Abstract
Due to the nonrenewable nature of neurons, recovery from viral infection of the central nervous system requires noncytopathic mechanisms for control of virus replication. Recovery from alphavirus encephalitis can occur without apparent neurological damage through the effects of antibody and gamma interferon (IFN-γ). To establish an in vitro cell culture system that will allow the study of mechanisms of IFN-γ-mediated control of Sindbis virus (SINV) replication in neurons, we have characterized the susceptibility to SINV infection and IFN-γ responsiveness of two neuronal cell lines that can be differentiated in vitro: CSM14.1, a rat nigral cell line, and NSC34, a mouse motor neuron cell line. Undifferentiated CSM14.1 and NSC34 cells were permissive for SINV and susceptible to virus-induced cell death. With differentiation, CSM14.1 cells reduced virus replication and became progressively resistant to virus-induced cell death, resulting in prolonged virus replication. NSC34 cells did not differentiate completely and became only partially resistant to SINV infection. Both CSM14.1 and NSC34 cells responded to pretreatment with IFN-γ by decreasing SINV replication. Differentiated CSM14.1 cells treated 24 h after infection with IFN-γ responded with increased cell viability and clearance of infectious virus. IFN-γ treatment sequentially altered the ratio of genomic to subgenomic viral RNA synthesis, promoted recovery of cellular protein synthesis, reduced viral protein synthesis, and inhibited viral RNA transcription within 24 h after treatment. We conclude that CSM14.1 cells provide an excellent model for the study of IFN-γ-mediated noncytolytic clearance of SINV from mature neurons.
Alphaviruses are an important cause of mosquito-borne encephalitis. Sindbis virus (SINV) is the prototype alphavirus in the Togaviridae family and is related to western equine encephalitis virus (23). SINV has a plus-strand, capped, and polyadenylated RNA genome of 11.7 kb which serves as mRNA for the nonstructural polyproteins P123 and P1234. The structural proteins, capsid, E1, and E2 are translated from a subgenomic RNA. E2 is synthesized as a precursor, pE2, that is cleaved to E2 and E3 late in replication to produce the mature E2 necessary for virions to be infectious. Heterodimers of E1 and E2 trimerize to form the spikes of the mature virion (60).
In humans, SINV infection causes clinical symptoms ranging from a minor influenza-like illness to polyarthritis and rash (35, 42). In mice, SINV infects neurons, resulting in a well-characterized encephalomyelitis (28). While neonatal mice die within the first few days after infection, adult immunocompetent mice clear SINV within 7 to 8 days (29, 36). In addition to age, the severity of disease in infected mice is dependent on the virus strain and genetic background of the mouse (28, 61, 62, 64). Innate and adaptive immune responses developed in response to SINV infection are critical for host survival and virus clearance (4, 6, 18, 39), and SINV-induced encephalitis is an excellent model system for study of the factors involved in determining the outcome of viral encephalomyelitis (4, 5, 17, 67, 68).
Recovery from virus infection of the central nervous system (CNS) is dependent upon elimination of the infecting virus and preservation of target cells. Elimination of virus-infected cells by major histocompatibility complex class I-restricted CD8+ cytotoxic T lymphocytes is an established and efficient strategy for eradicating virus from tissues. However, because neurons are terminally differentiated cells with very limited capacity for renewal, recovery from encephalomyelitis requires mechanisms for clearance of intracellular virus that do not harm the infected neurons (5, 17, 18, 48, 49).
The immune mediators involved in noncytolytic clearance of SINV from neuronal cells are incompletely understood. Type I interferon (IFN) is important for initial control of virus replication (6, 16, 55), and both humoral (6, 19, 39, 63) and cellular (4, 33) arms of the adaptive immune response play critical roles in facilitating clearance from the CNS. Passive transfer of SINV antibody to persistently infected immunodeficient SCID mice rapidly clears infectious virus from the CNS without neurologic sequelae (6, 39), and treatment of persistently infected mature dorsal root ganglia, neurons, and AT3-Bcl2 prostatic carcinoma cells with anti-E2 monoclonal antibody suppresses SINV replication (11, 12, 64). IFN-α/β acts synergistically with antiviral antibodies in vitro to facilitate virus clearance (11). T lymphocytes also contribute to the clearance of virus from certain populations of neurons. Viral RNA is reduced more slowly in brains of mice lacking CD8+ T cells (33), and IFN-γ can clear SINV from the spinal cord neurons of persistently infected immunodeficient mice (4).
Noncytolytic, cytokine-mediated clearance is increasingly recognized to be important for virus clearance. Tumor necrosis factor alpha, IFN-α/β, and IFN-γ control levels of hepatitis B virus replication in the liver and mediate clearance by T cells (20, 21, 22, 43, 69). IFN-γ protects the CNS from a number of neurotropic viruses (53), including SINV (4, 5, 17), measles virus (48, 49), hepatitis C virus (15), coronavirus (26), vesicular stomatitis virus (VSV) (10), yellow fever virus (41), and Theiler's murine encephalomyelitis virus (52) and mediates site-specific clearance of SINV without signs of paralysis or cytotoxicity (4). These in vivo studies suggest that the primary mechanism of T-cell-dependent viral clearance from neurons is mediated by IFN-γ through mechanisms that downregulate viral replication without damage to host cells (4, 9, 17, 49, 53).
The IFN-γ receptor is composed of two subunits, the ligand-binding IFN-γRα chain and the shorter, mostly intracellular, signaling IFN-γRβ chain (59). IFN-γ receptors are present on many cells, and the binding of IFN-γ homodimers results in activation of the Jak1/2 and STAT-1 signaling cascades (59). IFN-γ is important for activation of macrophages in defense against infection, but a wide variety of cells are responsive to induction of its direct antiviral activity. In the CNS, IFN-γ receptors are expressed at the highest levels on neurons and populations of neurons differ in the abundance of constitutively expressed receptors (51, 53, 66). Several regions of the CNS with high IFN-γ receptor expression receive projections from sensory neurons likely to be exposed to pathogens, suggesting that the constitutive distribution of the receptor may correlate with the role of IFN-γ in innate immunity and antiviral responses (53).
Although IFN-γ can induce an antiviral state, the actual downstream effector molecules responsible for noncytolytic resolution of viral infection are not known. Molecules implicated include RNA-dependent protein kinase (PKR), 2,5-oligoadenosine synthetase/RNase L, and nitric oxide (NO) synthetase (NOS)/NO, (10, 17, 20, 21, 22, 36, 43, 48, 49, 50, 53). However, IFN-γ regulates the transcription of many other cellular genes (20, 21, 36), and the specific molecules involved in inhibition of viral replication and in viral clearance remain poorly defined and may be different for different types of viruses and cells. Some of the difficulties in identifying these pathways relate to the complexity of the animal models, the redundancy of the antiviral pathways, and the lack of in vitro model systems for studying the process.
To initiate studies of molecular mechanisms of IFN-γ-dependent clearance of SINV from neurons, we have utilized the immortalized CSM14.1 rat neuronal cell line (13, 70) as an in vitro model system. CSM14.1 cells can be differentiated, develop prolonged SINV infection, and respond to IFN-γ by down regulating virus replication.
MATERIALS AND METHODS
Cell culture.
The rat CSM14.1 nigral neuronal cell line, immortalized with a temperature-sensitive simian virus 40 T antigen, was a gift from Dale E. Bredesen (Buck Institute for Age Research, Novato, Calif.) (13, 70). CSM14.1 cells were grown at the permissive temperature of 31°C in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2 mM glutamine (Gibco BRL). For differentiation, cells at 95% confluency in 12-well plates were shifted to the restrictive temperature of 39°C and growth medium was replaced with DMEM/1% FBS. Under these conditions, CSM14.1 cells stopped dividing and gradually differentiated to develop a mature neuronal phenotype (dCSM14.1 cells) (65).
