Abstract
Background and study aims There are rare data on the usefulness of endosonography-guided tissue acquisition (EUS-TA) in patients with pancreatic cystic lesions (PCLs). This study aimed to determine the accuracy of EUS-TA with ProCore 20G (PC20) for differentiating between mucinous neoplasia (MN) and non-MNs (n-MN) and identifying malignant PCLs, as well as its adverse events (AEs) in patients with PCLs without a classificatory diagnosis by imaging exams.
Patients and methods In this observational, retrospective, single-center study, all patients with PCL who underwent EUS-TA due to diagnostic doubts in imaging studies were consecutively recruited from June 2017 to December 2021. The outcomes were to determine the diagnostic accuracy of EUS-TA with PC20 for differentiating between MN and n-MN, identifying malignant PCLs, and the AEs.
Results Herein, 145 patients underwent EUS-TA, with 83 women (57.2%) and a mean age of 62.2 years. The mean size was 2.3 cm, with 81 patients (77.9%) having a PCL < 3.0 cm. The final diagnosis was made by EUS-TA (n = 81), surgery (n = 58), and follow-up (n = 6). The sensitivity, specificity, positive and negative predictive values, and accuracy for differentiating between MNs and n-MNs and identifying malignant PCLs were 92.6%, 98.4%, 98.7%, 91.3%, and 95.2% (kappa=0.9), and 92%, 99.2%, 95.8%, 98.3%, and 97.9% (kappa = 0.93), respectively. The AE rate was 2.7%, with no deaths in this cohort.
Conclusions EUS-TA with PC20 has high accuracy and technical success with a low AE rate for PCL diagnosis.
Keywords: Fine-needle aspiration/biopsy, Endoscopic ultrasonography, Intervention EUS, Pancreas, Tissue diagnosis
Introduction
Pancreatic cystic lesions (PCLs) are becoming more commonly detected as imaging techniques advance 1 2 . Incidental PCL findings on computed tomography (CT) or magnetic resonance cholangiopancreatography imaging (MRI/MRCP) reduce quality of life in patients over 65 years of age, increase risk of pancreatic ductal adenocarcinoma (PDA), and increase depressive states in patients when a certainty diagnosis is not obtained 3 . The benign PCLs are pancreatic pseudocysts (PP) and serous cystic neoplasms (SCNs). However, there are two types of potentially malignant PCLs (intraductal papillary mucinous neoplasia [IPMN] and mucinous cystic neoplasm [MCN]) as well as two types of malignant PCLs (cystic pancreatic-neuroendocrine tumor [cp-NET] and cystic-PDA [cp-DA]) 4 . Therefore, defining the nature of cysts is important for determining the best therapeutic strategy 5 .
Endoscopic ultrasound (EUS) is useful in classifying PCLs when conventional imaging methods such as CT and MRI/MRCP are inconclusive 6 . EUS provides morphological detail, facilitates aspiration of cystic fluid, and in some cases, can be used to obtain PCL wall cells 7 . Although biochemical and/or cytological analysis of the fluid is not always possible, when it is, it aids in differential diagnosis between mucinous neoplasms (MNs) and non-mucinous neoplasm (n-MNs) with low sensitivity and specificity without defining the nature of the cysts 6 .
New devices for obtaining MicroCore (MC) such as the ProCore 20G (PC20) were designed to overcome the limitations of conventional needles and the rigidity of the 19G needle to perform EUS-guided fine-needle aspiration (FNA). The PC20 (EchoTip Ultra Endoscopic Ultrasound, Cook Medical, Bloomington, Indiana, United States) is thicker than the 22G and thinner than the 19G, with a tissue-cutting bevel located on the shank, just above the tip of the needle. Another new device is the Moray microforceps that allow for obtaining histological specimens from the cyst wall, but its limitation is an increased risk of adverse events (AEs) in IPMN, and the possible advantage of using the PC20 for its design instead of the Moray 8 . The PC20 has not yet been tested in a large PCL cohort. However, in solid pancreatic lesions, its contribution has been made famous because of the increase in size and quality of samples with a smaller number of punctures and without the need to use the rapid on-site evaluation (ROSE) technique 9 10 11 . Theoretically, this needle could be used to obtain MC and aspirate cystic fluids due to its 20G caliber. This study aimed to determine the accuracy and safety of EUS-guided tissue acquisition (EUS-TA) in differentiating between MNs and n-MNs, as well as accurately classifying PCL histologically.
Patients and methods
Study characteristics and population
This was an observational, retrospective study with prospective data collection from patients consecutively treated in the Endoscopy Sector of Moriah Hospital. This study was approved by the Research Ethics Committee of the Federal University of São Paulo (no. 0471/2020). All patients signed an informed consent form before participating in the study. Data were collected from electronic medical records of patients with PCL undergoing EUS-TA from June 2017 to December 2021. Patients of any age with undiagnosed PCL identified by US, CT and MRI/MRCP, with growth ≥ 5 mm, elevated CA 19–9, history of pancreatic cancer in a first-degree relative, and pancreatitis during the follow-up were included. The presence or absence of PCL-related symptoms was recorded. Patients with known coagulation disorders who would be at risk of bleeding during the procedure were excluded.
EUS-TA
All procedures were performed using a Fujinon EG 580-UT linear echoendoscope (FUJIFILM Medical Systems, Wayne, New Jersey, United States). The ProCore 20G needle was used. All procedures were performed by experienced physicians. PCL in the head and/or the uncinate process were accessed through the duodenum, whereas cysts in the body and tail were approached through the stomach.
