Abstract
Background:
Risk stratification is a cornerstone of the Pediatric Infectious Diseases Society COVID-19 treatment guidance. This systematic review and meta-analysis aimed to define the clinical characteristics and comorbidities associated with critical COVID-19 in children and adolescents.
Methods:
Two independent reviewers screened the literature (Medline and EMBASE) for studies published through August 2023 that reported outcome data on patients aged ≤21 years with COVID-19. Critical disease was defined as an invasive mechanical ventilation requirement, intensive care unit admission, or death. Random effects models were used to estimate pooled odds ratios (OR) with 95% confidence intervals (CI), and heterogeneity was explored through subgroup analyses.
Results:
Among 10,178 articles, 136 studies met the inclusion criteria for review. Data from 70 studies, which collectively examined 172,165 children and adolescents with COVID-19, were pooled for meta-analysis. In previously healthy children, the absolute risk of critical disease from COVID-19 was 4% (95% CI, 1%–10%). Compared with no comorbidities, the pooled OR for critical disease was 3.95 (95% CI, 2.78–5.63) for presence of one comorbidity and 9.51 (95% CI, 5.62–16.06) for ≥2 comorbidities. Key risk factors included cardiovascular and neurological disorders, chronic pulmonary conditions (excluding asthma), diabetes, obesity, and immunocompromise, all with statistically significant ORs >2.00.
Conclusions:
While the absolute risk for critical COVID-19 in children and adolescents without underlying health conditions is relatively low, the presence of one or more comorbidities was associated with markedly increased risk. These findings support the importance of risk stratification in tailoring pediatric COVID-19 management.
Keywords: Predictors of severity, COVID-19, pediatric, comorbidities, risk factors
Summary
This systematic review with meta-analysis integrated data from 136 studies (172,165 patients) and identified diabetes; obesity; immunocompromise; and cardiovascular, neurological, and pulmonary disease as predictors of severe pediatric COVID-19. The presence of multiple comorbidities increases the risk of critical outcomes.
INTRODUCTION
While most pediatric COVID-19 cases are mild, a subset of children and adolescents experience severe outcomes [1]. Novel therapeutic and prophylactic agents have the potential to improve outcomes in these vulnerable children [2]. The Pediatric Infectious Diseases Society (PIDS) has established a COVID-19 Therapies Task Force that provides recommendations for preventing and managing SARS-CoV-2 in children and adolescents [3–5]. The latest guidance emphasizes the importance of risk stratification in clinical decision-making. Although previous reviews have identified comorbidities that put children at risk for worse outcomes [6–8], these were limited to studies from the early stages of the pandemic and focused on a narrow range of risk factors. As the pandemic has evolved, so has our understanding of how SARS-CoV-2 affects children. To develop effective risk stratification tools and therapeutic strategies, it is crucial to update the evidence base on the factors that predispose children and adolescents to severe disease. This systematic review and meta-analysis aimed to synthesize the best available evidence on the clinical characteristics and underlying health conditions associated with severe or critical pediatric COVID-19 outcomes.
METHODS
A systematic review of the published literature was conducted following established PRISMA guidelines and included searching Medline and EMBASE for eligible studies published from inception through August 25, 2023. The search terms were selected in consultation with a clinical information specialist (Supplementary Methods). Using the Covidence platform, three investigators (CRO, CA, CL) independently screened the titles and abstracts of all retrieved citations to identify potentially eligible studies.
We selected studies that included patients aged ≤21 years with confirmed COVID-19 and provided enough data to estimate the odds ratio (OR) of critical disease for a given risk factor. Studies defining critical disease as the need for invasive mechanical ventilation, admission to an intensive care unit, or death were considered. We excluded articles that were case reports or case series with fewer than thirty patients, those in languages other than English or Spanish, editorials, commentaries, opinions, or reviews. Studies that only provided data on post-acute sequelae of COVID-19, such as multisystem inflammatory syndrome in children and post-acute sequelae of SARS-CoV-2 infection, were also excluded, as were studies that used composite acute and post-acute outcomes that could not be disentangled.
The primary search strategy was supplemented by cross-checking reference lists from previous guidelines, systematic reviews, and meta-analyses. Further, articles recommended by subject matter experts on the PIDS Task Force were also considered for inclusion. Suggested references that did not meet the inclusion criteria but provided additional context, such as studies that only included children with a specific risk factor, were included in the narrative review but not synthesized as part of the meta-analysis. If there was an overlap in patient population between studies (i.e., used the same datasets or patient registries), the larger study was used in the meta-analysis.
Full-text reviews were conducted using standardized data extraction forms by two independent reviewers (CA, CL), and disagreements were resolved by discussion with a third (CRO). Quality assessment of each study was conducted using the Newcastle-Ottawa Scale [9]. Studies were weighted based on their variance, and random-effects models were used to compute pooled odds ratios (OR) with 95% confidence intervals (95%CI). Freeman–Tukey transformations were used to generate pooled prevalence estimates. Heterogeneity between studies was evaluated using the I2 statistic, and its source was explored with subgroup analyses when present. Two-sided P-values <.05 were defined as statistically significant. All analyses were conducted using STATA version 17.0. This study was exempt from ethics review as it used only previously published data. The study protocol was registered on PROSPERO (CRD 42023431457) and follows the PRISMA reporting guidelines.
RESULTS
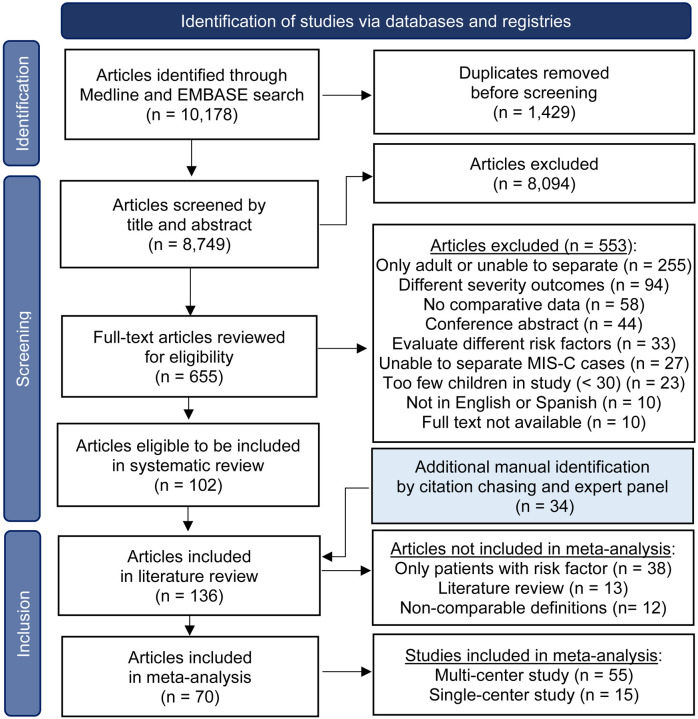
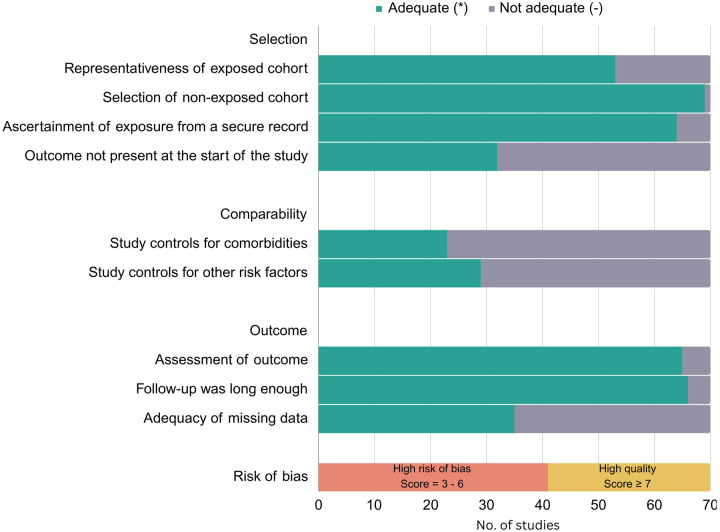
The initial search produced 10,178 articles. Following a screening of titles and abstracts, 655 articles were selected for full-text review. Out of these, 102 articles met the inclusion criteria. Through backward citation tracing and recommendations from subject matter experts, 34 additional articles were identified, yielding a total of 136 articles for the systematic review [6–8, 10–142]. The results of the search and study selection process are outlined in Figure 1. After excluding the single-arm or non-comparable studies, 70 studies were selected for the metaanalysis, which included data on 172,165 children from more than 20 countries. Supplemental Tables 1 and 2 summarize details of the studies included in the evidence base. Of the 70 studies included in the meta-analysis, 29 (41%) were judged to be of high quality using the Newcastle-Ottawa Scale (Figure 2). Uncertainty about the comparability of cohorts was the most commonly identified potential source of bias, given that analyses in only 23 (33%) studies controlled for other underlying comorbidities. Figure 3 provides an overview of the assessed risk factors, including sample sizes, pooled OR, and the I2 statistic. Further synthesis for each set of risk factors is provided in subsequent sections.
