Abstract
Background.
Patients with sentinel lymph node-positive (SLN+) melanoma are increasingly undergoing active nodal surveillance over completion lymph node dissection (CLND) since the Second Multicenter Selective Lymphadenectomy Trial (MSLT-II). Adherence to nodal surveillance in real-world practice remains unknown.
Methods.
In a retrospective cohort of SLN+ melanoma patients who underwent nodal surveillance at a single institution from July 2017 through April 2021, this study evaluated adherence to nodal surveillance ultrasound (US). Adherence to nodal US was compared with adherence to other surveillance methods based on receipt of adjuvant therapy. Early recurrence data were reported using descriptive statistics.
Results.
Among 109 SLN+ patients, 37 (34%) received US surveillance at recommended intervals. Of the 72 (66%) non-adherent patients, 16 were lost to follow-up, and 33 had planned follow-up at an outside institution without available records. More patients had a minimum of biannual clinic visits (83%) and cross-sectional imaging (53%) compared to those who were adherent with nodal US. The patients who received adjuvant therapy (60%) had fewer ultrasounds (p < 0.01) but more exams (p < 0.01) and a trend toward more cross-sectional imaging (p = 0.06). Of the overall cohort, 26 patients (24%) experienced recurrence at a median follow-up period of 15 months. Of these recurrences, 10 were limited to the SLN basin, and all of these isolated nodal recurrences were resectable.
Conclusions.
Pragmatic challenges to real-world delivery of nodal surveillance remain after MSLT-II, and adjuvant therapy appears to be associated with a decreased likelihood of US adherence. Understanding US utility alongside cross-sectional imaging will be critical as increasingly more patients undergo nodal surveillance and adjuvant therapy.
The proportion of melanoma patients who have positive sentinel lymph nodes managed with active nodal surveillance has drastically increased in the past 5 years since the release of the German Dermatologic Cooperative Oncology Group study (DeCOG-SLT) in 2016 and the Second Multicenter Selective Lymphadenectomy Trial (MSLT-II) in 2017. These large, multi-center randomized controlled trials demonstrated no difference in disease-specific survival (DSS) between positive sentinel lymph node (SLN+) patients managed with active surveillance and those treated with completion lymph node dissection (CLND).1–3 These findings have been further supported via multiple meta-analyses by other groups.4,5 Although the majority of the patients in MSLT-II and DeCOG-SLT did not receive adjuvant systemic therapy, this treatment modality has significantly expanded with the efficacy of anti-PD-1 antibodies and BRAF/MEK inhibitors in improving recurrence-free survival (RFS).6,7 However, no randomized trials have directly examined the impact of adjuvant systemic therapy on RFS for patients undergoing active nodal surveillance.
In the MSLT-II study protocol, active nodal surveillance consisted of examination and nodal ultrasound (US) every 4 months after a positive sentinel lymph node biopsy (SLNB) during years 1 and 2, then every 6 months during years 3 to 5.1 This frequent US surveillance facilitates early detection of nodal recurrence at a point where disease can be salvaged by regional surgery.8 It remains unknown, however, whether this high-intensity surveillance schedule is sustainable outside a highly regulated and resourced research protocol. These pragmatic challenges to surveillance imaging are likely exacerbated in settings of patients from rural areas seeking specialty surgical oncology care at larger hub facilities and then returning for ongoing adjuvant systemic therapy closer to home with local medical oncologists.
This study sought first to describe rates of adherence to nodal US and other common surveillance methods, including examination and cross-sectional imaging, in a single-institution retrospective cohort of SLN+ patients in the early post-MSLT-II era. Second, the study aimed to evaluate the impact of receipt of adjuvant systemic therapy on US surveillance adherence.
METHODS
Study Population
Approval of the study protocol was obtained from the University of Alabama at Birmingham Institutional Review Board. Cancer registry and billing data from the University of Alabama at Birmingham (UAB) O’Neal Cancer Center were used to retrospectively identify a cohort of patients with SLN+ melanoma who underwent SLNB and nodal surveillance from July 2017 to April 2021, to align with the publication of the MSLT-II trial. All the patients who had a positive SLNB during this period were included regardless whether they presented for their initial, or any, follow-up appointment at our institution or not.
The study excluded patients who underwent completion lymphadenectomy instead of nodal surveillance for positive SLN, had clinically involved lymph nodes at the time of presentation, or had evidence of distant metastasis on their preoperative or immediate postoperative staging workup. Demographic data were gathered including patient age, gender, race/ethnicity, distance from the treating institution, tumor and pathologic characteristics, and vital status at the time of data collection.
