Abstract
Background.
Fine particulate matter (PM2.5) and nitrogen dioxide (NO2) measures of ambient air pollution are associated with accelerated age-related cognitive impairment, and Alzheimer’s disease and related dementias (ADRD).
Objective.
We examined associations between air pollution, 4 cognitive factors, and the moderating role of apolipoprotein E (APOE) genotype in the understudied period of midlife.
Methods.
Participants were ~1100 men in the Vietnam Era Twin Study of Aging. Baseline cognitive assessments were from 2003 to 2007. Measures included past (1993–1999) and recent (3 years prior to baseline assessment) PM2.5 and NO2 exposure, in-person assessment of episodic memory, executive function, verbal fluency, and processing speed, and APOE genotype. Average baseline age was 56 years with a 12-year follow-up. Analyses adjusted for health and lifestyle covariates.
Results.
Performance in all cognitive domains declined from age 56 to 68. Higher PM2.5 exposures were associated with worse general verbal fluency. We found significant exposure-by-APOE genotype interactions for specific cognitive domains: PM2.5 with executive function and NO2 with episodic memory. Higher PM2.5 exposure was related to worse executive function in APOE-ε4 carriers, but not in non-carriers. There were no associations with processing speed.
Conclusion.
These results indicate negative effects of ambient air pollution exposure on fluency alongside intriguing differential modifications of cognitive performance by APOE genotype. APOE-ε4 carriers appeared more sensitive to environmental differences. The process by which air pollution and its interaction with genetic risk for ADRD affects risk for later life cognitive decline or progression to dementia may begin in midlife.
Keywords: air pollution, PM2.5, Nitrogen dioxide (NO2), cognition, aging, midlife, APOE genotype
INTRODUCTION
A 2020 Lancet commission report concluded that modification of 12 risk factors—including ambient air pollution—could reduce dementia incidence by as much as 40% [1]. Ambient air pollution (e.g., pollution coming from sources that use combustible fuels such as automobiles, power plants, and industries) acts as a notable public health hazard, especially in urban settings [2–5]. The World Health Organization estimated that 92% of the world population experiences excess exposure to fine particulate matter (PM) [5]; fine PM (particles with aerodynamic diameters less 2.5 microns) are considered particularly harmful to health. Although the Lancet commission’s estimated relative risk for dementia related to air pollution was relatively low (relative risk=1.1; 95% CI: [1.1–1.1]), the weighted population attributable fraction—the burden of air pollution accounting for other health risks—was higher (2.3%) due in part to the high prevalence of air pollution (75%). This places ambient air pollution above other Alzheimer’s disease and related dementia (ADRD) risk factors such as diabetes, physical inactivity, hypertension, alcohol consumption, and obesity. Of note, in regard to the present study, the Lancet report listed air pollution as a later life risk factor (age > 65) for ADRD, despite evidence that neurotoxicants such as those found in air pollution affect cognitive and brain health in childhood [6, 7].
Long-term exposure to air pollutants such as PM2.5 and nitrogen dioxide (NO2), increases risk for cardiovascular disease, stroke, inflammation, and neurotoxic reactions [6, 8–16]. PM2.5 exposure may accelerate brain-aging indicative of increased risk for AD [6, 9, 11, 17]. Air pollution levels are also associated with socioeconomic risk factors that predict poorer health and cognitive outcomes, increased stress, and higher risk for AD [3, 18]. There is not yet an identified threshold at the low end of the concentration range at which the effects disappear. Because these factors all have implications for cognitive functioning and AD, it is important to identify potentially modifiable risk factors as early in the life course as possible that may help to reduce risk of ADRD and thereby lighten its burden on the individual and society.
Small but consistent associations are found between ambient air pollution, ADRD, mild cognitive impairment (MCI), and accelerated cognitive decline; these associations are more likely found in studies of older women, adults over 65 years old, and in studies with higher levels of ambient air pollution [4, 19–29]. Studies focusing on specific cognitive abilities have not shown consistent patterns of association with air pollutants [3, 4, 8, 28], though some have found evidence for associations between some specific cognitive abilities and some air pollution components. In our review of the literature, we focused on adult cohorts with measures of specific cognitive abilities and fine particulate matter (PM2.5, PM10) and nitrogen oxides (NO2, NOx). Because of the relatively few studies examining air pollution and specific cognitive abilities, we included some older studies that used traffic density or distance from roadways as a proxy.
The most frequently researched cognitive ability is episodic memory (i.e., list learning tasks, story recall, visuospatial memory), presumably because episodic memory impairment is a prominent diagnostic feature of ADRD in older adults. Fewer than half of the samples found poorer memory associated with higher levels of air pollution [30–35], two reported better memory [33, 36], and 10 reported null or inconsistent associations [30, 32, 37–44]. Although research on ADRD has typically focused on memory, there is growing recognition of the importance of early deficits in executive function (EF)—a heterogeneous group of cognitive abilities that are very susceptible to aging and appear early in ADRD and MCI [45–48]—as a risk indicator. With regard to air pollution and EF, the heterogeneity of the abilities—encompassing working memory, set-shifting, and cognitive inhibition—and the tests used to assess EF make comparisons among studies challenging [45–48]. Among different samples evaluated, 4 found worse EF performance under conditions of higher air pollution [30, 34, 41, 49], 2 found better performance [38, 44], and 3 reported non-significant effects [30, 39, 50]. Most studies only measured one aspect of EF, often with only one test, or failed to adjust for related abilities (e.g., not adjusting for processing speed in Trails switching tests) [30, 32, 33, 37, 39, 40, 42]. Findings for verbal fluency and ambient air pollution are also mixed: associations range from worse [34, 35, 37, 42], to non-significant [32, 36, 39, 40, 44], to better performance [37, 41]. Other cognitive abilities such as processing speed and visuospatial ability are underrepresented in the air pollution literature. A few studies showed poorer processing speed [42, 44], but the majority found no association [30, 36, 39, 42, 51], while very few studies examined visuospatial ability [32, 34]. Most studies used only a single measure.
The opportunity for direct comparisons among studies is limited due to the widely varying assessments of air pollution and cognitive measures, and many cognitive measures were brief, screening, or global in nature. Sample characteristics such as age, sex, and socioeconomic status also vary widely. Finally, few studies of adults examine air pollution and cognitive abilities prior to old age with the majority being conducted in adults over 65 [3]. Despite recognition of the importance of the neurobiology of middle age [52], studies of ambient air pollution and cognitive functions in middle age—when interventions may crucially affect later ADRD outcomes—still constitute a major knowledge gap in this research.
Another knowledge gap in the air pollution/cognition literature is the extent to which genetic influences may moderate air pollution-cognition relationships [53]. Genetic factors such as APOE are non-modifiable risk factors that may influence the effect of modifiable risk factors such as air pollution. Thus, understanding the interacting effect of the genetic and environmental influences can highlight the importance of reducing the effect of air pollution in those who are genetically susceptible. Some research suggests that the apolipoprotein E (APOE) genotype affects ADRD risk by modifying susceptibility to environmental and health factors [53–57]. In a small autopsy study of children and young adults living in heavily polluted areas of Mexico City, APOE-ε4 allele carriers had accelerated beta amyloid 42 accumulation in the frontal cortex and hippocampus [6]. The authors proposed that older APOE-ε4 carriers might have additionally increased risk for developing ADRD if they resided in polluted environments [6]. In studies of older adults, however, 5 found no significant ambient air pollution-by-APOE genotype interaction [21, 58–61], and 1 found a significant interaction effect only on a measure of motor planning and execution [32]. One study reported significant moderation of the association between cognitive change and ambient air pollution (NO2, PM2.5, and PM10); APOE-ε4 carriers exposed to higher air pollution levels showed more pronounced cognitive decline, but cross-sectional findings were not addressed [59]. Mouse models of air pollution show more consistent adverse impact on carriers of human ApoE4 transgenes than ApoE3 [58, 62–67]. Because of their younger ages, these experimental studies suggest earlier/younger human ages should be examined where possible. Few air pollution studies examine the effects of APOE during midlife.
The APOE-ε4 allele is consistently associated with increased risk for accelerated cognitive aging, and ADRD in older adults [20, 68–71]. However, studies of ε4 carriers have found both preserved executive functioning and accelerated decline depending on life stage [20, 68, 72–76]. Whether APOE status modulates the effects of air pollution exposure on cognition in middle age remains unclear. We previously showed that APOE genotype was associated with significant decline in EF from middle to early old age [77]. In the present study, we examined whether effects of ambient air pollution on cognition—in particular on EF—from middle to old age are moderated by APOE genotype.
By using detailed residential PM2.5 and NO2 concentrations covering both past and recent air pollution exposures combined with in-depth cognitive assessments conducted from midlife to early old age, as well as APOE genotyping, this study provides a unique opportunity to address significant knowledge gaps in the ambient air pollution/cognition literature. VETSA’s extensive in-person evaluations, with multiple indicators of each cognitive domain in its day-long clinical neuropsychological protocol, allows us to replicate and extend previous research on specific cognitive domains. We first examined the extent to which specific cognitive factors—representing 4 cognitive domains of episodic memory, executive function, verbal fluency, and processing speed—were sensitive to the effect of past or recent ambient air pollution in midlife. In particular, we focused on episodic memory and executive functions as abilities most relevant to later ADRD. Second, we examined whether APOE genotype moderated the effects air pollution on cognitive functioning.
METHODS
Participants.
The Vietnam Era Twin Study of Aging (VETSA) is a longitudinal behavioral genetic study of cognitive and brain aging and risk for MCI and AD [78]. We randomly recruited participants from the Vietnam Era Twin Registry (VETR), a large nationally distributed registry of male-male twin pairs who served in the United States military at some point between 1965 and 1975 [79, 80]. All participants previously participated in the Harvard Twin Study of Substance Abuse [81], which included no exclusion criteria based on substance abuse or any diagnostic or other personal characteristic. At the baseline assessment (VETSA 1, data collection: 2003–2007; average age 56, range 51–61), inclusion criteria were that both members of a twin pair agreed to participate and were 50–59 years old at recruitment [82]. Follow-up inclusion criteria did not require participation of the cotwin (VETSA 3; 2016–2019; average age 68, range 61–73). Details of the sample ascertainment and data collections are described in detail elsewhere [78, 82]. Participants have comparable health, education, and lifestyle characteristics to American men in their age range [83]. Although all participants are veterans, most (80%) did not experience combat. From hereon we refer to the cognitive assessment waves as age 56 or age 68.
Procedures.
VETSA in-person assessments at both time points involved self-report questionnaires, medical history interviews, and in-depth neuropsychological testing. Assessments using identical protocols occurred at University of California, San Diego (UCSD) and Boston University (BU). The studies were approved by human subjects’ research protections review boards at the participating institutions. Participants provided written informed consent.