Figure 1.
PRISMA Flow diagram of the literature search
Figure 2. Risk of bias assessment for studies included in meta-analysis using the Newcastle-Ottawa Scale.
Legend:
Representativeness of exposed cohort: * indicates truly or somewhat representative of exposed cohort.
Selection of non-exposed cohort: * indicates non-exposed cases drawn from same community as the exposed cohort.
Ascertainment of exposure from secure record: * indicates taken from secure record.
Outcome not present at the start of the study: * indicates yes.
Study controls for comorbidities (most important factor): * indicates yes.
Study controls for other risk factors: * indicates yes.
Assessment of outcome: * indicated using record linkage.
Follow-up was long enough: * indicates all included patients were followed up until discharge from hospital or for > 1 month.
Adequacy of missing data: * Missing data unlikely to introduce bias (< 20).
Orange bar: High risk of bias = 3 – 6 *; Yellow bar: High quality ≥ 7 *
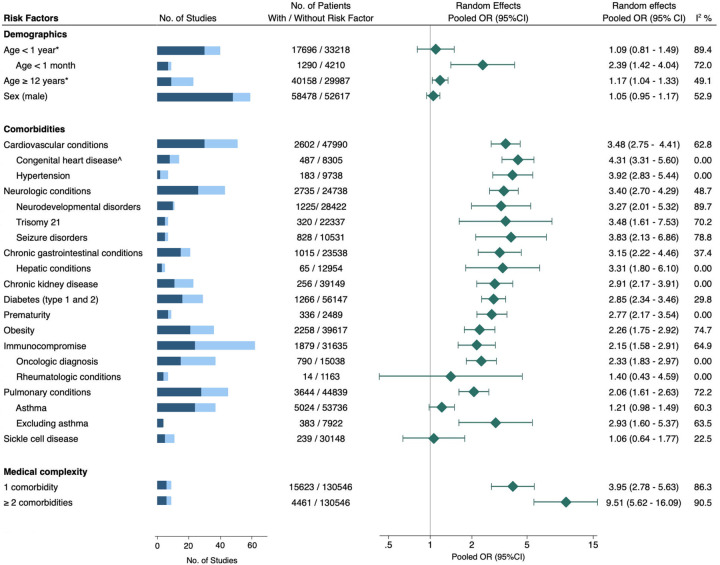
Figure 3. Association between risk factors and critical COVID-19 in children.
Blue bar = total number of studies evaluated, with darker shade indicating the subset of studies included in the meta-analysis.
Pooled OR (95%CI) = pooled odds ratio with 95% confidence interval, estimated using extracted data from published studies.
* = Reference age 1–11 years.
^ = Fixed effects model used.
Demographics
Of the 52 studies that considered age as a risk factor, 30 studies provided data on 17,696 children aged <1 year (10% critical; 95% CI, 6%–14%), 7 studies included information on 1,290 infants aged <1 month (20% critical; 95% CI, 8%–35%), and 9 studies had data on 40,158 children aged 12–21 years (19% critical; 95% CI, 8%–33%). While multiple studies suggested that age <1 year was a risk factor, we found this cutoff to be less consistently associated with increased risk and potentially influenced by younger infants (age <1 month) and prematurity, which were both stronger predictors of severity (Supplemental Figures 1–3). Increased odds of critical disease were also observed in adolescents relative to children aged 1–11 years, though the effect size was modest (OR 1.17; 95% CI, 1.04–1.33, Supplemental Figure 4). Evidence from 48 studies indicated that sex was not associated with an increased risk for progression to critical disease, with similar rates of critical outcomes in males and females (Supplemental Figure 5).
Cardiovascular and pulmonary conditions
Cardiovascular conditions considered across 51 studies included congenital heart disease, hypertension, heart failure, cardiomyopathies, valvular disease, septal defects, myocarditis, arrhythmias, and pulmonary hypertension. The rate of critical disease among 2,372 children with cardiovascular disease in these studies was 30% (95% CI, 23%–37%), and the pooled OR was 3.60 (95% CI, 2.81–4.61). On subgroup analysis, there was an increased rate of critical disease in children with congenital heart disease (OR 4.31; 95% CI, 3.31–5.60) and hypertension (OR 3.92; 95% CI, 2.83–5.44) (Supplementary Figures 6–8).
A total of 45 studies were evaluated to assess the effect of pulmonary conditions. As a group, pulmonary conditions were a risk factor for severity, with a pooled critical disease rate of 24% (95% CI, 17%–33%) and an OR of 2.15 (95% CI, 1.66–2.75) relative to children without pulmonary conditions (Supplementary Figure 9). In a sub-analysis of 24 studies that investigated whether asthma was a predictor, the pooled effect size was non-significant. In contrast, in the studies that excluded asthma from the definition of pulmonary comorbidities, the effect size was robust and statistically significant (Supplemental Figures 10–11).
Few studies delved into other pulmonary conditions aside from asthma. One study, by Martin et al. found a statistically significant association between tracheostomy and severe disease, even after adjusting for other comorbidities (aOR 1.49; 95% CI, 1.08–2.04) [21]. In a multicenter study of 945 children with COVID-19, none of the 14 children with cystic fibrosis had severe disease, and five out of nine children with bronchopulmonary dysplasia required inpatient care [37]. Sleep apnea as a comorbidity was independently assessed by Graff et al. in their single-center cohort study and was associated with hospitalization but not respiratory support once hospitalized [99].
Neurologic and psychiatric conditions
Pooling 26 studies involving 2,735 children with neurological conditions, the incidence of critical COVID-19 was 26% (95% CI, 16%–36%). These children had a threefold increased odds of critical illness compared to the general pediatric population (OR 3.40; 95% CI, 2.70–4.29; Supplemental Figure 12). Seizure disorder, in particular, showed a significant correlation with critical outcomes (Supplemental Figure 13), a relationship that persisted even in adjusted models (Supplemental Table 3). While a statistically significant correlation with critical disease was also seen with neurodevelopmental disorders, notable between-study heterogeneity was present (Supplemental Figure 14). This heterogeneity was primarily due to the largest study in this subgroup, which included 1,690 children with neurodevelopmental disorders and reported more hospitalizations but a lower-than-expected progression to critical illness (aRR 0.83; 95% CI, 0.70–0.98) in this cohort [107].
In a multicenter study that included 8,416 children with COVID-19 and mental health disorders, >90% had a mild or asymptomatic illness [14]. Parallel studies indicated that while conditions such as attention-deficit hyperactivity disorder, anxiety, and depression may be associated with an elevated risk of hospitalization, they do not necessarily correlate with increased likelihood of critical COVID-19 outcomes [12, 14, 107].
Diabetes and Obesity
We synthesized findings from 36 studies to examine the association of diabetes (type 1 and/or 2) and obesity (defined as a body mass index or weight above the 95th percentile for age and sex) with severe COVID-19 in children. Data from 15 studies involving 1,112 children with diabetes showed a 26% rate of critical outcomes (95% CI: 15%–39%). Meanwhile, analyses of 18 studies with 2,258 children with obesity indicated a 32% incidence of critical disease (95% CI: 24%–40%). A pooled analysis revealed two-fold higher odds of critical disease in children with either condition (Supplemental Figures 15, 16). Subgroup analysis by study quality showed that diabetes and obesity are each independent predictors of severity, with six high-quality studies reporting robust effect sizes even after controlling for confounders (Supplemental Table 3). Notably, one study of 21,591 hospitalized COVID-19 patients found a quadrupled mortality risk for children with co-existing diabetes and obesity [143].
Immunocompromise
Data on 1,879 children with COVID-19 who were classified as immunocompromised were extracted from 24 studies. Analysis of these data suggested that immunocompromise was a risk factor for critical COVID-19 (OR 2.15; 95% CI, 1.58–2.91; Supplemental Figure 17). These studies included a mixed group of immunocompromising conditions. However, restricting analyses to the 15 studies that only considered active oncologic diagnoses yielded a similar magnitude of risk (OR 2.33; 95% CI, 1.83–2.97). In contrast, studies of children with rheumatologic diseases did not show a significant correlation with critical COVID-19, though these studies were limited by small sample sizes (Supplemental Figures 18 and 19). Further analysis that considered data from an additional 7 case series, encompassing 1,278 children with rheumatologic conditions, revealed a pooled hospitalization rate of 6% (95% CI: 3%–10%) [62, 64, 65, 81, 95, 133, 134, 144–147]. The largest study in this subset, which included 607 children, did not find an independent correlation between hospitalization and the use of disease-modifying anti-rheumatic drugs or glucocorticoids [64].
