Abstract
Respiratory symptoms with rotavirus shedding in nasopharyngeal secretions have been reported in children with and without gastrointestinal symptoms (Zheng et al., 1991, J. Med. Virol. 34:29-37). To investigate if attenuated and virulent human rotavirus (HRV) strains cause upper respiratory tract infections or viremia in gnotobiotic pigs, we inoculated them with attenuated or virulent HRV intranasally, intravenously, or orally or via feeding tube (gavage) and assayed virus shedding. After oral or intranasal inoculation with attenuated HRV, the pigs remained asymptomatic, but 79 to 95% shed virus nasally and 5 to 17% shed virus rectally. After inoculation by gavage, no pigs shed virus nasally or rectally, but all pigs seroconverted with antibodies to HRV. No viremia was detected through postinoculation day 10. Controls inoculated intranasally with nonreplicating rotavirus-like particles or mock inoculated did not shed virus. In contrast, 100% of pigs inoculated with virulent HRV (oral, intranasal, or gavage) developed diarrhea, shed virus nasally and rectally, and had viremia. The infectivity of sera from the viremic virulent HRV-inoculated pigs was confirmed by inoculating gnotobiotic pigs orally with pooled HRV-positive serum. Serum-inoculated pigs developed diarrhea and fecal and nasal virus shedding and seroconverted with serum and intestinal HRV antibodies. Pigs inoculated intravenously with serum or intestinal contents from the viremic virulent HRV-inoculated pigs developed diarrhea, virus shedding, and viremia, similar to the orally inoculated pigs. This study provides new evidence that virulent HRV causes transient viremia and upper respiratory tract infection in addition to gastrointestinal infection in gnotobiotic pigs, confirming previous reports of rotavirus antigenemia (Blutt et al., Lancet 362:1445-1449, 2003). Our data also suggest that intestinal infection might be initiated from the basolateral side of the epithelial cells via viremia. Additionally, virus shedding patterns indicate a different pathogenesis for attenuated versus virulent HRV.
Group A rotaviruses are the most common cause of dehydrating diarrhea in infants and young children worldwide, with more than 2 million hospitalizations yearly and approximately 440,000 deaths. It is estimated that 82% of rotavirus deaths occur in children in the poorest countries (23). Rotavirus transmission occurs mainly by the fecal-oral route, although respiratory transmission has been suggested to occur (7).
Rotavirus infection was thought to be limited to the gastrointestinal tract. However, respiratory symptoms and rotavirus shedding in nasopharyngeal secretions have been reported in children with and without gastrointestinal symptoms (19, 26, 42). Rotavirus antigen was detected in the lung of 1 of 13 experimentally infected 3-week-old conventional pigs at postinoculation day 2 (30) and in liver and kidney specimens from immunodeficient children (9). Rotavirus RNA has also been detected in cerebrospinal fluid and blood of children with central nervous system disease (20, 34). Recently, Blutt and colleagues (2) detected rotavirus antigenemia in the serum of children, mice, rabbits, and calves. They further demonstrated that serum from infected mice induced rectal rotavirus antigen shedding after oral inoculation of rotavirus-negative adult mice with the serum. Previously, another enteric virus, the porcine enteric calicivirus (PEC), has also been associated with transient viremia (infectious virus in serum) after oral inoculation of gnotobiotic pigs (11).
We choose gnotobiotic pigs because they constitute an animal model of HRV-induced disease. Their gastrointestinal tract physiology and their development of mucosal immunity resemble that of humans. These similarities with HRV infections of infants allow us to establish correlations which could be applied for rotavirus vaccine development (14, 25)
The question addressed in our study was whether an attenuated human rotavirus and virulent HRV causes upper respiratory tract infections or viremia in naïve neonatal gnotobiotic pigs after various routes of inoculation. In this study we evaluated nasal and rectal virus shedding and viremia after oral, intranasal, feeding tube (gavage), and intravenous inoculation of neonatal gnotobiotic pigs with the Wa strain of attenuated HRV or virulent HRV. The presence of infectious virus in serum of gnotobiotic pigs after oral inoculation with Wa HRV was also investigated by oral and intravenous reinoculation of gnotobiotic pigs with a pool of the HRV-positive sera.
MATERIALS AND METHODS
Virus.
The attenuated cell culture-adapted Wa strain HRV (P1A [8]G1), derived from the 27th HRV passage in African Green monkey kidney cells (MA104) and the virulent Wa HRV from pooled intestinal contents of gnotobiotic pigs were used for inoculation of the gnotobiotic pigs at doses of 5 × 107 fluorescent focus-forming units (FFU) and 106 median infectious doses (ID50), respectively (40). The ID50 of the virulent Wa HRV inoculum for gnotobiotic pigs was previously determined to be at least 1 FFU, and the attenuated Wa HRV was previously determined to be 1.3 × 106 FFU (29, 35, 36).
Virus-like particles.
Recombinant baculoviruses expressing rotavirus proteins VP2 (bovine RF strain), provided by the late J. Cohen (Virologie Moleculaire et Structurale, UMR CNRS-INRA, Gif-sur-Yvette, France), and VP6 (human Wa strain) were used to produce 2/6 virus-like particles (VLPs) by coinfection of Sf9 (Spodoptera frugiperda) insect cells (6). The virus-like particles were purified as previously described and used at 250 μg/dose as a nonreplicating control inoculum (1, 39).
Inoculation of gnotobiotic pigs.
Near-term pigs were derived by surgery and maintained in gnotobiotic isolator units as described using separate isolator units for each treatment group (17). All pigs were seronegative for rotavirus antibodies prior to HRV exposure. At 3 to 5 days of age, pigs were assigned to one of the seven groups and inoculated as follows: single oral dose of attenuated HRV; single intranasal dose of attenuated HRV; single dose of attenuated HRV by gavage; single oral dose of virulent HRV; single intranasal dose of virulent HRV, single dose of virulent HRV by gavage; and single oral dose of minimal essential medium or intranasal dose of 2/6-virus-like particles (controls).
