Abstract
The Epstein-Barr virus (EBV) BGLF4 gene encodes a serine/threonine protein kinase (PK) that is expressed in the cytolytic cycle. EBV nuclear antigen 2 (EBNA2) is a key latency gene essential for immortalization of B lymphocytes and transactivation of viral and cellular promoters. Here we report that EBV PK phosphorylates EBNA2 at Ser-243 and that these two proteins physically associate. PK suppresses EBNA2's ability to transactivate the LMP1 promoter, and Ser-243 of EBNA2 is involved in this suppression. Moreover, EBNA2 is hyperphosphorylated during EBV reactivation in latently infected B cells, which is associated with decreased LMP1 protein levels. This is the first report about the effect of EBV PK on the function of one of its target proteins and regulation of EBNA2 phosphorylation during the EBV lytic cycle.
Epstein-Barr virus (EBV) is a human gammaherpesvirus with tropism for B cells and epithelial cells that establishes a lifelong persistent infection in more than 90% of the world's population. EBV produces both latent and lytic forms of infection. During latent infection, only a limited set of EBV genes is expressed. Among them, the Epstein-Barr nuclear antigen 2 (EBNA2) is a key latency gene; it regulates transcription of viral and cellular genes and is essential for immortalization of B lymphocytes (5, 14, 44). A principal target of EBNA2 is the EBV latent membrane protein 1 (LMP1) (1, 45), an oncoprotein whose expression is also essential for immortalization of B lymphocytes (22).
In most asymptomatic carriers of EBV, the virus periodically converts to the cytolytic form of infection, in which the full repertoire of viral genes is expressed. The EBV protein kinase (PK) encoded by the EBV BGLF4 gene is one of the lytic cycle gene products; it shows early kinetics during viral reactivation in latently infected Akata cells (11). EBV PK belongs to the group of herpesvirus-encoded protein kinases exemplified by UL13 of herpes simplex virus type 1, which is conserved in all herpesviruses (3, 30, 41). It has been identified as a Ser/Thr protein kinase by use of amino-acid-sequence alignment of regions conserved within the catalytic domains of protein kinases (3, 41) and is the only potential protein kinase identified in the EBV genome. The protein phosphorylates a number of viral and cellular proteins, including the EBV BMRF1 gene product (EA-D), the DNA polymerase processivity factor (4, 11); EBV nuclear antigen leader protein (EBNA-LP) (19), an EBV latency gene, which cotransactivates EBNA2-responsive promoters in an EBNA2-dependent manner (15, 33); PK itself (4, 9, 18); and the cellular translation-elongation factor 1 delta (EF-1δ) (18, 21). Most interestingly, EBV PK phosphorylates EBNA-LP (19) and EF-1δ (21) at the same sites as cdc2 kinase. In general, viral protein kinases of the UL13 group tend to target the same sites on the substrate as cdc2 (19-21). Although EBV PK phosphorylates important viral and cellular proteins, to date there is no evidence showing whether this phosphorylation influences functions of its target proteins.
EBNA2 is a phosphoprotein (12, 13); its phosphorylation by casein kinase II is important for growth transformation (24); however, whether constitutive phosphorylation of EBNA2 influences its transactivation function is unknown. We have discovered that phosphorylation of EBNA2 in latent infection is regulated during the cell cycle, with the protein being specifically hyperphosphorylated in mitosis. The result is that transcriptional activity of the hyperphosphorylated protein is suppressed (47). cdc2 kinase, the key regulator of mitosis (8, 28), is involved in hyperphosphorylation of EBNA2 in latently infected cells (47). Since EBV PK mimics cdc2 kinase in phosphorylating EBNA-LP and EF-1δ (19, 21), in this study we examine whether EBV PK, a viral lytic cycle protein, also phosphorylates EBNA2 and how this phosphorylation affects EBNA2 function.
EBV PK phosphorylates EBNA2 at Ser-243.
