Abstract
Recurrent urinary tract infections (UTIs) are a significant clinical problem for many women; however, host susceptibility factors have not been completely defined. The mouse model of induced UTI provides an experimental environment in which to identify specific host characteristics that are important in initial bacterial colonization of the urinary tract and in resolution of an infection. This study examined initial susceptibility, bacterial clearance, and host defense mechanisms during induction and resolution of Escherichia coli UTIs in genetically distinct strains of mice. Of the ten inbred strains tested, six (BALB/c, C3H/HeN, C57BL/6, DBA.1, DBA.2, and AKR) showed progressive resolution of bladder infections over a 14-day period. A constant, low-level bladder infection was observed in SWR and SJL mice. High bladder infection levels persisted over the 14-day study period in C3H/HeJ and C3H/OuJ mice. Kidney infection levels generally correlated with bladder infection levels, especially in C3H/HeJ and C3H/OuJ mice, the two most susceptible strains, in which infections became more severe with time after challenge. The degree of inflammation in bladder and kidneys, as well as antibody-forming cell responses, positively correlated with infection intensity in all strains except C3H/HeJ, which had minimal inflammation despite high infection levels. These results demonstrate two important aspects of host defense against UTI. First, the innate immune response to an infection in the bladder or kidneys consists primarily of local inflammation, which is followed by an adaptive response characterized in part by an antibody response to the infecting bacteria. Second, a UTI will be spontaneously resolved in most cases; however, in mice with specific genetic backgrounds, a UTI can persist for an extended length of time. The latter result strongly suggests that the presence or absence of specific host genes will determine how effectively an E. coli UTI will be resolved.
Urinary tract infections (UTIs) are one of the most common conditions seen in primary care, hospitals, and extended-care facilities (17). While UTIs can affect both men and women, they are far more prevalent in females. Approximately 50% of adult women report having had one or more UTIs, and some of these women will develop a history of repeated infections (17, 18). A number of studies have sought to define characteristics that make this patient population unusually susceptible to UTIs. Among host factors described thus far are increased numbers of receptors for uropathogenic Escherichia coli on vaginal and bladder epithelial cells (23), lowered urinary glycosaminoglycan excretion (19), low levels of cervicovaginal or urinary antibodies to uropathogens (12, 26, 27), hyporesponsiveness to antigens on uropathogenic E. coli (14), and specific ABO or Lewis erythrocyte antigen phenotypes (15, 20, 24).
Animal models provide a means to evaluate host factors that affect resistance to UTIs. Results from studies of mice and rats have revealed that a genetic component may be important in determining increased UTI susceptibility. A more severe UTI develops in the SWR and AKR strains than in BALB/c or C57BL/6 strains when mice are inoculated intravesically with Enterococcus faecalis (6, 7), and C3H/OuJ and C3H/HeJ mice are significantly less able to resolve an E. coli UTI than C3H/HeN mice (10). The objectives of the current study were to determine the extent to which the genetic backgrounds of other inbred strains affect induction and resolution of an E. coli UTI and to delineate the innate and adaptive immune responses elicited by bladder and kidney infections. Our data on the time course, local inflammatory responses, and antibody-mediated immunity to a UTI induced in different inbred mouse strains indicate the capacity of mice to resolve an E. coli UTI and provide further evidence that a genetic component is associated with host susceptibility and resistance to UTI.
MATERIALS AND METHODS
Mouse strains.
C3H/HeN mice were purchased from Harlan-Sprague Dawley (Indianapolis, Ind.). All other strains were obtained from the Jackson Laboratories (Bar Harbor, Maine). The mouse strains evaluated in this study do not have major, congenital immunodeficiencies, although unresponsiveness or hyporesponsiveness to specific mitogens or antigens has been reported. C3H/HeJ mice are unresponsive to the B-cell mitogen, lipopolysaccharide (21), and DBA.1, DBA.2, SJL, and SWR mice are low responders to the T-cell mitogen phytohemagglutinin (9). Poor responses to protein antigens such as bacteriophage have been reported for AKR and BALB/c mice (16), and DBA.1, DBA.2, and SWR strains have diminished antibody responses to ovomucoid (29). Synthetic antigens composed of amino acid polymers elicit poor antibody responses in DBA.1, DBA.2, C57BL/6, and SJL mice (2). Significant differences between mouse strains have been reported for an immune response to Salmonella outer membrane proteins. C3H/HeJ and A/J mice have strong responses, while BALB/c mice respond poorly (4).
