Abstract
A Borrelia burgdorferi N40 genomic expression library was screened with serum from actively infected mice to identify gene products that elicit protective immunity. A clone that contained a putative bicistronic operon containing two genes that encoded 20- and 22-kDa lipoproteins was identified and sequenced. These genes showed homology with the genes encoding decorin binding proteins DbpB and DbpA, respectively, of B. burgdorferi 297 and B31. N40-dbpA DNA hybridized with B. burgdorferi N40 DNA on a single 48-kb linear plasmid. Homologous genes could be amplified under various degrees of stringency by PCR or hybridized by Southern blotting from B. burgdorferi sensu stricto N40 and B31, and from B. burgdorferi sensu lato PBi and 25015, but not PKo. Recombinant N40-DbpB and N40-DbpA were reactive with antibody in serum from infected mice, and serum was more reactive against N40-DbpA than against B. burgdorferi N40 recombinant P39, OspC, or OspA. Sera from mice infected with B. burgdorferi sensu lato strains PKo and PBi were weakly reactive against N40-DbpB and N40-DbpA, and sera from mice infected with 25015 were moderately reactive, compared to sera from mice infected with B. burgdorferi N40. Hyperimmunization of mice with N40-DbpA, but not N40-DbpB, induced protective immunity against syringe challenge with cultured B. burgdorferi N40. DbpA may therefore be one of the antigens responsible for eliciting protective antibody known to exist in serum from infected mice. DNA amplification and serology suggest that DbpB and DbpA are likely to have homologs throughout the B. burgdorferi sensu lato family, but they are likely to be heterogeneous.
Infection of laboratory mice with Borrelia burgdorferi is persistent and multisystemic, resulting in intermittent polysynovitis and carditis reminiscent of human Lyme disease (3, 6, 8, 12). Despite persistent infection, sera from immunocompetent mice with active infection (immune sera) can passively protect naive mice against syringe challenge inoculation with cultured B. burgdorferi, using remarkably small amounts of immune sera and high-challenge doses of spirochetes (7, 9). Immune sera taken from mice with verified infection as early as 2 weeks after inoculation with <10 spirochetes are protective when passively transferred (9). Such early immune sera are reactive against a limited repertoire of antigens on B. burgdorferi immunoblots, including antigens of 41, 39, and 20 to 24 kDa (7, 10).
The dominant proteins (41, 39, and 20 to 24 kDa) recognized by antibody in immune sera during early infection are presumed to be flagellin, P39 (BmpA), and OspC, since antisera to recombinant forms of these proteins react in parallel on immunoblots with immune sera, and immune sera are reactive to these recombinant proteins (7, 10). Active or passive immunization against these proteins, however, all failed to induce protective immunity against syringe challenge of mice with cultured B. burgdorferi N40 spirochetes (9, 14, 26). This finding leads to the conclusion that proteins responsible for eliciting protective antibody in immune sera are expressed exclusively in vivo (and thus not reactive on immunoblots prepared from cultured spirochetes) or are proteins that comigrate with flagellin, P39, or OspC (and thus are obscured on immunoblots).
Both in vivo-expressed proteins and comigrating proteins are possibilities. A number of laboratories have demonstrated B. burgdorferi proteins that are expressed exclusively in vivo (1, 16, 27, 45, 46). There are also several comigrating immunogenic proteins, particularly in the 20- to 24-kDa (OspC) region, that are reactive with immune sera from human patients or experimental animals. None of these proteins, however, has been incriminated as an antigen capable of eliciting protective immunity (17, 29, 31, 32, 40, 47). We therefore embarked on a concerted effort to screen a B. burgdorferi genomic expression library with immune sera from infected mice to identify biologically relevant B. burgdorferi antigens, and this effort resulted in repeated cloning of the genes described in this report.
MATERIALS AND METHODS
Mice.
Three-week-old inbred C3H/HeN mice were obtained from the NCI Production Program, Frederick Cancer Research Center, Frederick, Md. Mice were killed with carbon dioxide gas and then exsanguinated.
B. burgdorferi.
The index isolate for these studies was a clonal strain of B. burgdorferi sensu stricto (cN40) that had been cloned by threefold limiting dilution and passage in mice as described previously (8). The cN40 strain is highly infectious and pathogenic for laboratory mice. Spirochetes or tissues from mice (urinary bladder, spleen, and skin from the inoculation site) were cultured in modified Barbour-Stoenner-Kelly (BSK II) medium (5) at 33°C as described previously (8). In addition, low-passage B. burgdorferi sensu lato isolates B. afzelii Pko, B. garinii PBi (provided by John Leong, University of Massachusetts, Worcester), 25015, a representative of the newly identified B. burgdorferi DN127 genomic group of U.S. isolates (2, 4), and B. burgdorferi B31 (provided by Alan Barbour, University of California, Irvine) were similarly cloned and mouse passaged in our laboratory. The classification of these clonal strains was verified by David H. Persing (Mayo Foundation, Rochester, Minn.) by pulsed-field gel electrophoresis as described previously (33).
Cloning of the operon.
A λZAP II B. burgdorferi N40 expression library was previously constructed (29) and provided by Richard A. Flavell (Yale University School of Medicine, New Haven, Conn.). The λZAP II phage contains pBluescript that can be excised and cloned directly with R408 helper phage (Stratagene, La Jolla, Calif.). The library was screened with immune sera from culture-positive mice at 90 days after intradermal inoculation with 102 B. burgdorferi cN40 cells. Phages were incubated with Escherichia coli; protein expression was induced with 10 mM isopropyl-1-thio-β-d-galactoside (IPTG); proteins were transferred to nitrocellulose membranes and then incubated with a 1:50 dilution of immune serum. After washing, membranes were incubated with a 1:5,000 dilution of alkaline phosphatase-conjugated goat anti-mouse immunoglobulin (Ig) antibodies (Sigma, St. Louis, Mo.), and bound antibodies were detected by color developed with nitroblue tetrazolium (Stratagene) and 5-bromo-4-chloro-3-indolylphosphate (Stratagene). Excision of the pBluescript plasmid from reactive clones was achieved by using the R408 helper phage. DNA from positive clones were screened for redundancy by cross-hybridization with known genes and clones, restriction enzyme analysis, or partial sequencing of the inserts (data not shown). DNA sequencing was performed at the W. M. Keck Foundation Biotechnology Resource Laboratory at Yale School of Medicine. DNA sequence was analyzed by using the MacVector program (Kodak, New Haven, Conn.).
Gene sequence comparison.