Measures.
Cognitive measures.
At mean ages 56 and 68, participants were administered the same extensive clinical neuropsychological battery comprising 16 standard cognitive tests comprising 24 subtests representing multiple cognitive domains. We used factor scores calculated for four cognitive domains (episodic memory, executive function, processing speed, and verbal fluency) based on our prior work which validated these latent variables [84–88]; factor scores comprising multiple tests are more robust than individual tests. Cognitive scores at age 68 were adjusted for practice effects prior to factor analysis; this approach adjusts for the expected improvement in performance among longitudinal research participants with previous exposure to the same tests [84–89].
Episodic memory factor. The episodic memory factor comprises 7 scores [84]: the total of the five learning trials, the short-delay free recall, and the long-delay free recall conditions from the Delis-Kaplan Executive Function System (D-KEFS) California Verbal Learning Test version 2 (CVLT) [90], and the immediate and delayed recall conditions from Visual Reproductions and Logical Memory subtests from the Wechsler Memory Scale (WMS)-III [91].
Executive function factor. An influential model of executive function—the unity/diversity model—has identified a robust common factor underlying executive function measures representing response inhibition, task-set switching, and working memory by modeling it as a common executive function latent factor [46, 47]. The common executive function factor reflects goal management abilities necessary for initiating and completing a task and to pursuing goal-directed actions despite distractions [77, 86].
The VETSA common executive function factor comprises scores from 6 measures that represent the 3 subdomains of executive function. Inhibition was assessed with the Stroop task [92, 93]. Set-shifting was assessed using the D-KEFS Trail Making Test switching trial adjusted for performance (i.e., speed) on the letter and number sequencing conditions and the D-KEFS category-switching subtest for verbal fluency adjusted for number of correct boys’ names and animals [94]. Working memory subdomain included 3 measures: the letter number sequencing, forward and backward digit span subtests from the Wechsler Memory Scale-III [91] and the reading span test [95].
In these analyses, we focus on the common executive function factor (EF). However, because a number of studies have examined working memory, we also created an additional working memory factor that allows more direct comparison with other studies that only assessed working memory based on previous research [77, 86, 96]. The working memory factor (WM) is based only on the 3 working memory tasks. Importantly, the working memory factor score used here reflects a contribution of common EF and working memory-specific abilities. It is thus highly correlated with common EF but still distinct because the 3 working memory measures all involve the ability to temporarily hold and manipulate information in one’s brain.
Verbal Fluency factor. The verbal fluency factor is based on our factor analysis of abilities in common across the multiple verbal fluency tests administered in VETSA (phonemic fluency & semantic fluency) [85]. The general verbal fluency factor (VF) [85] captured common variance on the D-KEFS verbal fluency phonemic (3 letters) and semantic conditions (3 categories, including the number of items generated in the switching task ignoring the number of switches) [94]. Because some air pollution studies only examined semantic fluency, we also created a separate semantic fluency factor score based on just the three D-KEFS semantic conditions. The semantic fluency factor has high overlap with the general fluency factor.
Processing speed factor. The processing speed factor comprises six processing speed indices [88]: the D-KEFS Trail Making number and letter sequencing conditions [94], word and color conditions of the Stroop task [92, 93], and a computerized version of simple reaction time [97]. Scores are coded so that high scores indicate better performance.
Air Pollution Exposure Assessment.
Address histories/geocoding. We combined 3 sources of residential addresses to create detailed address histories from 1993–2017: addresses provided to the study as part of ongoing study recruitment and retention, a mailed survey requesting addresses lived at between 1993 and 2018 for 6 months or longer, and LexisNexis. After we created detailed residential address histories, all geocodes were provided to the University of Washington Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESAAir) group. Geocodes data did not include dates or identification numbers and a large number of random geocodes were included to retain anonymity. MESAAir provided the VETR with PM2.5 and NO2 concentration levels at each geocode from 1993 to 2017. VETR staff then created individualized PM2.5 and NO2 histories for each participant by month. Since PM2.5 prior to 1999 was provided as an annual exposure, if an individual lived in different locations across a particular year, the air pollutant value for each location was time-weighted to the address history.
Creation of exposure concentration scores. For the data analyses, we created individualized past and recent exposure concentration scores for each participant for PM2.5 and/or NO2 exposure. The past score averaged pollutant data from 1993–1999. The recent score averaged pollutant data from the 36 months prior to a participant’s baseline cognitive testing date. We excluded participants if more than 20% of their pollution data were missing for the designated period. Out of the 1237 VETSA 1 participants, 1124 had usable exposure histories (91%); 112 (9%) did not have enough usable geocodes to create address histories or lived outside of the continental United States. Comparisons of the characteristics of participants with usable air pollution data with those who did not have usable data found only one significant difference for variables listed in Table 1; participants were more likely to be heavy drinkers in the group with more missing air pollution data [X2 (2, 1234) = 7.38, p=.025].
Table 1.
Descriptive statistics
| 1993–1999 | VETSA 1 | VETSA 3 | ||||
|---|---|---|---|---|---|---|
| Total N | Mean (STD)/N(%) | Total N | Mean (STD)/N(%) | Total N | Mean (STD)/N(%) | |
| Age Mean (STD) | 1123 | 55.9 (2.5) | 838 | 67.6 (2.4) | ||
| Ethnicity N (%) | 1144 | 1123 | 838 | |||
| White non-Hispanic | 1053 (92.1) | 1036 (92.3) | 770 (91.9) | |||
| Others | 91 (8) | 87 (7.8) | 68 (8.1) | |||
| Location (Urban/Rural) Mean N (%) | 1084 | 2.5 (1.9) | 1113 | 2.5 (1.9) | 831 | 2.6 (1.9) |
| Education Mean (STD) | 1144 | 13.9 (2.1) | 1123 | 13.9 (2.1) | 838 | 14 (2.1) |
| Family_income Mean (STD) | 1121 | 7.7 (3.2) | 1100 | 7.6 (3.2) | 827 | 7.8 (3.2) |
| APOE N (%) | 1136 | 1117 | 834 | |||
| APOE 4 non-carrier | 792 (69.7) | 786 (70.4) | 593 (71.1) | |||
| APOE 4 carrier | 344 (30.3) | 331 (29.6) | 241 (28.9) | |||
| PM2.5 (μm/m3) Mean (STD) | 1144 | 13.3 (2.8) | 1123 | 11.1 (2.8) | 838 | 11.1 (2.9) |
| NO2 (ppb) Mean (STD) | 1144 | 11.1 (6.4) | 1123 | 9.3 (5.2) | 824 | 9.3 (5.2) |
| Smoke Status | 1123 | 836 | ||||
| Never | 358 (31.9) | 279 (33.4) | ||||
| Former | 507 (45.2) | 446 (53.4) | ||||
| Current | 258 (23) | 111 (13.3) | ||||
| History of high cholesterol N (%) | 1121 | 337 (30.1) | 838 | 587 (70) | ||
| Hstory of Hypertension N (%) | 1121 | 643 (57.4) | 838 | 633 (75.5) | ||
| History of cardio/vascular event N (%) | 1121 | 131 (11.7) | 838 | 242 (28.9) | ||
| History of respiratory illness N (%) | 1121 | 96 (8.6) | 838 | 144 (17.2) | ||
| Drug abuse N (%) | 1120 | 14 (1.3) | 838 | 7 (0.8) | ||
| Alcohol use N (%) | 1121 | 837 | ||||
| No alcohol use in past 2 weeks | 386 (34.4) | 331 (39.6) | ||||
| >0 and <= 2 drinks per day | 581 (51.8) | 404 (48.3) | ||||
| > 2 drinks per day | 154 (13.7) | 102 (12.2) | ||||
| History of Stroke N (%) | 1121 | 22 (2) | 838 | 50 (6) | ||
| BMI Mean (STD) | 1119 | 29.3 (4.9) | 809 | 30 (5.4) | ||
| Depression Mean (STD) | 1115 | 8.2 (8.1) | 834 | 6.9 (7.5) | ||
Air pollution level concentrations: PM2.5 and NO2. The MESAAir statistical prediction of air pollution concentrations uses pollutant measurements from monitored locations in conjunction with multiple types of geographic, atmospheric, and physiochemical information as covariates in statistical models to predict the concentration of pollutants at a much larger number of unmonitored locations over a given timeframe104. The MESAAir fine scale national spatiotemporal model is an extension of universal kriging. Kriging, a Gaussian process regression, is a method by which data from a limited sampling of geographic data (e.g., a pollutant such as PM2.5 measured at a specific monitor) are interpolated to provide estimates of the value of that pollutant over a continuous spatial field. The universal kriging approach combines land-use regression (LUR) techniques with simple kriging methods tapping into the strengths of both. The model makes use of up to 400 geographic covariates based on geographic information systems (GIS) data. More specifically, the model combines information such as seasonal and systematic trends in emission sources, population, land use and near-source concentrations (i.e., the LUR construct; constant time-averaged spatial field) that vary over space and generates predicted concentration values. NO2 models may also include satellite data. The modeling is based on the assumption that pollutant concentrations exhibit systematic seasonal and secular trends that vary over space that can be used to predict other concentrations. The MESA Air model is unique because it is based on spatially-varying temporal pollution processes [98].
Initially the MESAAir models were developed for specific communities but have been extended to predict across the continental United States. By using spatial smoothing methods, the model also provides more realistic pollution surfaces, even in areas without adequate monitor coverage. Information from data-rich areas combined with other data can be interpolated to produce estimates even for sparsely populated regions with less monitoring infrastructure. To facilitate the extended models, MESAAir investigators divided the country into 9 climatic/topographic regions (for PM2.5 model) or three regions (for NO2) in order to account for subnational region-specific pollution processes and ensure each region contained supplemental monitors. Overall, the model reliably yields accurate predictions at specific geolocations; for PM2.5 the results prior to 1999 were predicted on an annual scale and after 1999, on a monthly scale. For NO2, results from 1993–2017 were predicted on a monthly scale. Values have been cross-validated with an R2 of 0.89 for PM2.5 and 0.87 for NO2 [98]. For regions with little or no monitor coverage, the R2 was 0.77. Given that the Environmental Protection Agency’s extensive monitoring system was only established in 1999, data prior to that period relied on ground-based data from 1999–2010 which was run through a spatiotemporal historical prediction model and back-extrapolated, resulting in R2 values ranging from 0.55 to 0.87 [98].
APOE.
At a participant’s first assessment certified phlebotomists drew blood that was used to determine APOE genotype; detailed methods were described previously [99]. In brief, APOE genotype was determined using polymerase chain reaction conditions and the HhaI restriction digest method in the laboratory at the Puget Sound VA Healthcare System. All genotypes were determined independently twice by laboratory personnel blind to initial genotype. We divided the sample into two groups: ε4 positive (individuals with 1 or 2 ε4 alleles) and ε4 negative (no ε4 alleles).
