Abstract
Introduction
The outcomes in advanced NSCLC have improved owing to the availability of more effective systemic and improved supportive care. This has increased the number of patients who seek treatment in the third line and beyond setting. We conducted this study to compare the quality of life (QoL), toxicity, and outcomes in patients receiving chemotherapy and EGFR tyrosine kinase inhibitors (TKIs) in this setting.
Methods
In this phase 3, randomized, open-label study, patients with stage III or IV NSCLC with disease progression on at least two prior lines of chemotherapy, with a life expectancy of at least 3 months, without prior EGFR TKI exposure, and stable brain metastases (if any) were included. Patients were randomized to receive chemotherapy (gemcitabine or docetaxel or paclitaxel or vinorelbine) or an EGFR TKI (erlotinib or gefitinib). The primary end point was the change in QoL at 8 to 10 weeks; the secondary outcomes were safety and overall survival (OS). Patients underwent clinical evaluation at every visit, and toxicity was assessed as per Common Terminology Criteria for Adverse Events version 4.03. A radiological tumor response assessment was done every 8 to 12 weeks from the start of therapy. The QoL was assessed using the EORTC QLQ C30 and LC13 questionnaires. The change in QoL scores was calculated as the difference between scores at baseline and scores at 8 to 10 weeks (Δ) for each QoL domain. The Mann-Whitney U test was used to compare the mean difference (Δ) for each domain. OS and progression-free survival (PFS) were determined using the Kaplan-Meier method and Cox proportional regression analysis.
Results
A total of 246 patients were enrolled in the study, with 123 in each arm. There was a male predominance with 69.1% male patients in the chemotherapy arm and 70.7% in the EGFR TKI arm. The median age of patients in the chemotherapy arm was 54 years and 55 years in the chemotherapy and EGFR TKI arms, respectively. There was no significant difference in the change in QoL at baseline and the second visit (Δ) in both arms in all domains of EORTC QLQ C30 except cognitive function (p = 0.0045) and LC13 except alopecia (0.01249). The mean Δ Global Health Status was −28 in the chemotherapy arm and −26.8 in the EGFR TKI arm; this was not statistically significant (p = 0.973). The median follow-up was 88.1 months (95% confidence interval [CI]: 39.04–137.15). On the intention-to-treat analysis, the median PFS was 3.13 months (95% CI: 2.15–4.11) in the chemotherapy arm and 2.26 months (95% CI: 2.1–2.43) in the EGFR TKI arm, with hazard ratio at 1.074 (95% CI: 0.83–1.38) (p = 0.58). There were 120 deaths in each arm. The median OS was 7.63 months (95% CI: 5.96–9.30) in the chemotherapy arm and 7.5 months in the EGFR TKI arm (95% CI: 5.85–9.14); hazard ratio at 1.033 (95% CI: 0.80–1.33) (p = 0.805). The toxicity profile was similar in both arms except for a significantly higher incidence of fatigue (p = 0.043), peripheral neuropathy (0.000), alopecia, hypokalemia (0.037), and pedal edema (0.007) in the chemotherapy arm and dry skin (p = 0.000) and skin rash (p = 0.019) in the EGFR TKI arm.
Conclusions
There was no significant difference in most QoL scales (except cognitive function and alopecia), OS, and PFS of patients with advanced NSCLC receiving an EGFR TKI as compared with chemotherapy TKI in the third-line setting. The toxicity profile is consistent with the known toxicities of the agents.
Keywords: Third line, Non–small cell lung cancer, EGFR TKI, Chemotherapy
Introduction
The treatment paradigm of lung cancer has changed with the availability of very effective first-line systemic therapy.1 The use of immunotherapy, identification of molecular drivers and effective targeted therapy, and better supportive care have significantly improved outcomes in patients with advanced NSCLC. This has increased the number of patients who receive treatment in the third line and beyond setting.2
Multiple studies have evaluated the efficacy of oral EGFR tyrosine kinase inhibitors (TKIs) such as gefitinib and erlotinib after progression on first- or second-line chemotherapy. The efficacy of erlotinib was revealed by BR.21 study, in which patients with advanced NSCLC who had failed first-line or second-line chemotherapy were randomized to receive erlotinib or best supportive care. The study revealed a statistically significant improvement in progression-free survival (PFS) and overall survival (OS) with erlotinib as compared with best supportive care. Erlotinib also improved tumor-related symptoms and important aspects of quality of life (QoL).3
The IDEAL-1 and IDEAL-2 studies revealed the activity of gefitinib in patients who had progressed on one or two prior lines of chemotherapy with at least one containing platinum.4,5 The response rates with gefitinib were 18.4% (250 mg/d) and 19% (500 mg/d) in the IDEAL-1 trial and 12% (250 mg/d) and 9% (500 mg/d) in the IDEAL-2 study. The patients also had significant improvement in symptoms, with a symptom improvement rate of 37% to 44%.4,5
The SIGN and INTEREST trials compared gefitinib with docetaxel in patients with pretreated advanced NSCLC in the second-line setting. Similar efficacy in terms of symptom improvement rates, response rates, and survival was observed with gefitinib as compared with docetaxel. Patients who received gefitinib also had fewer drug-related adverse events and better QoL than patients who received docetaxel.6,7
Even though molecular testing is increasingly used and a large number of patients receive targeted therapy or immunotherapy in the third-line setting and beyond. A significant number of patients in low- and middle-income countries do not have access to molecular testing,8 immunotherapy,9 and new targeted therapies.8,10 EGFR TKIs were used as a third-line therapy option when other standard treatments were exhausted or for patients in resource-limited settings with no access to new drugs.
As there was no head-to-head comparison of QoL and outcomes of chemotherapy versus EGFR TKIs in the third-line setting, we conducted this study in patients with advanced NSCLC in the third-line setting.
Materials and Methods
Study Design
This was a phase 3, open-label, parallel-arm, randomized controlled trial. The study was conducted at the Tata Memorial Centre, a tertiary cancer center in Mumbai, India. The study protocol was approved by the Institutional Ethics Committee of Tata Memorial Centre. All patients provided a written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonization. It was monitored by an independent data monitoring safety board. The study was registered with the Clinical Trials Registry India (CTRI/2014/09/005041).
Patient Selection
Adults with stage III or IV NSCLC with disease progression on at least two prior lines of chemotherapy, with an Eastern Cooperative Oncology Group performance status (ECOG PS) 1 to 2, with a life expectancy of at least 3 months, without prior EGFR TKI exposure, and with stable brain metastases (if any) were included. Patients also had to have adequate bone marrow function (absolute neutrophil count > 1500/μl, hemoglobin > 8 g/dl, and platelet count > 100,000/μl), renal function tests (creatinine < 2 mg/dl), and liver function tests (total bilirubin < 1.5 times the institutional upper limit of normal, aspartate aminotransferase, and alanine aminotransferase levels < 2 times the institutional upper limit of normal). Patients with prior EGFR TKI exposure, sensitizing EGFR mutations, a second primary tumor, uncontrolled infection, and uncontrolled comorbidities were excluded. Uncontrolled comorbidities were defined as any uncontrolled intercurrent illness including (but not limited to) diabetes mellitus, symptomatic congestive heart failure, unstable angina pectoris, cardiac arrhythmia, renal failure on dialysis, active gastrointestinal bleeding, cerebrovascular accident, inflammatory bowel disease, or psychiatric illness/social situations that would limit compliance with study requirements.