The nucleotide sequences of the two genes in the operon showed no homology with other genes, nor significant similarity with other proteins, in a search of GenBank at the time of these studies. However, a search of recent submissions to GenBank revealed a high degree of homology of the genes encoding the 20- and 22-kDa N40 proteins with B. burgdorferi 297 decorin binding protein (Dbp) genes dbpB (GenBank U75867) and dbpA (GenBank U75866), respectively. Furthermore, dbpB and dbpA sequences have been recently published for B. burgdorferi B31 (27a). The genes encoding the B. burgdorferi N40 20- and 22-kDa proteins are therefore herein referred to as N40-dbpB and N40-dbpA, respectively.
Expression and purification of recombinant proteins.
N40-dbpB and N40-dbpA genes, lacking the sequence encoding the hydrophobic N-terminal leader regions (amino acids 1 to 20 in N40-dbpB and amino acids 1 to 27 in N40-dbpA), were amplified by PCR using oligonucleotide primers based on their DNA sequences. The primers for the N40-dbpB gene corresponded to nucleotides 61 to 83 and 525 to 561 of the N40-dbpB gene. The primers for the N40-dbpA gene corresponded to nucleotides 49 to 75 and 549 to 582 of the N40-dbpB gene. Elimination of the signal sequences increased the likelihood that the recombinant proteins would be soluble when expressed, as previously described for the purification of OspA (22). Template DNA from the original reactive clone was denatured at 94°C for 1 min, annealed at 55°C for 1 min, and extended at 72°C for 1 min. This process was repeated for 30 cycles. The amplified N40-dbpB and N40-dbpA genes were cloned in frame with the glutathione S-transferase (GT) gene into pMX, a pGEX-2T vector (Pharmacia, Piscataway, N.J.) with a modified polylinker (37). The PCR-amplified DNA sequences were confirmed by sequence comparison with the original inserts. The primers for ospA corresponded to nucleotides 52 to 72 and 799 to 819 of the N40 ospA gene (25). Primers for ospC corresponded to nucleotides 58 to 69 and 616 to 627 of the N40 ospC gene (42). Primers for P39 were derived from the published sequence of B31 p39 (39), corresponding to nucleotides 55 to 72 and 997 to 1020. ospA, ospC, and p39 were PCR amplified and introduced into pMX as described above.
E. coli DH5α cells containing the recombinant plasmids were grown to an optical density at 600 nm of 0.5 (about 2 h), and the recombinant GT fusion proteins were induced with IPTG at a final concentration of 1 mM (2 h). Bacterial cells were centrifuged at 4,000 rpm for 20 min; pellets were washed with phosphate-buffered saline and (PBS) then dissolved in a 1/10 volume of PBS with 1% Triton X-100. The mixtures were sonicated and centrifuged at 10,000 rpm. Coomassie blue-stained agar gels showed that the GT-N40-DbpB and GT-N40-DbpA fusion proteins were soluble and in the supernatants. Therefore, supernatants containing GT-N40-DbpB or GT-N40-DbpA were loaded onto glutathione-Sepharose 4B columns (Pharmacia), 25 U of thrombin was added to the columns, and then the columns were incubated at room temperature for 2 h to remove the GT partner. N40-DbpA was eluted, free of its GT fusion partner, whereas N40-DbpB could not be cleaved with thrombin. Studies with N40-DbpB were therefore performed with the fusion protein GT-N40-DbpB. The GT partner was thrombin cleaved from OspA, OspC, and P39 recombinant proteins.
Localization of the N40-dbpB/A operon in the B. burgdorferi genome.
Pulsed-field gel electrophoresis was performed with total B. burgdorferi cN40 DNA as described previously (24), with minor modifications. DNA plugs containing approximately 108 B. burgdorferi cN40 were loaded onto an 0.8% agarose gel which was run in Tris-borate-EDTA buffer (0.025 M Tris, 0.5 mM EDTA, 0.025 M boric acid), using the CHEF-DRII system (Bio-Rad Laboratories, Richmond, Calif.). The gel was run at 14°C for 18 h at 198 V, with ramped pulse times from 1 to 30 s. Southern blot analyses were performed with N40-dbpA and ospA (25) DNA as probes as described previously (41).
To discriminate linear from circular plasmid DNA, plasmid DNA was electrophoresed and transferred to a nylon membrane as described previously (44). Radiolabeled probes were prepared for N40-dbpA and a gene located 3′ of an ospE homolog (p21) on a 32-kb circular plasmid (43, 45). The gene 3′ of p21 was amplified from an E. coli plasmid clone of this region (43), using oligonucleotide primers GATTTAAAACAAAATCCAGAAGGG and GATCACCACTTTGTTCTGCTGATTTTG. PCR conditions consisted of 20 cycles of 94°C for 1 min, 50°C for 30 s, and 72°C for 1 min. The PCR product was diluted 1:100 in distilled water, and 1 μl was subjected to a second round of amplification. The final PCR product was diluted in 2 ml of water and concentrated through a Centricon-100 microconcentrator (Amicon, Beverly, Mass.). Probes were radiolabeled with [α-32P]dATP (DuPont, Boston, Mass.) by random priming (Life Technologies, Gaithersburg, Md.). Probes were individually hybridized with membranes at 55°C in 6× SSC (1× SSC contains 0.15 M NaCl and 0.015 M sodium citrate)–0.5 g of nonfat dry milk per liter–0.1% sodium dodecyl sulfate–1 mM sodium pyrophosphate and washed at 55°C in 0.2× SSC.
PCR and Southern hybridization.
Similar conditions were used to PCR amplify target DNA prepared from B. burgdorferi N40, B31, PKo, PBi, and 25015. For PCR, annealing temperature was initially 55°C but was progressively decreased to 53 and 51°C for PKo, PBi, and 25015. Southern blotting was performed with the entire N40-dbpA gene hybridized against target DNA derived from each B. burgdorferi strain, using an enhanced chemiluminescence kit as specified by the manufacturer (Amersham Life Science, Buckinghamshire, England), at 42°C as hybridization and primary wash temperature.
Mouse hyperimmunization and challenge.
Mice were hyperimmunized subcutaneously with 20 μg of purified recombinant protein (GT-N40-DbpB, N40-DbpA, or GT) in complete Freund’s adjuvant and boosted twice at 14 and 28 days with 10 μg of protein in incomplete Freund’s adjuvant. Mice were bled 2 weeks after the last boost to test antibody reactivities. Serial 10-fold dilutions of sera were tested by immunoblotting using the respective recombinant proteins, and antibody reactivity was verified at a serum dilution of ≥1:100,000. Hyperimmunized mice were challenged with 104 cN40 spirochetes, intradermally. The median intradermal C3H mouse infectious dose of B. burgdorferi cN40 has been previously determined to be 1 to 10 spirochetes (6, 10). Mice were necropsied 2 weeks later, and tissues were cultured.