Preceding oral and gavage inoculation, pigs received 5 ml of 100 mM NaHCO3 to reduce gastric acidity. For oral inoculation, 5 ml of virus inoculum was slowly instilled into the mouth at the back of the throat using a needleless syringe. Inoculation via gavage was performed using a sterile feeding tube. The tubing was inserted orally with the pigs held in a vertical position. To assure the tube was in the stomach, milk was withdrawn with a syringe and 5 ml of virus inoculum was then injected using the syringe. Before removing the tubing, 10 ml of minimum essential medium was used to flush the tubing.
Nasal and rectal samples were obtained every day to monitor virus shedding, and blood samples were obtained every other day until postinoculation day 10 for detection of viremia. The samples were taken in the sequence blood, nasal swab, and then rectal swab, to avoid possible cross-contamination of samples. Two cotton-tipped wooden swabs were used per pig per day to obtain the rectal swabs. For nasal swabs, one calcium alginate fiber-tipped aluminum swab was used per nostril per day. After cleaning the intravenous collection site with alcohol wipes, whole-blood samples were collected. After clotting, the serum and clot were separated by centrifugation. The serum fraction was kept at −20°C until tested, and the clot was subjected to three freeze-and-thaw cycles and kept at −20°C until tested. Pigs from each group were euthanized at postinoculation days 1, 3, 5, 7, 10, and 21.
Detection of virus shedding and viremia by ELISA and CCIF.
Nasal and rectal swab samples and serum and blood clot were analyzed by antigen capture enzyme-linked immunosorbent assay (ELISA) and cell culture immunofluorescent assay (CCIF) to detect and quantitate HRV antigen and infectious rotavirus, respectively, as previously described (27, 28). Nasal and rectal swab samples were initially diluted 1:25 in serum-free minimum essential medium, then diluted 1:4 and serially diluted 10-fold thereafter. For assessment of viremia, serum and clot were tested by ELISA using undiluted samples and by CCIF using serial 10-fold dilutions. For both the ELISA and CCIF tests, nasal and rectal swab fluids and serum from mock-inoculated pigs were used as negative controls. CsCl-purified rotavirus or HRV-positive rectal swab fluids from previously tested HRV-inoculated pigs were used as positive controls.
The final CCIF titers were calculated based on the final dilution factor as the reciprocal of the highest dilution showing positive fluorescing cells. In antigen capture ELISA, half of the 96-well plates were coated with rotavirus-specific hyperimmune serum and the other half with a rotavirus-negative control serum. Samples were added in duplicate wells to both halves of the plate, and the ratio between the mean absorbance from the rotavirus-positive and rotavirus-negative serum-coated wells was calculated. The cutoff value was calculated as the mean absorbance from the negative coating wells plus 3 standard deviations. Samples with a ratio ≥2 and absorbance value higher than the cutoff value were considered positive. Antigen ELISA results are shown as the mean absorbance of replicate samples detected at 405 nm wavelength.
Reverse transcription-PCR.
Antigenemia detected by the antigen capture ELISA in undiluted serum was confirmed by reverse transcription-PCR. The RNA was extracted from serum of inoculated pigs by using Trizol according to the manufacturer's instructions (Life Technologies, Grand Island, N.Y.). The reverse transcription-PCR was conducted with primers to the VP7 gene as previously described by Gouvea et al. (10). Serum from mock-inoculated pigs and water were used as negative controls. Tissue-cultured rotavirus or HRV-positive rectal swab fluids from previously tested virulent HRV-inoculated pigs were used as positive controls.
Serum infectivity.
To determine if rotavirus present in the sera of HRV-inoculated pigs was infectious, the serum from pigs bled between postinoculation days 1 and 6 was pooled for each separate treatment group and filtered through 0.22-μm cellulose acetate filters. Three groups of pigs were inoculated orally, with each pig receiving 5 ml of the pooled serum from the attenuated HRV-, virulent HRV-, or mock-inoculated pigs.
Basolateral infection of the intestine.
To investigate if rotavirus, as demonstrated for PEC (11), could infect the intestinal epithelial cells via the basolateral side through the bloodstream and cause diarrhea, eight gnotobiotic pigs were inoculated intravenously (1 ml volume) via the anterior vena cava or jugular vein. Two pigs received pooled serum from the virulent HRV-inoculated pigs. Six other positive control pigs in separate isolator units received intestinal contents containing virulent HRV at a dose of 104 ID50 to 106 ID50. The inoculation site and any surface blood were wiped with 70% alcohol before and after injection to avoid the potential for oral infection of the pigs. Two of the eight pigs were euthanized at onset of diarrhea, and the remaining pigs were examined for 7 days for the presence of diarrhea or nasal or rectal virus shedding and euthanized at postinoculation day 21.
Isotype antibody ELISA.
Serum from pigs that did not shed virus and serum and intestinal contents from pigs inoculated intravenous with virulent HRV were tested by antibody-ELISA as previously described for seroconversion to immunoglobulin M (IgM), IgA, and IgG antibodies to HRV (1, 24, 32).
Statistical analysis.
The proportions of pigs that shed virus or developed viremia were compared using Fisher's exact test. One-way analysis of variance was used to compare mean duration of virus shedding and mean peak titers of virus shed between groups. Statistical significance was assessed at P < 0.05 throughout. Pearson's correlation coefficients were assessed for the level of nasal and rectal shedding and antigenemia in the three groups of pigs receiving virulent HRV. The analysis was limited to postinoculation days 1, 3, 5, and 7, where corresponding blood, nasal, and rectal samples were available. Pearson's correlation was also used to evaluate the correlation between levels of antigen shedding by ELISA (absorbance values) and titers of infectious virus (log-transformed values) by CCIF.