Randomization
Patients were randomized in a 1:1 manner to receive chemotherapy (gemcitabine or docetaxel or paclitaxel or vinorelbine) or EGFR TKI (erlotinib or gefitinib). Block randomization method was used with stratification for ECOG PS 0, 1, and 2.
End Points
The primary end point was the change (difference) in the QoL scores at 8 to 10 weeks from the baseline. The secondary end points were safety and OS. OS was measured as the time from the date of randomization to the date of death. PFS was measured from the date of randomization to the date of radiological or clinical disease progression or death.
Procedures
After randomization, the patients in the chemotherapy arm received one of the chemotherapy agents listed below, which they had not received in any prior line of treatment.
Chemotherapy dose and schedule:
-
•
Gemcitabine 1000 mg/m2 intravenous (IV) infusion (in 20–30 min) on days 1 and 8 of a 21-day cycle.
-
•
Docetaxel 75 mg/m2 as a 1-hour IV infusion on day 1 of a 21-day cycle.
-
•
Paclitaxel 80 mg/m2 as 1-hour IV infusion once a week.
-
•
Vinorelbine 30 mg/m2 IV once a week.
The patients on the EGFR TKI arm received either gefitinib or erlotinib (treating physician’s choice in consultation with the participant). The dose of erlotinib was 150 mg per oral once daily on an empty stomach, and the dose of gefitinib was 250 mg per oral once daily.
The study treatment was discontinued if there was radiological and/or clinical disease progression, grade 3 or 4 toxicities, toxicities that did not recover even after 2 weeks of cessation of therapy with optimum supportive care if the ECOG PS deteriorated during therapy, or if the patient wished to stop the treatment.
QoL Assessment
The QoL assessments were done at baseline and at every 8 to 10 weeks using the EORTC QLQ 30 (version 3) and LC13 questionnaires and their validated translations in Hindi and Marathi.
Safety and Efficacy Assessment
Patients underwent clinical evaluation at every visit, which included detailed history and physical examination by the physician, such as measurement of vital signs (pulse, blood pressure, temperature, etc.) and examination of various organ systems. Toxicities at each visit were assessed and graded as per Common Terminology Criteria for Adverse Events version 4.03.
Radiological tumor response assessments (contrast-enhanced computed tomography scans of the thorax and abdomen or whole-body positron emission tomography scan [if indicated]) were performed every 8 to 12 weeks from the start of therapy.
Statistical Analysis
Sample Size
The primary outcome is the change in the QoL score at 8 to 10 weeks. It was estimated that with 200 patients, the study will have 80% power to detect a significant difference between the two groups, with an alpha of 5% when the effect size is 0.4 for the EORTC Q 30 Global Health Status (GHS) scale.
Few studies evaluating systemic therapy in the third-line setting are conducted. Considering the challenges involved in conducting these studies, the investigators collectively decided that estimating the OS was equally important in this setting. The difference in QoL remained the primary outcome, and we re-estimated the sample size to adequately power the study to detect differences in OS between the arms. We assumed an OS of 4 months in the chemotherapy arm and expected an improvement in the OS to 6 months in the EGFR TKI arm. With 80% power, 5% alpha, and 1:1 randomization, the re-estimated sample size was 318 patients. The study protocol was amended with the revised sample size (for OS) and approved by the Institutional Ethics Committee.
QoL Analysis
The scores for all scales were calculated according to the EORTC scoring manual and range from 0 to 100.11 The change in QoL scores (Δ) was calculated as the difference between scores at baseline and scores at 8 to 10 weeks for each QoL scale for each patient in both arms. The mean difference (change) (Δ) was calculated for each arm. The Shapiro-Wilk test was used to test the normality of the continuous data. The data for mean difference (Δ) were not normally distributed, and hence the Mann-Whitney U test was used to compare the mean difference (Δ) for each scale. The effect size for each QoL scale was also calculated.
SPSS version 21 and R studio were used for the analysis. The “QoLMiss” package was used for the QoL analysis. Missing data were handled by imputation by the “QoLMiss” package.12
Analysis of Secondary End Points
Analysis of the study variables including toxicity was done using simple percentages. OS and PFS were determined using the Kaplan-Meier method, and Cox proportional regression analysis was used to determine the hazard ratios. SPSS version 21 (IBM) and R studio version 4.0.1 (Posit) were used for the analysis.
Results
Baseline Characteristics
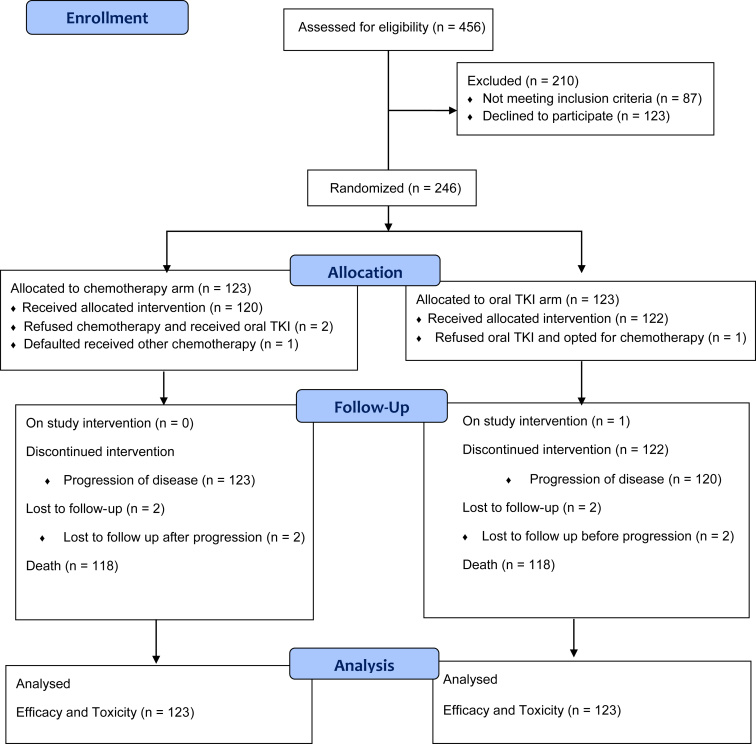
Between November 2014 and October 2020, 246 patients were enrolled in the study, 123 in each arm. There were 120 and 122 patients who received allocated treatment in the chemotherapy and EGFR TKI arms, respectively (Consolidated Standards of Reporting Trials; Fig. 1). There was a male predominance with 69.1% male patients in the chemotherapy arm and 70.7% in the EGFR TKI arm. The median age of patients in the chemotherapy arm was 54 years and 55 years in the chemotherapy arm and EGFR TKI arm, respectively. Smokers constituted 40.7% (50 patients) and 44.7% (55 patients) of the patients in the chemotherapy and EGFR TKI arms, respectively. Other baseline characteristics are found in Table 1. In both study arms, most of our patients were from outside the city of Mumbai and from lower socioeconomic backgrounds. In the chemotherapy arm, 68.3% (84 of 123) of the patients were registered under the “general” and “no charge” categories where cost of investigation and treatment is highly subsidized and funded by the government of India. Similarly, in the EGFR TKI arm, 75.6% (93 of 123) of the patients were registered in these categories (p = 0.323). This indicates that most patients are not able to cover cost of cancer care on their own and need financial assistance for treatment; hence, they seek treatment at state-owned tertiary care centers that offer subsidized treatment.
