single microinjection cannula aimed 1.0 mm dorsal to the NAc shell (+1.4 mm, ML +2.2 mm, DV −7.3 mm, 10° angle). Rats were single housed after surgery and allowed at least one week of recovery. During this time, rats were habituated to the testing chambers and handled daily. Microinjections of BDNF (0 or 0.125 μg) into the NAc shell were delivered using a PHD ULTRA syringe pump (Harvard Apparatus, Holliston, MA) in a volume of 1.0 μl administered over 2 minutes, with the injector left in place for an additional 2 minutes to allow sufficient diffusion. Recombinant human BDNF (Sigma-Aldrich, St. Louis, MO) was prepared in aCSF immediately prior to microinfusion. Doses of BDNF were chosen based on previously published reports (Lu et al. 2004; Vargas-Perez et al. 2014; Bobadilla et al. 2018; Haun et al. 2018).
Subjects were arbitrarily assigned to undergo one or seven microinfusion sessions for aCSF or BDNF (n = 61). Rats were then maintained in home cage for 2 days prior to beginning the ICSA protocol for EtOH reward in the NAc shell described for the previous experiment (Fig. 1, Bottom Panel). Rats were arbitrarily assigned to self-administer aCSF, 75 mg% EtOH, or 125 mg% EtOH with n = 6–8/pretreatment/EtOH concentration. During the first four sessions, subjects received their assigned doses of aCSF or EtOH. Only aCSF was available to self-infuse during sessions 5 and 6. During session 7, the original concentration of aCSF or EtOH was made available. The control group only received infusions of aCSF for all seven sessions.
Histology
Upon completion of the experiments, rats (n = 351) were euthanized and a 1% solution of bromophenol blue was infused into each cannula. Brains were quickly removed, frozen on dry ice, and stored at −80 °C until examined. Serial sections were collected with a thickness of 40 μm on a cryostat microtome for verification of microdialysis probe, microinjection, and/or ICSA placements. A blind code was assigned to each animal and maintained by the individual that carried out the experiments. Cannula placements were then determined by a second individual blinded to treatment groups to avoid any bias from the observer. Animals with cannula placements outside the ROI (n = 46) were excluded from the analysis.
Statistical Analysis
All analyses were carried out by an individual unaware of the treatment group assignments with a blind code to avoid potential bias. Microdialysis data are expressed as a percentage of basal extracellular dopamine and glutamate values to correct for subject-to-subject variability (Engleman et al. 2000). The Shapiro-Wilk test was used to determine the normality of data prior to parametric testing. Additionally, no data points were excluded following the Grubbs’ test for outliers. Basal baseline values for dialysate were calculated as the mean of the three samples collected prior to the challenge microinjection. The effects of pVTA EtOH, NIC, or EtOH+NIC microinjections on extracellular dopamine and glutamate were analyzed using a Time × Treatment mixed analysis of variance (ANOVA). Post-hoc comparisons to identify significance were Student’s two-tailed t for within group differences or Tukey’s b for between group differences. BDNF protein levels were normalized to total protein content for each sample and presented as a percentage of control treatments. Data were analyzed with mean BDNF pg/mg protein calculated for each treatment group. These values were then compared using a univariate ANOVA (p < 0.05). The ICSA data were analyzed with a Pretreatment × EtOH Dose × Session mixed ANOVA on the mean number of self-infusions during sessions 1–4. Additionally, responses on active and inactive levers were examined for each group with a Pretreatment × EtOH Dose × Session × Lever mixed ANOVA with repeated measures on session and lever. Post hoc tests were used when significant main effects were found (p < 0.05). Extinction was determined through comparison of active lever responses during sessions 4–6. Reinstatement was identified by comparing responses on the active lever during sessions 5–7. All analyses were carried out with IBM SPSS Statistics for Windows, Version 25.0. (IBM Corp., Armonk, NY).
The statistical procedures performed adhere to the algorithmic progression of data analysis recommended by Keppel and Zedeck (1989), Hayes (2005), Pedhazur (1998) and Jaccard and Becker (2010). Well-established statistical procedures were conducted as previously reported to determine relevant effects within the microdialysis and ICSA data sets (Keppel and Zedeck 1989; Toalston et al. 2014; Deehan et al. 2018; Waeiss et al. 2019). For example, significant interaction terms prevent further analysis of main effects (Keppel and Zedeck 1989; Hayes 2005; Pedhazur 1998; Jaccard and Becker 2010). Therefore, significant interaction terms were followed with the recommended decomposition by holding a single variable constant to examine the effects of the other independent variables on the dependent measure of the interaction term. Additional information and rationale on the statistical procedures utilized in the present study can be found in the supplemental materials.
Results
Microinjection-Microdialysis
Dopamine levels in the NAc shell dialysate had a mean of 1.31 ± 0.11 nM during baseline sample collection and were comparable to previous reports (Engleman et al. 2000; Ding et al. 2009; Deehan et al. 2018). Mean levels of glutamate during baseline were 1.42 ± 0.22 μM and are also consistent with past studies (Ding et al. 2016). Importantly, microdialysis can only provide an accurate assessment of extracellular neurotransmitter levels through a quantitative No-Net-Flux protocol that was not applied in the present study.
