Abstract
The abilities of a parent and mutant pair of Staphylococcus epidermidis strains, the slime-producing parent RP62A and its slime-negative mutant, to establish endocarditis in a rabbit model of aortic valve endocarditis and to accumulate and adhere to surfaces in vitro were compared. Vegetation titer and infection rate depended on the presence or absence of a catheter (P = 0.020) and on inoculum size (P < 0.001) but not on the infecting strain. The ability of the parent strain vis-à-vis its mutant to accumulate in vitro on surfaces as demonstrated in a slime test did not correlate with any enhancement in the development of endocarditis in the rabbit model. In vitro initial adherence rates were identical. Both isolates accumulated to the same reduced extent in vitro in the presence of serum, albumin, or gelatin. Adhesion was equally promoted by addition of fibronectin. These data suggest that the in vitro phenomenon of accumulation described as slime production in the absence of serum may not be an important virulence determinant in vivo.
Coagulase-negative staphylococci (CoNS), especially Staphylococcus epidermidis, are important causes of foreign-body infections (13, 22). It is generally assumed that the ability of S. epidermidis to adhere to and grow on polymer surfaces is related to production of an extracellular slime substance (2, 3, 20, 21). Series of reports claim an association between attachment to medical devices, slime production, and the pathogenesis of infection with CoNS of patients with indwelling medical devices (4, 12, 30). We define adherence as the initial step that allows the bacteria to anchor to the foreign body. Initial adherence may or may not be followed by an accumulation step that permits the formation of multilayers of colonies embedded in the glycocalyx or slime, anchored to the foreign-body surface. It has been speculated that the difficulty in eradicating CoNS foreign-body infections is due to slime production and that slime production can be used as a marker of pathogenicity (6). However, other reports claim that adherence and slime production play little or no role in the clinical outcome of infections (7, 14, 15, 19, 28). Fibronectin has been shown to be one of the major targets of Staphylococcus aureus in traumatized tissue, and microbial adhesion to fibronectin in vitro correlates with the production of endocarditis in rabbits (23). Unlike with S. aureus, interaction of CoNS with host-derived adhesins has not been well characterized. S. epidermidis binds to fibronectin-coated biomaterial but less avidly than S. aureus. It also recognizes the 29-kDa N-terminal fibronectin fragment that contains the primary S. aureus binding domain as well as laminin, vitronectin, and collagen (10, 26, 27).
We investigated the role of slime as a virulence factor in a rabbit model of aortic valve endocarditis with a parent and mutant pair of S. epidermidis strains that differ in their abilities to produce slime in vitro: slime-positive RP62A (3) and its slime-negative, adhesion-positive, accumulation-negative mutant M7 (11, 24). Whether pathogenicity in vivo correlated with slime production in vitro was examined. The abilities of the strains to bind to microtiter wells in the absence and presence of serum or proteins in serum and their growth kinetics were compared.
MATERIALS AND METHODS
Bacterial strains.
S. epidermidis RP62A (ATCC 35984) was kindly provided by G. Christensen, University of Missouri, Columbia. This strain is a known slime producer (3). The mutant strain M7 was obtained after chemical mutagenesis of S. epidermidis RP62A. The strains have been extensively characterized previously (11, 24).
Growth conditions and assessment of initial adherence.
Initial bacterial adherence was assessed by ATP bioluminescence (17). Briefly, bacteria grown on blood agar (Oxoid, Wesel, Germany) for 18 h at 37°C were suspended in phosphate-buffered saline (PBS; pH 7.2), adjusted to a final concentration of approximately 109 CFU/ml, and enumerated by colony counts on blood agar. Aliquots (200 μl) were placed into the wells of 96-well polystyrene tissue culture plates. All incubation steps were performed at room temperature. The plates were either directly incubated with the bacterial suspension as a blank or incubated after being coated with albumin (50 mg/ml), gelatin (20 mg/ml; Serva, Heidelberg, Germany), or pooled human serum (20%) in PBS for 1 h to suppress nonspecific adhesion. The coating solutions were then carefully aspirated, a fibronectin solution (20 μg/ml; Serva) was added for a further hour, and the mixtures were again aspirated. The bacterial suspension was carefully layered on blank or coated plates, which were then incubated for 90 min. Thereafter, the wells were washed three times with PBS. Two hundred microliters (2.5%, wt/vol) of trichloracetic acid was then added to each well to extract bacterial ATP and to inactivate ATP-degrading enzymes. The amount of ATP released was measured by an LKB-Wallac (Turku, Finland) model 1251 luminometer with ATP-monitoring reagent and ATP-Standard (LKB-Wallac). The amount of ATP in an extract of the original bacterial suspension in PBS of known CFU per milliliter (in linear dilutions) was used to generate a standard curve and to calculate the amounts of adherent bacterial cells. The mean value of five measurements per sample was taken as the final result. The experiments were repeated at least twice.