Figure 1.
CONSORT diagram. CONSORT, Consolidated Standards of Reporting Trials; TKI, tyrosine kinase inhibitor.
Table 1.
Baseline Characteristics
| Characteristics | Chemotherapy Arm (n = 123) n (%) | TKI Arm (n = 123) n (%) | p Value |
|---|---|---|---|
| Median age (in y) | 54 | 55 | - |
| Sex | |||
| Male | 85 (69.1) | 87 (70.7) | 0.889 |
| Female | 38 (30.9) | 36 (29.3) | |
| Patients with comorbidity | 63 (51.2) | 52 (42.3) | 0.201 |
| Hypertension | 15 (12.1) | 18 (14.6) | |
| Diabetes mellitus | 8 (6.5) | 10 (8.3) | |
| Ischemic heart disease | 0 (0) | 1 (0.8) | 0.066 |
| Tuberculosis | 7 (5.7) | 7 (5.7) | |
| Others | 6 (4.8) | 0 (0) | |
| Multiple comorbidities | 27 (21.9) | 15 (12.2) | |
| Smoking history | |||
| Smoker | 50 (40.7) | 55 (44.7) | 0.606 |
| Nonsmoker | 73 (59.3) | 68 (55.3) | |
| ECOG performance status | |||
| 0 | 1 (0.8) | 1 (0.8) | 1.000 |
| 1 | 93 (75.6) | 94 (76.4) | |
| 2 | 29 (23.6) | 28 (22.8) | |
| Histology | |||
| Adenocarcinoma | 97 (78.9) | 93 (75.6) | 0.649 |
| Squamous carcinoma | 26 (21.1) | 29 (23.6) | |
| Adenosquamous carcinoma | 0 (0) | 1 (0.8) | |
| Presence of driver mutationsa | |||
| EGFR | - | ||
| Sensitizing EGFR mutations (exon 19 del or exon 21 L858R) | 0 | 0 | |
| EGFR T790M | 1 (0.8)b | 0 | |
| EGFR exon 20 insertion | 0 | 1 (0.8)c | |
| ALK fusions | 2 (1.6) | 1 (0.8) | |
| ROS-1 fusions | 0 | 1 (0.8) | |
| Stage | |||
| IIIB | 3 (2.4) | 5 (4.1) | 0.722 |
| IV | 120 (97.6) | 118 (95.9) | |
| Sites of metastases | |||
| Lung | 111 (90.2) | 115 (93.5) | 0.485 |
| Liver | 38 (30.9) | 30 (24.4) | 0.318 |
| Bone | 61 (49.6) | 50 (40.7) | 0.200 |
| Braind | 13 (10.6) | 12 (9.8) | 1.000 |
| Adrenal | 22 (17.9) | 18 (14.6) | 0.605 |
| Lymph nodes | 106 (86.2) | 94 (76.4) | 0.070 |
| Pleural/pericardial effusion | 56 (45.5) | 45 (36.6) | 0.195 |
| Chemotherapy received on study | |||
| Gemcitabine | 6 | 1e | |
| Docetaxel | 1 | 0 | - |
| Paclitaxel | 102 | 0 | |
| Vinorelbine | 10 | 0 | |
| Irinotecan | 1 | 0 | |
| Pemetrexed + carboplatin | 1 | 0 | |
| EGFR TKI received on study | - | ||
| Gefitinib | 2e | 120 | |
| Erlotinib | 0 | 2 | |
| Prior systemic therapy in first line | |||
| Pemetrexed + platinum+ pembrolizumab | 1 (0.8) | 0 | |
| Pemetrexed + carboplatin | 84 (68.3) | 81 (65.9) | 0.083 |
| Pemetrexed+ cisplatin | 0 (0) | 5 (4.1) | |
| Paclitaxel+ cisplatin | 1 (0.8) | 0 (0) | |
| Paclitaxel + carboplatin | 18 (14.6) | 11 (8.9) | |
| Gemcitabine + carboplatin | 13 (10.6) | 22 (17.9) | |
| Gemcitabine | 2 (1.6) | 2 (1.6) | |
| Etoposide + carboplatin | 1 (0.8) | 0 (0) | |
| Vinorelbine + platinum | 2 (1.6) | 1 (0.8) | |
| Vinorelbine | 0 (0) | 1 (0.8) | |
| Prior systemic therapy in second line | |||
| Pemetrexed + carboplatin | 5 (4.1) | 1 (0.8) | |
| Pemetrexed | 1 (0.8) | 3 (2.4) | 0.494 |
| Paclitaxel+ carboplatin | 2 (1.6) | 4 (3.3) | |
| Gemcitabine + carboplatin | 12 (9.8) | 8 (6.5) | |
| Etoposide + carboplatin | 1 (0.8) | 0 | |
| Gemcitabine | 54 (43.9) | 54 (43.9) | |
| Vinorelbine | 7 (5.7) | 14 (11.4) | |
| Docetaxel | 21 (17.1) | 24 (19.5) | |
| Irinotecan | 3 (2.4) | 1 (0.8) | |
| Paclitaxel | 11 (8.9) | 11 (8.9) | |
| Paclitaxel + carboplatin+ bevacizumab | 2 (1.6) | 0 | |
| Docetaxel+ bevacizumab | 1 (0.8) | 1 (0.8) | |
| Docetaxel+ ramucirumab | 1 (0.8) | 0 | |
| Nivolumab | 2 (1.6) | 2 (1.6) |
ECOG, Eastern Cooperative Oncology Group; exon 19 del, exon 19 deletion; TKI, tyrosine kinase inhibitor.
Driver mutations detected during entire course of the patient’s illness, including those detected at initial diagnosis, at time of enrolment in the study, and after progression on the study.
EGFR T790M detected on repeat biopsy after progression on four lines of therapy.
Exon 20 insertion detected at initial diagnosis and persisted.
New or treated brain metastases.
Two patients randomized to the chemotherapy arm received gefitinib and one patient randomized to the EGFR TKI arm received chemotherapy.
