Abstract
Live-vector-based human immunodeficiency virus (HIV) vaccines are an integral part of a number of HIV vaccine regimens currently under evaluation. Live vectors that carry an intact gag gene are capable of eliciting HIV pseudovirion particle formation from infected host cells. The impact of pseudovirion particle formation on the immune response generated by live HIV vaccine vectors has not been established. In this study, a canarypox HIV vaccine candidate vector expressing HIV gag and env genes, vCP205, was modified by the introduction of a glycine-to-alanine coding change in the N-terminal myristylation site of gag to create Myr− vCP205. This substitution effectively eliminated particle formation without altering the level of protein production. vCP205 and Myr− vCP205 were then directly compared for the ability to induce HIV-specific immune responses in mice. The particle-competent vector vCP205 elicited higher levels of CD8+ T-cell responses, as indicated by gamma interferon enzyme-linked immunospot (ELISPOT) assay and intracellular cytokine staining. Humoral responses to Gag and Env were also markedly higher from animals immunized with the particle-competent vector. Furthermore, HIV-specific CD4+ T-cell responses were greater among animals immunized with the particle-competent vector. Using a human dendritic cell model of antigen presentation in vitro, vCP205 generated greater ELISPOT responses than Myr− vCP205. These results demonstrate that pseudovirion particle production by live-vector HIV vaccines enhances HIV-specific cellular and humoral immune responses.
A vaccine to protect individuals from human immunodeficiency virus (HIV) infection or disease is urgently needed to combat the ongoing epidemic of AIDS. A variety of approaches to generate HIV-specific cellular and humoral responses are presently under evaluation in preclinical development and in human trials. Among the most promising approaches are those employing live-virus-derived vector systems that express HIV proteins efficiently within host cells. Viral vectors presently undergoing testing in human HIV vaccine trials include poxviruses, adenoviruses, and alphaviruses, while a variety of additional vector systems are in earlier stages of development (6, 8, 31, 43). Vaccination regimens employing live viral vectors expressing HIV genes have provided protection from disease following simian-human immunodeficiency virus (SHIV) 89.6P challenge in macaques (1, 39, 41). The factors that influence the immunogenicity of live-virus-based HIV vaccines remain incompletely defined. One factor that may contribute to the immunogenicity of these vaccine vectors is their ability to generate HIV virus-like particles, or pseudovirions.
The HIV Gag protein drives the process of particle assembly and is sufficient to generate the production of virus-like particles, or pseudovirions, in the absence of additional viral gene products (16, 49). Thus, live vectors that include a complete gag gene and elicit sufficient production of Gag protein in the cytoplasm of infected cells will generate pseudovirions. Pseudovirion production has been demonstrated to occur in tissue culture cells infected by vaccinia virus vectors (22), modified vaccinia Ankara (MVA) vectors (34), canarypox (ALVAC) vectors (11, 12), and adenoviral vectors (28) expressing Gag proteins. Expression of Gag together with the envelope protein (Env) by live vaccine vectors leads to the release of pseudovirions bearing Env on their surfaces (12, 19, 21, 28).
Production of Gag or Gag-Env pseudovirions by live viral vectors in vivo may be beneficial to the immune response. Live vectors are likely to predominantly infect cells that are not professional antigen-presenting cells (APCs), such as skeletal muscle cells. Because these cells do not express class II molecules or costimulatory molecules on their surfaces, they are unlikely to function well as antigen-presenting cells. Uptake of released or particulate antigen by professional APCs, such as macrophages and dendritic cells, may then be required for the induction of potent adaptive immune responses to vector-encoded gene products. This uptake can be accomplished through the phagocytosis of particulate antigens released from vector-infected cells, followed by processing and presentation of vector-encoded antigen. We propose that pseudovirion formation by live vectors increases the availability of antigen to be taken up by professional APCs directly as particulate antigen and in this way enhances the production of a broad immune response. In support of this hypothesis, HIV particles have been shown to be taken up by dendritic cells in vitro, allowing HIV antigens to enter the class I presentation pathway through a process known as antigen cross-priming or the alternative class I presentation pathway (15, 25, 26). If this mechanism is relevant for HIV vaccine strategies, then we predict that a particle-competent vector would be superior in CD8+ T-cell responses to one that lacks this capacity.
In this study, we compared the immunogenicity of a poxvirus-based vector that elicits particle formation to that of a vector in which a single nucleotide substitution altered the myristylation receptor glycine residue of Gag and eliminated pseudovirion formation. We found that HIV-specific CD8+ T-cell responses were superior in mice receiving the particle-competent vector. The titers of antibodies to Gag and Env were also substantially higher in mice administered the particle-competent vector. Furthermore, the particle-competent vector markedly enhanced HIV-specific CD4+ T-cell responses. These results provide the first direct evidence that pseudovirion particle formation by live-vector HIV vaccines enhances HIV-specific humoral and cellular immune responses.
MATERIALS AND METHODS
Construction and purification of recombinant poxviruses.
The construction of vCP205 has been described (2, 11, 47). This recombinant canarypox vector includes gag and protease genes derived from HIV type 1LAI (HIV-1LAI), under the control of the vaccinia virus I3L promoter, and an env expression cassette consisting of gp120SU from HIV-1MN fused to the membrane-spanning domain of gp41TM from HIV-1LAI. The env expression cassette is under the control of the vaccinia H6 promoter. The gag/protease and env expression cassettes were placed between canarypox flanking regions in shuttle plasmid pHIV32, and homologous recombination was used to generate vCP205. To construct Myr− vCP205, a virus identical to parental vCP205 except for carrying a myristylation-deficient gag gene, an NcoI restriction site was first generated at the gag start codon using overlap extension PCR techniques (oligonucleotide sequences are available upon request). The myristyl acceptor glycine codon (GGT) of the gag gene in shuttle plasmid pHIV32 was then changed to an alanine codon (GCT) by inserting the NcoI-SphI fragment from plasmid pGPA1 (44) into the corresponding sites in pHIV32. The creation of the altered myristylation site and the integrity of the rest of the gag gene were confirmed by automated sequencing. Homologous-recombination techniques were then employed to generate recombinant canarypox viruses bearing the modified insert. Recombinant viruses were selected by plaque hybridization on chicken embryo fibroblasts (CEFs) using gag-specific oligonucleotide probes labeled with 32P. Recombinant isolates were then subjected to four rounds of plaque purification on CEFs. Viral stocks of vCP205 and Myr− vCP205 were generated by infection of CEFs at a multiplicity of infection (MOI) of 0.1; cells and supernatants were harvested at 72 h postinfection. The virus-containing preparation was subjected to freeze-thawing and sonication, and the resulting virus was purified by sedimentation through a 20% sucrose cushion. Virus pellets were resuspended in TNE (10 mM Tris-Cl, pH 8.0, 100 mM NaCl, 1 mM EDTA), the virus titer was determined by plaque-titration on CEFs, and stocks were aliquoted and stored at −80°C.
Analysis of protein and particle production by Myr− vCP205.
BSC-40 (African green monkey kidney) cells were obtained from Bernard Moss (National Institutes of Health [NIH]), and C2C12 cells (murine myoblasts) were obtained from the American Type Culture Collection (ATCC CRL-1772); both were maintained in Dulbecco's modified Eagle medium with 10% fetal calf serum, 100 U/ml of penicillin, and 100 μg/ml of streptomycin. BSC-40 cells or C2C12 cells were infected with ALVAC (no insert), vCP205, or Myr− vCP205 at an MOI of 10. Cells and supernatants were harvested at 72 h postinfection. The supernatants were subjected to centrifugation at 28,000 rpm in a Beckman SW28 rotor for 3 h at 4°C to pellet pseudovirion particles. Cell lysates and supernatant fractions were then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes for immunoblotting. Pooled HIV patient sera were used for detection, and the blot was developed using enhanced-chemiluminescence techniques. To directly examine myristic acid incorporation, BSC-40 cells were infected with viruses as before. Four hours postinfection, the culture medium was replaced by Dulbecco's modified Eagle medium containing 5% delipidated fetal calf serum and 50 μCi of [3H]myristic acid per milliliter (55 Ci/mmol; Amersham). The cells were incubated for an additional 8 h, and then Gag proteins were immunoprecipitated with HIV patient sera and analyzed by SDS-PAGE and autoradiography. In a separate experiment, the p24 contents of cell lysates and supernatants were measured at 36 h postinfection with vCP205 or Myr− vCP205 by p24 antigen capture enzyme-linked immunosorbent assay (ELISA) as previously described (12). For electron microscopy, BSC-40 and C2C12 cells were infected with vCP205 or Myr− vCP205 at an MOI of 10. Cells were harvested at 24 h postinfection, washed in phosphate-buffered saline (PBS), and fixed in 2.5% glutaraldehyde in PBS. Processing and analysis by transmission electron microscopy were performed as previously described (12). Imaging was performed using a Philips CM12 electron microscope.