The PCL was preferably punctured in a single pass to minimize the risk of infection and perforation from repeated punctures. Once the needle was in the lesion, a 10-cc syringe with a vacuum was used to aspirate the cyst contents. If solid components were repaired inside the cyst, they were specifically punctured with the needle to obtain the material. The cyst wall ( Fig. 1 ), vegetation, or nodulation was perforated by the needle to obtain tissue 12 . During the procedure, an intravenous dose of quinolone-derived broad-spectrum antibiotics was administered and maintained orally for 5 days.
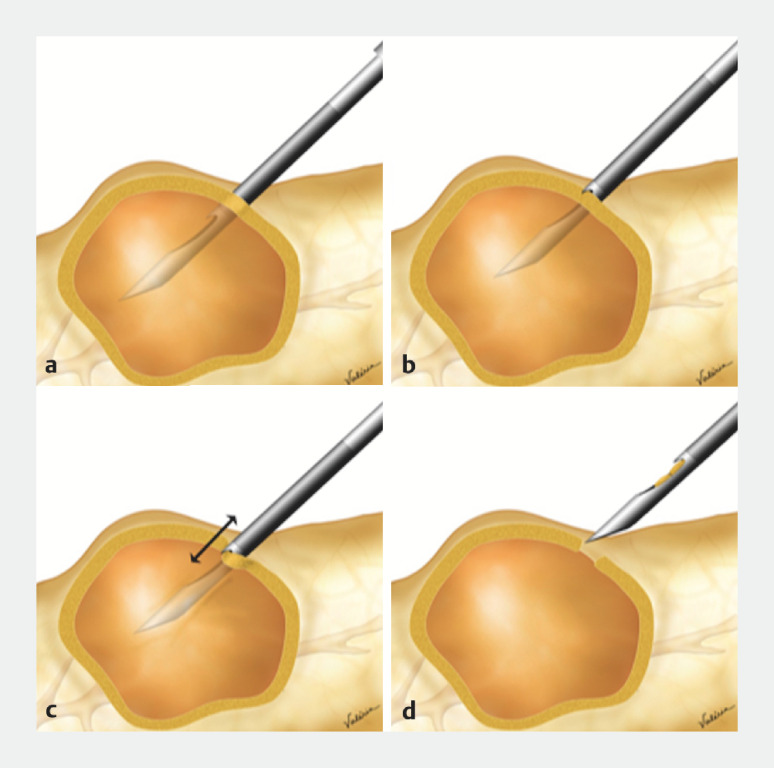
Fig. 1.
Schematic diagram of the endosonography-guided tissue acquisition (EUS-TA). Technique for aspirating cystic fluid and obtaining material from the pancreatic cystic lesions (PCL) wall. a Insertion of the PC20G. b Moment when the forward-bevel cuts the cyst wall. c Back-and-forth movement of the needle to remove various fragments from the wall. d Collecting the MicroCore (MC) inside the needle.
When PCL was identified, the following morphological characteristics were recorded: location, size, presence, and measurement of septa and/or nodules, calcifications, and communication and dilation of the main pancreatic duct (MD). EUS-TA with diagnostic intent was not performed in cases of interposed vessels in the needle path, suspicion of PP or "walled-off necrosis," as these could be referred to as interventional-EUS, or when the PCL identified by imaging examinations did not correspond to a cyst but to another anatomical structure.
Microhistology (McH) technique and biochemical analysis of cystic fluid
The MCs obtained by EUS-TA were fixed in 10% formalin for 6 to 24 hours and analyzed by a pathologist with experience in FNA following the pathology department's standard protocol. The residual fixative liquid in the biopsy flasks was sent for processing to collect tissues and make blocks with inclusion in agarose. The samples were centrifuged at 1500 RPM for 10 minutes. The tissue sediment was distributed in Eppendorf tubes and centrifuged again with 1.5 mL of 3% agarose. The resulting cone was cooled to 0.7°C and sent for standard and paraffin embedding processing by McH. Paraffin-embedded blocks and biopsies were cut into 3-μm-thick semi-serial histological sections at three different levels of depth and stained with hematoxylin and eosin 12 . Immunohistochemistry (IHC) was performed as an ancillary method in complex cases for a diagnostic definition or assessing the sample’s epithelial component.
When possible, the cystic fluid was sent for biochemical analysis, including measurements of amylase, carcinoeymbryonic antigen, and glucose levels using commercially available immunoassays.
McH characteristics of PCL classificatory diagnosis cp-DA
There was marked nuclear pleomorphism, an inconspicuous nucleolus with frequent mitoses, and stromal desmoplasia and necrosis. Perineural and angiolymphatic invasions were frequent 13 .
Pancreatic lymphangioma
These are benign and composed of lymphatic tissue that appear in children and are most likely congenital. They are uncommon in the abdomen and rare in the pancreas. The diagnosis is made by observing dilated cysts with capillary walls 14 .
Mucinous cyst neoplasia
This manifests as a solitary cyst, either unilocular or multilocular and unlike IPMN, does not communicate with the MD. The lining epithelium is mucus-secreting, flat or papillary, and has an ovarian mural stroma ( Fig. 2 ) 14 .
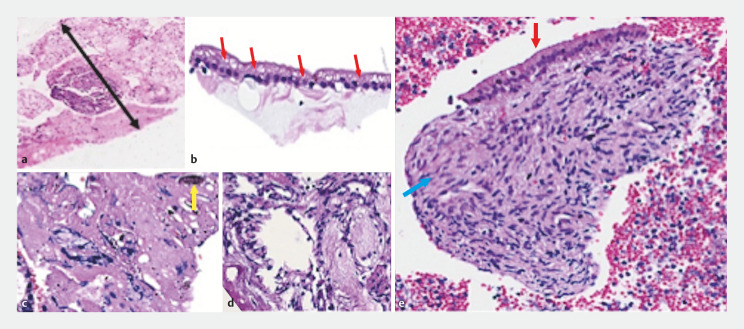
Fig. 2.
