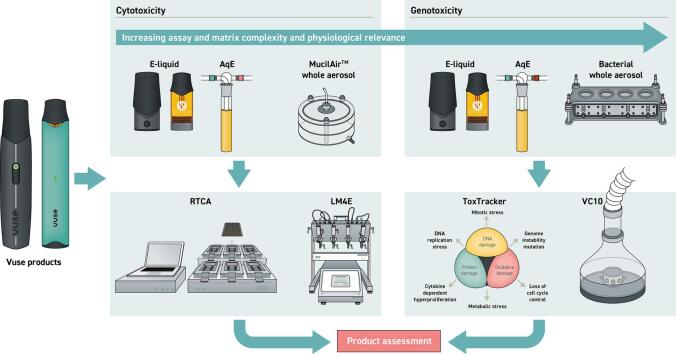
Graphical abstract
Keywords: Risk Assessment, In vitro, Cytotoxicity, Genotoxicity, Vaping
Highlights
-
•
Robust in vitro testing was conducted on e-liquid and aerosol test matrices.
-
•
Using various test matrices, cell lines and endpoints increase robustness of data.
-
•
E-cigarettes should be assessed as part of a stewardship assessment framework.
-
•
Differing heating technologies tested did not impact the e-cigarette toxicological profile.
Abstract
Interest in the toxicological assessment of iterations of e-cigarette devices, e-liquid formulations and flavour use is increasing. Here, we describe a multiple test matrix and in vitro approach to assess the biological impact of differing e-cigarette activation mechanism (button vs. puff-activated) and heating technology (cotton vs. ceramic wick). The e-liquids selected for each device contained the same nicotine concentration and flavourings. We tested both e-liquid and aqueous extract of e-liquid aerosol using a high throughput cytotoxicity and genotoxicity screen. We also conducted whole aerosol assessment both in a reconstituted human airway lung tissue (MucilAir) with associated endpoint assessment (cytotoxicity, TEER, cilia beat frequency and active area) and an Ames whole aerosol assay with up to 900 consecutive undiluted puffs. Following this testing it is shown that the biological impact of these devices is similar, taking into consideration the limitations and capturing efficiencies of the different testing matrices. We have contextualised these responses against previous published reference cigarette data to establish the comparative reduction in response consistent with reduced risk potential of the e-cigarette products tested in this study as compared to conventional cigarettes.
Introduction
A public policy of tobacco harm reduction including increased availability of reduced risk alternatives to traditional cigarettes is increasingly being adopted among countries aiming to reduce the projected public health burden of smoking. Accordingly, there has been increased regulatory interest in e-cigarettes (also known as electronic nicotine delivery systems (ENDS)) and their component devices and e-liquids. Regulatory concerns, include ensuring appropriate stewardship is conducted on these products to facilitate quality products to consumers that meet the potential for reduced harm, whilst simultaneously trying to ascertain the risks to a naïve vaping population. While currently there is no globally recognised standards on how to assess e-cigarette products (Belushkin et al., 2018), there have been proposed e-cigarette assessment frameworks which include generation of in vitro toxicology data to ensure products perform favourably in comparison to traditional cigarettes and contribute to a tobacco harm reduction strategy (Behar et al., 2018, Iskandar et al., 2016). Some standards relevant to e-cigarette assessment have been developed, such as the CORESTA recommended method 81 (CRM 81) recently adopted by ISO (CORESTA, 2015, ISO, 2018) that details how products should be activated and puffed for in vitro and analytical testing. These standards are not universally employed in testing approaches as researchers try to mimic human consumption behaviours more accurately. Although a standard for aerosol generation exists, which can harmonise emission and aerosol generation, how these aerosols are applied is equally important, and other than OECD test guidelines (such as NRU (OECD TG 432; Ames TG 471; IVMN TG 487; MLA TG 490), little standardisation exists for more mechanistic in vitro approaches.
With any proposed in vitro toxicological assessment framework there is a need to balance physiological relevance of both the cell system and the test matrix. The most physiologically relevant test system and test matrix are those that most closely model human exposure. Considering inhalation, 3D tissues and direct aerosol exposure represent the most physiological approach. Unfortunately, few validated models truly exist and those that do exist are time-consuming and costly to perform. Currently there is a drive to increase in vitro aerosol modelling approaches and validate new methods, whilst ensuring that alternative screening models exist to augment a testing strategy. Therefore, in combination with aerosol approaches, for inhalation exposure in this scenario and in the absence of more validated techniques, alternative testing approaches provide additional information to increase the weight of evidence in a comprehensive assessment framework, for comparative product risk. A review was recently published assessing various methods described in the literature to capture e-cigarette aerosol for in vitro testing (Smart and Phillips, 2021). This gives greater clarity of the potential limitations of the various test articles and therefore substantiates the need more a more comprehensive testing strategy, utilising multiple assays and test matrices.
For e-cigarette toxicological assessment the first tier of testing is often direct e-liquid exposure of cells in culture. E-–liquids are generally quite easy to source and to dilute into a test system and specialist equipment. However, the limitation of this approach is the physiological relevance of e-liquid and its for intended use; 1) consumers lungs are not exposed to non-aerosolised e-liquids: 2) due to the submerged nature of the exposure combined with the fact that most of the cytotoxicity measured in these assays is confounded by the osmolality shift caused by the high PG/VG content of the test samples, which has been widely discussed (Gonzalez-Suarez et al., 2016, Marescotti et al., 2020, Czekala et al., 2021a). Furthermore, diluting e-liquids to within physiological conditions (<1% e-liquid dilution) may limit the potential sensitivity of the assay to detect a response with meaningful relevance.
The next level of complexity involves evaluation of the toxicological effects of exposure to e-cigarette aerosol produced by interaction of e-liquid with an e-cigarette device. This evaluation can be conducted via capturing aerosol extracts, combining the ease of submerged monolayer culture with delivery of an aerosolised product. Multiple extracts can be created and frozen ahead of dosing, speeding up assay preparation. A limitation of this approach is the inability of the various phases of the aerosol to be appropriately captured, thus not providing a full representation of the test aerosol. Saturation of the test matrix solution also needs to be carefully considered, with various aerosol components shown to saturate at different levels, indicating that the test matrix may preferentially select certain compounds (Bozhilova et al., 2020). There are technical considerations when using this method, such as diligently preparing impingers and connecting tubing to make sure there are no leaks whilst ensuring minimal aerosol transit to maximise capture efficiency. However, this test matrix can be especially useful when adopting cell lines that cannot be easily exposed to whole aerosol.
The most physiologically representative test matrix for e-cigarette testing is direct aerosol exposure. Whole aerosol presents many complexities and technical considerations which have been previously reviewed (Thorne and Adamson, 2013, LI, X. , 2016). Briefly, these include the type of vaping or adapted smoking machine (such as from Vitrocell or Borgwaldt) that is used to actuate the device and deliver the aerosol to the test system, the attachment of the device to the machine, tubing lengths, exposure times, prevention of “dry-wicking” and reliable dosimetry methods to ensure appropriate exposure. Cost and complexity along with required training and maintenance limit the overall application of such approaches. Also, with the increase in biological relevance comes a reduced throughput trade off (Lacroix et al., 2018). Whole aerosol exposure systems and the associated biological approaches have limited throughput, thus leaving them only applicable to targeted studies. Some advancements such as clever use of experimental design (Bishop et al., 2022) and system developments, like the Vitrocell high throughput module (Keyser et al., 2022) have enabled a higher throughput for whole aerosol assessment, but still these throughput rates remain low when compared to submerged multi-well plate exposure-based assays.
From an assay perspective, monolayer (2D) cytotoxicity assays can provide faster results as cell lines often have little pre-culturing requirements and can be expanded to seed large numbers of plates for testing. These assays also generally are low cost, and vital stains such as neutral red, propidium iodide and MTT are also relatively inexpensive. Although more costly, high throughput methods such as xCELLigence real time cellular analysis (RTCA – a cell-impedance based technology) are now available that automate the monitoring of the cell response, offering a timesaving compared to traditional methods (Roshan Moniri, 2015, East et al., 2021). High throughput alternatives such as the ToxTracker assay also exist for genotoxicity screening. ToxTracker is a genotoxicity assay using mouse embryo stem cells expressing GFP (green fluorescence protein)-tagged markers for cellular processes associated with genotoxicity. Table 1 shows how the ToxTracker assay has been used in multiple industries for a variety of applications.
Table 1.
