Highlights
-
•
According to the latest ICTV 2023 publication, oropouche virus (OROV) is a member of bunyavirales order, peribunyaviridae family, orthobunyavirus geneus, the orthobunyavirus oropoucheense species.
-
•
The history of the epidemiological development of fever suggests that, given the global nature of environmental and climate change and the widespread migration of animals and humans, OROV is likely to expand beyond south america in the near future.
-
•
The proteins encoded by the different segments enables OROV to replicate efficiently in the host and to resist the host's immune response.
-
•
Phylogenetic analyses showed that OROV sequences are geographically distinct and have closer homologies with iquitos virus and perdoes virus.
-
•
Recent studies have utilised immunoinformatics approaches to develop epitope-based peptide vaccines against OROV.
Keywords: Oropouche virus, Epidemiology, Structure, Evolutionary, Immunity, Vaccine development
Abstract
The Oropouche virus is an important arthropod-borne virus in the Peribunyaviridae family that can cause febrile illnesses, and it is widely distributed in tropical regions such as Central and South America. Since the virus was first identified, a large number of related cases are reported every year. No deaths have been reported to date, however, the virus can cause systemic infections, including the nervous and blood systems, leading to serious complications. The transmission of Oropouche virus occurs through both urban and sylvatic cycles, with the anthropophilic biting midge Culicoides paraensis serving as the primary vector in urban areas. Direct human-to-human transmission of Oropouche virus has not been observed. Oropouche virus consists of three segments, and the proteins encoded by the different segments enables the virus to replicate efficiently in the host and to resist the host's immune response. Phylogenetic analyses showed that Oropouche virus sequences are geographically distinct and have closer homologies with Iquitos virus and Perdoes virus, which belong to the family Peribunyaviridae. Despite the enormous threat it poses to public health, there are currently no licensed vaccines or specific antiviral treatments for the disease it causes. Recent studies have utilised imJatobal virusmunoinformatics approaches to develop epitope-based peptide vaccines, which have laid the groundwork for the clinical use of vaccines. The present review focuses on the structure, epidemiology, immunity and phylogeny of Oropouche virus, as well as the progress of vaccine development, thereby attracting wider attention and research, particularly with regard to potential vaccine programs.
1. Introduction
Oropouche virus (OROV) is a member of the Bunyavirales order, Peribunyaviridae family, Orthobunyavirus geneus, the Orthobunyavirus oropoucheense species, characterized by a negative-sense, single-stranded RNA structure and spherical lipid envelope genome (Files et al., 2022; Valero. 2017; Kuhn et al., 2023). Comprising three single-stranded RNA segments, OROV is enveloped by a helical nucleocapsid that encodes essential components such as the virus RNA-dependent RNA polymerase (RdRp), viral surface glycoproteins (Gn and Gc), and nucleocapsid protein (N). OROV is classified within the Orthobunyavirus genus, which encompasses 129 viruses were assigned as the type species, and nine were classified as related unclassified. Specifically, OROV is categorized under the Simbu serogroup, which comprises 25 viruses divided into two phylogenetic subclades—Manzanilla and Oropouche (subclade A), and Simbu, Akabane, Sathuperi, Shamonda, and Shuni (subclade B) (Cardoso et al., 2015; Travassos da Rosa et al. 2017).
The virus was first identified in Trinidad and Tobago during an outbreak of febrile illness. Since then, the virus has been detected epidemics have occurred in several countries in South and Central America, including Brazil, Peru, and Panama (Gutierrez et al., 2020; Bonifay et al., 2023; Anderson et al., 1961). Up to now, more than 500,000 cases have been diagnosed, the actual number of cases is higher, as numerous instances may evade diagnosis or be misdiagnosed owing to the resemblance of clinical manifestations to other febrile diseases caused by co-circulating arboviruses (such as dengue, West Nile, yellow fever, Zika, chikungunya, Guama) (Culquichicón et al., 2017; Acrani et al., 2015). The high rate of underreporting of OROV has caused people to overlook the dangers, creating a huge potential for subsequent outbreaks and epidemics. The virus is transmitted to humans through the bite of infected Culicoides paraensis midges, which is the medium of transmission between people that found in forested areas and around water bodies (Romero-Alvarez et al. 2018; Feitoza et al., 2023). Once infected with OROV, people will experience periodic outbreaks of acute febrile illness, characterized by symptoms such as fever, headache, muscle pain, and joint pain (Vernal et al., 2019; Parra Barrera et al. 2023). An interesting fact is that symptoms can reappear one or two weeks after recovery in about 60 % of the patients. Additionally, there are reports of patients that showed hemorrhagic symptoms or neurological complications associated with OROV detection (Bastos Mde et al., 2012; Mourãão et al., 2009). There is currently no specific treatment or vaccine available for OROV, and the best approach is to manage the symptoms and provide supportive care (Sakkas et al., 2018). In recent years, there has been increasing interest in the development of a vaccine for OROV. Several research groups have been working on developing candidate vaccines using different approaches, including inactivated virus, live attenuated virus, and recombinant protein-based vaccines (Files et al., 2022). These vaccines have shown promising results in preclinical studies, and some have even advanced to clinical trials.
The potential of OROV for geographic spread, increasing the likelihood of its emergence in new areas, underscores its importance on an international public health scale. OROV will attract a lot of attention in the future due to frequent outbreaks of dengue-like symptoms such as fever and headache in the Americas (Sessions et al., 2023). This study provides a detailed interpretation of OROV from the perspectives of structural biology, epidemiology, and immunology, and offers an update on the current knowledge regarding the progress of OROV vaccine.
2. Epidemiology
The historical epidemiology of Oropouche fever underscores the impact of human-induced environmental and climatic changes on the spread and prevalence of hosts and vectors. OROV was first isolated a febrile patient in Trinidad, a forest worker (Anderson et al., 1961). During this incident, neutralizing antibodies were also detected in several other forest workers and in local monkey populations (Files et al., 2022). Five years later, OROV was isolated from mosquitoes in Trinidad and from a sloth and mosquito species in Brazil, both near a forested area during highway construction. The first recorded OROV fever outbreak occurred in Belém, a city in the most populous state of Para, known for its rainforests and the Amazon River, infecting approximately 11,000 people in1961 (Watts et al., 1997). During the same time period, seven outbreaks of OROV fever were reported in urban centers in Pará State, infecting around 30,000 people. From 1980 to 2005, sporadic cases or self-limited outbreaks were reported in the Brazilian Amazon regions, mainly in small villages, indicating potential silent circulation of the virus (Baisley et al., 1998). During the 2000s, OROV fever spread to other northern and northeastern Brazilian states and was first isolated from monkey species in southeastern Brazil, demonstrating OROV's potential to spread to new hosts and areas. In recent years, sporadic cases of OROV have been reported in non-endemic areas of Brazil. Interestingly, OROV was also detected in the Metropolitan region of Salvador, Bahia, located in the Northeast region of Brazil, specifically in serum, saliva, and urine samples from patients presenting with arbovirus infection symptoms. Furthermore, none of OROV-positive subjects in the report visited the documented OROV circulating areas in the month preceding symptom onset, providing evidence for the autochthonous circulation of the virus in non-endemic areas (Fonseca et al., 2020). In 2016, the first OROV fever case outside the Amazon region was reported, raising concerns about the occurrence of OROV fever out of the Amazon region. The broad distribution of the primary vector, coupled with anthropogenic environmental disturbances and extensive human and animal traveling, accentuate the potential for OROV to emerge in other territories (Rodrigues et al., 2011). The common features of the affected areas were the tropical climate, high frequency of rains, similar activities of the residents, unplanned urbanization, and very poor living standards. In 2020, a remote rainforest village in French Guiana witnessed an outbreak of a dengue-like syndrome. Of the 41 patients who developed symptoms, 23 (82.1 %) of the 28 tested were positive for OROV by PCR or microneutralization. The symptoms of this syndrome were similar to those of dengue fever, the patients presented with high fever, severe headache, pain behind the eyes, muscle and joint pain, rash, and mild bleeding. This finding was significant as it documented the emergence of OROV in French Guiana (Gaillet et al., 2021). During 2019–2022, investigators conducted health facility-based acute febrile illness (AFI) surveillance at four sites in Colombia (Cucuta, Cali, Villavicencio, Leticia). Phylogenetic analysis determined that at least two independent OROVs were introduced into Colombia (Ciuoderis et al., 2022). The associated results confirm OROV as a neglected pathogen and suggest increased surveillance to determine its burden as a cause of AFI in Colombia.