Chronic gastrointestinal conditions
Analysis of data from 15 studies, comprising 1,015 children with various chronic gastrointestinal (GI) diseases, showed an odds ratio (OR) of 3.15 for critical disease (95% CI: 2.22–4.46). The quality of these studies was moderate to low, and three studies that controlled for other comorbidities did not find GI diseases as a group to be independently associated with critical COVID-19 (Supplemental Table 3). The conditions included in the GI group included cirrhosis, pancreatic disease, esophageal disorders, inflammatory bowel disease, and dependency on parenteral or enteral feeding tubes. Of these, only feeding tube dependency and chronic liver disease were correlated with adverse outcomes (Supplemental Figures 20 and 21) [11, 110, 131, 141]. In contrast, no correlation with severity was noted for inflammatory bowel disease or esophageal disorders [66, 107].
Chronic kidney disease
A total of 23 studies reported outcomes of children with chronic kidney disease (CKD) and COVID-19. Twelve studies were excluded from the subsequent meta-analysis because they included overlapping patients from larger studies or could not differentiate pre-existing kidney disease from kidney injury acquired during hospitalization. Of the remaining 11 studies, most (82%) did not find a statistically significant increased risk, but the sample sizes were generally small (Supplemental Figure 22). A data-linkage study by Martinez-Valdez et al. contributed the most weight to the meta-analysis, showing a 26% mortality rate among 149 hospitalized children with CKD [112], but carried a risk of bias because comorbidities were self-reported and not operationally defined. In contrast, two other studies that adjusted for comorbidities did not corroborate an association between CKD and severe COVID-19. In a sensitivity analysis that considered data from CKD registries that were not eligible for the meta-analysis, the pooled rate of critical COVID-19 in a sample of 566 children with CKD was 11% (95% CI, 5%–17%), with fatalities predominantly occurring in low-income countries and among those with multiple comorbidities [148–150].
Sickle Cell Disease
Analysis from five studies of 239 children with sickle cell disease indicated a rate of critical disease of 15% (95% CI: 2%–35%), higher than otherwise reported baseline rates, but similar to comparison general pediatric study cohorts, with a pooled OR of 1.06 (CI 0.64–1.77). Supplemental Figure 23). Although the studies included in the meta-analysis had small sample sizes, these estimates are consistent with data from a large case series involving 590 children with sickle cell disease, which also noted a 15% ICU admission rate [151].
Medical Complexity
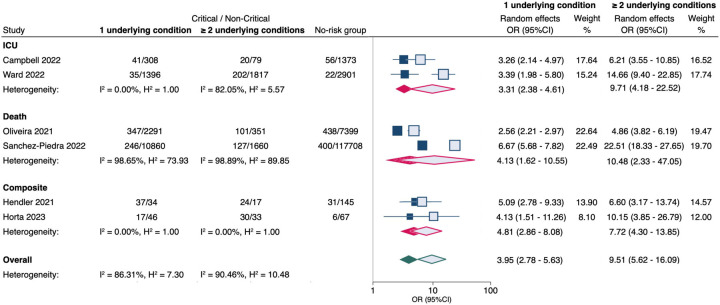
Six studies appraised medical complexity, defined as ≥2 pre-existing conditions, as a risk factor for severity. A synthesis of these data, involving 15,623 pediatric COVID-19 patients, revealed that, on average, the absolute risk of critical COVID-19 outcomes in children without comorbidity was 4% (95% CI, 1%–10%). The presence of any one comorbidity was associated with 3.95 times higher odds of critical disease (Figure 4). For children who had ≥2 chronic comorbidities, the odds of critical disease were even higher (OR 9.51; 95% CI, 5.62–16.06).
Figure 4. Meta-analysis of the association between the number of underlying comorbidities and critical COVID-19 in children.
No risk group = patients with no underlying conditions.
OR (95% CI) = odds ratio with 95% confidence interval, estimated using extracted data from published studies.
Filled markers = 1 underlying condition; Unfilled markers = ≥ 2 underlying conditions.
ICU = admission to intensive care unit; Composite = combined critical outcomes (ICU, IMV, CVS, or death).
DISCUSSION
In this systematic review and meta-analysis, we integrated data from 136 published manuscripts that collectively examined 172,165 children and adolescents with COVID-19. Based on our pooled data, we concluded that certain comorbidities are definite risk factors for critical COVID-19 outcomes. Specifically, cardiovascular and neurological disorders, chronic pulmonary conditions other than asthma, diabetes, obesity, and immunocompromise were all consistently associated with severe disease, exhibiting large effect sizes (OR >2.00) even when controlling for the presence of other comorbidities. While individual studies suggested potential associations between disease severity and conditions such as sickle cell disease, neurodisabilities, and chronic kidney, rheumatologic, or gastrointestinal diseases, the available evidence was not robust enough to draw definitive conclusions. We found a dose-response relationship when considering the total number of comorbidities, wherein multiple pre-existing conditions correlated with increased odds of critical disease compared with a single condition. We were able to corroborate certain findings of earlier evidence syntheses, but also contribute new insights and granularity into the conditions associated with COVID-19 severity in children. For example, in the most recent meta-analysis by Harwood et al., which included 57 studies and 21,549 patients, investigators reported that obesity was the only condition associated with increased odds of ICU admission and death independent of other comorbidities [6]. In contrast, our meta-analysis, which incorporated a substantially larger patient cohort and twice as many studies, further identified cardiovascular diseases, chronic pulmonary disorders, neurological conditions, and diabetes as independent predictors of severity. Some divergences from previous literature also emerged in these analyses. Where previous reviews indicated increased risks for both adolescents and children <1 year of age,[6] our data suggested that age was only a modest risk factor when no other risk factors were present, with the exception of age <1 month, for which evidence leaned towards increased severity.
The probability of critical illness from COVID-19 can be influenced by factors beyond those of pre-existing medical conditions. For instance, vaccination and immunity from prior infections can significantly mitigate risk in immunocompetent hosts [152–154]. Furthermore, the level of risk associated with specific medical conditions can vary depending on how well they are managed. For instance, there is evidence to suggest that diabetic children who maintain good glycemic control are at no greater risk than the general population [40, 78, 155, 156]. Similarly, children with congenital heart disease who have undergone surgical repair or no longer have a hemodynamically significant abnormality may be at lower risk [35, 157].
This study has some limitations to note. First, our findings are subject to the quality and biases of the included studies. We only analyzed observational studies, many of which were retrospective in nature. An inherent limitation of synthesizing data from non-randomized studies is the possibility that uncontrolled confounding could lead to biased pooled estimates. We attempted to mitigate this by focusing on high-quality studies that accounted for important confounders and only drawing inferences of definite risk when there was a sufficiently large magnitude of the effect (OR >2.00), as described in the GRADE conceptual framework [158]. Second, there was considerable heterogeneity across studies, particularly in how comorbidities were defined, introducing the possibility of misclassification bias. Lastly, the continuously evolving nature of the pandemic, with the introduction of novel variants, shifting disease- and vaccine-induced immunity, and the changing landscape of COVID-19 therapies, may affect the relevance of our conclusions over time.
The current management of COVID-19 in the pediatric population is a multifaceted issue, requiring a balanced assessment of the potential risks and benefits of various therapeutic agents, as well as a comprehensive evaluation of the myriad underlying risk factors that may predispose children to more severe disease. Although our study is subject to certain limitations, it contributes evidence on a number of risk factors that are clearly associated with a more severe trajectory of disease. Further research is needed to clarify the extent to which preexisting immunity and the management of these comorbidities modify these risks.
Supplementary Material
ACKNOWLEDGEMENTS
Funders:
This work was supported, in part, by the National Institutes of Health grant number K23AI159518 (Oliveira). Contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Conflict(s) of Interest:
C.R.O. receives grant support from the National Institutes of Health, grant numbers OT2HL161847, K23AI159518 and R01AI123204. G.M.M. receives research support from Astellas and SymBio Pharma. P.K.S. has received support to his institution from Gilead Sciences Inc., Merck Inc. and Allovir Inc. for participation in sponsored research. Z.I.W. has received support to his institution from Pfizer and Merck for participation in sponsored research. S.H.J has received support to his institution from Gilead for participation in sponsored research and has been a consultant for Bayer, outside the scope of this work.
