Summary
Background
Heart failure (HF) and dementia frequently co-exist with shared pathological mechanisms and risk factors. Our study aims to investigate the association between statin therapy and the risks of dementia and its subtypes among patients with HF.
Methods
The Hong Kong Clinical Data Analysis and Reporting System database was interrogated to identify patients with incident HF diagnosis from 2004 to 2018, using ICD 9/ICD 10 codes. Inverse probability of treatment weighting (IPTW) was used to balance baseline covariates between statin users (N = 54,004) and non-users (N = 50,291). The primary outcomes were incident all-cause dementia, including subtypes of Alzheimer's disease, vascular dementia, and unspecified dementia. Cox proportional-hazard model with competing risk regression was performed to estimate the sub-distribution hazards ratio (SHR) with corresponding 95% confidence intervals (CI) of the risks of all-cause dementia and its subtypes that are associated with statin use.
Findings
Of all eligible patients with HF (N = 104,295), the mean age was 74.2 ± 13.6 years old and 52,511 (50.3%) were male. Over a median follow-up of 9.9 years (interquartile range [IQR]: 6.4–13.0), 10,031 (9.6%) patients were diagnosed with dementia, among which Alzheimer's disease (N = 2250), vascular dementia (N = 1831), and unspecified dementia (N = 5950) were quantified separately. After IPTW, statin use was associated with a 20% lower risk of incident dementia compared with non-use (multivariable-adjusted SHR 0.80, 95% CI 0.76–0.84). Stratified by subtypes of dementia, statin use was associated with a 28% lower risk of Alzheimer's disease (SHR 0.72, 95% CI 0.63–0.82), 18% lower risk of vascular dementia (SHR 0.82, 95% CI 0.70–0.95), and a 20% lower risk of unspecified dementia (SHR 0.80, 95% CI 0.75–0.85).
Interpretation
In patients with HF, statin use was associated with a significantly lower risk of all-cause dementia and its subtypes, including Alzheimer's disease, vascular dementia, and unspecified dementia. Both randomized trials and experimental studies to validate the potential neuroprotective effect of statin are warranted.
Funding
No funding was provided for this study.
Keywords: Dementia, Statin, Heart failure, Cognitive impairment
Research in context.
Evidence before this study
We searched PubMed for studies in English from January 1995 until December 2023 using the search terms “statins”, “dementia”, “cognitive impairment”, “Alzheimer's disease”, “Vascular dementia”, or “heart failure”. Publications that investigated the association between statins and all-cause dementia and cause-specific dementia among patients with heart failure (HF) were barely evaluated. This was further extended to identify relevant articles limited to Asian patients. We found none that examined the impacts of statin use among patients with HF in large-scale Asian population.
Added value of this study
This is the first large population-based multi-center cohort study on HF which found that statin use was linked to a significantly 20% lower risk of all-cause dementia. Specifically, statin use demonstrated a 28% risk reduction in Alzheimer's disease, 18% risk reduction in vascular dementia, and 20% risk reduction in unspecified dementia. The results remained consistent across subgroups, including age, gender, comorbidities, and education levels, indicating that the cognitive benefits of statin may extend to a broad population of HF patients. Furthermore, lower serum LDL-C levels were associated with lower risk of dementia, suggesting a potential “dose response” benefit of LDL-C lowering in the prevention of incident dementia. Intriguingly, the benefit of statin use was observed even after adjusting for achieved LDL-C levels, suggesting potential additional pleiotropic effects of statins in modulating dementia beyond lipid-lowering benefits in HF.
Implications of all the available evidence
The burden of HF will increase further in tandem with the rapidly ageing demographics which portends poor prognosis. The findings have major clinical implications on the potential preventative strategies for dementia in patients with HF. Patients with statin use were at lower risk of all-cause dementia, Alzheimer's disease, vascular dementia or unspecified dementia compared with those without use of statins, independent of LDL-C levels. These findings can provide useful insights into the potential preventative strategies for dementia in patients with HF. The cognitive benefits of statin therapy in HF warrant evaluation in future randomized trials and experimental studies.
Introduction
Heart failure (HF) and dementia are common comorbidities in the older people. Globally, HF afflicts more than 64 million individuals1 and the prevalence continues to rise, attributable to a growing and ageing population. In recent studies, the investigative focus of HF outcomes has shifted from predominantly cardiovascular to non-cardiovascular morbidity and mortality, with dementia emerging as one of the key comorbidities.2,3 With global estimates of nearly 46.8 million cases,4 dementia constitutes a great proportion of the comorbidity burden especially in the older people. In this context, a prospective cohort study of patients with HF demonstrated that a lower cognitive test score is associated with increased risk of mortality and rehospitalization.5 A growing body of evidence has also shown that not only through shared risk factors but HF per se may drive the development of cognitive impairment6,7 or dementia.8,9 While knowledge on the underlying pathology of dementia in HF is limited, robust clinical studies that sought to explore effective interventional strategies to address this burden are also lacking. Recent clinical studies have yielded conflicting evidence on the association between statin use and the incidence of dementia. Clinical trials, including the HPS,10 PROSPER,11 and observational studies12, 13, 14, 15 did not support the causal role of statin use on preventing cognitive impairment in older people or in patients at high risk of vascular diseases. Conversely, several meta-analyses demonstrated that statin use was associated with a lower risk of all-cause dementia in a dose-response manner,16,17 as well as Alzheimer's disease and cognitive impairment.18 Further, experimental studies have suggested that statin might reduce the risk of dementia through various mechanisms, including the reduction of amyloid precursor proteins,19 and via its lipid-lowering and anti-inflammatory properties. However, it should be noted that the contradictory evidence might be attributed to the different study populations, selection bias, residual confounders, or reverse causation.
As such, detailed evaluation of the association between statin use and dementia incidence in the HF cohort would be of clinical interest. This study sought to investigate the impact of statin use on the risk of all-cause dementia, including Alzheimer's disease, vascular dementia, unspecified dementia in patients with HF using a territory-wide population with well-validated and prospectively updated data. Further, the mediation role of time-weighted Low-density lipoprotein-cholesterol (LDL-C) in this association would be evaluated.
Methods
Data sources
This is a retrospective cohort study conducted with data from the Clinical Data Analysis and Reporting System (CDARS), a territory-wide database developed by the Hong Kong Hospital Authority. As the statutory body and the singular provider of public healthcare services in Hong Kong, the Hospital Authority provides over 90% of in-hospitalisation services to the local population of 7.5 million,20 ensuring minimal loss to follow-up among patients admitted to public hospitals. CDARS prospectively collects patient information including, but not limited to, demographics, diagnoses, drug prescriptions and dispensing records, procedures, laboratory tests, episodes of hospital visits, outpatient and emergency department visits since 1993. Diagnostic data, specifically, were coded using the International Classification of Diseases, both Ninth Revision (ICD-9) and Tenth Revision (ICD-10), which demonstrated a high degree of accuracy in previous studies.21,22
Patient data (name and Hong Kong identification number) were de-identified in CDARS and unique reference numbers were generated. The study was approved by the institutional review board of the University of Hong Kong and the West Cluster of the Hong Kong Hospital Authority.
