Abstract
Transcription by the influenza virus RNA-dependent RNA polymerase is dependent on cellular RNA processing activities that are known to be associated with cellular RNA polymerase II (Pol II) transcription, namely, capping and splicing. Therefore, it had been hypothesized that transcription by the viral RNA polymerase and Pol II might be functionally linked. Here, we demonstrate for the first time that the influenza virus RNA polymerase complex interacts with the large subunit of Pol II via its C-terminal domain. The viral polymerase binds hyperphosphorylated forms of Pol II, indicating that it targets actively transcribing Pol II. In addition, immunofluorescence analysis is consistent with a new model showing that influenza virus polymerase accumulates at Pol II transcription sites. The present findings provide a framework for further studies to elucidate the mechanistic principles of transcription by a viral RNA polymerase and have implications for the regulation of Pol II activities in infected cells.
There is increasing evidence suggesting that RNA polymerase II (Pol II) transcription and RNA processing are coupled in vivo (11, 12, 27). An important role in orchestrating the coordinated sequence of processing steps has been assigned to the C-terminal domain (CTD) of the large subunit of Pol II. The CTD is phosphorylated differentially during the transcription cycle and interacts with a number of factors involved in all steps of mRNA maturation (26). Pol II transcription and RNA processing are influenced not only by cellular factors such as CTD kinases and phosphatases but also by viral proteins (30).
For influenza A virus, a functional link between viral transcription and cellular transcription by Pol II has been assumed for a long time (2, 7, 17, 21). Even though influenza viruses encode their own RNA-dependent RNA polymerase, cellular activities related to Pol II transcription are required for the production of mature viral mRNAs. Thus, viral mRNA transcription is initiated by use of short capped RNA oligonucleotides as primers, which are obtained by endonucleolytic cleavage of cellular pre-mRNAs by the viral polymerase complex (18, 25). In addition, two transcripts are spliced by the cellular splicing apparatus (17). Moreover, in an artificial system, influenza virus transcripts, which are normally polyadenylated by the viral polymerase, were cleaved and polyadenylated by the respective cellular machineries (7).
Despite this functional link, no biochemical evidence of interaction between influenza virus RNA polymerase and Pol II has yet been provided. Therefore, we set out to determine biochemically whether or not influenza virus polymerase complexes were associated with Pol II.
MATERIALS AND METHODS
Plasmids.
The pcDNA-PB1, pcDNA-PB2, and pcDNA-PA protein expression plasmids for the three polymerase subunits of influenza A/WSN/33 virus have been described elsewhere (6). Plasmid pcDNA-PB1-TAP was described previously (8). pcDNA-PB2-TAP was generated by replacing the green fluorescent protein (GFP) open reading frame (ORF) of pcDNA-PB2-GFP (8) with the tandem affinity purification (TAP) sequence (28). pcDNA-TAP-CTD, expressing the full-length CTD of the large subunit of mouse Pol II fused to a N-terminal TAP tag, was constructed by inserting the CTD sequence (a gift from P. Uguen, University of Oxford) between the NotI and XbaI sites of pcDNA3A (6). The N-terminal truncated TAP tag, consisting of two immunoglobulin G (IgG) binding domains of Staphylococcus aureus protein A and a tobacco etch virus (TEV) protease cleavage site, was inserted upstream of the CTD-coding region between the BamHI and NotI sites. pcDNA-GFP-CTD was generated by replacing the TAP ORF of pcDNA-TAP-CTD with the GFP ORF. To generate pcDNA-GFP-TAP, the TAP ORF derived from pcDNA-PB2-TAP by NotI-XbaI digestion was inserted into the pcDNA-GFP(NX) construct (8).
Antibodies.
The antibodies used were rabbit polyclonal anti-PA, anti-PB1, and anti-PB2 antibodies (gifts from T. Toyoda, Kurume University, Kurume, Japan); anti-CTD antibody 8WG16, anti-phosphoserine 2 CTD antibody H5, and anti-phosphoserine 5 CTD antibody H14 (Covance); anti-CTD antibody 4H8 (Upstate); anti-NP monoclonal antibody F8 (Advanced Immunochemical Inc.); rabbit polyclonal antibody against PCNA (Abcam); and rabbit polyclonal antibody against active caspase-3 (BioVision). Bacterially expressed His-tagged fragments of PB1 (amino acids [aa] 1 to 180), PB2 (aa 1 to 180), and PA (aa 490 to 716) were used to immunize rabbits to obtain polyclonal sera (Eurogentec; gifts from G. Brownlee, S. Carr, T. Deng, and T. Jung, University of Oxford).
Immunoprecipitations.