Infection induction and assessment.
Mice were infected by intravesical inoculation with 108 E. coli 1677 CFU delivered in 50 μl. Virulence characteristics of this strain include type 1 and P fimbriae, hemolysin, aerobactin, and the O6 serotype. Inoculation procedures and methods for the determination of bladder and kidney infection levels have been described previously (11, 13). Infection levels at 1, 3, 7, and 14 days after inoculation were defined as the numbers of viable E. coli cells per milligram of bladder or kidney tissue. The 14-day study period was based on previous experience with our mouse model, which indicated that an induced UTI will resolve within this length of time.
Histopathology.
When the bladder and both kidneys of each animal were removed for infection assessment, one-half of each organ was reserved for histopathology evaluation. Sections 5 μm thick were cut from paraffin-embedded tissue and stained with hematoxylin and eosin. The degree of inflammation over the total area of each section (both bladder and kidney) was graded by the same veterinary pathologist (A.G.-F.) by the criteria in Table 1. Slides with sections from untreated animals were identified and used to establish a baseline for uninfected tissue. Bladder and kidney sections from infected animals were read without knowledge of bacterial infection level or time point.
TABLE 1.
Histopathological grading scale for degree of inflammation
| Grade | Condition of:
|
|
|---|---|---|
| Bladder | Kidney | |
| 0 | Normal | Normal |
| 1 | Subepithelial cell inflammatory infiltration (focal and multifocal) | Focal inflammation (cellular infiltration and/or edema in the pelvis) |
| 2 | Edema and subepithelial inflammatory cell infiltration (diffuse) | Focal inflammation (more severe) from pelvis to medulla, with and without moderate edema |
| 3 | Marked subepithelial inflammatory cells with necrosis and neutrophils in and on bladder mucosal epithelium | Multifocal inflammation and cells from pelvis to medulla |
| 4 | Inflammatory cell infiltrate extends into muscle in addition to criteria for grade 3 | Extensive segmental inflammation and necrosis evident from pelvis to cortex |
| 5 | Loss of surface epithelium (necrosis with full-thickness inflammatory cell infiltration) | Diffuse tissue necrosis and inflammatory cell infiltration extending from pelvis to cortex |
AFC assay.
The antibody-mediated immune response to infection was evaluated by quantifying the number of splenic antibody-forming cells (AFCs) produced in response to E. coli 1677 by an enzyme-linked immunosorbent spot assay previously described (21). In brief, killed E. coli cells were used to coat plastic petri dishes and then were overlaid with a suspension of spleen cells. Localized deposition of anti-E. coli antibody was detected by incubating the plates with alkaline phosphatase-conjugated anti-immunoglobulin M (anti-IgM), followed by the addition of substrate and chromogen.
Statistical analyses.
Individual data sets were analyzed by the most appropriate method, and each statistical test is identified when a P value is cited. Analyses used were the Ward’s and centroid cluster methods, analysis of variance (ANOVA), analysis of covariance (ANCOVA), linear regression, Spearman’s rank correlation, and ordinal logistic regression (OLR) (14).
RESULTS
Bladder infections.
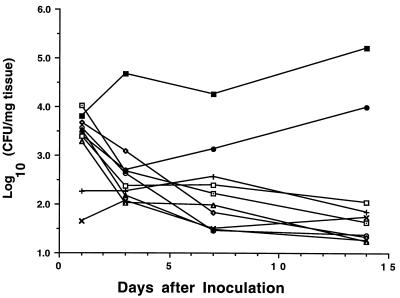
Figure 1 presents the time course of induced E. coli bladder infections in 10 different inbred strains of mice. Data on infection levels in C3H strains were previously reported (5) and are included here to provide a comprehensive view of UTI resolution for all of the strains tested. Cluster analyses were performed to determine which strains had similar or different patterns of infection resolution. The results in Fig. 1 indicate three basic patterns of infection outcomes: (i) progressively resolving from an initial high infection level, (ii) a constant low infection level, and (iii) not resolving from an initial high level of infection.
FIG. 1.
Time course of E. coli bladder infections in inbred mouse strains. Mice were inoculated intravesically with 108 E. coli CFU on day 0 and sacrificed at day 1, 3, 7, or 14. Each point represents the mean number of E. coli CFU in bladders from 5 or 6 mice. ○, AKR; ⊡, BALB/c; •, C3H/HeJ; ◊, C3H/HeN; ▪, C3H/OuJ; □, C57BL/6; ▴, DBA.1; ▵, DBA.2; ×, SJL; +, SWR.