Ultrasound-guided biopsies appearance of serous cystic neoplasia a ProCore 20-gauge caliber of the specimen (black arrow with two heads). b Simple cubic epithelium with vacuoles containing glycogen (red arrows). c Dense cicatricial stroma with hemosiderin (yellow arrow). d Loose myxoid stroma. e Mucinous cystic neoplasm. Flat epithelium with mucoproducing gastric foveolar pattern (red arrow) and dense ovarian pattern stroma (blue arrow).
Serous cyst neoplasia
This is covered by a delicate flat or cuboidal simple epithelium with clear cytoplasm rich in glycogen (periodic acid-Schiff +). Mitoses are absent and the nuclei are round and dense. The stroma usually exhibits a fibroblastic radial scar, but it can also be loose, myxoid, or have calcified foci. The trabeculae between the cysts show calcifications that form a radiating pattern ( Fig. 2 ) 15 .
Intraductal papillary mucinous neoplasia
Noninvasive IPMNs of MD and/or branch duct origin are usually > 5 mm in size and thus clinically detectable and are classified according to cell of origin into gastric/foveolar, intestinal, pancreaticobiliary, and oncocytic. Histologically, they are classified into low-grade and high-grade dysplasia, and invasive carcinoma (ICA). IHC associated with morphology increases subtyping, contributing to the indication of benign biological behavior when there is negativity for p53 protein expression ( Fig. 3 ) 16 .
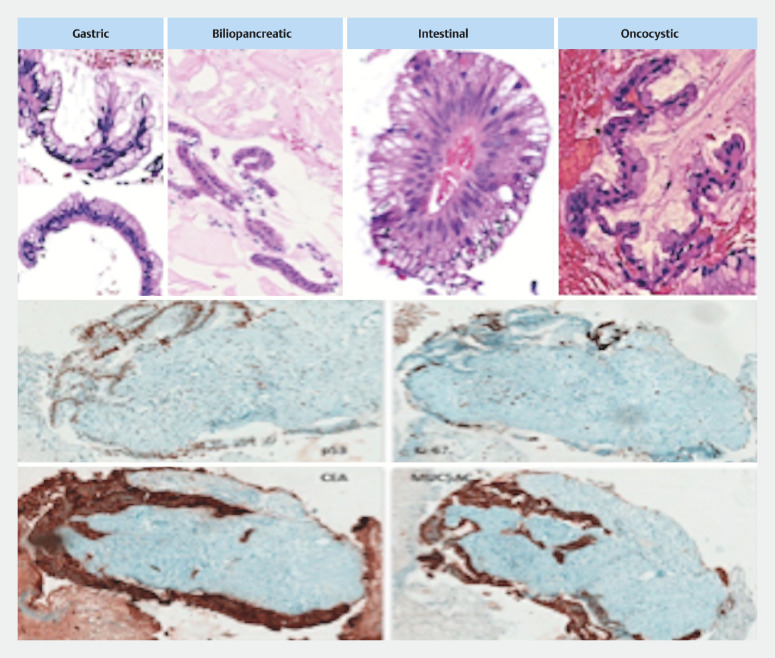
Fig. 3.
Ultrasound-guided biopsies of intraductal papillary mucinous neoplasia (IPMN) with their respective epithelia (gastric, biliopancreatic, intestinal and oncocytic). An invasive IPMN. Note the diffuse p53 positivity and the high rate of cell cluster proliferation (Ki-67). Invasion is best identified in the distribution and infiltration of desmoplastic area by carcinoembryonic antigen (CEA) and MUC5AC.
Solid pseudopapillary neoplasia
In ultrasound-guided biopsies, the approach was to create a concise panel that would allow for an accurate diagnosis. This panel includes beta-catenin, CD10, CD99, progesterone receptor, chromogranin A, and Ki-67. Tumor cell nuclei show strong beta-catenin (+), CD10 (+) in the cytoplasm, and CD99 (+) in the cytoplasm in the form of perinuclear dots. Progesterone receptor expression is strong and diffuse. Proliferative activity, as expressed by Ki-67 expression, is low, accounting for less than 2% of tumor cells 17 .
Pancreatic pseudocyst
The cyst wall may contain histiocytes, fibroblasts, giant cells, granulation tissue, and rarely eosinophils. The macrophage exudate in the fluid contains hemosiderin and blackened pigmented material from steatonecrosis in ultrasound-guided biopsies.
cp-NET
cp-NETs are typically solid, but in rare cases, they can present as cysts. Some cp-NETs have a unilocular cyst filled with serous fluid and surrounded by neoplastic tissue in the wall. They are differentiated by low mitotic activity and the absence of necrosis, perineural and lymph vascular invasion, or metastases. Based on a new World Health Organization classification (2019) of morphological characteristics, cp-NETs with Ki67 > 20% are classified as Grade 3 well-differentiated cp-NET and undifferentiated neuroendocrine carcinoma. The histological grading of cp-NETS is based on proliferative activity 18 .
Definitions
Diagnostic accuracy
Is the probability of EUS-TA with PC20 obtaining the PCL classificatory diagnosis described in the subchapter “McH characteristics for PCL classificatory diagnosis” for the differential diagnosis between MN vs. n-MN and benign vs malignant PCL.
Malignant PCL
Malignant PCLs were adenocarcinomas derived from IPMN, cp-DA, cp-NET, second primary neoplasms (SPNs), and a cystic pancreatic non-Hodgkin lymphoma.