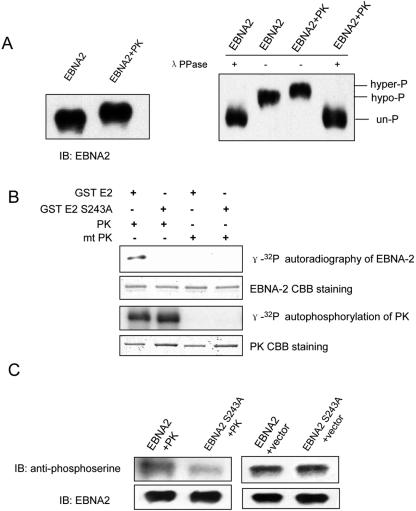
First, we studied whether EBNA2 is hyperphosphorylated when coexpressed with EBV PK. pSG5 EBNA2 (a gift from P. D. Ling) was transfected alone or cotransfected with pcDNA3-BGLF4-FLAG (29) into HeLa cells with the use of FuGENE6 (Roche), followed by immunoblotting with EBNA2 antibody (PE2, DAKO) as described previously (47). Migration of EBNA2 was retarded when cotransfected with PK (Fig. 1 A, left panel). To confirm that the shift in migration is due to phosphorylation, EBNA2 was immunoprecipitated with EBNA2 antibody from the cell lysates and treated with λ-phosphatase (λ-PPase)(NEB) at 30°C for 1 h as described before (47). As shown in Fig. 1A, right panel, λ-PPase treatment shifted both hypo- and hyperphosphorylated EBNA2 to its unphosphorylated form. The results indicate, therefore, that EBNA2 is hyperphosphorylated in vivo when coexpressed with EBV PK in HeLa cells.
FIG. 1.
EBV PK phosphorylates EBNA2 at Ser-243. (A) Hyperphosphorylation of EBNA2 when coexpressed with EBV PK in vivo. HeLa cells were transfected with pSG5-EBNA2 alone or cotransfected with pcDNA-BGLF4-FLAG and collected 48 h after transfection. Left panel, immunoblotting for EBNA2; right panel, EBNA2 immunoprecipitated with EBNA2 antibody from the cell lysates was subjected to immunoblotting directly (−) or after treatment with 2,000 units of λ-PPase (+). (B) In vitro kinase assay. Phosphorylation of purified GST-EBNA2 185-315aa (GST E2) or GST-EBNA2 185-315aa S243A (GST E2 S243A) by EBV PK (PK) or kinase-dead PK (mt PK) was carried out. The whole reaction mixtures were separated by SDS-polyacrylamide gel electrophoresis (4 to 20% gradient gels); after CBB staining, gels were exposed on a PhosphorImager. (C) Involvement of Ser-243 in phosphorylation of EBNA2 by PK in vivo. pSG5-EBNA2 wild type or the S243A mutant of EBNA2 was coexpressed with EBV PK or empty vector in HeLa cells. Whole-cell lysates were subjected to immunoprecipitation with EBNA2 antibody, washed extensively, blotted, and probed with mouse monoclonal phosphoserine antibody or EBNA2 antibody.
Second, we studied whether EBV PK can directly phosphorylate EBNA2 and which site is involved in this phosphorylation. Since EBV PK phosphorylates the same motif as cdc2 kinase in EBNA-LP (19) and EF-1δ (21), and Ser-243 of EBNA2 is a putative site of phosphorylation by cdc2, we tested whether EBV PK can directly phosphorylate EBNA2 at Ser-243 in vitro. An EBNA2 fragment encoding amino acids (aa) 185 to 315 (EBNA2 185-315aa) or aa 185 to 315 S243A (EBNA2 185-315aa S243A), in which Ser-243 was substituted with alanine, was expressed as a fusion protein with glutathione S-transferase (GST) in bacteria and purified by standard techniques (38). Purified protein was of the expected size, and its expression was confirmed by immunoblotting with GST antibody (data not shown). The BGLF4 gene or the K102I mutant of BGLF4, which has lost its kinase activity due to mutation of the invariant catalytic lysine, was cloned into baculovirus genomes as a fusion with GST and His6 tags (18, 19) (the viruses were a gift from Y. Kawaguchi) and expressed in insect cells following standard protocols (34). The protein purification and in vitro kinase assay protocols were described previously (18, 19). Purified GST-EBNA2 185-315aa, GST-EBNA2 185-315aa S243A, or GST (∼1 μg each) was incubated in the kinase assays for 30 min at 37°C with purified PK or PK K102I as a negative control. The reaction mixtures were separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels, and after staining with Coomassie bright blue (CBB) (Sigma), the gels were analyzed with a PhosphorImager (Molecular Dynamics). The results show that the GST-EBNA2 185-315aa fusion protein was phosphorylated by EBV PK, whereas GST-EBNA2 S243A was not (Fig. 1B). Autophosphorylation was selected as a measure of kinase activity. As expected, the kinase-dead mutant PK K102I phosphorylated neither itself nor EBNA2 (Fig. 1B). Purified GST was not phosphorylated by either PK or the kinase-dead mutant PK K102I, as published previously (19) (data not shown). CBB staining shows that essentially equal amounts of either protein were used in each kinase assay. Therefore, the results demonstrate that PK directly phosphorylates EBNA2 at Ser-243 in vitro.
