Abstract
Primary cilia are microtubule-based sensory organelles that project from the apical surface of most mammalian cells, including oligodendrocytes, which are myelinating cells of the central nervous system (CNS) that support critical axonal function. Dysfunction of CNS glia is associated with aging-related white matter diseases and neurodegeneration, and ciliopathies are known to affect CNS white matter. To investigate age-related changes in ciliary profile, we examined ciliary length and frequency in the retinogeniculate pathway, a white matter tract commonly affected by diseases of aging but in which expression of cilia has not been characterized. We found expression of Arl13b, a marker of primary cilia, in a small group of Olig2-positive oligodendrocytes in the optic nerve, optic chiasm, and optic tract in young and aged C57BL/6 wild-type mice. While the ciliary length and ciliated oligodendrocytes cells were constant in young mice in the retinogeniculate pathway, there was a significant increase in ciliary length in the anterior optic nerve as compared to the aged animals. Morphometric analysis confirmed specific increase in ciliation rate of CC1+/Olig2+ oligodendrocytes in aged mice compared with young mice. Thus, the prevalence of primary cilia in oligodendrocytes in the visual pathway and the age-related changes in ciliation suggest that they may play important roles in the white matter and age-associated optic neuropathies.
Keywords: Primary Cilia, Aging eye, Optic nerve, ARL13B, Mature Oligodendrocytes, Oligodendrocyte progenitor cells
INTRODUCTION
Primary cilia are conserved microtubule-enriched organelles that are present on most mammalian cell types, including neurons and glial cells1-10. Primary cilia serve as critical signaling hubs to regulate tissue homeostasis and cellular events, such as proliferation and differentiation9-25. Impairment of ciliary structure and function has been linked to many aging-associated neuronal disorders, including Alzheimer's disease, multiple sclerosis, and glaucoma24-34. Most research has focused on investigating the functions of neuronal cilia12, 35-38. However, there is an increasing interest in elucidating the functions of glial cilia, owing to their important roles in neurodegeneration—they affect neuronal synapse formation, inflammation, and circuit function 2, 3, 6, 39-43. Recent cilia studies have focused on their developmental roles in glial cells, but the distribution of ciliated cells in developed optic nerves and aging eyes remains underexplored6, 40, 41.
Advanced age is a risk factor for most neurodegenerative disorders44-49. The accelerated rate of loss of retinal ganglion cells in aging is a hallmark of glaucoma, a neurodegenerative disease of the optic nerve that leads to irreversible blindness50-56. In the brain, in addition to neuron loss, aging also significantly impacts the white matter, which predominantly consists of myelinated axons57-60. Previous studies have suggested that the degeneration of myelin sheaths contributes to age-associated white matter volume decline, and further results in demyelinating diseases in humans and rodents. In the central nervous system, myelin is formed and maintained by mature oligodendrocytes, which are derived from oligodendrocyte progenitor cells (OPCs). OPCs not only self-renew and maintain a small population in adulthood, but also can proliferate and differentiate into mature oligodendrocytes to sustain myelination and to modulate signal transduction. Despite the self-renewing ability of oligodendrocyte lineage cells, aging still negatively influences both OPCs and mature oligodendrocytes. Previous reports have demonstrated that aging impairs mature oligodendrocytes, and causes a decrease in myelin and its production, in mice and primates. In addition, aging has been suggested to degrade the ability of OPCs to remyelinate, proliferate, differentiate, and mature into oligodendrocytes. Overall, age-dependent changes in oligodendrocytes are prominent and may contribute to neurodegenerative diseases.
The loss of myelin in optic nerves can result in optic neuritis and contribute to glaucoma pathogenesis34, 61-63. Electron microscopic studies have characterized the degenerative changes in the myelination sheaths of rat and primate optic nerves and shown them to include dark cytoplasm and an increased number of paranodes60, 64. However, to the best of our knowledge, no direct evidence regarding the change in the number of oligodendrocytes in aged optic nerves has been published. Recent in vitro and in vivo reports have demonstrated that OPCs in mouse brains harbor primary cilia but lose them when differentiating into mature oligodendrocytes. Additionally, most ciliated oligodendrocytes have been proven to be OPCs, with only very few differentiated oligodendrocytes (promyelinating oligodendrocytes)6, 40, 41. Interestingly, depletion in oligodendrocytes of Kif3a transgenic mice has been observed to impair oligodendrocyte proliferation and differentiation through Shh signaling, and reduce motor function40. Additionally, the abnormal length of primary cilia in OPCs was found to be associated with impairment of oligodendrocyte differentiation6. However, the presence of ciliated oligodendrocytes and their ciliary profile in the aged optic nerve have not been investigated.
In the present study, we aimed to characterize primary cilia in oligodendrocytes throughout the mouse retinogeniculate pathway, from the anterior portion to the optic tract. The ciliation and ciliary length were examined by immunofluorescence analysis. Since defective primary cilia are tightly associated with aging, we further compared the cilia profile of oligodendrocytes, both mature oligodendrocyte and OPCs, between adult and aged optic nerves of C57BL/6 mice. Thus, for the first time, we thoroughly described the oligodendrocyte cilia along the rodent visual pathway in vivo, with the purpose of expanding our knowledge of ciliated glial cells in the optic nerve and age-associated diseases.
MATERIALS AND METHODS
Reagents
The following are the working conditions for antibodies (Table 1): anti-Centrin3 mouse antibody (IF 1:500) was purchased from Abnova (H00001070-M01); Myelin basic protein (MBP) (1:1000, rat; catalog number MAB386, Millipore Sigma, Burlington, MA, USA); Olig2 (1: 500, rabbit; catalog number AB9610, Millipore Sigma, Burlington, MA, USA), CC-1 (1:500, mouse, catalog number OP80-100UG, Millipore Sigma), and PDGFRα (1:150, CD140a, rat; catalog number 558774, BD Biosciences, San Jose, CA, USA). Anti-Arl13b mouse antibody (IF: 1:2000) was obtained from Antibodies Inc (N295B/66). Anti-IFT88 Rabbit Polyclonal Antibody (IF: 1:500) was obtained from Proteintech (13967-1-AP). The AlexaFluor 488, 647 and 594 IgG secondary antibodies (IF: 1:200, obtained from Life Technologies) were used to detect primary antibodies. DAPI and ProLong Gold Antifade Mount were obtained from Invitrogen.