Human embryonic kidney (293T) cells (about 6 × 106 cells) were infected with influenza A/WSN/33 virus at a multiplicity of infection (MOI) of 5 or were mock infected. Cells harvested at 6 h postinfection were resuspended in 600 μl of cell lysis buffer (50 mM Tris-HCl [pH 8.0], 200 mM NaCl, 2 mM MgCl2, 0.5% Igepal CA-630 [Sigma], 10 mM sodium fluoride, 10 μM sodium pervanadate, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride [PMSF], 25% glycerol, one Complete Mini EDTA-free protease inhibitor cocktail tablet [Roche]/10 ml) containing 60 U of Benzonase nuclease (Novagen) and incubated for 1 h at 4°C. Immunoprecipitations were performed in a final volume of 450 μl containing 150 μl of cell lysates, 5 mg of protein A-Sepharose (Sigma), and 7.5 μl of a rabbit polyclonal anti-PA antibody or 7.5 μl of a control antibody (active caspase-3 rabbit polyclonal antibody). The Protein A Sepharose was washed three times with 1 ml of wash buffer (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.1% Igepal CA-630, 1 mM PMSF). Bound proteins were released by heating samples at 100°C for 5 min in 80 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer.
Purification of TAP-tagged proteins.
TAP-CTD, PB2-TAP, PB1-TAP, or GFP-TAP and interacting proteins were purified by using the TAP method (28) as described previously (8). Briefly, cell lysates prepared from transfected 293T cells in the presence (for Fig. 2) or absence (for Fig. 3) of Benzonase nuclease were incubated with IgG-Sepharose and bound proteins released by cleavage with TEV protease (Invitrogen). Purified protein samples were analyzed on sodium dodecyl sulfate-8% polyacrylamide gels, followed by silver staining with the SilverXpress silver staining kit (Invitrogen) according to the manufacturer's instructions.
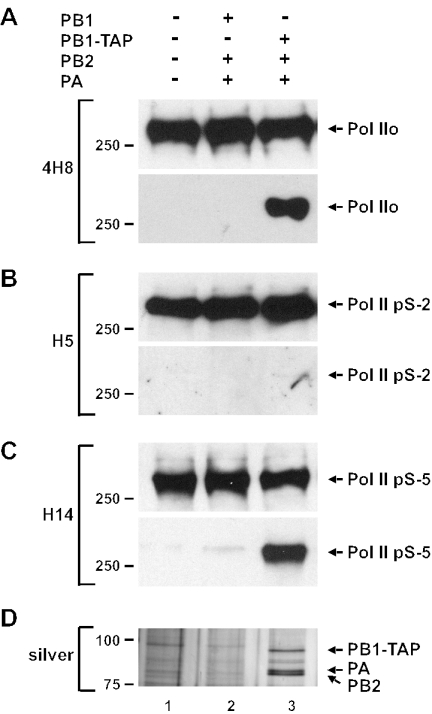
FIG. 2.
Recombinant influenza virus polymerase interacts with Pol II. Lysates from 293T cells expressing the three subunits of the influenza virus polymerase or the PB2 and PA subunits as well as PB1-TAP were purified on IgG Sepharose. (A to C) Total cell lysates (top panels) and purified samples (bottom panels) were analyzed by Western blotting with monoclonal anti-Pol II CTD antibody 4H8 (A), monoclonal anti-phosphoserine 2 Pol II antibody H5 (B), and monoclonal anti-phosphoserine 5 Pol II antibody H14 (C). (D) Purified material was analyzed on an 8% polyacrylamide gel, and proteins were detected by silver staining.
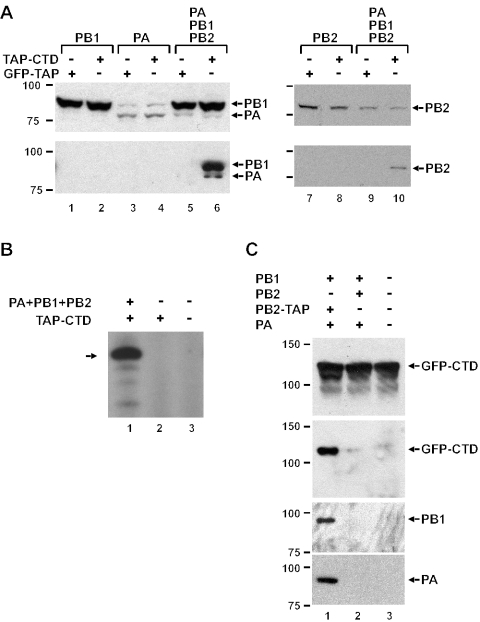
FIG. 3.