Outcomes
The primary end point for this study was adherence to nodal surveillance ultrasounds. For the purpose of this study, US adherence was considered as one nodal surveillance US per each 6-month interval during the study period as a pragmatic time interval to accommodate for delays in surveillance implementation in the early period after MSLT-II, which included nodal surveillance protocols recommending US every 4 months after SLNB. The secondary end points were adherence to surveillance with physical examination and cross-sectional imaging every 6 months, receipt of adjuvant systemic therapy, and disease recurrence.
The patients’ medical oncologists determined which patients were offered adjuvant systemic therapy. Time to disease recurrence was considered as the time from SLNB to melanoma recurrence at any site. Disease recurrence was determined by review of physician clinical documentation, imaging studies, and pathology results. Recurrence sites were defined as any involved sites at the time recurrence was initially diagnosed. Only the first instance of recurrence was included in the primary analysis if a patient experienced recurrent melanoma at multiple subsequent sites.
Nodal Surveillance
Surveillance methods and frequency were determined by the patients’ surgical oncologists and medical oncologists for this retrospective cohort. The vast majority of the surveillance nodal ultrasounds were completed at our institution, but when available, outside facility documentation of follow-up and surveillance imaging, including exams, ultrasounds, and cross-sectional imaging, were used for adherence calculations.
It is our institutional practice to surveil all SLN basins (i.e., not only the positive nodal basin) if patients mapped to more than one sentinel nodal basin. For surveillance of head and neck primary tumors with a positive SLN, we perform US to evaluate cervical nodal stations 2 to 5 and as indicated, stations 1 and 6, and/or the parotid gland.
Surveillance cross-sectional imaging included brain magnetic resonance imaging (MRI), computed tomography (CT) of the chest and/or abdomen/pelvis, and positron emission tomography (PET) scan. If a focused diagnostic nodal basin US was performed due to clinical concern of nodal recurrence, this was not counted as a surveillance nodal US for the purpose of US adherence calculations.
Statistical Analysis
The descriptive statistics used in this study included chisquare independence tests, two-sample t tests for data with parametric distribution, and Wilcoxon rank-sum tests for non-parametric data. Data are represented as median (interquartile range [IQR]) for continuous variables and number (percentage) for categorical measures. Statistical analyses were performed using Stata 15 statistical software (StataCorp LLC, College Station, TX, USA).
RESULTS
This study had 109 melanoma patients with a positive SLNB who did not undergo completion lymph node dissection during the study period. The cohort was predominantly male (56%) and white (94.5%), not Hispanic or Latino (89%), with a median age of 58.5 years.
The patients in this study had primary tumors with a median Breslow depth of 2.2 mm, and the majority (77%) had only one SLN+. The median greatest dimension of metastasis in the positive sentinel lymph node or nodes in this cohort was 1 mm. Of the 109 SLN+ patients, 57 (52.2%) had SLN metastasis of 1 mm or larger, with the largest metastasis 35 mm in the greatest dimension. Patient and tumor characteristics are further detailed in Table 1.
TABLE 1.
Cohort patient and tumor characteristicsa
| Characteristic | Cohort (n = 109) n (%) |
|---|---|
|
| |
| Age: years (IQR) | 58.5 (46.5–68) |
| Gender | 48 (44) |
| Female | 61 (56) |
| Male | |
| Race | 1 (0.9) |
| Asian | 1 (0.9) |
| Black | 1 (0.9) |
| Other | 3 (2.8) |
| Unknown | 103 (94.5) |
| White | |
| Ethnicity | 1 (0.9) |
| Hispanic or Latino | 97 (89.0) |
| Not Hispanic or Latino | 11 (10.1) |
| Unknown | |
| Travel distance: miles (IQR) | 76.6 (31–112.1) |
| Tumor location | 14 (12.8) |
| Head or neck | 19 (17.4) |
| Upper extremity | 45 (41.3) |
| Trunk | 31 (28.4) |
| Lower extremity | |
| Breslow depth: mm (IQR) | 2.2 (1.4–4.4) |
| Presence of ulceration | 46 (42.2) |
| Presence of microsatellites | 12(11) |
| No. of positive SLNs | 84 (77.0) |
| 1 | 21 (19.3) |
| 2 | 4 (3.7) |
| 3 | |
| Maximum tumor deposit in positive SLN: mm (IQR) | 1 (0.3–3) |
| Presence of extranodal extension | 7 (6.5) |
| Stage (AJCC 8th ed) | 1 (0.9) |
| 3A | 59 (54.1) |
| 3B | 46 (42.2) |
| 3C | 2 (1.8) |
| 3D | 1 (0.9) |
| Unavailable | |
| Received adjuvant systemic therapy | 62 (56.9) |
IQR Interquartile range, SLN Sentinel lymph node, AJCC American Joint Committee on Cancer
Data arepresented as median (IQR) for continuous measures and n (%) for categorical measures
Nodal Surveillance Adherence
Of the overall cohort, 37 patients (34%) received at least one surveillance nodal US in every 6-month interval after their SLNB. In the US non-adherent group (72 patients, 66%), 23 patients (21%) received inadequate surveillance, 16 patients (15%) were lost to follow-up, and 33 patients (30%) were lost to follow-up within our system but had documented planned outside medical oncologist follow-up without available records to confirm receipt of nodal surveillance.