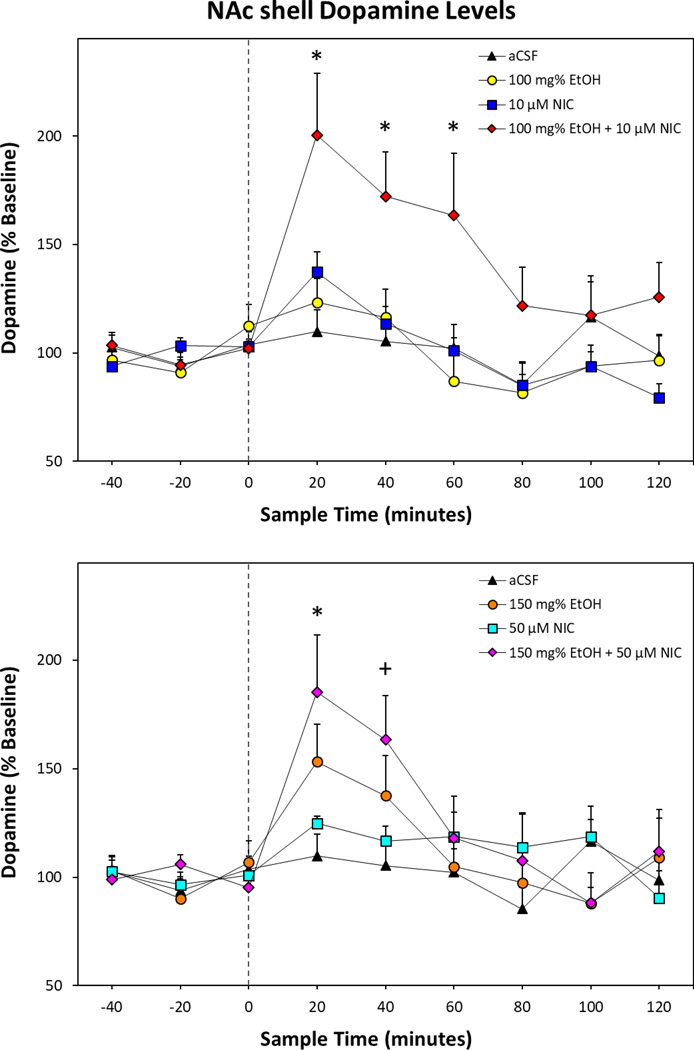
The overall analysis for stimulated dopamine release revealed a significant Time × Treatment interaction term (F48,400 = 2.38; p < 0.001; Fig. 2). Individual ANOVAs indicate a significant effect of Treatment during the first three sample periods following microinjection (F values > 2.49; p values < 0.04). Tukey’s b post-hoc comparisons found that only rats treated with combinations of EtOH and NIC (100 mg% EtOH+10 μM NIC; Fig. 2, Top Panel) and 150 mg% EtOH+50 μM NIC; Fig. 2, Bottom Panel) had significantly higher dopamine levels within the NAc shell than rats treated with aCSF during the first sample period. During the second and third 20-minute samples, rats treated with 100 mg% EtOH+10 μM NIC had significantly elevated dopamine levels compared to aCSF controls. Within-subject examination of individual microinjection groups revealed a significant effect of 150 mg% EtOH (F8,48 = 2.95; p = 0.009), 10 μM NIC (F8,56 = 7.15; p < 0.001), 50 μM NIC (F8,56 = 2.23; p = 0.038), 100 mg% EtOH+10 μM NIC (F8,72 = 9.12; p < 0.001), and 150 mg% EtOH+50 μM NIC (F8,48 = 6.51; p < 0.001). Compared to corresponding baseline levels, 10 μM NIC (t7 = 3.96, p = 0.005) and 50 μM NIC (t7 = 5.26, p = 0.001) significantly increased extracellular dopamine during the first sample to 137% and 125%, respectively. Additionally, 150 mg% EtOH and 150 mg% EtOH+50 μM NIC (t values > 2.61, p values < 0.04; Fig. 2, Bottom Panel) significantly increased dopamine levels over baseline during the first and second sample periods. Finally, post hoc comparisons of rats treated with 100 mg% EtOH+10 μM NIC indicated dopamine levels of the first three post microinjection samples were significantly higher than baseline (t values > 2.38, p values < 0.05; Fig. 2,Top Panel) at 201%, 172%, and 163%.
Figure 2.
Depicts the mean (+SEM) percent change in dopamine levels (n = 7–10 animals/group) within the NAc shell of rats receiving intra-pVTA infusions of EtOH and/or NIC. Top Panel: Mean (+SEM) dopamine levels for aCSF control, 100 mg% EtOH, 10 μM NIC, and 100 mg% EtOH+10 μM NIC. Bottom Panel: Mean (+SEM) dopamine levels for aCSF control, 150 mg% EtOH, 50 μM NIC, and 150 mg% EtOH+50 μM NIC. Treatment groups were analyzed together but presented separately for clarity. Asterisk (*) indicates significantly elevated dopamine levels in both EtOH+NIC treated groups compared to aCSF controls. Plus sign (+) indicates 150 mg% EtOH+50 μM NIC and 150 mg% EtOH treatment groups were significantly higher than respective baseline levels.
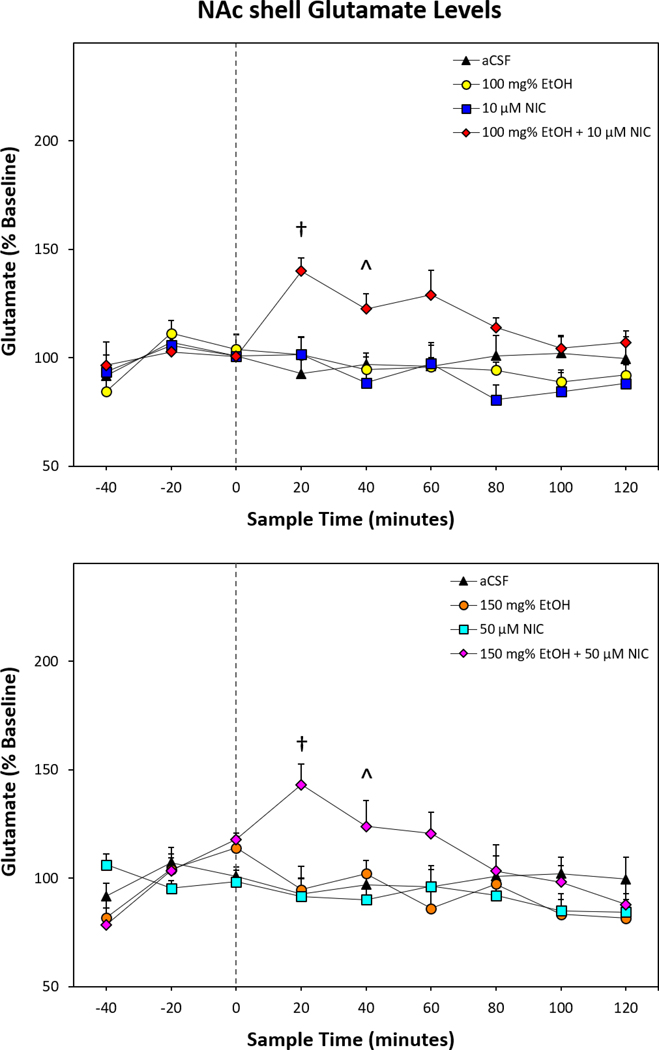
Repeated-Measure ANOVA of stimulated glutamate release indicated a significant effect of Time × Treatment interaction (F48,376 = 2.28; p < 0.001; Fig. 3). Reducing the interaction term to individual ANOVAs performed for each sample time period revealed significant differences between the treatment groups during the first four samples collected post-microinjection (F values > 2.52; p values < 0.04). Tukey’s b post hoc comparisons indicated during the first 20-minute sample period that rats microinjected with combinations of EtOH and NIC had significantly elevated glutamate levels compared to all other treatment groups. During the second 20-minute time period following microinjections, post hoc comparisons revealed glutamate levels in the NAc shell were higher in rats treated with combinations of EtOH and NIC compared to rats that received 10 μM NIC or 50 μM NIC. The third 20-minute time period following microinjections indicated glutamate levels in the NAc shell were elevated only in rats treated with 100 mg% EtOH+10 μM NIC compared to rats that received 150 mg% EtOH. The fourth post-microinjection sample collected indicated that glutamate levels were higher in rats that received 100 mg% EtOH+10 μM NIC compared to the 10 μM NIC treatment group. Within-subject examination of individual microinjection treatments revealed a significant effect of 100 mg% EtOH+10 μM NIC (F8,64 = 4.48; p < 0.001; Fig. 3, Top Panel) and 150 mg% EtOH+50 μM NIC (F8,40 = 7.88; p < 0.001; Fig. 3, Bottom Panel). Post hoc comparisons indicated glutamate levels within the NAc shell of the first post microinjection sample were significantly greater than baseline (t values > 3.97, p values < 0.003) at 140% and 143%.