Study participants
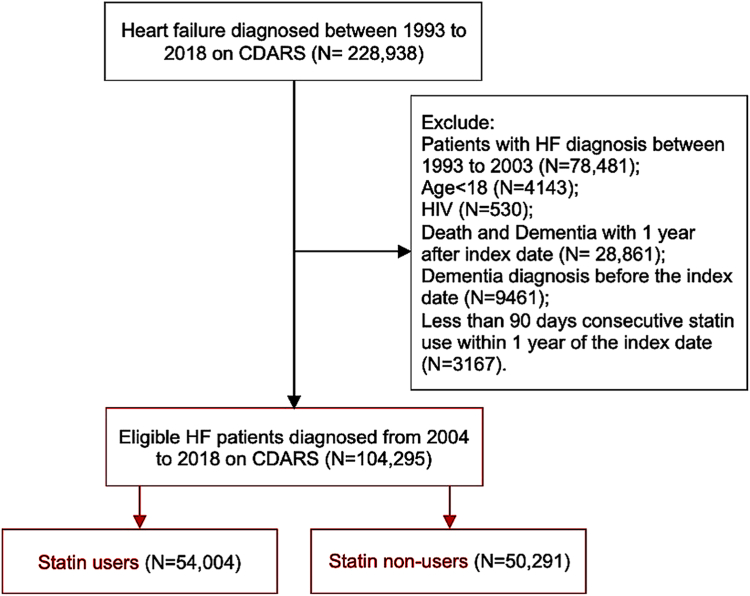
We searched for all patients aged 18 years old or above with HF (ICD-9: 398, 402, 404, 425, 428; ICD-10: I11.0, I13.0, I13.2, I50.0, I50.1, I50.9, I42, I43) as a primary diagnosis of hospitalization from 2004 to 2018 (N = 104,295). The index date was defined as the date of firstly diagnosed with HF. Patients diagnosed with HF between 1993 and 2003 (N = 78,481) were excluded to ensure that the enrolled patients had no prior history of HF. We further excluded patients who had dementia at baseline, or those who died within 1 year after the first diagnosis of HF (N = 28,861). Patients with a history of dementia, or those who were diagnosed with dementia within 1 year after the index date, with human immunodeficiency disease (HIV), and those with less than 90 days of consecutive statin use within the first year of index date (N = 3167) were also excluded (Fig. 1).
Fig. 1.
Flow chart of study cohort.
Exposures
Statin use was defined as ≥90 days of consecutive statin use22 beginning within the first year of the index date.21 Statin non-use was defined as the absence of statin prescription. We focused on the original treatment assignment as the exposure regardless of the change of treatment subsequently. Statin non-users who became users beyond 1 year after the index date (N = 2718) were excluded from the main analysis. The types of statins available in the public sector during the study period included simvastatin [in 35,578 patients (65.9%)], atorvastatin [in 15,450 (28.6%)], rosuvastatin [in 2420 (4.5%)], fluvastatin [in 556 (1.0%)]; lovastatin and pravastatin were hardly prescribed. Accordingly, we identified 54,004 statin users and 50,291 statin non-users after the index date.
Time-weighted average of LDL-C level was calculated to further evaluate the impact of lipid control on the association in this study cohort. The method of calculating time-weighted average LDL-C level was in accordance with that described in a previous publication.23 Among which, 83,125 (79.7%) patients had more than 2 measurements of post-HF LDL-C levels available. These patients were subsequently categorized based on the time-weighted LDL-C levels: LDL-C <1.8 mmol/L, 1.8 ≤ LDL-C < 2.6 mmol/L, and LDL-C ≥2.6 mmol/L in accordance with current guidelines. Patients with unrealistic LDL-C values (<0.75 mmol/L or >8 mmol/L) were excluded (N = 1580).
Covariates
We traced patients' records back to 3 years before the index date. Demographics including age at index date, sex; lifestyle factors including alcohol consumption and smoking; The comorbidities were defined based on the combination of ICD9/ICD10 codes which had been previously validated24, 25, 26 and medications prescribed, including hypertension, diabetes, obesity, stroke, anaemia, coronary artery disease (CAD), peripheral vascular disease (PVD), dyslipidaemia, atrial fibrillation (AF), other arrhythmias, cirrhosis, chronic renal failure (CRF), hearing loss, head injury, cancer, Parkinson's disease, rheumatism, depression, sleep apnea; Medications including antiplatelets, angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blocker (ARB), beta-blockers, calcium channel blockers (CCB), diuretics, statins, insulin, and other anti-diabetics; Education status (SES) including less than primary, primary, secondary, tertiary and higher education were retrieved from CDARS. Baseline drug use was defined as ≥90 days of consecutive drug use within 3 years before the index date. As a total of 29,905 patients had baseline statin use in our study, excluding these patients directly could potentially alter the characteristics of the entire study population and introduce selection bias. Therefore, we retained these patients in our primary analysis. In the sensitivity analysis, we excluded these patients to ensure the incident statin users. Details of the ICD-9 and ICD-10 codes used are listed in Supplementary Table 1.
Outcomes
The primary outcome was incident dementia, including subtypes of Alzheimer's disease, vascular dementia and unspecified dementia subsequent to HF diagnosis. The secondary outcome was all-cause mortality. Patients were followed-up until a diagnosis of dementia, death, or 31 December 2020, whichever came earlier.
Statistical analysis
Reporting of the present study is in compliance with Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement. Continuous variables were presented as mean and standard deviation (SD). Categorical variables were expressed as count and percentage. Differences between exposure groups were tested using independent t-test, ANOVA, or χ2 test, where appropriate. To address biases in treatment allocation due to lack of randomization, inverse probability of treatment weighting (IPTW) was adopted. All the baseline variables presented in Table 1 were included in calculating the weights for IPTW and the multivariable Cox model. After IPTW, covariates that were considered prognostically significant as well as those that might have influenced differences between exposure groups were considered insignificant if the standard mean difference (SMD) <0.2.27,28 The primary analyses focused on the association between statin use and the risk of all-cause dementia. The associations between statin and subtypes of dementia, including Alzheimer's disease, vascular dementia, unspecified dementia, were further calculated separately. We estimated the 10-year cumulative incidence by using the Cox proportional-hazards model adjusted for inverse probability of treatment weighting while accounting for competing risks. The multi-variable adjusted model included all of the confounders (Table 1) used to calculate the propensity score in “doubly robust adjustment”. A Fine–Gray model was used to account for competing risks, with all-cause mortality defined as the competing event.
Table 1.