The murine NSC34 cell line, produced by fusing mouse N18TB2 neuroblastoma cells with mouse motor neurons, was a gift from Neil Cashman (University of Toronto) (7, 14). NSC-34 cells were grown at 37°C in DMEM/10% FBS. For differentiation, cells were cultured at a density of 2 × 103 cells/cm2 in plates coated with poly-l-lysine and Matrigel (Becton-Dickinson Biosciences, Bedford, Mass.) and incubated with medium consisting of 1:1 DMEM and Ham's F12 (Gibco BRL) supplemented with 1% FBS, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2 mM glutamine (dNSC34 cells).
Virus infection and IFN-γ treatment of cells.
SINV strain 633 (SINV633; E2-55Q-172G) (62) expressing enhanced green fluorescent protein (eGFP) from a second subgenomic promoter was constructed by standard cloning DNA techniques as previously described (16, 24). Viral RNA was transcribed and transfected into BHK cells to produce stock virus. Amounts of SINV633 and SINV633-eGFP were assayed by plaque formation on BHK cells (24).
Differentiated CSM 14.1 cells were infected at a multiplicity of infection (MOI) of 1 (determined in BHK cells) with virus diluted in DMEM/1% FBS. For treatment with IFN-γ, medium was supplemented with rat recombinant IFN-γ (rIFN-γ; PBL Biomedical Laboratories, New Brunswick, N.J.). Differentiated NSC34 cells were infected at an MOI of 1 and treated with mouse rIFN-γ (Calbiochem Bioscience Inc., La Jolla, Calif.).
Cell viability was examined by trypan blue exclusion. Cells were trypsinized, washed once, and incubated with trypan blue at room temperature. Total numbers of viable cells/well were determined in triplicate.
Fluorescence microscopy.
Differentiated CSM14.1 cells were infected with SINV-eGFP or mock infected and treated with IFN-γ as described above. At 24, 48, 72, and 96 h after treatment, cells were washed with warm differentiation medium and examined with a Nikon Eclipse TE200 microscope. Green fluorescent (eGFP-positive cells) and phase-contrast pictures were captured and analyzed.
Analysis of protein synthesis.
Differentiated CSM14.1 cells were mock infected, SINV infected, or SINV infected and treated with IFN-γ as described above. At 2, 6, 12, 18, 24, 42, and 48 h after treatment, dCSM14.1 cells were incubated with methionine-free, cysteine-free DMEM (ICN, Aurora, Ohio) for 1 h, followed by methionine-free, cysteine-free DMEM containing 50 μCi/ml of 35S-translabel (ICN) for 1 h. Cells were then washed three times with ice-cold phosphate-buffered saline (PBS), lysed, and scraped in RIPA buffer (1% NP-40, 0.1% sodium dodecyl sulfate [SDS], 0.1% Na deoxycholate, 10 mM Tris-Cl [pH 7.0], 150 mM NaCl, 1 mM EDTA) with 25 μg of aprotinin/ml (Sigma-Aldrich, St. Louis, Mo.). All samples were stored at −80°C and then analyzed simultaneously.
Cell lysates were analyzed for incorporation of 35S into protein by precipitation with 10% trichloroacetic acid and counting in a scintillation counter. For analysis of the proteins synthesized, cell lysates (5 × 104 cpm/sample) were denatured by boiling for 5 min in 6× SDS loading buffer (0.5 M Tris [pH 6.8], 30% glycerol, 10% SDS, 0.12% bromophenol blue, 6% β-mercaptoethanol), followed by10% SDS-polyacrylamide gel electrophoresis (PAGE) and autoradiography.
For immunoprecipitation of SINV proteins, a 1:100 dilution of rabbit polyclonal antiserum against SINV (18) and immobilized protein A (Pierce, Rockford, Ill.) were used (11, 12). Cell lysates or supernatant fluids were incubated with SINV antiserum overnight at 4°C and then with 50 μl of protein A-conjugated beads at room temperature for 2 h. Immunocomplexed beads were washed three times with immunoprecipitation buffer (RIPA and 25 μg of aprotinin/ml), resuspended in 30 μl of 6× SDS loading buffer, and boiled for 10 min. Immunoprecipitated SINV structural proteins were analyzed by 10% SDS-PAGE.
For pulse-chase analysis of newly synthesized proteins, cells were washed once with PBS and twice with methionine-free, cysteine-free DMEM, incubated with 50 μCi of 35S-translabel/ml at 39°C for 30 min, and then washed twice with chase medium (DMEM/1% FBS with 15 mg of l-methionine/L). Cell lysates and supernatant fluids were collected at different times postchase and analyzed as described above. Supernatant fluids were ethanol precipitated (11, 12), immunoprecipitated, or loaded directly by equal volume onto a 10% SDS-PAGE gel.
Analysis of viral RNA synthesis.
Differentiated CSM14.1 cells were labeled for 2 h with DMEM containing 20 μCi of [5,6-3H]uridine/ml (NEN, Boston, Mass.) and 1 μg of actinomycin D/ml (Calbiochem, San Diego, Calif.). Cells were washed once with PBS, lysed with 250 μl of RNA STAT-60/well (RNAzol reagent; Tel-Test, Friendswood, Tex.), and scraped off. RNA was isolated with a standard chloroform extraction protocol, and samples were stored in isopropanol at −20°C overnight. The next day, RNA was precipitated with ethanol, stored at −80°C, and analyzed by agarose gel electrophoresis (11, 12, 16). Autoradiographs were imaged, and ratios of genomic to subgenomic RNA were determined using NIH Image.
RESULTS
Cell death and virus replication in undifferentiated and differentiated CSM14.1 neuronal cells.
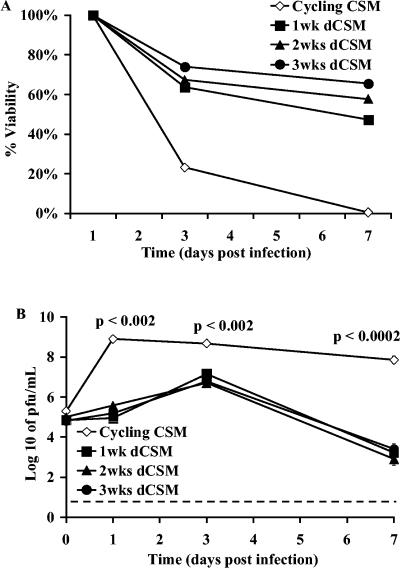
In vivo, SINV induces cell death in immature neurons but not in mature neurons (36). To identify a cell line that reflected these properties, we studied neuronal cell lines that could be differentiated in vitro. CSM14.1 is a rat nigral cell line, immortalized by a temperature-sensitive simian virus 40 T antigen (13, 70), that differentiates under restrictive culture conditions. To determine the effect of SINV infection on these cells, CSM14.1 cells that were undifferentiated and differentiated for various periods of time, from 1 to 3 weeks, were infected with SINV and viability assessed (Fig. 1A). Cycling, undifferentiated cells were susceptible to viral cytopathic effects, and all cells were dead by 7 days after infection. With differentiation, the cells ceased dividing and became increasingly resistant to SINV-induced cell death. The viability 7 days after infection of cells differentiated for 1 week was 47%, for 2 weeks was 58%, and for 3 weeks was 65%. As CSM14.1 cells differentiated, they remained susceptible to SINV infection (Fig. 1B), but virus growth was restricted and peak virus production was approximately 100-fold less than that of undifferentiated cells (P = 0.0013). This restriction is not due to differences in culture conditions because virus grows equally well in undifferentiated cells cultured under permissive and nonpermissive conditions (65).
FIG. 1.
SINV replication in undifferentiated and differentiated CSM14.1 (dCSM) cells. Cycling undifferentiated CSM14.1 cells and 1-, 2-, and 3-week differentiated CSM14.1 cells were analyzed for cell viability by trypan blue exclusion (A) and SINV replication (B). Cells were infected with SINV-eGFP at an MOI of 1. Viability is expressed as the percentage of live infected to live mock-infected cells. Each point represents the average and standard error of the mean of three individual wells. The dashed line indicates the limit of virus detection.