The trimeric influenza virus polymerase complex interacts with the CTD of Pol II. (A) Lysates of 293T cells either expressing individual viral polymerase subunits, as indicated, or coexpressing all three subunits, as well as TAP-CTD or GFP-TAP, were directly analyzed by Western blotting with a polyclonal anti-PB1 antibody (which cross-reacts with PA) (top left panel) or a polyclonal anti-PB2 antibody (top right panel) or were purified on IgG-Sepharose followed by Western blotting and probing with anti-PB1 (bottom left) or anti-PB2 (bottom right) antibodies. (B) Material purified on IgG-Sepharose from 293T cells expressing the three influenza virus polymerase subunits together with TAP-CTD or TAP-CTD alone was added to an in vitro transcription reaction mixture with a short RNA oligonucleotide template. Buffer instead of protein sample was used in the reaction mixture loaded in lane 3. The size of a marker RNA of 14 nucleotides (the expected product) is indicated by an arrow. (C) Lysates of 293T cells expressing PB1, PA, and either PB2 or PB2-TAP, as well as GFP-CTD, were analyzed by Western blotting with monoclonal anti-CTD antibody 8WG16 (top panel) or were purified on IgG-Sepharose followed by Western blotting and probing with anti-CTD antibody 8WG16 (second panel), polyclonal anti-PB1 (third panel), or polyclonal anti-PA (bottom panel) antibodies.
Transcription assay in vitro.
Transcription reactions in vitro with recombinant influenza RNA polymerase were performed as described previously (6). Briefly, influenza virus polymerase copurified with TAP-CTD was used to transcribe a short (14-nucleotide) viral RNA template in the presence of an ApG dinucleotide primer. Transcription products were extracted with phenol-chloroform (1:1), precipitated with ethanol, and analyzed on a 16% polyacrylamide gel containing 7 M urea. Transcription products were detected by autoradiography.
Immunofluorescence analysis.
MDCK cells grown on cover glasses were infected with influenza A/PR8/34 virus at an MOI of 5 or were mock infected. At 4 h postinfection, cells were fixed in 4% paraformaldehyde in 250 mM HEPES for 15 min at room temperature. Cells were permeabilized in 1% Triton X-100 in phosphate-buffered saline (PBS) for 10 min, blocked in PBS containing 4% normal donkey serum (Jackson ImmunoResearch Laboratories), and incubated with primary antibodies diluted in PBS containing 4% normal donkey serum as indicated in the figure legends. Secondary antibodies were donkey anti-mouse and donkey anti-rabbit polyclonal antibodies conjugated to Cy2 or Cy3 (Jackson ImmunoResearch Laboratories). DNA was stained with To-Pro-3 iodide (Molecular Probes). Cover glasses were mounted in Mowiol (Calbiochem) containing DABCO (Sigma). Images were acquired with a Radiance 2000 (Bio-Rad) confocal system attached to a Nikon Eclipse TE300 microscope with a 60×/1.40 oil objective. Individual channel (Cy2, Cy3, and To-Pro-3 iodide) images were recorded separately, employing Kalman filtering. Images were exported to Adobe Photoshop, contrast stretched, and overlaid electronically. Analysis of colocalization percentages was done with Metamorph software (Universal Imaging Corporation); pixels with fluorescence intensity values within the top 40% in each nucleus were identified by thresholding, and the percentages of colocalized pixels between two channels were determined from at least 30 nuclei per sample. The percentages of pixels in channel A colocalizing with pixels in channel B (A/B) are listed in Table 1. Student's t test was used to determine the statistical significance of differences in colocalization percentages. The same strategy was used to create the images showing colocalized pixels.
TABLE 1.
Colocalization between influenza virus proteins and Pol II
| Proteins | % Colocalizationa |
|---|---|
| NP/PA | 39.2 ± 15.7 |
| PA/NP | 17.8 ± 9.5 |
| Pol II/PA | 16.4 ± 10.2 |
| PA/Pol II | 37.1 ± 21.1 |
| NP/PCNA | 4.5 ± 3.0 |
| PCNA/NP | 10.5 ± 9.3 |
By Student's t test, P < 0.001 for all colocalization percentages compared to NP/PCNA or PCNA/NP, except for Pol II/PA compared to PCNA/NP (P < 0.02)
In situ extraction of nuclei.
MDCK cells grown on cover glasses were infected with influenza A/PR8/34 virus at an MOI of 1 or were mock infected. Extraction was done essentially as described previously (9). At 7 h postinfection, cells were washed once in cold PBS and once in cold CSK [10 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) (pH 6.8), 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 1 mM EGTA, 1.2 mM PMSF], permeabilized in CSK containing 0.5% Triton X-100 for 3 min on ice, washed in cold CSK, treated for 30 min at room temperature with RQ1 DNase (5 U/ml) in DB (10 mM PIPES [pH 6.8], 50 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 1 mM EGTA, 1.2 mM PMSF, 0.5% Triton X-100), and extracted for 2 min each on ice first in 0.25 M (NH4)2SO4 in DB and then in 2 M NaCl in DB. After each step, aliquots of cells were fixed in 4% paraformaldehyde. Staining for immunofluorescence was carried out as described above, and analysis was done on an MRC1024 confocal system attached to a Nikon Diaphot200 microscope with a 60×/1.40 oil objective.
RESULTS
Influenza A virus polymerase interacts with cellular RNA polymerase II.