Quality of Life
EORTC QLQ C30
Global Health Status
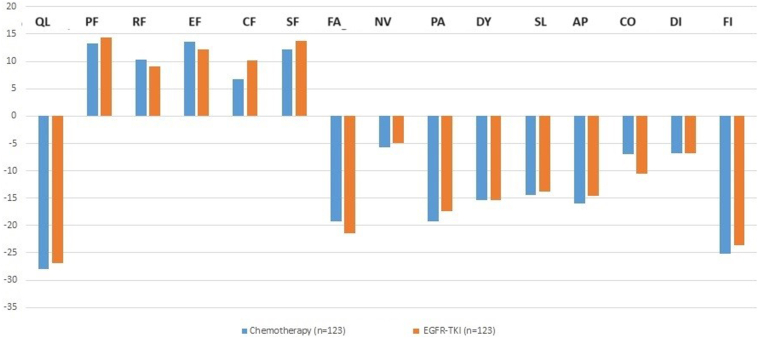
The difference in Global Health Status (GHS) scores at baseline and visit 2 (ΔGHS) was −28 in the chemotherapy arm and −26.8 in the EGFR TKI arm; this was not statistically significant (p = 0.973) (Table 2) (Fig. 2). The effect size for the difference in QoL between the two arms for GHS was small (Cohen’s d = 0.266) (Supplementary Table 1).
Table 2.
QoL—EORTC QLQ C30 and LC13
| QoL Scale | Mean Δ∗ (±SD) Chemotherapy Arm (n = 66) |
Mean Δ (±SD) TKI Arm (n = 69) | Difference in the Mean Δ Between the Two Arms (95% CI) | Mann-Whitney p Value |
|---|---|---|---|---|
| QLQ C30 | ||||
| Global health status (QoL) | −28 (±38.6) | −26.8 (±37.5) | −1.29 (−10.84 to 8.26) | 0.97319 |
| Physical function | 13.3 (±26.7) | 14.3 (±32.7) | −0.98 (−8.48 to 6.53) | 0.78251 |
| Emotional function | 10.3 (±29.5) | 9.1 (±32.7) | 1.22 (−6.6 to 9.04) | 0.66965 |
| Social function | 13.6 (±27.7) | 12.2 (±31.8) | 1.42 (−6.07 to 8.91) | 0.65483 |
| Cognitive function | 6.8 (±20.6) | 10.2 (±29.2) | −3.39 (−9.74 to 2.96) | 0.04531 |
| Role function | 12.2 (±28.6) | 13.7 (±34.3) | −1.49 (−9.41 to 6.43) | 0.47266 |
| Fatigue | −19.3 (±32.9) | −21.5 (±41.7) | 2.17 (−7.27 to −11.6) | 0.83484 |
| Nausea and vomiting | −5.7 (±17.2) | −5 (±22.8) | −0.68 (−5.74 to 4.39) | 0.9683 |
| Pain | −19.2 (±32.2) | −17.3 (±35) | −1.9 (−10.33 to −6.54) | 0.7634 |
| Dyspnea | −15.4 (±29.4) | −15.4 (±36.5) | 0 (−8.33 to 8.33) | 0.95813 |
| Insomnia | −14.4 (±35) | −13.8 (±43.7) | −0.54 (−10.49 to 9.4) | 0.89279 |
| Appetite loss | −16 (±33.4) | −14.6 (±43.1) | −1.36 (−11.05 to 8.34) | 0.82955 |
| Constipation | −7 (±24.6) | −10.6 (±28.4) | 3.52 (−3.15 to 10.2) | 0.47164 |
| Diarrhea | −6.8 (±20.9) | −6.8 (±26.3) | 0 (−5.96 to 5.96) | 0.41179 |
| Financial difficulty | −25.2 (±42.1) | −23.6 (±48.8) | −1.63 (−13.07 to 9.82) | 0.82736 |
| LC 13 | ||||
| Dyspnea | −13.9 (±27.7) | −12.6 (±31.5) | −1.26 (−8.72 to 6.19) | 0.99926 |
| Cough | −20.9 (±36.8) | −20.1 (±37.6) | −0.81 (−10.16 to 8.54) | 0.74383 |
| Hemoptysis | 0.5 (±10.4) | −0.8 (±17.8) | 1.36 (−2.32 to 5.03) | 0.34476 |
| Sore mouth | −4.1 (±17.9) | −5.1 (±25.3) | 1.08 (−4.42 to 6.59) | 0.33128 |
| Dysphagia | −8.1 (±20.6) | −3.5 (±24.1) | −4.61 (−10.24 to 1.02) | 0.29945 |
| Peripheral neuropathy | −4.1 (±29.7) | −10.3 (±33.4) | 6.23 (−1.71 to 14.17) | 0.06579 |
| Alopecia | −8.4 (±46.1) | −21.4 (±42.1) | 13.01 (1.93–24.09) | 0.01249 |
| Chest pain | −14.4 (±32) | −18.2 (±35.5) | 3.79 (−4.69 to 12.28) | 0.71135 |
| Pain in the arm | −11.9 (±31.4) | −14.6 (±33.1) | 2.71 (−5.39 to 10.81) | 0.33109 |
| Pain at other sites | −8.9 (±27.4) | −11.7 (±33.3) | 2.71 (−4.95 to 10.37) | 0.53923 |
Δ is the difference between the QoL scores at baseline and subsequent visit for each patient for a particular scale (Δ= QoL score V2- QoL score baseline). Mean Δ is the mean of these Δ values for a particular scale.
CI, confidence interval; QoL, quality of life; TKI, tyrosine kinase inhibitor.
Figure 2.
Bar graph representing the change in EORTC C30 scores at from baseline and 8 to 10 weeks. AP, appetite loss; CF, cognitive function; CO, constipation; DI, diarrhea; DY, dyspnea; EF, emotional function; FA, fatigue; FI, financial difficulty; NV, nausea and vomiting; PA, pain; PF, physical function; QL, global health status; RF, role function; SF, social function; SL, insomnia; TKI, tyrosine kinase inhibitor.
Function Scales
There was no significant difference in mean Δ scores of the physical function, role function, and social function scales between the chemotherapy arm and the EGFR TKI arm (Table 2). The effect size was also small for all these scales (Cohen’s d < 0.4) (Supplementary Table 1). There was a significant difference in the cognitive function scores at baseline and 8 to 10 weeks between the arms. The mean Δ for cognitive function scores in the chemotherapy arm was 6.8 (±20.6) and the EGFR TKI arm was 10.2 (±29.2), and the difference between Δ was −3.39 (p = 0.045) (Fig. 2).
Symptom Scales
There were no significant differences in the mean Δ scores for the any of the symptoms scales of the EORTC C30.