Six mouse strains (BALB/c, C3H/HeN, DBA.1, DBA.2, C57BL/6, and AKR) showed progressive resolution of the induced UTI. Mice from these strains had equivalent numbers of bladder CFU on day 1 (P = 0.44 by ANOVA). Infection levels steadily decreased over the next 2 weeks in an approximately linear fashion on a log-log scale (slopes ranged from −0.48 to −0.93; P < 0.001 in all cases; R2 ranged from 0.54 to 0.81 by linear regression).
Infection levels on day 1 in C3H/OuJ and C3H/HeJ mice were equivalent to those in mice of the six strains that resolved their infections (P = 0.60 by ANOVA). The C3H/OuJ mice, however, had UTIs that persisted and became more severe over the 14-day study period (slope = 0.41, P = 0.04, and R2 = 0.19 by linear regression). Following a decrease in infection level on day 3, C3H/HeJ mice had increasing levels over the remainder of the study (slope = 0.20, P = 0.40, and R2 = 0.04 by linear regression).
A continual, low-level infection was observed in SWR and SJL mice. Initial bladder infection levels in these animals were 1% of those seen in the other eight strains. Numbers of E. coli CFU in the bladder did not appreciably change for either strain over the 14-day study period.
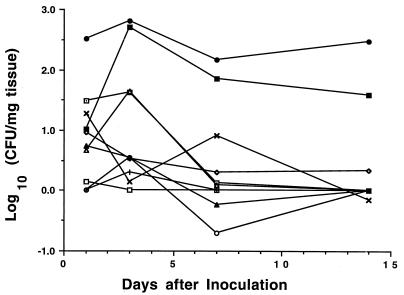
Kidney infections.
Figure 2 presents data on kidney E. coli CFUs in the 10 mouse strains for which bladder infection levels were also determined. Infection levels for all strains except C3H/HeJ were equivalent 1 day after inoculation (P > 0.05 by ANOVA). On day 3, C3H/OuJ and DBA.2 mice had higher levels of infection, with bacterial numbers in C3H/OuJ mice increasing to those in C3H/HeJ mice. The remaining strains (AKR, BALB/c, DBA.1, C3H/HeN, C57BL/6, SJL, and SWR) had either unchanged or lower numbers of kidney E. coli CFU at this time point. At 1 week after inoculation, mice of the last seven strains and DBA.2 mice had equivalent, minimal kidney infections that remained unchanged at day 14. In contrast, C3H/HeJ and C3H/OuJ mice had significantly higher infection levels at both of these later time points. Data for the C3H strains were previously reported (5) and are included for comparative purposes.
FIG. 2.
Time course of E. coli kidney infections in inbred mouse strains. Mice were inoculated intravesically with 108 E. coli CFU on day 0 and sacrificed at day 1, 3, 7, or 14. Each point represents the mean number of E. coli CFU in kidneys from 5 or 6 mice. Symbols are as defined for Fig. 1.
We further analyzed these data to determine whether the intensity of the kidney infection in an individual animal was related to the level of bladder infection. Kidney and bladder CFU for individual mice were analyzed by Spearman’s rank correlation test. The resulting correlation coefficient was found to be 0.419 (P = 0.0001), indicating a moderately high degree of association.
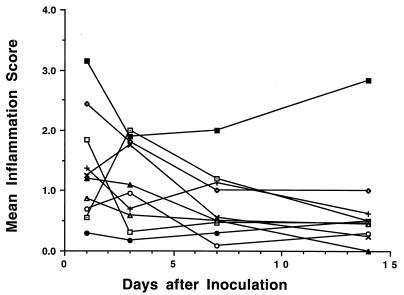
Bladder inflammatory responses.
Mouse strains in which bladder infections decreased over time (AKR, BALB/c, C3H/HeN, C57BL/6, DBA.1, and DBA.2) generally had moderate initial inflammatory responses (grade 1 to 2), all of which appeared to decrease with time after inoculation and decreasing numbers of E. coli CFU (Fig. 3). OLR indicated that the declines in inflammation were significant for C3H/HeN, C57BL/6, and DBA.1 mice (P < 0.05) and were nearly so for DBA.2 mice (P = 0.091). In contrast, C3H/HeJ mice had minimal inflammation throughout the 14-day study with no evidence of change over 14 days (P = 0.17 by OLR) even though the intensity of their bladder infections remained high during this period. The C3H/OuJ mice had an unchanging grade 2 to 3 inflammation throughout the course of infection (P = 0.59 by OLR). Bladder and kidney inflammatory responses were previously reported (5) and are included here for comparison.