RESULTS
Attenuated and virulent HRV were shed nasally in gnotobiotic pigs inoculated intranasally or orally, but only virulent HRV was shed nasally after gavage inoculation
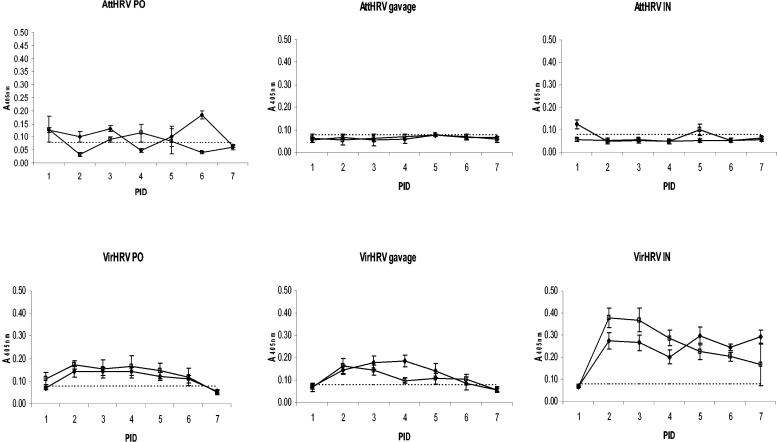
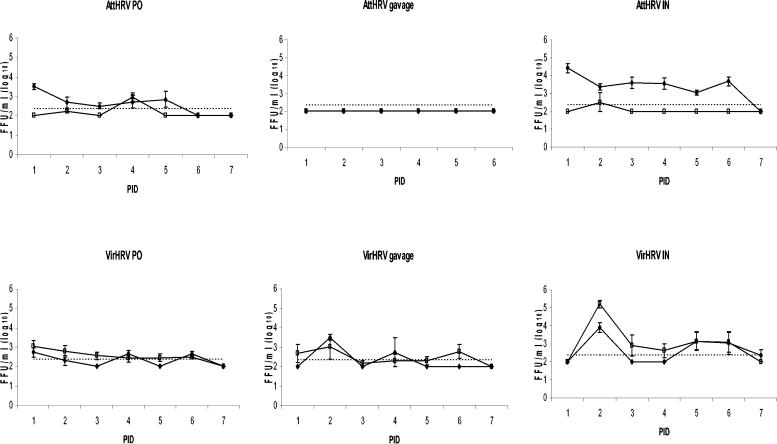
After inoculation with attenuated HRV (oral or intranasal), 79 to 95% of pigs shed virus in nasal specimens by either CCIF or ELISA test from postinoculation days 1 to 7. Pigs inoculated via gavage with attenuated HRV had no detectable nasal shedding (Table 1, Fig. 1 and 2) . In contrast, all pigs inoculated orally, intranasally, or via gavage with virulent HRV shed virus nasally. Nasal shedding was detected from postinoculation days 1 to 7 in the pigs inoculated with virulent HRV orally or by gavage and from postinoculation days 2 to 7 in pigs inoculated intranasally and lasted at least 1 day longer than rectal shedding in 60% of the pigs. The mean duration of nasal shedding was significantly longer (P < 0.05) in pigs inoculated with virulent HRV (3 to 5 days) than with attenuated HRV (1.4 to 1.7 days) (Table 1). There was no statistical difference in the average peak titer of virus shed between the two groups or the routes used, except for the pigs given attenuated HRV via gavage, which did not shed virus. No vomiting was observed in any pigs after inoculation via gavage. Therefore, regurgitation of virus from the stomach, resulting in nasal shedding, was unlikely in the virulent HRV-inoculated pigs.
TABLE 1.
Nasal and rectal virus shedding in gnotobiotic pigs after inoculation with attenuated and virulent Wa HRV by the oral (PO) and intranasal (IN) routes or by gavagea
| Treatment | No. of pigs | Nasal virus shedding
|
Rectal virus shedding
|
Virus in serumb (% of pigs)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| %c | Days to onset | Mean duration (days) | Peak titer (FFU/ml) | %b | Days to onset | Mean duration (days) | Peak titer (FFU/ml) | ELISA (antigen) | RT-PCR (RNA) | ||
| Attenuated HRV | |||||||||||
| IN | 23 | 95A | 2.4A | 1.7C | 3 × 104 | 5C | 3.5A | 1C | 3.4 × 103 | 0 | 0 |
| PO | 29 | 79A | 2.1A | 1.4C | 7 × 103 | 17B | 2.3AB | 0.9C | 1 × 103 | 0 | 0 |
| Gavage | 6 | 0B | 0B | 0D | 0C | 0D | 0 | 0 | |||
| Virulent HRV | |||||||||||
| IN | 6 | 100A | 2A | 5A | 1.5 × 104 | 100A | 2B | 4A | 1.8 × 105 | 100 | 100 |
| PO | 20 | 100A | 1.6A | 3.4B | 5 × 103 | 100A | 1.5B | 3B | 4 × 104 | 100 | 100 |
| Gavage | 8 | 100A | 2A | 3.5B | 3.4 × 103 | 100A | 1.8B | 3.2B | 6.8 × 104 | 100 | 100 |
| Control (MEM or VLP), PO or IN | 10 | 0B | 0B | 0D | 0C | 0D | 0 | 0 | |||
Values in the same column with different superscript letters differ significantly (Fisher's exact test, P < 0.05, or analysis of variance).
No infectious virus (viremia) was detected in the serum of any inoculated pig by CCIF; percent positive pigs determined by ELISA or RT-PCR.
% of pigs shedding virus by ELISA and/or CCIF.
FIG. 1.
Detection of nasal and rectal shedding in gnotobiotic pigs by ELISA after inoculation with attenuated (Att) and virulent (Vir) HRV. The dashed lines represent the cutoff value of the test (ELISA = 0.08). All control values were below the cutoff value and are not shown. Symbols: ♦, nasal samples; □, rectal samples. PID, postinoculation day; PO, oral; IN, intranasal.
FIG. 2.