EORTC LC13
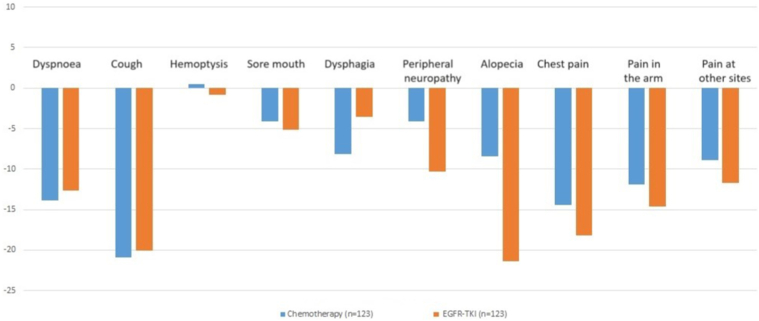
The mean difference in scores for alopecia (Δ) was −8.4 (SD ± 46.1) in the chemotherapy arm and −21.4 (SD ± 42.1) in the EGFR TKI arm; this was statistically significant (p = 0.012). The effect size for Δ alopecia between the two arms was also large (Cohen’s d = 2.311).
There were no significant differences in Δ for the other scales of the EORTC LC13 (Fig. 3).
Figure 3.
Bar graph representing the change in EORTC LC13 scores at from baseline and 8 to 10 weeks. TKI, tyrosine kinase inhibitor.
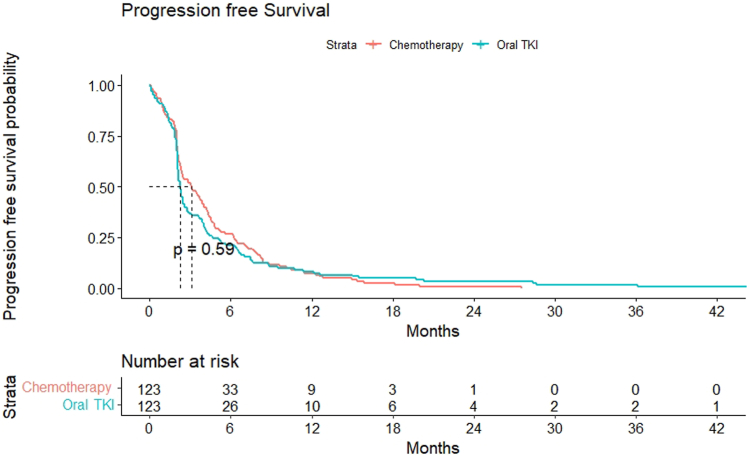
Progression-Free Survival
The median follow-up was 88.1 months (95% confidence interval [CI]: 39.04–137.15). On the intention-to-treat analysis, the median PFS was 3.13 months (95% CI: 2.15–4.11) in the chemotherapy arm and 2.26 months (95% CI: 2.1–2.43) in the EGFR TKI arm with hazard ratio at 1.074 (95% CI: 0.83–1.38) (p = 0.581) (Fig. 4).
Figure 4.
Kaplan-Meier curve for progression-free survival. TKI, tyrosine kinase inhibitor.
Overall Survival
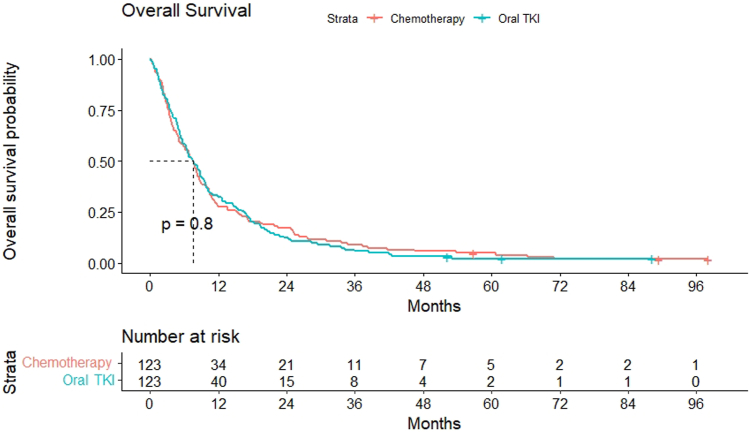
There were 120 deaths in each arm. The median OS was 7.63 months (95% CI: 5.96–9.30) in the chemotherapy arm and 7.5 months in the EGFR TKI arm (95% CI: 5.85–9.14) with hazard ratio at 1.033 (95% CI: 0.80–1.33) (p = 0.805) (Fig. 5).
Figure 5.
Kaplan-Meier curve for overall-free survival. TKI, tyrosine kinase inhibitor.
Compliance With Treatment
There were dose delays/interruptions in 55 (44.7%) and 15 patients (12.2%) in the chemotherapy and EGFR TKI arms, respectively. Dose reductions were required in 12.2% of the patients in the chemotherapy arm and 0% in the TKI arm (0.000). In the chemotherapy arm, 4.9% (six patients) each required 20% and 25% dose reductions and 1.6% (two patients) required 50% dose reduction (Supplementary Table 2).
Toxicity
The incidence of any-grade anemia, neutropenia, and thrombocytopenia was numerically higher in the chemotherapy arm as compared with the EGFR TKI arm, but this was not statistically significant. The incidence of grade 3 or higher hematological toxicities was low in both arms. Only one patient in the chemotherapy arm had febrile neutropenia (0.8%) (Table 3).
Table 3.