Animals and specimen processing.
Female BALB/c mice, 6 to 8 weeks old, were purchased from the Jackson Laboratory (Bar Harbor, Maine). Groups of five mice were immunized intramuscularly in the tibialis anterior muscle with 2 × 107 PFU of ALVAC, vCP205, or Myr− vCP205 virus. Immunizations were performed every 2 weeks for a total of eight (group 1) or five (group 2) injections. Blood samples were collected prior to each injection and at the time of necropsy. The animals were sacrificed 5 days after the final injection. Spleens were removed for lymphocyte isolation, and single-cell suspensions were generated by gently teasing them against a nylon mesh. The cells were treated with ACK lysis buffer (Quality Biological, Inc.) for 8 min at room temperature to lyse red blood cells and then were washed two times in RPMI 1640 medium. The number of cells was adjusted to 4 × 106/ml in R10 medium (RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml of penicillin, 100 μg/ml of streptomycin, 2 μM l-glutamine, 10 mM HEPES, 0.1 mM MEM nonessential amino acids, and 50 μM 2-mercaptoethanol).
IFN-γ ELISPOT assay.
Gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays were performed on fresh splenocytes essentially as previously described (38). Polyvinylidene difluoride-backed 96-well plates (MAIP S4510; Millipore, Bedford, Mass.) were coated with 10 μg/ml of the anti-IFN-γ monoclonal antibody (MAb) AN18 (no. 3321-3; Mabtech, Stockholm, Sweden) in PBS at 4°C overnight. After the plates were washed three times with PBS, each plate was blocked by the addition of 200 μl of R10 medium per well for at least 2 h at room temperature. The peptides employed were HIV Gag 197-205 (AMQMLKETI) (30), HIV Env (V3 loop) peptide (IGPGRAFYTT) (24), and control H-2d-restricted influenza virus NP peptide (TYQRTRALV) (9). The peptides were added directly to the wells in a volume of 50 μl, and then freshly isolated splenocytes were added at 200,000 cells/well in 50 μl of R10 medium in triplicate. The final concentration of the peptides in the screening assay was 10 μM. The plates were incubated for 18 to 20 h overnight at 37°C in 5% CO2. The plates were then washed, labeled with biotinylated anti-IFN-γ MAb R4-6A2 (2 μg/ml; Mabtech) in PBS containing 0.5% fetal bovine serum, and incubated at room temperature for 3 h. After additional washes, avidin-peroxidase complex (Vector Laboratories, Burlingame, Calif.) was added to each well in PBS containing 0.1% Tween 20 for 1 h at room temperature. The plates were washed, and spots representing individual IFN-γ-producing cells were detected after a 4-min color reaction using 100 μl of AEC substrate (20 mg of 3-amino-9-ethylcarbazol dissolved in 2.5 ml of dimethylformamide diluted 1:20 in 47.5 ml of sodium-acetate buffer plus 25 μl of 30% H2O2). IFN-γ spot-forming cells (SFC) were counted using a Zeiss Axioplan II ELISPOT reader system using KS version 4.3 software (Carl Zeiss, Thornwood, N.Y.). The results were expressed as the number of SFC per 106 input cells. The number of peptide-specific IFN-γ-secreting T cells was calculated by subtracting the background (no-peptide) control value from the established SFC count.
Intracellular-cytokine staining for detection of Gag-specific CD8+ T-cell responses.
Intracellular-cytokine staining assays were performed essentially as previously described (37). Single-cell splenocyte suspensions were cultured for 6 h in R10 medium supplemented with 5% recombinant interleukin 2 (IL-2) (Hemagen Diagnostic, Inc.) and 5 μg per ml of anti-CD28 antibody (no. 555028; BD Pharmingen) in the presence of 20 μM of Gag 197-205 peptide; 1 μl/ml of brefeldin A (BD Pharmingen) was added 1 h into the incubation period. The cells were then washed once with R10 medium and twice with PBS containing 2% bovine serum albumin (BSA) and 0.09% sodium azide. The cells were surface stained with anti-CD3-phycoerythrin and anti-CD8-avidin-peroxidase complex (BD Pharmingen) at 4°C for 30 min and then washed and subjected to intracellular cytokine staining using the Cytofix/Cytoperm kit in accordance with the manufacturer's recommendations (BD Pharmingen) using fluorescein isothiocyanate-conjugated rat anti-mouse IFN-γ MAb (clone XMG1.2). A total of 2 × 106 cells were stained in fluorescence-activated cell sorter buffer for 30 min at 4°C. Flow cytometry analysis was performed using a FACScalibur (Becton-Dickinson), and data were analyzed using FlowJo software (TreeStar, Inc., Ashland, Ohio).
Detection of CD4+ T-cell responses to Gag and Env proteins.
Splenocytes from unimmunized BALB/c mice were harvested and infected with recombinant vaccinia virus vP1291 (14) to generate antigen-presenting cells for the stimulation of cells from immunized mice. Splenocytes were infected with vP1291 at an MOI of 1 for 2 h. Complete R10 medium containing 5% human IL-2 was added after infection, and the cells were incubated at 37°C overnight. The cells were then harvested, washed twice with R10 medium, and irradiated with 20 Gy. Frozen splenocytes from immunized mice were thawed and added to APCs at a ratio of 1:4 (APCs:splenocytes) in a 96-well plate in R10 medium containing 5% human IL-2 and 5 μg/ml of anti-CD28 MAb. The cultures were incubated for 2 h at 37°C, and then 1 μl/ml of brefeldin A was added and incubation continued for an additional 5 h. After being washed with PBS-2% BSA, the cells were subjected to surface staining and intracellular staining processes as described above.
Measurement of Gag- and Env-specific antibodies.
Sera from immunized mice were analyzed by ELISA for the presence of antibodies specific for HIV gp120 and Pr55Gag. Maxisorp NUNC immunoplates (Nalge Nunc International) were coated overnight at room temperature with 1 ng of GagSF2 (obtained from Chiron Corp. through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH) or 20 ng of gp160MN (MicroGenSys, Inc., Meriden, Conn.) and then blocked for 1.5 h at 37°C with 3% BSA (Sigma) in PBS buffer. Sera were diluted in solution buffer (PBS with 1% BSA and 0.05% Tween 20) and incubated at 37°C for 2 h. Plates were washed four times with 0.2% Tween 20 in PBS and then incubated with horseradish peroxidase-conjugated goat anti-mouse antibody (no. 31446; 1:5,000 dilution; Pierce) for 1 hour at 37°C. Following four additional washes, 3,3′,5,5′ tetramethyl benzidine substrate was added at room temperature for 30 min, and the reaction was stopped by the addition of H2SO4. The optical density was measured at 450 nm and 650 nm as a reference using a ThermoMax microplate reader (Molecular Devices). The background reactivity of preimmune sera was subtracted from the values obtained for the postimmunization sera. The endpoint antibody titer was calculated as the highest serum dilution yielding a value greater than twice the background level.
Statistical analysis.