There were no statistically significant differences in US adherence based on patient or tumor characteristics, except for a statistically significant difference in the number of positive sentinel lymph nodes in the US non-adherent group (n = 1; IQR, 1–2) versus the US-adherent group (n = 1; IQR, 1–1) (p = 0.04). There was no statistically significant difference in US adherence based on travel distance to the treating institution (p = 0.40).
Comparisons of US adherence based on patient, tumor, and follow-up characteristics are further described in Table 2. Compared with nodal surveillance ultrasounds, more patients had one or more clinic visits with physical examination (n = 90, 83%) and cross-sectional imaging with CT or PET scan (n = 57, 52%) at least every 6 months after SLNB.
TABLE 2.
Comparison of ultrasound adherence based on patient, tumor, and follow-up characteristicsa
| Characteristic | US non-adherence (n = 72) n (%) |
US adherence (n = 37) n (%) |
p Value |
|---|---|---|---|
|
| |||
| Age: years (IQR) | 58.5 (46.5–68) | 59 (45–68) | 0.77 |
| Male gender | 43 (60) | 18 (49) | 0.27 |
| Travel distance: miles (IQR) | 79.2 (34–114.1) | 64.8 (28.5–104) | 0.44 |
| Tumor location | 9(12) | 5 (14) | 0.11 |
| Head or neck | 25 (35) | 6(16) | |
| Upper extremity | 29 (40) | 16 (43) | |
| Trunk | 9(12) | 10 (27) | |
| Lower extremity | |||
| Breslow depth: mm (IQR) | 2.5 (1.4–4.4) | 1.9 (1–4.3) | 0.24 |
| No. of positive SLNs (IQR) | 1 (1–2) | 1 (1–1) | 0.04 |
| Stage (AJCC 8th ed) | 0 (0) | 1 (3) | 0.36 |
| 3A | 39 (54) | 20 (54) | |
| 3B | 32 (44) | 14 (38) | |
| 3C | 1 (1) | 1 (3) | |
| 3D | 0 (0) | 1 (3) | |
| Unavailable | |||
| Received adjuvant systemic therapy | 44 (61) | 18 (49) | 0.07 |
| If lost to follow-up, did patient have planned surveillance with outside medical oncologist? | 33 (67) | 1 (9) | 0.22 |
| 6(12) | 5 (45) | ||
| Yes | 10 (20) | 5 (45) | |
| No | |||
| Unknown | |||
US Ultrasound, IQR Interquartile range, SLN Sentinel lymph node, AJCC American Joint Committee on Cancer
Data are presented as median (IQR) for continuous measures, and n (%) for categorical measures
After their SLN biopsy, 58 patients presented for at least one surveillance nodal US. Of those 58 patients, 88% (51 patients) presented for their initial surveillance US within 6 months after surgery. The majority of the patients (95 of 109 patients, 87.2%) in the cohort presented for at least one follow-up clinic visit with a melanoma provider (e.g., medical oncologist, surgical oncologist, or radiation oncologist), not including their postoperative visit. All but one of the 95 patients who went to at least one follow-up visit with any melanoma provider had their first visit within 6 months after surgery, with a median of 1 month from the date of surgery.
Impact of Adjuvant Therapy on Surveillance
Of the total cohort, 56% received adjuvant systemic therapy. The adjuvant therapy patients received more physical exams (p<0.01) and trended toward having more cross-sectional imaging (p = 0.06), but had fewer ultrasounds overall (p < 0.01) than the patients who did not undergo adjuvant systemic therapy. Although not statistically significant, the US non-adherent group showed a strong trend toward increased receipt of adjuvant therapy, with approximately two thirds (44 patients, 67%) of the US non-adherent group receiving adjuvant systemic therapy versus half of the US-adherent group (18 patients, 49%) (p = 0.07). The adjuvant therapy patients also were more likely to be adherent with at least one exam per 6-month period (p = 0.01), and trended toward at least one cross-sectional imaging study per 6-month period (p = 0.06) compared with the patients who did not receive adjuvant therapy (Fig. 1).
FIG. 1.