Compared to other arboviruses that prevailed in Central and South American countries, OROV is maintained in nature through two transmission cycles: the urban cycle and the sylvatic cycle. The urban cycle, known for causing sudden outbreaks of disease, is thought to mainly involve the biting midge Culicoides paraensis (Pinheiro et al., 1981). OROV, being an arbovirus, relies primarily on its midge vector (Culicoides paraensis) and certain mosquito vectors (Culex quinquefasciatus, Aedes aegypti, Ochlerotatus serratus) to maintain its urban cycle (Bonifay et al., 2023). The midge, a small fly with an average lifespan of 20 to 30 days, plays a crucial role. Notably, pathogen transmission is solely the responsibility of adult, female midges. This is because only female vectors require blood meals to support their egg production and maturation. Due to its historical association with numerous large-scale epidemics of the disease, this midge species is widely acknowledged as the primary vector responsible for OROV transmission. Moreover, Culicoides midges are a global public health concern because they serve as known vectors for several other arboviruses, such as Equine encephalitis and the Schmallenberg virus. Approximately 96 % of these midge species feed on blood from humans and wild mammals. This is significant because in the urban cycle, OROV can be transmitted to individuals susceptible to bites from infected vectors during blood meals. The Culex quinquefasciatus mosquito also plays a substantial role as an urban vector for OROV, particularly in tropical regions where it feeds on both humans and animals. To date, there have been no reported cases of human-to-human transmission of Oropouche fever. The main vertebrate hosts involved in the sylvatic cycle have not been fully identified, but there is evidence that wild birds, the three-toed sloth (Bradypus tridactylus), and certain species of New World non-human primates (NHP)-principally capuchin and howler monkeys-are involved (Tilston-Lunel et al., 2015; Nunes et al., 2005; Sciancalepore et al., 2022). The epidemiological history of OROV fever demonstrates the role of human-induced environmental and climatic changes on hosts’ and vectors’ spread and disease prevalence. Moreover, considering that environmental and climate changes, and extensive animal and human population migration are a global phenomenon, it will not be surprising for OROV to spread outside South America in the near future.
3. Viral genome and replication
OROV is an arbovirus belonging to the Peribunyaviridae family, Orthobunyavirus genus. Its structure, like that of other Peribunyaviridae family members, is characterized by a segmented, single-stranded RNA genome and several structural and non-structural proteins, each playing a crucial role in the virus's lifecycle (Nunes et al., 2019). OROV genome consists of three segments of single-stranded RNA, designated as Large (L), Medium (M), and Small (S). The L segment encodes the RdRp, a crucial enzyme for the replication of the viral genome. This polymerase synthesizes new copies of the viral genome in the cytoplasm of the infected cell (Aquino et al., 2003). The M segment, besides encoding the glycoproteins Gn and Gc, also encodes a non-structural protein, NSm. This protein is believed to play a role in the virus's assembly and budding process, although its exact function is not fully understood. The S segment encodes the nucleocapsid protein (N) and a non-structural protein (NSs) (Obijeski et al., 1976). The N protein forms the nucleocapsid structure by interacting with the viral RNA, encapsulating and protecting the viral genome segments. This protein is abundant in the viral particle and plays a significant role in viral replication and transcription, maintaining the integrity of the viral genome (Murillo et al., 2018). The NSs protein, while not incorporated into the viral particle, is instrumental in counteracting the host's antiviral response. This protein inhibits the host's interferon response, a crucial component of the innate immune system, facilitating viral replication (Elliott. 2014; Tilston-Lunel et al., 2015).
OROV is an enveloped, spherical virus, sized from 80 to 120 nm in diameter (Hellert et al., 2019). Its envelope is derived from the host cell membrane and is studded with spikes formed by two glycoproteins, Gn and Gc (Barbosa et al., 2023). These glycoproteins are integral to the structure and lifecycle of OROV, mediating the virus's entry, assembly, and release from host cells. OROV glycoproteins exhibit complex topology. They are type I transmembrane proteins, signifying that they possess a single pass transmembrane domain with their N-terminus on the exterior of the cell or virion and their C-terminus on the interior (Dias et al., 2022). The glycoproteins are synthesized as a precursor polyprotein that is co-translationally inserted into the endoplasmic reticulum (ER). This precursor is then cleaved by host proteases to produce the mature Gn and Gc proteins, which are transported to the Golgi apparatus where they undergo further modifications and are assembled into virions. The secretion of OROV glycoproteins is a critical phase in the virus life cycle and necessitates specific cellular components. The glycoproteins are transported from the ER to the Golgi apparatus in COPII-coated vesicles, a process mediated by the interaction of the glycoproteins with the COPII coat proteins Sec 24 and Sec 23. Additionally, the glycoproteins require the host protein ERGIC-53 for their transport from the ER to the Golgi. Once in the Golgi apparatus, the glycoproteins are packaged into virions and transported to the cell surface in vesicles for release (Aquinom et al., 2003; Barbosa et al., 2023).
OROV was isolated by inoculation in C6/36 and Vero cells, blindly transmitted for three generations, which showed significant cytopathic effects 5–7 days after inoculation in Vero cells (da Silva Ferreira et al. 2020; Moreli et al., 2001). Meanwhile, OROV can also infect a variety of other cells and cause apoptosis, such as liver cells, HeLa cells, astrocytes, but it does not infect astroglial cells, which are also neural cells. During the process of neural system infection, OROV infects and engulfs neurons through the infection of small glial cells, inducing neuronal apoptosis. Although astrocytes are also activated, they do not participate in the process of neuronal damage (Santos et al., 2012; Castro et al., 2022). Consequently, OROV also requires specific cellular components for its replication. The viral replication takes place in the cytoplasm of the infected cell, with the viral RdRp synthesizing new copies of the viral genome. The assembly and release of OROV are complex processes that are not wholly understood. It is believed that newly synthesized viral RNA and N protein interact in the cytoplasm to form nucleocapsids. These nucleocapsids then migrate to the plasma membrane, where they interact with the glycoproteins Gn and Gc, leading to the budding of new virus particles from the cell surface. The glycoproteins Gn and Gc are synthesized in the ER and undergo post-translational modifications in the Golgi apparatus before being transported to the plasma membrane, where they are incorporated into budding virions. OROV structure is further complicated by the presence of lipid rafts, microdomains in the host cell membrane enriched in cholesterol and sphingolipids (Files et al., 2022; Sakkas et al., 2018). These lipid rafts are believed to play a role in the assembly and budding of the virus, providing a platform for the concentration of viral proteins and the formation of new virus particles.