IFN-γ ELISPOT analysis of HIV-1 Gag- and Env-specific CD8+ T-cell responses by vCP205 versus Myr− vCP205 was conducted using linear mixed models (23) considering dependency among three repeated assays. The normalities of the model residuals were assessed graphically. The distributions of Gag-specific CD8+ T-cell responses measured by intracellular cytokine staining were compared among mice immunized with parental ALVAC, vCP205, or Myr− vCP205 using a general linear model, and comparisons between any two groups (ALVAC versus vCP205, ALVAC versus Myr− vCP205, and Myr− vCP205 versus vCP205) were performed using a contrast transformation only when we observed an overall difference in the general linear model. P values were corrected according to the Bonferroni method for multiple comparisons. The normalities of the model residuals were assessed graphically. HIV-specific CD4+ T-cell responses were compared among mice immunized with parental ALVAC, vCP205, or Myr− vCP205 in a similar manner. Analysis of antibody responses against Gag and Env were performed by comparing the areas under the curve (AUCs) between mice immunized with vCP205 or Myr− vCP205 using Wilcoxon-Mann-Whitney tests. A two-sided significance level of 0.05 was used for all statistical inferences, and SAS version 8.02 (SAS Institute, Cary, N.C.) was used for analysis.
Human DC antigen presentation assay.
Myeloid dendritic cells (DC) were derived from leukapheresis blood products from healthy HIV-seropositive donors carried out under an Institutional Review Board approved protocol (RV149). The study group was composed of healthy HIV-seropositive donors with normal CD4 counts and detectable viral loads. The leukapheresis product was layered over Ficoll-Hypaque (Amersham, Uppsala, Sweden) and centrifuged to isolate the mononuclear cells, which were allowed to adhere to 10-cm2-diameter tissue culture plates (Becton Dickinson, Franklin Lakes, N.J.) for 60 min at 37°C. Adherent cells were cultured in DC medium (RPMI-1640 [Quality Biological, Gaithersburg, Md.], 1% human serum AB [Gemini Bio-Products, Woodland, Calif.], 2 mM l-glutamine [Quality Biological], 2 μg/ml of gentamicin [American Pharmaceutical Partners, Schaumburg, Ill.]) overnight at 37°C with 5% CO2. IL-4 (R&D Systems, Minneapolis, Minn.) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Berlex, Montville, N.J.) (1,000 IU/ml each) were added to the media on days 1 and 3 to drive DC differentiation. On day 5, the DC were harvested and replated into six-well tissue culture dishes in DC medium with IL-4 and GM-CSF. To mature the DC, 5% (vol/vol) monocyte conditioned medium was added to the cells on day 6, as described previously (29). On day 7, cells were harvested into DC medium and subsequently used for infection experiments. For infection, DC were exposed to various vectors at an MOI of 5 or 10 (vector-DC) in small volumes of DC medium. Infections were carried out for 2 h at 37°C with 5% CO2. Following the infections, the cells were washed with medium and then resuspended to a concentration of 106/ml. A sample of each condition (105 DC) was separated and used for ELISPOT assay, and the remainder of the cells were returned to the incubator for 4 h. Infection and expression rates were determined using anti-canarypox and anti-HIV Gag antibodies and analyzed by flow cytometry (FACSCalibur and FlowJo software).
ELISPOTs for DC cell antigen presentation assay.
The functional effects of empty vector, vCP205, and Myr− vCP205 were assessed by IFN-γ ELISPOT assays. Briefly, 96-well ELISPOT plates (Multiscreen-IP plates; Millipore, Mass.) were prepared by precoating them with mouse anti-human IFN-γ antibody (MAb 1-D1K; Mabtech AB, Sweden) at 5 μg/ml in 100 μl of PBS for 3 h at 37°C. The plates were washed with PBS and blocked with complete medium for 1 hour at 37°C. Infected (or mock-infected) DC were distributed in triplicate wells at 2 × 104 cells per well. Autologous peripheral blood mononuclear cells were added to the wells at a 5:1 ratio. As a positive control, staphylococcal enterotoxin B was added to duplicate wells at 5 μg/ml (final concentration). The plates were incubated for 20 to 24 h at 37°C (5% CO2) and were then washed with PBS with 0.05% Tween 20 buffer and incubated for 2 hours with mouse anti-human IFN-γ antibody conjugated with biotin (7B6-1-biotin; Mabtech AB, Sweden). Development consisted of a 1-hour incubation with an avidin-horseradish peroxidase complex (Vectastain ABC kit; Vector Laboratories, Calif.), followed by washing (PBS) and incubation with peroxidase substrate AEC for 4 minutes (Vector Laboratories, Calif.).
RESULTS
Generation and characterization of a particle-incompetent poxvirus HIV vaccine vector.
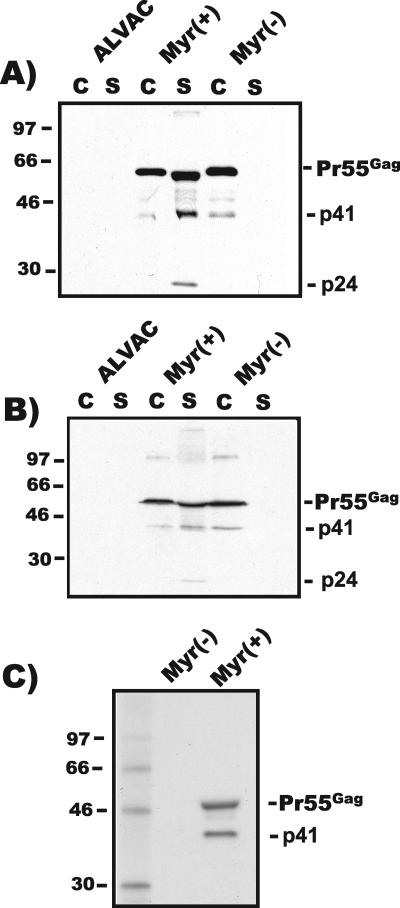
In order to determine whether HIV pseudovirion particle formation influences the immune response generated by live-vector HIV vaccines, we desired to create a matched pair of vectors differing only in their abilities to generate pseudovirion particles. Myristylation of the Gag protein is essential for particle formation, so we reasoned that the most effective and least disruptive method of generating a particle-incompetent vector was through mutation of the myristylation acceptor site. A glycine (GGT)-to-alanine (GCT) coding substitution at the extreme N terminus of the gag gene was engineered in a shuttle plasmid that had previously been used to create vaccine candidate vector vCP205. Using homologous-recombination methods, a novel recombinant canarypox vector bearing this substitution was generated. The resulting vector, Myr− vCP205, differs from vCP205 only by this single nucleotide change. Protein production by Myr− vCP205 was compared with that of vCP205 following infection of BSC-40 cells in order to establish that the myristic acid site mutation prevented particle formation. Parental ALVAC, lacking any HIV-specific sequences, was used as a control. Both vCP205 and Myr− vCP205 demonstrated expression of Pr55Gag in infected cell lysates (Fig. 1A). Pr55Gag was detected in cellular supernatants from cells infected with vCP205, in agreement with previous results (11). Myr− vCP205 did not generate particles, as indicated by the lack of detectable HIV proteins in cellular supernatants (Fig. 1A). Note that these Western blot assays were not optimized for demonstrating gp120. These data established that Myr− vCP205 was incapable of particle formation, as expected, due to the elimination of myristylation of Gag. vCP205 has been shown to efficiently produce pseudovirion particles from murine cells (11). Because we wished to examine the immunogenicity of vCP205 and Myr− vCP205 in a murine model, an identical particle release analysis was performed in murine myoblasts. In murine cells, as in simian cells, vCP205 infection led to release of antigen while Myr− vCP205 was completely incapable of particle formation and release (Fig. 1B). To further demonstrate the lack of myristylation of Gag produced by this vector, infected cells were radiolabeled with [3H]myristic acid. Figure 1C demonstrates that Pr55Gag produced by vCP205 was myristylated, while Gag produced by Myr− vCP205 was not.
FIG. 1.
Characterization of protein production by vCP205 and Myr− vCP205. (A) Western blot of cell lysates and extracellular particles. Supernatants and cells from BSC-40 cells infected with ALVAC, vCP205, or Myr− vCP205 were harvested and analyzed by Western blotting using pooled HIV+ patient sera 36 h postinfection. The lanes were loaded with equal proportions of total cell lysate (C) or total pelleted particles (S). Gag bands are indicated; a 41-kDa band (p41) represents an intermediate cleavage product (MACA). (B) Western blot of cell lysates and supernatants from murine C2C12 cells infected as in panel A. (C) [3H]myristic acid incorporation into Gag proteins expressed by vCP205 or Myr− vCP205. Cells infected with each virus were labeled with [3H]myristic acid, and cellular proteins were immunoprecipitated with pooled HIV patient sera. Analysis was performed by SDS-PAGE and fluorography. Molecular mass markers are indicated on the left in kilodaltons.