Coimmunoprecipitation analysis was performed to evaluate whether or not influenza A virus RNA polymerase was found in a complex with cellular Pol II. In order to obtain a cell lysate containing influenza virus RNA polymerase and both transcriptionally active, engaged Pol II and nonengaged Pol II, we prepared total cell lysates from infected 293T cells by including a promiscuous nuclease in the cell lysis buffer (see Materials and Methods). Nuclease treatment is known to result in the release of the actively transcribing, hyperphosphorylated form of Pol II (14). Cell lysates were incubated with an antibody against the PA subunit of the viral polymerase or a control antibody. Immunoprecipitated proteins were analyzed by Western blotting. The anti-PA antibody coprecipitated cellular Pol II (Fig. 1, top panel, lane 4) from lysates of infected cells but not from lysates of mock-infected cells (lane 3). The unrelated control antibody serving as a specificity control did not bring down Pol II from either lysate. An analogous coimmunoprecipitation experiment with a polyclonal antibody against the PB2 subunit gave similar results (data not shown).
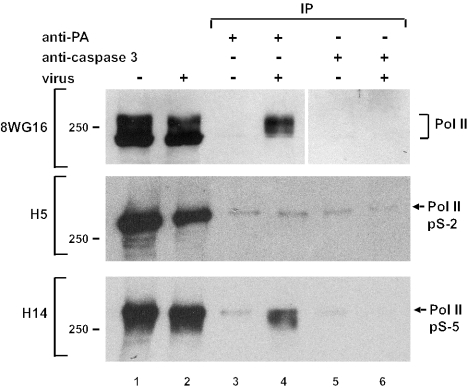
FIG. 1.
Coimmunoprecipitation of Pol II with influenza virus polymerase. Immunoprecipitations (IP) from lysates of 293T cells infected with influenza virus A/WSN/33 or mock infected were performed with a polyclonal anti-PA antibody (lanes 3 and 4) or with a polyclonal anti-caspase 3 antibody (lanes 5 and 6). Immunoprecipitates (lanes 3 to 6) and lysates (lanes 1 and 2) were analyzed by Western blotting with monoclonal anti-Pol II CTD antibody 8WG16, monoclonal anti-phosphoserine 2 Pol II antibody H5, and anti-phosphoserine 5 Pol II antibody H14, as indicated on the left.
Interestingly, we noticed that influenza virus polymerase brought down Pol II forms with slow mobility, whereas the fastest-migrating species was not detectable in the immunoprecipitate (Fig. 1). This suggested that it interacted with the hyperphosphorylated form of Pol II (Pol IIo) as well as with some intermediately phosphorylated species. To analyze the phosphorylation status of the coprecipitated Pol II in more detail, the immunoprecipitates were also probed with monoclonal antibodies H5 and H14 which recognize the phosphoserine 2 and phosphoserine 5 versions of the CTD, respectively. As shown in Fig. 1, the anti-PA antibody brought down the phosphoserine 5 form of Pol II recognized by antibody H14, but we were unable to detect significant levels above background of the phosphoserine 2 form recognized by antibody H5. Since phosphorylation of serine 5 of the CTD is associated with early events in the transcription cycle (11, 12, 26), these results suggest that the viral polymerase interacts with Pol II that is engaged in transcription.
We next tested whether the viral RNA polymerase complex alone, in the absence of other viral proteins and viral RNA, would interact with endogenous Pol II. For this purpose, 293T cells were transfected with three plasmids expressing the viral polymerase subunits, PB1, PB2, and PA, or with plasmids expressing a TAP-tagged PB1 (PB1-TAP) as well as PB2 and PA, or with empty vector plasmid. Lysates from the transfected cells were purified on IgG-Sepharose, which retains the TAP tag, and bound material was released by cleavage with TEV protease. Using this strategy, the PB2 and PA subunits copurified in approximately stoichiometric amounts with PB1-TAP (Fig. 2D), but all subunits were absent when only untagged subunits were expressed. Western blot analysis showed that Pol II, as detected by monoclonal antibody 4H8, which detects the CTD, also copurified with PB1-TAP (Fig. 2A, bottom panel, lane 3). Furthermore, the recombinant viral polymerase displayed the same specificity as the viral polymerase immunoprecipitated from infected cells; i.e., the phosphoserine 5 form of Pol II copurified with the recombinant polymerase, whereas the phosphoserine 2 form did not (Fig. 2B and C).
The trimeric complex of influenza virus polymerase interacts with the Pol II CTD.