Funding Statement
This work was supported, in part, by the National Institutes of Health grant number K23AI159518 (Oliveira). Contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
REFERENCES
- 1.Zimmermann P, Curtis N. Why Does the Severity of COVID-19 Differ With Age?: Understanding the Mechanisms Underlying the Age Gradient in Outcome Following SARS-CoV-2 Infection. Pediatr Infect Dis J. 2022. Feb 1;41(2):e36–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toussi SS, Hammond JL, Gerstenberger BS, Anderson AS. Therapeutics for COVID-19. Nat Microbiol. 2023. May;8(5):771–86. [DOI] [PubMed] [Google Scholar]
- 3.Chiotos K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, et al. Multicenter Interim Guidance on Use of Antivirals for Children With Coronavirus Disease 2019/Severe Acute Respiratory Syndrome Coronavirus 2. J Pediatric Infect Dis Soc. 2021. Feb 13;10(1):34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf J, Abzug MJ, Anosike BI, Vora SB, Waghmare A, Sue PK, et al. Updated Guidance on Use and Prioritization of Monoclonal Antibody Therapy for Treatment of COVID-19 in Adolescents. J Pediatric Infect Dis Soc. 2022. May 30;11(5):177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf J, Abzug MJ, Wattier RL, Sue PK, Vora SB, Zachariah P, et al. Initial Guidance on Use of Monoclonal Antibody Therapy for Treatment of Coronavirus Disease 2019 in Children and Adolescents. J Pediatric Infect Dis Soc. 2021. May 28;10(5):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harwood R, Yan H, Talawila Da Camara N, Smith C, Ward J, Tudur-Smith C, et al. Which children and young people are at higher risk of severe disease and death after hospitalisation with SARS-CoV-2 infection in children and young people: A systematic review and individual patient meta-analysis. EClinicalMedicine. 2022. Feb;44:101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi JH, Choi SH, Yun KW. Risk Factors for Severe COVID-19 in Children: A Systematic Review and Meta-Analysis. J Korean Med Sci. 2022. Feb 7;37(5):e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Q, Wang Z, Liu J, Wang X, Zhou Q, Li Q, et al. Risk factors for poor prognosis in children and adolescents with COVID-19: A systematic review and meta-analysis. EClinicalMedicine. 2021. Nov;41:101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Huang L, Wang D, Ren P, Hong Q, Kang D. The ROBINS-I and the NOS had similar reliability but differed in applicability: A random sampling observational studies of systematic reviews/meta-analysis. J Evid Based Med. 2021. May;14(2):112–22. [DOI] [PubMed] [Google Scholar]
- 10.Antoon JW, Grijalva CG, Thurm C, Richardson T, Spaulding AB, Teufel RJ 2nd, et al. Factors Associated With COVID-19 Disease Severity in US Children and Adolescents. J Hosp Med. 2021. Oct;16(10):603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armann J, Doenhardt M, Hufnagel M, Diffloth N, Reichert F, Haas W, et al. Risk factors for hospitalization, disease severity and mortality in children and adolescents with COVID-19: Results from a nationwide German registry. MedRxiv. 2021. [Google Scholar]
- 12.Catalan A, Aymerich C, Bilbao A, Pedruzo B, Perez JL, Aranguren N, et al. Psychosis and substance abuse increase the COVID-19 mortality risk. Psychol Med. 2022. Apr 12;53(9):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi SH, Choi JH, Lee JK, Eun BW, Song SH, Ahn B, et al. Clinical Characteristics and Outcomes of Children With SARS-CoV-2 Infection During the Delta and Omicron Variant-Dominant Periods in Korea. J Korean Med Sci. 2023. Mar 6;38(9):e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forrest CB, Burrows EK, Mejias A, Razzaghi H, Christakis D, Jhaveri R, et al. Severity of Acute COVID-19 in Children <18 Years Old March 2020 to December 2021. Pediatrics. 2022. Apr 1;149(4). [DOI] [PubMed] [Google Scholar]
- 15.Greenan-Barrett J, Aston S, Deakin CT, Ciurtin C. The impact of immunocompromise on outcomes of COVID-19 in children and young people-a systematic review and meta-analysis. Front Immunol. 2023;14:1159269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho M, Most ZM, Perl TM, Diaz MI, Casazza JA, Saleh S, et al. Incidence and Risk Factors for Severe Outcomes in Pediatric Patients With COVID-19. Hosp Pediatr. 2023. May 1;13(5):450–62. [DOI] [PubMed] [Google Scholar]
- 17.Jank M, Oechsle AL, Armann J, Behrends U, Berner R, Chao CM, et al. Comparing SARS-CoV-2 variants among children and adolescents in Germany: relative risk of COVID-19-related hospitalization, ICU admission and mortality. Infection. 2023. Feb 14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jone PN, John A, Oster ME, Allen K, Tremoulet AH, Saarel EV, et al. SARS-CoV-2 Infection and Associated Cardiovascular Manifestations and Complications in Children and Young Adults: A Scientific Statement From the American Heart Association. Circulation. 2022. May 10;145(19):e1037–e52. [DOI] [PubMed] [Google Scholar]
- 19.Leon-Abarca JA. Obesity and immunodeficiencies are the main pre-existing conditions associated with mild to moderate COVID-19 in children. Pediatr Obes. 2020. Dec;15(12):e12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu W, Yang L, Li X, Sun M, Zhang A, Qi S, et al. Early immune responses and prognostic factors in children with COVID-19: a single-center retrospective analysis. BMC Pediatr. 2021. Apr 17;21(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin B, DeWitt PE, Russell S, Anand A, Bradwell KR, Bremer C, et al. Characteristics, Outcomes, and Severity Risk Factors Associated With SARS-CoV-2 Infection Among Children in the US National COVID Cohort Collaborative. JAMA Netw Open. 2022. Feb 1;5(2):e2143151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarro-Olivos E, Padilla-Raygoza N, Flores-Vargas G, Gallardo-Luna MJ, Leon-Verdin MG, Lara-Lona E, et al. COVID-19-Associated Case Fatality Rate in Subjects Under 18 Years Old in Mexico, up to December 31, 2020. Front Pediatr. 2021;9:696425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouldali N, Yang DD, Madhi F, Levy M, Gaschignard J, Craiu I, et al. Factors Associated With Severe SARS-CoV-2 Infection. Pediatrics. 2021;147. [DOI] [PubMed] [Google Scholar]
- 24.Preston LE, Chevinsky JR, Kompaniyets L, Lavery AM, Kimball A, Boehmer TK, et al. Characteristics and Disease Severity of US Children and Adolescents Diagnosed With COVID-19. JAMA Netw Open. 2021. Apr 1;4(4):e215298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schober T, Caya C, Barton M, Bayliss A, Bitnun A, Bowes J, et al. Risk factors for severe PCR-positive SARS-CoV-2 infection in hospitalised children. BMJ Paediatr Open. 2022. Aug;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solorzano-Santos F, Miranda-Lora AL, Marquez-Gonzalez H, Klunder-Klunder M. Survival analysis and mortality predictors of COVID-19 in a pediatric cohort in Mexico. Front Public Health. 2022;10:969251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsankov BK, Allaire JM, Irvine MA, Lopez AA, Sauve LJ, Vallance BA, et al. Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int J Infect Dis. 2021. Feb;103:246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, et al. Epidemiology, Clinical Features, and Disease Severity in Patients With Coronavirus Disease 2019 (COVID-19) in a Children’s Hospital in New York City, New York. JAMA Pediatr. 2020. Oct 1;174(10):e202430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emes D, Huls A, Baumer N, Dierssen M, Puri S, Russell L, et al. COVID-19 in Children with Down Syndrome: Data from the Trisomy 21 Research Society Survey. J Clin Med. 2021. Oct 31;10(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahraman AB, Yildiz Y, Ciki K, Erdal I, Akar HT, Dursun A, et al. COVID-19 in inherited metabolic disorders: Clinical features and risk factors for disease severity. Mol Genet Metab. 2023. Jun;139(2):107607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chivers S, Cleary A, Knowles R, Babu-Narayan SV, Simpson JM, Nashat H, et al. COVID-19 in congenital heart disease (COaCHeD) study. Open Heart. 2023. Jul;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goss MB, Galvan NTN, Ruan W, Munoz FM, Brewer ED, O’Mahony CA, et al. The pediatric solid organ transplant experience with COVID-19: An initial multi-center, multi-organ case series. Pediatr Transplant. 2021. May;25(3):e13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachdeva S, Ramakrishnan S, Choubey M, Koneti NR, Mani K, Bakhru S, et al. Outcome of COVID-19-positive children with heart disease and grown-ups with congenital heart disease: A multicentric study from India. Ann Pediatr Cardiol. 2021. Jul-Sep;14(3):269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strah DD, Kowalek KA, Weinberger K, Mendelson J, Hoyer AW, Klewer SE, et al. Worse Hospital Outcomes for Children and Adults with COVID-19 and Congenital Heart Disease. Pediatr Cardiol. 2022. Mar;43(3):541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeh MJ, Bergersen L, Gauvreau K, Barry OM, Batlivala SP, Bjornlund E, et al. COVID-19 international experience in paediatric patients with congenital heart disease. Heart. 2023. Apr 12;109(9):710–8. [DOI] [PubMed] [Google Scholar]