Covariates.
Covariates were chosen based on prior research and included time (age 56, age 68 assessment), location, age, lifetime education, race/ethnicity, household income, smoking status, alcohol consumption status, depressive symptoms, self-reported physician diagnosis of cardiovascular disease, diabetes, high cholesterol, and/or stroke. Location was coded based on rural-urban continuum (RUC) codes that use zip code to categorize location by counties and recoded as: metropolitan (1= ≥ 1 million), urban/suburban (2= ≥ 50,000 to < 1 million), 3= rural (< 50,000) [100]. Education is a continuous measure reflecting years of formal education completed (i.e., completed high school = 12; completed college = 16; PhD/MD = 20). Race/ethnicity is categorized as 1 = White non-Hispanic versus 0 = Other. Cigarette smoking status is coded as 0 = never, 1 = former, 2 = current. Alcohol is coded as the extent in the past 2 weeks a person consumed beer, wine, and/or hard liquor: 0 = no alcohol; 1 = > 0 to ≤ 2 drinks per day, and 3 = > 2 drinks per day. Health information on cardiovascular conditions, stroke, diabetes, hypertension was self-reported in a medical history interview on the same day as cognitive testing (1 = present/0 = absent). Depressive symptoms were assessed with the Center for Epidemiologic Studies Depression Scale (CES-D) [101]. Education, household income, and depressive symptoms are modeled as continuous measures. Familial and adult socioeconomic status (SES) measures are a weighted combination of education and occupation.
Statistical analyses.
In preliminary analyses we divided PM2.5 and NO2 measures into quartiles and examined quartile differences in demographic measures and covariates to ascertain whether associations might be non-linear. There was little evidence of non-linearity (Supplemental Table S1 & S2) so we conducted analyses using continuous air pollution measures. In particular, APOE status did not differ by quartile.
Multivariate analyses were conducted with continuous PM2.5, NO2, and the 4 cognitive factors. We used generalized estimating equations implemented in SAS PROC GEE. GEE provides improved efficiency over mixed models and fits a marginal model to longitudinal data. The correlations between twins were taken into account in the working matrix, and the time effects (repeated measures at the age 56 and age 68 assessments) were estimated as a fixed effect in the output. In Model 1, we first examined the main effects of past and current PM2.5 or NO2 and APOE-ε4 status. Covariates included time, location, age, lifetime education, race/ethnicity, household income, smoking status, alcohol consumption status, depressive symptoms, and self-reported physician diagnosis of cardiovascular disease, diabetes, high cholesterol, and/or stroke. Models were conducted separately for past and recent PM2.5 and NO2. Tests of main effects in Model 1 test whether pollution exposure is related to cognitive function essentially averaged over both timepoints.
Second, in Model 2 we added air pollutant-by-APOE status and air pollutant-by-time interactions to Model 1. Model 2 tests whether pollution exposure effects differ as a function of APOE genotype, and whether the magnitude of pollution-cognition associations differed over time. For categorical moderators such as APOE in the current study, the concept of moderation is consistent with analytic interaction [102]. Although the participants are twins, these were non-twin analyses. Because these analyses treat the twins as individuals, GEE models control for the non-independence of the twins and address nested correlations (i.e., twins and age). In follow-up/sensitivity analyses, we also examined the working memory and semantic fluency sub-factors. To address the potential increased likelihood of multicollinearity in the models with interactions, we centered all the independent variables that formed the interaction terms in the models with APOE-by-pollutant interactions and calculated variance inflation factors (VIFs). VIFs for all the independent variables for each cognitive outcome were all less than 2, indicating no serious multicollinearity for any models.
RESULTS.
Descriptive analyses.
PM2.5 and NO2 declined steadily from 1993 to 2017 (Figure 1; Table 1). At past and recent timepoints, average PM2.5 levels were 13.37 μg/m3 (range 4.90–24.33) and 11.15 μg/m3 (range 2.58–24.37), respectively. These PM2.5 levels are below the 1997 Environmental Protection Agency (EPA) standards of 15 μg/m3. Average past NO2 was 11.14 ppb (range 1.60–44.74); average recent NO2 was 9.28 ppb (range 1.72–37.37); these NO2 levels were lower than EPA standard of annual average 53 ppb set in 1971. Paired t-tests indicate both PM2.5 and NO2 declined significantly within participant. Mean change from past to recent for PM2.5 was an average decrease of 2.12 μg/m3 (SD 1.73) [t (1070) = 41.99, p<0.0001] and for NO2 an average decrease of 1.18 ppb (SD 3.36) [t (1071) = 17.67, p<0.0001]. All of the cognitive factors showed significant effects of time indicating change (declines) in cognitive performance from mean age 56 to 68 (see Supplemental Tables S3–S6).
Figure 1.
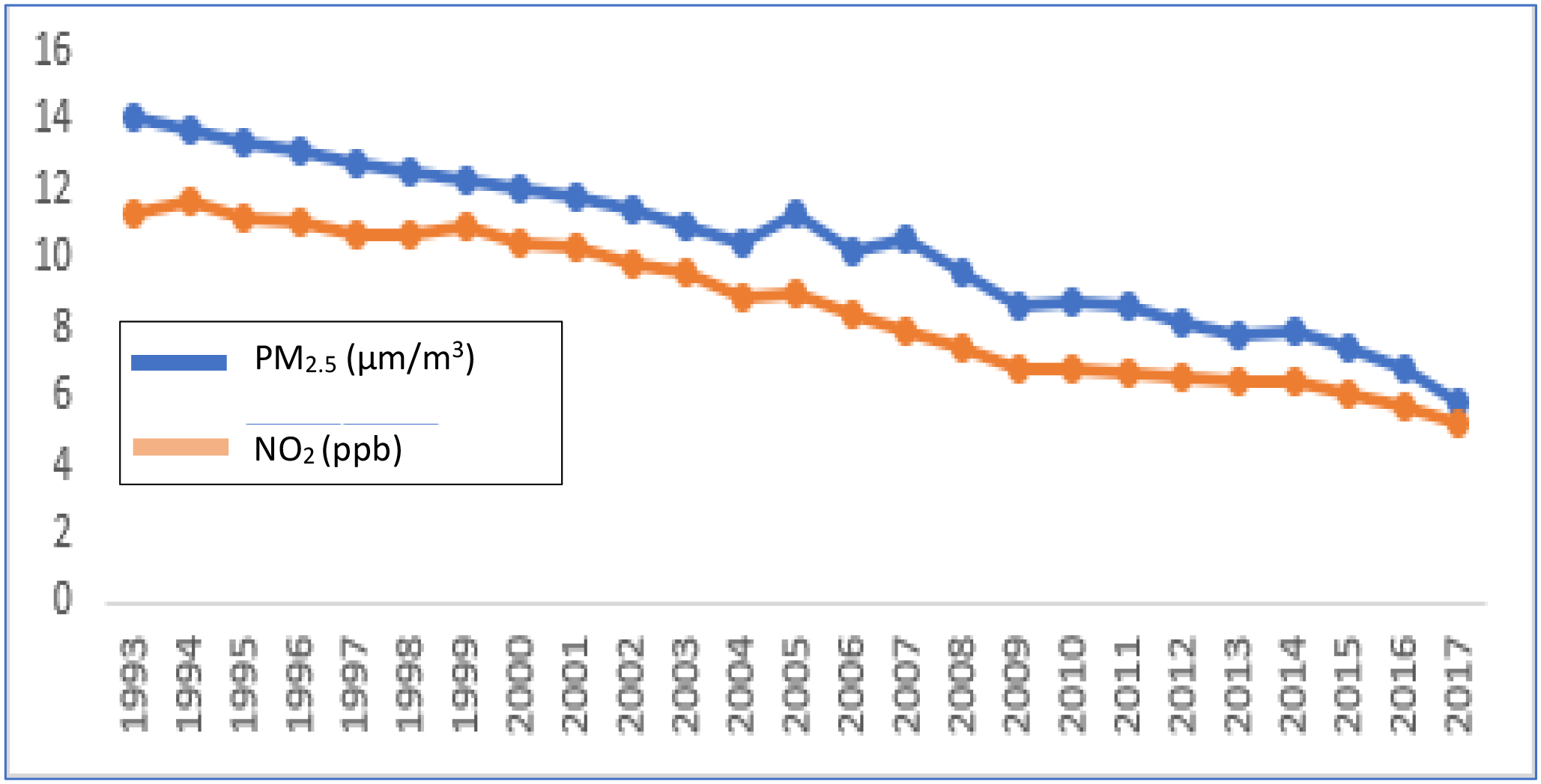
Average PM2.5 and NO2 by Year: 1993–2017.
The majority of participants (90.8%) were non-Hispanic White, with average lifetime education of 13.9 years. See Table 1 for descriptive statistics. Past and recent PM2.5 were correlated r=0.81 (p<.0001); past and recent NO2 were correlated r=0.86 (p<.0001). Within time correlations between the two air pollutants ranged from r=0.60 (p<.0001; past) to 0.46 (p<.0001; recent). In the GEE models, age, education, and depressive symptoms were consistently significantly associated with the cognitive factors, with the exception of the correlation between age and working memory (see Tables S3–S6).
Participants who did not return for the age 68 assessment included 78 deceased (6.3%) and 208 non-returnees for other reasons (16.8%). Comparisons of returnees with non-returnees of any type showed that non-returnees were significantly more likely to have been smokers, have cardiovascular conditions, had a stroke, lower education, and lower income at age 56 (all ps<.05 2-tailed). Non-returnees did not differ from returnees in PM2.5 or NO2 exposure, ethnicity, APOE genotype, BMI, respiratory problems, depressive symptoms, or urban/rural location. Past and/or recent air pollution exposure was no different for deceased participants compared with returnees.
We examined correlations of PM2.5 and NO2 with sociodemographic characteristics; associations varied by characteristic and pollutant (see Table 2). Parental SES was associated with men having higher PM2.5 but not NO2 concentrations in midlife. Living in a more urban setting at age 20 and having non-White race/ethnicity were both significantly associated with higher PM2.5 and NO2 in midlife. Living in a more urban setting at age 20 was also significantly associated with higher parental SES, adult SES, and adult income. At midlife, participants with more years of education and higher own SES lived in settings with significantly higher NO2 but not PM2.5. Thus, these air pollutants at midlife showed complex associations with earlier and midlife demographic factors.
Table 2.