Figure 3.
Depicts the mean (+SEM) percent change in glutamate levels (n = 6–10 animals/group) within the NAc shell of rats receiving intra-pVTA infusions of EtOH and/or NIC. Top Panel: Mean (+SEM) glutamate levels for aCSF control, 100 mg% EtOH, 10 μM NIC, and 100 mg% EtOH+10 μM NIC. Bottom Panel: Mean (+SEM) glutamate levels for aCSF control, 150 mg% EtOH, 50 μM NIC, and 150 mg% EtOH+50 μM NIC. Treatment groups were analyzed together but presented separately for clarity. Dagger (†) indicates significantly higher glutamate levels in both EtOH+NIC treated groups than all other treatment groups. Carrot (^) indicates significantly greater glutamate levels in both EtOH+NIC treated groups than those treated with 10 μM NIC or 50 μM NIC.
NAc shell BDNF following intra-pVTA drug pretreatment
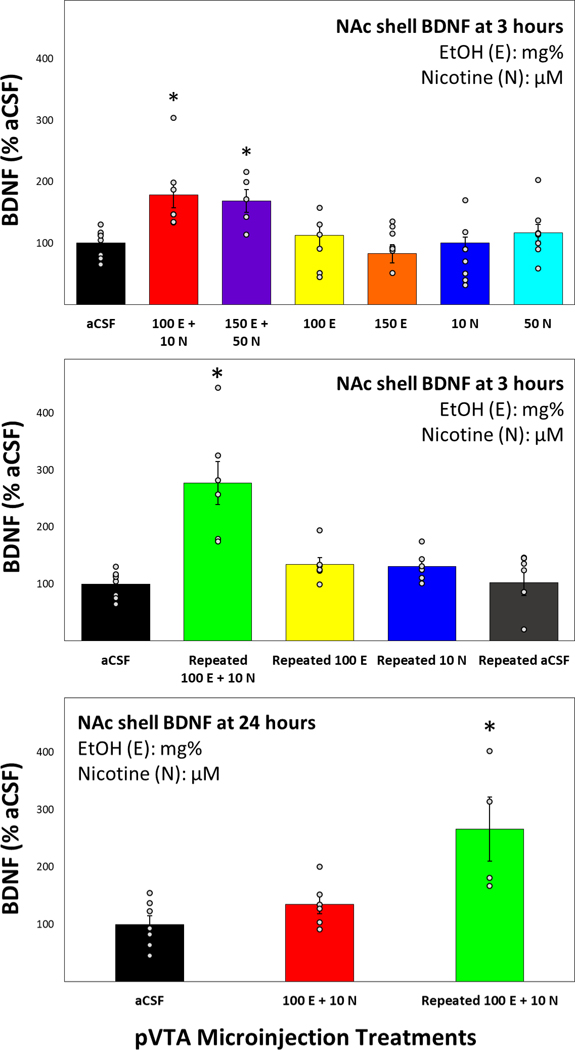
Previous research has demonstrated significant changes to neurotrophic gene expression within the NAc shell following microinjections of EtOH and NIC directly into the pVTA. In order to expand on these results, an ELISA kit was used to assess BDNF protein levels in the NAc shell of rats treated with sub- and suprathreshold doses of EtOH and/or NIC within the pVTA. The results indicate that only combinations of EtOH and NIC microinjected directly into the pVTA significantly increased BDNF protein in the NAc shell compared to aCSF 3 hours following treatment (F6,43 = 4.66, p = 0.001; Fig. 4, Top Panel). Microinjection of subthreshold or suprathreshold EtOH+NIC increased NAc shell BDNF to 179% and 168% of aCSF levels, respectively. Microinjections of EtOH only and NIC only showed no change to BNDF levels. The next experiment aimed to determine whether the significantly elevated BDNF is sustained over repeated exposure to EtOH+NIC or was a transient phenomenon. Repeated microinjection sessions of subthreshold EtOH+NIC into the pVTA were found to consistently increase the protein expression of BDNF in the NAc shell 3 hours after the final session over both acute and repeated aCSF treatments (F4,26 = 3.11, p = 0.032; Fig. 4, Middle Panel). Next, BDNF levels in the NAc shell were determined 24 hours after both acute and repeated microinjection sessions of EtOH+NIC. The data indicated that repeated microinjections of EtOH+NIC into the pVTA resulted in a lasting elevation of BDNF in the NAc shell at 24 hours that was not present after a single administration of EtOH+NIC (F2,21 = 11.37, p < 0.001; Fig. 4, Bottom Panel).
Figure 4.
Top Panel: Mean (+SEM) BDNF levels in the NAc shell relative to aCSF controls 3 hours following a single microinjection session into the pVTA (n = 5–8 animals/group). Middle Panel: Mean (+SEM) BDNF levels in the NAc shell relative to aCSF controls 3 hours following 7 microinjection sessions into the pVTA (n = 6–8 animals/group). Bottom Panel: Mean (+SEM) BDNF levels in the NAc shell relative to aCSF controls 24 hours following 1 or 7 microinjection sessions into the pVTA (n = 4–6 animals/group). Asterisk (*) indicates significantly greater BDNF levels compared to aCSF controls.