IFN-γ pretreatment protected undifferentiated and differentiated CSM14.1 cells from SINV infection and virus-induced cell death.
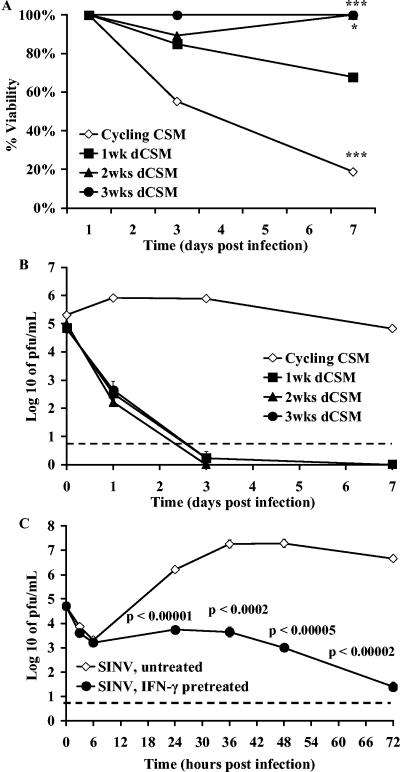
To determine whether undifferentiated and differentiated CSM14.1 cells could respond to IFN-γ with induction of an antiviral state, cells were pretreated with 100 U/ml of rat rIFN-γ 24 h before SINV infection (Fig. 2). Pretreatment with IFN-γ improved the viability of both undifferentiated and differentiated CSM14.1 cells (Fig. 2A) (cycling, P = 0.00043; 1 week, P = 0.07; 2 weeks, P = 0.02; 3 weeks, P = 0.0008), decreased SINV replication in undifferentiated cells, and prevented replication in differentiated cells (Fig. 2B). To determine whether replication was completely suppressed, virus growth was examined more closely over the first 72 h after infection of IFN-γ-pretreated 3-week differentiated CSM14.1 cells (Fig. 2C). Virus replication was suppressed at all time points (P < 0.00001). Therefore, both undifferentiated and differentiated CSM14.1 cells were able to respond to IFN-γ by induction of an antiviral state, but differentiated cells responded most completely.
FIG. 2.
Effect of treatment with IFN-γ before SINV infection. Cycling undifferentiated CSM14.1 cells and 1-, 2-, and 3-week differentiated CSM14.1 (dCSM) cells were treated with 100 U of IFN-γ/ml 24 h before infection and analyzed for cell viability by trypan blue exclusion (A) and SINV replication over 7 days (B) and over 3 days in 3-week differentiated cells (C). Cells were infected with SINV-eGFP at an MOI of 1. Cell viability was improved and virus replication was reduced in all groups compared to untreated cells (*, P < 0.05; **, P < 0.01; ***, P < 0.001; Student's t test). The dashed line indicates the limit of virus detection.
IFN-γ treatment of infected dCSM14.1 cells protected against cell death and suppressed virus replication.
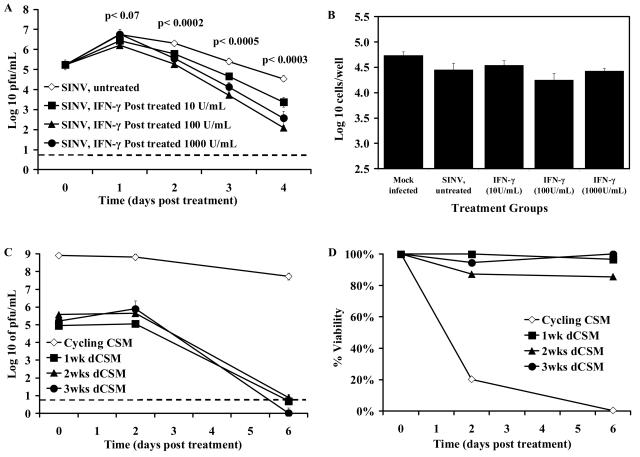
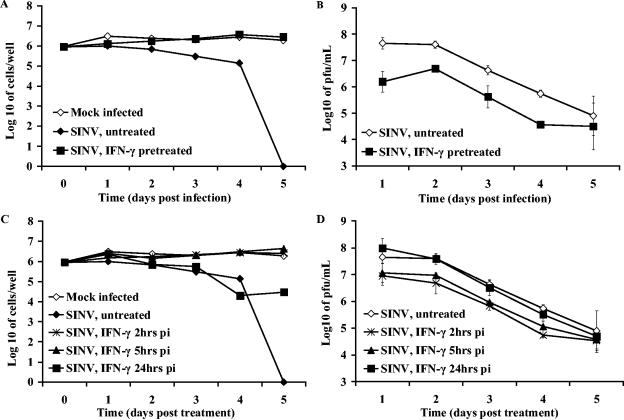
To determine whether SINV replication could be suppressed after viral infection had been established in differentiated CSM14.1 cells, cells were treated with 10, 100, and 1,000 U/ml of rat rIFN-γ 24 h after infection. At this time, most, if not all, cells were infected. SINV production and cell viability were monitored at different times after rIFN-γ treatment (Fig. 3A and B). Replication was significantly reduced by IFN-γ treatment (Fig. 3A), and this reduction was dose dependent, with 100 U/ml providing better suppression than 10 U/ml (P = 0.0003 at day 4 postinfection [p.i.]). The highest dose of 1,000 U/ml did not provide better suppression than 100 U/ml, and therefore 100 U/ml was used for subsequent studies. SINV-infected dCSM14.1 cells that were left untreated or treated with different doses of rIFN-γ remained viable, indicating that inhibition of viral replication in differentiated cells was nonlytic (Fig. 3B).
FIG. 3.
Effect of treatment with IFN-γ after SINV infection. Undifferentiated and differentiated CSM14.1 (dCSM) cells were infected with SINV-eGFP at an MOI of 1 and 24 h later treated with 0, 10, 100, or 1,000 U of rat rIFN-γ/ml. Virus production (A) and numbers of viable cells 8 days after treatment (B) of 2-week differentiated cells were determined. P values (Student's t test) compared SINV-infected untreated with rIFN-γ (100 U/ml)-treated cells (A). Undifferentiated cells and cells differentiated for various periods of time were treated with 100 U of IFN-γ, and virus production (C) and cell viability (D) were determined. Each point represents the average and standard error of the mean of three individual wells. The dashed line indicates the limit of virus detection.
To further assess the effects of treatment with rIFN-γ after infection on SINV-infected neurons, CSM14.1 cells, undifferentiated or differentiated for various lengths of time, were infected with SINV and treated with rIFN-γ 24 h after infection (Fig. 3C and D).
Replication of SINV in undifferentiated CSM14.1 cells was not affected by treatment with IFN-γ, while in differentiated neurons replication was suppressed so that infectious virus could no longer be detected 6 days after treatment (Fig. 3C). Treatment with IFN-γ after infection protected differentiated, but not undifferentiated, CSM14.1 cells from SINV-induced cell death (Fig. 3D). Effects were similar on cells differentiated for 1, 2, and 3 weeks. These data demonstrated that treatment with rIFN-γ after viral infection protected mature neurons and inhibited SINV replication by noncytolytic means.
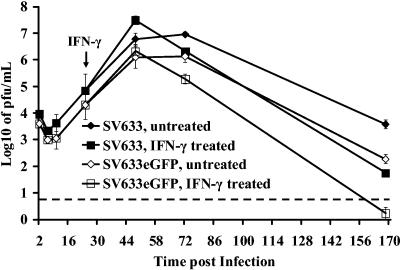
To determine whether the outcome would be the same with parental SINV633 as with the double subgenomic virus SINV633-eGFP, the effects of IFN-γ treatment 24 h after infection were determined. SINV633 replicated 25-fold better than SINV633-eGFP (Fig. 4), but cell viabilities were similar (data not shown). SINV633-infected, as well as SINV633-eGFP-infected differentiated CSM14.1 cells responded to IFN-γ treatment 24 h after infection by completely protecting mature neurons from cell death and restricting virus replication (Fig. 4).