Table1.
| Antibody | Immunogen | Host | Source | RRID | Concentration |
|---|---|---|---|---|---|
| Anti-Centrin3 | CETN3 (AAH05383, 1 a.a. ~ 100 a.a) | Mouse | Abnova | H00001070-M01 | 1-500 |
| Anti-Myelin basic protein (MBP) | Bovine myelin basic protein | Rat | Millipore Sigma | MAB386 | 1-1000 |
| Anti-Olig2 | Recombinant mouse Olig-2 | Rabbit | Millipore Sigma | AB9610 | 1-500 |
| Anti-CC-1 | a recombinant protein consisting of amino acids 1-226 of APC | Mouse | Millipore Sigma | OP80 | 1-500 |
| Anti-PDGFRα | Mouse PDGF Receptor α chain | Rat | BD Biosciences | 558774 | 1-150 |
| Anti-Arl13b | Fusion protein amino acids 208-427 (C-terminus) of mouse Arl13b | Mouse | Antibodies Inc | N295B/66 | 1-2000 |
| Anti-IFT88 | IFT88 fusion protein Ag4980 | Rabbit | Proteintech | 13967-1-AP |
Animals
This study utilized eyes from Arl13b-Cetn2 transgenic mice (Jackson Laboratory #027967, both female and male) and C57BL/6J mice (only male mice). The Arl13b-Cetn2 mouse was on a mixed background of FVB, C3H, BALB/c and C57BL/6. Three-month-old C57 mice are considered young, whereas 20-month-old C57 mice are considered aged. In adult Arl13b-Cetn2 transgenic mice, primary cilia were marked with mCherry labeling Arl13b protein and GFP targeting Centrin2. The animal facility maintained a 12:12 hour cycle of light and darkness for the mice. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Stanford University School of Medicine. All experimental procedures were conducted in compliance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Tissue preparation
Inhalation of CO2 was used to sacrifice mice before cervical dislocation. In this procedure, the eyes were removed, and the optic nerves were completely dissected. Dissection of the globe was performed with a razor blade in two locations: (1) approximately 1–2 mm behind the limbus, and (2) approximately 1 mm anterior to the optic nerve head. Using fine dissection scissors, the dura surrounding the optic nerve was delicately removed. O.C.T Tissue-Tek (Sakura Finetek) was used to mount the optic nerve and chiasm on dry ice after fixation in 4% PFA for 30 minutes. The cryopreserved optic nerves were cross-sectioned longitudinally at a thickness of 10 μm.
Immunofluorescence staining
Mounted sections were washed three times in PBS before incubation for two hours at room temperature in blocking buffer containing 10% goat serum and 0.3% Triton X-100. Incubation with primary antibodies was conducted overnight at 4°C after blocking optic nerve sections. The optic nerve sections were washed in 1XPBS (washed in blocking buffer for all aging samples) and then incubated for 1 hour at room temperature with secondary antibodies. DAPI was used to visualize nuclei. Afterwards, the slides were washed 3 times with 1XPBS (washed in blocking buffer for all aging samples) and mounted with ProLong Gold (Life Technologies). A Zeiss LSM880 confocal microscope was used for imaging (the images were taken every 0.3 μm in the z-plane).
Quantification
Images of optic nerve and chiasm sections were captured using a Zeiss LSM 880 scanning confocal microscope at 63× with 0.6× optical zoom. The maximum intensity projection images were generated from the Z-stacks at 0.3 μm intervals (around 20 slides per image). At least five optic nerves per group were captured for all analyses. Using Zen software, cell count and overlap were manually calculated from imaged stacks. Using Zen software, fluorescent micrographs were digitally processed only for brightness and contrast adjustment. Six different sites (from the globe to the chiasm) were examined for each of the sections obtained. The findings were compared within themselves and between young and aged eyes at various locations along the nerve.
Statistical analysis
Graphpad8 (Prism) was used for all statistical analyses in this study. Results are presented as mean values with standard deviation of measurement (SD). An unpaired t-test or one-way ANOVA was used for statistical analysis in comparisons of two or more groups as indicated. Normality of data was analyzed by Kolmogorov-Smirnov test. For one-way ANOVA, Turkey’s multiple comparison test was performed to compare among each selected zone. P-values less than 0.05 were considered statistically significant. All animals were used for research in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Availability of Data and Materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
RESULTS
Arl13b-positive primary cilia are present in adult Olig2-positive oligodendrocytes in the mouse anterior optic nerve.
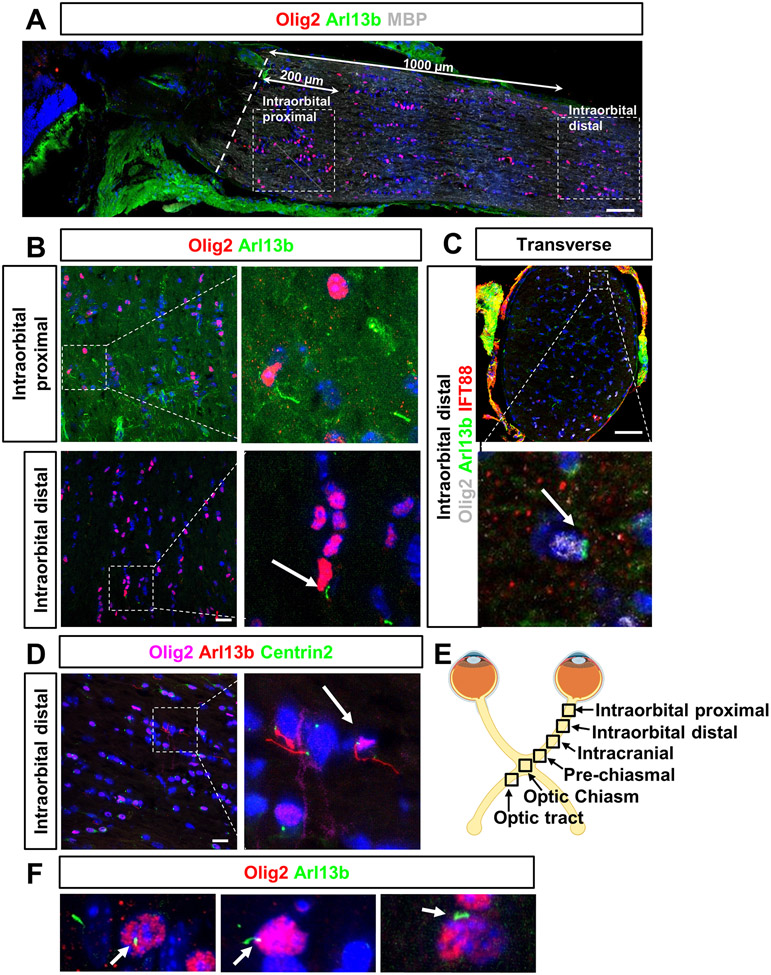
To investigate whether adult oligodendrocytes expressed primary cilia in mouse optic nerves, optic nerve sections were immunostained with antibodies to visualize oligodendrocytes and primary cilia. Olig2 is considered a pan-marker for oligodendrocyte lineage cells; it is present in both OPCs and mature oligodendrocytes65-70. Arl13b and IFT88 are employed as ciliary markers that label the ciliary axoneme and basal body, respectively10, 24, 38, 71, 72. Anterior optic nerves from adult C57BL/6 mice were collected and prepared as longitudinal and transverse sections (Fig. 1A-C, F). In longitudinal analysis, Olig2-positive oligodendrocytes were found in the myelinated portion of the optic nerve, confirmed by the positive myelin basic protein (MBP) signal. Two regions were chosen from the anterior optic nerve by confocal microscopy with 63x objective: 200 μm and 1000 μm away from the onset of myelination and named Intraorbital proximal and Intraorbital distal, respectively (Fig. 1A, 1E). In both regions, only a few Olig2-positive oligodendrocytes were observed to harbor Arl13b-positive primary cilia, which is consistent with previous studies6, 40, 41. Most primary cilia were found in non-Olig2-positive cells of the mouse optic nerve73. These findings were also observed in transverse sections (Intraorbital distal region) using two ciliary markers: Arl13b and IFT88 (Fig. 1C). Moreover, we further confirmed the presence of ciliated oligodendrocytes in a transgenic mouse strain: Arl13b-mCherry;Centrin2-GFP double transgenic mice, which genetically label the cilia3, 9, 38, 74. Immunostaining analysis similarly observed that few Olig2-positive cells were ciliated in the anterior optic nerves (Fig. 1D). Taken together, our data showed that only a small population of Olig2-positive oligodendrocytes expressed primary cilia in the adult mouse optic nerve, indicating that cilia may play a role in oligodendrocyte homeostasis during adulthood (such as to generate and maintain myelin).