Published applications of the ToxTracker Screening assay.
| Research application | Research area | Test products & Matrix | Reference |
|---|---|---|---|
| Screening | Tobacco | Complex mixtures (TPM, AqE, E-liquid from THP, e-cigarettes and MOP) | (Bishop et al., 2020, Czekala et al., 2021a, Smart et al., 2022) |
| Agriculture | Dicamba, 2–4 Dichlorophenoxyacetic acid & Glyphosphate (alone & in combination) Glyphosphate herbicides (3), & glyphosphate |
(Mesnage et al., 2021a) (Mesnage et al., 2021b) |
|
| Screening (Proof of concept) |
Food & Nutrition | Food grade particulates, Vegetable carbon (E153) and TiO2 (E171) |
(Brown et al., 2019) |
| Screening (mechanistic) | Biomedicine | Nucleoside analogues (21 compounds tested) |
(Brandsma et al., 2022) |
| Nanomaterials | Cobalt substances (11 compounds) Metal oxide nanoparticles |
(Derr et al., 2022) (Karlsson et al., 2014) |
|
| Follow-up studies | Cosmetics | Coumarin | (Baltazar et al., 2020) |
bbreviations: TPM = Total Particulate Matter, AqE = Aqueous Extract, e-cigarette = electronic cigarettes, THP = Tobacco Heating Product, MOP = Modern Oral Product.
However, currently there is still a perceived need to confirm any results from these high throughput screening assays in a more widely accepted assay such as the Ames assay. This is especially the case as the Ames assay can be successfully adapted to whole aerosol testing where the cells can withstand intense exposure scenarios (Thorne et al., 2018). However, experiments are often very time consuming and laborious due to the numbers of strains and the scoring involved and are also subject to operator subjectivity and the requirement for highly trained operators. The most physiologically relevant test system would involve reconstituted human lung tissues. These can be differentiated in house from normal human bronchial epithelial (NHBE) cells or purchased as fully differentiated tissues directly from suppliers such as Epithelix or MatTek. Either route is much more expensive than the use of 2D cells, while 3D tissues also have more defined shelf-life and restrictive in terms of numbers of samples that can be tested.
The aim of this study was to conduct a comprehensive in vitro data package using multiple test matrices, and endpoints to assess two e-cigarettes device iterations ePod3 to ePod. Such data would help inform the ongoing debate on product evolution and bridging (Gaca et al., 2022) whilst supporting tobacco harm reduction initiatives (Murphy et al., 2017). For this study two devices with contrasting mode of activation (button vs. puff) and wicking (cotton coil vs. ceramic) were assessed using a consistent Golden Tobacco e-liquid flavour. Test matrices assessed were e-liquid, AqE, and whole aerosol exposure, coupled with the biological endpoints of cytotoxicity, and genotoxicity via a recognised and novel method (Ames assay and ToxTracker, respectively). Each test matrix and assay showed similar results, suggesting that these devices show overall parity of response across the assays and matrix tested.
Materials and methods
Study design
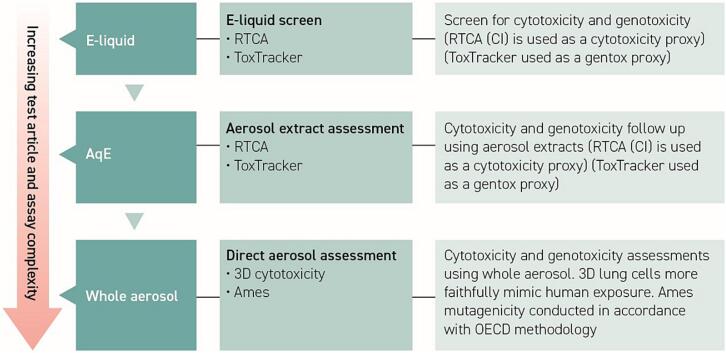
This manuscript covers two devices (Fig. 1), and a range of test matrices from e-liquid to whole aerosol, including an intermediate aerosol extract, thus assessing the complexities of direct exposure through to aerosolisation and capturing the conversion from liquid to aerosol and the potential formation of thermal degradation and breakdown products not captured in e-liquid only assessments. Direct e-liquid exposure assessments using RTCA and ToxTracker were considered proxies for cytotoxicity and genotoxicity respectively, were followed by aerosol captured approaches, using the same endpoints. The testing strategy was further complemented by 3D cytotoxicity and Ames mutagenicity aerosol assessments. Fig. 1 and Table 2 summarises the experimental design and assay conditions.
Fig. 1.
Schematic representation of the assessment approach. Study design represents increasing test article complexity from e-liquid to captured aerosol extract, and finally whole aerosol exposure, to capture the most complex interactions. Whole aerosol exposures are not limited by capture efficiency, matrix saturation or artefactual limitations, unlike e-liquids and captured aerosol extracts. The testing strategy starts with e-liquid screening using a 2D lung cell system (NCI-H292) and mechanistic assessment using mouse embryos and ToxTracker assay and is completed with whole aerosol assessments using 3D tissues (MucilAir) and an Ames mutagenicity whole aerosol-based methodology.
Table 2.
Overview of the assays used in this study and their parameters.
| Parameter |
Assay |
|||||
|---|---|---|---|---|---|---|
| Cytotoxicity | Oxidative stress, protein, and DNA damage | Mutagenicity | ||||
| Technique | RTCA | RTCA1 | MTT2 | TT3 | TT3 | Ames4 |
| Strategy | Cytotoxicity Proxy | Cytotoxicity Measure | Genotoxicity Screen | Mutagenicity Measure | ||
| Exposure | e-liquid5 | AqE6 | Whole aerosol | e-liquid5 | AqE6 | WA |
| Cells | 2D NCI-H292 |
2D NCI-H292 |
3D Reconstituted human airway tissues (MucilAir) |
2D Mouse embryo |
Bacterial strains TA98 TA100 TA97, TA104 WP2 uvrA pKM101 (E. coli) |
|
| Vaping Regimen | N/A | CRM 817 | CRM 817 | N/A | CRM 817 | CRM 817 |
| Total number of puffs | N/A | 200 | 900 | N/A | 200 | 9008 |
| Aerosol generator | N/A | Borgwaldt LM4E | Borgwaldt LM4E | N/A | Borgwaldt LM4E | Vitrocell VC 10 |
| Aerosol generator Serial number | N/A | Pump: 160,720,105 and 171,101,036 Puffing: 160,620,001 and 170,320,082 |
N/A | Pump: 160,720,105 and 171,101,036 Puffing:160,620,001 and 170,320,082 |
VC10/050614 | |
| Exposure type | N/A | Captured in 20 mLs DMEM | Undiluted Aerosol | N/A | Captured in 20 mLs DMEM | Undiluted Aerosol |
Abbreviations: RTCA = real time cell analysis; TT = ToxTracker; AqE = aqueous extracts; DMEM = Dulbecco’s modified eagles’ medium; WA = whole aerosol.
= Conducted in accordance with (Bishop et al., 2023).
= Conducted in line with (Bishop et al., 2019, Bishop et al., 2022).
= Conducted in accordance with (Hendriks et al., 2012, Hendriks et al., 2016, Smart et al., 2022).
= Conducted in line with (Thorne et al., 2018).
= e-liquid exposure 1–10 % to generate full curves for analysis (beyond 1 % is above physiological conditions).
= Limit of capture (200 puffs) defined by saturation of media. AgE generated in accordance with (Bozhilova et al., 2020).
= CRM 81; 55 mL puff, 3sec duration, 30sec interval, square wave puffing profile with 45-degree angle (1 sec pre activation for button pushing using ePen3) (CORESTA, 2015) also referred to as ISO 20768:2018 (ISO, 2018).
= 900 consecutive puffs delivered in vitro using an undiluted approach. For context average human daily consumption is approximately 250 puffs in a 12-hour period. 900 puffs delivered over a 3-hour exposure period represents an extreme testing scenario (approximately three times more than the average daily consumption in a quarter of the exposure time) (Thorne et al., 2018).
Materials
Unless otherwise stated all materials and reagents were purchased from Fisher Scientific (Loughborough, UK).
Test products
Vuse ePen3 (3rd Generation ePen) and ePod (1.0. 1st Generation ePod) e-cigarette devices and liquids were used for this study (www.vuse.com) (Nicoventures Trading Ltd., Globe House, London). Both products represented the last (ePen3) and newest versions (ePod) respectively, at the time of study design. Golden Tobacco e-liquids (18 mg/mL nicotine strength) were exclusively used. Golden tobacco represented a comparable formulation between devices. Moreover, smokers switching from cigarette smoke to e-cigarette use, in the first instance are more likely to use a tobacco- flavoured formulation, hence it’s selection in this study. ePen3 is powered by a 650-mAh battery, which is connected to a coil with resistance of 1.95–2.36 O, resulting in a power output of 5.9 W. The coil is made from a NiFe alloy whose resistance is strongly temperature dependent. The device uses a cotton wick to transport e-liquid (2 mL capacity) to the heated coil. ePod consists of a metallic outer device case, a printed circuit board to control the device, a lithium-ion rechargeable battery (350 mAh) and an e-cigarette cartridge. The voltage ranges from 2.2 to 3.1 V and is not adjustable by the user. The device has a power output of 6.5 ± 0.5 W. The electronic parts are switched on when a puff is taken. The cartridges or pods consist of a plastic case holding the ceramic wick material and a flat metal heating element (NiCr, 0.8 ∼ 1.4-ohm resistance). Each pod is pre-filled with Vuse e-liquid (1.9 mL) and is magnetically attached to the device. For a comparison between devices please refer to Fig. 2 and Table 3.