4. Reassortment and evolutionary analysis
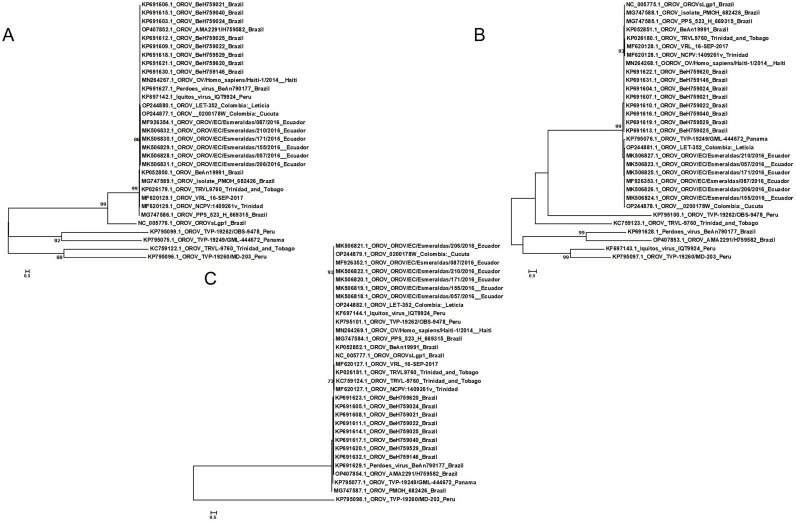
Sequences with all three segments complete and currently available worldwide were analyzed for homology and phylogenetic analysis (Table 1). For the 19 sequences, the L segment had the highest homology of 100 % and the lowest of 89.4 %, the M segment had between 82.9 % and 100 % homology, and the S segment 84.7 %−100 %. According to the results of evolutionary analysis, all OROV were divided into two groups (Fig. 1). Most of the sequences of strains from Brazil and Ecuador in three segments were grouped together, however, the strains from Trinidad and Tobago were more distantly related to the sequences of strains from other countries. In contrast, the sequence of the S segment from Trinidad and Tobago is closer to that of Brazil and Haiti. The strains from Peru exhibited different phylogenetic relationships in various segments, particularly in the S segment where two Peruvian strains showed closer kinship with strains from Brazil and Ecuador. However, the opposite was observed in the L and M segments. It is probable that the geographically differentiated distribution and genetic recombination of some segments result in reduced homology (Files et al., 2022; Gutierrez et al., 2020; Kilian et al., 2013).
Table 1.
Complete sequence characterisation of Oropouche viruses from different countries and regions.
| Virus | Host | Strain/code | Location | Isolation | Reference |
|---|---|---|---|---|---|
| OROV | Bradypus tridactylus | BeAn19991 | Brazil | Yes | Aquino, V.H., et al. (2004) |
| OROV | Homo sapiens | BeH759024BeH759021 | Brazil | Yes | Tilston-Lunel et al. (2015) |
| BeH759022BeH759025 | |||||
| BeH759040BeH759529 | |||||
| BeH759620BeH759146 | |||||
| OROV | Bradypus tridactylus Homo sapiens IFNAR-/- |
BeAn19991 TRVL-9760 TRVL9760 |
Brazil Trinidad and Tobago |
Yes | Acrani GO. et al. (2015) |
| OROV | Homo sapiens / |
OROV/EC/Esmeraldas/057/2016 155/2016 171/2016 206/2016 210/2016 087/2016 VRL 16-SEP-2017 |
Ecuador / |
Yes | Wise EL. et al. (2020); Emma L. et al. (2018) / |
| OROV | Homo sapiens | OV/Homo sapiens/Haiti-1/2014 | Haiti | Yes | Elbadry MA., et al. (2021) |
"/": the relevant content was not mentioned in the literature.
Fig. 1.
Phylogenetic tree of three segmentss of Oropouche Virus. A for L segments, B for M segments, C for S segments.
The segmented nature of OROV genome enhances the likelihood of genome reassortment events, a phenomenon that has been observed more generally in the bunyaviruses (Travassos da Rosa et al., 2017) and has been associated, in some cases, with a dramatic increase in disease severity, such as with Ngari virus, a reassortant between BUNV and Batai virus (Briese et al., 2006). Furthermore, reassortment appears to play an important role in the evolution and emergence of new viruses within OROV and related species, as has been described for the reassortant Perdoes virus (Tilston-Lunel et al., 2015) in Brazil, the Iquitos virus (IQTV) in Peru (Aguilar et al., 2011), and the Madre de Dios virus in Venezuela (Navarro et al., 2016). OROV genetic sequences remain scarce, with limited numbers. The existing genetic data exhibit a bias towards the shorter S segment, resulting in reduced phylogenetic resolution and an incomplete depiction of OROV evolution. The available sequences primarily represent viral diversity observed during human outbreaks, thus neglecting the dynamics of OROV in reservoir host species and vector populations. However, as sequencing technology advances swiftly, we will gain a comprehensive understanding of OROV propagation and genetic recombination.
5. Pathophysiology and clinical disease
After being bitten by an OROV-infected midge or mosquito, a 3–8-day incubation period precedes the onset of the disease (Sakkas et al., 2018; Pinheiro;Travassos da Rosa. 1981). The patient, from whom the virus was first isolated (Melajo Forest, Trinidad, 1955), reported symptoms such as fever, backache, and cough without a sore throat in an illness that lasted three days with no recurring symptoms. In subsequently documented cases, the most commonly reported symptom during acute disease is fever (∼39 °C), often accompanied by headache/retro-orbital pain, malaise, myalgia, arthralgia, nausea, vomiting, and photo-phobia. Less frequent symptoms encompass a rubella-like rash, meningitis, encephalitis, dizziness, anorexia, and other systemic manifestations. Hemorrhagic phenomena, such as epistaxis, gingival bleeding, and petechiae, or gastrointestinal manifestations such as diarrhea, are even more infrequent (Vasconcelos et al., 2009; Alvarez-Falconi et al., 2010).
During the acute disease, viremia peaks on day 2 after the onset of clinical symptoms and gradually decreases over the next several days31 (Cardoso et al., 2015). Elevated liver enzymes and leukopenia (values as low as 2000 leukocytes/mL) also manifest. In most individuals, the acute disease is relatively short, lasting from 2 to 7 days, but in some, particularly those with central nervous system involvement (meningitis and encephalitis), the disease can persist for 2–4 weeks and may include asthenia (loss of strength).
Recurrence is associated with a range of symptoms, including fever, headache, myalgia, asthenia, dizziness, and meningitis. The mechanisms responsible for the recurring disease remain undefined. Once the patient eventually recovers, there are no reported long-term sequelae or accounts of additional recurrence events at a later date, and, although the disease can be severe, no cases of human fatality have been reported. Currently, it is unclear whether certain OROV genotypes are more likely to produce more severe or unusual symptoms (Tesh, 1994).
6. Infection and immunity
OROV can lead to systemic infection and an inflammatory response in the central nervous system (CNS). The virus is highly detectable in blood from the onset of infection and can progressively reach the neural routes (Chiang et al., 2021; Santos et al., 2014). The disease is systemic and can affect the CNS in severe cases, with the virus being detected in cerebrospinal fluid. Research is primarily focused on understanding the mechanism by which OROV infects the CNS. Experimental animals such as mice and hamsters have been used to study the infection (Ribeiro Amorim et al., 2020; Almeida et al., 2021). In some studies, animals inoculated with OROV developed systemic infection with neurological motor impairment and paralysis, suggesting hematogenous transmission to the brain and liver (da Silva Menegatto et al., 2023).