Demographic measures: Correlations with PM2.5 AND NO2
| Past PM2.5 (93–99) | Recent PM2.5 (3 yrs prior to VETSA 1) | Past NO2 (93–99) | Recent NO2 (3 yrs prior to VETSA 1) | |
|---|---|---|---|---|
| Family of Origin Socioeconomic Status | −0.11 | −0.10 | −0.01 | −0.02 |
| Ethnicity (1= White non-Hispanic/0=other) | −0.12 | −0.06 | −0.19 | −0.16 |
| Age 20 Location (0=rural to 9=most urban; ~1969) | 0.16 | 0.11 | 0.30 | 0.32 |
| Education-Years of formal education | 0.00 | −0.02 | 0.10 | 0.12 |
| Age 20 General Cognitive Ability | −0.07 | −0.02 | −0.01 | 0.04 |
| Own Socioeconomic Status (age 56) | −0.01 | −0.03 | 0.07 | 0.08 |
| Own Income (age 56) | 0.04 | 0.04 | 0.05 | 0.04 |
| Own occupation (age 56) | −0.01 | 0.00 | 0.06 | 0.06 |
Items in bold are significant at than p<.05, two-tailed. With the exception of ethnicity, all measures are continuous variables.
Model testing.
Model 1: Associations between ambient air pollution, APOE-ε4 status, and cognitive functions adjusting for time, location, age, lifetime education, race/ethnicity, household income, smoking status, alcohol consumption status, depressive symptoms, and self-reported physician diagnosis of cardiovascular disease, diabetes, high cholesterol, and stroke. In Model 1, both past PM2.5 and recent PM2.5 were significantly associated with the general verbal fluency factor (β = −0.03, 95% CI [−0.05, −0.01]; β = −0.03, 95% CI [−0.05, −0.00] respectively; S3, S4). There were no significant main effects of recent or past PM2.5 or NO2 levels on the other three cognitive factors and no main effect of APOE genotype (see Supplemental tables S3–S6).
We also examined associations with the working memory and semantic fluency sub-factors. Associations for PM2.5 were not significant. Both past NO2 and recent NO2 levels were significantly associated with the semantic fluency factor (β= −0.01, 95% CI [−0.03, −0.00]; β = −0.02, 95% CI [−0.03, −0.01], respectively; Supplemental Tables S5, S6). However, examination of confidence intervals suggests there is no strong evidence supporting a major difference between the general fluency and semantic fluency factors for PM2.5 versus NO2 exposures. Neither past NO2 nor recent NO2 was associated with the working memory sub-factor.
Model 2: Model 1 plus air pollutant-by-APOE genotype and air pollutant-by-time interactions. The interactions of APOE genotype with both past and the recent PM2.5 were significant for executive function (past PM2.5-by-APOE: β= −0.06, 95% CI [−.10, −0.02]; recent PM2.5-by-APOE: β= −0.05, 95% CI [−.09, −0.01]; Table 3).
Table 3.
Summary of Results from Model 2 With Significant PM2.5 or NO2 by APOE Genotype Associations: Shown are Main Effects of Pollutant, APOE and Pollutant by APOE Interaction for the Significant Cognitive Factor
| Model 2 summary | beta Estimate se | 95% CI | Chi2 | p-value | |
|---|---|---|---|---|---|
| Past PM 2.5 *APOE and Executive Functions Factor | |||||
| Past PM2.5 | 0.02 | (0.01) | −0.0050, 0.0417 | 1.00 | 0.317 |
| APOE (REF ε4-) | 0.08 | (0.07) | −0.0464, 0.2128 | 1.57 | 0.209 |
| Past PM2.5*APOE | −0.06 | (0.02) | −0.0993,−0.0200 | 7.95 | 0.005 |
| Recent PM 2.5 *APOE and Executive Functions Factor | |||||
| Recent PM2.5 | 0.01 | (0.01) | −0.0070, 0.0365 | 0.83 | 0.362 |
| APOE (REF ε4-) | 0.09 | (0.07) | −0.0360, 0.2187 | 1.96 | 0.161 |
| Recent PM2.5*APOE | −0.05 | (0.02) | −0.0918,−0.0090 | 5.54 | 0.019 |
| Past PM 2.5 *APOE and Working Memory Sub-factor | |||||
| Past PM2.5 93–99 | 0.01 | (0.01) | −0.0108, 0.0404 | 0.84 | 0.360 |
| APOE (REF ε4-) | 0.09 | (0.07) | −0.0507, 0.2347 | 1.59 | 0.208 |
| Past PM2.5*APOE | −0.05 | (0.02) | −0.0927,−0.0111 | 5.82 | 0.016 |
| Recent PM 2.5 *APOE and Working Memory Sub-factor | |||||
| Recent PM2.5 | 0.01 | (0.01) | −0.0118, 0.0359 | 0.94 | 0.331 |
| APOE (REF ε4-) | 0.10 | (0.07) | −0.0440, 0.2350 | 1.79 | 0.181 |
| Recent PM2.5*APOE | −0.05 | (0.02) | −0.0896,−0.0053 | 4.73 | 0.030 |
| Past NO 2 *APOE and Episodic Memory Factor | |||||
| Past NO2 93–99 | −0.01 | (0.01) | −0.0200, 0.0067 | −0.98 | 0.329 |
| APOE (REF ε4-) | 0.01 | (0.08) | −0.1433, 0.1622 | 0.12 | 0.904 |
| Past NO2*APOE | 0.02 | (0.01) | 0.0043, 0.0422 | 2.41 | 0.016 |
| Recent NO 2 *APOE and Episodic Memory Factor | |||||
| Recent NO2 | −0.01 | (0.01) | −0.0235, 0.0063 | −1.13 | 0.259 |
| APOE (REF ε4-) | 0.03 | (0.08) | −0.1174, 0.1841 | 0.43 | 0.665 |
| Recent NO2*APOE | 0.03 | (0.01) | 0.0019, 0.0499 | 2.12 | 0.034 |
Notes. Summary results are from Model 2. Shown are the results for the pollutant, APOE ε4 status, and the pollutant by APOE interaction when the interaction is significant. Model 2 is run separately for the 4 pollutant measures predicting cognitive factors. Model 2 included the air pollutant, APOE ε4- status, the air pollutant by APOE ε4 interaction, air pollutant by time interaction covariates of time, location, age, lifetime education, race/ethnicity, household income, smoking status, alcohol consumption status, depressive symptoms, self-reported physician diagnosis of cardiovascular disease, diabetes, high cholesterol, and/or stroke. Full results for all Model 2 analyses (showing all variables in models) are in Supplemental Table 7.
Compared to APOE-ε4 non-carriers, ε4 carriers had steeper and more negative slopes indicating poorer executive function with increasing PM2.5 levels (Figure 2). In contrast, ε4 non-carriers had relatively flat slopes that were not significantly different from zero with regard to PM2.5. Interestingly, examining the confidence intervals (see Figure 3), APOE-ε4 carriers had significantly better executive functioning than the non-carriers when PM2.5 levels were low. Results were similar for working memory (past PM2.5-by-APOE: β= −0.05, 95% CI [−.09, −0.01]; recent PM2.5-by-APOE: β= −0.05, 95% CI [−.09, −0.01] Figure 3; Table 3). The PM2.5-by-APOE interactions were not significant for the other cognitive domains. There were no significant air pollutant-by-time interactions (see Table S7).
Figure 2.
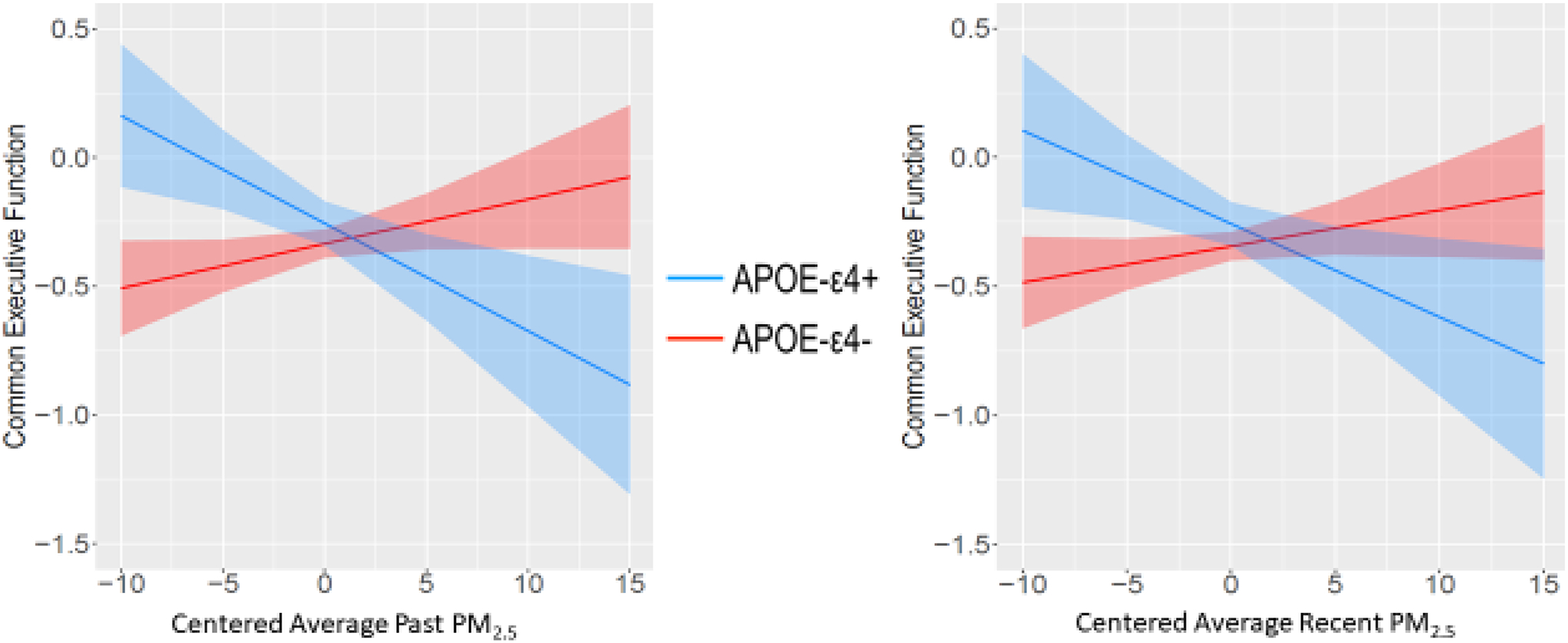
Executive Function Factor and PM2.5-by-APOE interaction
Figure 3.