Accumulation assay.
Bacterial growth in liquid culture and on a glass surface was assessed by sonication and by counting viable cells after serial dilutions were plated. Bacteria grown on blood agar for 18 h at 37°C were inoculated into PBS at a concentration of 2 × 109 CFU/ml. One milliliter of this suspension was added to 9 ml of tryptic soy broth (TSB) or TSB plus 20% human pooled serum in 25-ml glass beakers. Immediately and hourly thereafter for 8 h, and again after 24 h, the contents of the beakers were poured into centrifugation flasks. For collection of residual nonadherent cells from the beakers, the beakers were washed twice with 5 ml of PBS. The suspension was centrifuged for 5 min at 6,000 × g, and the pellet was then resuspended in 10 ml of PBS and the number of its CFU per milliliter was determined by serial dilution and plating. The washed beakers were filled with 10 ml of PBS and sonicated twice over ice for 45 s at 80 W (model 250 sonifier; Branson, Stuttgart, Germany) to release the glass-surface-adherent bacteria, which were then assessed as described above. The inner surface of each beaker was calculated to be approximately 10 cm2, allowing a direct correlation between CFU per milliliter and CFU per square centimeter.
Endocarditis model.
The inoculum was prepared from an overnight culture in TSB incubated at 37°C. Cells were harvested by centrifugation and resuspended in sterile 0.9% NaCl, and serial 10-fold dilutions over the range of 104 to 108 CFU/ml were prepared for injection. The number of CFU at each dilution was measured by quantitative culture.
To establish endocarditis, a polyethylene catheter was advanced via the carotid artery, positioned across the aortic valve, and secured in place. Twenty-four hours later, 1 ml of cell suspension was injected intravenously through a marginal ear vein. Eight rabbits for each strain had their catheters removed just before injection of bacteria. Twelve or 24 h later, rabbits were euthanized. Aortic valve vegetations were removed and quantitatively subcultured onto blood agar to determine the numbers of organisms remaining in vegetations. The number of organisms remaining in the vegetation of each rabbit, the vegetation titer, was expressed as log10 CFU per gram of vegetation. Data were analyzed by analysis of variance to determine statistically significant differences, defined as a P of <0.05.
RESULTS
Vegetation titer depended on the presence or absence of a catheter (P < 0.02) and inoculum size (P < 0.001 by analysis of variance) but not on the infecting strain (P = 0.984 by analysis of variance) (Tables 1 and 2). The 50% infective doses of CFU, approximately 105, were similar for the two strains.
TABLE 1.
Numbers of rabbits infected and their vegetation titers after a 24-h infection for all inocula
| Test parameter | No. of rabbits showing infection/total no. of rabbits infecteda with:
|
Mean vegetation titer (log10 CFU/g) ± SDb of rabbits (n) showing infection with:
|
||
|---|---|---|---|---|
| Parent | Mutant | Parent | Mutant | |
| Catheter in | 10/15 | 11/15 | 4.9 ± 1.4* (10) | 5.1 ± 1.5** (11) |
| Catheter out | 5/8 | 4/8 | 2.8 ± 1.4* (5) | 2.7 ± 1.1** (4) |
| Catheter in and out | 15/23 | 15/23 | 4.2 ± 1.7 (15) | 4.4 ± 1.8 (15) |
Mean inocula were 5.8 ± 1.2 CFU (n = 3) for the parent and 5.9 ± 1.5 CFU (n = 3) for the mutant.
*P < 0.02 by unpaired t test; **P < 0.02 by unpaired t test.
TABLE 2.