Detection of nasal and rectal shedding by CCIF assay in gnotobiotic pigs after inoculation with attenuated (Att) and virulent (Vir) HRV. The dashed line represents the cutoff value for the test (CCIF = 250 FFU/ml). A value of 100 FFU/ml was arbitrarily assigned to samples below the detection level of the test to be represented in the figure. All control values were below the cutoff value and are not shown. Symbols: ♦, nasal samples; □, rectal samples. PID, postinoculation day; PO, oral; IN, intranasal.
Significant positive correlations between levels of antigen shedding and titer of infectious virus were observed only in nasal specimens of the attenuated HRV group at postinoculation day 2 (R = 0.55, P = 0.013) and postinoculation day 6 (R = 0.52, P = 0.048). Levels of antigen shedding and titer of infectious virus was also significantly positively correlated for nasal and rectal samples from the three virulent HRV groups, mainly at postinoculation days 1, 2, 3, and 6 (R = 0.88, P = 0.0017; R = 0.888, P = 0.018; R = 0.966, P = 0.0017; and R = 0.6, P = 0.0051, respectively).
Attenuated HRV induced lower rates of rectal shedding, whereas virulent HRV induced rectal shedding in 100% of pigs independent of the inoculation route (Table 1; Fig. 1 and 2)
Only 5 to 17% of the pigs inoculated intranasally or orally with attenuated HRV shed virus rectally. Pigs inoculated with attenuated HRV via gavage had no detectable rectal rotavirus shedding, but they seroconverted with antibodies to HRV (data not shown). Rectal rotavirus shedding was present from postinoculation days 1 to 6 in all pigs inoculated with virulent HRV orally or by gavage and was detected from postinoculation days 2 to 7 in all pigs inoculated with virulent HRV intranasally. Pigs inoculated with attenuated HRV intranasally had significantly longer time to onset of rectal shedding than the virulent HRV-inoculated pigs.
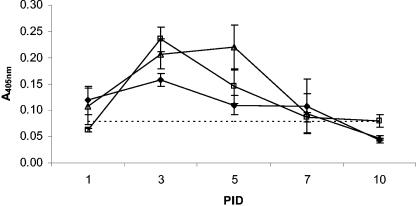
Inoculation of gnotobiotic pigs with virulent but not attenuated HRV caused a transient low level of antigenemia in serum.
Antigenemia and rotaviral RNA were detected in serum by ELISA and reverse transcription-PCR, respectively, but viremia could not be detected by CCIF (Table 1). This finding may be explained by interference of certain serum components with virus infectivity and replication in cell culture (see Discussion). The serum fraction but not the clot was positive for HRV antigen by ELISA. Antigenemia was not detected in any of the attenuated HRV-inoculated pigs by ELISA, nor was viral RNA detected by reverse transcription-PCR. All pigs inoculated with virulent HRV developed antigenemia between postinoculation days 1 and 7 (Fig. 3), and the presence of rotavirus genomic RNA in serum was confirmed by reverse transcription-PCR (Table 1).
FIG. 3.
Detection of antigenemia in gnotobiotic pigs by ELISA after inoculation with HRV. The dashed line represents the cutoff value (ELISA = 0.08). All control values were below the cutoff value and are not shown. Symbols: ⧫, virulent HRV, oral; ▵, virulent HRV, gavage; □, virulent HRV, intranasal.
Levels of nasal and rectal shedding correlated with antigenemia in pigs receiving virulent HRV by the different routes.
Significant positive correlations for levels of antigenemia and nasal and rectal shedding were observed mainly at postinoculation days 3 to 5. Positive correlations were observed in the virulent HRV intranasal and oral groups between rectal shedding and antigenemia (R = 0.96, P = 0.0018 and R = 0.64, P = 0.0057, respectively) at postinoculation day 3. In the virulent HRV oral group, positive correlations were found between nasal and rectal shedding at postinoculation days 3 and 5 (R = 0.53, P = 0.02 and R = 0.8, P = 0.0001, respectively). For the virulent HRV gavage group, a positive correlation between nasal and rectal shedding (R = 0.76, P = 0.02) was also seen at postinoculation day 5. For the virulent HRV intranasal group, a strong positive correlation between nasal and rectal shedding occurred at postinoculation day 7 (R = 0.92, P = 0.0077).
Intravenous inoculation of pigs with virulent HRV induces infection of the gut and diarrhea.
Pigs inoculated intravenously with 104 ID50 of virulent HRV shed virus nasally at postinoculation day 4 and rectally at postinoculation day 5 (data not shown), whereas two pigs receiving 106 ID50 of virulent HRV intravenously shed virus nasally and rectally as early as postinoculation day 3 (Table 2). Rotavirus antigens were found in the duodenum, jejunum, and ileum of such pigs (data not shown). The HRV-specific IgM, IgA, and IgG antibodies were detected in serum and intestinal contents of pigs inoculated intravenously with virulent HRV, reaching titers of 16,385, 8,192, and 8,192 in the serum, respectively, and 256, 128, and 16 in the intestinal contents, respectively, at postinoculation day 21.
TABLE 2.
Nasal and rectal virus shedding and antigenemia in gnotobiotic pigs after oral (PO) or intravenous (IV) inoculation with pooled sera or intestinal contents of gnotobiotic pigsa
| Treatment | No. of pigs | Nasal virus shedding
|
Rectal virus shedding
|
% of pigs with:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| %b | Mean days to onset | Mean duration (days) | Mean peak titer (FFU/ml) | %b | Mean days to onset | Mean duration (days) | Mean peak titer (FFU/ml) | Diarrhea | Viremia | ||
| Serumc PO | 4 | 100 | 2.8 | 4 | 2.8 × 103 | 100 | 1.8 | 4 | 2 × 104 | 100 | 100 |
| Serumc IV | 2 | 100 | 3 | 1 | <250 | 100 | 3 | 2 | 2 × 103 | 100 | 100 |
| Intestinal contentsc IV | 2 | 100 | 3 | 2 | 1 × 103 | 100 | 3 | 2 | 4 × 103 | 100 | 100 |
Pigs inoculated with pooled sera from attenuated HRV-exposed pigs (n = 3) and controls (n = 2) did not develop diarrhea or shed virus nasally or rectally
% of pigs shedding virus by ELISA and/or CCIF.