Adverse Events in Both Arms
| Adverse Events | Chemotherapy Arm (n = 123) |
Oral TKI Arm (n = 123) |
p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1 n (%) | Grade 2 n (%) | Grade 3 n (%) | Grade 4 n (%) | Grade 1 n (%) | Grade 2 n (%) | Grade 3 n (%) | Grade 4 n (%) | ||
| Clinical | |||||||||
| Fatigue | 24 (19.5) | 21 (17.1) | 20 (16.3) | 0 | 19 (15.4) | 20 (16.3) | 8 (6.5) | 0 | 0.043 |
| Anorexia | 26 (21.1) | 14 (11.4) | 2 (1.6) | 0 | 19 (15.4) | 9 (7.3) | 1 (0.8) | 0 | 0.364 |
| Peripheral neuropathy | 28 (22.8) | 12 (9.8) | 9 (7.3) | 0 | 3 (2.4) | 3 (2.4) | 0 | 0 | 0.000 |
| Oral mucositis | 8 (6.5) | 2 (1.6) | 2 (1.6) | 0 | 2 (1.6) | 2 (1.6) | 0 | 0 | 0.118 |
| Alopecia | 14 (11.4) | 11 (8.9) | 0 | 0 | 4 (3.3) | 0 | 0 | 0 | 0.000 |
| Diarrhea | 20 (16.3) | 2 (1.6) | 8 (6.5) | 0 | 16 (13) | 4 (3.3) | 4 (3.3) | 0 | 0.480 |
| Constipation | 11 (8.9) | 4 (3.3) | 0 | 0 | 6 (4.9) | 4 (3.3) | 2 (1.6) | 0 | 0.333 |
| Skin rash | 14 (11.4) | 8 (6.5) | 0 | 0 | 29 (23.6) | 9 (7.3) | 2 (1.6) | 0 | 0.019 |
| Dry skin | 4 (3.3) | 3 (2.4) | 0 | 0 | 30 (24.4) | 5 (4.1) | 1 (0.8) | 0 | 0.000 |
| Pruritis | 14 (11.4) | 1 (0.8) | 0 | 0 | 17 (13.8) | 7 (5.7) | 0 | 0 | 0.079 |
| Paronychia | 7 (5.7) | 4 (3.3) | 2 (1.6) | 0 | 6 (4.9) | 3 (2.4) | 0 | 0 | 0.514 |
| Myalgia | 10 (8.1) | 8 (6.5) | 2 (1.6) | 0 | 4 (3.3) | 2 (1.6) | 0 | 0 | 0.018 |
| Pedal edema | 12 (9.8) | 3 (2.4) | 2 (1.6) | 0 | 4 (3.3) | 0 | 0 | 0 | 0.021 |
| Weight loss | 11 (8.9) | 4 (3.3) | 0 | 0 | 8 (6.5) | 1 (0.8) | 0 | 0 | 0.304 |
| Laboratory | |||||||||
| Anemia | 67 (54.5) | 25 (20.3) | 4 (3.3) | 0 | 71 (57.7) | 21 (17.1) | 3 (2.4) | 1 (0.8) | 0.885 |
| Neutropenia | 12 (9.8) | 2 (1.6) | 1 (0.8) | 4 (3.3) | 5 (4.1) | 1 (0.8) | 2 (1.6) | 1 (0.8) | 0.210 |
| Thrombocytopenia | 11 (8.9) | 2 (1.6) | 0 | 1 (0.8) | 7 (5.7) | 0 | 1 (0.8) | 0 | 0.195 |
| Febrile neutropenia | - | - | 1 (0.8) | 0 | - | - | 0 | 0 | 1.000 |
| Raised bilirubin | 12 (9.8) | 2 (1.6) | 0 | 0 | 10 (8.1) | 2 (1.6) | 0 | 0 | 0.933 |
| Raised ALT | 32 (26) | 9 (7.3) | 2 (1.6) | 0 | 28 (22.8) | 8 (6.5) | 1 (0.8) | 0 | 0.855 |
| Raised AST | 26 (21.1) | 4 (3.3) | 2 (1.6) | 0 | 29 (23.6) | 5 (4.1) | 1 (0.8) | 0 | 0.888 |
| Hypoalbuminemia | 23 (18.7) | 11 (8.9) | 1 (0.8) | 0 | 35 (28.5) | 11 (8.9) | 1 (0.8) | 0 | 0.351 |
| Hyponatremia | 53 (43.1) | - | 10 (8.1) | 0 | 46 (37.4) | - | 7 (5.7) | 1 (0.8) | 0.460 |
| Hypokalemia | 11 (8.9) | 3 (2.4) | 1 (0.8) | 0 | 5 (4.1) | 0 | 0 | 0 | 0.037 |
| Hyperkalemia | 9 (7.3) | 1 (0.8) | 1 (0.8) | 0 | 7 (5.7) | 0 | 0 | 0 | 0.527 |
| Hypocalcemia | 24 (19.5) | 3 (2.4) | 0 | 0 | 17 (13.8) | 2 (1.6) | 0 | 0 | 0.518 |
| Hypomagnesemia | 35 (28.5) | 3 (2.4) | 0 | 0 | 38 (30.9) | 1 (0.8) | 0 | 0 | 0.569 |
ALT, alanine transaminase; AST, aspartate transaminase.
The non-hematological toxicity profile was similar in both arms except for a significantly higher incidence of all grades of fatigue (p = 0.043), peripheral neuropathy (0.000), alopecia, hypokalemia (0.037), and pedal edema (0.007) in the chemotherapy arm and dry skin (p = 0.000) and skin rash (p = 0.019) in the oral TKI arm (Table 3). This toxicity profile is consistent with the known toxicities of the drugs used in each arm.
Subsequent Therapy
A total of 138 patients (56.1%) received subsequent systemic therapy after disease progression on the study drug. In the chemotherapy arm, 66 of 123 patients (53.65%) received subsequent lines of therapy whereas the corresponding number in the EGFR TKI arm was 72 (58.53%). In the chemotherapy arm, 53.6%, 17.1%, and 8.9% of the patients received fourth, fifth, and sixth lines of therapy, respectively, whereas 58.5%, 21.1%, and 10.6% received fourth, fifth, and sixth lines of therapy in the EGFR TKI arm (Supplementary Table 3).
Driver Mutation Testing and Use of Targeted Therapy in the Study Population
Nearly all patients had molecular testing at some time point during the course of treatment, that is, either before the start of first- or second-line systemic therapy or before enrolment in this study (Supplementary Table 4). Molecular testing included qualitative real time polymerase chain reaction for EGFR mutations in exons 18, 19, 20, and 21, interphase fluorescence in situ hybridization (FISH) for ALK rearrangement, immunohistochemistry by Ventana D5F3 antibody assay for ALK, FISH for ROS-1, FISH for MET, and next-generation sequencing (NGS). The use of NGS increased over time as the availability of NGS and its use in clinical practice increased.
Furthermore, 28 patients did not undergo initial testing for oncogenic drivers, of which 20 patients (10 in each arm) had squamous cell carcinoma (SCC). There were 218 patients who were tested for EGFR either before first- or second-line therapy and did not have a sensitizing mutation in the EGFR gene. Only one patient had an EGFR exon 20 insertion at initial diagnosis (Table 1 and Supplementary Table 4). At that time, the drugs targeting EGFR exon 20 insertions such as amivantamab and poziotinib were not available, so she received standard chemotherapy in the first- and second-line settings. After progression on third-line therapy, she underwent a repeat biopsy and EGFR testing, which again revealed the exon 20 insertion, and she received osimertinib and poziotinib after progression on the study. None of the patients who underwent repeat biopsy and EGFR testing before enrolment in the study had a sensitizing EGFR mutation in exon 19 or 21. In one patient, the EGFR exon T790M mutation was detected after progression on the study. Only one patient had a ROS-1 fusion that was detected before first-line systemic therapy but did not receive any ROS-1–directed therapy. All three patients who were detected to have ALK rearrangements at the time or after progression on the study received ALK inhibitors (Supplementary Table 3). The details of mutational profile of the patients are found in Supplementary Table 4.
Use of Immune Checkpoint Inhibitors in the Study Population
Only five patients in the entire study population (2.03%) received immunotherapy in the first- and second-line setting before enrolment in this study. Three of 123 patients (2.4%) in the chemotherapy arm received immune checkpoint inhibitors (ICIs), one patient (0.8%) received pembrolizumab with pemetrexed plus carboplatin in the first line, and two (1.6%) received nivolumab in the second line. In the EGFR TKI arm, no patients received ICI in the first line and two patients (1.6%) received nivolumab in the second line. Only seven of 246 patients (2.84%) received an ICI, that is, nivolumab after progression on the study drug, three patients in the chemotherapy arm, and four patients in the oral TKI arm (Supplementary Table 3).