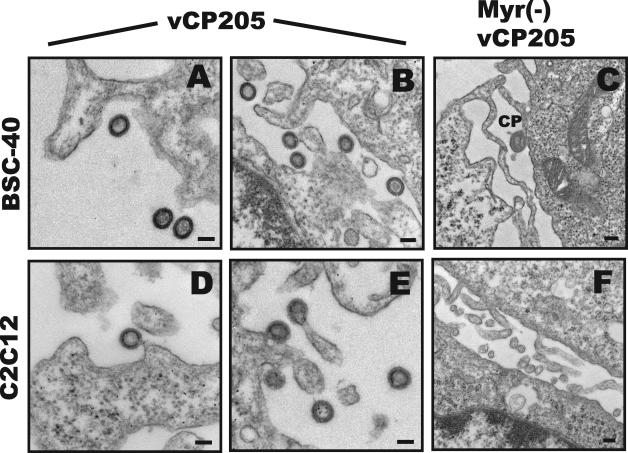
In order to confirm the lack of particle production by Myr− vCP205, we performed transmission electron microscopy on cells infected with the two vectors. Pseudovirions were readily detected on or near the surfaces of cells infected with vCP205 (Fig. 2A and B and D and E). As noted previously (12), the particles were predominantly immature in appearance. Particles were readily found budding from murine cells (Fig. 2D and E). No particles were found on or near the surfaces of BSC-40 or C2C12 cells infected with Myr− vCP205 (Fig. 2C and F). Canarypox virions were sometimes seen (Fig. 2C), as were cellular extensions that could sometimes appear circular when cut in cross section (Fig. 2F). However, the typical electron-dense appearance of immature Gag pseudovirion particles was not observed in >50 fields examined for each cell type. Of note, no intracellular virus-like particles were observed for vCP205 or Myr− vCP205.
FIG. 2.
Electron microscopic analysis of pseudovirion particle formation. (A) Pseudovirion particles produced by vCP205 in BSC-40 cells. Magnification, ×40,000. (B) Pseudovirion particles produced by vCP205. Magnification, ×33,000. (C) Representative view showing lack of particles formed by Myr− vCP205 in BSC-40 cells. Magnification, ×21,000. CP, canarypox particle. (D and E) Pseudovirion particle formation by vCP205 in murine myoblast C2C12 line. Magnification, ×40,000. (F) Representative micrograph showing lack of particle formation by Myr− vCP205 in C2C12 cells. Magnification, ×22,000. Bars represent 100 nm.
We considered the possibility that total protein production or protein stability might be altered by the myristylation site mutation. To address this, BSC-40 cells were infected with vCP205 or Myr− vCP205, and p24 antigen in cells and supernatants was quantified 36 h postinfection using a p24 antigen capture ELISA. As indicated in Table 1, p24 antigen was efficiently released into the supernatant from cells infected by vCP205, while only a minimal level of p24 was present in supernatants from Myr− vCP205. However, the total p24 production (cell plus supernatant) was not significantly different for the two vectors. This experiment was repeated several times, with harvest at 24 h, and in these experiments no p24 was found in the supernatant from Myr− vCP205-infected cultures, while a significant proportion was released from vCP205-infected cultures (data not shown). All of the p24 in the supernatant from vCP205-infected cells was in the form of viral particles, as indicated by a lack of any soluble p24 remaining following pelleting at 100,000 × g. We note that although the immunoreactivities of antibodies utilized in the p24 antigen capture ELISA may differ between Pr55Gag and p24, we saw no difference in intracellular cleavage between Myr+ and Myr− Gag, so the only potential bias in this measurement would be to provide a slight overestimate of the proportion of particulate, cleaved Gag. These data confirmed that vCP205 and Myr− vCP205 generated equivalent amounts of antigen expression, while vCP205 alone was capable of pseudovirion particle formation.
TABLE 1.
p24 antigen content of cell lysates and supernatants
| p24 antigen contenta
|
||
|---|---|---|
| Myr− vCP205 | vCP205 | |
| Cell lysate | 59.2 | 36.36 |
| Supernatant | 3.2 | 30.36 |
| Total | 62.4 | 66.72 |
Nanograms/106 cells.
Immunization regimen.
Two groups of mice were immunized with ALVAC, vCP205, or Myr− vCP205. Five mice were immunized with each vector in each of the two groups (for a total of 15 mice per group). Group 1 received eight total immunizations with vector, given at 2-week intervals. Group 2 received five immunizations according to a similar schedule. Vectors were injected intramuscularly without adjuvant. Serum was collected prior to each immunization, and sera and splenocytes were harvested 5 days following the final immunization for the assays described below.
CD8+ T-cell responses elicited by vCP205 versus Myr− vCP205.
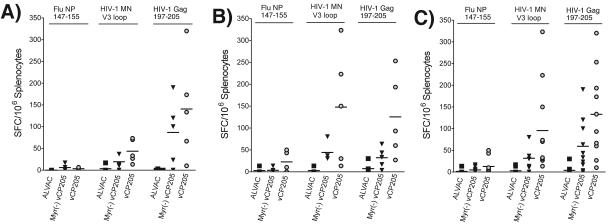
The purpose of this study was to compare the immunogenicity of a particle-competent vector (vCP205) with that of its particle-incompetent partner, Myr− vCP205. vCP205 has been noted in human trials to generate CD8+ T-cell responses and to prime for HIV-specific antibody responses (3, 13). We first focused on a comparison of the CD8+ T-cell responses produced upon immunization with each vector as measured by IFN-γ ELISPOT assay. In this assay, immunodominant Gag and Env peptides previously identified for BALB/c mice were used to stimulate freshly isolated splenocytes; an H-2d-restricted peptide from the influenza virus NP protein was included as a negative control. The level of SFC/million for control splenocytes in this assay was <20 (Fig. 3A, Flu peptide columns and ALVAC columns). The mean number of SFC elicited by Env peptide was 43 (±27) for vCP205 versus 19 (±17) for Myr− vCP205 (P = 0.11). A higher level of reactivity to the Gag peptide was seen, with a mean of 141 ± 118 for vCP205 and 87 ± 77 for Myr− vCP205 (P = 0.37). These responses were predominantly from CD8+ T cells, as demonstrated by depletion of CD8+ cells with anti-CD8 antibody coupled to magnetic beads (data not shown). Thus, both Env- and Gag-specific CD8+ T-cell responses were higher in animals immunized with the particle-competent vector, although the differences between these small groups did not attain statistical significance. A second group of mice received five immunizations (rather than eight, as in group 1), and demonstrated the same trend of greater responses from vCP205 revealed by the Gag peptide (125 ± 94 versus 32 ± 23; P = 0.05), as well as by the Env peptide (147 ± 131 versus 44 ± 20; P = 0.08) (Fig. 3B). When data from both groups of mice were combined in a multivariable mixed-regression model, differences between vCP205 and Myr− vCP205 in generating Env-specific responses reached statistical significance (P = 0.04), as did differences in Gag-specific responses (P = 0.05) (Fig. 3C). We conclude from these data that pseudovirion particle formation by vCP205 resulted in a moderate but significant enhancement of HIV-specific CD8+ T-cell responses.
FIG. 3.
IFN-γ ELISPOT analysis of HIV-1 Gag- and Env-specific T-cell responses following immunization with parental ALVAC, vCP205, or Myr− vCP205. Splenocytes were isolated 5 days after the final vector immunization and restimulated with 10 μM of the indicated peptides for 18 h. IFN-γ-expressing T cells were counted and expressed as the number of SFC per million input cells. (A and B) Groups of mice immunized eight and five times, respectively. (C) Combined analysis of IFN-γ ELISPOT results from both groups of mice. The horizontal lines in each column represent the means.
