Abstract
It is controversial whether the accessory human immunodeficiency virus type 1 (HIV-1) Nef protein inhibits or enhances apoptosis. To address this issue, we investigated the effect of Nef on programmed cell death with vectors or proviral HIV-1 constructs coexpressing Nef and green fluorescent protein from single bicistronic RNAs. This approach allows us to readily identify transfected or infected cells and to correlate cell death directly with Nef expression levels. We demonstrate that Nef does not significantly affect apoptosis in transfected or HIV-1-infected Jurkat T cells or primary human peripheral blood mononuclear cells. Unexpectedly, however, both nef+ and nef-defective HIV-1 infection blocked apoptosis in cells treated with UV light or etoposide but not cell death induced by CD95 antibody, TRAIL, Ly294002, or serum starvation. Our results show that HIV-1 infection inhibits DNA damage-induced but not death receptor-dependent cell death by a Nef-independent mechanism.
Human immunodeficiency virus type 1 (HIV-1) infection is characterized by the progressive loss of both CD4+ and CD8+ T lymphocytes, resulting in immunodeficiency associated with AIDS (35). Apoptosis seems to be a major cause of T-lymphocyte depletion. In vitro studies have shown that HIV-1 can directly induce apoptosis of infected primary CD4+ T cells (11). In HIV-1-infected individuals, both infected and uninfected cells undergo increased cell death (19, 32). However, most apoptotic cells detected in lymphatic tissues in vivo are uninfected bystander cells (10). Apoptosis of uninfected cells seems to be mainly caused by activation-induced cell death or by soluble HIV-1 gp120, Tat, Nef, and Vpu proteins (reviewed in references 2 and 16).
It has become clear, however, that HIV-1 not only enhances cell death but might also have evolved mechanisms that inhibit apoptosis, thereby prolonging the time of virion release by infected cells. Five viral proteins, Tat, Nef, Vpr, Vpu, and Env, were reported to inhibit programmed cell death (16). Possible antiapoptotic effects of Nef in particular have received a lot of recent interest. The nef gene of HIV-1 and simian immunodeficiency virus (SIV) is critical for the pathogenesis of AIDS (9, 26, 28). It has been proposed that Nef induces Fas ligand (CD95L) expression, permitting immune evasion by causing cytotoxic T-cell (CTL) apoptosis (49, 50). More recently, it has been suggested that endogenous Nef directly represses proapoptotic signaling in infected cells through different interactions with cellular kinases.
One study proposed that Nef might protect against CD95 and tumor necrosis factor alpha receptor-mediated death signaling by inhibiting apoptosis signal-regulating kinase 1 (ASK1) (13). A second report described that Nef-mediated activation of the phosphatidylinositol-3-kinase, and the p21-activated kinase results in Bad inactivation through phosphorylation (48). Consequently, the availability of the antiapoptotic Bcl-2 protein would be increased and enhance cell survival and hence virus production. Furthermore, it has been reported that Nef interacts directly with tumor suppressor protein p53 and protects cells against p53-mediated apoptosis (18).
However, controversy remains as to whether Nef inhibits or enhances apoptosis. Zauli et al. (51) described that Nef sensitizes CD4+ T cells to programmed death by upregulating surface expression of CD95 and CD95L. Contradictory to the results of Wolf et al. (48), others have reported that Nef enhances apoptosis by reducing the expression of the antiapoptotic proteins Bcl-2 and Bcl-XL (39). It has also been suggested that wild-type HIV-1 infection of primary CD4+ T lymphocytes causes apoptosis by inducing p53 phosphorylation and activation (14). Notably, a variety of activities other than effects on apoptosis might explain why Nef is critical for viral pathogenesis (reviewed in reference 3). Nef impairs recognition and attack by CTLs as well as stimulation of CD4+ helper T cells by down-regulating both the class I major histocompatibility complex (MHC-I) and mature MHC-II molecules and by up-regulating the invariant chain associated with immature MHC-II complexes (8, 44, 46). Furthermore, Nef alters T-cell receptor signaling by down-modulating cell surface expression of CD4 and CD28 (12, 47). Finally, Nef directly increases viral spread by enhancing virion infectivity and stimulating viral replication. Accumulating evidence suggests that these Nef functions are relevant for HIV-1 and SIVmac replication in infected human and simian hosts, respectively (7, 21, 33, 36, 43).
In this work, we further evaluated the controversial role of Nef in programmed cell death. Our data show that Nef has no significant effect on apoptosis in transfected or HIV-1-infected T cells. We also demonstrate, however, that HIV-1 infection blocks programmed cell death induced by UV light or etoposide, which cause DNA damage and usually p53-dependent but not death-receptor-induced apoptosis.
MATERIALS AND METHODS
Plasmids and proviral constructs.
Bicistronic cytomegalovirus-based pCG expression vector coexpressing the green fluorescent protein (GFP) and the NL4-3, NA7, or SIVmac239 nef allele and the NL4-3 proviral constructs carrying either functional (nef+) or defective (nef-defective) nef reading frames followed by an internal ribosome entry site (IRES) and the GFP gene have been previously described (17, 42). To generate proviral IRES-GFP vectors containing defective vpu or vpr genes, the intergenic region of NL4-3 mutants containing deletions in these genes (41) was cloned into the original proviral reporter constructs using the unique PflMI and StuI restriction sites.
Cell lines and transfection.
Jurkat cells were maintained in RPMI 1640 medium and 293T cells were grown in Dulbecco's modified Eagle's medium, both supplemented with 10% fetal calf serum and antibiotics. Peripheral blood mononuclear cells (PBMC) were isolated using lymphocyte separation medium (Organon Teknika Corporation) stimulated for 3 days with 3 μg of phytohemagglutinin per ml and cultured in RPMI 1640 medium with 15% fetal calf serum and 1 ng/ml interleukin-2. Transfection of Jurkat T cells was performed using the DMRIE-C reagent (Gibco-BRL) following the manufacturer′s instructions. At 18 h posttransfection, the cells were pelleted, washed with phosphate-buffered saline, and resuspended in fresh medium to remove phytohemagglutinin. Subsequently, the cells were maintained for an additional 18 h at 37°C and 5% CO2 before apoptosis was induced.