Figure 1.
Primary cilia are present in adult Olig2-positive oligodendrocytes in mouse anterior optic nerve.
Primary cilia are expressed in adult Olig2-positive oligodendrocytes throughout the mouse optic pathway.
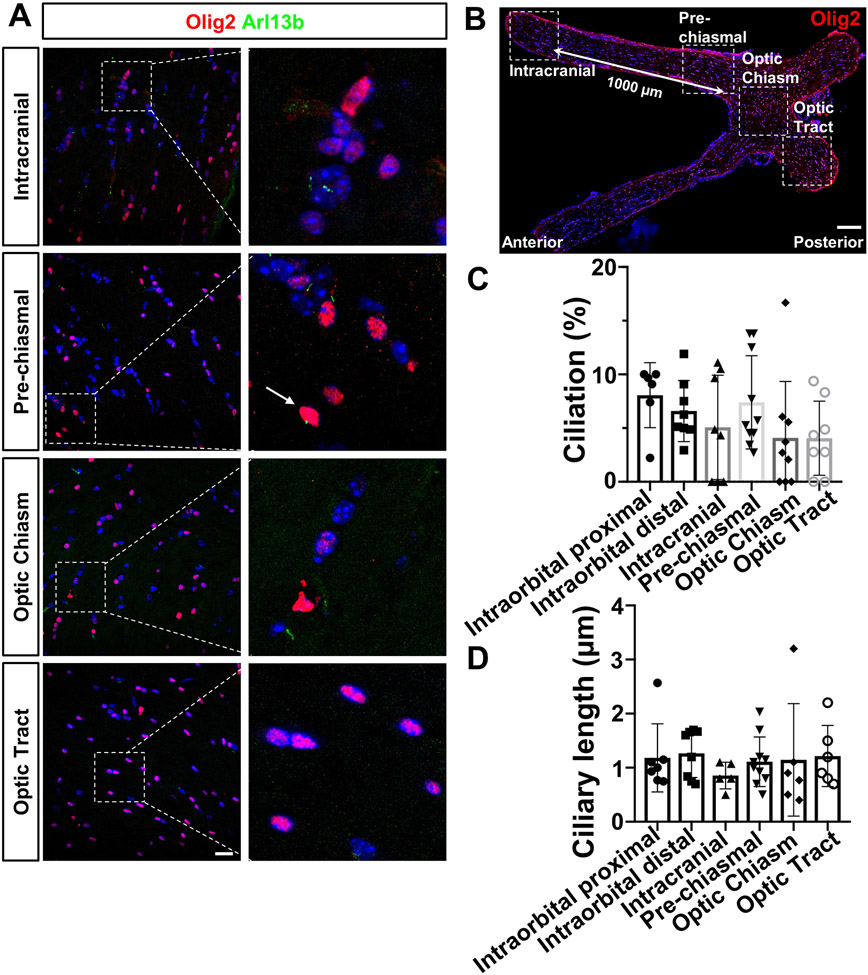
To further determine the distribution of ciliated oligodendrocytes along the visual pathway, the primary cilia profile was assessed in the posterior optic nerves, including the optic tract, in adult C57BL/6 mice (Fig. 2A-2B). In this study, several areas were captured to represent cilia features along optic nerves: intracranial (1000 μm away from pre-chiasmal), pre-chiasmal (an area just adjacent to optic nerve chiasm), optic chiasm and optic tract (an area just posterior to the optic chiasm, Fig. 2B, Fig. 1E). Olig2 and Arl13b antibodies were employed to visualize oligodendrocytes and primary cilia on longitudinal sections of posterior optic nerves. Ciliated Olig2-oligodendrocytes, though very few, were present throughout the optic nerves towards optic tract. The percentage of Olig2-positive cells that contained primary cilia (ciliation) and their ciliary length in each region, from the anterior optic nerve to the optic tract, were quantified. Quantitative analysis revealed that ciliation was greatest in the Intraorbital proximal region (8.06 ± 3.02%), and least in the optic tract region (4.06±3.45%), showing a trend of decreased ciliation from anterior to posterior. However, there were no significant differences in ciliation (n= 5-10, one-way ANOVA, F(5, 43)= 1.350, P=0.2618, Fig. 2C) or ciliary length (1.18±0.62 μm in Intraorbital proximal region and 1.14±1.04 μm in optic chiasm, n= 5-10, F(5, 36)= 0.3273, one-way ANOVA, P=0.8932, Fig. 2D) between any region. In summary, these results revealed that a subset of Olig2-positive oligodendrocytes expressed primary cilia along the visual pathway in adult mice, but it accounted for less than ~10% of the total population.
Figure 2.
Primary cilia are expressed throughout in adult Olig2-positive oligodendrocytes along mouse optic track.
Increased ciliary length in Olig2-positive oligodendrocytes of aged anterior optic nerve.