FIG. 4.
Comparison of SINV633 and SINV633-eGFP replication and response to IFN-γ in differentiated CSM14.1 cells. CSM14.1 cells differentiated for 3 weeks were infected with either double subgenomic recombinant SINV633-eGFP or SINV633. Cells were infected at an MOI of 1 and treated with IFN-γ (100 U/ml) 24 h p.i. Each point represents the average and standard error of the mean of three individual wells. The dashed line indicates the limit of virus detection.
Effect of IFN-γ on SINV infection of NSC34 cells.
In vivo studies indicate that motor neurons in the brainstem and spinal cord respond to the antiviral effects of IFN-γ better than cortical or hippocampal neurons (4). To determine whether cells with a motor neuron phenotype would provide a better in vitro model for studies of the effects of IFN-γ, the mouse neuroblastoma-motor neuron hybrid cell line NSC34 (7) was studied (Fig. 5). Unlike CSM14.1 cells, NSC34 cells do not completely leave the cell cycle under differentiation conditions and therefore do not become completely differentiated. NSC34 cells differentiated for 12 days replicated SINV to high titer, and all cells were dead by 5 days after infection (Fig. 5A). Pretreatment of dNSC34 motor neurons with mouse rIFN-γ protected them against SINV-induced cell death (Fig. 5A) and inhibited virus replication 31-fold compared to untreated cells (Fig. 5B) (P = 0.0043).
FIG. 5.
SINV infection and IFN-γ treatment of differentiated NSC34 cells. Differentiated NSC34 cells were infected with SINV-eGFP at an MOI of 1 and assayed for cell viability (A) and SINV replication (B) with or without treatment with 100 U/ml of mouse rIFN-γ for 24 h before infection. NSC34 cells were treated with 100 U of rIFN-γ/ml at 2, 5, and 24 h after SINV infection and analyzed for cell viability (C) and SINV replication (D). Cell viability was analyzed by trypan blue exclusion and displayed as log10 of the number of viable cells/well. Each point represents the average and standard error of the mean of three individual wells.
Differentiated NSC34 cells treated 2 or 5 h after infection were completely protected, remaining viable for 5 days (Fig. 5C), and virus replication was decreased approximately 10-fold (Fig. 5D). Treatment with rIFN-γ 24 h after infection was less effective, but there was substantial improvement in viability compared to untreated, SINV-infected cells (Fig. 5C). Overall, viral titers steadily decreased in the rIFN-γ treated cells, but unlike dCSM14.1 cells, virus was not cleared before the dNSC34 cells reached confluence and died. Because the continued proliferation and the mixed developmental stage of dNSC34 cells presented several confounding factors, subsequent studies were performed with dCSM14.1 cells differentiated for 2 to 3 weeks and treated with 100 U/ml of IFN-γ.
IFN-γ treatment of infected dCSM14.1 inhibited viral gene expression.
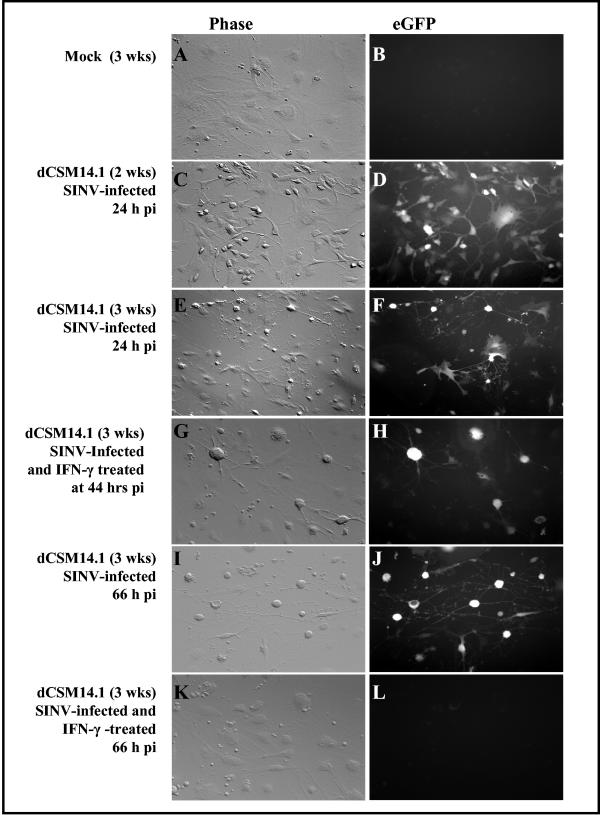
To investigate the effect of IFN-γ treatment on gene expression, dCSM14.1 cells were examined for expression of SINV-derived eGFP (Fig. 6). At the time of rIFN-γ treatment, 24 h after infection, there was extensive expression of eGFP, showing widespread infection of dCSM14.1 cells (Fig. 6D and F). At 20 h after rIFN-γ treatment (44 h p.i.), eGFP expression was still detected in rIFN-γ treated cells (Fig. 6H), but by 42 h after treatment (66 h p.i.) no eGFP was detected (Fig. 6L), while there was extensive eGFP expression in untreated cells (Fig. 6J). These results indicated that viral protein synthesis was inhibited in treated cells. Cells remained viable throughout (Fig. 6G and K).
FIG. 6.
Effect of IFN-γ on viral protein expression in dCSM14.1 cells. Differentiated CSM14.1 (dCSM14.1) cells were mock infected (A, B) or infected with SINV-eGFP (C-L). CSM14.1 cells differentiated for 2 or 3 weeks were examined by phase (C, E) and fluorescence (D, F) microscopy 24 h after infection just prior to rIFN-γ treatment. Cells differentiated for 3 weeks were examined 20 h (G, H) and 42 h after treatment (I to L). At 42 h after rIFN-γ treatment, eGFP expression was extensive in untreated dCSM14.1 cells (J), but eGFP could not be detected in cells that had been treated with rIFN-γ (L).
SINV protein synthesis is reduced and host cell protein synthesis is reinitiated after rIFN-γ treatment.
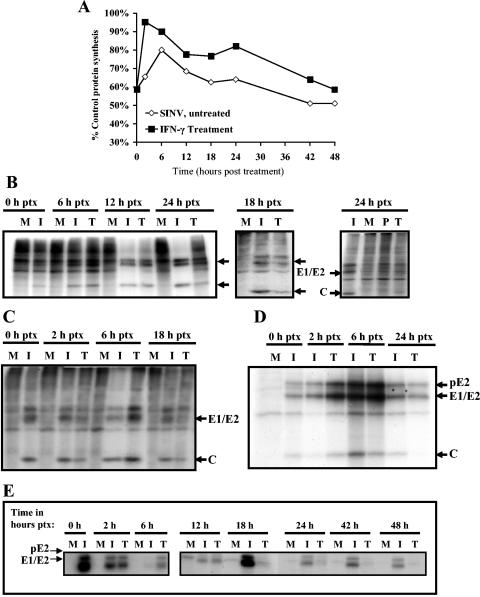
SINV infection inhibits host cell protein synthesis in mammalian cells (31). To identify changes in levels of viral and host protein synthesis in dCSM14.1 cells treated with rIFN-γ after SINV infection, we examined incorporation of 35S into newly synthesized proteins at various times after infection and treatment (Fig. 7). Before rIFN-γ treatment, SINV-infected cells had reduced overall protein labeling, consistent with a shutdown of host protein synthesis (Fig. 7A). At 2 h after treatment, there was an increase in the amount of protein synthesized in the rIFN-γ-treated cells and protein synthesis remained higher than in untreated cells (Fig. 7A). The types of synthesized proteins were analyzed by PAGE (Fig. 7B and C) and immunoprecipitation (Fig. 7D and E). There was an increase in the synthesis of both cellular and viral proteins by 6 h after IFN-γ treatment. Production of viral proteins in IFN-γ-treated cells then decreased below the levels synthesized in untreated cells, and synthesis of new viral proteins was undetectable 24 h after treatment. Cellular protein synthesis was restored in IFN-γ-treated cells and remained higher than in untreated cells.