Fig. 2.
Schematic of the products used in this study.
Table 3.
Comparisons of ePen3 and ePod technical specifications.
| Parameter | Device |
|
|---|---|---|
| ePen3 | ePod | |
| Characterisation Ref | (Cunningham et al., 2020, Bishop et al., 2022) | (Pinto et al., 2022) |
| E-cigarette classification | Pod Mod | Pod Mod |
| Classification description | Closed non-modifiable design compatible with single use pre-filled cartomisers | Closed non-modifiable design compatible with single use pre-filled cartomisers |
| e-liquid commercial flavour | Golden Tobacco | Golden Tobacco |
| Nicotine strength | 18 mg/mL | 18 mg/mL |
| PCODE (e-liquid) | RDVP000112 | LEPOD1.0_VGT18 |
| PCODE (e-liquid & Device) | EPEN3.0_VGT18 | EPOD1.0_VGT18 |
| Device activation | Button | Puff |
| Cartomiser specification | Coil and wick integrated into cartomiser | Coil and wick integrated into cartomiser |
| Size of device (HxWxD) | 121 mm x 26 mm x 12 mm | 104 mm x 19 mm x 10 mm |
| Wick material | Cotton | Ceramic |
| Power (W) | 5.9 | 6.5 |
| Coil resistance (ohm) | 1.5 | 1.4 |
| Battery capacity (mAh) | 650 | 350 |
| Mass* yields (mg/puff) | 8 | 6.5 |
Mass = ACM (aerosol collected mass).
Yields based on captured mass on a Cambridge filter pad under CRM 81 regime compared to an air blank.
Stewardship statement
Vuse e-cigarettes, ePod and ePen (technology and batteries, lithium ion -cell) are manufactured in accordance with the IECEE Certified Body Scheme operated by the IEC System of Conformity Assessment Schemes for Electrotechnical Equipment and Components - IECEE (web-link: https://certificates.iecee.org/ods/cb_hm.xsp). E-liquid formulations were risk assessed using established and documented approaches (Costigan and Lopez-Belmonte, 2017, Costigan and Meredith, 2015). Ingredients qualified as carcinogens, mutagens, known respiratory sensitisers and reprotoxicants were actively excluded from the formulation. Where flavourings have limited data available, toxicological thresholds of concern are applied to limit human exposure and minimise risk.
E-liquid exposure
E-liquid was extracted from commercially manufactured cartomisers for the study to ensure consistency of batches between the different test matrices and parts of the study. E-liquid was diluted in complete cell culture media between 0 and 10 % (v/v).
Aqueous extract (AqE) Collection
E-cigarette AqE generation has been previously described (Bozhilova et al., 2020), including impinger specification and design. Extracts were collected using an LM4E aerosol generator (Borgwaldt KC GmbH, Germany), a glass impinger and 20 mL of DMEM serum-free capture media. 200 puffs of e-cigarette aerosol were generated using ISO 20768 regimen (55 mL puff volume, over 3 s, every 30 s, with a square wave puffing profile (ISO, 2018). For ePen3, which is button activated, a 1 s pre-activation was employed. For ePod which is puff activated no pre-activation was used. 1 mL was taken for subsequent nicotine quantification. Foetal Bovine Serum (FBS) was added to the AqE aliquots prior to use to maintain the final concentration of 10 % (v/v).
Real-Time cell analysis (E-liquid and captured extracts)
As previously described (East et al., 2021) Real-time cell analysis (RTCA), the xCELLigence® multi-plate (MP) instrument (ACEA Biosciences; California, US with integrated (RTCA Software 2.1.0) software was used to determine Cell Index (CI) which is considered a proxy for cytotoxicity. The MP RTCA instrument has 6 cradles which allows 6 plates to be run simultaneously, with 3 samples per 96 well plate. The bottom of each 96 well E-Plate® contains a set of gold microelectrodes, attached to each well which allows the passing of an electric current. CI measures the electrode impedance at the electrode/solution interface, which is influenced by the presence of cells cultured in the wells and thus impeding electron flow. As described in the manufacturer’s protocol, E-Plates® were seeded 20,000 H292 cells/well and allowed to settle for 30 min before a background measurement was taken. Cells were allowed to proliferate for 24 h before dosing with test article for 24 h. Impedance (Cell Index) measurements were taken throughout the 48 h time period, producing a cell viability curve once analysed. 0.25 % (v/v) concentration of Triton-X100 was used to anchor curves when full cytotoxicity was not induced by the test article.
ToxTracker assay (E-liquid and captured extracts)
ToxTracker is a proprietary platform (Toxys B.V., Leiden, Netherlands) for the analysis of toxicological endpoints via the use of six validated reporter genes. Oxidative stress, (Nrf2-dependant Srxn1, and Nrf2-independent Blvrb genes), DNA damage (Bscl2, Rtkn), cell stress (Btg2) and protein damage / misfolding (Ddit3) cell lines and one wild type cell line were used as per the standard assay conditions (Hendriks et al., 2012, Hendriks et al., 2016). The cell lines were propagated in line with standardised Toxys protocols. As such, the cells were cultured on gelatine (Sigma, UK) coated dishes and passaged 3 times, with 6 media changes per week. Cell aliquots were used for a maximum of 6 weeks prior to exchange, with Geneticin (G418) at 200 µg/mL used as standard to preserve expression, with G418 excluded from the wild-type cell line. Assays were performed in 96 well plates (Costar, UK), each coated with gelatine prior to the addition of one row of each of the 7 cell lines. Two test articles were dosed in columns onto each plate, each at 5 concentrations plus a vehicle control, Cisplatin, Diethyl Maleate and Tunicamycin acted as positive controls for DNA damage, oxidative stress and protein damage respectively each dosed in columns at two concentrations, plus a vehicle control. Following 24 h exposure to the test articles, the cells were re-suspended in their wells by trypsinisation and the digestion was neutralised prior to reading the plate on a FACSCanto II multi reader (BD Bioscience). The FACSCanto II Cytometry setup and tracking (CS&T) process was performed before each run using standardisation beads. As a result, all gating and photomultiplier settings were persevered both intra and inter-experimentally. Readouts were assessed as a single cell population, gated by forward and side scatter, with a readout of the population cell count and a mean intensity for the FITC/GFP 488 nm channel. Data analysis was expressed as fold induction over the corresponding untreated cells. In addition, background subtractions were made using treated paired wild type cells to correct for potential autofluorescence of the added compounds.
Human bronchial epithelial cell culture (E-liquid and capture extracts)
NCI-H292 (American Type Culture Collection (ATCC) were cultured at 37 °C in a 5 % CO2 humidified atmosphere in Roswell Park Memorial Institute (RPMI) 1640 media supplemented with 10 % FBS, 2 mM Lglutamine and 50 U/mL penicillin and 50 µg/mL streptomycin. Cells were frozen in a cell bank at passage 79. Cyropreserved H292 were recovered and cultured for a further 21 passages before disposal.
Pseudo-stratified three-dimension human airway cell culture (Whole Aerosol)
The Borgwaldt LM4E puffing machine (Linear 4-port e-cigarette puffing machine, Borgwaldt-kc, Hamburg, Germany) was used in this study to apply undiluted e-cigarette aerosol to MucilAir cells, (pump module serial numbers: 160,720,105 and 171,101,036 puffing module serial numbers 160,620,001 and 170320082). The ePod devices were loaded into the mouthpieces and the cradles mounted at a 45⁰ angle, and button actuators were positioned, e-cigarettes were puffed to the ISO 20768 regimen (ISO, 2018). Triplicate inserts were exposed in a BAT exposure chamber (Patent Publication Number WO 03/100417) with 25 mL of cell culture media per chamber. Cartomisers were weighed prior to and post-exposure to provide a QC measure of device mass loss per puff. Following exposure at various time points, tissues were removed and placed into a recovery period, supplemented with 0.7 mL/well MucilAir media for 24 h (37 °C, 5 % CO2). Exposure media was collected from the chamber and stored at 2–8 ˚C for further nicotine quantification. MucilAir, 3D reconstituted human airway tissues, were purchased from Epithelix Sàrl (Plan-les-Oautes, Switzerland). Cells were of nasal origin, from a pool of 14 healthy non-smoking donors (batch ID MP0010) (Balogh Sivars et al., 2018). Cells arrived a week before exposures were scheduled to ensure acclimatisation after shipment. As per manufacturer’s instructions, cells were supplemented every 2–3 days with MucilAir media, and apical surface rinsed with phosphate buffered saline solution (PBS) once a week. Transepithelial electrical resistance (TEER) measurements were taken after each exposure using a resistance meter and Endohm-6 measurement chamber (World Precision Instruments, FL, USA).
MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium) cytotoxicity assay (Whole Aerosol)
MucilAir cytotoxicity was measured following 24 h recovery post-exposure. Cells were incubated in 0.5 mg/mL MTT reagent for 3 h to allow uptake of the dye at 37 ˚C, as per manufacturer’s instructions. Resulting formazan crystals were dissolved in MTT solubilisation solution with an overnight incubation at 2–8 ˚C. Apical and basal solutions were combined and 100 µL was pipetted into duplicate wells on a 96-well plate and using a spectrophotometer, read for optical density (OD) at 570 nm with a background subtraction at 690 nm. All results were normalised to the air control.
Cilia beat frequency
Following recovery, Cilia beat frequency (CBF) was analysed with Sisson-Ammons Video Analysis (SAVA) software (Sisson et al., 2003) using an inverted phase contrast microscope (Zeiss, Germany). Live cells were visualised on a heated stage at 100X magnification (10x ocular, 10x objective), focused to give a view of the cells and cilia. Two videos were recorded per well at 120 frames per second with a total of 512 frames per video. The software was used to calculate the percentage active area (AA) within the region of interest and CBF for all samples.
Ames (Whole Aerosol)
As previously described in (Thorne et al., 2018) The Vitrocell VC 10 smoking robot (Vitrocell Systems, Waldkirch, Germany) serial number VC10/050614 was used to expose bacteria to generated e-cigarette aerosol. Triplicate bacterial plates were exposed in the Vitrocell Ames 4 stainless steel exposure module. The trumpet height within the module was set to 2 mm above the agar surface and the entire aerosol stream was then diverted across the exposure interface by blocking the exhaust and creating a closed system and removing diluting airflow and vacuum. The resulting aerosol was subsequently positively exhausted out of the exposure module when the next puff was delivered. In this study, near-continuous aerosol delivery was generated by the serial puffing of four individual Vuse e-cigarettes at a 45⁰ angle to ISO 20768 regimen (ISO, 2018).
The Ames assay used in this study is a scaled-down modification of the standard 85 mm methodology and is described in detail in various studies (Aufderheide and Gressmann, 2007, Aufderheide and Gressmann, 2008, Thorne et al., 2015, Thorne et al., 2018). Briefly, whole aerosol exposures were performed in a scaled-down 35 mm plate format. Approximately 2 × 107 bacterial cells were mixed with 75 μL sodium phosphate buffer (pH 7.4) or a 10 % S9 mix, complemented with 40 μg/mL histidine and 48.8 μg/mL biotin mix. The bacterial cell suspension was plated directly onto Vogel-Bonner agar using a spread plate technique and incubated at 37 °C until dry (∼20 min) before transferring to Vitrocell Ames 4 exposure module for exposure at the AAI. The exposed agar plates were removed from the modules, sealed, inverted and incubated at 37 °C in the dark for 3 days prior to analysis. Concurrent controls (air, untreated and positive) were included with each exposure. Untreated controls (UTC) were maintained at room temperature for the duration of the exposure and then processed as per the rest of the experiment. Air controls were maintained in a separate exposure module as per the treatment condition and used as the “zero puff” benchmark. Positive controls were treated in a similar manner to the UTC. Each strain was assessed in the presence and absence of metabolic activation as shown by Table 4.
Table 4.
Ames Tester Strains.
| Bacteria | Strain | Mutant | Mutant Gene |
|---|---|---|---|
| S.typhimurium | TA971 | Frame-shift | Histidine |
| S.typhimurium | TA981 | Frame-shift | Histidine |
| S.typhimurium | TA1001 | Base-pair substitution | Histidine |
| S.typhimurium | TA1042 | Base-pair substitution | Histidine |
| E. coli | WP2 uvrA pKM1011 | Base-pair substitution | Tryptophan |
= Recommended by OECD guideline (TG 471).
= not recommended by OECD, used as a supplementary strain due to TA1535 not being applicable to the scaled down plate format (Thorne et al., 2015).
Determination of nicotine concentration
As a direct measurement of the nicotine concentration, post-exposure cell culture media was collected from the chamber and analysed, to give a quantification of exposure in situ. 495 µL extract media were spiked with 5 µL of d4-nicotine (10 ng/mL final concentration) and resuspended in 5 % acetonitrile in water before quantification by UPLC-MS/MS, as adapted from (Adamson et al., 2017).
Data analysis
Cytotoxicity curves were generated from the averaged data of three independent experiments, for RTCA (triplicate wells per concentration repeated on 3 independent occasions) and for whole aerosol (3 tissues per treatment condition, repeated on 3 independent occasions). RTCA Normalised Cell Index * Hour (%) values were calculated using the RTCA Software v. 2.0. Curve fitting and IC50 values calculation were performed in GraphPad Prism 9.0.0 using the function [Inhibitor] vs. response -- Variable slope (four parameters). For the ToxTracker assay, cell survival was calculated for the purpose of determining the range of extract concentrations that could be used for quantitative assessment of GFP induction values, following the assay manufacturer recommendations (Toxys B.V., Leiden, Netherlands). Briefly, cell survival was calculated by averaging the cell survival values for all six reporter cell lines per plate and extract concentration tested; only GFP inductions at concentrations that showed less than 75 % cytotoxicity were used for the ToxTracker analysis. GFP induction values were fitted using a four parameter log-logistic function (LL.4) using the drc package (Ritz et al., 2016) or a linear, quadratic, cubic or loess function as alternative fits. If the difference between the variance of the residuals of the LL.4 fit and that of an alternative fit was higher than 0.1, the alternative fit with the lowest residuals variance was used. In order to compare the GFP induction levels between ePen3 and ePod, the ratio between the highest and the lowest between the two product mean values was calculated for each concentration. Graphs were generated using the R software v.4.1.0 (R Core Team, 2022) using the drc package for curve fitting and the ggplot2 package (Wickham, 2016) for visualisation.
Results
RTCA
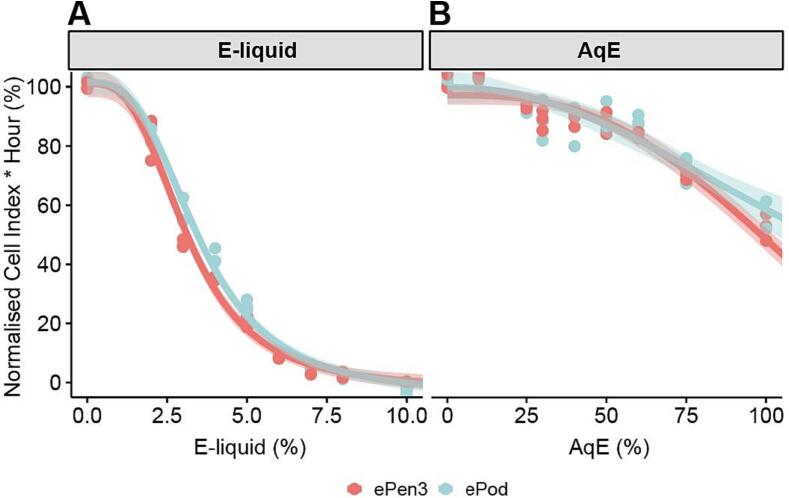
The first phase was to determine the effect of e-liquids on lung epithelial cells using the high-throughput screening tool, RTCA. Cell index was normalised to the media control. Fig. 3A shows the results of these experiments. The IC50 values of the two e-liquids were 3.17 and 3.55 % respectively and consistent between the two e-liquids, across the independent experiments. Fig. 3B shows the effect of capturing these e-liquids in culture media (AqE). In this assay the 100 % for both variants induced slightly less than 50 % reduction in cell viability as the predicted IC50 from the curve fit data was 110 and 116 %.
Fig. 3.
Cytotoxicity calculated by RTCA as Normalised Cell Index * Hour (%) of ePen3 or ePod e-liquid (A) or captured extracts (AqE) (B) in NCI-H292s. Data are averages of 3 triplicate wells per dose tested on 3 independent occasions. Graphs show individual data points, fit curve and 95 % confidence interval of the fit.