Induction of apoptosis.
For the induction of apoptosis, Jurkat T cells or PBMC were treated with six different stimuli: 10 to 20 μl/ml CD95 antibody (APO-I hybridoma supernatant, kindly provided by K.-M. Debatin), 50 ng/ml recombinant human TRAIL (R&D Systems), 50 μM Ly294002 (Promega), 50 μM etoposide (Sigma-Aldrich), 20 to 30 s of UV irradiation (5 to 7.5 J/m2), and serum starvation by maintaining cells in optimized minimal essential medium. The optimal time points for measurement and amounts of inductor used were determined by inducing apoptosis with different concentrations of inductor and measuring apoptosis in Jurkat cells over time by Nicoletti staining (34). The best time points for measurement of CD95 antibody, Trail, and Ly294002-induced apoptosis were 6 to 8 h postinduction. UV light-, etoposide-, and OMEM-induced apoptosis was measured 16 to 24 h postinduction.
Cell death analysis.
Apoptosis was detected using the annexin V apoptosis detection kit (BD Bioscience) as recommended by the manufacturer. Briefly, 5 × 105 cells were washed with phosphate-buffered saline and subsequently resuspended in 100 μl of annexin V binding buffer containing 5 μl of annexin V-phycoerythrin and 5 μl of 7-amino-actinomycin D (7-AAD). After 15 min of incubation in the dark, annexin V binding buffer was added to a total volume of 300 μl. Three-color flow cytometric analysis was performed using a FACSCalibur (Becton Dickinson). For measurement of caspase-3 and -7 activity, the Caspase-Glo 3/7 assay (Promega) was used. Briefly, 100 μl of cell suspension (1 × 104 to 2 × 104 cells) were mixed with 100 μl of Caspase-Glo 3/7 reagent and incubated for 1 h at room temperature. Luminescence was measured in white-walled 96-well dishes for 0.1 ms with the Berthold Orion luminometer.
Apoptosis in HIV-1-infected cells.
To generate pseudotyped viral particles, 293T cells were cotransfected with NL4-3 proviral constructs carrying open or defective vpr, vpu, and nef reading frames followed by an IRES and the eGFP gene, and a plasmid (pHIT-G) expressing the vesicular stomatitis virus G protein (VSV G) as described (42). The p24 antigen concentrations were quantified using an HIV-1 enzyme-linked immunosorbent assay provided by the National Institutes of Health AIDS Research and Reference Program; 106 Jurkat cells or PBMC were transduced with virus stocks containing 50 ng of p24 antigen and assayed for apoptosis 3 days later as described above. To inactivate HIV-1, the cells were fixed in 300 μl of annexin V binding buffer containing 3% paraformaldehyde prior to fluorescence-activated cell sorting analysis.
Fluorescence-activated cell sorting analysis of HIV-1-infected cells.
PBMC were transduced with VSV G-pseudotyped nef+ or nef-defective HIV-1 NL4-3 IRES-eGFP particles, and cell surface expression of MHC-I and CD4 was analyzed by fluorescence-activated cell sorting as described previously (42).
RESULTS
Nef does not inhibit apoptosis in Jurkat T cells.
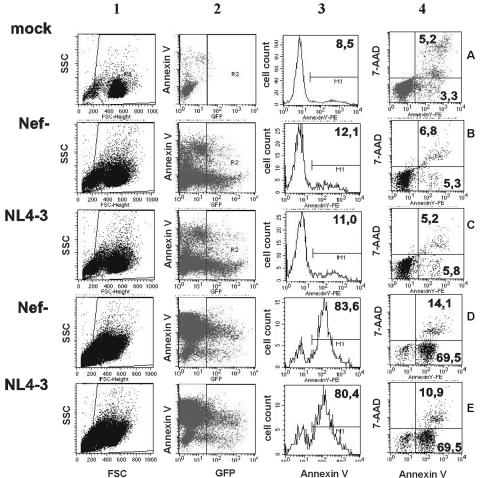
To study the effect of Nef on apoptosis, we transfected Jurkat T cells with plasmids coexpressing Nef and the GFP reporter protein from a single bicistronic RNA via an IRES element (17). This system has been used throughout to study various Nef functions because it allows us to quantitate the effect of Nef, e.g., on the surface expression of various receptors in a single transfection (17, 42). As depicted in Fig. 1, first gating is based on forward and side scatter for debris exclusion (lane 1). Subsequently, transfected cells were identified by their green fluorescence (Fig. 1, lane 2) and evaluated for annexin V binding (lane 3) and 7-AAD staining to distinguish between early (annexin V-positive/7-AAD-negative) and late (annexin V-positive/7-AAD-positive) stages of apoptosis (Fig. 1, lane 4). Predictably, treatment of Jurkat T cells expressing GFP alone (Fig. 1B) or together with Nef (Fig. 1C) with UV light resulted in a dramatic increase in cell death (Fig. 1D and E). Nef and GFP are expressed at correlating levels (42). Therefore, this experimental system allows us not only to readily identify the transfected cell population but also to evaluate apoptosis specifically in cells expressing different levels of Nef (17, 42).
FIG. 1.
Quantitation of apoptosis using bicistronic vectors coexpressing Nef and GFP. Flow cytometric analysis of uninduced (A to C) or 20-s UV light-treated (D and E) Jurkat cells transfected with constructs expressing GFP alone (B and D) or together with the NL4-3 Nef (C and E). Live cells were selected by forward and side scattering (panel 1). GFP expression allowed us to discriminate between transfected and untransfected cells (panel 2). GFP-positive cells and mock-transfected cells were evaluated for annexin V binding (panel 3), and triple staining for 7-AAD allowed us to distinguish between early and late apoptotic cells (panel 4). Numbers are the percentages of annexin V- and 7-AAD-positive cells. Similar results were obtained in three independent experiments.