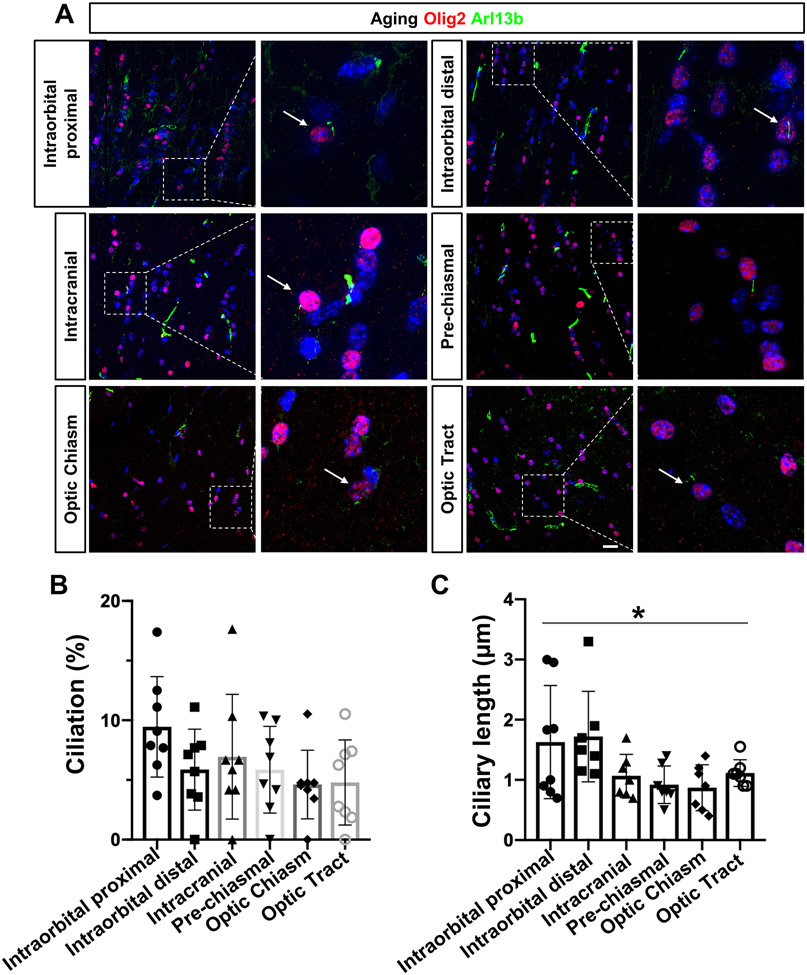
Previous studies have demonstrated that mature oligodendrocytes have a reduced capacity to maintain sufficient myelinogenesis in aging60, 75, 76. Given that defective cilia contribute to age-associated brain diseases, we sought to explore oligodendrocyte cilia in aging optic nerves. Longitudinal optic nerve sections from aged C57BL/6 mice were prepared and immunostained for Olig2 and Arl13b antibodies. Additionally, their ciliation and ciliary length from each captured region (same as adult C57BL/6 mice) were compared with those of young C57BL/6 mice. Immunostaining images revealed that Olig2-positive oligodendrocytes contained primary cilia in all areas of the visual pathway in aged nerves (Fig. 3A). Therefore, ciliation and ciliary length were compared among different regions within the aging nerves. As in adult C57BL/6 mice, a trend of decreased ciliation was observed in aging nerves, from anterior to posterior (Intraorbital proximal region: 9.45 ± 4.21%; Optic tract: 4.79±3.57%, Fig. 3B). However, there were no significant differences between any regions (n=8, one-way ANOVA, F(5, 42) = 1.678, P=0.1611, Fig. 3B). Interestingly, the ciliary length in the anterior portion of the optic nerve was significantly greater than that of the posterior portion (n=8, one-way ANOVA, F(5, 37)= 2.944, P=0.0246, Fig. 3C): the ciliary length in Intraorbital proximal was 1.63±0.94 μm, and 0.87±0.38 μm in the optic chiasm. Turkey’s multiple comparison test further indicated that none of the intergroups were significantly differences. Additionally, the cilia profile in each region was compared between young and aged optic nerves (Supplemental figure). Surprisingly, there were no significant differences in any regions, even for the ciliary length in the anterior portion of nerves, indicating that aging may have a limited effect on the primary cilia of oligodendrocytes along the visual pathway.
Figure 3.
Increased ciliary length in Olig2-positive oligodendrocytes of aged anterior optic nerve.
Decreased number of Olig2-positive oligodendrocytes and increased ciliation in CC1-positive oligodendrocytes in aged anterior optic nerve.
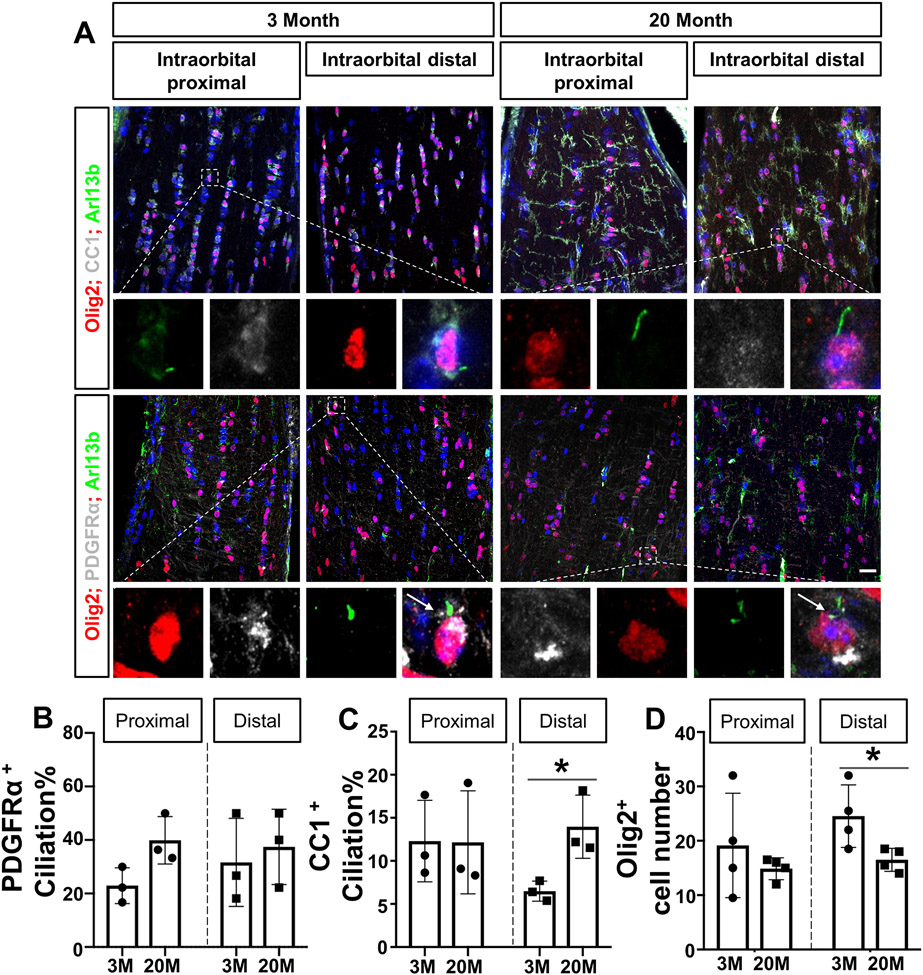
In the central nervous system, OPCs are maintained in adulthood, proliferating and differentiating through their lives to provide a continuous source of new mature oligodendrocytes6, 40, 41. To investigate whether the number of oligodendrocytes changed in the aged optic nerve, we assessed the number of Olig2-positive oligodendrocytes in the anterior portion of the optic nerve (Intraorbital distal area). The quantitative analysis showed a significant decrease of Olig2-positive oligodendrocytes in the aged optic nerves compared to the adult group (n=4, unpaired t-test, distal area: t(6)= 2.607, P=0.0403, Fig. 4D). The ciliary profile in mature oligodendrocytes and oligodendrocyte precursor cells (OPCs) was also examined in two groups. CC1 is a well-known marker for mature oligodendrocytes, whereas PDGFRα is an OPC marker66, 67, 70; antibodies were co-stained for CC1, Olig2, and Arl13b to investigate the cilia profile in mature oligodendrocytes, and for PDGFRα, Olig2 and Arl13b in OPCs. We studied longitudinal optic nerve sections from young adult and aged C57BL/6 mice (anterior portion, Intraorbital distal area). Interestingly, quantitative analysis showed a significant increase in ciliation of CC1+; Olig2+ oligodendrocytes in aged nerves compared to the young adult group (n=3, unpaired t-test, t(4)= 3.373, distal area: P=0.0280, Fig. 4C). However, there were no significant differences in the ciliation of PDGFRα+;Olig2+ OPCs between the aged and young adult groups (n=3, unpaired t test, t(4)= 2.638, proximal area: P=0.0577, distal area: t(4)= 0.4630, P=0.6675, Fig. 4B). Taken together, we observed fewer Olig2-positive oligodendrocytes and increased ciliation in CC1-positive oligodendrocytes in the aged anterior optic nerve.