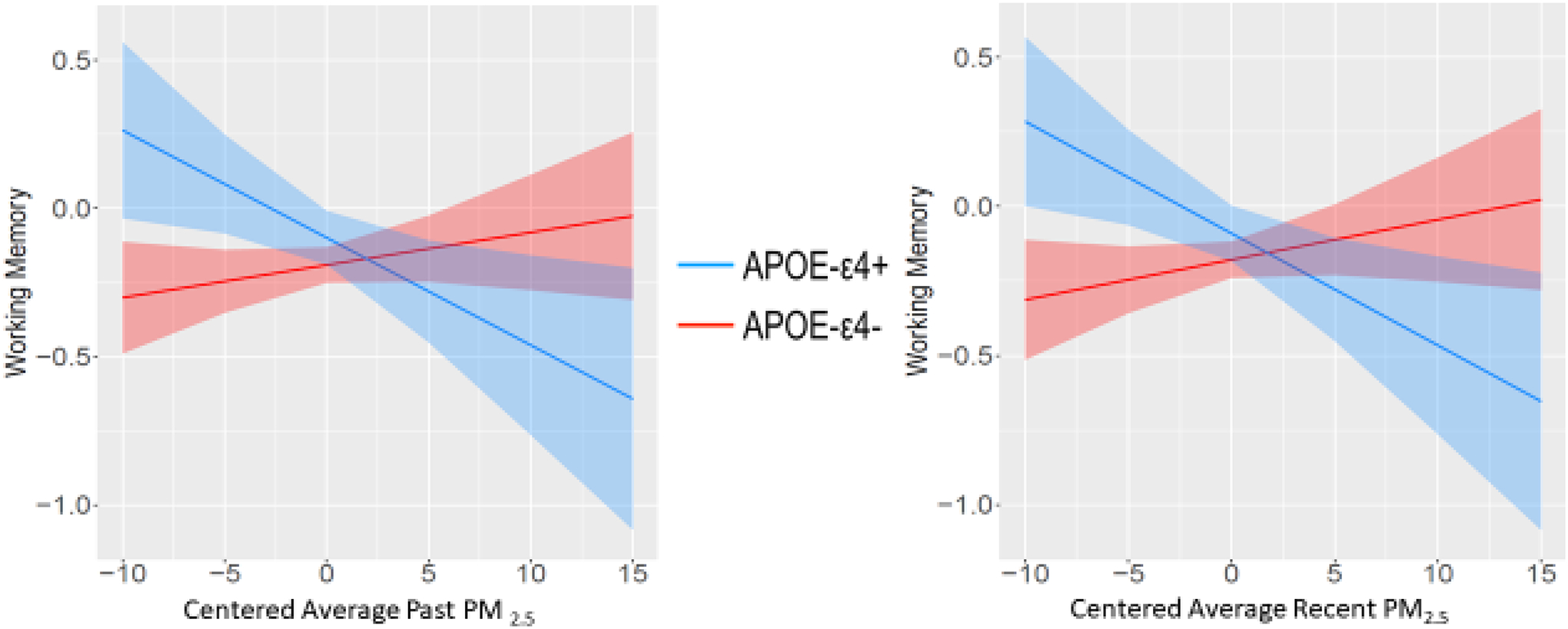
Working Memory Factor and PM2.5-by-APOE interaction
Past and recent NO2-by-APOE interactions for episodic memory were significant (past NO2-by-APOE: β = 0.02, 95% CI [.004, 0.04]; recent NO2 -by-APOE: β = 0.03, 95% CI [.001, 0.05], respectively; Table 3, Figure 4). Compared to APOE-ε4 non-carriers, ε4 carriers had positive slopes indicating better episodic memory with increasing NO2 levels. Among non-carriers, there were no associations between exposures and cognition. The NO2-by-APOE interactions were not significant for the other cognitive domains. There were no other significant NO2-by-APOE interactions (see Table S7).
Figure 4.
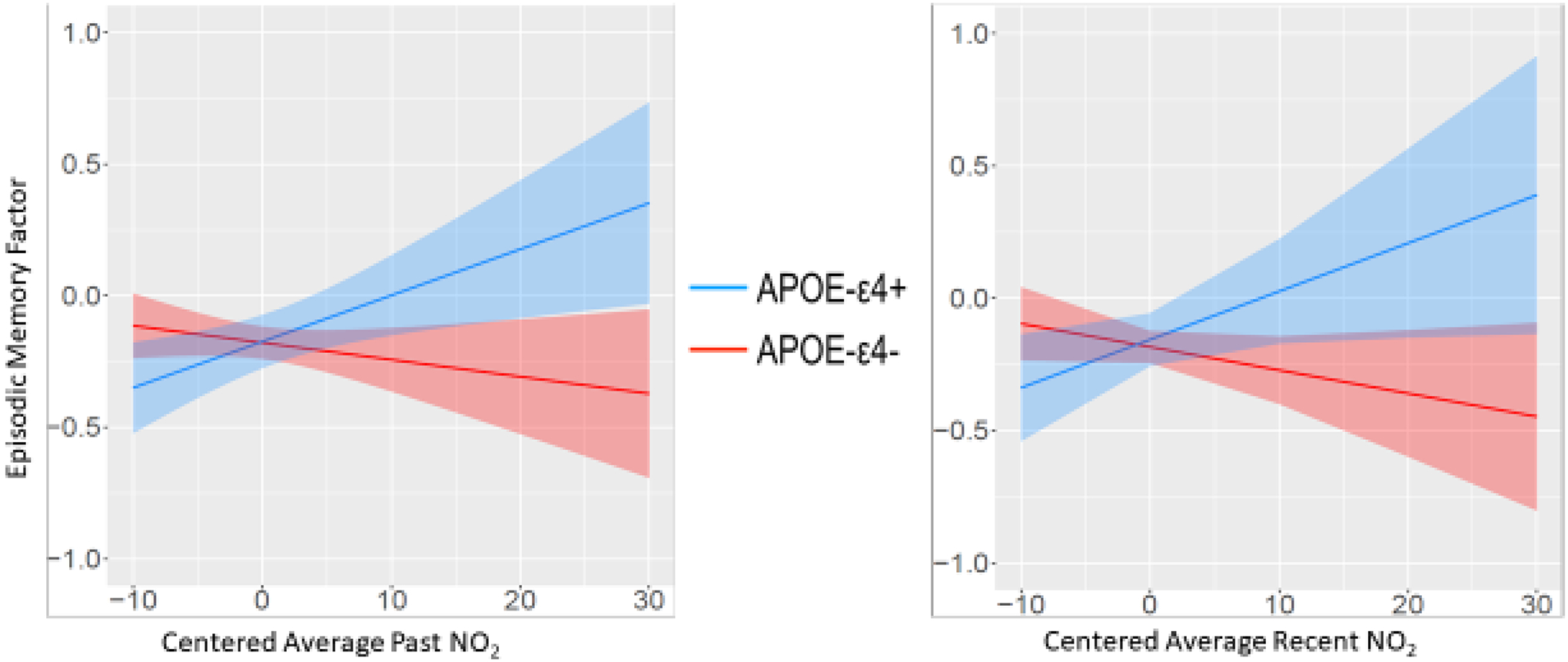
Episodic Memory Factor and NO2-by-APOE interaction
CONCLUSIONS
These findings from the VETSA sample demonstrate associations of PM2.5 and NO2 with certain cognitive functions from midlife to early old age, with certain associations differing as a function of APOE genotype. The only main effect was that individuals exposed to higher ambient air pollution had lower verbal fluency—general verbal fluency for PM2.5 and semantic fluency for NO2. These results are partially consistent with 4 studies of primarily older adults [34, 35, 37, 42], but 2 of those 4 studies only examined semantic fluency.
We found significant interactions between APOE genotype and air pollution in relation to executive function and episodic memory domains, suggesting the importance of examining complex modifiers that may begin to explain variability of previous findings in the literature. Although APOE-ε4 is the major risk allele for Alzheimer’s disease and APOE-ε4 may increase susceptibility to environmental and health factors [53–57, 71, 103], it also shows variability across the life course [20, 104]. Here, the interaction effect was such that as either past or recent as PM2.5 levels increased, executive function and working memory worsened in ε4 carriers, but not non-carriers. However, at very low levels of PM2.5 ε4 carriers actually had significantly better performance than non-carriers (Figure 2). While all participants had similar episodic memory performance at past lower levels of NO2, somewhat surprisingly, ε4 carriers had significantly better episodic memory at the highest levels of NO2. The steeper slopes of the ε4 carriers is consistent with evidence for APOE being a variability gene i.e., that it has variable sensitivity to environmental influences and that the ε4 allele is not always associated with more negative outcomes [54]. We do not know of other evidence that higher NO2 improves cognition. Previous literature has, for example, found that APOE ε4 carriers show either preserved functioning or accelerated decline in executive function depending on life stage [20, 68, 72–75, 105].
The complexity of these associations with ambient air pollution is evident in our finding that although the slopes were steeper for ε4 carriers, it remains unclear what could account for the opposite direction of these two sets of interaction effects. One possible explanation may lie in the different associations between NO2 and demographic factors. Participants with higher education and higher income experienced higher NO2 but not PM2.5 exposures in midlife, and also had better episodic memory. Men with higher education and income, as well as familial SES, were more likely to live in urban settings even at age 20. Given that PM2.5 exposures tend to be “flatter” across a metropolitan area and NO2—as a marker of traffic-related air pollution—has more fine area contrasts, NO2 may be more sensitive to SES gradients than PM2.5. These associations suggest there may be some spatial confounding as evidenced by associations between the air pollution measures and demographics. Although our statistical models adjusted for urban/rural location, education and income, it may be there are other differences not captured by our covariates that are associated with who resides in areas with higher pollution. In addition, while average levels of PM2.5 were relatively close to national EPA standards, average levels of NO2 were relatively low and well within the “good” range compared with EPA standards.
Limitations of this paper are that the sample is all male and mostly White non-Hispanic, so the results may not generalize to women and other ethnicities. Estimates of differences between white and non-white participants are based on a small subsample. We calculated air pollution measures based on residential address; however, at age 56 the majority of the men worked full-time, and many were still working at age 68. We did not have access to work geocodes. Thus, the residential addresses may not accurately reflect full exposures. Furthermore, the sample was a nationwide sample with about one-third residing in relatively rural areas where traffic-related ambient air pollution may be low and also less accurately assessed due to distances between pollution monitors. We also were limited to past and recent PM2.5 and NO2 and did not have pollution estimates at age 68. Finally, although we examined whether APOE genotype moderates the effects of air pollution, we did not examine other biological mechanisms. Different mechanisms have been proposed for why air pollution may be associated with risk for ADRD including vascular risk factors, other biological vulnerabilities, stress, behavioral and socioeconomic risks that may link residing in more polluted areas with poorer cognitive performance [3, 22, 53, 71, 106, 107].