ToxTracker
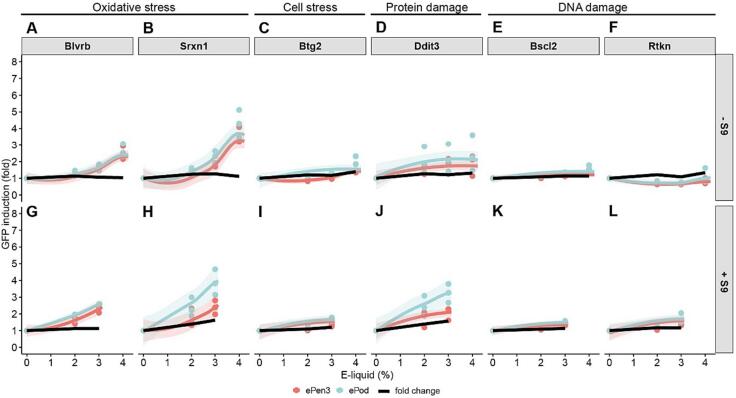
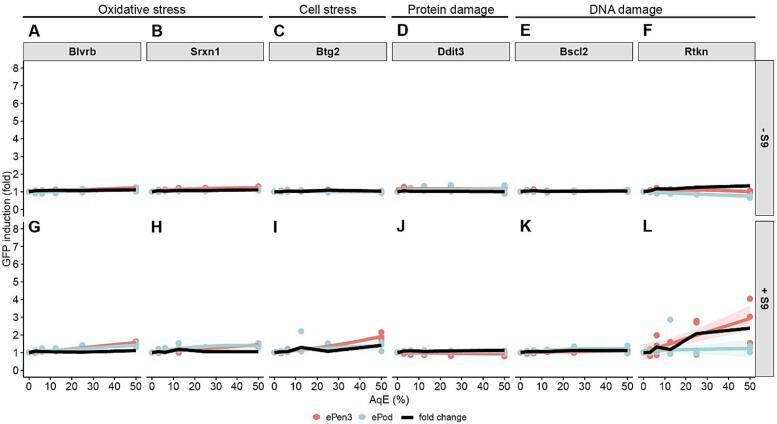
Fig. 4, Fig. 5 shows the e-liquid and AqE results for ToxTracker respectively. In this assay only the reduced dose range of 0–4 % was used in absence of S9 and the 0–3 % range in presence of S9, based on cell survival data. The top panel shows minus S9 and the bottom panel shows plus S9 results, which shows consistent responses. For both ePod and ePen3 both oxidative stress cell lines are positive (A, B, G & H). This is a minimal cell stress response and a protein damage response. Neither of the two DNA damage reporter cell lines were activated for 24 h submerged in diluted e-liquid.
Fig. 4.
ToxTracker results for ePen3 and ePod e-liquid expressed as % of the tested sample. Graphs for each cell line show data points, best fit and 95 % confidence interval of the fit. Black line shows fold change between ePen3 and ePod mean GFP induction values for each analysed concentration. Only concentrations where both tested products showed less than 75% cytotoxicity are displayed and were considered for data analysis.
Fig. 5.
ToxTracker results for ePen3 and ePod AqE expressed as % of the tested sample. Graphs for each product show data points, best fit and 95 % confidence interval of the fit. Black line shows fold change between ePen3 and ePod mean GFP induction values for each analysed concentration. Only concentrations where both tested products showed less than 75% cytotoxicity are displayed and were considered for data analysis.
When the assay was performed with captured aerosol extracts (AqE) none of the minus S9 treatments were over 2-fold or over the mean GFP induction values. For the plus S9 treatment there were small responses in panel G, H & I (oxidative stress and cell stress). However, these are not over 2-fold and therefore not considered biologically significant responses. For Rtkn there is an approximate 3-fold induction in ePen3 response at the highest concentration tested.
Whole aerosol cytotoxicity assessment
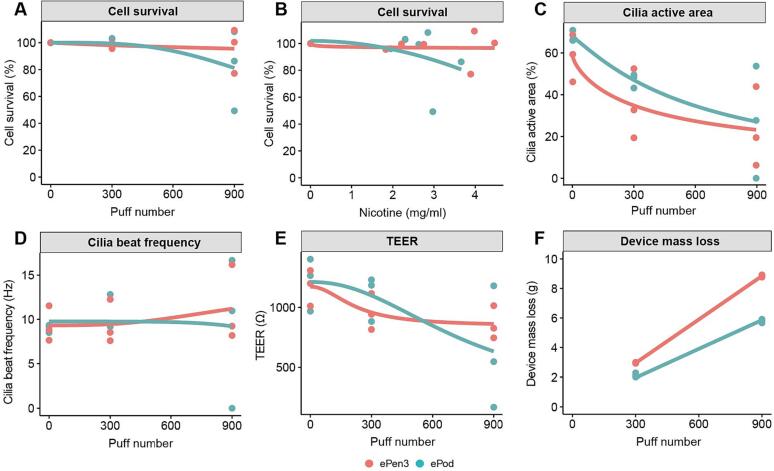
To further increase the physiological relevance of the test article, whole aerosol exposures were conducted with reconstituted human airway epithelium tissues (MucilAir) up to 900 consecutive puffs. The data from these experiments are shown in Fig. 6. Cell survival as determined by MTT is shown in Fig. 6A as a function of puff number and 6B as quantified nicotine concentration. There is very little loss in cell viability even at 900 consecutive puffs. There was slightly more nicotine captured by the ePen3 device which is echoed by the increase device mass loss recorded as shown in Fig. 6F and the device mass yields in Table 3 (ePen3 being 8 mg/puff and ePod 6.5 mg/puff). There is loss of cilia active area which is consistent between the two products (Fig. 6C), but beating frequency is unaffected (Fig. 6D). Barrier integrity, a pre-cursor to cytotoxicity is shown in Fig. 6E, where there is some reduction below 1000 Ohms at 900 puffs but is mostly maintained as shown in the cytotoxicity data.
Fig. 6.
Endpoint analysis of ePod and ePen3 (Golden Tobacco 18 mg/mL) in MucilAir. Data are cytotoxicity (MTT) by puff number (A); cytotoxicity by nicotine concentration (B); cilia active area (C); Cilia beat frequency (D); transepithelial electrical resistance (TEER) (E); and device mass loss (DML) (F). Data are averages from three independent experiments each containing three inserts.
Whole aerosol Ames assay
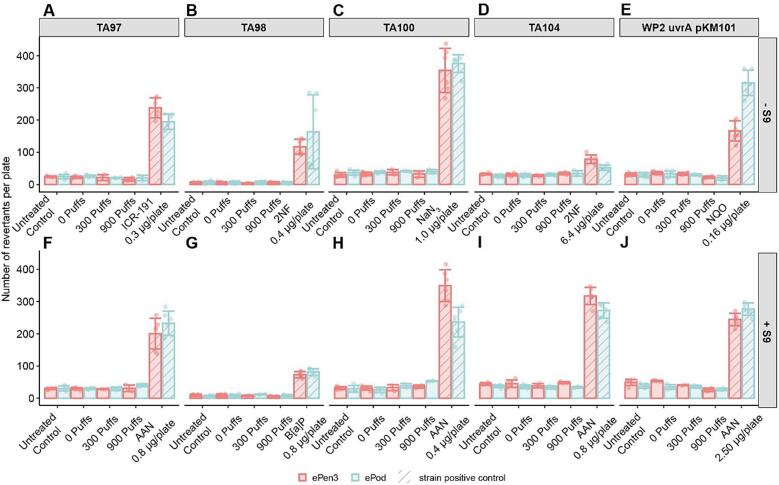
A whole aerosol Ames assay was conducted using the same exposure regimen as used in the MucilAir experiments. These data are shown in Fig. 7 for all strains; Fig. 7A-E in the absence of S9 and Fig. 7F-J in the presence of S9. In all strains and all conditions, the ePod and ePen3 aerosol was deemed non-mutagenic.
Fig. 7.
Revertant/plate data for Ames strains, TA98, TA100, TA97, E. coli and TA104 in the presence and absence of metabolic activation. A = TA97, B = TA98, C = TA100, D = TA104, E = E. coli. The concurrent positive control for each test product and test strain is shown in hashed bars.
Discussion
The aim of this study was to assess the biological impact of two Pod Mod-type e-cigarettes with differing heating technologies: coil and wick (cotton) vs. ceramic. An assessment strategy was employed to cover multiple test matrices and biological endpoints, focusing on screening assays such as RTCA and ToxTracker, followed up by cytotoxicity and genotoxicity assessments using whole aerosol. This study design utilises increasing test article complexity from e-liquid to aerosol extracts, and finally to whole aerosol exposure that captures the most complex interactions and allows assessment of potential thermal breakdown products generated during conversion from liquid to aerosol. Whole aerosol testing also allows for maximised exposure and a more direct link to consumer use (aerosol compared to an artificially generated test matrix). Aerosol exposures are not limited by capture efficiency and/or matrix saturation, or artefactual limitations (such the limitations associated with direct e-liquid exposure), unlike e-liquids and captured aerosol extracts. Aerosol exposure represents the most physiologically relevant test matrix but is limited by lower throughput and the requirement to utilise complex in vitro aerosol generating systems or bespoke alternatives (Li, 2016). E-liquid and captured extracts are easier to generate and evaluate in vitro but have less direct relevance to human exposure, and do not necessarily capture the complete process of aerosolisation or the exact ratios of chemicals present (Smart & Phillips, 2021). As described above, our testing strategy aims to capture e-liquid assessment all the way through to the aerosolisation process, utilising a 2D lung cell system (NCI-H292) and mechanistic assessment using mouse embryos and the ToxTracker assay, culminating with whole aerosol, 3D tissues (MucilAir) and a mutagenicity Ames-aerosol methodology.