EtOH reward in the NAc shell following intra-pVTA drug pretreatment
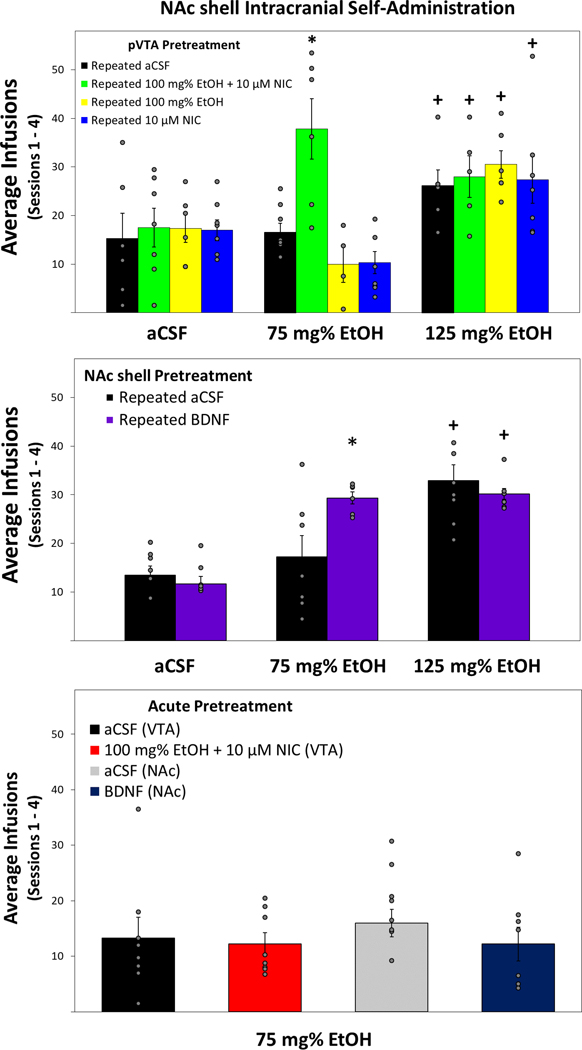
A mixed ANOVA was conducted on the average number of infusions received during the first four acquisition ICSA sessions of rats pretreated with intra-pVTA EtOH and/or NIC (Fig. 5, Top Panel). The analysis revealed a significant Pretreatment × EtOH Dose interaction (F6,77 = 3.89, p = 0.002). Reducing the interaction term was performed by examining the average number of infusions for each EtOH dose. Rats allowed to self-administer aCSF or 125 mg% EtOH showed no effect of pretreatment on the number of infusions received (F values < 0.59; p values > 0.63). Conversely, there was a significant difference in rats allowed to self-administer a subthreshold EtOH dose of 75 mg% between rats pretreated with repeated EtOH+NIC and those pretreated with repeated aCSF, EtOH only, or NIC only (F3,26 = 9.15, p < 0.001). Tukey’s b post hoc indicated that rats pretreated with repeated EtOH+NIC received significantly more self-infusions than all other groups (Fig. 5, Top Panel). In rats pretreated with repeated aCSF, EtOH, or NIC, there was a significant effect of EtOH dose on the number of self-infusions (F values > 6.27; p values < 0.01). Post hoc comparisons revealed that rats pretreated with repeated aCSF, EtOH, or NIC self-infused significantly more of the 125 mg% EtOH suprathreshold dose compared to infusions of aCSF or subthreshold 75 mg% EtOH. In rats pretreated with repeated EtOH+NIC, there was also a significant effect of EtOH dose on the number of self-infusions (F2,16 = 9.56, p = 0.002). Interestingly, post hoc comparisons indicated that rats given repeated EtOH+NIC pretreatment self-infused significantly more 125 mg% EtOH as well as the subthreshold 75 mg% EtOH dose compared to aCSF self-infusions.
Figure 5.
Top Panel: Mean (+SEM) number of self-infusions of EtOH during the first 4 ICSA sessions by rats pretreated with 7 microinjection sessions of aCSF, EtOH, NIC, or EtOH+NIC (n = 4–8 animals/group). Middle Panel: Mean (+SEM) number of self-infusions of EtOH during the first 4 ICSA sessions by rats pretreated with 7 microinjection sessions of aCSF or BDNF (n = 6–8 animals/group). Bottom Panel: Mean (+SEM) number of self-infusions of a subthreshold EtOH dose during the first 4 ICSA sessions by control rats pretreated with a single microinjection session of aCSF, EtOH+NIC, or BDNF (n = 8 animals/group). Asterisk (*) indicates significantly more self-infusions of 75 mg% EtOH compared to all other pretreatment groups. Plus sign (+) indicates significantly more self-infusions than corresponding pretreatment groups allowed to self-administer aCSF.
The number of active and inactive lever responses across the 7 ICSA sessions were examined and revealed a significant Pretreatment × EtOH Dose × Lever × Session interaction term (F36,456 = 3.90, p < 0.001; Supplementary Figs. 1–4). Overall analysis of inactive lever responses indicated no significant interaction Pretreatment × EtOH Dose × Session interaction (F36,456 = 0.83, p = 0.743). However, analysis of active lever responses revealed a significant Pretreatment × EtOH Dose × Session interaction (F36,456 = 2.69, p < 0.001). Further investigation of active lever responses by all pretreatment groups allowed to self-administer aCSF or 125 mg% EtOH into the NAc shell did not indicate significant interactions of Pretreatment × Session (F values < 0.73, p > 0.774). Examining active lever responses by all pretreatment groups allowed to self-administer 75 mg% EtOH revealed a significant Pretreatment × Session interaction (F18,156 = 5.27, p < 0.001). Performing individual ANOVAs on active lever responses for 75 mg% EtOH in each session indicated significant differences for all sessions (F3,26 values > 5.09, p < 0.007) except during extinction sessions 5 and 6 (F3,26 values < 1.09, p > 0.371). Tukey’s b post hoc analysis revealed that rats pretreated with repeated EtOH+NIC responded significantly more on the active lever for 75 mg% EtOH than all other groups during sessions 1–4 and 7.
EtOH reward in the NAc shell following repeated intra-NAc BDNF pretreatment
Next, a mixed ANOVA was conducted on the average number of infusions received over the first four ICSA sessions of rats pretreated with intra-NAc shell BDNF or aCSF (Fig. 5, Middle Panel). The analysis revealed a significant interaction of Pretreatment × EtOH Dose (F2,39 = 5.11, p = 0.011). The interaction term was then reduced by examining the average number of infusions for each EtOH dose. There was no effect of pretreatment found on number of infusions received by rats allowed to self-infuse aCSF or 125 mg% EtOH (F values < 0.74, p values > 0.406). However, there was a significant difference found in rats allowed to self-administer the subthreshold dose of 75 mg% EtOH between those pretreated with repeated BDNF and rats that received repeated aCSF (F1,11 = 6.05, p = 0.032). Tukey’s b post hoc analysis revealed rats pretreated with BDNF had significantly more self-infusions than aCSF pretreated rats. The Pretreatment × EtOH Dose interaction term was then reduced by investigating the average number of infusions for each pretreatment. In rats that received repeated intra-NAc shell infusions of aCSF there was a significant effect of EtOH dose on the number of self-infusions (F2,20 = 10.60, p = 0.001). Post hoc comparisons indicated that rats pretreated with repeated aCSF within the NAc shell self-infused significantly more 125 mg% EtOH compared to infusions of aCSF or subthreshold 75 mg% EtOH. Rats that underwent repeated BDNF infusions also showed a significant effect of EtOH dose on the number of self-infusions (F2,19 = 64.84, p < 0.001). Tukey’s b post hoc analysis revealed that unlike repeated aCSF pretreatment, repeated BDNF pretreated rats received significantly more self-infusions for both the subthreshold 75 mg% EtOH dose as well as the suprathreshold dose of 125 mg% EtOH.
Responses across the 7 ICSA sessions on the active and inactive levers revealed a significant overall interaction term of Pretreatment × EtOH Dose × Lever × Session (F12,228 = 2.26, p = 0.01; Supplementary Figs. 5 and 6). Reducing this interaction with analysis of inactive lever responses indicated no significant effect of Pretreatment × EtOH Dose × Session (F12,228 = 1.39, p = 0.173). Next, active lever responses were examined and further analysis indicated a significant Pretreatment × EtOH Dose × Session interaction (F12,228 = 2.19, p = 0.013). Additional analysis of active lever responses by rats allowed to self-administer aCSF into the NAc shell did not indicate a significant interaction of Pretreatment × Session (F6,84 = 0.61, p = 0.723). However, examination of active lever responses by BDNF or aCSF pretreated groups allowed to self-administer 75 mg% EtOH or 125 mg% EtOH revealed significant Pretreatment × Session interactions (F6,66 = 2.77, p = 0.021 and F6,78 = 2.64, p = 0.022; respectively). Though rats pretreated with repeated BDNF had higher mean responses on the active lever than those pretreated with aCSF during all sessions, individual ANOVAs performed on active lever responses for 75 mg% EtOH revealed only statistically significant differences during sessions 2 and 7 (F values > 12.07, p values < 0.005). Analysis of active lever responses for 125 mg% EtOH indicated session 5 was significantly different between BDNF and aCSF pretreated groups.