FIG. 3.
Bladder inflammation scores for inbred mouse strains during the course of E. coli UTIs. Mice were inoculated intravesically with 108 E. coli CFU on day 0 and sacrificed at day 1, 3, 7, or 14. The degree of inflammation over the total area of each section was graded according to the criteria in Table 1. Each point represents the mean inflammation score of 5 or 6 mice. Symbols are as defined for Fig. 1.
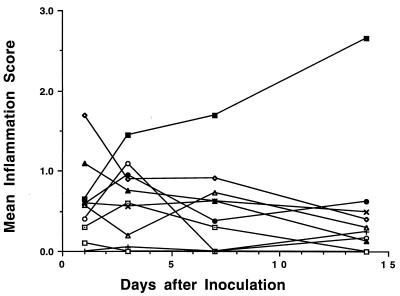
Kidney inflammation.
Kidney inflammation 1 day after inoculation was in the range of 0 to slightly greater than 1 for all mouse strains except C3H/HeN, which had a mean score of 1.7 (Fig. 4). Scores for all strains except C3H/OuJ were approximately 1 or less for the duration of the 14-day study period. Mean kidney inflammation scores for C3H/OuJ mice steadily increased to a maximum of 2.7 on day 14.
FIG. 4.
Kidney inflammation scores for inbred mouse strains during the course of E. coli UTIs. Mice were inoculated intravesically with 108 E. coli CFU on day 0 and sacrificed at day 1, 3, 7, or 14. The degree of inflammation over the total area of each section was graded according to the criteria in Table 1. Each point represents the mean inflammation score of 5 or 6 mice. Symbols are as defined for Fig. 1.
Splenic AFC responses.
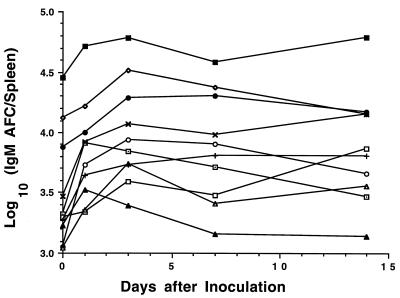
The numbers of IgM anti-E. coli AFCs/spleen at 1, 3, 7, and 14 days after inoculation for mice from 10 mouse strains with E. coli UTIs are presented in Fig. 5. All C3H strain mice had 4- to 10-fold more anti-E. coli AFCs prior to infection than mice of the other seven strains (P = 0.0005 by ANOVA). During the first 3 days of the induced UTI, anti-E. coli AFCs increased in mice of all strains except DBA.1; thereafter, IgM AFC responses tended to decline or remain unchanged for the remainder of the 14-day study.
FIG. 5.
Anti-E. coli splenic AFC responses in inbred mouse strains during the course of E. coli UTIs. Mice were inoculated intravesically with 108 E. coli CFU on day 0 and sacrificed at day 1, 3, 7, or 14. AFCs in the spleen were detected by an enzyme-linked immunosorbent spot assay using whole E. coli cells as the target antigens. Each point represents the mean inflammation score of 5 or 6 mice. Symbols are as defined for Fig. 1.
Correlations between infection course and host resistance.
Since infections of the bladder and kidney induce inflammatory and immune responses, we examined our data for correlations between bladder and kidney infection levels, inflammation scores, and anti-E. coli AFCs. Results of analyses using Spearman’s rank correlation coefficient and associated significance tests showed a moderate degree of association between bladder infection and inflammation (rs = 0.377, P = 0.0001) as well as between kidney infection and inflammation (rs = 0.446, P = 0.0001). Excluded from these analyses were C3H/HeJ mice since they did not show inflammatory responses despite high infection levels. Splenic AFC numbers were positively correlated with bladder and kidney infection levels (rs = 0.327, P = 0.0001 and rs = 0.424, P = 0.0001, respectively).