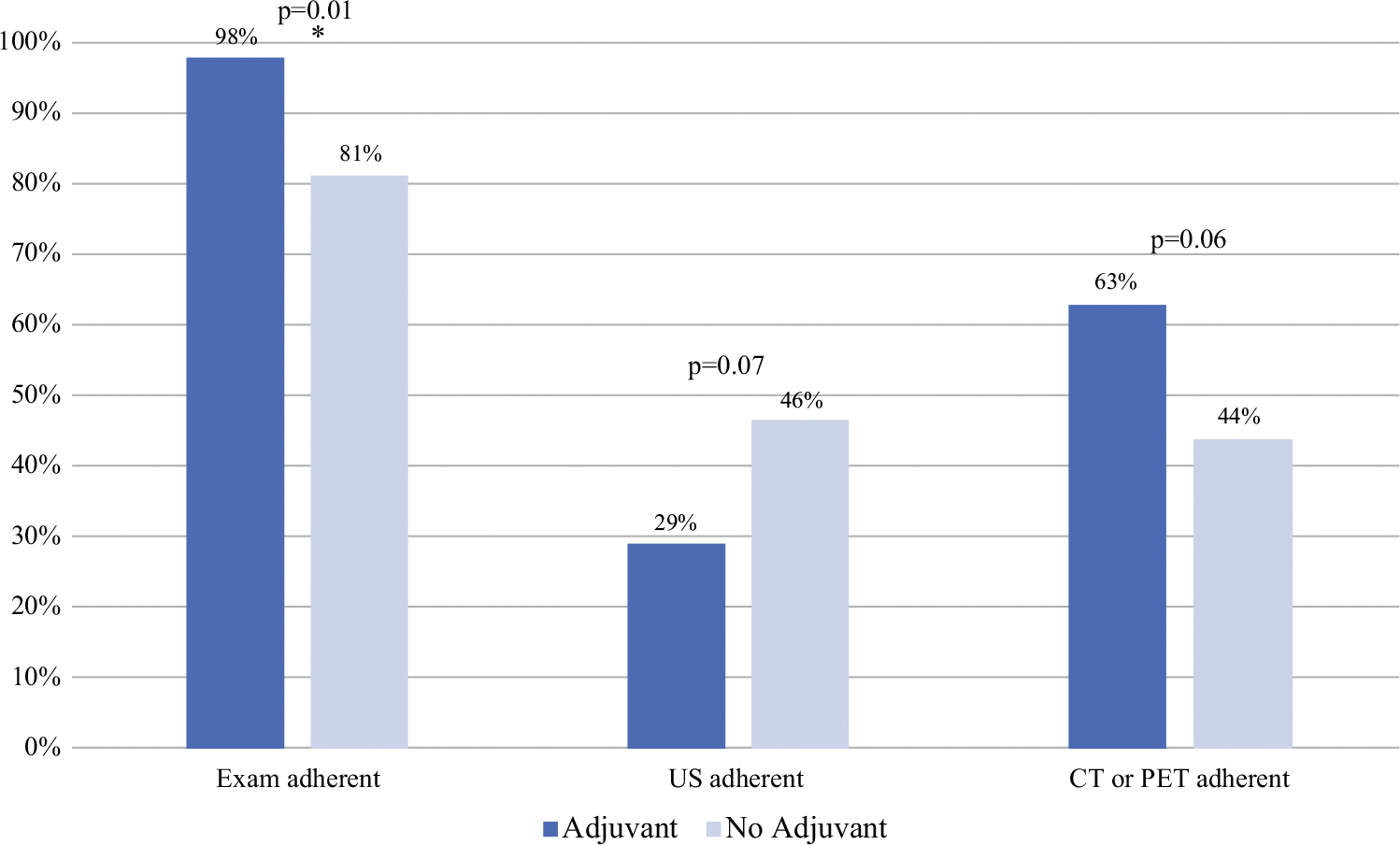
Difference in adherence to surveillance method based on receipt of adjuvant therapy
Disease Recurrence
During the study period, 26 patients (24%) experienced disease recurrence during a median follow-up interval of 15 months. Recurrence-free survival for the study population is represented as a Kaplan Meier curve in Fig. 2. Of the 26 patients who had recurrence at any site, 13 had nodal basin recurrence as a component of their recurrence, with 10 of these 13 patients having only nodal basin recurrence without involvement of any other site. Nine of the ten isolated nodal recurrences included the SLN basin that was positive at the time of SLNB. The involved nodal basins at initial recurrence included the groin (7 patients, all with lower-extremity primary tumors), the popliteal region (1 patient with a lower-extremity primary tumor), the axilla (4 patients: 3 with trunk primaries and 1 with an upper-extremity primary tumor), and postauricular and cervical nodal basins (1 patient who had a head and neck primary tumor with 2 positive sentinel lymph nodes). The distribution of initial disease recurrence sites is depicted in Fig. 3. Of the 10 patients with isolated nodal basin recurrences, 7 were detected with cross-sectional imaging, 1 with US, 1 via physical exam, and 1 by unknown means.