Despite its limitations, this study has many strengths. Unlike many previous studies examining air pollution and cognition, VETSA administered an in-depth in-person cognitive battery at 2 time points from midlife to early old age. There were multiple measures for each cognitive domain. In addition, we were able to calculate both past and recent air pollutant concentrations during midlife for 2 key air pollutants. The sample’s narrow age range at VETSA 1 (10 years) starting in midlife provides insights into an understudied age group. Effects of APOE genotype are complex, especially when taking the life course into account, so having a large sample in a narrow age range allowed us to focus on effects from midlife to early-old age. VETSA is also a nationwide sample providing heterogeneous exposures from very rural to very urban areas. Finally, our examination of the common executive function factor and its working memory sub-factor provide a more thorough investigation of executive function than in most prior studies.
In summary, these results reflect the complex interplay among APOE genotype, different air pollutants, cognitive abilities, and demographic factors across the life course. Also of import are the findings that the main contributors to cognitive function in midlife and later life were covariates reflecting influences such as education and depression, as well as age and time. Both education and depression are modifiable risk factors for ADRD and may interact with other risks such as air pollution [108, 109]; however, the possibility of meaningful modification of education will most likely be much earlier in life. Finally, it is important to consider the possibility of other modifiers, including other genes in the APOE cluster [110, 111]. Like prior studies, effect sizes in the present study were small. However, although research has found only small effects of air pollution on Alzheimer’s disease and mixed results for effects on cognition, as Livingston et al. point out [1], when taken in the context of the large proportion of the population exposed to high air pollution, the overall impact is increased.
Supplementary Material
Acknowledgements.
The U.S. Department of Veterans Affairs, Department of Defense; National Personnel Records Center, National Archives and Records Administration; National Opinion Research Center; National Research Council, National Academy of Sciences; and the Institute for Survey Research, Temple University provided invaluable assistance in the creation of the VETR. The Cooperative Studies Program of the U.S. Department of Veterans Affairs provided financial support for development and maintenance of the VETR. We would also like to acknowledge the continued cooperation and participation of the members of the VETR and their families.
Funding
The content is the sole responsibility of the authors and does not necessarily represent official views of the NIA, NIH, or VA. This work was supported by the National Institute on Aging at the National Institutes of Health: P01 AG055367, and R01s AG050595, AG022381, AG076838, AG037985, AG064955.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to report.
Data Availability
The data supporting the findings of this study are available on request from the corresponding author. The data are not available on a public website due to privacy or ethical restrictions established by the Vietnam Era Twin Registry (VETR). A data request form is available on the VETSA website (https://psychiatry.ucsd.edu/research/programs-centers/vetsa/index.html). Data requests can also be made at the VETR; the access process is described at: https://www.seattle.eric.research.va.gov/VETR/Investigator_Access.asp.
References
- [1].Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimaki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbaek G, Teri L, Mukadam N (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cao B, Chen Y, McIntyre RS (2021) Comprehensive review of the current literature on impact of ambient air pollution and sleep quality. Sleep Med 79, 211–219. [DOI] [PubMed] [Google Scholar]
- [3].Chandra M, Rai CB, Kumari N, Sandhu VK, Chandra K, Krishna M, Kota SH, Anand KS, Oudin A (2022) Air Pollution and Cognitive Impairment across the Life Course in Humans: A Systematic Review with Specific Focus on Income Level of Study Area. Int J Environ Res Public Health 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weuve J, Bennett EE, Ranker L, Gianattasio KZ, Pedde M, Adar SD, Yanosky JD, Power MC (2021) Exposure to Air Pollution in Relation to Risk of Dementia and Related Outcomes: An Updated Systematic Review of the Epidemiological Literature. Environ Health Perspect 129, 96001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].World Health Organization (2016) World Health Organization, Geneva Switzerland. [Google Scholar]
- [6].Calderon-Garciduenas L, Reed W, Maronpot RR, Henriquez-Roldan C, Delgado-Chavez R, Calderon-Garciduenas A, Dragustinovis I, Franco-Lira M, Aragon-Flores M, Solt AC, Altenburg M, Torres-Jardon R, Swenberg JA (2004) Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol 32, 650–658. [DOI] [PubMed] [Google Scholar]
- [7].Cardenas-Iniguez C, Burnor E, Herting MM (2022) Neurotoxicants, the Developing Brain, and Mental Health. Biol Psychiatry Glob Open Sci 2, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Clifford A, Lang L, Chen R, Anstey KJ, Seaton A (2016) Exposure to air pollution and cognitive functioning across the life course - A systematic literature review. Environ Res 147, 383–398. [DOI] [PubMed] [Google Scholar]
- [9].Bhatt DP, Puig KL, Gorr MW, Wold LE, Combs CK (2015) A pilot study to assess effects of long-term inhalation of airborne particulate matter on early Alzheimer-like changes in the mouse brain. PLoS One 10, e0127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Campbell A, Oldham M, Becaria A, Bondy SC, Meacher D, Sioutas C, Misra C, Mendez LB, Kleinman M (2005) Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology 26, 133–140. [DOI] [PubMed] [Google Scholar]
- [11].Durga M, Devasena T, Rajasekar A (2015) Determination of LC50 and sub-chronic neurotoxicity of diesel exhaust nanoparticles. Environ Toxicol Pharmacol 40, 615–625. [DOI] [PubMed] [Google Scholar]
- [12].Escalante-Membrillo C, Gonzalez-Maciel A, Reynoso-Robles R, Gonzalez-Pina R (2005) Brain thiobarbituric acid-reactive substances in rats after short periods of ozone exposure. Environ Res 99, 68–71. [DOI] [PubMed] [Google Scholar]
- [13].Guerrero AL, Dorado-Martinez C, Rodriguez A, Pedroza-Rios K, Borgonio-Perez G, Rivas-Arancibia S (1999) Effects of vitamin E on ozone-induced memory deficits and lipid peroxidation in rats. Neuroreport 10, 1689–1692. [DOI] [PubMed] [Google Scholar]
- [14].Casanova R, Wang X, Reyes J, Akita Y, Serre ML, Vizuete W, Chui HC, Driscoll I, Resnick SM, Espeland MA, Chen JC (2016) A Voxel-Based Morphometry Study Reveals Local Brain Structural Alterations Associated with Ambient Fine Particles in Older Women. Front Hum Neurosci 10, 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen JC, Wang X, Wellenius GA, Serre ML, Driscoll I, Casanova R, McArdle JJ, Manson JE, Chui HC, Espeland MA (2015) Ambient air pollution and neurotoxicity on brain structure: Evidence from Women’s Health Initiative Memory Study. Ann Neurol 78, 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wilker EH, Preis SR, Beiser AS, Wolf PA, Au R, Kloog I, Li W, Schwartz J, Koutrakis P, DeCarli C, Seshadri S, Mittleman MA (2015) Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke 46, 1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Calderon-Garciduenas L, Torres-Jardon R (2015) The impact of air pollutants on the brain. JAMA Psychiatry 72, 529–530. [DOI] [PubMed] [Google Scholar]
- [18].Hajat A, Diez-Roux AV, Adar SD, Auchincloss AH, Lovasi GS, O’Neill MS, Sheppard L, Kaufman JD (2013) Air pollution and individual and neighborhood socioeconomic status: evidence from the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect 121, 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Carey IM, Anderson HR, Atkinson RW, Beevers SD, Cook DG, Strachan DP, Dajnak D, Gulliver J, Kelly FJ (2018) Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open 8, e022404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gharbi-Meliani A, Dugravot A, Sabia S, Regy M, Fayosse A, Schnitzler A, Kivimaki M, Singh-Manoux A, Dumurgier J (2021) The association of APOE epsilon4 with cognitive function over the adult life course and incidence of dementia: 20 years follow-up of the Whitehall II study. Alzheimers Res Ther 13, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Parra KL, Alexander GE, Raichlen DA, Klimentidis YC, Furlong MA (2022) Exposure to air pollution and risk of incident dementia in the UK Biobank. Environ Res 209, 112895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ (2019) Air Pollution and Dementia: A Systematic Review. J Alzheimers Dis 70, S145–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen H, Kwong JC, Copes R, Hystad P, van Donkelaar A, Tu K, Brook JR, Goldberg MS, Martin RV, Murray BJ, Wilton AS, Kopp A, Burnett RT (2017) Exposure to ambient air pollution and the incidence of dementia: A population-based cohort study. Environ Int 108, 271–277. [DOI] [PubMed] [Google Scholar]
- [24].Power MC, Adar SD, Yanosky JD, Weuve J (2016) Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. Neurotoxicology 56, 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tzivian L, Dlugaj M, Winkler A, Weinmayr G, Hennig F, Fuks KB, Vossoughi M, Schikowski T, Weimar C, Erbel R, Jockel KH, Moebus S, Hoffmann B, Heinz Nixdorf Recall study Investigative G (2016) Long-Term Air Pollution and Traffic Noise Exposures and Mild Cognitive Impairment in Older Adults: A Cross-Sectional Analysis of the Heinz Nixdorf Recall Study. Environ Health Perspect 124, 1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lo YC, Lu YC, Chang YH, Kao S, Huang HB (2019) Air Pollution Exposure and Cognitive Function in Taiwanese Older Adults: A Repeated Measurement Study. Int J Environ Res Public Health 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim H, Noh J, Noh Y, Oh SS, Koh SB, Kim C (2019) Gender Difference in the Effects of Outdoor Air Pollution on Cognitive Function Among Elderly in Korea. Front Public Health 7, 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Delgado-Saborit JM, Guercio V, Gowers AM, Shaddick G, Fox NC, Love S (2021) A critical review of the epidemiological evidence of effects of air pollution on dementia, cognitive function and cognitive decline in adult population. Sci Total Environ 757, 143734. [DOI] [PubMed] [Google Scholar]