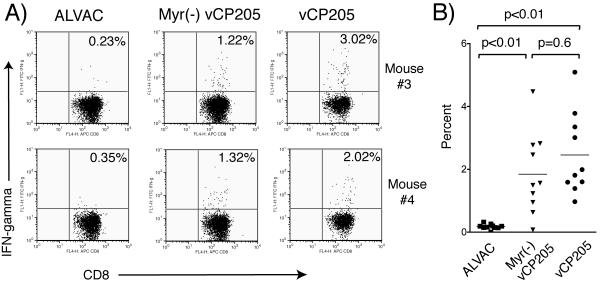
We next performed intracellular-cytokine staining to further analyze the observed differences in CD8+ T-cell responses. Frozen splenocytes from both groups of immunized mice were incubated with Gag 197-205 peptide, and the frequency of IFN-γ-specific cells within the CD8+ T-cell population was determined using an intracellular-cytokine staining protocol for IFN-γ. CD8+ responses specific for Gag were detected in splenocytes from mice immunized with either vector (Fig. 4). Representative dot plots from two animals in each study arm are shown in Fig. 4A; combined results from all animals are presented graphically in Fig. 4B. Consistent with IFN-γ ELISPOT results, a higher number of IFN-γ-staining CD8+ T cells were seen in animals immunized with vCP205 than in those immunized with Myr− vCP205. Differences between Myr− vCP205 and vCP205 in this assay, although showing a trend in agreement with the ELISPOT results, did not reach statistical significance (P = 0.60).
FIG. 4.
Gag-specific CD8+ T-cell responses measured by intracellular cytokine staining. Splenocytes derived from mice immunized with parental ALVAC, vCP205, or Myr− vCP205 were restimulated with 20 μM Gag peptide 197-205 for 6 h. The splenocytes were then stained with avidin-peroxidase complex-labeled anti-CD8 for cell surface staining and fluorescein isothiocyanate-labeled anti-IFN-γ using an intracellular staining protocol. (A) Representative dot plots from two animals in each immunization group are shown. (B) Results from all animals are shown. The horizontal lines represent the mean percentage of IFN-γ-secreting CD8+ T cells in each group.
Gag- and Env-specific humoral responses elicited by vCP205versus Myr− vCP205.
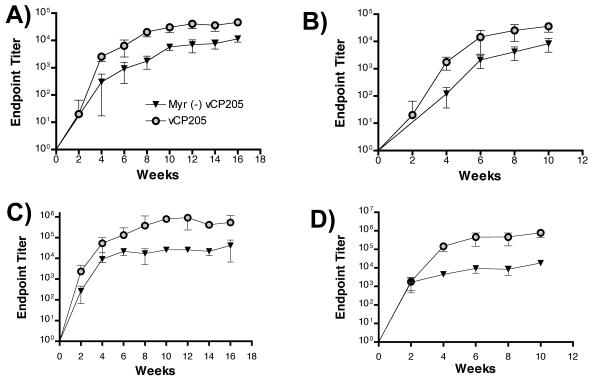
Recombinant poxvirus vectors have been shown to generate strong CD8+ T-cell responses and are often included in vaccine regimens primarily for this purpose. We initiated this study with the major hypothesis that CD8+ T-cell responses would be enhanced by pseudovirion particle formation through particle uptake and presentation via the exogenous class I presentation pathway. In measuring anti-Gag antibody production over the course of the immunization protocols, however, we were surprised to find significant differences in antibody titers. vCP205 elicited endpoint binding antibody titers to Gag that were significantly higher than those generated by Myr− vCP205 in both groups of immunized mice (Fig. 5A and B). The mean AUC for anti-Gag responses in group 1 was 320,040 ± 69,600 for vCP205 and 58,280 ± 11,297 for Myr− vCP205 (P = 0.03). Results from animals in group 2 were similar: AUCs of 119,400 ± 51,868 for vCP205 and 21,040 ± 9,210 for Myr− vCP205 (P = 0.03). The difference became statistically significant in both groups after two immunizations. We next measured responses to the HIV-1 envelope glycoprotein, as these responses should have more biological relevance. Envelope-specific responses were enhanced >10-fold in those animals immunized with vCP205 (Fig. 5C and D). The mean areas under the curve for anti-Env responses were 5,844,000 ± 4,810,213 for vCP205 and 282,920 ± 66,376 for Myr− vCP205 in group 1 (P = 0.03). Group 2 responses were similar: AUCs of 2,886,120 ± 3,445,022 for vCP205 and 65,400 ± 42,186 for Myr− vCP205 (P = 0.03). The difference became statistically significant after four immunizations. These data demonstrated that humoral immune responses were greatly enhanced by the ability of a live-vector HIV vaccine to generate pseudovirion particles.
FIG. 5.
Antibody responses against Gag and Env elicited in mice immunized with vCP205 or Myr− vCP205. Sera were collected before priming and 2 weeks after each injection. The data shown represent the reciprocal endpoint dilution titers showing specific binding to antigen. (A) Anti-Gag responses from group 1 mice, presented as mean ± standard deviation of reciprocal endpoint titers. (B) Anti-Gag responses from group 2 mice. (C) Anti-Env responses in group 1 mice. (D) Anti-Env responses in group 2 mice.
We note that a plateau was reached in the titers of antibody responses seen in all groups after the fourth immunization, with either modest or no increases thereafter (especially notable in Fig. 5B to D). This likely represents the effect of antivector immunity, which was not specifically measured in this study.
HIV-specific CD4+ T-cell responses measured by intracellular cytokine staining.
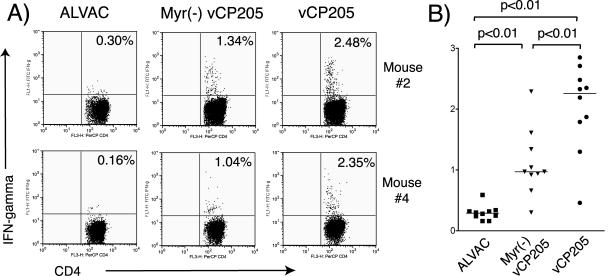
The marked enhancement in humoral immune responses observed in mice immunized with vCP205 suggested to us that HIV-specific CD4+ T-cell responses may have been enhanced by particle formation. To investigate this possibility, we compared HIV-specific CD4+ T-cell responses to Env and Gag using intracellular-cytokine staining. Splenocytes from unimmunized BALB/c mice were infected with a vaccinia virus expressing HIV Gag and Env and irradiated to serve as APCs. Splenocytes from immunized animals were incubated with these APCs, and CD4+ T cells expressing IFN-γ were quantified. Shown in Fig. 6A are representative results from two animals in each group, with cumulative results from all animals plotted in Fig. 6B. CD4+ T-cell responses in cells from animals immunized with vCP205 of Myr− vCP205 were significantly greater than those of animals immunized with empty vector (ALVAC). Remarkably, Gag- and Env-specific CD4+ T cells were significantly increased in animals immunized with vCP205 compared to Myr− vCP205 (2.0 ± 0.71 versus 1.1 ± 0.54; P < 0.01). We conclude that CD4+ T-cell responses were strongly enhanced by the ability to generate pseudovirion particles. Taken together, these studies indicate that CD8+ T-cell responses, humoral responses, and CD4+ T-cell responses are all enhanced by the ability of a live vector to generate pseudovirion particles.
FIG. 6.
HIV-specific CD4+ T-cell responses measured by intracellular-cytokine staining. Splenocytes from naïve BALB/c mice were infected with vaccinia virus expressing Env and Gag to function as APCs. Cryopreserved splenocytes from immunized mice were restimulated with APCs at the ratio of 4 to 1 for 7 h. The splenocytes were then subjected to surface labeling for CD4 and intracellular cytokine staining for IFN-γ. (A) Results from two representative mice from groups immunized with ALVAC, Myr− vCP205, and vCP205. (B) Cumulative results from the three groups of mice. The horizontal lines represent the mean responses for each group.
Human DC infected by particle-competent vCP205 function as more efficient antigen-presenting cells.