Immunoblots.
GT-N40-DbpB, N40-DbpA, GT, or B. burgdorferi lysates were resolved in sodium dodecyl sulfate–12% polyacrylamide gels by electrophoresis and transferred to nitrocellulose membranes, which were cut into strips, as described previously (7, 10). Strips were probed with immune sera or antisera to the appropriate recombinant protein. Secondary antibody was alkaline phosphatase-conjugated goat anti-mouse IgG (Stratagene).
Enzyme-linked immunosorbant assay (ELISA).
Recombinant GT-N40-DbpB, N40-DbpA, OspA, OspC, P39, or GT (200 μl of each at 1 μg/ml) in carbonate coating buffer (pH 9.6) were plated in 96-well plates (ICN Pharmaceuticals, Inc., Costa Mesa, Calif.) (18) for 90 min at room temperature and then washed with PBS–0.1% Tween 20 (PBST) three times. Triplicate samples of each mouse serum, including uninfected normal mouse serum as a control, were diluted 1:80 in PBST and added to the plates at 37°C for 45 min then washed three times with PBST. This serum dilution and antigen concentration were optimized for maximal sensitivity and specificity for immune serum compared to normal mouse serum, with at least 3 standard deviations (SD) above normal serum or control antigen background. Sheep anti-mouse IgM (μ chain specific) or IgG (whole molecule) linked with peroxidase (Sigma) (1:10,000) was added to the plates at 37°C for another 45 min. Plates were washed with PBST three times, and p-nitrophenyl phosphate (1 mg/ml) was added to the plates. Plates were incubated at 37°C for 1 h and read on a Titertek Multiskan (ICN, Costa Mesa, Calif.) with an optical density at 405 nm. Values were subtracted from background reactivity against GT for GT-N40-DbpB or with normal mouse serum for N40-DbpA, OspA, OspC, and P39.
Nucleotide sequence accession numbers.
N40-dbpB and N40-dbpA sequence data have been submitted to the GenBank nucleotide sequence database (accession no. U69553 and U63932, respectively).
RESULTS
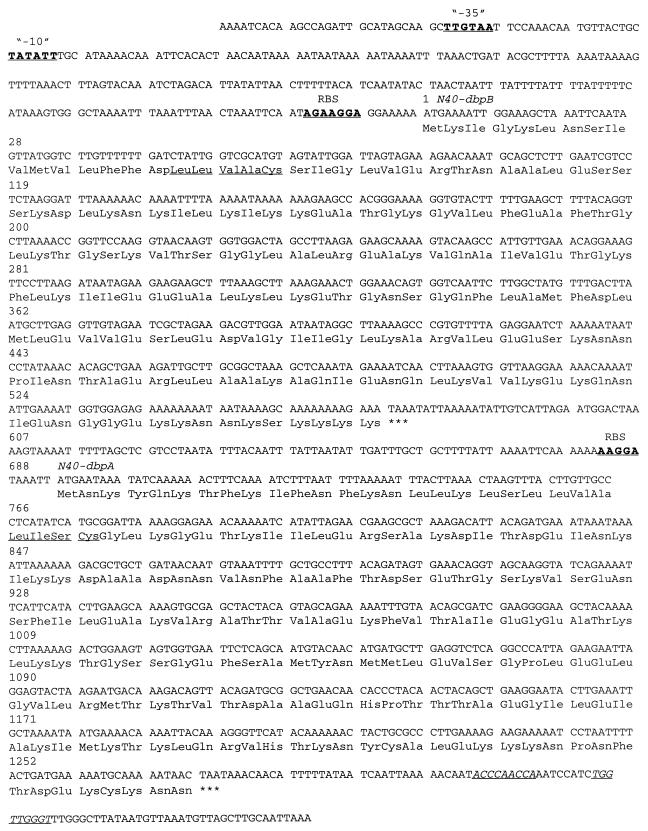
Immune sera were used to screen the B. burgdorferi N40 genomic expression library, yielding a large number of reactive clones. A positive clone was sequenced and analyzed. The sequence revealed a putative bicistronic operon encoding two genes with a presumed promoter −35 (TTGTAA) and −10 (TATATT) region located 233 and 208 bp, respectively, upstream from the start codon (Fig. 1). A putative ribosome binding site (AGAAGGA) was 7 bp upstream from the start codon. A second open reading frame was 122 bp downstream from the first open reading frame. There was a putative second ribosome binding site (AAGGA) 6 bp upstream from the start codon of the second open reading frame and a hairpin structure (a putative transcription terminator) 33 bp downstream from the stop codon of second open reading frame.
FIG. 1.
DNA and amino acid sequences of the immunoreactive clone containing a bicistronic operon encoding N40-dbpB and N40-dbpA. Putative −35 and −10 regions and putative ribosome binding sites (RBS) are indicated by underlining and boldface letters. Stop codons after N40-dbpB and N40-dbpA are indicated by asterisks. A possible hairpin structure at the end of the N40-dbpA sequence is indicated by underlining and italic letters. Signal peptidase II consensus sequences are underlined.