We used three different nef alleles to study their potential effects on apoptosis: (i) the HIV-1 NL4-3 nef, which has been well characterized in previous studies, including those proposing antiapoptotic effects of Nef (13, 18); (ii) NA7 nef, originally derived from an AIDS patient and known to be highly active in established in vitro assays for Nef function (17, 20, 42, 47); and (iii) the SIVmac239 nef, because this allele has been initially used to demonstrate that Nef is critical for efficient viral replication and AIDS pathogenesis in infected rhesus macaques (26).
Jurkat cells expressing GFP alone or in conjunction with the HIV-1 NL4-3 and NA7 or SIVmac239 Nefs were treated with a variety of known inducers of programmed cell death. As expected, CD95 monoclonal antibody, TRAIL, the phosphatidylinositol-3-kinase inhibitor Ly294002, serum starvation, and UV light induced apoptosis in a dose-dependent manner (data not shown) and concentrations within the linear range were used in subsequent experiments. However, in contrast to published data (13), we found that treatment of Jurkat T cells with up to 200 ng/ml tumor necrosis factor alpha (Pan Biotech) did not result in significant induction of apoptosis. Therefore, the effect of Nef on tumor necrosis factor alpha-induced apoptosis in Jurkat T cells could not be investigated further.
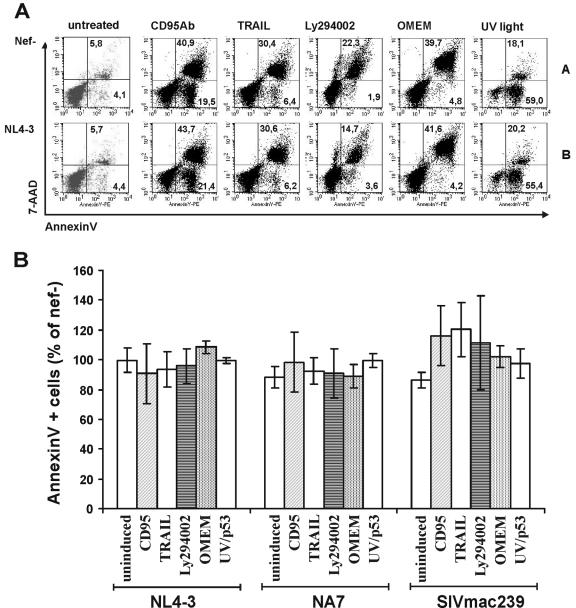
As shown in Fig. 2A, treatment with CD95 monoclonal antibody (60.4% annexin V-positive cells), TRAIL (36.8%), Ly294002 (24.2%), and UV light (77.1%) or serum starvation (44.5%) enhanced the frequency of apoptotic Jurkat T cells compared to untreated cultures (9.9%). Notably, NL4-3 Nef expression had no marked effect on cell death (Fig. 2A, compare panel A with panel B). On average, the NL4-3 and NA7 Nefs marginally reduced and the 239 wild-type Nef slightly enhanced CD95-, TRAIL-, and UV light-induced apoptosis (Fig. 2B). However, these subtle differences were not significant. Similarly, no significant effects of these Nef proteins on programmed cell death were observed when we analyzed the fluorescence-activated cell sorting data distinguishing between early (annexin V-positive/7-AAD-negative) and late (annexin V-positive/7-AAD-positive) apoptotic cells (data not shown). We also evaluated whether Nef might stimulate Bad phosphorylation to induce antiapoptotic signals (48). Intracellular staining showed, however, that the levels of total and phosphorylated Bad did not differ significantly in Nef-expressing Jurkat cells compared to cells expressing only GFP or between cells expressing different levels of Nef (data not shown).
FIG. 2.
Expression of HIV-1 or SIVmac Nef does not significantly affect apoptosis. (A) One million Jurkat T cells were transfected with vectors expressing GFP alone (A) or in conjunction with the NL4-3 (B) nef allele, and apoptosis was induced by 20 μl of CD95 antibody, 50 ng/ml TRAIL, 50 μM Ly294002, serum starvation (OMEM), or 20 s of UV light as described in Materials and Methods. Transfected GFP-positive cells were stained for annexin V and 7-AAD to identify early and late apoptotic cells as shown in Fig. 1. (B) Percentages of apoptotic annexin V-positive T Jurkat cells expressing the indicated nef alleles relative to transfected cells expressing only GFP after treatment with the indicated inducers. Shown are average values ± standard deviation obtained from five independent experiments.
HIV-1 infection inhibits UV light-mediated apoptosis independently of Nef function.
Our data do not support a relevant role for HIV-1 or SIVmac Nef in modulating cell death in transfected Jurkat T cells. However, Nef might influence apoptosis in HIV-1-infected cells only in the presence of other viral proteins. To evaluate this possibility, we generated Env-expressing, replication-competent, and env-defective NL4-3 proviral constructs carrying either an intact or a disrupted nef gene followed by an IRES element and the eGFP reporter gene. Viral particles were pseudotyped with VSV-G to increase the proportion of infected cells. Control experiments revealed that these eGFP reporter viruses express high levels of functional Nef and efficiently down-modulate CD4 and MHC-I from the surface of infected Jurkat T cells (data not shown).