- [29].Semmens EO, Leary CS, Fitzpatrick AL, Ilango SD, Park C, Adam CE, DeKosky ST, Lopez O, Hajat A, Kaufman JD (2022) Air pollution and dementia in older adults in the Ginkgo Evaluation of Memory Study. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kulick ER, Wellenius GA, Boehme AK, Joyce NR, Schupf N, Kaufman JD, Mayeux R, Sacco RL, Manly JJ, Elkind MSV (2020) Long-term exposure to air pollution and trajectories of cognitive decline among older adults. Neurology 94, e1782–e1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Petkus AJ, Younan D, Widaman K, Gatz M, Manson JE, Wang X, Serre M, Vizuete W, Chui H, Espeland MA, Resnick S, Chen JC (2020) Exposure to fine particulate matter and temporal dynamics of episodic memory and depressive symptoms in older women. Environ Int 135, 105196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schikowski T, Vossoughi M, Vierkotter A, Schulte T, Teichert T, Sugiri D, Fehsel K, Tzivian L, Bae IS, Ranft U, Hoffmann B, Probst-Hensch N, Herder C, Kramer U, Luckhaus C (2015) Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women. Environ Res 142, 10–16. [DOI] [PubMed] [Google Scholar]
- [33].Shin J, Han SH, Choi J (2019) Exposure to Ambient Air Pollution and Cognitive Impairment in Community-Dwelling Older Adults: The Korean Frailty and Aging Cohort Study. Int J Environ Res Public Health 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wellenius GA, Boyle LD, Coull BA, Milberg WP, Gryparis A, Schwartz J, Mittleman MA, Lipsitz LA (2012) Residential proximity to nearest major roadway and cognitive function in community-dwelling seniors: results from the MOBILIZE Boston Study. J Am Geriatr Soc 60, 2075–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Salinas-Rodriguez A, Fernandez-Nino JA, Manrique-Espinoza B, Moreno-Banda GL, Sosa-Ortiz AL, Qian ZM, Lin H (2018) Exposure to ambient PM2.5 concentrations and cognitive function among older Mexican adults. Environ Int 117, 1–9. [DOI] [PubMed] [Google Scholar]
- [36].Ilango SD, Gonzalez K, Gallo L, Allison MA, Cai J, Isasi CR, Hosgood DH, Vasquez PM, Zeng D, Mortamais M, Gonzalez H, Benmarhnia T (2021) Long-Term Exposure to Ambient Air Pollution and Cognitive Function Among Hispanic/Latino Adults in San Diego, California. J Alzheimers Dis 79, 1489–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen JH, Kuo TY, Yu HL, Wu C, Yeh SL, Chiou JM, Chen TF, Chen YC (2020) Long-Term Exposure to Air Pollutants and Cognitive Function in Taiwanese Community-Dwelling Older Adults: A Four-Year Cohort Study. J Alzheimers Dis 78, 1585–1600. [DOI] [PubMed] [Google Scholar]
- [38].Crous-Bou M, Gascon M, Gispert JD, Cirach M, Sanchez-Benavides G, Falcon C, Arenaza-Urquijo EM, Gotsens X, Fauria K, Sunyer J, Nieuwenhuijsen MJ, Luis Molinuevo J, Study A (2020) Impact of urban environmental exposures on cognitive performance and brain structure of healthy individuals at risk for Alzheimer’s dementia. Environ Int 138, 105546. [DOI] [PubMed] [Google Scholar]
- [39].Gatto NM, Henderson VW, Hodis HN, St John JA, Lurmann F, Chen JC, Mack WJ (2014) Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology 40, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rocha D, Suemoto CK, Souza Santos I, Lotufo PA, Bensenor I, Gouveia N (2020) Vehicular traffic density and cognitive performance in the ELSA-Brasil study. Environ Res 191, 110208. [DOI] [PubMed] [Google Scholar]
- [41].Tonne C, Elbaz A, Beevers S, Singh-Manoux A (2014) Traffic-related air pollution in relation to cognitive function in older adults. Epidemiology 25, 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zare Sakhvidi MJ, Yang J, Lequy E, Chen J, de Hoogh K, Letellier N, Mortamais M, Ozguler A, Vienneau D, Zins M, Goldberg M, Berr C, Jacquemin B (2022) Outdoor air pollution exposure and cognitive performance: findings from the enrolment phase of the CONSTANCES cohort. Lancet Planet Health 6, e219–e229. [DOI] [PubMed] [Google Scholar]
- [43].Ailshire JA, Crimmins EM (2014) Fine particulate matter air pollution and cognitive function among older US adults. Am J Epidemiol 180, 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cullen B, Newby D, Lee D, Lyall DM, Nevado-Holgado AJ, Evans JJ, Pell JP, Lovestone S, Cavanagh J (2018) Cross-sectional and longitudinal analyses of outdoor air pollution exposure and cognitive function in UK Biobank. Sci Rep 8, 12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Buckner RL (2004) Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron 44, 195–208. [DOI] [PubMed] [Google Scholar]
- [46].Friedman NP, Miyake A (2017) Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex 86, 186–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Miyake A, Friedman NP (2012) The Nature and Organization of Individual Differences in Executive Functions: Four General Conclusions. Curr Dir Psychol Sci 21, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Junquera A, Garcia-Zamora E, Olazaran J, Parra MA, Fernandez-Guinea S (2020) Role of Executive Functions in the Conversion from Mild Cognitive Impairment to Dementia. J Alzheimers Dis 77, 641–653. [DOI] [PubMed] [Google Scholar]
- [49].Ailshire JA, Clarke P (2015) Fine particulate matter air pollution and cognitive function among U.S. older adults. J Gerontol B Psychol Sci Soc Sci 70, 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ranft U, Schikowski T, Sugiri D, Krutmann J, Kramer U (2009) Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res 109, 1004–1011. [DOI] [PubMed] [Google Scholar]
- [51].Chen JC, Schwartz J (2009) Neurobehavioral effects of ambient air pollution on cognitive performance in US adults. Neurotoxicology 30, 231–239. [DOI] [PubMed] [Google Scholar]
- [52].Finch CE (2009) The neurobiology of middle-age has arrived. Neurobiol Aging 30, 515–520; discussion 530–533. [DOI] [PubMed] [Google Scholar]
- [53].Finch CE, Haghani A (2021) Gene-Environment Interactions and Stochastic Variations in the Gero-Exposome. J Gerontol A Biol Sci Med Sci 76, 1740–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Reynolds CA, Gatz M, Christensen K, Christiansen L, Dahl Aslan AK, Kaprio J, Korhonen T, Kremen WS, Krueger R, McGue M, Neiderhiser JM, Pedersen NL, Interplay of G, Environment across Multiple Studies c (2016) Gene-Environment Interplay in Physical, Psychological, and Cognitive Domains in Mid to Late Adulthood: Is APOE a Variability Gene? Behav Genet 46, 4–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sachs-Ericsson N, Corsentino E, Collins N, Sawyer K, Blazer DG (2010) Problems meeting basic needs moderate the association between the APOE epsilon4 allele and cognitive decline. Aging Ment Health 14, 138–144. [DOI] [PubMed] [Google Scholar]
- [56].Sachs-Ericsson NJ, Sawyer KA, Corsentino EA, Collins NA, Blazer DG (2010) APOE epsilon4 allele carriers: Biological, psychological, and social variables associated with cognitive impairment. Aging Ment Health 14, 679–691. [DOI] [PubMed] [Google Scholar]
- [57].Garcia AR, Finch C, Gatz M, Kraft T, Eid Rodriguez D, Cummings D, Charifson M, Buetow K, Beheim BA, Allayee H, Thomas GS, Stieglitz J, Gurven MD, Kaplan H, Trumble BC (2021) APOE4 is associated with elevated blood lipids and lower levels of innate immune biomarkers in a tropical Amerindian subsistence population. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cacciottolo M, Wang X, Driscoll I, Woodward N, Saffari A, Reyes J, Serre ML, Vizuete W, Sioutas C, Morgan TE, Gatz M, Chui HC, Shumaker SA, Resnick SM, Espeland MA, Finch CE, Chen JC (2017) Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry 7, e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kulick ER, Elkind MSV, Boehme AK, Joyce NR, Schupf N, Kaufman JD, Mayeux R, Manly JJ, Wellenius GA (2021) Long-term exposure to ambient air pollution, APOE-epsilon4 status, and cognitive decline in a cohort of older adults in northern Manhattan. Environ Int 136, 105440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Oudin A, Andersson J, Sundstrom A, Nordin Adolfsson A, Oudin Astrom D, Adolfsson R, Forsberg B, Nordin M (2019) Traffic-Related Air Pollution as a Risk Factor for Dementia: No Clear Modifying Effects of APOEvarepsilon4 in the Betula Cohort. J Alzheimers Dis 71, 733–740. [DOI] [PubMed] [Google Scholar]
- [61].Younan D, Wang X, Millstein J, Petkus AJ, Beavers DP, Espeland MA, Chui HC, Resnick SM, Gatz M, Kaufman JD, Wellenius GA, Whitsel EA, Manson JE, Rapp SR, Chen JC (2022) Air quality improvement and cognitive decline in community-dwelling older women in the United States: A longitudinal cohort study. PLoS Med 19, e1003893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cacciottolo M, Morgan TE, Finch CE (2021) Age, sex, and cerebral microbleeds in EFAD Alzheimer disease mice. Neurobiol Aging 103, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cacciottolo M, Morgan TE, Saffari AA, Shirmohammadi F, Forman HJ, Sioutas C, Finch CE (2020) Traffic-related air pollutants (TRAP-PM) promote neuronal amyloidogenesis through oxidative damage to lipid rafts. Free Radic Biol Med 147, 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Haghani A, Johnson RG, Woodward NC, Feinberg JI, Lewis K, Ladd-Acosta C, Safi N, Jaffe AE, Sioutas C, Allayee H, Campbell DB, Volk HE, Finch CE, Morgan TE (2020) Adult mouse hippocampal transcriptome changes associated with long-term behavioral and metabolic effects of gestational air pollution toxicity. Transl Psychiatry 10, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Haghani A, Thorwald M, Morgan TE, Finch CE (2021) The APOE gene cluster responds to air pollution factors in mice with coordinated expression of genes that differs by age in humans. Alzheimers Dement 17, 175–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Haghani A, Johnson R, Safi N, Zhang H, Thorwald M, Mousavi A, Woodward NC, Shirmohammadi F, Coussa V, Wise JP Jr., Forman HJ, Sioutas C, Allayee H, Morgan TE, Finch CE (2020) Toxicity of urban air pollution particulate matter in developing and adult mouse brain: Comparison of total and filter-eluted nanoparticles. Environ Int 136, 105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Shkirkova K, Lamorie-Foote K, Connor M, Patel A, Barisano G, Baertsch H, Liu Q, Morgan TE, Sioutas C, Mack WJ (2020) Effects of ambient particulate matter on vascular tissue: a review. J Toxicol Environ Health B Crit Rev 23, 319–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Caselli RJ, Dueck AC, Locke DE, Hoffman-Snyder CR, Woodruff BK, Rapcsak SZ, Reiman EM (2011) Longitudinal modeling of frontal cognition in APOE epsilon4 homozygotes, heterozygotes, and noncarriers. Neurology 76, 1383–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Baxter LC, Rapcsak SZ, Shi J, Woodruff BK, Locke DE, Snyder CH, Alexander GE, Rademakers R, Reiman EM (2009) Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med 361, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, Alexander GG (2004) Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology 62, 1990–1995. [DOI] [PubMed] [Google Scholar]