Numbers of rabbits infected and their vegetation titers after a 24-h infection as functions of inoculum
| Inoculum range (log10 CFU) | No. of rabbits showing infection/total no. of rabbits infected with:
|
Mean vegetation titer (log10 CFU/g)a ± SD of rabbits (n) showing infection with:
|
||
|---|---|---|---|---|
| Parent | Mutant | Parent | Mutant | |
| 7.0–8.0 | 4/4 | 5/6 | 5.8 ± 1.4 (4) | 5.8 ± 0.6 (5) |
| 6.0–6.9 | 5/8 | 5/6 | 4.9 ± 0.8 (5) | 5.1 ± 1.6 (5) |
| 5.0–5.9 | 2/4 | 4/6 | 2.6 ± 0.2 (2) | 2.4 ± 1.0 (4) |
| 4.0–4.9 | 3/6 | 1/4 | 3.1 ± 1.6 (3) | 2.7 (1) |
| <104 | 1/1 | 0/1 | 1.4 (1) | <1 (1) |
Differences in vegetation titers depended on the inoculum (P < 0.001) but not the strain (P > 0.5) by analysis of variance.
An inoculum of 2 × 105 CFU of each strain was administered to two groups of 10 rabbits with catheters left in place to determine whether levels of infectivity might differ between strains at approximately the 50% infectious inoculum. Vegetations were removed 12 h after inoculation to assess whether slime production might be associated with differences in levels of adherence early in infection. Eight rabbits infected with the parent strain had detectable organisms within vegetations versus nine rabbits infected with the mutant (Table 3). Vegetation sizes and bacterial densities were virtually identical for the two groups.
TABLE 3.
Results of a single low-inoculum experiment in which rabbits with catheters left in were euthanized and vegetations were obtained 12 h after infection
| Strain | Inoculum (log10 CFU) (105) | No. of rabbits showing infection/total no. of rabbits infected | Mean vegetation titer (log10 CFU/g) ± SD of rabbits (n) showing infection |
|---|---|---|---|
| Parent | 2 | 8/10 | 3.4 ± 1.8 (8) |
| Mutant | 2 | 9/10 | 3.4 ± 1.6 (9) |
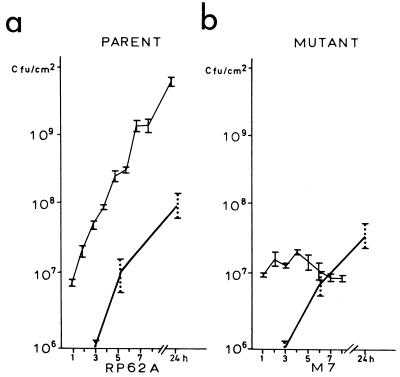
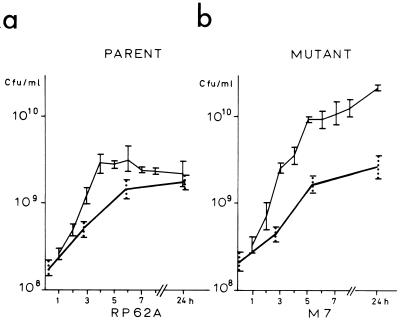
In order to understand the lack of difference in levels of infectivity between these strains, we investigated their growth kinetics on glass surfaces (Fig. 1) and in a broth suspension (Fig. 2) in the presence and absence of serum. In the absence of serum, the growth kinetics of the parent and mutant on glass showed a marked difference. The number of CFU of the parent strain, RP62A, steadily increased when the strain was recovered from the surface (Fig. 1a), whereas the number of CFU of the mutant strain, M7, after initial adherence to the surface did not increase (Fig. 1b). Strain M7, however, was recovered from the broth (Fig. 2b). In the presence of serum, however, this difference in levels of surface accumulation was abolished, with both strains being recovered in equivalent numbers from the glass surfaces of the beakers.
FIG. 1.
Growth kinetics of the parent strain S. epidermidis RP62A (a) and mutant M7 (b) on a glass surface. —, growth in the presence of 20% human pooled serum; , growth in the absence of serum. The vertical bars indicate deviations between results of two experiments; the numbers of CFU were determined five times in each experiment.
FIG. 2.
Growth kinetics of S. epidermidis RP62A (a) and mutant M7 (b) in TSB. —, growth in the presence of 20% human pooled serum; , growth in the absence of serum. The vertical bars indicate deviations between results of two experiments; the numbers of CFU were determined five times in each experiment.