FIG. 2.
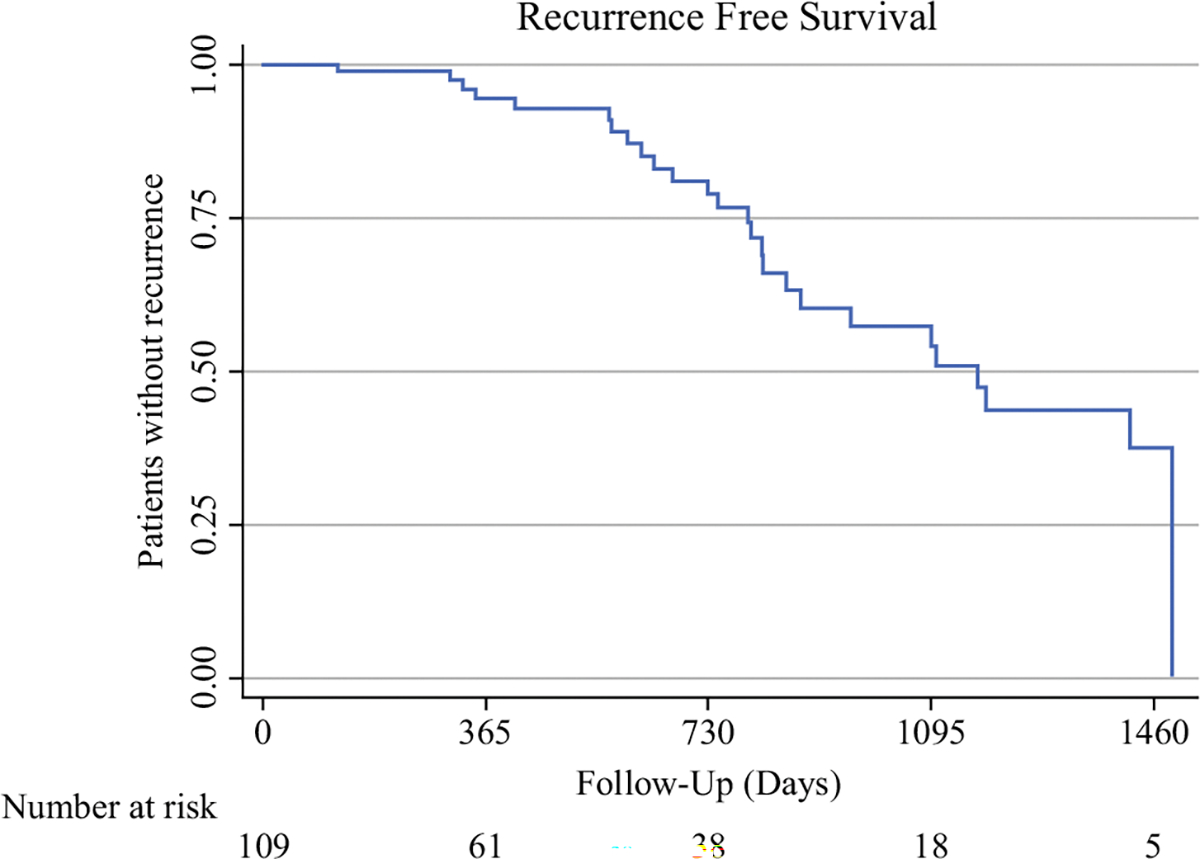
Recurrence-free survival of the study cohort, represented as the percentage of study patients without recurrence at a given follow-up interval. The number of patients at risk of recurrence at each 1-year follow up interval are displayed below each time interval
FIG. 3.