The results presented above suggested that the interaction of Pol II with influenza virus polymerase was mediated by the phosphorylated CTD of Pol II. To confirm this, a plasmid coding for a fusion (TAP-CTD) of the TAP tag (28) and the CTD of mouse Pol II, which differs from the human CTD by only one amino acid, was transfected into 293T cells together with plasmids expressing the three influenza virus polymerase subunits. As controls, we substituted the TAP-CTD plasmid for a plasmid expressing GFP-TAP. Lysates of transfected cells were purified by using IgG-Sepharose; bound proteins were released by cleavage with TEV protease. Western blot analysis showed that the influenza virus polymerase subunits copurified with TAP-CTD (Fig. 3A, bottom panels, lanes 6 and 10), indicating that the CTD was sufficient for the interaction between Pol II and influenza virus polymerase. These results could be due to interaction of Pol II with the trimeric influenza virus polymerase complex or with the individual polymerase subunits. To distinguish between these possibilities, 293T cells were also transfected with a plasmid expressing TAP-CTD as well as with single plasmids expressing individual subunits and processed as before. None of the individually expressed influenza virus polymerase subunits copurified with TAP-CTD, suggesting that the trimeric complex was the entity recognized by the CTD (Fig. 3A, bottom panels, compare lanes 2, 4, and 8 with lanes 6 and 10). We also tested all possible combinations of coexpression of two subunits; again, copurification of influenza virus RNA polymerase subunits was observed only when all three subunits were coexpressed (data not shown), lending further support to the idea that the trimeric polymerase complex interacts with Pol II. It should be noted that these results do not exclude indirect interaction between the CTD and the influenza virus RNA polymerase complex. In vitro transcription reactions were performed in order to ascertain whether the polymerase complexes copurifying with TAP-CTD were functional. As shown in Fig. 3B, only the sample purified from cell lysates containing TAP-CTD and polymerase subunits yielded the expected transcript. Since it is known that only the complex of all three subunits is active in this viral transcription assay (3), this again indicated that the viral holoenzyme was interacting with the CTD.
In a reverse experiment, we tested whether the CTD could be copurified with viral polymerase. Cells were transfected with plasmids expressing a fusion of GFP and CTD (GFP-CTD) as well as the influenza virus polymerase subunits PA, PB1, and PB2 or a fusion of PB2 and the TAP tag (PB2-TAP), and lysates were purified on IgG-Sepharose. As expected, the untagged influenza virus polymerase subunits PA and PB1 were present, after purification, only in the sample containing PB2-TAP (Fig. 3C). More importantly, GFP-CTD copurified with the polymerase complex but was absent when PB2-TAP was replaced with the untagged PB2 (Fig. 3C, second panel, compare lanes 1 and 2).
Evidence for colocalization between influenza virus polymerase and cellular Pol II.
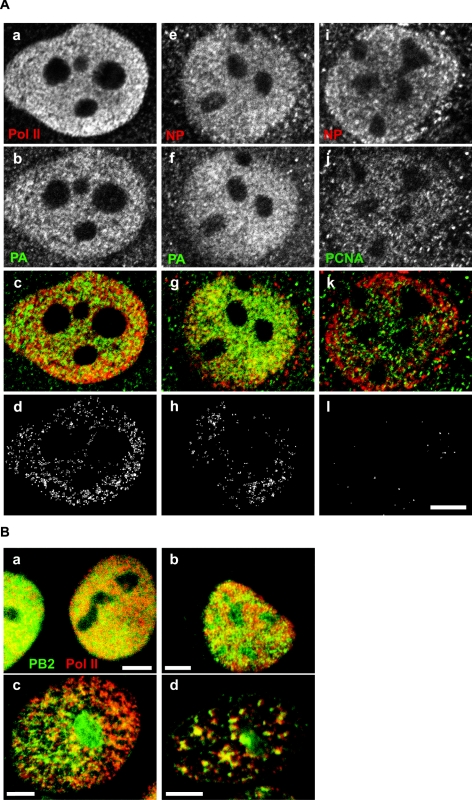
In order to determine whether influenza virus polymerase was associated with cellular Pol II sites, we performed double immunofluorescence labeling of the viral PA protein and Pol II in MDCK cells infected with PR8 virus. As shown in Fig. 4A, panels a to d, a portion of the PA signal overlapped with the signal for Pol II. Viral RNPs consisting of viral genomic RNAs and NP are templates for the viral RNA polymerase. In immunofluorescence analysis, we observed partial colocalization between the polymerase subunit PA and NP (Fig. 4A, panels e to h), as expected. The fact that colocalization was only partial is presumably due to the presence of free NP not bound to viral RNAs and, possibly, free polymerase subunits or polymerase complexes not engaged in RNA synthesis. As a control, we performed double labeling with anti-NP and anti-PCNA antibodies; the latter protein plays multiple roles in DNA replication, DNA repair, and cell cycle control but does not colocalize with Pol II transcription sites (14). As expected, the degree of overlap between NP and PCNA was low (Fig. 4A, panels i to l). Statistical analysis of the degree of overlap confirmed that the overlap between influenza virus polymerase and Pol II was significantly higher than the overlap between NP and PCNA (Table 1).
FIG. 4.