The virus is thought to penetrate the blood-brain barrier (BBB) through a Trojan-horse mechanism, where the pathogen is carried through the bloodstream inside infected phagocytes, thereby going unrecognized to the target organs/tissues. Once inside, it can replicate, eluding any immune response and crossing any barriers such as the BBB. However, a neural route of brain invasion may also be involved due to the observed viral accumulation within neurons (Bastos Mde et al., 2012). Experimental infections in mice have demonstrated severe manifestations of encephalitis related to the extended spread of OROV through the brain parenchyma (Santos et al., 2014). Despite the severe CNS disease, histopathology was mild in the brain and spinal cord with little inflammation, indicating that replication in neurons may occur with relatively little functional impairment. The hepatotropic nature of OROV is described in several studies, though there are no reports on hepatitis manifestations in OROV fever patients (Almeida et al., 2021; Bastos et al., 2014). Spleen hyperplasia with the absence of OROV recovery or antigen detection in the spleen has also been reported.
The host immune response to OROV infection is a crucial aspect in controlling and resolving the infection. The virus's replication in host cells leads to the production of viral proteins that are recognized by the host's immune system, triggering an immune response. The immune response to OROV infection involves both the innate and adaptive immune systems. The innate immune response serves as the first line of defense against OROV infection. Studies have shown that the innate immune response, characterized by the production of interferon and pro-inflammatory cytokines, is activated early in OROV infection (de Oliveira et al., 2019). The containment of viral replication relies heavily on the innate immune response to viral infections. At the onset of a viral infection, cellular intrinsic components, otherwise known as pattern recognition receptors (PRRs), identify pathogen-associated molecular patterns (PAMPs). This identification initiates signaling cascades that result in the production of several cytokines, with interferons (IFNs) being particularly crucial for the containment of viral replication. OROV negative genome, a significant PAMP, can be detected by either Toll-like receptors (TLR) located at the endosome membrane or RIG-like receptors within the cytoplasm (Elliott, 2014; Haller et al., 2006). Antigen-presenting cells (APCs), which are highly expressed in the blood, play a vital role in recognizing PAMPs and triggering the IFN response via interferon-stimulated genes (ISGs). APCs also contribute significantly to the evolution of adaptive immunity (Abbas et al., 2005; Mellman, 2013). In a recent study, it is revealed that certain microRNAs, specifically miR-217 and miR-576–3p, act as proviral factors during Oropouche infection. MicroRNAs are small non-coding RNA molecules that function in RNA silencing and post-transcriptional regulation of gene expression. The study indicates that these specific microRNAs promote OROV infection, suggesting they might be manipulated by the virus to enhance its replication and survival. The proviral role of these microRNAs could be a strategy used by OROV to evade the host immune response, further complicating the immune response to the virus. Despite the host's immune response, OROV has evolved various strategies to evade the immune system. For instance, the virus can modulate the host's immune response by interfering with cytokine production or signaling, or by inhibiting the function of immune cells. The virus can also hide inside cells or form immune complexes with antibodies to evade the immune response. In addition to the immune system's role, cellular host factors also play a vital role in OROV infection. A study revealed that the cellular host factor Lrp1 mediates OROV infection. Lrp1, or low-density lipoprotein receptor-related protein 1, is a multifunctional receptor involved in endocytosis and signal transduction. The study demonstrated that Lrp1 is essential for OROV entry into host cells, providing another potential target for therapeutic intervention (Schwarz et al., 2022).
7. Vaccine development
The recent outbreak of OROV around the world and the virus's evolutionary dynamics in South America have underscored the urgent need for effective vaccines against OROV. The first preclinical study on OROV vaccines was based on an immunoinformatics analysis that identified several potential T and B cell epitopes within OROV M-segment polyprotein (Barbosa et al., 2023). These epitopes can stimulate an immune response against the virus, offering a promising strategy for vaccine development.
Several strategies for vaccine development are being considered, including live attenuated, chemically inactivated, DNA-vectored, and protein-subunit immunization strategies (Ayers et al., 2022). One of the most promising candidates is a live attenuated vaccine that has shown efficacy in animal models. This vaccine is based on the attenuated OROV strain BeAn19991, which has been shown to provide protection against multiple strains of the virus. The vaccine was shown to be safe and immunogenic in a phase I clinical trial conducted in healthy volunteers. The vaccine was well-tolerated, and no serious adverse events were reported. The study also showed that the vaccine induced a robust immune response, with high levels of neutralizing antibodies detected in all vaccinated individuals. Furthermore, a recent study also explored the use of a replication-competent vesicular stomatitis virus (VSV) expressing OROV glycoproteins as a potential vaccine candidate (Stubbs et al., 2021). This candidate vaccine was shown to protect mice from wild-type challenge, demonstrating its potential for human use. Meanwhile, the development of a reverse genetics system for OROV is expected to significantly benefit vaccine development. This system allows for the manipulation of the virus's genetic material, enabling the design of vaccines that can elicit a strong and specific immune response against OROV. Several strategies for attenuation have been explored, including the deletion of nonstructural proteins or specific regions within the untranslated regions (UTRs), interchanging UTRs between segments, exchanging coding regions of the M segment with related Orthobunyaviruses, and introducing mutations in the N (Adhikari et al., 2018). These approaches are designed to decrease the virulence of the virus while preserving its capacity to elicit an immune response.
The development of OROV vaccines may also be informed by attempts to develop vaccines against other members of the Simbu serogroup, such as Schmallenberg virus (SBV) (Afonso et al., 2014), Aino virus (Yeh et al., 2021), and Akabane virus (AKAV) (Ogawa et al., 2022). These viruses are important veterinary pathogens, and vaccines against them have been developed and approved for use in the European Union. These vaccines have been shown to reduce or prevent viremia in cattle and sheep, demonstrating their effectiveness (Bridgen et al., 2001; Kraatz et al., 2015). A candidate vaccine for SBV-AKAV, which is a bivalent protein-subunit comprising the N-termini of the Gc proteins from both SBV and AKAV, has been developed. This vaccine, expressed in HEK-293T cells and covalently linked, was administered subcutaneously in two doses of 50 μg each, three weeks apart. It demonstrated protection against a cattle-passaged field strain of SBV, with animals showing no clinical symptoms or detectable viral RNA in blood or organs, and neutralizing antibody titers of at least 20 at the time of challenge (Wernike et al., 2017). Additionally, two DNA-vectored vaccine candidates, encoding either N or the ectodomain of Gc, were shown to be protective in IFNAR−/− mice, preventing weight loss and reducing viremia compared to mock-vaccinated controls (Puntasecca et al., 2021). Although neither vaccine candidate stimulated detectable neutralizing antibody, the N vaccine triggered high levels of SBV-specific binding antibody, and the Gc vaccine led to the proliferation of SBV-specific CD8+ T cells.
The development of a vaccine for OROV is a challenging task due to the genetic diversity of the virus and the need for a vaccine that provides broad protection against multiple strains. Currently, there are no licensed vaccines for OROV, but several vaccines are in development or have been tested in clinical trials, which will provide a promising theoretical basis for the prevention of Oropouche fever.