Figure 4.
Decreased number of Olig2-positive oligodendrocytes and increased ciliation in CC1-positive oligodendrocytes in aged anterior optic nerve.
DISCUSSION
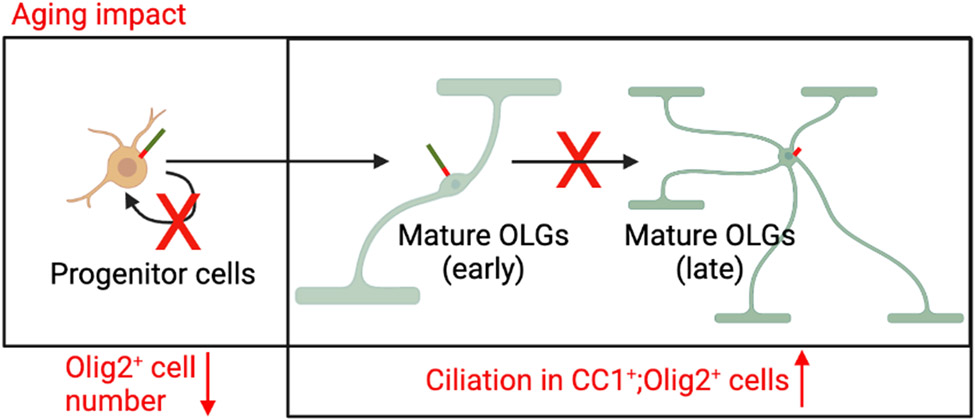
In this study, we describe the primary cilia found on oligodendrocytes of mouse optic nerves. We observed a small population of ciliated oligodendrocytes in young adult and aged mouse optic nerves in vivo. In the aged optic nerves, ciliary length was greater in the anterior than the posterior portion. Surprisingly, the number of cilia did not differ between aged and young adult oligodendrocytes in any of the regions examined77. Furthermore, we discovered that most of these ciliated oligodendrocytes were OPCs, which is consistent with previous in vitro studies41. Collectively, this study suggests the potentially important role of primary cilia in oligodendrocytes in adulthood and age-associated optic neuropathies.
Primary cilia are postulated to be involved in the differentiation of myelinating cells6, 40, 41. Previous studies have indicated that primary cilia may promote the myelination process through regulating hedgehog signaling in Schwann cells, which produce myelin in the peripheral nervous system78. Interestingly, disassembly of primary cilia was also found in mature Schwann cells, as they were found to be assembled only in immature Schwann cells. In the central nervous system, most ciliated oligodendrocytes were OPCs in mouse brain (in vivo and vitro) and rat optic nerves (in vitro), which is consistent with our results from mouse optic nerve tissue6, 40, 41. Moreover, primary cilia in oligodendrocytes play important roles in OPC differentiation. Specifically, an in vitro study has shown that the sonic hedgehog signaling pathway regulating cilia, is involved in OPC proliferation in rat cultured OPCs41. Another recent study has shown that impaired cilia assembly in mouse OPCs (deletion of Kif3a) can result in defective OPC proliferation and oligodendrogenesis40. Interestingly, the association between defective oligodendrocyte differentiation and aging and multiple sclerosis, a classic demyelinating disease, may imply the involvement of primary cilia in its pathogenesis6. To best of our knowledge, there are no direct data to support the role of primary cilia in differentiated oligodendrocytes in age-associated diseases in CNS. Our present study provided evidence of ciliated oligodendrocytes, most of them OPCs, in young adult and aging optic nerves. Additionally, we observed that approximately 10% of CC1-positive mature oligodendrocytes are ciliated, indicating a potential role of cilia in adulthood. Therefore, ciliary length as well as control of ciliary biogenesis, including assembly and disassembly during oligodendrocyte differentiation, provides a potential target for the treatment of demyelinating disorders.
The alteration of ciliary length can be an indicator of downstream signaling events in various cell types39, 79-82. For instance, it has been shown that the length of primary cilia, which are known to be mechanosensory, reflects the ratio of the Ca2+ signaling response. Ciliary length reductions are associated with compression in chondrocytes, flow in endothelial cells, and necrosis in kidney cells39, 79-82. In oligodendrocytes, the increased length of primary cilia in OPCs is suggested to be linked with impairment of oligodendrocyte differentiation6. The process of OPCs proliferation and differentiation is fine-tuned by intrinsic and extracellular signals. The unique receptors embedded especially on the ciliary membrane allow cilia to detect and transduce extracellular signals into cells and coordinate cell signaling pathways11, 13-23. In the present study, we found an increased ciliary length of oligodendrocytes localized in the anterior portion of the aged optic nerve. We hypothesize that increased length of primary cilia in the aged optic nerve may function to improve sensitivity and correctly detect extracellular cues, such as signaling by Ca2+ or Shh pathways. Based on previous studies40, 41, increased ciliary length may also indicate impairment of myelination in aged oligodendrocytes, especially in the anterior portion of aged optic nerves. To test this hypothesis and further investigate the molecular mechanisms involved in ciliary length regulation in oligodendrocytes caused by aging, future studies are needed to elucidate the mechanisms for the key roles played by primary cilia in adult glial cells and demyelinating diseases. In addition to the alterations associated with aging, future studies should investigate demyelinating diseases that involve optic nerves, such as glaucoma induced by ocular hypertension and optic nerve crush. Since previous studies have demonstrated that activated Shh signaling plays a critical role in remyelination of oligodendrocytes in multiple sclerosis6, the sonic hedgehog signaling pathway appears to be a promising candidate mechanism causing demyelination and remyelination of the optic nerve.
In summary, this study provides a thorough characterization of ciliated oligodendrocytes in adult and aged mouse optic nerves. Our findings provide insight into the potential roles of primary cilia for maintaining myelination in differentiated oligodendrocytes in age-associated diseases, especially in the anterior portion of optic nerves. Future investigations should be performed to determine the molecular mechanisms of ciliary length regulation and their role in demyelinating diseases.
Supplementary Material
Figure S1
Comparison of primary cilia profile of visual pathway in young and aged C57BL/6 mice.
Quantification of ciliation and ciliary length of Olig2-positive oligodendrocytes in optic nerves in each region. Data were statistically analyzed using an unpaired Student’s t-test (*p < 0.05): (A) Ciliation: n=6-8, t(12)= 0.6833, P=0.5074; length: n=7-8, t(13)= 1.067, P=0.3056; (B) ciliation: n=8, t(14)= 0.4576, P=0.6543; length: n=7-8, t(13)= 1.456, P=0.1691; (C) ciliation: n=8, t(14)= 0.7434, P=0.4695; length: n=5-7, t(10)= 1.135, P=0.2827; (D) ciliation: n=8-10, t(16)= 0.8040, P=0.4332; length: n=7-10, t(15)= 0.9514, P=0.3565; (E) ciliation: n=8-9, t(15)= 0.2559, P=0.8015; length: n=6-7, t(11)= 0.6498, P=0.5292; (F) ciliation: n=8, t(14)= 0.4123, P=0.6863; length: n=6-7, t(11)= 0.4449, P=0.6650;
Figure 5.