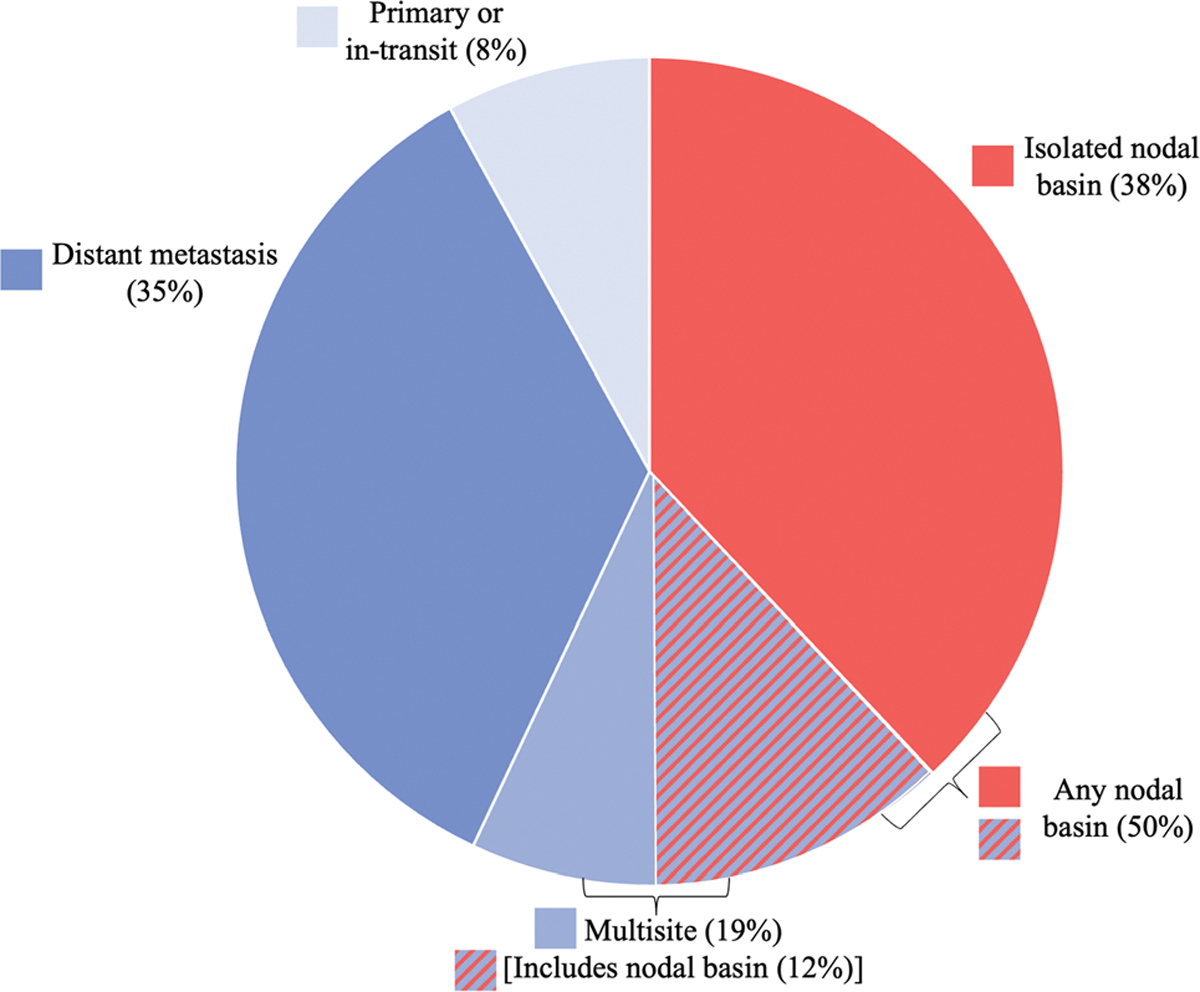
Distribution of the sites of the initial disease recurrence
All the isolated nodal basin recurrences were resectable, and 8 of the 10 occurred within the first 2 years after SLN biopsy. Although all the isolated nodal basin recurrences were resectable at the time of initial detection, only eight patients proceeded with therapeutic lymph node dissection (TLND). The disease burden at time of TLND for these patients is described in Table 3. The remaining two patients with resectable isolated nodal recurrence did not undergo TLND due to election for neoadjuvant systemic therapy before TLND, with subsequent disease progression including distant metastasis during systemic therapy.
TABLE 3.
Disease burden at the time of TLND for patients with initial isolated nodal recurrence who underwent TLND
| Patient age (years), sex | Primary tumor site | Positive SLN basin | Nodal basin with recurrence | Nodes positive/nodes removed | Greatest dimension of metastasis (mm) | ENE present |
|---|---|---|---|---|---|---|
|
| ||||||
| 45, Female | Lower extremity | Groin | Groin | 1/4 | 30 | No |
| 45, Female | Lower extremity | Groin | Popliteal | 0/1 | N/A | N/A |
| 72, Female | Upper extremity | Axilla | Axilla | 2/17 | 11 | Yes |
| 55, Male | Trunk | Axilla | Axilla | 5/23 | NR | NR |
| 71, Male | Trunk | Groin | Groin | 1/14 | NR | No |
| 59, Male | Lower extremity | Groin | Groin | 6/19 | 35 | Yes |
| 65, Male | Lower extremity | Groin | Groin | 1/4 | 10 | No |
| 65, Male | Lower extremity | Groin | Groin | 4/4 | 35 | Yes |
TLND Therapeutic lymph node dissection, SLN Sentinel lymph node, ENE Extranodal extension, N/A Not applicable, NR Not reported
DISCUSSION
In this retrospective cohort study of early post-MSLT-II active nodal surveillance at a single institution, only one third (34%) of the cohort received adequate nodal surveillance with US. Deeper examination of ongoing challenges to active nodal surveillance is critical, with the rates of surveillance significantly increasing in the last half decade.