The numbers of CFU of both isolates adhering to microtiter plates, as measured by the bioluminescence assay, were drastically reduced in the presence of serum, gelatin, or albumin compared to the number of CFU adhering to noncoated polymer, which was considered 100% adherence (Table 4). This technique has previously been shown to correlate with fluorescence microscopic examination (17). Over a 90-min period, the numbers of CFU adhering to the polymer did not differ between the parent and mutant strains. The addition of fibronectin to serum in the assay enhanced the level of adherence by threefold. The addition of antifibronectin antibodies blocked adherence by 50% (data not shown), suggesting the presence of fibronectin-binding sites.
TABLE 4.
Results of bacterial adhesion to microtiter plates coated with serum, albumin, gelatin, and serum plus fibronectin
| Microtiter plate coating | Adhesiona with:
|
|||
|---|---|---|---|---|
| Parent strain RP62A
|
Mutant strain M7
|
|||
| CFU | % | CFU | % | |
| None | 5.08 ± 0.78 (106) | 100 | 5.01 ± 0.18 (106) | 100 |
| Serum | 1.67 ± 0.18 (105) | 3 | 1.60 ± 0.25 (105) | 3 |
| Albumin | 1.45 ± 0.17 (105) | 3 | 1.4 ± 0.05 (105) | 3 |
| Gelatin | 1.73 ± 0.35 (105) | 3 | 2.0 ± 0.01 (105) | 4 |
| Serum + FNb | 4.42 ± 0.11 (105) | 9 | 6.6 ± 0.25 (105) | 13 |
Adhesion is expressed as CFU per microtiter plate as determined by the bioluminescence method. The number of CFU determined on the noncoated microtiter plate was considered 100% adhesion.
FN, fibronectin.
DISCUSSION
The role of slime in virulence is controversial. Early animal experiments emphasized slime as a virulence factor (1, 4). Genetically unrelated strains belonging to one or more CoNS species were compared, and the association of slime with virulence was reported but without consideration for the presence in most of the isolates of other factors (e.g., enzymes like protease, hemolysins, and lipase) (8) that have to do more with the strain than with the species. Further, the terminology describing the ability of CoNS to “stick” to medical devices is very confusing and authors refer to adherence and accumulation too often as one step. Every CoNS, including many non-S. epidermidis organisms that have been tested in our laboratory, representing hundreds of strains (19a), was able to adhere to various plastic and glass materials within minutes of incubation, and recovery of the strains from the surfaces was possible only by ultrasonication for quantification or DNA extraction via bioluminescence. The strains, however, differed in their abilities to sustain growth on a solid surface. Strains—mostly S. epidermidis strains—able to accumulate, i.e., sustain growth on a solid surface by forming multilayers, are identified as slime producers by the test of Christensen et al. (3). Strains that cannot sustain growth on a solid surface and can be recovered in the broth are slime-negative strains. Using a parent and a mutant strain differing in their abilities to accumulate on surfaces in vitro (and therefore classified as slime-positive and slime-negative according to the test of Christensen et al. [3]), we could demonstrate that this parameter had no measurable effect on induction of endocarditis in a rabbit model. The possibility that the mutant strain reverted to a slime-positive phenotype in vivo was excluded. The mutant has previously been shown not to express a 140-kDa protein relevant for accumulation, and antibodies selectively raised against this protein inhibit accumulation of the wild type to surfaces (11, 24). Levels of initial adhesion in the absence of serum were equally high for the two strains. The presence of serum, albumin, or gelatin coating the microtiter plates significantly reduced the number of CFU able to adhere to the plates by reducing nonspecific binding, as was previously shown also for medical devices (10, 27). The subsequent coating of the microtiter plates with fibronectin over the serum layer enhanced S. epidermidis wild-type and mutant adherence to similar extents. Again, accumulation of the wild type was significantly reduced in the presence of serum in comparison to accumulation in the serum-free media (29). In the presence of serum, the wild type has no advantage over the slime-negative mutant. This phenomenon may account for the lack of differences in levels of virulence in vivo. Both the parent and mutant strains exhibited similar levels of adherence and accumulation properties in the presence of serum proteins.