Influenza virus polymerase partially colocalizes with Pol II. (A) MDCK cells infected with influenza A/PR8/34 virus for 4 h were immunostained with antibodies against the proteins indicated and analyzed by confocal microscopy. Panels c, g, and k show electronic overlays of the images displayed in the corresponding panels above. Yellow indicates overlap of the two fluorescent signals. Panels d, h, and l show the pixels that have signal within the top 40% of fluorescence intensity in both channels (colocalized pixels). (B) A portion of influenza virus polymerase is insoluble upon high-salt extraction. MDCK cells infected with influenza A/PR8/34 virus for 7 h were either directly fixed (a) or subjected to stepwise extractions; cells were permeabilized in CSK plus 0.5% Triton X-100 (b), then treated with DNase and extracted in 0.25 M (NH4)2SO4 (c), and finally extracted in 2 M NaCl (d). Electronic overlays of the two separate channels (PB2 is pseudocolored green, and Pol II is pseudocolored red) are shown. Bars, 5 μm.
As described above (Fig. 1), in coimmunoprecipitation, influenza virus polymerase interacted preferentially with a slowly migrating, hyperphosphorylated form(s) of Pol II. Pol IIo, the active, engaged form of Pol II, is found mainly in an insoluble nuclear fraction by biochemical fractionation. We therefore investigated whether influenza virus polymerase shared solubility properties with Pol IIo by performing in situ extractions on living cells, followed by double immunofluorescence labeling for the phosphoserine 2 form of Pol II and influenza virus polymerase. As shown in Fig. 4B, stepwise extractions of cells revealed a more pronounced granular nuclear pattern for hyperphosphorylated Pol II. Significantly, influenza virus PB2 protein also resisted, at least in part, high-salt extraction conditions and strongly colocalized with the hyperphosphorylated active form of Pol II.
DISCUSSION
It has been proposed that influenza virus transcription has a functional link with Pol II transcription (7). In this report, we show by using biochemical and imaging approaches that influenza virus RNA polymerase is indeed associated with Pol II. It was found that the CTD of the large subunit of Pol II was sufficient for the interaction with influenza virus polymerase. Moreover, the hyperphosphorylated form of Pol II, Pol IIo, was enriched in immunoprecipitates together with influenza virus polymerase. The CTD is a versatile protein-protein interaction platform that mediates the association of RNA processing complexes with active Pol II and thus ensures that RNA processing events occur cotranscriptionally (1, 11, 12, 27). It is therefore possible that influenza virus has coopted the CTD in order to gain access to transcriptionally active Pol II. In this respect, it is of particular interest that in coimmunoprecipitation experiments only the H14-reactive, phosphoserine 5 form of Pol IIo interacted with influenza virus polymerase (Fig. 1). Serine 5 of the CTD is phosphorylated early upon transcription initiation and plays a role in recruitment and stimulation of capping enzymes (11, 12). By targeting specifically this form of Pol II, influenza virus polymerase would bind at a stage in the Pol II transcription cycle when the capping process of pre-mRNAs is taking place. Thus, influenza virus polymerase would be at the right place at the right time for stealing capped RNA oligonucleotides from cellular pre-mRNAs.
Our data do not rule out that the interaction of influenza virus RNA polymerase with Pol II is indirect and is mediated by factors that bind both the hyperphosphorylated CTD and the trimeric influenza virus polymerase complex (Fig. 5). Since the phosphoserine 5 form of Pol II was enriched in immunoprecipitations with anti-influenza virus polymerase antibodies, proteins involved in capping, or perhaps the cap binding complex, may play such a role. Recently, a number of nuclear host proteins have been reported to interact with influenza virus proteins (4, 10, 13, 22-24), two of which (hsp90 and hCLE) bind to viral polymerase subunits. These could mediate indirect interaction between the viral polymerase and Pol II.
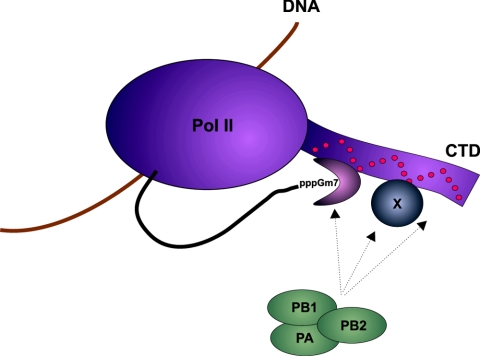
FIG. 5.
Model of influenza virus RNA polymerase interacting with Pol II. The trimeric viral polymerase complex (PA, PB1, and PB2) interacts with the hyperphosphorylated form of Pol II either directly or indirectly by binding to the cellular capping machinery or to an as-yet-unknown protein (X) that interacts with the CTD. Phosphorylated serine 5 residues of the CTD are represented as red circles. The drawing is not to scale.