FIG. 7.
Effect of IFN-γ on viral and host cell protein synthesis in dCSM14.1 cells. Protein synthesis was assessed by 35S incorporation by infected differentiated CSM (dCSM) cells labeled for 1 h at various times after treatment. (A) Incorporation of 35S into trichloroacetic acid-precipitable intracellular proteins. (B, C) Total protein synthesis, as analyzed in two separate experiments by PAGE, in mock-infected (M), SINV-infected (I), and SINV-infected/rIFN-γ-treated (T) dCSM14.1 cells at 0, 6, 12, 18, and 24 h (B) or 0, 3, 6, and 12 h (C) posttreatment (ptx). IFN-γ treatment of dCSM14.1 cells 24 h prior to infection (P) was also analyzed in the 24-h ptx gel (72 h after treatment, 48 h after infection) (B). (D, E) Immunoprecipitation of 35S-labeled viral structural proteins from lysates of mock-infected (M), SINV-infected (I), and SINV-infected/rIFN-γ-treated (T) dCSM14.1 cells from two separate experiments at 0, 3, 6, and 24 h (D) or 0, 2, 6, 12, 18, 24, 42, and 48 h (E) after treatment. Results displayed are representative of multiple experiments. C, capsid.
Viral RNA transcription is reduced after rIFN-γ treatment.
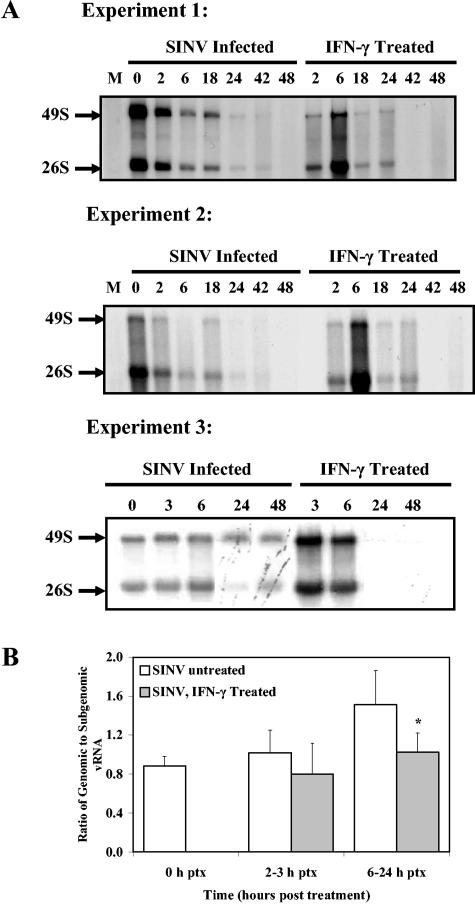
Several steps of viral replication are possible targets for intracellular antiviral factors. Because all dCSM14.1 cells were infected at the time of treatment, initial steps of entry were not involved. RNA labeling showed that viral RNA transcription was active at the time of rIFN-γ treatment, 24 h after infection (Fig. 8A, time zero). At 6 h there was a general increase in viral RNA synthesis in IFN-γ-treated cells, but by 18 h viral RNA transcription was substantially reduced in IFN-γ-treated cells compared with untreated cells, and by 42 h after treatment viral RNA synthesis was no longer detectable in treated cells (Fig. 8A). The ratio of genomic to subgenomic RNA averaged 0.88 in cells prior to treatment and increased to 1.53 in untreated cells over 24 h of study, while in IFN-γ-treated cells the ratios plateaued at 1.02 (P = 0.045) (Fig. 8B).
FIG. 8.
Viral RNA (vRNA) transcription in SINV-infected dCSM14.1 cells with and without rIFN-γ treatment. CSM14.1 cells were mock infected (M) or SINV infected for 24 h (0 h after treatment) and then treated with 100 U/ml of rIFN-γ. Cells with or without treatment were labeled with [3H]uridine for 2 h in the presence of actinomycin D at 2, 6, 18, 24, 42, and 48 h (experiments 1 and 2) or 3, 6, 24, and 48 h (experiment 3) after treatment. Newly synthesized RNA was examined by agarose gel electrophoresis and autoradiography (A). Ratios of genomic to subgenomic RNA were determined using NIH Image and averaged from six separate experiments (B). *, P = 0.045 (Student's t test).
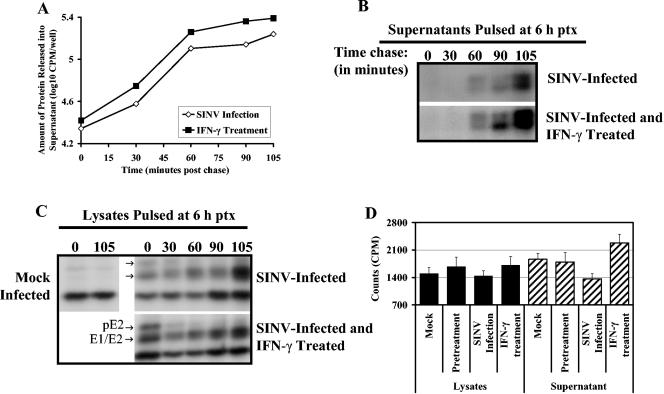
Release of viral proteins is not affected at 6 h after rIFN-γ treatment.
Treatment of SINV-infected cells with anti-E2 monoclonal antibodies results in inhibition of virus budding from infected cells and accumulation of immature particles at the plasma membrane (11, 12). To determine whether increased levels of accumulated viral structural proteins within cells 6 h after treatment (Fig. 7) indicated a deficiency in viral protein release, cells were labeled at 6 h after treatment and chased for different periods of time (Fig. 9). Newly synthesized protein released into supernatant fluids during the course of the pulse-chase suggested that more protein was being synthesized and released in the rIFN-γ-treated cells than in untreated cells (Fig. 9A). 35S-labeled viral proteins appeared in the supernatant fluid as early as 1 h after chase and were equal in IFN-γ-treated and untreated cells (Fig. 9B). However, by 90 min after chase there was a higher level of labeled viral protein released from IFN-γ-treated cells, consistent with the increase in viral protein synthesis at this time (Fig. 7). The decrease and disappearance of the precursor E2 (pE2) band from immunoprecipitated cell lysates at 30 to 60 min after chase indicated that viral structural proteins were processed efficiently in both treated and untreated cells (Fig. 9C). Analysis of cellular lysates and supernatant fluids at 15 h postchase showed that treated cells continued to release higher levels of labeled protein (Fig. 9D).
FIG. 9.
Pulse-chase analysis of protein synthesized 6 h after IFN-γ treatment. Cells were labeled with 35S for 30 min at 6 h after rIFN-γ treatment and analyzed at different times after chase. (A) Analysis of total labeled proteins released into the supernatant fluid of untreated or treated cells, 0 to 105 min after chase. (B) Immunoprecipitation of viral proteins from supernatant fluids. Top row are supernatant fluids from SINV-infected cells, and bottom row are supernatant fluids from SINV-infected, IFN-γ-treated cells. (C) Analysis of viral protein processing by immunoprecipitation of viral proteins from cell lysates. Top row are lysates of SINV-infected cells, and bottom row SINV-infected and rIFN-γ-treated cells. (D) Total labeled protein in cell lysates and supernatant fluids at 15 h after chase. Samples were quantitated by scintillation counts in triplicate. ptx, posttreatment.