8. Conclusion
OROV is currently a major public health problem with geographical limitations, causing persistent endemicity and periodic outbreaks in much of South America. It has been a neglected disease due to its similarity in onset to arboviral diseases such as dengue fever. Therefore, information on the distribution, prevalence and incidence of the disease in humans, animals and vector hosts is not well documented. However, its widespread prevalence and severe neurological complications have attracted extensive attention from researchers in recent years. Similar to other vector-borne diseases, the emergence and prevalence of OROV fever is the result of an ecological imbalance worldwide. This imbalance was largely a result of the rapid and massive movement of people and goods around the world, as well as global warming. Considering that environmental, climatic, and demographic changes are a global phenomenon, it is not surprising that OROV will spread beyond the Americas in the near future. Due to the expansion of the epidemic range and the segmented nature of the genome, the likelihood of OROV genome reassortment events has significantly increased. This phenomenon is more commonly observed in Bunyaviruses and is associated with a rapid increase in disease severity in certain cases. Furthermore, reassortment appears to play an important role in the evolution and emergence of new viruses within OROV and related species, as has been described for the reassortant Perdoes virus in Brazil, the Iquitos virus (IQTV) in Peru, and the Madre de Dios virus in Venezuela.
In conclusion, given the genetic diversity of the virus and the requirement for a vaccine that offers extensive protection against various strains, formulating a vaccine for OROV presents a significant challenge. Nevertheless, the use of immunoinformatics approaches, VSV chimeras, and reverse genetics systems offers promising strategies for vaccine development. Of course, further research is needed to optimize these vaccines and evaluate their efficacy and safety in human clinical trials. The development of an OROV vaccine will both have a significant impact on public health, especially in areas where the virus is endemic, and will facilitate the related development of vaccines for other viruses, laying a deep foundation for additional vaccine research for arboviruses.
Funding
This research was supported by the National Natural Science Foundation of China (82104053), Natural Science Foundation of Shandong Province (ZR2019BH035), the Science and Technology Development Program in Weifang (2020GX014).
CRediT authorship contribution statement
Yuli Zhang: Methodology, Validation, Formal analysis. Xiao Liu: Investigation, Methodology, Validation. Zhen Wu: Methodology, Formal analysis. Shuo Feng: Investigation, Conceptualization, Formal analysis. Ke Lu: Investigation, Conceptualization, Formal analysis. Wenbing Zhu: Methodology, Validation, Formal analysis. Hengyi Sun: Formal analysis, Writing – original draft, Funding acquisition. Guoyu Niu: Investigation, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing, Resources, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Figures were created with MEGA.
Contributor Information
Hengyi Sun, Email: Sunhengyi1987@wfmc.edu.cn.
Guoyu Niu, Email: niugy@wfmc.edu.cn.
Data availability
No data was used for the research described in the article.
References
- Abbas A.K., Sharpe A.H. Dendritic cells giveth and taketh away. Nat. Immunol. 2005;6:227–228. doi: 10.1038/ni0305-227. [DOI] [PubMed] [Google Scholar]
- Acrani G.O., Tilston-Lunel N.L., Spiegel M., Weidmann M., Dilcher M., Andrade da Silva D.E., Nunes M.R.T., Elliott R.M. Establishment of a minigenome system for Oropouche virus reveals the S genome segment to be significantly longer than reported previously. J. Gen. Virol. 2015;96:513–523. doi: 10.1099/jgv.0.000005. [DOI] [PubMed] [Google Scholar]
- Adhikari U.K., Tayebi M., Rahman M.M. Immunoinformatics approach for epitope-based peptide vaccine design and active site prediction against polyprotein of emerging oropouche virus. J. Immunol. Res. 2018;2018 doi: 10.1155/2018/6718083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso A., Conraths F. Schmallenberg virus. Prev. Vet. Med. 2014;116:337–338. doi: 10.1016/j.prevetmed.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Aguilar P.V., Barrett A.D., Saeed M.F., Watts D.M., Russell K., Guevara C., Ampuero J.S., Suarez L., Cespedes M., Montgomery J.M., Halsey E.S., Kochel T.J. Iquitos virus: a novel reassortant orthobunyavirus associated with human illness in Peru. PLoS Negl. Trop. Dis. 2011;5:e1315. doi: 10.1371/journal.pntd.0001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida G.M., Souza J.P., Mendes N.D., Pontelli M.C., Pinheiro N.R., Nogueira G.O., Cardoso R.S., Paiva I.M., Ferrari G.D., Veras F.P., Cunha F.Q., Horta-Junior J.A.C., Alberici L.C., Cunha T.M., Podolsky-Gondim G.G., Neder L., Arruda E., Sebollela A. Neural infection by Oropouche virus in adult human brain slices induces an inflammatory and toxic response. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.674576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Falconi P.P., Ríos Ruiz B.A. [Oropuche fever outbreak in Bagazan, San Martin, Peru: epidemiological evaluation, gastrointestinal and hemorrhagic manifestations] Rev. Gastroenterol. Peru. 2010;30:334–340. [PubMed] [Google Scholar]
- Anderson C.R., Spence L., Downs W.G., Aitken T.H. Oropouche virus: a new human disease agent from Trinidad, West Indies. Am. J. Trop. Med. Hyg. 1961;10:574–578. doi: 10.4269/ajtmh.1961.10.574. [DOI] [PubMed] [Google Scholar]
- Aquino V.H., Moreli M.L., Moraes Figueiredo L.T. Analysis of oropouche virus L protein amino acid sequence showed the presence of an additional conserved region that could harbour an important role for the polymerase activity. Arch. Virol. 2003;148:19–28. doi: 10.1007/s00705-002-0913-4. [DOI] [PubMed] [Google Scholar]
- Ayers V.B., Huang Y.S., Dunlop J.I., Kohl A., Brennan B., Higgs S., Vanlandingham D.L. Replication kinetics of a candidate live-attenuated vaccine for Cache Valley virus in Aedes albopictus. Vector Borne Zoonotic. Dis. 2022;22:553–558. doi: 10.1089/vbz.2022.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baisley K.J., Watts D.M., Munstermann L.E., Wilson M.L. Epidemiology of endemic Oropouche virus transmission in upper Amazonian Peru. Am. J. Trop. Med. Hyg. 1998;59:710–716. doi: 10.4269/ajtmh.1998.59.710. [DOI] [PubMed] [Google Scholar]
- Barbosa N.S., Concha J.O., daSilva L.L.P., Crump C.M., Graham S.C. Oropouche virus glycoprotein topology and cellular requirements for glycoprotein secretion. J. Virol. 2023;97 doi: 10.1128/jvi.01331-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos M.S., Lessa N., Naveca F.G., Monte R.L., Braga W.S., Figueiredo L.T., Ramasawmy R., Mourão M.P. Detection of Herpesvirus, Enterovirus, and Arbovirus infection in patients with suspected central nervous system viral infection in the Western Brazilian Amazon. J. Med. Virol. 2014;86:1522–1527. doi: 10.1002/jmv.23953. [DOI] [PubMed] [Google Scholar]