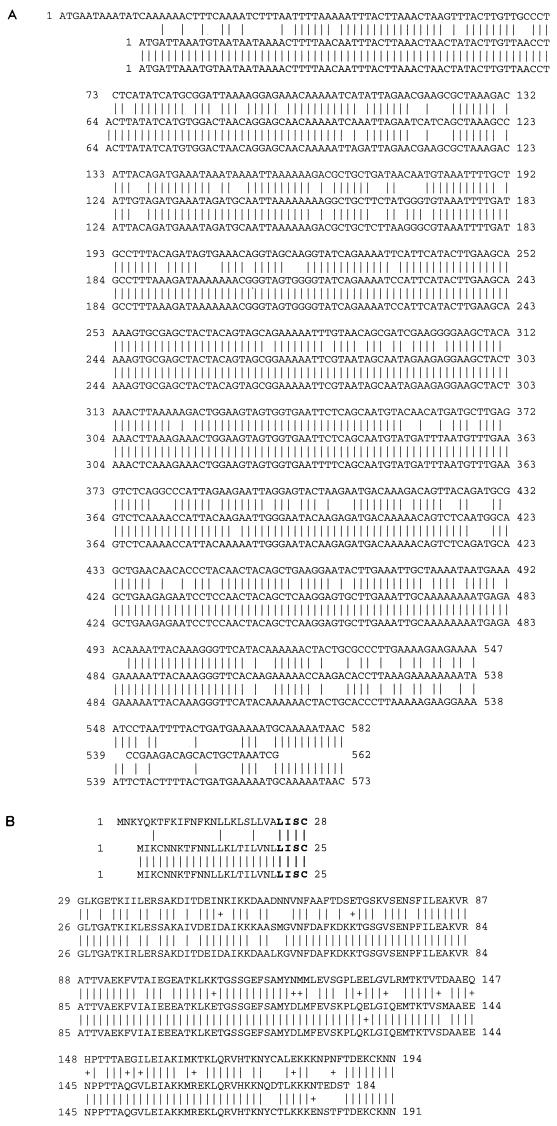
Sequence comparison with GenBank and with recently published nucleotide sequence data on B. burgdorferi B31 (27a) indicated that the first open reading frame (N40-dbpB) showed high identity with dbpB of strains 297 and B31 (both 99%) and the second open reading frame (N40-dbpA) showed high identity with dbpA of strains 297 (85%) and B31 (84%). N40-dbpA had significant differences from dbpA of B. burgdorferi 297 and B31, particularly in the leader sequence and the carboxyl terminus, whereas the central regions of the genes were conserved (Fig. 2). There was 30% identity on the DNA level and 25% similarity on the amino acid level between N40-dbpB and N40-dbpA. Both N40-DbpB and N40-DbpA had a predicted hydrophobic region in center of the N-terminus leader sequence, followed by signal peptidase consensus sequences of Leu-Leu-Val-Ala-Cys for N40-DbpB and Leu-Ile-Ser-Cys for N40-DbpA, suggesting that they may be lipoproteins. The pIs of N40-DbpB and N40-DbpA were 9.4 and 9.1, respectively, indicating they were both basic proteins. They were lysine rich, 15.5% in N40-DbpB and 13.9% in N40iDbpA, as found with other B. burgdorferi proteins, such as P55 (S1) (23), S2 (23), OspA and OspB (13, 30), and OspF (30).
FIG. 2.
Sequence comparison of DbpA of B. burgdorferi sensu stricto isolates N40 (top row), 297 (center row), and B31 (bottom row), based on data published in GenBank for 297 and in reference 27a for B31. (A) Nucleic acid comparison among dbpAs. The vertical lines between rows represent identity among all three strains. Empty spaces in sequence represent missing nucleotides compared to other strains. Note the difference in the N40 leader sequence. (B) Amino acid comparison among dbpAs. Vertical lines between rows represent identity among all three strains, whereas a + represents amino acid similarity between two strains. Bold print represents the signal peptidase II consensus sequence.
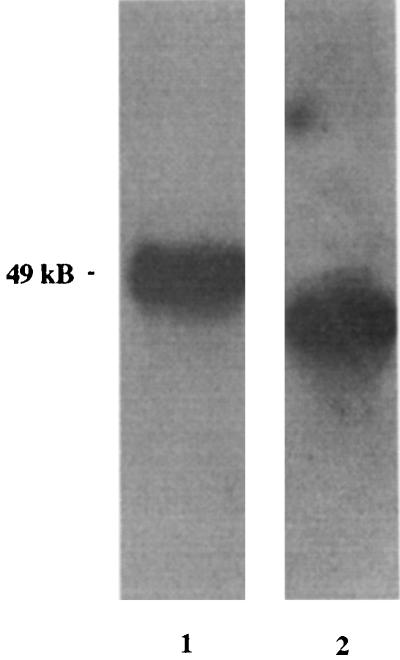
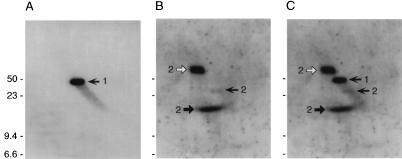
To determine where the N40-dbpB/A operon was located in the B. burgdorferi N40 genome, we performed pulsed-field gel electrophoresis with total B. burgdorferi cN40 DNA followed by Southern blotting, probing with N40-dbpA DNA and ospA DNA as the control. ospA hybridized with an approximately 49-kb plasmid, and N40-dbpA hybridized with a band slightly below the ospA-containing plasmid (Fig. 3). Next, a two-dimensional agarose gel subjected to Southern blotting was examined to determine if the plasmid containing N40-dbpA was linear or circular. Both linear and open circular DNAs are able to resolve the supercoiling added by the intercalated chloroquine due to their open ends. Supercoiled circular DNA is unable to resolve this change in supercoiling, resulting in an altered mobility in the second dimension. N40-dbpA DNA hybridized with a linear 48-kb plasmid (Fig. 4A and C). N40-dbpA hybridized only to a single linear plasmid, suggesting that there were no other homologs or related gene families located elsewhere within the N40 genome. On the other hand, the gene located 3′ of the p21 gene hybridized with three DNA species, indicative of the mobility patterns of circular plasmid DNA (Fig. 4B and C).
FIG. 3.
Southern blot of total B. burgdorferi N40 DNA separated by pulsed-field gel electrophoresis and probed with ospA (lane 1) and N40-dbpA (lane 2). ospA hybridized with a 49-kb plasmid, whereas N40-dbpA hybridized with a lower-molecular-size (ca. 48-kb) plasmid. Both lanes were cut from the same blot, with clear separation in molecular weights between hybridization products on lanes 1 and 2, indicating that ospA and N40-dbpA do not comigrate.
FIG. 4.
Southern blot analysis of B. burgdorferi N40 plasmids separated by two-dimensional agarose gel electrophoresis. (A) Hybridization with an N40-dbpA probe. The single DNA species is indicated by a left-facing arrow and a numeral 1. (B) Hybridization with a probe derived from a gene located immediately 3′ of the p21 gene, a gene located on a 32-kb circular plasmid. This probe hybridized with three DNA species, each labeled with a numeral 2: supercoiled circular plasmid (solid right-facing arrow); nicked, open circular plasmid (open right-facing arrow); and linearized plasmid (solid left-facing arrow). (C) Alignment of the blots shown in panels A and B. Note that the linear and open circular DNAs migrate on one axis, while supercoiled DNA migrates on a different axis. Sizes are indicated in kilobases.