DISCUSSION
IFN-γ is an important cytokine for the noncytolytic control of SINV infection of neurons in vivo (4), but in vitro models for study of mechanisms of virus clearance have not been available. We used a rat neuronal cell culture system, CSM14.1 cells (13, 70), that could be differentiated in vitro. CSM14.1 cells were resistant to SINV-induced death and continued to produce virus for many days after infection. CSM14.1 cells were responsive to IFN-γ so that pretreatment prevented replication of SINV in differentiated neurons and decreased replication in undifferentiated neurons by about 1,000-fold. IFN-γ treatment improved the survival of undifferentiated and differentiated neuron cells treated before or after infection. When treated with IFN-γ 24 h after infection, SINV-infected differentiated CSM14.1 cells cleared virus infection. This was associated with a rapid decrease in the ratio of genomic to subgenomic RNA synthesis. A transient increase in synthesis of total viral RNA and protein at 6 h after treatment was followed by cessation of viral protein and RNA synthesis and restoration of host cell protein synthesis. There was no inhibition of viral protein processing or release of viral proteins by IFN-γ treatment. These data indicated that IFN-γ treatment activated signaling cascades and antiviral factors that led to a series of cellular changes that directly affected viral RNA transcription, viral protein synthesis, and virus production by infected neurons.
Age of the host is a critical factor determining the outcome of SINV infection and neuronal maturity correlates with resistance to viral replication and virus-induced cell death (29, 36, 38). While young mice succumb to lethal encephalitis, adult mice are able to clear viral infection with pathology in the CNS that includes mononuclear cell infiltration but does not include neuronal damage or clinical signs of disease. Cycling immature CSM14.1 and NSC34 cells were susceptible to SINV-induced cytotoxicity, while mature CSM14.1 cells were substantially more resistant. IFN-γ treatment, either before or after infection, provided some protection to immature CSM14.1 cells, but mature CSM14.1 cells were completely protected from SINV-induced death. Likewise, SINV replication was not completely eliminated in undifferentiated CSM14.1 cells, as it was in the differentiated CSM14.1 cells. These data suggest that IFN-γ-inducible factors that promote cell survival might differ from those that mediate viral clearance and that mature neurons respond more completely to IFN-γ treatment than immature neurons. The cellular factors expressed during neuronal maturation that may affect responsiveness to IFN-γ are at present unknown.
Many neuronal populations within the CNS constitutively express receptors for IFN-γ (45, 51, 66), but functional responses differ substantially depending on the cell type, neuronal maturity, and length of exposure to this cytokine and others that may be simultaneously expressed during an in vivo immune response. In vitro, IFN-γ can decrease proliferation, promote differentiation (2, 25, 27, 30), improve survival (8, 49, 52), and induce an antiviral state in selected neurons (9, 10, 40, 44, 46, 58). For instance, some neuroblastoma cell lines and embryonic neurons respond to IFN-γ by decreasing proliferation and promoting differentiation (2, 25, 27, 30). IFN-γ treatment of cultured embryonic rat sympathetic neurons inhibits initial dendrite outgrowth and in mature neurons induces retraction of existing dendrites (32). Long-term exposure to IFN-γ can result in neurodegeneration, demyelination, and excitotoxic damage in vitro (1) and in vivo (47, 54). In these studies, the effect of IFN-γ on cells of the rat nigral cell line CSM14.1 were protective and antiviral.
Noncytolytic clearance of viral infection depends on the ability of the infected cell to activate the required pathways for inhibition of viral replication, as well as the susceptibility of the virus to activated pathways (20, 21). SINV replication is susceptible to intracellular antiviral factors, and mature neurons are able to activate pathways that result in noncytolytic clearance of virus infection. SINV replication can be inhibited in many types of cells by pretreatment with IFN-α/β, and the present studies have shown that pretreatment and postinfection treatment with IFN-γ have a direct antiviral effect on SINV-infected mature neurons. However, the downstream signaling events and effector molecules responsible either for preventing or resolving SINV infection are not understood. Antiviral proteins known to play important roles in inhibition of replication of other viruses include PKR, 2,5-oligoadenosine synthetase/RNase L, double-stranded RNA-specific adenosine deaminase, NOS/NO, and the Mx GTPase proteins (9, 53). However, mice lacking PKR, RNase L, and Mx1 mount IFN-dependent antiviral responses and inhibit SINV (56), VSV, and encephalomyocarditis virus (71) replication in vivo and in vitro. NOS-dependent mechanisms play critical roles in inhibiting VSV, poliovirus, and herpes simplex virus type 1 replication in neurons (34, 53), but inhibition of SINV and influenza virus replication is not NOS dependent (34, 50).
SINV downregulated translation of cellular proteins and increased viral protein synthesis in differentiated CSM14.1 cells in a manner typical of the response of other types of cells to SINV infection. However, the cells survived infection and responded to IFN-γ by shutting down virus replication. Sequential changes in SINV-infected neurons were recognized after IFN-γ treatment. Between 3 and 6 h, there was a general increase in cellular protein synthesis, viral protein synthesis, and viral RNA transcription. This period of improved cellular function was followed by a gradual elimination of viral protein and RNA synthesis. This suggested that multiple mechanisms were activated and converged to inhibit virus replication and that de novo cellular protein synthesis might be required for complete inhibition of SINV replication. The only antiviral protein that has been clearly associated with inhibition of alphavirus replication is zinc finger antiviral protein (ZAP) (3). ZAP inhibits the translation of nonstructural proteins off incoming genomic RNA by an unknown mechanism, making ZAP a candidate for establishing the antiviral state in uninfected neurons. Decreased production of nonstructural proteins would limit synthesis of new minus-strand RNAs because the P123 polyprotein, plus nsP4, is required for minus-strand synthesis. P123 is processed by nsP2, and fully processed nsP1, nsP2, and nsP3 plus nsP4 show a preference for the subgenomic promoter, and changes in abundance could affect the relative amounts of genomic and subgenomic RNA synthesized (37, 57). However, minus-strand synthesis is normally shut off early in alphavirus infection and genomic and subgenomic plus-strand RNAs continued to be produced. Therefore, clearance of established infection appears to require unidentified, IFN-γ-induced effector molecules that function at later stages of viral replication. Further study will be needed to define these effects more clearly.
In this study we have presented an in vitro cell system that can replicate the in vivo responses to SINV infection and IFN-γ-mediated clearance. Our data suggest that suppression of genomic viral RNA synthesis and reactivation of cellular protein synthesis are involved in abolishing established SINV infection and in the promotion of survival of differentiated neurons infected with SINV.
Acknowledgments
We thank Dale E. Bredesen and Neal R. Cashman for kindly providing the CSM14.1 and NSC34 cell lines; Douglas Kerr, Ching G. Ng, Calvin Chue, Patty S. Vernon, and Ron Knight for helpful discussions and experimental design assistance; and Ian T. Kerr for providing valuable discussions and technical support.
This work was supported by research grant NS38932 and training grant AI07417 from the National Institutes of Health.