DISCUSSION
Variability in the induction and resolution of UTIs in genetically different mice inoculated with the same E. coli strain indicates that genetic makeup is an important factor in determining host susceptibility and defense against infection. Significant infections were induced and resolved spontaneously in most strains examined; however, SJL and SWR mice had the least-severe UTIs, and C3H/HeJ and C3H/OuJ mice had intense infections that persisted throughout the 2-week study period. The specific host characteristics that contribute to differences in the induction and resolution of UTIs in the strains we have studied here are not known at this time. In SWR and SJL mice, where uropathogenic E. coli did not cause severe bladder and kidney infections, host genetic factors may decrease the capacity of E. coli to adhere to bladder epithelial cells. It is also conceivable that in both these strains, as in nonsusceptible women, there is a low density of cellular receptors for uropathogenic E. coli. Alternatively, bladder mucin may bind to bacterial cellular receptors and block adherence to the bladder epithelium.
In mice that progressively resolved bladder and kidney infections, it appeared that local inflammation and induction of an antibody-mediated immune response to E. coli were temporally related and associated with infection resolution. The most intense bladder inflammatory responses tended to occur within the first 1 to 3 days after inoculation and were followed by a marked increase in splenic anti-E. coli AFCs. Kidney inflammatory responses were also observed within the first 3 days after inoculation and coincided with the appearance of AFCs. It is likely that a combination of these innate and adaptive responses to infection was responsible for UTI resolution, as has been reported for a diuresing mouse model of E. coli UTI (3, 25). Here, an influx of polymorphonuclear neutrophils into the bladder was followed by the appearance of mononuclear inflammatory cells within 1 week after intravesical inoculation. Anti-E. coli antibodies were subsequently detected on bacteria recovered from the urine. The phagocytosis of bacteria by mononuclear cells such as macrophages reduces the number of bacteria in infected organs and is also an important first step in generating adaptive responses. Bacterial antigens processed by macrophages activate T lymphocytes which, in turn, participate in the stimulation of antibody-producing B cells. With the exception of those in C3H/HeJ mice, the AFC responses seen in mice of the strains tested were very likely initiated through the bladder inflammatory response. In C3H/HeJ mice, inflammation in the kidney may have facilitated the recruitment of antigen-presenting macrophages.
The C3H strains in which infections persisted in the presence of significant inflammatory or AFC responses provide an opportunity to identify apparent deficits in their defense mechanisms against an induced E. coli UTI. In particular, C3H/OuJ mice had intense bladder and kidney inflammation as well as the largest numbers of splenic AFCs produced in response to E. coli. This observation indicates that an inflammatory response alone is not sufficient to resolve an E. coli UTI and suggests some deficit in the adaptive response. In this regard, antibodies induced by the infection may not be specific for antigenic sites on bacterial virulence factors. The C3H/OuJ mice may be capable of making antibodies to many different E. coli antigens so that AFCs are detected, but they may be unable to produce antibodies which specifically inhibit bacterial adherence to epithelial cells. It is also conceivable that C3H/OuJ mice may have a defect in transporting antibodies into the urine or in phagocytosing opsonized bacteria. We observed that C3H/HeJ mice had high infection levels in the presence of large numbers of anti-E. coli AFCs but did not have intense inflammation in the bladder. This result supports the concept that lipopolysaccharide responsiveness is important for initiating cytokine-mediated inflammatory responses to gram-negative bacteria at mucosal surfaces (1, 8). Whether C3H/HeJ and C3H/OuJ mice have common deficits in antibody-mediated immunity to E. coli or in other host defense mechanisms remains to be determined.
The data presented here add to that in previous reports by other investigators on differences between inbred mouse strains in susceptibility to chronic pyelonephritis. As noted above (3, 4), SWR, AKR, and C57BL/6 mice develop chronic pyelonephritis lasting up to 12 weeks after intravesical inoculation with enterococci; however, we found that these strains did not develop any significant kidney infections following similar inoculation with E. coli. There has also been a report of chronic pyelonephritis in BALB/c mice infected with E. coli (5), in contrast to our finding of minimal kidney infections in this strain by 1 week after inoculation. Mice in that study and ours were inoculated with equivalent numbers of E. coli CFU, but three separate inoculations over a 16-week period were needed in that study to achieve chronic kidney infections. Our studies achieved kidney infections spanning at least 2 weeks in C3H/OuJ mice following a single inoculation and also showed that kidney pathology increased with length of infection time. Taken together, these various studies point to the important finding that there were marked differences in the induction and clearance of lower- and upper-tract infections in mice and that these differences are dependent upon the bacterial strain, mouse strain, and inoculation methods used.