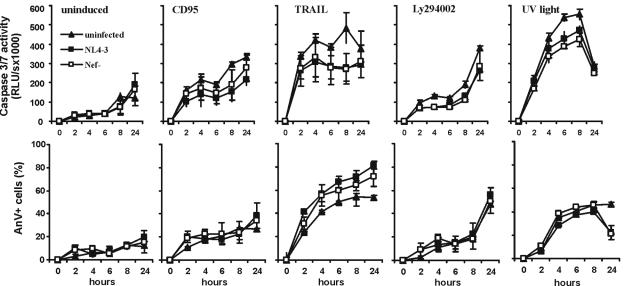
First, we studied the time course of activation of apoptosis in Jurkat cells infected with nef+ and nef-defective HIV-1 NL4-3 eGFP reporter viruses. Increases in caspase activities in the cellular extracts and in the percentages of cells undergoing apoptosis were measured in uninduced cells and after treatment with CD95, TRAIL, Ly294002, and UV light. Nef expression did not significantly affect the patterns of caspase activation in infected Jurkat cells (Fig. 3, upper panel). Caspase activities were generally lower in HIV-1-infected than in uninfected cell cultures. This is most likely due to reduced cell numbers, because virus infection inhibited cell growth. In comparison, the increases in the number of annexin V-positive apoptotic cells did not differ between uninfected cells and cells transduced with nef+ or nef-defective HIV-1 variants after induction with CD95 monoclonal antibody or Ly294002 (Fig. 3, lower panel). After treatment with TRAIL, however, apoptosis was moderately reduced in uninfected cells versus HIV-1-infected cells. This result is consistent with previous reports showing that TRAIL is more efficient in inducing cell death of virus-infected cells (22, 31). In contrast, HIV-1 infection protected Jurkat cells against UV light-induced cell death. Comparable numbers of apoptotic cells were detected early after treatment, but at 24 h most of the HIV-1-infected cells recovered, whereas the uninfected cells died (Fig. 3).
FIG. 3.
Expression of Nef in HIV-1-infected Jurkat cells does not inhibit CD95-, TRAIL-, Ly294002-, or UV light-induced apoptosis. One million Jurkat cells were transduced with nef+ or nef-defective HIV-1 NL4-3 particles pseudotyped with the VSV G Env protein. At 3 days posttransduction, the cells were treated with the indicated inducers, and aliquots of the cultures were removed at the indicated time points. Cells were lysed and assayed for caspase-3 and -7 activity (upper panel). GFP expression allowed us to discriminate between uninfected cells and those transduced with the nef+ and nef-defective HIV-1 NL4-3 proviral constructs. Apoptosis of GFP-positive cells was assessed by annexin V (AnV, lower panel) staining as described for the bicistronic Nef-GFP expression constructs. All transductions were done in triplicate. Shown are average numbers ± standard deviation. Similar results were obtained in an independent experiment.
HIV-1 infection inhibits DNA damage-induced apoptosis of PBMC.
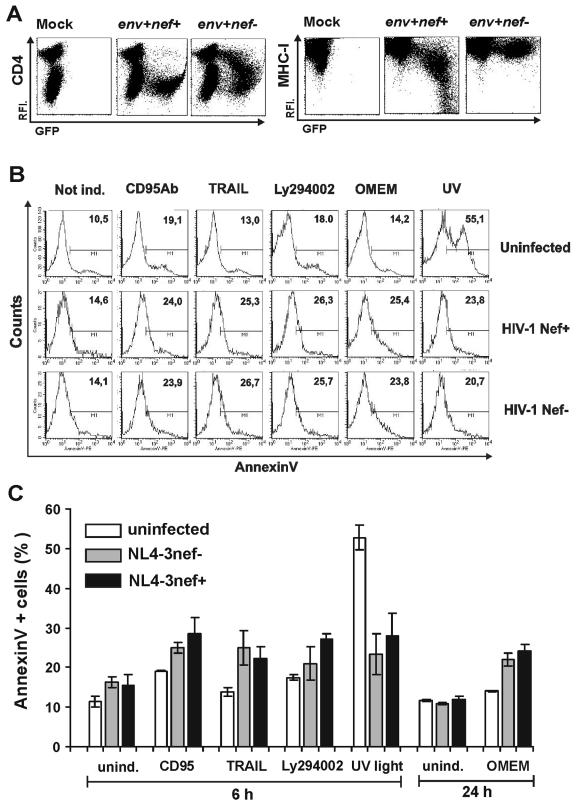
The data described above suggest that HIV-1 protects cells against apoptosis induced by UV radiation. However, results obtained with transformed Jurkat T cells might not have direct relevance for HIV-1 infection of primary cells. Therefore, we next evaluated whether HIV-1 infection also affects cell death in human PBMC. First, we tested whether NL4-3-IRES-eGFP reporter viruses are active in other established assays for Nef function in infected PBMC. Transduction with the nef+ but not with the nef-defective virus resulted in a dramatic reduction of surface CD4 even at low Nef expression levels (Fig. 4A, left). Similar to the results obtained in Jurkat cells, MHC-I was also efficiently down-regulated, although higher levels of Nef expression were required (Fig. 4A, right).
FIG. 4.
HIV-1 infection blocks UV light-induced apoptosis by a Nef-independent mechanism. (A) CD4 (left panel) and MHC-I (right panel) surface expression of mock-transduced PBMC or of cells transduced with eGFP-expressing VSV-G-pseudotyped HIV-1 NL4-3 particles containing intact or disrupted nef genes. (B) Uninfected and HIV-1-infected PBMC were not treated or apoptosis was induced by CD95 antibody, TRAIL, Ly294002, serum starvation, or UV light. The numbers give the percentages of annexin V (AnV)-positive cells in the total uninfected cell population (upper panel) or in the HIV-1-infected eGFP-positive population in the presence and absence of Nef. (C) Percentages of annexin V-positive HIV-1-infected PBMC after the indicated treatment. Shown are average values ± standard deviation obtained from three independent transductions. Similar results were obtained in an independent experiment.
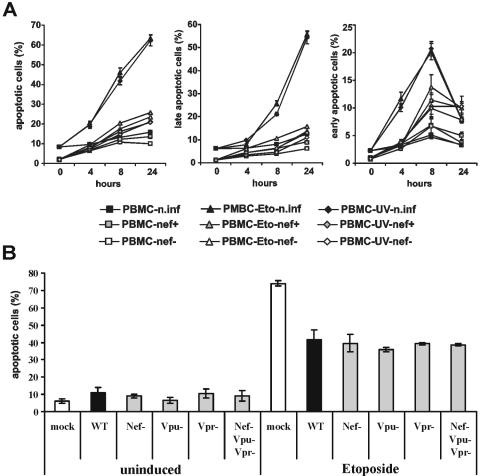
Quantitation of cell death demonstrated that HIV-1-infected PBMC showed slightly to moderately enhanced levels of apoptosis after treatment with CD95 monoclonal antibody, TRAIL, or Ly294002 or after serum starvation (Fig. 4B). In agreement with the results obtained using Jurkat T cells, expression of Nef did not reduce the frequency of apoptotic PBMC. Concordant with published data (22, 31), TRAIL efficiently induced apoptosis only of HIV-1-infected but not of uninfected cells (Fig. 4C). Finally, the studies in PBMC confirmed that HIV-1 infection efficiently inhibits apoptosis induced by UV light (Fig. 4B and C). Treatment of uninfected PBMC cultures resulted in an increase of annexin V-positive cells of about 10% to 55%, whereas only about 20% to 24% of HIV-1-infected cells became apoptotic.