In a tiered in vitro testing strategy, there are clear advantages for using screening assays to increase the overall weight of evidence, coupled with more traditional approaches. The utility of RTCA has been shown across multiple studies as a high throughput alternative to traditional methods such as neutral red uptake (NRU) and MTT assays for monolayer cell lines (Roshan Moniri, 2015, East et al., 2021). Due to the proliferative state of the cells during the monitoring of the assay, RTCA provides additional sensitivity compared to non-proliferative assays. In the case of e-liquid and captured extract testing this added sensitivity is an advantage. ToxTracker is also used as a mechanistic screening tool in tobacco, agricultural, cosmetics, pharmaceuticals and nanomaterial research (Table 1). However, osmolality is a confounding variable in these screening assessments should be addressed. High proportions of humectants (PG and VG) present in e-liquid formulations can cause in vitro cellular physiological osmolality ranges to be exceeded during exposure. Ultimately, this results in cell disruption and loss, and responses that are artefacts of in vitro exposure rather than an actual response to chemicals present in the e-liquids. It has been widely reported that e–liquid concentrations over 1 % exceed physiological range (Gonzalez-Suarez et al., 2016, Marescotti et al., 2020, Czekala et al., 2021). Osmolality is also a factor in captured aerosol assessments and has previously been reported by Bozhilova et al in creating a 200 puff ePen3 aerosol extract (with an 18 mg/mL tobacco flavour e-liquid), as used in this study. Extract dilutions of 75 % and above exceeded physiological osmolality and results should be interpreted with caution (Bozhilova et al., 2020). It can be hypothesised that ePod, being a slightly lower delivery device, would be inside the acceptable range. The ToxTracker experiments in this study assessed test concentrations below 75 %. However, RTCA experiments were conducted up to 100 % captured extract to achieve a full cytotoxicity dose–response curve to facilitate an IC50 calculation, so responses over 75 % should be interpreted with caution.
The results of the study indicate that ePod and ePen e-cigarettes perform similarly, with comparable results across the tiered testing approach. This was initially shown with e-liquid analysis, which in this tiered testing approach represented the most direct and simplified exposure method. When considering artefactual responses because of physiological changes in the test media due to shifts in osmolality, both e-liquid formulations gave comparable results in terms of toxicity for RTCA. The same was observed for ToxTracker endpoints: DNA damage, oxidative stress and protein damage (+/- S9) following e-liquid exposure. These results are reassuring as both e-liquids irrespective of device were comparable in their complexity and flavourings (Golden Tobacco 18 mg/mL). The results for both assays using e-liquid exposure can be caveated by the observation above, in that responses were only observed outside normal cellular physiological ranges (>1% e-liquid dose). The next stage of this tiered assessment framework focused on an aerosol extracts (termed AqE), where soluble components are captured in an aqueous trap, in this instance cell culture medium, but other studies use other capture mediums such as H2O and PBS (Smart & Phillips, 2021). This test matrix increases complexity beyond simple e-liquid exposures and captures the aerosolisation process and any potentially harmful products derived via thermal breakdown or conversion at high temperature from a liquid to a gas. A limitation of this approach is that not all chemicals are captured at the same levels of efficiency, thus leading to a test matrix that is more complex than e-liquid assessments alone but is still potentially not entirely representative of the whole aerosol. Extracts are also prone to saturation of the aqueous matrix, further limiting their usefulness. Using RTCA, both e-cigarette aerosols showed similar cytotoxicity responses. In the ToxTracker assay, almost all endpoints were negative (+/-S9) for extracts. There was one observed difference between the two captured aerosols. In the presence of metabolic activation, for RTKN (DNA damage endpoint), ePen showed a positive dose related response while no such response was observed for ePod captured aerosol under any treatment condition. Previous chemical analysis has not reported any significantly higher levels in undesired constituents for ePen (Margham et al., 2016, Cunningham et al., 2020, Pinto et al., 2022), making it difficult to ascertain what is driving this response. For example, although a positive RTKN - DNA damaging endpoint was observed in ToxTracker, it is not clear whether this response is transitory in nature and whether the cells can recover, which is an inherent limitation of the in vitro setting and screening nature of the study. Finally, to really ascertain whether the DNA damaging element is true-positive observation follow up work focused on DNA damaging assays such as the Ames assay or in vitro micronucleus would provide greater reassurance. In a study conducted by Thorne et al., 2018, ePen3 was assessed using an Ames-aerosol air-agar approach up to 900 continuous puffs. None of the OECD approved strains assessed demonstrated an increase in mutation frequencies in response to ePen aerosols. This suggests that the DNA damaging observation noted in this study is transitory and/or does not lead to a mutagenic event.
To complement the screening approaches, undiluted whole aerosol was assessed for cytotoxicity and mutagenicity using the MTT and Ames assays, respectively. In this instance, fully differentiated human 3D cells were assessed in combination with an MTT cytotoxicity measure and functional endpoints (such CBF, AA, TEERs). The Ames assay was used in combination with whole aerosol, +/- S9 and five tester strains to assess the mutagenic potential of both e-cigarette aerosols in the absence of trapping or capture methodologies. Both whole aerosol approaches have been well documented and previously discussed (Bishop et al., 2022, Bishop et al., 2019, Thorne et al., 2018). The results corroborated previous observations from the screening assays that both aerosols were comparable. For example, the cytotoxicity profiles were comparable up to 900 puffs of undiluted e-cigarette aerosol. At 900 puffs both aerosols showed some induction of toxicity but both equally variable, which is unsurprising given the nature of the exposure scenario, confirmed by similar observation in the functional endpoints. Using an Ames aerosol approach, no mutagenic activity was observed in either aerosol under any treatment condition up to 900 puffs of undiluted e-cigarette exposure. This is adds additional confidence in the conclusion that the in vitro DNA damaging response observed in ToxTracker for ePen captured aerosol did not translate into a mutagenic event, indicating that the damage observed was either repaired, not sufficient to cause a mutation or alternatively activating a different DNA damage pathway. In some cases, heightened potencies have been recorded in vitro using the ToxTracker assay compared to in vivo responses (Wills et al., 2021), which could also explain the observation. Further research would be required to explore this observation in alternative assays and assessment scenarios. For this study, using the most complex assays and aerosol generating systems, no clear mutagenic activity was observed for either e-cigarette aerosol. Similarly, cytotoxicity responses in a human derived, differentiated cell system were observed only at the highest and most concentrated condition (900 puffs). No meaningful biological difference was observed between either e-cigarette aerosol.
Limitations of this study design are that we have only tested products using 18 mg/mL nicotine strength, because at the time of the study conduct this is the highest marketed nicotine strength for these products. Lower strengths of 6 mg/mL and 0 mg/mL were available. We also only tested one comparable flavour across both devices, with the intent of assessing the closest possible e-liquid formulation across both platforms, to remove differences in formulation as a factor from this assessment. Furthermore, we also have only assessed a 24 h exposure time / recovery time and therefore only measured the acute effects of product exposure. Longer term repeated exposures have been investigated (Haswell et al., 2021, Czekala et al., 2021b) but are technically challenging with aerosol exposure, due to ensuring sterility of the cells throughout multiple exposure runs, resource requirements, maintenance of machinery and dosimetry methods across the study. Finally, no direct cigarette comparator was included in the study, limiting any potential harm reduction comparison from e-cigarette to cigarette and other next generation products. However, from the literature we have contextualised the e-cigarette responses to give an indication of the potency of e-cigarette aerosol to that of cigarette smoke, under comparable conditions and testing parameters. The results obtained in this study when compared to previously published reference cigarette smoke responses indicate > 95 % lower levels of responses in all cases (Bishop 2020, Bishop 2023, Thorne 2016).
Conclusions
The data shown in this study demonstrate the comparability of responses measured from the e-cigarette products tested. These differences need to be contextualised against multiple test matrices to ensure consistency of results. Here we have seen a potential mechanistic difference between devices when using a captured aerosol that was not represented by whole aerosol data. This fundamentally demonstrates the importance of a matrix-based testing strategy and to ensure results are appropriately considered with due regard to test article nuances. Collectively, these data are consistent with the observations that e-cigarettes offer reduced risk potential when used as alternatives to conventional cigarette smoking as part of a tobacco harm reduction policy adding to the growing weight of evidence. This study provides additional in vitro biological data on the responsiveness of ePen3 and ePod devices, when coupled with emissions (Cunningham et al., 2020, Pinto et al., 2022), human behaviour (Prasad et al., 2022), clinical (Gale et al., 2022) and post market surveillance, provide a comprehensive overall assessment to ascertain their potential risk profile (Murphy et al., 2017). Further studies are required to substantiate the potential risk, which includes clinical and post market surveillance. Although e-cigarette use may represent a potential reduced harm product to those that smoke, for non-smokers, a better understanding of the inherent risk of these products is required. Some in vitro studies are already showing that e-cigarette use is not without risk, and the risk profile although significantly reduced compared to smoking does not increase with the addition of complex flavours providing a robust stewardship process governs the development of these products (Bishop 2023). In addition, this study adds to the growing evidence for the use of bridging approaches to demonstrate biological equivalence between product iterations (Gaca et al., 2022). Which will in turn reduce the regulatory burden on assessing each individual product as standalone.