REFERENCES
- 1.Bal-Price, A., and G. C. Brown. 2001. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J. Neurosci. 21:6480-6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barish, M. E., N. B. Mansdorf, and S. S. Raissdana. 1991. Gamma-interferon promotes differentiation of cultured cortical and hippocampal neurons. Dev. Biol. 144:412-423. [DOI] [PubMed] [Google Scholar]
- 3.Bick, M. J., J. W. Carroll, G. Gao, S. P. Goff, C. M. Rice, and M. R. MacDonald. 2003. Expression of the zinc-finger antiviral protein inhibits alphavirus replication. J. Virol. 77:11555-11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binder, G. K., and D. E. Griffin. 2001. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science 293:303-306. [DOI] [PubMed] [Google Scholar]
- 5.Binder, G. K., and D. E. Griffin. 2003. Immune-mediated clearance of virus from the central nervous system. Microbes Infect. 5:439-448. [DOI] [PubMed] [Google Scholar]
- 6.Byrnes, A. P., J. E. Durbin, and D. E. Griffin. 2000. Control of Sindbis virus infection by antibody in interferon-deficient mice. J. Virol. 74:3905-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cashman, N. R., H. D. Durham, J. K. Blusztajn, K. Oda, T. Tabira, I. T. Shaw, S. Dahrouge, and J. P. Antel. 1992. Neuroblastoma × spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev. Dyn. 194:209-221. [DOI] [PubMed] [Google Scholar]
- 8.Chang, J. Y., D. P. Martin, and E. M. Johnson, Jr. 1990. Interferon suppresses sympathetic neuronal cell death caused by nerve growth factor deprivation. J. Neurochem. 55:436-445. [DOI] [PubMed] [Google Scholar]
- 9.Chesler, D. A., J. L. Munoz-Jordan, N. Donelan, A. Garcia-Sastre, and C. S. Reiss. 2003. PKR is not required for interferon-gamma inhibition of VSV replication in neurons. Viral Immunol. 16:87-96. [DOI] [PubMed] [Google Scholar]
- 10.Chesler, D. A., and C. S. Reiss. 2002. The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev. 13:441-454. [DOI] [PubMed] [Google Scholar]
- 11.Despres, P., J. W. Griffin, and D. E. Griffin. 1995. Antiviral activity of alpha interferon in Sindbis virus-infected cells is restored by anti-E2 monoclonal antibody treatment. J. Virol. 69:7345-7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Despres, P., J. W. Griffin, and D. E. Griffin. 1995. Effects of anti-E2 monoclonal antibody on Sindbis virus replication in AT3 cells expressing bcl-2. J. Virol. 69:7006-7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durand, M., D.C. Chugani, M. Mahmoudi, and M. E. Phelps. 1990. Characterization of neuron-like cell line immortalized from primary rat mesencephalon cultures. Soc. Neurosci. Abstr. 16:40. [Google Scholar]
- 14.Durham, H. D., S. Dahrouge, and N. R. Cashman. 1993. Evaluation of the spinal cord neuron × neuroblastoma hybrid cell line NSC-34 as a model for neurotoxicity testing. Neurotoxicology 14:387-395. [PubMed] [Google Scholar]
- 15.Frese, M., V. Schwarzle, K. Barth, N. Krieger, V. Lohmann, S. Mihm, O. Haller, and R. Bartenschlager. 2002. Interferon-gamma inhibits replication of subgenomic and genomic hepatitis C virus RNAs. Hepatology 35:694-703. [DOI] [PubMed] [Google Scholar]
- 16.Frolova, E. I., R. Z. Fayzulin, S. H. Cook, D. E. Griffin, C. M. Rice, and I. Frolov. 2002. Roles of nonstructural protein nsP2 and alpha/beta interferons in determining the outcome of Sindbis virus infection. J. Virol. 76:11254-11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin, D. E. 2003. Immune responses to RNA-virus infections of the CNS. Nat. Rev. Immunol. 3:493-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin, D. E., B. Levine, W. R. Tyor, and D. N. Irani. 1992. The immune response in viral encephalitis. Semin. Immunol. 4:111-119. [PubMed] [Google Scholar]
- 19.Griffin, D. E., S. Ubol, P. Despres, T. Kimura, and A. Byrnes. 2001. Role of antibodies in controlling alphavirus infection of neurons. Curr. Top. Microbiol. Immunol. 260:191-200. [DOI] [PubMed] [Google Scholar]
- 20.Guidotti, L. G. 2002. The role of cytotoxic T cells and cytokines in the control of hepatitis B virus infection. Vaccine 4:A80-A82. [DOI] [PubMed] [Google Scholar]
- 21.Guidotti, L. G., and F. V. Chisari. 2000. Cytokine-mediated control of viral infections. Virology 273:221-227. [DOI] [PubMed] [Google Scholar]
- 22.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 23.Hahn, C. S., S. Lustig, E. G. Strauss, and J. H. Strauss. 1988. Western equine encephalitis virus is a recombinant virus. Proc. Natl. Acad. Sci. USA 85:5997-6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardwick, J. M., and B. Levine. 2000. Sindbis virus vector system for functional analysis of apoptosis regulators. Methods Enzymol. 322:492-508. [DOI] [PubMed] [Google Scholar]
- 25.Higuchi, T., A. Tanaka, H. Hiratani, H. Watanabe, and J. Imanishi. 1989. Variant human neuroblastoma cell lines resistant to the differentiation-inducing effect of interferon-gamma. Cell Struct. Funct. 14:439-445. [DOI] [PubMed] [Google Scholar]
- 26.Hooks, J. J., Y. Wang, and B. Detrick. 2003. The critical role of IFN-gamma in experimental coronavirus retinopathy. Investig. Ophthalmol. Vis. Sci. 44:3402-3408. [DOI] [PubMed] [Google Scholar]
- 27.Improta, T., A. M. Salvatore, A. Di Luzio, G. Romeo, E. M. Coccia, and P. Calissano. 1988. IFN-gamma facilitates NGF-induced neuronal differentiation in PC12 cells. Exp. Cell Res. 179:1-9. [DOI] [PubMed] [Google Scholar]
- 28.Jackson, A. C., T. R. Moench, D. E. Griffin, and R. T. Johnson. 1987. The pathogenesis of spinal cord involvement in the encephalomyelitis of mice caused by neuroadapted Sindbis virus infection. Lab. Investig. 56:418-423. [PubMed] [Google Scholar]
- 29.Johnson, R. T., H. F. McFarland, and S. E. Levy. 1972. Age-dependent resistance to viral encephalitis: studies of infections due to Sindbis virus in mice. J. Infect. Dis. 125:257-262. [DOI] [PubMed] [Google Scholar]
- 30.Jonakait, G. M., R. Wei, Z. L. Sheng, R. P. Hart, and L. Ni. 1994. Interferon-gamma promotes cholinergic differentiation of embryonic septal nuclei and adjacent basal forebrain. Neuron 12:1149-1159. [DOI] [PubMed] [Google Scholar]
- 31.Kaariainen, L., and M. Ranki. 1984. Inhibition of cell functions by RNA-virus infections. Annu. Rev. Microbiol. 38:91-109. [DOI] [PubMed] [Google Scholar]
- 32.Kim, I. J., H. N. Beck, P. J. Lein, and D. Higgins. 2002. Interferon gamma induces retrograde dendritic retraction and inhibits synapse formation. J. Neurosci. 22:4530-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura, T., and D. E. Griffin. 2000. The role of CD8+ T cells and major histocompatibility complex class I expression in the central nervous system of mice infected with neurovirulent Sindbis virus. J. Virol. 74:6117-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komatsu, T., Z. Bi, and C. S. Reiss. 1996. Interferon-gamma induced type I nitric oxide synthase activity inhibits viral replication in neurons. J. Neuroimmunol. 68:101-108. [DOI] [PubMed] [Google Scholar]
- 35.Kurkel, S. 2004. Causative agent of Pogosta disease isolated from blood and skin lesions. Emerg. Infect. Dis. 10:889-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labrada, L., X. H. Liang, W. Zheng, C. Johnston, and B. Levine. 2002. Age-dependent resistance to lethal alphavirus encephalitis in mice: analysis of gene expression in the central nervous system and identification of a novel interferon-inducible protective gene, mouse ISG12. J. Virol. 76:11688-11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemm, J. A., T. Rumenapf, E. G. Strauss, J. H. Strauss, and C. M. Rice. 1994. Polypeptide requirements for assembly of functional Sindbis virus replication complexes: a model for the temporal regulation of minus- and plus-strand RNA synthesis. EMBO J. 13:2925-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine, B., J. E. Goldman, H. H. Jiang, D. E. Griffin, and J. M. Hardwick. 1996. Bc1-2 protects mice against fatal alphavirus encephalitis. Proc. Natl. Acad. Sci. USA 93:4810-4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine, B., J. M. Hardwick, B. D. Trapp, T. O. Crawford, R. C. Bollinger, and D. E. Griffin. 1991. Antibody-mediated clearance of alphavirus infection from neurons. Science 254:856-860. [DOI] [PubMed] [Google Scholar]