Baseline characteristics of the heart failure cohort and grouped by statin use or non-use.
| Baseline characteristics | Overall | Statin non-use | Statin use | SMD before IPTW | SMD after IPTW |
|---|---|---|---|---|---|
| No. | 104,295 | 50,291 | 54,004 | ||
| Age (mean ± SD) | 74.2 ± 13.6 | 75.6 ± 14.6 | 73.0 ± 12.4 | 0.191 | 0.011 |
| Sex (male, %) | 52,511 (50.3) | 23,774 (47.3) | 28,737 (53.2) | 0.119 | 0.011 |
| Smoking (%) | 22,957 (22.0) | 10,582 (21.0) | 12,375 (22.9) | 0.045 | <0.001 |
| Alcohol (%) | 2740 (2.6) | 1389 (2.8) | 1351 (2.5) | 0.016 | 0.005 |
| Comorbidities | |||||
| Hypertension (%) | 47,151 (45.2) | 20,060 (39.9) | 27,091 (50.2) | 0.208 | 0.067 |
| Diabetes (%) | 28,197 (27.0) | 9175 (18.2) | 19,022 (35.2) | 0.391 | 0.087 |
| Obesity (%) | 1406 (1.3) | 393 (0.8) | 1013 (1.9) | 0.096 | 0.001 |
| Stroke (%) | 9986 (9.6) | 3740 (7.4) | 6246 (11.6) | 0.141 | 0.051 |
| Anemia (%) | 11,852 (11.4) | 6666 (13.3) | 5186 (9.6) | 0.115 | 0.103 |
| CAD (%) | 33,050 (31.7) | 8741 (17.4) | 24,309 (45.0) | 0.625 | 0.111 |
| PVD (%) | 12,732 (12.2) | 5586 (11.1) | 7146 (13.2) | 0.065 | 0.069 |
| Dyslipidemia (%) | 13,498 (12.9) | 2021 (4.0) | 11,477 (21.3) | 0.537 | 0.149 |
| Atrial fibrillation (%) | 26,353 (25.3) | 13,924 (27.7) | 12,429 (23.0) | 0.108 | 0.007 |
| Other arrythmias (%) | 5865 (5.6) | 2466 (4.9) | 3399 (6.3) | 0.061 | 0.01 |
| Cirrhosis (%) | 828 (0.8) | 533 (1.1) | 295 (0.5) | 0.058 | 0.001 |
| Chronic renal failure (%) | 9018 (8.6) | 3893 (7.7) | 5125 (9.5) | 0.062 | 0.079 |
| Other chronic kidney diseases (%) | 17,025 (16.3) | 6875 (13.7) | 10,150 (18.8) | 0.139 | 0.077 |
| Hearing loss (%) | 1157 (1.1) | 533 (1.1) | 624 (1.2) | 0.009 | 0.002 |
| Head injury (%) | 8077 (7.7) | 4159 (8.3) | 3918 (7.3) | 0.038 | 0.006 |
| Cancer (%) | 9424 (9.0) | 5031 (10.0) | 4393 (8.1) | 0.065 | 0.009 |
| Parkinson's disease (%) | 856 (0.8) | 544 (1.1) | 312 (0.6) | 0.056 | 0.004 |
| Rheumatism (%) | 7408 (7.1) | 4076 (8.1) | 3332 (6.2) | 0.075 | 0.006 |
| Depression (%) | 2558 (2.5) | 1186 (2.4) | 1372 (2.5) | 0.012 | 0.001 |
| Sleep apnea (%) | 2500 (2.4) | 866 (1.7) | 1634 (3.0) | 0.086 | 0.026 |
| Drugs | |||||
| Aspirin (%) | 48,001 (46.0) | 23,195 (46.1) | 24,806 (45.9) | 0.004 | 0.002 |
| ACE inhibitors (%) | 30,094 (28.9) | 11,288 (22.4) | 18,806 (34.8) | 0.276 | 0.021 |
| ARB (%) | 8562 (8.2) | 2462 (4.9) | 6100 (11.3) | 0.236 | 0.005 |
| Beta-blockers (%) | 34,445 (33.0) | 12,075 (24.0) | 22,370 (41.4) | 0.378 | 0.030 |
| CCB (%) | 39,615 (38.0) | 16,297 (32.4) | 23,318 (43.2) | 0.224 | 0.015 |
| Diuretics (%) | 31,476 (30.2) | 14,644 (29.1) | 16,832 (31.2) | 0.045 | 0.054 |
| Statins (%) | 29,905 (28.7) | 2110 (4.2) | 27,795 (51.5) | 1.241 | 0.127 |
| Insulin (%) | 8175 (7.8) | 2085 (4.1) | 6090 (11.3) | 0.270 | 0.046 |
| Other anti-DM drugs (%) | 23,628 (22.7) | 7490 (14.9) | 16,138 (29.9) | 0.366 | 0.053 |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; CAD, coronary artery disease; CCB, calcium channel blocker; DM, diabetes; IPTW, inverse probability of treatment weighting; No., number; PVD, Peripheral vascular disease; SMD, standard mean difference.
Subgroup analyses were performed by age groups (age ≤65, 65 < age ≤ 75, 75 < age ≤ 85, age >85 years old), sex (male and female), education levels, comorbidities (with or without hypertension, diabetes, obesity, stroke, anemia, and coronary artery disease), and statin sub-types (lipophilic statin vs. hydrophilic statin). We also conducted several sensitivity analyses including (i) a traditional multivariable Cox proportional hazards regression model without accounting for IPTW or competing risk; (ii) an alternative 1:1 propensity score matching instead of IPTW; (iii) excluding death and incident dementia within 2 years after the index date to minimise reverse causation; (iv) excluding participants with baseline statin use before the index date to ensure new statin use after HF diagnosis; (v) modelling statin use as a time-varying variable to reduce immortal time bias; (vi) including statin use initiated beyond the first year of the index date (N = 56,722); and (vii) examining the association between statin use and traffic accidents, as a falsification endpoint (excluding participants with a history of traffic accidence before HF diagnoses, N = 1218). Furthermore, considering socioeconomic status as a crucial confounder for dementia incidence, we performed additional analyses in a subgroup with detailed education records (N = 63,020). Patients were stratified into 4 groups based on education levels, including less than primary education (N = 16,334), primary education (N = 25,089), secondary education (N = 17,705), and tertiary and higher education (N = 3892). The association between statin use and incident dementia was evaluated within each education stratum. To determine the role of LDL-C in relation to incident dementia, we further evaluated the association in three LDL-C strata: LDL-C ≤1.8 mmol/L; 1.8 < LDL-C ≤ 2.6 mmol/L; LDL-C >2.6 mmol/L. For all analyses, 95% confidence intervals (95% CI) are reported and P-values <0.05 denoted statistical significance. All the statistical analyses were performed using R version 4.1.3.
Results
Study cohort
Between 2004 and 2018, 104,295 eligible patients with HF were included in our study. The mean age was 74.2 ± 13.6 years, with 50.3% male. Nearly half of the population had hypertension, one-third had CAD, a quarter had diabetes or AF. 54,004 statin users and 50,291 statin non-users were defined (Table 1). After IPTW, covariates were generally well-balanced with SMD <0.2. Although statin users still had a higher propensity for anemia, coronary artery disease, and dyslipidemia. The baseline characteristics of the weighted cohort were described in Supplementary Table 2. During a median follow-up of 9.9 years [interquartile range (IQR), 6.4–13.0], 10,031 patients developed dementia, including 2250 cases of Alzheimer's disease, 1831 vascular dementia, and 5950 unspecified dementia (Table 2).
Table 2.