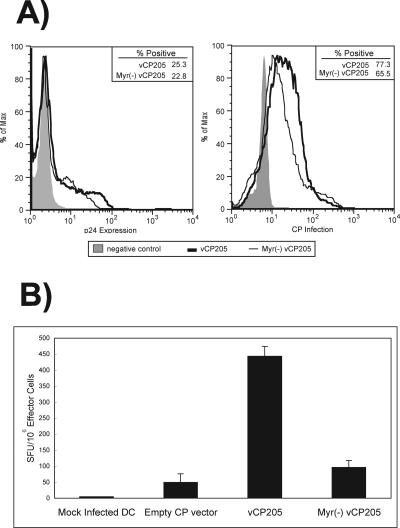
We hypothesized that a particle-competent live vector would also result in more efficient antigen presentation in human cells. To test this hypothesis, we used an in vitro antigen presentation method that has been well characterized (29). Peripheral blood was obtained from HIV-infected volunteer donors, and monocyte-derived DC were prepared by culturing in the presence of GM-CSF and IL-4. The DC were then infected with vCP205 or Myr− vCP205. The efficiencies of infection (Fig. 7A, right) and of antigen production (Fig. 7A, left) in human DC were similar for the two vectors; 50 to 65% of DC were infected, and 23 to 25% of DC expressed p24, consistent with previously reported results (29). Next, autologous peripheral blood mononuclear cells were added at a 5:1 ratio and cocultivated with the canarypox-loaded dendritic cells for 24 h, and the IFN-γ-secreting cells were quantified by IFN-γ ELISPOT. Myr− vCP205 stimulated antigen-specific T cells to a level that was slightly above that of the empty-vector control (Fig. 7B). The level of antigen-specific cells stimulated to secrete IFN-γ was significantly greater when vCP205 was used to infect DCs than with Myr− vCP205 (Fig. 7B). We conclude that the expression of myristylated Gag, and potentially the production of pseudovirions by human dendritic cells, enhanced activation and cytokine secretion by HIV-specific T cells in this in vitro model.
FIG. 7.
In vitro human DC antigen presentation by vCP205 and Myr− vCP205. (A) Expression of HIV p24/Gag protein (left) and rates of infection (right) in DC infected with vCP205, Myr−vCP205, or control. The negative control for p24 expression was DC infected with empty canarypox vector. The negative control for infection was mock-infected DC. (B) IFN-γ ELISPOT analysis of T-cell responses following coculture with DC infected with empty canarypox vector, vCP205, or Myr− vCP205 or mock infected. Data are shown as the mean value from triplicate wells plus standard deviation.
DISCUSSION
Live-vector-based HIV vaccine candidates have yielded promising results in preclinical testing. Attenuated poxvirus vectors have induced partial protection against pathogenic strains of simian immunodeficiency virus (4, 32, 34, 35). Recombinant adenoviral vectors, MVA-based vectors, and vesicular stomatitis virus vectors have been successfully utilized in vaccination regimens to protect macaques against disease following challenge with SHIV 89.6P (1, 10, 39). Live vectors have generated substantial levels of HIV or simian immunodeficiency virus-specific CD8+ T-cell responses in these trials, and this response is thought to be the most likely immune mechanism involved in the protection exhibited in this model system (27). Live vectors can also induce humoral immunity when used alone or can prime for humoral responses when employed in a prime-boost vaccination regimen. The potent production of HIV antigens by current generations of vectors makes it likely that any vector that carries an intact wild-type gag gene will generate pseudovirion particles in vivo. However, it has not been proven that the production of pseudovirions is beneficial to the immune response. Indeed, some vectors currently in human trials, such as the Venezuelan equine encephalitis virus-based vector under evaluation in human trials in the United States and South Africa (6-8), express a nonmyristylated Gag protein that is incapable of particle formation. Similarly, a vaccine construct expressed in both DNA and recombinant MVA forms that is currently in human trials in England and Kenya was engineered in a manner that eliminates particle formation (20). While these approaches maintain maximal intracellular antigen expression, they may not be ideal if released particulate antigen enhances HIV-specific immunity. The present study was initiated to determine if pseudovirion formation contributes to the cellular and humoral immune responses generated by live-vector HIV vaccines.
Our results support the hypothesis that pseudovirion particle formation is beneficial to the immune response to live-vector HIV vaccine candidates. Using an avipox vector system, we demonstrated an increase in HIV-specific CD8+ T-cell responses when a particle-competent vector (vCP205) was employed compared to a particle-deficient vector (Myr− vCP205). These differences in CD8+ T-cell responses reached statistical significance for groups of 10 mice. The resulting antibody responses were clearly superior for the particle-competent vector, and differences were again statistically significant. CD4+ T-cell responses were likewise significantly increased for the particle-competent vector compared to the particle-deficient vector. These results support a model in which pseudovirion particles produced in an immunized host can extend the immune response through uptake of exogenous antigen by APCs.
ELISPOT results from a human DC-T-cell coculture system demonstrated a greater stimulation of HIV-specific T cells when dendritic cells were infected with the particle-competent vector vCP205. This finding suggests that the enhanced immunogenicity of particle-competent vectors seen in mice may translate into humans. In this in vitro antigen presentation system, the loading of major histocompatibility complex molecules on dendritic cells with HIV antigen may have been superior for those cell cultures infected with vCP205 due to cross-priming by pseudovirions, resulting in enhanced stimulation of Gag- or Env-specific T cells. Previous studies using this same virus and in vitro system have demonstrated that the primary population responding to the vCP205-loaded DC is CD8+ T cells (29). However, this experiment utilized direct infection of DC, and it remains possible that myristylated Gag produced directly in the infected APCs was more efficiently processed and presented than myristylation-deficient Gag. It will be important in future studies to derive the precise mechanism by which myristylated Gag and/or pseudovirion production enhances HIV-specific T-cell responses in this in vitro model system.
The results reported in this study suggest a strategy for designing live-vector HIV vaccines with enhanced immunogenicity. While these results bear repeating in the context of other live vectors, they suggest that particle formation by live vectors is beneficial. In order to optimize particle formation, Gag molecules bearing intact essential assembly domains should be employed. The I (interaction) domain of Gag overlies the nucleocapsid region and contributes to particle formation (40). The L (late) domain is located within the p6 region at the C-terminal end of Gag and contributes to particle budding through interactions with TSG101 and other components of cellular endosomal sorting pathways (17, 46, 48). At least three elements (myristylated N terminus, I domain, and L domain) are therefore required in a vector designed to generate pseudovirions efficiently. It may be possible to design live-vector HIV vaccines in which the production of pseudovirion particles is enhanced through the addition of optimized Gag molecules. Accessory molecules that enhance particle assembly, such as the HIV-1 Vpu protein (18), could be included in live-vector vaccines and coexpressed with Gag to maximize particle formation. Our results emphasize the need to consider the functional domains of HIV proteins that are included in live-vector designs, in addition to consideration of which epitopes are most desirable for inclusion.
Several potential mechanisms can be invoked to explain the enhanced cellular and humoral immune responses seen with the myristylation-competent vaccine vector in the murine immunogenicity model. Antigen-presenting cells, such as DC and macrophages, may take up pseudovirion particles after their formation and release from muscle cells. The alternative class I presentation pathway (antigen cross-presentation) could then allow the particle-associated proteins to be proteolytically processed and loaded on class I molecules. This pathway has been proposed as a major means of presentation for viral antigens (42). Indeed, HIV particles themselves can be processed by DC and stimulate proliferation of HIV-specific T cells in cell culture model systems (26). The alternative class I pathway could explain the enhanced CD8+ T-cell responses seen following immunization with the myristylation-competent vector in this study. We note, however, that the pseudovirions generated by the vectors in this study do not have a fusion-competent envelope glycoprotein, and fusion-competent particles may be necessary for optimal cross-presentation by HIV virus-like particles (5). Notably, a recent study of cross-priming in vivo suggests that stable proteins or protein aggregates may serve as ideal substrates for transfer to professional APCs for CD8+ T-cell cross-priming (33). Gag proteins in particulate “aggregates” may serve this purpose well, and the pseudovirions may have been taken up in this form in an envelope-independent manner. However, our results would also support a role for the uptake and processing of pseudovirions through the class II presentation pathway, leading to the generation of greater HIV-specific CD4+ T-cell populations that support HIV-specific B-cell responses and provide help for HIV-specific CD8+ T-cell responses. The more striking difference seen in CD4+ T-cell responses (compared with differences in CD8+ T-cell responses) suggests that this is the primary mechanism underlying the superior immunogenicity of vCP205 in this study. We conclude that a broad immune response to HIV antigens produced by live-vector HIV vaccines can be facilitated by pseudovirion particle formation, probably primarily through a more robust HIV-specific CD4+ T-cell response.