- 40.Linda, H., H. Hammarberg, S. Cullheim, A. Levinovitz, M. Khademi, and T. Olsson. 1998. Expression of MHC class I and beta2-microglobulin in rat spinal motoneurons: regulatory influences by IFN-gamma and axotomy. Exp. Neurol. 150:282-295. [DOI] [PubMed] [Google Scholar]
- 41.Liu, T., and T. J. Chambers. 2001. Yellow fever virus encephalitis: properties of the brain-associated T-cell response during virus clearance in normal and gamma interferon-deficient mice and requirement for CD4+ lymphocytes. J. Virol. 75:2107-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malherbe, H., M. Strickland-Cholmley, and A. L. Jackson. 1963. Sindbis virus infection in man. Report of a case with recovery of virus from skin lesions. S. Afr Med. J. 37:547-552. [PubMed] [Google Scholar]
- 43.McClary, H., R. Koch, F. V. Chisari, and L. G. Guidotti. 2000. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J. Virol. 74:2255-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLaren, F. H., C. N. Svendsen, P. Van der Meide, and E. Joly. 2001. Analysis of neural stem cells by flow cytometry: cellular differentiation modifies patterns of MHC expression. J. Neuroimmunol. 112:35-46. [DOI] [PubMed] [Google Scholar]
- 45.Neumann, H., H. Schmidt, A. Cavalie, D. Jenne, and H. Wekerle. 1997. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha. J. Exp. Med. 185:305-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oshiro, S., Y. Liu, T. Fukushima, K. Asotra, and K. L. Black. 2001. Modified immunoregulation associated with interferon-gamma treatment of rat glioma. Neurol. Res. 23:359-366. [DOI] [PubMed] [Google Scholar]
- 47.Owens, T., H. Wekerle, and J. Antel. 2001. Genetic models for CNS inflammation. Nat. Med. 7:161-166. [DOI] [PubMed] [Google Scholar]
- 48.Patterson, C. E., J. K. Daley, and G. F. Rall. 2002. Neuronal survival strategies in the face of RNA viral infection. J. Infect. Dis. 186(Suppl. 2):S215-S219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patterson, C. E., D. M. Lawrence, L. A. Echols, and G. F. Rall. 2002. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J. Virol. 76:4497-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reiss, C. S., and T. Komatsu. 1998. Does nitric oxide play a critical role in viral infections? J. Virol. 72:4547-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robertson, B., G. Kong, Z. Peng, M. Bentivoglio, and K. Kristensson. 2000. Interferon-gamma-responsive neuronal sites in the normal rat brain: receptor protein distribution and cell activation revealed by Fos induction. Brain Res. Bull. 52:61-74. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez, M., L. J. Zoecklein, C. L. Howe, K. D. Pavelko, J. D. Gamez, S. Nakane, and L. M. Papke. 2003. Gamma interferon is critical for neuronal viral clearance and protection in a susceptible mouse strain following early intracranial Theiler′s murine encephalomyelitis virus infection. J. Virol. 77:12252-12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rottenberg, M., and K. Kristensson. 2002. Effects of interferon-γ on neuronal infections. Viral Immunol. 15:247-260. [DOI] [PubMed] [Google Scholar]
- 54.Rowell, J. F., and D. E. Griffin. 2002. Contribution of T cells to mortality in neurovirulent Sindbis virus encephalomyelitis. J. Neuroimmunol. 127:106-114. [DOI] [PubMed] [Google Scholar]
- 55.Ryman, K. D., W. B. Klimstra, K. B. Nguyen, C. A. Biron, and R. E. Johnston. 2000. Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J. Virol. 74:3366-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryman, K. D., L. J. White, R. E. Johnston, and W. B. Klimstra. 2002. Effects of PKR/RNase L-dependent and alternative antiviral pathways on alphavirus replication and pathogenesis. Viral Immunol. 15:53-76. [DOI] [PubMed] [Google Scholar]
- 57.Shirako, Y., and J. H. Strauss. 1994. Regulation of Sindbis virus RNA replication: uncleaved P123 and nsP4 function in minus-strand RNA synthesis, whereas cleaved products from P123 are required for efficient plus-strand RNA synthesis. J. Virol. 68:1874-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song, G. Y., G. DeJong, and W. Jia. 1999. Cell surface expression of MHC molecules in glioma cells infected with herpes simplex virus type-1. J. Neuroimmunol. 93:1-7. [DOI] [PubMed] [Google Scholar]
- 59.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 60.Strauss, J. H., E. G. Strauss, and R. J. Kuhn. 1995. Budding of alphaviruses. Trends Microbiol. 3:346-350. [DOI] [PubMed] [Google Scholar]
- 61.Thach, D. C., T. Kimura, and D. E. Griffin. 2000. Differences between C57BL/6 and BALB/cBy mice in mortality and virus replication after intranasal infection with neuroadapted Sindbis virus. J. Virol. 74:6156-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tucker, P. C., E. G. Strauss, R. J. Kuhn, J. H. Strauss, and D. E. Griffin. 1993. Viral determinants of age-dependent virulence of Sindbis virus for mice. J. Virol. 67:4605-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tyor, W. R., S. Wesselingh, B. Levine, and D. E. Griffin. 1992. Long term intraparenchymal Ig secretion after acute viral encephalitis in mice. J. Immunol. 149:4016-4020. [PubMed] [Google Scholar]
- 64.Ubol, S., P. C. Tucker, D. E. Griffin, and J. M. Hardwick. 1994. Neurovirulent strains of alphavirus induce apoptosis in bcl-2-expressing cells: role of a single amino acid change in the E2 glycoprotein. Proc. Natl. Acad. Sci. USA 91:5202-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vernon, P. S., and D. E. Griffin. 2005. Characterization of an in vitro model of alphavirus infection of immature and mature neurons. J. Virol. 79:3438-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vikman, K., B. Robertson, G. Grant, A. Liljeborg, and K. Kristensson. 1998. Interferon-gamma receptors are expressed at synapses in the rat superficial dorsal horn and lateral spinal nucleus. J. Neurocytol. 27:749-759. [DOI] [PubMed] [Google Scholar]
- 67.Wesselingh, S. L., and D. E. Griffin. 1994. Local cytokine responses during acute and chronic viral infections of the central nervous system. Semin. Virol. 5:457-463. [Google Scholar]
- 68.Wesselingh, S. L., B. Levine, R. J. Fox, S. Choi, and D. E. Griffin. 1994. Intracerebral cytokine mRNA expression during fatal and nonfatal alphavirus encephalitis suggests a predominant type 2 T cell response. J. Immunol. 152:1289-1297. [PubMed] [Google Scholar]
- 69.Wieland, S. F., R. G. Vega, R. Müller, C. Evans, B. Hilbush, L. G. Guidotti, G. J. Sutcliffe, P. G. Schultz, and F. V. Chisari. 2003. Searching for interferon-induced genes that inhibit hepatitis B virus replication in transgenic mouse hepatocytes. J. Virol. 77:1227-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhong, L. T., T. Sarafian, D. J. Kane, A. C. Charles, S. P. Mah, R. H. Edwards, and D. E. Bredesen. 1993. Bcl-2 inhibits death of central neural cells induced by multiple agents. Proc. Natl. Acad. Sci. USA 90:4533-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou, A., J. M. Paranjape, S. D. Der, B. R. Williams, and R. H. Silverman. 1999. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology 258:435-440. [DOI] [PubMed] [Google Scholar]