AEs and follow-up
AEs were all unexpected events that occurred during or after the procedure and caused morbidity or mortality. AEs were recorded and graded (I, II, III, IV, and V) by the AGREE classification 19 . All patients were followed up by the attending physicians for at least 1 year, and in cases of malignant PCL, until the final outcome of the evolution.
Gold standard of comparison
In surgical cases, the pathology of the resected samples was the gold standard. For patients who did not have surgery, the final diagnosis was obtained considering clinical deterioration, death, and/or imaging studies consistent with metastatic disease after 12 months of follow-up. Likewise, in the case of a negative McH diagnosis, the negative gold standard was consistent clinical results and imaging studies after 12 months of follow-up. In addition to cp-DA, cp-NETs were considered positive for malignancy.
Statistical analysis
Descriptive analyses of the variables were performed, showing the absolute (n) and relative (%) frequency distributions for the qualitative variables and the main summary measures, such as the mean, standard deviation, median, minimum, and maximum values, for the quantitative variables. The association between qualitative variables was analyzed using the chi-squared test or Fisher's exact test, when appropriate. The qualitative variables were compared in relation to the distribution of quantitative variables using the student’s t -test for independent groups or the nonparametric Mann-Whitney test.
The diagnostic test was evaluated by calculating sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), and accuracy, as well as the kappa concordance index (k) in relation to the gold standard. The significance level was set at 5%. Statistical analyses were performed using IBM Statistical Package for the Social Sciences software, Version 25, and the free software R, Version 3.6.2.
Results
Population characteristics
Herein, 145 patients underwent EUS-TA with the PC20. Their mean age was 62.2 years (21–91 years), with 83 women (57.2%). There were 52 symptomatic patients (35.8%), with 45 (31.0%) having abdominal pain, 17 (11.7%) having acute pancreatitis (AP), six (4.1%) having diarrhea, and six (4.1%) having jaundice.
EUS-TA findings in pancreatic cystic lesions
None of the patients referred for EUS-TA were excluded. EUS findings revealed PCLs with a mean size of 2.3 cm (0.6–10 cm), with 81 patients (77.9%) having PCLs < 3.0 cm and 21 (20.2%) having PCLs > 3.0 cm. The most frequent site of cysts was the head in 81 (56%), the body in 41 (28%), the tail in 13 (9%), and more than one segment in 10 cases (7%). The MD had an average diameter of 0.39 (0.3–3.1 cm), and 76 (52.4%) of the PCLs communicated with it ( Table 1 ).
Table 1 Demographic characteristics and EUS findings in the study patients.
| Clinical characteristics | N (median) | % |
| EUS, endoscopic ultrasound; EUS-TA, endosonography-guided tissue acquisition; PCL, pancreatic cystic lesions; MD, main pancreatic duct. | ||
| Gender | ||
|
83 | 57.2 |
|
62 | 42.8 |
| Age, median (range, SD) | 62.2 | (21–91, 15.9) |
| Indications for EUS-TA (PC) | ||
| Asymptomatic | 93 | 64.1 |
| Symptoms | 52 | 35.8 |
|
45 | 31 |
|
17 | 11.7 |
|
6 | 4.1 |
|
6 | 4.1 |
| EUS PCL features ( n = 145) | N , median | Range |
| Cyst location number (%) | ||
|
81 | 56 |
|
41 | 28 |
|
13 | 9 |
|
10 | 7 |
| Cyst size, median (range) cm | 2.24 | (0.4–10) |
|
115 | 79 |
|
30 | 21 |
| MD comunication | ||
|
76 | 52.4 |
|
69 | 47.6 |
| MD size, median (range) cm | 0.39 | (0.3–3.10) |
|
105 | 72.4 |
|
40 | 27.6 |
The transduodenal route for puncture was used in 81 patients (56%), while the gastric route was used in 64 (44%). In about 73% of patients, a single puncture was performed, with an average of 1.2 punctures (1–2), and the material was sufficient to perform the IHC in 81 patients (55.8%).
Cystic fluid analysis as a final diagnosis
Cystic fluids could be obtained for biochemical analysis in 57 patients who underwent puncture (39.3%). The mean fluid volume was 14.1 mL (2–150 mL). CEA levels > 192 ng/mL were suspicious for presence of MNs in 22 patients (40.7%), which was confirmed in 11 (50%). CEA levels < 5 ng/mL were suspicious for presence of n-MNs in 13 patients (24%), which was confirmed in 12 (92.3%). However, CEA levels ranging from 5 to 192 ng/mL were inconclusive in 19 patients (35.3%).
Amylase levels were < 250 U/L in 15 (28.3%) and > 250 U/L in 38 patients (71.7%) with suspected PP, which was not confirmed in any of them. Amylase also showed no significance with EUS-TA ( P = 0.371). Glucose levels were suspicious for MN in 10 patients (50%), which was confirmed in 30% of cases, and for n-MN in 10 cases (50%), which was confirmed in 60% of cases ( Table 2 ).