These results utilizing poxvirus-based vectors may not be applicable to all live-vector-based vaccine approaches. For example, vectors that directly infect APCs with high efficiency might benefit more from increased intracellular antigen concentration rather than from enhanced release of antigen. Further studies will be required to test the hypothesis that myristylation-deficient Gag may be superior in this setting. Similarly, the route of vector delivery may be an important determinant of the efficacy of pseudovirion formation in generating enhanced immune responses. Routes that enhance the recruitment and direct infection of APCs, such as intradermal inoculation, may not demonstrate the marked effect seen in our study with intramuscular administration, where it is more likely that antigen cross-presentation is important. The innate immune responses elicited by an immunogen within muscle tissue is another factor that may alter the ability of myocytes to function themselves in antigen presentation. For example, major histocompatibility complex class II production on myocytes can be induced by the use of immunostimulatory DNA sequences (45). We acknowledge the limitations involved in applying results from a murine model to the design of vaccines that are intended for use in humans. We also recognize the fact that the qualitative, as well as the quantitative, nature of the immune responses generated by a vaccine must be carefully considered in current HIV vaccine design (36). Nevertheless, this study suggests that the ability to form pseudovirion particles is an important consideration in HIV vaccine design and provides a basis for further studies in primates and humans.
In summary, we have demonstrated that pseudovirion particle formation by live-vector HIV vaccines is beneficial to the immune response. This property should be a consideration in the design of future generations of live-vector-based HIV vaccines. Further investigation into the precise mechanism underlying the enhanced immunogenicity of particle-competent live-vector HIV vaccines is warranted.
Acknowledgments
This work was supported by a Basic Research Grant from the Elizabeth Glaser Pediatric AIDS Foundation (P.S. and X.C.) and by NIH R01 AI52007. Statistical support was provided through the Vanderbilt-Meharry Center for AIDS Research (CFAR) (NIH P30 AI054999).
Facilities and collaborations supporting this work were provided by the Vanderbilt-Meharry CFAR. We thank the Vanderbilt Sequencing Core Facility, the Vanderbilt Flow Cytometry Core Facility, the Vanderbilt Electron Microscopy Core Facility, and Elvin Woodruff for expert technical assistance. We thank Spyros Kalams for helpful comments during the performance and presentation of this work.
REFERENCES
- 1.Amara, R. R., F. Villinger, S. I. Staprans, J. D. Altman, D. C. Montefiori, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J. Virol. 76:7625-7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belshe, R. B., G. J. Gorse, M. J. Mulligan, T. G. Evans, M. C. Keefer, J. L. Excler, A. M. Duliege, J. Tartaglia, W. I. Cox, J. McNamara, K. L. Hwang, A. Bradney, D. Montefiori, K. J. Weinhold, et al. 1998. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. AIDS 12:2407-2415. [DOI] [PubMed] [Google Scholar]
- 3.Belshe, R. B., C. Stevens, G. J. Gorse, S. Buchbinder, K. Weinhold, H. Sheppard, D. Stablein, S. Self, J. McNamara, S. Frey, J. Flores, J. L. Excler, M. Klein, R. E. Habib, A. M. Duliege, C. Harro, L. Corey, M. Keefer, M. Mulligan, P. Wright, C. Celum, F. Judson, K. Mayer, D. McKirnan, M. Marmor, and G. Woody. 2001. Safety and immunogenicity of a canarypox-vectored human immunodeficiency virus type 1 vaccine with or without gp120: a phase 2 study in higher- and lower-risk volunteers. J. Infect. Dis. 183:1343-1352. [DOI] [PubMed] [Google Scholar]
- 4.Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J. Virol. 72:4170-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buseyne, F., S. Le Gall, C. Boccaccio, J. P. Abastado, J. D. Lifson, L. O. Arthur, Y. Riviere, J. M. Heard, and O. Schwartz. 2001. MHC-I-restricted presentation of HIV-1 virion antigens without viral replication. Nat. Med. 7:344-349. [DOI] [PubMed] [Google Scholar]
- 6.Caley, I. J., M. R. Betts, N. L. Davis, R. Swanstrom, J. A. Frelinger, and R. E. Johnston. 1999. Venezuelan equine encephalitis virus vectors expressing HIV-1 proteins: vector design strategies for improved vaccine efficacy. Vaccine 17:3124-3135. [DOI] [PubMed] [Google Scholar]
- 7.Caley, I. J., M. R. Betts, D. M. Irlbeck, N. L. Davis, R. Swanstrom, J. A. Frelinger, and R. E. Johnston. 1997. Humoral, mucosal, and cellular immunity in response to a human immunodeficiency virus type 1 immunogen expressed by a Venezuelan equine encephalitis virus vaccine vector. J. Virol. 71:3031-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, N. L., A. West, E. Reap, G. MacDonald, M. Collier, S. Dryga, M. Maughan, M. Connell, C. Walker, K. McGrath, C. Cecil, L. H. Ping, J. Frelinger, R. Olmsted, P. Keith, R. Swanstrom, C. Williamson, P. Johnson, D. Montefiori, and R. E. Johnston. 2002. Alphavirus replicon particles as candidate HIV vaccines. IUBMB Life 53:209-211. [DOI] [PubMed] [Google Scholar]
- 9.Deliyannis, G., D. C. Jackson, N. J. Ede, W. Zeng, I. Hourdakis, E. Sakabetis, and L. E. Brown. 2002. Induction of long-term memory CD8+ T cells for recall of viral clearing responses against influenza virus. J. Virol. 76:4212-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earl, P. L., L. S. Wyatt, D. C. Montefiori, M. Bilska, R. Woodward, P. D. Markham, J. D. Malley, T. U. Vogel, T. M. Allen, D. I. Watkins, N. Miller, and B. Moss. 2002. Comparison of vaccine strategies using recombinant env-gag-pol MVA with or without an oligomeric Env protein boost in the SHIV rhesus macaque model. Virology 294:270-281. [DOI] [PubMed] [Google Scholar]
- 11.Fang, Z. Y., I. Kuli-Zade, and P. Spearman. 1999. Efficient human immunodeficiency virus (HIV)-1 Gag-Env pseudovirion formation elicited from mammalian cells by a canarypox HIV vaccine candidate. J. Infect. Dis. 180:1122-1132. [DOI] [PubMed] [Google Scholar]
- 12.Fang, Z. Y., K. Limbach, J. Tartaglia, J. Hammonds, X. Chen, and P. Spearman. 2001. Expression of vaccinia E3L and K3L genes by a novel recombinant canarypox HIV vaccine vector enhances HIV-1 pseudovirion production and inhibits apoptosis in human cells. Virology 291:272-284. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari, G., C. Berend, J. Ottinger, R. Dodge, J. Bartlett, J. Toso, D. Moody, J. Tartaglia, W. I. Cox, E. Paoletti, and K. J. Weinhold. 1997. Replication-defective canarypox (ALVAC) vectors effectively activate anti-human immunodeficiency virus-1 cytotoxic T lymphocytes present in infected patients: implications for antigen-specific immunotherapy. Blood 90:2406-2416. [PubMed] [Google Scholar]
- 14.Ferrari, G., W. Humphrey, M. J. McElrath, J. L. Excler, A. M. Duliege, M. L. Clements, L. C. Corey, D. P. Bolognesi, and K. J. Weinhold. 1997. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc. Natl. Acad. Sci. USA 94:1396-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonteneau, J. F., D. G. Kavanagh, M. Lirvall, C. Sanders, T. L. Cover, N. Bhardwaj, and M. Larsson. 2003. Characterization of the MHC class I crosspresentation pathway for cell associated antigens by human dendritic cells. Blood 102:4448-4455. [DOI] [PubMed] [Google Scholar]
- 16.Freed, E. O. 1998. HIV-1 Gag proteins: diverse functions in the virus life cycle. Virology 251:1-15. [DOI] [PubMed] [Google Scholar]