- Bastos Mde S., Figueiredo L.T., Naveca F.G., Monte R.L., Lessa N., Pinto de Figueiredo R.M., Gimaque J.B., Pivoto João G., Ramasawmy R., Mourão M.P. Identification of Oropouche Orthobunyavirus in the cerebrospinal fluid of three patients in the Amazonas, Brazil. Am. J. Trop. Med. Hyg. 2012;86:732–735. doi: 10.4269/ajtmh.2012.11-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifay T., Turnier P.Le, Epelboin Y., Carvalho L., De Thoisy B., Djossou F., Duchemin J.B., Dussart P., Enfissi A., Lavergne A., Mutricy R., Nacher M., Rabier S., Talaga S., Talarmin A., Rousset D., Epelboin L. Review on main arboviruses circulating on French Guiana, an ultra-peripheric European region in South America. Viruses. 2023;15:1268. doi: 10.3390/v15061268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgen A., Weber F., Fazakerley J.K., Elliott R.M. Bunyamwera bunyavirus nonstructural protein NSs is a nonessential gene product that contributes to viral pathogenesis. Proc. Natl. Acad. Sci. USA. 2001;98:664–669. doi: 10.1073/pnas.98.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briese T., Bird B., Kapoor V., Nichol S.T., Lipkin W.I. Batai and Ngari viruses: M segment reassortment and association with severe febrile disease outbreaks in East Africa. J. Virol. 2006;80:5627–5630. doi: 10.1128/jvi.02448-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso B.F., Serra O.P., Heinen L.B., Zuchi N., Souza V.C., Naveca F.G., Santos M.A., Slhessarenko R.D. Detection of Oropouche virus segment S in patients and inCulex quinquefasciatus in the state of Mato Grosso, Brazil. Mem. Inst. Oswaldo. Cruz. 2015;110:745–754. doi: 10.1590/0074-02760150123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro F.L., Brustolini O.J.B., Geddes V.E.V., Souza J., Alves-Leon S.V., Aguiar R.S., Vasconcelos A.T.R. Modulation of HERV expression by four different Encephalitic arboviruses during infection of human primary astrocytes. Viruses. 2022;14:2505. doi: 10.3390/v14112505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang J.O., Azevedo R.S., Justino M.C.A., Matos H.J., Cabeça H.L.S., Silva S.P., Henriques D.F., Silva E.V.P., Andrade G.S.S., Vasconcelos P.F., Martins L.C., Azevedo R.S.S. Neurological disease caused by Oropouche virus in northern Brazil: should it be included in the scope of clinical neurological diseases? J Neurovirol. 2021;27:626–630. doi: 10.1007/s13365-021-00987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuoderis K.A., Berg M.G., Perez L.J., Hadji A., Perez-Restrepo L.S., Aristizabal L.C., Forberg K., Yamaguchi J., Cardona A., Weiss S., Qiu X., Hernandez-Ortiz J.P., Averhoff F., Cloherty G.A., Osorio J.E. Oropouche virus as an emerging cause of acute febrile illness in Colombia. Emerg. Microbes Infect. 2022;11:2645–2657. doi: 10.1080/22221751.2022.2136536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culquichicón C., Cardona-Ospina J.A., Patiño-Barbosa A.M., Rodriguez-Morales A.J. Bibliometric analysis of Oropouche research: impact on the surveillance of emerging arboviruses in Latin America. F1000Res. 2017;6:194. doi: 10.12688/f1000research.10936.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Ferreira R., de Toni Aquino da Cruz L.C., de Souza V.J., da Silva Neves N.A., de Souza V.C., Filho L.C.F., da Silva Lemos P., de Lima C.P.S., Naveca F.G., Atanaka M., Nunes M.R.T., Slhessarenko R.D. Insect-specific viruses and arboviruses in adult male culicids from Midwestern Brazil. Infect. Genet. Evol. 2020;85 doi: 10.1016/j.meegid.2020.104561. [DOI] [PubMed] [Google Scholar]
- da Silva Menegatto M.B., Ferraz A.C., Lima R.L.S., Almeida L.T., de Brito R.C.F., Reis A.B., Carneiro C.M., de Lima W.G., de Mello Silva B., de Magalhães J.C., Magalhães C.L.B. Oropouche virus infection induces ROS production and oxidative stress in liver and spleen of mice. J. Gen. Virol. 2023;104:1857. doi: 10.1099/jgv.0.001857. [DOI] [PubMed] [Google Scholar]
- de Oliveira E., Azevedo R., Coelho-Dos-Reis J.G., Antonelli L., Ferreira M.S., Campi-Azevedo A.C., Costa-Silva M.F., Martins L.C., Chiang J.O., Teixeira-Carvalho A., Martins-Filho O.A., Vasconcelos P.F.C. IFN-α as a time-sensitive biomarker during Oropouche virus infection in early and late seroconverters. Sci. Rep. 2019;9:17924. doi: 10.1038/s41598-019-54223-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias H.G., de Lima R.C., Barbosa L.S., Souza T.M.A., Badolato-Correa J., Maia L.M.S., Ferreira R.D.S., Neves N., Costa M.C.S., Martins L.R., Souza E.M., Carvalho M.D.S., Araujo-Oliveira A., Marques W.A., Sabino-Santos G., Marques M.S., Macedo G.C., Nantes W.A.G., Santos F.M., Netto C.C., Morgado T.O., Bianchini M.A., Correa S.H.R., Almeida J.R., Campos L.P., Souza I.M., Barreto W.T.G., Porfírio G., Alencar J.A.F., Herrera H.M., Shlessarenko R.D., Cunha R.V.D., Azeredo E.L., Salyer S.J., Komar N., Pauvolid-Corrêa A., Dos Santos F.B. Retrospective molecular investigation of Mayaro and Oropouche viruses at the human-animal interface in West-central Brazil, 2016-2018. PLoS One. 2022;17 doi: 10.1371/journal.pone.0277612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R.M. Orthobunyaviruses: recent genetic and structural insights. Nat. Rev. Micro. 2014;12:673–685. doi: 10.1038/nrmicro3332. [DOI] [PubMed] [Google Scholar]
- Feitoza L.H.M., de Carvalho L.P.C., da Silva L.R., Meireles A.C.A., Rios F.G.F., Silva G.S., de Paulo P.F.M., Pessoa F.A.C., de Medeiros J.F., Julião G.R. Influence of meteorological and seasonal parameters on the activity of Culicoides paraensis (Diptera: ceratopogonidae), an annoying anthropophilic biting midge and putative vector of Oropouche Virus in Rondônia, Brazilian Amazon. Acta Trop. 2023;243 doi: 10.1016/j.actatropica.2023.106928. [DOI] [PubMed] [Google Scholar]
- Files M.A., Hansen C.A., Herrera V.C., Schindewolf C., Barrett A.D.T., Beasley D.W.C., Bourne N., Milligan G.N. Baseline mapping of Oropouche virology, epidemiology, therapeutics, and vaccine research and development. NPJ Vaccines. 2022;7:38. doi: 10.1038/s41541-022-00456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca L., Carvalho R.H., Bandeira A.C., Sardi S.I., Campos G.S. Oropouche virus detection in Febrile Patients' saliva and urine samples in Salvador, Bahia, Brazil. Jpn. J. Infect. Dis. 2020;73:164–165. doi: 10.7883/yoken.JJID.2019.296. [DOI] [PubMed] [Google Scholar]