Table 2 EUS-TA results in relation to biochemical analysis and McH results.
| EUS-TA | N | % |
| EUS-TA, endosonography-guided tissue acquisition; McH, microhistology; CEA, carcinoembryonic antigen; MN, mucinous cystic neoplasia; n-MN, non-mucinous cystic neoplasia; PP, pancreatic pseudocyst; CA,cancer antigen. | ||
| Cystic fluid number (%) | 57 | 39.3 |
| Cystic fluid, median (range) mL | 14.1 | (2–150) |
| CEA (ng/mL), n | 54 | 94.7 |
| > 192 (MN detected) | 22 | 40.7 |
| 5–192 (Inconclusive) | 19 | 35,3 |
| < 5 (n-MN detected) | 13 | 24 |
| CA19.9 (U/mL), n | 52 | 91.2 |
| > 140.000 (MN detected) | 7 | 13.4 |
| < 140.000 (inconclusive) | 45 | 86.5 |
| Amylase (U/L) | 53 | 93 |
| < 250 (no PP) | 15 | 28.3 |
| > 250 (PP? or MN) | 38 | 71.7 |
| Glucose (mg/dL) | 20 | 35 |
| < 50 (MN detected) | 10 | 50 |
| > 50 (n-MN detected) | 10 | 50 |
Final diagnosis and results of EUS-TA microhistology
The final diagnosis obtained by EUS-TA (n = 81), surgery (n = 58), and follow-up (n = 6) revealed benign MNs in 67 patients (54 IPMNs and 13 MNs), malignant MNs in 14 (all of them IPMNs), benign n-MNs in 53 (37 serous cystadenoma, 6 PPs, 4 retention cysts, 2 cystic lymphangiomas, and 4 walled-off necrosis), and malignant n-MN in 11 (5 cp-DAs, 3 cp-NETs, and 2 SPNs). Eight (5.5%) of the patients who underwent EUS-TA with PC20 were not classified (6 negatives, 1 scar, and 1 macrophage exudate). The diagnosis obtained by EUS-TA was MN in 64 of 67 patients (95.5%), malignant MN in 11 of 14 (78.5%), and SPN in 36 of 37 (97.2%).
Table 3 shows the number of true positives, true negatives, false positives, and false negatives for each diagnosis obtained, measuring the performance and concordance for each of the PCLs identified by McH. All classificatory diagnoses were found to have high-performance values, with sensitivities of 78.5% (k = 0.642) and 95.5% (k = 0.639) for malignant IPMN and MN, respectively. The type of epithelium detected was significantly associated with both communications with the MD ( P = 0.004) and MN diagnosis by EUS-TA ( P < 0.001). For statistical purposes, IPMNs and MNs were considered MN, and all others found were considered n-MNs, whereas malignant IPMNs, cp-DAs, cp-NETs, and SPNs were considered malignant.
Table 3 Pathology results compared with the final diagnosis obtained by surgery or patient follow-up.
| EUS-TA diagnosis | McH | Final diagnosis |
| EUS-TA, endosonography-guided tissue acquisition; McH, microhistology; MN, mucinous cystic neoplasia; IPMN, intraductal papillary mucinous neoplasia; n-MN, non-mucinous cystic neoplasia. | ||
| Mucinous cystic neoplasia (MN) | ||
| IPMN | 52 | 54 |
| Mucinous cystadenoma | 12 | 13 |
| Ductal ectasia | 2 | 0 |
| Malignant MN | ||
| IPMN | 11 | 14 |
| Non-mucinous neoplasia (n-MN) | ||
| Serous cystic neoplasia | 36 | 37 |
| Pancreatic pseudocyst | 5 | 6 |
| Chronic pancreatitis (retention cyst) | 4 | 4 |
| Lymphangioma cystic | 2 | 2 |
| Walled off necrosis | 3 | 4 |
| Malignant n-MN | ||
| Cystic pancreatic ductal adenocarcinoma | 5 | 5 |
| Cystic pancreatic-neuroendocrine tumor | 3 | 3 |
| Pancreatic non-Hodgkin Lymphoma | 1 | 1 |
| Solid pseudopapillary neoplasia | 1 | 2 |
| Non-diagnostic | ||
| Negative | 6 | 0 |
| Cicatricial scar | 1 | 0 |
| Macrophagic exudate | 1 | 0 |
| Total | 145 | 145 |
The sensitivity, specificity, PPV, and NPV, and accuracy with 95% confidence intervals (CIs) for differentiating between MN and n-MN were 92.6% (95% CI: 86.9%-98.3%), 98.4% (95% CI: 95.4%-101.5%), 98.7% (95% CI: 96.1%-101.2%), 91.3% (95% CI: 84.7%-98%), and 95.2% (95% CI: 91.7%-98.7%), respectively, with a k value of 0.90 (very good). For the diagnosis of malignancy, the results were 92% (95% CI: 81.4%-102.6%), 99.2% (95% CI: 97.5–100.8%), 95.8% (95% CI: 87.8%-103.8%), 98.3% (95% CI: 96.1%-100.6%), and 97.9% (95% CI: 95.6%-100.2%), respectively, with a k value of 0.93 (very good).
Accuracy of EUS-TA
A total of 58 patients underwent surgery after EUS and pathological diagnosis was regarded as the gold standard. There were 24 malignant cysts by pathology and four were misdiagnosed by EUS-TA. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of EUS-TA in differentiating benign and malignant lesions were 88% (21/24), 91% (31/34), 88% (21/24), 91% (31/34) and 90% (52/58), respectively. When evaluating the capacity of classificatory diagnosis of PCLs, the accuracy of EUS-TA was 90% (52/58).
AE frequency
The overall rate of AEs in this sample was 2.7%. Three patients had bleeding and one had AP. All bleeding episodes occurred immediately after the procedure, with two episodes occurring at the puncture site and one intracystic (SCN) documented during EUS and all resolved uneventfully. The AP episode occurred 24 hours after the EUS-TA in a branch duct-IPMN. All were treated conservatively, and there were no fatalities in this cohort.