PCR and Southern blotting were performed to evaluate if other B. burgdorferi sensu lato strains carry N40-dbpB- and N40-dbpA-homologous genes. Target DNA was prepared from cloned PKo (B. afzelii), PBi (B. garinii), 25015 (genomic group DN127), B31, and N40 (B. burgdorferi sensu stricto). Using primers defined above, N40-dbpB was PCR amplified from N40 under high-stringency conditions (55°C) and could be amplified from B31 at 53°C and PBi at 51°C. Further reduction of annealing temperatures could not amplify N40-dbpB from 25015 or PKo. On the other hand, N40-dbpA was PCR amplified from all five strains tested at 55°C. Because N40-dbpB could not be PCR amplified from 25015 or PKo, we next performed Southern blotting with the entire N40-dbpB as the probe. The N40-dbpB gene hybridized with all B. burgdorferi senso lato strains except PKo.
When recombinant GT-N40-DbpB and N40-DbpA were probed with 90-day immune sera by immunoblotting (data not shown), both were strongly reactive, suggesting that N40-DbpB and N40-DbpA were expressed during infection of mice. The infecting dose of spirochetes (102) used to generate the immune serum has been shown to be low enough that the input spirochetes were antigenically subliminal, and the ensuing antibody response represented response to antigens expressed by replicating spirochetes within mice (7). Antiserum to N40-DbpA reacted against a ca. 22-kDa protein in B. burgdorferi cN40 lysates that migrated below a protein of ca. 23 to 24 kDa reactive with antiserum to OspC and parallel with a ca. 22-kDa protein reactive with immune sera from actively infected mice (Fig. 5). Antiserum to GT-N40-DbpB reacted against a ca. 20-kDa protein in B. burgdorferi lysates that paralleled reactivity of immune serum to a protein of similar size. We next examined N40-DbpA expression in cultured N40 and whether the expression was constant through different passages. N40-DbpA antiserum was used to probe B. burgdorferi cN40 whole-cell lysates prepared from cN40 in vitro passages 4, 10, 30, and 50. N40-DbpA was consistently expressed throughout the different passages.
FIG. 5.
Immunoblots of B. burgdorferi lysates, derived from gels that separate 20- to 24-kDa proteins, probed with 90-day immune serum (lane 1) and antisera to OspC (lane 2), N40-DbpA (lane 3), and GT-N40-DbpB (lane 4). Note relationship of reactivity of different antisera against comigrating native B. burgdorferi proteins.
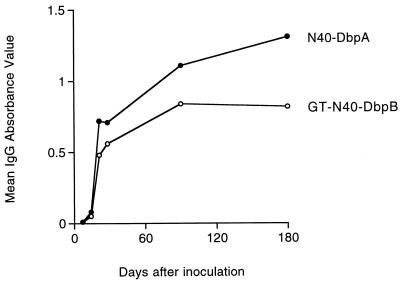
Seroconversion to N40-DbpB and N40-DbpA in infected mice was examined by ELISA. GT-N40-DbpB and N40-DbpA recombinant proteins were probed with sera collected from B. burgdorferi cN40-infected mice (infection verified by culture at each interval) at 0, 7, 14, 21, 28, 90, and 180 days (one mouse per interval) after inoculation with 103 spirochetes. IgM reactivity to GT-N40-DbpB and N40-DbpA was low (data not shown), but IgG reactivity to both GT-N40-DbpB and N40-DbpA rose rapidly during the course of infection, remaining high for 6 months (Fig. 6). Reactivity against GT-N40-DbpB appeared weaker than that against N40-DbpA by ELISA, but results must be interpreted with the caveat that the N40-DbpB antigen was diluted because of its GT fusion partner.
FIG. 6.
IgG ELISA response of B. burgdorferi-infected mice against GT-N40-DbpB and N40-DbpA at intervals of infection. Data points represent single mice per interval, but the same sera were reacted against each protein.
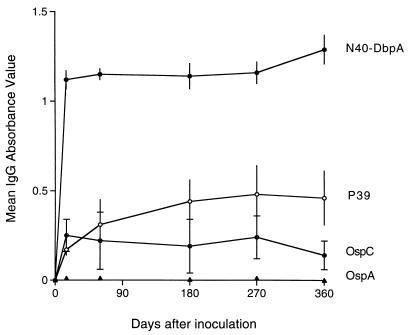
Individual sera from a larger series of culture-positive mice (5 mice on days 15 and 60, 27 mice on day 180, 23 mice on day 270, and 25 mice on day 360) inoculated with 103 B. burgdorferi cN40 cells were examined for reactivity to N40-DbpA compared to other hallmark B. burgdorferi cN40 recombinant proteins, including OspA, OspC, and P39. Antibody reactivity to N40-DbpA arose within 15 days, with maintenance of high reactivity throughout the course of persistent infection, relative to antibody reactivity to the other antigens (Fig. 7). As previously reported (10), mice infected with this dose of inoculum did not seroconvert to OspA. Reactivity to OspC was consistently evident but at considerably lower levels compared to N40-DbpA. Furthermore, there was variation in antibody reactivity to OspC among individual mice compared to the other antigens, exemplified by the standard deviations depicted in Fig. 7. Reactivity to P39 was intermediate.
FIG. 7.
IgG ELISA response of immune sera from B. burgdorferi-infected mice against N40-DbpA, relative to reactivity of the same sera against recombinant OspA, OspC, and P39, at intervals of infection. Data are represented as means ± SD.
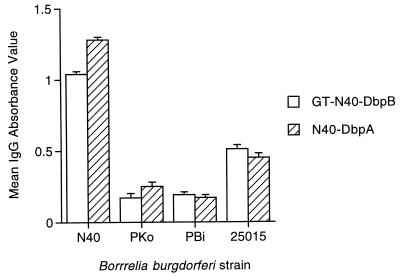
Reactivity of B. burgdorferi cN40-DbpA and GT-N40-DbpB among mice infected with genetically diverse strains of B. burgdorferi was examined by ELISA, using 90-day sera from culture-positive mice inoculated with 102 to 103 cN40, PKo, PBi, or 25015 cells. Mice infected with genetically distant PKo and PBi reacted against N40 GT-N40-DbpB and N40-DbpA but at significantly lower levels compared to N40-infected mice, and mice infected with 25015 reacted against GT-N40-DbpB and N40-DbpA with intermediate levels (Fig. 8), suggesting some degree of cross-reactivity but antigenic divergence among B. burgdorferi sensu lato isolates. Further studies are needed to examine the epitope specificity of these cross-reacting antibodies.
FIG. 8.