Third, we studied whether Ser-243 of EBNA2 is involved in PK phosphorylation of EBNA2 in vivo. pSG5 EBNA2 or pSG5 EBNA2 S243A was cotransfected with pcDNA3-BGLF4-FLAG (29) into HeLa cells. Cell lysates were immunoprecipitated with EBNA2 antibody, and the immunocomplex was probed with mouse monoclonal phosphoserine antibody (Sigma, P-3430) or EBNA2 antibody as described before (24). As shown in Fig. 1C, substitution of serine at Ser-243 resulted in a clear decrease in overall EBNA2 serine phosphorylation when coexpressed with PK (left panel) but not with empty vector (right panel). Immunoblotting of EBNA2 shows essentially the same amount of EBNA2 in each lane (Fig. 1C). The results indicate that Ser-243 of EBNA2 is involved in hyperphosphorylation of EBNA2 when coexpressed with EBV PK in vivo.
Association of EBV PK with EBNA2.
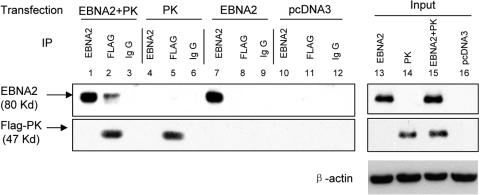
EBNA2 is a nuclear protein (35, 39). Recently we have demonstrated the nuclear localization of EBV PK (10). Thus, next we explored whether PK and EBNA2 can physically associate. HeLa cells were transfected with either pSG5-EBNA2 or pcDNA-BGLF4-FLAG (29) or both. Transfection with pcDNA3 served as a negative control. Aliquots of cell lysates from each transfection were subjected to immunoprecipitation with EBNA2 and FLAG (M2) antibodies or with normal mouse immunoglobulin G as a negative control, and immunocomplexes were probed with EBNA2 or FLAG antibodies. As shown in Fig. 2, lane 2, EBNA2 can be coimmunoprecipitated with PK. We consistently detected less EBNA2 coimmunoprecipitated with PK compared with the amount precipitated with EBNA2 antibody. PK could not be immunoprecipitated with the EBNA2 antibody (Fig. 2, lane 1), perhaps because the binding site is masked by the EBNA2 antibody; a similar situation was encountered in attempts to coimmunoprecipitate cdc2 kinase with EBNA2 antibody (6, 7, 47). The results indicate that EBNA2 and EBV PK physically associate.
FIG. 2.
Coimmunoprecipitation of EBNA2 and EBV PK. HeLa cells were transfected with pSG5-EBNA2 (EBNA2) (lanes 7, 8, 9) or pcDNA-BGLF4-FLAG (PK) alone (lanes 4, 5, 6) or cotransfected with both plasmids (EBNA2+PK) (lanes 1, 2, 3). Transfection with pcDNA3 served as a negative control (lanes 10, 11, 12). Cells were collected 48 h posttransfection, and aliquots of cell lysates from each transfection were subjected to immunoprecipitation with antibodies against EBNA2 or FLAG; normal mouse immunoglobulin G was used as a negative control. Immunocomplexes were resolved in 10% SDS-polyacrylamide gel electrophoresis, and immunoblots were carried out for EBNA2 and FLAG from the same gel. Whole-cell lysates before immunoprecipitation served as an input control (lanes 13, 14, 15, 16), and β-actin was used as a loading control.
EBV PK suppresses EBNA2 transactivation of the LMP1 promoter.