- 36.Zareef R, Salameh E, Hammoud R, Tannouri T, Bitar F, Arabi M. COVID-19 in congenital heart disease patients: what did we learn?! Front Cardiovasc Med. 2023;10:1235165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moeller A, Thanikkel L, Duijts L, Gaillard EA, Garcia-Marcos L, Kantar A, et al. COVID-19 in children with underlying chronic respiratory diseases: survey results from 174 centres. ERJ Open Res. 2020. Oct;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021. Mar;22(2):202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demeterco-Berggren C, Ebekozien O, Rompicherla S, Jacobsen L, Accacha S, Gallagher MP, et al. Age and Hospitalization Risk in People With Type 1 Diabetes and COVID-19: Data From the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2022. Jan 18;107(2):410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mann EA, Rompicherla S, Gallagher MP, Alonso GT, Fogel NR, Simmons J, et al. Comorbidities increase COVID-19 hospitalization in young people with type 1 diabetes. Pediatr Diabetes. 2022. Nov;23(7):968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abu Shanap M, Sughayer M, Alsmadi O, Elzayat I, Al-Nuirat A, Tbakhi A, et al. Factors that predict severity of infection and seroconversion in immunocompromised children and adolescents with COVID-19 infection. Front Immunol. 2022;13:919762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Averbuch D, de la Camara R, Tridello G, Knelange NS, Bykova TA, Ifversen M, et al. Risk factors for a severe disease course in children with SARS-COV-2 infection following hematopoietic cell transplantation in the pre-Omicron period: a prospective multinational Infectious Disease Working Party from the European Society for Blood and Marrow Transplantation group (EBMT) and the Spanish Group of Hematopoietic Stem Cell Transplantation (GETH) study. Bone Marrow Transplant. 2023. May;58(5):558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behr GG, Raphael JP, Price AP, Pandit-Taskar N. Clinical and imaging experience with COVID-19 in nonvaccinated children with cancer. Clin Imaging. 2022. Oct;90:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belsky JA, Tullius BP, Lamb MG, Sayegh R, Stanek JR, Auletta JJ. COVID-19 in immunocompromised patients: A systematic review of cancer, hematopoietic cell and solid organ transplant patients. J Infect. 2021. Mar;82(3):329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhatt NS, Sharma A, St Martin A, Abid MB, Brown VI, Diaz Perez MA, et al. Clinical Characteristics and Outcomes of COVID-19 in Pediatric and Early Adolescent and Young Adult Hematopoietic Stem Cell Transplant Recipients: A Cohort Study. Transplant Cell Ther. 2022. Oct;28(10):696 e1– e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dominguez-Rojas JA, Vasquez-Hoyos P, Perez-Morales R, Monsalve-Quintero AM, Mora-Robles L, Diaz-Diaz A, et al. Association of Cancer Diagnosis and Therapeutic Stage With Mortality in Pediatric Patients With COVID-19, Prospective Multicenter Cohort Study From Latin America. Front Pediatr. 2022;10:885633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Qushayri AE, Benmelouka AY, Dahy A, Hashan MR. COVID-19 outcomes in paediatric cancer: A large scale pooled meta-analysis of 984 cancer patients. Rev Med Virol. 2022. Sep;32(5):e2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fonseca EV, Pardo CA, Linares A, Lopez JF, Camacho G, Aponte NH, et al. Clinical Characteristics and Outcomes of a Cohort of Pediatric Oncohematologic Patients With COVID-19 Infection in the City of Bogota, Colombia. Pediatr Infect Dis J. 2021. Jun 1;40(6):499–502. [DOI] [PubMed] [Google Scholar]
- 49.Haeusler GM, Ammann RA, Carlesse F, Groll AH, Averbuch D, Castagnola E, et al. SARS-CoV-2 in children with cancer or after haematopoietic stem cell transplant: An analysis of 131 patients. Eur J Cancer. 2021. Dec;159:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hammad M, Shalaby L, Sidhom I, Sherief N, Abdo I, Soliman S, et al. Management and Outcome of Coronavirus Disease 2019 (COVID-19) in Pediatric Cancer Patients: A Single Centre Experience from a Developing Country. Clin Lymphoma Myeloma Leuk. 2021. Nov;21(11):e853–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ljungman P, Tridello G, Pinana JL, Ciceri F, Sengeloev H, Kulagin A, et al. Improved outcomes over time and higher mortality in CMV seropositive allogeneic stem cell transplantation patients with COVID-19; An infectious disease working party study from the European Society for Blood and Marrow Transplantation registry. Front Immunol. 2023;14:1125824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madhusoodhan PP, Pierro J, Musante J, Kothari P, Gampel B, Appel B, et al. Characterization of COVID-19 disease in pediatric oncology patients: The New York-New Jersey regional experience. Pediatr Blood Cancer. 2021. Mar;68(3):e28843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meena JP, Kumar Gupta A, Tanwar P, Ram Jat K, Mohan Pandey R, Seth R. Clinical presentations and outcomes of children with cancer and COVID-19: A systematic review. Pediatr Blood Cancer. 2021. Jun;68(6):e29005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Millen GC, Arnold R, Cazier JB, Curley H, Feltbower R, Gamble A, et al. COVID-19 in children with haematological malignancies. Arch Dis Child. 2022. Feb;107(2):186–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morford M, Green RF, Drzymalla E, Okasako-Schmucker DL, So CN, Hill AL, et al. Brief Summary of Findings on the Association Between Underlying Primary Immunodeficiency and Severe COVID-19 Outcomes. CDC COVID-19 Scientific Brief. [Google Scholar]
- 56.Mukkada S, Bhakta N, Chantada GL, Chen Y, Vedaraju Y, Faughnan L, et al. Global characteristics and outcomes of SARS-CoV-2 infection in children and adolescents with cancer (GRCCC): a cohort study. Lancet Oncol. 2021. Oct;22(10):1416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicastro E, Verdoni L, Bettini LR, Zuin G, Balduzzi A, Montini G, et al. COVID-19 in Immunosuppressed Children. Front Pediatr. 2021;9:629240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parker RS, Le J, Doan A, Aguayo-Hiraldo P, Pannaraj PS, Rushing T, et al. COVID-19 outcomes in children, adolescents and young adults with cancer. Int J Cancer. 2022. Dec 1;151(11):1913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rouger-Gaudichon J, Bertrand Y, Boissel N, Brethon B, Ducassou S, Gandemer V, et al. COVID19 and acute lymphoblastic leukemias of children and adolescents: Updated recommendations (Version 2) of the Leukemia Committee of the French Society for the fight against Cancers and leukemias in children and adolescents (SFCE). Bull Cancer. 2021. May;108(5):490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rouger-Gaudichon J, Thebault E, Felix A, Phulpin A, Paillard C, Alimi A, et al. Impact of the First Wave of COVID-19 on Pediatric Oncology and Hematology: A Report from the French Society of Pediatric Oncology. Cancers (Basel). 2020. Nov 17;12(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schlage S, Lehrnbecher T, Berner R, Simon A, Toepfner N. SARS-CoV-2 in pediatric cancer: a systematic review. Eur J Pediatr. 2022. Apr;181(4):1413–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alqanatish J, Almojali A, Alfadhel A, Albelali A, Ahmed A, Alqahtani A, et al. COVID-19 and Pediatric Rheumatology: A Comprehensive Study from a Leading Tertiary Center in Saudi Arabia. J Epidemiol Glob Health. 2023. Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kamdar KY, Kim TO, Doherty EE, Pfeiffer TM, Qasim SL, Suell MN, et al. COVID-19 outcomes in a large pediatric hematology-oncology center in Houston, Texas. Pediatr Hematol Oncol. 2021. Nov;38(8):695–706. [DOI] [PubMed] [Google Scholar]