It is known that drugs that interfere with Pol II transcription inhibit influenza virus (16, 20, 21, 29). This has been interpreted as reflecting the need for ongoing cellular transcription which continuously provides capped pre-mRNAs (2). While we assume this interpretation to be correct, we add a new dimension to the link between Pol II transcription and influenza virus RNA synthesis. By being in a complex with active, engaged Pol IIo, influenza virus could make use of the locally higher concentrations of capped pre-mRNAs and preassembled splicing factors. Similarly, it has been proposed that cellular transcription factories provide high local concentrations of polymerases, promoters, and other factors, which leads to effective and productive transcription of genes tethered to such factories (5). This scenario predicts influenza virus polymerase to be found near or at Pol II transcription sites in the nucleus. Our immunofluorescence analysis indeed supports this prediction since we observed significant colocalization between Pol II and viral polymerase. Not surprisingly, colocalization was only partial, because not all engaged Pol II molecules are expected to interact with viral polymerase. Moreover, our influenza virus polymerase-specific antibodies do not distinguish between engaged and transcriptionally inactive forms, e.g., viral RNPs destined for nuclear export. Interestingly, the solubility characteristics of Pol IIo and a portion of influenza virus polymerase were found to be similar in that both were resistant to high-salt extraction conditions. This indicates that influenza virus polymerase is associated with structures or complexes that are firmly attached to nuclear substructures. In accordance with this finding, it was reported previously that influenza virus RNA synthesis took place in so-called “nuclear cages” or in the nuclear matrix fraction (15, 19). Similarly, it has been demonstrated that high-salt-resistant Pol IIo, which can be solubilized by treatment with RNase, is the active fraction of Pol II (14).
Aside from the obvious implications of the interaction between influenza virus polymerase and Pol II for the production of viral mRNAs, an intriguing issue is the potential consequence of this interaction for the function of Pol II. Although recently it has been proposed that the NS1 protein of influenza virus is a major player in shutting down host protein synthesis by interfering with the host polyadenylation machinery (4, 24), it is tempting to speculate that binding of the viral polymerase to the CTD might contribute to virus-induced host shutoff by resulting in abortive cellular mRNA transcription. The viral polymerase might interfere with the function of the CTD either by affecting its phosphorylation status or by competing with cellular proteins that normally bind to the CTD.
In conclusion, this work extends the current model of influenza virus polymerase action in the nucleus of the infected cell by proposing that the functional interplay between viral polymerase and cellular RNA processing events is mediated, either directly or indirectly, through a physical interaction between influenza virus polymerase and active, transcribing Pol II. While this model suggests an attractive mechanism for the interplay between a viral and a host RNA polymerase, the full significance of this observation remains to be determined.
Acknowledgments
We thank T. Deng, T. Jung, S. Carr, T. Toyoda, and P. Uguen for reagents; J. Robinson for sequencing; A. Akoulitchev, G. Brownlee, P. Cook, S. Murphy, and F. Iborra for helpful discussions; A. Akoulitchev, N. Proudfoot, and F. Vreede for critical reading of the manuscript; and A. Wilson, M. Shaw, and N. White for advice on and help with imaging.
This work was supported by the MRC (senior nonclinical fellowship G117/457 to E.F).
REFERENCES
- 1.Bentley, D. 2002. The mRNA assembly line: transcription and processing machines in the same factory. Curr. Opin. Cell Biol. 14:336-342. [DOI] [PubMed] [Google Scholar]
- 2.Bouloy, M., S. J. Plotch, and R. M. Krug. 1978. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc. Natl. Acad. Sci. USA 75:4886-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlee, G. G., and J. L. Sharps. 2002. The RNA polymerase of influenza A virus is stabilized by interaction with its viral RNA promoter. J. Virol. 76:7103-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, Z., Y. Li, and R. M. Krug. 1999. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3′-end processing machinery. EMBO J. 18:2273-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook, P. R. 2002. Predicting three-dimensional genome structure from transcriptional activity. Nat. Genet. 32:347-352. [DOI] [PubMed] [Google Scholar]