EBNA2 is a key transactivator of a number of viral and cellular gene promoters, including the promoter for LMP1, the principal EBV oncoprotein. Using the LMP1 promoter as a prototype, first we studied whether EBV PK can influence its transactivation by EBNA2. The LMP-1 promoter construct pGL2 (−512/+72)-luciferase (a gift from E. Kieff) was cotransfected with pSG5-EBNA2, pHD/BGLF4, or pHD/K128Q (11) in different combinations into DG75, an EBV-negative BL cell line, as described before (47). The K128Q mutation in pHD/K128Q corresponds to the K102I mutation in EBV PK expressed in insect cells (18, 19). In both mutations, the invariant lysine 102 is changed in order to destroy kinase activity. The pRL-TK plasmid (Promega), which contains the Renilla luciferase gene, was cotransfected as an internal standard. Cells were collected 48 h after transfection, and cell lysates were prepared according to Promega's protocol. Luciferase activity was measured with a luminometer (Promega) and normalized against the activity of the Renilla luciferase gene. As shown in Fig. 3A, neither PK nor kinase-dead PK alone activates LMP1 promoter activity. However, EBV PK suppresses EBNA2's ability to transactivate the LMP1 promoter (P < 0.01), whereas the kinase-dead PK has no such effect. Immunoblotting revealed that EBNA2 became hyperphosphorylated when coexpressed with PK but not with kinase-dead PK (Fig. 3A). The results indicate, therefore, that EBV PK suppresses EBNA2 function and that the kinase activity of PK is involved in this effect. Second, we studied whether Ser-243 of EBNA2, is involved in suppression of EBNA2 transactivation by PK. Similar promoter-reporter assays were carried out as described above to compare transactivation of pLMP1 by EBNA2 and EBNA2 S243A in the absence or presence of EBV PK. As shown in Fig. 3B, there is no significant difference between transactivation of the LMP1 promoter by EBNA2 and EBNA2 S243A in the absence of PK. However, in the presence of PK, substitution of Ser-243 with alanine (EBNA2 S243A) partly restored EBNA2 transactivation of pLMP1 that is suppressed by PK (P < 0.01). Also, the phosphomimetic mutation of EBNA2 Ser-243 to glutamic acid or aspartic acid (24-26, 37, 46) resulted in suppressed induction of endogenous LMP1 expression in transfected P3HR1 cells (unpublished results). Thus, these data suggest that Ser-243 of EBNA2 is involved at least partly in suppression of its transactivation of pLMP1 by PK, although there might be other functionally important EBV PK phosphorylation sites in EBNA2.
FIG. 3.
Phosphorylation of EBNA2 by EBV PK suppresses transactivation of the LMP1 promoter. (A) LMP-1 promoter construct pGL2 (−512/+72)-luciferase was cotransfected with pSG5 EBNA2 (EBNA2), pHD/BGLF4 (PK), or pHD/K128Q (PK K128Q) in different combinations; pRL-TK plasmid was cotransfected as an internal standard. Levels of transactivation of the LMP-1 promoter are normalized to the vector control. Each data point represents the average of three independent experiments, each done in triplicate. Error bars represent the means ± standard errors. Immunoblotting shows the EBNA2 expression level in each transfection; β-actin was used as a loading control. (B) The EBNA2 S243A mutant was tested similarly as detailed in panel A.
Hyperphosphorylation of EBNA2 and decreased LMP1 protein levels during EBV reactivation in type III latency.
EBNA2 is a latent gene product, whereas EBV PK is expressed and active in the viral lytic cycle. Since EBNA2 is hyperphosphorylated when coexpressed with PK and hyperphosphorylation of EBNA2 by PK suppresses its transactivation of pLMP1, next we studied whether EBNA2 is hyperphosphorylated and the LMP1 protein level is decreased during EBV reactivation in type III latency cells. A tetracycline-inducible system for expression of the BZLF1 protein in B95-8 (LCL) cells was used for this purpose as described previously, since expression of BZLF1 drives EBV reactivation and the lytic cycle in latently infected cells (23). As shown in Fig. 4, after induction of cells with doxycycline, migration of EBNA2 is retarded compared with that in noninduced cells. A λ PPase assay confirmed that the shift in EBNA2 migration was caused by hyperphosphorylation of EBNA2 similar to that shown in Fig. 1A, right panel (data not shown). Hyperphosphorylation of the EBV BMRF1 gene product (EA-D), which is a substrate of EBV PK (4, 11), was detected by immunoblotting with EA-D antibody (Capricorn Products) and used to confirm the induction of the lytic cycle and PK activity. The data indicate that EBNA2 is hyperphosphorylated during EBV reactivation when EBV PK is active. As shown in Fig. 4, the endogenous LMP1 protein level is decreased after the induction of the lytic cycle. Doxycycline by itself has no effect on either EBNA2 phosphorylation or LMP1 expression in B95-8 cells (data not shown). The results show in vivo the correlation between hyperphosphorylation of EBNA2 and decreased LMP1 protein levels upon cytolytic induction of latently infected cells.