- [71].Martens YA, Zhao N, Liu CC, Kanekiyo T, Yang AJ, Goate AM, Holtzman DM, Bu G (2022) ApoE Cascade Hypothesis in the pathogenesis of Alzheimer’s disease and related dementias. Neuron 110, 1304–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Reas ET, Laughlin GA, Bergstrom J, Kritz-Silverstein D, Barrett-Connor E, McEvoy LK (2019) Effects of APOE on cognitive aging in community-dwelling older adults. Neuropsychology 33, 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, Berry-Kravis E, Bach J, Fox JH, Evans DA, Bennett DA (2002) The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6-year period. Arch Neurol 59, 1154–1160. [DOI] [PubMed] [Google Scholar]
- [74].Taylor WD, Boyd B, Turner R, McQuoid DR, Ashley-Koch A, MacFall JR, Saleh A, Potter GG (2017) APOE epsilon4 associated with preserved executive function performance and maintenance of temporal and cingulate brain volumes in younger adults. Brain Imaging Behav 11, 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Cacciaglia R, Molinuevo JL, Falcon C, Sanchez-Benavides G, Gramunt N, Brugulat-Serrat A, Esteller M, Moran S, Fauria K, Gispert JD, study A (2019) APOE-epsilon4 risk variant for Alzheimer’s disease modifies the association between cognitive performance and cerebral morphology in healthy middle-aged individuals. Neuroimage Clin 23, 101818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chang L, Douet V, Bloss C, Lee K, Pritchett A, Jernigan TL, Akshoomoff N, Murray SS, Frazier J, Kennedy DN, Amaral DG, Gruen J, Kaufmann WE, Casey BJ, Sowell E, Ernst T, Pediatric Imaging N, Genetics Study C (2016) Gray matter maturation and cognition in children with different APOE epsilon genotypes. Neurology 87, 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gustavson DE, Reynolds CA, Hohman TJ, Jefferson AL, Elman JA, Panizzon MS, Neale MC, Logue MW, Lyons MJ, Franz CE, Kremen WS (2022) Alzheimer’s Disease Polygenic Scores Predict Changes in Episodic Memory and Executive Function Across 12 Years in Late Middle Age. J Int Neuropsychol Soc, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kremen WS, Franz CE, Lyons MJ (2019) Current Status of the Vietnam Era Twin Study of Aging (VETSA). Twin Res Hum Genet 22, 783–787. [DOI] [PubMed] [Google Scholar]
- [79].Eisen SA, Ture WR, Goldberg J, Henderson W, Robinette CD (1987) The Vietnam Era Twin (VET) Registry: Method of construction. Acta Geneticae Medicae et Gemellologiae 36, 61–66. [DOI] [PubMed] [Google Scholar]
- [80].Henderson WG, Eisen SE, Goldberg J, True WR, Barnes JE, Vitek M (1990) The Vietnam Era Twin Registry: A resource for medical research. Public Health Reports 105, 368–373. [PMC free article] [PubMed] [Google Scholar]
- [81].Tsuang MT, Bar JL, Harley RM, Lyons MJ (2001) The Harvard Twin Study of Substance Abuse: What we have learned. Harvard Review of Psychiatry 9, 267–279. [PubMed] [Google Scholar]
- [82].Kremen WS, Thompson-Brenner H, Leung YM, Grant MD, Franz CE, Eisen SA, Jacobson KC, Boake C, Lyons MJ (2006) Genes, environment, and time: the Vietnam Era Twin Study of Aging (VETSA). Twin Res Hum Genet 9, 1009–1022. [DOI] [PubMed] [Google Scholar]
- [83].Schoeneborn CA, Heyman KM (2009) Health characteristics of adults aged 55 years and over: United States, 2004–2007. In National Health Statistics Reports National Center for Health Statistics, Hyattsville, MD. [PubMed] [Google Scholar]
- [84].Gustavson DE, Elman JA, Sanderson-Cimino M, Franz CE, Panizzon MS, Jak AJ, Reynolds CA, Neale MC, Lyons MJ, Kremen WS (2020) Extensive memory testing improves prediction of progression to MCI in late middle age. Alzheimers Dement (Amst) 12, e12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Gustavson DE, Panizzon MS, Elman JA, Franz CE, Beck A, Reynolds CA, Jacobson KC, Xian H, Toomey R, Lyons MJ, Kremen WS (2018) Genetic and Environmental Influences on Verbal Fluency in Middle Age: A Longitudinal Twin Study. Behav Genet 48, 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Gustavson DE, Panizzon MS, Franz CE, Friedman NP, Reynolds CA, Jacobson KC, Xian H, Lyons MJ, Kremen WS (2018) Genetic and environmental architecture of executive functions in midlife. Neuropsychology 32, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gustavson DE, Panizzon MS, Kremen WS, Reynolds CA, Pahlen S, Nygaard M, Wod M, Catts VS, Lee T, Gatz M, Franz CE, Consortium I (2021) Genetic and Environmental Influences on Semantic Verbal Fluency Across Midlife and Later Life. Behav Genet 51, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sanderson-Cimino M, Panizzon MS, Elman JA, Gustavson DE, Franz CE, Reynolds CA, Toomey R, Lyons MJ, Kremen WS (2019) Genetic and environmental architecture of processing speed across midlife. Neuropsychology 33, 862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Elman JA, Jak AJ, Panizzon MS, Tu XM, Chen T, Reynolds CA, Gustavson DE, Franz CE, Hatton SN, Jacobson KC, Toomey R, McKenzie R, Xian H, Lyons MJ, Kremen WS (2018) Underdiagnosis of mild cognitive impairment: A consequence of ignoring practice effects. Alzheimers Dement (Amst) 10, 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Delis DC, Kramer JH, Kaplan E, Ober BA (2000) California Verbal Learning Test (CVLT-2), Psychological Corporation, San Antonio, TX. [Google Scholar]
- [91].Wechsler D (1997) Wechsler Memory Scale (WMS-III), Psychological Corporation, San Antonio, TX. [Google Scholar]
- [92].Golden CJ (2003) Stroop Color and Word Test, Multi-Health Systems. [Google Scholar]
- [93].Golden C, Freshwater S (2002), Wood Dale, IL. [Google Scholar]
- [94].Delis DC, Kaplan E, Kramer JH (2001) Delis-Kaplan Executive Function System (D-KEFS), Psychological Corporation, San Antonio, TX. [Google Scholar]
- [95].Daneman M, Carpenter PA (1980) Individuals differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior 19, 450–466. [Google Scholar]
- [96].Gustavson DE, Panizzon MS, Elman JA, Franz CE, Reynolds CA, Jacobson KC, Friedman NP, Xian H, Toomey R, Lyons MJ, Kremen WS (2018) Stability of genetic and environmental influences on executive functions in midlife. Psychol Aging 33, 219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Teng E (1990) The 3RT Test: Three reaction time tasks for IBM PC computers. Behavior research methods 22, 389–392. [Google Scholar]
- [98].Kirwa K, Szpiro AA, Sheppard L, Sampson PD, Wang M, Keller JP, Young MT, Kim SY, Larson TV, Kaufman JD (2021) Fine-Scale Air Pollution Models for Epidemiologic Research: Insights From Approaches Developed in the Multi-ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Curr Environ Health Rep 8, 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Lyons MJ, Genderson M, Grant MD, Logue M, Zink T, McKenzie R, Franz CE, Panizzon M, Lohr JB, Jerskey B, Kremen WS (2013) Gene-environment interaction of ApoE genotype and combat exposure on PTSD. Am J Med Genet B Neuropsychiatr Genet 162B, 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kremen WS, Seidman LJ, Faraone SV, Tsuang MT (2008) IQ decline in cross-sectional studies of schizophrenia: methodology and interpretation. Psychiatry Res 158, 181–194. [DOI] [PubMed] [Google Scholar]
- [101].Radloff LS (1977) The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1, 385–401. [Google Scholar]
- [102].Tang W, Yu Q, Crits-Cristoph P, Tu XM (2009) A new analytic framework for moderation analysis --- Moving beyond analytic interactions. Journal of Data Science 7, 313–329. [PMC free article] [PubMed] [Google Scholar]
- [103].Slayday RE, Gustavson DE, Elman JA, Beck A, McEvoy LK, Tu XM, Fang B, Hauger RL, Lyons MJ, McKenzie RE, Sanderson-Cimino ME, Xian H, Kremen WS, Franz CE (2021) Interaction between Alcohol Consumption and Apolipoprotein E (ApoE) Genotype with Cognition in Middle-Aged Men. J Int Neuropsychol Soc 27, 56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Reynolds CA, Smolen A, Corley RP, Munoz E, Friedman NP, Rhee SH, Stallings MC, DeFries JC, Wadsworth SJ (2019) APOE effects on cognition from childhood to adolescence. Neurobiol Aging 84, 239 e231–239 e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Licher S, Ahmad S, Karamujic-Comic H, Voortman T, Leening MJG, Ikram MA, Ikram MK (2019) Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nat Med 25, 1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Finch CE, Kulminski AM (2019) The Alzheimer’s Disease Exposome. Alzheimers Dement 15, 1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Moulton PV, Yang W (2012) Air pollution, oxidative stress, and Alzheimer’s disease. J Environ Public Health 2012, 472751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Petkus AJ, Resnick SM, Wang X, Beavers DP, Espeland MA, Gatz M, Gruenewald T, Millstein J, Chui HC, Kaufman JD, Manson JE, Wellenius GA, Whitsel EA, Widaman K, Younan D, Chen JC (2022) Ambient air pollution exposure and increasing depressive symptoms in older women: The mediating role of the prefrontal cortex and insula. Sci Total Environ 823, 153642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Petkus AJ, Wang X, Beavers DP, Chui HC, Espeland MA, Gatz M, Gruenewald T, Kaufman JD, Manson JE, Resnick SM, Stewart JD, Wellenius GA, Whitsel EA, Widaman K, Younan D, Chen JC (2021) Outdoor air pollution exposure and inter-relation of global cognitive performance and emotional distress in older women. Environ Pollut 271, 116282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kulminski AM, Loiko E, Loika Y, Culminskaya I (2022) Pleiotropic predisposition to Alzheimer’s disease and educational attainment: insights from the summary statistics analysis. Geroscience 44, 265–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Nazarian A, Loika Y, He L, Culminskaya I, Kulminski AM (2022) Genome-wide analysis identified abundant genetic modulators of contributions of the apolipoprotein E alleles to Alzheimer’s disease risk. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available on request from the corresponding author. The data are not available on a public website due to privacy or ethical restrictions established by the Vietnam Era Twin Registry (VETR). A data request form is available on the VETSA website (https://psychiatry.ucsd.edu/research/programs-centers/vetsa/index.html). Data requests can also be made at the VETR; the access process is described at: https://www.seattle.eric.research.va.gov/VETR/Investigator_Access.asp.