Author contributions
The study was designed by DT, the work was conducted by EB, SB, FM, NE, TJ, AB, RE & DS. The data was analysed by FM, DT & EB and the manuscript prepared by DT & EB. All authors approved the final version.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank David Corkill for his support in generating the e-cigarette compliance statement. Anthony Cunningham for his technical device inputs and Tomasz Jaunky for support with generation of AqE samples.
Data availability
Data will be made available on request.
References
- Adamson J., Li X., Cui H., Thorne D., Xie F., Gaca M.D. Nicotine Quantification In Vitro: A Consistent Dosimetry Marker for e-Cigarette Aerosol and Cigarette Smoke Generation. Appl. Vitro Toxicol. 2017;3:14–27. [Google Scholar]
- Aufderheide M., Gressmann H. A modified Ames assay reveals the mutagenicity of native cigarette mainstream smoke and its gas vapour phase. Exp. Toxicol. Pathol. 2007;58:383–392. doi: 10.1016/j.etp.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Aufderheide M., Gressmann H. Mutagenicity of native cigarette mainstream smoke and its gas/vapour phase by use of different tester strains and cigarettes in a modified Ames assay. Mutation Research/genetic Toxicol. Environ. Mutagenesis. 2008;656:82–87. doi: 10.1016/j.mrgentox.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Balogh Sivars K., Sivars U., Hornberg E., Zhang H., Branden L., Bonfante R., Huang S., Constant S., Robinson I., Betts C.J., Aberg P.M. A 3D Human Airway Model Enables Prediction of Respiratory Toxicity of Inhaled Drugs In Vitro. Toxicol. Sci. 2018;162:301–308. doi: 10.1093/toxsci/kfx255. [DOI] [PubMed] [Google Scholar]
- Baltazar M.T., Cable S., Carmichael P.L., Cubberley R., Cull T., Delagrange M., Dent M.P., Hatherell S., Houghton J., Kukic P., Li H., Lee M.-Y., Malcomber S., Middleton A.M., Moxon T.E., Nathanail A.V., Nicol B., Pendlington R., Reynolds G., Reynolds J., White A., Westmoreland C. A Next-Generation Risk Assessment Case Study for Coumarin in Cosmetic Products. Toxicol. Sci. 2020;176:236–252. doi: 10.1093/toxsci/kfaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar R.Z., Wang Y., Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob Control. 2018;27:325–333. doi: 10.1136/tobaccocontrol-2016-053472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belushkin M., Esposito M., Jaccard G., Jeannet C., Korneliou A., Tafin Djoko D. Role of testing standards in smoke-free product assessments. Regul. Toxicol. Pharm. 2018;98:1–8. doi: 10.1016/j.yrtph.2018.06.021. [DOI] [PubMed] [Google Scholar]
- Bishop E., Haswell L., Adamson J., Costigan S., Thorne D., Gaca M. An approach to testing undiluted e-cigarette aerosol in vitro using 3D reconstituted human airway epithelium. Toxicol. in Vitro. 2019;54:391–401. doi: 10.1016/j.tiv.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Bishop E., East N., Bozhilova S., Santopietro S., Smart D., Taylor M., Meredith S., Baxter A., Breheny D., Thorne D., Gaca M. An approach for the extract generation and toxicological assessment of tobacco-free ‘modern’ oral nicotine pouches. Food Chem. Toxicol. 2020;145 doi: 10.1016/j.fct.2020.111713. [DOI] [PubMed] [Google Scholar]
- Bishop E., Terry A., East N., Breheny D., Gaca M., Thorne D. A 3D comparison of two undiluted e-cigarette aerosol generating systems. Toxicol. Lett. 2022;358:69–79. doi: 10.1016/j.toxlet.2022.01.002. [DOI] [PubMed] [Google Scholar]
- Bishop E., East N., Miazzi F., Fiebelkorn S., Breheny D., Gaca M., Thorne D. A contextualised e-cigarette testing strategy shows flavourings do not impact lung toxicity in vitro. Toxicol. Lett. 2023;380:1–11. doi: 10.1016/j.toxlet.2023.03.006. [DOI] [PubMed] [Google Scholar]
- Bozhilova S., Baxter A., Bishop E., Breheny D., Thorne D., Hodges P., Gaca M. Optimization of aqueous aerosol extract (AqE) generation from e-cigarettes and tobacco heating products for in vitro cytotoxicity testing. Toxicol. Lett. 2020;335:51–63. doi: 10.1016/j.toxlet.2020.10.005. [DOI] [PubMed] [Google Scholar]
- Brandsma I., Derr R., Zhang G., Moelijker N., Hendriks G., Østerlund T. Genotoxicity assessment of potentially mutagenic nucleoside analogues using ToxTracker®. Toxicol. Lett. 2022;362:50–58. doi: 10.1016/j.toxlet.2022.04.002. [DOI] [PubMed] [Google Scholar]
- Brown D.M., Danielsen P.H., Derr R., Moelijker N., Fowler P., Stone V., Hendriks G., Møller P., Kermanizadeh A. The mechanism-based toxicity screening of particles with use in the food and nutrition sector via the ToxTracker reporter system. Toxicol. In Vitro. 2019;61 doi: 10.1016/j.tiv.2019.104594. [DOI] [PubMed] [Google Scholar]
- R CORE TEAM 2022. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- CORESTA. 2015. Routine analytical machine for e-cigarette aerosol generation and collection - definitions and standard conditions [Online]. Available: https://www.coresta.org/sites/default/files/technical_documents/main/CRM_81.pdf [Accessed].
- Costigan S., Lopez-Belmonte J. An approach to allergy risk assessments for e-liquid ingredients. Regul. Toxicol. Pharm. 2017;87:1–8. doi: 10.1016/j.yrtph.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Costigan S., Meredith C. An approach to ingredient screening and toxicological risk assessment of flavours in e-liquids. Regul. Toxicol. Pharm. 2015;72:361–369. doi: 10.1016/j.yrtph.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Cunningham A., McAdam K., Thissen J., Digard H. The Evolving E-cigarette: Comparative Chemical Analyses of E-cigarette Vapor and Cigarette Smoke. Frontiers. Toxicology. 2020;2 doi: 10.3389/ftox.2020.586674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czekala L., Chapman F., Simms L., Rudd K., Trelles Sticken E., Wieczorek R., Bode L.M., Pani J., Moelijker N., Derr R., Brandsma I., Hendriks G., Stevenson M., Walele T. The in vitro ToxTracker and Aneugen Clastogen Evaluation extension assay as a tool in the assessment of relative genotoxic potential of e-liquids and their aerosols. Mutagenesis. 2021;36:129–142. doi: 10.1093/mutage/geaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czekala L., Wieczorek R., Simms L., Yu F., Budde J., Sticken E.T., Rudd K., Verron T., Brinster O., Stevenson M., Walele T. Multi-endpoint analysis of human 3D airway epithelium following repeated exposure to whole electronic vapor product aerosol or cigarette smoke. Current Res. Toxicol. 2021;2:99–115. doi: 10.1016/j.crtox.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derr R., Moelijker N., Hendriks G., Brandsma I. A tiered approach to investigate the inhalation toxicity of cobalt substances. Tier 2 b: Reactive cobalt substances induce oxidative stress in ToxTracker and activate hypoxia target genes. Regul. Toxicol. Pharm. 2022;129 doi: 10.1016/j.yrtph.2022.105120. [DOI] [PubMed] [Google Scholar]
- East N., Bishop E., Breheny D., Gaca M., Thorne D. A screening approach for the evaluation of tobacco-free 'modern oral' nicotine products using Real Time Cell Analysis. Toxicol Rep. 2021;8:481–488. doi: 10.1016/j.toxrep.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaca M., Williamson J., Digard H., Adams L., Hawkridge L., Proctor C. Bridging: Accelerating Regulatory Acceptance of Reduced-Risk Tobacco and Nicotine Products. Nicotine Tob Res. 2022;24:1371–1378. doi: 10.1093/ntr/ntac041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale N., Haswell L.E., McEwan M., Azzopardi D., Thissen J., Hardie G. Biomarkers of Exposure and Potential Harm in Exclusive Users of Electronic Cigarettes and Current, Former and Never-Smokers: A Cross-Sectional Study Protocol. J. Health Environ. Res. 2022;8(2):116–127. [Google Scholar]
- Gonzalez-Suarez I., Martin F., Marescotti D., Guedj E., Acali S., Johne S., Dulize R., Baumer K., Peric D., Goedertier D., Frentzel S., Ivanov N.V., Mathis C., Hoeng J., Peitsch M.C. In Vitro Systems Toxicology Assessment of a Candidate Modified Risk Tobacco Product Shows Reduced Toxicity Compared to That of a Conventional Cigarette. Chem. Res. Toxicol. 2016;29:3–18. doi: 10.1021/acs.chemrestox.5b00321. [DOI] [PubMed] [Google Scholar]
- Haswell L.E., Smart D., Jaunky T., Baxter A., Santopietro S., Meredith S., Camacho O.M., Breheny D., Thorne D., Gaca M.D. The development of an in vitro 3D model of goblet cell hyperplasia using MUC5AC expression and repeated whole aerosol exposures. Toxicol. Lett. 2021;347:45–57. doi: 10.1016/j.toxlet.2021.04.012. [DOI] [PubMed] [Google Scholar]
- Hendriks G., Atallah M., Morolli B., Calleja F., Ras-Verloop N., Huijskens I., Raamsman M., van de Water B., Vrieling H. The ToxTracker assay: novel GFP reporter systems that provide mechanistic insight into the genotoxic properties of chemicals. Toxicol. Sci. 2012;125:285–298. doi: 10.1093/toxsci/kfr281. [DOI] [PubMed] [Google Scholar]
- Hendriks G., Derr R.S., Misovic B., Morolli B., Calleja F.M., Vrieling H. The Extended ToxTracker Assay Discriminates Between Induction of DNA Damage, Oxidative Stress, and Protein Misfolding. Toxicol. Sci. 2016;150:190–203. doi: 10.1093/toxsci/kfv323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskandar A.R., Gonzalez-Suarez I., Majeed S., Marescotti D., Sewer A., Xiang Y., Leroy P., Guedj E., Mathis C., Schaller J.P., Vanscheeuwijck P., Frentzel S., Martin F., Ivanov N.V., Peitsch M.C., Hoeng J. A framework for in vitro systems toxicology assessment of e-liquids. Toxicol. Mech. Methods. 2016;26:389–413. doi: 10.3109/15376516.2016.1170251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO 2018. ISO 20768:2018. Vapour products — Routine analytical vaping machine — Definitions and standard conditions.