Schematic image of one hypothesis about how aging impacted oligodendrocytes cilia profile.
SIGNIFICANCE STATEMENT.
This study provides evidence of the presence of primary cilia in mature oligodendrocytes within the mouse optic nerve. We further demonstrate alterations in the ciliary profile of oligodendrocytes in the aged mouse optic nerve, suggesting their involvement in aging-associated neuropathies.
Acknowledgments:
We thank Drs. Yannan Li, Siyu Chen and Zhiquan Liu for technical contributions.
Funding:
This work was supported by NIH/NEI K08-EY022058 (Y.S.), R01-EY025295 (Y.S.), R01-EY032159 (Y.S.), R01-EY-023295 (Y.H.), R01-EY024932 (Y.H.), R01-EY26877 (Y.H.), R01-EY28106 (Y.H.), K99EY034932(K.N.).an unrestricted grant from Research to Prevent Blindness, New York, NY to the Moran Eye Center. VA merit CX001481 (Y.S.), Children’s Health Research Institute Award (Y.S.). Research for Prevention of Blindness Unrestricted grant (Stanford Ophthalmology), International Retinal Research Foundation (K.N.). and BrightFocus Foundation (K.N.).
Footnotes
Competing interests: The authors declare no financial/non-financial competing interest.
REFERENCE
- 1.Alvarez-Satta M, Moreno-Cugnon L, Matheu A. Primary cilium and brain aging: role in neural stem cells, neurodegenerative diseases and glioblastoma. Ageing Res Rev. Jul 2019;52:53–63. doi: 10.1016/j.arr.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Sterpka A, Chen X. Neuronal and astrocytic primary cilia in the mature brain. Pharmacol Res. Nov 2018;137:114–121. doi: 10.1016/j.phrs.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sterpka A, Yang J, Strobel M, Zhou Y, Pauplis C, Chen X. Diverged morphology changes of astrocytic and neuronal primary cilia under reactive insults. Mol Brain. Mar 2 2020;13(1):28. doi: 10.1186/s13041-020-00571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anvarian Z, Mykytyn K, Mukhopadhyay S, Pedersen LB, Christensen ST. Cellular signalling by primary cilia in development, organ function and disease. Nat Rev Nephrol. Apr 2019;15(4):199–219. doi: 10.1038/s41581-019-0116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs JL, Schwark HD. Neuronal primary cilia: a review. Cell Biol Int. 2004;28(2):111–8. doi: 10.1016/j.cellbi.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Ki SM, Jeong HS, Lee JE. Primary Cilia in Glial Cells: An Oasis in the Journey to Overcoming Neurodegenerative Diseases. Front Neurosci. 2021;15:736888. doi: 10.3389/fnins.2021.736888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louvi A, Grove EA. Cilia in the CNS: the quiet organelle claims center stage. Neuron. Mar 24 2011;69(6):1046–60. doi: 10.1016/j.neuron.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong CK, Han YG, Shah JK, Obernier K, Guinto CD, Alvarez-Buylla A. Primary cilia are required in a unique subpopulation of neural progenitors. Proc Natl Acad Sci U S A. Aug 26 2014;111(34):12438–43. doi: 10.1073/pnas.1321425111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarado JA, Dhande OS, Prosseda PP, et al. Developmental distribution of primary cilia in the retinofugal visual pathway. J Comp Neurol. May 1 2021;529(7):1442–1455. doi: 10.1002/cne.25029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowal TJ, Dhande OS, Wang B, et al. Distribution of prototypical primary cilia markers in subtypes of retinal ganglion cells. J Comp Neurol. Aug 2022;530(12):2176–2187. doi: 10.1002/cne.25326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veland IR, Lindbaek L, Christensen ST. Linking the Primary Cilium to Cell Migration in Tissue Repair and Brain Development. Bioscience. Dec 1 2014;64(12):1115–1125. doi: 10.1093/biosci/biu179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnett JB, Lupu FI, Eggenschwiler JT. Proper ciliary assembly is critical for restricting Hedgehog signaling during early eye development in mice. Dev Biol. Oct 1 2017;430(1):32–40. doi: 10.1016/j.ydbio.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amador-Arjona A, Elliott J, Miller A, et al. Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory. J Neurosci. Jul 6 2011;31(27):9933–44. doi: 10.1523/JNEUROSCI.1062-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blitzer AL, Panagis L, Gusella GL, Danias J, Mlodzik M, Iomini C. Primary cilia dynamics instruct tissue patterning and repair of corneal endothelium. Proc Natl Acad Sci U S A. Feb 15 2011;108(7):2819–24. doi: 10.1073/pnas.1016702108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen ST, Pedersen SF, Satir P, Veland IR, Schneider L. The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr Top Dev Biol. 2008;85:261–301. doi: 10.1016/S0070-2153(08)00810-7. [DOI] [PubMed] [Google Scholar]
- 16.Christensen ST, Veland IR, Schwab A, Cammer M, Satir P. Analysis of primary cilia in directional cell migration in fibroblasts. Methods Enzymol. 2013;525:45–58. doi: 10.1016/B978-0-12-397944-5.00003-1. [DOI] [PubMed] [Google Scholar]
- 17.Goto H, Inoko A, Inagaki M. Cell cycle progression by the repression of primary cilia formation in proliferating cells. Cell Mol Life Sci. Oct 2013;70(20):3893–905. doi: 10.1007/s00018-013-1302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han YG, Alvarez-Buylla A. Role of primary cilia in brain development and cancer. Curr Opin Neurobiol. Feb 2010;20(1):58–67. doi: 10.1016/j.conb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higginbotham H, Eom TY, Mariani LE, et al. Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev Cell. Nov 13 2012;23(5):925–38. doi: 10.1016/j.devcel.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones TJ, Adapala RK, Geldenhuys WJ, et al. Primary cilia regulates the directional migration and barrier integrity of endothelial cells through the modulation of hsp27 dependent actin cytoskeletal organization. J Cell Physiol. Jan 2012;227(1):70–6. doi: 10.1002/jcp.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masyuk AI, Huang BQ, Ward CJ, et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol. Oct 2010;299(4):G990–9. doi: 10.1152/ajpgi.00093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rondanino C, Poland PA, Kinlough CL, et al. Galectin-7 modulates the length of the primary cilia and wound repair in polarized kidney epithelial cells. Am J Physiol Renal Physiol. Sep 2011;301(3):F622–33. doi: 10.1152/ajprenal.00134.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider L, Cammer M, Lehman J, et al. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem. 2010;25(2-3):279–92. doi: 10.1159/000276562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prosseda PP, Alvarado JA, Wang B, et al. Optogenetic stimulation of phosphoinositides reveals a critical role of primary cilia in eye pressure regulation. Sci Adv. May 2020;6(18):eaay8699. doi: 10.1126/sciadv.aay8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran M, Kolesnikova M, Kim AH, et al. Clinical characteristics of high myopia in female carriers of pathogenic RPGR mutations: a case series and review of the literature. Ophthalmic Genet. Aug 26 2022:1–9. doi: 10.1080/13816810.2022.2113544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braun DA, Hildebrandt F. Ciliopathies. Cold Spring Harb Perspect Biol. Mar 1 2017;9(3)doi: 10.1101/cshperspect.a028191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartill V, Szymanska K, Sharif SM, Wheway G, Johnson CA. Meckel-Gruber Syndrome: An Update on Diagnosis, Clinical Management, and Research Advances. Front Pediatr. 2017;5:244. doi: 10.3389/fped.2017.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. Apr 21 2011;364(16):1533–43. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JE, Gleeson JG. A systems-biology approach to understanding the ciliopathy disorders. Genome Med. Sep 26 2011;3(9):59. doi: 10.1186/gm275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang SF, Kowal TJ, Ning K, et al. Review of Ocular Manifestations of Joubert Syndrome. Genes (Basel). Dec 4 2018;9(12)doi: 10.3390/genes9120605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waters AM, Beales PL. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol. Jul 2011;26(7):1039–56. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kowal TJ, Prosseda PP, Ning K, et al. Optogenetic Modulation of Intraocular Pressure in a Glucocorticoid-Induced Ocular Hypertension Mouse Model. Transl Vis Sci Technol. May 3 2021;10(6):10. doi: 10.1167/tvst.10.6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma X, Ning K, Jabbehdari S, et al. Oculocerebrorenal syndrome of Lowe: Survey of ophthalmic presentations and management. Eur J Ophthalmol. Sep 2020;30(5):966–973. doi: 10.1177/1120672120920544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S, Zhou J, Bu J, et al. Ectodysplasin A protein promotes corneal epithelial cell proliferation. J Biol Chem. Aug 11 2017;292(32):13391–13401. doi: 10.1074/jbc.M117.803809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green JA, Mykytyn K. Neuronal primary cilia: an underappreciated signaling and sensory organelle in the brain. Neuropsychopharmacology. Jan 2014;39(1):244–5. doi: 10.1038/npp.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guemez-Gamboa A, Coufal NG, Gleeson JG. Primary cilia in the developing and mature brain. Neuron. May 7 2014;82(3):511–21. doi: 10.1016/j.neuron.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youn YH, Han YG. Primary Cilia in Brain Development and Diseases. Am J Pathol. Jan 2018;188(1):11–22. doi: 10.1016/j.ajpath.2017.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ning K, Sendayen BE, Kowal TJ, et al. Primary Cilia in Amacrine Cells in Retinal Development. Invest Ophthalmol Vis Sci. Jul 1 2021;62(9):15. doi: 10.1167/iovs.62.9.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wann AK, Knight MM. Primary cilia elongation in response to interleukin-1 mediates the inflammatory response. Cell Mol Life Sci. Sep 2012;69(17):2967–77. doi: 10.1007/s00018-012-0980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cullen CL, O'Rourke M, Beasley SJ, et al. Kif3a deletion prevents primary cilia assembly on oligodendrocyte progenitor cells, reduces oligodendrogenesis and impairs fine motor function. Glia. May 2021;69(5):1184–1203. doi: 10.1002/glia.23957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falcon-Urrutia P, Carrasco CM, Lois P, Palma V, Roth AD. Shh Signaling through the Primary Cilium Modulates Rat Oligodendrocyte Differentiation. PLoS One. 2015;10(7):e0133567. doi: 10.1371/journal.pone.0133567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. Nov 6 2008;60(3):430–40. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Li S, Zhou J, Zhang L, et al. Ectodysplasin A regulates epithelial barrier function through sonic hedgehog signalling pathway. J Cell Mol Med. Jan 2018;22(1):230–240. doi: 10.1111/jcmm.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzales MM, Garbarino VR, Pollet E, et al. Biological aging processes underlying cognitive decline and neurodegenerative disease. J Clin Invest. May 16 2022;132(10)doi: 10.1172/JCI158453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou Y, Dan X, Babbar M, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. Oct 2019;15(10):565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 46.Hung CW, Chen YC, Hsieh WL, Chiou SH, Kao CL. Ageing and neurodegenerative diseases. Ageing Res Rev. Nov 2010;9 Suppl 1:S36–46. doi: 10.1016/j.arr.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Thal DR, Del Tredici K, Braak H. Neurodegeneration in normal brain aging and disease. Sci Aging Knowledge Environ. Jun 9 2004;2004(23):pe26. doi: 10.1126/sageke.2004.23.pe26. [DOI] [PubMed] [Google Scholar]
- 48.Walker L, McAleese KE, Erskine D, Attems J. Neurodegenerative Diseases and Ageing. Subcell Biochem. 2019;91:75–106. doi: 10.1007/978-981-13-3681-2_4. [DOI] [PubMed] [Google Scholar]
- 49.Wyss-Coray T, Ageing neurodegeneration and brain rejuvenation. Nature. Nov 10 2016;539(7628):180–186. doi: 10.1038/nature20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ehrlich JR, Moroi SE. Glaucoma, Cognitive Decline, and Healthy Aging. JAMA Ophthalmol. Jul 1 2017;135(7):740–741. doi: 10.1001/jamaophthalmol.2017.1278. [DOI] [PubMed] [Google Scholar]
- 51.Garway-Heath DF, Wollstein G, Hitchings RA. Aging changes of the optic nerve head in relation to open angle glaucoma. Br J Ophthalmol. Oct 1997;81(10):840–5. doi: 10.1136/bjo.81.10.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guedes G, Tsai JC, Loewen NA. Glaucoma and aging. Curr Aging Sci. Jul 2011;4(2):110–7. doi: 10.2174/1874609811104020110. [DOI] [PubMed] [Google Scholar]
- 53.Jammal AA, Berchuck SI, Thompson AC, Costa VP, Medeiros FA. The Effect of Age on Increasing Susceptibility to Retinal Nerve Fiber Layer Loss in Glaucoma. Invest Ophthalmol Vis Sci. Nov 2 2020;61(13):8. doi: 10.1167/iovs.61.13.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu B, McNally S, Kilpatrick JI, Jarvis SP, O'Brien CJ. Aging and ocular tissue stiffness in glaucoma. Surv Ophthalmol. Jan - Feb 2018;63(1):56–74. doi: 10.1016/j.survophthal.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Ramirez AI, Fernandez-Albarral JA, Hoz R, et al. Microglial changes in the early aging stage in a healthy retina and an experimental glaucoma model. Prog Brain Res. 2020;256(1):125–149. doi: 10.1016/bs.pbr.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 56.Li S, Ning K, Zhou J, et al. Sleep deprivation disrupts the lacrimal system and induces dry eye disease. Exp Mol Med. Mar 2 2018;50(3):e451. doi: 10.1038/emm.2017.