The impact of statin use on dementia incidence.a
| Dementia and subtypes | No. of events/total number | 10-Year cumulative incidence (%) | SHR (95% CI)b |
|
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| All types of dementia | 10,031 | |||
| Statin non-use | 5757/50,291 | 11.0 | Ref. | Ref. |
| Statin use | 4274/54,004 | 7.3 | 0.73 (0.70–0.76) | 0.80 (0.76–0.84) |
| Alzheimer's disease | 2250 | |||
| Statin non-use | 1374/50,291 | 2.6 | Ref. | Ref. |
| Statin use | 876/54,004 | 1.5 | 0.64 (0.58–0.69) | 0.72 (0.63–0.82) |
| Vascular dementia | 1831 | |||
| Statin non-use | 954/50,291 | 1.8 | Ref. | Ref. |
| Statin use | 877/54,004 | 1.5 | 0.91 (0.83–1.00) | 0.82 (0.70–0.95) |
| Unspecified dementia | 5950 | |||
| Statin non-use | 3429/50,291 | 6.4 | Ref. | Ref. |
| Statin use | 2521/54,004 | 4.3 | 0.73 (0.69–0.77) | 0.80 (0.75–0.85) |
Abbreviations: Ref., reference; SHR, sub-distribution hazards ratio; CI, confidence interval.
Inverse probability of treatment weighting (IPTW) was used to balance the covariates between statin users and non-users. A multivariable-adjusted model further accounted for the demographic covariates, comorbidities, and medications.
We calculated the sub-distribution hazards ratio (SHR) and 95% confidence interval (95% CI) using the Fine Gray's test for equality of the cumulative functions between each exposure group after accounting for competing risks of all-cause mortality.
Dementia incidence
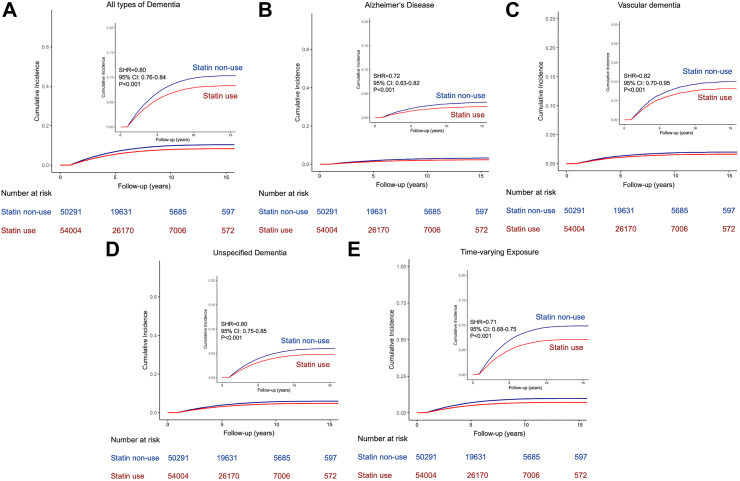
The 10-year cumulative incidence of overall dementia was 11.0% among statin non-users and 7.3% among statin users. Statin users had a 20% lower risk of dementia than non-users after multivariable adjustment with competing risk regression (adjusted SHR, 0.80; 95% CI 0.76–0.84).
With regard to subtypes of dementia, statin use consistently reduced the risk of Alzheimer's disease, vascular dementia, and unspecified dementia. For Alzheimer's disease, the 10-year cumulative incidence was 1.5% among statin users and 2.6% among non-users. Statin users had a 28% lower risk of Alzheimer's disease than non-users (adjusted SHR, 0.72; 95% CI 0.63–0.82). The 10-year cumulative incidence of vascular dementia was 1.5% among statin users and 1.8% among non-users. Statin users had an 18% lower risk of vascular dementia than non-users (adjusted SHR, 0.82; 95% CI 0.70–0.95). For unspecified dementia, the 10-year cumulative incidence was 4.3% among statin users and 6.4% among non-users, with a 20% lower risk with statin use (adjusted SHR, 0.80; 95% CI 0.75–0.85) (Table 2, Fig. 2).
Fig. 2.
(A) The impact of statin use on all types of dementia incidence compared with statin non-use; (B) The impact of statin use on Alzheimer's disease incidence compared with statin non-use; (C) The impact of statin use on vascular dementia incidence compared with statin non-use; (D) The impact of statin use on unspecified dementia incidence compared with statin non-use; (E) The impact of statin use on all types of dementia incidence compared with statin non-use after modeling statin use as a time-varying exposure. All the analyses were calculated after IPTW and competing risk regression. The inset shows the same data on an expanded y axis.
Subgroups
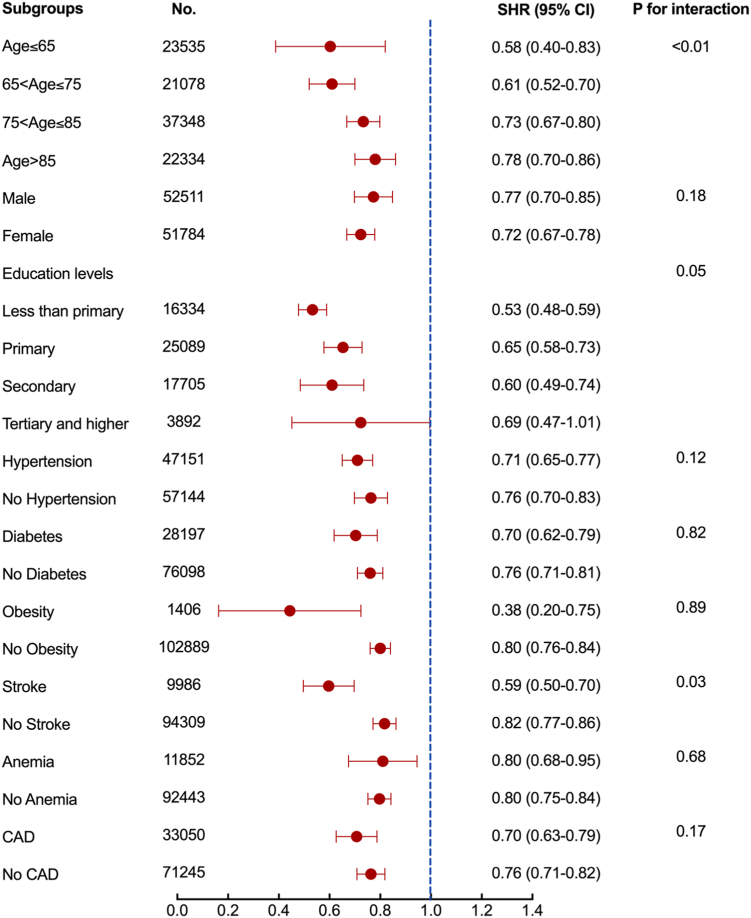
Due to the close association between education and dementia, we performed subgroup analyses in 63,020 participants with detailed education records. The baseline characteristics of these patients are summarized in Supplementary Table 3. Patients were divided into 4 groups based on their education level: less than primary education (N = 16,334), primary education (N = 25,089), secondary education (N = 17,705), and tertiary and higher education (N = 3892). After correction for education in the multivariate regression model, statin use remained associated with a lower risk of dementia (SHR 0.60, 95% CI 0.56–0.64, Table 3). These results were consistent across subgroups of education levels. Specifically, statin use was associated with a 47% lower risk of dementia (SHR 0.53, 95% CI 0.48–0.59) compared with statin non-use in patients with less than primary education, 35% lower risk (SHR 0.65, 95% CI 0.58–0.73) in patients with primary education, and 40% lower risk (SHR 0.60, 95% CI 0.49–0.74) in patients with secondary education. In patients with tertiary or higher education, statin use was marginally associated with a 31% lower risk of dementia, with a wide confidence interval owing to the small number of events (SHR 0.69, 95% CI 0.47–1.01) (Fig. 3).