FIG. 4.
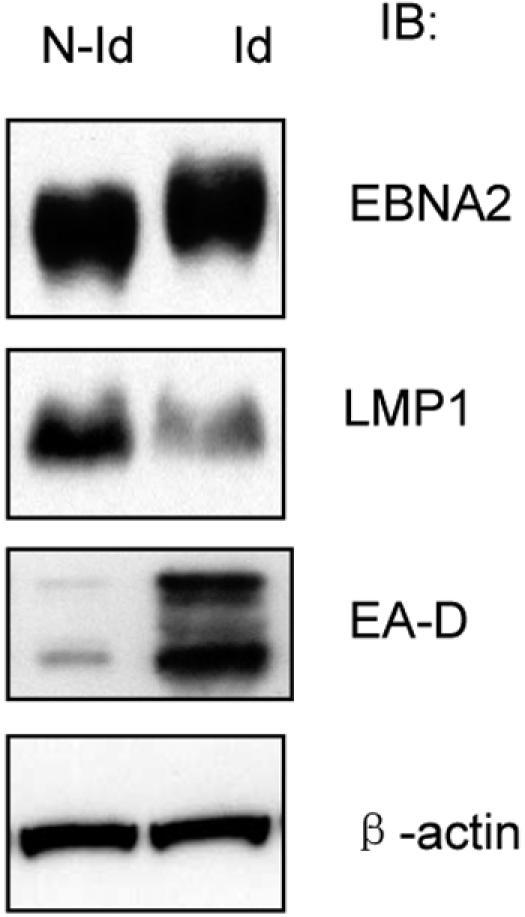
Hyperphosphorylation of EBNA2 and decreased LMP1 protein level during EBV reactivation. The tetracycline-inducible cell line B95-8 BZLF1 was induced with doxycycline at a final concentration of 1 μg/ml for 72 h, and immunoblots of EBNA2, LMP1, and EA-D were carried out in induced (Id) and noninduced (N-Id) cells. β-Actin served as a loading control.
Several UL13 homologs of herpesvirus-encoded protein kinases target the same substrates and even the same phosphorylation sites as cdc2 kinase (19, 21). Phosphorylation of EBNA2 by EBV PK and cdc2 kinase (47) provides additional evidence of targeting the same substrate by a herpesvirus-encoded protein kinase and cdc2. A number of viral and cellular gene products were reported to be phosphorylated by EBV PK (4, 11, 18, 19, 21); however, none of these reports demonstrated the effect of the EBV PK on functions of its target proteins. To the best of our knowledge, this is the first report that demonstrates such an effect. Based on the present results and our report that hyperphosphorylation of EBNA2 in mitosis suppresses its function (47), it is tempting to predict that EBV PK and cdc2 kinase have similar effects on EBNA2 function and phosphorylate the same sites on EBNA2. EBNA2, the key EBV latency gene, has been known to be a phosphoprotein for more than a decade, but only recently has it been shown that EBNA2 function is regulated by phosphorylation in latently infected cells (24, 47). The evidence presented here is the first indication of possible regulation of EBNA2 function through phosphorylation during the EBV lytic cycle.
LMP1 is generally viewed as a latency protein. Regulation of the LMP1 promoter has been extensively studied in EBV latency (15, 16, 32, 45); however, whether and how the LMP1 promoter is regulated during the lytic cycle are unknown. Recently a possible role for LMP1 in the lytic cycle has emerged. It has been reported that LMP1 inhibits induction and progression of the EBV lytic cycle (2, 36), the benefit of which in vivo could be that cytotoxicity of this viral product for the host is limited and, at any given time, viral antigen release may be below a threshold for antigen presentation and an efficient immune response. Thus, regulation of LMP1 promoter activity during the lytic cycle is of considerable potential interest. Suppression of LMP1 promoter activity in response to hyperphosphorylation of EBNA2 by PK and decreased endogenous LMP1 protein levels in lyticly induced type III latency cells suggest that the LMP1 promoter may be down-regulated during the lytic cycle. Thus, hyperphosphorylation of EBNA2 by PK might contribute to the promotion of lytic cycle progression by inhibiting LMP1 expression and providing a more precise level of regulation as the lytic cycle ultimately progresses.