In one recent multi-institutional, international retrospective study from the early post-MSLT-II period, 84% of a cohort of 1154 patients underwent active nodal surveillance instead of completion lymph node dissection.8 That study showed no differences in all-site RFS or DSS based on nodal management, consistent with findings from MSLT-II.
Additional studies, including a single-institution retrospective cohort study from Bredbeck et al.9 and another examination of CLND versus active surveillance rates in a National Cancer Database population from 2012–2017 by Broman et al.10, have confirmed this trend of rising proportions of active surveillance for SLN+ patients. Qualitative work also has supported this rapid implementation of new evidence. In a December 2018 survey of melanoma surgeons reported by Downs et al.,11 only 5% of the 65 surgeon respondents stated that they would routinely recommend CLND, with 55% recommending CLND for selected cases.
Alongside this increase in active surveillance over CLND is a concordant rise in the frequency of adjuvant systemic therapy, particularly for SLN+ patients. In the current study, the majority of the overall cohort (56%) received adjuvant systemic therapy. These patients were more likely to be adherent with examinations every 6 months and trended toward less adherence with ultrasounds than the patients who did not receive adjuvant treatment. Although this rate of adjuvant therapy is much higher than for the patients in MSLT-II or DeCOG-SLT, it is consistent with rates from other studies that have followed these randomized trials.8,10 One recent study by Farrow et al.12 demonstrated that the majority of SLN+ patients who did not undergo CLND were subsequently treated with adjuvant systemic therapy, with a 1-year RFS of 82%, similar to landmark adjuvant therapy trials that required CLND before adjuvant treatment as part of their study protocol.
Adjuvant therapy, overseen by medical oncologists who more frequently surveil with cross-sectional imaging, may represent an unexpected barrier to consistent delivery of adequate nodal surveillance with US if patients are followed regularly only by their medical oncologists as part of adjuvant treatment and not by their surgical oncologists. In this study, nearly half (46%) of the US non-adherent group was lost to follow-up at our institution but had planned follow-up by an outside medical oncologist without available records.
As a large tertiary care center for Alabama and surrounding states with a National Cancer Institute-designated Comprehensive Cancer Center that includes multidisciplinary melanoma specialists, our institution frequently receives referrals from medical oncologists and dermatologists across Alabama and surrounding states for melanoma surgical cancer care. Based on discussions with each patient and their oncology providers, these patients then often are referred back to their local medical oncologist for administration of adjuvant therapy if indicated, and may or may not return to our institution for ongoing surveillance.
No statistically significant patient characteristics were associated with decreased US adherence, including no difference in travel distance, although this may have been due to the small sample size in this single-institution study. Although a statistically significant association was observed between US non-adherence and having more positive sentinel lymph nodes, the clinical significance of this is unknown.
Although the patients in this study were found to have received more cross-sectional imaging than ultrasounds, multiple studies have demonstrated the importance of US in monitoring for nodal recurrence. A meta-analysis from Xing et al.13 demonstrated that US had the highest sensitivity (96%; confidence interval [CI], 85–99%) and specificity (99%; 95% CI, 95–100%) for lymph node surveillance compared with the slightly inferior PET sensitivity (87%; 95% CI, 53–93%) and specificity (98%; 95% CI, 93–100%) for nodal surveillance. Another study found that regardless whether adjuvant therapy is received or not, most nodal recurrences are initially detected on examination or by US.14 To date, no prospective comparison of US with cross-sectional imaging for detection of nodal recurrence has been performed.
Additionally, the accessibility of specialized nodal US performance at smaller or more rural centers is an important consideration in discussions comparing real-world feasibility of surveillance methods due to ongoing difficulty in standardizing the quality of nodal US studies and their interpretation by experienced radiologists. In the current study, all surveillance nodal ultrasounds except for one US were performed at our large tertiary care hospital. Nodal basin ultrasounds at our institution are performed at the same outpatient imaging facility by trained US technicians who follow standardized protocols to detect suspicious findings based on those described in the MSLT-II study protocol.1 Although this degree of standardization is ideal, the patients in this study traveled a median distance of 77 miles (range, 5–249 miles) to have these studies completed, which is notable considering the recommended frequency of nodal surveillance. While non-melanoma centers may be geographically closer to patients from rural areas, nodal surveillance ultrasounds performed at these sites may be highly variable in quality because these centers are less likely to perform a sufficiently high volume of nodal ultrasounds to provide sonographers and interpreting radiologists with the experience necessary to detect subtle signs of nodal metastasis.