Our data suggested that HIV-1 specifically blocks UV light-induced apoptosis, which involves DNA damage and is usually dependent on p53 (25, 30). However, UV irradiation might also activate pathways other than the DNA damage-related pathway to induce apoptosis (40). Therefore, we performed time course studies in which we induced apoptosis of HIV-1-infected PBMC not only by UV light but also by etoposide (an inhibitor of topoisomerase II), which also causes DNA damage and activation of p53 (24). Our results demonstrate that HIV-1 infection blocks UV light- and etoposide-induced cell death with equal efficiency (Fig. 5A). The protective effects were independent of Vpr, Vpu, and Nef function, since cells transduced with wild-type HIV-1 NL4-3 or with mutant forms containing individual or combined deletions in these accessory genes showed similar levels of etoposide-induced apoptosis (Fig. 5B). Notably, PBMC were consistently protected more efficiently than Jurkat T cells against cell death induced by UV radiation.
FIG. 5.
HIV-1 infection inhibits cell death induced by etoposide independently of Vpu, Vpr, or Nef function. (A) One million PBMC were transduced with VSV G-pseudotyped HIV-1 particles and treated with etoposide or UV light 3 days later. Apoptosis of uninfected and eGFP-positive HIV-1-infected cells was assessed by triple annexin V and 7-AAD staining at the indicated time points as described in the legend to Fig. 1. Shown are average numbers ± standard deviation obtained from triple transductions. Left, total percentage of annexin-V-positive cells; middle, percentage of annexin V-positive/7-AAD-positive cells; and right, percentage of annexin V-positive/7-AAD-negative cells. (B) Apoptosis of uninfected PBMC and cells infected with NL4-3 wild-type virus (WT) or HIV-1 variants with accessory gene deletions. Cells were either not treated (left panel) or treated with 50 μM etoposide for 24 h. Shown are average percentages of annexin-V-positive cells ± standard deviation obtained from transductions of PBMC derived from two different donors.
Finally, to exclude the possibility that GFP expression alone might have protective effects, we transfected or transduced Jurkat cells with plasmids or full-length proviral constructs expressing GFP. Treatment of GFP-positive transfected cells with etoposide enhanced the frequency of apoptotic cells by 35.1 ± 1.7% (n = 3), whereas the same dose increased cell death of HIV-1-infected cells by only about 15.9 ± 1.7% (n = 3; P = 0.001; data not shown). Thus, etoposide-induced cell death was also significantly inhibited by HIV-1 infection.
DISCUSSION
In this study, we show that expression of Nef in transfected or HIV-1-infected Jurkat T cells or PBMC does not significantly affect the frequency of apoptotic cells. We used the NL4-3, NA7, and SIVmac239 nef alleles, which are all active in established in vitro assays for Nef function, such as modulation of cell surface expression of various human receptors and enhancement of virus infectivity and replication. Notably, the SIVmac239 Nef was used to demonstrate that Nef is a major determinant of viral pathogenicity in the SIV-macaque model (26) and the NL4-3 Nef mediates efficient HIV-1 replication and CD4+ T-cell depletion in ex vivo-infected human lymphoid tissue (15). Thus, these HIV-1 and SIV Nefs should be able to perform all functions critical for efficient viral replication in the infected host and for AIDS pathogenesis.
Our approaches allow us to readily quantitate the effects of Nef on CD4, MHC-I, MHC-II, and invariant chain (Ii) surface expression and on HIV and SIV replication and infectivity. Nevertheless, we consistently detected comparable numbers of apoptotic cells in the presence and absence of Nef after induction of cell death by a variety of stimuli. Accordingly, endogenous Nef does not exert significant pro- or antiapoptotic effects in Jurkat T cells or PBMC. Our findings are in agreement with the accumulating evidence suggesting that other Nef functions are critical for efficient viral replication and disease progression in vivo (7, 21, 33, 36, 43). While our data do not confirm a relevant role of Nef in apoptosis, we provide strong evidence that HIV-1 infection efficiently blocks DNA damage-induced, i.e., most likely p53-mediated but not death receptor-dependent cell death. The underlying mechanisms and the relevance of this antiapoptotic effect in HIV-1-infected individuals remain to be elucidated, but the prolonged life span of infected cells could clearly play an important role in viral replication and hence in AIDS pathogenesis.
Both enhancing and inhibitory effects of Nef on programmed cell death have been documented (13, 18, 39, 48, 51). Most of the discrepancies between our results and previous findings might be explained by different constructs, methods, and cell lines used. We used bicistronic vectors and proviral constructs to quantitate apoptosis specifically in the GFP-positive transfected and HIV-1-infected cell populations and to distinguish between cells expressing different levels of Nef. Thus, our approach allows us to correlate HIV-1 infection and Nef expression levels quantitatively with apoptotic features of the cells. Furthermore, the HIV-1 IRES-eGFP constructs express unmodified forms of all viral proteins. Therefore, we believe that our data reflect “normal” HIV-1 infection more closely than those of other studies.
It has been proposed that activation of p21-activated kinase by Nef stimulates Bad phosphorylation to induce antiapoptotic signals (48). Most of their experiments were performed in Jurkat cells with CD8-Nef fusion proteins that might affect cell signaling and death differently from normal Nef. Notably, it has been proposed that expression of the CD8-Nef chimera in Jurkat T cells both inhibits and enhances their apoptotic death (5). Untagged Nef was used to show that Nef might protect HIV-1-infected cells against apoptosis induced by serum starvation (48). The apparent discrepancies with our results may be related to matters of interpretation. Nef is known to cause T-cell activation (45) and the moderately enhanced levels of living cells in starvation medium might be due to Nef-induced cell proliferation rather than antiapoptotic effects.