- Gaillet M., Pichard C., Restrepo J., Lavergne A., Perez L., Enfissi A., Abboud P., Lambert Y., Ma L., Monot M., Demar M., Djossou F., Servas V., Nacher M., Andrieu A., Prudhomme J., Michaud C., Rousseau C., Jeanne I., Duchemin J.B., Epelboin L., Rousset D. Outbreak of oropouche virus in French Guiana. Emerg. Infect. Dis. 2021;27:2711–2714. doi: 10.3201/eid2710.204760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez B., Wise E.L., Pullan S.T., Logue C.H., Bowden T.A., Escalera-Zamudio M., Trueba G., Nunes M.R.T., Faria N.R., Pybus O.G. Evolutionary dynamics of oropouche virus in South America. J. Virol. 2020;94 doi: 10.1128/jvi.01127-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O., Kochs G., Weber F. The interferon response circuit: induction and suppression by pathogenic viruses. Virology. 2006;344:119–130. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellert J., Aebischer A., Wernike K., Haouz A., Brocchi E., Reiche S., Guardado-Calvo P., Beer M., Rey F.A. Orthobunyavirus spike architecture and recognition by neutralizing antibodies. Nat Commun. 2019;10:879. doi: 10.1038/s41467-019-08832-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian P., Valdes J.J., Lecina-Casas D., Chrudimský T., Růžek D. The variability of the large genomic segment of Ťahyňa orthobunyavirus and an all-atom exploration of its anti-viral drug resistance. Infect. Genet. Evol. 2013;20:304–311. doi: 10.1016/j.meegid.2013.09.023. [DOI] [PubMed] [Google Scholar]
- Kraatz F., Wernike K., Hechinger S., König P., Granzow H., Reimann I., Beer M. Deletion mutants of Schmallenberg virus are avirulent and protect from virus challenge. J. Virol. 2015;89:1825–1837. doi: 10.1128/jvi.02729-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J.H., Abe J., Adkins S., Alkhovsky S.V., Avšič-Županc T., Ayllón M.A., Bahl J., Balkema-Buschmann A., Ballinger M.J., Kumar Baranwal V., Beer M., Bejerman N., Bergeron É., Biedenkopf N., Blair C.D., Blasdell K.R., Blouin A.G., Bradfute S.B., Briese T., Brown P.A., Buchholz U.J., Buchmeier M.J., Bukreyev A., Burt F., Büttner C., Calisher C.H., Cao M., Casas I., Chandran K., Charrel R.N., Kumar Chaturvedi K., Chooi K.M., Crane A., Dal Bó E., Carlos de la Torre J., de Souza W.M., de Swart R.L., Debat H., Dheilly N.M., Di Paola N., Di Serio F., Dietzgen R.G., Digiaro M., Drexler J.F., Duprex W.P., Dürrwald R., Easton A.J., Elbeaino T., Ergünay K., Feng G., Firth A.E., Fooks A.R., Formenty P.B.H., Freitas-Astúa J., Gago-Zachert S., Laura García M., García-Sastre A., Garrison A.R., Gaskin T.R., Gong W., Gonzalez J.J., de Bellocq J., Griffiths A., Groschup M.H., Günther I., Günther S., Hammond J., Hasegawa Y., Hayashi K., Hepojoki J., Higgins C.M., Hongō S., Horie M., Hughes H.R., Hume A.J., Hyndman T.H., Ikeda K., Jiāng D., Jonson G.B., Junglen S., Klempa B., Klingström J., Kondō H., Koonin E.V., Krupovic M., Kubota K., Kurath G., Laenen L., Lambert A.J., Lǐ J., Li J.M., Liu R., Lukashevich I.S., MacDiarmid R.M., Maes P., Marklewitz M., Marshall S.H., Marzano S.L., McCauley J.W., Mirazimi A., Mühlberger E., Nabeshima T., Naidu R., Natsuaki T., Navarro B., Navarro J.A., Neriya Y., Netesov S.V., Neumann G., Nowotny N., Nunes M.R.T., Ochoa-Corona F.M., Okada T., Palacios G., Pallás V., Papa A., Paraskevopoulou S., Parrish C.R., Pauvolid-Corrêa A., Pawęska J.T., Pérez D.R., Pfaff F., Plemper R.K., Postler T.S., Rabbidge L.O., Radoshitzky S.R., Ramos-González P.L., Rehanek M., Resende R.O., Reyes C.A., Rodrigues T.C.S., Romanowski V., Rubbenstroth D., Rubino L., Runstadler J.A., Sabanadzovic S., Sadiq S., Salvato M.S., Sasaya T., Schwemmle M., Sharpe S.R., Shi M., Shimomoto Y., Kavi Sidharthan V., Sironi M., Smither S., Song J.W., Spann K.M., Spengler J.R., Stenglein M.D., Takada A., Takeyama S., Tatara A., Tesh R.B., Thornburg N.J., Tian X., Tischler N.D., Tomitaka Y., Tomonaga K., Tordo N., Tu C., Turina M., Tzanetakis I.E., Maria Vaira A., van den Hoogen B., Vanmechelen B., Vasilakis N., Verbeek M., von Bargen S., Wada J., Wahl V., Walker P.J., Waltzek T.B., Whitfield A.E., Wolf Y.I., Xia H., Xylogianni E., Yanagisawa H., Yano K., Ye G., Yuan Z., Zerbini F.M., Zhang G., Zhang S., Zhang Y.Z., Zhao L., Økland A.L. Annual (2023) taxonomic update of RNA-directed RNA polymerase-encoding negative-sense RNA viruses (realm Riboviria: kingdom Orthornavirae: phylum Negarnaviricota) J. Gen. Virol. 2023;104 doi: 10.1099/jgv.0.001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Dendritic cells: master regulators of the immune response. Cancer Immunol. Res. 2013;1:145–149. doi: 10.1158/2326-6066.Cir-13-0102. [DOI] [PubMed] [Google Scholar]
- Moreli M.L., Aquino V.H., Figueiredo L.T. Identification of Simbu, California and Bunyamwera serogroup bunyaviruses by nested RT-PCR. Trans. R. Soc. Trop. Med. Hyg. 2001;95:108–113. doi: 10.1016/s0035-9203(01)90354-2. [DOI] [PubMed] [Google Scholar]
- Mourãão M.P., Bastos M.S., Gimaqu J.B., Mota B.R., Souza G.S., Grimmer G.H., Galusso E.S., Arruda E., Figueiredo L.T. Oropouche fever outbreak, Manaus, Brazil, 2007-2008. Emerg. Infect. Dis. 2009;15:2063–2064. doi: 10.3201/eid1512.090917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo J.L., Cabral A.D., Uehara M., da Silva V.M., Dos Santos J.V., Muniz J.R.C., Estrozi L.F., Fenel D., Garcia W., Sperança M.A. Nucleoprotein from the unique human infecting Orthobunyavirus of Simbu serogroup (Oropouche virus) forms higher order oligomers in complex with nucleic acids in vitro. Amino Acids. 2018;50:711–721. doi: 10.1007/s00726-018-2560-4. [DOI] [PubMed] [Google Scholar]
- Navarro J.C., Giambalvo D., Hernandez R., Auguste A.J., Tesh R.B., Weaver S.C., Montañez H., Liria J., Lima A., Travassos da Rosa J.F., da Silva S.P., Vasconcelos J.M., Oliveira R., Vianez J.L., Jr., Nunes M.R. Isolation of Madre de Dios Virus (Orthobunyavirus; Bunyaviridae), an oropouche virus species reassortant, from a monkey in Venezuela. Am. J. Trop. Med. Hyg. 2016;95:328–338. doi: 10.4269/ajtmh.15-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes M.R., Martins L.C., Rodrigues S.G., Chiang J.O., Azevedo Rdo S., da Rosa A.P., Vasconcelos P.F. Oropouche virus isolation, southeast Brazil. Emerg. Infect. Dis. 2005;11:1610–1613. doi: 10.3201/eid1110.050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes M.R.T., de Souza W.M., Savji N., Figueiredo M.L., Cardoso J.F., da Silva S.P., da Silva de Lima C.P., Vasconcelos H.B., Rodrigues S.G., Ian Lipkin W., Vasconcelos P.F.C., Palacios G. Oropouche orthobunyavirus: genetic characterization of full-length genomes and development of molecular methods to discriminate natural reassortments. Infect. Genet. Evol. 2019;68:16–22. doi: 10.1016/j.meegid.2018.11.020. [DOI] [PubMed] [Google Scholar]