IgG ELISA response of immune sera from mice infected for 90 days with B. burgdorferi sensu lato strains PKo (B. afzelii), PBi (B. garinii), and 25015 compared to B. burgdorferi N40. Data represent means and SD derived from groups of five mice.
In an effort to incriminate N40-DbpB or N40-DbpA as a potential antigen capable of eliciting protective immunity against syringe-borne challenge with cultured spirochetes in immunized mice, we hyperimmunized mice against GT-N40-DbpB, N40-DbpA, or GT (Table 1). Hyperimmunization with N40-DbpA, but not GT-N40-DbpB (or GT), protected mice against syringe challenge with cultured B. burgdorferi cN40 spirochetes. Notably, the single N40-DbpA-hyperimmunized mouse that became infected upon challenge was culture positive only at the site of challenge inoculation. A confirmatory immunization experiment with N40-DbpA as the antigen was performed with a lower (103) intradermal challenge dose. We obtained similar results: one of five immunized mice was infected upon challenge. In contrast to the first experiment, the single infected mouse had culture-positive blood, urinary bladder, and inoculation site.
TABLE 1.
Protection of mice by hyperimmunization with N40-DbpA, but not GT-N40-DbpB or GT, against intradermal challenge of C3H mice with 104 B. burgdorferi spirochetes
| Immunogen | No. of positive samples/no. of cultured samples
|
|||
|---|---|---|---|---|
| Urinary bladder | Spleen | Inoculation site | Cumulativea | |
| Expt 1 | ||||
| N40-DbpA | 0/5 | 0/5 | 1/5 | 1/5 |
| GT | 3/4 | 1/4 | 3/3 | 4/4b |
| Expt 2 | ||||
| GT-N40-DbpB | 2/4 | 2/4 | 5/5 | 5/5 |
| GT | 4/5 | 2/5 | 5/5 | 5/5 |
All sites combined.
Chi square, P < 0.01 compared to N40-DbpA-immunized group.
DISCUSSION
We describe herein 20- and 22-kDa B. burgdorferi N40 proteins, encoded on a 48-kb linear plasmid that appears to be distinct from the 49-kb plasmid that bears ospA/B. Sequence comparisons with information available in GenBank for B. burgdorferi 297 and in the recently published genome of B. burgdorferi B31 (27a) indicate that these proteins represent the N40 version of dbpB and dbpA. In contrast to B. burgdorferi B31, wherein the dbpB/A operon is on the same linear plasmid as ospA/B, our data indicate that the N40-dbpB/A operon is located on a linear plasmid other than the one containing ospA/B. Since the genome of only one B. burgdorferi strain (B31) is known, further work is needed to examine the plasmid relationship of the dbpB/A operon with the ospA/B operon among other strains of B. burgdorferi sensu stricto, as well as sensu lato.
N40-DbpB and -A proteins both elicit strong and early antibody responses during the course of persistent infection, suggesting that they are coexpressed in the mammalian host. Our studies suggest that these proteins are expressed by B. burgdorferi within the conditions of in vitro culture as well as in the infected mouse. This is in keeping with the hypothesis that N40-DbpB and N40-DbpA comigrate with or migrate near other B. burgdorferi proteins, and reactivity of immune serum can be misinterpreted by presumed reactivity to other proteins, such as OspC (21). Indeed, reactivity in the ca. 20- to 24-kDa region can also be obscured by the known reactivity of immune serum to other ca. 20- to 24-kDa proteins (17, 29, 31, 32, 40, 47). Our results, albeit based on immune sera from mice infected with a single strain of B. burgdorferi, suggest that N40-DbpA may be preferable to OspC as a serodiagnostic recombinant protein because N40-DbpA (and N40-DbpB) elicits an early and relatively strong antibody response throughout persistent infection, whereas antibody to OspC is consistently present, but at considerably lower and variable levels, particularly during the persistent phase of infection.
It is becoming increasingly apparent that B. burgdorferi expresses proteins differentially under various environmental conditions. OspA is now known to be expressed in culture but is significantly lost or downregulated during tick feeding and in the mammalian host (15, 20), whereas OspC is expressed in culture and in flat ticks but is upregulated under different culture conditions, at elevated temperatures, during tick feeding, and in the mammalian host (34, 36). Additional proteins, exemplified by P35 and P37, are expressed exclusively in the mammalian host (27). A number of other proteins, including EppA (16), P21 (45), BbK2.10 (1), and P22 (46), likewise appear to be expressed in the mammalian host. N40-DbpB and N40-DbpA seem to represent proteins that are expressed preferentially, but not exclusively, in the mammalian host, analogous to P39 and OspC (34, 38).
Although immunoblots prepared with B. burgdorferi lysates can be labeled with OspC antiserum and immune sera, including Lyme disease patient sera, are reactive against recombinant OspC antigen (9, 48), our ELISA data suggest that N40-DbpA, although it does not comigrate identically with OspC, may be responsible for the strong 22-kDa reactivity observed on immunoblots attributed to OspC, particularly in blots in which the proteins in this region are not fully separated. Indeed, in a study examining the immunoblot profiles of naturally infected dogs, we found a high rate of reactivity to 22-kDa proteins, which was suspected to be due to OspC reactivity, but the sera failed to react or reacted inconsistently to recombinant OspC antigen (11). These observations warrant reexamination of immunoblot data with human or dog sera that have been presumed to be reactive against native 22-kDa proteins in B. burgdorferi lysates presumed to be OspC (21).
Our previous studies failed to demonstrate induction of protective activity with flagellin, P39, or OspC in mice challenged with cultured B. burgdorferi N40, whereas passive or active immunization against PKo OspC has been shown to be protective against PKo challenge in mice (9, 14) and gerbils (35). These results suggest that the protective activity that is readily demonstrable in immune sera from mice actively infected with B. burgdorferi N40 is directed against some other, undefined protein(s). We therefore tested the ability of recombinant N40-DbpB and N40-DbpA to induce a protective immune response in hyperimmunized mice and showed that mice actively immunized with recombinant N40-DbpA, but not N40-DbpB, resisted syringe-borne challenge inoculation with cultured N40 spirochetes.
These preliminary studies lend credence to the possibility that N40-DbpA represents an antigen responsible for protective immunity in immune sera from mice actively infected with B. burgdorferi N40. Whether N40-DbpA induces protective immunity against tick-borne challenge is beyond the scope of this study and remains to be determined. However, we recently found that immune serum, which can passively protect naive mice against high-dose syringe-borne challenge with cultured organisms, failed to protect mice challenged with host-adapted spirochetes (derived from in vivo-implanted chambers or skin transplants from infected donor mice) or by tick-borne inoculation (19). Despite these caveats, N40-DbpA appears to be a candidate for the one or more antigens involved in active infection that is responsible for eliciting protective immunity against syringe-borne cultured B. burgdorferi, whatever the relevance of that phenomenon may be to natural infection.