Discussion
The risk of an incidental PCL turning malignant is 0.01% per year, and the risk of progression to ICA is 0.24% per year. When compared with the general population with no PCLs, the risk of malignancy is 22.5 times higher 20 . PCLs with malignant potential account for 15% to 45% of all resected lesions, with MNs having moderate potential, high-grade dysplasia and ICA found in 10% to -40% of resected lesions, and IPMN in up to 42% 21 . EUS is ideal for evaluating PCL detected by CT and/or MRI/MRCP. However, determining whether it is malignant or benign based only on the images obtained is unlikely. SCNs can be observed and/or no longer followed, whereas MCNs and IPMNs warrant follow-up or surgery as they have the potential for malignancy 21 22 . Two retrospective studies have suggested that approximately one-third of patients with incidental PCLs treated surgically were misdiagnosed prior to the procedure 23 24 . The wrong diagnosis increases the economic and medical burden, lowers quality of life, and harms a patient's physical and mental health by requiring unnecessary surgery 3 .
Rigid criteria for performing EUS-FNA for PCLs in various guidelines remain controversial, particularly regarding cyst size, a fact that has increasingly been neglected 4 . In our study, the average size of punctured PCLs was 2.24 cm (0.4–10 cm), with 79% of them being < 3.0 cm, indicating that size was not the primary indication for performing EUS-TA, but rather, diagnostic doubt based on the imaging. Another criterion for EUS-TA was the growth of PCLs, elevation of CA 19–9, familial PDA or appearance of worrisome features in patients being followed up due to a diagnosis of BD-IPMN based on the morphological characteristics of MRI/CT. EUS-FNA should be performed if the results alter the therapeutic strategy to be adopted 25 , especially for PCLs with described high-risk factors or those with no defined diagnosis, which occurred throughout our series 4 . The average age of our cohort was 62.2 years, and most PCLs were found in the head (56%), with normal MD (72.4%) and communication with the MD (52.4%). A PCL diagnosis is challenging. Obtaining adequate cytological samples with current methods, such as FNA, suffers from low sensitivity for malignant cytology and pooled specificity, due to high false-negative rates or inadequate samples 26 . Another meta-analysis of 1,438 patients undergoing EUS-FNA with cytological analysis showed sensitivity and specificity of 54% and 93%, respectively. This study showed that for EUS-FNA to be effective it needs to be analyzed in conjunction with imaging tests, thus becoming a useful diagnostic tool for the correct identification of MNs 27 .
Tissue acquisition needles are excellent and safe for obtaining histological samples from PCLs, as evidenced in our study. In that series, a single-pass biopsy was satisfactory in 80% of cases, whereas a second pass was required in the remaining cases. The technical success rate was 100%. This result was probably due to the large lumen and flexibility of the needle, which facilitated its introduction even when the endoscope was at an angle. This was achieved by coating the needle with polytetrafluorethylene, a smooth and flexible material. In addition, to reduce resistance when crossing the tissue, the cutting edges of the needle have a forward-facing bevel and a Menghini-type tip design, facilitating its introduction into the cyst wall 28 .
EUS-FNA has a high specificity (88%-97%) in diagnosis of malignant PCLs by analyzing cystic fluid amylase, CEA, and glucose levels, as well as genetic sequencing 26 27 . However, in clinical practice, CEA is widely used to distinguish MNs from n-MNs, and a value of ≥ 192 ng/mL is considered the best cutoff for obtaining the best yield. This was not confirmed in our study, which found MNs in 50% of cases in which cystic fluid was collected (n = 22). Cytology results to classify MN had sensitivity, specificity, and accuracy of 35%, 83% and 59%, respectively. The sensitivity of cytology for diagnosing a malignant PCL was 22% 6 . These results are much lower when compared with the EUS-TA described in this study, thus making the latter a simple option for classificatory diagnosis of PCLs.
Park et al. found that cystic fluid glucose levels were lower in MNs than n-MNs (5 vs. 82 mg/dL, P = 0.002) 29 . In our sample, high glucose levels confirmed n-MNs in 60% of cases in which cystic fluids could be collected. A combination of low glucose and elevated CEA levels can predict MNs 30 . However, the low concentration of tumor cells in the cystic fluid alters the accuracy and sensitivity of EUS-FNA for cystic fluid acquisition and cytological analysis. This was demonstrated in our case series, in which adequate amounts of liquid were obtained for biochemical analysis in only 39.3% of cases. In another study, the cyst fluid sent for cytology provided to have adequate cellular material for an intention-to-diagnose yield of 31% (44/143), and sufficient fluid for biochemical analysis was obtained in 49% of cases, similar to our study 31 .
Therefore, obtaining MC from the cystic wall for the McH examination is important for a classificatory diagnosis. A meta-analysis revealed that EUS-FNA has a 51% sensitivity and a 94% specificity for malignant cytology 26 . Another randomized controlled study of EUS-FNA in PCLs with ROSE and without ROSE revealed that most cytology results from EUS-FNA of PCLs are negative or nondiagnostic. There was no difference using EUS-FNA without on-site cytology compared with ROSE when measuring total diagnostic yield (22.2% vs 13%) and number of nondiagnostic specimens (50% vs 54%) 32 . Our study demonstrated diagnostic efficacy for differentiating between MNs and n-MNs and identifying malignant PCLs of 95.2% and 97.9%, respectively, which are higher than the results found in the literature, and the number of nondiagnostic specimens was eight of 145 (5%).
EUS-FNA is a safe method for diagnosing PCL 33 . Since the advent of EUS-FNB for PCLs, AEs have been reported. Phan et al. reported the first use of EUS-FNB in a PCL case 34 . The lateral pore structure of the PC20 allows for more tissues to be obtained while also increasing the chance of cutting the MD, at least theoretically. Luo et al. reported a case of PCL in which the patient developed severe pancreatitis with respiratory failure after EUS-FNB 35 . In our study, AEs occurred in 2.7% of the patients, with three cases of mild bleeding after the puncture that were all clinically treated and one case of mild AP that was also clinically treated.