- 64.Kearsley-Fleet L, Chang ML, Lawson-Tovey S, Costello R, Fingerhutova S, Svestkova N, et al. Outcomes of SARS-CoV-2 infection among children and young people with pre-existing rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2022. Jul;81(7):998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sengler C, Eulert S, Minden K, Niewerth M, Horneff G, Kuemmerle-Deschner J, et al. Clinical manifestations and outcome of SARS-CoV-2 infections in children and adolescents with rheumatic musculoskeletal diseases: data from the National Paediatric Rheumatology Database in Germany. RMD Open. 2021. Jul;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brenner EJ, Pigneur B, Focht G, Zhang X, Ungaro RC, Colombel JF, et al. Benign Evolution of SARS-Cov2 Infections in Children With Inflammatory Bowel Disease: Results From Two International Databases. Clin Gastroenterol Hepatol. 2021. Feb;19(2):394–6 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freitas AF, Pugliese RPS, Feier F, Miura IK, Danesi VLB, Oliveira EN, et al. Impact of COVID-19 Infection on Children and Adolescents after Liver Transplantation in a Latin American Reference Center. Microorganisms. 2022. May 15;10(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nicastro E, Ebel NH, Kehar M, Czubkowski P, Ng VL, Michaels MG, et al. The Impact of Severe Acute Respiratory Syndrome Coronavirus Type 2 on Children With Liver Diseases: A Joint European Society for Pediatric Gastroenterology, Hepatology and Nutrition and Society of Pediatric Liver Transplantation Position Paper. J Pediatr Gastroenterol Nutr. 2022. Jan 1;74(1):159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arlet JB, de Luna G, Khimoud D, Odievre MH, de Montalembert M, Joseph L, et al. Prognosis of patients with sickle cell disease and COVID-19: a French experience. Lancet Haematol. 2020. Sep;7(9):e632–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoogenboom WS, Alamuri TT, McMahon DM, Balanchivadze N, Dabak V, Mitchell WB, et al. Clinical outcomes of COVID-19 in patients with sickle cell disease and sickle cell trait: A critical appraisal of the literature. Blood Rev. 2022. May;53:100911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoogenboom WS, Fleysher R, Soby S, Mirhaji P, Mitchell WB, Morrone KA, et al. Individuals with sickle cell disease and sickle cell trait demonstrate no increase in mortality or critical illness from COVID-19 - a fifteen hospital observational study in the Bronx, New York. Haematologica. 2021;1–6(11):3014–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mucalo L, Brandow AM, Dasgupta M, Mason SF, Simpson PM, Singh A, et al. Comorbidities are risk factors for hospitalization and serious COVID-19 illness in children and adults with sickle cell disease. Blood Adv. 2021. Jul 13;5(13):2717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abdolsalehi MR, Madani S, Mahmoudi S, Navaeian A, Khodabandeh M, Hosseinpour Sadeghi R, et al. Association of body mass index with COVID-19 outcome in a pediatric tertiary referral hospital in Iran. Arch Pediatr. 2023. Jul;30(5):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alharbi M, Kazzaz YM, Hameed T, Alqanatish J, Alkhalaf H, Alsadoon A, et al. SARS-CoV-2 infection in children, clinical characteristics, diagnostic findings and therapeutic interventions at a tertiary care center in Riyadh, Saudi Arabia. J Infect Public Health. 2021. Apr;14(4):446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Antunez-Montes OY, Escamilla MI, Figueroa-Uribe AF, Arteaga-Menchaca E, Lavariega-Sarachaga M, Salcedo-Lozada P, et al. COVID-19 and Multisystem Inflammatory Syndrome in Latin American Children: A Multinational Study. Pediatr Infect Dis J. 2021. Jan;40(1):e1–e6. [DOI] [PubMed] [Google Scholar]
- 76.Araujo da Silva AR, Fonseca CGB, Miranda J, Travassos BV, Baião CR, Silva KD, et al. 2021.
- 77.Armann JP, Diffloth N, Simon A, Doenhardt M, Hufnagel M, Trotter A, et al. Hospital Admission in Children and Adolescents With COVID-19. Dtsch Arztebl Int. 2020. May 22;117(21):373–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Banull NR, Reich PJ, Anka C, May J, Wharton K, Kallogjeri D, et al. Association between Endocrine Disorders and Severe COVID-19 Disease in Pediatric Patients. Horm Res Paediatr. 2022;95(4):331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bayesheva D, Boranbayeva R, Turdalina B, Fakhradiyev I, Saliev T, Tanabayeva S, et al. COVID-19 in the paediatric population of Kazakhstan. Paediatr Int Child Health. 2021. Feb;41(1):76–82. [DOI] [PubMed] [Google Scholar]
- 80.Bellino S, Punzo O, Rota MC, Del Manso M, Urdiales AM, Andrianou X, et al. COVID-19 Disease Severity Risk Factors for Pediatric Patients in Italy. Pediatrics. 2020. Oct;146(4). [DOI] [PubMed] [Google Scholar]
- 81.Bhavsar SM, Clouser KN, Gadhavi J, Anene O, Kaur R, Lewis R, et al. COVID-19 in Pediatrics: Characteristics of Hospitalized Children in New Jersey. Hosp Pediatr. 2021. Jan;11(1):79–87. [DOI] [PubMed] [Google Scholar]
- 82.Bolanos-Almeida CE, Espitia Segura OM. Clinical and Epidemiologic Analysis of COVID-19 Children Cases in Colombia PEDIACOVID. Pediatr Infect Dis J. 2021. Jan;40(1):e7–e11. [DOI] [PubMed] [Google Scholar]
- 83.Bundle N, Dave N, Pharris A, Spiteri G, Deogan C, Suk JE, et al. COVID-19 trends and severity among symptomatic children aged 0–17 years in 10 European Union countries, 3 August 2020 to 3 October 2021. Euro Surveill. 2021. Dec;26(50). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cairoli H, Raiden S, Chiolo MJ, Di Lalla S, Ferrero F, Colaboradores. Patients assisted at the Department of Medicine of a pediatric hospital at the beginning of the COVID-19 pandemic in Buenos Aires, Argentina. Arch Argent Pediatr. 2020. Dec;118(6):418–26. [DOI] [PubMed] [Google Scholar]
- 85.Campbell JI, Dubois MM, Savage TJ, Hood-Pishchany MI, Sharma TS, Petty CR, et al. Comorbidities Associated with Hospitalization and Progression Among Adolescents with Symptomatic Coronavirus Disease 2019. J Pediatr. 2022. Jun;245:102–10 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chao JY, Derespina KR, Herold BC, Goldman DL, Aldrich M, Weingarten J, et al. Clinical Characteristics and Outcomes of Hospitalized and Critically Ill Children and Adolescents with Coronavirus Disease 2019 at a Tertiary Care Medical Center in New York City. J Pediatr. 2020. Aug;223:14–9 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choudhary R, Webber BJ, Womack LS, Dupont HK, Chiu SK, Wanga V, et al. Factors Associated With Severe Illness in Patients Aged <21 Years Hospitalized for COVID-19. Hosp Pediatr. 2022. Sep 1;12(9):760–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Ceano-Vivas M, Martin-Espin I, Del Rosal T, Bueno-Barriocanal M, Plata-Gallardo M, Ruiz-Dominguez JA, et al. SARS-CoV-2 infection in ambulatory and hospitalised Spanish children. Arch Dis Child. 2020. Aug;105(8):808–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Desai A, Mills A, Delozier S, Cabrera Aviles C, Edwards A, Dirajlal-Fargo S, et al. Pediatric Patients with SARS-CoV-2 Infection: Clinical Characteristics in the United States from a Large Global Health Research Network. Cureus. 2020. Sep 12;12(9):e10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Du H, Dong X, Zhang JJ, Cao YY, Akdis M, Huang PQ, et al. Clinical characteristics of 182 pediatric COVID-19 patients with different severities and allergic status. Allergy. 2021. Feb;76(2):510–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Farrar DS, Drouin O, Moore Hepburn C, Baerg K, Chan K, Cyr C, et al. Risk factors for severe COVID-19 in hospitalized children in Canada: A national prospective study from March 2020-May 2021. Lancet Reg Health Am. 2022. Nov;15:100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Farzan S, Rai S, Cerise J, Bernstein S, Coscia G, Hirsch JS, et al. Asthma and COVID-19: An early inpatient and outpatient experience at a US children’s hospital. Pediatr Pulmonol. 2021. Aug;56(8):2522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fattahi P, Abdi S, Saeedi E, Sirous S, Firuzian F, Mohammadi M, et al. In-hospital mortality of COVID-19 in Iranian children and youth: A multi-centre retrospective cohort study. J Glob Health. 2022. Nov 12;12:05048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fernandes DM, Oliveira CR, Guerguis S, Eisenberg R, Choi J, Kim M, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Clinical Syndromes and Predictors of Disease Severity in Hospitalized Children and Youth. J Pediatr. 2021. Mar;230:23–31 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fisler G, Izard SM, Shah S, Lewis D, Kainth MK, Hagmann SHF, et al. Characteristics and risk factors associated with critical illness in pediatric COVID-19. Ann Intensive Care. 2020. Dec 19;10(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Funk AL, Florin TA, Kuppermann N, Tancredi DJ, Xie J, Kim K, et al. Outcomes of SARS-CoV-2-Positive Youths Tested in Emergency Departments: The Global PERN-COVID-19 Study. JAMA Netw Open. 2022. Jan 4;5(1):e2142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giacomet V, Barcellini L, Stracuzzi M, Longoni E, Folgori L, Leone A, et al. Gastrointestinal Symptoms in Severe COVID-19 Children. Pediatr Infect Dis J. 2020. Oct;39(10):e317–e20. [DOI] [PubMed] [Google Scholar]