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu H, Yang Y, Xia Y, et al. Aging of cerebral white matter. Ageing Res Rev. Mar 2017;34:64–76. doi: 10.1016/j.arr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nasrabady SE, Rizvi B, Goldman JE, Brickman AM. White matter changes in Alzheimer's disease: a focus on myelin and oligodendrocytes. Acta Neuropathol Commun. Mar 2 2018;6(1):22. doi: 10.1186/s40478-018-0515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ouyang Y, Cui D, Yuan Z, et al. Analysis of Age-Related White Matter Microstructures Based on Diffusion Tensor Imaging. Front Aging Neurosci. 2021;13:664911. doi: 10.3389/fnagi.2021.664911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters A. The effects of normal aging on myelinated nerve fibers in monkey central nervous system. Front Neuroanat. 2009;3:11. doi: 10.3389/neuro.05.011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kimura A, Namekata K, Guo X, Noro T, Harada C, Harada T. Targeting Oxidative Stress for Treatment of Glaucoma and Optic Neuritis. Oxid Med Cell Longev. 2017;2017:2817252. doi: 10.1155/2017/2817252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarrazin N, Chavret-Reculon E, Bachelin C, et al. Failed remyelination of the nonhuman primate optic nerve leads to axon degeneration, retinal damages, and visual dysfunction. Proc Natl Acad Sci U S A. Mar 8 2022;119(10):e2115973119. doi: 10.1073/pnas.2115973119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xue J, Zhu Y, Liu Z, et al. Demyelination of the Optic Nerve: An Underlying Factor in Glaucoma? Front Aging Neurosci. 2021;13:701322. doi: 10.3389/fnagi.2021.701322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sugiyama I, Tanaka K, Akita M, Yoshida K, Kawase T, Asou H. Ultrastructural analysis of the paranodal junction of myelinated fibers in 31-month-old-rats. J Neurosci Res. Nov 1 2002;70(3):309–17. doi: 10.1002/jnr.10386. [DOI] [PubMed] [Google Scholar]
- 65.Jiang L, Shen F, Degos V, et al. Oligogenesis and oligodendrocyte progenitor maturation vary in different brain regions and partially correlate with local angiogenesis after ischemic stroke. Transl Stroke Res. Sep 2011;2(3):366–75. doi: 10.1007/s12975-011-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mesentier-Louro LA, Shariati MA, Dalal R, et al. Systemic hypoxia led to little retinal neuronal loss and dramatic optic nerve glial response. Exp Eye Res. Apr 2020;193:107957. doi: 10.1016/j.exer.2020.107957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rivers LE, Young KM, Rizzi M, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. Dec 2008;11(12):1392–401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valerio-Gomes B, Guimaraes DM, Szczupak D, Lent R. The Absolute Number of Oligodendrocytes in the Adult Mouse Brain. Front Neuroanat. 2018;12:90. doi: 10.3389/fnana.2018.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang H, Xu L, Lai C, et al. Region-specific distribution of Olig2-expressing astrocytes in adult mouse brain and spinal cord. Mol Brain. Feb 17 2021;14(1):36. doi: 10.1186/s13041-021-00747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wegener A, Deboux C, Bachelin C, et al. Gain of Olig2 function in oligodendrocyte progenitors promotes remyelination. Brain. Jan 2015;138(Pt 1):120–35. doi: 10.1093/brain/awu375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ning K, Song E, Sendayen BE, et al. Defective INPP5E distribution in NPHP1-related Senior-Loken syndrome. Mol Genet Genomic Med. Jan 2021;9(1):e1566. doi: 10.1002/mgg3.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alvarado JA, Dhande OS, Prosseda PP, et al. Developmental distribution of primary cilia in the retinofugal visual pathway. J Comp Neurol. Sep 17 2020;doi: 10.1002/cne.25029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ono K, Gotoh H, Nomura T, et al. Ultrastructural characteristics of oligodendrocyte precursor cells in the early postnatal mouse optic nerve observed by serial block-face scanning electron microscopy. PLoS One. 2022;17(12):e0278118. doi: 10.1371/journal.pone.0278118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bangs FK, Schrode N, Hadjantonakis AK, Anderson KV. Lineage specificity of primary cilia in the mouse embryo. Nat Cell Biol. Feb 2015;17(2):113–22. doi: 10.1038/ncb3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sams EC. Oligodendrocytes in the aging brain. Neuronal Signal. Sep 2021;5(3):NS20210008. doi: 10.1042/NS20210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X, Huang N, Xiao L, Wang F, Li T. Replenishing the Aged Brains: Targeting Oligodendrocytes and Myelination? Front Aging Neurosci. 2021;13:760200. doi: 10.3389/fnagi.2021.760200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palla AR, Hilgendorf KI, Yang AV, et al. Primary cilia on muscle stem cells are critical to maintain regenerative capacity and are lost during aging. Nat Commun. Mar 17 2022;13(1):1439. doi: 10.1038/s41467-022-29150-6. The remaining authors declare no competing interests. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoshimura K, Takeda S. Hedgehog signaling regulates myelination in the peripheral nervous system through primary cilia. Differentiation. Feb 2012;83(2):S78–85. doi: 10.1016/j.diff.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 79.Bougault C, Aubert-Foucher E, Paumier A, et al. Dynamic compression of chondrocyte-agarose constructs reveals new candidate mechanosensitive genes. PLoS One. 2012;7(5):e36964. doi: 10.1371/journal.pone.0036964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han SJ, Jang HS, Kim JI, Lipschutz JH, Park KM. Unilateral nephrectomy elongates primary cilia in the remaining kidney via reactive oxygen species. Sci Rep. Feb 29 2016;6:22281. doi: 10.1038/srep22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Upadhyay VS, Muntean BS, Kathem SH, Hwang JJ, Aboualaiwi WA, Nauli SM. Roles of dopamine receptor on chemosensory and mechanosensory primary cilia in renal epithelial cells. Front Physiol. 2014;5:72. doi: 10.3389/fphys.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verghese E, Ricardo SD, Weidenfeld R, et al. Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol. Oct 2009;20(10):2147–53. doi: 10.1681/ASN.2008101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Comparison of primary cilia profile of visual pathway in young and aged C57BL/6 mice.
Quantification of ciliation and ciliary length of Olig2-positive oligodendrocytes in optic nerves in each region. Data were statistically analyzed using an unpaired Student’s t-test (*p < 0.05): (A) Ciliation: n=6-8, t(12)= 0.6833, P=0.5074; length: n=7-8, t(13)= 1.067, P=0.3056; (B) ciliation: n=8, t(14)= 0.4576, P=0.6543; length: n=7-8, t(13)= 1.456, P=0.1691; (C) ciliation: n=8, t(14)= 0.7434, P=0.4695; length: n=5-7, t(10)= 1.135, P=0.2827; (D) ciliation: n=8-10, t(16)= 0.8040, P=0.4332; length: n=7-10, t(15)= 0.9514, P=0.3565; (E) ciliation: n=8-9, t(15)= 0.2559, P=0.8015; length: n=6-7, t(11)= 0.6498, P=0.5292; (F) ciliation: n=8, t(14)= 0.4123, P=0.6863; length: n=6-7, t(11)= 0.4449, P=0.6650;
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.