From virulent HRV-inoculated pigs.
Virulent HRV induces not only antigenemia but also viremia assessed by in vivo virus infectivity assay of pooled serum from virulent HRV-inoculated pigs.
The infectivity of virus in serum from the pigs with antigenemia was confirmed by inoculating the pigs orally with a pool of antigen ELISA-positive sera from the virulent HRV-inoculated pigs. All pigs inoculated orally with the pooled serum from the virulent HRV-inoculated pigs developed diarrhea, rectal and nasal virus shedding, and transient antigenemia (Table 2) and seroconverted to HRV. The pigs in this group developed IgM (256 and 256), IgA (8,192 and 128), and IgG (32,786 and 181) antibody responses in serum and intestinal contents, respectively, at postinoculation day 21. None of the pigs inoculated orally with sera from attenuated HRV- or mock-inoculated pigs shed virus or seroconverted with antibodies to HRV.
DISCUSSION
In this study we demonstrated nasal shedding after inoculation of gnotobiotic pigs with attenuated HRV and virulent HRV. To clarify if the presence of HRV in the nasal tract was a result of replication of attenuated and virulent HRV in the respiratory tract/oral cavity or was only residual inoculum remaining in the nasopharyngeal region, gnotobiotic pigs were also orally inoculated with a gavage tube. All pigs inoculated with virulent HRV by the oral, gavage, or intranasal route shed virus nasally; only pigs inoculated with attenuated HRV by the intranasal and oral routes, not via gavage, shed virus. These findings suggest that in pigs inoculated via gavage, virulent HRV may have reached the nasal epithelial cells secondarily via the blood circulation, since viremia was evident in all virulent HRV-inoculated pigs but not in the attenuated HRV-inoculated pigs. For the attenuated HRV-inoculated pigs, direct contact of the nasal or oral mucosal surfaces with the virus delivered orally or intranasally may be a requirement for infection of these cells. Moreover, the finding of rotavirus antigen in nasal epithelial cells of both attenuated HRV- and virulent HRV-infected pigs confirms the likely active replication of rotavirus in these cells and the upper respiratory tract. Nasal virus shedding was observed as early as postinoculation day 1 in the virulent HRV-inoculated pigs, with no statistical difference in the mean time to onset of nasal and rectal shedding in this group. Based on this observation and the lower percentage of rectal virus shedding in the attenuated HRV group compared to nasal shedding, it is unlikely that the nasal shedding was from fecal-nasal contamination inside the isolator unit.
Zhaori et al. (41) detected HRV antigen in 27.6% of tracheal aspirates of children with a clinical diagnosis of pneumonia, of which only two cases had diarrhea with rotavirus detectable in feces. In gnotobiotic pigs, 100% of the pigs showed nasal and rectal virus shedding when exposed to virulent HRV. For attenuated HRV-inoculated pigs, nasal virus shedding was more prominent than rectal shedding (oral or intranasal but not gavage), and infectious virus was detected by CCIF in the nasal swabs. However, no antigenemia (or viremia confirmed by serum inoculation) was evident in the attenuated HRV-inoculated pigs, suggesting a different pathogenesis for virulent HRV versus attenuated HRV. The latter probably requires efficient oronasal replication for further spread to the gut, an observation potentially important for the design and delivery of attenuated HRV vaccines. Moreover, it is possible that, based on the higher titers of virus shed nasally and the longer duration, the attenuated HRV strain may represent a temperature-sensitive mutant of HRV with greater replication in the upper respiratory tract. Of interest, Mebus et al. (16a) reported that attenuation of the neonatal calf diarrhea virus vaccine strain of bovine rotavirus was achieved by serial passage in cell culture at lowered temperatures (29 to 30°C). Alternatively, attenuated HRV may be less stable in the presence of gastrointestinal enzymes, leading to reduced intestinal infectivity and rectal shedding, as evident from the complete absence of rotavirus shedding in pigs inoculated with attenuated HRV directly into the stomach via gavage.
Nasal virus shedding in the attenuated HRV-inoculated pigs was detected by CCIF and to a lesser extent by ELISA. These findings indicate the presence of viable virus particles and potentially less free or degraded viral antigens, since fewer proteolytic enzymes are present in respiratory compared to intestinal secretions. In the virulent HRV-inoculated pigs, nasal shedding was more readily detected by ELISA than CCIF, probably signifying a higher level of free antigen but also not excluding the fact that the virulent HRV strain is more fastidious than the attenuated HRV strain for cell culture replication (37). We were unable to detect infectious virus (viremia) in serum by CCIF in either the attenuated HRV- or virulent HRV-inoculated pigs, possibly for the same reason, but also because of the inhibitory effects of the pig serum components on virulent HRV infectivity in cell culture, as observed similarly for fetal bovine serum (5, 33, 37). Serum components such as lipoproteins may have protease inhibitor effects on the activity of the proteolytic enzymes, which are essential for in vitro replication of rotavirus (8, 15). However, the in vivo infectivity was not affected, since diarrhea and virus shedding was promptly induced by oral inoculation with the positive serum. The gastric enzymes and bile acids may have contributed to overcome the inhibitory affects of the serum. Moreover, the serum may have increased the initial stability of the virus throughout the gut, increasing the efficiency of infection.