These data suggest that slime production as demonstrated in vitro by the test of Christensen et al. is not an important virulence determinant during the early phase of infection as bacteria attach to and accumulate on damaged valves or catheter material in the rabbit endocarditis model. Our experiments using isogenic strains of S. epidermidis that grossly differ in levels of slime production in vitro allow a more direct determination of the contribution of slime production to virulence in vivo. Our findings agree with those of Stekelberg et al., who described no effect of slime production on the treatment of experimental S. epidermidis endocarditis (25). The results differ from those of a subcutaneous catheter infection model and an intraperitoneal infection model with mice, in which the importance of extracellular slime in establishing infection was shown (5, 16). The use of genetically engineered mutants may clarify the role of slime as a factor associated with virulence. The recent availability in our laboratory (9) and in that of others (18) of slime-negative mutants derived by transposon insertional mutagenesis from a slime-positive parent (9, 18) promises to increase our understanding of the role of slime as a virulence factor.
REFERENCES
- 1.Baddour L M, Christensen G D, Hester M G, Bisno A L. Production of experimental endocarditis by coagulase-negative staphylococci: variability in species virulence. J Infect Dis. 1984;150:721–727. doi: 10.1093/infdis/150.5.721. [DOI] [PubMed] [Google Scholar]
- 2.Bayston R, Penny S R. Excessive production of mucoid substance in Staphylococcus S IIA: a possible factor in colonization of Hopter shunts. Dev Med Child Neurol Suppl. 1972;14:25–28. doi: 10.1111/j.1469-8749.1972.tb09769.x. [DOI] [PubMed] [Google Scholar]
- 3.Christensen G D, Simpson W A, Bisno A L, Heachey E H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen G D, Parisi J T, Bisno A L, Simpson W A, Beachey E H. Characterization of clinically significant strains of coagulase-negative staphylococci. J Clin Microbiol. 1983;18:258–262. doi: 10.1128/jcm.18.2.258-269.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen G D, Simpson W A, Bisno A L, Beachey E H. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect Immun. 1983;40:407–410. doi: 10.1128/iai.40.1.407-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davenport D S, Massanari R M, Pfaller M A, Bale M J, Strees S A, Hierholzer W J. Usefulness of a test for slime production as a marker for clinically significant infections with coagulase-negative staphylococci. J Infect Dis. 1986;153:332–339. doi: 10.1093/infdis/153.2.332. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Mitoma F, Harding G K M, Hoban D J, Roberts R S, Low D E. Clinical significance of a test for slime production in ventriculoperitoneal shunt infections caused by coagulase-negative staphylococci. J Infect Dis. 1987;156:555–560. doi: 10.1093/infdis/156.4.555. [DOI] [PubMed] [Google Scholar]
- 8.Gemmel C G, Schumacher-Perdreau F. Extracellular toxins and enzymes elaborated by coagulase-negative staphylococci. Pathogenic and taxonomic potential. In: Mardh P A, Schleifer K H, editors. Coagulase-negative staphylococci. Stockolm, Sweden: Alquist & Wiksell International; 1986. pp. 109–121. [Google Scholar]
- 9.Heilmann C, Gerke C, Perdreau-Remington F, Götz F. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect Immun. 1996;64:277–282. doi: 10.1128/iai.64.1.277-282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrmann M, Vaudaux P E, Pittet D, Auckenthaler R, Lew P E, Schumacher-Perdreau F, Peters G, Waldvogel F A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988;158:693–670. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- 11.Hussain M, Herrmann M, von Eiff C, Perdreau-Remington F, Peters G. A 140-kDa extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect Immun. 1997;65:519–524. doi: 10.1128/iai.65.2.519-524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishak M A, Gröschel D H M, Mandell G L, Wenzel R P. Association of slime with pathogenicity of coagulase-negative staphylococci causing nosocomial septicemia. J Clin Microbiol. 1985;22:1025–1029. doi: 10.1128/jcm.22.6.1025-1029.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karchmer A W, Bisno A L. Infections of prosthetic heart and vascular grafts. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. Washington, D.C: American Society for Microbiology; 1989. pp. 129–159. [Google Scholar]
- 14.Kotilainen P. Association of coagulase-negative staphylococcal slime production and adherence with the development and outcome of adult septicemias. J Clin Microbiol. 1990;28:2779–2785. doi: 10.1128/jcm.28.12.2779-2785.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kristinson K G, Spencer R C, Brown C B. Clinical importance of production of slime by coagulase-negative staphylococci in chronic ambulatory dialysis. J Clin Microbiol. 1986;28:2779–2785. doi: 10.1136/jcp.39.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowy F D, Hammer S M. Staphylococcus epidermidis infections. Ann Intern Med. 1983;99:834–839. doi: 10.7326/0003-4819-99-6-834. [DOI] [PubMed] [Google Scholar]
- 17.Ludwicka A, Switalski L L, Lundin A, Pulverer G, Wadstroem T. Bioluminescent assay for measurement of bacterial attachment to polyethylene. J Microbiol Methods. 1985;4:169–177. [Google Scholar]
- 18.Muller E, Hübner J, Gutierrez N, Takeda S, Goldmann D A, Pier G B. Isolation and characterization of transposon mutants of Staphylococcus epidermidis deficient in capsular polysaccharide/adhesion and slime. Infect Immun. 1993;61:551–558. doi: 10.1128/iai.61.2.551-558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patrick C C, Plaunt M R, Hetherington S V, May S M. Role of the Staphylococcus epidermidis slime layer in experimental tunnel tract infections. Infect Immun. 1992;60:1363–1367. doi: 10.1128/iai.60.4.1363-1367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Perdreau-Remington, F. Unpublished data.