Discussion
The treatment paradigm in advanced NSCLC has changed; patients are living longer with effective first-line therapy and often go on to receive at least two or three lines of therapy. Even in older studies, a significant proportion of patients received palliative systemic therapy in the third-line setting and beyond. In the randomized phase 3 trial that compared pemetrexed with docetaxel as second-line treatment, more than 40% of the patients went on to receive third-line therapy post-study.13 With the increasing use of NGS leading to detection of rare molecular drivers, and the development of new drugs, survival in NSCLC has further improved.
Symptom improvement in lung cancer is important in the palliative setting. In previous studies comparing docetaxel with gefitinib in the second-line setting, symptom improvement rates using the FACT-L questionnaire were 36.8% and 26.0% with gefitinib and docetaxel, respectively.6 Reduction of symptoms and improvement in QoL are important aspects of cancer care especially in advanced disease. Hence, QoL is an important end point for studies in palliative settings. The primary outcome of this study was to look for the change in QoL from baseline to the second visit (8–10 wk) after starting treatment. We considered 8 to 10 weeks as an appropriate time point to assess the change in QoL as the time to have response to systemic therapy in most patients is approximately 2 months. Our study revealed no difference in the QoL scores of patients receiving chemotherapy versus EGFR TKIs in most symptom scales except alopecia, where patients in the chemotherapy arm had worse QoL scores (p = 0.012). This is expected as alopecia is a known adverse effect of chemotherapy but not found with gefitinib/erlotinib. The change in the GHS score was similar in both arms indicating that the use of chemotherapy or EGFR TKI did not affect the overall QoL in patients.
The change in the function scales from baseline was not statistically significant for the physical function, emotional function, role function, and social function scales, but there was a small but statistically significant difference in the scores for cognitive function. The mean Δ for cognitive function scores in the chemotherapy arm was 6.8 (±20.6) and the EGFR TKI arm was 10.2 (±29.2); the difference between Δ was −3.39 (p = 0.045) (Table 2). Overall, in both arms, there seemed to be an increase in the scores at 8 to 10 weeks, which indicates improvement in cognitive function. Nevertheless, the improvement was higher in the EGFR TKI arm as compared with the chemotherapy arm. Although it was statistically significant, the magnitude of the difference in scores is small.
Chemotherapy-induced cognitive impairment could be a contributing factor to this change.14 Another possible reason for this could be the missing QoL data for the 8 to 10 weeks of visit which could have affected the scores. In addition, QoL is a patient-reported outcome, and multiple factors such as disease-related symptoms and treatment toxicity affect the reporting of QoL by the patients. In many settings, the QoL may not correlate with the reported toxicities.
Although the hematological toxicity was numerically higher in the chemotherapy arm, it was not significant and the incidence of grade 3 or higher toxicities was small. In nonhematologic toxicity, fatigue, peripheral neuropathy, alopecia, myalgia, and pedal edema were significantly higher in the chemotherapy arm. Nevertheless, the skin toxicities of EGFR TKIs were significant (skin rash and dry skin). The toxicity profile correlated with QoL scores for alopecia. The toxicity profile of patients in this study is consistent with the known toxicities of chemotherapy and EGFR TKIs.
There was no significant difference in the PFS and OS with chemotherapy and EGFR TKIs. This is consistent with that reported in the literature in the second-line setting which revealed similar survival with gefitinib and docetaxel.6,7 This lack of improvement in PFS and OS could be due to the inherent aggressive biology of driver mutation-negative NSCLC which has progressed on two or more lines of systemic therapy.
The study population consisted of predominantly driver mutation-negative adenocarcinomas and was not enriched with patients with oncogenic drivers which could have affected the results. Only two patients had oncogenic driver mutations detected before starting third-line therapy. Of these, one had an EGFR exon 20 insertion and received chemotherapy which was the standard of care at the time. The other had a ROS-1 fusion detected but could not access ROS-1–directed therapy. Two patients were tested for ALK rearrangements, at the time of screening for this study; however, owing to delayed availability of the ALK interphase FISH results, they were included in the study and later received ALK inhibitors at disease progression. Furthermore, 28 patients did not have mutation testing done before previous lines of treatment, and of these, 71.4% (20 of 28) patients had SCCs (Supplementary Table 4). During the initial phase of the study, molecular testing was not routinely done in all SCCs on the basis of guidelines at the time. Later on, when data from our center revealed that 5.8% of SCC had therapeutically relevant EGFR mutations, we started testing for oncogenic drivers, especially EGFR in SCC.15
A significant number of patients—more than 50% in both arms—went on to receive fourth-line therapy; however, a very small number of patients received ICIs. Only five of 246 patients in the entire study population (2.03%) received an ICI in the first- and second-line setting before enrolment in this study. In addition, only seven of 246 patients (2.84%) received an ICI, that is, nivolumab, after progression on the study drug (Supplementary Table 3). Most of the patients included in this study could not afford to receive ICIs as third-line therapy or had either already progressed on an ICI. Although this may be considered a limitation of this study, this reflects the real-world scenario in our setting and many resource-constrained settings in low- and middle-income countries where access to immunotherapy is limited. Data from our center reveal that only 2.4% of patients with thoracic malignancies (including lung cancer) actually received ICIs when indicated.9 This has slightly improved over the years, but even today, access is still an issue. Few patients in resource-limited settings like ours have access to comprehensive genomic testing and drugs such as ICIs, new targeted agents such as antibody-drug conjugates, and bispecific monoclonal antibodies.8, 9, 10 Many targeted agents for NSCLC such as selpercatinib and trastuzumab deruxtecan are not available in India at present, whereas others (amivantamab, alectinib, lorlatinib, etc.) are out of reach of most patients owing to the cost.
At the time this study was conceptualized, EGFR TKIs were acceptable in later lines of therapy in patients with EGFR wild-type NSCLC.3, 4, 5 Hence, we thought of comparing EGFR TKIs with chemotherapy. The advent of new targeted agents and ICIs has changed the treatment paradigm in NSCLC.1 EGFR TKIs are no longer considered appropriate therapy for patients with wild-type EGFR and have been removed as an option from international treatment guidelines. This may make it this study seems irrelevant now, but even today, there is no standard-of-care third-line systemic therapy for driver mutation-negative NSCLC, and few studies have specifically addressed this question. The practice in our country has changed with the times, and EGFR TKIs are now not routinely used in driver mutation-negative patients in later lines. Now, they are used only when all standard therapy options are exhausted or when targeted therapy and ICIs cannot be used owing to lack of access or affordability.
Although the study did not find an improvement in QoL or survival with EGFR TKIs, it provides valuable insights. It highlights the fact that all patients with NSCLC receiving treatment in the third-line setting are not the same. More studies are needed to understand the disease biology and mechanisms of poor response to therapy; also testing for oncogenic drivers in this setting is important to improve outcomes.
One limitation of our study is the missing QoL data for the 8 to 10 weeks of time point, which could have affected the change in QoL. The other limitations include very limited use of ICIs in the study population, small number of patients undergoing rebiopsy, and molecular testing at time of randomization to the study.