A final analysis aimed to determine whether single microinjection sessions of intra-NAc shell BDNF, intra-pVTA 100 mg% EtOH+10 μM NIC, and corresponding aCSF controls altered subthreshold EtOH self-administration within the NAc shell (Fig. 5, Bottom Panel). The results indicate no significant difference between pretreatment groups and self-infusion rates at similar levels to control groups from previous experiments (F3,28 = 1.32, p = 0.287; Fig. 5).
Discussion
The results of the present study indicate that co-administration of EtOH+NIC directly into the pVTA induced a unique neurochemical response within the NAc shell ( Figs. 2–4). A single intra-pVTA microinjection session of EtOH+NIC produced significant increases in dopamine, glutamate, and BDNF that were not present for either drug alone. Moreover, repeated exposure to EtOH+NIC resulted in a significant elevation of endogenous BDNF in the NAc shell lasting at least 24 hours that was not seen after a single pretreatment session. This repeated intra-pVTA exposure to EtOH+NIC also produced an enhancement of EtOH reward within the NAc shell (Fig. 5). Repeated microinfusions of exogenous BDNF into the NAc shell recapitulated the increased sensitivity to the reinforcing properties of EtOH within the NAc shell produced by intra-pVTA pretreatment with EtOH+NIC. Collectively, these results indicate that co-administration of EtOH+NIC interacts within the pVTA to generate distinct responses and subsequent neuroadaptations of the NAc shell via BDNF that augment EtOH reward in that region.
It is well established that exposure to EtOH or NIC elevates VTA neuronal activity and is associated increased dopamine release within the NAc shell (Di Chiara and Imperato 1988; Lecca et al. 2006; Ding et al. 2009; Robinson et al. 2009). Furthermore, combinations of EtOH and NIC have been shown to interact within the VTA to enhance dopaminergic activity and increase NAc shell dopamine release beyond the levels of either drug alone (Fig. 2; Clark and Little 2004; Tizabi et al. 2002, 2007). The mechanisms responsible for the exaggerated dopamine response produced by EtOH+NIC within the pVTA could be through interactions with shared neurotransmitter systems in addition to independent actions. A specific site of interaction between nicotine and EtOH within the pVTA occurs at the α6-containing nicotinic receptors, which modulate that activity of AMPA receptor function (Engle et al. 2015). Other neuronal nicotinic acetylcholine receptors (nAChRs) have been investigated as sites contributing to the disproportionate co-use of EtOH and NIC (Tizabi et al. 2002, 2007; Van Skike et al. 2016; Adams 2017). Within the VTA, nAChRs are expressed on dopamine, GABA, and glutamate neurons and the distinct subunit combinations alter channel properties as well as agonist binding. Importantly, the high-affinity α4β2 is most densely expressed on dopaminergic VTA neurons while the α7-homomeric nAChRs are primarily located on excitatory glutamatergic terminals that synapse on dopamine neurons. Both of which have been demonstrated to increase dopamine signaling via activation by EtOH and/or NIC (reviewed in Morel et al. 2018). Evidence suggests that EtOH may potentiate the actions of most nAChRs while inhibiting nicotine-induced receptor desensitization (Aistrup et al. 1999; Marszalec et al. 1999). Furthermore, another member of the Cys-loop ion channel superfamily, the serotonin-3 (5-HT3) receptor, has been implicated as a convergent site of action for EtOH and NIC (Lovinger and White 1991; Breitinger et al. 2001; Rodd et al. 2007, 2010; Hauser et al. 2014b). EtOH increases the excitatory action of 5-HT3 receptor activation and promotes stimulated dopamine release within the mesolimbic system (Campbell et al. 1996; Rodd et al. 2010). Together, evidence suggests intra-pVTA co-administration of EtOH and NIC interact and promote enhanced dopamine release within the NAc shell over either drug alone through convergent actions on specific nAChRs and 5-HT3 receptors.
It has been suggested that glutamatergic inputs of the NAc play a critical role in the initiation and learning of drug-seeking behaviors (Spencer et al. 2016). These projections into the NAc are involved in stimuli response to previously established associations and cues (Scofield et al. 2016). Several studies have demonstrated alterations in basal extracellular glutamate levels or clearance rates within the NAc following both voluntary and forced chronic exposure to EtOH and/or NIC (Fig. 3; Reid et al. 2000; Liechti et al. 2007; Lallemand et al. 2011; Ding et al. 2013; Griffin et al. 2014; Carcoba et al. 2018). The significant increase seen here of stimulated glutamate release within the NAc shell of only naïve rats exposed to intra-pVTA EtOH+NIC suggests a transient alteration in post-synaptic glutamate signaling. The prior studies examining NAc shell basal glutamate levels demonstrated changes in glutamatergic regulatory genes or proteins following chronic, repeated exposure or withdrawal conditions providing support for the reported results (Ding et al. 2013; Griffin et al. 2014; Carcoba et al. 2018). The present study reveals a response to EtOH+NIC under acute conditions by naïve rats suggesting a unique pVTA activation profile that occurs immediately and is a distinct response than either drug alone. The simultaneous increase of both dopamine and glutamate, specific to EtOH+NIC, provides some explanation for the subsequent gene changes and neurochemical alterations within the NAc shell found in previous work (Deehan et al. 2015; Truitt et al. 2015).
Co-administration of both EtOH+NIC combinations into the pVTA results in significantly increased BDNF protein in the NAc shell at three hours post-treatment (Fig. 4). Repeated exposure to EtOH+NIC was found to result in BDNF increases lasting more than 24 hours, which was not observed following equivalent treatment of EtOH or NIC alone (Fig. 4). These results are consistent with mounting research implicating a role for BDNF in the regulation of drug-related behaviors (Fig. 5; Lu et al. 2004; Logrip et al. 2009; Vargas-Perez et al. 2014; Bobadilla et al. 2018; Haun et al. 2018).