Table 3.
Sensitivity analyses.
| Sensitivity analyses | Statin non-use (ref.) | Statin use (multi-adjusted SHR, 95% CI)a |
|---|---|---|
| Principal result | 1 | 0.80 (0.76–0.84) |
| Cox regression without competing risk | 1 | 0.62 (0.59–0.65) |
| 1:1 propensity score matching | 1 | 0.84 (0.80–0.88) |
| Exclude death and dementia within 2 years after the index date | 1 | 0.81 (0.77–0.86) |
| Exclude baseline statin use to define statin new users | 1 | 0.83 (0.78–0.88) |
| Statin use was modeled as a time-varying exposure | 1 | 0.71 (0.68–0.75) |
| Adjust for education | 1 | 0.60 (0.56–0.64) |
Abbreviations: CI, confidence interval; No., number; Ref., reference; SHR, sub-distribution hazards ratio.
We calculated the SHR and 95% CI using the Fine Gray's test for equality of the cumulative functions between each exposure group after accounting for competing risks of all-cause mortality.
Fig. 3.
Subgroup analysis for the impact of statin use on dementia incidence. We calculated the SHR and 95% CI after IPTW and competing risk regression with each subgroup.
Since age is an important confounder for dementia, we divided patients into 4 age groups. The SHR (95% CI) for dementia in statin users was 0.58 (0.40–0.83) in the less than 65 years old age group, 0.61 (0.52–0.70) in the 65–75 age group, 0.73 (0.67–0.80) in the 75–85 age group, and 0.78 (0.70–0.86) in more than 85 years old age group compared with statin non-users. When stratified by sex, statin use was associated with a 23% lower risk in males (SHR 0.77, 95% CI 0.70–0.85) and a 28% lower risk in females (SHR 0.72, 95% CI 0.67–0.78). Further, the association of statin use with a lower risk of incident dementia was consistent across subgroups of patients with or without hypertension, diabetes, obesity, stroke, anemia, and CAD (Fig. 3). Upon stratifying the statin sub-types into lipophilic and hydrophilic categories, we found that patients prescribed lipophilic statin exhibited lower SHRs compared to those receiving. Nonetheless, the differences did not reach statistical significance. It is noteworthy that the results should be interpreted with caution due to the limited sample size in the hydrophilic statin subgroup (Supplementary Table 4).
Low-density lipoproteins (LDL-C)
To evaluate the role of LDL-C levels in relation to incident dementia, we limited the analyses to 83,125 participants with more than two LDL-C records after HF diagnosis and modeled the LDL-C as a time-weighted covariate. Compared to patients with time-weighted LDL-C ≤1.8 mmol/L, those with 1.8–2.6 mmol/L LDL-C had a 21% higher risk of dementia incidence (SHR 1.21, 95% CI 1.13–1.29), while those with more than 2.6 mmol/L LDL-C incurred a 51% higher risk (SHR 1.51, 95% CI 1.41–1.61, Supplementary Table 5). To evaluate the potential pleiotropic effects of statin on dementia, we performed Cox hazards analyses with competing risk regression within each LDL-C group. The SHRs for the association of statin use with dementia incidence were 0.61 (95% CI 0.49–0.76) for LDL-C ≤1.8 mmol/L, 0.71 (95% CI 0.63–0.80) for LDL-C 1.8–2.6 mmol/L and 0.84 for LDL-C of more than 2.6 mmol/L (95% CI 0.77–0.91), separately (Supplementary Table 6).
All-cause mortality
A total of 62,945 (60.4%) patients died. Among which, 29,054 were statin users while 33,891 were statin non-users. Statin use was associated with a 30% (HR 0.70, 95% CI 0.69–0.72) lower risk of all-cause mortality.
Sensitivity analyses
Without IPTW and competing risk regression, a traditional Cox proportional hazards regression model consistently showed that statin use was associated with a 38% lower risk of dementia incidence compared with non-use (SHR 0.62, 95% CI 0.59–0.65). Instead of IPTW, an alternative 1:1 propensity score matching (Supplementary Table 7) showed a consistent result with SHR 0.84 (95% CI 0.80–0.88). To reduce reverse causation, we excluded those who were dead or developed incident dementia within 2 years after the index date, a 19% lower risk of dementia was observed among statin users compared with non-user (SHR 0.81, 95% CI 0.77–0.86). After excluding those with statin use before the index date, newly statin use was associated with a 17% lower risk of dementia (SHR 0.83, 95% CI 0.78–0.88) compared with non-use. To minimize immortal time bias, statin use was modeled as a time-varying exposure and showed a 29% lower risk (SHR 0.71, 95% CI 0.68–0.75) (Table 3, Fig. 2E). After adding statin use initiated beyond the first year of the index date, statin use remained associated with lower risks of dementia incidence and its sub-types (Table 4). Further, we chose traffic accidents as a negative control. No significant difference between statin users and non-users was shown with regard to traffic accidents (SHR 1.25, 95% CI 0.90–1.74, Supplementary Table 8).
Table 4.
Sensitivity analysis on the impact of statin use with dementia incidence and its sub-types (including statin use initiated beyond the first year of the index date).
| Dementia and subtypes | Statin non-use (ref.) | Statin use (multi-adjusted SHR, 95% CI)a |
|---|---|---|
| No. | 47,573 | 56,722 |
| All-cause dementia | 1 | 0.86 (0.82–0.90) |
| Alzheimer's disease | 1 | 0.84 (0.76–0.93) |
| Vascular dementia | 1 | 0.88 (0.78–0.99) |
| Unspecified dementia | 1 | 0.84 (0.79–0.89) |
Abbreviations: CI, confidence interval; No., number; Ref., reference; SHR, sub-distribution hazards ratio.
We calculated the SHR and 95% CI using the Fine Gray's test for equality of the cumulative functions between each exposure group after accounting for competing risks of all-cause mortality.
Discussion
In this territory-wide cohort study of 104,295 patients with HF, statin use was demonstrated to be associated with a 20% lower risk of all-cause dementia. Specifically, statin use was associated with a 28% risk reduction in Alzheimer's disease, 18% risk reduction in vascular dementia, and 20% risk reduction in unspecified dementia. The results were consistent across subgroups of age, gender, comorbidities, education levels and LDL-C levels, indicating that the cognitive benefits of statin may apply to a broad population of patients with HF.