The onset of antigenemia and nasal and rectal shedding in the virulent HRV-inoculated pigs occurred at postinoculation day 1 for both orally and gavage-inoculated pigs, but in intranasally inoculated pigs, nasal shedding was detected 1 day later (postinoculation day 2). Nasal shedding was also observed when pigs were inoculated via gavage. This infectivity pattern suggests that the virulent HRV reached the nasal cells via the bloodstream. How rotavirus reaches the blood after oral, intranasal, or gavage inoculation remains unknown. Some studies suggest that rotavirus may be taken up by macrophages or other antigen-presenting cells in the gut-associated lymphoid tissue and then enter the bloodstream (3). Osborne et al. (22) showed an increase in the blood flow through villi at 72 h postinfection during rotavirus infection of mice. Cedgard et al. (4) demonstrated that blood flow in the gut was doubled after exposing the intestinal mucosa to bacterial toxins. Whether the viral enterotoxin NSP4 is capable of activating the same mechanism and increasing blood flow, leading to transport or leakage of rotavirus into serum or increased uptake by antigen-presenting cells, requires further investigation.
Another possible way for virulent HRV to penetrate the gut barrier from the lumenal side would be by the destruction of enterocytes in the villi, exposing the basement membrane, followed by transit into the blood and then followed by transit to the nasal cavity with replication in the nasal epithelial cells. This explanation is consistent with the villous atrophy induced by virulent HRV but not attenuated HRV in gnotobiotic pigs (35). We also observed significant positive correlations between levels of rectal shedding but not nasal shedding and antigenemia, which might imply that the occurrence of antigenemia depends on the magnitude of virus replication in the gut.
In the attenuated HRV-inoculated pigs, no antigenemia was detected, so the probable mechanism for the infectivity pattern observed would be that attenuated HRV initially replicated in the nasal epithelial cells (after intranasal inoculation) or in the tonsils and pharyngeal tissues with transit to the nasal tissue (after oral inoculation), followed by swallowing of virus and introduction into the stomach with the limited viral replication seen in the epithelial cells of the gut (35). The low percentage of rectal shedding detected in this study corroborated the study by Ward et al. (35). The inability to detect rectal virus shedding in these piglets could be explained by a low level of virus replication which was still capable of initiating a rotavirus-specific IgM antibody response.
The ID50 of attenuated HRV Wa is 1.3 × 106 FFU, as previously determined by Ward et al. (35) based on seroconversion, since little or no shedding was detectable. Currently, candidate rotavirus vaccines are being evaluated in doses of 105 FFU to 4 × 105 FFU in humans (12). The ID50 of the attenuated HRV Wa used in this study is higher than that of the candidate HRV vaccines but lower than that of the virulent HRV. The high dose of virulent HRV was necessary to induce 100% diarrhea with the heterologous HRV in the age-matched naïve piglets. The aim of this study was not to compare attenuated virus to virulent virus in terms of ID50 but to mimic the use of the attenuated virus as a vaccine and the high dose of virulent virus to mimic the natural rotavirus infection and disease.
Our results provide a new perspective on the pathogenesis of virulent HRV in a gnotobiotic pig model, confirming extraintestinal infection or spread of a HRV strain (nasal, viremia) in an animal rotavirus disease model. Additional studies are in progress to delineate if extraintestinal infection by virulent HRV is limited mainly to viremia and nasal shedding or if virulent HRV may spread to and replicate in other organs.
Our findings have clinical implications. The role of rotavirus in upper respiratory tract infections of children requires additional study, as does the presence of rotavirus viremia in children and its possible consequence related to detection of HRV in the cerebrospinal fluid of children with central nervous system disease (16, 38). The diarrhea associated with rotavirus typically lasts from 2 to 8 days, but immunodeficiency, immunosuppression, and malnutrition are risk factors for severe and prolonged symptoms and chronic infection (9, 16). The incidence of rotavirus infection is similar in both developed and developing countries, but the morbidity and mortality of the disease are much greater in developing countries. How extraintestinal spread of rotavirus contributes to disease severity and induction of protective immunity has yet to be determined.
Svensson et al. (31) and Jourdan et al. (13) showed that rotavirus infects differentiated Caco-2 cells in vitro both apically and basolaterally. The results of our study, in which administration of virulent HRV by the intravenous route resulted in infection of intestinal epithelial cells, corroborates such in vitro findings. We demonstrated diarrhea, rectal shedding, HRV antigen in duodenum, jejunum, and ileum as well as seroconversion to HRV and the presence of IgM, IgA, and IgG antibodies to HRV in the intestinal contents of the pigs inoculated intravenously with virulent HRV. Our finding provides new in vivo evidence for the idea that rotavirus may infect from the basolateral surface. Guo et al. (11) also observed infection of the intestine after intravenous inoculation of neonatal gnotobiotic pigs with a porcine enteric calicivirus, Cowden strain. Other investigators have suggested a pathway similar to the one described for reovirus (21), whereby virus in the blood reaches the ileum and infects crypt cells, possibly by attaching to the basolateral membrane (2, 18).
To our knowledge, this study is the first to describe infection of the gastrointestinal tract by a virulent rotavirus strain via viremia from the blood and rotavirus shedding from the upper respiratory tract in neonatal animals. Whether the same event will occur after exposure to different rotavirus serotypes and the role of cross-protective antibodies in avoiding viremia are still to be determined. The implications of our findings of the distinct pathogenesis and virus distribution patterns of virulent HRV versus attenuated HRV for protective immunity and vaccine development require further study. For vaccination studies, detection of viremia may be another parameter to evaluate vaccine protection induced by the candidate vaccines against circulating virulent HRV strains.
Acknowledgments
We thank Juliette Hanson, Richard McCormick, Veronica Costantini, Qiuhong Wang, Menira Souza Dias, Peggy Lewis, and Tiffany Kinney for technical assistance and the late Jean Cohen (Virologie Moleculaire et Structurale) for providing the strain RF VP2 baculovirus clone used for the virus-like particles.
Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University. This work was supported by grants from the National Institutes of Health, NIAID (R01AI3356-08 and R01AI37111). Marli S. P. Azevedo was a fellow of Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq), Brasilia, Brazil.