Besides pLMP1, EBNA2 upregulates all the other latency gene promoters, including Cp (27, 43), which drives the transcription of all the EBNAs; it also upregulates pLMP2A (31) as well as a number of key cellular genes (17, 27, 40, 42). It is reasonable to predict that other EBNA2-responsive promoters may also be similarly influenced by EBV PK through phosphorylation of EBNA2. Thus, although the significance of PK-induced suppression of EBNA2 functions in the cytolytic cycle is yet to be established, it may shed light on an area of EBV biology that is largely unexplored.
Acknowledgments
We thank Jeffery Lin and E. Kieff for pGL2 (−512/+72) and LMP1p-Luc plasmids and Paul D. Ling for the pSG5 EBNA2 plasmid, Y. Kawaguchi for providing the EBV BGLF4 and the BGLF4 K102I mutant expressing recombinant baculoviruses, M. Marschall for the pcDNA-BGLF4-FLAG plasmid, T. Tsurumi for the BZLF1 tetracycline-inducible stable cell line B95-8, and Shannon Kenney, Julia Shackelford, and Matthew G. Davenport for valuable discussion of the data.
This work was supported in part by grants from the National Cancer Institute (CA 19014) and from the National Institutes of Health (HL 64851).
REFERENCES
- 1.Abbot, S. D., M. Rowe, K. Cadwallader, A. Ricksten, J. Gordon, F. Wang, L. Rymo, and A. B. Rickinson. 1990. Epstein-Barr virus nuclear antigen 2 induces expression of the virus-encoded latent membrane protein. J. Virol. 64:2126-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler, B., E. Schaadt, B. Kempkes, U. Zimber-Strobl, B. Baier, and G. W. Bornkamm. 2002. Control of Epstein-Barr virus reactivation by activated CD40 and viral latent membrane protein 1. Proc. Natl. Acad. Sci. USA 99:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chee, M., G. Lawrence, and B. Barrell. 1989. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J. Gen. Virol. 70:1151-1160. [DOI] [PubMed] [Google Scholar]
- 4.Chen, M. R., S. J. Chang, H. Huang, and J. Y. Chen. 2000. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J. Virol. 74:3093-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, J. I., F. Wang, J. Mannick, and E. Kieff. 1989. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc. Natl. Acad. Sci. USA 86:9558-9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dillner, J., B. Kallin, G. Klein, H. Jornvall, H. Alexander, and R. Lerner. 1985. Antibodies against synthetic peptides react with the second Epstein-Barr virus-associated nuclear antigen. EMBO J. 4:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dillner, J., V. Wendel-Hansen, G. Kjellstrom, B. Kallin, and A. Rosen. 1988. Purification and characterization of the Epstein-Barr virus nuclear antigen 2 using monoclonal antipeptide antibody. Int. J. Cancer 42:721-727. [DOI] [PubMed] [Google Scholar]
- 8.Furukawa, Y., S. Iwase, Y. Terui, J. Kikuchi, T. Sakai, M. Nakamura, S. Kitagawa, and M. Kitagawa. 1996. Transcriptional activation of the cdc2 gene is associated with Fas-induced apoptosis of human hematopoietic cells. J. Biol. Chem. 271:28469-28477. [DOI] [PubMed] [Google Scholar]
- 9.Gershburg, E., K. Hong, and J. S. Pagano. 2004. Effects of maribavir and selected indolocarbazoles on Epstein-Barr virus protein kinase BGLF4 and on viral lytic replication. Antimicrob. Agents Chemother. 48:1900-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gershburg, E., M. Marschall, K. Hong, and J. S. Pagano. 2004. Expression and localization of the Epstein-Barr virus-encoded protein kinase. J. Virol. 78:12140-12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gershburg, E., and J. S. Pagano. 2002. Phosphorylation of the Epstein-Barr virus (EBV) DNA polymerase processivity factor EA-D by the EBV-encoded protein kinase and effects of the l-riboside benzimidazole 1263W94. J. Virol. 76:998-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grasser, F. A., S. Gottel, P. Haiss, B. Boldyreff, O. G. Issinger, and N. Mueller-Lantzsch. 1992. Phosphorylation of the Epstein-Barr virus nuclear antigen 2. J. Virol. 186:1694-1710. [DOI] [PubMed] [Google Scholar]
- 13.Grasser, F. A., P. Haiss, and N. Mueller-Lantzsch. 1991. Biochemical characterization of Epstein-Barr virus nuclear antigen 2A. J. Virol. 65:3779-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammerschmidt, W., and B. Sugden. 1989. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature 340:393-397. [DOI] [PubMed] [Google Scholar]