The key limitations of this study included its relatively small sample of patients from a single institution. As previously discussed, we had a high rate of patients lost to follow-up who had planned follow-up with an outside medical oncologist for consideration of adjuvant therapy but never returned to our institution for ongoing nodal surveillance. With limited facilities in our state that regularly perform nodal surveillance ultrasounds, we suspect that the inability to obtain this surveillance imaging locally may have been a barrier for some patients. Perhaps unsurprisingly, 7 of the 10 isolated nodal recurrences in this study were detected on cross-sectional imaging. Additionally, this study evaluated an early post-MSLT-II population. Adherence to nodal surveillance has likely improved in subsequent years, which we plan to evaluate in future work.
Moving forward, we hope to further understand barriers to adequate nodal surveillance, which likely include factors associated with patients, providers, and the surveillance imaging methods themselves. In addition, future study into the utility of nodal US alongside cross-sectional imaging for patients undergoing adjuvant therapy would provide valuable insight for the management of this growing population of SLN+ patients receiving adjuvant therapy during active nodal surveillance, and for the roles of both the medical and surgical oncologists in their surveillance.
CONCLUSIONS
Pragmatic challenges to the consistent delivery of nodal surveillance US remain despite evidence from MSLT-II of equivalent disease-specific survival for SLN+ patients undergoing active surveillance. Receipt of adjuvant therapy appears to be associated with a deceased likelihood of surveillance via nodal US, with cross-sectional imaging favored. Understanding barriers to nodal US and its utility alongside cross-sectional imaging will be critical as this population is increasingly managed with both active surveillance and adjuvant therapy.
Footnotes
DISCLOSURE There are no conflicts of interest.
REFERENCES
- 1.Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in Melanoma. N Engl J Med. 2017;376:2211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomized, phase 3 trial. Lancet Oncol. 2016;17:757–67. [DOI] [PubMed] [Google Scholar]
- 3.Leiter U, Stadler R, Mauch C, et al. Final analysis of DeCOG-SLT trial: no survival benefit for complete lymph node dissection in patients with melanoma with positive sentinel node. J Clin Oncol. 2019;37:3000–8. [DOI] [PubMed] [Google Scholar]
- 4.Angeles CV, Kang R, Shirai K, Wong SL. Meta-analysis of completion lymph node dissection in sentinel lymph node-positive melanoma. BJS. 2019;106:672–81. [DOI] [PubMed] [Google Scholar]
- 5.Klemen ND, Han G, Leong SP, et al. Completion lymphadenectomy for a positive sentinel node biopsy in melanoma patients is not associated with a survival benefit. J Surg Oncol. 2019;119:1053–9. [DOI] [PubMed] [Google Scholar]
- 6.Kwak M, Farrow NE, Salama AKS, et al. Updates in adjuvant systemic therapy for melanoma. J Surg Oncol. 2019;119:222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813–23. [DOI] [PubMed] [Google Scholar]
- 8.Broman KK, Hughes T, Dossett L, et al. Active surveillance of patients who have sentinel node positive melanoma: an international, multi-institution evaluation of adoption and early outcomes after the Multicenter Selective Lymphadenectomy Trial II (MSLT-2). Cancer. 2021;127:2251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bredbeck BC, Mubarak E, Zubieta DG, et al. Management of the positive sentinel lymph node in the post-MSLT-II era. J Surg Oncol. 2020;122:1778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broman KK, Richman J, Bhatia S. Evidence and implementation gaps in management of sentinel node-positive melanoma in the United States. Surgery. 2022. 10.1016/j.surg.2021.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downs JS, Subramaniam S, Henderson MA, et al. A survey of surgical management of the sentinel node positive melanoma patient in the post-MSLT2 era. J Surg Oncol. 2021;124:1544–50. [DOI] [PubMed] [Google Scholar]
- 12.Farrow NE, Raman V, Williams TP, Nguyen KY, Tyler DS, Beasley GM. Adjuvant therapy is effective for melanoma patients with a positive sentinel lymph node biopsy who forego completion lymphadenectomy. Ann Surg Oncol. 2020;27:5121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing Y, Bronstein Y, Ross MI, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011;103:129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broman KK, Bettampadi D, Pérez-Morales J, et al. Surveillance of sentinel node-positive melanoma patients who receive adjuvant therapy without undergoing completion lymph node dissection. Ann Surg Oncol. 2021;28:6978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