BDNF is part of the neurotrophin family and via binding with tropomyosin receptor kinase B (TrkB) promotes neuronal development, modulates synaptic function, and regulates synaptic plasticity (Park and Poo 2013; Nakahata and Yasuda 2018). A number of studies have examined BDNF in response to acute exposure, seeking, dependence, and relapse to EtOH or NIC (Logrip et al. 2015; Machaalani and Chen 2018). However, preclinical research has demonstrated a relationship between BDNF expression and drug-related changes in the brain that is dependent upon a variety of factors including duration of exposure, drug dose, and ROI (Lu et al. 2004; Logrip et al. 2009; Vargas-Perez et al. 2014; Bobadilla et al. 2018; Haun et al. 2018). In order to determine whether the associated neurochemical changes in the current study had any significant impact on reward-related behaviors, rats were allowed to self-administer EtOH into the NAc shell following single or repeated intra-pVTA microinjections of aCSF, EtOH, NIC, or EtOH+NIC. EtOH reward in the NAc shell was only significantly altered in rats pretreated with EtOH+NIC in the pVTA. Specifically, the average number of self-infusions for 75 mg% EtOH during the first 4 sessions by aCSF, EtOH, or NIC pretreated rats was 10–17 infusions/session. In contrast, rats pretreated with EtOH+NIC self-infused 75 mg% EtOH significantly more with an average of almost 38/session into the NAc shell. The apparent shift in sensitivity to EtOH reward within the NAc shell suggests pVTA co-exposure to EtOH+NIC results in unique alterations with the mesolimbic dopamine system.
Rats pretreated with repeated BDNF were the only group that readily self-infused 75 mg% EtOH into the NAc shell. Single infusion sessions of aCSF, BDNF, or EtOH+NIC did not alter sensitivity to EtOH reward. Additionally, there was no effect of repeated BDNF pretreatment on self-infusions for aCSF or 125 mg% EtOH, a dose known to be reinforcing within the NAc shell. Taken together, the data indicate that direct pretreatment of the NAc shell with exogenous BDNF is sufficient to recapitulate previous results demonstrating repeated exposure to EtOH+NIC within the pVTA increases the sensitivity to the rewarding properties of EtOH in the NAc shell.
Though the current experiments provide insight into a potential mechanism underlying the enhanced EtOH reward following repeated exposure to EtOH+NIC, there are some important limitations. It is critical to note that administration of EtOH or NIC directly into the NAc shell, or systemically, can also enhance local dopamine release through independent mechanisms (Tizabi et al. 2002, 2007; Kleijn et al. 2011; Jonsson et al. 2014). Through the isolation of the mesolimbic pathway with intra-pVTA microinjections and NAc shell microdialysis, the contribution of other circuits to the neurochemical response and altered behavior has been limited. Future work involving systemic drug administration is needed to better delineate the relationship of EtOH+NIC exposure and enhanced EtOH reward. Additionally, one caveat of these studies is that the elevation of BDNF within the NAc shell following microinjections of EtOH+NIC into the pVTA was not blocked directly through pharmacological manipulation. Thus, although BDNF infusions into the NAc shell mimicked the effects of intra-pVTA EtOH+NIC and enhanced EtOH reinforcement in the NAc shell, other factors could be involved in mediating the observed effects.
Collectively, the results of the present study support previous work demonstrating that co-administration of EtOH+NIC produces a unique initial physiological response which facilitates the development of neuroadaptations within the mesolimbic reward pathway ( Figs. 2–4; Tizabi et al. 2002, 2007; Truitt et al. 2015). Multiple reports have indicated that naltrexone and varenicline, the pharmacological ‘gold standards’ for treating AUD and nicotine dependence, both fail to alter the concurrent co-administration of EtOH+NIC (Van Skike et al. 2016; Waeiss et al. 2018; Maggio et al. 2018). The current results provide clear preclinical data indicating that EtOH+NIC co-administration results in a unique neurological cascade that is not observed following comparable exposure to EtOH or NIC alone. Evaluating potential pharmacotherapeutics for the treatment of AUD or nicotine dependence using a single drug of abuse fails to reflect the reality of how humans ingest EtOH and NIC. This method of investigating individual drugs of abuse is likely a major reason that efficacious treatments for these substance disorders has not occurred. Future research should focus on co-exposure models to better elucidate the specific effects of EtOH+NIC within the CNS if better pharmacotherapeutics are to be developed.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Alcohol Abuse and Alcoholism (NIAAA) grants AA007462, AA024612, AA020396, and AA012262.
Abbreviations used:
- 5-HT
serotonin
- aCSF
artificial cerebrospinal fluid
- AUD
alcohol use disorder
- BDNF
brain-derived neurotrophic factor
- EMIT
electrolytic microinfusion transducer
- ELISA
enzyme-linked immunosorbent assay
- EtOH
ethanol
- HPLC
high-performance liquid chromatography
- ICSA
intracranial self-administration
- NAc
nucleus accumbens
- NIC
nicotine
- nAChR
nicotinic acetylcholine receptor
- PND
post-natal dat
- pVTA
posterior ventral tegmental area
- ROI
region of interest
- RRID
Research Resource Identifier (see scicrunch.org)
- Trk
tropomyosin receptor kinase
Footnotes
conflict of interest disclosure
The authors declare no conflicts of interest.
References
- Adams S (2017) Psychopharmacology of Tobacco and Alcohol Comorbidity: a Review of Current Evidence. Curr Addict Rep 4(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aistrup GL, Marszalec W, Narahashi T (1999) Ethanol modulation of nicotinic acetylcholine receptor currents in cultured cortical neurons. Mol Pharmacol 55(1):39–49. [DOI] [PubMed] [Google Scholar]
- Bell RL, Hauser S, Rodd ZA, Liang T, Sari Y, McClintick J, Rahman S, Engleman EA (2016) A Genetic Animal Model of Alcoholism for Screening Medications to Treat Addiction. Int Rev Neurobiol 126:179–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobadilla A, Garcia KC, Chareunsouk V, Hyde J, Camacho DM, Heinsbroek JA, Kalivas PW (2018) Accumbens brain-derived neurotrophic factor (bdnf) transmission inhibits cocaine seeking. Addict Biol [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo JK, Husten C (2000) Sociocultural influences on smoking and drinking. Alcohol Res Health 24:225–32. [PMC free article] [PubMed] [Google Scholar]
- Breitinger HG, Geetha N, Hess GP (2001) Inhibition of the serotonin 5-HT3 receptor by nicotine, cocaine, and fluoxetine investigated by rapid chemical kinetic techniques. Biochemistry 40(28):8419–29. [DOI] [PubMed] [Google Scholar]
- Campbell AD, Kohl RR, McBride WJ (1996) Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol 13(6):569–74. [DOI] [PubMed] [Google Scholar]
- Carcoba LM, Flores RJ, Natividad LA, O’Dell LE (2018) Amino acid modulation of dopamine in the nucleus accumbens mediates sex differences in nicotine withdrawal. Addict Biol 23(5):1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, Little HJ (2004) Interactions between low concentrations of ethanol and nicotine on firing rate of ventral tegmental dopamine neurones. Drug Alcohol Depend 75(2):199–206. [DOI] [PubMed] [Google Scholar]