Further studies are needed to examine homologs of these proteins in other strains of B. burgdorferi sensu lato and the generality of their role in eliciting host protective immunity against challenge with cultured spirochetes during active infection. Indirect evidence (PCR amplification, Southern blotting, and serology) suggests that there appear to be homologs in distantly related strains of B. burgdorferi sensu lato, but that they are likely to be genetically and antigenically diverse. N40-DbpB and its gene appeared to possess greater diversity, as immune sera from mice infected with genetically distant B. burgdorferi isolates were least reactive to N40-DbpB relative to N40-DbpA. Furthermore N40-dbpB-specific primers did not amplify genes from DNA of distantly related B. burgdorferi isolates under stringent conditions, but N40-dbpA-specific primers did. Reduction of stringency (lower annealing temperatures) resulted in PCR amplification of distantly related PBi but not PKo. Southern blotting with the entire N40-dbpB gene revealed hybridization with all B. burgdorferi strains except PKo. Sequencing is obviously needed for direct comparison of gene homologs, but several lines of evidence suggest their presence. The fact that homologs appear to be present, that the genes are single genes within the genome (at least N40 and B31), and that they are located on large linear plasmids (at least N40 and B31) attest to the likelihood that these genes play an important role in the life of B. burgdorferi and can be possibly exploited for preventive or therapeutic immunity.
An intriguing discovery is that B. burgdorferi N40-DbpB and N40-DbpA possess a high degree of identity with B. burgdorferi 297 and B. burgdorferi B31 DbpB and DbpA. The gene products of B. burgdorferi 297 dbpA and dbpB were reported to be 20- and 19-kDa proteins (28), whereas N40-dbpA and N40-dbpB gene products are 22- and 20-kDa proteins. B. burgdorferi 297 DbpB and DbpA were discovered by ligand binding assays, whereas N40-DbpB and N40-DbpA were revealed through an entirely different approach, using genomic library screening with reactive immune serum. Although the gene products differ slightly in size, they are likely to be the same. Thus, we can now ascribe function (decorin binding) with a biologic effect (antibody-mediated protective immunity against cultured spirochetes), and our collective observations reinforce the importance of these proteins in Lyme disease.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI-26815 and AI-45253 from the National Institute of Allergy and Infectious Diseases and in part by the Mathers Foundation.
We thank Debby Beck, Rhonda Bangham, and Yen Zhang for technical support.
REFERENCES
- 1.Akins D R, Porcella S F, Popova T G, Shevchenko D, Baker S I, Li M, Norgard M V, Radolf J D. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol Microbiol. 1995;18:507–520. doi: 10.1111/j.1365-2958.1995.mmi_18030507.x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson J F, Barthold S W, Magnarelli L A. Infectious but nonpathogenic isolate of Borrelia burgdorferi. J Clin Microbiol. 1990;28:2693–2699. doi: 10.1128/jcm.28.12.2693-2699.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong A L, Barthold S W, Persing D H, Beck D S. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am J Trop Med Hyg. 1992;47:249–258. doi: 10.4269/ajtmh.1992.47.249. [DOI] [PubMed] [Google Scholar]
- 4.Assous M V, Postic D, Paul G, Nevot P, Baranton G. Individualisation of two genomic groups among American Borrelia burgdorferi sensu lato. FEMS Microbiol Lett. 1994;121:93–98. doi: 10.1111/j.1574-6968.1994.tb07081.x. [DOI] [PubMed] [Google Scholar]
- 5.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold S W. Infectivity of Borrelia burgdorferi relative to route of inoculation and genotype in laboratory mice. J Infect Dis. 1991;163:419–420. doi: 10.1093/infdis/163.2.419. [DOI] [PubMed] [Google Scholar]
- 7.Barthold S W, Bockenstedt L K. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect Immun. 1993;61:4696–4702. doi: 10.1128/iai.61.11.4696-4702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barthold S W, deSouza M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:951–971. [PMC free article] [PubMed] [Google Scholar]
- 9.Barthold S W, Feng S, Bockenstedt L K, Fikrig E, Feen K. Protective and arthritis-resolving activity in serum from mice infected with Borrelia burgdorferi. Clin Infect Dis. 1997;25:S9–S17. doi: 10.1086/516166. [DOI] [PubMed] [Google Scholar]
- 10.Barthold S W, Fikrig E, Bockenstedt L K, Persing D H. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63:2255–2261. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barthold S W, Levy S A, Fikrig E, Bockenstedt L K, Smith A L. Serologic response of naturally exposed or vaccinated dogs to Borrelia burgdorferi, the agent of Lyme borreliosis. J Am Vet Med Assoc. 1995;207:1435–1440. [PubMed] [Google Scholar]
- 12.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease following intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 13.Bergstrom S, Bundoc V, Barbour A G. Molecular analysis of linear plasmid encoded major surface proteins, OspA and OspB, of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 1989;3:479–485. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 14.Bockenstedt L K, Hodzic E, Feng S, Bourell K W, deSilva A, Montgomery R, Radolf J D, Barthold S W. Borrelia burgdorferi strain-specific OspC-mediated immunity in mice. Infect Immun. 1997;65:4661–4667. doi: 10.1128/iai.65.11.4661-4667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burkot T R, Piesman J, Wirtz R A. Kinetics of the Borrelia burgdorferi outer surface protein A (OspA) in the tick, Ixodes scapularis. In: Cevinini R, Sambri V, LaPlaca M, editors. Advances in Lyme borreliosis research. Proceedings of the VI International Conference on Lyme Borreliosis. Bologna, Italy: University of Bologna; 1994. pp. 224–227. [Google Scholar]
- 16.Champion C I, Blanco D R, Skare J T, Haake D A, Giladi M, Foley D, Miller J N, Lovett M A. A 9.0 kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect Immun. 1994;62:2653–2661. doi: 10.1128/iai.62.7.2653-2661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman J L, Benach J L. Characterization of antigenic determinants of Borrelia burgdorferi shared by other bacteria. J Infect Dis. 1992;165:658–666. doi: 10.1093/infdis/165.4.658. [DOI] [PubMed] [Google Scholar]