REFERENCES
- 1.Azevedo, M. S. P., L. Yuan, C. Iosef, K.-O. Chang, Y. Kim, T. Van Nguyen, and L. J. Saif. 2004. Magnitude of serum and intestinal antibody responses induced by sequential replicating and nonreplicating rotavirus vaccines in gnotobiotic pigs and correlation with protection. Clin. Diagn. Lab. Immunol. 11:12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blutt, S. E., C. D. Kirkwood, V. Parreno, K. L. Warfield, M. Ciarlet, M. K. Estes, K. Bok, R. F. Bishop, and M. E. Conner. 2003. Rotavirus antigenaemia and viraemia: a common event? Lancet 362:1445-1449. [DOI] [PubMed] [Google Scholar]
- 3.Brown, K. A., and P. A. Offit. 1998. Rotavirus-specific proteins are detected in murine macrophages in both intestinal and extraintestinal lymphoid tissues. Microb. Pathog. 24:327-331. [DOI] [PubMed] [Google Scholar]
- 4.Cedgard, S., D. A. Hallback, M. Jodal, O. Lundgren, and S. Redfors. 1978. The effects of cholera toxin on intramural blood flow distribution and capillary hydraulic conductivity in the cat small intestine. Acta Physiol. Scand. 102:148-158. [DOI] [PubMed] [Google Scholar]
- 5.Christy, C., D. Vosefski, and H. P. Madore. 1990. Comparison of three enzyme immunoassays to tissue culture for the diagnosis of rotavirus gastroenteritis in infants and young children. J. Clin. Microbiol. 28:1428-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford, S. E., M. Labbe, J. Cohen, M. H. Burroughs, Y. J. Zhou, and M. K. Estes. 1994. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J. Virol. 68:5945-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennehy, P. H. 2000. Transmission of rotavirus and other enteric pathogens in the home. Pediatr. Infect. Dis. J. 19:S103-105. [DOI] [PubMed] [Google Scholar]
- 8.Ericson, B. L., D. Y. Graham, B. B. Mason, and M. K. Estes. 1982. Identification, synthesis, and modifications of simian rotavirus SA11 polypeptides in infected cells. J. Virol. 42:825-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilger, M. A., D. O. Matson, M. E. Conner, H. M. Rosenblatt, M. J. Finegold, and M. K. Estes. 1992. Extraintestinal rotavirus infections in children with immunodeficiency. J. Pediatr. 120:912-917. [DOI] [PubMed] [Google Scholar]
- 10.Gouvea, V., R. I. Glass, P. Woods, K. Taniguchi, H. F. Clark, B. Forrester, and Z. Y. Fang. 1990. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 28:276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo, M., J. Hayes, K. O. Cho, A. V. Parwani, L. M. Lucas, and L. J. Saif. 2001. Comparative pathogenesis of tissue culture-adapted and wild-type Cowden porcine enteric calicivirus (PEC) in gnotobiotic pigs and induction of diarrhea by intravenous inoculation of wild-type PEC. J. Virol. 75:9239-9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoshino, Y., M. Wagner, X. Y. Yan, I. Perez-Schael, and A. Z. Kapikian. 2003. Horizontal transmission of rhesus monkey rotavirus-based quadrivalent vaccine during a phase 3 clinical trial in Caracas, Venezuela. J. Infectious Diseases. 187:791-800. [DOI] [PubMed] [Google Scholar]
- 13.Jourdan, N., J. Cotte Laffitte, F. Forestier, A. L. Servin, and A. M. Quero. 1995. Infection of cultured human intestinal cells by monkey RRV and human Wa rotavirus as a function of intestinal epithelial cell differentiation. Res. Virol. 146:325-331. [DOI] [PubMed] [Google Scholar]
- 14.Kim, Y. 1975. Developmental immunity in the piglets. Birth Defects. 11:549-557. [PubMed] [Google Scholar]
- 15.Lopez, S., C. F. Arias, J. R. Bell, J. H. Strauss, and R. T. Espejo. 1985. Primary structure of the cleavage site associated with trypsin enhancement of rotavirus SA11 infectivity. Virology 144:11-19. [DOI] [PubMed] [Google Scholar]
- 16.Lynch, M., W. J. Shieh, K. Tatti, J. R. Gentsch, T. Ferebee-Harris, B. Jiang, J. Guarner, J. S. Bresee, M. Greenwald, S. Cullen, H. D. Davies, C. Trevenen, S. R. Zaki, and R. I. Glass. 2003. The pathology of rotavirus-associated deaths, using new molecular diagnostics. Clin. Infect. Dis. 37:1327-1333. [DOI] [PubMed] [Google Scholar]
- 16a.Mebus, C. A., R. G. White, E. P. Bass, and M. J. Twiehaus. 1973. Immunity to neonatal calf diarrhea virus. J. Am. Vet. Med. Assoc. 163:880-883. [Google Scholar]
- 17.Meyer, R. C., E. H. Bohl, and E. M. Kohler. 1964. Procurement and maintenance of germ-free seine for microbiological investigations. Appl. Microbiol. 12:295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mossel, E. C., and R. F. Ramig. 2002. Rotavirus genome segment 7 (NSP3) is a determinant of extraintestinal spread in the neonatal mouse. J. Virol. 76:6502-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nigro, G., and M. Midulla. 1983. Acute laryngitis associated with rotavirus gastroenteritis. J. Infect. 7:81-82. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura, S., H. Ushijima, H. Shiraishi, C. Kanazawa, T. Abe, K. Kaneko, and Y. Fukuyama. 1993. Detection of rotavirus in cerebrospinal fluid and blood of patients with convulsions and gastroenteritis by means of the reverse transcription polymerase chain reaction. Brain Dev. 15:457-459. [DOI] [PubMed] [Google Scholar]
- 21.Organ, E., and D. Rubin. 1998. Pathogenesis of reovirus gastrointestinal and hepatobiliary disease. Curr. Top. Microbiol. Immunol. 233:163-177. [DOI] [PubMed] [Google Scholar]
- 22.Osborne, M. P., S. J. Haddon, K. J. Worton, A. J. Spencer, W. G. Starkey, D. Thornber, and J. Stephen. 1991. Rotavirus-induced changes in the microcirculation of intestinal villi of neonatal mice in relation to the induction and persistence of diarrhea. J. Pediatr. Gastroenterol. Nutr. 12:111-120. [DOI] [PubMed] [Google Scholar]
- 23.Parashar, U. D., E. G. Hummelman, J. S. Bresee, M. A. Miller, and R. I. Glass. 2003. Global illness and deaths caused by rotavirus disease in children. Emerging Infect. Dis. 9:565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parreno, V., D. C. Hodgins, L. de Arriba, S. Y. Kang, L. Yuan, L. A. Ward, T. L. To, and L. J. Saif. 1999. Serum and intestinal isotype antibody responses to Wa human rotavirus in gnotobiotic pigs are modulated by maternal antibodies. J. Gen. Virol. 80:1417-1428. [DOI] [PubMed] [Google Scholar]
- 25.Phillips, R., and M. Tumbleson. 1986. Models, p. 437-440. In M. Tumbleson (ed.), Swine in biomedical research. Plenum Press, New York, N.Y.