In conclusion, this study has demonstrated that genetically distinct inbred mice differ in initial susceptibility to an E. coli UTI and ability to resolve an induced infection. Significant UTIs were induced in the majority of mouse strains evaluated, and mice in all but two strains (C3H/HeJ and C3H/OuJ) were able to successfully reduce the number of bacteria in bladder and kidneys over a 2-week period. We have also demonstrated that an antibody-mediated immune response is elicited by an E. coli UTI in mice, consistent with results for nonhuman primates (13). The observation that C3H/HeJ and C3H/OuJ mice had intense, protracted infections despite significant anti-E. coli AFC responses suggests that these animals may have one or more genetically determined deficits in antibody responses to specific E. coli virulence factors or in other host defense mechanisms. A clearer delineation of these deficits by using the mouse model of UTI will increase our understanding of genetic factors that may determine UTI resistance and susceptibility in humans.
REFERENCES
- 1.de Man P, van Kooten C, Aarden L, Engberg I, Linder H, Edén C S. Interleukin-6 induced at mucosal surfaces by gram-negative bacterial infection. Infect Immun. 1989;57:3383–3388. doi: 10.1128/iai.57.11.3383-3388.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchs S, Mozes E, Maoz A, Sela M. Thymus independence of a collagen-like synthetic peptide and of collagen, and the need for thymus and bone marrow-cell cooperation in the immune response to gelatin. J Exp Med. 1974;139:148–158. doi: 10.1084/jem.139.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillon G, Small M, Medalia O, Aronson M. Sequential study of bacterial clearance in experimental cystitis. J Med Microbiol. 1984;18:319–326. doi: 10.1099/00222615-18-3-319. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez C R, Mejia M V, Paniagua J, Ortiz-Navarette V, Ramirez G, Isibasi A. Immune response to porins isolated from Salmonella typhi in different mouse strains. Arch Med Res. 1995;26:S99–S103. [PubMed] [Google Scholar]
- 5.Gupta R, Ganguly N K, Ahuja V, Joshi K, Sharma S. An ascending non-obstructive model for chronic pyelonephritis in BALB/c mice. J Med Microbiol. 1995;43:33–36. doi: 10.1099/00222615-43-1-33. [DOI] [PubMed] [Google Scholar]
- 6.Guze P A, Karavodin L M, Bonavida B, Kalmanson G M, Ishida K, Targan S, Guze L B. Strain-dependent differences in susceptibility of mice to experimental pyelonephritis. J Infect Dis. 1985;152:416–419. doi: 10.1093/infdis/152.2.416. [DOI] [PubMed] [Google Scholar]
- 7.Guze P A, Kalmanson G M, Ishida K, Guze L B. Strain-dependent differences in susceptibility of mice to experimental ascending pyelonephritis. J Infect Dis. 1987;156:513–525. doi: 10.1093/infdis/156.3.523. [DOI] [PubMed] [Google Scholar]
- 8.Hedges S, Agace W, Svensson M, Sjogren A, Ceska M, Svanborg C. Uroepithelial cells are part of a mucosal cytokine network. Infect Immun. 1994;62:2315–2321. doi: 10.1128/iai.62.6.2315-2321.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heininger H J, Taylor B A, Hards E J, Meyer H. Heritability of the phytohaemagglutinin responsiveness of lymphocytes and its relationship to leukemogenesis. Cancer Res. 1975;35:825–831. [PubMed] [Google Scholar]
- 10.Hopkins W J, Gendron-Fitzpatrick A, McCarthy D O, Haine J E, Uehling D T. Lipopolysaccharide-responder and nonresponder C3H mouse strains are equally susceptible to an induced Escherichia coli urinary tract infection. Infect Immun. 1996;64:1369–1372. doi: 10.1128/iai.64.4.1369-1372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopkins W J, Hall J A, Conway B P, Uehling D T. Induction of urinary tract infection by intraurethral inoculation with Escherichia coli: refining the murine model. J Infect Dis. 1995;171:462–465. doi: 10.1093/infdis/171.2.462. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins W J, Uehling D T. Resolution time of Escherichia coli cystitis is correlated with levels of preinfection antibody to the infecting Escherichia coli strain. Urology. 1995;45:42–46. doi: 10.1016/s0090-4295(95)96444-4. [DOI] [PubMed] [Google Scholar]