As technology evolves, EUS-TA and confocal laser endomicroscopy (EUS-nCLe) performed through a 19-gauge needle 36 , as well as the use of Moray forceps for ultrasound-guided biopsy, have been applied to PCLs, but the enormous economic cost increase that these techniques entail for diagnosis cannot be ignored 37 . Furthermore, there is no evidence to support their role in determining or excluding the presence of neoplasms before surgery.
Our study has some limitations. First, it was a single-center study with a small population sample. Second, the results of EUS specimen acquisition can be influenced by the significant experience of endoscopists and the pathology sector in analyzing this type of material. Third, because not all patients underwent surgery, the gold standard confirmation criterion for PCL diagnosis was not based on postoperative pathology, which would be ideal.
Conclusions
In conclusion, EUS-TA with PC20 has a high technical success rate, high diagnostic accuracy, and low rate of AEs. Larger, multicenter, randomized, controlled, and prospective studies are required to confirm these findings.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Schraibman V, Goldman SM, Ardengh JC et al. New trends in diffusion-weighted magnetic resonance imaging as a tool in differentiation of serous cystadenoma and mucinous cystic tumor: a prospective study. Pancreatology. 2011;11:43–51. doi: 10.1159/000324565. [DOI] [PubMed] [Google Scholar]
- 2.Zerboni G, Signoretti M, Crippa S et al. Systematic review and meta-analysis: prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology. 2019;19:2–9. doi: 10.1016/j.pan.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Marinelli V, Secchettin E, Andrianello S et al. Psychological distress in patients under surveillance for intraductal papillary mucinous neoplasms of the pancreas: the “Sword of Damocles” effect calls for an integrated medical and psychological approach a prospective analysis. Pancreatology. 2020;20:505–510. doi: 10.1016/j.pan.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka M, Fernández-Del Castillo C, Kamisawa T et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–753. doi: 10.1016/j.pan.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Dumonceau JM, Deprez PH, Jenssen C et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline – updated January 2017. Endoscopy. 2017;49:695–714. doi: 10.1055/s-0043-109021. [DOI] [PubMed] [Google Scholar]
- 6.Brugge WR, Lewandrowski K, Lee-Lewandrowski E et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Vaiciunas S, Taglieri E, Micelli-Neto O et al. Endoscopic ultrasound-guided fine-needle aspiration microhistology in asymptomatic and symptomatic pancreatic cystic lesions. Pancreas. 2020;49:584–590. doi: 10.1097/MPA.0000000000001525. [DOI] [PubMed] [Google Scholar]
- 8.Facciorusso A, Kovacevic B, Yang D et al. Predictors of adverse events after endoscopic ultrasound-guided through-the-needle biopsy of pancreatic cysts: a recursive partitioning analysis. Endoscopy. 2022;54:1158–1168. doi: 10.1055/a-1831-5385. [DOI] [PubMed] [Google Scholar]
- 9.Ardengh JC, Brunaldi VO, Brunaldi MO et al. Is the new procore 20G double forward-bevel needle capable to obtain better histological samples by endoscopic ultrasound for diagnosing solid pancreatic lesions? Arq Bras Cir Dig. 2021;33:e1554. doi: 10.1590/0102-672020200004e1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armellini E, Manfrin E, Trisolini E et al. Histologic retrieval rate of a newly designed side-bevelled 20G needle for EUS-guided tissue acquisition of solid pancreatic lesions. U Eur Gastroenterol J. 2019;7:96–104. doi: 10.1177/2050640618804443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Riet PA, Larghi A, Attili F et al. A multicenter randomized trial comparing a 25-gauge EUS fine-needle aspiration device with a 20-gauge EUS fine-needle biopsy device. Gastrointest Endosc. 2019;89:329–339. doi: 10.1016/j.gie.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Ardengh JC, Lopes CV, de Lima LF et al. Cell block technique and cytological smears for the differential diagnosis of pancreatic neoplasms after endosonography-guided fine-needle aspiration. Acta Gastroenterol Latinoam. 2008;38:246–251. [PubMed] [Google Scholar]
- 13.Compton CC. Protocol for the examination of specimens from patients with endocrine tumors of the pancreas, including those with mixed endocrine and acinar cell differentiation: a basis for checklists. Cancer Committee of the College of American Pathologists. Arch Pathol Lab Med. 2000;124:30–36. doi: 10.5858/2000-124-0030-PFTEOS. [DOI] [PubMed] [Google Scholar]
- 14.Khandelwal M, Lichtenstein GR, Morris JB et al. Abdominal lymphangioma masquerading as a pancreatic cystic neoplasm. J Clin Gastroenterol. 1995;20:142–144. doi: 10.1097/00004836-199503000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Rampy BA, Waxman I, Xiao SY et al. Serous cystadenoma of the pancreas with papillary features: a diagnostic pitfall on fine-needle aspiration biopsy. Arch Pathol Lab Med. 2001;125:1591–1594. doi: 10.5858/2001-125-1591-SCOTPW. [DOI] [PubMed] [Google Scholar]