Wolf et al. proposed that the antiapoptotic activity of Nef requires the interaction with p21-activated kinase and might be important for efficient viral replication in vivo. Others have reported, however, that the proline motif in Nef which is critical for p21-activated kinase binding is required for the induction of programmed cell death (51). Notably, the selective pressure for the interaction of Nef with p21-activated kinase in SIVmac-infected macaques is weak, and a significant number of infected animals progress to AIDS in the absence of restorative changes (6, 27, 29). Consistent with a limited relevance for viral pathogenesis, many HIV-1 Nefs interact with p21-activated kinase only in the presence of an active p21 GTPase but do not activate p21-activated kinase themselves (38). It will be of some interest to further clarify the relevance of p21-activated kinase association or activation for Nef function. However, other Nef functions, such as down-modulation of CD4 and MHC-I, might be more relevant for efficient viral replication in the infected host (21, 33, 43).
It is controversial whether Nef increases or inhibits CD95-mediated T-cell death (13, 51). Geleziunas et al. (13) reported that HIV-1 and SIV Nef inhibit Fas- and tumor necrosis factor alpha-mediated apoptosis by inhibiting apoptosis signal-regulating kinase 1. We found, however, that Nef did not reduce death receptor-mediated apoptosis after treatment with CD95 antibody or TRAIL. Also different from their data, in our experiments, treatment of Jurkat T cells or PBMC with up to 200 ng/ml tumor necrosis factor alpha did not result in significant induction of apoptosis. However, some of the effects described in the study of Geleziunas and coworkers are difficult to evaluate. For example, the percentages of apoptotic cells were usually only given as a percentage of mock-transfected cells. Since no absolute numbers were provided, it remains unclear how efficiently apoptosis was induced compared to untreated control cells. It is also difficult to comprehend why only cells staining positive for annexin V but negative for 7-AAD were analyzed in the previous study. Since many apoptotic cells might be already at a late stage, these measurements might greatly underestimate the total frequency of cell death. Early studies described that Nef is cytotoxic (5). Thus, the results of Geleziunas et al. could also be consistent with a more rapid killing in the presence of Nef because many cells would stain 7-AAD positive and therefore be excluded from further analysis.
While our results demonstrate that endogenous Nef has no significant effect on apoptosis in transfected or HIV-1-infected Jurkat cells or stimulated PBMC, they do not imply that Nef does not affect programmed cell death in the infected host. For example, efficient down-modulation of MHC-I (Fig. 4) should clearly reduce CTL-mediated apoptosis of HIV-1-infected cells (8, 44). Similarly, removal of cell surface CD4 by Nef (Fig. 4A) should protect infected T cells against HIV-1 Env-induced apoptosis. Furthermore, the effect of Nef on CD4 and/or T-cell receptor signaling might affect the death of virus-infected cells in the human host (1, 4, 23). To further elucidate the effects of Nef on cell survival or death, it seems important to perform studies under conditions that more closely mimic the in vivo situation, e.g., in the SIV-macaque model or in ex vivo-infected human lymphoid tissue.
Concordant with previous studies, we found that HIV-1-infection enhances TRAIL-mediated apoptosis (22, 31). More remarkably, however, our results demonstrate that both nef+ and nef-defective HIV-1 infection specifically blocks apoptosis induced by UV radiation or etoposide, both of which cause DNA damage and are usually p53 dependent. Recently, it has been shown that HIV-1 Env-induced apoptosis is largely mediated by p53 (37). Lymphatic tissues are the major sites of HIV-1 replication in vivo. In such an environment, where the density of virus-infected cells is high, the ability of HIV-1 to inhibit apoptosis induced by interactions between Env-expressing cells or by soluble Env might be critical for efficient viral replication and spread. Another possible reason why HIV-1 has evolved mechanisms to block p53-induced apoptosis is that proviral integration requires double-stranded DNA breaks and might be sufficient to induce cell death by the p53-initiated mitochondrion-mediated pathway (14).
Our data are evidence that HIV-1 infection protects cells against p53-mediated apoptosis but do not support previous findings suggesting that Nef might play a role in this process (18). Greenway et al. showed that the N-terminal part of Nef binds to p53 in coimmunoprecipitation and enzyme-linked immunosorbent assay-based assays, decreases its half-life and steady-state expression levels, and inhibits UV light-mediated cell death in Molt-4 cells. In contrast, others have reported that infection with Nef-expressing HIV-1 increases p53 activity (14). We did not observe significant effects of Nef alone on UV light-induced apoptosis in Jurkat T cells or PBMC. It remains to be clarified whether the effects of Nef might be cell type dependent. Using p53-eCFP and Nef-eYFP fusions, we did not find evidence for an interaction between these proteins in living cells (data not shown). Our results do not exclude the possibility that Nef and p53 might interact in HIV-1-infected cells at levels below the detection limit of our assay and that binding can be shown in other assays. However, we believe that the different cellular localization and the lack of an effect of Nef on UV light-induced apoptosis clearly argue against a relevant role of Nef in blocking p53 function.
In summary, our results have two important implications. First, we demonstrate that endogenous Nef does not significantly protect HIV-1-infected cells against programmed death, suggesting that other aspects of Nef function are critical for efficient viral replication in vivo. Second, our results indicate that HIV-1 efficiently blocks DNA damage-induced apoptosis but not death receptor-dependent cell death. Our preliminary results indicate that Vpu, Vpr, and Nef do not contribute to this effect. Further studies will be necessary to clarify the precise mechanism by which HIV-1 protects cells against p53-mediated death and to evaluate the relevance of this effect for virus spread and AIDS pathogenesis in the infected host.
Acknowledgments
We thank Thomas Mertens for constant support, Nicola Bailer and Sophie Aftring for excellent technical assistance, Ingrid Bennett for critical reading of the manuscript, Klaus-Michael Debatin for the APO-I hybridoma supernatant, and Simone Fulda and Lisa Wiesmüller for helpful discussions.