Cognitive impairment is common in patients with HF, with estimates of its prevalence ranging from 10 to 68%.7,29 When present, it confers a worse prognosis, with loss of independence, impaired quality of life, and a higher risk of mortality. For mild HF, the predominant morphological feature of brain injury includes atrophy of the medial temporal lobe, accompanied by significant cognitive declines in attention and memory.7 Other reports have also implicated multiple aspects of higher cerebral dysfunction in chronic HF, including domains of cognition, language, psychomotor function, and visuospatial acuity.29,30 Although the precise pathophysiological processes involved are still under debate, events including poor perfusion, micro-embolism, ischaemic syndromes, cerebral inflammation and endothelial dysfunction have been proposed to play a role.31,32 Additionally, shared risk factors, including vascular comorbidities of atrial fibrillation and diabetes, may further predispose patients with HF to dementia.33,34 Given HF's strong association with cognitive impairment and its assorted complications, potential strategies to reduce the risk of dementia in patients with HF are urgently needed. Accordingly, emerging evidence has suggested that higher LDL-C concentrations were associated with an increased dementia risk35 independent of vascular risk factors.36 A recent Mendelian Randomization Study37 also suggested that genetically low LDL-C levels may reduce the risk of Alzheimer's disease. Our study, based on serum time-weighted LDL-C, further demonstrates that increasing LDL-C was associated with a stepwise increase in dementia risk. Particularly, a serum time-weighted LDL-C between 1.8 and 2.6 mmol/L or more than 2.6 mmol/L was associated with a 21% or 51% higher risk of incident dementia compared with a time-weighted LDL-C of less than 1.8 mmol/L. These results unveil LDL-C control as a potential target for preventing dementia and provide a crucial rationale for exploring lipid-lowering therapies to prevent the progression of cognitive impairment.
It is noteworthy that current guidelines do not recommend the routine use of statins in patients with HF unless there is an underlying indication. Prior studies have demonstrated the neuroprotective benefits of statin in patients with ischemic heart disease,38 atrial fibrillation,39 post-stroke,40 diabetes,41 and the older people.42 Our results corroborate previous studies, suggesting the benefits of statin use on reducing the risk of dementia and extend these findings for the first time to a large population-based cohort of patients with HF.16,17,43 In a sample of Medicare Beneficiaries between 2006 and 2013, statin use was associated with a reduced risk of Alzheimer's disease, with significant variation across statin molecules, sex, and race/ethnicity.43 In a nationwide study, statin users had a 22% lower risk of incident dementia, contingent on the potency and duration of use.17 However, these studies have been limited by imbalanced exposure groups, a relatively short follow-up (given the long duration of dementia development), and the lack of detailed information on education levels and the prescription of key medications.17,43,44 Our study, with vigorous statistical adjustment and detailed clinical, education, laboratory, and medication information, provides reasonably strong observational evidence of the potential cognitive-protective benefits of statin in HF populations. In contrast, in a longitudinal study involving community-dwelling elderly individuals with a follow-up period exceeding 6 years, statin therapy did not demonstrate any substantial improvement in memory or cognition.45 Similarly, among older adults, statin therapy did not exhibit any association with incident dementia, mild cognitive impairment (MCI), or declines in specific cognitive domains.12 These observations could likely be attributed to differences in cohorts, with community-dwelling participants vs. patients with HF (in our study). Among older patients, our findings also showed a weaker association among older vs. younger patients.
Reasons behind the stronger association in individuals who were younger and with lower LDL levels are unclear. Older individuals are more likely to have multiple comorbidities and other risk factors for dementia, such as hypertension, diabetes, or cardiovascular disease. These conditions may contribute to the development of dementia, making it harder for statins alone to provide substantial protection. Our result was consistent with the analysis of UK biobank which showed that the benefit of statin was stronger in individuals under 65, suggesting that statin therapy in earlier life could be more beneficial for cognitive function.46 Indeed, our study showed that the hazard ratio for statin use and dementia incidence was comparatively lower among patients with lower LDL-C levels. However, the differences observed between LDL-C groups (LDL ≤1.8, 1.8 < LDL ≤ 2.6, LDL >2.6 mmol/L) did not reach statistical significance. Nevertheless, we hypothesized that in addition to lipid-lowering effect, statins may also have anti-inflammatory and antioxidant properties that may further protect the brain from impairment and thus reduce the risk of dementia. Future randomized studies and experimental trials to verify the cognitive protective effects of statin are warranted. Moreover, the association between statin use and dementia incidence showed no significant difference between those with or without hypertension or coronary artery disease (CAD) at baseline. These findings provided compelling evidence suggesting that statins may employ additional cognitive protective mechanisms, beyond their lipid-lowering effects, in reducing the risk of dementia. Possible mechanisms contributing to this protective effect may include but are not limited to anti-inflammatory effects, antioxidant properties, and modulation of amyloid-beta metabolism. However, it is noteworthy that there was an interaction with stroke, but not coronary artery disease. Our hypothesis was that patients with stroke experience direct damage to the cerebral regions whereas CAD may not significantly affect the brain.
While it may be compelling to attribute statin's benefit solely to its lipid-lowering effect, our study results suggest otherwise, as the neuroprotective benefit of statin in HF was observed even after adjusting for extent of LDL-C-lowering. Accordingly, the reduction of dementia risk is likely mediated through both LDL-C-dependent and LDL-C-independent pleiotropic pathways. While the brain cholesterol metabolism is largely segregated from their systemic counterpart by the Blood Brain Barrier (BBB), oxysterols such as 24S-hydroxycholesterol and 27-hydroxycholesterol have been shown capable of passing the BBB and mediating the progression of dementia via LDL-C-dependent mechanisms.47,48 Specifically, statins may directly increase apoE receptor-mediated clearance of Aβ,49 or indirectly disrupt amyloidogenic processing of Aβ (via lowering cholesterol levels),48 thereby preventing Aβ accumulation and subsequent development of Alzheimer's disease. For LDL-C-independent pathways, statins may exert anti-oxidant, anti-thrombotic, anti-inflammatory, and vasodilatory effects that may prevent cognitive impairment and dementia.50 These postulations are further supported by a German study that found a significant neurocognitive benefit of statin use despite a null association between baseline LDL-C levels and dementia risk.42 Based on time-weighted LDL-C levels, which account for multiple LDL-C levels over follow-up, our study provides a more robust and accurate evaluation of LDL-C concentration to substantiate the pleiotropic effects of statin use for preventing dementia in patients with HF.
Strengths of this study include the use of a territory-wide, well-validated, and prospectively updated electronic healthcare database (CDARS) with records of all demographics, comorbidities, medications, laboratory tests, and outcomes, precluding selection bias and residual confounding common in observational studies. Reverse causation was attenuated by accounting for the time duration between index date and endpoints. Various statistical approaches were also applied to address potential biases, including sensitivity analysis and a negative control. We further modeled statin use as a time-varying exposure to minimize misclassification and immortal time bias.