- 26.Prince, D. S., C. Astry, S. Vonderfecht, G. Jakab, F. M. Shen, and R. H. Yolken. 1986. Aerosol transmission of experimental rotavirus infection. Pediatr. Infect. Dis. 5:218-222. [DOI] [PubMed] [Google Scholar]
- 27.Saif, L., L. Yuan, L. Ward, and T. To. 1997. Comparative studies of the pathogenesis, antibody immune responses, and homologous protection to porcine and human rotaviruses in gnotobiotic piglets. Adv. Exp. Med. Biol. 412:397-403. [DOI] [PubMed] [Google Scholar]
- 28.Saif, L. J., D. R. Redman, K. L. Smith, and K. W. Theil. 1983. Passive immunity to bovine rotavirus in newborn calves fed colostrum supplements from immunized or nonimmunized cows. Infect. Immun. 41:1118-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saif, L. J., L. A. Ward, L. Yuan, B. I. Rosen, and T. L. To. 1996. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Arch. Virol. Suppl. 12:153-161. [DOI] [PubMed] [Google Scholar]
- 30.Shaw, D. P., L. G. Morehouse, and R. F. Solorzano. 1989. Experimental rotavirus infection in three-week-old pigs. Am. J. Vet. Res. 50:1961-1965. [PubMed] [Google Scholar]
- 31.Svensson, L., B. B. Finlay, D. Bass, C. H. von Bonsdorff, and H. B. Greenberg. 1991. Symmetric infection of rotavirus on polarized human intestinal epithelial (Caco-2) cells. J. Virol. 65:4190-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.To, T. L., L. A. Ward, L. Yuan, and L. J. Saif. 1998. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Gen. Virol. 79:2661-2672. [DOI] [PubMed] [Google Scholar]
- 33.Urasawa, T., S. Urasawa, and K. Taniguchi. 1981. Sequential passages of human rotavirus in MA-104 cells. Microbiol. Immunol. 25:1025-1035. [DOI] [PubMed] [Google Scholar]
- 34.Ushijima, H., K. Q. Xin, S. Nishimura, S. Morikawa, and T. Abe. 1994. Detection and sequencing of rotavirus VP7 gene from human materials (stools, sera, cerebrospinal fluids, and throat swabs) by reverse transcription and PCR. J. Clin. Microbiol. 32:2893-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward, L. A., B. I. Rosen, L. Yuan, and L. J. Saif. 1996. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J. Gen. Virol. 77:1431-1441. [DOI] [PubMed] [Google Scholar]
- 36.Ward, L. A., L. Yuan, B. I. Rosen, T. L. To, and L. J. Saif. 1996. Development of mucosal and systemic lymphoproliferative responses and protective immunity to human group A rotaviruses in a gnotobiotic pig model. Clin. Diagn. Lab. Immunol. 3:342-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward, R. L., D. R. Knowlton, and M. J. Pierce. 1984. Efficiency of human rotavirus propagation in cell culture. J. Clin. Microbiol. 19:748-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida, A., T. Kawamitu, R. Tanaka, M. Okumura, S. Yamakura, Y. Takasaki, H. Hiramatsu, T. Momoi, M. Iizuka, and O. Nakagomi. 1995. Rotavirus encephalitis: detection of the virus genomic RNA in the cerebrospinal fluid of a child. Pediatr. Infect. Dis. J. 14:914-916. [PubMed] [Google Scholar]
- 39.Yuan, L., C. Iosef, M. S. Azevedo, Y. Kim, Y. Qian, A. Geyer, T. V. Nguyen, K. O. Chang, and L. J. Saif. 2001. Protective immunity and antibody-secreting cell responses elicited by combined oral attenuated Wa human rotavirus and intranasal Wa 2/6-VLPs with mutant Escherichia coli heat-labile toxin in gnotobiotic pigs. J. Virol. 75:9229-9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan, L., L. A. Ward, B. I. Rosen, T. L. To, and L. J. Saif. 1996. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Virol. 70:3075-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhaori, G. T., L. T. Fu, Y. H. Xu, Y. R. Guo, Z. J. Peng, and W. S. Shan. 1991. Detection of rotavirus antigen in tracheal aspirates of infants and children with pneumonia. Chin. Med. J. 104:830-833. [PubMed] [Google Scholar]
- 42.Zheng, B. J., R. X. Chang, G. Z. Ma, J. M. Xie, Q. Liu, X. R. Liang, and M. H. Ng. 1991. Rotavirus infection of the oropharynx and respiratory tract in young children. J. Med. Virol. 34:29-37. [DOI] [PubMed] [Google Scholar]