This work was supported by grants from the Deutsche Forschungsgemeinschaft, the Wilhelm-Sander-Stiftung, and the Landesforschungsschwerpunkt Apoptose Baden-Württemberg.
REFERENCES
- 1.Algeciras, A., D. H. Dockrell, D. H., Lynch, and C. V. Paya. 1998. CD4 regulates susceptibility to Fas ligand- and tumor necrosis factor-mediated apoptosis. J. Exp. Med. 187:711-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alimonti, J. B., T. B. Ball, and K. R. Fowke. 2003. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J. Gen. Virol. 84:1649-1661. [DOI] [PubMed] [Google Scholar]
- 3.Arora, V. K., B. L. Fredericksen, and J. V. Garcia. 2002. Nef: agent of cell subversion. Microbes Infect. 4:189-199. [DOI] [PubMed] [Google Scholar]
- 4.Banda, N. K., J. Bernier, D. K. Kurahara, R. Kurrle, N. Haigwood, R. P. Sekaly, and T. H. Finkel. 1992. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J. Exp. Med. 176:1099-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baur, A. S., E. T. Sawai, P. Dazin, W. J. Fantl, C. Cheng-Mayer, and B. M. Peterlin. 1994. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity 1:373-384. [DOI] [PubMed] [Google Scholar]
- 6.Carl, S., A. J. Iafrate, S. M. Lang, N. Stolte, K. Matz-Rensing, D. Fuchs, C. Stahl-Hennig, J. Skowronski, and F. Kirchhoff. 2000. Simian immunodeficiency virus containing mutations in N-terminal tyrosine residues and in the PxxP motif in Nef replicates efficiently in rhesus macaques. J. Virol. 74:4155-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carl, S., T. C. Greenough, M. Krumbiegel, M. Greenberg, J. Skowronski, J. L. Sullivan, and F. Kirchhoff. 2001. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J. Virol. 75:3657-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 9.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. Mc Phee, A. L. Greenway, A. Ellett, and C. Chatfield. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 10.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129-134. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi, R. T., B. K. Chen, S. E. Straus, J. K. Dale, M. J. Lenardo, and D. Baltimore. 1998. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J. Exp. Med. 187:1113-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent down-regulation of cell-surface CD4 by nef. Nature 350:508-511. [DOI] [PubMed] [Google Scholar]
- 13.Geleziunas, R., W. Xu, K. Takeda, H. Ichijo, and W. C. Greene. 2001. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410:834-838. [DOI] [PubMed] [Google Scholar]
- 14.Genini, D., D. Sheeter, S. Rought, J. J. Zaunders, S. A. Susin, G. Kroemer, D. D. Richman, D. A. Carson, J. Corbeil, and L. M. Leoni. 2001. HIV induces lymphocyte apoptosis by a p53-initiated, mitochondrial-mediated mechanism. FASEB J. 15:5-6. [DOI] [PubMed] [Google Scholar]
- 15.Glushakova, S., J. C. Grivel, K. Suryanarayana, P. Meylan, J. D. Lifson, R. Desrosiers, and L. Margolis. 1999. Nef enhances human immunodeficiency virus replication and responsiveness to interleukin-2 in human lymphoid tissue ex vivo. J. Virol. 73:3968-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gougeon, M. L. 2003. Apoptosis as an HIV strategy to escape immune attack. Nat. Rev. Immunol. 3:392-404. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg, M. E., A. J. Iafrate, and J. Skowronski. 1998. The SH3 domain binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17:2777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenway, A. L., D. A. McPhee, K. Allen, R. Johnstone, G. Holloway, J. Mills, A. Azad, S. Sankovich, and P. Lambert. 2002. Human immunodeficiency virus type 1 Nef binds to tumor suppressor p53 and protects cells against p53-mediated apoptosis. J. Virol. 76:2692-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossman, Z., M. Meier-Schellersheim, A. E. Sousa, R. M. Victorino, and W. E. Paul. 2002. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat. Med. 8:319-323. [DOI] [PubMed] [Google Scholar]
- 20.Iafrate, A. J., S. Bronson, and J. Skowronski. 1997. Separable functions of Nef disrupt two aspects of T cell receptor machinery: CD4 expression and CD3 signaling. EMBO J. 16:673-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iafrate, A. J., S. Carl, S. Bronson, C. Stahl Hennig, T. Swigut, J. Skowronski, and F. Kirchhoff. 2000. Disrupting surfaces of Nef required for down regulation of CD4 and for enhancement of virion infectivity attenuates simian immunodeficiency virus replication in vivo. J. Virol. 74:9836-9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeremias, I., I. Herr, T. Boehler, and K. M. Debatin. 1998. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur. J. Immunol. 28:143-152. [DOI] [PubMed] [Google Scholar]
- 23.Kabelitz, D., T. Pohl, and K. Pechhold. 1995. T cell apoptosis triggered via the CD3/T cell receptor complex and alternative activation pathways. Curr. Top Microbiol. Immunol. 200:1-14. [DOI] [PubMed] [Google Scholar]
- 24.Karpinich, N. O., M. Tafani, R. J. Rothman, M. A. Russo, and J. L. Farber. 2002. The course of etoposide-induced apoptosis from damage to DNA and p53 activation to mitochondrial release of cytochrome c. J. Biol. Chem. 277:16547-16552. [DOI] [PubMed] [Google Scholar]
- 25.Kastan, M. B., O. Onyekwere, D. Sidransky, B. Vogelstein, and R. W. Craig. 1991. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 51:6304-6311. [PubMed] [Google Scholar]