- 15.Harada, S., and E. Kieff. 1997. Epstein-Barr virus nuclear protein LP stimulates EBNA-2 acidic domain-mediated transcriptional activation. J. Virol. 71:6611-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johannsen, E., E. Koh, G. Mosialos, X. Tong, E. Kieff, and S. R. Grossman. 1995. Epstein-Barr virus nuclear protein 2 transactivation of the latent membrane protein 1 promoter is mediated by Jκ and PU.1. J. Virol. 69:253-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaiser, C., G. Laux, D. Eick, N. Jochner, G. W. Bornkamm, and B. Kempkes. 1999. The proto-oncogene c-myc is a direct target gene of Epstein-Barr virus nuclear antigen 2. J. Virol. 73:4481-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato, K., Y. Kawaguchi, M. Tanaka, M. Igarashi, A. Yokoyama, G. Matsuda, M. Kanamori, K. Nakajima, Y. Nishimura, M. Shimojima, H. T. T. Phung, E. Takahashi, and K. Hirai. 2001. Epstein-Barr virus-encoded protein kinase BGLF4 mediates hyperphosphorylation of cellular elongation factor 1δ (EF-1δ): EF-1δ is universally modified by conserved protein kinases of herpesviruses in mammalian cells. J. Gen. Virol. 82:1457-1463. [DOI] [PubMed] [Google Scholar]
- 19.Kato, K., A. Yokoyama, Y. Tohya, H. Akashi, Y. Nishiyama, and Y. Kawaguchi. 2003. Identification of protein kinases responsible for phosphorylation of Epstein-Barr virus nuclear antigen leader protein at serine-35, which regulates its coactivator function. J. Gen. Virol. 84:3381-3392. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi, Y., and K. Kato. 2003. Protein kinases conserved in herpesviruses potentially share a function mimicking the cellular protein kinase cdc2. Rev. Med. Virol. 13:331-340. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi, Y., K. Kato, M. Tanaka, M. Kanamori, Y. Nishiyama, and Y. Yamanashi. 2003. Conserved protein kinases encoded by Herpesviruses and cellular protein kinase cdc2 target the same phosphorylation site in eukaryotic elongation factor 1δ. J. Virol. 77:2359-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaye, K. M., K. M. Izumi, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA. 90:9150-9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudoh, A., M. Fujita, T. Kiyono, K. Kuzushima, Y. Sugaya, S. Izuta, Y. Nishiyama, and T. Tsurumi. 2003. Reactivation of lytic replication from B cells latently infected with Epstein-Barr virus occurs with high S-phase cyclin-dependent kinase activity while inhibiting cellular DNA replication. J. Virol. 77:851-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwiatkowski, B., S. Y. J. Chen, and W. H. Schubach. 2004. CKII site in Epstein-Barr virus nuclear protein 2 controls binding to hSNF5/Ini1 and is important for growth transformation. J. Virol. 78:6067-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, J.-S., K. M. Collins, A. L. Brown, C.-H. Lee, and J. H. Chung. 2000. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature 404:201-204. [DOI] [PubMed] [Google Scholar]
- 26.Lee, S. Y., M. R. Wenk, Y. Kim, A. C. Nairn, and P. De Camilli. 2004. Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc. Natl. Acad. Sci. USA. 101:546-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling, P. D., J. J. Hsieh, I. K. Ruf, D. R. Rawlins, and S. D. Hayward. 1994. EBNA-2 upregulation of Epstein-Barr virus latency promoters and the cellular CD23 promoter utilizes a common targeting intermediate, CBF1. J. Virol. 68:5375-5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowe, M., C. Rabouille, N. Nakamura, R. Watson, M. Jackman, E. Jamsa, D. Rahman, D. J. C. Pappin, and G. Warren. 1998. Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell 94:783-793. [DOI] [PubMed] [Google Scholar]
- 29.Marschall, M., M. Stein-Gerlach, M. Freitag, R. Kupfer, M. van den Bogaard, and T. Stamminger. 2002. Direct targeting of human cytomegalovirus protein kinase pUL97 by kinase inhibitors is a novel principle for antiviral therapy. J. Gen. Virol. 83:1013-1023. [DOI] [PubMed] [Google Scholar]
- 30.McGeoch, D. J., and A. J. Davison. 1986. Alphaherpesviruses possess a gene homologous to the protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res. 14:1765-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meitinger, C., L. J. Strobl, G. Marschall, G. W. Bornkamm, and U. Zimber-Strobl. 1994. Crucial sequences within the Epstein-Barr virus TP1 promoter for EBNA2-mediated transactivation and interaction of EBNA2 with its responsive element. J. Virol. 68:7497-7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ning, S., A. M. Hahn, L. E. Huye, and J. S. Pagano. 2003. Interferon regulatory factor 7 regulates expression of Epstein-Barr virus latent membrane protein 1: a regulatory circuit. J. Virol. 77:9359-9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nitsche, F., A. Bell, and A. Rickinson. 1997. Epstein-Barr virus leader protein enhances EBNA-2-mediated transactivation of latent membrane protein 1 expression: a role for the W1W2 repeat domain. J. Virol. 71:6619-6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O′Reilly, D. R., L. K. Miller, and V. A. Luckow. 1992. Baculovirus expression vectors: a laboratory manual. W. H. Freeman and Company, New York, N.Y.