- Karlsson H.L., Gliga A.R., Calléja F.M.G.R., Gonçalves C.S.A.G., Wallinder I.O., Vrieling H., Fadeel B., Hendriks G. Mechanism-based genotoxicity screening of metal oxide nanoparticles using the ToxTracker panel of reporter cell lines. Part. Fibre Toxicol. 2014;11:41. doi: 10.1186/s12989-014-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser B.M., Leverette R., Hollings M., Seymour A., Weidman R.A., Berquette C.A., Jordan K. Characterization of Aerosol Deliveries from Combustible Cigarettes, Heated Tobacco Products, and Electronic Nicotine Delivery Systems Using the Vitrocell Ames 48. Applied in Vitro Toxicology. 2022;8:39–49. doi: 10.1016/j.toxrep.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix G., Koch W., Ritter D., Gutleb A.C., Larson S.T., Loret T., Zanetti F., Constant S., Chortarea S., Rothen-Rutishauser B., Hiemstra P.S., Frejafon E., Hubert P., Gribaldo L., Kearns P., Aublant J., Diabate S., Weiss C., de Groot A., Kooter I. Air-Liquid Interface In Vitro Models for Respiratory Toxicology Research: Consensus Workshop and Recommendations. Applied in Vitro Toxicology. 2018;4:91–106. doi: 10.1089/aivt.2017.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, x. In vitro toxicity testing of cigarette smoke based on the air-liquid interface exposure: A review. Toxicol in Vitro. 2016;36:105–113. doi: 10.1016/j.tiv.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Marescotti D., Mathis C., Belcastro V., Leroy P., Acali S., Martin F., Dulize R., Bornand D., Peric D., Guedj E., Ortega Torres L., Biasioli M., Fuhrimann M., Fernandes E., Frauendorfer F., Gonzalez Suarez I., Sciuscio D., Ivanov N.V., Peitsch M.C., Hoeng J. Systems toxicology assessment of a representative e-liquid formulation using human primary bronchial epithelial cells. Toxicol. Rep. 2020;7:67–80. doi: 10.1016/j.toxrep.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margham J., McAdam K., Forster M., Liu C., Wright C., Mariner D., Proctor C. Chemical Composition of Aerosol from an E-Cigarette: A Quantitative Comparison with Cigarette Smoke. Chem. Res. Toxicol. 2016;29:1662–1678. doi: 10.1021/acs.chemrestox.6b00188. [DOI] [PubMed] [Google Scholar]
- Mesnage R., Brandsma I., Moelijker N., Zhang G., Antoniou M.N. Genotoxicity evaluation of 2,4-D, dicamba and glyphosate alone or in combination with cell reporter assays for DNA damage, oxidative stress and unfolded protein response. Food Chem. Toxicol. 2021;157 doi: 10.1016/j.fct.2021.112601. [DOI] [PubMed] [Google Scholar]
- Mesnage, R., Ibragim, M., Mandrioli, D., Falcioni, L., Tibaldi, E., Belpoggi, F., Brandsma, I., Bourne, E., Savage, E., Mein, C. A. & Antoniou, M. N. 2021b. Comparative toxicogenomics of glyphosate and Roundup herbicides by mammalian stem cell-based genotoxicity assays and molecular profiling in Sprague-Dawley rats. bioRxiv, 2021.04.12.439463. [DOI] [PMC free article] [PubMed]
- Murphy J., Gaca M., Lowe F., Minet E., Breheny D., Prasad K., Camacho O., Fearon I.M., Liu C., Wright C., McAdam K., Proctor C. Assessing modified risk tobacco and nicotine products: Description of the scientific framework and assessment of a closed modular electronic cigarette. Regul. Toxicol. Pharmacol. 2017;90:342–357. doi: 10.1016/j.yrtph.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Pinto M.I., Thissen J., Hermes N., Cunningham A., Digard H., Murphy J. Chemical characterisation of the vapour emitted by an e-cigarette using a ceramic wick-based technology. Sci. Rep. 2022;12:16497. doi: 10.1038/s41598-022-19761-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K., Gray A., Edward L. Puffing topography and mouth level exposure of two closed-system Vype e-cigarettes. Int. J. Sci. Rep. 2022;8:163–172. [Google Scholar]
- Ritz C., Baty F., Streibig J.C., Gerhard D. Dose-Response Analysis Using R. PLOS ONE. 2016;10:e0146021. doi: 10.1371/journal.pone.0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan Moniri M., Young A., Reinheimer K., Rayat J., Dai L.-J., Warnock G.L. Dynamic assessment of cell viability, proliferation and migration using real time cell analyzer system (RTCA) Cytotechnology. 2015;67:379–386. doi: 10.1007/s10616-014-9692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson J.H., Stoner J.A., Ammons B.A., Wyatt T.A. All-digital image capture and whole-field analysis of ciliary beat frequency. J. Microsc. 2003;211:103–111. doi: 10.1046/j.1365-2818.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- Smart D.E., Bozhilova S., Miazzi F., Haswell L.E., Gaca M.D., Thorne D., Breheny D. Application of ToxTracker for the toxicological assessment of tobacco and nicotine delivery products. Toxicol. Lett. 2022;358:59–68. doi: 10.1016/j.toxlet.2022.01.005. [DOI] [PubMed] [Google Scholar]
- Smart D.J., Phillips G. Collecting e-cigarette aerosols for in vitro applications: A survey of the biomedical literature and opportunities to increase the value of submerged cell culture-based assessments. J. Appl. Toxicol. 2021;41:161–174. doi: 10.1002/jat.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne D., Adamson J. A review of in vitro cigarette smoke exposure systems. Exp. Toxicol. Pathol. 2013;65:1183–1193. doi: 10.1016/j.etp.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Thorne D., Kilford J., Hollings M., Dalrymple A., Ballantyne M., Meredith C., Dillon D. The mutagenic assessment of mainstream cigarette smoke using the Ames assay: A multi-strain approach. Mutation Research/Genetic Toxicol. Environ. Mutagenesis. 2015;782:9–17. doi: 10.1016/j.mrgentox.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Thorne D., Hollings M., Seymour A., Adamson J., Dalrymple A., Ballantyne M., Gaca M. Extreme testing of undiluted e-cigarette aerosol in vitro using an Ames air-agar-interface technique. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018;828:46–54. doi: 10.1016/j.mrgentox.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Wickham, H. 2016. ggplot2 - Elegant Graphics for Data Analysis, Springer Cham.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.