This study has several limitations. Firstly, there was the noticeably high percentage of patients diagnosed with unspecified dementia. In addition to the potential for coding inconsistencies, databases based on administrative registries frequently face a critical challenge that a considerable proportion of dementia cases are categorized as unspecified.51, 52, 53 However, our results align with the distribution of differential causes of dementia identified among nursing home residents with dementia,51 as well as those observed in German primary care.52 The analyses of patients in ambulatory medical care showed that unspecified dementia was diagnosed by non-specialists in 62% of patients and by specialists (neurologists/psychiatrists) in 46% of patients.53 Education and serum LDL-C levels can only be retrieved in a subgroup of the HF population leading to potential selection bias. Detailed information of left ventricular ejection fraction was missing, and the differential benefit of statin use in reducing the risk of dementia in heart failure with preserved or reduced ejection fraction cannot be evaluated. Nonetheless, the incidence of dementia in HF has been shown to be increased regardless of the ejection fraction.2 Moreover, we were unable to determine the precise dosage of statin medication prescribed to each individual, thus impeding our ability to conduct a dose-response analysis. However, it is noteworthy that the negative association between statin use and dementia incidence was observed across all dosage levels of statin therapy.42 CDARS, as an administrative registry, may present a potential underestimation of the prevalence of alcohol consumption and obesity in our study population. However, the association between statin use and dementia incidence in patients with HF was independent of the aforementioned variables, as per our findings. As this is not an interventional study, causality cannot be established. Furthermore, there is prescription bias; patients with heart failure who were prescribed statins inherently differ from those who do not receive such treatment. Residual confounders remained even after IPTW and multi-variable adjustment.
Conclusion
In this territory-wide cohort study of patients with HF, statin use was significantly associated with lower risks of all-cause dementia and its subtypes, across multiple subgroups and potentially occurring via LDL-C-dependent and LDL-C-independent mechanisms. These findings can provide useful insights to informing the design and rationale of future randomized trials to provide definitive answer to this research question. The cognitive benefits of statins in HF warrant evaluation in future randomized studies.
Contributors
QW Ren and KH Yiu designed the study. QW Ren collected, analysed the data and drafted the manuscript. TH Teng and YK Tse provided assistance with data validation, analysis and manuscript drafting. CT Tsang, SY Yu, MZ Wu, XL Li, D Hung, HF Tse and CSP Lam critically revised the manuscript and provided the final approval. QW Ren and KH Yiu accessed and verified the data of this study and were responsible for the decision to submit the manuscript.
Data sharing statement
Data could be obtained upon request.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
There is no conflict of interest.
Acknowledgements
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.101006.
Appendix A. Supplementary data
References
- 1.James S.L., Abate D., Abate K.H., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon J.A., Moffitt P., Perez-Moreno A.C., et al. Cognitive impairment and heart failure: systematic review and meta-analysis. J Card Fail. 2017;23:464–475. doi: 10.1016/j.cardfail.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Vishwanath S., Qaderi V., Steves C.J., et al. Cognitive decline and risk of dementia in individuals with heart failure: a systematic review and meta-analysis. J Card Fail. 2022;28:1337–1348. doi: 10.1016/j.cardfail.2021.12.014. [DOI] [PubMed] [Google Scholar]
- 4.https://www.alzint.org/u/WorldAlzheimerReport2015.pdf
- 5.Holm H., Bachus E., Jujic A., et al. Cognitive test results are associated with mortality and rehospitalization in heart failure: Swedish prospective cohort study. ESC Heart Fail. 2020;7:2948–2955. doi: 10.1002/ehf2.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almeida O.P., Garrido G.J., Beer C., et al. Cognitive and brain changes associated with ischaemic heart disease and heart failure. Eur Heart J. 2012;33:1769–1776. doi: 10.1093/eurheartj/ehr467. [DOI] [PubMed] [Google Scholar]
- 7.Frey A., Sell R., Homola G.A., et al. Cognitive deficits and related brain lesions in patients with chronic heart failure. JACC Heart Fail. 2018;6:583–592. doi: 10.1016/j.jchf.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Adelborg K., Horvath-Puho E., Ording A., et al. Heart failure and risk of dementia: a Danish nationwide population-based cohort study. Eur J Heart Fail. 2017;19:253–260. doi: 10.1002/ejhf.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu C., Winblad B., Marengoni A., et al. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 10.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebocontrolled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 11.Shepherd J., Blauw G.J., Murphy M.B., et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Z., Ryan J., Ernst M.E., et al. Effect of statin therapy on cognitive decline and incident dementia in older adults. J Am Coll Cardiol. 2021;77:3145–3156. doi: 10.1016/S0735-1097(21)04500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zandi P.P., Sparks D.L., Khachaturian A.S., et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry. 2005;62:217–224. doi: 10.1001/archpsyc.62.2.217. [DOI] [PubMed] [Google Scholar]
- 14.Ancelin M.L., Carriere I., Barberger-Gateau P., et al. Lipid lowering agents, cognitive decline, and dementia: the three-city study. J Alzheimers Dis. 2012;30:629–637. doi: 10.3233/JAD-2012-120064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arvanitakis Z., Schneider J.A., Wilson R.S., et al. Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology. 2008;70:1795–1802. doi: 10.1212/01.wnl.0000288181.00826.63. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X., Wen J., Zhang Z. Statins use and risk of dementia: a dose-response meta analysis. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000011304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou C.Y., Chou Y.C., Chou Y.J., et al. Statin use and incident dementia: a nationwide cohort study of Taiwan. Int J Cardiol. 2014;173:305–310. doi: 10.1016/j.ijcard.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Chu C.S., Tseng P.T., Stubbs B., et al. Use of statins and the risk of dementia and mild cognitive impairment: a systematic review and meta-analysis. Sci Rep. 2018;8:5804. doi: 10.1038/s41598-018-24248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong J.H., Yum K.S., Chang J.Y., et al. Dose-specific effect of simvastatin on hypoxia-induced HIF-1alpha and BACE expression in Alzheimer's disease cybrid cells. BMC Neurol. 2015;15:127. doi: 10.1186/s12883-015-0390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.https://www.scribd.com/document/514060182/HASR-2018-2019-HAO
- 21.Cheung K.S., Chan E.W., Wong A.Y.S., et al. Statins were associated with a reduced gastric cancer risk in patients with eradicated Helicobacter pylori infection: a territory-wide propensity score matched study. Cancer Epidemiol Biomarkers Prev. 2020;29:493–499. doi: 10.1158/1055-9965.EPI-19-1044. [DOI] [PubMed] [Google Scholar]