- 6.Fodor, E., M. Crow, L. J. Mingay, T. Deng, J. Sharps, P. Fechter, and G. G. Brownlee. 2002. A single amino acid mutation in the PA subunit of the influenza virus RNA polymerase inhibits endonucleolytic cleavage of capped RNAs. J. Virol. 76:8989-9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fodor, E., A. Mikulasova, L. J. Mingay, L. L. Poon, and G. G. Brownlee. 2000. Messenger RNAs that are not synthesized by RNA polymerase II can be 3′ end cleaved and polyadenylated. EMBO Rep. 1:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fodor, E., and M. Smith. 2004. The PA subunit is required for efficient nuclear accumulation of the PB1 subunit of the influenza A virus RNA polymerase complex. J. Virol. 78:9144-9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He, D. C., J. A. Nickerson, and S. Penman. 1990. Core filaments of the nuclear matrix. J. Cell Biol. 110:569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirayama, E., H. Atagi, A. Hiraki, and J. Kim. 2004. Heat shock protein 70 is related to thermal inhibition of nuclear export of the influenza virus ribonucleoprotein complex. J. Virol. 78:1263-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirose, Y., and J. L. Manley. 2000. RNA polymerase II and the integration of nuclear events. Genes Dev. 14:1415-1429. [PubMed] [Google Scholar]
- 12.Howe, K. J. 2002. RNA polymerase II conducts a symphony of pre-mRNA processing activities. Biochim. Biophys. Acta 1577:308-324. [DOI] [PubMed] [Google Scholar]
- 13.Huarte, M., J. J. Sanz-Ezquerro, F. Roncal, J. Ortín, and A. Nieto. 2001. PA subunit from influenza virus polymerase complex interacts with a cellular protein with homology to a family of transcriptional activators. J. Virol. 75:8597-8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iborra, F. J., A. E. Escargueil, K. Y. Kwek, A. Akoulitchev, and P. R. Cook. 2004. Molecular cross-talk between the transcription, translation, and nonsense-mediated decay machineries. J. Cell Sci. 117:899-906. [DOI] [PubMed] [Google Scholar]
- 15.Jackson, D. A., A. J. Caton, S. J. McCready, and P. R. Cook. 1982. Influenza virus RNA is synthesized at fixed sites in the nucleus. Nature 296:366-368. [DOI] [PubMed] [Google Scholar]
- 16.Lamb, R. A., and P. W. Choppin. 1977. Synthesis of influenza virus polypeptides in cells resistant to alpha-amanitin: evidence for the involvement of cellular RNA polymerase II in virus replication. J. Virol. 23:816-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamb, R. A., and C. M. Horvath. 1991. Diversity of coding strategies in influenza viruses. Trends Genet. 7:261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamb, R. A., and R. M. Krug. 2001. Orthomyxoviridae: the viruses and their replication, p. 1487-1530. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 19.Lopez-Turiso, J. A., C. Martinez, T. Tanaka, and J. Ortín. 1990. The synthesis of influenza virus negative-strand RNA takes place in insoluble complexes present in the nuclear matrix fraction. Virus Res. 16:325-337. [DOI] [PubMed] [Google Scholar]
- 20.Mahy, B. W., N. D. Hastie, and S. J. Armstrong. 1972. Inhibition of influenza virus replication by alpha-amanitin: mode of action. Proc. Natl. Acad. Sci. USA 69:1421-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mark, G. E., J. M. Taylor, B. Broni, and R. M. Krug. 1979. Nuclear accumulation of influenza viral RNA transcripts and the effects of cycloheximide, actinomycin D, and alpha-amanitin. J. Virol. 29:744-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Momose, F., C. F. Basler, R. E. O′Neill, A. Iwamatsu, P. Palese, and K. Nagata. 2001. Cellular splicing factor RAF-2p48/NPI-5/BAT1/UAP56 interacts with the influenza virus nucleoprotein and enhances viral RNA synthesis. J. Virol. 75:1899-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Momose, F., T. Naito, K. Yano, S. Sugimoto, Y. Morikawa, and K. Nagata. 2002. Identification of Hsp90 as a stimulatory host factor involved in influenza virus RNA synthesis. J. Biol. Chem. 277:45306-45314. [DOI] [PubMed] [Google Scholar]
- 24.Nemeroff, M. E., S. M. Barabino, Y. Li, W. Keller, and R. M. Krug. 1998. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′end formation of cellular pre-mRNAs. Mol. Cell 1:991-1000. [DOI] [PubMed] [Google Scholar]
- 25.Neumann, G., G. G. Brownlee, E. Fodor, and Y. Kawaoka. 2004. Orthomyxovirus replication, transcription, and polyadenylation. Curr. Top. Microbiol. Immunol. 283:121-143. [DOI] [PubMed] [Google Scholar]
- 26.Palancade, B., and O. Bensaude. 2003. Investigating RNA polymerase II carboxyl-terminal domain (CTD) phosphorylation. Eur. J. Biochem. 270:3859-3870. [DOI] [PubMed] [Google Scholar]
- 27.Proudfoot, N. J., A. Furger, and M. J. Dye. 2002. Integrating mRNA processing with transcription. Cell 108:501-512. [DOI] [PubMed] [Google Scholar]
- 28.Puig, O., F. Caspary, G. Rigaut, B. Rutz, E. Bouveret, E. Bragado-Nilsson, M. Wilm, and B. Seraphin. 2001. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods 24:218-229. [DOI] [PubMed] [Google Scholar]
- 29.Spooner, L. L., and R. D. Barry. 1977. Participation of DNA-dependent RNA polymerase II in replication of influenza viruses. Nature 268:650-652. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi, Y., S. Delehouzee, and H. Handa. 2002. HIV and hepatitis delta virus: evolution takes different paths to relieve blocks in transcriptional elongation. Microbes Infect. 4:1169-1175. [DOI] [PubMed] [Google Scholar]