- 20.Peters G, Locci R, Pulverer G. Microbial colonization of prosthetic devices. II. Scanning electron microscopy of naturally infected intravenous catheters. Zentbl Bakteriol Hyg B. 1981;172:293–299. [PubMed] [Google Scholar]
- 21.Peters G, Locci R, Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982;146:479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- 22.Rupp M E, Archer G L. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin Infect Dis. 1994;19:231–243. doi: 10.1093/clinids/19.2.231. [DOI] [PubMed] [Google Scholar]
- 23.Scheld W M, Strunck R W, Balian G, Galderone R A. Microbial adhesion to fibronectin in vitro correlates with production of endocarditis in rabbits. Proc Soc Exp Biol Med. 1985;180:474–482. doi: 10.3181/00379727-180-42205. [DOI] [PubMed] [Google Scholar]
- 24.Schumacher-Perdreau F, Heilmann C, Peters G, Götz F, Pulverer G. Comparative analysis of a biofilm-forming Staphylococcus epidermidis strain and its adhesion-positive, accumulation-negative mutant M7. FEMS Microbiol Lett. 1994;117:71–78. doi: 10.1111/j.1574-6968.1994.tb06744.x. [DOI] [PubMed] [Google Scholar]
- 25.Stekelberg J M, Keating M R, Rouse M S, Wilson W R. Lack of extracellular slime effect on treatment outcome of Staphylococcus epidermidis experimental endocarditis. J Antimicrob Chemother. 1989;23:117–121. doi: 10.1093/jac/23.1.117. [DOI] [PubMed] [Google Scholar]
- 26.Valentin-Weigand P, Timmis K N, Chhatwal G S. Role of fibronectin in staphylococcal colonization of fibrin thrombi and plastic surfaces. J Med Microbiol. 1993;38:90–95. doi: 10.1099/00222615-38-2-90. [DOI] [PubMed] [Google Scholar]
- 27.Vaudaux P, Pittet D, Haeberli A, Huggler E, Nydegger U E, Lew D P, Waldvogel F A. Host factors selectively increase staphylococcal adherence on inserted catheters: a role for fibronectin and fibrinogen or fibrin. J Infect Dis. 1989;160:865–875. doi: 10.1093/infdis/160.5.865. [DOI] [PubMed] [Google Scholar]
- 28.West T E, Walshe J J, Krol C P, Amsterdam D. Staphylococcal peritoneal dialysis. J Clin Microbiol. 1986;23:809–812. doi: 10.1128/jcm.23.5.809-812.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilcox M H, Schumacher-Perdreau F. Lack of evidence for increased adherent growth in broth or human serum of clinically significant coagulase-negative staphylococci. J Hosp Infect. 1994;26:239–250. doi: 10.1016/0195-6701(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 30.Younger J J, Christensen G D, Bartley D L, Simmons H, Barett F. Coagulase-negative staphylococci isolated from cerebrospinal fluid shunts: importance of slime production, species identification, and shunt removal to clinical outcome. J Infect Dis. 1987;156:548–554. doi: 10.1093/infdis/156.4.548. [DOI] [PubMed] [Google Scholar]