- 13.Hopkins W J, Uehling D T, Balish E. Local and systemic antibody responses accompany spontaneous resolution of experimental cystitis in cynomolgus monkeys. Infect Immun. 1987;55:1951–1956. doi: 10.1128/iai.55.9.1951-1956.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopkins W J, Xing Y, Dahmer L A, Balish E, Uehling D T. Western blot analysis of anti-Escherichia coli serum immunoglobulins in women susceptible to recurrent urinary tract infections. J Infect Dis. 1995;172:1612–1616. doi: 10.1093/infdis/172.6.1612. [DOI] [PubMed] [Google Scholar]
- 15.Kinane D F, Blackwell C C, Brettle R P, Weir D M, Winstanley F P, Elton R A. ABO blood group, secretor state, and susceptibility to recurrent urinary tract infection in women. Br Med J. 1982;285:7–9. doi: 10.1136/bmj.285.6334.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kölsch E, Diller E, Weber G, Davies A J S. Genetics of the immune response. I. The immune response to the phage fd in high and low responding inbred strains of mice. Eur J Immunol. 1971;1:201–210. doi: 10.1002/eji.1830010310. [DOI] [PubMed] [Google Scholar]
- 17.Kunin C M. Urinary tract infections: detection, prevention, management. Baltimore, Md: Williams and Wilkins; 1997. [Google Scholar]
- 18.Neu H C. Urinary tract infections. Am J Med. 1992;92:63S–70S. doi: 10.1016/0002-9343(92)90312-y. [DOI] [PubMed] [Google Scholar]
- 19.Parsons C L. Pathogenesis of urinary tract infections: bacterial adherence, bladder defense mechanisms. Urol Clin N Am. 1986;13:563–568. [PubMed] [Google Scholar]
- 20.Ratner J J, Thomas V L, Forland M. Relationships between human blood groups, bacterial pathogens, and urinary tract infections. Am J Med Sci. 1986;292:87–91. doi: 10.1097/00000441-198608000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Rosenstreich D L, Glode L M. Differences in cell mitogen responsiveness between closely related strains of mice. J Immunol. 1975;115:777–780. [PubMed] [Google Scholar]
- 22.SAS Institute, Inc. SAS/STAT user’s guide, version 6. Cary, N.C: SAS Institute, Inc.; 1989. [Google Scholar]
- 23.Schaeffer A J, Jones J M, Dunn J K. Association of in vitro Escherichia coli adherence to vaginal and buccal epithelial cells with susceptibility of women to recurrent urinary tract infections. N Engl J Med. 1981;304:1062–1066. doi: 10.1056/NEJM198104303041802. [DOI] [PubMed] [Google Scholar]
- 24.Sheinfeld J, Schaeffer A J, Cordon-Cardo C, Rogatko A, Fair W R. Association of the Lewis blood-group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773–777. doi: 10.1056/NEJM198903233201205. [DOI] [PubMed] [Google Scholar]
- 25.Sivan Y, Griffel B, Medalia O, Aronson M. Comparative histology of the mouse bladder following initial infection and re-infection with Escherichia coli. J Pathol. 1982;138:353–364. doi: 10.1002/path.1711380406. [DOI] [PubMed] [Google Scholar]
- 26.Stamey T A, Wehner N, Mihara G, Condy M. The immunologic basis of recurrent bacteriuria: role of cervicovaginal antibody in enterobacterial colonization of the introital mucosa. Medicine (Baltimore) 1978;57:47–56. doi: 10.1097/00005792-197801000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Tuttle J P, Jr, Sarvas H, Koistinen J. The role of vaginal immunoglobulin A in girls with recurrent urinary tract infections. J Urol. 1978;120:742–744. doi: 10.1016/s0022-5347(17)57347-4. [DOI] [PubMed] [Google Scholar]
- 28.Uehling D T, James L J, Hopkins W J, Balish E. Immunization against urinary tract infection with a multi-valent vaginal vaccine. J Urol. 1991;146:223–226. doi: 10.1016/s0022-5347(17)37756-x. [DOI] [PubMed] [Google Scholar]
- 29.Vaz N M, Phillips-Quagliata J M, Levine B B, Vaz E M. H-2 linked genetic control of immune responsiveness to ovalbumin and ovomucoid. J Exp Med. 1971;134:1335–1348. doi: 10.1084/jem.134.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]