- 35.Petti, L., C. Sample, and E. Kieff. 1989. Subnuclear localization and phosphorylation of Epstein-Barr virus latent infection nuclear proteins. Virology 176:563-574. [DOI] [PubMed] [Google Scholar]
- 36.Prince, S., S. Keating, C. Fielding, P. Brennan, E. Floettmann, and M. Rowe. 2003. Latent membrane protein 1 inhibits Epstein-Barr virus lytic cycle induction and progress via different mechanisms. J. Virol. 77:5000-5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rayet, B., Y. Fan, and C. Gelinas. 2003. Mutations in the v-Rel transactivation domain indicate altered phosphorylation and identify a subset of NF-κB-regulated cell death inhibitors important for v-Rel transforming activity. Mol. Cell. Biol. 23:1520-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Expression of cloned genes in Escherichia coli. In C. Nolan (ed.), Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor.
- 39.Sauter, M., and N. Mueller-Lantzsch. 1987. Characterization of an Epstein-Barr virus nuclear antigene 2 variant (EBNA2B) by specific sera. Virus Res. 8:152. [DOI] [PubMed] [Google Scholar]
- 40.Schlee, M., T. Krug, O. Gires, R. Zeidler, W. Hammerschmidt, R. Mailhammer, G. Laux, G. Sauer, J. Lovric, and G. W. Bornkamm. 2004. Identification of Epstein-Barr virus (EBV) nuclear antigen 2 (EBNA2) target proteins by proteome analysis: activation of EBNA2 in conditionally immortalized B cells reflects early events after infection of primary B cells by EBV. J. Virol. 78:3941-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, R. F., and T. F. Smith. 1989. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J. Virol. 63:450-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spender, L. C., G. H. Cornish, B. Rowland, B. Kempkes, and P. J. Farrell. 2001. Direct and indirect regulation of cytokine and cell cycle proteins by EBNA-2 during Epstein-Barr virus infection. J. Virol. 75:3537-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sung, N. S., S. Kenney, D. Gutsch, and J. S. Pagano. 1991. EBNA-2 transactivates a lymphoid-specific enhancer in the Bam H1 C promoter of Epstein-Barr virus. J. Virol. 65:2164-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, F., C. Gregory, C. Sample, M. Rowe, D. Liebowitz, R. Murray, A. Rickinson, and E. Kieff. 1990. Epstein-Barr virus latent membrane protein (LMP-1) and nuclear protein 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP-1 cooperatively induce CD23. J. Virol. 64:2309-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, F., S. F. Tsang, M. G. Kurilla, J. I. Cohen, and E. Kieff. 1990. Epstein-Bar virus nuclear antigen 2 transactivates latent membrane protein LMP-1. J. Virol. 64:3407-3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yokoyama, A., M. Tanaka, G. Matsuda, K. Kato, M. Kanamori, H. Kawasaki, H. Hirano, I. Kitabayashi, M. Ohki, K. Hirai, and Y. Kawaguchi. 2001. Identification of major phosphorylation sites of Epstein-Barr virus nuclear antigen leader protein (EBNA-LP): ability of EBNA-LP to induce latent membrane protein 1 cooperatively with EBNA-2 is regulated by phosphorylation. J. Virol. 75:5119-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yue, W., M. G. Davenport, J. Shackelford, and J. S. Pagano. 2004. Mitosis-specific hyperphosphorylation of Epstein-Barr virus nuclear antigen 2 suppresses its function. J. Virol. 78:3542-3552. [DOI] [PMC free article] [PubMed] [Google Scholar]