- 26.Kestler, H. W., D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 27.Khan, I. H., E. T. Sawai, E. Antonio, C. J. Weber, C. P. Mandell, P. Montbriand, and P. A. Luciw. 1998. Role of the SH3-Ligand domain of simian immunodeficiency virus Nef in interaction with Nef-associated kinase and simian AIDS in rhesus macaques. J. Virol. 72:5820-5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Absence of intact nef sequences in a long-term, nonprogressing survivor of HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 29.Lang, S. M., A. J. Iafrate, C. Stahl-Hennig, E. M. Kuhn, T. Niβlein, M. Haupt, G. Hunsmann, J. Skowronski, and F. Kirchhoff. 1997. Association of simian immunodeficiency virus nef with cellular serine/threonine kinases is dispensable for the development of AIDS in rhesus macaques. Nat. Med. 3:860-865. [DOI] [PubMed] [Google Scholar]
- 30.Lu, X., and D. P. Lane. 1993. Differential induction of transcriptionally active p53 following UV or ionizing radiation: defects in chromosome instability syndromes? Cell 75:765-778. [DOI] [PubMed] [Google Scholar]
- 31.Lum, J. J., A. A. Pilon, J. Sanchez-Dardon, B. N. Phenix, J. E. Kim, J. Mihowich, K. Jamison, N. Hawley-Foss, D. H. Lynch, and A. D. Badley. 2001. Induction of cell death in human immunodeficiency virus-infected macrophages and resting memory CD4 T cells by TRAIL/Apo2l. J. Virol. 75:11128-11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCune, J. M. 2001. The dynamics of CD4+ T-cell depletion in HIV disease. Nature 410:974-979. [DOI] [PubMed] [Google Scholar]
- 33.Münch, J., N. Stolte, D. Fuchs, C. Stahl Hennig, and F. Kirchhoff. 2001. Efficient class I MHC down regulation by simian immunodeficiency virus Nef is associated with a strong selective advantage in infected rhesus macaques. J. Virol. 75:10532-10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicoletti, I., G. Migliorati, M. C. Pagliacci, F. Grignani, and C. Riccardi. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods. 139:271-279. [DOI] [PubMed] [Google Scholar]
- 35.Pantaleo, G., and A. S. Fauci. 1995. Apoptosis in HIV infection. Nat. Med. 1:118-120. [DOI] [PubMed] [Google Scholar]
- 36.Patel, P. G., M. T. Yu Kimata, J. E. Biggins, J. M. Wilson, and J. T. Kimata. 2002. Highly pathogenic simian immunodeficiency virus mne variants that emerge during the course of infection evolve enhanced infectivity and the ability to downregulate CD4 but not class I major histocompatibility complex antigens. J. Virol. 76:6425-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perfettini, J. L., T. Roumier, M. Castedo, N. Larochette, P. Boya, B. Raynal, V. Lazar, F. Ciccosanti, R. Nardacci, J. Penninger, M. Piacentini, and G. Kroemer. 2004. NF-κB and p53 are the dominant apoptosis-inducing transcription factors elicited by the HIV-1 envelope. J. Exp. Med. 199:629-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pulkkinen, K., G. H. Renkema, F. Kirchhoff, and K. Saksela. 2004. Nef associates with PAK2 in a p21-GTPase-dependent dynamic activation complex with lipid rafts. J. Virol. 78:12773-12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rasola, A., D. Gramaglia, C. Boccaccio, and P. M. Comoglio. 2001. Apoptosis enhancement by the HIV-1 Nef protein. J. Immunol. 166:81-88. [DOI] [PubMed] [Google Scholar]
- 40.Rosette, C., and M. Karin. 1996. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factors and cytokine receptors. Science 274:1194-1197. [DOI] [PubMed] [Google Scholar]
- 41.Rucker, E., J. Munch, J. C. Grivel, F. Kirchhoff, F., and L. Margolis. 2004. Vpr and Vpu are important for efficient HIV-1 replication and CD4+ T cell depletion in human lymphoid tissue ex vivo. J. Virol. 78:12689-12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schindler, M., S. Wuerfl, P. Benaroch, T. C. Greenough, R. Daniels, P. Easterbrook, M. Brenner, J. Münch, and F. Kirchhoff. 2003. Down-modulation of mature MHC class II and up-regulation of invariant chain cell surface expression are well conserved functions of human and simian immunodeficiency virus nef alleles. J. Virol. 77:10548-10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schindler, M., J. Münch, C. Stahl-Hennig, J. Skowronski, and F. Kirchhoff. 2004. Comprehensive Analysis of Nef Functions Selected in Simian Immunodeficiency Virus-Infected Macaques. J. Virol. 78:10588-10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 45.Simmons, A., V. Aluvihare, and A. McMichael. 2001. Nef triggers a transcriptional program in T cells imitating single-signal T cell activation and inducing HIV virulence mediators. Immunity 14:763-777. [DOI] [PubMed] [Google Scholar]
- 46.Stumptner-Cuvelette, P., S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA. 98:12144-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swigut, T., N. Shody, and J. Skowronski. 2001. Mechanism for down regulation of CD28 by Nef. EMBO J. 20:1593 1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf, D., V. Witte, B. Laffert, K. Blume, E. Stromer, S. Trapp, P. d'Aloja, A. Schurmann, and A. S. Baur. 2001. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat. Med. 7:1217-1224. [DOI] [PubMed] [Google Scholar]
- 49.Xu, X. N., G. R. Screaton, F. M. Gotch, T. Dong, R. Tan, N. Almond, B. Walker, R. Stebbings, K. Kent, S. Nagata, J. E. Stott, and A. J. McMichael. 1997. Evasion of cytotoxic T lymphocyte (CTL) responses by nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J. Exp. Med. 186:7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu, X. N., B. Laffert, G. R. Screaton, M. Kraft, D. Wolf, W. Kolanus, J. Mongkolsapay, A. J. McMichael, and A. S. Baur. 1999. Induction of Fas ligand expression by HIV involves the interaction of Nef with the T cell receptor zeta chain. J. Exp. Med. 189:1489-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zauli, G., D. Gibellini, P. Secchiero, H. Dutartre, D. Olive, S. Capitani, and Y. Collette. 1999. Human immunodeficiency virus type 1 Nef protein sensitizes CD4+ T lymphoid cells to apoptosis via functional upregulation of the CD95/CD95 ligand pathway. Blood 93:1000-1010. [PubMed] [Google Scholar]