- Deehan GJ, Hauser SR, Waeiss RA, Knight CP, Toalston JE, Truitt WA, McBride WJ, Rodd ZA (2015) Co-administration of ethanol and nicotine: the enduring alterations in the rewarding properties of nicotine and glutamate activity within the mesocorticolimbic system of female alcohol-preferring (P) rats. Psychopharmacology 232(23), 4293–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deehan GA Jr, Hauser SR, Getachew B, Waeiss RA, Engleman EA, Knight CP, McBride WJ, Truitt WA, Bell RL, Rodd ZA (2018) Selective breeding for high alcohol consumption and response to nicotine: locomotor activity, dopaminergic in the mesolimbic system, and innate genetic differences in male and female alcohol-preferring, non-preferring, and replicate lines of high-alcohol drinking and low-alcohol drinking rats. Psychopharmacology 235(9) 2755–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, McBride WJ. (2009) Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res 33: 1571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Engleman EA, Rodd ZA, McBride WJ (2012) Ethanol increases glutamate neurotransmission in the posterior ventral tegmental area of female wistar rats. Alcohol Clin Exp Res 36(4), 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. (2013) Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring ( P) rats. Addict Biol 18(2):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Ingraham CM, Rodd ZA, McBride WJ (2016) Alcohol drinking increases the dopamine-stimulating effects of ethanol and reduces D₂ auto-receptor and group II metabotropic glutamate receptor function within the posterior ventral tegmental area of alcohol preferring (P) rats. Neuropharmacology 109:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzanti BA, Yamamoto BK (1988) An improved and rapid HPLC-EC method for the isocratic separation of amino acid neurotransmitters from brain tissue and microdialysis perfusates. Life Sci 43(11), 913–922. [DOI] [PubMed] [Google Scholar]
- Engle SE, McIntosh JM, Drenan RM (2015) Nicotine and ethanol cooperate to enhance ventral tegmental area AMPA receptor function via α6-containing nicotinic receptors. Neuropharmacology 91:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, McBride WJ, Wilber AA, Shaikh SR, Eha RD, Lumeng L, Li TK, Murphy JM. (2000) Reverse microdialysis of a dopamine uptake inhibitor in the nucleus accumbens of alcohol-preferring rats: effects on dialysate dopamine levels and ethanol intake. Alcohol Clin Exp Res 24(6), 795–801. [PubMed] [Google Scholar]
- Engleman EA, Ingraham CM, McBride WJ, Lumeng L, Murphy JM (2006) Extracellular dopamine levels are lower in the medial prefrontal cortex of alcohol-preferring rats compared to Wistar rats. Alcohol 38:5–12. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Ding ZM, Oster SM, Toalston JE, Bell RL, Murphy JM, McBride WJ, Rodd ZA (2009) Ethanol is self-administered into the nucleus accumbens shell, but not the core: evidence of genetic sensitivity. Alcohol Clin Exp Res 33(12):2162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhöfel S (2006) An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health 29(3):162–71. [PMC free article] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li T-K (1994) Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol 11:557–564. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS (2015) Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72(8):757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS (2017) Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 74(9):911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC (2014) Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology 39(3):707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Grant BF (2015) The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol 50(11):1609–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NL, Skelly MJ, Grosserode EK, Lowes DC, Zeric T, Phister S, Salling MC (2017) Effects of acute alcohol on excitability in the CNS. Neuropharmacology 1;122:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun HL, Griffin WC, Lopez MF, Solomon MG, Mulholland PJ, Woodward JJ, McGinty JF, Ron D, Becker HC (2018) Increasing Brain-Derived Neurotrophic Factor (BDNF) in medial prefrontal cortex selectively reduces excessive drinking in ethanol dependent mice. Neuropharmacology 140, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Bracken AL, Deehan GA Jr, Toalston JE, Ding ZM, Truitt WA, Bell RL, McBride WJ, Rodd ZA (2014a) Selective breeding for high alcohol preference increases the sensitivity of the posterior VTA to the reinforcing effects of nicotine. Addict Biol 19(5), 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Deehan GA Jr, Toalston JE, Bell RL, McBride WJ, Rodd ZA (2014b) Enhanced alcohol-seeking behavior by nicotine in the posterior ventral tegmental area of female alcohol-preferring (P) rats: modulation by serotonin-3 and nicotinic cholinergic receptors. Psychopharmacology (Berl) 231(18), 3745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2005) Statistical methods for communication science. New York, New York. Lawrence Erlbaum Associates. [Google Scholar]
- Jaccard J, Becker MA (2010) Statistics for the behavioral sciences. Belmont, California. Wadsworth. [Google Scholar]
- John U, Meyer C, Rumpf HJ, Schumann A, Thyrian JR, Hapke U (2003) Strength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sample. Alcohol Alcohol 38(6):606–12. [DOI] [PubMed] [Google Scholar]
- Jonsson S, Adermark L, Ericson M, Söderpalm B (2014) The involvement of accumbal glycine receptors in the dopamine-elevating effects of addictive drugs. Neuropharmacology 82:69–75. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR (2006) Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 296(1):56–63. [DOI] [PubMed] [Google Scholar]
- Keppel G, Zedeck S (1989). A series of books in psychology. Data analysis for research designs: Analysis of variance and multiple regression/correlation approaches. New York, NY, US: W H Freeman/Times Books/ Henry Holt & Co. [Google Scholar]
- Kleijn J, Folgering JH, van der Hart MC, Rollema H, Cremers TI, Westerink BH (2011) Direct effect of nicotine on mesolimbic dopamine release in rat nucleus accumbens shell. Neurosci Lett 493(1–2):55–8. [DOI] [PubMed] [Google Scholar]
- Koob GF (2013) Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci 13:3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand F, Ward RJ, De Witte P, Verbanck P (2011) Binge drinking +/− chronic nicotine administration alters extracellular glutamate and arginine levels in the nucleus accumbens of adult male and female Wistar rats. Alcohol Alcohol 46(4):373–82. [DOI] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Gronli J, Spiga S, Di Chiara G (2006) Preferential increase of extracellular dopamine in the rat nucleus accumbens shell as compared to that in the core during acquisition and maintenance of intravenous nicotine self-administration. Psychopharmacology 184: 435–46. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A (2007) Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci 27(34):9077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D (2009) Escalating ethanol intake is associated with altered corticostriatal BDNF expression. J Neurochem 109(5):1459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Barak S, Warnault V, Ron D (2015) Corticostriatal BDNF and alcohol addiction. Brain Res 1628(Pt A):60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G (1991) Ethanol potentiation of 5-hydroxytryptamine 3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol 40(2):263–70. [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y (2004) A Single Infusion of Brain-Derived Neurotrophic Factor into the Ventral Tegmental Area Induces Long-Lasting Potentiation of Cocaine Seeking after Withdrawal. J Neurosci 24(7) 1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaalani R, Chen H (2018) Brain derived neurotrophic factor (BDNF), its tyrosine kinase receptor B (TrkB) and nicotine. Neurotoxicology 65:186–195. [DOI] [PubMed] [Google Scholar]