- 98.Gotzinger F, Santiago-Garcia B, Noguera-Julian A, Lanaspa M, Lancella L, Calo Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020. Sep;4(9):653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Graff K, Smith C, Silveira L, Jung S, Curran-Hays S, Jarjour J, et al. Risk Factors for Severe COVID-19 in Children. Pediatr Infect Dis J. 2021. Apr 1;40(4):e137–e45. [DOI] [PubMed] [Google Scholar]
- 100.Gujski M, Jankowski M, Rabczenko D, Gorynski P, Juszczyk G. Characteristics and Clinical Outcomes of 116,539 Patients Hospitalized with COVID-19-Poland, March-December 2020. Viruses. 2021. Jul 27;13(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gupta MK, Bhardwaj P, Goel AD, Saurabh S, Misra S. Trends of Epidemiological and Demographic Indicators of COVID-19 in India. J Infect Dev Ctries. 2021. May 31;15(5):618–24. [DOI] [PubMed] [Google Scholar]
- 102.Hendler JV, Miranda do Lago P, Muller GC, Santana JC, Piva JP, Daudt LE. Risk factors for severe COVID-19 infection in Brazilian children. Braz J Infect Dis. 2021. Nov-Dec;25(6):101650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hillesheim D, Tomasi YT, Figueiro TH, Paiva KM. Severe Acute Respiratory Syndrome due to COVID-19 among children and adolescents in Brazil: profile of deaths and hospital lethality as at Epidemiological Week 38, 2020. Epidemiol Serv Saude. 2020;29(5):e2020644. [DOI] [PubMed] [Google Scholar]
- 104.Horta M, Ribeiro GJC, Campos NOB, de Oliveira DR, de Almeida Carvalho LM, de Castro Zocrato K, et al. ICU Admission, Invasive Mechanical Ventilation, and Mortality among Children and Adolescents Hospitalized for COVID-19 in a Private Healthcare System. Int J Pediatr. 2023;2023:1698407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kainth MK, Goenka PK, Williamson KA, Fishbein JS, Subramony A, Barone S, et al. Early Experience of COVID-19 in a US Children’s Hospital. Pediatrics. 2020;146(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kalyanaraman Marcello R, Dolle J, Grami S, Adule R, Li Z, Tatem K, et al. Characteristics and outcomes of COVID-19 patients in New York City’s public hospital system. PLoS One. 2020;15(12):e0243027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kompaniyets L, Agathis NT, Nelson JM, Preston LE, Ko JY, Belay B, et al. Underlying Medical Conditions Associated With Severe COVID-19 Illness Among Children. JAMA Netw Open. 2021. Jun 1;4(6):e2111182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kufa T, Jassat W, Cohen C, Tempia S, Masha M, Wolter N, et al. Epidemiology of SARS-CoV-2 infection and SARS-CoV-2 positive hospital admissions among children in South Africa. Influenza Other Respir Viruses. 2022. Jan;16(1):34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leung C, Su L, Simoes ESAC, Arocha LS, de Paiva KM, Haas P. Risk for Severe Illness and Death among Pediatric Patients with Down Syndrome Hospitalized for COVID-19, Brazil. Emerg Infect Dis. 2023. Jan;29(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Madani S, Shahin S, Yoosefi M, Ahmadi N, Ghasemi E, Koolaji S, et al. Red flags of poor prognosis in pediatric cases of COVID-19: the first 6610 hospitalized children in Iran. BMC Pediatr. 2021. Dec 10;21(1):563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mania A, Faltin K, Mazur-Melewska K, Malecki P, Jonczyk-Potoczna K, Lubarski K, et al. Clinical Picture and Risk Factors of Severe Respiratory Symptoms in COVID-19 in Children. Viruses. 2021. Nov 25;13(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martinez-Valdez L, Richardson Lopez Collada V, Castro-Ceronio LE, Rodriguez Gutierrez AM, Bautista-Marquez A, Hernandez-Avila M. Risk factors for COVID-19 hospitalisations and deaths in Mexican children and adolescents: retrospective cross-sectional study. BMJ Open. 2022. Jun 17;12(6):e055074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moreira A, Chorath K, Rajasekaran K, Burmeister F, Ahmed M, Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. 2021. May;180(5):1659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Murillo-Zamora E, Jiménez-Álvarez A. Supervivencia en pacientes pediátricos con neumonía en enfermedad por coronavirus 2019. Revista Médica del Instituto Méxicano del Seguro Social. 2022;60(5):511–6. [PMC free article] [PubMed] [Google Scholar]
- 115.Murillo-Zamora E, Trujillo X, Huerta M, Rios-Silva M, Lugo-Radillo A, Mendoza-Cano O. Decreased survival in children inpatients with COVID-19 and antibiotic prescription. BMC Infect Dis. 2022. Jun 10;22(1):532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nguyen PNT, Thuc TT, Hung NT, Thinh LQ, Minh NNQ, Duy DQ, et al. Risk factors for disease severity and mortality of children with Covid-19: A study at a Vietnamese Children’s hospital. J Infect Chemother. 2022. Oct;28(10):1380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oliveira EA, Colosimo EA, Simoes ESAC, Mak RH, Martelli DB, Silva LR, et al. Clinical characteristics and risk factors for death among hospitalised children and adolescents with COVID-19 in Brazil: an analysis of a nationwide database. Lancet Child Adolesc Health. 2021. Aug;5(8):559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oliveira EA, Mak RH, Colosimo EA, Mendonça ACQ, Vasconcelos MA, Martelli Júnior H, et al. Risk factors for COVID 19 related mortality in hospitalized children and adolescents with diabetes mellitus: An observational retrospective cohort study. Pediatric Diabetes. 2022;23(6):763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oliveira MCL, Colosimo EA, Vasconcelos MA, Martelli-Junior H, Mak RH, Silva LR, et al. The association between pre-existing asthma and reduced risk of death among children and adolescents hospitalized with COVID-19 in Brazil. Pediatr Pulmonol. 2023. Mar;58(3):727–37. [DOI] [PubMed] [Google Scholar]
- 120.Oliveira MCL, Simoes ESAC, Colosimo EA, Campos MK, Martelli-Junior H, Silva LR, et al. Clinical Impact and Risk Factors of Mortality in Hospitalized Children and Adolescents With Hematologic Diseases and COVID-19: An Observational Retrospective Cohort Study. J Pediatr Hematol Oncol. 2023. Apr 1;45(3):e315–e22. [DOI] [PubMed] [Google Scholar]
- 121.Parri N, Magista AM, Marchetti F, Cantoni B, Arrighini A, Romanengo M, et al. Characteristic of COVID-19 infection in pediatric patients: early findings from two Italian Pediatric Research Networks. Eur J Pediatr. 2020. Aug;179(8):1315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qian G, Zhang Y, Xu Y, Hu W, Hall IP, Yue J, et al. Reduced inflammatory responses to SARS-CoV-2 infection in children presenting to hospital with COVID-19 in China. EClinicalMedicine. 2021. Apr;34:100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020. May 26;323(20):2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rivas-Ruiz R, Roy-Garcia IA, Ureña-Wong K, Aguilar-Ituarte F, Vázquez-De Anda GF, Gutiérrez-Castrellón P, et al. Factores asociados a muerte en niños con COVID-19 en México. Gaceta Médica de México. 2020;156(6). [DOI] [PubMed] [Google Scholar]