- Obijeski J.F., Bishop D.H., Murphy F.A., Palmer E.L. Structural proteins of La Crosse virus. J. Virol. 1976;19:985–997. doi: 10.1128/jvi.19.3.985-997.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y., Eguchi M., Shimoji Y. Two Akabane virus glycoprotein Gc domains induce neutralizing antibodies in mice. J. Vet. Med. Sci. 2022;84:538–542. doi: 10.1292/jvms.21-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra Barrera E.L., Reales-González J., Salas D., Reyes Santamaría E., Bello S., Rico A., Pardo L., Parra E., Rodriguez K., Alarcon Z., Guerra Vega A.P., Porras M.A., Gomez-Rangel S.Y., Duarte C., Moreno J. Fatal acute undifferentiated febrile illness among clinically suspected leptospirosis cases in Colombia, 2016-2019. PLoS Negl. Trop. Dis. 2023;17 doi: 10.1371/journal.pntd.0011683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro F.P., Travassos da Rosa A.P., Travassos da Rosa J.F., Ishak R., Freitas R.B., Gomes M.L., LeDuc J.W., Oliva O.F. Oropouche virus. I. A review of clinical, epidemiological, and ecological findings. Am. J. Trop. Med. Hyg. 1981;30:149–160. [PubMed] [Google Scholar]
- Puntasecca C.J., King C.H., LaBeaud A.D. Measuring the global burden of chikungunya and Zika viruses: a systematic review. PLoS Negl. Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Amorim M., Cornejo Pontelli M., Fabiano de Souza G., Primon Muraro S., de Toledo-Teixeira D.A., Forato J., Bispo-Dos-Santos K., Barbosa N.S., Cavalheiro Martini M., Lorencini Parise P., Vieira A., Paier Milanez G., Lamberti Pinto daSilva L., Jaychand Lalwani P., Santos Farias A., Ramirez Vinolo M.A., Sesti-Costa R., Arruda E., Proenca-Modena J.L. Oropouche virus infects, persists and induces IFN response in human peripheral blood mononuclear cells as identified by RNA PrimeFlowTM and qRT-PCR assays. Viruses. 2020;12:785. doi: 10.3390/v12070785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues A.H., Santos R.I., Arisi G.M., Bernardes E.S., Silva M.L., Rossi M.A., Lopes M.B., Arruda E. Oropouche virus experimental infection in the golden hamster (Mesocrisetus auratus) Virus Res. 2011;155:35–41. doi: 10.1016/j.virusres.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Romero-Alvarez D., Escobar L.E. Oropouche fever, an emergent disease from the Americas. Microbes Infect. 2018;20:135–146. doi: 10.1016/j.micinf.2017.11.013. [DOI] [PubMed] [Google Scholar]
- Sakkas H., Bozidis P., Franks A., Papadopoulou C. Oropouche fever: a review. Viruses. 2018;10:175. doi: 10.3390/v10040175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R.I., Almeida M.F., Paula F.E., Rodrigues A.H., Saranzo A.M., Paula A.E., Silva M.L., Correa V.M., Acrani G.O., Neder L., Arruda E. Experimental infection of suckling mice by subcutaneous inoculation with Oropouche virus. Virus Res. 2012;170:25–33. doi: 10.1016/j.virusres.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Santos R.I., Bueno-Júnior L.S., Ruggiero R.N., Almeida M.F., Silva M.L., Paula F.E., Correa V.M., Arruda E. Spread of Oropouche virus into the central nervous system in mouse. Viruses. 2014;6:3827–3836. doi: 10.3390/v6103827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M.M., Price D.A., Ganaie S.S., Feng A., Mishra N., Hoehl R.M., Fatma F., Stubbs S.H., Whelan S.P.J., Cui X., Egawa T., Leung D.W., Amarasinghe G.K., Hartman A.L. Oropouche orthobunyavirus infection is mediated by the cellular host factor Lrp1. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2204706119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciancalepore S., Schneider M.C., Kim J., Galan D.I., Riviere-Cinnamond A. Presence and multi-species spatial distribution of oropouche virus in Brazil within the one health framework. Trop. Med. Infect. Dis. 2022;7:111. doi: 10.3390/tropicalmed7060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions Z., Bobrowski T., Martin H.J., Beasley J.T., Kothari A., Phares T., Li M., Alves V.M., Scotti M.T., Moorman N.J., Baric R., Tropsha A., Muratov E.N. Praemonitus praemunitus: can we forecast and prepare for future viral disease outbreaks? FEMS Microbiol. Rev. 2023;47 doi: 10.1093/femsre/fuad048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs S.H., Cornejo Pontelli M., Mishra N., Zhou C., de Paula Souza J., Mendes Viana R.M., Lipkin W.I., Knipe D.M., Arruda E., Whelan S.P.J. Vesicular stomatitis virus chimeras expressing the Oropouche virus glycoproteins elicit protective immune responses in mice. mBio. 2021;12 doi: 10.1128/mBio.00463-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh R.B. The emerging epidemiology of Venezuelan hemorrhagic fever and Oropouche fever in tropical South America. Ann. N. Y. Acad. Sci. 1994;740:129–137. doi: 10.1111/j.1749-6632.1994.tb19863.x. [DOI] [PubMed] [Google Scholar]
- Tilston-Lunel N.L., Acrani G.O., Randall R.E., Elliott R.M. Generation of recombinant Oropouche viruses lacking the nonstructural protein NSm or NSs. J. Virol. 2015;90:2616–2627. doi: 10.1128/jvi.02849-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilston-Lunel N.L., Hughes J., Acrani G.O., da Silva D.E., Azevedo R.S., Rodrigues S.G., Vasconcelos P.F., Nunes M.R., Elliott R.M. Genetic analysis of members of the species Oropouche virus and identification of a novel M segment sequence. J. Gen. Virol. 2015;96:1636–1650. doi: 10.1099/vir.0.000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travassos da Rosa J.F., de Souza W.M., Pinheiro F.P., Figueiredo M.L., Cardoso J.F., Acrani G.O., Nunes M.R.T. Oropouche virus: clinical, epidemiological, and molecular aspects of a neglected Orthobunyavirus. Am. J. Trop. Med. Hyg. 2017;96:1019–1030. doi: 10.4269/ajtmh.16-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero N. [Oropouche Virus: what is it and how it is transmitted?] Invest. Clin. 2017;58:1–2. [PubMed] [Google Scholar]
- Vasconcelos H.B., Azevedo R.S., Casseb S.M., Nunes-Neto J.P., Chiang J.O., Cantuária P.C., Segura M.N., Martins L.C., Monteiro H.A., Rodrigues S.G., Nunes M.R., Vasconcelos P.F. Oropouche fever epidemic in Northern Brazil: epidemiology and molecular characterization of isolates. J. Clin. Virol. 2009;44:129–133. doi: 10.1016/j.jcv.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Vernal S., Martini C.C.R., da Fonseca B.A.L. Oropouche virus-associated aseptic Meningoencephalitis, Southeastern Brazil. Emerg. Infect. Dis. 2019;25:380–382. doi: 10.3201/eid2502.181189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D.M., Phillips I., Callahan J.D., Griebenow W., Hyams K.C., Hayes C.G. Oropouche virus transmission in the Amazon River basin of Peru. Am. J. Trop. Med. Hyg. 1997;56:148–152. doi: 10.4269/ajtmh.1997.56.148. [DOI] [PubMed] [Google Scholar]
- Wernike K., Aebischer A., Roman-Sosa G., Beer M. The N-terminal domain of Schmallenberg virus envelope protein Gc is highly immunogenic and can provide protection from infection. Sci. Rep. 2017;7:42500. doi: 10.1038/srep42500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J.Y., Ga Y.J. Seroepidemiology of Aino virus in farmed and free-ranging Cervids in the Republic of Korea. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.702978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.