- 16.Basturk O, Hong SM, Wood LD et al. A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015;39:1730–1741. doi: 10.1097/PAS.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ardengh JC, Lopes CV, Venco FE et al. Diagnosis of pancreatic solid pseudopapillary neoplasms using cell-blocks and immunohistochemical evaluation of endoscopic ultrasound-guided fine needle aspiration biopsy specimens. Cytopathology. 2021;32:50–56. doi: 10.1111/cyt.12905. [DOI] [PubMed] [Google Scholar]
- 18.Kongkam P, Al-Haddad M, Attasaranya S et al. EUS and clinical characteristics of cystic pancreatic neuroendocrine tumors. Endoscopy. 2008;40:602–605. doi: 10.1055/s-2007-995740. [DOI] [PubMed] [Google Scholar]
- 19.Nass KJ, Zwager LW, van der Vlugt M et al. Novel classification for adverse events in GI endoscopy: the AGREE classification. Gastrointest Endosc. 2022;95:1078–1085. doi: 10.1016/j.gie.2021.11.038. [DOI] [PubMed] [Google Scholar]
- 20.Lee BS, Nguyen AK, Tekeste TF et al. Long-term follow-up of branch-duct intraductal papillary mucinous neoplasms with No change in first 5 years of diagnosis. Pancreatology. 2021;21:144–154. doi: 10.1016/j.pan.2020.10.040. [DOI] [PubMed] [Google Scholar]
- 21.McCarty TR, Paleti S, Rustagi T. Molecular analysis of EUS-acquired pancreatic cyst fluid for KRAS and GNAS mutations for diagnosis of intraductal papillary mucinous neoplasia and mucinous cystic lesions: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93:1019–1.033E8. doi: 10.1016/j.gie.2020.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Sakorafas GH, Smyrniotis V, Reid-Lombardo KM et al. Primary pancreatic cystic neoplasms revisited. Part III. Intraductal papillary mucinous neoplasms. Surg Oncol. 2011;20:e109–e118. doi: 10.1016/j.suronc.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Giannone F, Crippa S, Aleotti F et al. Improving diagnostic accuracy and appropriate indications for surgery in pancreatic cystic neoplasms: the role of EUS. Gastrointest Endosc. 2022;96:648–65600. doi: 10.1016/j.gie.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Roldán J, Harrison JM, Qadan M et al. Evolving trends in pancreatic cystic tumors: a 3-decade single-center experience with 1290 resections. Ann Surg. 2023;277:491–497. doi: 10.1097/SLA.0000000000005142. [DOI] [PubMed] [Google Scholar]
- 25.Ardengh JC, Lopes CV, de Lima-Filho ER et al. Impact of endoscopic ultrasound-guided fine-needle aspiration on incidental pancreatic cysts. A prospective study. Scand J Gastroenterol. 2014;49:114–120. doi: 10.3109/00365521.2013.854830. [DOI] [PubMed] [Google Scholar]
- 26.Wang QX, Xiao J, Orange M et al. EUS-guided FNA for diagnosis of pancreatic cystic lesions: a meta-analysis. Cell Physiol Biochem. 2015;36:1197–1209. doi: 10.1159/000430290. [DOI] [PubMed] [Google Scholar]
- 27.Thornton GD, McPhail MJ, Nayagam S et tal. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology. 2013;13:48–57. doi: 10.1016/j.pan.2012.11.313. [DOI] [PubMed] [Google Scholar]
- 28.Barresi L, Tacelli M, Ligresti D et al. Tissue acquisition in pancreatic cystic lesions. Dig Liver Dis. 2019;51:286–292. doi: 10.1016/j.dld.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Park WG, Wu M, Bowen R et al. Metabolomic-derived novel cyst fluid biomarkers for pancreatic cysts: glucose and kynurenine. Gastrointest Endosc. 2013;78:295–30200. doi: 10.1016/j.gie.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopes CV. Cyst fluid glucose: an alternative to carcinoembryonic antigen for pancreatic mucinous cysts. World J Gastroenterol. 2019;25:2271–2278. doi: 10.3748/wjg.v25.i19.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Jong K, Poley JW, van Hooft JE et al. Endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions provides inadequate material for cytology and laboratory analysis: initial results from a prospective study. Endoscopy. 2011;43:585–590. doi: 10.1055/s-0030-1256440. [DOI] [PubMed] [Google Scholar]
- 32.Estrada P, Benson M, Gopal D et al. Cytology with rapid on-site examination (ROSE) does not improve diagnostic yield of EUS-FNA of pancreatic cystic lesions. Diagn Cytopathol. 2019;47:1184–1189. doi: 10.1002/dc.24291. [DOI] [PubMed] [Google Scholar]
- 33.Du C, Chai NL, Linghu EQ et al. Incidents and adverse events of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cystic lesions. World J Gastroenterol. 2017;23:5610–5618. doi: 10.3748/wjg.v23.i30.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phan J, Dawson D, Sedarat A et al. Clinical utility of obtaining endoscopic ultrasound-guided fine-needle biopsies for histologic analyses of pancreatic cystic lesions. Gastroenterology. 2020;158:475–4770. doi: 10.1053/j.gastro.2019.10.048. [DOI] [PubMed] [Google Scholar]
- 35.Luo S, Li DF, Guo L et al. Severe acute pancreatitis caused by endoscopic ultrasonography-guided fine-needle biopsy of a pancreatic solid pseudopapillary neoplasm. Endoscopy. 2021;53:E322–E323. doi: 10.1055/a-1275-9603. [DOI] [PubMed] [Google Scholar]
- 36.Facciorusso A, Buccino VR, Sacco R. Needle-based confocal laser endomicroscopy in pancreatic cysts: a meta-analysis. Eur J Gastroenterol Hepatol. 2020;32:1084–1090. doi: 10.1097/MEG.0000000000001728. [DOI] [PubMed] [Google Scholar]
- 37.Nakai Y, Iwashita T, Park DH et al. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204–1214. doi: 10.1016/j.gie.2014.10.025. [DOI] [PubMed] [Google Scholar]