- 125.Saatci D, Ranger TA, Garriga C, Clift AK, Zaccardi F, Tan PS, et al. Association Between Race and COVID-19 Outcomes Among 2.6 Million Children in England. JAMA Pediatr. 2021. Sep 1;175(9):928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sanchez-Piedra C, Gamino-Arroyo AE, Cruz-Cruz C, Prado-Galbarro FJ. Impact of environmental and individual factors on COVID-19 mortality in children and adolescents in Mexico: An observational study. Lancet Reg Health Am. 2022. Apr;8:100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sedighi I, Fahimzad A, Pak N, Khalili M, Shokrollahi MR, Heydari H, et al. A multicenter retrospective study of clinical features, laboratory characteristics, and outcomes of 166 hospitalized children with coronavirus disease 2019 (COVID-19): A preliminary report from Iranian Network for Research in Viral Diseases (INRVD). Pediatr Pulmonol. 2022. Feb;57(2):498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sena GR, Lima TPF, Vidal SA, Duarte M, Bezerra PGM, Fonseca Lima EJ, et al. Clinical Characteristics and Mortality Profile of COVID-19 Patients Aged less than 20 years Old in Pernambuco - Brazil. Am J Trop Med Hyg. 2021. Feb 18;104(4):1507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Simoes ESAC, Vasconcelos MA, Colosimo EA, Mendonca ACQ, Martelli-Junior H, Silva LR, et al. Outcomes and risk factors of death among hospitalized children and adolescents with obesity and COVID-19 in Brazil: An analysis of a nationwide database. Pediatr Obes. 2022. Sep;17(9):e12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sorg AL, Hufnagel M, Doenhardt M, Diffloth N, Schroten H, von Kries R, et al. Risk for severe outcomes of COVID-19 and PIMS-TS in children with SARS-CoV-2 infection in Germany. Eur J Pediatr. 2022. Oct;181(10):3635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sousa BLA, Brentani A, Costa Ribeiro CC, Dolhnikoff M, Grisi S, Ferrer APS, et al. Non-communicable diseases, sociodemographic vulnerability and the risk of mortality in hospitalised children and adolescents with COVID-19 in Brazil: a cross-sectional observational study. BMJ Open. 2021. Sep 6;11(9):e050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Storch-de-Gracia P, Leoz-Gordillo I, Andina D, Flores P, Villalobos E, Escalada-Pellitero S, et al. Espectro clínico y factores de riesgo de enfermedad complicada en niños ingresados con infección por SARS-CoV-2. Anales de Pediatría. 2020;93:323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Swann OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020. Aug 27;370:m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ungar SP, Solomon S, Stachel A, Shust GF, Clouser KN, Bhavsar SM, et al. Hospital and ICU Admission Risk Associated With Comorbidities Among Children With COVID-19 Ancestral Strains. Clinical Pediatrics. 2023 2023. Oct;62(9):1048–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.van der Zalm MM, Lishman J, Verhagen LM, Redfern A, Smit L, Barday M, et al. Clinical Experience With Severe Acute Respiratory Syndrome Coronavirus 2-Related Illness in Children: Hospital Experience in Cape Town, South Africa. Clin Infect Dis. 2021. Jun 15;72(12):e938–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vasconcelos MA, Mendonca ACQ, Colosimo EA, Nourbakhsh N, Martelli-Junior H, Silva LR, et al. Outcomes and risk factors for death among hospitalized children and adolescents with kidney diseases and COVID-19: an analysis of a nationwide database. Pediatr Nephrol. 2023. Jan;38(1):181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Verma S, Lumba R, Dapul HM, Gold-von Simson G, Phoon CK, Lighter JL, et al. Characteristics of Hospitalized Children With SARS-CoV-2 in the New York City Metropolitan Area. Hosp Pediatr. 2021. Jan;11(1):71–8. [DOI] [PubMed] [Google Scholar]
- 138.Wanga V, Gerdes ME, Shi DS, Choudhary R, Dulski TM, Hsu S, et al. Characteristics and Clinical Outcomes of Children and Adolescents Aged <18 Years Hospitalized with COVID-19 — Six Hospitals, United States, July–August 2021. Morbidity and Mortality Weekly Report. 2021. December 31, 2021;70(51–52):1766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ward JL, Harwood R, Smith C, Kenny S, Clark M, Davis PJ, et al. Risk factors for PICU admission and death among children and young people hospitalized with COVID-19 and PIMS-TS in England during the first pandemic year. Nat Med. 2022. Jan;28(1):193–200. [DOI] [PubMed] [Google Scholar]
- 140.Wong-Chew RM, Noyola DE, Villa AR. [Clinical characteristics and mortality risk factors in patients aged less than 18 years with COVID-19 in Mexico and Mexico City]. An Pediatr (Barc). 2022. Aug;97(2):119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Woodruff RC, Campbell AP, Taylor CA, Chai SJ, Kawasaki B, Meek J, et al. Risk Factors for Severe COVID-19 in Children. Pediatrics. 2022;149(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yayla B, Aykac K, Ozsurekci Y, Ceyhan M. Characteristics and Management of Children With COVID-19 in a Tertiary Care Hospital in Turkey. Clinical Pediatrics. 2021;60(3):170 – 7. [DOI] [PubMed] [Google Scholar]
- 143.Oliveira EA, Mak RH, Colosimo EA, Mendonca ACQ, Vasconcelos MA, Martelli-Junior H, et al. Risk factors for COVID-19-related mortality in hospitalized children and adolescents with diabetes mellitus: An observational retrospective cohort study. Pediatr Diabetes. 2022. Sep;23(6):763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sozeri B, Ulu K, Kaya-Akca U, Haslak F, Pac-Kisaarslan A, Otar-Yener G, et al. The clinical course of SARS-CoV-2 infection among children with rheumatic disease under biologic therapy: a retrospective and multicenter study. Rheumatol Int. 2022. Mar;42(3):469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Haslak F, Varol SE, Gunalp A, Kaynar O, Yildiz M, Adrovic A, et al. Comparisons of Clinical Features and Outcomes of COVID-19 between Patients with Pediatric Onset Inflammatory Rheumatic Diseases and Healthy Children. J Clin Med. 2022. Apr 9;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wakiguchi H, Kaneko U, Sato S, Imagawa T, Narazaki H, Miyamae T. Clinical Features of COVID-19 in Pediatric Rheumatic Diseases: 2020–2022 Survey of the Pediatric Rheumatology Association of Japan. Viruses. 2023. May 20;15(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Melo AT, Bernardo M, Pinheiro F, Rodrigues M, Torres R, Mourao AF, et al. Assessment of the outcomes of SARS-CoV-2 infection in children and young people followed at Portuguese pediatric rheumatology clinics. ARP Rheumatol. 2022. Oct 1;1(ARP Rheumatology, n masculine3 2022):205–9. [PubMed] [Google Scholar]
- 148.Morello W, Vianello FA, Bulgaro C, Montini G. Epidemiology, severity, and risk of SARS-CoV-2-related relapse in children and young adults affected by idiopathic nephrotic syndrome: a retrospective observational cohort study. Pediatr Nephrol. 2023. Apr;38(4):1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Marlais M, Wlodkowski T, Al-Akash S, Ananin P, Bandi VK, Baudouin V, et al. COVID-19 in children treated with immunosuppressive medication for kidney diseases. Arch Dis Child. 2020. Dec 21;106(8):798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weinbrand-Goichberg J, Ben Shalom E, Rinat C, Choshen S, Tzvi-Behr S, Frishberg Y, et al. COVID-19 in children and young adults with kidney disease: risk factors, clinical features and serological response. J Nephrol. 2022. Jan;35(1):121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mucalo L, Brandow AM, Singh A. A perspective on the sickle cell disease international COVID-19 registry. Best Pract Res Clin Haematol. 2022. Sep;35(3):101385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ryoma Kani AW, Masao Iwagami, Hisato Takagi, Jun Yasuhara, Toshiki Kuno. Efficacy and Safety of mRNA COVID-19 Vaccines in Children Aged 5–11 Years: A Systematic Review and Meta-Analysis. Journal of the Pediatric Infectious Diseases Society. 2023. November 2023;12:S5. [Google Scholar]
- 153.Oliveira CR, Niccolai LM, Sheikha H, Elmansy L, Kalinich CC, Grubaugh ND, et al. Assessment of Clinical Effectiveness of BNT162b2 COVID-19 Vaccine in US Adolescents. JAMA Netw Open. 2022. Mar 1;5(3):e220935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lin DY, Gu Y, Xu Y, Zeng D, Wheeler B, Young H, et al. Effects of Vaccination and Previous Infection on Omicron Infections in Children. N Engl J Med. 2022. Sep 22;387(12):1141–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Al-Sayyar A, Hulme KD, Thibaut R, Bayry J, Sheedy FJ, Short KR, et al. Respiratory Tract Infections in Diabetes - Lessons From Tuberculosis and Influenza to Guide Understanding of COVID-19 Severity. Front Endocrinol (Lausanne). 2022;13:919223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Karavanaki K, Rodolaki K, Soldatou A, Karanasios S, Kakleas K. Covid-19 infection in children and adolescents and its association with type 1 diabetes mellitus (T1d) presentation and management. Endocrine. 2023. May;80(2):237–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ammar LA, Nassar JE, Bitar F, Arabi M. COVID-19 in Cyanotic Congenital Heart Disease. Can J Infect Dis Med Microbiol. 2023;2023:5561159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011. Dec;64(12):1311–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.