- 22.Ren Q.W., Yu S.Y., Teng T.K., et al. Statin associated lower cancer risk and related mortality in patients with heart failure. Eur Heart J. 2021;42:3049–3059. doi: 10.1093/eurheartj/ehab325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y., Pletcher M.J., Vittinghoff E., et al. Association between cumulative low-density lipoprotein cholesterol exposure during young adulthood and middle age and risk of cardiovascular events. JAMA Cardiol. 2021;6:1406–1413. doi: 10.1001/jamacardio.2021.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwok W.C., Tam T.C.C., Sing C.W., et al. Validation of diagnostic coding for bronchiectasis in an electronic health record system in Hong Kong. Pharmacoepidemiol Drug Saf. 2023;32:1077–1082. doi: 10.1002/pds.5638. [DOI] [PubMed] [Google Scholar]
- 25.Ye Y., Hubbard R., Li G.H., et al. Validation of diagnostic coding for interstitial lung diseases in an electronic health record system in Hong Kong. Pharmacoepidemiol Drug Saf. 2022;31:519–523. doi: 10.1002/pds.5421. [DOI] [PubMed] [Google Scholar]
- 26.Kwok W.C., Tam T.C.C., Sing C.W., et al. Validation of diagnostic coding for asthma in an electronic health record system in Hong Kong. J Asthma Allergy. 2023;16:315–321. doi: 10.2147/JAA.S405297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung K.S., Chan E.W., Chen L., et al. Diabetes increases risk of gastric cancer after Helicobacter pylori eradication: a territory-wide study with propensity score analysis. Diabetes Care. 2019;42:1769–1775. doi: 10.2337/dc19-0437. [DOI] [PubMed] [Google Scholar]
- 28.Cheung K.S., Chan E.W., Wong A.Y.S., et al. Metformin use and gastric cancer risk in diabetic patients after Helicobacter pylori eradication. J Natl Cancer Inst. 2019;111:484–489. doi: 10.1093/jnci/djy144. [DOI] [PubMed] [Google Scholar]
- 29.Sauve M.J., Lewis W.R., Blankenbiller M., et al. Cognitive impairments in chronic heart failure: a case controlled study. J Card Fail. 2009;15:1–10. doi: 10.1016/j.cardfail.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Alwerdt J., Edwards J.D., Athilingam P., et al. Longitudinal differences in cognitive functioning among older adults with and without heart failure. J Aging Health. 2013;25:1358–1377. doi: 10.1177/0898264313505111. [DOI] [PubMed] [Google Scholar]
- 31.Gruhn N., Larsen F.S., Boesgaard S., et al. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–2533. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- 32.Taylor J., Stott D.J. Chronic heart failure and cognitive impairment: co-existence of conditions or true association? Eur J Heart Fail. 2002;4:7–9. doi: 10.1016/s1388-9842(01)00182-9. [DOI] [PubMed] [Google Scholar]
- 33.Xu W.L., Qiu C.X., Wahlin A., et al. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63:1181–1186. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- 34.Bunch T.J. Atrial fibrillation and dementia. Circulation. 2020;142:618–620. doi: 10.1161/CIRCULATIONAHA.120.045866. [DOI] [PubMed] [Google Scholar]
- 35.Iwagami M., Qizilbash N., Gregson J., et al. Blood cholesterol and risk of dementia in more than 1·8 million people over two decades: a retrospective cohort study. Lancet Healthy Longev. 2021;2:e498–e506. doi: 10.1016/S2666-7568(21)00150-1. [DOI] [PubMed] [Google Scholar]
- 36.Schilling S., Tzourio C., Soumare A., et al. Differential associations of plasma lipids with incident dementia and dementia subtypes in the 3C study: a longitudinal, population-based prospective cohort study. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benn M., Nordestgaard B.G., Frikke-Schmidt R., et al. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer's disease and Parkinson's disease: Mendelian randomisation study. BMJ. 2017;357:j1648. doi: 10.1136/bmj.j1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim M.Y., Jung M., Noh Y., et al. Impact of statin use on dementia incidence in elderly men and women with ischemic heart disease. Biomedicines. 2020;8:30. doi: 10.3390/biomedicines8020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chao T.F., Liu C.J., Chen S.J., et al. Statins and the risk of dementia in patients with atrial fibrillation: a nationwide population-based cohort study. Int J Cardiol. 2015;196:91–97. doi: 10.1016/j.ijcard.2015.05.159. [DOI] [PubMed] [Google Scholar]
- 40.Yang Z., Toh S., Li X., et al. Statin use is associated with lower risk of dementia in stroke patients: a community-based cohort study with inverse probability weighted marginal structural model analysis. Eur J Epidemiol. 2022;37:615–627. doi: 10.1007/s10654-022-00856-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J.M., Chen T.H., Chuang H.C., et al. Statin reduces the risk of dementia in diabetic patients receiving androgen deprivation therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2019;22:276–283. doi: 10.1038/s41391-018-0091-4. [DOI] [PubMed] [Google Scholar]
- 42.Zingel R., Bohlken J., Riedel-Heller S., et al. Association between low-density lipoprotein cholesterol levels, statin use, and dementia in patients followed in German general practices. J Alzheimers Dis. 2021;79:37–46. doi: 10.3233/JAD-201176. [DOI] [PubMed] [Google Scholar]
- 43.Zissimopoulos J.M., Barthold D., Brinton R.D., et al. Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurol. 2017;74:225–232. doi: 10.1001/jamaneurol.2016.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H., Cui Y., Zhao Y., et al. Effects of sartans and low-dose statins on cerebral white matter hyperintensities and cognitive function in older patients with hypertension: a randomized, double-blind and placebo-controlled clinical trial. Hypertens Res. 2019;42:717–729. doi: 10.1038/s41440-018-0165-7. [DOI] [PubMed] [Google Scholar]
- 45.Samaras K., Makkar S.R., Crawford J.D., et al. Effects of statins on memory, cognition, and brain volume in the elderly. J Am Coll Cardiol. 2019;74:2554–2568. doi: 10.1016/j.jacc.2019.09.041. [DOI] [PubMed] [Google Scholar]
- 46.Alsehli A.M., Olivo G., Clemensson L.E., et al. The cognitive effects of statins are modified by age. Sci Rep. 2020;10:6187. doi: 10.1038/s41598-020-63035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu M., Zhai Y., Liang X., et al. Connecting the dots between hypercholesterolemia and Alzheimer's disease: a potential mechanism based on 27-hydroxycholesterol. Front Neurosci. 2022;16 doi: 10.3389/fnins.2022.842814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petek B., Villa-Lopez M., Loera-Valencia R., et al. Connecting the brain cholesterol and renin-angiotensin systems: potential role of statins and RAS-modifying medications in dementia. J Intern Med. 2018;284:620–642. doi: 10.1111/joim.12838. [DOI] [PubMed] [Google Scholar]
- 49.Shinohara M., Sato N., Shimamura M., et al. Possible modification of Alzheimer's disease by statins in midlife: interactions with genetic and non-genetic risk factors. Front Aging Neurosci. 2014;6:71. doi: 10.3389/fnagi.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loera-Valencia R., Goikolea J., Parrado-Fernandez C., et al. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer's disease: potential novel targets for treatment. J Steroid Biochem Mol Biol. 2019;190:104–114. doi: 10.1016/j.jsbmb.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Huber M., Kolzsch M., Rapp M.A., et al. Antipsychotic drugs predominate in pharmacotherapy of nursing home residents with dementia. Pharmacopsychiatry. 2012;45:182–188. doi: 10.1055/s-0031-1301285. [DOI] [PubMed] [Google Scholar]
- 52.Eichler T., Thyrian J.R., Hertel J., et al. Rates of formal diagnosis in people screened positive for dementia in primary care: results of the DelpHi-trial. J Alzheimers Dis. 2014;42:451–458. doi: 10.3233/JAD-140354. [DOI] [PubMed] [Google Scholar]
- 53.Kaduszkiewicz H., Wiese B., Steinmann S., et al. [Diagnosing and diagnosis coding of dementias in claims data from German statutory health insurance] Psychiatr Prax. 2014;41:319–323. doi: 10.1055/s-0033-1349505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.