- 17.Garrus, J. E., U. K. von Schwedler, O. W. Pornillos, S. G. Morham, K. H. Zavitz, H. E. Wang, D. A. Wettstein, K. M. Stray, M. Cote, R. L. Rich, D. G. Myszka, and W. I. Sundquist. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55-65. [DOI] [PubMed] [Google Scholar]
- 18.Gottlinger, H. G., T. Dorfman, E. A. Cohen, and W. A. Haseltine. 1993. Vpu protein of human immunodeficiency virus type 1 enhances the release of capsids produced by gag gene constructs of widely divergent retroviruses. Proc. Natl. Acad. Sci. USA 90:7381-7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haffar, O., J. Garrigues, B. Travis, P. Moran, J. Zarling, and S. L. Hu. 1990. Human immunodeficiency virus-like, nonreplicating, gag-env particles assemble in a recombinant vaccinia virus expression system. J. Virol. 64:2653-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanke, T., A. J. McMichael, M. Mwau, E. G. Wee, I. Ceberej, S. Patel, J. Sutton, M. Tomlinson, and R. V. Samuel. 2002. Development of a DNA-MVA/HIVA vaccine for Kenya. Vaccine 20:1995-1998. [DOI] [PubMed] [Google Scholar]
- 21.Kang, C. Y., L. Luo, M. A. Wainberg, and Y. Li. 1999. Development of HIV/AIDS vaccine using chimeric gag-env virus-like particles. Biol. Chem. 380:353-364. [DOI] [PubMed] [Google Scholar]
- 22.Karacostas, V., K. Nagashima, M. A. Gonda, and B. Moss. 1989. Human immunodeficiency virus-like particles produced by a vaccinia virus expression vector. Proc. Natl. Acad. Sci. USA 86:8964-8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laird, N. M., and J. H. Ware. 1982. Random-effects models for longitudinal data. Biometrics 38:963-974. [PubMed] [Google Scholar]
- 24.Lapham, C., B. Golding, J. Inman, R. Blackburn, J. Manischewitz, P. Highet, and H. Golding. 1996. Brucella abortus conjugated with a peptide derived from the V3 loop of human immunodeficiency virus (HIV) type 1 induces HIV-specific cytotoxic T-cell responses in normal and in CD4+ cell-depleted BALB/c mice. J. Virol. 70:3084-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsson, M., J. F. Fonteneau, and N. Bhardwaj. 2003. Cross-presentation of cell-associated antigens by dendritic cells. Curr. Top. Microbiol. Immunol. 276:261-275. [DOI] [PubMed] [Google Scholar]
- 26.Larsson, M., J. F. Fonteneau, M. Lirvall, P. Haslett, J. D. Lifson, and N. Bhardwaj. 2002. Activation of HIV-1 specific CD4 and CD8 T cells by human dendritic cells: roles for cross-presentation and non-infectious HIV-1 virus. AIDS 16:1319-1329. [DOI] [PubMed] [Google Scholar]
- 27.Letvin, N. L. 2002. Strategies for an HIV vaccine. J. Clin. Investig. 110:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo, L., Y. Li, and C. Yong Kang. 2003. Budding and secretion of HIV Gag-Env virus-like particles from recombinant human adenovirus infected cells. Virus Res. 92:75-82. [DOI] [PubMed] [Google Scholar]
- 29.Marovich, M. A., J. R. Mascola, M. A. Eller, M. K. Louder, P. A. Caudrelier, R. El-Habib, S. Ratto-Kim, J. H. Cox, J. R. Currier, B. L. Levine, C. H. June, W. B. Bernstein, M. L. Robb, B. Schuler-Thurner, R. M. Steinman, D. L. Birx, and S. Schlesinger-Frankel. 2002. Preparation of clinical-grade recombinant canarypox-human immunodeficiency virus vaccine-loaded human dendritic cells. J. Infect. Dis. 186:1242-1252. [DOI] [PubMed] [Google Scholar]
- 30.Mata, M., P. J. Travers, Q. Liu, F. R. Frankel, and Y. Paterson. 1998. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J. Immunol. 161:2985-2993. [PubMed] [Google Scholar]
- 31.McMichael, A. J., and T. Hanke. 2003. HIV vaccines 1983-2003. Nat. Med. 9:874-880. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson, C., G. Sutter, L. Walther-Jallow, P. ten Haaft, L. Akerblom, J. Heeney, V. Erfle, P. Bottiger, G. Biberfeld, and R. Thorstensson. 2002. Immunization with recombinant modified vaccinia virus Ankara can modify mucosal simian immunodeficiency virus infection and delay disease progression in macaques. J. Gen. Virol. 83:807-818. [DOI] [PubMed] [Google Scholar]
- 33.Norbury, C. C., S. Basta, K. B. Donohue, D. C. Tscharke, M. F. Princiotta, P. Berglund, J. Gibbs, J. R. Bennink, and J. W. Yewdell. 2004. CD8+ T cell cross-priming via transfer of proteasome substrates. Science 304:1318-1321. [DOI] [PubMed] [Google Scholar]
- 34.Ourmanov, I., C. R. Brown, B. Moss, M. Carroll, L. Wyatt, L. Pletneva, S. Goldstein, D. Venzon, and V. M. Hirsch. 2000. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 74:2740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pal, R., D. Venzon, N. L. Letvin, S. Santra, D. C. Montefiori, N. R. Miller, E. Tryniszewska, M. G. Lewis, T. C. VanCott, V. Hirsch, R. Woodward, A. Gibson, M. Grace, E. Dobratz, P. D. Markham, Z. Hel, J. Nacsa, M. Klein, J. Tartaglia, and G. Franchini. 2002. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pantaleo, G., and R. A. Koup. 2004. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 10:806-810. [DOI] [PubMed] [Google Scholar]
- 37.Peters, C., X. Peng, D. Douven, Z. K. Pan, and Y. Paterson. 2003. The induction of HIV Gag-specific CD8+ T cells in the spleen and gut-associated lymphoid tissue by parenteral or mucosal immunization with recombinant Listeria monocytogenes HIV Gag. J. Immunol. 170:5176-5187. [DOI] [PubMed] [Google Scholar]
- 38.Rock, M. T., and J. E. Crowe, Jr. 2003. Identification of a novel human leucocyte antigen-A*01-restricted cytotoxic T-lymphocyte epitope in the respiratory syncytial virus fusion protein. Immunology 108:474-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 40.Sandefur, S., V. Varthakavi, and P. Spearman. 1998. The I domain is required for efficient plasma membrane binding of human immunodeficiency virus type 1 Pr55Gag. J. Virol. 72:2723-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 42.Sigal, L. J., S. Crotty, R. Andino, and K. L. Rock. 1999. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature 398:77-80. [DOI] [PubMed] [Google Scholar]
- 43.Spearman, P. 2003. HIV vaccine development: lessons from the past and promise for the future. Curr. HIV Res. 1:101-200. [DOI] [PubMed] [Google Scholar]
- 44.Spearman, P., J. J. Wang, N. Vander Heyden, and L. Ratner. 1994. Identification of human immunodeficiency virus type 1 Gag protein domains essential to membrane binding and particle assembly. J. Virol. 68:3232-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stan, A. C., S. Casares, T. D. Brumeanu, D. M. Klinman, and C. A. Bona. 2001. CpG motifs of DNA vaccines induce the expression of chemokines and MHC class II molecules on myocytes. Eur. J. Immunol. 31:301-310. [DOI] [PubMed] [Google Scholar]
- 46.Strack, B., A. Calistri, S. Craig, E. Popova, and H. G. Gottlinger. 2003. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114:689-699. [DOI] [PubMed] [Google Scholar]
- 47.Tartaglia, J., J. L. Excler, R. El Habib, K. Limbach, B. Meignier, S. Plotkin, and M. Klein. 1998. Canarypox virus-based vaccines: prime-boost strategies to induce cell-mediated and humoral immunity against HIV. AIDS Res. Hum. Retrovir. 14(Suppl. 3):S291-S298. [PubMed] [Google Scholar]
- 48.von Schwedler, U. K., M. Stuchell, B. Muller, D. M. Ward, H. Y. Chung, E. Morita, H. E. Wang, T. Davis, G. P. He, D. M. Cimbora, A. Scott, H. G. Krausslich, J. Kaplan, S. G. Morham, and W. I. Sundquist. 2003. The protein network of HIV budding. Cell 114:701-713. [DOI] [PubMed] [Google Scholar]
- 49.Wills, J. W., and R. C. Craven. 1991. Form, function, and use of retroviral Gag proteins. AIDS 5:639-654. [DOI] [PubMed] [Google Scholar]