- 18.Craft J E, Grodzicki R L, Steere A C. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984;149:789–795. doi: 10.1093/infdis/149.5.789. [DOI] [PubMed] [Google Scholar]
- 19.deSilva A, Fikrig E, Hodzic E, Telford III S R, Barthold S W. Immune evasion by tick-borne and host-adapted Borrelia burgdorferi. J Infect Dis. 1998;177:395–400. doi: 10.1086/514200. [DOI] [PubMed] [Google Scholar]
- 20.deSilva A, Telford S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 22.Dunn J J, Lade B N, Barbour A G. Outer surface protein A (OspA) from the Lyme disease spirochete, Borrelia burgdorferi: high level expression and purification of a soluble recombinant form of OspA. Protein Expression Purif. 1990;1:159–168. doi: 10.1016/1046-5928(90)90011-m. [DOI] [PubMed] [Google Scholar]
- 23.Feng S L, Das S, Lam T, Flavell R A, Fikrig E. A 55-kilodalton antigen encoded by a gene on a Borrelia burgdorferi 49-kilobase plasmid is recognized by antibodies in sera from patients with Lyme disease. Infect Immun. 1995;63:3459–3466. doi: 10.1128/iai.63.9.3459-3466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferdows M S, Barbour A G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci USA. 1989;86:5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fikrig E, Barthold S W, Kantor F S, Flavell R A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- 26.Fikrig E, Barthold S W, Marcantonio N, Deponte K, Kantor F S, Flavell R A. Roles of OspA, OspB, and flagellin in protective immunity to Lyme borreliosis in laboratory mice. Infect Immun. 1992;60:657–661. doi: 10.1128/iai.60.2.657-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fikrig E, Barthold S W, Sun W, Feng W, Telford III S R, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 27a.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J-F, Fleishman R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 28.Guo B P, Norris S J, Rosenberg L C, Hook M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam T T, Nguyen T K, Fikrig E, Flavell R A. A chromosomal Borrelia burgdorferi gene encodes a 22-kDa putative lipoprotein (P22) that is serologically recognized in Lyme disease. J Clin Microbiol. 1994;32:876–883. doi: 10.1128/jcm.32.4.876-883.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam T T, Nguyen T K, Montgomery R R, Kantor F S, Fikrig E, Flavell R A. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeFebvre R B, Probert W S, Perng G-C. Characterization of a chromosomal gene and the antigen it expresses from the Lyme disease agent, Borrelia burgdorferi. J Clin Microbiol. 1993;31:2146–2151. doi: 10.1128/jcm.31.8.2146-2151.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luft B J, Jiang W, Munoz P, Dattwyler R J, Gorevic P D. Biochemical and immunological characterization of the surface proteins of Borrelia burgdorferi. Infect Immun. 1989;57:3637–3645. doi: 10.1128/iai.57.11.3637-3645.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathiesen D A, Oliver J H, Kolbert C P, Tullson E, Johnson B J, Campbell G L, Mitchell P D, Reed K D, Telford III S R, Anderson J F, Lane R S, Persing D H. Genetic heterogeneity of Borrelia burgdorferi in the United States. J Infect Dis. 1997;175:98–107. doi: 10.1093/infdis/175.1.98. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery R A, Feen K, Malawista S E, Bockenstedt L K. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preac-Mursic V, Wilske B, Patsouris E, Javris S, Will S, Southchek E, Rainhardt S, Lehert S, Klockmann U, Mehrain P. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against B. burgdorferi infection. Infection. 1992;20:342–349. doi: 10.1007/BF01710681. [DOI] [PubMed] [Google Scholar]
- 36.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of outer surface protein on Borrelia burgdorferi during tick-feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sears J E, Fikrig E, Nakagawa T Y, Deponte K, Marcantonio N, Kantor F S, Flavell R A. Molecular mapping of OspA-mediated immunity against Borrelia burgdorferi, the agent of Lyme disease. J Immunol. 1991;147:1995–2001. [PubMed] [Google Scholar]
- 38.Simpson W J, Burgdorfer W, Schrumpf M E, Karstens R H, Schwan T G. Antibody to a 39-kilodalton Borrelia burgdorferi antigen (P39) as a marker for infection in experimentally and naturally inoculated animals. J Clin Microbiol. 1991;29:236–243. doi: 10.1128/jcm.29.2.236-243.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson W J, Cieplak W, Schrumpf M E, Barbour A G, Schwan T G. Nucleotide sequence and analysis of the gene in Borrelia burgdorferi encoding the immunogenic P39 antigen. FEMS Microbiol Lett. 1994;119:381–388. doi: 10.1111/j.1574-6968.1994.tb06917.x. [DOI] [PubMed] [Google Scholar]
- 40.Simpson W J, Schrumpf M E, Hayes S F, Schwan T G. Molecular and immunological analysis of a polymorphic periplasmic protein of Borrelia burgdorferi. J Clin Microbiol. 1991;29:1940–1948. doi: 10.1128/jcm.29.9.1940-1948.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 42.Stevenson B, Bockenstedt L K, Barthold S W. Expression and gene sequence of outer surface protein C of Borrelia burgdorferi reisolated from chronically infected mice. Infect Immun. 1994;62:3568–3571. doi: 10.1128/iai.62.8.3568-3571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevenson B, Casjens S, van Vugt R, Porcella S F, Tilly K, Bono J L, Rosa P. Characterization of cp18, a naturally truncated member of the cp32 family of Borrelia burgdorferi plasmids. J Bacteriol. 1997;179:4285–4291. doi: 10.1128/jb.179.13.4285-4291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevenson B, Tilly K, Rosa P A. A family of genes located on four separate 32-kilobase circular plasmids in Borrelia burgdorferi B31. J Bacteriol. 1996;178:3508–3516. doi: 10.1128/jb.178.12.3508-3516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suk K, Das S, Sun W, Jwang B, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallich R, Simon M M, Hofmann H, Moter S E, Schaible U E, Kramer M D. Molecular and immunological characterization of a novel polymorphic lipoprotein of Borrelia burgdorferi. Infect Immun. 1993;61:4158–4166. doi: 10.1128/iai.61.10.4158-4166.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilske B, Fingerle V, PreacMursic V, JaurisHeipke S, Hoffman A, Loy H, Pfister H W, Rossler D, Soutschek E. Immunoblot using recombinant antigens derived from different genospecies of Borrelia burgdorferi sensu lato. Med Microbiol Immunol. 1994;183:43–59. doi: 10.1007/BF00193630. [DOI] [PubMed] [Google Scholar]