In conclusion, there was no significant difference in the QoL, OS, and PFS of patients with advanced NSCLC receiving EGFR TKIs as compared with chemotherapy in the third-line setting. The toxicity profile was consistent with the known toxicities of the drugs.
CRediT Authorship Contribution Statement
Vanita Noronha: Conceptualization, Formal analysis, Methodology, Project administration, Investigation, Resources, Writing—original draft, Writing—review and editing.
Nandini S. Menon: Conceptualization, Data curation, Formal analysis, Methodology, Investigation, Writing—original draft, Writing—review and editing.
Vijay Maruti Patil: Conceptualization, Formal analysis, Methodology, Project administration, Investigation, Writing—original draft, Writing—review and editing.
M. V. Chandrakanth: Conceptualization, Methodology, Writing—review and editing.
Sucheta More: Data curation, Project administration, Resources, Writing—review and editing.
Aditya Dhanawat: Writing—review and editing.
Oindrila Roy Chowdhary: Data curation, Formal analysis, Writing—review and editing.
Ajaykumar Chandrabhan Singh: Data curation, Writing—review and editing.
Supriya Goud: Data curation, Project administration, Resources, Writing—review and editing.
Srushti Shah: Data curation, Project administration, Resources, Writing—review and editing.
Naveen Karuvandan: Data curation, Writing—review and editing.
Kunal Naishadh Jobanputra: Data curation, Writing—review and editing.
Darshit Kalpeshkumar Shah: Data curation, Writing—review and editing.
Minit Jalan Shah: Data curation, Writing—review and editing.
Rupjyoti Sarma: Data curation, Writing—review and editing.
Dhwaniben Patel: Data curation, Writing—review and editing.
Ritam Joarder: Data curation, Writing—review and editing.
Prashant Kumar: Data curation, Writing—review and editing.
Anupa John: Data curation, Writing—review and editing.
Jaspreet Kaur: Data curation, Writing—review and editing.
Saurabh Bagra: Data curation, Writing—review and editing.
Nilendu Purandare: Investigation, Writing—review and editing.
Amit Janu: Investigation, Investigation, Writing—review and editing.
Abhishek Mahajan: Investigation, Writing—review and editing.
Kumar Prabhash: Conceptualization, Formal analysis, Methodology, Project administration, Investigation, Resources, Writing—original draft, Writing—review and editing.
Footnotes
Drs. Noronha and Menon contributed equally to this work.
Disclosure Dr. Noronha reports receiving grants from Dr. Reddy’s Laboratories, Amgen, Sanofi, and Tata Memorial Centre Research Administration Council, outside of the submitted work. Dr. Prabhash reports receiving grants from Tata Memorial Centre Research Administration Council, the Indian Cooperative Oncology Network, and Glenmark Pharmaceuticals, during the conduct of the study; grants from Dr. Reddy’s Laboratories, Fresenius Kabi India, and Roche Holding, outside of the submitted work. Dr. Menon reports receiving grants from AstraZeneca and Aurigene Oncology Limited outside of the submitted work. The remaining authors declare no conflict of interest.
Cite this article as: Noronha V, Menon NS, Patil VM, et al. A comparative study evaluating the quality of life and survival outcomes in patients receiving chemotherapy versus oral tyrosine kinase inhibitor in the third line and beyond setting for advanced NSCLC. JTO Clin Res Rep. 2024;5:100622.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2023.100622.
Supplementary Data
References
- 1.Prabhash K., Vora A., Limaye S., et al. Treatment of advanced non-small-cell lung cancer: first line, maintenance, and second line– Indian consensus statement update (Under the aegis of Lung Cancer Consortium Asia, Indian Cooperative Oncology Network, Indian Society of Medical and Pediatric Oncology, Molecular Oncology Society, and Association of Physicians of India) Cancer Res Stat Treat. 2021;4:279–314. [Google Scholar]
- 2.Syrigos K.N., Saif M.W., Karapanagiotou E.M., Oikonomopoulos G., De Marinis F. The need for third-line treatment in non-small cell lung cancer: an overview of new options. Anticancer Res. 2011;31:649–659. [PubMed] [Google Scholar]
- 3.Shepherd F.A., Rodrigues Pereira J., Ciuleanu T., et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 4.Fukuoka M., Yano S., Giaccone G., et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol. 2003;41:1162–1171. doi: 10.1200/JCO.22.02499. [DOI] [PubMed] [Google Scholar]
- 5.Kris M.G., Natale R.B., Herbst R.S., et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 6.Cufer T., Vrdoljak E., Gaafar R., Erensoy I., Pemberton K., SIGN Study Group Phase II, open-label, randomized study (SIGN) of single-agent gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) non-small-cell lung cancer. Anti Cancer Drugs. 2006;17:401. doi: 10.1097/01.cad.0000203381.99490.ab. [DOI] [PubMed] [Google Scholar]
- 7.Kim E.S., Hirsh V., Mok T., et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 8.Febbraro M., Gheware A., Kennedy T., Jain D., et al. Barriers to access: global variability in implementing treatment advances in lung cancer. Am Soc Clin Oncol Educ Book. 2022:666–672. doi: 10.1200/EDBK_351021. [DOI] [PubMed] [Google Scholar]
- 9.Patil V., Abraham G., Ravikrishna M., et al. Retrospective analysis: checkpoint inhibitor accessibility for thoracic and head and neck cancers and factors influencing it in a tertiary centre in India. Ecancermedicalscience. 2022;16:1464. doi: 10.3332/ecancer.2022.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leighl N.B., Sharon Nirmalakumar S., Doreen A., et al. An arm and a leg: the rising cost of cancer drugs and impact on access. Am Soc Clin Oncol Educ Book. 2021:e1–e12. doi: 10.1200/EDBK_100028. [DOI] [PubMed] [Google Scholar]
- 11.EORTC QLQ C−30 scoring manual and supplementary modules. 3rd edition. https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf
- 12.Pal A., Pradhan S., Mishra A., Chatruvedi P., Bhattacharjee A. QoLMiss: package for repeatedly measured quality of life of cancer patients data. IntechOpen. https://www.intechopen.com/online-first/1139703
- 13.Pujol J.L., Shaharyar S., Kortsik C., et al. Post study docetaxel in non-small cell lung cancer (NSCLC) patients after discontinuation from a randomized phase III trial of pemetrexed versus docetaxel: an exploratory analysis. J Clin Oncol. 2004;22(suppl 14) 7135–7135. [Google Scholar]
- 14.Schagen S.B., Klein M., Reijneveld J.C., et al. Monitoring and optimising cognitive function in cancer patients: present knowledge and future directions. EJC Suppl. 2014;12:29–40. doi: 10.1016/j.ejcsup.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi A., Mishra R., Desai S., et al. Molecular characterization of lung squamous cell carcinoma tumors reveals therapeutically relevant alterations. Oncotarget. 2021;12:578–588. doi: 10.18632/oncotarget.27905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.