- Maggio SE, Saunders MA, Nixon K, Prendergast MA, Zheng G, Crooks PA, Dwoskin LP, Bell RL, Bardo MT (2018) An improved model of ethanol and nicotine co-use in female P rats: Effects of naltrexone, varenicline, and the selective nicotinic α6β2* antagonist r-bPiDI. Drug Alcohol Depend 193:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalec W, Aistrup GL, Narahashi T (1999) Ethanol-nicotine interactions at alpha-bungarotoxin-insensitive nicotinic acetylcholine receptors in rat cortical neurons. Alcohol Clin Exp Res 23(3):439–45. [PubMed] [Google Scholar]
- McBride WJ, Schultz JA, Kimpel MW, McClintick JN, Wang M, You J, Rodd ZA (2009) Differential effects of ethanol in the nucleus accumbens shell of alcohol-preferring (P), alcohol-non-preferring (NP) and Wistar rats: A proteomics study. Pharmacol Biochem Behav 92(2), 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Falba T, O’Malley SS, Sindelar J, O’Connor PG (2007) Smoking status as a clinical indicator for alcohol misuse in US adults. Arch Internal Med 167:716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH (1972) Drinking patterns during work-contingent and noncontingent alcohol acquisition. Psychosom Med 34(2):139–64. [DOI] [PubMed] [Google Scholar]
- Morel C, Montgomery S, Han MH. (2018) Nicotine and alcohol: the role of midbrain dopaminergic neurons in drug reinforcement. Eur J Neurosci [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motschman CA, Gass JC,Wray JM, Germeroth LJ, Schlienz NJ, Munoz DA,Moore FE, Rhodes JD, Hawk LW, Tiffany ST (2016) Selection criteria limit generalizability of smoking pharmacotherapy studies differentially across clinical trials and laboratory studies: a systematic review on varenicline. Drug Alcohol Depend 169:180–189. [DOI] [PubMed] [Google Scholar]
- Nakahata Y, Yasuda R (2018) Plasticity of Spine Structure: Local Signaling, Translation and Cytoskeletal Reorganization. Front Synaptic Neurosci 29;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals. (2011) The National Academies Collection: Reports funded by National Institutes of Health In Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academies Press (US) National Academy of Sciences. [Google Scholar]
- Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14(1):7–23. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) The Rat Brain in Stereotaxic Coordinates, 4th ed. Academic Press, New York. [Google Scholar]
- Pedhazur EJ (1997) Multiple regression in behavioral research (3rd ed.). Orlando, FL: Harcourt Brace. [Google Scholar]
- Reid MS, Fox L, Ho LB, Berger SP (2000) Nicotine stimulation of extracellular glutamate levels in the nucleus accumbens: neuropharmacological characterization. Synapse 35(2):129–36. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Howard EC, McConnell S, Gonzales RA, Wightman RM (2009) Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol Clin Exp Res 33(7):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Melendez RI, Kuc KA, Lumeng L, Li T-K, Murphy JM, McBride WJ. (2004) Comparison of Intracranial Self-Administration of Ethanol Within the Posterior Ventral Tegmental Area Between Alcohol-Preferring and Wistar Rats. Alcohol Clin Exp Res 28(8), 1212–1219. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Gryszowka VE, Toalston JE, Oster SM, Ji D, Bell RL, McBride WJ (2007) The reinforcing actions of a serotonin-3 receptor agonist within the ventral tegmental area: evidence for subregional and genetic differences and involvement of dopamine neurons. J Pharmacol Exp Ther 321(3):1003–12. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Oster SM, Toalston JE, Pommer TJ, McBride WJ, Murphy JM (2010) Serotonin-3 receptors in the posterior ventral tegmental area regulate ethanol self-administration of alcohol-preferring (P) rats. Alcohol 44(3):245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ (2000) Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology 149:217–224. [DOI] [PubMed] [Google Scholar]
- Saha TD, Grant BF, Chou SP, Kerridge BT, Pickering RP, Ruan WJ (2018) Concurrent use of alcohol with other drugs and DSM-5 alcohol use disorder comorbid with other drug use disorders: Sociodemographic characteristics, severity, and psychopathology. Drug Alcohol Depend 187:261–269. [DOI] [PubMed] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, Roberts-Wolfe D, Kalivas PW (2016) The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol Rev 68(3):816–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA (2010) Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 35, 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R (2009) Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev 89(2):649–705. [DOI] [PubMed] [Google Scholar]
- Spencer S, Scofield M, Kalivas PW (2016) The good and bad news about glutamate in drug addiction. J Psychopharmacol 30(11):1095–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisurapanont M, Jarusuraisin N (2005) Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol 8(2):267–80. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RJ, Louis VA, Taylor RE (2002) Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res 26(3), 394–399. [PubMed] [Google Scholar]
- Tizabi Y, Bai L, Copeland RJ, Taylor RE (2007) Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol 42(5), 413–416. [DOI] [PubMed] [Google Scholar]
- Toalston JE, Deehan GA Jr., Hauser SR, Engleman EA, Bell RL, Murphy JM, Truitt WA, McBride WJ, Rodd ZA (2014) Reinforcing properties and neurochemical response of ethanol within the posterior ventral tegmental area are enhanced in adulthood by periadolescent ethanol consumption. J Pharmacol Exp Ther 351: 317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt WA, Hauser SR, Deehan GJ, Toalston JE, Wilden JA, Bell RL, McBride WJ, Rodd ZA (2015) Ethanol and nicotine interaction within the posterior ventral tegmental area in male and female alcohol-preferring rats: evidence of synergy and differential gene activation in the nucleus accumbens shell. Psychopharmacology 232(3), 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Skike CE, Maggio SE, Reynolds AR, Casey EM, Bardo MT, Dwoskin LP, Prendergast MA, Nixon K (2016) Critical needs in drug discovery for cessation of alcohol and nicotine polysubstance abuse. Prog Neuropsychopharmacol Biol Psychiatry 65:269–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Perez H, Bahi A, Bufalino MR, Ting-A-Kee R, Maal-Bared G, Lam J, Fahmy A, Clarke L, Blanchard JK, Larsen BR, Steffensen S, Dreyer J-L, van der Kooy D (2014) BDNF signaling in the VTA links the drug-dependent state to drug withdrawal aversions. J Neurosci 34(23) 7899–7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeiss RA, Knight CP, Hauser SR, Pratt LA, McBride WJ, Rodd ZA (2019) Therapeutic challenges for concurrent ethanol and nicotine consumption: naltrexone and varenicline fail to alter simultaneous ethanol and nicotine intake by female alcohol-preferring (P) rats. Psychopharmacology (Berl) doi: 10.1007/s00213-019-5174-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeiss RA, Knight CP, Carvajal GB, Bell RL, Engleman EA, McBride WJ, Hauser SR, Rodd ZA (2019) Peri-adolescent alcohol consumption increases sensitivity and dopaminergic response to nicotine during adulthood in female alcohol-preferring (P) rats: Alterations to α7 nicotinic acetylcholine receptor expression. Behav Brain Res 30;376:112190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Pilver CE, Hoff RA, Mazure CM, McKee SA (2013) Changes in smoking for adults with and without alcohol and drug use disorders: longitudinal evaluation in the US population. Am J Drug Alcohol Abuse 39, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2018) Global status report on alcohol and health 2018. http://www.who.int/substance_abuse/publications/global_alcohol_report/en/ (Accessed May 1, 2019).
- Yadav AK, Velaga NR (2019) Laboratory analysis of driving behavior and self-perceived physiological impairment at 0.03%, 0.05% and 0.08% blood alcohol concentrations. Drug Alcohol Depend 17;205:107630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.